Abstract
Background
In male pre-pubertal cancer patients, radiation and chemotherapy impact future fertility by eradication of spermatogonial stem cells (SSCs). In macaques, spermatogenesis could be regenerated by intratesticular transplantation of SSCs, but only a small percentage of spermatozoa produced were of donor origin. Transient hormone suppression with a GnRH antagonist (GnRH-ant) enhanced spermatogenic recovery from transplanted SSCs.
Objectives
To evaluate donor-derived and endogenous spermatogenic recovery after SSC transplantation into irradiated monkeys and to test whether hormone suppression around the time of transplantation facilitates spermatogenic recovery.
Materials and methods
Testes of 15 adult rhesus monkeys were irradiated with 7 Gy and 4 months later transplanted, to one of the testes, with cryopreserved testicular cells containing SSCs from unrelated monkeys. Monkeys were either treated with GnRH-ant for 8 weeks before transplantation, GnRH-ant from 4 weeks before to 4 weeks after transplantation, or with no GnRH-ant. Tissues were harvested 10 months after transplantation.
Results
Two of the 15 monkeys, a control and a pre-transplantation GnRH-ant–treated, showed substantially higher levels of testicular spermatogenesis and epididymal sperm output in the transplanted side as compared to the untransplanted. Over 84% of epididymal spermatozoa on the transplanted side had the donor genotype and were capable of fertilizing eggs after intracytoplasmic sperm injection forming morulae of the donor paternal origin. Low levels of donor spermatozoa (~1%) were also identified in the epididymis of three additional monkeys. Transplantation also appeared to enhance endogenous spermatogenesis.
Discussion and conclusion
We confirmed that SSC transplantation can be used for restoration of fertility in male cancer survivors exposed to irradiation as a therapeutic agent. The success rate of this procedure, however, is low. The success of filling the tubules with the cell suspension, but not the GnRH-ant treatment, was related to the level of colonization by transplanted cells.
Keywords: transplantation, radiation, spermatogenesis, GnRH antagonist, ICSI
1 |. INTRODUCTION
Cancer treatment regimens endanger the fertility of men of reproductive age. Adult men have the option to cryopreserve spermatozoa that can be used in the future to achieve pregnancy.1 However, there are currently no standard-of-care options to preserve the fertility of pre-pubertal boys who are not producing spermatozoa. According to recent US cancer statistics,2 each year more than 3000 boys are diagnosed with cancers that require treatment with high-dose chemotherapy or irradiation involving the genital region, of which 86% survive. As 43% of them have treatment-induced sterility,3 each year an additional 1200 young men will become sterile due to cancer therapy as children. In addition to cancer survivors, over several hundred boys receive hematopoietic stem cell transplants each year for non-malignant conditions4 and the myeloablative conditioning therapy also presents a risk of infertility.5 Whereas adults have the option of cryopreserving semen before therapy to ensure that they can produce offspring, pre-pubertal or peripubertal patients, who cannot supply an appropriate semen sample, do not currently have any fertility preservation choices that have proven effective. This is a significant human health concern,6 and most parents are indeed interested in preserving fertility on behalf of their children with cancer.7 The development of new methods of fertility preservation to prevent these effects or restore normal reproductive function after cytotoxic treatment is of great importance to these young male cancer survivors.
If spermatogonial stem cells (SSCs) are completely lost after gonadotoxic therapy, the only way to preserve future fertility of pre-pubertal males is by harvesting tissue or a cell suspension containing SSCs prior to therapy and cryopreservation. Indeed, it is the current clinical practice to cryopreserve the testicular tissues before gonadotoxic therapies in boys in various centers in the world8–11 hoping that a satisfactory technique will be developed to produce spermatozoa from the SSCs present in this tissue. SSC transplantation to the seminiferous tubules is one of the techniques that have the potential to restore spermatogenesis in vivo from an individual’s own testis. Spermatozoa can be obtained from the testis, epididymis, or the ejaculate and have been successfully used to produce live offspring with rodents12–14 and goats,15 and embryos with non-human primates.16
Preparation of the recipient is also important to obtain optimal spermatogenic recovery after spermatogonial transplantation.17 It has been shown that in mice,18 rats,19 and macaques20 that transient suppression of gonadotropins and testosterone with GnRH antagonists (GnRH-ant) or similar agents enhances recovery from the transplanted SSCs. Specifically, in adult macaques we showed that transient hormone suppression started immediately after irradiation and continued until just before autologous transplantation of SSCs marked by lentivirus infection, enhanced spermatogenic recovery from the donor SSCs (Figure 1A).20 However, due to low efficiency of the lentivirus infection, the reliable identification of donor-derived spermatozoa was not possible, and hence, their fertilizing ability could not be ascertained. Secondly, although hormone suppression with GnRH-ant was used prior to SSC transplantation, it is not known whether continuing hormone suppression during the post-transplantation period is also beneficial, as was shown in mice.18 Since in the previous study,20 hormone suppression was started immediately after irradiation prior to the complete radiation-induced depletion of germ cells, it is not clear whether hormone suppression treatment initiated after cancer treatment-induced germ cell depletion, a situation similar to that of the cancer survivors needing fertility preservation procedures, also enhances the fertility outcome after SSC transplantation. In the current study, we tested whether transient hormone suppression after irradiation-induced depletion of germ cells but before transplantation of cryopreserved testicular cells, as well as hormone suppression after transplantation, can enhance spermatogenic recovery from donor-derived SSCs. Transplantation was performed using rhesus testicular cells from an allogeneic source so that the donor-derived and endogenous spermatogenesis could be quantified using unique microsatellite markers that differed between the donor-recipient pairs. Similarly, we used microsatellite markers, to assess whether the embryos produced by intracytoplasmic sperm injection (ICSI) of rhesus oocytes were donor-derived.
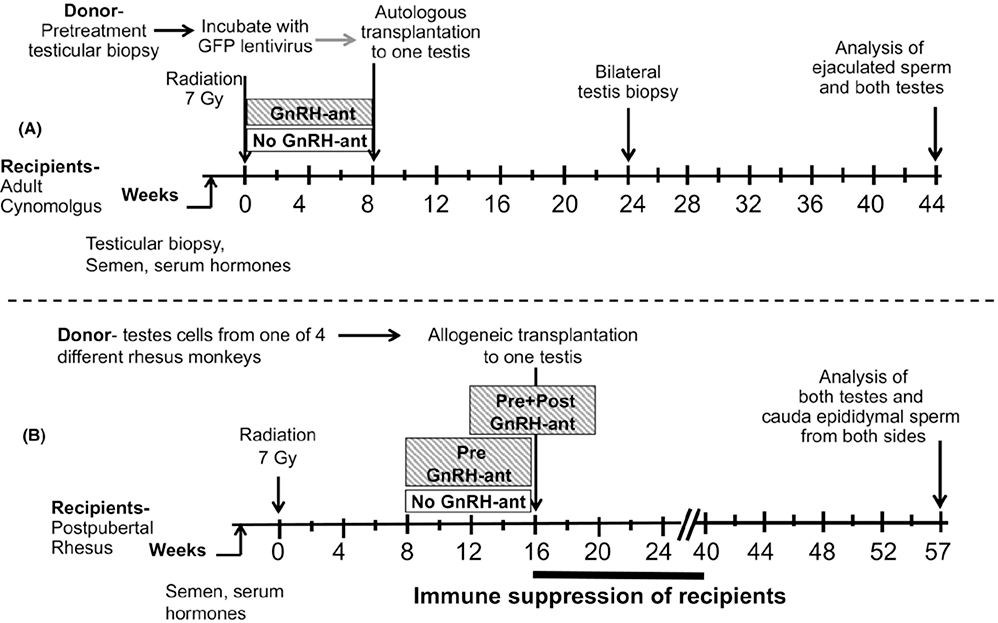
FIGURE 1.
