Abstract
Malignant gliomas, including glioblastoma (GBM) as the most aggressive type of adult CNS tumors, are notoriously resistant to current standard of care treatments, including surgery, systemic chemotherapy, and radiation therapy (RT). This lack of effective treatment options highlights the urgent need for novel therapies, including immunotherapies. The overarching goal of immunotherapy is to stimulate and activate the patient’s immune system in a targeted manner to kill tumor cells. The success of immunotherapeutic interventions in other cancer types has led to interest in and evaluation of various experimental immunotherapies in patients with malignant gliomas. However, these primary malignant brain tumors present a challenge because they exist in a vital and sensitive organ with a unique immune environment. The challenges and current status of experimental immunotherapeutic approaches, including vaccines, immune-checkpoint blockade, chimeric antigen receptor T-cell therapy, and oncolytic viruses will be discussed, as well as the potential for combinatorial therapies.
Keywords: checkpoint, glioblastoma, glioma, immunotherapy, vaccine
The Immune Microenvironment of Glioblastoma
Although the CNS has traditionally been classified as an immune-privileged site, it has become clear that immunosurveillance is actively occurring in the brain. Egress of antigen-presenting cells from the cerebral spinal fluid (CSF) into the deep cervical lymph nodes are able to prime T and B cells against CNS antigens. However, this does not widely occur in the brain parenchyma because there are no clear drainage routes for potential antigen-presenting cells to lymph nodes.1 Furthermore, in the CNS parenchyma of a healthy brain, the primary resident immune cells are microglia, specialized macrophages, and CNS border–associated macrophages. In this context, microglia function in immune surveillance and synapse pruning but may not be capable of functioning as antigen-presenting cells.1,2 Furthermore, although T cells can be found in the CNS, there are extremely low levels of infiltration by CD4 + helper and CD8 + cytotoxic T cells in the healthy brain, as compared with other organs.
The immune microenvironment of the brain may undergo drastic remodeling in the presence of a tumor. For example, downregulation of sphingosine 1-phosphate receptor 1 has been shown to result in T-cell sequestration in the bone marrow of patients with brain lesions, including glioblastoma (GBM) tumors. 3This further decreases the level of T-cell infiltration.
In addition to an overall lack of T-cell infiltration, GBMs have a high infiltration of myeloid cells. Myeloid cells comprise the majority of the innate immune response, and differentiate into monocytes, dendritic cells, macrophages, and neutrophils, etc. These cells function to protect the host by releasing inflammatory cytokines. In the setting of GBM, certain myeloid populations become polarized toward protumor function. These are myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs).4,5 MDSCs are closely related to monocytes and neutrophils, and are not typically present in healthy tissues; infiltration of MDSCs occurs in cancer or pathological conditions associated with inflammation. The primary function of MDSCs is T-cell suppression.4 TAMs consist both of infiltrating bone marrow–derived macrophages and brain-resident microglia. In fact, microglia and macrophages have distinct immunosuppressive signatures, and their relative levels change throughout tumor progression, with levels of TAMs increasing as the tumor grows.6 Tumor-infiltrating TAMs that migrate from the blood show an increase in immunosuppressive markers, such as programmed death ligand 1 (PD-L1), FLT3, and TGFβ compared with CNS-resident microglia.6 Additionally, Osteopontin (OPN) has been shown to be an important factor that mediates TAM infiltration in gliomas and promotes the M2 protumor macrophage phenotype.7,8 Tumor-infiltrating MDSCs suppress cytotoxic T-cell activity by expressing indoleamine 2,3-dioxygenase 1 (IDO), Arginase (ARG1), and inducible nitric oxide synthase. 9IDO depletes tryptophan and ARG1 depletes arginine, which inhibits T-cell proliferation and activation. 10IL-10 and TGFβ, soluble factors secreted by MDSCs and TAMs, respectively, also are known to be mediators of immunosuppression.11,12 MDSCs and TAMs constitute a large number of intratumoral immune cells in GBM patient samples, ranging from 30% to 60% of all cells composing the tumor.13 Patient-derived MDSCs and TAMs have been shown to suppress the activation of T cells in vitro. The strong presence of immunosuppressive myeloid cells paired with a paucity of antigen-presenting cells, such as dendritic cells (DCs), renders the tumor myeloid compartment a promising target for immunotherapies.
Additionally, GBMs have fewer somatic mutations in comparison to other tumor sites, such as lung cancer and melanoma.14 A paucity of tumor antigens may contribute to many GBM tumors being “poorly immunogenic,” limiting the immune responses initiated by antigen-presenting cells.
Corticosteroid Use and Immunotherapy
Corticosteroids are commonly used both before and after tumor-resection surgery to control cerebral edema.15,16 Dexamethasone, a synthetic glucocorticoid, is primarily used for GBM patients but has potent immunosuppressive properties. In preclinical models, dexamethasone administration led to the inhibition of naive T-cell differentiation and proliferation by upregulating cytotoxic T lymphocyte antigen 4 (CTLA-4).17 Immunotherapy Response Assessment in Neuro-Oncology guidelines recommend that patients undergoing immunotherapy should receive as little corticosteroid treatment as possible to maximize immunotherapeutic efficacy.18 With the development of immunotherapies for GBM, safe reduction of corticosteroids may become pertinent to the success of existing, novel, and combination therapies.
Current Approaches
Vaccines
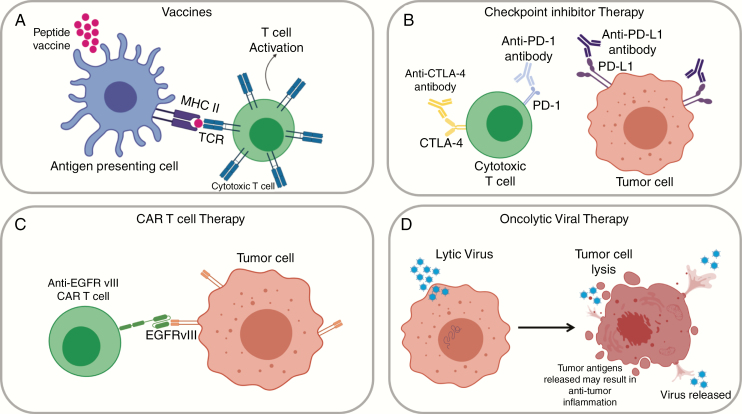
Vaccines aim to stimulate an intrinsic immune response to the tumor, with the primary goal of increasing effector cell activation and infiltration. There are a wide variety of vaccine approaches that are currently being evaluated in GBM, but they can be fundamentally categorized into 2 classes: peptide/DNA vaccines, and cell-based vaccines (Fig. 1A; Table 1). Peptide and DNA vaccines involve the injection of tumor-specific antigens or nucleic acids, often paired with immune-stimulatory molecules to strengthen the adaptive immune response to the tumor. Tumor-specific antigens are present on tumor cells but not on normal healthy cells. Cell-based therapies rely on antigen-presenting cells, such as DCs, to induce an adaptive immune response. DCs function as a mediator between the innate and adaptive arms of the immune system, and are professional antigen-presenting cells capable of activating potent T- and B-cell responses. In this arm of vaccine therapy, patient-derived DCs are isolated from patient blood, matured, and loaded with tumor antigen. Subsequently, the DCs are injected back into the patient. In addition to DC-based vaccines, tumor lysates or fixed tumor cells have been directly injected.19 Of all current immunotherapies for GBM, vaccines have been one of the most thoroughly investigated strategies to date. Although more comprehensive reviews of vaccine approaches have been published,20–23 a few recent approaches are discussed here.
