Abstract
Tumor metastasis is the major cause of mortality from cancer. Metabolic rewiring and the metastatic cascade are highly intertwined, co-operating to promote multiple steps of cancer metastasis. Metabolites generated by cancer cells influence the metastatic cascade, encompassing epithelial-mesenchymal transition (EMT), survival of cancer cells in circulation, and metastatic colonization at distant sites. A variety of molecular mechanisms underlie the prometastatic effect of tumor-derived metabolites, such as epigenetic deregulation, induction of matrix metalloproteinases (MMPs), promotion of cancer stemness, and alleviation of oxidative stress. Conversely, metastatic signaling regulates expression and activity of rate-limiting metabolic enzymes to generate prometastatic metabolites thereby reinforcing the metastasis cascade. Understanding the complex interplay between metabolism and metastasis could unravel novel molecular targets, whose intervention could lead to improvements in the treatment of cancer. In this review, we summarized the recent discoveries involving metabolism and tumor metastasis, and emphasized the promising molecular targets, with an update on the development of small molecule or biologic inhibitors against these aberrant situations in cancer.
Subject terms: Cancer metabolism, Metastasis
Introduction
It has been appreciated since the early days of oncology research that the metabolic profiles of tumor cells differ from normal cells. Thompson et al. organized known cancer-associated metabolic phenotypes into six hallmarks: (1) dysregulated uptake of glucose and amino acids, (2) use of opportunistic modes of nutrient acquisition, (3) use of glycolysis/TCA cycle intermediates for biosynthesis and NADPH production, (4) increased demand for nitrogen, (5) alterations in metabolite-driven gene regulation, and (6) metabolic interactions with the microenvironment [1]. Cancer cells have high metabolic demands and rewire cellular metabolism to support malignant properties.
Cancer metastasis is the primary cause of cancer-associated mortality. However, our current understanding of the biology of metastases and development of druggable targets against tumor metastasis lags behind that for primary cancers. Metastasis is a multistep process consisting of a series of sequential events: (1) local invasion into the tumor-associated stroma, (2) intravasation into hematopoietic, lymphatic systems or peritoneum, (3) survival in circulation, (4) extravasation into pre-metastatic niches at distant organs, and (5) colonization to form metastases [2, 3]. Metastatic sites often present different metabolic challenges for the cancer cells with respect to nutrient and oxygen availability, as well as oxidative stress [4].
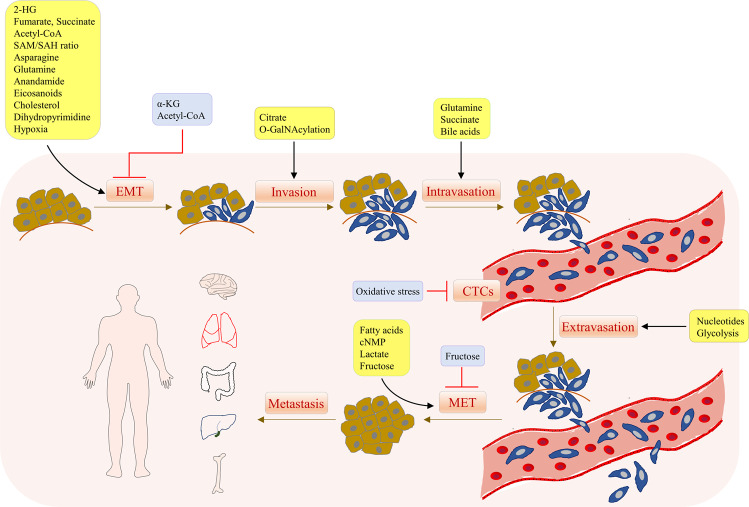
Emerging evidence indicates a complex interplay between tumor metastasis and metabolic rewiring in cancer (Fig. 1). Metabolic rewiring could drive metastasis by (1) generating oncometabolites to hijack metastatic signaling cascades via regulating gene expression, (2) generating metabolites/cofactors that act as agonists/antagonists for functional proteins involved in metastasis, and (3) modulating metabolic demands of cancer cells, thus allowing adaptation to the various stages of metastatic cascade. On the other hand, metastatic-associated signaling can influence cellular metabolism by directly affecting the expression and activity of metabolic enzymes. In this review, we provide a summary of molecular mechanisms linking cancer metastasis and cell metabolism, their underlying roles in metastasis and highlight the potential metabolic targets that could be harnessed to suppress cancer metastasis.
Fig. 1. Metabolites modulate the tumor metastasis cascade.
Overall summary of the role of metabolites in different stages of the tumor metastasis cascade, including epithelial-mesenchymal transition (EMT), invasion, intravasation, survival in circulation, extravasation and outgrowth into detectable metastasis.
Effects of metabolic rewiring on metastatic signaling cascades in cancer
Metabolites regulating epithelial-mesenchymal transition to facilitate tumor invasion
Epithelial-Mesenchymal transition (EMT) is a critical initiating step in the metastatic cascades. Epithelial cells that underwent EMT lose their polarity and gain the ability to infiltrate neighboring tumor-associated stroma. EMT is characterized by loss of epithelial marker E-cadherin, together with increased mesenchymal markers vimentin and N-cadherin [5]. EMT is primarily driven by the transcription factors SNAIL1/2, zinc-finger E-box-binding (ZEB1/2), and basic helix-loop-helix transcription factors (TWIST1/2) that repress epithelial marker genes whilst inducing expression of mesenchymal markers. These transcription factors are overexpressed in response to extracellular mitogenic signals. Tumor microenvironment, such as inflammation and hypoxia [5, 6], are also triggers for EMT. Below we outlined metabolites that could modulate EMT and drives the initial steps of metastatic progression.
2-hydroxyglutarate (2-HG)
2-HG is an oncometabolite identified to accumulate in tumors harboring mutations in isocitrate dehydrogenase 1 or 2 (IDH1/2). IDH1/2 are TCA cycle enzymes that catalyzes a two-step reaction for oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG). IDH1 (R132) or IDH2 (R140/R172) mutations abolish their original catalytic activity but are accompanied with gain-of-function in conversion of α-KG to 2-HG, leading to very high 2-HG levels (5–35 mM) [7]. Some IDH1/2-wildtype tumors evolved alternative mechanisms for 2-HG production. In breast cancer, glutaminolysis drives L-2-HG biosynthesis via wildtype IDH2. L-2-HG can also be derived from α-KG via lactate dehydrogenase 1 or malate dehydrogenase at acidic pH [8]. Besides, deficiency of 2-hydroxyglutarate dehydrogenases elevates 2-HG by preventing its conversion back to α-KG [8].
2-HG is structurally similar to α-KG and behaves as a competitive antagonist to inhibit α-KG-dependent dioxygenases (>60) involved in diverse biological functions [9, 10]. The roles of 2-HG in epigenetic deregulation have received intensive attention. 2-HG inhibits the activity of DNA demethylases (Ten-eleven Translocation, TET) and histone demethylases (Jumonji C, e.g., KDMs), leading to aberrant DNA and histone methylation. These α-KG-dependent dioxygenases have Km for α-KG at physiological concentrations, making their activities susceptible to fluctuations in α-KG or 2-HG [11]. Recent work has shown that 2-HG-triggered DNA and histone methylation are involved in tumor metastasis.
2-HG is an inducer ZEB1-mediated EMT [12]. Comparison of isogenic cell lines expressing mutant or wildtype IDH1/2 revealed that cancer cells expressing mutant IDH1/2 exhibited reversible EMT phenotype with a fibroblast-like morphology, along with reduced E-cadherin but increased levels of fibronectin, vimentin and N-cadherin. Exogenous 2-HG recapitulated induction of EMT in IDH1/2 wildtype cells, implying a direct role for this metabolite in EMT. ZEB1 knockdown or miR200 re-expression reverted IDH1/2-mutant cells to an epithelial phenotype, suggesting that the effect of 2-HG on EMT is dependent on ZEB1/miR-200 pathway. Colvin et al. [8] showed that D-2HG, but not L-2HG, increased the trimethylation of H3K4, an active transcription mark, at ZEB1 promoter, leading to ZEB1 expression and EMT. Accordingly, higher D-2HG in colorectal tumors was associated with distant organ metastasis. 2-HG thus promotes a prometastatic phenotype via epigenetic activation of ZEB expression.
Succinate and fumarate
Succinate and fumarate are also competitive antagonists for α-KG-dependent dioxygenases. Inactivating mutations of fumarate hydratase (FH) and succinate dehydrogenase (SDH) drive the accumulation of fumarate and succinate, respectively. SDH catalyzes the oxidation of succinate to fumarate. Mutations in SDH complex are found in gastrointestinal stromal tumors, renal cell carcinoma, phaeochromocytomas syndrome (PCC), or paraganglioma (PGL), leading to succinate accumulation [9]. FH mediates reversible conversion between fumarate and malate. FH mutations can be found in renal cell carcinoma, where its loss-of-function leads to elevated levels of fumarate [9].
Recent evidence showed that succinate and fumarate accumulation contribute to SNAIL-, TWIST- and ZEB-mediated EMT via the inhibition of DNA and/or histone demethylases. In PCC, PGL and ovarian cancers, SDHB mutation or knockdown led to histone methylation and increased expression of EMT markers LOXL2, TWIST1/2, TCF3, and metalloproteases (MMPs) [13–15]. In CRC cells, SDHB knockdown also activated TGF-β signaling and SNAIL-dependent EMT [16]. Sciacovelli et al. [17] revealed that FH-deficient cells displayed features of EMT. Mechanistically, fumarate suppresses DNA demethylases, resulting in CpG methylation of an antimetastatic microRNA-200ba429 cluster. Transcriptional silencing of miR-200 cluster induced expression of vimentin, SNAIL2, ZEB1, and ZEB2. Apart from epigenetic alterations, increased fumarate upon FH loss in hereditary leiomyomatosis and renal cell cancer (HLRCC) modulated NFE2-related factor 2, which promoted EMT via activation of NOTCH1 [18–20]. Indeed, FH-deficient HLRCC is associated with tumor metastasis. Fumarate and succinate are thus pro-metastastic metabolites that drives EMT through epigenetic dysregulation and activation of EMT-associated signaling pathways.
α-Ketoglutarate
α-KG functions as a metastasis suppressor through counteracting effects of 2-HG, succinate, or fumarate. α-KG dehydrogenase complex is the key modulator of cellular α-KG by catalyzing conversion of α-KG to succinyl-CoA. Up-regulation of α-KG dehydrogenase in breast cancer confers oncogenic properties as it diminished α-KG levels. Conversely, inhibition of this enzyme mediate α-KG accumulation to suppress tumor progression and metastasis in p53−/− pancreatic ductal adenocarcinoma and breast cancer [21, 22]. Mechanistically, α-KG promotes TETs-mediated promoter demethylation and re-expression of antimetastatic miR-200 cluster, thereby down-regulating ZEB1/CtBP1-MMP3 axis and EMT phenotype [22]. Collectively, these studies highlight the α-KG-to-succinate/fumarate/2-HG ratios as pivotal regulators of EMT phenotype and metastasis in multiple cancer types.