Study design. A, Design of previous study20 to illustrate how the current study will differ. B, Design of the current study. The monkeys were evaluated before treatment and periodically after exposure to radiation, hormone suppression, and transplantation. Evaluation included sampling of serum and semen and measurements of testis volume. Starting 8 or 12 weeks after testicular irradiation, two groups underwent GnRH antagonist–mediated hormone suppression. At 16 weeks after irradiation, they received allogeneic transplantation of cryopreserved testis tubular cells into one testis, followed by 6 months of immune suppression
2 |. MATERIALS AND METHODS
2.1 |. Animals
Fifteen male rhesus monkeys (Macaca mulatta) were purchased from Michale E. Keeling Center for Comparative Medicine and Research, MD Anderson Cancer Center, Bastrop, Texas, as recipient monkeys for testicular cell transplantation to the testis. They were peripubertal (age: 42–59 months) at the time of purchase and were housed in pairs at the MD Anderson Cancer Center, Houston, Texas, in steel cages with a sliding panel between two adjacent compartments to allow social interaction with another companion of the same study group. The animals were fed Harlan Tekland Primate diet #7195 with daily enrichment foods, such as seeds, peanuts, fruits, and vegetables; the environment was maintained at a constant temperature (75°F-80°F) and humidity (40%−55%) with a 12-hour light/12-hour dark cycle.
Four unrelated 45- to 46-month-old rhesus monkeys, maintained in the animal facility of Magee-Womens Research Institute, were used as donors of testicular cells. All animal care and treatment protocols were approved by the Institutional Animal Care and Use Committees of MD Anderson Cancer Center and Magee-Womens Research Institute.
2.2 |. Experimental design
After we confirmed that the recipient monkeys had reached puberty, based on the individual testis volume being ≥ 5 cm3 and serum testosterone levels of ≥1.5 ng/ml,21 they were given 7 Gy of testicular irradiation (Figure 1B). Five of these irradiated monkeys were treated with GnRH-ant for 8 weeks, starting 8 weeks after irradiation until the time of transplantation (pre–GnRH-ant), and five others were treated with GnRH-ant starting 12 weeks after irradiation, so that the treatment lasted from 4 weeks before to 4 weeks after transplantation (pre + post–GnRH-ant). Five control monkeys (no GnRH-ant) were sham-injected with bacteriostatic water. At 16 weeks after irradiation, all monkeys received allogeneic transplantation of cryopreserved testicular cells from one of the four donor monkeys into the seminiferous tubules of one testis. All the recipients were then given anti-CD154 for immune suppression for 24 weeks. The testes and epididymis were harvested 41 weeks after transplantation for analyses.
2.3 |. General surgical and post-surgical procedures
For spermatogonial transplantation, the monkeys were first sedated with an IM injection of ketamine (10–25 mg/kg) and then anesthetized with 1%−3% isoflurane in oxygen. Before all surgical manipulations, 2% lidocaine (IM) was instilled into the surgical site to provide local anesthesia. All surgical procedures were performed under aseptic conditions. Each animal received an analgesic (buprenorphine, 0.01–0.03 mg/kg body weight) prior to and at the end of the day of surgery, and two times per day for up to 3 days as needed by appearance of the animal under constant monitoring. In addition, at the discretion of the Clinical Veterinarian, daily IM injections of Baytril antibiotic (5 mg/kg) were given for a week post-surgery.
2.4 |. Irradiation
For irradiation, the monkeys were anesthetized with ketamine and anesthesia was maintained with isoflurane. Testes were irradiated using a cobalt-60 gamma-irradiator20,22 with an 8 × 8 or a 10 × 10 cm field size, depending on the individual scrotal areas. Tissue-equivalent bolus material (5 mm thick) was placed over the scrotum to provide a build-up layer. Both posteroanterior and anteroposterior positions were used, and half of the dose was given in each position. The monkeys were irradiated at a total calculated dose of 7 Gy at a rate of 108 cGy/min, with a source-to-skin distance of 80.0 cm measured to the bolus. The 7-Gy dose was previously shown in cynomolgus macaques to effectively eliminate nearly all of the germ cells including most of the SSCs.20
2.5 |. GnRH antagonist treatment
The GnRH-ant Acyline was obtained from the Contraceptive Development Program of the NICHD, Rockville, MD, USA. Stock solutions of Acyline (2 mg/ml) in 5% aqueous mannitol were prepared and stored at 4°C for a maximum of 1 week. The monkeys were given twice-weekly subcutaneous injections of Acyline on Mondays and Thursdays at doses of 200 μg/kg and 300 μg/kg, respectively.20,22
2.6 |. Semen and Blood collection
Semen was obtained from anesthetized monkeys by electro-ejaculation using a rectal probe (Beltron Instruments, Longmont, CO, USA), as described previously.20 The sample was allowed to liquefy at 37°C for an hour before spermatozoa were counted in the exudate using a hemocytometer. Sperm counts were expressed per total ejaculate (volume of exudate plus remaining coagulum).
Blood (5–10 ml) was drawn by venipuncture of the saphenous vein with the animal under ketamine/1%−3% isoflurane sedation. Serum was separated and stored at −20°C.
In general, blood and semen sampling was done at monthly intervals with the following exceptions. Prior to irradiation, blood was often drawn every 2 weeks to carefully assess pubertal development. After irradiation, semen collection was done only once (at 8 weeks), to assess the effects of radiation on sperm production; semen collection was resumed at 8 weeks after transplantation. Blood was drawn more frequently during and immediately after GnRH-ant treatment, to assess the effects of that treatment on hormone levels.
2.7 |. Testicular measurements
Testis volume was determined by measuring the length and width of each testis within the scrotum of anesthetized monkey with calipers and modeling the testis as a prolate ellipsoid, applying the following formula: testis volume = π × width2 × length/6.
2.8 |. Preparation of testis cells for transplantation
Cells were recovered from the testicular parenchyma of four rhesus monkeys (Table S1) at Magee-Womens Research Institute as done previously.23,24 Castrated testes were cut into small pieces, which were digested with collagenase type IV (1 mg/ml; Sigma) in Hanks balanced salt solution (HBSS; Invitrogen) for 5–10 minutes at 37°C. The seminiferous tubules were sedimented by centrifugation at 100 g and washed in HBSS to remove interstitial cells. The tubular tissue was further digested with trypsin (2.0 mg/ml trypsin plus 1 mM EDTA; Invitrogen) and DNase I (1.4 mg/ml; Sigma) in HBSS for 10–15 minutes at 37°C. Digestions were quenched with 10% fetal bovine serum (FBS) and filtered through a 70-μm nylon mesh. Cells were pelleted by centrifugation at 600 g and resuspended in minimum essential medium alpha (MEMα; Invitrogen) containing 10% FBS. Three of the monkeys had spermatozoa in the suspension and were designated as pubertal; one monkey had no spermatozoa and also had an average testis weight of only 1.2 g and thus was designated as pre-pubertal. The cells were resuspended at 40 × 106 per milliliter in medium (MEMα + 10% FBS) and aliquoted in cryovials, and an equal volume of freezing medium (MEMα + 20% FBS + 20% dimethyl sulfoxide [DMSO]) was added dropwise. These vials containing cells at a concentration of 20 × 106 per milliliter were frozen in −1°C/minute controlled-rate freezing containers (Nunc, Rochester, NY), transferred to −80°C, and then stored in liquid nitrogen. Cryopreserved cells were shipped on liquid nitrogen to Houston where they were prepared for transplantation as in our previous study.20 To minimize potential variability in the transplantation outcome due to the origin of donor cells, cells from the three pubertal donors were uniformly assigned to recipient monkeys among all the three groups (Table S1). The cells from the pre-pubertal monkey were assigned to one of the sham-control recipients. Xenotransplantation of the testis cell suspensions prepared from each donor monkey into nude mice recipients23 revealed that the number of colonies formed was not significantly different between the donor samples.
