Abstract
Diabetes mellitus (DM) negatively affects the development and progression of chronic liver diseases (CLD) of various etiologies. Concurrent DM and CLD are also associated with worse clinical outcomes with respect to mortality, the occurrence of hepatic decompensation, and the development of hepatocellular carcinoma (HCC). Unfortunately, early diagnosis and optimal treatment of DM can be challenging, due to the lack of established clinical guidelines as well as the medical complexity of this patient population. We conducted an exploratory review of relevant literature to provide an up-to-date review for internists and hepatologists caring for this patient population. We reviewed the epidemiological and pathophysiological associations between DM and CLD, the impact of insulin resistance on the progression and manifestations of CLD, the pathogenesis of hepatogenic diabetes, as well as the practical challenges in diagnosis and monitoring of DM in this patient population. We also reviewed the latest clinical evidence on various pharmacological antihyperglycemic therapies with an emphasis on liver disease-related clinical outcomes. Finally, we proposed an algorithm for managing DM in patients with CLD and discussed the clinical and research questions that remain to be addressed.
Keywords: End stage liver disease, Diabetes mellitus, Liver cirrhosis, Insulin resistance, Non-alcoholic fatty liver disease, Liver diseases
Core Tip: Diabetes is an independent risk factor for the development and progression of chronic liver disease (CLD) of various etiologies. Concurrent diabetes and CLD predict worse clinical outcomes, including hepatic decompensation, hepatocellular carcinoma (HCC), and complications following liver transplantation. Traditional glycemic markers, including fasting glucose, oral glucose tolerance test, and hemoglobin A1c, are not accurate in patients with severe CLD. Metformin and α-glucosidase inhibitors are associated with significant benefits beyond glycemic control, including reductions in HCC risk and incidence of hepatic encephalopathy. Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors may exert a hepatic protective effect irrespective of the degree of glycemic control.
INTRODUCTION
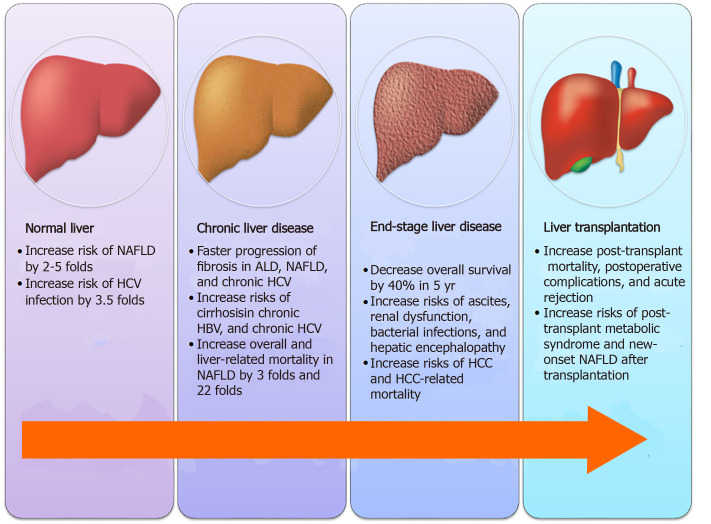
The liver plays a major role in maintaining blood glucose homeostasis, including glycogenesis and lipogenesis under feeding conditions as well as glycogenolysis and gluconeogenesis under fasting conditions. The liver is also the primary site of insulin clearance. Not surprisingly, diabetes mellitus (DM), a metabolic disease characterized by impaired blood glucose regulation and altered insulin sensitivity, is strongly associated with the development, progression, and consequence of chronic liver diseases (CLD), as illustrated in Figure 1.
Figure 1.
Impact of diabetes on various stages of chronic liver diseases. NAFLD: Non-alcoholic fatty liver disease; HCV: Hepatitis C virus; ALD: Alcoholic liver disease; HCC: Hepatocellular carcinoma.
DIABETES AND CHRONIC LIVER DISEASES
Insulin resistance, occurring in the context of metabolic syndrome, is a well-established independent pathophysiological driver for the development of non-alcoholic fatty liver disease (NAFLD)[1]. An early study on non-obese patients revealed that fasting insulin level and index of insulin resistance were nearly double in those with NAFLD compared to healthy controls[2]. Subsequent cross-sectional studies have shown that as much as 69%-87% of patients with type 2 DM (T2DM) have evidence of NAFLD on imaging or histology[3,4], while 21%-45% of patients with NAFLD were also found to have DM[5]. It is generally accepted that the presence of T2DM increases the risk of developing NAFLD by 2-5 folds[6]. Indeed the most recent practice guidelines from the American Diabetes Association (ADA) now make specific recommendations regarding screening patients with prediabetes or T2DM for NAFLD[7], representing a step up from prior guidelines published by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), which only included a qualified recommendation about screening high-risk patients due to significant gaps in knowledge concerning the treatment of NAFLD at the time[8,9].
The impact of insulin resistance and DM holds true for other etiologies of CLD as well. Insulin resistance, for example, was found to be an independent predictor of fibrosis in non-diabetic patients with chronic hepatitis C virus (HCV) infection[10]. Hyperinsulinemia and hyperglycemia have also been associated with the development of more severe fibrosis in patients with chronic HCV infection[11,12]. A recent Taiwanese nationwide cohort study has further identified new-onset DM as an independent predictor for cirrhosis and decompensation in patients with chronic hepatitis B virus (HBV) or chronic HCV[13,14]. In alcoholic liver disease (ALD), BMI and fasting blood glucose levels have been demonstrated to correlate with fibrosis score after adjusting for daily alcohol intake and the total duration of alcohol use[15].
The relationship between DM and HCV infection is particularly noteworthy because of its possible two-way association. There is epidemiological evidence to suggest that diabetic patients are nearly 3.5 folds more likely to acquire HCV infection[16]. Conversely, HCV infection is associated with a 1.5-1.7 fold excess risk of new-onset T2DM development[17,18]. Although the exact mechanisms of action remain elusive, it is postulated that the metabolic disturbances associated with DM may favor HCV survival as evidenced by reports that insulin resistance positively correlates with HCV viral load[10]. Meanwhile, insulin resistance and DM are also increasingly being recognized as metabolic extrahepatic manifestations of chronic HCV infection. HCV may exert a direct cytopathic effect on pancreatic islets and induce β-cell death via a caspase 3-dependent pathway[19,20]. In addition, HCV core protein has been shown to promote ubiquitination of insulin receptor substrate (IRS)-1 and -2 in transfected hepatoma cells and a transgenic mouse model[21,22], thereby interrupting hepatic insulin signaling resulting in hepatic insulin resistance. Finally, dipeptidyl peptidase-4 (DPP-4) expression was found to be upregulated by HCV nonstructural protein while interferon therapy led to decrease serum DPP-4 activity in HCV-infected patients[23,24], providing support for another mechanism of HCV-induced insulin resistance via the glucagon-like peptide-1 (GLP-1) pathway[25].