Acetyl-CoA
Acetyl-CoA is an important intermediate at the crossroads of glycolysis, glutaminolysis, and fatty acid metabolism. Cytosolic and nuclear acetyl-CoA levels are maintained by four metabolic pathways: (1) direct synthesis from acetate and CoA via acetyl-CoA synthetase short-chain family (ACSS); (2) conversion from citrate to acetyl-CoA by ATP citrate lyase (ACLY); (3) its catabolism to acetate and CoA by acyl-CoA thioesterase (ACOT); and (4) irreversible carboxylation from Acetyl-CoA to malonyl-CoA by Acetyl-CoA carboxylase (ACC) [9]. Deregulation of acetyl-CoA has been reported in multiple cancers [23–33], and it contribute to malignant phenotypes.
Acetyl-CoA is involved in epigenetic regulation by functioning as a cofactor for histone acetyltransferase (HATs). Intracellular acetyl-CoA levels fall within Km range of HATs. Increased Acetyl-CoA triggers metastasis by inducing histone acetylation, leading to an open chromatin permissive for gene expression. Acetyl-CoA was shown to be involved in hepatocellular carcinoma (HCC) metastasis [24]. ACOT12, which catabolizes acetyl-CoA, was silenced in HCC and associated with metastasis and poor prognosis [24]. In HCC cells, ACOT12 knockdown induced acetyl-CoA levels, which promoted histone acetylation via KAT2A to induce TWIST2 expression. TWIST2 in turn up-regulated N-cadherin and reduced E-cadherin, thereby contributing to EMT and metastasis in animal models. Acetate addition to boost acetyl-CoA also promoted TWIST2-mediated EMT, underscoring the role of this metabolite in HCC metastasis [24]. In breast cancer, TGF-β signaling was shown to induce ACC1 phosphorylation and inactivation, thus, preventing acetyl-CoA flux toward fatty acid biosynthesis and resulting in elevated acetyl-CoA [26, 34]. Acetyl-CoA directly promoted acetylation and nuclear translocation of Smad2 transcription factor to mediate SNAIL1/2, EMT, and tumor metastasis. In line with the role of acetyl-CoA in EMT induction, silencing of ACLY to abolish acetyl-CoA biosynthesis was sufficient to reverse EMT markers induced by ACC1 silencing, accompanied by blunted invasive capacity [26]. Contrary to the above results, ACSS2 overexpression in HCC was found to mediate acetylation of HIF-2α to inhibit EMT under hypoxia [35]. Therefore, the effect of acetyl-CoA is dependent on its target proteins and the tumor microenvironment.
SAM/SAH ratio
DNA (DNMTs) and histone methyltransferases (HMTs) utilize a common methyl donor: S-adenosylmethionine (SAM), a product of one-carbon cycle. Donation of methyl group from SAM releases S-adenosyl-homocysteine (SAH), an inhibitor of DNMTs/HMTs. SAM/SAH ratio dictates methyltransferase activities [9].
SAM was shown to suppress tumor cell invasion in vitro and tumor metastasis in vivo by inducing promoter methylation and silencing of prometastatic genes such as urokinase-type plasminogen activator (uPA) [36]. This inhibited the activation of its receptor uPAR and downstream ERK, PI3K/Akt, Src, and Rac1 [36]. SAM/SAH ratio also influences histone methylation. Nicotinamide N-methyltransferase (NNMT) requires SAM as a methyl donor for converting nicotinamide to 1-methylnicotinamide. NNMT overexpression resulted in SAM depletion and substantially inhibited histone methylation at H3K4, H3K9, H3K27, and H4K20 [9]. Decreased histone methylation then regulated signaling pathways associated with acquisition of a more aggressive phenotype. Consistently, NNMT knockdown decreased expression of EMT-related genes MMP9, SNAI2, vimentin, WNT5B, ZEB1/2; and suppressed cell invasion and migration in squamous cell carcinoma [37]. Aberrant methylation of DNA/histone driven by SAM thus has contrasting effects on EMT to that induced by demethylation blockade via 2-HG/succinate/fumarate.
Asparagine and glutamine
Asparagine and glutamine are nonessential amino acids; however, cancer cells demonstrate acquired dependence on these two amino acids. Asparagine synthetase (ASNS) mediates the de novo synthesis of asparagine from aspartate and it is up-regulated in multiple cancers [38]. Utilizing a focused shRNA library, Knott et al. [39] identified ASNS as the top essential gene for 4T1 breast cancer cell migration in vitro and lung metastasis in vivo. Silencing of ASNS reduced intracellular asparagine and suppressed cell invasion, an effect rescued by asparagine. Asparagine was shown to promote EMT via up-regulation of TWIST. Treatment with L-asparaginase or dietary asparagine restriction suppressed breast cancer metastasis in vivo, whilst excess dietary asparagine or ASNS ectopic expression exacerbated tumor metastasis. The effect of asparagine is specific to metastasis, as growth of the primary tumor was not affected.
Asparagine also facilitates the expression of glutamine synthetase (GLUL), which sustains cell proliferation and protein synthesis by de novo glutamine biosynthesis [40]. While glutamine is readily available in the circulation, its levels in metastatic niche is low. By promoting GLUL, asparagine mediated tumor cell survival in the distant organs and promoted outgrowth to form metastasis [40, 41]. Up- regulation of GLUL could also directly induce EMT in HCC [42]. Hence, asparagine and glutamine might function co-operatively to fuel tumor metastasis.
Anandamide
Anandamide (AEA) is an unsaturated fatty acid derivative derived from arachidonic acid (AA). AEA turnover is regulated by fatty acid amide hydrolase (FAAH) that degrades AEA to ethanolamine and AA. FAAH is up-regulated in CRC, prostate, and lung cancers, and it drives tumorigenic phenotypes [43]. Inhibition of FAAH or AEA addition exerted an inhibitory effect on Wnt/β-catenin mediated EMT in breast cancer, suggesting AEA as an antimetastatic metabolite [44].
Eicosanoids
Eicosanoids such as prostaglandins, thromboxanes, leukotrienes, lipoxins, HETEs and EETs, are signaling molecules derived from AA via the action of cyclo-oxygenases (COX), lipoxygenases and cytochrome P450 epoxygenases. As ligands of peroxisome proliferators-activated receptors (PPAR), AEA, and eicosanoids both activate PPARα, PPARβ/δ, and PPARγ, which bind to peroxisome proliferator hormone response elements (PPREs) of SNAILs, ZEBs or TWISTs to regulate their expression and control tumor metastasis [45–52].
Prostaglandin E2 (PGE2) is a key proinflammatory PG. PGE2 upregulated TAMs and MDSCs contributed to immunosuppression and EMT-mediated lung metastasis to mouse lungs [53]. Apart from modulating EMT, PGE2 derived from COX-2 induced expression of MIR675–5p, which inhibited p53 and promoted CRC metastasis [54]. PGE2 is involved in metastatic lymphangiogenesis in breast cancer [55]. PGE2 also induced CSC markers expression and promoted EP4/NF-κB-mediated liver metastasis [56]. PGE2 thus have diverse effects on different stages of metastasis. Consistent with prometastatic effect of PGE2, combination of a selective COX-2 inhibitor, celecoxib, and VEGF inhibitor Axitinib, caused a striking decrease in the metastasis of HCT116 cells to the liver in mice models [57].
Cholesterol
Cholesterol, together with lipids, forms lipid rafts on the cell membrane to regulate the activities of cell surface receptors. Reduced membrane fluidity due to altered cholesterol flux reduced cell motility, stem cell-like properties, and EMT, thus, suppressing tumor cell metastasis in vivo [58]. Concordantly, cholesterol treatment in vitro induced mesenchymal-like morphological features of metastatic prostate cancer cells in vitro, and cholesterol-fed mice formed markedly more liver metastatic nodules than mice fed normal chow diet [59]. Mechanistically, cholesterol induced lipid-rafts stabilize adipocyte plasma membrane-associated protein, which together with epidermal 35 growth factor receptor substrate 15-related protein (EPS15R), reduced endocytosis-mediated EGFR degradation. EGFR in turn activated ERK1/2 to trigger EMT. Targeting of HMG-CoA reductase (HMGCR), a rate-limiting enzyme for cholesterol biosynthesis, greatly down-regulated spontaneous lung metastasis by suppressing expression of MMPs [60].
Dihydropyrimidine
Dihydropyrimidine dehydrogenase (DPYD) is the rate limiting enzyme for pyrimidine degradation by catalyzing the breakdown of uracil/thymine to dihydrouracil [DHU] and dihydrothymine [DHT]. A comprehensive analysis of 1704 metabolic genes in 978 human cancer cells, followed by functional analysis using an RNAi library identified that DPYD is associated with a mesenchymal phenotype [61]. DPYD depletion abrogated EMT in HMLE cells expressing Twist, as exemplified by suppression of mesenchymal markers Vimentin, N-cadherin, ZEB1/2, and SNAIL1/2. DPYD knockdown abrogated EMT-induced invasion of lung tissues by HMLE-Twist cells, suggesting that DPYD is essential for EMT-induced metastasis. Notably, DPYD positively regulated intracellular dihydropyrimidine-to-pyrimidine ratio that correlates with mesenchymal character. Dihydropyrimidines supplementation restored EMT in DPYD depleted cells, confirming their direct involvement in EMT [61]. DPYD was overexpressed in HCC cells with high metastatic potential, and gain-/loss-of-function studies confirmed the role of DPYD in HCC metastasis [62]. Consistently, expression of DPYD is associated with poor survival of HCC patients. Nevertheless, mechanisms whereby dihydropyrimidine induce EMT remains unknown. Further investigation is required to identify the direct molecular target of these metabolites.
Hypoxia
In solid tumors, hypoxia, or low oxygen tension, is a hallmark feature of the tumor microenvironment arising from an imbalance between increased consumption of oxygen by cancer cells combined with inadequate oxygen supply. In response to hypoxia, hypoxia-inducible factors (HIFs) and their downstream gene networks are triggered to allow adaptation to low oxygen conditions [63, 64]. HIF-1α is a driving factor for tumor angiogenesis and mediates EMT-associated metastasis [63]. HIF-1α induced EMT through increased expression of vimentin, fibronectin, N-cadherin, snail, twist, TIMP-1 and -2, and pERK in mouse fibroblasts [65]. HIF-1 transactivates EMT inducing factors, TWIST and ZEB1, by binding directly to hypoxia-response element (HRE) in the promoters of TWIST and ZEB1 [66, 67]. Repression of TWIST under hypoxia or in HIF1α-overexpressing cells prevented EMT and abolished metastatic phenotypes [66, 68]. ZEB1 inhibition abrogated HIF-1α-induced EMT and invasion in colorectal cancer cells [67], inferring a critical role of this transcription factor in mediating its prometastatic effect. Moreover, HIF signaling indirectly promotes EMT through induction of Notch, TGF-β, integrin-linked kinase, Wnt, Hedgehog, and AXL receptor tyrosine kinase signaling [63]. Accordingly, hypoxia and HIF-1α are major promoting factors in EMT-mediated metastasis.
