Abstract
Steroid‐refractory chronic graft‐vs‐host disease (cGvHD) contributes to morbidity after allogeneic hematopoietic stem cell transplantation. Here, we report on 11 patients with severe, refractory cGvHD treated with repeated infusions of allogeneic bone marrow‐derived mesenchymal stromal cells (MSC) over a 6‐ to 12‐month period. Six patients responded to MSC treatment following National Institutes of Health response criteria, accompanied by improvement in GvHD‐related symptoms and quality of life. This response was durable, with systemic immunosuppressive therapy withdrawn from two responders, and a further two free from steroids and tapering calcineurin inhibitors. All responders displayed a distinct immune phenotype characterized by higher levels of naïve T cells and B cells before treatment compared with the nonresponders, and a significantly higher fraction of CD31+ naïve CD4+ T cells. MSC treatment was associated with significant increases in naïve T cells, B cells, and Tregs 7 days after each infusion. Skin biopsies showed resolution of epidermal pathology. CXCL9 and CXCL10 showed differential responses in responder and nonresponder patients. Our data support the use of MSC infusions as treatment for steroid‐refractory cGvHD with durable responses. We propose CXCL9 and CXCL10 as early biomarkers for responsiveness to MSC treatment. Our results highlight the importance of the MSC recipient immune phenotype in promoting treatment response. This trial was registered at www.ClinicalTrials.gov as #NCT01522716.
Keywords: cellular therapy, clinical trials, hematopoietic stem cell transplantation, mesenchymal stem cells, thymus
A, In this clinical phase II study, 11 patients with severe, refractory chronic graft‐vs‐host disease (cGvHD) were treated with repeated monthly infusions of mesenchymal stromal cell (MSC). B, Six patients responded with a reduction in disease severity as measured by the National Institutes of Health score. Immunological analysis revealed that response was associated with an immune profile with increased naïve CD4+ T‐cell and Treg numbers. In addition, a better thymic function in the responders was suggested based on increased ratio of recent thymic emigrants among naïve CD4+ T cells. Short‐term after each infusion we observed an increase in CD4+ T‐cell and Treg numbers in the responders. Skin histology improved in both groups with reduced epidermal inflammation. Our results support the use of MSC infusions for refractory cGvHD and suggest the recipient immune system phenotype as a predictor of responsiveness. R, responder; NR, nonresponder.
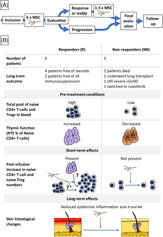
Significance statement.
Novel treatments for steroid‐refractory chronic graft‐vs‐host disease (cGvHD) are greatly needed. Mesenchymal stromal cells (MSC) represent a promising option, but clinical trials are lacking and their mechanism of action in vivo remains unknown. In this trial, 11 patients with severe, refractory cGvHD were treated with repeated infusions of MSCs. Six patients responded with reductions in disease severity and doses of immunosuppression as well as improved quality of life, suggesting MSCs as a feasible option in this situation. This study found markers that could predict responsiveness to treatment already after the first MSC infusion, several months before clinical improvement was evident. All responders displayed a distinct immune phenotype characterized by higher levels of naïve T and B cells and a significantly higher fraction of recent thymic emigrants among naïve CD4+ T cells. CXCL9 and CXCL10 levels increased after infusion in responder patients.
1. INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment for several hematological malignancies. HSCT introduces an alloimmune reaction, mediated by the donor immune system, responsible for the treatment efficacy via a graft‐vs‐tumor effect. Graft‐vs‐host disease (GvHD) represents the reverse side of the coin—an undesired immunological reaction against the healthy tissue of the recipient. GvHD is divided into acute and chronic forms. In recent years, short‐term and overall survival rates after HSCT have greatly improved. 1 Despite this, chronic GvHD (cGvHD) continues to be a major threat, which impacts on the quality of life, as well as life expectancy in transplanted patients. 2
First‐line treatment of cGvHD is corticosteroids, with or without the addition of calcineurin inhibitors. 3 , 4 The immunological heterogeneity of cGvHD is highlighted by the fact that only about 50% of patients respond to this treatment. 5 For the remaining patients, no established second‐line treatment exists, and many different agents are used with varying success. 5 , 6 The most well documented second‐line treatment is extracorporeal photopheresis (ECP), with reported response rates of about 60%. 7 Only ECP has been evaluated in a randomized controlled study which actually, failed to reach its primary endpoint. 8 ECP is generally well tolerated, but logistically challenging with frequent hospital visits. Other promising candidates in this setting are ruxolitinib, which is currently evaluated in a phase III randomized trial, and ibrutinib, which was recently approved by the US Food and Drug Administration for treatment of refractory cGvHD based on a single‐arm phase II trial. 9 , 10
Bone marrow‐derived mesenchymal stromal cells (MSC) display immunomodulatory properties. 11 Following the first report of successful MSC therapy in aGvHD, many studies have confirmed that it is a safe and valuable treatment for patients suffering from severe, refractory aGvHD. 12 , 13 , 14 Due to the multiple modulatory effects that MSCs exert on both innate and adaptive immune functions, the cells represent a promising treatment option also for cGvHD. 11 , 15 To date, published clinical studies on MSC adoptive transfer for cGvHD are scarce but indicate substantial benefit for the patients. 16 , 17 , 18 , 19 Although these studies are characterized by heterogeneity with regard to disease characteristics and treatment protocol, a treatment schedule with repeated infusions seems to be advantageous. 16 , 18 Further, these studies indicate long‐standing effects in vivo. For example, Peng et al showed that MSC treatment of cGvHD patients induced clinical responses and an increased number of interleukin (IL)‐10 positive Bregs, 3 months after infusion. Weng et al saw increased ratios of CD5+ CD19+/CD5− CD19+ B cells and CD8+ CD28−/CD8+ CD28+ T cells at 3 months, and maximal responses at a median of 233 days after treatment initiation. 16 , 17 However, neither study addressed early immune responses after MSC infusion.
Clinical experience shows that extended treatment periods are often necessary to achieve improvement in cGvHD. For moderate to severe disease, at least a year of treatment is usually recommended, and the median treatment duration is up to 3.5 years. 20 Further, clinical responses to cGvHD treatment at 3 and 6 months poorly correlate with long‐term success. 21 Duration of response is of major importance for the clinical relevance of the treatment. The aim must be a long‐lasting improvement in symptoms, enabling a tapering of the total immunosuppression to reduce the risks of infections and toxicity, as well as to improve quality of life. Of note, persisting lesions without active inflammation, such as the sicca syndrome or stable skin fibrosis, are not uncommon in cGvHD. Patients that have only such lesions remaining are defined as having “inactive” instead of “resolved” cGvHD status. 22 Since the National Institutes of Health (NIH) criteria require complete resolution of symptoms to classify a patient as being a complete responder, a partial responder may have a clinically and immunologically inactive phenotype. 23 Further, due to the gradual slow‐progressing nature of the disease, clinical evaluation of the chosen treatment is not usually possible until several months have passed.
