Abstract
Techniques that enable longitudinal tracking of cell fate after myocardial delivery are imperative for optimizing the efficacy of cell‐based cardiac therapies. However, these approaches have been underutilized in preclinical models and clinical trials, and there is considerable demand for site‐specific strategies achieving long‐term expression of reporter genes compatible with safe noninvasive imaging. In this study, the rhesus sodium/iodide symporter (NIS) gene was incorporated into rhesus macaque induced pluripotent stem cells (RhiPSCs) via CRISPR/Cas9. Cardiomyocytes derived from NIS‐RhiPSCs (NIS‐RhiPSC‐CMs) exhibited overall similar morphological and electrophysiological characteristics compared to parental control RhiPSC‐CMs at baseline and with exposure to physiological levels of sodium iodide. Mice were injected intramyocardially with 2 million NIS‐RhiPSC‐CMs immediately following myocardial infarction, and serial positron emission tomography/computed tomography was performed with 18F‐tetrafluoroborate to monitor transplanted cells in vivo. NIS‐RhiPSC‐CMs could be detected until study conclusion at 8 to 10 weeks postinjection. This NIS‐based molecular imaging platform, with optimal safety and sensitivity characteristics, is primed for translation into large‐animal preclinical models and clinical trials.
Keywords: cardiomyocytes, CRISPR/Cas9, electrophysiology, imaging, in vivo tracking, iPSCs, NIS, PET, rhesus macaque, sodium iodide symporter
Site‐specific, CRISPR/Cas9‐mediated delivery of the sodium/iodide symporter (NIS) gene allowed sensitive in vivo tracking of rhesus macaque induced pluripotent stem cell‐derived cardiomyocytes (RhiPSC‐CMs) in a mouse model of myocardial infarction. The effect of NIS expression on cardiomyocyte electrophysiological function was also carefully investigated as a critical step before large animal and potential clinical applications.
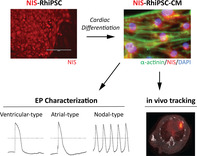
Significance statement.
In vivo imaging techniques are imperative to guide clinical translation of cell‐based therapeutics; however, current strategies are limited by immunogenicity and potential genotoxicity. In this proof‐of‐principle study, site‐specific delivery of the sodium/iodide symporter (NIS) gene via CRISPR/Cas9 enabled sensitive in vivo tracking of induced pluripotent stem cell‐derived cardiomyocytes (iPSC‐CM) in a clinically relevant model of myocardial infarction, and NIS‐positive iPSC‐CMs retained electrophysiological characteristics comparable to controls. Featuring a superior safety profile, this approach offers wider applications in both the preclinical and clinical development of cardiac cell therapies.
1. INTRODUCTION
Imaging approaches enabling noninvasive longitudinal monitoring of cardiac cellular therapies are imperative for assessment of the safety and efficiency endpoints required for clinical translation, including engraftment, persistence, localization, and correlation with functional and structural outcomes. However, applicable long‐term cell tracking technologies are lacking, particularly for application in relevant immunocompetent large animal models, where approaches using optical scanning of xenogeneic fluorescent proteins are not applicable. 1 The sodium/iodide symporter (NIS), a transmembrane protein expressed normally at high levels in the thyroid and stomach, but with limited expression in other tissues, 2 has many advantageous properties for in vivo cell fate tracking. NIS is nonimmunogenic and allows multimodality molecular imaging with various safe and clinically available radiotracers. Furthermore, NIS has been shown to enable sensitive and specific in vivo identification of transplanted cells in the injured heart via radiolabeled substrate injection followed by either positron emission tomography (PET) or single‐photon emission computed tomography. 3 , 4 , 5 However, these prior studies suffer from issues that could impede translation to the clinic, such as reliance upon clinically irrelevant donor cell types, inefficient plasmid transfection, use of nonintegrating adenoviral vectors offering only transient expression, or application of potentially genotoxic or silenced lentiviral vectors to introduce the NIS gene. In addition, there has been limited assessment of the effect of NIS expression on cardiomyocyte physiology. Hence, development of strategies that use site‐specific gene introduction methods offering safe and durable transgene expression would overcome these limitations, accelerating utilization of in vivo imaging techniques.
We have previously used CRISPR/Cas9 targeted genome editing to introduce transgenes into the adeno‐associated virus site 1 (AAVS1) safe harbor locus in rhesus macaque induced‐pluripotent stem cells (RhiPSCs), a site known to enable robust and stable gene expression in both pluripotent and differentiated cells, while minimizing genotoxicity. 6 , 7 We now report the utility of this genome targeting approach to introduce the NIS gene into RhiPSCs at the AAVS1 site, and demonstrate the feasibility and relevance of this model to the preclinical development of regenerative cardiac therapies. In addition, for the first time, we present a thorough and clinically oriented electrophysiological (EP) description of NIS‐positive induced pluripotent stem cell‐derived cardiomyocytes (iPSC‐CMs). RhiPSCs were selected for this study as they, unlike human iPSCs (hiPSCs), have the additional advantage of being directly compatible with long‐term preclinical studies in clinically relevant nonhuman primate (NHP) large animal models. RhiPSCs resemble hiPSCs in terms of colony morphology, expression of pluripotency‐associated transcription factors and surface markers, and are dependent on similar growth factors to maintain their pluripotency. 8 , 9 In addition, RhiPSCs and hiPSCs have been shown to confer comparable cardioprotection in another rodent model of myocardial infarction, and via similar mechanisms. 10 Moreover, NHPs bear key anatomical, physiological, and immunologic similarities to humans that would enable closer translation of strategies that optimize therapeutic efficacy. In this regard, we describe a new NIS‐based platform that has the potential to greatly enhance utilization of molecular imaging techniques in both preclinical and clinical trials of regenerative cardiac cell therapies.
2. MATERIALS AND METHODS
2.1. Animal use
All animals used in this study were housed and handled in accordance with protocols approved by the Institutional Animal Care and Use Committees of the NHLBI and NCI.
2.2. RhiPSC generation and maintenance
Rhesus CD34+ hematopoietic stem and progenitor cells or skin fibroblasts were isolated and reprogrammed as previously described. 6 , 11 , 12 RhiPSCs were cultured either on mouse embryonic fibroblast (GlobalStem) feeders or growth factor‐reduced Matrigel (BD Biosciences)‐coated plates.
2.3. Generation of NIS‐RhiPSCs via CRISPR/Cas9‐mediated genomic editing
The rhesus NIS transgene was removed from the pLV‐SFFV‐NIS‐PGK‐Puro plasmid (Imanis Life Sciences) via digestion with restriction enzymes BamHI and NotI, and cloned into the rhesus AAVS1‐CAG‐copGFP donor plasmid (Addgene #84209). The all‐in‐one CRISPR/Cas9 vector containing the high‐fidelity eSpCas9 (Addgene #79145), the previously reported rhesus AAVS1 guide RNA sequence, and the NIS donor plasmid were introduced into RhiPSCs via nucleofection, and puromycin selection was performed to enrich targeted cells (Figure 1A). 6 , 13 Clones with NIS integration at the intended rhesus AAVS1‐like site were identified as previously described. 6 , 13
FIGURE 1.