Metabolites that modulate MMPs and invasive front infiltration
Matrix metalloproteinase (MMP) is one of major players to facilitate local invasion and invasive front infiltration [3]. The endothelial basement membrane (BM) presents a mechanical barrier that prohibits cancer cells infiltration into neighboring stroma and extravasation after cell engraftment at distant organs [3]. MMPs, proteinases that degrade ECM proteins, are crucially involved in overcoming BM barrier [69]. Cancer cells up-regulate MMPs to promote uncontrolled proteolysis and degradation of BM, thereby promoting infiltration [3]. Citrate and O-Glycosylation are two metabolites that modulate MMPs to promote metastasis [3, 70, 71], as described below.
Citrate
Up-regulation of phosphofructokinase (PFKP) and citrate synthase (CS) were found to enhance the activity and expression of MMP2 and MMP9 to promote breast cancer metastasis [71]. PFKP induced glycolysis, which in concert with CS activity to generate excess intracellular citrate. Citrate accumulation via PFKP, CS, or exogenous citrate triggers AKT/ERK signaling to up-regulate MMP2 and MMP9 expression, leading to enhanced cell invasion and metastasis of triple-negative breast cancer [71].
O-GalNAcylation
O-GalNAcylation is a post-translational modification critical for MMPs activity. O-N-acetylgalactosamine transferases (GALNTs), which is frequently overexpressed in cancers [70, 72], catalyzes protein O-GalNAcylation involving the attachment of O-linked GalNAc to serine or threonine residues of secreted proteins or cell surface mucins to form N-acetylgalactosamine (Tn carbohydrate antigen) [73, 74]. The core 1 synthase, glycoprotein-N-acetylgalactosamine 3-beta-galactosyltransferase 1 (C1GALT1) catalyzes the second step of O-glycosylation by addition of galactose to Tn that forms core 1 carbohydrate structure [73]. Contrary to GALNTs, C1GALT1 is inactivated in cancer [72–75]. Increased GALNTs or the loss of C1GALT1 cause enhanced O-GalNAcylation and Tn in tumors [70], which in turn modulates MMPs [76]. Overexpression of GALNT14 resulted in up-regulation of MMP-2 in MCF-7 cells, while silencing of GALNT14 suppressed MMP-2 [72]. O-GalNAcylation also modifies MMP-14 activity. In liver tumors, expression of an ER-targeted GALNT1 increased Tn and enabled efficient matrix degradation and tissue invasion of cancer cells via O-GalNAcylation of MMP14 [70]. Both elevated GALNTs or dysregulated C1GALT1 could lead to hyper-O-GalNAcylation of MMPs and are common features of metastatic cancers.
Pro-angiogenesis metabolites promoting intravasation into hematopoietic systems
Intravasation is governed by invasive capacities of cancer cells and tumor-associated angiogenesis, which are typically leaky with high permeability. Hypoxic and nutrient deficient microenvironment is a major trigger for tumor-driven angiogenesis [77]. Succinate and glutamine are metabolites with known associations with angiogenesis.
Glutamine
M2-like macrophages (TAMs) are commonly associated with tumors and they possess pro-angiogenic, immunosuppressive and metastatic function. Palmieri et al. [78] demonstrated that glutamine metabolism in macrophages plays a key role in macrophage polarization and promotion of tumor metastasis. Inhibition of glutamine synthetase (GS) reversed M2 phenotype (TAMs) and promoted polarization to the M1 phenotype. GS blockade reduced intracellular glutamine, and glutamine uptake was channeled toward succinate biosynthesis through gamma-aminobutyric acid shunt. Succinate stabilized HIF-1α to enhance glycolysis flux and promote reversion to M1 phenotype. Macrophage-specific deletion of GS in macrophages promoted cytotoxic T-cell infiltration, reduced angiogenesis, and suppressed tumor metastasis, indicating that targeting GS in macrophages might be useful for metastasis treatment.
Succinate
In contrast to its antimetastatic role in macrophages, succinate promotes angiogenesis via two mechanisms. First is by stabilization of HIF in cancer cells through inhibition of HIF prolyl-hydroxylase, an α-KG-dependent dioxygenase [79]. This leads to HIF1α activation and consequent up-regulation of HREs target genes involved in angiogenesis, such as VEGF, VEGFR1/2, PLGF-A, PDGF-B, FGF, MMPs, and Angiopoietin-1/2 [80]. The second mechanism involved activation of the Succinate Receptor 1 (SUNCR1), a G-protein coupled receptor for succinate. Upon binding to succinate, SUNCR1 up-regulates VEGF by activating ERK1/2 and STAT3 signaling in a HIF1α-independent manner [13]. Accordingly, succinate may exert a pro- or anti-angiogenic effect and tumor metastasis depending on the cellular context of succinate accumulation.
Lymphangiogenesis leading to intravasation into lymphatics systems
Emerging evidence indicates that lymphatic vessels density in the vicinity of primary tumors correlates with lymph node metastasis. Mechanistic studies highlighted that HIF-1α is a major transcription factor that induces lymphangiogenesis via mediating VEGF-A/-C expression [81].
Bile acids
Lymph nodes present a unique microenvironment enriched with fatty acids. A recent study showed that adaptation of melanoma to lymph node microenvironment requires a shift to fatty acid oxidation. Interestingly, lymph node metastatic melanoma acquired the ability to produce bile acids from cholesterol through CYP7A1 [82]. Bile acids bind to vitamin D receptor and activate yes-associated protein (YAP), which in turn mediated expression of fatty acid oxidase (FAO) and enabled metabolic adaptation in metastatic melanoma extravasated to the lymph node. Besides, YAP forms a complex with HIF-1α in the nucleus and sustains HIF-1α stability to induce its downstream genes including VEGFs and glycolysis under hypoxia [83]. Targeting these metabolic adaptations might help prevent lymphatic metastasis.
Survival of circulating tumor cells (CTCs) in vasculature transition or fluid circulation
Following invasion into the vasculature, the next step of metastatic cascade is survival in the circulation as circulating tumor cells (CTCs). Few CTCs survive in circulation. CTCs must adapt to environmental stresses, including shear forces, oxidative stress, and escape immunosurveillance to survive [3].
Oxidative stress
Detachment of cells from the extracellular matrix is associated with increased oxidative stress. Piskounova et al. [84] systematically evaluated metabolic status in melanoma cells in subcutaneous tumors and in metastatic nodules, showing that the latter had lower GSH/GSSG ratio and increased ROS levels. Administration of antioxidants in vivo promotes tumor metastasis, without any effect on growth of primary tumors, suggesting that oxidative stress represents a major barrier for CTCs. Metastasized melanoma cells have altered expression of folate metabolism enzymes, showing increased NADPH-regenerating enzymes ALDH1L2 and MTHFD1, together with suppression of NADPH-consuming MTHFR. This conserved NADPH, which provided reducing power to regenerate GSH and protected against ROS to promote CTCs survival [84]. Melanoma cells also increased fatty acid oxidation to overcome metabolic stress in CTCs. Li et al. [85] found that ROS induced ERK2-dependent mitochondrial translocation of Nur77, where Nur77 bind and maintained the activity of TPβ, a rate-limiting enzyme of FAO under oxidative stress. This prevented the depletion of NADPH, restricted a surge in ROS, maintained ATP levels and promoted survival of melanoma CTCs. Together, rewiring of metabolic pathways to combat oxidative stress for CTCs survival, and targeting antioxidative mechanisms might be a promising approach to suppress metastasis.
Extravasation into “fertile soil” at distant premetastatic niches
CTCs in circulation can potentially be deposited at any distant organs; nevertheless, it is observed that CTCs exhibit tropism and tend to form metastasis in specific organs. Both passive extravasation and active homing contributes to tumor extravasation, the first step in metastatic colonization.
Nucleotides
Adenine nucleotides play a potential role in extravasation. Tumor cells activated platelets release dense ATP granules, which activates endothelial purinergic P2Y2 receptors and induces the opening of the endothelial BM. This is a critical step necessary for transmigration of tumor cells through endothelial cell layer shortly after vascular arrest. Dihydropyrimidine, apart from promoting EMT, is involved in extravasation. Depletion of DYPD reduced ability of HMLER-Twist-ER cells to enter the mouse lung by 95%, a process measured by GFP staining that was significantly rescued by ectopically expression of mouse DPYD (mDPYD) [61].
Glycolysis
Tumor cells-released exosomes participate in metastatic cascade, as they encapsulate prometastatic signaling molecules and deliver them to distant organs. Exosomes release is energy intensive and requires aerobic glycolysis. Pyruvate kinase isozyme type M2 (PKM2), which is up-regulated in tumor cells, modulates exosomes secretion by direct phosphorylation of SNAP-23 (Ser95), a key step for synaptosome/ SNARE complex formation and exosome release [86]. Indeed, the deregulation of PKM2 has been shown to promote metastasis in different settings [87].
Metastatic colonization
The final stage of metastasis involves outgrowth of cancer cells in distant organs. Metastasis initiating-cells (MIC) or cancer stem cells (CSC) are critical in this process, as they possess metabolic versatility to adapt to the microenvironment, and the ability to self-renew and sustain tumorigenesis; non-CSCs could not initiate tumorigenesis. CSCs have demonstrated distinct metabolic programs involved in the maintenance of stemness characteristics. Outgrowth of a metastatic colony also involved reversion of cells to an epithelial phenotype in a process known as the mesenchymal-epithelial transition, and analogous to EMT, involved extensive metabolic rewiring.
Fatty acids
Pascual et al. [88] identified a role of fatty acid metabolism in regulation of MICs/CSCs. CD36 is a fatty acid receptor that facilitates import of long-chain fatty acids. A subset of CD44high/CD36high cells with metastatic capacity was isolated from oral carcinoma. CD36high CSCs consumed high levels of environmental palmitic acid, and promoted tumor-initiating ability at distant sites. Metastasis of CD36high CSCs was induced by palmitate or high-fat diet, while CD36 blockade with neutralization antibodies impairs tumor metastasis. It is likely that the increased utilization of fatty acids, an energy-dense fuel, could increase the survival of CSCs in distant metastatic niche [89].
cNMP
Up-regulation of phosphodiesterase (PDE) in CSCs correlates with increased tumorigenesis, poor prognosis, and tumor metastasis [90]. PDEs are the members of a highly conserved superfamily of enzymes that degrade canonical and noncanonical cyclic nucleotides (cNMPs) [91]. PDEs work in conjunction with adenylate/guanylate cyclases to regulate secondary messengers of G protein-coupled receptor signaling [92]. PDE5 inhibition increased cGMP and cAMP, which stimulates to cAMP/PKA signaling to induce differentiation of CSCs to non-stem tumor cells [90]. Inhibition of PDE5 using a chemical inhibitor has been shown to elevate cGMP which activated cGMP-dependent PKG to attenuate TAZ-mediated gene transcription and suppressed stemness in prostate CSCs [93]. PDE3 also plays a role in CSCs. In pancreatic cancer cells expressing 67-kDa laminin receptor-dependent cGMP inducer, a PDE3 inhibitor in combination with epigallocatechin-3-O-gallate induced cGMP, which impair CSCs properties [94]. In a separate study, cGMP was shown to suppress CD44high pancreatic CSCs [95]. PDEs thus regulates CSCs via modulating cNMPs.