In this paper, we report the results of a clinical trial of repeated MSC infusions for the treatment of refractory, severe cGvHD. In contrast to previously reported MSC trials for cGvHD, we chose an extended protocol of repeated MSC infusions for 6 to 9 months. In line with experience from ECP treatment, where response can be delayed for up to 24 weeks, no patient was determined a nonresponder until at least 6 months of treatment had been administered. 24 To enable earlier guidance of treatment choice in future trials, we investigated early biomarkers that may predict the success of MSC therapy.
The treatment induced long‐term partial responses in six out of 11 patients resulting in reduction of cGvHD symptoms, increased patient‐reported quality of life as well as a substantial reduction of immunosuppressive therapy. The clinical effects were paralleled by reduced inflammatory cytokine levels and skin histology in the responders. Importantly, we observed that the size of the naïve T‐cell pool associates with the long‐term responsiveness to MSC therapy, thus highlighting that the recipient immune phenotype may influence MSC treatment response.
2. MATERIALS AND METHODS
2.1. Study design
This was a prospective, single‐arm, single‐center, phase II study for treatment with MSC in cGvHD. Inclusion criteria were diagnosed cGvHD of grade moderate to severe, refractory to or not tolerating 3 months standard treatment of calcineurin inhibitor plus high dose steroids. 25 Patients with active malignancy were excluded. No major change in systemic immunosuppression was allowed for 6 weeks prior to enrolment. Calcineurin inhibitors and steroids were allowed for systemic immunosuppression concomitantly with MSC therapy if clinically indicated. The protocol was approved by the regional ethical committee in Stockholm and registered with ClinicalTrials.gov (ClinicalTrials.gov Identifier NCT01522716). Written informed consent was obtained at enrolment.
2.2. Study patients
The study included 11 patients with severe refractory cGvHD from Karolinska University Hospital, Stockholm, Sweden: 6 females and 5 males, with a median age of 50 years (range 20‐61). Patients underwent HSCT for hematological malignancies. All patients had received mobilized peripheral stem cells: 1 patient from an unrelated matched donor and the other 10 patients from matched sibling donors. Median time from cGvHD diagnosis to study enrolment was 18 months (range 6‐70), and median number of previous systemic cGvHD treatments was 4 (range 1‐10). Patients were affected by cGvHD in a median of five different organs (range 3‐6), of which NIH organ score was ≥2 in a median of 4 (range 1‐5). The 10‐point global severity score median was 8 (range 6‐9). 23 The most commonly affected organs were skin (n = 10), eyes (9), and joints (8). All patients had received previous lines of treatment, but following inclusion criteria a minimum of 6‐week washout period was required before initiation of MSC therapy. See Table 1 for patient characteristics.
TABLE 1.
Patient Characteristics at time of study inclusion
| Patient | Age | Diagnosis | Conditioning regimen | GvHD prophylaxis | Preceeding aGvHD | CMV infection | Months from HSCT to cGvHD diagnosis | Months from cGvHD to inclusion | Previous lines of cGvHD therapy | Prednisone dose at inclusion (mg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | AML | Flu+Bu | CyA+Mtx | No | No | 25 | 14 | CyA+prednisone, MMF, ECP, Mtx | 10 |
| 2 | 61 | Myelo‐fibrosis | Flu+Bu | CyA+Mtx+ATG | Yes | No | 5 | 11 | CyA+ prednisone, tacrolimus+sirolimus, imatinib, ECP | 50 |
| 3 | 56 | CLL | Flu+Cy+TBI | Tacrolimus+sirolimus | — | — | 9 | 18 | Tacrolimus+ prednisone, Rituximab | 7.5 |
| 4 | 50 | AML | Bu+Cy | CyA+Mtx | — | — | 7 | 6 | CyA+ prednisone | 50 |
| 5 | 38 | AML | Bu+Cy | CyA+Mtx | Yes | Yes | 11 | 37 | CyA+ prednisone, Mtx | 15 |
| 6 | 30 | HL | Flu+Cy+TBI | CyA+Mtx | Yes | No | 8 | 34 | CyA+ prednisone, Mtx, rituximab, ECP, MMF | 3.75 |
| 7 | 41 | MM | Flu+TBI | CyA+Mtx | No | No | 3 | 9 | CyA+prednisone, infliximab | 30 |
| 8 | 20 | AML | Bu+Cy | CyA+Mtx | Yes | Yes | 7 | 57 | CyA+ prednisone, sirolimus, MMF, infliximab, rituximab, tacrolimus, ECP, imatinib, chloroquin, DSC | 15 |
| 9 | 53 | AML | Bu+Cy | CyA+MMF | Yes | No | 18 | 42 | Tacrolimus+ prednisone, thalidomide, imatinib, ECP | 0 |
| 10 | 60 | AML | Flu+Bu | CyA+Mtx | Yes | No | 7 | 70 | CyA+MMF, prednisone, rituximab, Mtx | 0 |
| 11 | 29 | ALL | Cy+TBI | Tacrolimus+sirolimus | No | No | 15 | 6 | Tacrolimus+ prednisone, imatinib | 12.5 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; cGvHD, chronic graft‐vs‐host disease; CLL, chronic lymphocytic leukemia; CyA, cyclosporin A; DSC, decidual stromal cells; ECP, extracorporeal photopheresis; HL, Hodgkins lymphoma; HSCT, hematopoietic stem cell transplantation; MM, multiple myeloma; MMF, mycophenolate mofetil; Mtx, methotrexate.
2.3. Study treatment
At the time of study, MSCs were classified in Sweden as a tissue and MSCs from the bone marrow of healthy, third‐party donors were harvested and expanded following a procedure approved by the Swedish National Board of Health and Welfare according to the European Tissues and Cells Directive. The MSC production (described in detailed in the Supplemental Material) was carried out as previously described. 26 Donors provided written informed consent before the harvest. All doses were cryopreserved until administration. The characteristics of the MSC donors and grafts are shown in Table S1. A dose of 2 × 106 MSC per kg was thawed and infused at 4‐ to 6‐week intervals. A minimum of six doses were given. In patients with a response to treatment after six doses, an additional 1 to 3 doses were infused, while patients with progressive disease were taken off MSC infusions, to allow for alternative second‐line treatments. All patients continued on their previous immunosuppressive regime, which was tapered as clinically indicated. The patients were followed regularly for cytomegalovirus (CMV) DNA‐emia with a quantitative PCR as previously described and received prophylaxis against Pneumocystis jirovecii with either co‐trimoxazole or inhaled pentamidine and against varicella zoster virus with acyclovir or valaciclovir. 27 Fungal prophylaxis with posaconazol was generally recommended, but due to side effects some patients received fluconazole or no prophylaxis.
