Abstract
Introduction
Plantar fasciitis (PF) is reported to be the most common cause of plantar heel pain. Acupuncture has been used for patients experiencing PF, but evidence of the efficacy of acupuncture on PF is limited. The primary objective of this trial is to compare combined acupuncture and sham acupuncture (SA) versus waitlist control for improving the level of pain experienced by patients suffering from chronic PF.
Methods and analysis
This will be a two-centre, parallel-group, sham and no-treatment controlled, assessor-blinded randomised trial. We will randomly allocate 120 participants with chronic PF to acupuncture, SA and waitlist control groups at a ratio of 2:1:1. Participants in the acupuncture and SA groups will receive a 30 min acupuncture or SA treatment for a total of 12 sessions over 4 weeks, with a 12-week follow-up. Participants in the waitlist control group will not undergo treatment for a period of 16 weeks but instead will have the option of 4 weeks (12 sessions) of acupuncture free of charge at the end of the follow-up period. The primary outcome will be the treatment response rate 4 weeks after randomisation, assessed as a minimum of 50% improvement in the worst pain intensity during the first steps in the morning compared with the baseline. All analyses will be performed with a two-sided p value of <0.05 considered significant following the intention-to-treat principle.
Ethics and dissemination
The study has been approved by the Ethical Committee of the Guang’anmen Hospital, China Academy of Chinese Medical Sciences (approval no. 2019-210-KY). The results will be disseminated through presentation at a peer-reviewed medical journal, the relevant conferences and scientific meetings.
Trial registration
Keywords: complementary medicine, pain management, clinical trials
Strengths and limitations of this study.
This study is the first randomised controlled trial comparing combined acupuncture and sham acupuncture versus waitlist control for pain relief in participants with chronic plantar fasciitis.
The advantages to this study include sham acupuncture and waitlist control design, objective measurements (ie, pressure pain threshold, plantar fascia thickness), strict quality control and evaluation of participants’ expectation regarding acupuncture.
The 2:1:1 allocation ratio used in this trial could facilitate recruitment and enhance patient adherence by allowing more patients to receive acupuncture.
Acupuncturists and participants in the waitlist control group will not be blinded, which may cause bias.
A high dropout rate may exist in the waitlist group because participants expect to receive acupuncture treatment when they join the trial.
Background
Plantar fasciitis (PF), which presents with heel pain and tenderness particularly at the plantar aspect of the calcaneal tuberosity1 on the initiation of weight bearing, is one of the most prevalent complaints encountered by foot and ankle specialists. It is reported that 1 in 10 people suffer from inferior heel pain within their lifetime2 and this condition is attributed to PF in 80% of cases.3 PF predominantly affects elderly and middle-aged individuals4 and is more frequent in runners or those whose employment requires standing.5 The exact aetiology of PF is multifactorial and not completely understood. Physical–mechanical overload and micro-tears within the fascia6 could be involved in the development of PF, resulting in localised inflammation and degeneration of the proximal plantar aponeurosis.7
The available treatment options for PF mainly include non-operative treatments (eg, plantar fascia and gastrocnemius soleus muscle stretching, heel cups, arch supports, night splints, shockwave therapy, non-steroidal anti-inflammatory drugs (NSAIDs), local corticosteroid injections) and operative management.8 However, no consensus has been reached regarding the most beneficial treatment method for PF.9 Although conservative treatment of PF is successful in the vast majority of cases10 and many PF cases are self-limiting and eventually enter remission, it can take up to months or even years for patients to recover.11 Moreover, approximately 10% to 20% of patients are recalcitrant to conventional treatments, resulting in foot pain and/or disabilities for years.12
Acupuncture, an integral part of traditional Chinese medicine (TCM), is a technique where the acupoints located on specific body areas are pierced with fine needles for therapeutic purposes based on the principles of TCM.13 Acupuncture has been used in the management of PF and other musculoskeletal pain–related conditions for thousands of years. Mechanistic studies have revealed that acupuncture can induce an analgesic response via the release of neuropeptides (eg, enkephalin, dynorphin, β-endorphin and endomorphin).14 Two recent systematic reviews15 16 found that acupuncture may reduce pain intensity and improve plantar function for patients with PF. However, there were methodological problems with the small sample sizes, lack of control with a placebo/waitlist group or no adjustment for the confounding effects of patients who received combination treatments in the design of the included acupuncture literature. Therefore, the placebo effects of acupuncture and spontaneous remission of PF cannot be excluded and the beneficial effects of acupuncture for PF remain in need of further assessment.