Fig. 1.
Immunotherapy concepts for glioblastoma (GBM). A, Vaccines can be peptide/DNA based or cell based. Their goal is to promote antigen presentation and T-cell infiltration. Shown here is a peptide vaccine, which may encompass both broad GBM antigens and patient-specific antigens. B, Checkpoint inhibitor therapy relies on antibodies to inhibit immune-checkpoint molecules. Programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) are expressed on T cells and promote self-tolerance in healthy tissues. In GBM, this mechanism is exploited by tumor cells that express programmed death ligand 1 (PD-L1). Immune-checkpoint inhibitors bind to these molecules and limit T-cell inhibition. C, Chimeric antigen receptor (CAR) T-cell therapy uses genetically engineered T cells to target tumor-specific or associated antigens. CAR T-cell activation does not rely on MHC-dependent antigen presentation, and efficiently targets surface antigens, such as epidermal growth factor receptor variant III (EGFRvIII). D,) Viral therapies use both lytic and nonlytic viruses to kill tumor cells and induce an immune response. These viruses may directly kill tumors cells by lysis or encode enzymes that convert prodrugs into cytotoxic chemotherapies. Figures were created with BioRender.com.
Table 1.
Completed and Ongoing Clinical Trials for Glioblastoma Immunotherapy
| Vaccines | |||||
|---|---|---|---|---|---|
| Clinical Trial Name | Description | No. of Participants | Primary Outcome Measure | Phase | Study Completed |
| NCT01480479 (ACT IV) | Rindopepimut (CDX-110) + TMZ; newly diagnosed EGFRvIII GBM patients | 745 | Overall survival | 3 | Yes |
| NCT02193347 (RESIST) | IDH peptide vaccine (PEPIDH1M) + TMZ; recurrent grade II glioma patients | 24 | % patients with unacceptable (grade 3) toxicity | 1 | No |
| NCT02960230 | H3.3K27M peptide vaccine; children with newly diagnosed DIPG/other gliomas | 29 | % patients with AEs; overall survival at 12 mo | 1 | No |
| NCT01920191 (IMA-950) | IMA950 Multipeptide vaccine + poly-ICLC; newly diagnosed GBM patients | 19 | Tolerability and safety | 2/3 | Yes |
| NCT02924038 | Varlilumab (CDX-1127) + IMA950/poly-ICLC; low-grade glioma patients | 30 | AEs; CD4 + and CD8 + T-cell responses | 1 | No |
| NCT02149225 (GAPVAC) | APVAC 1 and 2 + GM-CSF + poly-iCLC + TMZ; newly diagnosed GBM patients | 16 | AEs | 1 | Yes |
| NCT02287428 (NeoVax) | Neoantigen cancer vaccine (NeoVax) + RT + pembrolizumab MGMT unmethylated; newly diagnosed GBM patients | 46 | AEs; No. of patients with actionable peptides; No. of patients able to receive post-RT vaccine therapy | 1 | No |
| NCT01280552 (ICT-107) | Randomized, double-blind, controlled study of ICT-107 with maintenance TMZ; newly diagnosed GBM following resection | 124 | Overall survival | 2 | Yes |
| NCT00045968 (DCVax-L) | Randomized, double-blind, controlled study of DCVax-L; newly diagnosed GBM following resection | 348 | PFS | 3 | Yes |
| NCT03018288 (HSPPC-96) | Randomized, double-blind study of RT + TMZ and pembrolizumab with and without HSPPC-96; newly diagnosed GBM | 108 | 1-y overall survival | 2 | No |
| Checkpoint Inhibitors | |||||
| Clinical Trial Name | Description | No. of Participants | Primary Outcome Measure | Phase | Study Completed |
| NCT02017717 (CheckMate 143) | Nivolumab alone nivolumab + ipilimumab comparator: bevacizumab; recurrent GBM patients | 626 | Safety and tolerability: Overall survival | 3 | Yes |
| NCT02617589 (CheckMate 498) | Nivolumab + RT comparator: TMZ + RT MGMT unmethylated; newly diagnosed GBM patients | 550 | Overall survival | 3 | No |
| NCT02667587 (CheckMate 548) | Nivolumab + TMZ + RT comparator: TMZ + RT MGMT unmethylated; newly diagnosed GBM patients | 693 | PFS; overall survival | 3 | No |
| NCT02337491 | Pembrolizumab alone pembrolizumab + bevacizumab; recurrent GBM patients | 80 | Pembrolizumab maximum tolerated dose; pembrolizumab dose-limiting toxicity at 6 mo. PFS | 2 | Yes |
| NCT02313272 | Hypofractionated stereotactic irradiation + pemobrolizumab + bevacizumab; recurrent HGG patients | 32 | Pembrolizumab maximum tolerated dose | 1 | No |
| CAR T-cell therapy | |||||
| Clinical Trial Name | Description | No. of Participants | Primary Outcome Measure | Phase | Study Completed |
| NCT02208362 | IL13Rα2-specific CAR T cells; refractory or recurrent malignant glioma patients | 92 | AEs (grade 3 or higher); dose-limiting toxicity; incidence of toxicities (including neurological); | 1 | No |
| NCT02209376 | EGFRvIII CAR T cells; EGFRvIII GBM patients | 11 | No. of AEs (2 y) | 1 | Yes |
| NCT01109095 | HER2 virus-specific CAR T cells | 16 | Dose-limiting toxicity after T-cell infusion | 1 | Yes |
| NCT02442297 | HER2 CAR T cells | 28 | Dose-limiting toxicity after T-cell infusion | 1 | No |
| Oncolytic Viral therapy | |||||
| Clinical Trial Name | Description | No. of Participants | Primary Outcome Measure | Phase | Study Completed |
| NCT00805376 (DNX-2401) | DNX-2401 (conditionally replication-competent adenovirus) ± surgery; recurrent HGG patients | 37 | Maximum tolerated dose | 1 | Yes |
| NCT03896568 (Ad5-DNX-2401) | Ad5-DNX-2401 (oncolytic adenovirus) in bone marrow human mesenchymal stem cells; recurrent HGG patients | 36 | Maximum tolerated dose Incidence adverse events | 1 | No |
| NCT01491893 (PVSRIPO) | Recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO); recurrent GBM patients | 61 | Maximum tolerated dose; dose-limiting toxicities; recommended phase 2 dose | 1 | No |
| NCT02414165 (Toca 5) | Toca 511 (retroviral replicating vector encoding cytosine deaminase) + Toca FC (flucytosine) comparator: lomustine, TMZ, bevacizumab; recurrent HGG patients | 403 | Overall survival | 2/3 | Yes |
| NCT02457845 (G207) | HSV G207 (first-generation oncolytic HSV-1) + RT; children with recurrent supratentorial brain tumors | 18 | Safety and tolerability (grade 3 or higher AEs) | 1 | Yes |