DIABETES AND END-STAGE LIVER DISEASES
Once CLD progresses to end-stage liver disease (ESLD), the presence of DM continues to predict more severe diseases and worse clinical outcomes. On the one hand, the prevalence of DM positively correlates with the severity of liver disease based on the Child-Pugh (CP) class or Model for End-Stage Liver Disease (MELD) score[26,27]. On the other hand, multiple prospective studies on patients with compensated/ decompensated cirrhosis of various etiologies have consistently demonstrated lower survival, as much as a 40% reduction in 5 years, in the diabetic group compared to the non-diabetic group[28-30]. There is evidence that even the presence of subclinical impaired glucose tolerance (IGT) predicts worse short-term and long-term survival in cirrhotic patients[29,31,32]. It is noteworthy that the higher mortality in these diabetic patients is due not to the classical DM-related micro-/macrovascular diseases, but complications of liver failure[33]. Clinically, baseline DM status was shown to be independently associated with the development of ascites, renal dysfunction, and bacterial infections during long-term follow-up in a cohort of cirrhotic patients with chronic HCV infection[34]. DM is also associated with more severe hepatic encephalopathy in cirrhotic patients independent of the severity of liver disease[35].
Most importantly, the incidence of hepatocellular carcinoma (HCC) and HCC-specific mortality are nearly double in patients with preexisting DM based on a meta-analysis of 28 prospective epidemiological studies[36]. The risk for HCC is further increased by up to 10 folds when combined with alcohol consumption and/or viral hepatitis[37,38]. The impact of DM on the risk for HCC is particularly dramatic in patients with certain single nucleotide polymorphisms in the patatin-like phospholipase domain containing 3 (PNPLA3) gene, which was known to increase the risk for HCC in alcoholic cirrhosis[39]. A case-control study of nearly 500 HCC patients revealed an adjusted odds ratio of 19.11 for diabetic patients with the GG genotype compared to that of 2.65 for non-diabetic controls with the same genotype[40]. Remarkably, cirrhosis is only present in 50% of patients with NAFLD-related HCC[41], suggesting that the mechanism of hepatocarcinogenesis in the context of metabolic syndrome may be different from the classical mechanism involved in cirrhosis. It is postulated that hyperinsulinemia is central to the pathophysiological effects of DM on HCC development by upregulating growth signaling pathways[42], promoting an inflammatory milieu[43], activating hepatic progenitor cells[44], and stimulating angiogenesis[45].
Regardless of the etiology of underlying CLD, the diagnosis of HCC carries a poor prognosis with median survival measured in the order of months[46]. A recent meta-analysis of over 9000 HCC cases concluded that DM status is associated with lower overall/disease-free survival in patients receiving hepatic resection as well as lower overall survival in patients receiving non-surgical treatments[47]. This reduction in overall survival may partly be attributable to an increased risk of postoperative complications, including hepatic decompensation, intractable ascites, and intraperitoneal infection, as demonstrated by several regression studies on HCC patients undergoing hepatic resection[48,49]. Similarly, there is evidence that diabetic patients undergoing transarterial chemoembolization (TACE) are at an increased risk for hepatic decompensation, liver abscess formation, and prolonged acute renal failure[50,51].
HEPATOGENIC DIABETES
It is worth noting that not only is DM a risk factor for ESLD but it can also be a complication of ESLD. Hepatogenic diabetes is characterized by the development of hyperinsulinemia, insulin resistance, and β-cell dysfunction after the onset of cirrhosis, often in the absence of traditional risk factors, such as a family history of DM or obesity. As illustrated in Figure 2, it is postulated that hyperinsulinemia occurs in cirrhosis as a result of decreased insulin clearance by the damaged liver and increased portosystemic shunting. This notion is supported by evidence that hyperinsulinemia worsens after transjugular intrahepatic portosystemic shunt (TIPS) placement in diabetic patients[52], yet improves with portosystemic shunt occlusion by balloon-occluded retrograde transvenous obliteration (BRTO)[53]. There is also evidence that cirrhotic patients may develop exaggerated β-cell responsiveness to glucose as well as pancreatic islet hypertrophy, resulting in increased insulin secretion[54].
Figure 2.
Mechanisms of action of hepatogenic diabetes.
Sustained hyperinsulinemia can, by means of an array of negative feedback mechanisms[55-57], give rise to insulin resistance in peripheral adipose tissues and skeletal muscles, resulting in reduced insulin-stimulated non-oxidative glucose disposal[58]. The hyperglycemic effect of reduced glucose disposal can be further amplified by cirrhosis-induced sarcopenia and impaired glucose effectiveness[59]. Moreover, certain etiologies of CLD may exert additional disease-specific mechanisms of insulin resistance, in a similar fashion to HCV-induced DM[21]. Chronic alcohol exposure, for instance, may inhibit insulin signaling pathways by altering intracellular calcium level within hepatocytes[60], resulting in impaired insulin-mediated suppression of hepatic glucose production[61]. Finally, there is evidence that hepatic expression of DPP-4 is increased and GLP-1 is decreased in both chronic HCV and NAFLD[25,62,63], resulting in hepatic insulin resistance and IGT.
As in the pathogenesis of T2DM, the development of pancreatic β-cell dysfunction signals the body’s diminishing capacity to compensate for worsening insulin resistance and marks the progression from IGT to frank DM[27]. Similar to the development of insulin resistance, a number of disease-specific mechanisms of β-cell dysfunction may be operating even before the onset of cirrhosis. Chronic alcohol consumption and hereditary hemochromatosis, for instance, cause down-regulation of glucokinase and increased oxidative stress, respectively, leading to increased β-cell apoptosis and decreased glucose-induced insulin secretion[64,65]. In NAFLD, the combination of chronic hyperglycemia and elevated free fatty acid levels lead to dysfunction and death of pancreatic β-cells by glucolipotoxicity[66]. Meanwhile, chronic HCV infection is thought to induce pancreatic islet destruction via a combination of autoimmune-mediated and direct cytopathic mechanisms as abovementioned[19,20,67]. Eventually, the development of cirrhosis and hepatic decompensation further exacerbates β-cell dysfunction due to the accumulation of advanced glycation end products[68], which can inhibit glucose-stimulated insulin secretion and induce apoptosis of β-cells[69,70].
