Abstract
Objective:
The objective of this study was the development of AMPREDICT-Mobility, a tool to predict the probability of independence in either basic or advanced (iBASIC or iADVANCED) mobility 1 year after dysvascular major lower extremity amputation.
Methods:
Two prospective cohort studies during consecutive 4-year periods (2005–2009 and 2010–2014) were conducted at seven medical centers. Multiple demographic and biopsychosocial predictors were collected in the periamputation period among individuals undergoing their first major amputation because of complications of peripheral arterial disease or diabetes. The primary outcomes were iBASIC and iADVANCED mobility, as measured by the Locomotor Capabilities Index. Combined data from both studies were used for model development and internal validation. Backwards stepwise logistic regression was used to develop the final prediction models. The discrimination and calibration of each model were assessed. Internal validity of each model was assessed with bootstrap sampling.
Results:
Twelve-month follow-up was reached by 157 of 200 (79%) participants. Among these, 54 (34%) did not achieve iBASIC mobility, 103 (66%) achieved at least iBASIC mobility, and 51 (32%) also achieved iADVANCED mobility. Predictive factors associated with reduced odds of achieving iBASIC mobility were increasing age, chronic obstructive pulmonary disease, dialysis, diabetes, prior history of treatment for depression or anxiety, and very poor to fair self-rated health. Those who were white, were married, and had at least a high-school degree had a higher probability of achieving iBASIC mobility. The odds of achieving iBASIC mobility increased with increasing body mass index up to 30 kg/m2 and decreased with increasing body mass index thereafter. The prediction model of iADVANCED mobility included the same predictors with the exception of diabetes, chronic obstructive pulmonary disease, and education level. Both models showed strong discrimination with C statistics of 0.85 and 0.82, respectively. The mean difference in predicted probabilities for those who did and did not achieve iBASIC and iADVANCED mobility was 33% and 29%, respectively. Tests for calibration and observed vs predicted plots suggested good fit for both models; however, the precision of the estimates of the predicted probabilities was modest. Internal validation through bootstrapping demonstrated some overoptimism of the original model development, with the optimism-adjusted C statistic for iBASIC and iADVANCED mobility being 0.74 and 0.71, respectively, and the discrimination slope 19% and 16%, respectively.
Conclusions:
AMPREDICT-Mobility is a user-friendly prediction tool that can inform the patient undergoing a dysvascular amputation and the patient’s provider about the probability of independence in either basic or advanced mobility at each major lower extremity amputation level.
Choosing the optimum amputation level for the dysvascular/diabetic patient requiring amputation is challenging for both the physician and the patient. It is a decision that must integrate the combined risks of failed residual limb healing, impaired functional mobility, and mortality. Unfortunately, there are no laboratory tests that predict healing, nor are there existing models that predict functional outcome or mortality.1
This uncertainty has led to inadequate shared decision-making in the preoperative period as well as significant variability in amputation level practices.2 Having adequate evidence to inform the risks and benefits of different amputation level options is critical to this process and can facilitate the incorporation of the patient’s values and preferences into the decision. The variability in current amputation level selection may be reflective of how the risks of mortality, reduced mobility, and ream-putation are balanced in different geographic regions and health systems. In the United States, the below-knee amputation (BKA)/above-knee amputation (AKA) ratio in the Veterans Health Administration was reported to be 1.6 during 1994–2001 and 1.5 during 2002–2003, whereas in a comparable time period (1996) in a Medicare population, it was 0.81.3–5 In England’s National Health Care, the BKA/AKA ratios were 0.73 and 1.2 in different health districts between 2003 and 2008.6 The complexity of decision-making is increased considering transmetatarsal amputations (TMAs). TMA has been advocated because it is thought to result in a greater probability of preservation of function.7 However, the anticipated gains in functional outcome may be compromised by revision rates that may be as high as 45% to 57%.8,9 These data confirm the complexity and variability in amputation level selection as well as the need for patient-specific prediction models to better inform the surgeon and patient so these can be incorporated into shared decision-making.
The objective of this study was to develop and to validate a patient-specific predictive model of mobility outcome (AMPREDICT-Mobility) in individuals undergoing their first major lower extremity amputation (LEA) because of complications of diabetes or peripheral arterial disease (PAD). The model was developed to predict the probability that an individual will achieve independence in basic or advanced mobility 12 months after amputation at each LEA level on the basis of a spectrum of demographic, comorbidity, psychological, and social predictors collected during the periamputation period. The broader goal of this prediction model is to provide surgeons and patients with the necessary evidence to inform mobility prognosis at each anatomic amputation level, to improve shared decision-making, and to reduce variability in current amputation level decision-making.
METHODS
Study design.
Two multisite prospective cohort studies were conducted on individuals undergoing their first major LEA because of complications of PAD or diabetes. The first study was conducted between 2005 and 2009 at four sites: two Veterans Administration medical centers (located in Seattle and Denver), a Seattle-area university hospital, and a Seattle-based level I trauma center. The second study was conducted between 2010 and 2014 at four Veterans Administration medical centers (located in Seattle, Portland, Houston, and Dallas). To increase study power and to expand the generalizability of the model, both data sets were combined, ensuring a broad geographic and temporal range. Study operations and data elements collected were comparable for each study. The decision to perform TMA, BKA, or AKA was made at each site per usual care. Participants were assessed in-person or by telephone within 6 weeks after the definitive amputation procedure for baseline data and 12 months postsurgically. Additional data were gathered by systematic review of the medical records, and aspects of interview data were verified against the medical record. All assessments were performed by a trained study coordinator designated for each site. These studies were conducted in accordance with the procedures approved by human subjects review boards at each participating institution. All participants provided informed consent.
Participants.
In the first prospective study, 239 potential participants were screened for participation. In the second prospective study, 415 potential participants were screened for participation. Participants were eligible if (1) they were 18 years of age or older and (2) they were awaiting (or underwent in the last 6 weeks) a first major LEA (ie, TMA, BKA, or AKA) related to complications of diabetes or PAD. Participants were excluded if (1) they had inadequate cognitive or language function to consent or to participate defined by more than four errors on the Short Portable Mental Status Questionnaire or (2) they were nonambulatory before the amputation for reasons unrelated to PAD or diabetes. Among the potential participants in the first study, 136 (57%) met study criteria; 87 participants (64% of eligible) agreed and were able to participate (Fig 1). Among the potential participants in the second study, 198 (48%) met study criteria; 113 subjects (57% of eligible) agreed and were able to participate (Fig 2). A total of 200 participants made up the combined baseline study population.
Fig 1.