| NCT03152318 (rQNestin) | rQNestin34.5v0.2 (oncolytic HSV-1) + cyclophosphamide; recurrent malignant glioma patients | 108 | Maximum tolerated dose | 1 | No |
| NCT00390299 | MV-CEA (carcinoembryonic antigen-expressing measles virus); recurrent GBM patients | 23 | Maximum tolerated dose; no. and severity of all AEs; no. and severity of grade 3 + AEs; overall toxicity | 1 | Yes |
| NCT01301430 (ParvOryx01) | H-1PV; progressive primary or recurrent GBM patients | 18 | Safety and tolerability | 1/2a | Yes |
| NCT03714334 (DNX-2440) | DNX-2440 conditionally replication-competent adenovirus armed with OX40 ligand (T-cell stimulator); recurrent GBM patients | 24 | Treatment-emergent AEs | 1 | No |
| NCT02062827 (M032-HSV-1) | M032-HSV-1 (second-generation oncolytic HSV) armed with IL-12 (immune-stimulatory); recurrent progressive/GBM patients | 36 | Maximum tolerated dose | 1 | No |
| Combination Therapies | |||||
| Clinical Trial Name | Description | No. of Participants | Primary Outcome Measure | Phase | Study Completed |
| NCT03726515 | CART-EGFRvIII + pembrolizumab; newly diagnosed EGFRvIII MGMT-unmethylated GBM patients | 7 | No. of patients with treatment-related AEs | 1 | No |
| NCT03422094 | NeoVax + ipilumumab/nivolumab; newly diagnosed unmethylated GBM | 30 | Safety and tolerability; identification of tumor-specific neoantigens | 1 | No |
| NCT02798406 (CAPTIVE) | DNX-2401 + pembrolizumab; recurrent GBM patients | 49 | Objective response rate (tumor size reduction) | 2 | No |
| NCT03665545 (IMA950-106) | Pembrolizumab + IMA950/poly-ICLC; relasping GBM | 24 | Treatment-emergent AEs | 2/3 | No |
All information taken from www.clinicaltrials.gov. Information current as of February 9, 2020.
Abbreviations: AEs, adverse events; DIPG, diffuse intrinsic pontine glioma; EGFRvIII, epidermal growth factor receptor variant III; GBM, glioblastoma; H-1PV, H-1 parvovirus; HGG, high-grade glioma; HSPPC-96, heat-shock protein peptide complex-96; HSV, herpes simplex virus; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine-DNA methyltransferase; PFS, progression-free survival; RT, radiation therapy; TMZ, temozolomide.
Epidermal growth factor receptor variant III (EGFRvIII) is a GBM-specific antigen that is present in approximately 20% of GBM patients. Such specificity allows for minimal off-target toxicities, but its heterogeneous expression throughout the tumor remains a difficulty. In a phase 2 trial, a peptide vaccine against EGFRvIII, rindopepimut, was paired with temozolomide (TMZ). Although this trial reported promising results and verified safety, the phase 3 trial (NCT01480479) did not show any increase in overall survival.24 Because the single antigen-targeting vaccine relies on stable widespread expression of the EGFRvIII epitope throughout the tumor, antigen loss and variable expression between tumor cells and patients may have contributed to the outcomes of the phase 3 trial.25,26
Isocitrate dehydrogenase mutation (IDH1R132H) has also become a target for peptide-based vaccines. A current investigational trial (NCT02193347) is evaluating safety and immunological activity of a vaccine (PEPIDH1M) targeted to the IDHR132H mutation seen in patients with IDHR132H + World Health Organization grade III and IV recurrent glioma.27
An ongoing trial (NCT02960230) is investigating an H3.3K27M epitope-specific vaccine for pediatric diffuse intrinsic pontine glioma (DIPG) and non-DIPG glioma patients.28 More than 70% of DIPG patients harbor the H3.3K27M mutation in histone 3 variant 3; the H3.3K27M mutation serves as a negative prognostic marker because patients with the mutation have decreased survival outcomes.29,30 The use of Poly-ICLC adjuvant along with synthetic H3.3K27M as a peptide-based vaccine may increase antigen presentation against the H3.3K27M epitope and facilitate cytotoxic T-cell infiltration. Both IDHR132H and H3.3K27M mutations are 2 very early mutations in glioma development and are reported to be present uniformly throughout the tumor tissue, making them attractive therapeutic targets with low to minimum risk of antigen-loss escape, unlike EGFRvIII-targeting vaccines discussed earlier.27,29,31
In addition to approaches that target a single peptide, vaccines that target multiple peptides are also being developed and tested. For example, IMA-950 is a peptide vaccine that combines 11 common GBM-derived antigens. The vaccine is composed of 9 CD8 + T-cell antigens and 2 CD4 + T-cell antigens. The 11 peptides are immunogenic and are targeted toward increased cytotoxic T-cell and helper T-cell function in GBM patients. In a phase 1 trial, IMA-950 plus granulocyte-macrophage colony stimulating factor (GM-CSF) was given with standard therapy (radiation therapy [RT] + TMZ). Ninety percent of patients were classified as responders, with 50% responding to multiple peptides. Responders produced specific peripheral CD8 + T-cell responses to the tumor-associated antigens. Interestingly, steroid administration did not affect immune response to the IMA-950 antigens.32 Results of the phase 1/2 trial (NCT01920191) were published in 2019 and reported safety of the IMA-950 vaccine paired with the Poly-ICLC adjuvant. Patients exhibited T-cell responses both to single and multiple peptides; median survival was reported at 19 months.33 Two current trials using the IMA-950 vaccine are ongoing: in conjunction with pembrolizumab for relapsing GBM (NCT03665545) and in conjunction with varlilumab for grade II low-grade gliomas (NCT02924038). Because of patients’ relatively intact immune system, vaccine-specific responses in patients with low-grade glioma have been extremely promising.34 Slow growth of their tumors (ie, low-grade glioma) also allows for repeated vaccinations, which may be necessary to mount high levels of vaccine-reactive T responses. As such, vaccine approaches may be particularly suitable for patients with World Health Organization grade II low-grade glioma.