We designed a randomised controlled trial to evaluate the efficacy of acupuncture, compared with sham acupuncture (SA) or being on a waitlist control group, for patients with chronic PF for >6 months. Given that clinical and experimental results have shown that SA can induce a significant alleviation of pain similar to verum acupuncture17 due to non-specific effects (eg, acupuncture expectations), the primary hypothesis in this trial was that combined acupuncture and SA will result in larger improvements in heel pain than no acupuncture treatment in patients with chronic PF. The secondary hypothesis examined whether acupuncture can reduce heel pain intensity more effectively than SA or no acupuncture.
Methods and design
Study design
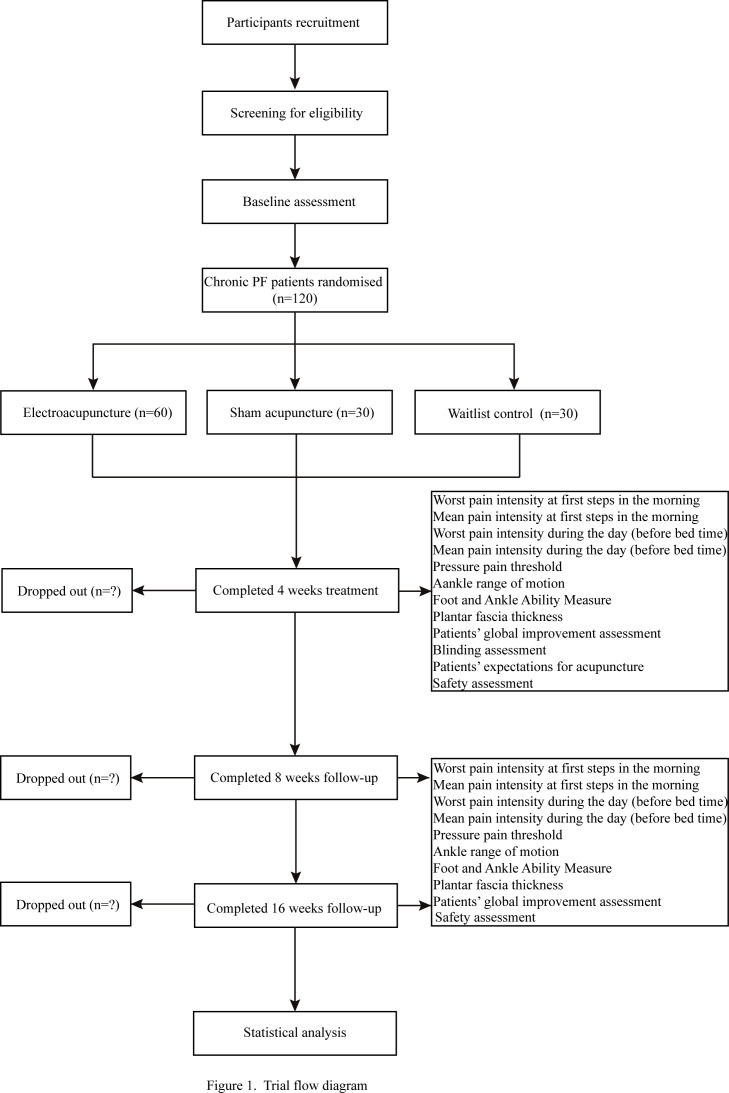
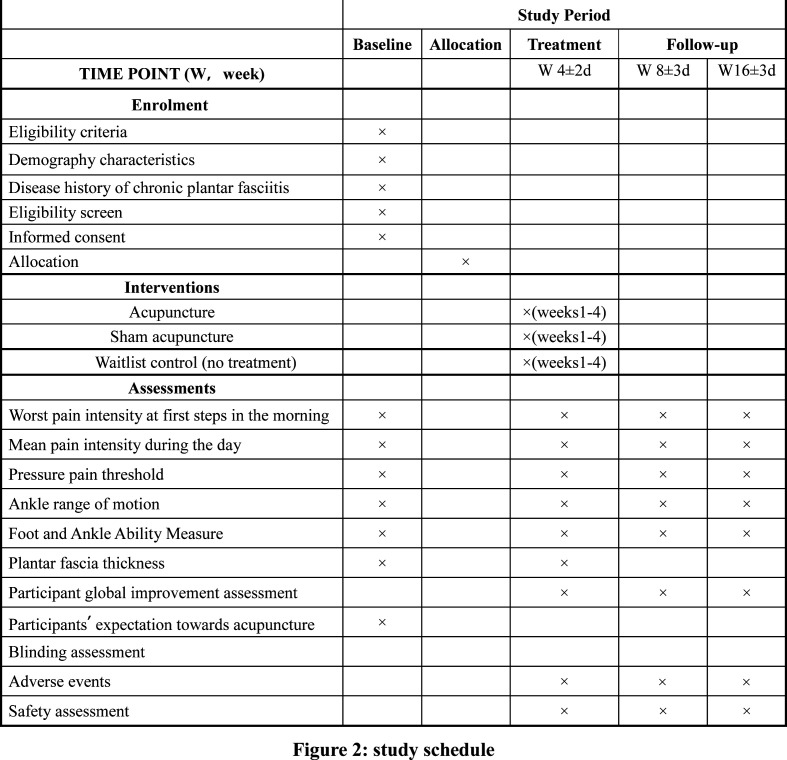
This will be a two-centre, parallel-group, sham and no-treatment controlled, assessor-blinded randomised trial comprising three arms with a 2:1:1 allocation rate. We will design the protocol in accordance with standard protocol items including the Recommendations for Interventional Trials18 and the Standards for Reporting Interventions in the Clinical Trials of Acupuncture19 guidelines. The study flow chart and study schedule are shown in figures 1 and 2.