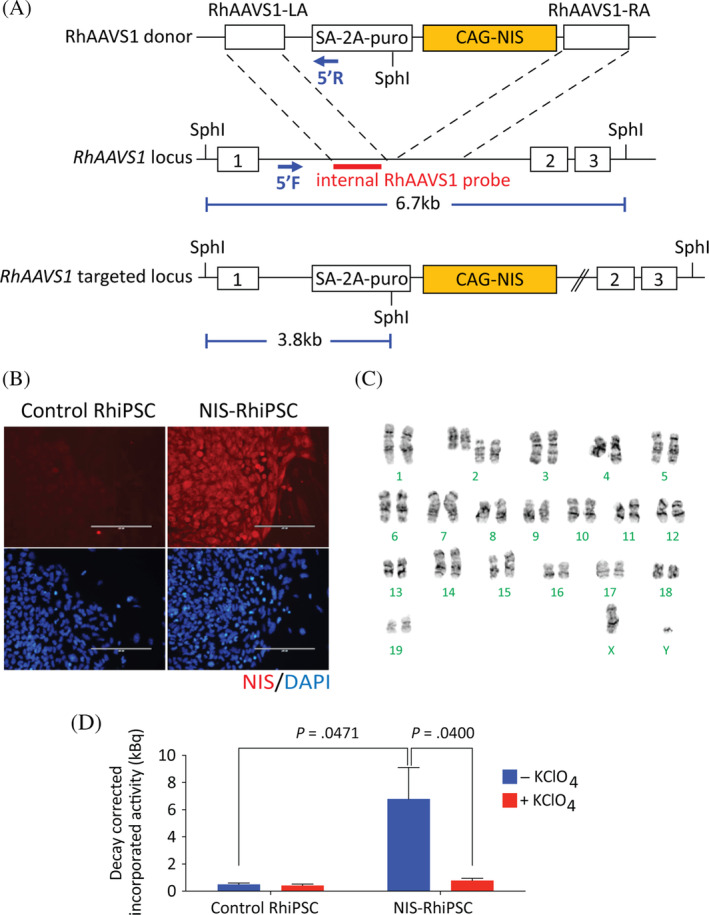
Functional NIS expression in NIS‐RhiPSCs. A, Schematic of the NIS donor plasmid construct (RhAAVS1‐CAG‐RhNIS) and the AAVS1 target site in the rhesus genome (RhAAVS1) between exons 1 and 2. The donor plasmid contains RhAAVS1 left (LA) and right (RA) homology arms flanking an excisable puromycin selection cassette and the RhNIS cDNA driven by a CAG promotor. B, Immunostaining confirmed expression of RhNIS (red) in NIS‐RhiPSCs, compared to no expression in parental RhiPSCs. Counterstaining with DAPI (blue) reveals ~100% RhNIS positivity in cultured NIS‐RhiPSCs. Scale bar = 200 μm. C, Karyotype NIS‐RhiPSCs retained normal. D, Uptake assay showing functionality of RhNIS in NIS‐RhiPSCs. Statistical analysis of in vitro 18F‐TFB uptake between NIS‐positive (n = 7) and control cells (n = 5), without KClO4 was performed with a t test for independent samples. Statistical analysis of in vitro 18F‐TFB uptake in NIS‐positive cells, with and without KClO4 (n = 7) was performed with a paired t test. Error bars represent mean ± SEM. 18F‐TFB, 18F‐tetrafluoroborate; AAVS1, adeno‐associated virus site 1; DAPI, 4′,6‐diamidine‐2′‐phenylindole dihydrochloride; RhiPSCs, rhesus macaque induced pluripotent stem cells
2.4. Teratoma assay
Standard teratoma assays to document pluripotency were conducted using NIS‐positive and control parental RhiPSCs in nonobese diabetic severe combined immunodeficient Il2rg −/− (NSG) mice (Jackson Laboratories) as described.
2.5. In vitro function of NIS
In vitro substrate transport function of NIS‐positive RhiPSCs was determined using 18F‐tetrafluoroborate (18F‐TFB). Cells cultured on Matrigel were dissociated with Accutase (STEMCELL Technologies) and 1 × 106 cells were resuspended in 500 μL of either uptake buffer, 10 mM 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES) in 1x Hanks' Balanced Salt Solution (HEPES/HBSS, Thermo Fisher Scientific) or inhibitor buffer, 10 mM HEPES/HBSS supplemented with 2 mM potassium perchlorate (KClO4). 18F‐TFB (1.11 MBq, 30 μCi) was added to the cell suspension and allowed to incubate for 1 hour at 37°C. Following incubation, cells were washed three times with 10 mM HEPES/HBSS and the residual radioactivity within the pellet was quantitated via γ‐counter (Wizard2 Automatic Gamma Counter; Perkin Elmer) in counts per minute (CPM). As a radioactivity standard, one tenth of the activity used for the assay (111 kBq, 3 μCi) was added to three empty tubes and measured in parallel with the cell samples 1 hour later. The activity taken up by the cells was decay corrected to the starting time of the incubation to minimize experiment‐to‐experiment decay differences occurred between the start of the assay and activity measurement. Decay‐corrected incorporated activity (kBq) of each sample was derived by dividing its CPM with the mean CPM of the standards, and then multiplied by 111 (kBq). In Figures 1 and 3, decay‐corrected incorporated activity per million cells was expressed as kBq.
FIGURE 3.
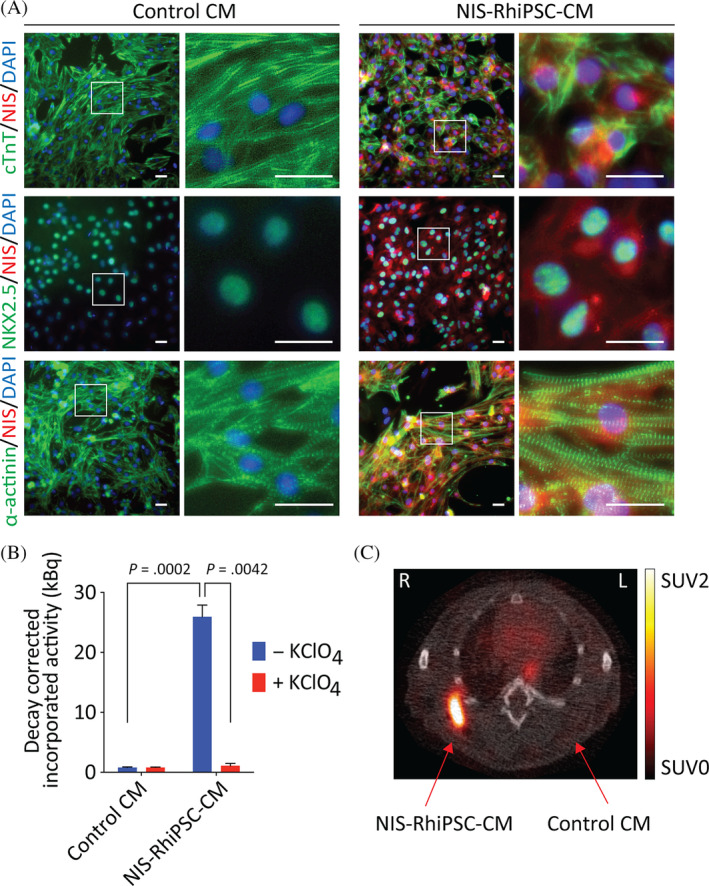
NIS‐RhiPSC‐CMs retain functional NIS expression. A, Immunostaining at day 20 of cardiac differentiation shows phenotypically normal RhiPSC‐CMs with preserved NIS expression (red in all panels). NIS‐RhiPSC‐CMs exhibit characteristic staining patterns for CTnT (first row, green), NKX2.5 (second row, green), and α‐actinin (third row, green). Per CTnT and α‐actinin costaining, there were no detectable derangements in sarcomeric structures. All replicates were counterstained with DAPI (blue). Scale bar = 50 μm. B, NIS‐RhiPSC‐CMs retained expression of functional RhNIS per 18F‐TFB uptake assay. NIS‐RhiPSC‐CMs exhibited significantly greater uptake of 18F‐TFB vs control RhiPSC‐CMs (n = 3). Tracer uptake was again ablated in the presence of KClO4, confirming NIS‐dependence. Error bars represent mean ± SEM. C, Intramuscular injection of 2 million NIS‐RhiPSC‐CMs and control (NIS‐negative) RhiPSC‐CMs with subsequent 18F‐TFB intravenous injection and imaging via PET/CT 1 hour later (n = 3). Transverse slices through NIS‐RhiPSC‐CM injection sites at upper limb show readily visible cell‐derived signals. No detectable signal was observed following injection of control RhiPSC‐CMs. 18F‐TFB, 18F‐tetrafluoroborate; CT, computed tomography; DAPI, 4′,6‐diamidine‐2′‐phenylindole dihydrochloride; PET, positron emission tomography; RhiPSC‐CMs, rhesus macaque induced pluripotent stem cell‐derived cardiomyocytes
2.6. Cardiac differentiation
Cardiac differentiation of RhiPSCs was performed as previously described. 12 Briefly, RhiPSCs were dissociated and cultured with Activin A (10 ng/mL, R&D), bone morphogenic protein 4 (BMP4; 10 ng/mL, R&D), and fibroblast growth factor 2 (FGF2; 10 ng/mL, Peprotech) for 3 days. Wnt signaling inhibitor IWP2 (3 μM, Tocris) was then added for an additional 5 days. Following differentiation, cells were maintained in insulin (20 μg/mL, Sigma)‐containing medium for further maturation.
2.7. Immunohistochemistry
Cells were fixed in 2% paraformaldehyde (PFA) in phosphate‐buffered saline (PBS) for 30 minutes, followed by incubation in blocking buffer (PBS + 4% bovine serum albumin) for 30 minutes. Fixed cells were then incubated with primary antibody followed by incubation with a fluorescently labeled secondary antibody, each for 1 hour at room temperature. Last, 4′,6‐diamidine‐2′‐phenylindole dihydrochloride (DAPI) was applied to labeled cells and allowed to incubate for 10 minutes. Specific primary antibodies and dilutions used were: antibodies against human NIS (rabbit, 1:200, Imanis Life Sciences), CTnT (cardiac troponin T; mouse, 1:1000, DSHB), α‐actinin (mouse, 1:1000, Sigma), and NKX2.5 (mouse, 1:200, Abcam). Secondary labeling was performed with an anti‐rabbit Alexa Fluor 555 (1:1000, Thermo Fisher Scientific) or an anti‐mouse Alexa Fluor 488 (1:1000, Thermo Fisher Scientific).