Another class of GBM vaccine therapies encompass personalized vaccine approaches that target neoantigens. The GAPVAC 101 phase 1 trial (NCT02149225) exploited both unmutated antigens (APVAC1 arm) and neoantigens (APVAC2 arm) to individualized patient-specific vaccines. APVAC2 vaccines were chosen from transcriptomes and immunopeptidomes of patient tumor samples. Poly-ICLC and GM-CSF were used as adjuvants. The vaccines were injected intradermally during TMZ maintenance therapy. The study found that APVAC1 peptides were immunogenic and resulted in specific effector and memory T cells. Additionally, APVAC2-vaccinated neoepitopes resulted in a CD4 + T-cell response, but no CD8 + T-cell activation.35 Another phase 1/1b trial (NCT02287428) used a personalized neoantigen vaccine in conjunction with RT and pembrolizumab for newly diagnosed GBM patients. In contrast, patients who did not receive dexamethasone during vaccine priming had strong immune responses against neoantigens of interest and increased CD4 + and CD8 + T-cell infiltration. In contrast, patients who received dexamethasone did not generate an IFN immune-stimulating response. Median progression-free survival was 7.6 months and median overall survival was 16.8 months.36 Although these personalized vaccine approaches may address interpatient heterogeneity and may induce truly tumor-specific (ie, neoantigen-specific) immune responses, the magnitude of vaccine-specific T-cell responses appears relatively low35 compared to the levels that can be achieved by adoptive-cell transfer approaches. Further engineering and combination with potent immunoadjuvants may be necessary to improve the potency of vaccine approaches.
A phase 2 (NCT 01280552), double-blind, placebo-controlled trial for the DC vaccine ICT-107 was also conducted. The study used autologous DCs that target 6 GBM tumor-associated antigens. The trial resulted in significantly increased progression-free survival in the treatment group with a marked immunological response in a subset (HLA-A2) of patients.37 A phase 3 trial was begun but suspended in 2017 because of inadequate funding.
DCVax-L, another DC-based vaccine, was evaluated in a phase 3, randomized, double-blind, placebo-controlled trial. DCVax-L uses autologous DCs pulsed with tumor lysate. Patients received standard-of-care surgery and radiotherapy with concurrent TMZ. Following standard-of-care treatment, patients were randomly assigned to receive TMZ plus DCVax-L or TMZ plus placebo. At recurrence, all patients were eligible to receive DCVax-L without unblinding. Median overall survival was 23.1 months, with a subgroup of patients (n = 100) exhibiting a 40.5-month median overall survival.38
Heat-shock protein peptide complex-96 (HSPPC-96) is another vaccine-based approach. HSPPC-96 contains tumor-derived glycoprotein-96 and triggers both innate and adaptive immune responses. HSPs bind tumor-associated antigens and can be taken up by antigen presenting cells. HSPs, with HSPPC-96 most common in glioma, are a promising target for innate immune activation. Patient-derived HSP complexes are purified and directly injected as a personalized vaccine. 39In a phase 1 trial (NCT02122822), HSPPC-96 was deemed safe for GBM patients. A randomized, double-blind, placebo-controlled phase 2 trial (NCT03018288) is currently ongoing evaluating radiotherapy plus TMZ and pembrolizumab with or without HSPPC-96.
Immune-Checkpoint Inhibitors
Immune-checkpoint inhibitors, defined as antibodies that block pathways reducing antitumor T-cell activation, have had success in multiple cancer types, including melanoma and non–small cell lung cancer.40–42 The most successful inhibitors have been aimed at immune-checkpoint inhibitor proteins programmed cell death protein 1 (PD1), PD-L1, and CTLA-4 (Fig. 1B; Table 1). PD1 and CTLA-4 are expressed on T cells, and act as immunomodulatory checkpoints to promote self-tolerance and reduce aberrant T-cell–mediated inflammation. 42CTLA-4 can suppress naive and memory T-cell activation by blocking signaling of costimulatory molecules. 43In healthy brain tissue, PD-L1 is expressed by a subset of immune cells, including macrophages and microglia, and its expression is further upregulated in the GBM microenvironment.44,45 Additionally, PD-L1 has been shown to be expressed by antigen-presenting cells, tumor cells, and parenchymal cells in GBM patients.44,46,47 Interaction of PD1 and PD-L1 leads to reduced proliferation of antigen-specific effector T cells and reduced apoptosis of T-regulatory cells, leading to a dampened adaptive immune response.48
Preclinical animal models of anti-PD1 therapy showed promising results in an orthotopically implanted GL-261 GBM model.49,50 However, the GL261 model has a higher mutational load than human GBM samples. Mutational load has been conjectured to play a large role in the success of immune-checkpoint inhibitors. Successes in melanoma and non–small cell lung cancer are strongly correlated with the high mutational loads of these tumor types.14 SB28, another GBM model, has been shown to more accurately reflect human GBM tumors because it has fewer mutations and low MHC class I expression and CD8 + T cell infiltration.51 A study by Genoud et al found that combination anti-PD1/anti-CTLA-4 checkpoint-inhibitor therapy was curative in more than 50% of mice with GL261 tumors but had no impact on SB28 tumors.51 The disparity in mutational load between patient-derived tumors, as reflected by the SB28 and GL261 models, may help to explain the outcomes of immune-checkpoint inhibitors in clinical trials.