Lactate
Lactate dehydrogenase-A (LDHA) is up-regulated in multiple cancers and is important for CSCs [96]. LDHA catalyzes NADH-dependent reduction of pyruvate to lactate and regenerates NAD+ required for sustaining glycolysis [97]. Blockade of fermentative glycolysis diminished tumor initiating capacity of CSCs. Consistently, knockdown or pharmacological inhibition of LDHA suppressed cancer stemness, as determined by tumorsphere assay and decreased CD24/44 expression [97].
Fructose
Dysregulation of fructose metabolism was found to play a major role in the metastatic colonization of the liver in CRC patients [98]. Aldolase B, which catalyzes reversible conversion of fructose-1,6-bisphosphate to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate, is overexpressed in metastatic lesions as compared to primary tumors [99]. Aldolase B induced incorporation of fructose into glycogen and lipids, both essential for sustaining highly proliferative cells. Aldolase B knockdown in CRC cell lines suppressed liver metastases following cecal transplantation [100]. Moreover, dietary fructose restriction diminished tumor metastasis but had no effect on the primary tumor. Aldolase B is commonly up-regulated in liver metastasis from several cancers [100], and targeting Aldolase B or fructose restriction might be useful for suppressing outgrowth of liver metastasis
Metastatic signaling-mediated regulation of metabolic gene expression and activity in cancer
Transcriptional factors
Transcriptional factors modulate expression and activity of metabolic genes directly by regulating their transcription or indirectly by deregulation of metastatic cascades. For example, EMT-associated transcriptional factors (SNAIL, TWIST, and ZEB) and HIFs can mediate rewiring. WNT/SNAIL signaling inhibits mitochondrial respiration by repressing cytochrome C oxidase (COX), the terminal enzyme of mitochondrial respiratory chain, thereby inducing the glycolytic switch [101]. SNAIL also targets fructose-1,6-bisphosphatase (FBP1), a rate-limiting enzyme which catalyzes splitting of F1,6BP into fructose 6-phosphate and inorganic phosphate in gluconeogenesis. The Snail-G9a-Dnmts complex induces promoter methylation and transcription silencing of FBP1, thus promoting glycolysis and inhibiting mitochondrial respiration. A recent study also revealed that ZEB1 could occupy promoter region of FBP1 and attenuate its expression [102]. Yang et al. [103] also showed that TWIST overexpressing cancer cells have up-regulated levels of glycolytic enzymes including LDHA, PKM2, HK2, and G6PD.
HIF signaling drives multiple steps of metastatic cascades [104]. HIF1 reduces mitochondria biomass and upregulates PKM2 to suppress oxidative respiration [105]. HIF1α induces pyruvate dehydrogenase kinase 1 (PDK1), which phosphorylates and inhibits pyruvate dehydrogenase (PDH) to block entry of acetyl-CoA into Krebs cycle, thereby repressing mitochondria respiration [105]. Prolonged hypoxia up-regulates expression of GLUT1, a glucose transporter, via HIF-1 [105]. These results suggest that EMT transcription factors and HIF1 conferred a glycolytic phenotype in cancer cells to trigger metastasis.
RTK signaling
Receptor tyrosine kinases (RTKs) are overexpressed in cancers. Elevated RTKs are associated with the activation of downstream signaling molecules including MAPK, JAK/STAT, and PI3K/Akt that promoted cancer aggressiveness and metastasis [106]. With respect to the metastatic cascade, AKT, for example, enhances glycolysis flux and activates pentose phosphate pathway to generate NADPH to antagonize increased ROS in metastasis. AKT also promotes inhibition of PDH, thus favouring LDH-mediated biosynthesis of lactate key to maintaining cancer stemness. Activation of ERK1/2 or suppression of AMPK/JNK signaling also mediate a metabolic switch from oxidative respiration to glycolysis in cancer cells in order to fulfill energetic and biosynthetic requirements for tumor metastasis [107].
Integrins signaling
Integrin proteins including ITGA1, ITGB4, and ITGB5, might be receptors of ECM on cell membranes. ITGB4/FAK/glycogen synthase kinase 3β signaling enhances SOX2, a transcription factor involved in EMT induction, and also induces HIF1α to activate glycolytic gene expression including HK2, GLUT1, and LDHA and modulate glucose metabolism [108]. Alternatively, ITGB4 could bind to PD-L1 to promote SNAI1 and AKT/GSK3β signaling, which in turn activates glycolysis and tumor metastasis [109].
Therapeutic opportunities targeting the metastasis-metabolism crosstalk in cancer
Glycolysis inhibitors
Accumulating evidence indicates that inhibition of glycolysis could be effective for the targeting of metastatic cancers [110]. Glycolysis inhibitors targeting hexokinase (HK) includes 2-deoxyglucose (2-DG), Lonidamine (LND) and 3-bromopyruvate (3-BrPA) (Table 1). A mitochondria-targeted LND demonstrated an antimetastatic effect in vitro and in vivo by inhibition of bioenergetics, induction of ROS and inactivation of AKT/mTOR/p70S6K signaling. Glycolysis blockade with 2- DG or 3-BrPA reversed NAD(P)H:quinone oxidoreductase-1 (NQO1)-induced EMT by inhibiting vimentin, Snail, Slug and Twist, and up-regulating E-cadherin, leading to attenuated NQO1-induced glycolysis and metastasis in breast cancer cells [111]. Lonidamine and 2-DG are currently in Phase I-III clinical trials of multiple metastatic cancers [112–114].
Table 1.
Alleviation of cancer metastasis using metabolic enzyme inhibitors.
| Inhibitor | Target gene | Mode of action | Clinical use/trials | NCT | Reference |
|---|---|---|---|---|---|
|
Glycolysis inhibitors Lonidamine (LND), Mitochondria-targeted lonidamine (Mito-LND), 2-Deoxyglucose (2-DG) |
HK1, HK2 | LND/Mito-LND induces ROS and autophagy to block lung cancer migration amd invasion. Mito-LND inhibits p-P70S6K and p-AKT to suppress EMT. 2-DG abrogates NQO1/PKLRdependent glycolysis and tumor metastasis by reversing EMT. |
Lonidamine (Phase II/III); 2-deoxyglucose (Phase I/II) |
[111, 146, 147] | |
| 3-Bromopyruvate (3-BrPA) | GAPDH | 3-BrPA inhibits GAPDH, acetyl-CoA production and attenuates NQ01/ PKLR signaling axis-enhanced tumor glycolysis and metastasis via EMT. | N.A. | N.A. | [111] |
|
3PO, PFK-158 (3PO derivative) |
PFKFB3 | 3PO treatment leads to PFKFB3 inactivation and lowered lactate levels. Blockage of glycolysis by targeting PFKFB3 could suppress the migration and invasion of HNSCC cells and reduce growth of both primary and metastatic melanoma tumors. |
ACT-PFK-158 (Phase I) |
NCT02044861 | [116–118] |
| Glycogenolysis and gluconeogenesis inhibitors | |||||
| Dexamethasone | G6PC | Dexamethasone restores gluconeogenesis and inhibits HCC growth and angiogenesis by enhancing G6PC and PEPCK expression. | Phase I/II/III | [138] | |
|
Chlorogenic acid, AD4-015 |
G6PT | G6PT blockers represses tumor metastasis by suppressing the expression of MMP-2 and MMP-9. |
Chlorogenic acid (Phase I/II/III) |
NCT03751592NCT02245204NCT02728349NCT03758014 | [139, 140] |
| TCA cycle inhibitors: | |||||
|
AG-120 (Ivosidenib), AG-881, AGI-5198, AGI-5027 |
Mutant IDH1 | IDH1 inhibitors suppressed 2-HG levels in IDH1-mutant cells. IDH1 inhibtors inhibited SNAIL-dependent EMT and invasive ability of IDH1-mutant cells. |
AG-120 (Approved for AML) AG-881 (Phase I) |
NCT02073994NCT02989857NCT02481154 | [125, 148] |
|
AG-221 (Enasidenib), AG-881, AGI-6780 |
Mutant IDH2 | IDH2 inhibitors suppressed 2-HG production in IDH2-mutant cells to inhibit liver progenitor cell expansion, development of premalignant biliary lesions, and progression to metastatic IHCC. |
AG-221 (Approved for AML; Phase I/II) |
NCT02273739NCT03515512NCT02481154 | [125, 149, 150] |
| Lipid metabolism inhibitors: | |||||
| Omeprazole | FASN | Omeprazole significantly decreased cancer cell invasion and metastasis to the lung and the expression of at least two prometastatic genes, MMP-9 and CXCR4. | N.A. | N.A. | [128, 151] |
| Cholesterol metabolism inhibitors: | N.A. | ||||
|
Simvastatin, Pravastatin |
HMGCR | Statins inhibit cholesterol biosynthesis and protein prenylyation. Pravastatin treatment greatly reduced the occurrence and extent of spontaneous lung metastasis by decrease the expression of several MMPs including MMP-2, pro-MMP-2, TIMP-2, MMP-14, and MMP-9. |
Simvastatin (Phase II/III); Pravastatin (Phase II/III) |
[60, 129, 152] | |
| SAM cycle inhibitors: | |||||
|
DZNep, Adenosine dialdehyde |
SAHH | DZNep and adenosine dialdehyde increased SAH-to-SAM ratio and blocked EZH2-mediated H3K4me3 and transcription of SNAIL and PRC2, leading to reversal of EMT. | N.A. | N.A. | [131, 132] |
| Nucleotide metabolism inhibitors: | |||||
|
MY-5445, Sildenafil, Tadalafil |
PDE5 | Accumulation of cGMP by PDE5 inhibition upregulates cAMP-dependent PKA activity resulting in a reduction of CSCs involved in metastasis and resistance development. |
Sildenafil (Phase I/II/III); Tadalafil (Phase II) |
NCT01817751NCT00142506 |
[90] |
N.A. not applicable.
