Abstract
STUDY QUESTION
Is endosialin a specific marker of human stem Leydig cells (SLCs) with the ability to differentiate into testosterone-producing Leydig cells (LCs) in vitro and in vivo?
SUMMARY ANSWER
Endosialin is a specific marker of human SLCs which differentiate into testosterone-producing LCs in vitro and in vivo.
WHAT IS KNOWN ALREADY
Human SLCs have been identified and isolated using the marker platelet-derived growth factor receptor α (PDGFRα) or nerve growth factor receptor (NGFR). However, the specificity was not high; thus, LCs and germ cells could be mistakenly sorted as SLCs if PDGFRα or NGFR was used as a marker for human SLCs isolation.
STUDY DESIGN, SIZE, DURATION
Firstly, we re-evaluated the specificity of PDGFRα and NGFR for SLCs in adult human testes. Then we analysed the previously published single-cell sequencing data and found that endosialin may identify human SLCs. Subsequently, we sorted endosialin+ cells from four human donors and characterized their self-renewal and multipotent properties. To assess whether endosialin+ cells have the potential to differentiate into functional LCs in vitro, these cells were stimulated by differentiation-inducing medium. We next assessed the in vivo regenerative potential of human endosialin+ cells after xenotransplantation into the testes of immunodeficient mice.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Single-cell sequencing analysis, immunofluorescence and flow cytometry were used to characterize human testis tissues. In vitro colony formation, multipotent differentiation (adipogenic, osteogenic and chondrogenic) and Leydig cell-lineage induction were used to assess stem cell activity. Xenotransplantation into 3-week-old immunodeficient mice was used to determine in vivo regenerative potential. Endpoint measures included testosterone measurements, cell proliferation, immunofluorescence, flow cytometry and quantitative RT–PCR.
MAIN RESULTS AND THE ROLE OF CHANCE
The results indicate that endosialin is a specific marker of SLCs compared with PDGFRα and NGFR. Additionally, endosialin+ cells isolated from human testes show extensive proliferation and differentiation potential in vitro: their self-renewal ability was inferred by the formation of spherical clones derived from a single cell. Moreover, these cells could differentiate into functional LCs that secreted testosterone in response to LH in a concentration-dependent manner in vitro. These self-renewal and differentiation properties reinforce the proposal that human testicular endosialin+ cells are SLCs. Furthermore, transplanted human endosialin+ cells appear to colonize the murine host testes, localize to peritubular and perivascular regions, proliferate measurably and differentiate partially into testosterone-producing LCs in vivo.
LARGE SCALE DATA
NA.
LIMITATIONS, REASONS FOR CAUTION
Owing to the difficulty in collecting human testis tissue, the sample size was limited. The functions of endosialin on SLCs need to be elucidated in future studies.
WIDER IMPLICATIONS OF THE FINDINGS
A discriminatory marker, endosialin, for human SLCs purification is a prerequisite to advance research in SLCs and logically promote further clinical translation of SLCs-based therapies for male hypogonadism.
STUDY FUNDING/COMPETING INTEREST(S)
A.P.X. was supported by the National Key Research and Development Program of China (2017YFA0103802 and 2018YFA0107200). C.D. was supported by the National Natural Science Foundation of China (81971314) and the Natural Science Foundation of Guangdong Province, China (2018B030311039). The authors declare no conflict of interest.
Keywords: stem Leydig cells / male hypogonadism / endosialin / testosterone / testis
Introduction
Leydig cells (LCs) that reside in the testicular interstitium are primarily responsible for testosterone production (Teerds and Huhtaniemi, 2015; Kaufman et al., 2019). Male hypogonadism occurs when LCs fail to restore serum testosterone levels, caused by congenital, acquired or systemic disorders (Basaria, 2014). Administration of exogenous testosterone, known as testosterone replacement therapy, is the most widely used treatment for hypogonadism, and it can help to reverse many of the symptoms caused by low testosterone levels (Bhasin et al., 2018). However, exogenous testosterone replacement can induce many adverse effects, such as polycythaemia (Ponce et al., 2018), sleep apnoea (Fernández-Balsells et al., 2010), infertility (Patel et al., 2019) and increased risks of cardiovascular events (Spitzer et al., 2013). Notably, exogenous testosterone has yet to mimic the physiological patterns of testosterone secretion (Giannoulis et al., 2012). Stem Leydig cells (SLCs), which are capable of regenerating new LCs through proliferation and differentiation, may play a critical role in maintaining LCs homoeostasis in the adult testis (Ge et al., 2006; Jiang et al., 2014; Landreh et al., 2014; Yu et al., 2017). SLCs from rodents have been successfully isolated, expanded and differentiated in vitro (Ge et al., 2006; Jiang et al., 2014). Recent studies have demonstrated that SLCs can replace senescent and chemically disrupted LCs to produce testosterone, indicating that SLCs transplantation is a promising therapy for hypogonadism (Ge et al., 2006; Jiang et al., 2014; Zang et al., 2017; Arora et al., 2019). In contrast to rodent SLCs, human SLCs remain largely uncharacterized and have not yet been used in the clinic, mainly because of the lack of an effective isolation system (Landreh et al., 2014; Zhang et al., 2017; Eliveld et al., 2019). Thus, there is an urgent demand for practical methods for the identification and isolation of human SLCs.
Several options for identifying and isolating putative human SLCs have been described in the past few years. Using a testicular biopsy explant culture system, Landreh et al. (2014) discovered that human testicular peritubular cells can host putative SLCs that exhibit steroidogenic potential under forskolin stimulation in vitro. However, peritubular cells are mixtures of different types of cells in addition to SLCs, such as myoid cells and perhaps LCs (Landreh et al., 2014). Therefore, this method for isolation of human SLCs is suboptimal. Until recently, our group (Zhang et al., 2017) sorted putative human SLCs for the first time through flow cytometric detection of a cell surface protein nerve growth factor receptor (NGFR). The NGFR+ SLCs exhibited the clonogenic self-renewal capacity and showed multi-lineage differentiation potential. More importantly, the putative human SLCs differentiated into functional LCs that expressed LCs lineage markers and produced testosterone in vitro and in vivo. These findings suggested that NGFR could serve as a surface marker of human SLCs. Unfortunately, it has been demonstrated that NGFR is also expressed on pachytene spermatocytes and round spermatids (Jin et al., 2006; Levanti et al., 2006; Lin et al., 2015), which leads to germ cell infiltration in the NGFR-based sorting system and thus lowers the purity of the SLCs population, reducing the utility of the isolated cells in clinical applications. Eliveld et al. (2019) isolated a similar putative SLCs population from human testes through detection of platelet-derived growth factor receptor α (PDGFRα). These cells showed characteristics of mesenchymal stem cells (MSCs) and were capable of differentiating into steroidogenic cells with LCs characteristics. Nevertheless, it is worth noting that PDGFRα is also expressed on LCs at several developmental stages (Brennan, 2003; Ge et al., 2006; Schmahl et al., 2008). Therefore, the purity problem of the putative human SLCs remains to be solved. Furthermore, none of these studies thoroughly examined markers of other testicular cell types in the populations claimed to be SLCs, nor did they provide evidence of whether SLCs retained their crucial adult stem cell characteristics after being transplanted in vivo (Li et al., 2018). To solve the problem of SLCs impurity, which may weaken therapeutic effects and introduce safety problems, distinctive cell surface markers for the identification and isolation of human SLCs are needed.
To achieve this goal, single-cell RNA sequencing (scRNA-seq) approaches can be used to effectively delineate cell types and reveal cell markers (Rennert et al., 2016; Griffiths et al., 2018). We analysed the previously published single-cell transcriptome data on human testes (Sohni et al., 2019) and found that the positive rate of endosialin was the highest on putative SLC populations. Endosialin, also referred to as CD248, is a type I transmembrane glycoprotein ubiquitously expressed in normal somatic tissues and during development (Opavsky et al., 2001; Lax et al., 2010; Hong et al., 2019). Endosialin is also expressed on some MSCs (Naylor et al., 2012) and cancer stem cells (Rouleau et al., 2012; Sun et al., 2015), indicating that endosialin might serve as a surface marker of stem cells. However, the expression pattern of endosialin in human testes and whether endosialin can serve as a discriminatory marker of SLCs remain to be determined. Here, we systematically examined endosialin in adult human testes and subsequently evaluated the feasibility of applying endosialin to identify and isolate human SLCs. In addition, we conducted an in vitro differentiation culture assay and introduced an in vivo xenotransplantation model to assess the regenerative potential of endosialin+ SLCs.
Materials and methods
Human testis samples
Adult human testis samples were obtained from two brain-dead donors (56 and 60 years old) and two prostate cancer patients undergoing bilateral orchidectomy (57 and 67 years old). Informed consent was obtained from all patients receiving surgery and from the family members of the brain-dead donors. Ethical approval was obtained from the ethics committee of the First Affiliated Hospital of Sun Yat-sen University (Assurance # 2019-148).
Animals
Three-week-old male NOD-Prkdcem26Cd52Il2rgem26Cd22/Nju (NCG) mice were obtained from the Model Animal Research Center of GemPharmatech Co., Ltd (Nanjing, China). All mice were maintained under controlled temperature (24 ± 1°C) and relative humidity (50–60%) with an alternating 12-h light/12-h dark cycle and were given free access to a standard rodent diet and drinking water. All procedures were approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University (Assurance # 2019-013).