DIABETES AND LIVER TRANSPLANTATION
In many cases, orthotopic liver transplantation (OLT) remains the only curative option for patients with ESLD. Given the pathogenic effects of insulin resistance on oxidative and inflammatory stresses, it is not surprising that multiple large-scale retrospective studies have repeatedly demonstrated pre-transplant DM to be associated with decreased post-transplant survival, independent of BMI or MELD score[71-73]. Pre-transplant DM is also an independent predictor for postoperative complications, including cardiovascular events, infections, renal dysfunction, and acute graft rejection[71]. There is even evidence that the donor’s history of DM correlates with increased recipient’s mortality as well as increased risk of graft failure[73], suggesting that DM can exert biologically-significant damages to the liver without causing clinically-detectable hepatic dysfunction.
Similarly, the presence of post-transplant DM, which occurs in about 30% of patients undergoing OLT[74], has been linked to increased mortality as well as a higher incidence of postoperative complications and acute rejection[73,75,76]. Although preexisting overt DM may disappear in more than half of the patients after OLT[77], pre-transplant DM remains one of the most important predictors for post-transplant DM[78]. This observation is consistent with the findings that peripheral insulin resistance is improved, but not completely normalized, after OLT[79]. Other recognized risk factors for new-onset DM after transplantation, which affects 7%-18% of OLT patients[77,80], include male gender[78,80], use of tacrolimus[80,81], and HCV infection[78,81]. Besides DM, metabolic syndrome and its other individual components, namely hypertension, dyslipidemia, and obesity, are also found to be highly prevalent post-transplant[82]. Not only does it increases the incidence of cardiovascular events[83], but the presence of post-transplant metabolic syndrome may also contribute to the development of new-onset NAFLD after transplantation for non-NAFLD cirrhosis[84]. While there have been case reports of combined liver and islet transplantation in cirrhotic patients with concurrent type 1 DM[85], the safety and utility of this technique in patients with hepatogenic diabetes or T2DM is yet to be determined[86].
TRADITIONAL GLYCEMIC MARKERS FOR PATIENTS WITH LIVER DISEASES
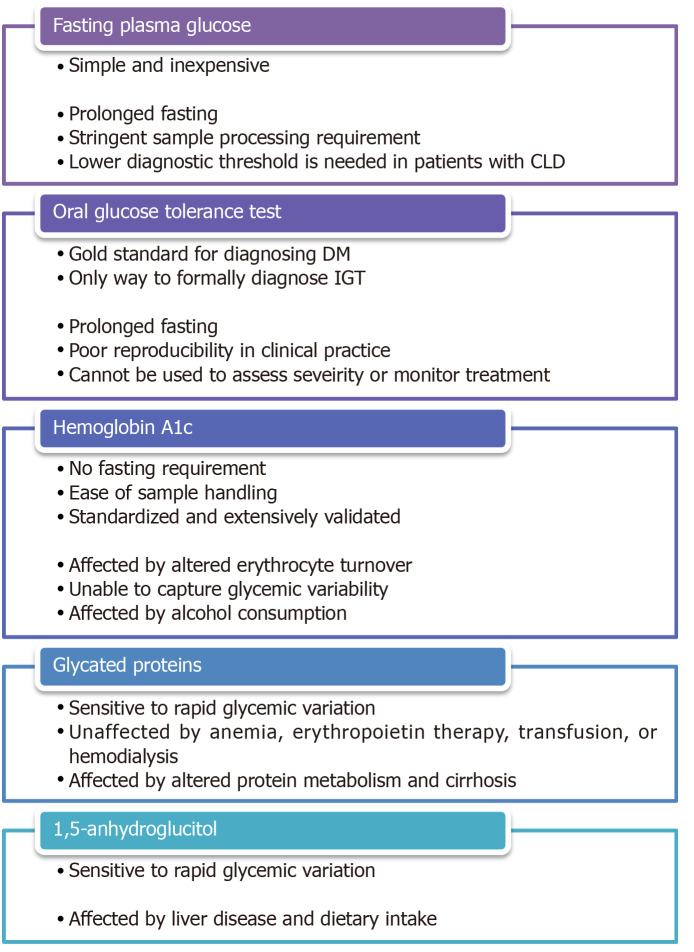
Given the abovementioned detrimental effects of DM on the development and progression of CLD as well as the manifestation and management of ESLD, it is reasonable to hypothesize that early diagnosis and optimal treatment of DM in patients with CLD would be beneficial. Nonetheless, IGT and DM were identified in as much as 38.5% and 19.2%, respectively, of patients with normal fasting plasma glucose (FPG) and compensated cirrhosis of various etiologies in a small prospective study[87], which alluded to the practical challenges of managing DM in this patient population. The first challenge is to establish an accurate diagnosis and to assess disease severity. As summarized in Figure 3, the utility and accuracy of most glycemic markers are restricted in patients with CLD.
Figure 3.
Pros and cons of various glycemic markers. CLD: Chronic liver diseases; DM: Diabetes mellitus; IGT: Impaired glucose tolerance.
Fasting plasma glucose
FPG is a simple and inexpensive test for diagnosing DM but its widespread adoption is limited by the need for prolonged fasting and stringent processing requirements[88]. Although the test offers a reasonable intra-individual reproducibility[89], FPG can be affected by stress, acute illness, and alcohol consumption. Moreover, the optimal FPG threshold for diagnosing DM in patients with CLD/ESLD remains controversial. Certain studies have reported that using thresholds of 100-125 mg/dL and ≥ 126 mg/dL, the sensitivities of FPG in detecting prediabetes and DM in high-risk populations were as low as 28.9% and 55.7%, respectively[90,91]. In a study on patients with HCV cirrhosis, 21% of patients with FPG < 110 mg/dL met the diagnostic criteria for DM based on oral glucose tolerance test (OGTT) and regression analysis showed that a threshold of 107 mg/dL, instead of 126 mg/dL should be used for diagnosing DM in these patients[92]. It is unclear if the same threshold can be applied to patients with other etiologies or severities of liver diseases.
Oral glucose tolerance test
OGTT is often considered the gold standard for diagnosing DM. It is the preferred method for detecting gestational DM, cystic fibrosis-related DM, and post-transplant DM[93]. Furthermore, it is the only way to formally diagnose IGT, which may be particularly relevant to cirrhotic patients because of the implications on short-term and long-term prognosis as abovementioned[29,31,32]. The major drawbacks of OGTT are the need for prolonged fasting as well as the length and complexity of the test, which may contribute to the poor reproducibility of OGTT in clinical practice[94]. Most importantly, OGTT cannot be used to assess disease severity or treatment effectiveness because of practical limitations and the lack of established OGTT-based glycemic targets.