2.4. Study assessment
Global and organ‐specific evaluation was carried out according to the 2014 NIH criteria with one addition: In case of sclerodermatous disease a reduction in total sclerotic body surface area (BSA) by at least 25% was considered partial organ‐specific response (PR). 23 Evaluation was performed after every three MSC doses until the end of treatment. The primary endpoint was clinical response at the end of treatment. The time point “end of treatment” was defined as after six infusions if the patient was classified as nonresponder (NR) at that time, or after the final infusion if further infusions were administered. Patients taken off the study before six infusions had been administered were considered nonresponders. A final formal evaluation was made 12 months after the last dose of MSC and patients were then followed at the routine outpatient clinic. Patient‐reported measures were cGvHD‐related symptoms on the Lee symptom scale, global severity scale and quality of life on the Functional Assessment of Cancer Therapy‐Bone Marrow Transplant (FACT‐BMT) scale. 28 , 29
2.5. Blood collection
Venous blood samples were collected before each infusion, at 1 to 3 hours, 24 hours, 2 to4 days, and 7 days after each infusion. For details of the plasma and peripheral blood mononuclear cell separation, refer to Supplementary Methods.
2.6. Peripheral blood mononuclear cell and cytokine analysis
Cells were stained with florescence‐coupled monoclonal antibodies as detailed in Supplementary Methods. For intracellular staining, cells were surface stained for desired cell surface markers, fixed, permeabilized (Fixation and Permeabilization Kit, eBioscience, San Diego, California) and stained according to the manufacturer's instructions. Cells were acquired using an LSRFortessa (Becton Dickinson and Company, San Jose, California). Data were analyzed using the FlowJo X software. 30 The levels of selected cytokines and chemokines were assessed on seven patients (patients 1, 2, 5‐9) at time points before, and 1‐3 hours and 24 hours after infusions 1, 2, and 6. For details, refer to Supplementary Methods.
2.7. Tissue biopsies
Skin biopsies were taken before and after completion of MSC treatment. Paraffin‐formalin fixed biopsies were routinely histologically stained and blindly evaluated by a dermatopathologist for features indicative of cGvHD‐induced tissue damage, including inflammation, vacuolization, apoptosis, and fibrosis. 31
2.8. Micro‐RNA (miRNA) analysis
Circulating plasma miRNA were analyzed in seven patients (patients 1, 2, 5‐9) before and at two time points (1‐3 hours and 24 hours) after the first MSC infusion. Total RNA isolation and analysis were conducted at Exiqon Services (Vedbaek, Denmark). For details, refer to Supplementary Methods.
2.9. Statistics
The primary outcome measure was change in disease activity from inclusion to after the final MSC infusion, according to NIH criteria. Secondary outcome measures included change in disease activity as measured by histological examination and immunological analysis, change in self‐assessed disease activity and quality of life, safety (frequency of complications, infections, and relapse), and freedom from steroids at 1 year after MSC treatment.
Immunological assessment was performed on those patients that completed at least six MSC infusions. Absolute levels of cell subsets and cytokines were compared using Student's t test and relative levels were compared using Wilcoxon rank‐sum test. For miRNA analysis, comparisons were performed using a paired t test with Benjamini‐Hochberg correction for multiple analyses. Long‐term cytokine changes were analyzed with repeated measures ANOVA. For the time‐dependent changes in PBMC subset counts, a linear mixed effects analysis of long‐ and short‐term effects of MSC infusions were performed. This also allowed for responder vs nonresponder comparisons. For detailed information, refer to Supplementary Methods. All statistical tests were considered significant at the .05 level. As initial analysis was hypothesis‐generating, to be confirmed with further studies, no further correction was carried out for multiple analyses to avoid type II errors. Analysis was performed using the R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. MSC treatment induced long‐term partial responses in refractory cGvHD patients
Eleven patients were included. Two patients were excluded before receiving six MSC infusions: One patient died due to progressive cGvHD after receiving only one MSC infusion. One patient with chronic lymphocytic leukemia (CLL) discontinued the study after three infusions due to increasing CD19 recipient chimerism. The other nine patients received at least 6 infusions.
According to NIH criteria, six patients showed an overall PR at end of treatment and were classified as responders. The remaining five were either prematurely excluded (n = 2) or displayed a mixed response (n = 3) with progression of symptoms in some organs and were classified as nonresponders (Figure 1). In the responders, continued improvement was noted at each follow‐up during MSC treatment. For five patients, the response was durable, with further improvement observed at final evaluation 12 months after finishing MSC treatment (Figure S1) and three patients were free of steroids at that time point. With a median follow‐up time of 76 months (range 34‐99) from inclusion, two patients have discontinued all systemic immunosuppression, and two are free of steroids and tapering calcineurin inhibitors (Figure 1 and Figure S2). Organ responses were seen in joints (n = 8), skin (n = 4), eyes (n = 4), mouth (n = 3), gastrointestinal tract, and liver (n = 1 each). MSC treatment was well tolerated without immediate side effects. Serious adverse events recorded before the final evaluation included five events of grade 3 infection and two incidents of dysplasia (1 skin and 1 cervical), as well as one patient discontinued the study after seven infusions due to increasing M‐protein levels. All events are summarized in Table S3. Patient‐reported symptoms were measured using the Lee scale and responders showed a mean reduction in Lee symptom score of 7.75 points, or 16% compared with baseline values. 28 Quality of life was evaluated using the FACT‐BMT questionnaire, with a mean increase in FACT‐BMT total score of 4.7 points or 6% compared to baseline values in the responder group. 29 To conclude, MSC infusions were well tolerated and resulted in sustained and clinically relevant improvement in severe cGvHD symptoms in 6 of 11 patients with disease refractory to primary as well as several secondary treatments.
FIGURE 1.