Isolation and culture of endosialin+ cells from human testes
Human primary endosialin+ cells were isolated from the testis tissues of clinical donors. In detail, the testes were mechanically cut and enzymatically disassociated with 1 mg/ml type IV collagenase (Gibco, Grand Island, NY, USA) and 200 μg/ml DNase I (Roche, Indianapolis, IN, USA) in Dulbecco’s modified Eagle medium/nutrient mixture F-12 (DMEM/F12; 1:1, Gibco) at 37°C for 20 min with slow shaking (100 cycles/min). The samples were filtered through a 50 μm filter and centrifuged at 256 g for 4 min at 4°C. The cell pellets were rinsed two times with Ca2+-/Mg2+-free phosphate-buffered saline and then incubated with an anti-endosialin antibody conjugated with AF647 (BD Biosciences, Franklin Lakes, NJ, USA) and an isotype antibody in the dark for 20 min. The endosialin+ cells were enriched by flow cytometry (Influx Cell Sorter, BD Biosciences) followed by culture in expansion medium composed of DMEM/F12 (Gibco), 1% non-essential amino acids (Gibco), 1% GlutaMAX (Gibco), 1 × insulin-transferrin-selenium (ITS) supplement (Gibco), 1% N2 (Gibco), 2% B27 (Gibco), 20 ng/ml PDGF-BB (PeproTech, NJ, USA), oncostatin M (PeproTech), basic fibroblast growth factor (PeproTech), epidermal growth factor (PeproTech), 1 ng/ml leukaemia inhibitory factor (Millipore, Bedford, MA, USA), 1 nM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA) and 0.1 mM mercaptoethanol (Gibco). The cells were cultured at 37°C with 5% CO2, and the medium was changed every 3 days.
Cell proliferation assay
Cell proliferation was assayed as previously reported (Mou et al., 2016). Briefly, endosialin+ cells at passage 1 (P1) were seeded into 24-well plates at a density of 1 × 104 cells per well and cultured with expansion medium for 7 days before being digested with 1 mg/ml type IV collagenase (Gibco) in PBS and re-seeded at a density of 1 × 104 cells per well. Cell proliferation was examined using a Cellometer Auto T4 automated cell counter (Nexcelom Bioscience, Lawrence, MA, USA) when the cells were passaged every 6–8 days.
In vitro differentiation of endosialin+ cells
For adipogenic differentiation, we applied a previously reported method (Jiang et al., 2014). Endosialin+ cells were cultured in high-glucose DMEM (H-DMEM, Gibco) supplemented with 10% foetal bovine serum (FBS, HyClone, Logan, UT, USA), 10 μg/ml insulin (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), 200 μM indomethacin (Sigma-Aldrich) and 500 μM 3-isobutyl-1-methylxanthine (Sigma-Aldrich). The cells were maintained in differentiation culture for 2 weeks, and the medium was changed every 3 days. The adipogenically differentiated cells were confirmed by Oil Red O staining (Sigma-Aldrich) and quantitative RT–PCR of adipogenic lineage-specific genes (primers are listed in Supplementary Table SI).
For osteogenic differentiation, we applied a previously reported method (Jiang et al., 2014). Endosialin+ cells were seeded in H-DMEM (Gibco) containing 20% FBS (HyClone), 10 mM β-glycerophosphate (Sigma-Aldrich), 100 μg/ml ascorbic acid and 100 nM dexamethasone (Sigma-Aldrich). The cells were cultured for up to 2 weeks, and the medium was changed every 3 days. Osteogenic differentiation was confirmed by Alizarin Red S (Sigma-Aldrich) staining and quantitative RT–PCR for osteogenic lineage-specific genes (primers are listed in Supplementary Table SI).
For chondrogenic differentiation, we applied a previously reported method (Jiang et al., 2014). Endosialin+ cells were induced by using a cell pellet culture system. The cells were suspended in a 15 ml conical tube (NEST, Wuxi, China) containing 3 ml of induction medium consisting of DMEM (Gibco) with 3% FBS (HyClone), 1× ITS (Gibco), 1 mM pyruvate (Sigma-Aldrich) and 10 ng/ml transforming growth factor-β3 (PeproTech). The endosialin+ cells were maintained in culture for 4 weeks, and the medium was changed every 3 days. Chondrogenic differentiation was confirmed by Alcian Blue (Sigma-Aldrich) staining and quantitative RT–PCR for chondrogenic lineage-specific genes (primers are listed in Supplementary Table SI).
For LCs differentiation, we modified a previously described method (Ge et al., 2006). In brief, endosialin+ cells were induced in LCs differentiation-inducing medium (DIM) containing phenol red-free M199 (Gibco), 2% FBS (HyClone), 0.5 μM Smoothened agonist (EMD Millipore), 1 ng/ml LH (R&D, Minneapolis, USA), 1 nM thyroid hormone (T3, Sigma-Aldrich), 50 ng/ml insulin-like growth factor 1 (IGF1, PeproTech), 10 ng/ml platelet-derived growth factor AA (PDGF-AA, PeproTech), 1× ITS (Gibco) and 2.5 μM 25-hydroxycholesterol (25-HC, Sigma-Aldrich). By the end of 2 weeks, steroidogenic differentiation was confirmed by immunostaining and quantitative RT–PCR for LCs lineage-specific markers (primers and antibodies are listed in Supplementary Tables SI and SII). The ability of the cells to produce testosterone was assessed after 3 h of incubation with M199 (Gibco) containing 10 ng/ml LH (R&D), 2.5 μM 25-HC (Sigma-Aldrich) and 1× ITS (Gibco). The cell supernatants were collected on the 2nd, 4th, 7th, 10th and 14th days of culture for evaluation of testosterone concentrations. To test whether the testosterone secretion of the cells was regulated by LH, the cells were stimulated with 0.001, 0.01, 0.1, 1, 10 and 100 ng/ml LH (R&D) for 3 h before the supernatants were collected and stored at −20°C until analyses, as reported previously (Wang et al., 2018).
RNA isolation and quantitative RT–PCR
Total RNA was extracted using a RNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. RNA purity was determined by 260/280 ratio (1.87–1.93) and 260/230 ratio (2.18–2.34) using NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, DE, USA). Reverse transcription was performed using a High-Capacity RNA-to-cDNATM Kit (Thermo Fisher Scientific). Quantitative RT–PCR was performed using SYBR PCR Master Mix (Roche) and a Light Cycler 480 Detection System (Roche). To check primers, a melting curve was generated to confirm a single peak and rule out the possibility of non-specific product or primer–dimer formation. GAPDH was amplified as control, and the target gene expression levels were calculated using the ΔCt methods and expressed as relative expression. The primers are listed in Supplementary Table SI.
Lentiviral vector construction and infection
Lentiviral vectors expressing red fluorescent protein (RFP) driven by the EF-1α promoter were constructed as previously described (Zang et al., 2017). The endosialin+ cells were incubated with the virus overnight, and then the medium was replaced with fresh complete medium. The transfection efficiency was about 60% and RFP-labelled cells were purified by flow cytometry for the subsequent experiments. Before transplantation, over 97% of these cells were RFP and endosialin double positive.
Transplantation of human endosialin+ cells
To investigate the potential of the sorted human endosialin+ cells to differentiate into LCs in vivo, we transplanted them into the testes of 3-week-old immunodeficient NCG mice. Approximately 2 × 105 RFP-labelled endosialin+ cells in 15 μl of PBS were injected into the testicular interstitium of each recipient under whole-body anaesthesia using Avertin (Sigma-Aldrich). The control animals received testicular injections of PBS at the same volume. Four weeks after transplantation, the animals were sacrificed after being anaesthetized excessively. Bilateral testes were collected for flow cytometry (Influx Cell Sorter, BD Biosciences) or fixed in 4% paraformaldehyde (PFA) for immunofluorescence analyses.
Flow cytometry analysis of endosialin+ cells
Flow cytometry analysis was used to examine the marker expression of endosialin+ cells. The cells were disassociated, filtered and incubated with monoclonal fluorescent-labelled antibodies against various human antigens, including endosialin, PDGFRα, NGFR and Nestin (antibodies are listed in Supplementary Table SII). Irrelevant isotype-identical antibodies were used as negative controls. The cells were incubated for 20 min with the appropriate antibodies in the dark at room temperature and then detected by flow cytometry (CytoFLEX, Beckman Coulter, Krefeld, Germany). The data were analysed with FlowJo software (BD Biosciences).
Immunofluorescence staining
The cultured cells were fixed with 4% PFA for 10 min at room temperature and rinsed in PBS. For intracellular protein detection, permeabilization was performed with 0.2% Triton X-100 in PBS for 15 min. Cells were incubated with 3% BSA (Sigma-Aldrich) for 30 min at room temperature, and incubated overnight with primary antibodies at 4°C. Following incubation, the cells were rinsed five times with PBS and incubated with secondary antibodies (Invitrogen, Carlsbad, CA, USA) at room temperature for an hour. After being rinsed five times with PBS, the cells were stained with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) for 5 min, after which the DAPI was removed and replaced with mounting medium (DAKO, Glostrup, Denmark). For the negative control, we stained cells in the absence of the primary antibodies (second-antibody-only control). All the primary and secondary antibodies are listed in Supplementary Table SII.
Endosialin+ cells were seeded into 6-well plates at a density of 2 × 105 cells per well and cultured with expansion medium for 7 days to develop into spheres; then the formed spheres were fixed with 4% PFA for 15 min. For intracellular protein detection, permeabilization was performed with 0.2% Triton X-100 in PBS for 15 min. Then, non-specific binding of antibodies was blocked with 3% BSA for 30 min at room temperature, and incubated overnight with the relevant primary antibodies at 4°C. Next, the sections were washed with PBS five times and then incubated with the appropriate secondary antibodies for 45 min at room temperature. The nuclei were counterstained with DAPI for 5 min then the DAPI solution was removed and replaced with mounting medium (DAKO). For the negative control, we stained spheres in the absence of the primary antibodies (second-antibody-only control). All the primary and secondary antibodies are listed in Supplementary Table SII.
Human and mouse testis tissue was fixed with 4% PFA at 4°C for 2 h. The tissues were then dehydrated with 30% sucrose solution in PBS at 4°C for 24 h. Afterwards, the tissues were soaked in Tissue-Tek O.C.T. Compound (Sakura Finetek, Torrance, CA, USA) for 30 min and then frozen for cryosectioning at 10 μm thickness using a frozen slicer (Leica CM1950, Germany). For intracellular protein detection, sections were permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) for 15 min. Next, non-specific binding of antibodies was blocked with 3% BSA (Sigma-Aldrich) for 45 min at room temperature and then incubated overnight with primary antibodies at 4°C. Then, the sections were incubated with secondary antibodies at room temperature for 45 min in the dark and subsequently incubated for 5 min with DAPI. Finally, the specific fluorescence was visualized and photographed using an LSM800 confocal microscope (Zeiss, Jena, Germany) or a Leica DMi8 microscope (Leica, Wetzlar, Germany). For the negative control, all sections were stained in the absence of the primary antibodies (second-antibody-only control). All the primary and secondary antibodies are listed in Supplementary Table SII.