Alternative approaches target PFK1 that catalyzes the second rate-limiting step in glycolysis. PFK1 is allosterically activated by fructose-2,6-bisphosphate, a product of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatases (PFKFB1-4). PFKFB3 isoform, which has greater kinase activity than phosphatase activity, is overexpressed in cancer, thereby increasing fructose-2,6-bisphosphate and allosteric activation of PFK1 [115]. 3PO, a PFKFB3 inhibitor, blocked glycolysis and inhibited cell migration/invasion of HNSCC cells [116]. 3PO treatment of endothelial cells also exerts an antimetastatic effect through promoting cell quiescence and tumor vessel normalization [117, 118]. ACT-PFK-158, a derivative of 3PO, is under Phase I clinical trials for advanced solid malignancies (Table 1) [119]. Glycolysis inhibitors thus holds promise in the reversal of metastatic cascades in cancer.
IDH1/2 inhibitors
Inhibition of IDH1/2 currently in Phase I/II/III clinical trials has been pursued as a strategy to suppress the production of oncometabolite 2-HG (Table 1) [120–124]. A series of mutant IDH1R132H inhibitors have been reported to inhibit mutant IDH1R132H in a nanomolar range and exhibited selectivity over wildtype IDH1 [9]. Treatment of mutant IDH1R132C-expressing hepatoblasts with AGI-5027 (ML309) attenuated 2HG, inhibited liver progenitor cells and restored cell differentiation to halt progression to metastatic intrahepatic cholangiocarcinoma (IHCC) [125]. Besides, treatment of human fibrosarcoma HT1080 cells expressing heterozygous IDH1R132C mutation with AGI-5198 attenuated mTORC1/2 activity, whose oncogenic activation stimulates angiogenesis and metastasis [126]. These proof-of-concept studies indicate that targeting mutant IDH1/IDH2 has potential clinical application as a differentiation therapy to suppress cancer metastasis.
Lipid metabolism inhibitors
Endogenous FA biogenesis is tandemly activated by ACC and fatty acid synthase (FASN), which constitutes a metastatic stimulus that drives tumor progression [127]. Omeprazole, a proton pump inhibitor, was shown to inhibit the thioesterase domain of FASN and prevent release of free palmitate from acyl carrier protein. The blockade of FASN by Omeprazole reduced palmitate and dose-dependently inhibited breast cancer cell invasion and metastasis [128].
Cholesterol metabolism inhibitors
HMG-CoA reductase catalyzes the conversion of HMG-CoA to mevalonate, the rate limiting step of cholesterol biosynthesis. Statins, inhibitors of HMG-CoA reductase, have been shown to inhibit cell viability, stemness, tumor growth and metastasis through modulation of Sonic Hedgehog (Shh)-related gene expression [129, 130]. Pravastatin treatment greatly reduces the occurrence and extent of spontaneous lung metastasis by repressing MMPs, suggesting a beneficial effect of statins in slowing tumor progression and metastasis [60].
SAM cycle inhibitors
SAM blockers inhibit DNMTs and HMTs activities and will likely influence DNA and histone methylation-induced metastasis. SAH hydrolase catalyzes the hydrolysis of SAH into adenosine and homocysteine. As SAH caused by-product inhibition of DNMTs and HMTs, targeting SAH hydrolase compromises DNMTs/HMTs activities. DZNep, a SAH hydrolase inhibitor, blocks EZH2-mediated H3K4me3 and transcription of SNAIL and PRC2, leading to reversal of EMT [131]. Adenosine dialdehyde is another inhibitor of SAH hydrolase, and it down-regulated MMP-9 expression and invasion of cancer cells [132].
Nucleotide-metabolic inhibitors
Targeting PDEs can regulate second messengers of G protein-coupled receptor signaling involved in metastatic cascades [92]. MY-5445, a PDE5 inhibitor, induced cGMP and PKA signaling, resulting in a reduction of CSCs involved in metastasis [84]. Another PDE5 inhibitor, Sildenafil, approved by FDA for pulmonary arterial hypertension, has been tested in Phase I/II/III trials for advanced solid tumors. Sildenafil was shown to suppress postoperative metastasis by targeting surgery-induced myeloid derived suppressor cell (MDSC)-dependent inhibition of NK cell cytotoxicity (Table 1) [133–136]. Sildenafil down-regulated ARG1, IL4Ra and ROS to inhibit MDSC-mediated suppression of NK and T cells. Targeting PDE5 with Tadalafil also alleviated MDSC and NK cells-associated metastasis in phase II trials of metastatic head and neck squamous cell carcinoma and oesophagus cancer (Table 1) [136, 137]. Thus, PDE5 is a potential target for antimetastatic therapy.
Glycogenolysis and gluconeogenesis inhibitors
Inhibition of glucose-6-phosphate translocase (G6PT), which transports G-6-P from cytoplasm into ER lumen, has been pursued as a strategy to regulate glycogenolysis and gluconeogenesis [138]. Inhibition of G6PT antagonizes tumor metastasis through the inhibition of MMP2 [139], MMP9 [140], and angiogenesis. G6PT inhibitors including chlorogenic acid, mumbaistatin, and AD4-015 are currently under evaluation in cancer treatment. Chlorogenic acid is being tested in Phase I/II/III trials for advanced lung cancer and glioblastoma (Table 1) [141–144].
Conclusion and 5-year view
Crosstalk between cellular metabolism and metastatic cascades are the consequences of adaptation to unique nutrition requirement for invasion/metastasis and to overcome environment stress in the circulation and metastatic niche. As we have summarized, the tumor metastatic proteome and transcriptome are dynamically modulated by the metabolome. Metabolome-induced signaling cascades may drive tumor aggression and metastasis via diverse pathways involved in every step of the metastasis cascade (Figs. 1 and 2). Here we have outlined potential strategies targeting the crosstalk between metastatic signaling and cell metabolism for selective blockage of metastasis (Tables 1 and 2). Currently, much experimental research involved in vitro cell culture systems, which poorly reflects the complexity of metastasis in vivo that involved not only metastatic tumor cells, but also stromal and immune components. In the future, preclinical studies need to take into consideration the tumor microenvironment to validate potential targets, which ultimately will contribute to identification of novel molecular targets for suppression of metastasis.
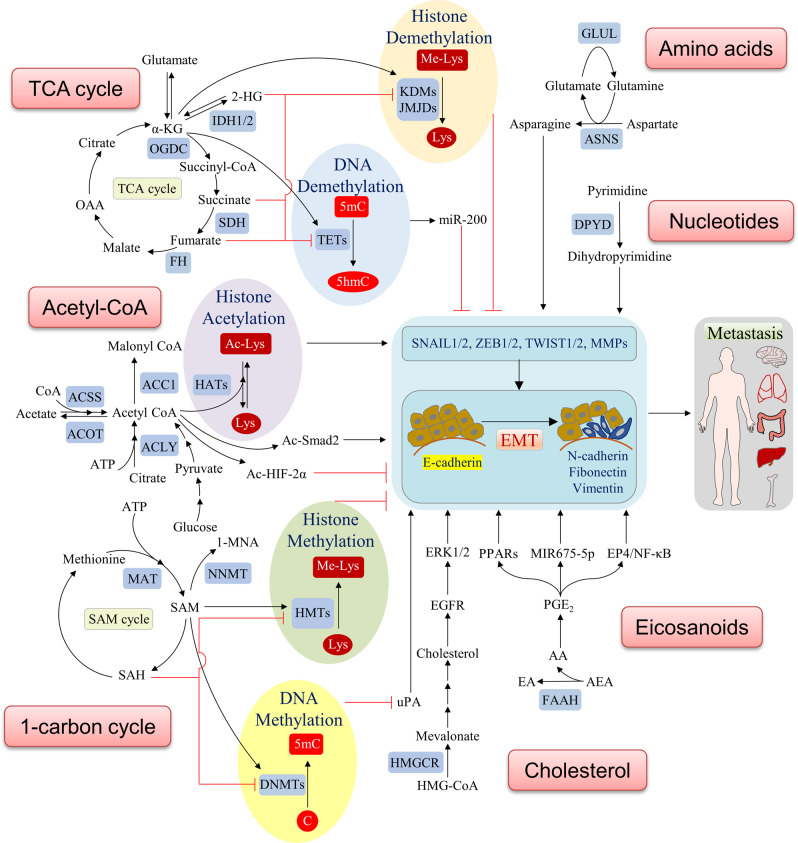
Fig. 2. Molecular mechanisms of metabolites-mediated regulation of EMT.
Many metabolites contribute to rewiring of metastatic pathway in cancer progression.
Table 2.
Regulation of cancer metabolism by antimetastatic drugs.
| Inhibitor | Target gene | Mode of action | Clinical use/trials | NCT | Reference |
|---|---|---|---|---|---|
| Transcription factor inhibitors: | |||||
| Tigecycline | MYC | Tigecycline inhibits cell migration/invasion. It also suppresses mitochondrial OxPhos, glycolysis and extracellular acidification in various neoplasms. | Phase I | NCT01332786 | [153, 154] |
| TH-302 (Evofosfamide), EO9 (Apaziquone) | HIF-1α | HIF-1α inhibitors blocked a shift of OxPhos to anaerobic glycolysis for decreasing ROS levels, and eventually inhibited metastatic colonization to the lungs. |
TH-302 (Phase I/II/III); EO9 (Phase I/II/III) |
[155–157] | |
| Fatostatin | SREBP1/2 | Fatostatin lowers expression of SREBP-regulated enzymes for fatty acid and cholesterol synthesis of FA, and inhibited prostate tumor growth and distant lymph node metastasis. | N.A. | N.A. | [158] |
| Kinase inhibitors: | |||||
|
Cetuximab, Erlotinib Panitumumab, |
EGF-EGFR | Anti-EGFR antibodies and inhibitors are commonly used in metastatic cancers and they reversed the Warberg effect by inhibiting HIF-1-regulated glycolysis. | Approved for multiple cancers | N.A. | [106] |
|
Bevacizumab (Avastin), Sunitinib (Sutent) |
VEGF-VEGFR | Anti-VEGF antibodies are commonly used in metastatic cancers and they also suppressed glucose metabolism. | Approved for multiple cancers | N.A. | [106, 159–161] |
| Trametinib | MEK | Trametinib blocks RAS-RAF-MEK-ERK MAPK cascades mediated-EMT and suppresses pHi and glycolysis in response to hypoxia. | Approved for Braf mutant melamona; (Phase I/II) | [162, 163] | |
| Dasatinib,Saracatenib | SRC | Saracatinib and dasatinib suppress migration of mesenchymal-like HNSCC cells. SRC inhibitor blocks c-Myc translation and glycolysis. | Approved for CML and resistant ALL; (Phase I/II/III) | [159, 164–166] | |
| Integrin inhibitors: | |||||
| Cilengitide | αvβ3/αvβ5- integrin | Cilengitide treatment of breast cancer bone metastasis resulted in a significant reduction in fluorine-18 fluorodeoxyglucose uptake. | Cilengitide (Phase II) | [167, 168] | |
| Epigenetic regulators | |||||
|
Sodium butyrate, SAHA (Vorinostat), LBH589 (Panobinostat), JNJ-26481585 (Quisinostat) |
HDACs | HDAC inhibitors has been shown to reduced glucose uptake, glycolytic flux and lactate metabolism. Inhibition of HDAC11, a binding/transport protein for free fatty acids/acyl-CoA also impact fatty acid metabolism. | Vorinostat (Approved for Cutaneous T-cell lymphoma), Panobinostat (Approved for Multiple myeloma) | [169–171] |
N.A. not applicable.