2.8. Patch clamp
RhiPSC‐CMs were dissociated and plated onto glass coverslips as single cells as previously described. 12 For action potential (AP) measurements, cells were inserted into a small‐volume chamber and bathed in Tyrode's solution (140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 10 mM HEPES, 10 mM glucose, pH 7.4) and maintained at 33°C to 35°C. Spontaneous and evoked APs were recorded using a patch clamp amplifier (Axopatch 200B, Molecular Devices) in whole‐cell current‐clamp mode. Pipettes (1‐3 MΩ) were filled with a standard internal solution. Calcium (Ca2+) transients were simultaneously measured as previously described, 12 using a fluorescent Ca2+ indicator (Cal‐520, AAT‐Bioquest). Recorded fluorescence was corrected for background and normalized to the baseline value (ΔF/F 0). In some cases, recordings were performed 3 minutes after addition of sodium iodide (NaI), in which 10% of the well medium was replaced with a 10x concentrated NaI stock solution warmed to 37°C, yielding a final concentration of 1 mM NaI.
2.9. Optical imaging
AP parameters of RhiPSC‐CMs were measured as described. 14 Briefly, RhiPSC‐CMs were plated onto 96‐well glass‐bottom plates (MatTek, P96G‐1.5‐5‐F) precoated with Matrigel at 1 × 105 cells per well. The effects of NaI on AP parameters were studied 2 to 5 days after plating, or on days 15 to 20 of cardiac differentiation. On the day of the testing, RhiPSC‐CMs maintenance medium was replaced with optically transparent Dulbecco's Modified Eagle Medium (FluoroBrite, Thermo Fisher Scientific) and cells were stained with 6 μM di‐4‐ANEPPS (Thermo Fisher Scientific) for 1 minute and allowed to re‐equilibrate in dye‐free FluoroBrite at 37°C, 5% CO2 for 2 hours before baseline recordings. Spontaneous RhiPSC‐CM APs were measured using ratiometric fluorescence recordings by a plate‐based optical system (CellOPTIQ, Clyde Biosciences). AP recordings of 20‐second duration were obtained from each well at baseline and 45 minutes after NaI addition. Each dose of NaI was assessed in multiple wells (n ≥ 10) by replacing 10% of the well medium with 10x concentrated NaI stock solution warmed to 37°C. Vehicle controls (NaI‐free medium) were included on each plate (n ≥ 10). The Fridericia formula was used to calculate the AP duration corrected for the beating rate (APDC). Rate‐corrected baseline and vehicle‐controlled NaI‐induced changes in AP duration at 90% repolarization were calculated (ΔΔAPD90C).
2.10. Induction of myocardial infarction and intramyocardial cell injection
Myocardial infarction in 3‐month‐old NSG mice was induced via 90‐minute ligation of the left anterior descending (LAD) coronary artery, followed by reperfusion. 2 × 106 NIS‐RhiPSC‐CMs in 30 μL pro‐survival media consisting of 50% 10 μM ROCK inhibitor in maintenance medium and 50% Matrigel were injected into the myocardium at the border zone of the infarct area immediately prior to reperfusion. Control injections consisted only of vehicle.
2.11. Animal imaging and analysis
18F‐TFB was synthesized in a radiochemical yield of 18% to 50% (n = 50, uncorrected for decay) and greater than 99% radiochemical purity, on par with other preclinical and clinical studies 15 , 16 , 17 in which 18F‐TFB was used. All animal imaging was performed using micro‐PET/computed tomography imager (μPET/CT; BioPET, Bioscan), and images were acquired 1 hour after intravenous injection of 3.7 MBq 18F‐TFB. An optimal wait time of 60 minutes was chosen based on a preliminary study, wherein superior visualization of NIS‐RhiPS‐derived cells was achieved compared with imaging at 90 minutes. A 250 to 700 keV energy window was used, and 5‐minute emission scans per bed position for a total of two bed positions were acquired. The acquired PET images were reconstructed using a three‐dimensional ordered‐subsets expectation maximization (3D OSEM) algorithm. PET images reconstructed using a 2D OSEM algorithm were analyzed using MiM (MiM Software, Inc.) for image quantitation. Regions of interest were drawn at the boundary of signal at each teratoma or implanted cells at the heart on axial images, and both volumetric and radioactivity information was recorded. VivoQuant (Build 3.0, Invicro) was used to merge maximum intensity projection PET images and CT. The total lesion 18F‐TFB uptake, which is equivalent to total lesion glycolysis in 18F‐fluorodeoxyglucose imaging, was calculated by multiplying the volume of lesion with increased 18F‐TFB uptake (mL) and its mean standardized uptake value (SUV). The total lesion 18F‐TFB uptake is a normalized value taking into account both the injected dose and body weight of the mouse.
2.12. Histological analysis
For immunohistochemical analysis on frozen sections of explanted murine tissues, samples were fixed with 4% PFA and blocked in 3% goat serum. All primary and secondary antibodies and their respective concentrations were used as above in the immunohistochemistry section, with the sole exception of CTnT, which was concentrated to 1:200. Detection was performed via Ventana Benchmark XT (Ventana Medical Systems) using the UltraView DAB Detection Kit. NIS‐immunostained slides were analyzed by MetaMorph software.
2.13. Statistical analysis
The Student's t test was used to compare in vitro 18F‐TFB uptake, with and without KClO4, between NIS‐positive and control cells, as well as to compare AP parameters of RhiPSC‐CMs obtained by patch clamp. In the event the assumptions of normality or equal variances were unsatisfied, the Wilcoxon signed‐rank test was performed. A P value of <.05 was selected to determine significance.
3. RESULTS
3.1. CRISPR/Cas9‐engineered RhiPSCs demonstrate functional NIS expression
Following targeted knock‐in of the rhesus NIS (SLC5A5) cDNA into the “safe harbor” AAVS1 locus via CRISPR/Cas9‐mediated homologous recombination (Figure 1A and Figure S1), clones with high level NIS transgene expression were identified via immunostaining (Figure 1B) and used for all subsequent studies. NIS‐RhiPSCs continued to form colonies that were morphologically indistinguishable from parental nontransgenic RhiPSCs and maintained normal karyotypes (Figure 1C). The functionality of the expressed NIS transporter was evaluated by cellular uptake of 18F‐TFB from culture media, with significantly greater uptake observed in NIS‐RhiPSCs compared to NIS‐negative control RhiPSCs (P = .0471, Figure 1D). 18F‐TFB uptake was attenuated to background levels in the presence of KClO4, an established NIS inhibitor, 18 indicating tracer uptake was specific and NIS‐dependent (P = .0400).
3.2. NIS‐RhiPSC‐derived teratomas trackable in vivo
To demonstrate the utility of this platform for longitudinal in vivo imaging, 5 × 106 NIS‐RhiPSCs were injected subcutaneously into the hind limb of NSG mice and serially imaged for 6 weeks with PET/CT (Figure 2A). NIS‐RhiPSC‐derived teratomas could be visualized on imaging as early as 2 weeks postinjection, 4 weeks prior to the development of a palpable mass, while NIS‐negative RhiPSC‐derived teratomas were not visible at any of the imaging time points. The size, morphology, and spatial distribution of NIS‐positive teratomas could be followed longitudinally, and both tumor‐to‐muscle mean SUV ratios and total lesion 18F‐TFB uptake of the tumors increased over the follow‐up period in each replicate (Figure 2B). At 4 to 6 weeks, teratomas were harvested and subjected to histopathological analysis. NIS‐RhiPSC‐derived teratomas were indistinguishable from control RhiPSC‐derived teratomas, exhibiting all three germ layers on histologic analysis (Figure S2). Persistent RhNIS expression was also demonstrated by immunohistochemical staining of NIS‐RhiPSC‐derived teratomas. Of note, some heterogeneity was observed in the level of NIS expression between different cell types within the teratoma, although all cells expressed NIS above background. This finding was consistent with previously observed heterogeneity in expression of other marker genes knocked into the AAVS1 site in RhiPSCs. 6
FIGURE 2.