Hemoglobin A1c
Since it was first proposed as a measure of glucose intolerance in 1976[95], glycated hemoglobin (A1c) has evolved into one of the most broadly deployed tests for diagnosing and monitoring DM. Its major advantages are the absence of a fasting requirement and the relative ease of sample handling. A1c is particularly useful as a marker for treatment effectiveness because it reflects average blood glucose over a period of months instead of a single point in time. While it remains a useful glycemic marker in most patients with mild liver diseases, the accuracy and validity of A1c in patients with advanced liver diseases remained controversial. A small study involving 15 patients with compensated cirrhosis found 40% of subjects to have A1c results below the non-diabetic reference range[96]. Another retrospective study on a cohort decompensated cirrhotic patients undergoing liver transplantation evaluation revealed a similar discordance between measured A1c and average blood glucose[97]. The poor diagnostic performance of A1c is attributable to the well-described curvilinear correlation between A1c and erythrocyte turnover[98], which can occur in patients with CLD/ESLD as a result of hemorrhage related to portal hypertension and coagulopathy, hemolysis caused by splenomegaly as well as impaired erythropoiesis due to marrow suppression and nutritional deficiency[99]. A1c value can also be affected unpredictably by blood transfusion[100,101]. More importantly, the inherent biochemical characteristics of the glycation process limit the ability of A1c to capture the excessive blood glucose lability exhibited by patients with CLD, as demonstrated in a recent study that compared A1c against continuous glucose monitoring in diabetic patients with cirrhosis[102]. This shortcoming may have crucial clinical implications since glycemic variability was shown to be an independent predictor for fibrosis in NAFLD as well as cardiovascular disease and all-cause mortality in the general population[103,104]. Finally, there is evidence that alcohol consumption is independently associated with lower A1c values, even adjusting for confounding factors such as FPG, obesity, and anemia[105-107], likely as a result of an increase in the size of the hemoglobin A1 fraction[108].
NON-TRADITIONAL GLYCEMIC MARKERS FOR PATIENTS WITH LIVER DISEASES
Glycated proteins
Glycated albumin (GA) and fructosamine are ketoamines that are formed by non-enzymatic glycation of glucose to serum proteins in a similar fashion to the glycation of hemoglobin. Due to the shorter half-life of albumin and other serum proteins, the faster rate of albumin glycation compared to hemoglobin glycation, and the direct exposure of serum proteins to serum glucose, GA and fructosamine are typically taken to reflect glycemic control over 2-3 wk[109,110]. GA has proven to be a particularly useful glycemic marker in patients with chronic kidney disease and/or on dialysis because, unlike A1c, GA level is not affected by anemia, erythropoietin therapy, blood transfusion, or hemodialysis[111,112]. Not surprisingly, the accuracy of GA and fructosamine is negatively impacted by disease states that affect protein metabolisms. Indeed, an older study on a small cohort of cirrhotic patients demonstrated an inverse correlation between fructosamine levels and serum albumin levels [113]. A more recent study comparing fructosamine against continual glucose monitoring confirmed the poor association in diabetic patients with cirrhosis[102]. Several entities, including albumin-corrected fructosamine, total protein-corrected fructosamine, and CLD-A1c, have been proposed in an attempt to improve the diagnostic performance of GA and fructosamine by correcting for variations in serum protein concentrations[114,115]. Unfortunately, the validity of these entities outside the study populations has not been externally or prospectively verified.
Serum 1,5-anhydroglucitol
Serum 1,5-anhydroglucitol (1,5-AG) is a dietary monosaccharide that is normally reabsorbed by the proximal renal tubules, but its reabsorption is competitively inhibited by glucosuria in the setting of hyperglycemia. Serum 1,5-AG was found to be a sensitive day-to-day glycemic marker in diabetic patients as well as a sensitive diagnostic test in those at high risk of DM[116,117]. Nonetheless, there is evidence that 1,5-AG metabolism is affected in patients with liver diseases[118,119]. There is also a concern that dietary intake may affect serum 1,5-AG level[120], which is particularly relevant to the cirrhotic population due to the high prevalence of malnutrition.
MANAGEMENT OF DIABETES IN PATIENTS WITH LIVER DISEASES
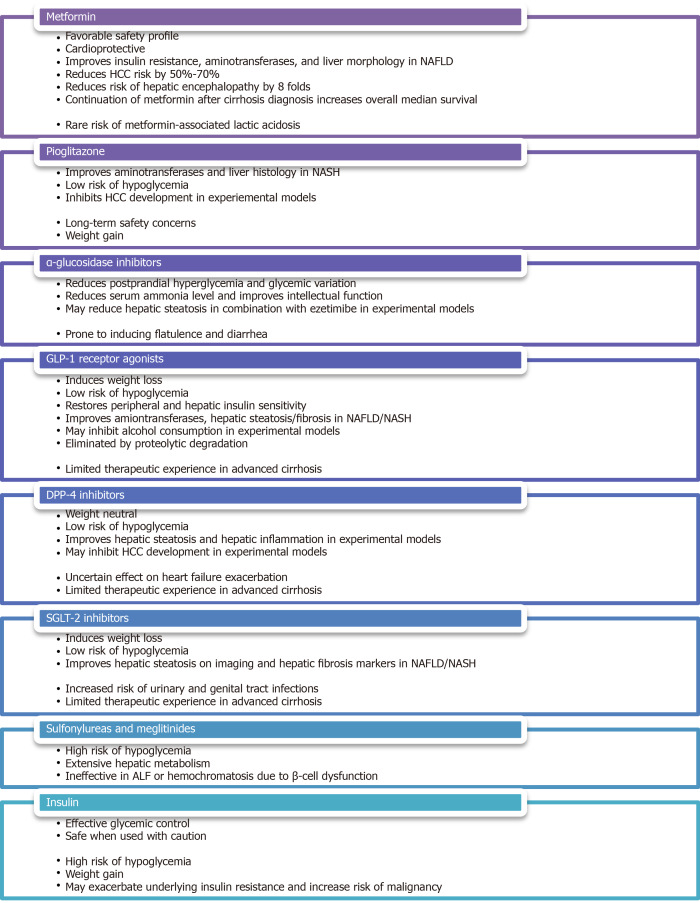
The second challenge in the management of DM in patients with liver diseases is to identify a safe and effective treatment strategy for this medically complicated population, especially those with decompensated cirrhosis. It is well accepted that basic lifestyle interventions, such as healthful diet, physical activity, alcohol/smoking cessation as well as psychosocial support are fundamental to the medical management of both DM and liver diseases. Nonetheless, antihyperglycemic medications are often needed when patients fail to achieve targeted glycemic control through lifestyle interventions alone. Attention must be paid to consider the unique mechanisms of action, the side effect profiles, and the implications on liver diseases associated with the use of these medications, as summarized in Figure 4. An up-to-date summary, with a focus on liver-disease related outcomes, of the major clinical trials involving these medications is provided in Supplementary Table 1.