2.9 |. Allogeneic transplantation
Transplantation of cells into one testis was done essentially as described previously.16,20 Briefly, cells were suspended at 54–140 × 106 viable cells/ml in MEMα containing 10% FBS, 0.4 mg trypan blue/ml, 20% (v/v) Optison ultrasound contrast agent (GE Healthcare, Waukesha, WI), 1% antibiotic-antimycotic (a combination of penicillin, streptomycin, and amphotericin B; Gibco), and 0.1 mg DNase I/ml in a total volume of as much as 1 ml. The cells were transplanted via ultrasound-guided injections into the rete testis. A 13-MHz linear superficial probe and a MicroMaxx ultrasound machine (Sonosite, Bothell, WA) were used to visualize the rete testis space and to guide a 25-gauge, 2” spinal needle into the space. Cells were injected under slow constant pressure and chased with saline solution. Approximately 45 × 106 cells were injected into the testis (Table 1, Table S1). The contralateral testis was sham-transplanted at the same time by injection of the suspension medium with all constituents except the cells. A transplantation efficiency score was recorded for each transplantation, based on an estimate of the percentage of circumference of tubules, going outward from the rete testis, that were filled by donor cell suspension, as visualized by ultrasound. The scores were as follows: 5 = >80%; 4 = 60%−80%; 3 = 40%−60%; 2 = 20%−40%; and 1 = <20%.
TABLE 1.
Average baseline recipient monkey characteristics at time of irradiation and donor cell transplantation data for the three different GnRH antagonist treatment groups
| GnRH-antagonist injection | Age (months) | Serum T (ng/ml)b | Average testis volume (ml)b | Viable cells injected (millions) | Viability (%) | Transplant efficiency score(1–5) |
|---|---|---|---|---|---|---|
| No GnRH-anta | 54 ± 3 | 2.7 ± 0.4 | 16 ± 4 | 54 ± 8 | 73 ± 2 | 3.4 ± 0.5 |
| Pre-transplantationa | 58 ± 3 | 2.0 ± 0.1 | 18 ± 3 | 39 ± 6 | 77 ± 2 | 3.2 ± 0.7 |
| Pre- and post-transplantationa | 59 ± 2 | 2.3 ± 0.4 | 17 ± 3 | 36 ± 3 | 73 ± 4 | 2.6 ± 0.8 |
No differences were observed between GnRH treatment groups in the values of any of the parameters (ANOVA, P > .05).
Average of last two measurements.
To prevent T cell–mediated rejection of the transplanted cells, the recipient was treated with human/mouse chimeric anti-CD154 IgG 5C8 (NIH Nonhuman Primate Reagent Resource, University of Massachusetts Medical School, Boston, MA) at 20 mg/kg on days −1, 0, 3, 10, 18, 28, and monthly thereafter for additional 5 months.25
2.10 |. Hormone assays
Testosterone was assayed using a radioimmunoassay (RIA) kit KIR1709 (Immuno-Biological Laboratories America, Minneapolis, MN). We used our own testosterone standard for the assay that was diluted in the zero standard provided in the kit.26 The detection limit of the assay is 0.05 ng/ml. The intra- and inter-assay coefficients of variation were 5% and 16%, respectively.
Circulating concentrations of FSH and luteinizing hormone (LH) were determined by RIA at the Endocrine Technologies Support Core, Oregon National Primate Research Center, Beaverton. The sensitivities of both the FSH and LH assays were 0.05 ng/ml with 100 μl samples. The intra-assay coefficients of variation were 12.5% and 8.2%, respectively, for FSH and LH.
2.11 |. Histological and Immunohistochemical procedures
The monkey testis tissue was fixed in Bouin solution and embedded in paraffin, and sections were stained with periodic acid-Schiff reagent and hematoxylin. At least three sections, chosen from different regions, were assessed by systematic scanning across the entire section and a minimum of 2469 tubules were scored per testis. The recovery of germ cells was evaluated by calculating the tubule differentiation index (TDI), which represents the percentage of seminiferous tubule cross sections containing at least one differentiated germ cell type (B spermatogonia or later stages). In addition, the extent of the progression of germ cell differentiation was assessed by determining the percentages of tubules with germ cells that contained spermatocytes, round spermatids, or elongating/elongated spermatids as the latest germ cell type present.
For fluorescent immunostaining, paraffin sections of testes, fixed in 4% paraformaldehyde, were used. After deparaffinization and antigen retrieval,22 sections were stained with the following primary antibodies. Goat anti-VASA (1:200; R&D Systems, AF2030) marks all germ cells.27 Rabbit anti-acrosin (1:300; Novus, NBP1–85407) marks the acrosomes of spermatids.26 Sections were counterstained with DAPI (Vector Laboratories). Positive immunoreactivity was validated by omission of the primary antibody.
2.12 |. Epididymal sperm isolation
The cauda epididymis from each side was minced thoroughly in 1 ml PBS with 0.1% glucose (PBSG). Five ml of PBSG was added, and the suspension was filtered through a 100-μm cell strainer into a 50-ml tube, and the strainer was washed with an additional 3 ml of PBSG. The spermatozoa were counted using a hemocytometer and then isolated from somatic cells using Percoll density gradient centrifugation as follows. The cell suspension containing 13.5% Percoll was carefully layered on top of 4 ml of 31.5% Percoll in a 10-ml tube and centrifuged (912 g, 20 min) at room temperature, with the brake on low. The supernatant was aspirated carefully, and the pellet was resuspended in 5 ml PBS. After a second count, the spermatozoa were centrifuged again (2800 g, 5 min) and the pellet was frozen at −80°C and stored at that temperature. At the time of preparation, we had only planned to use the spermatozoa for microsatellite analysis; later, we decided to perform intracytoplasmic sperm injections (ICSI) to test their fertilization potential, even though they were frozen without any cryopreservative in the medium.
2.13 |. Preparation of DNA from the spermatozoa, blood, and testicular tissues
To genotype the recipients’ microsatellite loci, genomic DNA was prepared from non-coagulated blood using the DNeasy Blood & Tissue Kit from Qiagen (Cat No.: 69504). To genotype the donor, DNA, from 2-mm3 pieces of testicular tissues, was extracted with High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH).22
To extract DNA from spermatozoa, the pellets suspended in saline sodium citrate buffer were treated with 0.2% sodium dodecyl sulfate (SDS) to lyse remaining non-sperm cells. The sperm sample was digested using the lysis solution from the Gentra Puregene Kit (Qiagen, Germantown, MD; Cat. No. 158906), with the addition of proteinase K and dithiothreitol (DTT) to final concentrations of 2 mg/ml and 10 mM, respectively. The protein was removed using the Qiagen precipitation solution (Cat. No. 158910), and the DNA in the supernatant was precipitated using cold isopropanol. The pellet was resuspended in water by heating for 75 minutes at 55°C.
2.14 |. DNA microsatellite analysis
Genomic DNA was used for microsatellite repeat fingerprinting as done previously.22 The donors and recipients were initially tested with a panel of 29 microsatellites; of these, D7S513, D7S794, D11S925, D3S1768, D4S2365, D5S1457, D13S765, D17S1300, D2S1333, D8S1106, and D15S823 proved informative for distinguishing recipient and donor tissue. Microsatellites were amplified, and the PCR products were separated by capillary electrophoresis on ABI 3730 DNA Analyzer (Applied Biosystems). To avoid cross-contamination, all steps in genotyping the post-transplant epididymal sperm DNA samples were performed separately from the genotyping of donor and recipient animal DNA samples. Fragment size analysis and genotyping were done with the computer software STRand. The contribution of the donor spermatozoa in the post-transplant epididymal DNA sample was calculated from the peak heights of the donor- and recipient-specific alleles, which reflect the relative fluorescence unit (RFU) measurements for each allele. To determine parental origin and sex of ICSI embryos, genotyping was done as above except that before PCR the cells were put through the whole-genome amplification (WGA) process using the REPLI-g kit, which contains reagents and primers that will replicate most of the cell genome, producing sufficient DNA for testing: https://www.qiagen.com/us/service-and-support/learning-hub/technologies-and-research-topics/wga/replig-principal-procedure/. The full panel of 29 microsatellites was used to determine the parental origin of the embryos. To determine the gender of the embryos, the primers 5′-CCCTGGGGCTCTGTAAAGAATAGTG-3′ and 5′-ATCAGAGCTTAAACTGGGAAGCTG-3′ were used to amplify sequences from the amelogenin gene which differ on the X and Y chromosomes.