With numerous drugs targeting metabolism in clinical development, we will have the tools to be able to effectively target these abnormalities in cancer in the next 5 years. L-asparaginase, novel drugs inhibiting asparagine, for instance, is approved by FDA for ALL and undergoing phase II trials for metastatic pancreatic cancer [145]. A caveat of targeted therapies, as exemplified by the application of receptor tyrosine kinase inhibitors, is that they are helpful only when their target(s) are the main drivers of metastatic carcinogenesis. To fully realize the potential of metabolic/antimetastatic modulators, future clinical trials should incorporate analysis of biomarkers to unravel metastatic proteome, transcriptome and metabolomic markers that allow the selection of subsets of patients that might benefit most from targeted treatments. Given the extensive association between metastatic cascade and metabolic pathway, perhaps the combination approaches involving metastatic signaling and metabolic regulators may achieve synergistic metastasis inhibition. Development of rationale drug combinations involving metabolic inhibitors, antimetastatic agents, together with traditional chemotherapeutics will likely have the greatest impact on future cancer management.
Acknowledgements
This project was supported by funds from National Natural Science Foundation of China (NSFC; 81772501); Research Grants Council-General Research Fund (RGC-GRF; 14101917, 14108718, 14163817 and 14110819), Hong Kong; Heath and Medical Research Fund (HMRF) (06170686); Science and Technology Program Grant Shenzhen (JCYJ20170413161534162).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Ng JM, Wong CC, Ng EKW, Yu J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene. 2018;37:4903–20. doi: 10.1038/s41388-018-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schild T, Low V, Blenis J, Gomes AP. Unique metabolic adaptations dictate distal organ-specific metastatic colonization. Cancer Cell. 2018;33:347–54. doi: 10.1016/j.ccell.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastushenko I, Blanpain C. EMT transition states during tumour progression and metastasis. Trends Cell Biol. 2019;29:212–26. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–59. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colvin H, Nishida N, Konno M, Haraguchi N, Takahashi H, Nishimura J, et al. Oncometabolite D-2-hydroxyglurate directly induces epithelial-mesenchymal transition and is associated with distant metastasis in colorectal cancer. Sci Rep. 2016;6:36289. doi: 10.1038/srep36289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CC, Qian Y, Yu J. Interplay between epigenetics and metabolism in oncogenesis: mechanisms and therapeutic approaches. Oncogene. 2017;36:3359–74. doi: 10.1038/onc.2016.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vatrinet R, Leone G, De Luise M, Girolimetti G, Vidone M, Gasparre G, et al. The alpha-ketoglutarate dehydrogenase complex in cancer metabolic plasticity. Cancer Metab. 2017;5:3. doi: 10.1186/s40170-017-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassian AR, Lin F, Barrett R, Liu Y, Jiang W, Korpal M, et al. Isocitrate dehydrogenase (IDH) mutations promote a reversible ZEB1/microRNA (miR)-200-dependent epithelial-mesenchymal transition (EMT) J Biol Chem. 2012;287:42180–94. doi: 10.1074/jbc.M112.417832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalla Pozza E, Dando I, Pacchiana R, Liboi E, Scupoli MT, Donadelli M, et al. Regulation of succinate dehydrogenase and role of succinate in cancer. Semin Cell Dev Biol. 2020;98:4–14. doi: 10.1016/j.semcdb.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–52. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Aspuria P-JP, Lunt SY, Väremo L, Vergnes L, Gozo M, Beach JA, et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2014;2:21. doi: 10.1186/2049-3002-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Chen Y, Wu G. SDHB deficiency promotes TGFbeta-mediated invasion and metastasis of colorectal cancer through transcriptional repression complex SNAIL1-SMAD3/4. Transl Oncol. 2016;9:512–20. doi: 10.1016/j.tranon.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, da Costa AS, Gaude E, et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;537:544–7. doi: 10.1038/nature19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–8. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- 19.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiuru M, Launonen V. Hereditary leiomyomatosis and renal cell cancer (HLRCC) Curr Mol Med. 2004;4:869–75. doi: 10.2174/1566524043359638. [DOI] [PubMed] [Google Scholar]
- 21.Morris JP, Yashinskie JJ, Koche R, Chandwani R, Tian S, Chen CC, et al. Alpha-Ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019;573:595–9. doi: 10.1038/s41586-019-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atlante S, Visintin A, Marini E, Savoia M, Dianzani C, Giorgis M, et al. Alpha-ketoglutarate dehydrogenase inhibition counteracts breast cancer-associated lung metastasis. Cell Death Dis. 2018;9:756. doi: 10.1038/s41419-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen J, Min X, Shen M, Hua Q, Han Y, Zhao L, et al. ACLY facilitates colon cancer cell metastasis by CTNNB1. J Exp Clin Cancer Res. 2019;38:401. doi: 10.1186/s13046-019-1391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu M, Zhu WW, Wang X, Tang JJ, Zhang KL, Yu GY, et al. ACOT12- dependent alteration of acetyl-CoA drives hepatocellular carcinoma metastasis by epigenetic induction of epithelial-mesenchymal transition. Cell Metab. 2019;29:886–900. doi: 10.1016/j.cmet.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Yao L, Guo X, Gui Y. Acetyl-CoA Synthetase 2 promotes cell migration and invasion of renal cell carcinoma by upregulating lysosomal-associated membrane protein 1 expression. Cell Physiol Biochem. 2018;45:984–92. doi: 10.1159/000487293. [DOI] [PubMed] [Google Scholar]
- 26.Rios Garcia M, Steinbauer B, Srivastava K, Singhal M, Mattijssen F, Maida A, et al. Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 2017;26:842–55. doi: 10.1016/j.cmet.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Qian X, Hu J, Zhao J, Chen H. ATP citrate lyase expression is associated with advanced stage and prognosis in gastric adenocarcinoma. Int J Clin Exp Med. 2015;8:7855. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Bollu LR, Tozzi F, Ye X, Bhattacharya R, Gao G, et al. ATP citrate lyase mediates resistance of colorectal cancer cells to SN38. Mol Cancer Ther. 2013;12:2782–91. doi: 10.1158/1535-7163.MCT-13-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, et al. ATP citrate lyase: activation and therapeutic implications in non–small cell lung cancer. Cancer Res. 2008;68:8547–54. doi: 10.1158/0008-5472.CAN-08-1235. [DOI] [PubMed] [Google Scholar]
- 30.Hur H, Kim YB, Ham IH, Lee D. Loss of ACSS2 expression predicts poor prognosis in patients with gastric cancer. J Surg Oncol. 2015;112:585–91. doi: 10.1002/jso.24043. [DOI] [PubMed] [Google Scholar]
- 31.Bae JM, Kim JH, Oh HJ, Park HE, Lee TH, Cho NY, et al. Downregulation of acetyl-CoA synthetase 2 is a metabolic hallmark of tumour progression and aggressiveness in colorectal carcinoma. Mod Pathol. 2017;30:267–77. doi: 10.1038/modpathol.2016.172. [DOI] [PubMed] [Google Scholar]
- 32.Wen H, Lee S, Zhu WG, Lee OJ, Yun SJ, Kim J, et al. Glucose-derived acetate and ACSS2 as key players in cisplatin resistance in bladder cancer. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:413–21. doi: 10.1016/j.bbalip.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the Acetyl-CoA-carboxylase-α gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–25. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 34.Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumour growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108–19. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L, Kong Y, Cao M, Zhou H, Li H, Cui Y, et al. Decreased expression of acetyl-CoA synthase 2 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Cancer Sci. 2017;108:1338–46. doi: 10.1111/cas.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahin M, Sahin E, Gumuslu S, Erdogan A, Gultekin M. DNA methylation or histone modification status in metastasis and angiogenesis-related genes: a new hypothesis on usage of DNMT inhibitors and S-adenosylmethionine for genome stability. Cancer Metastasis Rev. 2010;29:655–76. doi: 10.1007/s10555-010-9253-0. [DOI] [PubMed] [Google Scholar]
- 37.Hah YS, Cho HY, Jo SY, Park YS, Heo EP, Yoon TJ. Nicotinamide Nmethyltransferase induces the proliferation and invasion of squamous cell carcinoma cells. Oncol Rep. 2019;42:1805–14. doi: 10.3892/or.2019.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomelino CL, Andring JT, McKenna R, Kilberg MS. Asparagine synthetase: function, structure, and role in disease. J Biol Chem. 2017;292:19952–8. doi: 10.1074/jbc.R117.819060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554:378–81. doi: 10.1038/nature25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM, et al. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab. 2018;27:428–38. doi: 10.1016/j.cmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo M, Brooks M, Wicha MS. Asparagine and glutamine: co-conspirators fueling metastasis. Cell Metab. 2018;27:947–9. doi: 10.1016/j.cmet.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Liu P, Lu D, Al-Ameri A, Wei X, Ling S, Li J, et al. Glutamine synthetase promotes tumour invasion in hepatocellular carcinoma through mediating epithelial–mesenchymal transition. Hepatol Res. 2020;50:246–57. doi: 10.1111/hepr.13433. [DOI] [PubMed] [Google Scholar]
- 43.Brunetti L, Loiodice F, Piemontese L, Tortorella P, Laghezza A. New approaches to cancer therapy: combining fatty acid amide hydrolase (FAAH) inhibition with peroxisome proliferator-activated receptors (PPARs) activation. J Med Chem. 2019;62:10995–1003. doi: 10.1021/acs.jmedchem.9b00885. [DOI] [PubMed] [Google Scholar]
- 44.Laezza C, D’Alessandro A, Paladino S, Maria Malfitano A, Chiara Proto M, Gazzerro P, et al. Anandamide inhibits the Wnt/beta-catenin signalling pathway in human breast cancer MDA MB 231 cells. Eur J Cancer. 2012;48:3112–22. doi: 10.1016/j.ejca.2012.02.062. [DOI] [PubMed] [Google Scholar]
- 45.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181–95. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfer A, Ramaswamy S. MYC and metastasis. Cancer Res. 2011;71:2034–37. doi: 10.1158/0008-5472.CAN-10-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ham SA, Yoo T, Hwang JS, Kang ES, Lee WJ, Paek KS, et al. Ligand-activated PPARδ modulates the migration and invasion of melanoma cells by regulating Snail expression. Am J Cancer Res. 2014;4:674. [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo X, Xu W, Xu M, Tian R, Moussalli MJ, Mao F, et al. Metastasis regulation by PPARD expression in cancer cells. JCI Insight. 2017;2:e91419. doi: 10.1172/jci.insight.91419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montagner A, Delgado MB, Tallichet-Blanc C, Chan JS, Sng MK, Mottaz H, et al. Src is activated by the nuclear receptor peroxisome proliferator-activated receptor beta/delta in ultraviolet radiation-induced skin cancer. EMBO Mol Med. 2014;6:80–98. doi: 10.1002/emmm.201302666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin LC, Hsu SL, Wu CL, Hsueh CM. TGFbeta can stimulate the p(38)/beta- catenin/PPARgamma signaling pathway to promote the EMT, invasion and migration of non-small cell lung cancer (H460 cells) Clin Exp Metastasis. 2014;31:881–95. doi: 10.1007/s10585-014-9677-y. [DOI] [PubMed] [Google Scholar]