Nivolumab, an anti-PD-1 antibody, has been used most widely in clinical trials.52 The phase 3 clinical trial CheckMate 143 (NCT02017717) was one of the first large trials to evaluate the effectiveness of anti–PD-1 antibodies in GBM. The trial compared bevacizumab (a VEGF inhibitor) to either nivolumab monotherapy or nivolumab/ipilimumab (anti–CTLA-4 antibody) combination therapy in recurrent GBM patients.53 As of 2017, nivolumab alone did not result in prolonged overall survival compared to bevacizumab; both arms of the trial resulted in a 42% 12-month overall survival. The trial resulted in significantly higher adverse effects in patients receiving the combination therapy. This arm of the trial (combination therapy) was discontinued and PD-1 monotherapy was continued.53
Standard GBM treatments, such as TMZ and RT, further compound tumor-mediated immunosuppression by causing widespread lymphopenia, with a dramatic reduction in CD4 + T cells.54 Lymphopenia compounded with poor T-cell infiltration may also contribute to poor responses to immune-checkpoint inhibitor therapy. Further, the efficiency of targeting biologics, such as antibodies, to the brain through the blood-brain barrier is another likely confounding factor in the failure of these therapies in GBM. Another phase 3 trial (NCT02617589), CheckMate 498, evaluated nivolumab plus RT and TMZ plus RT in O6-methylguanine-DNA methyltransferase (MGMT)-unmethylated newly diagnosed GBM patients. The trial did not meet its primary end point of overall survival.55 A subsequent and ongoing phase 3 trial, CheckMate 548 (NCT02667587), is comparing nivolumab plus RT with TMZ plus radiotherapy in patients with MGMT-methylated tumors. The study did not show statistically significant improvement in progression-free survival and therefore missed one of its primary end points; overall survival assessment is ongoing.56
In a 2019 study by Zhao et al, authors investigated factors that lead to positive or negative response to anti–PD-1 therapy in recurrent GBM patients. They analyzed whether response to anti–PD-1 (pembrolizumab and nivolumab) therapy is associated with certain patient biomarkers and immune expression signatures. The authors profiled 66 patients and over time and collected DNA, RNA, and performed tissue imaging. This information was paired with clinical outcomes to determine a trend of immunotherapy response. Patients who responded to anti–PD-1 treatment had significant enrichment of MAPK pathway members, such as BRAF and PTPN11. Nonresponders had an enrichment of PTEN mutations resulting in immunosuppressive gene signatures.57
Another 2019 study by Cloughesy and colleagues showed that patients who received neoadjuvant pembrolizumab and continued adjuvant therapy after surgery had improved overall survival compared with patients who received adjuvant pembrolizumab alone. The authors concluded that neoadjuvant anti–PD-1 therapy results in increased infiltration of lymphocytes into the tumor, and this is a major factor contributing to improved overall survival. Interestingly, presurgical and postsurgical tumor volume and dexamethasone administration were not identified as factors affecting patient outcome.58
Owing to their promising results in other tumor types, checkpoint blockades remain a viable target for treating GBM. Biomarker screening of patients and selective use of checkpoint blockades may result in durable responses for a subset of patients. Combination therapies pairing checkpoint blockades with bevacizumab (NCT02337491) or laser ablation (NCT02313272) are being evaluated.
Chimeric Antigen Receptor T Cells
Chimeric antigen receptor (CAR)-based therapies rely on genetically modified T cells that express CARs engineered to recognize cancer-associated cell surface antigens59 (Fig 1C; Table 1). They have been previously shown to be efficacious in lymphoma and acute leukemia patients.59,60 The CAR is a hybrid of an antigen recognition domain, generally derived from a single-chain antibody, fused to the T-cell activation domain (CD3ζ); the addition of one or more costimulatory receptor intracellular domains (4-1BB, CD28, OX40) is common to increase T-cell activation and response.61 Interestingly, CAR T cells are activated by direct binding to the target antigen but independently of antigen presentation by the MHC, which is responsible for processing and presenting internal cellular antigens. Because CAR T cells do not rely on MHC presentation, they can recognize cell surface antigens and are useful in targeting specific tumor antigens that may not be efficiently presented in the context of a particular patient’s MHC repertoire.59,62
A promising target for CAR T therapy is IL-13Rα2. It is overexpressed in approximately 75% of GBM tumors and is linked to increased tumor invasiveness.63 In a clinical trial (NCT02208362) of CAR T-cell therapy targeting IL-13Rα2, there was a dramatic response (~ 80% average tumor shrinkage of all 7 lesions) in 1 patient receiving intratumoral and intraventricular infusion of these CAR T cells. The patient had no detectable lesions or spine metastases for 7.5 months following CAR treatment. However, 4 new lesions became detectable at 228 days after the first CAR T-cell infusion. The emergence of new lesions was likely due to the outgrowth of cells not expressing surface IL-13Rα2.64
Another clinical trial (NCT02209376) employed a single intravenous infusion of autologous T cells engineered to express a CAR against EGFRvIII mutation in 10 recurrent patients with EGFRvIII + GBM.34 The EGFRvIII mutation results in deletion of exons 2 through 7 and creates an immunogenic GBM-specific antigen.65 The single dose of CAR T cells against EGFRvIII was delivered intravenously and did not result in cytokine release syndrome (CRS). Systemic CRS occurs when immune cells (B cells, T cells, DCs, and macrophages) become activated and release inflammatory cytokines, resulting in further immune activation. CRS may be life-threatening and is a major concern in adoptive T-cell therapies.66,67 All patients in this study demonstrated detectable transient expansion of CART-EGFRvIII cells in peripheral blood. In 5 of 7 patients who underwent tumor resection post–CAR T-cell infusion, trafficking of CAR T cells to tumor sites and reduction of EGFRvIII expression levels were noted. As reported, CAR T-cell infiltration was associated with robust induction of inhibitory molecules, such as IDO1, PD-L1, and FoxP3, and infiltration by regulatory T cells.68 Similar to results seen in the IL-13Rα2 CAR T study, 64the phase 1 EGFRvIII-targeted trial revealed a significantly decreased expression of the CAR-targeted antigen expression in recurrent tumor cells. These results suggest immunoediting of the tumor and outgrowth of tumor cells without EGFRvIII expression.
A third potential target for CAR T-cell therapy is human epidermal growth factor receptor 2 (HER2). HER2 is a receptor tyrosine kinase that is overexpressed in some GBM tumors and other cancer types, making it a possible CAR target. Two phase 1 trials have targeted HER2 (NCT02442297, NCT01109095). In the completed study (NCT01109095), investigators used virus-specific (CMV, Epstein-Barr, or adenovirus) T cells to express the CAR construct. This allows for enhanced immune activation by presentation of latent viral antigens. Although the trial was deemed safe, expansion of HER2-CAR T cells did not occur in the blood.69 In the currently ongoing trial (NCT02442297), non–virus-specific autologous T cells expressing the HER2 CAR are being tested.
These data imply that development of successful CAR T therapy for GBM will require further engineering and/or integration of strategies to improve CAR T homing and persistence in the GBM tissue, overcome local immunosuppression, and address marked antigenic heterogeneity of GBM.