Hormone measurements
The hormone levels in the cell culture supernatants were measured using a commercially available ELISA kit (R&D) according to the manufacturer’s instructions. The absorbance at 450 nm was measured using an ELISA microtiter plate reader (Tecan, Switzerland). The hormone concentrations were evaluated according to a standard curve constructed by plotting the absorbance of each reference standard against its corresponding concentration. The coefficient of variation of this ELISA kit is 2.9–4% for intra-assay precision and 5.6–6.8% for inter-assay precision. The detectable dose of testosterone ranges from 0.012 to 0.041 ng/ml and the mean minimum detectable dose was 0.030 ng/ml.
Karyotype assay
G-band chromosomal analyses were conducted at P20 by DAAN Gene Co., Ltd (Guangzhou, China).
Single-cell sequence analysis
Seurat software (http://satijalab.org/seurat/, R package, v.3.1.0) was used to perform cell clustering analysis on the adult testis scRNA-seq dataset GSM3526588 (Gene Expression Omnibus [GEO]: GSE124263) (Sohni et al., 2019). First, the feature table, barcode table and matrix table were all loaded into R with the Read10X function. Seurat objects were created, normalized and scaled from these data with the default settings. Specifically, cells for which fewer than 17% of the reads mapped to the mitochondrial genome and fewer than 4000 genes were expressed were retained. Uniform Manifold Approximation and Projection (UMAP) and clustering analyses were performed. Next, we filtered out the cells that had no PDGFRA, or NGFR expression. The remaining cells were then subjected to clustering analysis again. Two separate clusters were found under the pipeline listed in the Guided Tutorial (http://satijalab.org/seurat/vignettes.html). Several markers were used to identify each cell cluster, and a differential gene test was used to find marker genes in each cluster. The genes from the differential test table that were associated with the cell surface (GO:0009986) and had the highest cell cluster proportions were selected.
Statistical analysis
All data are presented as the mean ± SEM and were analysed using SPSS 20.0 software (IBM SPSS Statistics, Armonk, NY, USA). We have performed the Shapiro–Wilk test for normality in each experiment. Statistical differences between samples were assessed with independent-samples Student’s t-tests or nonparametric tests (Mann–Whitney U tests). Differences were considered significant when P < 0.05 (*P < 0.05, **P < 0.01 and ***P < 0.001).
Results
Expression profile of endosialin in human testes
To re-evaluate whether the cell surface markers PDGFRα and NGFR are distributed specifically in human SLCs, we detected their expression patterns in adult human testes by immunostaining. Consistent with previous findings, some PDGFRα+ cells were immunopositive for the LCs markers hydroxysteroid dehydrogenase 3β (HSD3β) (Supplementary Fig. S1A) and cytochrome P450 family 17 subfamily A member 1 (CYP17A1) (Supplementary Fig. S1B). Similarly, an immunofluorescence assay revealed that NGFR was co-expressed with the germ cell markers DEAD-box helicase 4 (DDX4) (Supplementary Fig. S1C) and peanut agglutinin in germ cells (Supplementary Fig. S1D) of human testes. These results confirm that the cell surface markers PDGFRα and NGFR are not adequate markers for the specific identification and isolation of human SLCs.
To identify potential specific cell-surface markers of human SLCs, we analysed the PDGFRA+ NGFR+ subsets from previously published single-cell sequencing data (Supplementary Fig. S2A and B). Surprisingly, clustering analysis revealed two major subpopulations (Supplementary Fig. S2C). One of them was identified to contain peritubular myoid cells (Cluster 1), and the other was determined to possibly contain putative human SLCs (Cluster 0) (Supplementary Fig. S2D). Furthermore, we examined the expression of cell surface proteins and found that the proportion of endosialin+ cells was the highest in Cluster 0 (Supplementary Fig. S2E), indicating that endosialin might be of greatest use for purifying human SLCs.
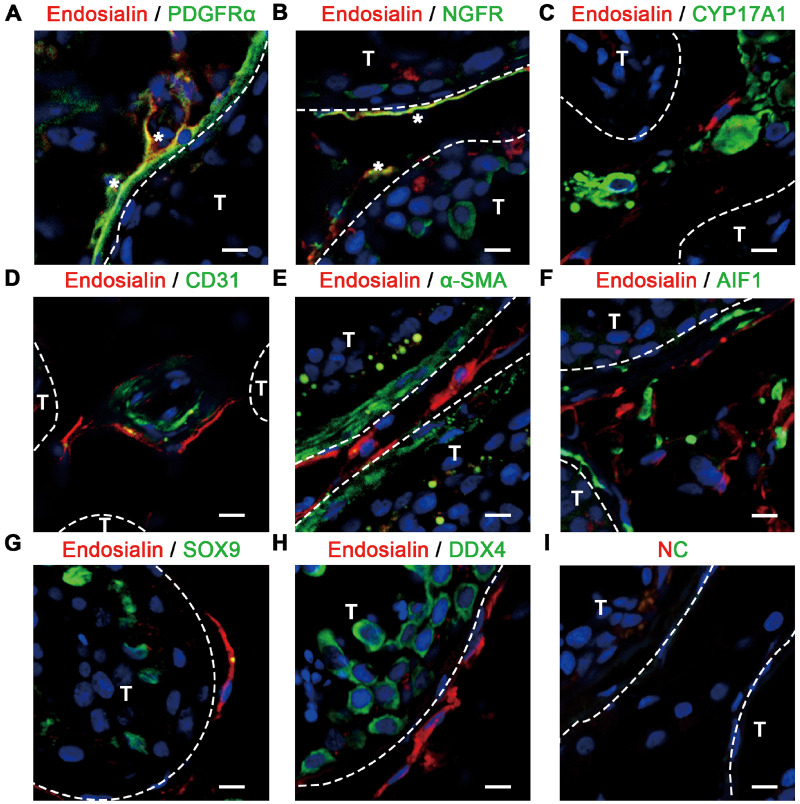
Subsequently, we examined the expression profile of endosialin in adult human testes to assess whether endosialin could be used as a putative marker of human SLCs. Immunofluorescence detection was performed on testes samples from three donors, and the results confirmed that endosialin was expressed exclusively in the interstitial areas of the testes (Fig. 1). Further examinations revealed that endosialin was present in a subset of cells positive for PDGFRα (Fig. 1A) and NGFR (Fig. 1B). In contrast, endosialin was not co-expressed with the LCs marker CYP17A1 (Fig. 1C), or the endothelial cell marker CD31 (Fig. 1D) or the peritubular myoid cell marker alpha-smooth muscle actin (α-SMA) (Fig. 1E). Moreover, we observed that endosialin+ cells did not express the macrophage marker allograft inflammatory factor 1 (AIF1) (Fig. 1F), the Sertoli cell marker SRY-box transcription factor 9 (SOX9) (Fig. 1G) or the germ cell marker DDX4 (Fig. 1H). Collectively, these data indicate that endosialin might be a more specific marker for human SLCs than the currently used molecules.
Figure 1.
Expression pattern of endosialin in human testes, as shown by immunofluorescence. (A–C) Endosialin was co-expressed with the reported SLCs markers PDGFRα (A) and NGFR (B) but did not co-localize with the LCs marker CYP17A1 (C) in the interstitial region. Co-expression is marked with white asterisks in (A) and (B). (D, E) Endosialin+ cells were located in the perivascular region (endothelial cells are marked with CD31) (D) and peritubular region (peritubular myoid cells are marked with α-SMA) (E) of the human testes. (F–H) Endosialin-expressing cells did not express the macrophage marker AIF1 (F), the Sertoli cell marker SOX9 (G) or the germ cell marker DDX4 (H). (I) The negative control stained in the absence of the primary antibodies is shown. Nuclei were counterstained with DAPI. T and interstitium were indicated by dotted lines. The scale bars denote 10 μm. The images are representative of results obtained from patient samples (n = 3). AIF1, allograft inflammatory factor 1; CYP17A1, cytochrome P450 family 17 subfamily A member 1; DAPI, 4,6-diamidino-2-phenylindole; DDX4, DEAD-box helicase 4; LCs, Leydig cells; NC, negative control; NGFR, nerve growth factor receptor; PDGFRα, platelet-derived growth factor receptor α; SLCs, stem Leydig cells; SOX9, SRY-box transcription factor 9; T, seminiferous tubules; α-SMA, alpha-smooth muscle actin.