Figure 1.
Trial flow diagram.
Figure 2.
Study schedule.
Study setting and recruitment
This trial is planned to be conducted at Guang’anmen Hospital, China Academy of Chinese Medical Sciences, and Yantai Hospital of Traditional Chinese Medicine from March 2020 to March 2022. A total of 120 participants will be publicly recruited through the use of posters and hospital webs in the two participating hospitals. The duration of the trial for each participant will be 17 weeks: 1-week baseline, 4-week treatment and 12-week follow-up.
Randomisation and blinding
The eligible participants who sign an informed consent form will complete a 1-week baseline assessment before randomisation, which includes foot symptoms (ie, worst pain intensity at first steps in the morning, mean pain intensity during the day), functionality and ultrasound examinations (see figure 2). Participants’ expectation towards acupuncture will be assessed in the acupuncture and SA groups at baseline by asking participants: “Do you think acupuncture will be helpful to improve your chronic PF?” Participants will choose one of the following answers: “Extremely helpful”, “Very helpful”, “Helpful”, “Not help at all” and “Unclear”. Participants will be randomised into the acupuncture group, SA group or waitlist (no acupuncture) group at a ratio of 2:1:1 using simple randomisation. Randomisation will be generated with the PROC PLAN in SAS 9.4 (SAS Institute, Cary, NC, USA). Details of the group allocation will be concealed on cards inside sealed opaque envelopes by the staff member responsible for the allocation. A research coordinator, who will not be involved in the treatment and outcome assessments, will be responsible for contacting participants and allocating them to their assigned group. Participants in the acupuncture and SA groups, together with efficacy evaluators and data analysts, will be blinded to the group assignments. Participants in the waitlist control group and acupuncturists will not be blinded.
Participants
Participants with a diagnosis of PF by an orthopaedist on clinical grounds will be included in the study only if they meet all of the following inclusion criteria and do not fulfil any of the exclusion criteria. Diagnosis of PF will be made according to the guidelines described by the Orthopaedic Section of the American Physical Therapy Association.20 The following clinical findings will be used to diagnose PF: plantar medial heel pain during the initial steps after a period of inactivity but also worse pain following prolonged weight bearing, heel pain precipitated by a recent increase in weight-bearing activity, physical examination findings (heel pain with palpation of the proximal insertion of the plantar fascia, limited ankle range of motion), abnormal foot posture index, high body mass index, as well as a positive windlass test and negative tarsal tunnel tests.
Inclusion criteria
Age ≥18 years and ≤75 years;
History of plantar medial heel pain for at least 6 months before enrolment;
Reported an average worst pain intensity at first steps in the morning over the last 7 days of at least 50 mm on a 100 mm visual analogue scale (VAS) before enrolment;
Failure to respond to conservative treatment for ≥1 month, including any of the following modalities: stretching exercises, NSAIDs, shockwave therapy, dry needling and orthotics;
Ability to comply with the study protocol, understand the medical information forms as well as having provided informed consent.
Exclusion criteria
History of calcaneus fracture, calcaneal bone tumour or cyst, plantar fascia rupture or having a significant foot deformity (clubfoot, pes cavus or pes calcaneovalgus);
Previous injection (corticosteroid, platelet-rich plasma, lidocaine needling), or radiation, or surgery to plantar fascia within 6 months preceding enrolment;
Lumbosacral radiculopathy or peripheral neuropathy around the ankle joint such as nerve entrapment tarsal tunnel syndrome or Achilles tendinopathy;
Systemic disorders like rheumatoid arthritis, gout, Reiter syndrome, type 1 or 2 diabetes mellitus, osteoporosis, spondyloarthritis or osteomyelitis;
Joint, bone or skin infection in the affected foot;
Clinically significant cardiovascular disorder, severe hepatic/renal insufficiency or coagulation disorder at baseline as determined by the investigator;
Known phobia to acupuncture or receiving acupuncture treatment within 4 weeks prior to enrolment.