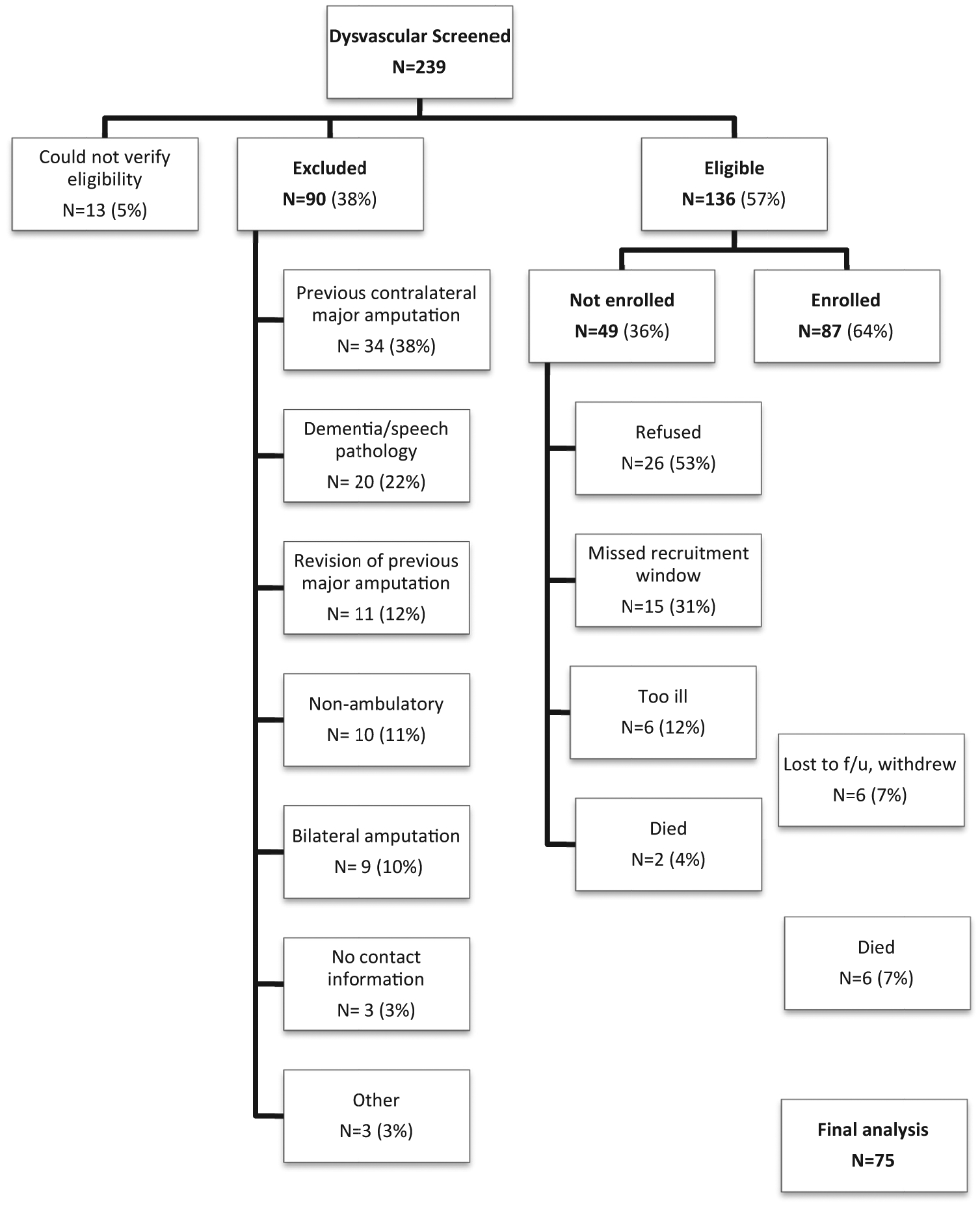
Consolidated Standards of Reporting Trials (CONSORT) diagram depicting total numbers excluded, not enrolled, enrolled, and final 12-month follow-up (f/u) for cohort I.
Fig 2.
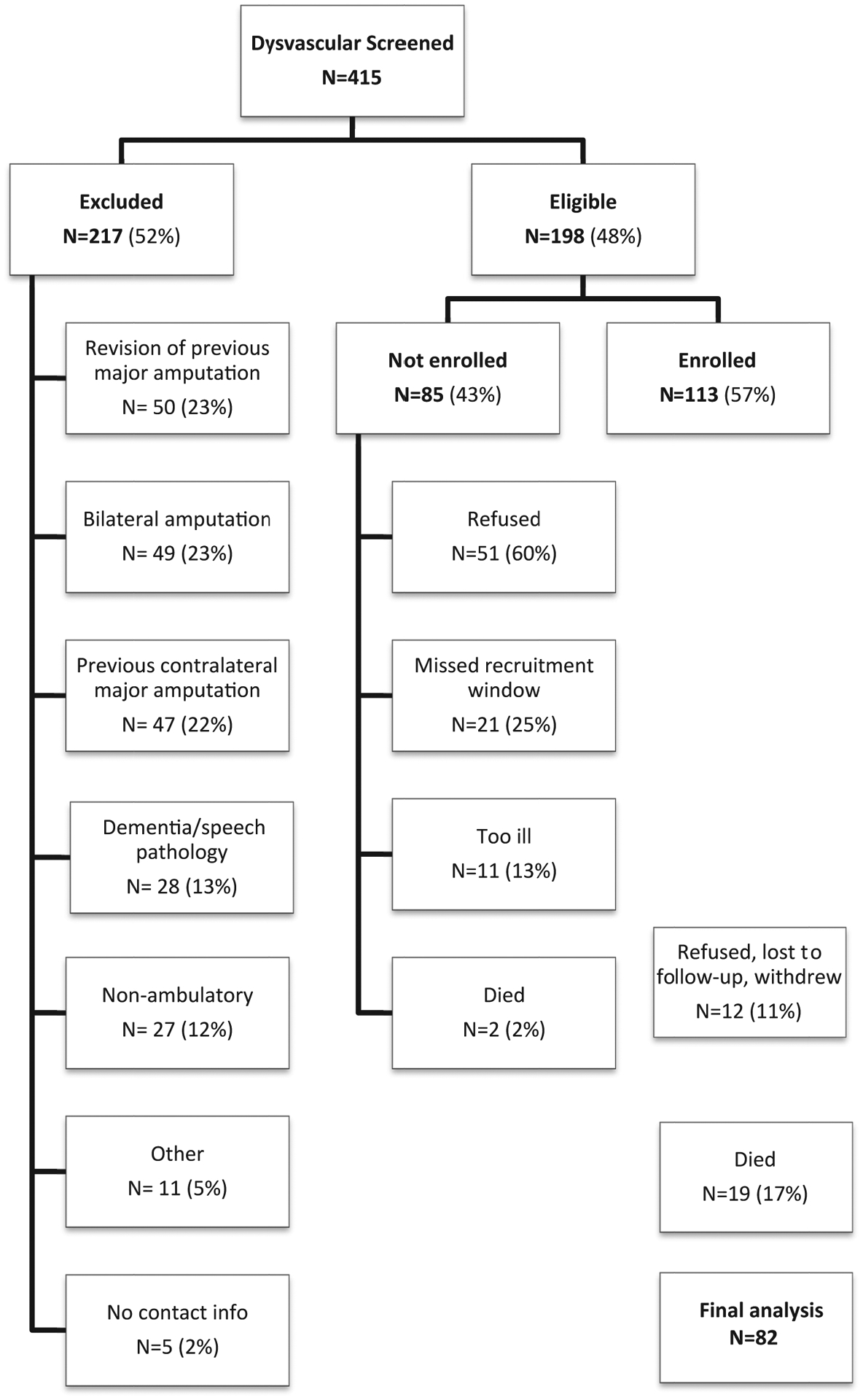
Consolidated Standards of Reporting Trials (CONSORT) diagram depicting total numbers excluded, not enrolled, enrolled, and final 12-month follow-up for cohort II.