Isolation and identification of endosialin+ cells in vitro
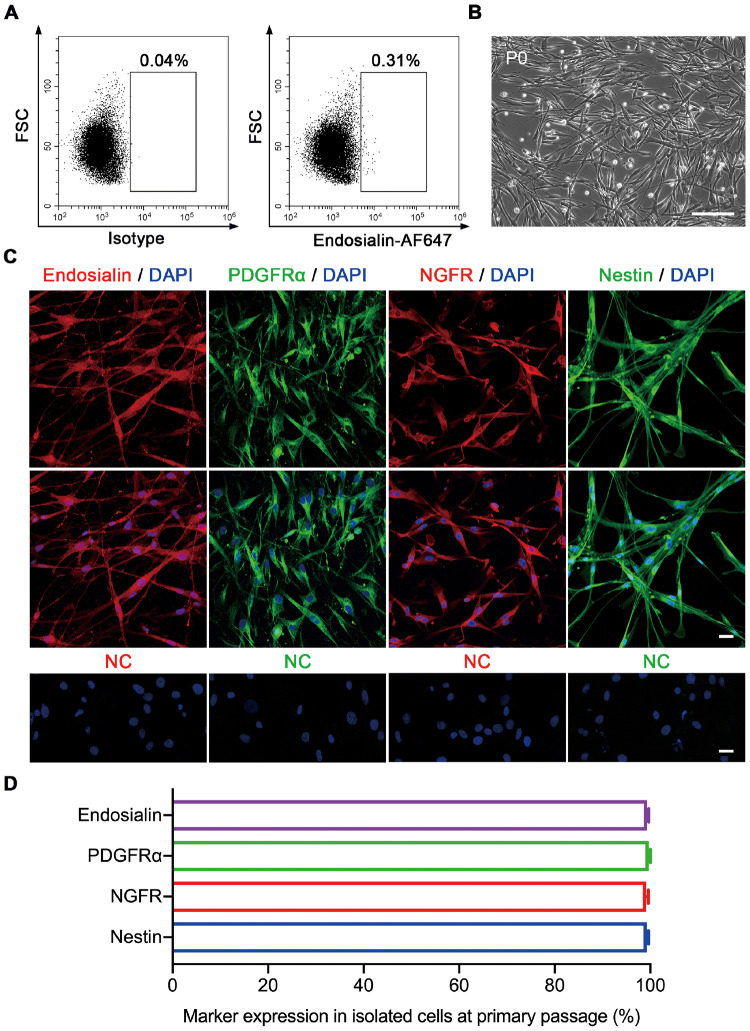
To further study endosialin+ cells, human testicular cells were sorted using fluorescence-activated cell sorting (FACS). Endosialin+ cells constituted 0.31 ± 0.03% of the total testicular cell population (Fig. 2A). These freshly isolated endosialin+ cells were then cultured on plastic dishes in expansion medium at a density of 2 × 105 cells/ml. One day later, the attached endosialin+ cells maintained the typical cellular morphology of SLCs (Fig. 2B). Subsequently, the sorted cells were characterized by immunostaining at the primary passage when they propagated to 50–60% confluence on plastic dishes. They robustly expressed endosialin (99.3 ± 0.32%), PDGFRα (99.7 ± 0.15%), NGFR (98.5 ± 0.21%) and Nestin (99.1 ± 0.27%) (Fig. 2C and D). In addition, the sorted cells were also positive for the transcription factors nuclear receptor subfamily 2 group F member 2 (NR2F2) (98.5 ± 0.66%) and aristaless-related homeobox (ARX) (93.1 ± 0.97%) (Supplementary Fig. S3B), which have been reported to be expressed in SLCs and to be important for testis formation (Miyabayashi et al., 2013; Kilcoyne et al., 2014). In accordance with the in vivo results shown in Fig. 1, endosialin+ cells showed negligible CYP17A1, CD31, α-SMA, AIF1, SOX9 or DDX4 staining (Supplementary Fig. S3C–J), indicating that the isolated SLC cell population was not contaminated with LCs, endothelial cells, peritubular myoid cells, macrophages, Sertoli cells or germ cells. Overall, these results strongly support the hypothesis that endosialin may be an excellent marker for human SLCs.
Figure 2.
Isolation and identification of endosialin+ cells from human testes. (A) Endosialin+ cells were isolated by FACS from adult human testes (n = 4). Left: isotype controls. Right: stained samples. (B) Representative phase-contrast micrograph of endosialin+ cells at P0 (n = 4). The scale bar denotes 200 μm. (C) Endosialin+ cells expressed the SLCs markers PDGFRα, NGFR and Nestin (n = 3). The nuclei were counterstained with DAPI. NC stained in the absence of the primary antibody. The scale bars denote 25 μm. (D) The percentages of endosialin+, PDGFRα+, NGFR+ and Nestin+ cells among isolated cells were analysed. The data are presented as the mean ± SEM (n = 3). FACS, fluorescence-activated cell sorting; FSC, forward scatter; P0, primary culture.
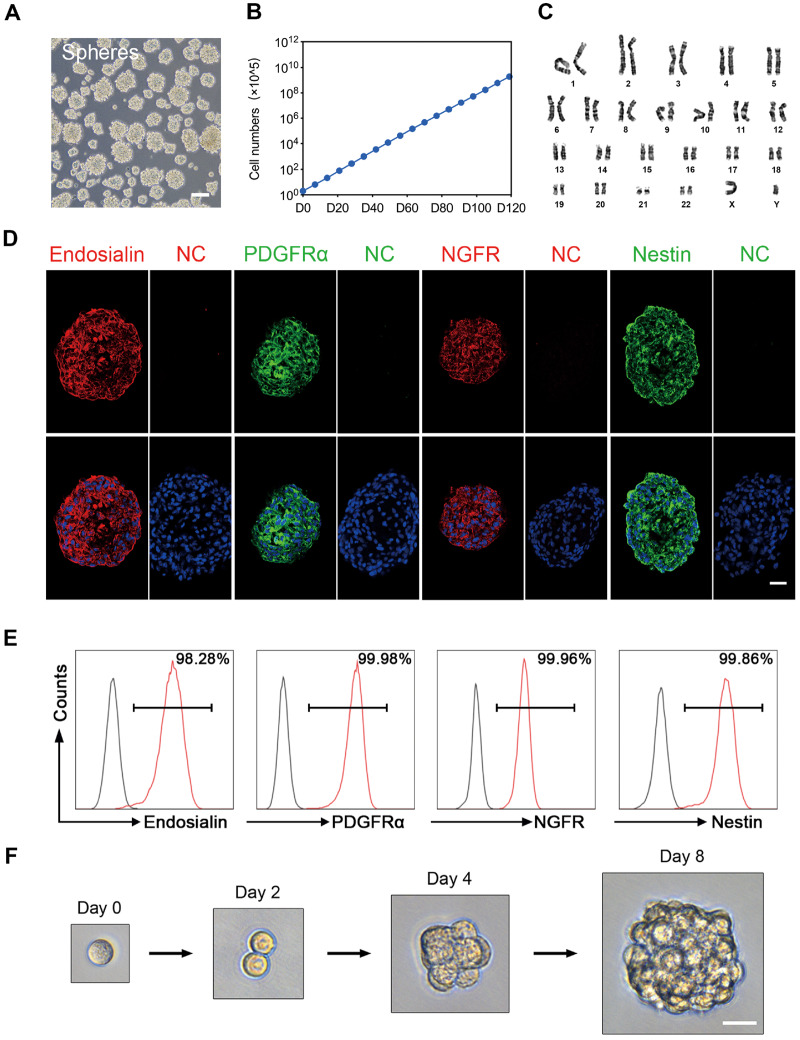
It has been reported that SLCs are capable of forming spheres that exhibit continuous proliferation (Jiang et al., 2014). When endosialin+ cells were cultured in serum-free expansion medium, most of these cells showed sphere formation capacity and could be passaged every 6–8 days (Fig. 3A). Notably, spheres derived from endosialin+ cells could be continuously grown for over 4 months, with cell counts reaching 1015 at P20 (Fig. 3B). More importantly, we did not observe any obvious chromosomal elimination, displacement or imbalance after long-term culture (Fig. 3C). There were no signs of tumourigenicity 3 months after endosialin+ cells obtained at P20 were injected s.c. into 8-week-old immunodeficient NCG mice, but tumours did grow in mice injected with MA-10 Leydig tumour cells (data not shown).
Figure 3.
Proliferation and self-renewal capacity of endosialin+ cells. (A) Phase-contrast micrograph of floating spheres at P3 growing from human testicular endosialin+ cells in serum-free expansion medium (n = 3). The scale bar denotes 100 μm. (B) Proliferation curve of endosialin+ cells cultured in expansion medium over different passages. The initial cell count was 2 × 105. The data are expressed as the mean ± SEM (n = 3). (C) Karyotypic stability of expanded endosialin+ cells up to P20 was analysed. (D) Immunofluorescence staining for the indicated markers (Endosialin, PDGFRα, NGFR and Nestin) in endosialin+ cell spheres. The nuclei were counterstained with DAPI. NC stained in the absence of the primary antibody. The scale bars denote 25 μm. (E) FACS analysis for detection of the indicated markers (Endosialin, PDGFRα, NGFR and Nestin) in endosialin+ cell spheres (n = 3). (F) Representative images of a clonal sphere growing from a single cell, as observed using bright-field microscopy (n = 3). The scale bar denotes 25 μm. P3, passage 3; P20, passage 20.
To examine whether the culture conditions maintained the human SLCs in an undifferentiated state, we analysed the endosialin+ spheres by immunofluorescence and flow cytometry assays. Immunofluorescence staining showed that P5 spheres expressed endosialin and the established SLCs markers PDGFRα, NGFR and Nestin (Fig. 3D). The results were confirmed by flow cytometry analysis, in which almost all the cells (>98%) expressed endosialin, PDGFRα, NGFR and Nestin (Fig. 3E). To investigate the self-renewal capacity of these cells, we carried out single-cell sphere formation assays in which single-cell suspensions derived from P5 spheres were seeded into 96-well plates. The seeded single cells divided and formed spheres after 8 days of culture (Fig. 3F). A sphere with a diameter ≥50 μm in a given well was counted as one clone. The clonogenic efficiency of endosialin+ cells was 18.6 ± 4.4%. These results demonstrate that endosialin+ cells can continuously expand and exhibit self-renewal capacity in vitro.
Multipotent properties of human endosialin+ cells
Human SLCs have been confirmed to possess multi-lineage differentiation potential in vitro (Zhang et al., 2017; Eliveld et al., 2019). To characterize the multipotency of the sorted endosialin+ cells, we induced these cells to differentiate under the defined conditions (Supplementary Fig. S4A). Adipogenesis was confirmed by the presence of lipid-rich vacuoles stained with Oil Red O (Supplementary Fig. S4B). Consistently, the mRNA levels of adipogenic markers, including fatty acid-binding protein 4 and peroxisome proliferator-activated receptor-γ (Supplementary Fig. S4E) were increased. Compared with the undifferentiated cells, the differentiated cells exhibited the deposition of Alizarin Red-positive mineralized matrix after 2 weeks of induction (Supplementary Fig. S4C). We further found that the expression levels of osteogenic markers, including alkaline phosphatase and runt-related transcription factor 2, were highly upregulated (Supplementary Fig. S4E). Four weeks after chondrogenic induction, the pellets stained intensely stained with Alcian Blue (Supplementary Fig. S4D). In addition, the mRNA expression levels of the chondrogenic genes collagen type 2 alpha 1 chain and aggrecan were also highly upregulated (Supplementary Fig. S4E). Collectively, these results suggest that endosialin+ cells possess multi-lineage differentiation potential.