Interventions
Acupuncture group
The acupuncture protocol was developed by the consensus of three experts based on the meridian theory of TCM and was used in our previous trial.21 Licensed acupuncturists with more than 2 years of acupuncture experience will perform the treatment. We will apply needles to two Ashi points (the two most severe tender points in the most sensitive area over the anteromedial aspect of the heels, according to the participant’s perceived pain on palpation) as well as the Chengshan (BL57), Taixi (KI3) and Kunlun (BL60) acupoints in this trial. The position of the aforementioned acupoints will be based on the nomenclature and location of acupuncture points22 designated by the National Standard of the People’s Republic of China (GB/T 12 346-2006). Sterile disposable stainless-steel needles (Hwato brand; Suzhou Medical Appliance Factory, Suzhou, China; 0.3 mm×40 mm) will be used. With the patient in a prone position, the local skin will be routinely sterilised, followed by pasting a 10 mm diameter and 5 mm thick sterile adhesive pad (Hwato brand; Suzhou Medical Appliance Factory) onto each selected acupoint. Ashi points will be perpendicularly inserted through the pad to the plantar fascia layer with a depth of approximately 15–20 mm depending on the location. BL57, KI3 and BL60 will be punched perpendicularly 10–15 mm deep into the skin through the pad. All needles except the Ashi points will be manually stimulated with small, equal manipulations of lifting, thrusting, twirling and rotating to achieve De qi (a sensation including soreness, numbness, distention and heaviness).23 Needles will be retained for 30 min per treatment. During each treatment, every needle will be manipulated three times every 10 min.
SA group
In the SA group, sham Ashi (0.5 cun away from Ashi, one ‘cun’ is equivalent to the greatest width of the individual patients’ thumb, ~1.5 cm), sham BL57 (0.5 cun lateral to the true BL57 horizontally), sham KI3 (midway between the true KI3 and the heel tendon) and sham BL60 (midway between the true BL60 and the heel tendon) will be used. The treatment protocol will be similar to that of the acupuncture group. The Hwato brand disposable blunt-tipped needles (size 0.30×25 mm) will be inserted at the sham points through the adhesive pads attached to the skin without skin penetration. The needles will then be lifted, thrust, twirled and rotated evenly three times every 10 min. No specific De qi response will be elicited.
Waitlist control group
Participants will receive no treatment for their heel pain for a period of 16 weeks after randomisation, and subsequently have the option of 4 weeks (12 sessions) of acupuncture free of charge at the end of the follow-up period.
The intervention will last for 30 min in the acupuncture and SA groups, and will be performed three times per week for a total of 12 sessions in four consecutive weeks. If participants suffer pain bilaterally, the acupuncturists will treat both sides and evaluate the more severe side. Participants in all groups will be treated and (or) evaluated separately. Participants in all groups will be advised to use soft heel foot wear, not to stand for a long time and not to walk barefoot during the 17-week study period.
Rescue medication
Additional therapies for heel pain during the entire study period will be prohibited. However, the investigator will be permitted to prescribe ibuprofen (sustained release type, 300 mg/T; Tianjin Smith Kline & French Laboratories, Tianjin, China) as rescue medication no more than 2 days per week up to the maximum daily dose if unbearable heel pain occurs. Participants will be required not to take rescue medication within 72 hours before the baseline and outcome measurements. In the event rescue medication needs to be taken after the baseline measurement, the participant will postpone the next visit to the treatment centre.
Outcome measures
Primary outcome
The primary outcome used in this trial will be the proportion of participants with a treatment response 4 weeks after randomisation, defined as a minimum of 50% improvement in the worst pain intensity during the first steps in the morning compared with the baseline. The average worst pain intensity over the last 3 days will be used for analysis in this trial. Pain intensity will be measured using a 0–100 VAS, with 0 indicating no pain and 100 indicating maximal pain. Participants who must resort to additional treatments other than rescue medication will be classified as non-responders. In addition, the responder rate at weeks 8 and weeks 16 will also be assessed.