Oncolytic Viral Therapy
The use of viral vectors for gene therapy of GBM has been evaluated for more than 3 decades70,71 (Fig 1D; Table 1). However, the failure of most clinical trials to show therapeutic efficacy using conventional replication-defective viral vectors has resulted in investigation into tumor-selectively replicating viruses, or oncolytic viruses.72 Oncolytic viruses are able to selectively replicate in and kill cancer cells. Cancer cells, including CNS tumors, have defects in innate cellular immune defenses that allow for replication, production of viral proteins, and budding or lysis.73 Viruses used as oncolytic agents are commonly attenuated or contain deletions in virulence factors, blocking their ability to infect and spread through healthy tissues.73 Therefore, oncolytic viruses may preferentially infect tumor cells, which commonly have defects in interferon signaling, which activates antiviral defense pathways that normally block viral replication.74
However, replication-competent oncolytic viruses, as well as conventional replication-defective viral vectors, both can result in activation of the adaptive immune system. Through activation of toll-like receptor and pathogen-associated molecular pattern sensors, viral infection can stimulate DCs to produce type I IFNs that result in a proinflammatory immune response. 74,75IFN upregulation causes production of the cytokines CXCL9, CXCL10, and CXCL11, which are regulators of T-cell trafficking and infiltration.76,77 In addition to cytokine signaling, viral-induced cell lysis can create physical space for T-cell infiltration by disrupting tissue architecture and extracellular matrix, and causes the release of endogenous tumor antigens.78
Several viruses, including adenovirus, measles virus (MV), polio virus, parvovirus, HSV, and retroviral replicating vectors (RRVs) have been engineered to treat GBM.79,80
A phase 1 clinical trial (NCT00805376) using the replication-competent oncolytic adenovirus DNX-2401 was conducted in patients with recurrent glioma.81 The DNX-2401 virus contains a deletion in the E1A gene, which inhibits viral replication in nonmalignant cells with a functional retinoblastoma pathway. This virus has been shown to cause glioma cell death and enhanced antitumor immunity in preclinical models.82,83 In the clinical trial, patients received either (A) a single intratumoral injection of the virus via an implanted catheter or (B) an initial intratumoral injection followed by tumor resection and subsequent further viral injections into the walls of the resection cavity. Viral replication was observed in the tumors of group B patients. Patients in group A showed reduction in tumor size, and 20% of patients survived more than 3 years. Tumor necrosis and CD8 + T-cell infiltration were detected, but no change in the immunosuppressive molecules PD-1, PD-L1, and IDO-1H were detected. A current phase 1 clinical trial (NCT03896568) in recurrent GBM is evaluating safety of allogeneic bone marrow-derived human mesenchymal stem cells with the DNX-2401 virus.
A recent clinical trial (NCT01491893) using recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO) showed the safety and encouraging preliminary efficacy of the virus in recurrent GBM patients.84 PVSRIPO recognizes CD155, a poliovirus receptor often expressed on GBM cells, and is attenuated by being engineered with a different internal ribosome entry site sequence from rhinovirus. Its tumor selectivity is due to the lack of innate intracellular immunity in GBM cells. The trial resulted in increased survival in patients at 24 and 36 months compared to historical controls.59
Another approach, combining virotherapy and gene therapy, uses a nonlytic RRV. The virus, Toca 511 (vocimagene amiretrorepvec), integrates into the host genome and spreads efficiently through rapidly proliferating cells, and encodes yeast cytosine deaminase as a prodrug activator, which converts the prodrug 5-fluorocytosine (Toca FC) into the potent chemotherapeutic drug 5-fluorouracil.83,85 As the virus integrates permanently into the cancer cell genome, oral administration of the prodrug can achieve tumor-cell killing over multiple cycles. Preclinical studies showed prolonged survival, intratumoral infiltration of T cells, and a decrease in immunosuppressive myeloid cells after prodrug conversion.86,87 Phase 1 clinical trials evaluating Toca 511 in recurrent high-grade glioma patients established the safety of the virus and persistence of RRV in the tumor, and promising evidence of therapeutic benefit.88 In 2019, a phase 3 trial was completed in recurrent GBM patients undergoing resection. The trial missed its primary end point, but a patient subset analysis has yet to be published.89
HSV, MV, and parvovirus have also been used in oncolytic viral therapies. HSV-based therapies include HSV1716, G207 (NCT02457845), rQNestin 34.5 (NCT03152318), and G47Δ viruses.90,91 Recently it has been reported that G47Δ was able to achieve a 1-year survival rate of 92% in recurrent GBM patients after repeated stereotactic intratumoral injections on rerecurrence.92 An ongoing phase 1 trial using oncolytic MV (Carcinoembryonic Antigen-Expressing Measles Virus) is being assessed in recurrent GBM patients (NCT00390299). A phase 1/2a clinical trial using an oncolytic parvovirus (ParvOryx01) (NCT01301430) resulted in a proinflammatory response and tumor infiltration with activated CD8 + cytotoxic T cells.93,94
Encouraging survival data (ie, long tail in survival curves) suggest that oncolytic viruses may be truly effective in subpopulations of patients. Therefore, it is critically important to find solid biomarkers for selecting patient populations who are more likely to respond.
Future Directions and Combination Therapies
With the lack of success in immune monotherapies, it has become clear that combination therapies are a promising path forward. Because most of the current immunotherapies target only one immunological mechanism, there is a clear need to develop coordinated approaches to attack the comprehensive aspects of immune mechanisms Table 1.
Vaccine-based immunotherapies may be a useful and promising treatment for gliomas, especially in combination with other therapies, such as adjuvant and/or checkpoint blockade therapy. The flexibility of a vaccine approach allows for the development both of patient-specific and broad, common-tumor, antigen-targeted therapies.
As a monotherapy, immune-checkpoint inhibitors have not resulted in improved survival outcomes in malignant gliomas, unlike some other tumor types. However, they remain an extremely viable target for combination therapies. For example, cytokine therapies to increase T-cell infiltration or myeloid-based approaches may result in increased efficacy of immune-checkpoint inhibitors. Further, it has been reported that EGFRvIII CAR T-cell therapy resulted in marked upregulation of PD-L1 in the tumor microenvironment; accordingly, combining adoptive T-cell therapy with an anti–PD-L1 antibody may show promise as a combination therapy.68 This is currently being evaluated in a combination EGFRvIII CAR T and pembrolizmuab phase 1 clinical trial in newly diagnosed GBM patients (NCT03726515). Another ongoing pilot study is evaluating the safety and immunogenicity of NeoVax (a personalized neoantigen-based vaccine) plus ipilimumab or nivolumab (NCT03422094).