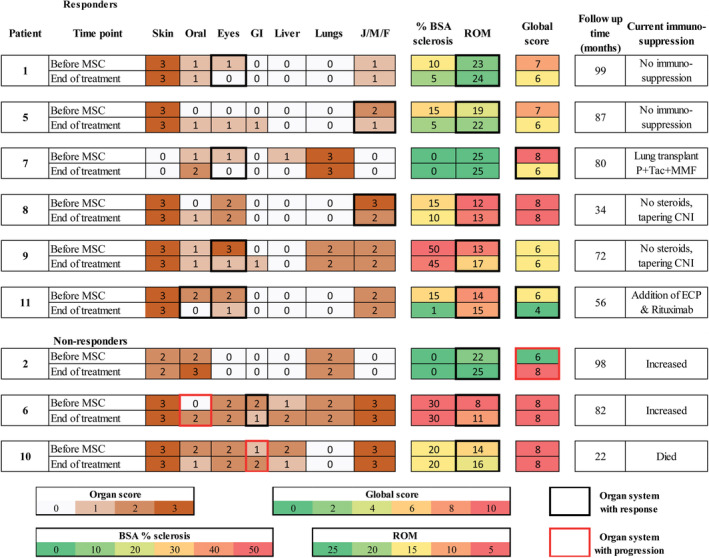
Heatmap of individual responses: NIH organ score, National Institutes of Health (NIH) global score, range of motion (ROM), and body surface area (BSA) percentage involved with sclerosis. Time points are at study enrolment and at the end of MSC treatment. Black boxes denote organs with response and red boxes denote organs with progression. CNI, calcineurin inhibitors; ECP, extracorporeal photopheresis; F, fascia; J, joints; MMF, mycophenolate mofetil; M, muscles; P, prednisolone; Tac, tacrolimus
3.2. Distinct immune profile with more naïve T cells and naïve B cells in responders compared with nonresponders
One aim of this study was to search for a possible immune profile correlating with response to MSC therapy for cGvHD. The flow cytometry analysis did not reveal any differences in the percentages or absolute counts of total lymphocytes, CD19+ B cells, CD3+ T cells, or CD4+ or CD8+ T cells between responders and nonresponders (Table S5 and Figure S3). However, further analysis of T‐cell subsets revealed that responders had significantly higher percentage of naïve CD4+ T cells defined as CD4+ CD27+ CD45RA+ compared to the nonresponders, whose T cells were dominated by non‐naïve subsets (Figure 2B). 34 Moreover, prior to the first infusion, the C‐C chemokine receptor 7 (CCR7) was expressed on a significantly higher proportion of CD4+ T cells in patients that responded to MSC treatment (Figure S4). 35 Also, the absolute number of naïve T cells was higher in responders but in contrast, no differences were seen in the absolute number of any memory T‐cell subsets (Figure 2C and Table S5). We investigated B‐cell subsets using two different reported gating strategies. 36 , 37 The percentage of CD19+ IgD+ CD38low naïve B cells was higher in responders than in nonresponders (Figure S5C). 36 Further, the absolute number of CD19+ IgD+ CD21hiCD27‐ and CD19+ IgD+ CD38low naïve B cells was also higher in responder patients (Figure 2E and Figure S5C). In contrast, distributions and absolute numbers of memory B cells were similar between the groups. Importantly, the differences in the T‐cell and B‐cell compartments remained stable throughout the entire course of the study (Figure 2 and Figure S5). To conclude, we found an association between the distribution of naïve CD4+ T cells and B cells, and clinical response to MSC treatment in our study.
FIGURE 2.
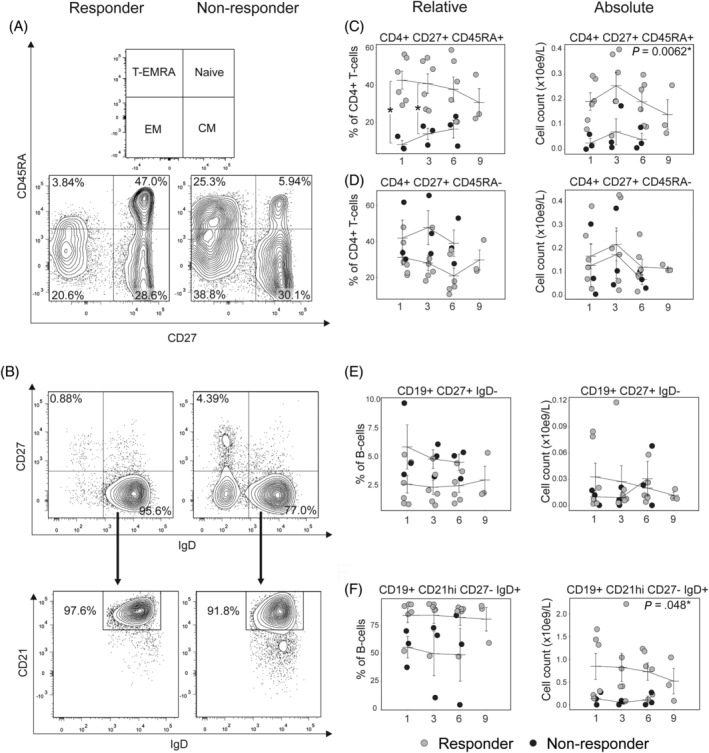
Responders have higher absolute and relative levels of naïve CD4+ T cells and naïve B cells compared with nonresponders. A and B, Representative plots of the flow cytometry analysis. CD4+ T cells were divided into naïve, CM, EM, and T‐EMRA based on CD27 and CD45RA. 32 Naïve B cells were defined as CD19+CD27‐IgD+CD21hi. CD21 was used to exclude CD27‐CD21low B cells, a population previously suggested to contain aberrant B cells in cGvHD. 33 C and D, Relative and absolute numbers of naïve CD4+ T cells were higher in R compared with NR throughout the study. There were no differences in CM CD4+ T‐cell numbers. E and F, Memory B cells were not different between R and NR. Absolute numbers of naïve B cells were higher in R compared with NR throughout the study. P‐values in the absolute number graphs represent the P‐values for the Responder factor in the linear mixed effects analysis. P‐values for relative numbers with Wilcoxon rank‐sum test. *P < .05. Error bars show mean ± SEM. CM, central memory T‐cell; EM, effector memory T‐cell; T‐EMRA, terminally differentiated effector memory T cells
3.3. Long‐term improvements in inflammatory cytokine profile and epidermal pathology after MSC infusions
We performed a broad range analysis of cytokines and chemokines before, and 1 month and 5 months after the first MSC infusion (infusions 1, 2, and 6). The levels of the chemokines CXCL9, CXCL10, CXCL12, and the cytokines TNFα and IL‐6 were similar in both patient groups before the first infusion. All of them increased in nonresponders during the first 5 months of treatment, while remaining stable in responders. The chemokines CXCL2 and CCL2 decreased in responders (Figure 3A). CXCL10 changes were completely segregated between the groups, increasing in all nonresponders, but in none of the responders, 5 months after the first infusion (Figure S5). The increase in inflammatory environment in nonresponders coincided with the progression of cGvHD symptoms and consequently the patients were classified as nonresponders after 6 months of treatment.
FIGURE 3.