Predictor variables.
Predictors were chosen on the basis of three main criteria: (1) clinical expert consensus on predictive importance of specific variables; (2) literature support for the predictive importance of specific variables; and (3) they could be easily obtained before amputation in the clinical/surgical setting. Baseline measures included age, gender, marital status, race (self-reported and coded as white or nonwhite because of very low proportion of nonwhite), education level, living environment, body mass index (BMI), self-rated health, tobacco use, several comorbid medical conditions, history of anxiety or depression, and level of amputation.
The anatomic level of amputation (ie, TMA, BKA, or AKA) was determined from the medical record, as was the primary etiology (diabetes vs PAD). The presence or absence of the following specific comorbid conditions or procedures was self-reported and then verified in the medical record: diabetes, previous lower extremity arterial reconstruction, traumatic brain injury, hypertension, joint replacement, chronic obstructive pulmonary disease (COPD), currently on dialysis, previous heart attack, heart failure, and stroke. If the condition was not reported but identified in the medical record, the participants were counted as having the condition. If the condition was self-reported but not identified in the record, the participants were counted as having the condition. We also asked participants whether they had participated in individual or group psychotherapy, whether they were taking medications for mood, and whether they had a history of treatment for anxiety or depression. We assessed the degree of social support using the brief version of the Modified Social Support Survey, a measure of perceived social support developed initially as part of the Medical Outcomes Study and subsequently shortened (to 5 items from 18) as part of the Multiple Sclerosis Quality of Life Inventory.10,11 Possible total scores range from 0 to 100, with higher scores indicating greater perceived social support. Participants were considered smokers if they endorsed smoking “every day” or “some days” before amputation and nonsmokers if they endorsed “not smoke at all.” All baseline assessment measures are presented in Table I.
Table I.
Baseline sociodemographic, general health, and health behavior data by study population and combined
| Variable | Cohort I (n = 87) | Cohort II (n = 113) | Combined (N = 200) |
|---|---|---|---|
| Amputation level | |||
| TMA | 27 (31) | 26 (23) | 53 (27) |
| BKA | 52 (60) | 59 (52) | 111 (56) |
| AKA | 8 (9) | 28 (25) | 36 (18) |
| Age, years, mean (SD) | 62.1 (8.7) | 63.5 (8.1) | 62.9 (8.4) |
| BMI, kg/m2, mean (SD) | 31.0 (7.4) | 28.2 (7.1) | 29.4 (7.3) |
| Female | 6 (7) | 2 (2) | 8 (4) |
| Marital status | |||
| Not married/partner | 38 (44) | 56 (50) | 94 (47) |
| Married/partner | 48 (55) | 57 (50) | 105 (53) |
| Race | |||
| White | 73 (84) | 79 (70) | 152 (76) |
| Nonwhite | 14 (16) | 34 (30) | 48 (24) |
| Education level | |||
| Less than high-school graduate | 5 (6) | 8 (7) | 13 (6) |
| High-school graduate or higher | 81 (94) | 105 (93) | 186 (94) |
| Living status | |||
| Home alone | 26 (30) | 24 (21) | 50 (25) |
| Home with spouse/other | 52 (60) | 77 (68) | 129 (65) |
| SNF/nursing home | 7 (8) | 11 (10) | 18 (9) |
| Other | 2 (2) | 1 (1) | 3 (2) |
| Diabetes | 75 (86) | 81 (72) | 156 (78) |
| Stroke | 17 (20) | 28 (25) | 45 (23) |
| Heart attack | 29 (33) | 27 (24) | 56 (28) |
| Heart failure | 22 (25) | 35 (31) | 57 (29) |
| Dialysis | 8 (9) | 12 (11) | 20 (10) |
| COPD | 9 (10) | 19 (17) | 28 (14) |
| Lower extremity arterial reconstruction | 32 (37) | 46 (41) | 78 (39) |
| Traumatic brain injury | 22 (25) | 10 (9) | 32 (16) |
| Joint replacement | 8 (9) | 7 (6) | 15 (8) |
| Hypertension | 59 (68) | 86 (76) | 145 (73) |
| Treated for anxiety/depression | 30 (34) | 40 (35) | 70 (35) |
| Smoker | 33 (38) | 28 (25) | 61 (31) |
| Psychotherapy | 11 (13) | 22 (19) | 33 (17) |
| Mood-altering drugs | 23 (26) | 28 (25) | 51 (26) |
| Modified Social Support Survey score, mean (SD) | 67.6 (28.6) | 75.0 (27.3) | 71.9 (28.0) |
| Self-rated health | |||
| Good or very good | 35 (40) | 39 (35) | 74 (37) |
| Fair, poor, or very poor | 51 (59) | 74 (65) | 125 (63) |
AKA, Above-knee amputation; BKA, below-knee amputation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; SD, standard deviation; SNF, skilled nursing facility; TMA, transmetatarsal amputation.
Data are presented as number (%) unless otherwise indicated.
Primary outcome measure: Locomotor Capabilities Index 5-level (LCI-5) scale.