In vitro differentiation of human testicular endosialin+ cells into LCs
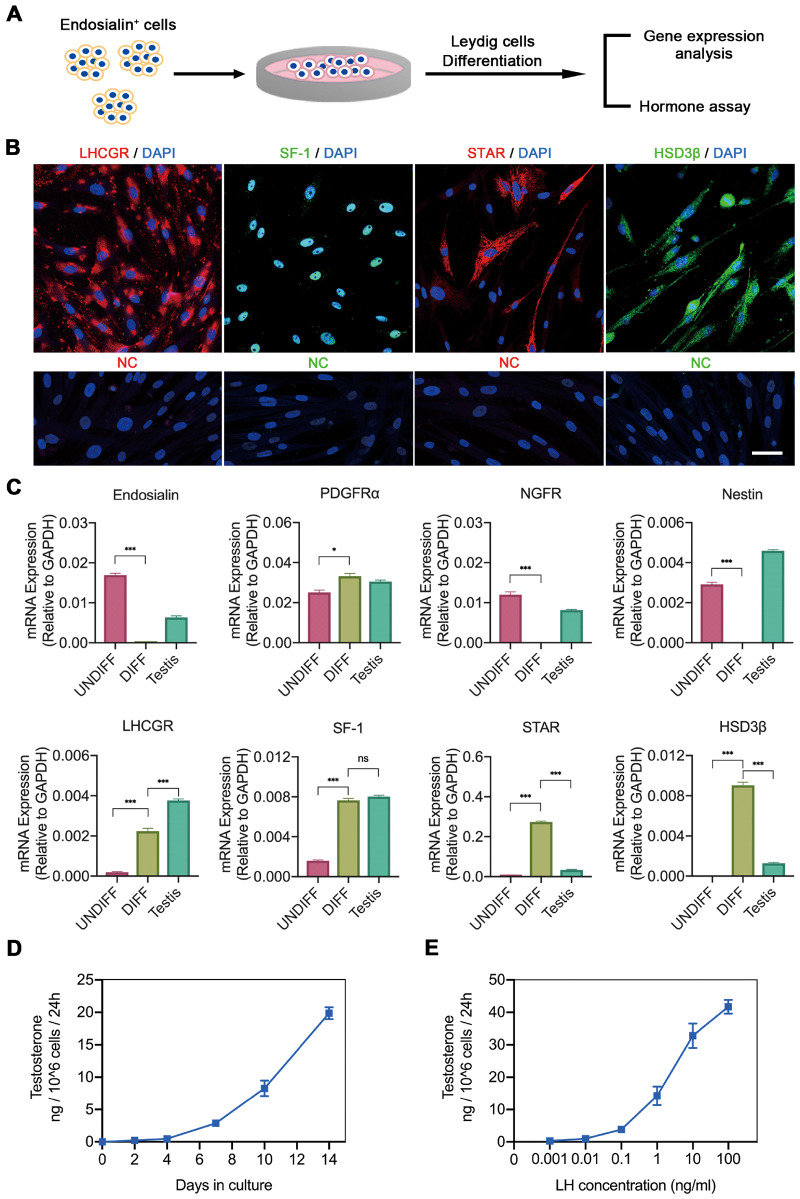
To assess whether endosialin+ cells have the potential to differentiate into functional LCs in vitro, we stimulated the cells with DIM (Fig. 4A). After 2 weeks of culture in DIM, the majority of endosialin+ cells exhibited signs of differentiation, as indicated by immunofluorescence staining for proteins involved in testosterone biosynthesis, such as LH receptor (LHCGR), steroidogenic factor 1 (SF-1), steroidogenic acute regulatory protein (STAR) and HSD3β (Fig. 4B). In addition, differentiated endosialin+ cells were examined by quantitative RT–PCR for the LCs markers LHCGR, SF-1, STAR and HSD3β, as well as endosialin and the known SLCs markers PDGFRα, NGFR and Nestin. The expression levels of the indicated genes were compared with those in undifferentiated endosialin+ cells and human testis tissues. As expected, the transcript levels of endosialin, NGFR and Nestin were significantly lower in differentiated endosialin+ cells than in undifferentiated cells (Fig. 4C). However, the expression of PDGFRα was higher in differentiated endosialin+ cells than in undifferentiated cells, in accordance with our previous results (Supplementary Fig. S1A and B). Similarly, upregulation of LHCGR, SF-1, STAR and HSD3β was detected. Culture supernatants were harvested for the determination of testosterone production at the indicated time points. The data showed that testosterone synthesis gradually increased over time after LCs lineage differentiation of endosialin+ cells (Fig. 4D), increasing to approximately 20 ng of testosterone per 1 × 106 cells in 24 h. Furthermore, the testosterone production assay reflected that differentiated endosialin+ cells responded to LH in a concentration-dependent manner, indicating that these testosterone-producing LCs were regulated by upstream gonadotrophin (Fig. 4E). The ability of endosialin+ cells to differentiate into LCs reinforces the idea that endosialin+ cells in human testes are SLCs.
Figure 4.
Potential of human testicular endosialin+ cells to differentiate towards LCs lineages. (A) Schematic of the experimental procedure used to induce LCs lineage differentiation. (B) After 14 days of differentiation in DIM, endosialin+ cells expressed the LCs lineage-specific markers LHCGR, SF-1, STAR and HSD3β, as shown by immunofluorescence (n = 3). The nuclei were counterstained with DAPI. NC stained in the absence of the primary antibody. The scale bar denotes 50 μm. (C) DIFF were examined by quantitative RT–PCR analysis of the expression of the indicated markers. The expression levels of each gene were compared with those in UNDIFF and human testis tissue. The data are expressed as the mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ns = no significance. (D) Testosterone production was analysed by ELISA during LCs lineage induction in DIM. (E) Testosterone production of DIFF stimulated by LH increased in a concentration-dependent manner, as analysed by ELISA. The data are expressed as the mean ± SEM (n = 3). DIFF, differentiated endosialin+ cells; DIM, differentiation-inducing medium; HSD3β, hydroxysteroid dehydrogenase 3β; LHCGR, LH receptor; SF-1, steroidogenic factor 1; STAR, steroidogenic acute regulatory protein; Testis, human testis tissues; UNDIFF, undifferentiated endosialin+ cells.
Characteristics of endosialin+ cells in vivo
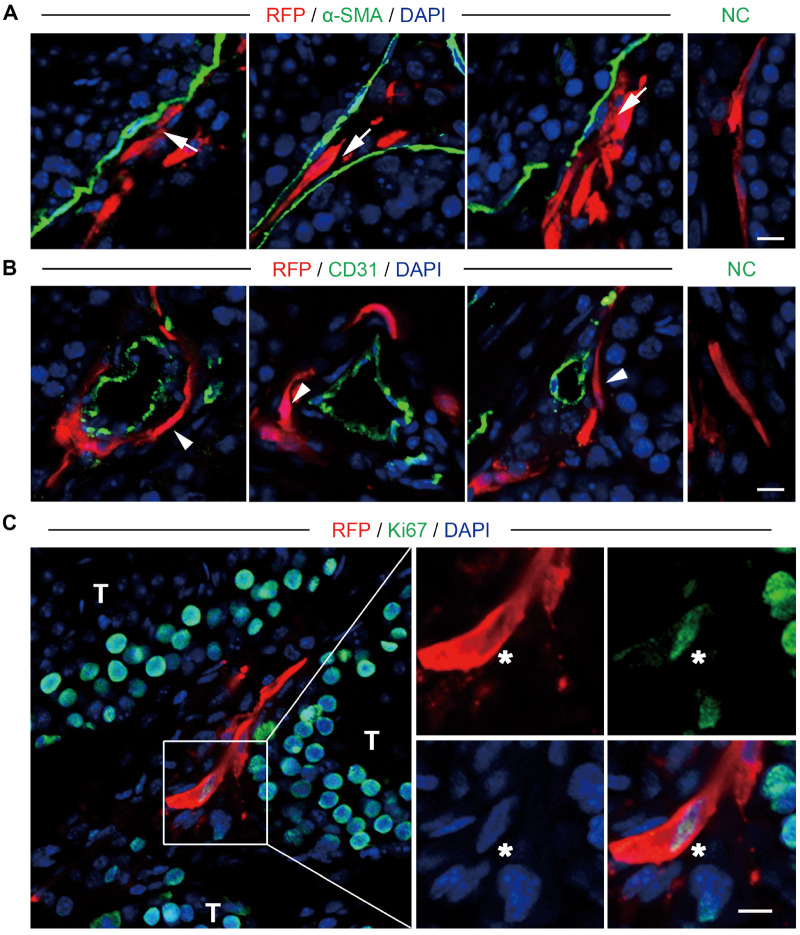
Having confirmed that the human testicular endosialin+ cells were multipotent, with the ability to differentiate into LCs in vitro, we next assessed the in vivo properties of endosialin+ cells after xenotransplantation into the testes of immunodeficient NCG mice. To trace the transplanted cells in vivo, we cocultured the endosialin+ cells with lentiviruses expressing RFP under the control of the elongation factor 1α promoter. The infected cells stably expressed RFP and proliferated as floating clonal spheres; thus, they were used in the following experiments (Supplementary Fig. S5). We then transplanted the RFP-labelled cells into the testes of eight prepubescent (3 weeks old) mice. To investigate the fate of endosialin+ cells, we harvested the testes for immunofluorescence analysis and flow cytometry assays 4 weeks after transplantation. Immunostaining indicated that the RFP-labelled cells were exclusively located in the interstitial area in the testes (Supplementary Fig. S6). In addition, some RFP-labelled cells were found locating in peritubular (Fig. 5A) and perivascular regions (Fig. 5B;Supplementary Video S1) consistent with the fact that SLCs are located around both seminiferous tubules and blood vessels in adult testes. Furthermore, the transplanted endosialin+ cells were perfused by host vessels, as evidenced by anti-CD31 immunostaining (Fig. 5B;Supplementary Video S1). In addition, 8.34 ± 1.65% of the RFP-labelled cells were positive for the pan-cell-cycle marker Ki67 (Fig. 5C), indicating the measurable proliferative activity of the transplanted endosialin+ cells.