2.15 |. Intracytoplasmic sperm injection
Controlled ovarian stimulation was performed on two female rhesus macaques as previously described.28,29 Oocytes were collected and fertilized with spermatozoa by ICSI, and resulting embryos were cultured as described.30–32 Additional details are provided in the Supporting Methods. Following ICSI and in vitro development, individual embryos were frozen and sent from the Oregon National Primate Research Center to the Veterinary Genetics Laboratory, University of California, Davis, for microsatellite analysis.
2.16 |. Statistical analysis
The serum FSH levels, testis volume, and testis weights are presented as arithmetic mean ± SEM. The serum testosterone, LH levels, epididymal sperm numbers, and TDI were represented as means ± SEM calculated from log-transformed values. When only two groups were being compared (transplanted and sham-transplanted testes within a given treatment group), the significance of differences between treatments was evaluated by the paired-samples t test and, when the distributions deviated from a normal distribution (Shapiro-Wilk test), also by the non-parametric Wilcoxon matched-pair signed-rank test. To analyze the relative increase in the endpoints after transplantation (transplant/sham transplant ratio), one-sample t test and one-sample Wilcoxon matched-pair signed-rank test were used. When multiple treatment groups were being compared, a one-way analysis of variance (ANOVA) test was performed to test whether there were significant differences between the groups (ANOVA P < .05) and then individual groups were compared using a post hoc Tukey test. Again, when the distributions deviated from normal, the Kruskal-Wallis one-way ANOVA tests were used to test whether there were significant differences between groups (P < .05), and then, individual groups were compared by Mann-Whitney tests. All significance values were based on 2-tailed calculations. Analyses were performed with the IBM SPSS (version 23) statistical package.
3 |. RESULTS
3.1 |. Serum and semen data
We used the experimental design shown in Figure 1B to determine the benefits of spermatogonial transplantation alone and adding transient hormone suppression treatment given either before spermatogonial transplantation or both before and after transplantation. Early post-pubertal monkeys, aged 47–63 months, with testis volumes of 5.9–27.3 cm3, and testosterone levels of 1.5–4.1 ng/ml (Table S1), were used as recipients. Spermatozoa were observed in the ejaculates of 9 out of the 10 monkeys that provided adequate ejaculates; the remaining 5 failed to give ejaculates (Table S2). The monkeys were assigned to the three treatment groups so that the distributions of ages, testes sizes, and testosterone levels were similar in the different groups (Table 1). One group of five monkeys were treated with a GnRH-ant for 8 weeks just prior to the time of transplantation (pre–GnRH-ant), and another group of 5 monkeys were treated both before and after transplantation (pre + post–GnRH-ant) for periods of 4 weeks each (Figure 1B). A group of five irradiated monkeys receiving no GnRH-ant served as controls and were transplanted at the same time as the other two groups.
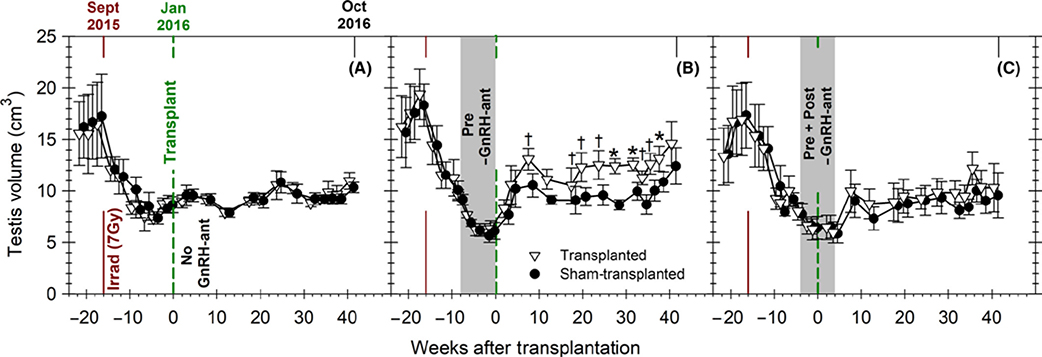
As expected from our previous study with cynomolgus monkeys,20 serum FSH, LH, and testosterone levels were markedly suppressed during GnRH-ant treatment, and when the treatment was stopped, they reverted to normal levels for irradiated monkeys (Figure S1). The reductions in testes volume were consistent with the loss of germ cells due to radiation and hormone suppression, and somatic cell hypotrophy due to hormone suppression33 (Figure 2). In monkeys treated with GnRH-ant before the transplantation, but not in the other two groups, the average of volume of the transplanted testes was greater than that of the contralateral testis at 10 weeks after transplantation, but remained consistently greater only 20 weeks after transplantation.
FIGURE 2.
Changes in testis volume during the study. Testis volume in groups of monkeys that were irradiated and received testicular germ cell transplantation. The monkeys received (A) no GnRH-ant treatment (n = 5), (B) GnRH-ant treatment before transplantation (n = 5), and (C) GnRH-ant treatment both before and after transplantation (n = 5). The continuous dark red and the dashed green lines represent the times of the irradiation and transplantation, respectively. The gray shaded area in B and C represents the duration of the GnRH-ant treatment. Declines in testis volumes due to irradiation and additionally due to GnRH-ant treatment were observed. Differences between the sizes of testes between the transplanted (∇) and sham-transplanted (•) were detected beginning 10 weeks after transplantation only in the group treated with GnRH-ant prior to transplantation. Comparison of differences between the transplanted vs sham-transplanted testis volume (paired-samples t test: *P < .01; †P < .05)
Although the ability to obtain semen ejaculates was not consistent in these monkeys (Table S2), likely due to the seasonality observed in these monkeys and also the use of anesthesia might make the procedure less efficient, some trends could be observed. Whereas the yield of spermatozoa was 0.85 million (average of log-transformed data) per non-azoospermic ejaculate before irradiation, it was only 0.10 million at 7 weeks after irradiation and at 24–28 weeks after irradiation, all samples were azoospermic (Table S2). Between 36 and 54 weeks after irradiation (20–38 weeks after transplantation), some monkeys (about 4/15 at each time point) showed the presence of a few spermatozoa in the ejaculate. During this interval, only one monkey had a count above 1 million (2.7 million) at one time point and the average yield of spermatozoa was only 1200 per non-azoospermic ejaculate.
3.2 |. Tissue harvest results
At the end of the study, 41 weeks after transplantation, tissues were harvested, testis weights and epididymal sperm counts were assessed, and testicular histology was analyzed. Histology revealed that the somatic cell structure of the testis was unaffected by irradiation, and we did not observe any indications of inflammatory reactions. However, most of the tubule cross sections were Sertoli cell–only with the complete absence of germ cells (Figure S2A,B,B′”). In both the transplanted and sham-transplanted testes, a small percentage of tubules showed differentiating germ cells (Figure S2A,C,D), which was quantified as the tubule differentiation index (TDI). In the differentiating tubules, spermatogenesis usually proceeded to the spermatid stages, as confirmed with immunofluorescent staining for VASA and acrosin (Figure S3). Round and elongating spermatids were observed in 90% and 76% of differentiating tubules, respectively (Table S3); these percentages were independent of the hormonal treatment or whether the testes were transplanted or sham-transplanted. Spermatozoa were observed in all epididymides examined, and about 10% showed non-progressive motility.