Figure 4.
Pros and cons of various antihyperglycemic medications. NAFLD: Non-alcoholic fatty liver disease; HCC: Hepatocellular carcinoma; NASH: Nonalcoholic steatohepatitis.
Metformin
Metformin, which is often considered first-line oral therapy in T2DM due to its favorable safety profile and cardioprotective effects[121], has been studied in both diabetic and non-diabetic patients with biopsy-proven NAFLD[122-128]. Despite the limitations of these small, open-label, or non-randomized published trials, the overall evidence indicates that metformin use is associated with improvement in insulin resistance, aminotransferase levels, and liver morphology[129]. Furthermore, several large retrospective studies on diabetic patients with CLD of various etiologies have demonstrated a 50%-70% reduction in HCC risk among those treated with metformin[130-132], with one recent case-control study showing a dose-dependent association[133]. Similar results, including a reduction in 5-year HCC incidence, liver-related mortality, and transplantation, were obtained in a prospective study involving specifically diabetic patients with HCV cirrhosis[134]. Additionally, metformin has been shown to reduce the incidence of overt hepatic encephalopathy by 8 folds through inhibition of glutaminase activity[135]. Most strikingly, a retrospective study on 250 diabetic patients with cirrhosis of various etiologies revealed that the continuation of metformin after cirrhosis diagnosis nearly doubles the overall median survival across all Child-Pugh classes[136].
Despite its remarkable morbidity and mortality benefits, metformin is often withheld from patients with liver diseases due to an exaggerated concern for metformin-associated lactic acidosis (MALA). It is worth noting, however, that metformin is not intrinsically hepatotoxic and can be safely administered even in the context of mildly abnormal transaminases[137]. In fact, animal studies have revealed a protective potential of metformin against acetaminophen-induced hepatotoxicity[138,139]. Additionally, MALA is an exceedingly rare condition with an estimated incidence of < 10 per 100000 patient-years of exposure in patients without significant renal impairment[140]. A recent retrospective study of over 132000 patients with newly diagnosed T2DM found no significant difference in the risk of lactic acidosis in patients prescribed metformin compared to those prescribed other antihyperglycemic medications or no medication[141]. This is in contrast to its predecessor, phenformin, which carries a significantly higher risk of lactic acidosis even at therapeutic drug levels[142]. Finally, it is important to bear in mind that although metformin can increase lactate production by promoting intestinal glucose utilization via the anaerobic pathway as well as inhibiting hepatic lactate uptake and gluconeogenesis[143,144], lactate is not toxic in itself and hyperlactatemia does not directly cause the clinical condition of lactic acidosis[145].
Pioglitazone
The thiazolidinedione pioglitazone, an insulin-sensitizing peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist, is another extensively studied antihyperglycemic agent that shows promise in the treatment of nonalcoholic steatohepatitis (NASH). A small pilot study on non-diabetic patients with biopsy-proven NASH resulted in normalization of aminotransferase levels in 72% of patients as well as improvement in histological features of hepatic steatosis, hepatocellular injury, parenchymal inflammation, and fibrosis in 67% of patients after 48 wk of treatment[146]. Similar results except for a decrease in fibrosis score, which failed to achieve statistical significance in two studies[147,148], were replicated in subsequent randomized, placebo-controlled trials of various study designs involving both diabetic and non-diabetic patients[147-150]. It is interesting to note that although its beneficial effects are greater in patients with T2DM, pioglitazone use is associated with a reduction in NAFLD activity score and resolution of NASH in 46% and 26%, respectively, of patients without frank DM[151]. Given its low risk of inducing hypoglycemia, pioglitazone may be uniquely suited in the treatment of selected NASH patients with normoglycemia at baseline. Finally, there is limited in vitro and in vivo data suggesting that pioglitazone may inhibit HCC development[152,153], but these findings have not been confirmed in human studies[154].
Lingering concerns regarding the long-term safety of thiazolidinedione therapy stemmed from potential troglitazone-related hepatotoxicity, rosiglitazone-related cardiovascular risks, and pioglitazone-related bladder cancer[155-157], remain a barrier to the widespread use of pioglitazone in clinical practice. This is particularly relevant in the face of findings that long-term rosiglitazone or pioglitazone therapy does not impart additional improvement in metabolic or hepatic parameters compared to short-term therapy[150,158]. A recently published expert panel statement, however, does recommend the use of pioglitazone, either alone or in combination with a statin or ezetimibe, for the primary or secondary prevention of cardiovascular disease as well as the avoidance of cirrhosis, liver transplantation, or HCC in patients with NAFLD/NASH[159]. Another barrier to the use of pioglitazone is its propensity to cause significant weight gain, which may be dose-dependent[160], as demonstrated by multiple systematic reviews and meta-analyses[161,162]. Finally, other than a small pilot study that showed a reduction in hepatic steatosis on imaging in patients with human immunodeficiency virus (HIV)/HCV coinfection[163], the use of pioglitazone in other etiologies of CLD has not been thoroughly investigated.
α-glucosidase inhibitors
Although α-glucosidase inhibitors, such as acarbose, voglibose, and miglitol, enjoy limited adoption amongst the general diabetic population due to its gastrointestinal side effects of flatulence and diarrhea, they deserved to be recognized as a pluripotent antihyperglycemic agent in patients with CLD. A randomized, placebo-controlled trial of acarbose in diabetic patients with compensated cirrhosis, who are prone to postprandial hyperglycemia partly due to decreased hepatic glucose uptake and impaired glycogenesis[164], demonstrated a significant reduction in fasting glycemia, postprandial glycemia, mean glycemic variation, and A1c in the treatment group[165]. By favoring saccharolytic, instead of proteolytic, intestinal bacterial flora, acarbose treatment has also been shown to reduce serum ammonia level as well as to improve intellectual function in a randomized, placebo-controlled trial, crossover study[166], making it an effective regimen for the consolidated treatment of hyperglycemia and mild hepatic encephalopathy. In a small pilot study of diabetic patients with biopsy-confirmed NASH, miglitol was found to reduce aminotransferase levels, hepatic steatosis, and histological inflammation after 12 months of therapy[167]. Moreover, voglibose was found to be effective in minimizing pioglitazone-induced weight gain in the general diabetic population[168]. It is important to note that although there are rare case reports of acarbose-related acute hepatitis[169], the medication is generally felt to be safe in patients with CLD/ESLD since it is exclusively metabolized in the gastrointestinal tract and has a very low systemic bioavailability[170]. This notion is supported by a Taiwanese nationwide retrospective study, which discovered no increased risk of liver injury amongst diabetic patients with severe renal insufficiency regardless of the presence or absence of CLD[171].