Mobility was assessed using the LCI-5 at 12-month follow-up; 14-items are graded on a 5-level ordinal scale ranging from “unable to perform the activity” (0 points) to “able to perform independently without assistance” (4 points).12 Possible scores for the LCI-5 range from 0 to 56 points, with higher scores representing higher function. Among amputees, the LCI-5 has well-established internal consistency, test-retest reliability, and content, discriminant, and criterion validity. Two subscales were generated from the measure (Table II), namely, independent (i) in basic (iBASIC) mobility (seven basic items) and independent (i) in advanced (iADVANCED) mobility (seven advanced items).13 iBASIC mobility or iADVANCED mobility was achieved if a participant was able to perform all of the tasks associated with the subscale independently with or without ambulatory aids. These were the two primary outcomes for our prediction models. All but one individual who achieved iADVANCED also achieved iBASIC mobility. This individual was independent without the use of an assistive device for six of seven basic mobility elements (the exception was that the person required assistance for stepping down a sidewalk curb).
Table II.
Items included in iBASIC and iADVANCED mobility, Locomotor Capabilities Index (LCI)a
| iBASIC mobility | iadvanced mobility |
|---|---|
| Get up from a chair | Pick up an object from the floor when you are standing up with your prosthesis |
| Walk in the house | Get up from the floor (eg, if you fell) |
| Walk outside on even ground | Walk outside on uneven ground (eg, grass, gravel, slope) |
| Go upstairs with a handrail | Go down a few steps (stairs) without a handrail |
| Go downstairs with a handrail | Go up a few steps (stairs) without a handrail |
| Step up a sidewalk curb | Walk outside in inclement weather (eg, snow, rain, ice) |
| Step down a sidewalk curb | Walk while carrying an object |
Independence requires ability to perform all tasks with or without mobility aids.
Statistical analysis.
All predictors considered for inclusion in the development models and their format and categorization are presented in Table I. Age and BMI were centered (at 60 years and 30 kg/m2, respectively) to aid in the interpretation of the model coefficients. In modeling the association with mobility outcomes, we also considered quadratic terms in age and BMI to accommodate possible nonlinear relationships. Although we recognize the potential for factors such as patient age, BMI, marital status, and presence of COPD to modify the impact of amputation level on mobility outcomes, because of sample size constraints, especially in the AKA group, we did not consider interaction terms in the primary models. In fact, the optimism-adjusted area under the curve estimates were lower when interaction terms were included. The main effects of amputation level were forced to be retained. Other variables were retained with a P value ≤ .20. To quantify the discrimination of each model, we estimated the C statistic and the discrimination slope. Calibration was assessed using the Hosmer-Lemeshow (H-L) goodness-of-fit test and plots of the observed proportions against estimated probabilities using a lowess smooth curve for visualization. Outliers in the box plots of predicted probabilities were inspected for clinical plausibility. The developed models were internally validated with bootstrap sampling to obtain estimates of the optimism of the C statistic and the difference in predicted probabilities for those who did and did not achieve iBASIC and iADVANCED mobility (ie, the discrimination slope). Bootstrap samples were drawn with replacement and with the same size as the original sample. Model selection was carried out for each bootstrap sample and model performance assessment compared with that on the original sample. This was repeated 500 times to obtain stable estimates of the average optimism of the C statistic and discrimination slope for each model.
To demonstrate the clinical utility of AMPREDICT-Mobility, the estimated probabilities (and associated 95% prediction intervals) of achieving iBASIC and iADVANCED mobility at 1 year after amputation were considered in hypothetical clinical scenarios and included in the Appendix (online only). Statistical analyses were performed using Stata 9.0.14
RESULTS
Baseline characteristics.
Among the 87 participants enrolled in the first cohort, 4 participants (5%) formally withdrew, 2 (2%) were lost to follow-up, and 6 (7%) died during the 12-month follow-up period; 75 participants completed their 12-month interview (86%; Fig 1). Among the 113 subjects enrolled in the second cohort, 5 subjects (4%) formally withdrew during the course of the study, 1 subject (~1%) refused the 12-month interview, 6 (5%) were lost to follow-up, and 19 subjects (17%) died during the 12-month follow-up period; 82 subjects (73%) completed their 12-month interview (Fig 2). Table I summarizes the baseline characteristics of both cohorts. In total, 157 subjects (79%) completed their 12-month follow-up and were included in the two prediction models.
LCI-5 scores and achievement of iBASIC and iADVANCED mobility.
The mean LCI-5 score at 12-month follow-up was 36.1 (standard deviation, 17.1; range, 0–56). Among the 157 subjects in the combined sample who completed their 12-month follow-up, 54 (34%) did not achieve iBASIC mobility; 103 (66%) achieved iBASIC mobility, and of these, 51 (32%) also achieved iADVANCED mobility. Differences in achieving iBASIC mobility by amputation level were statistically significant (χ2,P = .007), with 83%, 62%, and 48% of TMA, BKA, and AKA amputees achieving this level of mobility. A statistically significant difference across amputation levels was not observed in those achieving iADVANCED mobility, with 39%, 33%, and 20% of TMA, BKA, and AKA amputees achieving that level (χ2, P = .26).
Prediction model development.
The selected logistic regression models for iBASIC and iADVANCED mobility with regression coefficients are presented in Table III, and the variables retained in the final models are listed in Fig 3. Predictive factors associated with reduced odds of achieving iBASIC mobility were increasing age, COPD, dialysis, diabetes, prior history of treatment for depression or anxiety, and very poor to fair self-rated health. Those who were white, were married, and had at least a high-school degree had a higher probability of achieving iBASIC mobility. The odds of achieving iBASIC mobility increased with increasing BMI up to 30 kg/m2 and decreased with increasing BMI thereafter. In secondary analyses, we considered including in the prediction model selected interaction terms for amputation level with age, BMI, marital status, and presence of COPD. However, the interaction terms either were not selected or were in directions that were contrary to our understanding of the roles of these variables. In addition, there was little gain in predictive value when the interaction terms were included. The estimated C statistic was 0.85, and the H-L goodness-of-fit test indicated adequate calibration (P = .07). The predicted probabilities for those who did and did not achieve iBASIC mobility are illustrated in Fig 4, A and show good separation of the two groups. The difference in mean predicted probability was 33% and the difference in medians was >40%, demonstrating good discrimination. Whereas 75% of subjects who achieved iBASIC mobility had estimated probabilities >70%, we observed seven outliers (6.8% of subjects who achieved this level of mobility) who had a probability of <40% for achieving iBASIC mobility and yet successfully achieved it. The plot of predicted vs observed probabilities indicated good fit of the model.
Table III.