Figure 5.
Localization and proliferation of endosialin+ cells after transplantation into the testes of mice. (A, B) Immunostaining showed the accumulation of RFP-labelled endosialin+ cells in the testicular interstitium tissues of recipient mice. RFP-labelled cells resided in both peritubular (A, white arrows) and perivascular (B, white arrowheads) regions in the testes of mice. Peritubular myoid cells were marked by α-SMA (A), and endothelial cells were marked by CD31 (B). The scale bars denote 10 μm. (C) Proliferation of the transplanted cells, as demonstrated by staining for Ki67 (indicated by asterisks). NC stained in the absence of the primary antibody. The scale bar denotes 20 μm. The nuclei were counterstained with DAPI. The images represent the results obtained from recipient mice (n = 3). RFP, red fluorescent protein.
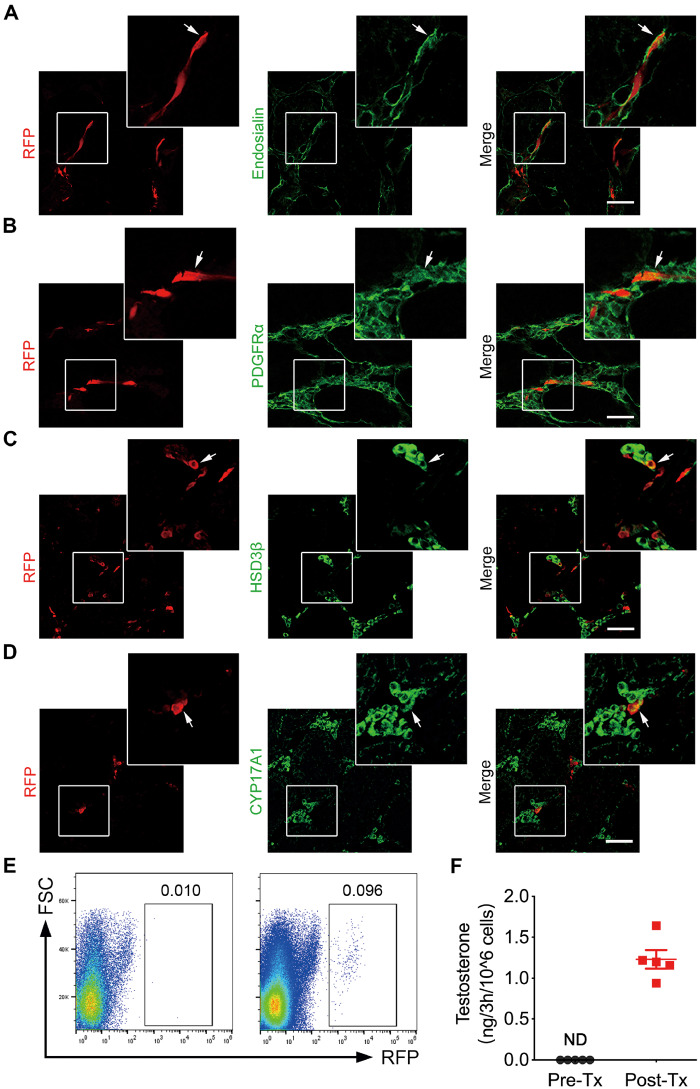
Previous studies have reported that SLCs can differentiate into LCs after being transplanted into the testes (Ge et al., 2006; Jiang et al., 2014). Surprisingly, approximately 60–80% of the RFP-labelled cells retained expression of endosialin (72.94 ± 4.31%) (Fig. 6A), indicating that most of the transplanted cells retained their stem cell characteristics. In addition, a small portion of the grafted cells gave rise to LCs, as evidenced by the expression of HSD3β (10.20 ± 2.97%), CYP17A1 (8.33 ± 1.36%), STAR (11.56 ± 3.71%) and CYP11A1 (10.46 ± 3.49%) (Fig. 6C and D; Supplementary Fig. S7). Nonetheless, almost all of the transplanted cells still expressed PDGFRα (95.32 ± 2.47%) (Fig. 6B), which has been reported to be expressed either on SLCs or LCs lineages within testes. Furthermore, no signs of tumour formation were detected in any of the animals tested at 4 weeks.
Figure 6.
Identification of the transplanted endosialin+ cells in the testes of mice. (A, B) RFP-labelled endosialin+ cells transplanted into the testes of NCG mice were assessed 4 weeks after transplantation. Immunofluorescence staining showed that some of the engrafted cells retained expression of endosialin (A) and PDGFRα (B) (indicated by arrows). (C, D) Immunostaining showed that a portion of the transplanted cells differentiated into HSD3β+ (C) and CYP17A1+ LCs (D; indicated by arrows). The scale bars denote 50 μm. The nuclei were counterstained with DAPI. Representative images obtained from recipient mice are shown (n = 3). (E) The images represent the detection and isolation of transplanted RFP-labelled cells from recipient testes in the different groups; left: nontransplantation controls (n = 2); right: RFP-labelled cells transplanted samples (n = 5) as determined by FACS. (F) The testosterone produced by sorted RFP-labelled cells 4 weeks Post-Tx and endosialin+ cells Pre-Tx was detected using ELISA. The data are shown as the mean ± SEM (n = 5). NCG, NOD-Prkdcem26Cd52Il2rgem26Cd22/Nju; ND, not detectable; Pre-Tx, before transplantation; Post-Tx, after transplantation.
To further confirm the LCs differentiation of the endosialin+ cells in vivo, the testes of recipient mice were dissociated into single cells, and RFP-labelled cells were sorted by flow cytometry (Fig. 6E). Subsequently, the RFP-labelled cells before and after transplantation were seeded into separate plastic dishes. After being cultured in M199 supplemented with 10 ng/ml LH and 2.5 μM 25-hydroxycholesterol for 3 h, the supernatants were collected for testosterone quantification. Notably, testosterone production in the post-transplantation cells was 1.232 ± 0.16 ng per 1 × 106 cells in 3 h, while that in the control cells was undetectable.
Taken together, these observations indicate that human testicular endosialin+ cells possess the characteristics expected of SLCs in vivo.
Discussion
In this study, we sought to examine whether endosialin can serve as a specific surface marker for the identification and isolation of human SLCs. We first revealed the expression profile of endosialin in adult human testes. Then, we isolated and characterized endosialin+ cells that exhibited persistent proliferative capacity and LCs differentiation potential in vitro and in vivo. The results collectively suggest that endosialin is an ideal marker for the identification and isolation of human SLCs, which has latent value for further research on human SLCs and advances in cell replacement therapies for future clinical applications related to male hypogonadism.
Transplantation of heterogeneous cell populations might cause safety risks and reduce the effectiveness of treatment, suggesting that increasing purity is a key objective for stem cell research (Tohyama et al., 2013). Previous studies have reported several markers of human SLCs, including PDGFRα (Eliveld et al., 2019), NGFR (Zhang et al., 2017) and Nestin (Zhang et al., 2017). Although Nestin seems to be exclusively expressed on SLCs in human testes, it cannot be applied for isolation of living human SLCs, since it is an intracellular cytoskeletal protein (Jiang et al., 2014). NGFR is located in germ cells in adult human testes (Jin et al., 2006; Levanti et al., 2006; Lin et al., 2015) and PDGFRα is expressed on LCs (Brennan, 2003; Schmahl et al., 2008); thus, germ cells or LCs could be mistakenly sorted as SLCs if NGFR or PDGFRα is used as a marker for human SLCs isolation. As revealed in our prior study, NGFR+ cells constitute approximately 1.79% of testicular cells (Zhang et al., 2017), while the PDGFRα+ cells in this study accounted for approximately 1.5% (data not shown). Both of these percentages are obviously higher than that of the endosialin+ population (0.3% of testicular cells), to some degree reflecting that the use of either NGFR or PDGFRα may overestimate the proportion of actual SLCs. Consistently, these flow cytometric results were confirmed by immunofluorescence analysis. As we first illustrated in the current study, endosialin largely overlapped with the former SLCs markers PDGFRα and NGFR in adult human testes. Endosialin+ cells negligibly expressed any of the known markers for other types of testicular cells, including LCs (CYP17A1), endothelial cells (CD31), peritubular myoid cells (α-SMA), macrophages (AIF1), Sertoli cells (SOX9) and germ cells (DDX4). These results suggested that endosialin may serve as a discriminatory cell surface marker of human SLCs, potentially improving the specificity and feasibility of human SLCs isolation.
Given the specificity of endosialin for SLCs, we further determined that endosialin+ cells sorted by FACS exhibited properties of typical SLCs, including proliferation, self-renewal and the potential to differentiate into functional LCs in vitro (Ge et al., 2006; Jiang et al., 2014). Here, we found that endosialin+ cells continuously proliferated under cultivation in the expansion medium. Additionally, endosialin+ cells possessed self-renewal ability, inferred by the formation of spherical clones derived from a single cell. Moreover, these cells could differentiate into functional LCs that secreted considerable testosterone in response to LH in a concentration-dependent manner in vitro. These self-renewal and differentiation properties reinforce the idea that human testicular endosialin+ cells are SLCs.