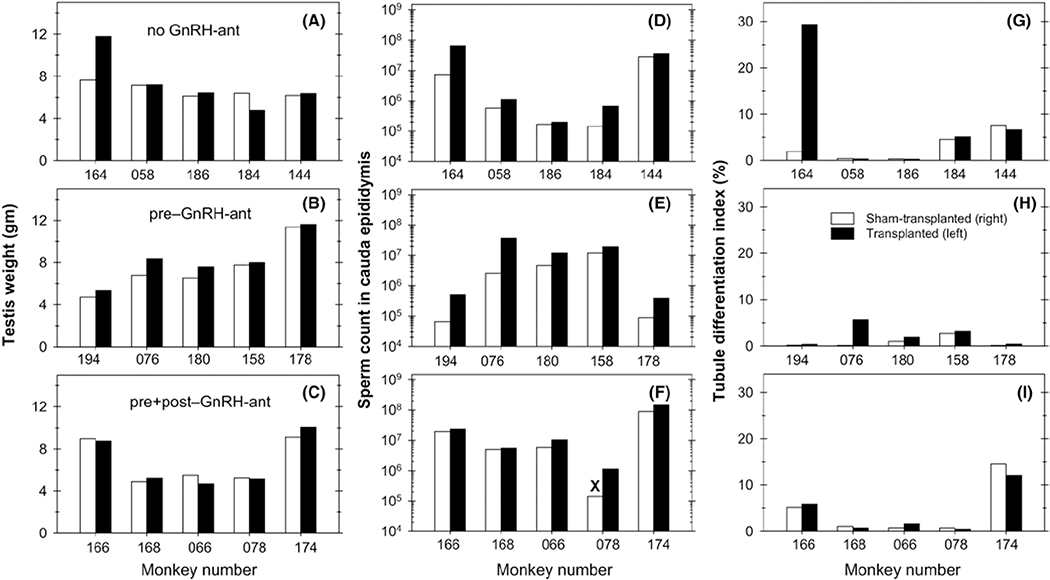
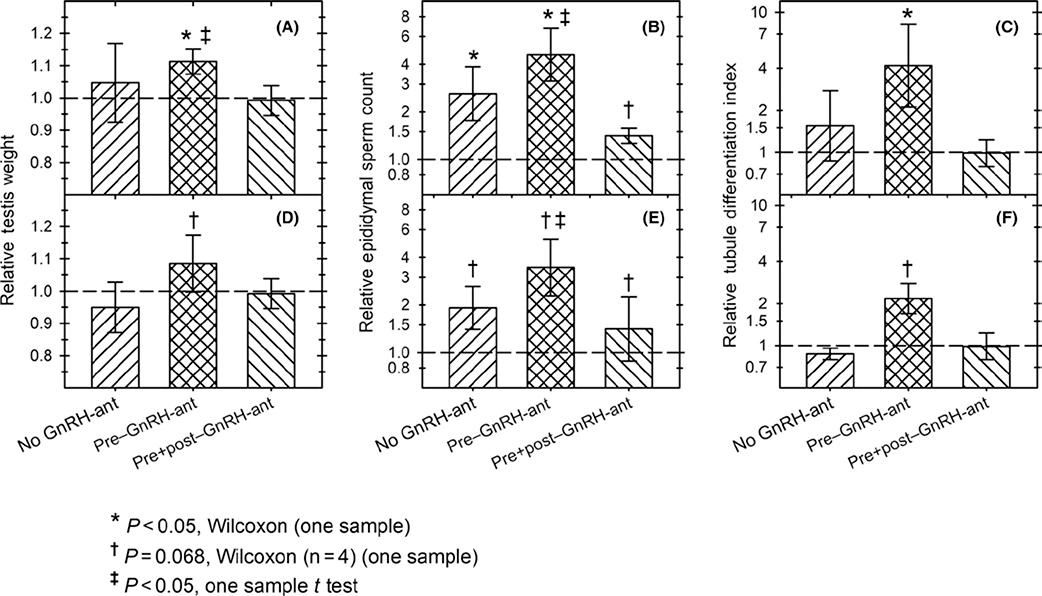
The testis weights, sperm counts, and TDI values obtained in individual animals are presented in Figure 3. For all three endpoints, there was a trend toward higher values in the transplanted testes or the epididymal side corresponding to the transplanted testis than in the corresponding sham-transplanted testis side. When the data from all 15 monkeys were pooled, the sperm counts in the transplanted side were consistently higher (P < .001, Wilcoxon) than those on the non-transplanted side (Figure 4); the increases in testis weights and TDI did not reach statistical significance (P ~ 0.1–0.2). Statistical analyses of the three treatment groups separately, confirmed that for the pre–GnRH-ant treatment group, testis weights, sperm counts, and TDI showed significantly greater increases in the transplanted vs the sham-transplanted side (P < .05, Wilcoxon test) (Figure 5A-C). In the other treatment groups, this difference was only significant for the sperm count endpoint for the no GnRH-ant group (Figure 5B). Non-parametric analysis of variance showed that the differences in increase in TDI in different treatment groups (Figure 5C) were very close to statistical significance (P = .059, Kruskal-Wallis). The higher recovery in the pre–GnRH-ant group was significantly different from the pre + post–GnRH-ant group (P = .03, Mann-Whitney), but only differed from the no GnRH-ant control group at P = .08 (Figure 5C). These results suggest that GnRH-ant given before transplantation, but not after transplantation, may be beneficial for subsequent spermatogenic recovery.
FIGURE 3.
Spermatogenic endpoints in individual monkeys. Data for testis weights (A-C), number of spermatozoa in the cauda epididymis (D-F), and tubule differentiation indices (G-I) in the transplanted (solid bars) and sham-transplanted testes (open bars) and the corresponding epididymides for individual monkeys in the three experimental groups: (A, D, G) no GnRH-ant–treated; (B, E, H) pre-GnRH-ant–treated; and (C, F, I) pre + post-GnRH-ant–treated monkeys. “X” indicates that the value is only a lower limit as part of the sample was spilled
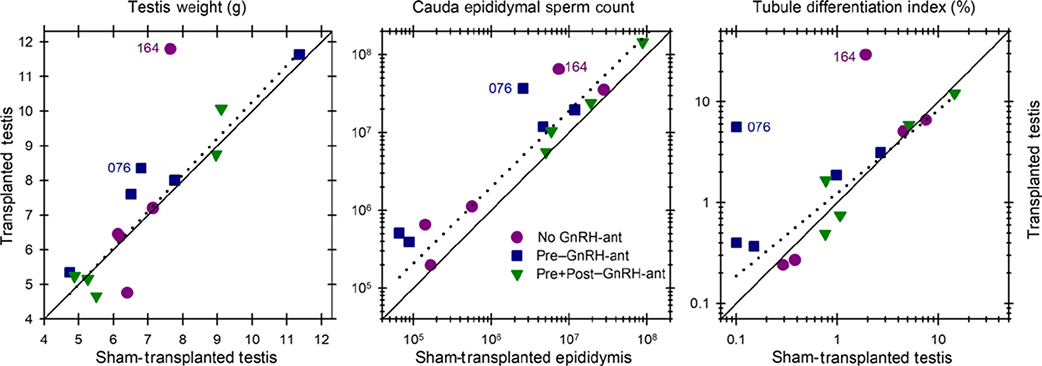
FIGURE 4.
The correlations of spermatogenic endpoints between the transplanted and sham-transplanted testes. The correlations are shown for (A) testes weights, (B) cauda epididymal sperm count, and (C) tubule differentiation indices. The diagonal continuous line represents equivalent response on the two sides. The correlations calculated on the 13 monkeys that did not show appreciable numbers of donor spermatozoa are shown by the dashed lines. Note that these 13 animals without appreciable levels of donor-derived spermatogenesis show a trend toward higher values in the transplanted side than in the sham-transplanted side. Only the differences between the transplanted vs sham-transplanted sides based on the sperm count data from the 13 monkeys were significant (P = .002, Wilcoxon test)
FIGURE 5.