Logistic regression coefficients (95% confidence intervals) for variables in the 12-month iBASIC and iADVANCED obility prediction models (N = 157)
| iBASIC mobility | iadvanced mobility | |||
|---|---|---|---|---|
| Risk factor | Coefficient (95% confidence interval) | P value | Coefficient (95% confidence interval) | P value |
| Amputation level | ||||
| TMA | Reference | Reference | ||
| BKA | −1.12 (−2.19 to −0.054) | .04 | 0.016 (−0.904 to 0.936) | .97 |
| AKA | −2.80 (−4.36 to −1.25) | <.01 | −1.30 (−2.69 to −0.081) | .07 |
| Age,a years | −0.125 (−0.187 to −0.063) | <.01 | −0.138 (−0.205 to −0.071) | <.01 |
| BMI,b kg/m2 | NR | −0.064 (−0.125 to −0.003) | .04 | |
| BMI squared | −0.008 (−0.014 to −0.002) | .01 | NR | |
| Race | 1.10 (0.155–2.04) | .02 | 2.01 (0.799–3.21) | <.01 |
| Marital status | 0.995 (0.133–1.86) | .02 | 1.16 (0.296–2.03) | .01 |
| Education level | 1.28 (−0.678 to 3.24) | .20 | NR | |
| Diabetes | −1.76 (−3.07 to −0.439) | .01 | NR | |
| Dialysis | −1.19 (−2.56 to 0.180) | .09 | −1.02 (−1.46 to 0.409) | .16 |
| COPD | −1.74 (−2.95 to −0.519) | .01 | NR | |
| Treatment for anxiety or depression | −0.796 (−1.70 to 0.107) | .08 | −1.56 (−2.54 to −0.587) | <.01 |
| Self-rated health | −0.713 (−1.61 to 0.719) | .12 | −1.19 (−2.08 to −0.307) | .01 |
| Intercept | 2.57 (−0.058 to 5.19) | .06 | −1.34 (−2.62 to −0.030) | .05 |
AKA, Above-knee amputation; BKA, below-knee amputation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NR, not retained in model; TMA, transmetatarsal amputation.
Race (white vs nonwhite [reference]), marital status (married/partner vs single [reference]), education level (high-school diploma and above vs less than high school [reference]), self-rated health (very poor to fair vs good to very good [reference]).
Age centered at 60 years.
BMI centered at 30 kg/m2.
Fig 3.

Predictors for achieving iBASIC and iADVANCED mobility. AKA, Above-knee amputation; BKA, below-knee amputation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; TMA, transmetatarsal amputation.
Fig 4.
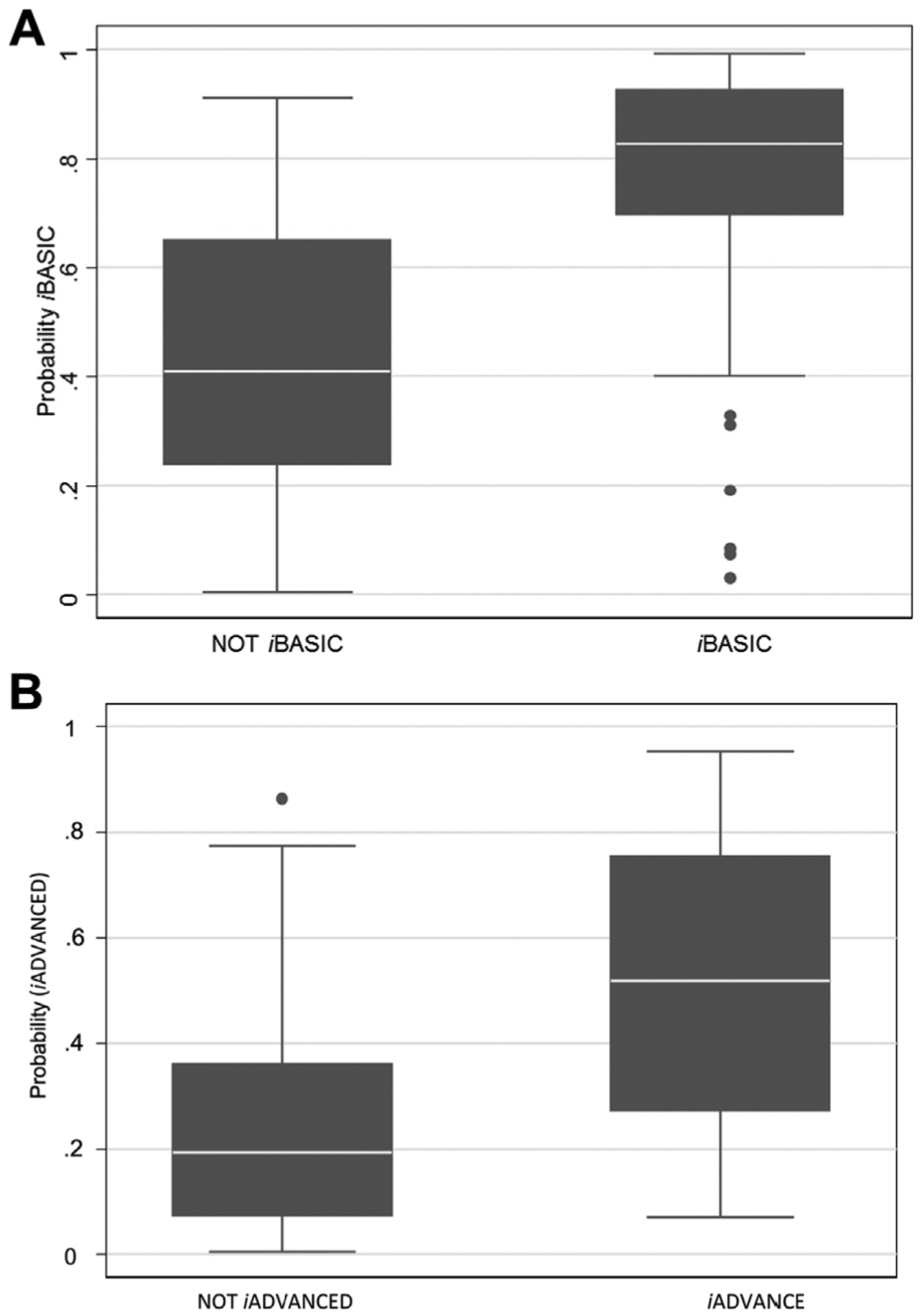
Predicted probability of achieving iBASIC mobility (A) and iADVANCED mobility (B).