Secondary outcomes
The secondary outcomes are as follows:
Changes in the VAS score for worst pain intensity during the first steps in the morning from baseline to 4, 8 and 16 weeks after randomisation;
Changes in the VAS score for mean pain intensity during the day from baseline to 4, 8 and 16 weeks after randomisation;
Changes in the pressure pain threshold (PPT) at the most painful area from baseline to 4, 8 and 16 weeks after randomisation. PPT is defined as the minimum pressure detected when the sensation of pressure first changes to a sensation of pain.24 PPT will be tested with a pressure algometer (Fabrication Enterprises, White Plains, NY; from 1 kg/cm2 to 5 kg/cm2) using a metal probe with a 0.5 cm2 rubber disc by a trained researcher. PPT will be measured when the participant is lying supine in a relaxed position with the affected foot hanging over the edge of the bed. When measuring the PPT, the rubber disc will be placed perpendicularly on the painful spot and pressure will be applied at a rate of approximately 0.1 kg/cm2/s through the metal probe of the pressure algometer. Participants will be informed to report when the initial pain sensation occurs, and the readings of the algometer will be recorded. The score will be determined by averaging three repeated measurements with 30 s between each trial. All values below 1 kg/cm2 will be reported as 0.5 kg/cm2.
Changes in the ankle range of motion (AROM) from baseline to 4, 8 and 16 weeks after randomisation: The examiner will measure the AROM including dorsiflexion and plantar flexion in two positions (flexed knee and extended knee) using a digital goniometer (Tangxia Electronic Instrument Factory, Dongguan, from 0° to 360°). For the flexed-knee assessment, the participant will sit in a relaxed station with the popliteal space at the edge of the table and their knees with 90° of flexion. For the extended-knee assessment, the participant will be seated on a treatment table with the knees fully extended (0°) and the feet hanging off the end of the table. The axis of the goniometer will be placed at the lateral malleolus. The stationary arm will be placed parallel to the fifth metatarsal and the moving arm placed parallel to the centre of the fibular head. The ankle will be passively moved from a neutral starting position into dorsiflexion and plantar flexion until a firm end feel is elicited25 and the readings of the goniometer will be registered. The mean score of three trials with 10 s between each examination will be calculated and used for analysis.
Changes in the Foot and Ankle Ability Measure (FAAM) total score and subscale scores from baseline to 4, 8 and 16 weeks after randomisation: The FAAM is a self-reported questionnaire concerning 21 activities of daily living (ADL) items and eight sports subscale items.26 Each item is scored on a 0–4 point Likert scale anchored by 0 (unable to do) and 4 (no difficulty at all), with higher total scores indicating a higher level of function. The FAAM has a maximum potential score of 116 (84 ADL and 32 sport subscales). The obtained score (total, ADL and sport subscale scores) is divided by the maximum potential score and multiplied by 100 to obtain a percentage. If the patient does not respond, the specific question will be left blank and not be a part of the final value of the questionnaire. In this trial, we will use the previously validated Chinese version of the FAAM.27
Change in plantar fascia thickness (PFT) from baseline to 4 weeks after randomisation: PFT will be measured at the thickest point closest to the calcaneal insertion in its medial portion using ultrasound. The ultrasound scan will be performed using an 8–12 MHz linear probe with the patient in the prone position at the baseline and at 4 weeks after randomisation.
Participant global assessment of improvement: Participants will be asked to rate their global improvement using a 7-point scale. The improvement will be scaled from 1 (complete recovery) to 7 (vastly worse), with 2 being obvious improvement, 3 being a little improvement, 4 being no change, 5 being a little worse and 6 being obviously worse. The proportions of participants with different degrees of improvement will be assessed at 4, 8 and 16 weeks after randomisation. Scales of participant global assessment of improvement with seven response categories have been rated as relatively easy to use and show good reliability and validity.28
Participants’ expectation towards acupuncture at baseline: at baseline, participants in the acupuncture and SA groups will be asked the following question: “Do you think acupuncture will be helpful to improve your chronic PF?” Participant will choose one of the following answers: “Extremely helpful”, “Very helpful”, “Helpful”, “Not help at all” and “Unclear”.
The proportion of participants who have maintained blinding during treatment in the acupuncture and SA groups: Participants’ blindness to the mode of acupuncture will be assessed 5 min after the end of any treatment in the fourth week by asking the patients the following question: “Which of the two acupuncture modalities do you think you received, acupuncture or SA?” Participants will choose one of the following answers: “Acupuncture”, “SA” or “Unclear”. Prior to the question, patients will be informed that they may have received one of two modalities: acupuncture with a deeper insertion or SA with no skin penetration.
Safety assessment
The adverse events (AEs) during the entire study will be recorded and described as acupuncture-related AEs and non–acupuncture-related AEs. Acupuncture-related AEs include fainting, broken needle, unbearable pain during acupuncture (VAS ≥8, using VAS from 0 (no pain) to 10 (worst pain imaginable)) and other unintended signs or symptoms after acupuncture (eg, localised haematoma or infection, nausea, dizziness, vomiting, headache, palpitations). Detailed information on AEs including the name, onset, end date, intensity, correlation with acupuncture and outcomes will be documented in the case report form. Investigators will immediately report serious AEs (eg, requiring hospitalisation, causing disability or impaired ability to work) to the Medical Ethics Committee of Guang’anmen Hospital, and stop the clinical trial until further instruction is given.