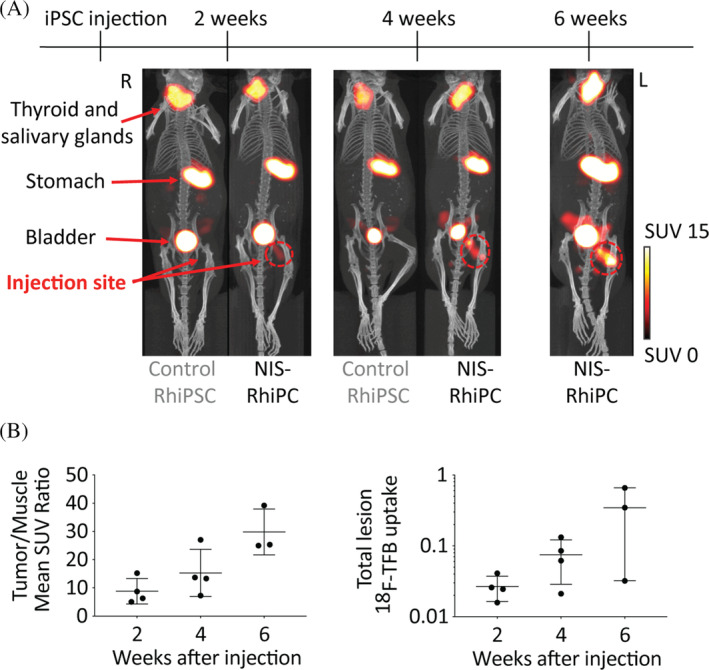
NIS enables in vivo tracking of RhiPSC‐derived teratomas. A, Delivery of RhNIS into RhiPSCs enables longitudinal in vivo tracking of NIS‐RhiPSC‐derived teratomas by PET/CT. After inoculating 5 million cells, NIS‐RhiPSC‐derived teratomas could be visualized as early as 2 weeks postinjection, and continued growth was observed at both 4 and 6 weeks. 18F‐TFB signals were observed as expected in organs with known endogenous NIS expression, such as the thyroid, salivary glands, stomach, seminal vesicles, and also in the urinary bladder, indicating renal excretion of 18F‐TFB. Two additional infero pelvic foci of 18F‐TFB uptake were observed in male mice, possibly representing perianal glands. Maximal intensity projection images of PET merged with CT are shown. B, Tumor/muscle mean SUV ratios (left) and total lesion 18F‐TFB uptake (right) increased over the follow‐up period in all replicates (n = 4), however one mouse underwent sacrifice at 4 weeks postinjection for histopathological examination. Error bars represent mean ± SD. 18F‐TFB, 18F‐tetrafluoroborate; CT, computed tomography; PET, positron emission tomography; RhiPSCs, rhesus macaque induced pluripotent stem cells; SUV, standardized uptake value
3.3. NIS‐RhiPSC‐CMs retain functional NIS expression
NIS‐RhiPSCs and parental control RhiPSCs underwent cardiac differentiation as previously described. 12 The overall kinetics and efficiency of cardiac differentiation of NIS‐RhiPSCs were similar to that of parental RhiPSCs. NIS‐RhiPSC‐derived cardiomyocytes maintained characteristic cellular morphology, could be obtained with high purity, and exhibited spontaneous beating in culture. NIS‐RhiPSC‐CMs manifested expected expression of canonical cardiac markers, including CTnT, NKX2.5, and α‐actinin (Figures and S3) at levels indistinguishable from cardiomyocytes differentiated from parental RhiPSCs. There were no apparent derangements in sarcomeric structures in NIS‐positive vs NIS‐negative RhiPSC‐CMs, and ectopic NIS expression did not appear to alter the cardiogenic potential of RhiPSCs. No differentiation‐induced NIS silencing was observed in any of the independent cardiomyocyte differentiation experiments (n = 5; Figure 3A). Like undifferentiated NIS‐RhiPSCs, NIS‐RhiPSC‐CMs demonstrated significantly greater 18F‐TFB uptake than control RhiPSC‐CMs (P = .0002), with expected attenuation by KClO4, a specific NIS inhibitor (P = .0042, Figure 3B).
To confirm that NIS‐RhiPSC‐CMs were detectable in vivo and assess sensitivity, we carried out intramuscular (IM) injections of various numbers of NIS‐RhiPSC‐CMs or control RhiPSC‐CMs, followed by immediate 18F‐TFB administration and PET/CT imaging 1 hour later (n = 3). In each of the mice assessed, regions that underwent injections of control parental RhiPSC‐CMs exhibited no detectable signal, while signals were reliably detected in sites where NIS‐RhiPSC‐CMs were introduced (Figure 3C). Of note, as few as 1 × 105 NIS‐RhiPSC‐CMs could be detected in vivo following local IM injection (Figure S4).
3.4. EP characterization of NIS‐RhiPSC‐CMs
To investigate the impact of NIS expression upon EP parameters of NIS‐RhiPSC‐CMs, a combination of whole‐cell patch clamp and optical imaging was used. At day 20 to 25 of differentiation, patch clamp recording documented the presence of nodal‐, atrial‐, and ventricular‐like cardiomyocytes by established AP morphological features, and there were no gross perturbations in baseline AP morphology in NIS‐RhiPSC‐CMs compared to parental control RhiPSC‐CMs (Figure 4A, upper panel). Consistent with prior studies on both human and rhesus iPSC‐CMs, 12 , 19 ventricular‐type CMs constituted the majority of the cells after differentiation (Figure 4B). NIS expression in RhiPSC‐CMs did not induce significant deviations in most AP parameters such as baseline resting potential, AP amplitude, or AP duration at 90% repolarization (APD90) when compared with controls across all CM subtypes. Of note, V max was found to be significantly higher in NIS‐positive atrial‐like cells (23.30 ± 3.91 vs 9.92 ± 0.73 [V/s]; P = .0405) compared to control atrial‐like cells; however, this trend was not observed among nodal‐ or ventricular‐type CMs. Overall, each of the aforementioned EP indices was comparable to previously reported ranges for human and NHP PSC‐CMs at similar stages of development. 12 , 20 , 21 , 22 , 23 Finally, simultaneous recording of calcium (Ca2+) flux revealed well‐coupled Ca2+ transients to their respective APs in each of the cardiomyocyte subtypes (Figure 4A, lower panel).
FIGURE 4.
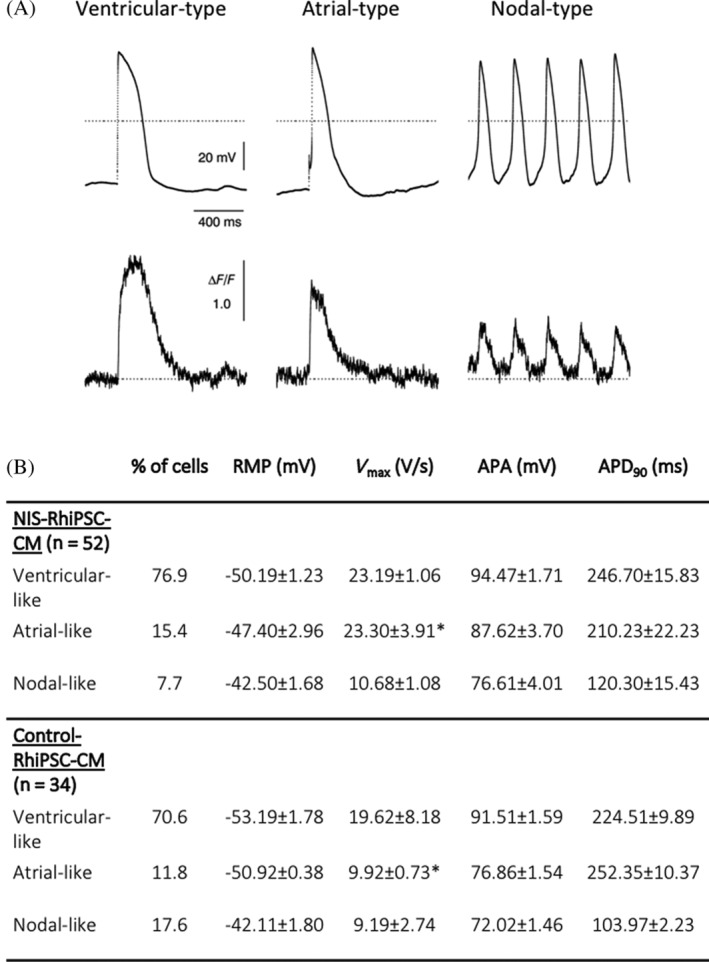
Electrophysiological characteristics of NIS‐RhiPSC‐CMs. A, Coupling of action potential (top) and Ca2+ flux (bottom), shown via simultaneous recording by patch clamp and Ca2+ fluorescence imaging of three different cardiomyocyte subtypes derived from NIS‐RhiPSCs. B, Baseline electrophysiological parameters of NIS‐RhiPSC‐CMs and control RhiPSC‐CMs by whole‐cell patch clamp in each of the CM subtypes, expressed as mean ± SEM. Statistical analysis between NIS‐RhiPSC‐CMs and control RhiPSC‐CMs was performed with a t test for independent samples. *P < .05. RhiPSC‐CMs, rhesus macaque induced pluripotent stem cell‐derived cardiomyocytes
Although there is evidence to suggest that NIS participates in sodium flux in an anion‐independent manner, and thus might influence EP characteristics even at baseline, NIS current has been shown to be largely anion‐dependent. 24 Hence, we next assessed the impact of iodide on EP parameters in NIS‐positive and control CMs. Iodide was selected to maximize the sensitivity of these and subsequent studies, as it has been demonstrated to yield higher NIS‐dependent transmembrane potentials than TFB or other NIS‐transported anions. 24 Preliminary data were gathered by whole‐cell patch clamp, in which ventricular‐like NIS‐RhiPSC‐CMs (n = 5) were exposed to 1 mM NaI, more than 3000‐fold greater than the upper limit of the normal serum level of free iodide. 24 , 25 , 26 Even at these levels of NaI, there were no appreciable derangements in overall spontaneous AP morphology or in each of the measured EP parameters vs pre‐exposure baseline (Figure 5A‐D). To determine whether the presence of NaI altered Ca2+ handling, beating single NIS‐RhiPSC‐CMs (n = 3) were again exposed to the Ca2+ indicator Cal‐520. Peak fluorescence amplitude, as well as the rate of fluorescence decline (CaD50), were similar in the presence and absence of 1 mM NaI, indicating that NaI did not exert a significant effect on intracellular Ca2+ levels or on overall Ca2+ handling compared to baseline (Figure 5E‐G).