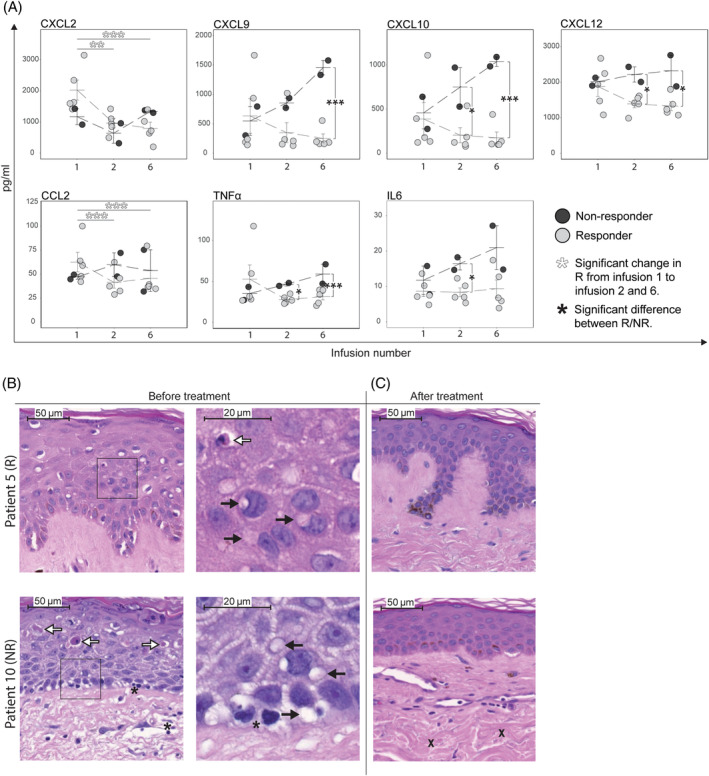
Mesenchymal stromal cell (MSC) treatment induces improvements in inflammatory cytokine profile in responders and in epidermal skin pathology in both responders and nonresponders. A, Multiplex analysis of plasma samples taken before each MSC infusion is presented. Levels of several pro‐inflammatory chemokines, as well as TNFα and IL6 increased in NR and decreased in R after two and six MSC infusions (1 and 5 months after treatment start, respectively), compared to before the first infusion. P‐values for within group changes using repeated measures ANOVA. P‐values for comparing NR and R at different time points with t test. *P < .05, **P < .005, ***P < .0005. B, Blinded pathological review of skin biopsies taken before and after treatment revealed improvements with mainly epidermal changes in both R and NR after treatment. For details, see Table S5. Representative images of one R (patient 5) and one NR (patient 10) showing mononuclear infiltration (asterisks), extensive vacuolization of KC (black arrows) as well as apoptotic and Civatte bodies in the epithelium (white arrows) before treatment. C, The epidermal pathology regressed in both R and NR after treatment with little or no remaining vacuolization and few apoptotic cells, but in the NR dermal sclerosis progressed (X). CXCL, chemokine (C‐X‐C motif) ligand; IL, interleukin; KC, keratinocytes; LP, lamina propria; MCP, monocyte chemotactic protein; NR, nonresponder; R, responder; TNF, tumor necrosis factor
To assess tissue effects of MSC on cGvHD pathology, puncture biopsies were taken from skin before and after completed treatment in six patients (one nonresponders and five responders). Blinded histopathological analysis revealed dermal sclerosis and epidermal inflammation and degeneration with mononuclear infiltration, extensive vacuolization of keratinocytes, as well as apoptotic and Civatte bodies before treatment in all patients (Figure 3B, Table S4). However, after treatment there was an almost complete resolution of epidermal changes in most of responders and nonresponders, while sclerosis remained unchanged in responders but progressed in nonresponders.
3.4. MSC infusions‐induced rapid release of miRNA in all patients, and short‐term increases in naïve lymphocyte and Treg numbers in responders
Short‐term effects of MSC infusions on the immune cell populations, as well as cytokines and miRNA levels were evaluated. MSC infusion induced an increase in four miRNAs out of 372 analyzed at 1 to 3 hours after infusion in both responders and nonresponders (Figure 4A). However, we did not see changes in the plasma level of cytokines or chemokines at these time points (data not shown). At the cellular level, the percentage of lymphocyte subsets did not change after infusion (data not shown); however, in responder patients, we found a significant increase in the absolute number of total lymphocytes 1 day and 7 days after each MSC infusion (Table S5). Interestingly, the increased lymphocytes consisted mainly of naïve and central memory (CM) CD4+ as well as naïve CD8+ T cells and naïve B cells (Table S5 and Figure 4C). However, absolute numbers of effector memory CD8+ T cells increased as well, within 7 days after each infusion (Table S5). By the next infusion, the absolute number of cells returned to baseline again. Nonresponders had no significant lymphocyte changes after any infusion.
FIGURE 4.
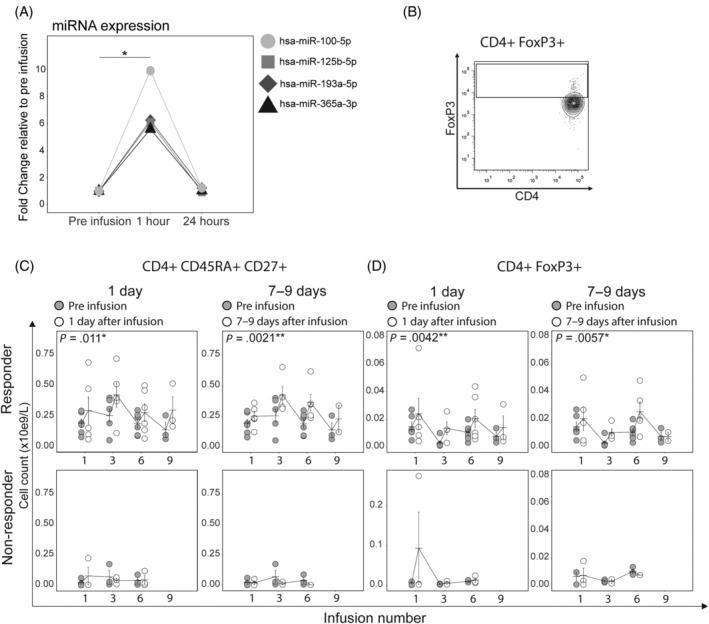
Short‐term increases in naïve miRNA, T cells, and Tregs after infusion. A, Four circulating miRNAs were significantly up regulated in R and NR plasma at 1 hour after mesenchymal stromal cell (MSC) infusion 1 and returned to baseline levels 24 hours after infusion. t Test with Benjamini‐Hochberg correction. B, Representative plots of the flow cytometry analysis. Tregs are defined as CD4+ FoxP3+ T cells. Naïve CD4+ T cells are defined as in Figure 2 (CD4+ CD45RA+ CD27+). C and D, Absolute number of naïve CD4+ T cells and Tregs increase in R 1 and 7 days after each MSC infusion compared with before infusion. These short‐term increases are not seen in NR. X‐axes display infusion number. The P‐values are derived from the mixed effects model and represent the significance of the factor Days since last infusion. *P < .05, **P < .005. Error bars show mean ± SEM. R, responder; NR, nonresponder; Treg, regulatory T cell
MSCs have been shown to induce Tregs in vivo. 38 Therefore, we investigated the short‐ and long‐term increases in these populations in our study. We found that the absolute number of Tregs increased 1 day and 7 days after infusion in responder but not in nonresponder patients (Figure 4D). To analyze the functionality of Tregs we set‐up a panel described previously by Sakaguchi's group, in which FoxP3+CD4+ cells were functionally divided into naïve Tregs (CD45RA+FoxP3low), activated Tregs (CD45RA‐FoxP3hi) and cytokine producing non‐Tregs (CD45RA‐FoxP3low) based on expression of CD45RA and FoxP3. 39 Analysis of these subsets revealed that responders had a significantly higher proportion of naïve Tregs, while the nonresponders had higher proportion of non‐Tregs (Figure S7).