Sample size calculation
Based on the results of a previous study,12 a sample size of 120 participants will be enrolled to provide 80% power to detect a difference of 35% between the combined acupuncture group and waiting-list group in the proportion of participants with treatment response 4 weeks after randomisation at a two-sided significance level of 0.05. The proportion of participants with treatment response after 4 weeks was assumed to be roughly 12% for the waiting-list group,12 with an anticipated 10% loss to follow-up.
Statistical analysis
The null hypothesis is that the proportion of participants with treatment response 4 weeks after randomisation will be the same for the combined acupuncture groups and waiting-list group. Data will be presented as mean±SD for quantitative variables and frequencies (number of cases), with relative frequencies (percentages) for categorical variables. The primary outcome analysis will use the Cochran-Mantel-Haenszel test to compare the response rate between the combined acupuncture groups and the waiting-list group. If the result of this analysis is significant, hierarchical testing will be applied to the acupuncture group versus waiting-list group, SA group versus waiting-list group and acupuncture group versus SA group. For normally distributed quantitative variables, a repeated-measures analysis of variance with multiple comparisons post hoc test will be performed using baseline as a covariate when comparing more than two groups and an unpaired t-test when comparing two groups. For non-normally distributed quantitative variables, the non-parametrical Kruskal-Wallis test and Mann-Whitney U test will be performed. For categorical variables, the χ2 test will be used. CIs for the difference between treatments will be calculated at the 95% level. A two-tailed test will be applied for all available data, and a p value <0.05 will be considered statistically significant. All analyses in this trial will be performed using SPSS software V.20.0 on the basis of the intention-to-treat population, which will include participants who had been randomised. Missing data will be completed as the last value observed before dropout. Only the analysis of primary outcome will be considered in a confirmatory manner. No adjustment will be made for multiple comparisons as those analyses of secondary outcomes will be interpreted as exploratory.
Quality control
To ensure the quality of the trial, all the relevant staff will be uniformly trained before the trial on the purpose and content of the trial (eg, diagnosis of chronic PF, inclusion and exclusion criteria, intervention procedures and outcome measures). Licensed acupuncturists with at least 2 years’ acupuncture experience will perform the treatment. Throughout the trial, strict three-level monitoring will be conducted for data quality control. Dropouts and withdrawals including the reasons will be recorded during the trial. Paper-based study data will be stored in locked file cabinets under the management of the investigators. Electronic records will be stored in a Structured Query Language (SQL) server database on a limited-access, secure server maintained by the Guang’anmen Hospital, China Academy of Chinese Medical Sciences.
Patient and public involvement
The research question of whether combined acupuncture and SA will result in larger improvements in heel pain than no acupuncture treatment for patients with chronic PF was first proposed by the investigator after encountering a patient who received SA and reported a similar improvement in heel pain as another patient who received routine acupuncture in the clinic. Patients were not involved in conceiving or implementing the study.
Ethics and dissemination
This trial will be conducted in accordance with the principles of the Declaration of Helsinki. The study has been registered at the ClinicalTrials.gov (NCT04185259) and approved by the Ethical Committee of the Guang’anmen Hospital, China Academy of Chinese Medical Sciences (approval no. 2019-210-KY). All participants must sign the informed consent form prior to randomisation, and they will be permitted to withdraw at any time during the trial, with or without reasons being provided. Any amendment or other change of the protocol will need to be approved by the Ethical Committee of the Guang’anmen Hospital, China Academy of Chinese Medical Science, and agreed to by the co-researchers.
Following analysis of the data, the findings of this study will be submitted for publication in a peer-reviewed medical journal. The results will also be disseminated through presentation at the relevant conferences and scientific meetings.
Discussion
Although several reviews and RCTs12 15 16 29 have been published that focus on acupuncture for PF, owing to the lack of a placebo control, non-specific physiology effects of needling and spontaneous remission of PF cannot be excluded. To date, this is the first randomised trial with three parallel arms, assessing whether combined acupuncture and SA compared with no treatment control produce a significant reduction in pain intensity in chronic PF. We anticipate that this study will determine the efficacy of acupuncture for patients with chronic PF, and improve the care of these patients in the clinic.
Though most patients with PF will achieve significant improvement in symptoms within 1 year regardless of treatment,30 many will seek treatment before then. Patients often choose other treatment options when they cannot obtain a satisfactory outcome from conservative treatment (eg, muscle stretching, heel cups, arch supports, night splints, shockwave therapy, NSAIDs). In this trial, we recruited only chronic participants who had failed to respond to conservative treatment prior to participation. The results can be generalised to patients experiencing chronic refractory PF.