FIGURE 5.
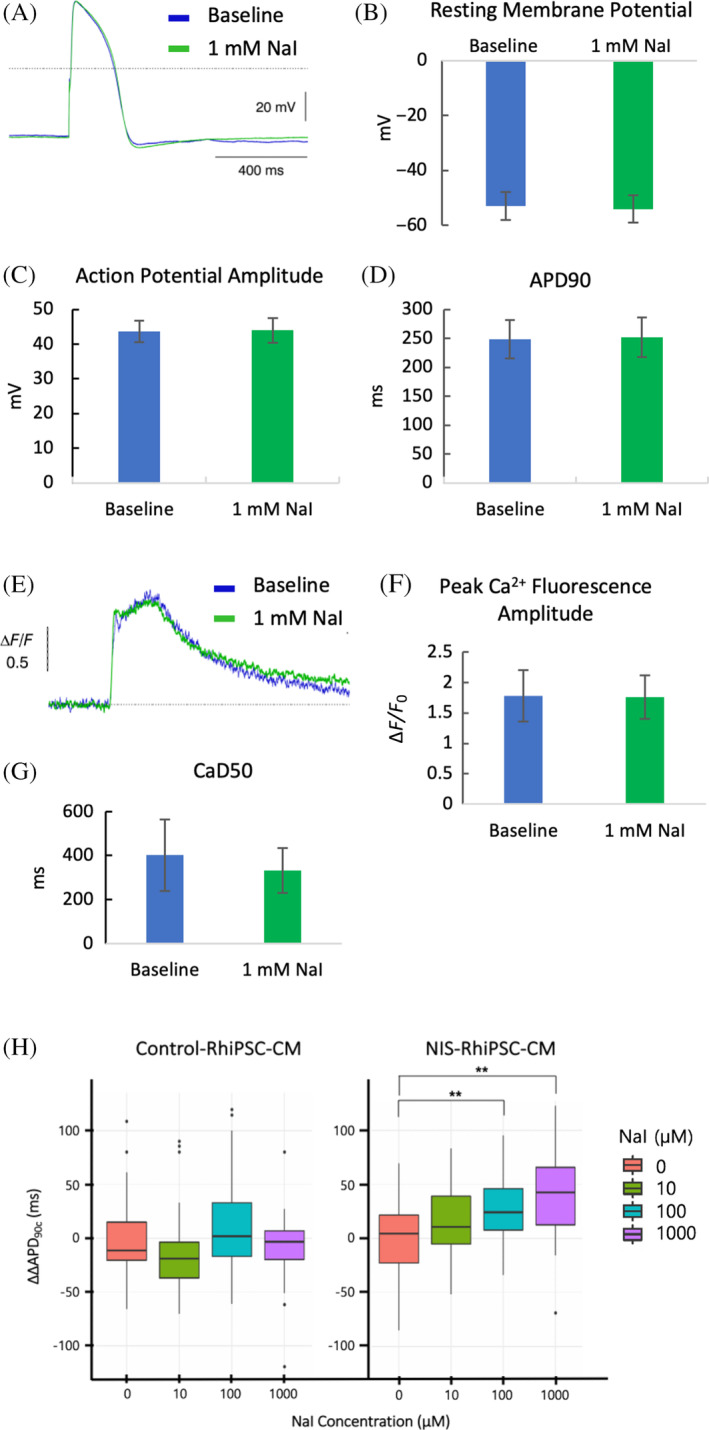
Electrophysiological (EP) characteristics of NIS‐RhiPSC‐CMs after addition of sodium iodide (NaI). A, Representative AP tracings at pre‐exposure baseline (blue) and 3 minutes after addition of 1 mM NaI (green) in V‐type NIS‐RhiPSC‐CMs (n = 5). Images obtained via whole‐cell patch clamp. B‐D, Graphic representation of key EP parameters in NIS‐RhiPSC‐CMs, both before and during incubation with 1 mM NaI (n = 5), expressed as mean ± SEM. E, Representative Ca2+ transients at pre‐exposure baseline (blue) and 3 minutes after addition of 1 mM NaI (green) in V‐type NIS‐RhiPSC‐CMs (n = 3). F,G, Graphic representation of peak Ca2+ fluorescence and time to 50% Ca2+ fluorescence decay (CaD50) in NIS‐RhiPSC‐CMs, both before and during incubation with 1 mM NaI (n = 3), expressed as mean ± SEM. H, Optical imaging with voltage‐sensitive dyes revealed a progressive increase in ΔΔAPD90C in NIS‐RhiPSC‐CMs (n = 96) relative to baseline with escalating levels of NaI, becoming significant at 100 μM NaI and remaining significant at 1000 μM NaI. No significant deviations were observed in control RhiPSC‐CMs (n = 107) at any of the NaI levels assessed. Data are represented as box plots, wherein the middle line, box ends, and whiskers represent the median, first and third quartiles, and minimum and maximum values, respectively. Dots on either side of the whiskers are outliers. **P < .01. AP, action potential; RhiPSC‐CMs, rhesus macaque induced pluripotent stem cell‐derived cardiomyocytes
As only small numbers of individual cells can be assessed using patch clamp approaches, we also used high‐throughput optical imaging with voltage‐sensitive dyes (VSD) to further investigate EP differences between control and NIS‐positive RhiPSC‐CMs. These techniques permit evaluation of EP parameters in much larger populations of cells, and have previously enabled detection of action potential duration (APD) prolongation in iPSC‐CMs after administration of drugs known to induce clinical QT prolongation, 27 a significant risk factor for lethal ventricular arrhythmias. Hence, we elected to focus on APD90 for these studies. The VSD analyses were carried out under escalating NaI concentrations, and APs were obtained both at baseline and after treatment. To control for variation in spontaneous beating rate between wells, APD90 measurements were normalized to the observed rate in each well (APD90C), then corrected for vehicle‐induced change from baseline (ΔΔAPD90C). Consistent with the results obtained by patch clamp, no perturbations in ΔΔAPD90C were observed between NIS‐RhiPSC‐CMs and control at baseline (P = .61), nor were any significant differences in NIS‐RhiPSC‐CMs relative to baseline at 10 μM NaI, approximately 30‐fold the physiologic serum level of free iodide. However, after 45‐minute treatment with 100 μM NaI, a concentration 300‐fold higher than physiologic levels, we observed a significant increase in ΔΔAPD90C in NIS‐RhiPSC‐CMs relative to baseline (P < .01; Figure 5H). This finding was also noted following incubation with 1000 μM NaI (P < .01; Figure 5H); however, the magnitude of these deviations was small at both of these supraphysiologic concentrations, on average 17% and 24% of baseline for each of the aforementioned NaI levels, respectively. Notably, no significant change in ΔΔAPD90C was seen in control RhiPSC‐CMs at any level of NaI compared to baseline (Figure 5H, left panel). Additionally, forms of triggered activity, such as early or delayed after depolarizations, were not observed at any of the NaI levels assessed. Taken together, these results indicate that NIS does not significantly impact EP parameters of RhiPSC‐CMs at baseline, nor at iodide levels up to 30‐fold higher than normal serum levels. However, when exposed to massively supraphysiologic levels of NaI, NIS‐positive CMs exhibited a small dose‐dependent increase in ΔΔAPD90C, suggestive of an effect of NIS‐dependent ion transport.
3.5. Longitudinal in vivo tracking of transplanted NIS‐RhiPSC‐CMs via PET/CT
Myocardial infarction was induced in immunodeficient mice by 90‐minute ligation of the LAD followed by reperfusion. Immediately following reperfusion, 2 × 106 NIS‐RhiPSC‐CMs (n = 10) or vehicle alone (n = 4) were injected into the infarct zone. For in vivo imaging of the injected hearts, serial μPET/CT was performed every 1 to 4 weeks. Table S1 summarizes the results. No signals were visualized after injection of vehicle alone. In the cohort that underwent cell injection, transplanted cells were clearly and quantifiably visualized via PET/CT in six out of 10 animals during the follow‐up period (Figures 6A‐C and S5 Video S1).
FIGURE 6.