To conclude, MSC infusion induced a rapid release of several miRNAs in all treated patients. This was followed by an increase in naïve lymphocytes and regulatory T cells in responders, but not in nonresponder patients.
3.5. The responders have a better thymic function compared to nonresponders
The next question addressed was whether the higher numbers and the short‐term increases in naïve T cells were due to mobilization from the thymus or due to increased proliferation of the circulating naïve T‐cell pool. To measure thymic function, we used the proportion naïve CD4+ T cells expressing CD31 (CD4+ CD45RA+ CD31+), a subset with significantly higher signal joint T‐cell receptor excision circle content, compared with CD4+ CD45RA+ CD31− T cells. 40 We found that responders had a significantly higher proportion of CD31+ cells among naïve CD4+ T cells compared with nonresponders (Figure 5A). We did not see a difference in proliferation (Ki67+ cells) either among total naïve T cells or CD31+ cells between the groups (Figure 5B). Together, this data suggested a better thymic function in patients responding to MSC treatment.
FIGURE 5.
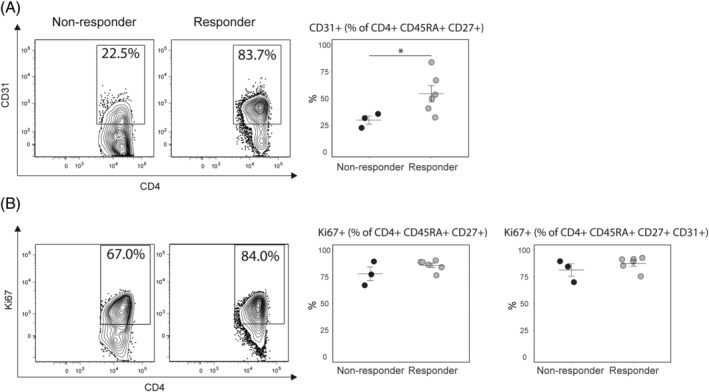
Thymic function and proliferation of naïve CD4+ T cells. A, Thymic function was assessed by measuring the proportion of CD31+ cells among naïve CD4+ T cells. We found a higher percentage of CD31+ naïve CD4+ T cells in R compared to NR before infusion, indicating better thymic function. B, Proliferation of naïve CD4+ T cells was assessed using levels of Ki67. The levels of Ki67 were not different between the responders and the nonresponders. Data from infusion 3. P‐values calculated using the Wilcoxon rank‐sum test. *P < .05. Error bars show mean ± SEM
4. DISCUSSION
The results of this study are encouraging in demonstrating that, with MSC therapy, long‐term responses can be achieved in patients with severe cGvHD refractory to several lines of previous treatment. The clinical response was strictly evaluated according to NIH criteria and was accompanied by a reduction in systemic immunosuppression, as well as improvement in patient‐reported measures of GvHD‐related symptoms and quality of life; demonstrating that MSC treatment led to a factual improvement for the patients.
MSC treatment was well tolerated without side effects, requiring considerably less time and effort for the patients than ECP, with clinic visits as sparingly as every 4 weeks. Close surveillance of infections is essential, as in all cGvHD patients. The occurrence of two cases of disease relapse calls for further evaluation, though these were seen in CLL and multiple myeloma, two diseases that are known for a high frequency of relapse after HSCT.
Immunological analysis resulted in three major observations: (a) a higher percentage and number of naïve lymphocyte populations in responder patients before MSC treatment; (b) transient increases in the absolute number of naïve and regulatory lymphocytes in responders but not in nonresponders, shortly after MSC infusions; and (c) long‐term reductions in levels of pro‐inflammatory cytokines in the plasma of responders but not nonresponders, after multiple MSC infusions.
T‐cell progenitors migrate from the bone marrow to the thymus where they undergo selection and expansion. 41 In our study, responders had significantly higher proportion of CD31+ naïve T cells (an accepted marker of human thymic function), supporting a better thymic function underlying their enlarged naïve T‐cell pool. 40 The cause of the decreased thymic function in nonresponders in our study was probably multifactorial. During HSCT, the conditioning regimens and aGvHD disrupt thymic function, and a recent study showed that the number of CD4+ T cells was higher in de novo cGvHD compared with cGvHD preceded by aGvHD. 41 , 42 When analyzed separately, neither age, conditioning regimen, nor preceding aGvHD segregated responders from nonresponders in our study. However, different combinations of these factors might underlie the thymic disruption in each nonresponder patient, and our study is too limited in size to allow such multivariate analyses to be performed. Regardless of the underlying mechanism, this distinct immunological difference between the groups has clinical validity. The significantly higher levels of CD4+ CCR7+ clearly distinguished responders before the first infusion, suggesting CCR7 as a useful biomarker to predict response to MSC infusions in cGvHD.
There are several possible mechanisms underlying the short‐term increases in naïve lymphocytes observed after each MSC infusion in our study. First, MSCs have been shown to promote hematopoietic stem cell (HSC) engraftment and lymphocyte reconstitution after HSCT, suggesting an underlying interaction between MSCs and HSCs, and explaining the observed increases in naïve T cells and B cells. 43 , 44 , 45 Secondly, previous murine studies have shown that infused MSCs engraft into the thymus, expressing multiple cytokines and promoting T‐cell reconstitution. 46 , 47 It is possible that the MSC infusions in our study stimulated thymic naïve T‐cell output either through direct homing to the organ or through secretion of soluble factors. The inability of nonresponders to mount this response could be explained by a lack of sufficient baseline thymic function. The miRNAs hsa‐miR‐193a and hsa‐miR‐125b, which increased significantly in both patient groups 1‐3 hours after infusion, are predicted to regulate the proteins thymocyte selection associated family member (THEMIS)1 and THEMIS2 respectively, crucial for the intrathymic maturation of thymocytes. 48
Defective immune tolerance is an important mechanism underlying the pathogenesis of cGvHD. 49 MSCs improve peripheral tolerance by stimulating the conversion of naïve T cells into Tregs. 38 , 50 In our study, Tregs increased in the peripheral blood of responders shortly after each MSC infusion. Despite this, there was no accumulation of Tregs after repeated infusions. This can be due to homeostatic regulation. 51 Another possible explanation is that the regulatory cells leave the circulation and home to inflamed tissues, which might partially explain the long‐term improvement in skin fibrosis and decrease in pro‐inflammatory cytokines. 52 It was interesting to find that responders had elevated levels of CD45RA+FoxP3low Tregs. This subset of Tregs proliferates upon activation and exerts potent suppressive functions. 39 In comparison, Tregs in nonresponders were dominated by a subset lacking the expression of CD45RA (CD45RA‐FoxP3low). Not all FoxP3+ T cells are regulatory. For example, FoxP3 expression have been demonstrated on effector memory T cells, and CD45RA‐FoxP3low T cells were described as non‐Tregs expressing FoxP3, due to their lack of suppressive function. 39 , 53 In conclusion, in the responders, MSC infusions caused a short‐term increase in the absolute number of preexisting Tregs with suppressive activity. These effects were not seen in the nonresponder group.