The prediction model for iADVANCED mobility included the same predictors as the model for iBASIC mobility with the exception of diabetes, COPD, and education level. Education level did not have a strong association with iBASIC mobility (P = .2 in the final prediction model), and COPD came close to inclusion in the iADVANCED model (P = .2005). The C statistic was 0.82, and the H-L goodness-of-fit test indicated good calibration (P = .49). The predicted probabilities for those who did and did not achieve iADVANCED mobility are illustrated in Fig 4, B and show good separation of the two groups. The difference in mean predicted probability was 29% and the difference in medians was >30%, demonstrating good discrimination. We observed one outlier who had a probability of 86% for achieving iADVANCED mobility and failed to do so. The plot of predicted vs observed probabilities demonstrated good model fit.
Prediction model validation.
The bootstrapping procedure provided estimates of the optimism of the estimated C statistic and discrimination slope of each model. Bootstrap estimates of the optimism for the C statistic were 0.11 for both iBASIC and iADVANCED mobility models and for the discrimination slope 0.14 and 0.13, respectively. This demonstrated some overoptimism of the original model development, with the optimism-adjusted C statistic for iBASIC and iADVANCED mobility being 0.74 and 0.71, respectively, and the discrimination slope 19% and 16%, respectively.
DISCUSSION
The primary goal of this investigation was to develop and internally validate a set of mobility prediction models for use among patients with first major dysvascular LEA (AMPREDICT-Mobility) that uses baseline patient factors, including amputation level, to predict iBASIC mobility and iADVANCED mobility 12 months after dysvascular LEA.
Prediction modeling is currently being used in many aspects of medicine, including cancer care, the evaluation of risk of death after myocardial infarction, diabetes care, and spinal cord injury.15–19 The current movement in health care toward shared decision-making requires not only general population evidence but evidence that supports individual probabilities of risks and benefits.
AMPREDICT-Mobility uses two prediction models that enable the prediction of probable independence in all mobility subtasks included in iBASIC and iADVANCED. The prediction models are patient specific and use easily obtainable preamputation variables. There are no existing predictive models of mobility outcome after amputations that allow comparison with AMPREDICT-Mobility. However, previously published retrospective and cross-sectional studies have demonstrated increasing age associated with adverse functional and mobility outcomes.20–23 Anxiety and depression are common after amputation and can adversely affect quality of life.24,25 Some studies suggest that there is no relationship between depression and prosthetic use, whereas others have found that depression was predictive of poorer mobility outcomes.23,26 These studies describe the association between anxiety/depression after amputation and postamputation outcomes, whereas the current predictive model uses pre-existing anxiety and depression. Self-rated health has not been examined in amputee outcomes. It is a complex multidimensional measure that has many underlying determinants that may vary by study population.27,28 The validity of self-rated health and its contribution to the prediction of mobility outcome in amputees are reflected by its association with disability, health care utilization, and mortality.29 Dialysis has been associated with lower functional outcome scores and reduced prosthetic use.30,31 The effect of BMI on amputee mobility outcome is controversial. Kalbaugh et al found no effect on mobility, whereas Rosenberg et al did find reduced prosthetic use with increased BMI.32,33 The effect of BMI on probable mobility outcome in the two prediction models reflects some of the differing results seen in the literature. In the iBASIC model, a quadratic effect of BMI was associated with an increased probability of iBASIC mobility with increasing BMI up to 30 kg/m2 and decreased probability thereafter; in the iADVANCED model, increasing BMI reduced the probability of independence over the entire range of BMI. Racial factors and mobility outcome after amputation have not been evaluated in the literature; however, African American racial background has been associated with increasing rates of amputation, reduced survival, and increased odds of having a higher level of amputation.34,35 Similarly, the effect of marital status has not been studied, although social integration, which may be a surrogate for marital status, has been associated with improved function.36
It is important to consider not only the predictors that were incorporated into the predictive model but also the potential predictors that were not included. This study was unique in that baseline perioperative variables also included key individual medical comorbidities, smoking, social support, psychotherapy, treatment for mental health disorders, and revascularization surgery and joint arthroplasty. Perhaps surprisingly, comorbid medical conditions such as prior myocardial infarction, diagnosis of congestive heart failure, and prior stroke were not retained in the models. Intuitively, one would consider these factors influential in mobility outcome; however, a prior systematic review of the literature also did not support these associations.37
The inclusion of amputation level in the models allows the clinician and patient to obtain a probability of achieving iBASIC and iADVANCED mobility at each major level. Interestingly, amputation level had a large effect on achieving iBASIC mobility. Amputation at the BKA and AKA levels compared with the TMA level had an adverse impact on the probability of achieving iBASIC mobility. The BKA level compared with TMA had little effect on achieving iADVANCED mobility, whereas the AKA level had an adverse effect.
Several limitations of the current study are worthy of note. The sample was restricted to participants with at least a minimum level of ambulatory function before their initial amputation and adequate cognitive capacity to participate in an interview. Furthermore, the demographics of the participating institutions were such that some subpopulations were under-represented. For example, the numbers of women and those with a low educational level were small, making it difficult to generalize the model to these populations.
It is well known that significant associations with an outcome are not sufficient to ensure accurate prediction.38 Although our prediction models for iBASIC and iADVANCED mobility show good discrimination and calibration, the predicted probabilities for some covariate patterns have wide prediction intervals (see case studies in Appendix, online only). Despite this being the largest prospectively enrolled study of dysvascular amputees with 12-month longitudinal follow-up, the sample size was modest and contributed to the relatively wide prediction intervals. Nevertheless, the prediction models do provide a common language for communication of anticipated mobility after amputation and provide useful evidence on expected mobility to inform patients and providers. Although we have adjusted for optimism in assessing model performance by internal validation, ideally these models should be externally validated in the future with larger sample sizes.
Our predictive model was developed using the assessment of self-rated health in the immediate postoperative period; therefore, it may not reflect the self-rated health during the immediate preoperative period, when the prediction model would be used. However, participants were asked to recall their self-rated health before the amputation. Although we have not established the validity of this method, prior published research indicates that the proportion of individuals with diabetes who report fair, poor, and very poor ratings of health is similar.39,40 Furthermore, prior research does indicate that during a hospitalization for an acute medical event, the recall of self-rated health before the event is still predictive of key outcomes.41 Whereas the LCI can be divided into a basic and advanced scale, the basic scale does not include very basic mobility elements, such as bed and toilet transfers or wheeled mobility. Therefore, in counseling a patient with the AMPREDICT-Mobility model, it will be important that it be done with a full knowledge of what mobility activities are being predicted.