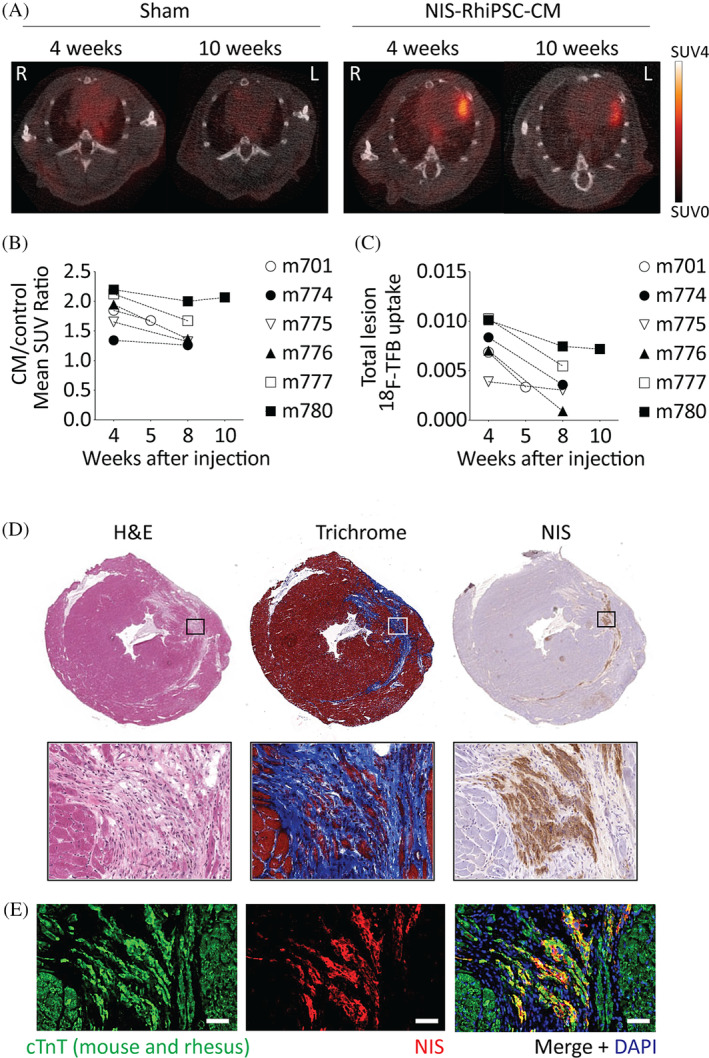
Longitudinal in vivo tracking of NIS‐RhiPSC‐CMs by μPET/CT. A, Intramyocardial signals within the putative infarct zone can be readily visualized at both 4 and 10 weeks in the NIS‐RhiPSC‐CM injection animal (right) vs the sham vehicle injection animal (left). Representative PET/CT images are shown. Display settings shared by all images. B,C, Quantification 18F signals of implanted NIS‐RhiPSC‐CMs in PET‐positive animals over time. D, Histologic analysis of explanted PET‐positive hearts revealed small left anterior descending artery‐territory infarcts by H&E (left), shown more clearly by trichrome staining (middle). Immunostaining for NIS highlights engraftment of NIS+ cells throughout the infarct region (right) in this representative image. Images in top and bottom panels obtained at 40X and 320X, respectively. E, Costaining of NIS (red) and CTnT (green) confirmed engraftment of NIS‐RhiPSC‐CMs in infarct zone. CTnT is expressed in both mouse and rhesus cardiomyocytes. All images in (D, E) were obtained from the same injected mouse shown in panel (A), right. Scale bar = 50 μm. μPET, micro‐positron emission tomography; CT, computed tomography; RhiPSC‐CMs, rhesus macaque induced pluripotent stem cell‐derived cardiomyocytes
In the PET‐positive animals, intracardiac signals were putatively derived from engrafted NIS‐positive CMs within the left ventricular myocardium. This was confirmed via microscopic analysis of the explanted hearts, with NIS+/CTnT+ double‐positive cells observed throughout the LAD‐territory scar (Figure 6D,E). Virtually all NIS+ cells were also positive for CTnT, and there was no evidence for teratoma formation. In addition, NIS expression appeared homogenous across the cardiomyocytes within the infarct scars, implying no loss of NIS expression over time in maturing and engrafted cardiomyocytes. Hearts from one of the four mice in the cell‐injection cohort that failed to exhibit a detectable PET signal (PET‐negative) also underwent histopathological examination, revealing minimal engraftment of NIS+ cells relative to its PET‐positive counterparts (Figure S6 Table S1).
Tissues from the remaining three PET‐negative mice did not undergo further histopathological analysis. No teratomas or other undifferentiated cells were observed in the tissue sections, and no ectopic PET signals consistent with extra‐cardiac tumor formation were observed throughout the follow‐up period.
4. DISCUSSION
Molecular imaging techniques that enable long‐term tracking of cells after myocardial delivery are crucial for optimizing the safety and therapeutic efficacy of cardiac cellular therapies. NIS is a nonimmunogenic endogenous gene that can be repurposed to monitor persistence and localization of cellular therapies via standard clinical imaging modalities. In the current study, we demonstrate for the first time that CRISPR/Cas9‐mediated introduction of the NIS reporter gene enables stable longitudinal imaging of iPSC‐CMs after engraftment into infarcted myocardium, and permits sensitive detection of teratomas developing from undifferentiated engrafted iPSCs.
We have previously shown that marker transgenes such as green fluorescent protein (GFP) and human truncated CD19 can be robustly and stably expressed following CRISPR/Cas9‐mediated targeted introduction at the AAVS1 safe harbor locus, a site in the genome permitting high level constitutive expression without genotoxicity. 6 However, neither of these transgenes is suitable for high‐resolution longitudinal in vivo imaging, thus we used the CRISPR/Cas9 genome editing system to introduce the NIS transgene into the AAVS1 site. Expression of NIS was sufficiently robust and stable to allow sensitive and specific noninvasive imaging of engrafted cells in live animals via PET/CT. In contrast to lentiviral transduction, which may result in multiple copies per cell, CRISPR/Cas9 targeted transgene introduction into the AAVS1 locus results in only one, or at most two, copies per cell. Despite this limited copy number per cell, our methodology resulted in an in vivo threshold of detection of as few as 1 × 105 injected cells—on par with prior studies using lentiviral vectors for NIS integration. 4 , 5 Use of CRISPR/Cas9 AAVS safe harbor targeting also overcomes two potential drawbacks of using lentiviral vectors to deliver NIS, specifically genotoxicity related to semi‐random genome integration and developmental silencing of lentiviral vector‐encoded transgenes. 28 , 29 , 30 , 31
In the present study, NIS expression did not appear to affect differentiation capacity of iPSCs into all three germ layers in NIS‐iPSC‐derived teratomas. Furthermore, consistent with a prior study using rat cardiac‐derived stem cells, 5 there were no changes in the efficiency of cardiomyocyte differentiation from NIS‐iPSCs. This is important as delivery of large numbers of cells, at least 108 to 109, are likely required to facilitate cardiac regeneration in humans based on large‐animal data, 32 , 33 , 34 and any reduction in differentiation efficiency could impose significant feasibility constraints upon large‐scale cell production protocols. Differentiation‐induced NIS silencing was not observed. NIS‐RhiPSC‐CMs exhibited intact expression of key cardiomyocyte markers and structural features, and successfully engrafted in infarcted myocardium after local injection. Most importantly, despite small infarct sizes, NIS‐positive iPSC‐CMs could be detected and localized in the heart for up to 10 weeks postinjection via PET/CT.
Prior investigation has demonstrated that adenoviral transfection of NIS into rat hearts does not result in significant myocardial injury or dysfunction relative to a control GFP‐carrying vector, 35 However, although NIS is known to be electrogenic, 24 the impact of ectopic NIS expression on EP parameters in cardiomyocytes has not been thoroughly assessed. This is an important step as any derangement in impulse conduction might predispose to lethal arrhythmia formation in more advanced in vivo models, or even clinical trials. Overall, characteristic AP parameters between NIS‐positive and control CMs at baseline were very similar, particularly for ventricular and nodal type cells, and within reported normal ranges. As NIS current is anion‐dependent, 24 we then examined key EP features in the presence of iodide. By patch clamp, we did not observe any notable derangements in overall AP morphology, or in any of the EP parameters assessed for ventricular cardiomyocyes with NaI exposure. However, the cell numbers in the patch clamp studies were limited, and AP tracings were obtained shortly following NaI administration. By subjecting control and NIS‐positive CMs to high‐throughput optical imaging with VSD, a progressive prolongation of the ΔΔAPD90C in NIS‐RhiPSC‐CMs with increasing amounts of NaI was revealed, becoming significant at 100 μM NaI. Of note however, this level of iodide represents greater than 300‐fold the reported normal serum level of free iodide. 24 , 25 , 26 As it was not evident in control CMs, this finding possibly reflects increased iodide‐driven inward Na+ current via NIS, leading to a protracted repolarization phase. However, additional studies are needed to clarify the precise mechanism of the observed APD90 prolongation in NIS‐RhiPSC‐CMs. It must be noted that the effect size of the observed APD90C prolongation was small, hence while the increase in ΔΔAPD90C at these levels of NaI was significant, it was substantially lower than has been observed in other in vitro iPSC‐CM models of drug‐induced or genetically induced QT prolongation. 27 , 36 , 37 In addition, we did not observe other key findings reported in channelopathic iPSC‐CM lines, such as Ca2+‐transient irregularities and early after depolarizations, 36 , 38 even in the presence of massively supraphysiologic NaI. Taken together, this data indicate that ectopic expression of NIS is not likely to significantly impact the EP parameters of iPSC‐CMs under in vivo conditions.