Effect of transplantation on spermatogenic endpoints. Ratio of the values for the transplanted side to the sham-transplanted side for (A, D) testis weights, (B, E) epididymal sperm numbers, and (C, F) tubule differentiation indices (TDI) at the time of harvest, in groups of irradiated monkeys without GnRH-ant treatment or with two different GnRH-ant treatments. Panels A-C show calculations based on all 15 monkeys; values in D-F were calculated on 13 monkeys after excluding the two monkeys that had appreciable donor-derived spermatozoa (#164 from no GnRH-ant and #076 from pre–GnRH-ant group) in order to show effects of transplantation on endogenous spermatogenesis. The relative epididymal sperm counts and TDI are plotted using the log-transformed data. A-C: n = 5 in all groups except in B, n = 4 for sperm count of the pre + post–GnRH-ant group; D-F: n = 4 for the no GnRH-ant and pre–GnRH-ant groups and for the pre + post–GnRH-ant group in E; n = 5 for the pre + post–GnRH-ant group in D and F. In all cases, statistics were computed in one-sample tests comparing the ratios to “1.” *P < .05, Wilcoxon test; †P = .068, Wilcoxon test; ‡P < .05, t test
Although there was variability in all three endpoints, this was due to the differences in the degree of response to irradiation and/or recovery after irradiation, as it was observed even in the sham-transplanted testes. It was clear that the response of the two testes was correlated as most of the points fell close to the diagonal line representing equivalent response (Figure 4). It was striking that for all three endpoints, the same two animals (#164 and #076, which belonged to the no GnRH-ant and pre–GnRH-ant group, respectively) showed much higher levels of spermatogenesis in the transplanted vs. the sham-transplanted testis. As will be shown below, in these two animals nearly all of the spermatozoa produced were derived from the donor cells, whereas all of the others produced either no donor spermatozoa or about 1% donor-derived spermatozoa. The correlations between transplanted and sham-transplanted testes, calculated on the 13 monkeys that did not show appreciable numbers of donor spermatozoa, are shown by the dashed lines, and the correlation coefficients, r2, were all about 0.9. The general trend for these 13 animals without appreciable levels of donor-derived spermatogenesis was to show higher values in the transplanted side than in the non-transplanted side. This was highly significant (P = .002, Wilcoxon) for sperm counts (Figure 4B). When the treatment groups were analyzed separately (Figure 5D-F), the sperm count ratio between the transplanted and sham-transplanted sides approached statistically significant differences (P = .068, Wilcoxon) in all three groups (Figure 5E). Furthermore, for the pre–GnRH-ant group, sperm counts were shown to be significantly higher in the transplanted side (P = .02, t test), and the increases in testis weights and TDI (Figure 5F) approached significance (P = .068, Wilcoxon). In addition, the increase in TDI in the pre–GnRH-ant treatment group resulting from transplantation was significantly greater than that in the no GnRH-ant group (P < .05, Kruskal-Wallis and post hoc Mann-Whitney). As these monkeys did not show appreciable donor spermatozoa, these results suggest that there might be an effect of transplantation of donor cells on the ability of endogenous spermatogenesis to recover, which may be enhanced by hormone suppression prior to transplantation.
3.3 |. Microsatellite analysis of donor spermatogenesis
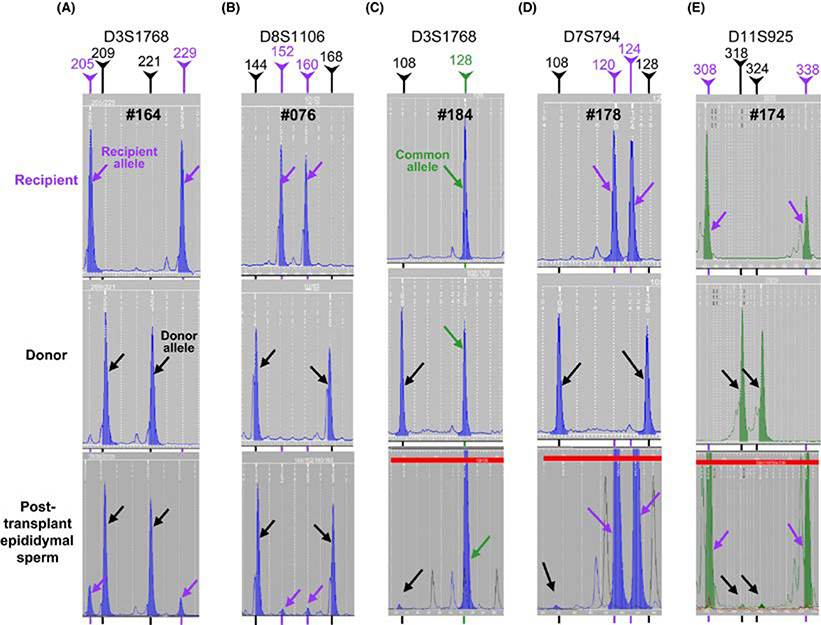
We then evaluated the presence of donor spermatozoa in the cauda epididymis corresponding to the transplanted testis in all the monkeys by microsatellite analysis. The two recipients that showed significantly enhanced spermatogenesis in the transplanted versus the sham-transplanted side, #164 and #076, had very high contributions of spermatozoa derived from the donor SSCs, 84% and 93%, respectively (Figure 6A,B). The donor contributions in these monkeys were confirmed by analyzing the peaks of the alleles unique to the donor-recipient pairs for multiple loci (Figure S4 and Table S4), which also averages for any variations in PCR amplification efficiency between alleles. As observed earlier,22 the proportions of donor- and recipient-specific allele peaks in these chimeric samples using different microsatellite loci were very similar (Table S4) confirming that the allele peak heights truly represent the percentages of cells with donor and recipient genotypes. In addition to the above two animals, small donor peaks averaging 1.7%, 0.4%, and 1.0% of the DNA in the samples were observed in the sperm samples from additional no GnRH-ant–treated (#184), pre-GnRH-ant–treated (#178), and a pre + post-GnRH-ant–treated (#174) monkeys, respectively (Figure 6C-E). These percentages were low, but they were confirmed with several different microsatellite loci (Figure S5 and Table S5). Although there were 1.6- to 4-fold increases in the epididymal spermatozoa yield in the transplanted side compared with the sham-transplanted side in these three monkeys (Figure 3D-F), the small percentages of donor spermatozoa cannot account for such increases. Sperm samples from the cauda of the transplanted side in the other 10 monkeys and from the cauda corresponding to the side of the sham-transplanted testis in the monkeys tested did not show any evidence of donor DNA (Table S4).
FIGURE 6.
Microsatellite analyses of the spermatozoa retrieved from the cauda epididymis corresponding to the transplanted testis from the five recipient monkeys with donor spermatozoa (lower panels). Tissues from the recipient (blood) and the donor (testis) were also analyzed (upper panels) to identify the source of the alleles observed in the sperm. For each monkey, the profiles from one representative locus are displayed. Note the unique donor (black lines and arrows) and recipient (purple lines and arrows) alleles as well as alleles common to both the donor and the recipient (green line and arrows). The relative peak heights of these alleles were used to calculate the donor contribution in the sperm sample (Table S5). Monkeys #164 and #076 produced 84% and 93% donor spermatozoa, respectively, and the remaining 3 monkeys (#184, #178, and #174) had about 1% donor spermatozoa
3.4 |. Intracytoplasmic sperm injection
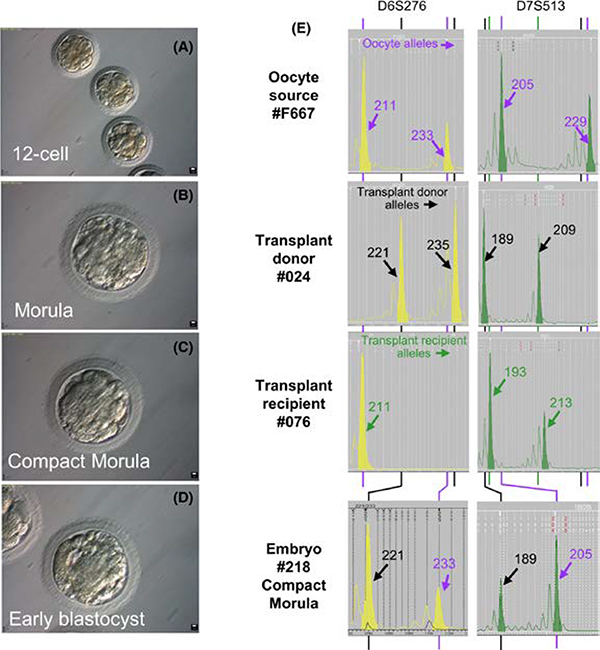
To test whether the spermatozoa derived from the donor could be functionally normal with the capacity to fertilize the eggs and produce embryos, we injected these spermatozoa into oocytes retrieved from rhesus macaques. From two females, 46 oocytes were injected with spermatozoa from recipient monkey #076, which had 93% donor-derived spermatozoa in the epididymis, and 11 of these oocytes were successfully fertilized (Table 2). After culture for 7 days, one embryo reached blastula stage, as expected for healthy embryos, six embryos reached morula, and four were arrested at about a 12-cell stage (Figure 7A-D, Table 2). Microsatellite analysis was successful for eight of these embryos and revealed that the paternal source for five of them was monkey #024 (Figure 7E), whose testicular cells were used as donor cells for transplantation. Although there was variability in the ratios of the peak height of the sperm allele to that of the oocyte-derived allele because of the whole-genome amplification, the average of the ratios was 0.90 ± 0.37, indicating that the sperm DNA replicated after fertilization and fully contributed to the embryo. However, the remaining three embryos developed as a result of parthenogenesis, as all informative alleles were hemizygous and were derived from the egg donor (Figure S6). All three parthenotes appeared to arrest at day 6 at the morula stage or earlier, whereas 2 of the 5 embryos with male contribution progressed to the compact morula stage at day 7. In addition, we fertilized three oocytes by ICSI with spermatozoa from #164, two of which reached compact morula stage after culture for 7 days. These compact morulae were not genotyped, but were transferred to a surrogate female, which did not result in pregnancy.