In this study, pain intensity measured with VAS during the first steps in the morning will be used as the primary outcome. This variable has been used in previous trials12 21 and is a meaningful subject outcome measure for the assessment of PF improvement. In addition, we will also use PPT and PFT as objective secondary outcomes. PPT is an essential evaluation tool for patients suffering from many musculoskeletal disorders including PF and provides a reliable process for measuring participants’ responses to mechanical stimuli.31 Compared with normal asymptomatic patients, patients with PF often exhibit a thickened plantar fascia on ultrasound.32 Therefore, a PFT evaluation would provide information to detect the anatomical changes that occur in the plantar fascia after acupuncture.
The strengths of this study include a sham control (non-penetrating at non-acupuncture point) and waitlist control design, objective measurements (ie, PPT, PFT), strict quality control and evaluation of the participants’ expectations regarding acupuncture. We chose sham acupuncture as a placebo treatment for this study to confirm the specific physiological effect of needling because sham acupuncture may be preferable, particularly for Chinese patients who are familiar with the general procedure of acupuncture. Several limitations to this trial need to be acknowledged. First, it will be impossible to blind the acupuncturists and participants in the waitlist control group, which is a general problem in non-pharmacological interventional trials and can cause bias. Second, a high dropout rate may exist in the waitlist group because participants expect to receive acupuncture treatment when they join the trial. Third, the follow-up period will not exceed 12 weeks, which will not allow for detection of the long-term effects of acupuncture for chronic PF. Fourth, our approach will enable us to draw conclusions about the selected acupuncture points but not about individualised treatments.
Ethical approval and consent to participate
The study has received approval from the Institutional Review Boards of Guang’anmen Hospital in China (approval no. 2019-210-KY, Tel +86-10-88001552), and all investigators complied with the Helsinki Declaration.
Supplementary Material
Acknowledgments
The authors appreciate the support and efforts from people who will be included in this study.
Footnotes
WW, SL and YL contributed equally.
Contributors: WW and ZL conceived the idea and designed this trial. WW, ZZ and ZL developed the acupuncture protocol to this article. WZ, ZZ, SL and LL will be responsible for the recruitment, acupuncture and assessment, respectively. YL will be responsible for statistical analysis. This manuscript was drafted by WW and SL, and was revised by YL and ZL. All authors read and approved the final draft of the manuscript.
Funding: This RCT is funded by China Academy of Chinese Medical Sciences (grant no. ZZ13-YQ-019).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gill LH. Plantar fasciitis: diagnosis and conservative management. J Am Acad Orthop Surg 1997;5:109–17. 10.5435/00124635-199703000-00006 [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum AJ, DiPreta JA, Misener D. Plantar heel pain. Med Clin North Am 2014;98:339–52. 10.1016/j.mcna.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 3.Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg 2008;16:338–46. 10.5435/00124635-200806000-00006 [DOI] [PubMed] [Google Scholar]
- 4.Cotchett MP, Landorf KB, Munteanu SE, et al. Effectiveness of trigger point dry needling for plantar heel pain: study protocol for a randomised controlled trial. J Foot Ankle Res 2011;4:5. 10.1186/1757-1146-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhbari B, Salavati M, Ezzati K, et al. The use of dry needling and myofascial meridians in a case of plantar fasciitis. J Chiropr Med 2014;13:43–8. 10.1016/j.jcm.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cione JA, Cozzarelli J, Mullin CJ. A retrospective study of radiofrequency thermal lesioning for the treatment of neuritis of the medial calcaneal nerve and its terminal branches in chronic heel pain. J Foot Ankle Surg 2009;48:142–7. 10.1053/j.jfas.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 7.Thapa D, Ahuja V. Combination of diagnostic medial calcaneal nerve block followed by pulsed radiofrequency for plantar fascitis pain: a new modality. Indian J Anaesth 2014;58:183–5. 10.4103/0019-5049.130824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petraglia F, Ramazzina I, Costantino C. Plantar fasciitis in athletes: diagnostic and treatment strategies. A systematic review. Muscles Ligaments Tendons J 2017;7:107–18. 10.11138/mltj/2017.7.1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uygur E, Aktaş B, Eceviz E, et al. Preliminary report on the role of dry needling versus corticosteroid injection, an effective treatment method for plantar fasciitis: a randomized controlled trial. J Foot Ankle Surg 2019;58:301–5. 10.1053/j.jfas.2018.08.058 [DOI] [PubMed] [Google Scholar]