According to criteria in previous reports, putative SLCs should have the ability to colonize the interstitium and subsequently replenish LCs after being transplanted in vivo (Ge et al., 2006; Zang et al., 2017; Zhang et al., 2017). To examine the in vivo characteristics of endosialin+ cells, we chose 3-week-old immunodeficient mice as recipients to avoid interference from immunological rejection. Similar to the findings in earlier reports (Ge et al., 2006; Jiang et al., 2014), transplanted RFP-labelled endosialin+ cells colonized the testicular interstitium and differentiated into functional LCs. Beyond that, we observed that the transplanted cells homed to a specific location in which SLCs usually reside (Li et al., 2016; Kumar and DeFalco, 2018; Chen et al., 2020) and that they were perfused by host vessels, forming an organic whole (Chong et al., 2014). Additionally, the majority of endosialin+ cells retained characteristics of stem cells, as indicated by the expression of the putative SLCs markers endosialin and PDGFRα, which had not been examined for SLCs in previous studies. Only a small proportion of the RFP-labelled cells differentiated into LCs. Meanwhile, the testosterone production of the transplanted cells was not as high as those in vitro, in accordance with the low differentiation ratio in vivo determined by immunofluorescence assays. A possible explanation for this finding is that the microenvironment in 3-week-old mouse testes was not perfect for the differentiation of human SLCs since SLCs commit to a differentiation pathway to form LCs lineages by postpartum day 11 in mice (Ye et al., 2017; Chen et al., 2020). Surprisingly, although the transplanted endosialin+ cells survived and exhibited measurable cell cycle activity, the retrieved RFP-labelled cells accounted for only 10% of the total transplanted cells 4 weeks after transplantation. We speculate that this might have been due to species differences or immune rejection; these possibilities remain to be thoroughly assessed in future studies. Similarly, even with the use of CD4 antibodies to suppress the immune system, human PDGFRα+ SLCs do not survive over 20 weeks in LH receptor-knockout mouse testes (Eliveld et al., 2019). Given these findings, there is an urgent need to further identify animal models that are optimal for evaluating in vivo functions of human SLCs.
Although most of the previous studies focused on LCs differentiation potential after SLCs transplantation (Ge et al., 2006; Jiang et al., 2014; Arora et al., 2019), it has been reported that a subpopulation of LC progenitors derived from foetal gonad could give rise to adult LCs, peritubular myoid cells and so on (Shima et al., 2018). Moreover, we demonstrated that endosialin+ cells showed multipotency with the ability to differentiate into adipocytes, osteoblasts and chondrocytes in vitro, which raises the possibility that these cells may be able to give rise to other testicular interstitial cell types. As illustrated by in vivo immunostaining data, transplanted RFP-labelled cells did not express the known markers for peritubular myoid cells (α-SMA) or endothelial cells (CD31). These results suggested that the transplanted endosialin+ cell population did not develop into myoid cells or endothelial cells in the present study. However, at this point, we could not rule out the possibility that endosialin+ human SLCs may give rise to other testicular interstitial cell types given the appropriate niche signals, especially in certain testicular disease states. These possibilities remain to be thoroughly assessed in future studies.
Despite the fact that endosialin+ human SLCs have the potential to differentiate into functional LCs in vitro and in vivo, we admit that this SLCs population might not explain the whole story. Researchers have defined the ancestors of LCs from various perspectives or at different developmental stages. To the best of our knowledge, several cell types besides SLCs have been proposed to differentiate into adults LCs, as follows. First, previous studies have provided evidence that pericytes are the progenitors of the LCs in the testes (Davidoff et al., 2004; Chen et al., 2019; Davidoff, 2019). In the present study, the self-renewal and differentiation properties of endosialin+ human SLCs were highly similar to the characteristics of pericytes (Armulik et al., 2011; Wong et al., 2015). Moreover, a pericyte marker PDGFRβ (Armulik et al., 2011) is co-expressed with endosialin both in testis tissues and in cultured endosialin+ spheres (data not shown), implying that the endosialin+ cell population overlaps with the pericyte population in human testes to some degree. Second, adult LCs are thought to derive from the trans-differentiation of foetal LCs (FLCs) (Prince, 2001). Using lineage tracing of FLCs, Shima et al. (2018) suggested that some FLCs undergo dedifferentiation during foetal and neonatal stages and serve as adult LC progenitor cells. Another piece of evidence comes from the observation of expression of NR2F2 in human endosialin+ cells and rodent SLCs (Kilcoyne et al., 2014) since NR2F2 was initially considered a marker for subpopulations of FLCs (van den Driesche et al., 2012; Teerds and Huhtaniemi, 2015). However, we could not analyse the relation between endosialin and FLCs owing to a lack of foetal testicular tissues. Third, LCs are thought to have a mesenchymal origin (Hardy et al., 1989). Several studies have shown that MSCs are present in the testes of both human beings and rodents (Chikhovskaya et al., 2012; Ahmed et al., 2017). Nevertheless, to our knowledge, it has yet to be investigated whether testicular MSCs could be induced into LCs in vitro or in vivo. We and other groups have demonstrated that PDGFRα+ (Eliveld et al., 2019) and NGFR+ (Zhang et al., 2017) human SLCs are multipotent cells with MSCs properties, similar to endosialin+ human SLCs in the present study. In addition, endosialin+ human SLCs are positive for the MSCs minimal criteria markers CD73, CD90 and CD105 (data not shown). In addition, using lineage tracing models, Liu et al. (2016) provided evidence that the GLI1+/CYP17A1− non-steroidogenic progenitor cells retain their undifferentiated state during the foetal stage and become an adult LC population in post-pubertal testis. Besides, Kumar and DeFalco (2018) demonstrated that Nestin-expressing cells are adult LC progenitors during normal development. In the present study, endosialin+ cells also expressed GLI1 (data not shown) and Nestin. Nevertheless, to date, the development of LCs from stem cells is still a matter of debate, and it remains unclear whether these distinct cell populations share a common stem cell ontogeny, especially in human. It is anticipated that future studies would resolve these important issues.
The surface protein endosialin has been reported to be expressed in various tissues including muscle (Naylor et al., 2014), brain, thymus (Lax et al., 2012) and liver (Mogler et al., 2015). In the current study, we clarified the expression pattern of endosialin in adult human testes and revealed its superiority over two other markers for the identification and isolation of human SLCs. However, whether endosialin could serve as a surface marker of stem cells in other tissues needs to be further determined. In addition, cell surface proteins usually function as regulators of stem cell fate (Alexander et al., 2016; Tomellini et al., 2019). As previously reported, endosialin can regulate MSCs proliferation and maintain a promigratory phenotype (Naylor et al., 2012), and endosialin expressed on a population of thymic MSCs is involved in controlling thymic growth during postnatal development and infection-dependent thymic regeneration (Lax et al., 2012). In addition, endosialin expression in MSCs is required for wound healing of myofibroblasts (Hong et al., 2019). These findings imply a role of endosialin in tissue remodelling and repair. Moreover, endosialin helps regulate pericyte and hepatic stellate cell proliferation stimulated by PDGF-BB; these processes are blunted by the absence of endosialin, indicating that endosialin expression is necessary for PDGF signalling, at least in these cells (Tomkowicz et al., 2010; Mogler et al., 2015; Wilhelm et al., 2016). Since PDGF-BB is by far the most potent SLCs proliferation stimulator (Li et al., 2016) and endosialin is negligibly expressed once SLCs differentiate into LCs, we suspect that endosialin may play roles in determining the balance between SLCs self-renewal and differentiation. However, the effects of endosialin on SLCs cell biology are not yet fully understood and need to be elucidated in future studies.
In conclusion, we have identified endosialin as a more specific marker of SLCs compared with the currently used molecules. Additionally, we found that endosialin+ cells isolated from human testes show characteristics of SLCs with the ability to undergo extensive proliferation and differentiate into testosterone-producing LCs in vitro. Furthermore, transplanted endosialin+ cells appear to colonize the host interstitium, localize to peritubular and perivascular regions, be perfused by host vessels, proliferate measurably and differentiate partially into functional LCs in vivo. Thus, the present study identifies a promising discriminatory surface marker for SLCs identification and isolation, which is likely to give insight into the characterization of human SLCs and promote potential clinical applications of cell replacement therapies for male hypogonadism.
Supplementary Material
Acknowledgement
We especially want to thank the donors and their families, who made this study possible.
Authors’ roles
All authors fulfil the criteria for authorship. C.D. and A.P.X. designed the study and revised the manuscript critically. K.X., X.F. and R.D. conducted the experiments, collected and analysed the data. Y.M. and Q.K. participated in interpreting the data. K.X. drafted the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFA0103802, 2018YFA0107200 to A.P.X.), the National Natural Science Foundation of China (481971314 to C.D.) and the Natural Science Foundation of Guangdong Province, China (42018B030311039 to C.D.).
Conflict of interest
The authors declare no conflict of interest.