In this context, although CAR T cells present a promising opportunity for tumor-specific targeted therapy, antigen escape and intratumoral heterogeneity remain a hindrance. As with other monotherapies, CAR T-cell therapy would likely be enhanced by combinatory approaches to increase T-cell homing, persistence, and function as well as antigen spreading in the tumor.
Another promising strategy in the developing field of oncolytic virotherapy is arming these viruses with immunoregulatory genes to enhance their cytotoxic potency and immunostimulatory effects. For example, a phase 1 clinical trial is investigating the DNX-2440 virus, which is the DNX-2401 adenovirus armed with OX40 Ligand (NCT03714334).95 As another example, an HSV-based virus, M032-HSV-1, is armed with IL-12 to further activate antitumor immunity and limit angiogenesis (NCT02062827).96
Combination therapies using immune-checkpoint inhibitors along with oncolytic viruses are also being investigated. For example, a current phase 2 trial is combining DNX-2401 and pembrolizumab (an anti–PD-1 checkpoint inhibitor) in recurrent GBM patients. Furthermore, oncolytic viruses are also being used to deliver checkpoint inhibitor agents for expression directly within the tumor, thereby mitigating the potential for systemic autoimmune adverse effects. Mitchell et al described an RRV that carries a single-chain variable fragment (scFv)–PD-L1. On infection with the virus, target cells release the scFv–PD-L1 that blocks PD-1 binding, resulting in a robust antitumor immune response in preclinical models.97
For the success of any immunotherapeutic strategy, a major goal is to increase antitumor T-cell activity and antigen presentation within the tumor, although the microheterogeneity of GBM presents a challenge because this predisposes to immunoediting and tumor recurrence. The profoundly immunosuppressive nature of these tumors must also be overcome or altered to evoke a sustained and targeted antitumor immunity.98,99 Combination therapies to activate T cells and simultaneously eliminate or reprogram suppressive myeloid cells is a promising avenue of investigation. Inducing a coordinated antitumor response in T cells, DC, and macrophages is also being investigated.100
Conclusions
Many immunotherapeutic approaches have been evaluated in patients with GBM, including checkpoint inhibitors, CAR T cells, oncolytic viral therapy, and vaccines. The limited success of these therapies, in comparison with results obtained in other tumor types, presents a challenge for the field of cancer immunotherapy. The microheterogeneity of glioma cells even within the same tumor, the strong presence of immunosuppressive cells in the tumor microenvironment, and the paucity of infiltrating T cells may limit effective and durable antitumor responses. In addition, better homing and function of antitumor effector cells, as well as increased epitope spreading, would likely contribute to the effectiveness both of monotherapies and combination therapies for GBM tumors. Combinatorial approaches are being pursued and are likely to show improved therapeutic results in clinical trials.
It is important to recognize that some promising outcomes in early-phase, single-arm trials failed in subsequent pivotal trials with randomized design (eg, rhindopepimut, Toca511 as discussed earlier). Comparison with historical data for single-arm studies of immunotherapy has been known to have typical pitfalls, such as selection of eligible patients with low tumor burden and no or low corticosteroid use. Investigators should be aware of these limitations and incidences in the past single-arm trials, and should conduct appropriate comparative trials before declaring any treatment as anything more than promising.
Funding
This work was funded by National Institutes of Health/National Institute of Neurological Disorders and Stroke 1R35 NS105068 [H.O.], National Institutes of Health/National Cancer Institute 1R01CA222965 [H.O.], Department of Defense CA181015P1 [N.K.], and Clinical Translation Award from the Alliance for Cancer Gene Therapy [A.C.G.T., N.K.].
Conflict of interest statement.
References
- 1. Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123–131. [DOI] [PubMed] [Google Scholar]
- 2. Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48(2):380–395.e6. [DOI] [PubMed] [Google Scholar]
- 3. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. [DOI] [PubMed] [Google Scholar]
- 6. Müller S, Kohanbash G, Liu SJ, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei J, Marisetty A, Schrand B, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wei J, Chen P, Gupta P, et al. Immune biology of glioma associated macrophages and microglia: functional and therapeutic implications. Neuro Oncol. 2020;22(2):180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huettner C, Czub S, Kerkau S, et al. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 1997;17(5A):3217–3224. [PubMed] [Google Scholar]
- 12. Bodmer S, Strommer K, Frei K, et al. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989;143(10):3222–3229. [PubMed] [Google Scholar]
- 13. Hussain SF, Yang D, Suki D, et al. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. ; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain . Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(pt 5):1458–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cenciarini M, Valentino M, Belia S, et al. Dexamethasone in glioblastoma multiforme therapy: mechanisms and controversies. Front Mol Neurosci. 2019;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kong Z, Wang Y, Ma W. Vaccination in the immunotherapy of glioblastoma. Hum Vaccin Immunother. 2018;14(2):255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weller M, Roth P, Preusser M, et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017;13(6):363–374. [DOI] [PubMed] [Google Scholar]
- 21. Sprooten J, Ceusters J, Coosemans A, et al. Trial watch: dendritic cell vaccination for cancer immunotherapy. Oncoimmunology. 2019;8(11):e1638212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Platten M, Bunse L, Riehl D, et al. Vaccine strategies in gliomas. Curr Treat Options Neurol. 2018;20(5):11. [DOI] [PubMed] [Google Scholar]
- 23. Mohme M, Neidert MC, Regli L, et al. Immunological challenges for peptide-based immunotherapy in glioblastoma. Cancer Treat Rev. 2014;40(2):248–258. [DOI] [PubMed] [Google Scholar]
- 24. Schuster J, Lai RK, Recht LD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17(6):854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9(11):4247–4254. [PubMed] [Google Scholar]
- 27. Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. [DOI] [PubMed] [Google Scholar]
- 28. Chheda ZS, Kohanbash G, Okada K, et al. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. 2018;215(1):141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 30. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang PF, Liu N, Song HW, et al. IDH-1R132H mutation status in diffuse glioma patients: implications for classification. Oncotarget. 2016;7(21):31393–31400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rampling R, Peoples S, Mulholland PJ, et al. A Cancer Research UK First Time in Human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016;22(19):4776–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Migliorini D, Dutoit V, Allard M, et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro Oncol. 2019;21(7):923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7(275):275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. [DOI] [PubMed] [Google Scholar]
- 36. Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wen PY, Reardon DA, Armstrong TS, et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25(19):5799–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liau LM, Ashkan K, Tran DD, et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ji N, Zhang Y, Liu Y, et al. Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial. JCI Insight. 2018;3(10):e99145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Queirolo P, Boutros A, Tanda E, et al. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: a model of cancer immunotherapy. Semin Cancer Biol. 2019;59:290–297. [DOI] [PubMed] [Google Scholar]