Finally, the iBASIC prediction model had seven outliers. Of the 103 subjects who achieved iBASIC mobility, these subjects were predicted not to achieve this and did achieve it. Examination of patient characteristics did not reveal a defined pattern to explain this finding. The majority of these participants were diabetic, were not married, and rated their health fair to poor. The effects of these factors are complex and multidimensional; therefore, their effect in different individuals may vary.42,43
CONCLUSIONS
The absence of prediction models has contributed to the challenges that medical providers face in communicating the risks and benefits of different amputation levels on anticipated mobility outcome. AMPREDICT-Mobility is a novel predictive tool that was built on a wide spectrum of biopsychosocial factors existing at the time of amputation surgical decision-making. It is designed to quantify the probability that either iBASIC or iADVANCED mobility will be achieved, depending on the amputation level, to inform communication between the patient and surgeon during the preoperative period. Future application may involve an on-line calculator or smart phone application that can be used in the clinical environment.
Supplementary Material
Acknowledgments
This material is based on work supported by the U.S. Department of Veterans Affairs, Office of Research and Development, Rehabilitation Research and Development (Merit Review A41241 [J.M.C., principal investigator] and Career Development Award B4927W [A.P.T., principal investigator]). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States government.
APPENDIX (online only).
The following clinical case examples illustrate the potential utility of AMPREDICT-Mobility to inform a dialogue between surgeon and patient. Because the patient’s risk of failure of healing and mortality risk are unknown, the surgeon will have to discuss these factors on the basis of their clinical assessment and the population risks that are described in the literature. Each of these clinical cases and the results of the prediction model should be used in conjunction with the mobility characteristics defined in iBASIC and iADVANCED. Each model will allow a determination of whether the mobility subtasks can be performed independently—it does not inform whether they will be done with or without ambulatory aids or, if ambulatory aids are used, with what type of ambulatory aids.
Hypothetical cases applying the AMPREDICT-Mobility prediction model for decision-making
Case 1: Typical patient requiring a diabetes-related amputation at the transtibial or transmetatarsal level.
A 68-year-old white man with a high-school education and a body mass index (BMI) of 30 presents with an infected first metatarsophalangeal foot ulcer with deformity of the remaining toes. His peripheral pulses are not palpable, and vascular evaluation shows that he has unreconstructible vascular disease. He has a history of diabetes and end-stage renal disease with dialysis. Before the development of the foot ulcer, he was ambulatory, living independently alone in a single-level home with four-stairs access to the outside. He has a history of depression and is currently being treated with antidepressants. When asked, he reports that he would rate his overall health fair. He is being considered for a possible transmetatarsal amputation (TMA) vs below-knee amputation (BKA), and he is wondering what his functional level of mobility might be if he has an amputation at each level. Would he be able to return home? You are uncertain about what the probability of healing a TMA might be but appreciate that a BKA would have a greater probability of healing.
After incorporating the patient’s characteristics into the model, you are able to say that at 1 year after the amputation, the patient has about a 23% chance that he would achieve independence with basic mobility (ie, independently be able to do things like walk in the home, climb stairs with a handrail, or step up or down a curb) if he had a TMA, whereas if he had a BKA, it would be about 10% (Supplementary Table I). This patient would have a limited potential to independently climb the stairs necessary to access his home, and the choice of amputation level would not make a very large difference in his probable success. The probability of achieving independent advanced mobility is very low (about 1%) regardless of amputation level.
Case 2: Healthy patient requiring a diabetes-related amputation at the transtibial or transmetatarsal level.
A 62-year-old white male patient with a high-school education and a BMI of 25 presents with an infected first metatarsophalangeal foot ulcer with underlying osteomyelitis of the first and second meta-tarsals. He has a history of diabetes but otherwise is relatively healthy. Before the development of the foot ulcer, he was ambulatory at home and in the community, living independently with his spouse. When asked, he reports that he would rate his overall health good. He is being considered for a possible TMA vs BKA, and he is wondering what his functional level of mobility might be if he has an amputation at each level. Does it matter what amputation level he chooses?
In this case example, the patient has a very good probability of achieving iBASIC mobility and also has about a 67% chance of achieving iADVANCED mobility (Supplementary Table II). He will therefore likely be able to do things like walk outside on irregular terrain and in inclement weather. Of note, there is little difference in the probability of achieving iADVANCED mobility with a BKA compared with a TMA.
Case 3. You have evaluated a 74-year-old black man with 2 years of college education and a BMI of 27 who presents with severe rest pain in his foot.
He has had a number of revascularization procedures in the prior 4 years, and at this time there are no further revascularization options. You are considering either a possible BKA or AKA. This patient has a prior history of chronic obstructive pulmonary disease, myocardial infarction, treatment for anxiety/depression, but no diabetes. He lives alone in a wheelchair-accessible apartment; although he was ambulatory with a single-point cane, it was limited to short-distance ambulation by claudication. When asked how he would rate his overall health, he states that it is good. He is wondering what the difference in his mobility might be if he had either amputation procedure. Table III provides the probabilities that can be discussed with the patient.