References
- Ahmed M, Ghabriel M, Amleh A. Enrichment, propagation, and characterization of mouse testis-derived mesenchymal stromal cells. Cell Reprogram 2017;19:35–43. [DOI] [PubMed] [Google Scholar]
- Alexander MS, Rozkalne A, Colletta A, Spinazzola JM, Johnson S, Rahimov F, Meng H, Lawlor MW, Estrella E, Kunkel LM. et al. CD82 is a marker for prospective isolation of human muscle satellite cells and is linked to muscular dystrophies. Cell Stem Cell 2016;19:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011;21:193–215. [DOI] [PubMed] [Google Scholar]
- Arora H, Zuttion MSSR, Nahar B, Lamb D, Hare JM, Ramasamy R. Subcutaneous Leydig stem cell autograft: a promising strategy to increase serum testosterone. Stem Cells Transl Med 2019;8:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S. Male hypogonadism. Lancet 2014;383:1250–1263. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018;103:1715–1744. [DOI] [PubMed] [Google Scholar]
- Brennan J. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 2003;17:800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zhao X, Guan X, Chen H. Origin and regulation of stem Leydig cells in the adult testis. Curr Opin Endocr Metab Res 2019;6:49–53. [Google Scholar]
- Chen P, Zirkin BR, Chen H. Stem Leydig cells in the adult testis: characterization, regulation and potential applications. Endocr Rev 2020;41:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhovskaya JV, Jonker MJ, Meissner A, Breit TM, Repping S, van Pelt AMM. Human testis-derived embryonic stem cell-like cells are not pluripotent, but possess potential of mesenchymal progenitors. Hum Reprod 2012;27:210–221. [DOI] [PubMed] [Google Scholar]
- Chong JJH, Yang X, Don CW,, Minami E, Liu Y-W, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS. The Pluripotent Microvascular Pericytes Are the Adult Stem Cells Even in the Testis Pericyte Biology in Different Organs. Cham: Springer International Publishing, 2019, 235–267. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol 2004;167:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliveld J, van den Berg EA, Chikhovskaya JV, van Daalen SKM, de Winter-Korver CM, van der Veen F, Repping S, Teerds K, van Pelt AMM. Primary human testicular PDGFRα+ cells are multipotent and can be differentiated into cells with Leydig cell characteristics in vitro. Hum Reprod 2019;34:1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Balsells MM,, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT. et al. Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010;95:2560–2575. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM,, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci U S A 2006;103:2719–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. time to talk hormones? Endocr Rev 2012;33:314–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JA, Scialdone A, Marioni JC. Using single-cell genomics to understand developmental processes and cell fate decisions. Mol Syst Biol 2018;14:e8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology 1989;124:762–770. [DOI] [PubMed] [Google Scholar]
- Hong Y-K, Lee Y-C, Cheng T-L, Lai C-H, Hsu C-K, Kuo C-H, Hsu Y-Y, Li J-T, Chang B-I, Ma C-Y. et al. Tumor endothelial marker 1 (TEM1/Endosialin/CD248) enhances wound healing by interacting with platelet-derived growth factor receptors. J Invest Dermatol 2019;139:2204–2214.e7. [DOI] [PubMed] [Google Scholar]
- Jiang MH, Cai B, Tuo Y, Wang J, Zang ZJ, Tu X, Gao Y, Su Z, Li W, Li G. et al. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res 2014;24:1466–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Arai KY, Shimizu K, Kojima C, Itoh M, Watanabe G, Taya K. Cellular localization of NGF and its receptors trkA and p75LNGFR in male reproductive organs of the Japanese monkey, Macaca fuscata fuscata. Endocrine 2006;29:155–160. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Lapauw B, Mahmoud A, T'Sjoen G, Huhtaniemi IT. Aging and the male reproductive system. Endocr Rev 2019;40:906–972. [DOI] [PubMed] [Google Scholar]
- Kilcoyne KR, Smith LB, Atanassova N, Macpherson S, McKinnell C, van den Driesche S, Jobling MS, Chambers TJG, De Gendt K, Verhoeven G. et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci U S A 2014;111:E1924–E1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DL, DeFalco T. A perivascular niche for multipotent progenitors in the fetal testis. Nat Commun 2018;9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreh L, Spinnler K, Schubert K, Häkkinen MR, Auriola S, Poutanen M, Söder O, Svechnikov K, Mayerhofer A. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. J Clin Endocrinol Metab 2014;99:E1227–E1235. [DOI] [PubMed] [Google Scholar]
- Lax S, Hardie DL, Wilson A, Douglas MR, Anderson G, Huso D, Isacke CM, Buckley CD. The pericyte and stromal cell marker CD248 (endosialin) is required for efficient lymph node expansion. Eur J Immunol 2010;40:1884–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S, Ross EA, White A, Marshall JL, Jenkinson WE, Isacke CM, Huso DL, Cunningham AF, Anderson G, Buckley CD. CD248 expression on mesenchymal stromal cells is required for post-natal and infection-dependent thymus remodelling and regeneration. FEBS Open Bio 2012;2:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanti MB, Germanà A, de Carlos F, Ciriaco E, Vega JA, Germanà G. Effects of increased nerve growth factor plasma levels on the expression of TrkA and p75 in rat testicles. J Anat 2006;208:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huang L, Zeng J, Lin W, Li K, Sun J, Huang W, Chen J, Wang G, Ke Q. et al. Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells. Mol Psychiatry 2018;23:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Z, Jiang Z, Guo J, Zhang Y, Li C, Chung J, Folmer J, Liu J, Lian Q. et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci U S A 2016;113:2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Ding X-F, Shi C-G, Zeng D, QuZong S, Liu S-H, Wu Y, LuoBu G, Fan M, Zhao YQ. Nerve growth factor promotes human sperm motility in vitro by increasing the movement distance and the number of A grade spermatozoa. Andrologia 2015;47:1041–1046. [DOI] [PubMed] [Google Scholar]
- Liu C, Rodriguez K, Yao HHC. Mapping lineage progression of somatic progenitor cells in the mouse fetal testis. Development 2016;143:3700–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabayashi K, Katoh-Fukui Y, Ogawa H, Baba T, Shima Y, Sugiyama N, Kitamura K, Morohashi K-I. Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. PLoS One 2013;8:e68050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogler C, Wieland M, König C, Hu J, Runge A, Korn C, Besemfelder E, Breitkopf Heinlein K, Komljenovic D, Dooley S. et al. Hepatic stellate cell‐expressed endosialin balances fibrogenesis and hepatocyte proliferation during liver damage. EMBO Mol Med 2015;7:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, Zhang B, Solomon GM, Turner B, Bihler H. et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 2016;19:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AJ, Azzam E, Smith S, Croft A, Poyser C, Duffield JS, Huso DL, Gay S, Ospelt C, Cooper MS. et al. The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice. Arthritis Rheum 2012;64:3334–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AJ, McGettrick HM, Maynard WD, May P, Barone F, Croft AP, Egginton S, Buckley CD. A differential role for CD248 (Endosialin) in PDGF-mediated skeletal muscle angiogenesis. PLoS One 2014;9:e107146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opavsky R, Haviernik P, Jurkovicova D, Garin MT, Copeland NG, Gilbert DJ, Jenkins NA, Bies J, Garfield S, Pastorekova S. et al. Molecular characterization of the mouse Tem1/endosialin gene regulated by cell density in vitro and expressed in normal tissues in vivo. J Biol Chem 2001;276:38795–38807. [DOI] [PubMed] [Google Scholar]
- Patel AS, Leong JY, Ramos L, Ramasamy R. Testosterone is a contraceptive and should not be used in men who desire fertility. World J Mens Health 2019;37:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, Salcido-Montenegro A, Benkhadra R, Prokop LJ, Bhasin S. et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metabol 2018;103:1745–1754. [DOI] [PubMed] [Google Scholar]
- Prince FP. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol 2001;168:213–216. [DOI] [PubMed] [Google Scholar]
- Rennert RC, Januszyk M, Sorkin M, Rodrigues M, Maan ZN, Duscher D, Whittam AJ, Kosaraju R,, Chung MT, Paik K. et al. Microfluidic single-cell transcriptional analysis rationally identifies novel surface marker profiles to enhance cell-based therapies. Nat Commun 2016;7:11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau C, Sancho J, Campos-Rivera J, Teicher BA. Endosialin expression in side populations in human sarcoma cell lines. Oncol Lett 2012;3:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl J, Rizzolo K, Soriano P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev 2008;22:3255–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima Y, Miyabayashi K, Sato T, Suyama M, Ohkawa Y, Doi M, Okamura H, Suzuki K. Fetal Leydig cells dedifferentiate and serve as adult Leydig stem cells. Development 2018;145:dev169136. [DOI] [PubMed] [Google Scholar]
- Sohni A, Tan K, Song H-W, Burow D, de Rooij DG, Laurent L, Hsieh T-C, Rabah R, Hammoud SS, Vicini E. et al. The neonatal and adult human testis defined at the single-cell level. Cell Reports 2019;26:1501–1517.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol 2013;9:414–424. [DOI] [PubMed] [Google Scholar]
- Sun D-X, Liao G-J, Liu K-G, Jian HAN. Endosialin-expressing bone sarcoma stem-like cells are highly tumor-initiating and invasive. Mol Med Rep 2015;12:5665–5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerds KJ, Huhtaniemi IT. Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum Reprod Update 2015;21:310–328. [DOI] [PubMed] [Google Scholar]
- Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y. et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013;12:127–137. [DOI] [PubMed] [Google Scholar]
- Tomellini E, Fares I, Lehnertz B, Chagraoui J, Mayotte N, MacRae T, Bordeleau M-È, Corneau S, Bisaillon R, Sauvageau G. Integrin-α3 is a functional marker of ex vivo expanded human long-term hematopoietic stem cells. Cell Reports 2019;28:1063–1073.e5. [DOI] [PubMed] [Google Scholar]
- Tomkowicz B, Rybinski K, Sebeck D, Sass P, Nicolaides NC, Grasso L, Zhou Y. Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther 2010;9:908–915. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Walker M, McKinnell C, Scott HM, Eddie SL, Mitchell RT, Seckl JR, Drake AJ, Smith LB, Anderson RA. et al. Proposed role for COUP-TFII in regulating fetal Leydig cell steroidogenesis, perturbation of which leads to masculinization disorders in rodents. PLoS One 2012;7:e37064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang S, Wang Z, Chen F, Chen P, Zhao X, Lin H, Ge R, Zirkin B, Chen H. Long-term maintenance of luteinizing hormone-responsive testosterone formation by primary rat Leydig cells in vitro. Mol Cell Endocrinol 2018;476:48–56. [DOI] [PubMed] [Google Scholar]
- Wilhelm A, Aldridge V, Haldar D, Naylor AJ, Weston CJ, Hedegaard D, Garg A, Fear J, Reynolds GM, Croft AP. et al. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism. Gut 2016;65:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S-P, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther 2015;151:107–120. [DOI] [PubMed] [Google Scholar]
- Ye L, Li X, Li L, Chen H, Ge R-S. Insights into the development of the adult Leydig cell lineage from stem Leydig cells. Front Physiol 2017;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Zhang P, Dong W, Zeng W, Pan C. Identification of stem Leydig cells derived from pig testicular interstitium. Stem Cells Int 2017;2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang ZJ, Wang J, Chen Z, Zhang Y, Gao Y, Su Z, Tuo Y, Liao Y, Zhang M, Yuan Q. et al. Transplantation of CD51+ stem Leydig cells: a new strategy for the treatment of testosterone deficiency. Stem Cells 2017;35:1222–1232. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang J, Deng C, Jiang MH, Feng X, Xia K, Li W, Lai X, Xiao H, Ge R-S. et al. Transplanted human p75-positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell Death Dis 2017;8:e3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.