TABLE 2.
Developmental stage and genotype of cultured embryos 7 days after ICSI with spermatozoa from recipient monkey #076 (93% donor-derived spermatozoa from donor #024)
| Egg donor number | Oocytes injected with sperm | Zygotes formed | Embryo number | Stage of development (day 7) | Genotyping result |
|---|---|---|---|---|---|
| F296 | 36 | 6 | 212 | Early blastocyst | Faileda |
| 214 | Morula | Parthenote | |||
| 216 | Morula | Parthenote | |||
| 217 | Morula | XX donor | |||
| 215 | 12- to 16-cell | XY donor | |||
| 213 | 12-cell | Faileda | |||
| F667 | 10 | 5 | 218 | Compact morula | XY donor |
| 219 | Compact morula | XX donor | |||
| 220 | Compact Morula | Embryo lost | |||
| 221 | 12-cell | Parthenote | |||
| 222 | 12-cell | XY donor |
Initial assays done without whole-genome amplification.
FIGURE 7.
Embryos produced by ICSI from spermatozoa from a monkey with 93% of donor-derived spermatozoa. Epididymal spermatozoa corresponding to the transplanted testis of recipient #076 was used to fertilize rhesus oocytes by intracytoplasmic sperm injection (ICSI). The subsequent in vitro culture resulted in embryos at the 12-cell (A), morula (B), compact morula (C), and blastocyst (D) stages. (E) Microsatellite DNA fingerprinting of a representative embryo, and comparison with the oocyte, donor, and recipient profiles. Microsatellite loci are noted above each column of electropherograms, and the alleles specific for the oocyte donor (represented by purple font and arrow) transplant donor (represented by black font and arrow) and transplant recipient (represented by green font and arrow) are indicated on each electropherogram panel
Although we succeeded in producing embryos using the spermatozoa from both monkeys with donor-derived spermatozoa, the embryos, with one exception that reached early blastula, did not progress beyond the compact morula stage. We assume that these results are due to the improper preparation of the spermatozoa for ICSI, as they were frozen in PBS at an uncontrolled freezing rate and were stored at −80°C rather than in liquid nitrogen. Sperm analysis from #076 at the time of ICSI indeed revealed that they were immotile, with non-intact plasma membranes, and showed no mitochondrial activity. Also, it was likely that the freezing damage to the spermatozoa resulted in failure of the male nucleus to replicate yielding the three parthenotes.
4 |. DISCUSSION
The most striking result of the current study was the production of almost exclusively donor-derived spermatogenesis in the transplanted testes of two monkeys. In these monkeys, there were about 50 million spermatozoa in the cauda epididymis in the transplanted side and about 90% of them were donor-derived. Thus, in these monkeys the large increases in testis weight, tubule differentiation index, and sperm count on the transplanted vs. the sham-transplanted side (Figure 3) must be a result of spermatogenesis derived from donor SSCs. However, there were only 2/15 monkeys that showed this high level of recovery from the donor cells, and only 5/15 had any evidence of donor spermatozoa.
In a previous study with adult busulfan-treated rhesus monkeys,16 ejaculated spermatozoa from donor cells were detected in 2/6 recipients of allogeneic cells, whereas they were detected in 9/12 recipients of lentivirus-transfected autologous cells. However, the highest percentage of donor spermatozoa in those ejaculates, which could only be determined in the allogeneic transplants, was only 12%, in contrast to 93% in the current study. In studies with autologously transplanted irradiated monkeys, 2/5 irradiated adult cynomolgus monkeys34 and 1/6 pre-pubertal/pubertal rhesus monkeys35 showed appreciable increases in testis volumes and tubule differentiation indices in the testis receiving the transplants, indicating that the transplanted cells were responsible for this increase. In our previous study with irradiated adult cynomolgus monkeys transplanted with lentivirus-transfected autologous cells,20 6/12 showed detectable lentivirus-transfected donor germ cells or spermatozoa, two of which also showed major increases in tubule differentiation indices in the transplanted testis. Thus, the present study represents the highest percentage of transplant-derived spermatozoa; however, the frequency of success was similar or slightly lower than in previous studies.
It is possible that the low frequency of success is because, despite immune suppression treatment, allogeneic transplantation may not be as effective as autologous transplantation. A lower success rate with allogeneic transplantation was suggested previously,16 2/6 for allogeneic vs 9/12 for autologous, indicating a trend, but the difference was not statistically significant (P = .14, Fisher’s exact test). Nevertheless, we should consider the possibility of residual immunological surveillance or an unknown direct negative effect of the immunosuppression contributing to the low frequency of successful allogeneic transplants.
We next examined specific factors associated with the transplantation that may have accounted for the reasons that the two monkeys showed excellent colonization with donor cells, three showed low levels of colonization, and 10 showed no colonization. Several possible factors, including recipient characteristics, which donor cells were used, day of the transplantation, cells injected, and cell viability (Table S1), showed no correlations with success. However, we noted that of the three monkeys that had a transplant efficiency score of five, two showed excellent colonization and one showed low levels of colonization. Statistical analysis confirmed that the transplant efficiency score was indeed significantly correlated with success (P = .031, Spearman’s correlation).
In our previous study,20 hormone suppression with GnRH-ant clearly enhanced the spermatogenic recovery in the transplanted testes, as assessed by detection of donor spermatozoa in ejaculates or testes of 5 out of 6 of the GnRH-ant–treated as compared with only 1 of 6 of the monkeys not given GnRH-ant. In the current study, of the two monkeys showing robust donor spermatogenesis, only one was treated with GnRH-ant prior to transplantation and the other received no GnRH-ant. Furthermore, the three monkeys that showed about 1% donor spermatogenesis were evenly distributed among the three treatment groups.
Several possibilities exist to explain the apparent lack of enhancement of success by GnRH-ant treatment in the current study. The delay in starting the GnRH-ant treatment (Figure 1), which was started immediately after irradiation in the previous study, until 8 weeks after irradiation to allow depletion of endogenous spermatogenesis in the current study, is one possibility. The need for immunosuppression when transplanting allogeneic cells in the current study might also be a factor affecting the ability to stimulate recovery by hormone suppression. As androgens are known to dampen immune responses,36 GnRH-ant–induced androgen suppression should increase immune responses in males. Even though anti-CD154 treatment was started a day before, there might be some residual immune system activity at the time of transplantation. Any residual immunoactivity against allogeneic donor cells should be higher in the GnRH-suppressed animals and could offset any benefit the hormone suppression might have on colonization and recovery.
The use of allogeneic donor cells enabled us to quantify the percentage of donor spermatozoa in the epididymis and to identify the 13 animals in which spermatogenesis was 99% or 100% derived from endogenous stem cells. The testis weights, epididymal sperm counts, and TDI showed some statistically significant and some nearly significant enhancements in the transplanted testes as opposed to the sham-transplanted testes of pre-GnRH-ant–treated group and nearly significant enhancements of epididymal sperm count in all three treatment groups (Figure 5D-F). The enhancement of TDI was significantly greater in the pre–GnRH-ant group than the no GnRH-ant treatment group. Thus, we conclude that there is a trend for transplantation to enhance endogenous spermatogenesis and this may be particularly effective when hormone suppression with GnRH-ant is given just prior to transplantation.