In responders, the cytokine analysis revealed significant long‐term decreases of the chemokines CXCL2 and CCL2, mainly produced by pro‐inflammatory monocytes. 54 In contrast, in nonresponders, levels of cytokines (TNFα, IL‐6) and chemokines (CXCL9, CXCL10, CXCL12), associated with the pro‐inflammatory monocyte phenotype increased during the study. MSCs are known to interact with monocytes and have been shown to induce skewing of these cells toward an anti‐inflammatory phenotype. 15 Our findings suggest a long‐term MSC‐mediated skewing of monocytes in responders. Alternatively, these long‐term alterations in cytokine concentrations may not be MSC‐specific, but could simply reflect decreased systemic inflammation in the responders in contrast to continued progression of inflammation in nonresponders. In nonresponders, the absence of monocyte skewing led to a progressive increase of cytokines associated with pro‐inflammatory monocytes. A recent paper suggested that MSC efficacy for treating aGvHD was caused by anti‐inflammatory monocyte skewing. 55 The distinct lymphocyte phenotypes between responders and nonresponders seen in our study, as well as the observed changes in monocyte‐derived cytokines and chemokines support these findings and the hypothesis that the immune phenotype of the recipient is the major determinant of MSC treatment response. In addition, the differential levels of CXCL10 at 5 months after initiating MSC treatment, suggests it as a candidate biomarker for response.
5. CONCLUSION
Our study suggests that repeated MSC infusions constitute a safe and valuable treatment option for patients with severe steroid‐refractory cGvHD. Further, our findings indicate that success of MSC treatment is influenced by the immunological characteristics of the individual patient. More specifically, responsiveness was associated with the recipient having a sufficient naïve lymphocyte pool and being able to generate ample numbers of naïve T cells and naïve Tregs. The single‐arm design and the small number of patients prevent us from drawing definitive conclusions. Therefore, additional preferably randomized clinical studies are needed to confirm the clinical effects observed and to shed further light on the underlying mechanisms.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
L.V.B.: study design, patient evaluations, clinical data collection, and manuscript writing; E.B: flow cytometry experiments, data analysis, and manuscript writing; N.H.: result discussion and manuscript revision; G.A., K.G.L.: patient evaluations; C.M.: treatment administration and sample collection; P.P.: dermatopathological evaluation; P.L.: patient accrual; N.K.: supervision of the flow cytometry work, data analysis, and manuscript writing; K.L.B.: principal investigator, study design, patient selection, MSC donation and expansion, and manuscript writing. All authors contributed to the discussion of the manuscript.
Supporting information
Data S1. Supporting information.
ACKNOWLEDGMENTS
This study was supported by grants from Cancerfonden (11 0315), Swedish Research Council (K2011‐??X‐20742‐04‐6), VINNOVA (2010‐00501), Stockholms Läns Landsting (ALF) (20110152), Cancerföreningen i Stockholm, Swedish Society of Medicine, Tobias Foundation and Karolinska Institutet. The sponsors of this study are public or nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data. We also thank Evren Alici, MD, PhD for his valuable insights in discussing results and during manuscript writing, as well as for his generous donations of laboratory material. E.B. and L.V.B. were PhD candidates at Karolinska Institutet. This work is submitted in partial fulfillment of the requirement for the PhD.
Boberg E, von Bahr L, Afram G, et al. Treatment of chronic GvHD with mesenchymal stromal cells induces durable responses: A phase II study. STEM CELLS Transl Med. 2020;9:1190–1202. 10.1002/sctm.20-0099
Lena von Bahr and Erik Boberg contributed equally to this study.
Funding information Karolinska Institutet; Tobias Foundation; Swedish Society of Medicine; Cancerföreningen i Stockholm; Stockholms Läns Landsting, Grant/Award Number: 20110152; VINNOVA, Grant/Award Number: 2010‐00501; Swedish Research Council, Grant/Award Number: K2011‐??X‐20742‐04‐6; Cancerfonden, Grant/Award Number: 11 0315
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic‐cell transplantation. N Engl J Med. 2010;363(22):2091‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duell T, van Lint MT, Ljungman P, et al. Health and functional status of long‐term survivors of bone marrow transplantation. EBMT working party on late effects and EULEP study group on late effects. European Group for Blood and Marrow Transplantation. Ann Intern Med. 1997;126(3):184‐192. [DOI] [PubMed] [Google Scholar]
- 3. Ruutu T, Gratwohl A, de Witte T, et al. Prophylaxis and treatment of GVHD: EBMT‐ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014;49(2):168‐173. [DOI] [PubMed] [Google Scholar]
- 4. Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft‐versus‐host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48‐51. [DOI] [PubMed] [Google Scholar]
- 5. Wolff D, Schleuning M, von Harsdorf S, et al. Consensus conference on clinical practice in chronic GVHD: second‐line treatment of chronic graft‐versus‐host disease. Biol Blood Marrow Transplant. 2011;17(1):1‐17. [DOI] [PubMed] [Google Scholar]
- 6. Arai S, Pidala J, Pusic I, et al. A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clin Cancer Res. 2016;22(2):319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malik MI, Litzow M, Hogan W, et al. Extracorporeal photopheresis for chronic graft‐versus‐host disease: a systematic review and meta‐analysis. Blood Res. 2014;49(2):100‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft‐versus‐host disease. Blood. 2008;112(7):2667‐2674. [DOI] [PubMed] [Google Scholar]
- 9. Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft‐versus‐host disease after failure of prior therapy. Blood. 2017;130(21):2243‐2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. A Study of Ruxolitinib vs Best Available Therapy (BAT) in Patients With Steroid‐refractory Chronic Graft vs. Host Disease (GvHD) After Bone Marrow Transplantation (REACH3). Vol 2020 Wilmington: Incyte Corporation; 2017. [Google Scholar]
- 11. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392‐402. [DOI] [PubMed] [Google Scholar]
- 12. Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439‐1441. [DOI] [PubMed] [Google Scholar]
- 13. Lalu MM, McIntyre L, Pugliese C, et al.; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta‐analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernardo ME, Fibbe WE. Mesenchymal stromal cells and hematopoietic stem cell transplantation. Immunol Lett. 2015;168(2):215‐221. [DOI] [PubMed] [Google Scholar]
- 15. Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol Lett. 2015;168(2):140‐146. [DOI] [PubMed] [Google Scholar]
- 16. Peng Y, Chen X, Liu Q, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636‐646. [DOI] [PubMed] [Google Scholar]