GLP-1 receptor agonists
GLP-1 receptor agonists, such as exenatide and liraglutide, constitute an increasingly popular class of incretin-based therapy for the treatment of T2DM thanks to their ability to induce weight loss and their lower risk of hypoglycemia. The observations that glucose-induced GLP-1 secretion and hepatic GLP-1 receptor expression are deficient in NAFLD patients suggest GLP-1 agonism may exert a direct effect on hepatocytes and may play a role in the treatment of NAFLD[62,172,173]. Several small open-label studies have demonstrated reductions in hepatic steatosis, aminotransferase levels, and liver fibrosis score after treatment with liraglutide in diabetic patients with imaging findings of hepatic steatosis[174-176]. These findings were further supported by a Japanese single-arm, open-label study, and a British double-blinded, randomized, placebo-controlled trial of liraglutide on patients with biopsy-proven NASH. The former showed significant improvement in histological inflammation and fibrosis in 60%-70% of subjects after 96 wk of therapy[177], while the latter showed histological resolution of NASH in 39% of subjects after 48 wk of therapy[178]. A reduction in hepatic triglyceride content was also observed in a small, open-label, randomized study of exenatide in diabetic patients with imaging findings suggestive of NAFLD[179]. With regard to other etiologies of CLD, GLP-1 receptor agonists were found to exhibit an inhibitory effect on alcohol consumption in animal models[180,181]. It has also been reported that fasting serum GLP-1 levels were decreased in patients with chronic HCV, but not those with chronic HBV[25]. It is worth noting, however, that patients with HBV-, HCV-, or alcohol-related liver diseases were excluded from the original Liraglutide Effect and Action in Diabetes trials[182]. The effects of GLP-1 agonism on other liver disease-related clinical outcomes, such as encephalopathy and HCC development, have yet to be thoroughly evaluated. Since GLP-1 receptor agonists are eliminated by proteolytic degradation and glomerular filtration, no dosage adjustment is necessary for patients with any degree of hepatic impairment[183]. Caution is advised in patients with advanced cirrhosis given limited therapeutic experiences in this vulnerable population.
DPP-4 inhibitors
DPP-4 is a ubiquitously expressed membrane-bound and circulating glycoprotein that acts enzymatically on a variety of substrates, including GLP-1, gastric inhibitory polypeptide (GIP), insulin-like growth factor-1 (IGF-1), neuropeptide Y and substance P, as well as interacts non-enzymatically with many ligands, including adenosine deaminase, caveolin-1, and CD45[184]. As such, DPP-4 inhibitors, such as linagliptin, saxagliptin, sitagliptin, and vildagliptin are thought to act upstream of GLP-1 by slowing its degradation, but they may also exert diverse metabolic, immunologic, and neurologic effects via other GLP-1-independent pathways. Similar to GLP-1 receptor agonists, DPP-4 inhibitors are considered weight neutral and carry a low risk of hypoglycemia[185], but they have the added advantage of being oral agents. In the context of liver diseases, hepatic DPP-4 expression was found to be upregulated in patients with NAFLD while serum DPP-4 activity was reported to correlate with the histopathologic grade of NASH as well as serological markers of liver damage[186-188]. In addition, DPP-4 inhibition by specific inhibitors was demonstrated in several animal models of NAFLD to attenuate hepatic steatosis[189,190]. There were two open-label trials of sitagliptin, one reported a reduction in intrahepatic lipid content in diabetic patients with clinical NAFLD, another reported improvements in hepatic steatosis and ballooning in patients with biopsy-proven NASH irrespective of DM status[176,191]. Unfortunately, improvements in hepatic steatosis, liver fibrosis, or NAFLD activity score were not observed in other randomized, placebo-controlled trials of sitagliptin[192,193]. It is worth noting that the hepatic protective effects of DPP-4 inhibitors, which may be mediated through direct actions on hepatocytes via GLP-1 receptors[172], appear to occur irrespective of the degree of glycemic control[187]. Moreover, the observations in obese patients of increased expression of membrane-bound DPP-4 on visceral adipose tissue-derived dendritic cells as well as increased release of soluble DPP-4 as adipokine from visceral adipocytes support the notion that DPP-4 inhibition may play a role in modulating adipose tissue inflammation and regulating adipose tissue metabolism[194,195], likely through a GLP-1-independent pathway[196]. Finally, alteration in DPP-4 expression was observed in rat hepatoma cell lines and human HCC specimens[197,198], raising the possibility that DPP-4 activity may influence HCC development. It is prudent, however, to mention that although DPP-4 inhibitors were shown to be protective against cardiovascular diseases by improving endothelial function and reducing inflammation[199,200], a recent meta-analysis of five clinical trials revealed a slightly increased risk of hospital admission for heart failure attributable to DPP-4 inhibitor use amongst diabetic patients with existing cardiovascular risk factors[201]. Most DPP-4 inhibitors, except vildagliptin, are considered safe in patients with mild or moderate hepatic impairment, although caution is advised in patients with severe hepatic impairment due to limited therapeutic experiences[183].
Sodium-glucose cotransporter-2 inhibitors
Instead of directly altering insulin availability or insulin sensitivity, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, exert their antihyperglycemic effect by blocking proximal renal tubular glucose reabsorption, thus leading to increased glucose excretion in the form of glucosuria. SGLT-2 inhibitors carry a low risk of hypoglycemia and are capable of inducing modest weight loss as well as lowering blood pressure[202]. They have been demonstrated in several animal models of NAFLD/NASH to improve hepatic steatosis and liver fibrosis[203,204]. Improvement in hepatic steatosis as well as reductions in various serological or imaging parameters of liver injury were also observed in clinical trials of several SGLT-2 inhibitors in diabetic NAFLD patients[205-211]. Most impressively, preliminary evidence indicates SGLT-2 inhibitors may slow the proliferation of hepatoma cell lines and hinder xenograft tumor growth in nude mice independent of their antihyperglycemic effect, suggesting that SGLT-2 inhibitors may attenuate HCC development via some unidentified mechanisms of action[212]. The use of SGLT-2 inhibitors in other etiologies of CLD has not been thoroughly investigated. Long-term safety data on the use of SGLT-2 inhibitors in cirrhotic patients is relatively scarce given limited therapeutic experiences, but canagliflozin and ertugliflozin are generally considered safe in patients with mild or moderate hepatic impairment[213,214]. Limited data is showing that empagliflozin can be used without dosage adjustment across all degrees of hepatic impairment[215], while dose reduction may be necessary for dapagliflozin in patients with severe hepatic impairment[216].