- 41. Rausch MP, Hastings KT. Immune checkpoint inhibitors in the treatment of melanoma: from basic science to clinical application. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Brisbane, AU: Codon Publications; 2017. [PubMed] [Google Scholar]
- 42. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Topalian SL, Sharpe AH. Balance and imbalance in the immune system: life on the edge. Immunity. 2014;41(5):682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang I, Tihan T, Han SJ, et al. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010;17(11):1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100(9):5336–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. [DOI] [PubMed] [Google Scholar]
- 47. Huang J, Liu F, Liu Z, et al. Immune checkpoint in glioblastoma: promising and challenging. Front Pharmacol. 2017;8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17(8):1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 51. Genoud V, Marinari E, Nikolaev SI, et al. Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology. 2018;7(12):e1501137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8(53):91779–91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grossman SA, Ye X, Lesser G, et al. ; NABTT CNS Consortium . Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bristol-Myers Squibb announces phase 3 CheckMate -498 study did not meet primary endpoint of overall survival with Opdivo (nivolumab) plus radiation in patients with newly diagnosed MGMT-unmethylated glioblastoma multiforme. Press release. Bristol-Myers Squibb. May 9, 2019. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did. Accessed February 10. [Google Scholar]
- 56. Bristol-Myers Squibb provides update on phase 3 Opdivo (nivolumab) CheckMate -548 trial in patients with newly diagnosed MGMT-methylated glioblastoma multiforme. Press release. Bristol-Myers Squibb. September 5, 2019. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-provides-update-phase-3-opdivo-nivolumab-. Accessed February 10, 2020. [Google Scholar]
- 57. Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dotti G, Gottschalk S, Savoldo B, et al. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–113. [DOI] [PubMed] [Google Scholar]
- 62. Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thaci B, Brown CE, Binello E, et al. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro Oncol. 2014;16(10):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. An Z, Aksoy O, Zheng T, et al. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bonifant CL, Jackson HJ, Brentjens RJ, et al. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2019;25(4):e123–e127. [DOI] [PubMed] [Google Scholar]
- 68. O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399) pii: eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ahmed N, Brawley V, Hegde M, et al. HER2-Specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. [DOI] [PubMed] [Google Scholar]
- 71. Foreman PM, Friedman GK, Cassady KA, et al. Oncolytic virotherapy for the treatment of malignant glioma. Neurotherapeutics. 2017;14(2):333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lichty BD, Breitbach CJ, Stojdl DF, et al. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–567. [DOI] [PubMed] [Google Scholar]
- 73. Muik A, Stubbert LJ, Jahedi RZ, et al. Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res. 2014;74(13):3567–3578. [DOI] [PubMed] [Google Scholar]
- 74. Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6(7):821–825. [DOI] [PubMed] [Google Scholar]
- 75. Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Berghuis D, Santos SJ, Baelde HJ, et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8(+) T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223(3):347–357. [DOI] [PubMed] [Google Scholar]
- 77. Brown MC, Holl EK, Boczkowski D, et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci Transl Med. 2017;9(408):eaan4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Twumasi-Boateng K, Pettigrew JL, Kwok YYE, et al. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer. 2018;18(7):419–432. [DOI] [PubMed] [Google Scholar]
- 79. Allen C, Paraskevakou G, Liu C, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther. 2008;8(2):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rajaraman S, Canjuga D, Ghosh M, et al. Measles virus-based treatments trigger a pro-inflammatory cascade and a distinctive immunopeptidome in glioblastoma. Mol Ther Oncolytics. 2019;12:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jiang H, Clise-Dwyer K, Ruisaard KE, et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS One. 2014;9(5):e97407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20(9):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Desjardins A, Gromeier M, Herndon JE II, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ostertag D, Amundson KK, Lopez Espinoza F, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hiraoka K, Inagaki A, Kato Y, et al. Retroviral replicating vector-mediated gene therapy achieves long-term control of tumor recurrence and leads to durable anticancer immunity. Neuro Oncol. 2017;19(7):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mitchell LA, Lopez Espinoza F, Mendoza D, et al. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017;19(7):930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tocagen reports results of Toca 5 phase 3 trial in recurrent brain cancer. Press release. Tocagen. September 12, 2019. Accessed February 10, 2020.https://ir.tocagen.com/news-releases/news-release-details/tocagen-reports-results-toca-5-phase-3-trial-recurrent-brain. [Google Scholar]
- 90. Aghi MK, Chiocca EA. Phase Ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol Ther. 2009;17(1):8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ulasov IV, Borovjagin AV, Schroeder BA, et al. Oncolytic adenoviruses: a thorny path to glioma cure. Genes Dis. 2014;1(2):214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Todo T. Abstract ATIM-14. Results of phase II clinical trial of oncolytic herpes virus G47Δ in patients with glioblastoma. Neuro-Oncology 2019;21(suppl 6):vi4. [Google Scholar]
- 93. Geletneky K, Hajda J, Angelova AL, et al. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol Ther. 2017;25(12):2620–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Angelova AL, Barf M, Geletneky K, Unterberg A, Rommelaere J. Immunotherapeutic potential of oncolytic H-1 parvovirus: hints of glioblastoma microenvironment conversion towards immunogenicity. Viruses. 2017;9(12):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shibahara I, Saito R, Zhang R, et al. OX40 ligand expressed in glioblastoma modulates adaptive immunity depending on the microenvironment: a clue for successful immunotherapy. Mol Cancer. 2015;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Patel DM, Foreman PM, Nabors LB, et al. Design of a phase I clinical trial to evaluate M032, a genetically engineered HSV-1 expressing IL-12, in patients with recurrent/progressive glioblastoma multiforme, anaplastic astrocytoma, or gliosarcoma. Hum Gene Ther Clin Dev. 2016;27(2):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mitchell LA, Yagiz K, Hofacre A, et al. PD-L1 checkpoint blockade delivered by retroviral replicating vector confers anti-tumor efficacy in murine tumor models. Oncotarget. 2019;10(23):2252–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ding AS, Routkevitch D, Jackson C, et al. Targeting myeloid cells in combination treatments for glioma and other tumors. Front Immunol. 2019;10:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McGranahan T, Therkelsen KE, Ahmad S, et al. Current state of immunotherapy for treatment of glioblastoma. Curr Treat Options Oncol. 2019;20(3):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]