Last, a principal utility of NIS‐based imaging platforms lies in their translatability. NIS is compatible with a wide range of safe and effective molecular imaging modalities and radiotracers, including several already used in the clinic, such as 124I and 99mTc pertecnetate (99mTcO4 −). Here, we elected to pursue PET imaging with 18F‐TFB based on overall superior sensitivity and appreciably shorter half‐life (110 minutes) compared to other NIS‐compatible radiotracers. 39 In addition to enabling more frequent imaging, this short half‐life minimizes radiation exposure to both the patient and graft. Notably, prior studies have shown that the interaction between NIS and TFB is significantly less electrogenic than with iodide‐based preparations. Hence, as our data indicate significant iodide loads can provoke dose‐dependent APD prolongation, 18F‐TFB is a conceivably safer option in imaging platforms using NIS. However, while it must be noted that 18F‐TFB is not yet FDA approved for molecular imaging, it has been safely used in recent clinical studies, 40 , 41 and other NIS‐compatible radiotracers can be used while awaiting approval.
Several limitations must be acknowledged. First, only imaging outcomes after cell transplantation were robustly assessed in this study, hence we are unable to evaluate the functional impact of NIS‐iPSC‐CMs on the infarcted hearts. However, multiple prior investigations in large animals have demonstrated the ability of PSC‐CMs to impart structural or functional improvement after transplantation into infarcted hearts. 32 , 33 , 34 Additionally, although the findings in this study indicate that NIS is not likely to significantly impact EP parameters in iPSC‐CMs under physiologic conditions, their application in large animal studies is required for definitive assessment of both baseline and iodide‐evoked arrhythmogenicity. To facilitate clinical translation by showing viability in platform compatible with downstream NHP models, this study used RhiPSC‐CMs. Although RhiPSC‐CMs and hiPSC‐CMs are phenotypically and functionally similar, including comparable reference ranges for EP characteristics, further investigation is necessary to investigate the impact of NIS expression in hiPSC‐CMs before application in future clinical cardiomyocyte cell therapy trials.
5. CONCLUSION
In this study, site‐specific expression of the sodium‐iodide symporter via the AAVS1 safe harbor locus was shown to enable sensitive, longitudinal, and readily quantifiable in vivo tracking of iPSC‐CMs in a clinically relevant model of myocardial infarction, without provocation of significant differences in EP parameters when compared with control. This approach holds significant promise for application in downstream clinical and preclinical trials of cardiac cell therapies.
CONFLICT OF INTEREST
K.‐W.P. is a cofounder, co‐owner, and holds equity in Imanis Life Sciences. Imanis Life Sciences has not funded any aspect of this research. The other authors declared no potential conflict of interest.
AUTHOR CONTRIBUTIONS
J.W.O., R.Y.: collection and assembly of data, data analysis and interpretation, manuscript writing; N.S., M.K., K.B., D.P., R.V., M.P., H.S., Y.L., F.B., X.Z., R.E.S.: collection and assembly of data, data analysis and interpretation; S.P., K.‐W.P., M.H., P.L.C., J.Z.: data analysis and interpretation; M.B.: data analysis and interpretation, financial support; S.H.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; C.E.D.: conception and design, data interpretation, manuscript writing, financial support.
Supporting information
Figure S1 Southern blot analysis showed the targeted and random integrations in NIS‐RhiPSCs. RI, random integration; TI, targeted integration (=3.8 kb); WT, wild type (=6.7 kb).
Figure S2. Equivalence of NIS‐RhiPSC and control RhiPSC‐derived teratomas. Representative images of control RhiPSC‐derived (left) and NIS‐RhiPSC‐derived teratomas (right). Histopathologic analysis by H&E discloses normal trilineage germline tissues, demonstrating intact pluripotency in both groups. The lower panel depicts representative NIS staining. The majority of cells present in the NIS‐RhiPSC‐derived teratomas expressed NIS, including mature cells derived from all three germ layers. Images obtained at 160X. Scale bar = 500 μm
Figure S3. CTnT and NIS co‐staining on NIS‐RhiPSC‐CMs. Images shown here are single or double channel images of cTnT/NIS/DAPI immunohistochemistry in Figure 3A. Scale bar = 50 μm
Figure S4. NIS‐RhiPSC‐CM in vivo detection sensitivity. (A) Representative maximal intensity projection images of PET merged with CT after intramuscular injection of escalating numbers of NIS‐RhiPSC‐CMs (n = 3 for each cell number). (B) Representative μPET/CT images of the same animals in “A,” highlighting cell injection sites. Clear signals obtained following injection with as few as 1x105 NIS‐RhiPSC‐CMs.
Figure S5. Maximal intensity projection PET/CT Images after NIS‐RhiPSC‐CM Injection. Images were obtained at 10 weeks after injection of NIS‐RhiPSC‐CMs (right) and vehicle only (left). Right panel highlights intracardiac NIS‐RhiPSC‐CMs. Additional sites of endogenous NIS expression (thyroid, salivary glands, stomach, and seminal vesicles) and radiotracer accumulation (bladder) are readily appreciated in both projections. Display settings shared by images.
Figure S6. Histological analysis of engraftment of NIS‐RhiPSC‐CMs in injected murine hearts. PET imaging was performed at 8 weeks, and hearts were harvested for histology at 10 weeks. Relative infarct and graft sizes were estimated using MetaMorph software based on trichrome staining and NIS immunostaining, respectively. Overall, PET‐positive hearts exhibited greater cell engraftment than PET‐negative heart (m772). Graft and infarct sizes expressed as percentage of left ventricle (LV).
Table S1 Intramyocardial delivery of NIS‐RhiPSC‐CMs following myocardial infarction in mice
Video S1 Video of maximal intensity projection of μPET/CT images after NIS‐RhiPSC‐CM injection. Video derived from the NIS‐RhiPSC‐CMs injected animal in Figure S5. In this model, μPET/CT enables rapid three‐dimensional localization of engrafted NIS‐RhiPSC‐CMs in the infarcted murine heart 10 weeks following injection, in addition to other regions of endogenous NIS expression.
ACKNOWLEDGMENTS
We are grateful to Stefan Cordes for his generous advice and help with statistical analyses. We thank Shiqin Judy Yu for assisting with iPSC culture. We also thank NHLBI core facilities, including the Animal Surgery and Resources Core for surgical support, the Pathology Core for tissue processing, and the Light Microscopy Core for imaging analyses, as well as the NCI Clinical Research Center Animal Facility staff for provision of excellent animal care. This research was supported by funding from the Division of Intramural Research program of the NHLBI and NCI. J. W. O. was supported by the Medical Research Scholars Program of the NIH.
Ostrominski JW, Yada RC, Sato N, et al. CRISPR/Cas9‐mediated introduction of the sodium/iodide symporter gene enables noninvasive in vivo tracking of induced pluripotent stem cell‐derived cardiomyocytes. STEM CELLS Transl Med. 2020;9:1203–1217. 10.1002/sctm.20-0019
John W. Ostrominski and Ravi Chandra Yada contributed equally to this study.
Funding information Medical Research Scholars Program of the NIH; Division of Intramural Research program of the NHLBI and NCI
Contributor Information
So Gun Hong, Email: sogun.hong@nih.gov.
Cynthia E. Dunbar, Email: dunbarc@nhlbi.nih.gov.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from corresponding authors upon request.