Sulfonylureas and meglitinides
The second- and third-generation sulfonylureas, including glipizide, glyburide, gliclazide, and glimepiride, and the meglitinides, repaglinide, and nateglinide, are insulin secretagogues that stimulate insulin secretion from pancreatic β-cells. While these medications are highly effective antihyperglycemic agents capable of lowering A1c by 1%-2%[217], they also carry a high risk of hypoglycemia[218]. Patients with hepatic impairment are particularly susceptible due to reduced drug inactivation and elevated free drug concentration because both sulfonylureas and meglitinides are extensively metabolized by the liver and are tightly bound to serum proteins. The risk of hypoglycemia is further exacerbated in patients with cirrhosis as a result of impaired hepatic gluconeogenesis, reduced hepatic insulin clearance, and peripheral hyperinsulinism[219]. Alcohol consumption can predispose patients to hypoglycemia in a similar fashion by impairing gluconeogenesis and glycogenolysis[220]. Conversely, these medications may be rendered ineffective in chronic ALD or hemochromatosis due to β-cell dysfunction and apoptosis[64,65]. Most notably, several case-control studies have meta-analyses revealed an increased odds of HCC development by up to 3 folds amongst patients with T2DM treated with sulfonylureas[131,221-223], possibly as a result of hyperinsulinemia. Expert opinions advise that insulin secretagogues be avoided or used with extreme caution in patients with CLD/ESLD[224].
Insulin
Despite the ever-expanding list of antihyperglycemic medications, insulin and insulin analogs remain the safest and most effective glycemic therapy in patients with DM. They can be used, with caution, even in patients with decompensated cirrhosis or severe hepatic impairment[225,226]. Not surprisingly, hypoglycemia is a major limiting side effect. On the one hand, cirrhosis can induce or exacerbate a state of hyperinsulinemia and insulin resistance as abovementioned[219], which may increase a patient’s insulin requirement. On the other hand, cirrhotic patients may exhibit an exaggerated response to insulin due to impaired hepatic gluconeogenesis and sarcopenia[227]. These opposing mechanisms of actions, together with symptoms of nausea, bloating, and poor appetite experienced by many patients with CLD, make it difficult to predict a patient’s day-to-day exogenous insulin requirement. Furthermore, some cirrhotic patients, who are on β-blockers for variceal bleeding prophylaxis, may experience an impaired hormonal counterregulatory response to insulin, resulting in delayed recovery from hypoglycemia[228]. As such, expert opinions recommend the use of short-acting insulin analogs as well as frequent dose adjustment and close glucose monitoring to minimize the risk of hypoglycemia[229].
Weight gain of 3 to 9 kg associated with the initiation of insulin therapy, attributable to the intrinsic anabolic properties of insulin, the central effects of insulin on appetite and reward regulation, the non-physiological pharmacokinetics of exogenous insulin, and the behavioral changes related to fear of hypoglycemia[230], is a well-documented phenomenon amongst the general diabetic population[231]. This weight gain can be particularly detrimental to patients with metabolic syndrome as it is predominantly represented by fat mass and is likely to further exacerbate the underlying insulin resistance, oxidative stress, and systemic inflammation[232], leading to worsening metabolic diseases as well as increased risk of malignancy. Similar to the case of sulfonylureas, data from observational studies suggest an association between insulin therapy and HCC development amongst patients with T2DM[130,131]. Further studies are needed to further delineate the oncogenic risk of insulin therapy versus the cardiometabolic risk of uncontrolled hyperglycemia. Nonetheless, it may be prudent to reserve insulin therapy in patients with CLD to those who are unable to receive or inadequately managed by other antihyperglycemic medications.
GLYCEMIC TARGETS IN PATIENTS WITH LIVER DISEASES
After selecting an appropriate glycemic marker and formulating an effective antihyperglycemic therapy plan, the third challenge in the management of DM in patients with CLD is to determine a reasonable glycemic target. For the general non-pregnant adult population, the ADA recommends an A1c goal of < 7%[233], which is largely based on several landmark studies demonstrating decreased rates of microvascular complications, such as retinopathy, neuropathy, and nephropathy, in patients undergoing intensive glycemic control[234,235]. It is important to note, however, that the higher mortality in patients with concurrent DM and CLD is predominantly attributable to complications of liver failure, not DM-related micro-/macrovascular diseases[33]. Unfortunately, except for a small cohort study on NAFLD patients showing an association between improvement in A1c and improvement in liver fibrosis on biopsy[236], the impact of glycemic control on the natural history of various CLD etiologies has not been thoroughly investigated. It is also unclear if the degree of glycemic control directly correlates with the incidence or severity of liver disease complications. Although the ADA does recommend that less stringent A1c goals be considered in selected patients with limited life expectancy, extensive comorbid conditions, advanced micro-/macrovascular complications, or difficult to manage diabetes[233], there is little data to guide the clinical decision-making process in practice. Further studies are desperately needed to help determine the optimal glycemic targets, in relation to the etiology of liver disease and the degree of decompensation, in patients with CLD.