Recent evidence has shown that transplantation of mesenchymal or endothelial cells into the testis interstitium or tubules, either before or 5 weeks after busulfan injection, can enhance the subsequent recovery of spermatogenesis from surviving endogenous stem cells.37–40 In the current study, a mixture of rhesus monkey testicular cells were injected into the irradiated testes, and it is likely that there are mesenchymal and endothelial cells present in the suspension, which might stimulate recovery of spermatogenesis from surviving endogenous SSCs. Further evidence that transplanted testicular cells can stimulate endogenous spermatogenesis is derived from our studies in rats41 and monkeys,22 in which transplanted germ cell suspensions that formed de novo tubular structures in the interstitium enhanced spermatogenic differentiation from endogenous surviving stem cells in adjoining tubules.
In conclusion, we have demonstrated that SSC transplantation can be used to restore spermatogenesis and sperm production after nearly complete loss of endogenous germ cells including SSCs by irradiation, but so far the success rate is low. GnRH-ant treatment around the time of transplantation did not significantly enhance donor-derived spermatogenic recovery and sperm production. Our results suggest that precise delivery of the cells into the rete and filling of the tubules is the major factor in achieving efficient recovery of spermatogenesis from the transplanted cells and might benefit by improved imaging techniques. Comparisons with other non-human primate studies indicated that higher success rates may be expected in the autologous transplants, as proposed in the clinical scenario, than in the allogeneic experimental models. Thus in the non-human primate model, the improvement in the ability to genetically mark the donor stem cells prior to autologous transplantation is most important. Additional non-human primate studies, particularly those involving donor cells from immature monkey testes, pre-pubertal irradiation of the recipients, and the in vitro expansion of SSC from the small amounts of donor material, comparable to that available from immature boys (median = 175 mg),8 are necessary before these methods are applicable to young male patients who have undergone cancer therapy and cryopreserved testicular cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by research grants P01 HD075795 from NIH/NICHD to KO and Cancer Center Support Grant P30 CA016672 from NIH/NCI to the University of Texas MD Anderson Cancer Center. We would like to acknowledge the outstanding work of Leisa Moore who assisted in managing the treatment of the monkeys, sample collections, and animal health issues and also Cathy Ramsey for her valuable assistance in the ovarian stimulation, embryo culture, and ICSI procedures. We sincerely thank Dr Min S. Lee, National Institute for Child Health and Human Development, for providing the Acyline.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: P01 HD075795; National Cancer Institute, Grant/Award Number: P30 CA016672
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Woodruff TK. The Oncofertility Consortium-addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015. MD: National Cancer Institute; Bethesda; 2018. [Google Scholar]
- 3.Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CIBMTR. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR Summary slides; 2018. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/Pages/index.aspx
- 5.Sanders JE, Buckner CD, Leonard JM, et al. Late effects on gonadal function of cyclophosphamide, total-body irradiation, and marrow transplantation. Transplantation. 1983;36:252–255. [DOI] [PubMed] [Google Scholar]
- 6.Kenney LB, Antal Z, Ginsberg JP, et al. Improving male reproductive health after childhood, adolescent, and young adult cancer: Progress and future directions for survivorship research. J Clin Oncol. 2018;36:2160–2168. [DOI] [PubMed] [Google Scholar]
- 7.Sadri-Ardekani H, Akhondi M-M, Vossough P, et al. Parental attitudes toward fertility preservation in boys with cancer: context of different risk levels of infertility and success rates of fertility restoration. Fertil Steril. 2013;99:796–802. [DOI] [PubMed] [Google Scholar]
- 8.Valli-Pulaski H, Peters KA, Gassei K, et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum Reprod. 2019;34:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassei K, Orwig KE. Experimental methods to preserve male fertility and treat male factor infertility. Fertil Steril. 2016;105:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picton HM, Wyns C, Anderson RA, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30:2463–2475. [DOI] [PubMed] [Google Scholar]
- 11.Giudice MG, de Michele F, Poels J, Vermeulen M, Wyns C. Update on fertility restoration from prepubertal spermatogonial stem cells: How far are we from clinical practice? Stem Cell Res. 2017;21:171–177. [DOI] [PubMed] [Google Scholar]
- 12.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Renfree MB, Short RV. Successful intra- and interspecific male germ cell transplantation in the rat. Biol Reprod. 2003;68:961–967. [DOI] [PubMed] [Google Scholar]
- 14.Ryu BY, Orwig KE, Avarbock MR, Brinster RL. Stem cell and niche development in the postnatal rat testis. Dev Biol. 2003;263:253–263. [DOI] [PubMed] [Google Scholar]
- 15.Honaramooz A, Behboodi E, Megee SO, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. [DOI] [PubMed] [Google Scholar]
- 16.Hermann B, Sukhwani M, Winkler F, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Shao SH, Weng CC, Wei C, Meistrich ML. Hormonal suppression restores fertility in irradiated mice from both endogenous and donor-derived stem spermatogonia. Toxicol Sci. 2010;117:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Shao S, Meistrich ML. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211:149–158. [DOI] [PubMed] [Google Scholar]
- 20.Shetty G, Uthamanthil RK, Zhou W, et al. Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys. Andrology. 2013;1:886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology. 1985;116:1341–1350. [DOI] [PubMed] [Google Scholar]
- 22.Shetty G, Mitchell JM, Lam TNA, et al. Donor spermatogenesis in de novo formed seminiferous tubules from transplanted testicular cells in rhesus monkey testis. Hum Reprod. 2018;33: 2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann BP, Sukhwani M, Lin C-C, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in Rhesus macaques. Hum Reprod. 2009;24:1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5: 686–693. [DOI] [PubMed] [Google Scholar]
- 26.Fayomi AP, Peters K, Sukhwani M, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci USA. 2000;97:9585–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. [DOI] [PubMed] [Google Scholar]
- 29.Bishop CV, Reiter TE, Erikson DW, et al. Chronically elevated androgen and/or consumption of a Western-style diet impairs oocyte quality and granulosa cell function in the nonhuman primate periovulatory follicle. J Assist Reprod Genet. 2019;36:1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewitson L, Dominko T, Takahashi D, et al. Unique checkpoints during the first cell cycle of fertilization after intracytoplasmic sperm injection in rhesus monkeys. Nat Med. 1999;5:431–433. [DOI] [PubMed] [Google Scholar]
- 31.Mitalipov S, Kuo H-C, Byrne J, et al. Isolation and characterization of novel rhesus monkey embryonic stem cell lines. Stem Cells. 2006;24:2177–2186. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey C, Hanna C. In vitro culture of rhesus macaque (Macaca mulatta) embryos. Methods Mol Biol. 2019;2006:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter KL, Shetty G, Meistrich ML. Testicular edema is associated with spermatogonial arrest in irradiated rats. Endocrinology. 2006;147:1297–1305. [DOI] [PubMed] [Google Scholar]
- 34.Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17:55–62. [DOI] [PubMed] [Google Scholar]
- 35.Jahnukainen K, Ehmcke J, Quader MA, et al. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011;26:1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gubbels Bupp MR, Jorgensen TN. Androgen-Induced Immunosuppression. Front Immunol. 2018;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang RF, Liu TH, Zhao K, Xiong CL. Enhancement of mouse germ cell-associated genes expression by injection of human umbilical cord mesenchymal stem cells into the testis of chemical-induced azoospermic mice. Asian J Androl. 2014;16:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhang DH, Kim B-J, Kim BG, et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat Commun. 2018;9:4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadam P, Ntemou E, Baert Y, Van Laere S, Van Saen D, Goossens E. Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice. Stem Cell Res Ther. 2018;9:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gauthier-Fisher A, Kauffman A, Librach CL. Potential use of stem cells for fertility preservation. Andrology. 2019. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Shao S, Shetty G, Meistrich ML. Donor Sertoli cells transplanted into irradiated rat testes stimulate partial recovery of endogenous spermatogenesis. Reproduction. 2009;137:497–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.