- 51.Lim JCW, Kwan YP, Tan MS, Teo MHY, Chiba S, Wahli W, et al. The role of PPARbeta/delta in melanoma metastasis. Int J Mol Sci. 2018;19:2860.. doi: 10.3390/ijms19102860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen B, Chu ES, Zhao G, Man K, Wu CW, Cheng JT, et al. PPARgamma inhibits hepatocellular carcinoma metastases in vitro and in mice. Br J Cancer. 2012;106:1486–94. doi: 10.1038/bjc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang T, Jing B, Xu D, Liao Y, Song H, Sun B, et al. PTGES/PGE2 signaling links immunosuppression and lung metastasis in Gprc5a-knockout mouse model. Oncogene. 2020;39:3179–94. doi: 10.1038/s41388-020-1207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cen B, Lang JD, Du Y, Wei J, Xiong Y, Bradley N, et al. Prostaglandin E2 induces miR675-5p to promote colorectal tumour metastasis via modulation of p53 expression. Gastroenterology. 2019;158:971–84. doi: 10.1053/j.gastro.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest. 2014;124:3901–12. doi: 10.1172/JCI73777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149:1884–95. doi: 10.1053/j.gastro.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6:242ra284. doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo Y, Li L, et al. Candidate antimetastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Cancer Res. 2016;76:2037–49. doi: 10.1158/0008-5472.CAN-15-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang S, Wang X, Song D, Liu X, Gu Y, Xu Z, et al. Cholesterol induces epithelial-to-mesenchymal transition of prostate cancer cells by suppressing degradation of EGFR through APMAP. Cancer Res. 2019;79:3063–75. doi: 10.1158/0008-5472.CAN-18-3295. [DOI] [PubMed] [Google Scholar]
- 60.Taras D, Blanc JF, Rullier A, Dugot-Senant N, Laurendeau I, Vidaud M, et al. Pravastatin reduces lung metastasis of rat hepatocellular carcinoma via a coordinated decrease of MMP expression and activity. J Hepatol. 2007;46:69–76. doi: 10.1016/j.jhep.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 61.Shaul YD, Freinkman E, Comb WC, Cantor JR, Tam WL, Thiru P, et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell. 2014;158:1094–109. doi: 10.1016/j.cell.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu WP, Liu ZY, Zhao YM, He XG, Pan Q, Zhang N, et al. Dihydropyrimidine dehydrogenase predicts survival and response to interferon-alpha in hepatocellular carcinoma. Cell Death Dis. 2018;9:69. doi: 10.1038/s41419-017-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–80. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu GZ, Jin MZ, Dai JX, Sun W, Feng JH, Jin WL. Reprogramming of the tumour in the hypoxic niche: the emerging concept and associated therapeutic strategies. Trends Pharm Sci. 2017;38:669–86. doi: 10.1016/j.tips.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Kuo YL, Jou IM, Jeng SF, Chu CH, Huang JS, Hsu TI, et al. Hypoxia-induced epithelial-mesenchymal transition and fibrosis for the development of breast capsular contracture. Sci Rep. 2019;9:10269. doi: 10.1038/s41598-019-46439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 67.Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, et al. HIF-1alpha promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS ONE. 2015;10:e0129603. doi: 10.1371/journal.pone.0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle. 2008;7:2090–6. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- 69.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumour microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen AT, Chia J, Ros M, Hui KM, Saltel F, Bard F. Organelle specific O-glycosylation drives MMP14 activation, tumour growth, and metastasis. Cancer Cell. 2017;32:639–53. doi: 10.1016/j.ccell.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Peng M, Yang D, Hou Y, Liu S, Zhao M, Qin Y, et al. Intracellular citrate accumulation by oxidized ATM-mediated metabolism reprogramming via PFKP and CS enhances hypoxic breast cancer cell invasion and metastasis. Cell Death Dis. 2019;10:228. doi: 10.1038/s41419-019-1475-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Huanna T, Tao Z, Xiangfei W, Longfei A, Yuanyuan X, Jianhua W, et al. GALNT14 mediates tumour invasion and migration in breast cancer cell MCF-7. Mol Carcinogenesis. 2015;54:1159–71. doi: 10.1002/mc.22186. [DOI] [PubMed] [Google Scholar]
- 73.Chugh S, Barkeer S, Rachagani S, Nimmakayala RK, Perumal N, Pothuraju R, et al. Disruption of C1galt1 gene promotes development and metastasis of pancreatic adenocarcinomas in mice. Gastroenterology. 2018;155:1608–24. doi: 10.1053/j.gastro.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hung J-S, Huang J, Lin Y-C, Huang M-J, Lee P-H, Lai H-S, et al. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2. Oncotarget. 2014;5:2096. doi: 10.18632/oncotarget.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tzeng SF, Tsai CH, Chao TK, Chou YC, Yang YC, Tsai MH, et al. O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J. 2018;32:6869–82. doi: 10.1096/fj.201800687. [DOI] [PubMed] [Google Scholar]
- 76.Boon L, Ugarte-Berzal E, Vandooren J, Opdenakker G. Glycosylation of matrix metalloproteases and tissue inhibitors: present state, challenges and opportunities. Biochem J. 2016;473:1471–82. doi: 10.1042/BJ20151154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 78.Palmieri EM, Menga A, Martin-Perez R, Quinto A, Riera-Domingo C, De Tullio G, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumour metastasis. Cell Rep. 2017;20:1654–66. doi: 10.1016/j.celrep.2017.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 80.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–33. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji RC. Hypoxia and lymphangiogenesis in tumour microenvironment and metastasis. Cancer Lett. 2014;346:6–16. doi: 10.1016/j.canlet.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Lee CK, Jeong SH, Jang C, Bae H, Kim YH, Park I, et al. Tumour metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–9. doi: 10.1126/science.aav0173. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Li Y, Ma Y, Yang L, Wang T, Meng X, et al. Yes-associated protein (YAP) binds to HIF-1alpha and sustains HIF-1alpha protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J Exp Clin Cancer Res. 2018;37:216. doi: 10.1186/s13046-018-0892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–91. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li XX, Wang ZJ, Zheng Y, Guan YF, Yang PB, Chen X, et al. Nuclear receptor Nur77 facilitates melanoma cell survival under metabolic stress by protecting fatty acid oxidation. Mol Cell. 2018;69:480–92. doi: 10.1016/j.molcel.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041. doi: 10.1038/ncomms14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng TY, Yang YC, Wang HP, Tien YW, Shun CT, Huang HY, et al. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene. 2018;37:1730–42. doi: 10.1038/s41388-017-0086-y. [DOI] [PubMed] [Google Scholar]
- 88.Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–5. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 89.Celia-Terrassa T, Kang Y. Metastatic niche functions and therapeutic opportunities. Nat Cell Biol. 2018;20:868–77. doi: 10.1038/s41556-018-0145-9. [DOI] [PubMed] [Google Scholar]
- 90.Klutzny S, Anurin A, Nicke B, Regan JL, Lange M, Schulze L, et al. PDE5 inhibition eliminates cancer stem cells via induction of PKA signaling. Cell Death Dis. 2018;9:192. doi: 10.1038/s41419-017-0202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baillie GS, Tejeda GS, Kelly MP. Therapeutic targeting of 3’,5’-cyclic nucleotide phosphodiesterases: inhibition and beyond. Nat Rev Drug Discov. 2019;18:770–96. doi: 10.1038/s41573-019-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gulati S, Palczewski K, Engel A, Stahlberg H, Kovacik L. Cryo-EM structure of phosphodiesterase 6 reveals insights into the allosteric regulation of type I phosphodiesterases. Sci Adv. 2019;5:eaav4322.. doi: 10.1126/sciadv.aav4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu N, Mei L, Fan X, Tang C, Ji X, Hu X, et al. Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway. Cancer Lett. 2016;378:38–50. doi: 10.1016/j.canlet.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 94.Kumazoe M, Takai M, Hiroi S, Takeuchi C, Yamanouchi M, Nojiri T, et al. PDE3 inhibitor and EGCG combination treatment suppress cancer stem cell properties in pancreatic ductal adenocarcinoma. Sci Rep. 2017;7:1917. doi: 10.1038/s41598-017-02162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumazoe M, Takai M, Bae J, Hiroi S, Huang Y, Takamatsu K, et al. FOXO3 is essential for CD44 expression in pancreatic cancer cells. Oncogene. 2017;36:2643–54. doi: 10.1038/onc.2016.426. [DOI] [PubMed] [Google Scholar]
- 96.Mishra D, Banerjee D. Lactate dehydrogenases as metabolic links between tumour and stroma in the tumour microenvironment. Cancers. 2019;11:E750. doi: 10.3390/cancers11060750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, et al. Targeting lactate dehydrogenase–a inhibits tumourigenesis and tumour progression in mouse models of lung cancer and impacts tumour-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bu P, Chen KY, Xiang K, Johnson C, Crown SB, Rakhilin N, et al. Aldolase B-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell Metab. 2018;27:1249–62. doi: 10.1016/j.cmet.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lincet H, Icard P. How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions? Oncogene. 2015;34:3751–9. doi: 10.1038/onc.2014.320. [DOI] [PubMed] [Google Scholar]
- 100.Li Q, Li Y, Xu J, Wang S, Xu Y, Li X, et al. Aldolase B overexpression is associated with poor prognosis and promotes tumour progression by epithelial-mesenchymal transition in colorectal adenocarcinoma. Cell Physiol. Biochem. 2017;42:397–406. doi: 10.1159/000477484. [DOI] [PubMed] [Google Scholar]
- 101.Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, et al. Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res. 2012;72:3607–17. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, Wang J, Xing H, Li Q, Zhao Q, Li J. Down-regulation of FBP1 by ZEB1-mediated repression confers to growth and invasion in lung cancer cells. Mol Cell Biochem. 2016;411:331–40. doi: 10.1007/s11010-015-2595-8. [DOI] [PubMed] [Google Scholar]
- 103.Yang L, Hou Y, Yuan J, Tang S, Zhang H, Zhu Q, et al. Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signaling pathways. Oncotarget. 2015;6:25755–69. doi: 10.18632/oncotarget.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang R, Zong X. Aberrant cancer metabolism in epithelial-mesenchymal transition and cancer metastasis: Mechanisms in cancer progression. Crit Rev Oncol Hematol. 2017;115:13–22. doi: 10.1016/j.critrevonc.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR. Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol. 2019;7:4. doi: 10.3389/fcell.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Butti R, Das S, Gunasekaran VP, Yadav AS, Kumar D, Kundu GC. Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer. 2018;17:34. doi: 10.1186/s12943-018-0797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Papa S, Choy PM, Bubici C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene. 2019;38:2223–40. doi: 10.1038/s41388-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gan L, Meng J, Xu M, Liu M, Qi Y, Tan C, et al. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin beta4/FAK/SOX2/HIF-1alpha signaling pathway in gastric cancer. Oncogene. 2018;37:744–55. doi: 10.1038/onc.2017.363. [DOI] [PubMed] [Google Scholar]
- 109.Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y, et al. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin beta4/SNAI1/SIRT3 signaling pathway. Oncogene. 2018;37:4164–80. doi: 10.1038/s41388-018-0252-x. [DOI] [PubMed] [Google Scholar]
- 110.Warburg O. Uber den Stoffwechsel der Karzinomezellen. Biochem Z. 1924;152:309–44. [Google Scholar]
- 111.Yang Y, Zhu G, Dong B, Piao J, Chen L, Lin Z. The NQO1/PKLR axis promotes lymph node metastasis a.nd breast cancer progression by modulating glycolytic reprogramming. Cancer Lett. 2019;453:170–83. doi: 10.1016/j.canlet.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 112.NCT00435448.