- 17. Weng JY, Du X, Geng SX, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45(12):1732‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou H, Guo M, Bian C, et al. Efficacy of bone marrow‐derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft‐versus‐host disease: clinical report. Biol Blood Marrow Transplant. 2010;16(3):403‐412. [DOI] [PubMed] [Google Scholar]
- 19. Perez‐Simon JA, Lopez‐Villar O, Andreu EJ, et al. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft‐versus‐host disease: results of a phase I/II clinical trial. Haematologica. 2011;96(7):1072‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flowers ME, Martin PJ. How we treat chronic graft‐versus‐host disease. Blood. 2015;125(4):606‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin PJ, Storer BE, Carpenter PA, et al. Comparison of short‐term response and long‐term outcomes after initial systemic treatment of chronic graft‐versus‐host disease. Biol Blood Marrow Transplant. 2011;17(1):124‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT‐NIH‐CIBMTR task force position statement on standardized terminology & guidance for graft‐versus‐host disease assessment. Bone Marrow Transplant. 2018;53(11):1401‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft‐versus‐host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: IV. The 2014 response criteria working group report. Biol Blood Marrow Transplant. 2015;21(6):984‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greinix HT, van Besien K, Elmaagacli AH, et al.; UVADEX Chronic GVHD Study Group. Progressive improvement in cutaneous and extracutaneous chronic graft‐versus‐host disease after a 24‐week course of extracorporeal photopheresis–results of a crossover randomized study. Biol Blood Marrow Transplant. 2011;17(12):1775‐1782. [DOI] [PubMed] [Google Scholar]
- 25. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945‐956. [DOI] [PubMed] [Google Scholar]
- 26. Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid‐resistant, severe, acute graft‐versus‐host disease: a phase II study. Lancet. 2008;371(9624):1579‐1586. [DOI] [PubMed] [Google Scholar]
- 27. Ljungman P, Boeckh M, Hirsch HH, et al.; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87‐91. [DOI] [PubMed] [Google Scholar]
- 28. Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft‐versus‐host disease. Biol Blood Marrow Transplant. 2002;8(8):444‐452. [DOI] [PubMed] [Google Scholar]
- 29. McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy‐Bone Marrow Transplant (FACT‐BMT) scale. Bone Marrow Transplant. 1997;19(4):357‐368. [DOI] [PubMed] [Google Scholar]
- 30. FlowJo Software Team . FlowJo. Ashland, OR: Tree Star, Inc; 2015. [Google Scholar]
- 31. Shulman HM, Cardona DM, Greenson JK, et al. NIH consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21(4):589‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larbi A, Fulop T. From "truly naive" to "exhausted senescent" T cells: when markers predict functionality. Cytometry A. 2014;85(1):25‐35. [DOI] [PubMed] [Google Scholar]
- 33. Sarantopoulos S, Ritz J. Aberrant B‐cell homeostasis in chronic GVHD. Blood. 2015;125(11):1703‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Mitri D, Azevedo RI, Henson SM, et al. Reversible senescence in human CD4+CD45RA+CD27‐ memory T cells. J Immunol. 2011;187(5):2093‐2100. [DOI] [PubMed] [Google Scholar]
- 35. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708‐712. [DOI] [PubMed] [Google Scholar]
- 36. Peng Y, Chen X, Liu Q, et al. Alteration of naive and memory B‐cell subset in chronic graft‐versus‐host disease patients after treatment with mesenchymal stromal cells. Stem Cells Translational Med. 2014;3(9):1023‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol. 2012;12(3):191‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol. 2013;91(1):12‐18. [DOI] [PubMed] [Google Scholar]
- 39. Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899‐911. [DOI] [PubMed] [Google Scholar]
- 40. Kohler S, Thiel A. Life after the thymus: CD31+ and CD31‐ human naive CD4+ T‐cell subsets. Blood. 2009;113(4):769‐774. [DOI] [PubMed] [Google Scholar]
- 41. Krenger W, Blazar BR, Hollander GA. Thymic T‐cell development in allogeneic stem cell transplantation. Blood. 2011;117(25):6768‐6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bohmann EM, Fehn U, Holler B, et al. Altered immune reconstitution of B and T cells precedes the onset of clinical symptoms of chronic graft‐versus‐host disease and is influenced by the type of onset. Ann Hematol. 2017;96(2):299‐310. [DOI] [PubMed] [Google Scholar]
- 43. Stenger EO, Krishnamurti L, Galipeau J. Mesenchymal stromal cells to modulate immune reconstitution early post‐hematopoietic cell transplantation. BMC Immunol. 2015;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Batorov EV, Shevela EY, Tikhonova MA, et al. Mesenchymal stromal cells improve early lymphocyte recovery and T cell reconstitution after autologous hematopoietic stem cell transplantation in patients with malignant lymphomas. Cell Immunol. 2015;297(2):80‐86. [DOI] [PubMed] [Google Scholar]
- 45. Koç ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous‐blood stem cells and culture‐expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high‐dose chemotherapy. J Clin Oncol. 2000;18(2):307‐316. [DOI] [PubMed] [Google Scholar]
- 46. Liu G, Wang L, Pang T, et al. Umbilical cord‐derived mesenchymal stem cells regulate thymic epithelial cell development and function in Foxn1(−/−) mice. Cell Mol Immunol. 2014;11(3):275‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu KX, Wang MH, Fan C, Wang L, Guo M, Ai HS. CM‐DiI labeled mesenchymal stem cells homed to thymus inducing immune recovery of mice after haploidentical bone marrow transplantation. Int Immunopharmacol. 2011;11(9):1265‐1270. [DOI] [PubMed] [Google Scholar]
- 48. Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43(Database Issue):D146‐D152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cooke KR, Luznik L, Sarantopoulos S, et al. The biology of chronic graft‐versus‐host disease: a task force report from the National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease. Biol Blood Marrow Transplant. 2017;23(2):211‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase‐1‐mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117(18):4826‐4835. [DOI] [PubMed] [Google Scholar]
- 51. Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T‐cell homeostasis: steady‐state maintenance and modulation during inflammation. Immunol Rev. 2014;259(1):40‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14(10):1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kmieciak M, Gowda M, Graham L, et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ariel A, Serhan CN. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front Immunol. 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Galleu A, Riffo‐Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient‐mediated immunomodulation. Sci Transl Med. 2017;9(416).–. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