Footnotes
Additional material for this article may be found online at www.jvascsurg.org.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Landry GJ, Silverman DA, Liem TK, Mitchell EL, Moneta GL. Predictors of healing and functional outcome following transmetatarsal amputations. Arch Surg 2011;146:1005–9. [DOI] [PubMed] [Google Scholar]
- 2.Dillon MP, Fatone S, Quigley M. Describe the outcomes of dysvascular partial foot amputation and how these compare to transtibial amputation: a systematic review protocol for the development of shared decision-making resources. Syst Rev 2015;4:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz CP, Eidt JF, Capps C, Kirtley L, Moursi MM. Major lower extremity amputations at a Veterans Affairs hospital. Am J Surg 2003;186:449–54. [DOI] [PubMed] [Google Scholar]
- 4.Bates B, Stineman MG, Reker DM, Kurichi JE, Kwong PL. Risk factors associated with mortality in veteran population following transtibial or transfemoral amputation. J Rehabil Res Dev 2006;43:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil 2005;86: 480–6. [DOI] [PubMed] [Google Scholar]
- 6.Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJ. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg 2010;97:1348–53. [DOI] [PubMed] [Google Scholar]
- 7.Mueller MJ, Salsich GB, Strube MJ. Functional limitations in patients with diabetes and transmetatarsal amputations. Phys Ther 1997;77:937–43. [DOI] [PubMed] [Google Scholar]
- 8.Stone PA, Back MR, Armstrong PA, Flaherty SK, Keeling WB, Johnson BL, et al. Midfoot amputations expand limb salvage rates for diabetic foot infections. Ann Vasc Surg 2005;19: 805–11. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TH, Gordon IL, Whalen D, Wilson SE. Transmetatarsal amputation: predictors of healing. Am Surg 2006;72:973–7. [PubMed] [Google Scholar]
- 10.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–14. [DOI] [PubMed] [Google Scholar]
- 11.Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG. Multiple sclerosis quality of life inventory: a user’s manual. New York: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 12.Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil 2004;85:743–8. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier-Gagnon C, Grise MC, Lepage Y. The Locomotor Capabilities Index: content validity. J Rehabil Outcomes Meas 1998;2:40–6. [Google Scholar]
- 14.Stata Statistical Software [computer program]. Version Release 9. College Station, Tex: StataCorp LP; 2005. [Google Scholar]
- 15.Bevilacqua JL, Kattan MW, Fey JV, Cody HS 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol 2007;25:3670–9. [DOI] [PubMed] [Google Scholar]
- 16.Weiser MR, Landmann RG, Kattan MW, Gonen M, Shia J, Chou J, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol 2008;26:380–5. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs DR Jr, Kroenke C, Crow R, Deshpande M, Gu DF, Gatewood L, et al. PREDICT: a simple risk score for clinical severity and long-term prognosis after hospitalization for acute myocardial infarction or unstable angina: the Minnesota heart survey. Circulation 1999;100:599–607. [DOI] [PubMed] [Google Scholar]
- 18.Iezzoni L Risk and outcomes Risk adjustment for measuring health care outcomes. 2nd ed Chicago: Health Administration Press; 1997. p. 1–41. [Google Scholar]
- 19.Wilson JR, Grossman RG, Frankowski RF, Kiss A, Davis AM, Kulkarni AV, et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J Neurotrauma 2012;29:2263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson VJ, Kondziela S, Gottschalk F. Pre and postamputation mobility of trans-tibial amputees: correlation to medical problems, age and mortality. Prosthet Orthot Int 1995;19:159–64. [DOI] [PubMed] [Google Scholar]
- 21.Munin MC, Seligman K, Dew MA, Quear T, Skidmore ER, Gruen G, et al. Effect of rehabilitation site on functional recovery after hip fracture. Arch Phys Med Rehabil 2005;86: 367–72. [DOI] [PubMed] [Google Scholar]
- 22.Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, et al. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg 2003;38:7–14. [DOI] [PubMed] [Google Scholar]
- 23.Schoppen T, Boonstra A, Groothoff JW, de Vries J, Goeken LN, Eisma WH. Physical, mental, and social predictors of functional outcome in unilateral lower-limb amputees. Arch Phys Med Rehabil 2003;84:803–11. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Ripley D, Pentland B, Todd I, Hunter J, Hutton L, et al. Depression and anxiety symptoms after lower limb amputation: the rise and fall. Clin Rehabil 2009;23:281–6. [DOI] [PubMed] [Google Scholar]
- 25.Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int 2008;32:231–43. [DOI] [PubMed] [Google Scholar]
- 26.Larner S, van Ross E, Hale C. Do psychological measures predict the ability of lower limb amputees to learn to use a prosthesis? Clin Rehabil 2003;17:493–8. [DOI] [PubMed] [Google Scholar]
- 27.Cott CA, Gignac MA, Badley EM. Determinants of self rated health for Canadians with chronic disease and disability. J Epidemiol Community Health 1999;53:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W, Watanabe M, Tanimoto Y, Shibutani T, Kono R, Saito M, et al. Factors associated with good self-rated health of non-disabled elderly living alone in Japan: a cross-sectional study. BMC Public Health 2007;7:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norvell DC, Turner AP, Williams RM, Hakimi KN, Czerniecki JM. Defining successful mobility after lower extremity amputation for complications of peripheral vascular disease and diabetes. J Vasc Surg 2011;54:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arneja AS, Tamiji J, Hiebert BM, Tappia PS, Galimova L. Functional outcomes of patients with amputation receiving chronic dialysis for end-stage renal disease. Am J Phys Med Rehabil 2015;94:257–68. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg 2005;42:227–35. [DOI] [PubMed] [Google Scholar]
- 32.Kalbaugh CA, Taylor SM, Kalbaugh BA, Halliday M, Daniel G, Cass AL, et al. Does obesity predict functional outcome in the dysvascular amputee? Am Surg 2006;72:707–12;discussion:712–3. [PubMed] [Google Scholar]
- 33.Rosenberg DE, Turner AP, Littman AJ, Williams RM, Norvell DC, Hakimi KM, et al. Body mass index patterns following dysvascular lower extremity amputation. Disabil Rehabil 2013;35:1269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newhall K, Spangler E, Dzebisashvili N, Goodman DC, Goodney P. Amputation rates for patients with diabetes and peripheral arterial disease: the effects of race and region. Ann Vasc Surg 2016;30:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefebvre KM, Chevan J. The persistence of gender and racial disparities in vascular lower extremity amputation: an examination of HCUP-NIS data (2002–2011). Vasc Med 2015;20:51–9. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins AT, Pallangyo AJ, Herman AM, Schaumeier MJ, Smith AD, Hevelone ND, et al. The effect of social integration on outcomes after major lower extremity amputation. J Vasc Surg 2016;63:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sansam K, Neumann V, O’Connor R, Bhakta B. Predicting walking ability following lower limb amputation: a systematic review of the literature. J Rehabil Med 2009;41: 593–603. [DOI] [PubMed] [Google Scholar]
- 38.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 2004;159:882–90. [DOI] [PubMed] [Google Scholar]
- 39.Dankner R, Olmer L, Kaplan G, Chetrit A. The joint association of self-rated health and diabetes status on 14-year mortality in elderly men and women [published online ahead of print May 2, 2016]. Qual Life Res doi: 10.1007/s11136-016-1291-9. [DOI] [PubMed] [Google Scholar]
- 40.McDaid O, Hanly MJ, Richardson K, Kee F, Kenny RA, Savva GM. The effect of multiple chronic conditions on self-rated health, disability and quality of life among the older populations of Northern Ireland and the Republic of Ireland: a comparison of two nationally representative cross-sectional surveys. BMJ Open 2013;3:e002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerber Y, Benyamini Y, Goldbourt U, Drory Y; Israel Study Group on First Acute Myocardial Infarction. Prognostic importance and long-term determinants of self-rated health after initial acute myocardial infarction. Med Care 2009;47:342–9. [DOI] [PubMed] [Google Scholar]
- 42.Badawi G, Gariepy G, Page V, Schmitz N. Indicators of self-rated health in the Canadian population with diabetes. Diabet Med 2012;29:1021–8. [DOI] [PubMed] [Google Scholar]
- 43.Hoppmann CA, Gerstorf D. Biobehavioral pathways underlying spousal health dynamics: its nature, correlates, and consequences. Gerontology 2014;60:458–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