- 10.Osman AM, El-Hammady DH, Kotb MM. Pulsed compared to thermal radiofrequency to the medial calcaneal nerve for management of chronic refractory plantar fasciitis: a prospective comparative study. Pain Physician 2016;19:E1181–7. [PubMed] [Google Scholar]
- 11.Young CC, Rutherford DS, Niedfeldt MW. Treatment of plantar fasciitis. Am Fam Physician 2001;63:477–8. [PubMed] [Google Scholar]
- 12.Kumnerddee W, Pattapong N. Efficacy of electro-acupuncture in chronic plantar fasciitis: a randomized controlled trial. Am J Chin Med 2012;40:1167–76. 10.1142/S0192415X12500863 [DOI] [PubMed] [Google Scholar]
- 13.Cheuk DKL, Yeung WF, Chung KF, et al. Acupuncture for insomnia (review). Cochrane Database Syst Rev 2012;12:CD005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JY, Rapson LM. Acupuncture in the management of pain of musculoskeletal and neurologic origin. Phys Med Rehabil Clin N Am 1999;10:531–45. 10.1016/S1047-9651(18)30179-7 [DOI] [PubMed] [Google Scholar]
- 15.Clark RJ, Tighe M. The effectiveness of acupuncture for plantar heel pain: a systematic review. Acupunct Med 2012;30:298–306. 10.1136/acupmed-2012-010183 [DOI] [PubMed] [Google Scholar]
- 16.Thiagarajah AG. How effective is acupuncture for reducing pain due to plantar fasciitis? Singapore Med J 2017;58:92–7. 10.11622/smedj.2016143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundeberg T, Lund I, Sing A, et al. Is placebo acupuncture what it is intended to be? Evid Based Complement Alternat Med 2011;2011:1–5. 10.1093/ecam/nep049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacPherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (stricta): extending the CONSORT statement. J Evid Based Med 2010;3:140–55. 10.1111/j.1756-5391.2010.01086.x [DOI] [PubMed] [Google Scholar]
- 20.Martin RL, Davenport TE, Reischl SF, et al. Heel pain-plantar fasciitis: revision 2014. J Orthop Sports Phys Ther 2014;44:A1–33. 10.2519/jospt.2014.0303 [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Liu Y, Zhao J, et al. Electroacupuncture versus manual acupuncture in the treatment of plantar heel pain syndrome: study protocol for an upcoming randomised controlled trial. BMJ Open 2019;9:e026147. 10.1136/bmjopen-2018-026147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standardization Administration of the People’s Republic of China GB/ T12346-2006, Nomenclature and Location of Acupuncture Points [S], 2006. [Google Scholar]
- 23.Zhou K, Fang J, Wang X, et al. Characterization of De Qi with electroacupuncture at acupoints with different properties. J Altern Complement Med 2011;17:1007–13. 10.1089/acm.2010.0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain 1987;30:115–26. 10.1016/0304-3959(87)90089-3 [DOI] [PubMed] [Google Scholar]
- 25.Fong C-M, Blackburn JT, Norcross MF, et al. Ankle-dorsiflexion range of motion and landing biomechanics. J Athl Train 2011;46:5–10. 10.4085/1062-6050-46.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin R. Foot and ankle ability measure (FAAM). Available: http://www.healthsciences.duq.edu/pdf/FAAM12-051.pdf[Accessed 23 May 2019].
- 27.González-Sánchez M, Li GZ, Ruiz Muñoz M, et al. Foot and ankle ability measure to measure functional limitations in patients with foot and ankle disorders: a Chinese cross-cultural adaptation and validation. Disabil Rehabil 2017;39:2182–9. 10.1080/09638288.2016.1219772 [DOI] [PubMed] [Google Scholar]
- 28.Preston CC, Colman AM. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent preferences. Acta Psychol 2000;104:1–15. 10.1016/S0001-6918(99)00050-5 [DOI] [PubMed] [Google Scholar]
- 29.Zhang SP, Yip T-P, Li Q-S. Acupuncture treatment for plantar fasciitis: a randomized controlled trial with six months follow-up. Evid Based Complement Alternat Med 2011;2011:1–10. 10.1093/ecam/nep186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monteagudo M, Maceira E, Garcia-Virto V, et al. Chronic plantar fasciitis: plantar fasciotomy versus gastrocnemius recession. Int Orthop 2013;37:1845–50. 10.1007/s00264-013-2022-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saban B, Masharawi Y. Pain threshold tests in patients with heel pain syndrome. Foot Ankle Int 2016;37:730–6. 10.1177/1071100716642038 [DOI] [PubMed] [Google Scholar]
- 32.Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc 2011;101:385–9. 10.7547/1010385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.