- 113.NCT00096707.
- 114.NCT00188929.
- 115.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–49. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li HM, Yang JG, Liu ZJ, Wang WM, Yu ZL, Ren JG, et al. Blockage of glycolysis by targeting PFKFB3 suppresses tumour growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:7. doi: 10.1186/s13046-016-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumour vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016;30:968–85. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conradi LC, Brajic A, Cantelmo AR, Bouche A, Kalucka J, Pircher A, et al. Tumour vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis. 2017;20:599–613. doi: 10.1007/s10456-017-9573-6. [DOI] [PubMed] [Google Scholar]
- 119.NCT02044861.
- 120.NCT02273739.
- 121.NCT03515512.
- 122.NCT02073994.
- 123.NCT02989857.
- 124.NCT02481154.
- 125.Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, et al. Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–4. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carbonneau M, Gagné LM, Lalonde ME, Germain MA, Motorina A, Guiot MC, et al. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat Commun. 2016;7:12700. doi: 10.1038/ncomms12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76. doi: 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tripathi SC, Fahrmann JF, Vykoukal JV, Dennison JB, Hanash SM. Targeting metabolic vulnerabilities of cancer: Small molecule inhibitors in clinic. Cancer Rep. 2019;2:e1131. doi: 10.1002/cnr2.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li J, Gu D, Lee SS, Song B, Bandyopadhyay S, Chen S, et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016;35:6378–88. doi: 10.1038/onc.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yin Y, Liu L, Zhao Z, Yin L, Bauer N, Nwaeburu CC, et al. Simvastatin inhibits sonic hedgehog signaling and stemness features of pancreatic cancer. Cancer Lett. 2018;426:14–24. doi: 10.1016/j.canlet.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Crea F, Hurt EM, Mathews LA, Cabarcas SM, Sun L, Marquez VE, et al. Pharmacologic disruption of Polycomb Repressive Complex 2 inhibits tumourigenicity and tumour progression in prostate cancer. Mol Cancer. 2011;10:40. doi: 10.1186/1476-4598-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim JH, Kim JH, Kim SC, Yi YS, Yang WS, Yang Y, et al. Adenosine dialdehyde suppresses MMP-9-mediated invasion of cancer cells by blocking the Ras/Raf-1/ERK/AP-1 signaling pathway. Biochem Pharmacol. 2013;86:1285–300. doi: 10.1016/j.bcp.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 133.NCT02466802.
- 134.NCT01817751.
- 135.NCT00142506.
- 136.Tai LH, Alkayyal AA, Leslie AL, Sahi S, Bennett S, Tanese de Souza C, et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology. 2018;7:e1431082. doi: 10.1080/2162402X.2018.1431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.NCT03993353.
- 138.Wang Z, Dong C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5:30–45. doi: 10.1016/j.trecan.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 139.Belkaid A, Currie JC, Desgagnes J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for microsomal glucose-6-phosphate translocase in brain tumour progression. Cancer Cell Int. 2006;6:7. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tahanian E, Lord-Dufour S, Das A, Khosla C, Roy R, Annabi B. Inhibition of tubulogenesis and of carcinogen-mediated signaling in brain endothelial cells highlight the antiangiogenic properties of a mumbaistatin analog. Chem Biol Drug Des. 2010;75:481–8. doi: 10.1111/j.1747-0285.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 141.NCT03751592.
- 142.NCT02245204.
- 143.NCT02728349.
- 144.NCT03758014.
- 145.Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA Drug Approval Summary: Pegaspargase (Oncaspar) for the First-Line Treatment of Children With Acute Lymphoblastic Leukemia (ALL) Oncologist. 2007;12:991–8. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]
- 146.Cheng G, Zhang Q, Pan J, Lee Y, Ouari O, Hardy M, et al. Targeting lonidamine to mitochondria mitigates lung tumourigenesis and brain metastasis. Nat Commun. 2019;10:2205. doi: 10.1038/s41467-019-10042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagarajan A, Malvi P, Wajapeyee N. Oncogene-directed alterations in cancer cell metabolism. Trends Cancer. 2016;2:365–77. doi: 10.1016/j.trecan.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu WS, Chan SH, Chang HT, Li GC, Tu YT, Tseng HH, et al. Isocitrate dehydrogenase 1–snail axis dysfunction significantly correlates with breast cancer prognosis and regulates cell invasion ability. Breast Cancer Res. 2018;20:25. doi: 10.1186/s13058-018-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Waitkus MS, Diplas BH, Yan H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell. 2018;34:186–95. doi: 10.1016/j.ccell.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Punekar S, Cho DC. Novel therapeutics affecting metabolic pathways. Am Soc Clin Oncol Educ Book. 2019;39:e79–e87. doi: 10.1200/EDBK_238499. [DOI] [PubMed] [Google Scholar]
- 151.Jin UH, Lee SO, Pfent C, Safe S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer. 2014;14:498. doi: 10.1186/1471-2407-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lim SH, Kim TW, Hong YS, Han SW, Lee KH, Kang HJ, et al. A randomised, double-blind, placebo-controlled multi-centre phase III trial of XELIRI/FOLFIRI plus simvastatin for patients with metastatic colorectal cancer. Br J Cancer. 2015;113:1421–6. doi: 10.1038/bjc.2015.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu B, Guo Y. Inhibition of mitochondrial translation as a therapeutic strategy for human ovarian cancer to overcome chemoresistance. Biochem Biophys Res Commun. 2019;509:373–8. doi: 10.1016/j.bbrc.2018.12.127. [DOI] [PubMed] [Google Scholar]
- 154.Dong Z, Abbas MN, Kausar S, Yang J, Li L, Tan L, et al. Biological functions and molecular mechanisms of antibiotic tigecycline in the treatment of cancers. Int J Mol Sci. 2019;20:E3577. doi: 10.3390/ijms20143577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharm Ther. 2016;164:152–69. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 156.Fallah J, Rini BI. HIF Inhibitors: status of current clinical development. Curr Oncol Rep. 2019;21:6. doi: 10.1007/s11912-019-0752-z. [DOI] [PubMed] [Google Scholar]
- 157.Rey S, Schito L, Wouters BG, Eliasof S, Kerbel RS. Targeting hypoxia-inducible factors for antiangiogenic cancer therapy. Trends Cancer. 2017;3:529–41. doi: 10.1016/j.trecan.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 158.Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez-Billalabeitia E, et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat Genet. 2018;50:206–18. doi: 10.1038/s41588-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201–18. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Incio J, Tam J, Rahbari NN, Suboj P, McManus DT, Chin SM, et al. PlGF/VEGFR-1 signaling promotes macrophage polarization and accelerated tumour progression in obesity. Clin Cancer Res. 2016;22:2993–3004. doi: 10.1158/1078-0432.CCR-15-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumour growth. Mol Cancer Ther. 2011;10:2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 162.Caunt CJ, Sale MJ, Smith PD, Cook SJ. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat Rev Cancer. 2015;15:577–92. doi: 10.1038/nrc4000. [DOI] [PubMed] [Google Scholar]
- 163.McDonald PC, Chafe SC, Brown WS, Saberi S, Swayampakula M, Venkateswaran G, et al. Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia. Gastroenterology. 2019;157:823–37. doi: 10.1053/j.gastro.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 164.Kolli-Bouhafs K, Sick E, Noulet F, Gies JP, De Mey J, Ronde P. FAK competes for Src to promote migration against invasion in melanoma cells. Cell Death Dis. 2014;5:e1379. doi: 10.1038/cddis.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lang L, Shay C, Xiong Y, Thakkar P, Chemmalakuzhy R, Wang X, et al. Combating head and neck cancer metastases by targeting Src using multifunctional nanoparticle-based saracatinib. J Hematol Oncol. 2018;11:85. doi: 10.1186/s13045-018-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sundberg TB, Choi HG, Song JH, Russell CN, Hussain MM, Graham DB, et al. Small-molecule screening identifies inhibition of salt-inducible kinases as a therapeutic strategy to enhance immunoregulatory functions of dendritic cells. Proc Natl Acad Sci USA. 2014;111:12468–73. doi: 10.1073/pnas.1412308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bäuerle T, Komljenovic D, Merz M, Berger MR, Goodman SL, Semmler W. Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int J Cancer. 2011;128:2453–62. doi: 10.1002/ijc.25563. [DOI] [PubMed] [Google Scholar]
- 168.Cheng C, Komljenovic D, Pan L, Dimitrakopoulou-Strauss A, Strauss L, Bäuerle T. Evaluation of treatment response of cilengitide in an rxperimental model of breast cancer bone metastasis using dynamic PET with 18F-FDG. Hell J Nucl Med. 2011;14:15–20. [PubMed] [Google Scholar]
- 169.Yang J, Jin X, Yan Y, Shao Y, Pan Y, Roberts LR, et al. Inhibiting histone deacetylases suppresses glucose metabolism and hepatocellular carcinoma growth by restoring FBP1 expression. Sci Rep. 2017;7:43864. doi: 10.1038/srep43864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leslie PL, Chao YL, Tsai YH, Ghosh SK, Porrello A, Van Swearingen AED, et al. Histone deacetylase 11 inhibition promotes breast cancer metastasis from lymph nodes. Nat Commun. 2019;10:4192. doi: 10.1038/s41467-019-12222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kutil Z, Novakova Z, Meleshin M, Mikesova J, Schutkowski M, Barinka C. Histone deacetylase 11 Is a fatty-acid deacylase. ACS Chem Biol. 2018;13:685–93. doi: 10.1021/acschembio.7b00942. [DOI] [PubMed] [Google Scholar]