REFERENCES
- 1. Chen IY, Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation. 2011;123(4):425‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ravera S, Reyna‐Neyra A, Farrandino G, et al. The sodium/iodide symporter (NIS): molecular physiology and preclinical and clinical applications. Annu Rev Physiol. 2017;79:261‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee AR, Woo SK, Kang SK, et al. Adenovirus‐mediated expression of human sodium‐iodide symporter gene permits in vivo tracking of adipose tissue‐derived stem cells in a canine myocardial infarction model. Nucl Med Biol. 2015;42:621‐629. [DOI] [PubMed] [Google Scholar]
- 4. Templin C, Zweigerdt R, Schwanke K, et al. Transplantation and tracking of human‐induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 2012;126(4):430‐439. [DOI] [PubMed] [Google Scholar]
- 5. Terrovitis J, Kwok KF, Lautamaki R, et al. Ectopic expression of the sodium‐iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single‐photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008;52(20):1652‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong S, Yada RC, Choi K, et al. Rhesus iPSC safe harbor gene‐editing platform for stable expression of transgenes in differentiated cells of all germ layers. Mol Ther. 2017;25(1):44‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papapetrou EP, Schambach A. Gene insertion into genomic safe harbors for human gene therapy. Mol Ther. 2016;24(4):678‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu H, Zhu F, Yong J, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3(6):587‐590. [DOI] [PubMed] [Google Scholar]
- 9. Hong S, Winkler T, Wu C, et al. Path to the clinic: assessment of iPSC‐based cell therapies in vivo in a nonhuman primate model. Cell Rep. 2014;7(4):1298‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao X, Chen H, Xiao D, et al. Comparison of non‐human primate versus human induced pluripotent stem cell‐derived cardiomyocytes for treatment of myocardial infarction. Stem Cell Reports. 2018;10(2):422‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yada RC, Hong S, Lin Y, et al. Rhesus macaque iPSC generation and maintenance. Curr Protoc Stem Cell Biol. 2017;41:4A.11.1‐4A.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin Y, Liu H, Klein M, et al. Efficient differentiation of cardiomyocytes and generation of calcium‐sensor reporter lines from nonhuman primate iPSCs. Sci Rep. 2018;8(1):5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yada RC, Ostrominski JW, Tunc I, et al. CRISPR/Cas9‐based safe‐harbor gene editing in rhesus iPSCs. Curr Protoc Stem Cell Biol. 2017;43:5A.11.1‐5A.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hortigon‐Vinagre MP, Zamora V, Burton FL, Green J, Gintant GA, Smith GL. The use of ratiometric fluorescence measurements of the voltage sensitive dye Di‐4‐ANEPPS to examine action potential characteristics and drug effects on human induced pluripotent stem cell‐derived cardiomyocytes. Toxicol Sci. 2016;154(2):320‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang H, Bansal A, Pandey MK, et al. Synthesis of 18F‐tetrafluoroborate via radiofluorination of boron trifluoride and evaluation in a murine C6‐glioma tumor model. J Nucl Med. 2016;57(9):1454‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SB, Lee HW, Lee H, et al. Tracking dendritic cell migration into lymph nodes by using a novel PET probe (18)F‐tetrafluoroborate for sodium/iodide symporter. EJNMMI Res. 2017;7(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samnick S, Al‐Momani E, Schmid JS, et al. Initial clinical investigation of [18F]Tetrafluoroborate PET/CT in comparison to [124I]Iodine PET/CT for imaging thyroid cancer. Clin Nucl Med. 2018;43(3):162‐167. [DOI] [PubMed] [Google Scholar]
- 18. Dingli D, Peng KW, Harvey ME, et al. Image‐guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641‐1646. [DOI] [PubMed] [Google Scholar]
- 19. Sanchez‐Freire V, Lee AS, Hu S, et al. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol. 2014;64(5):436‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11(8):855‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell‐derived cardiomyocytes. Stem Cells. 2013;31(5):829‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Klos M, Wilson GF, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111(9):1125‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sayed N, Liu C, Wu JC. Translation of human‐induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol. 2016;67(18):2161‐2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eskandari S, Loo DD, Dai G, et al. Thyroid Na+/I‐symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272(43):27230‐27238. [DOI] [PubMed] [Google Scholar]
- 25. Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154(1):65‐82. [DOI] [PubMed] [Google Scholar]
- 26. Michalke B, Schramel P, Hasse S. Separation of free iodide from other I‐species in human serum ‐ quantification in serum pools and individual samples. Anal Bioanal Chem. 1996;354(5‐6):576‐579. [DOI] [PubMed] [Google Scholar]
- 27. Blinova K, Stohlman J, Vicente J, et al. Comprehensive translational assessment of human‐induced pluripotent stem cell derived cardiomyocytes for evaluating drug‐induced arrhythmias. Toxicol Sci. 2017;155(1):234‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cesana D, Ranzani M, Volpin M, et al. Uncovering and dissecting the genotoxicity of self‐inactivating lentiviral vectors in vivo. Mol Ther. 2014;22(4):774‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herbst F, Ball CR, Tuorto F, et al. Extensive methylation of promoter sequences silences lentiviral transgene expression during stem cell differentiation in vivo. Mol Ther. 2012;20(5):1014‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sadelain M, Papapetrou EP, Bushman FD. Safe harbours for the integration of new DNA in the human genome. Nat Rev Cancer. 2011;12(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 31. Wu C, Hong SG, Winkler T, et al. Development of an inducible caspase‐9 safety switch for pluripotent stem cell‐based therapies. Mol Ther Methods Clin Dev. 2014;1:14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chong JJ, Yang X, Don CW, et al. Human embryonic‐stem‐cell‐derived cardiomyocytes regenerate non‐human primate hearts. Nature. 2014;510(7504):273‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu YW, Chen B, Yang X, et al. Human embryonic stem cell‐derived cardiomyocytes restore function in infarcted hearts of non‐human primates. Nat Biotechnol. 2018;36(7):597‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shiba Y, Gomibuchi T, Seto T, et al. Allogeneic transplantation of iPS cell‐derived cardiomyocytes regenerates primate hearts. Nature. 2016;538(7625):388‐391. [DOI] [PubMed] [Google Scholar]
- 35. Lee KH, Bae JS, Lee SC, et al. Evidence that myocardial Na/I symporter gene imaging does not perturb cardiac function. J Nucl Med. 2006;47(11):1851‐1857. [PubMed] [Google Scholar]
- 36. Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471(7337):225‐229. [DOI] [PubMed] [Google Scholar]
- 37. Moretti A, Bellin M, Welling A, et al. Patient‐specific induced pluripotent stem‐cell models for long‐QT syndrome. N Engl J Med. 2010;363(15):1397‐1409. [DOI] [PubMed] [Google Scholar]
- 38. Maizels L, Huber I, Arbel G, et al. Patient‐specific drug screening using a human induced pluripotent stem cell model of catecholaminergic polymorphic ventricular tachycardia type 2. Circ Arrhythm Electrophysiol. 2017;10(6):e004725. [DOI] [PubMed] [Google Scholar]
- 39. Jauregui‐Osoro M, Sunassee K, Weeks AJ, et al. Synthesis and biological evaluation of [(18)F]tetrafluoroborate: a PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter. Eur J Nucl Med Mol Imaging. 2010;37(11):2108‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang H, Schmit NR, Koenen AR, et al. Safety, pharmacokinetics, metabolism and radiation dosimetry of (18)F‐tetrafluoroborate ((18)F‐TFB) in healthy human subjects. EJNMMI Res. 2017;7(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Doherty J, Jauregui‐Osoro M, Brothwood T, et al. (18)F‐tetrafluoroborate, a PET probe for imaging sodium/iodide symporter expression: whole‐body biodistribution, safety, and radiation dosimetry in thyroid cancer patients. J Nucl Med. 2017;58(10):1666‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Southern blot analysis showed the targeted and random integrations in NIS‐RhiPSCs. RI, random integration; TI, targeted integration (=3.8 kb); WT, wild type (=6.7 kb).
Figure S2. Equivalence of NIS‐RhiPSC and control RhiPSC‐derived teratomas. Representative images of control RhiPSC‐derived (left) and NIS‐RhiPSC‐derived teratomas (right). Histopathologic analysis by H&E discloses normal trilineage germline tissues, demonstrating intact pluripotency in both groups. The lower panel depicts representative NIS staining. The majority of cells present in the NIS‐RhiPSC‐derived teratomas expressed NIS, including mature cells derived from all three germ layers. Images obtained at 160X. Scale bar = 500 μm
Figure S3. CTnT and NIS co‐staining on NIS‐RhiPSC‐CMs. Images shown here are single or double channel images of cTnT/NIS/DAPI immunohistochemistry in Figure 3A. Scale bar = 50 μm
Figure S4. NIS‐RhiPSC‐CM in vivo detection sensitivity. (A) Representative maximal intensity projection images of PET merged with CT after intramuscular injection of escalating numbers of NIS‐RhiPSC‐CMs (n = 3 for each cell number). (B) Representative μPET/CT images of the same animals in “A,” highlighting cell injection sites. Clear signals obtained following injection with as few as 1x105 NIS‐RhiPSC‐CMs.
Figure S5. Maximal intensity projection PET/CT Images after NIS‐RhiPSC‐CM Injection. Images were obtained at 10 weeks after injection of NIS‐RhiPSC‐CMs (right) and vehicle only (left). Right panel highlights intracardiac NIS‐RhiPSC‐CMs. Additional sites of endogenous NIS expression (thyroid, salivary glands, stomach, and seminal vesicles) and radiotracer accumulation (bladder) are readily appreciated in both projections. Display settings shared by images.
Figure S6. Histological analysis of engraftment of NIS‐RhiPSC‐CMs in injected murine hearts. PET imaging was performed at 8 weeks, and hearts were harvested for histology at 10 weeks. Relative infarct and graft sizes were estimated using MetaMorph software based on trichrome staining and NIS immunostaining, respectively. Overall, PET‐positive hearts exhibited greater cell engraftment than PET‐negative heart (m772). Graft and infarct sizes expressed as percentage of left ventricle (LV).
Table S1 Intramyocardial delivery of NIS‐RhiPSC‐CMs following myocardial infarction in mice
Video S1 Video of maximal intensity projection of μPET/CT images after NIS‐RhiPSC‐CM injection. Video derived from the NIS‐RhiPSC‐CMs injected animal in Figure S5. In this model, μPET/CT enables rapid three‐dimensional localization of engrafted NIS‐RhiPSC‐CMs in the infarcted murine heart 10 weeks following injection, in addition to other regions of endogenous NIS expression.
Data Availability Statement
The data that support the findings of this study are available from corresponding authors upon request.