CONCLUSION
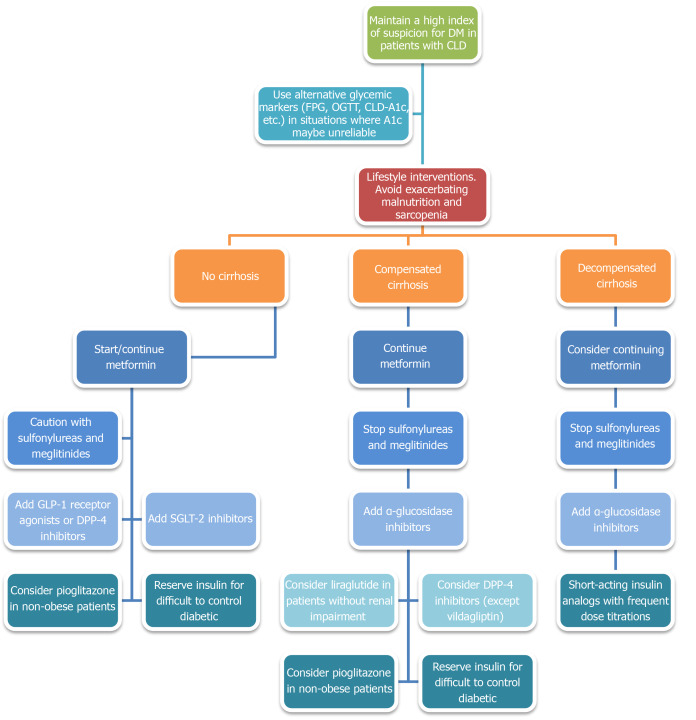
Given the abovementioned evidence, it is clear that DM plays a significant role in the development and progression of CLD. DM can also occur as a consequence of or be exacerbated by CLD. Most importantly, concurrent DM and CLD are associated with worse clinical outcomes, including reduced survival, more severe liver failure-related complications, and a higher incidence of HCC and HCC-specific mortality. It is, therefore, imperative that practitioners astutely identify and closely monitor the development of DM in any patient with CLD, bearing in mind that A1c may not be accurate in patients with advanced liver diseases. Alternative glycemic markers, such as FPG, OGTT, or CLD-A1c, may be needed to establish the diagnosis in patients with moderate-to-severe anemia, frequent transfusion requirement, chronic alcohol consumption, and/or fluctuating A1c levels. Once the diagnosis of DM is confirmed, efforts should be made to incorporate the management of DM into the patient’s overall treatment plan, with aims to slow the progression of CLD and to reduce the risk of liver failure-related complications. A proposed treatment algorithm is presented in Figure 5. Similar to the general diabetic population, lifestyle interventions involving a calorie-restricted diet and moderate-intensity exercise should be considered first-line treatment. If antihyperglycemic pharmacotherapy is deemed necessary, metformin should be included as the backbone, unless otherwise contraindicated, given its favorable safety profile, chemopreventive effect, and mortality benefit. GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors may be considered, even in patients with mild-to-moderate hepatic impairment, given its low risk of hypoglycemia, weight-neutral metabolic profile, and protective effect on hepatic steatosis and hepatic fibrosis. α-glucosidase inhibitors may be useful in patients with concurrent postprandial hyperglycemia and mild hepatic encephalopathy as a way to minimize polypharmacy. Insulin therapy should be reserved for patients who failed other antihyperglycemic medications and should entail frequent dose adjustment and close glucose monitoring to minimize the risk of hypoglycemia. Sulfonylureas and meglitinides should be avoided in most instances.
Figure 5.
Proposed diabetes treatment algorithm in patients with chronic liver diseases. CLD: Chronic liver diseases; DM: Diabetes mellitus; FPG: Fasting plasma glucose; OGTT: Oral glucose tolerance test; SGLT-2: Sodium-glucose cotransporter-2; GLP-1: Glucagon-like peptide-1; DPP-4: Dipeptidyl peptidase-4.
The aforementioned evidence also highlighted the importance of recognizing the impact of insulin resistance and DM on other etiologies of CLD besides NAFLD. A recent consensus statement endorsed by a panel of international experts has revitalized the decades-long effort to revise the definition and nomenclature of NAFLD[237], acknowledging the heterogeneity of the disease in relation to its natural history, risk factors, clinical manifestations, and responses to treatment[238]. The newly proposed definition of metabolic dysfunction-associated fatty liver disease (MAFLD) no longer requires the exclusion of alcohol consumption or other concomitant liver diseases. It also serves to promote research on patients with coexisting metabolic and alcoholic liver disease, who were previously excluded from most NAFLD studies but are in fact at high risk of disease progression[239].
FUTURE DIRECTIONS
Despite our improved understanding of the interplay between DM and CLD, thanks largely to research in the pathophysiology and management of NAFLD, many pressing clinical questions remain to be addressed. First, an alternative glycemic marker, whose diagnostic accuracy is not affected by altered erythrocyte turnover or excessive glycemic variability, is desperately needed for diagnosing and monitoring DM in patients with advanced liver diseases. Ideally, the test could be performed without prolonged fasting and the result could be easily converted back to an A1c-equivalent value. Second, the optimal glycemic target for slowing CLD progression and preventing liver disease complications while minimizing the risk of hypoglycemia needs to be established. It is reasonable to suspect that patients with various degrees of decompensation would benefit from different glycemic targets. Third, a serological marker for DM-related liver diseases akin to the use of urine albumin excretion to screen for diabetic nephropathy should be investigated. A recent study has shown, for example, that the circulating level of osteopontin (OPN), a soluble pluripotent glycophosphoprotein and a proinflammatory cytokine, may be useful in diagnosing NAFLD in patients with DM[240]. Fourth, given the impact of DM on the progressive of CLD and liver disease complications, it would be interesting to see if the inclusion of a glycemic marker in the calculation of the MELD score would improve its predictive value for the short-term survival and liver disease severity. Fifth, the long-term safety and efficacy of the novel antihyperglycemic medications, including GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors, need to be thoroughly investigated, especially in the non-NAFLD patient populations and with regard to liver disease-related clinical outcomes, such as encephalopathy and HCC development. Sixth, the oncogenic risk of insulin therapy in the context of insulin resistance and chronic hyperinsulinemia must be further evaluated. Seventh, considering the medical complexity of patients with CLD, the risk for drug-induced liver injury from polypharmacy, and the impact of glycemic control on transplant survival and complications, it is worth debating if DM in patients with CLD would be best managed by internists, endocrinologists, or hepatologists.
It is also important to acknowledge and address a number of systemic barriers in order to facilitate advances in this area of research. Clinical studies on the management of DM have traditionally focused on the micro-/macrovascular complications of prolonged hyperglycemia instead of liver disease-related clinical outcomes. In addition, patients with CLD are often excluded from clinical trials of novel medications due to overwhelming concerns regarding hepatic dysfunction and drug-induced liver injury. Conversely, there is an emphasis on the manifestations of portal hypertension as well as the relatively short-term changes in morbidity and mortality, instead of the systemic and long-term effects of insulin resistance, in the management of CLD. While the rapidly worsening epidemic of metabolic syndrome has certainly called to attention the impact of DM on the natural history of liver diseases, most research efforts, especially in terms of novel treatment options, remain restricted to the traditional definitions of NAFLD/NASH. We hope that the evolution from NAFLD to MAFLD would help broaden future studies in this area to include other etiologies of CLD as well over the next 5 to 10 years.
Footnotes
Conflict-of-interest statement: The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; and American Gastroenterological Association.
Peer-review started: July 8, 2020
First decision: July 30, 2020
Article in press: August 15, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Firneisz G, Tanaka N, S-Editor: Gong ZM L-Editor: A P-Editor: Li JH
Contributor Information
Waihong Chung, Division of Gastroenterology, Department of Medicine, Rhode Island Hospital, Providence, RI 02905, United States. waihongchung@gmail.com.
Kittichai Promrat, Division of Gastroenterology and Hepatology, Providence VA Medical Center, Providence, RI 02908, United States.
Jack Wands, Liver Research Center, The Warren Alpert Medical School of Brown University, Providence, RI 02903, United States.
References
- 1.Marceau P, Biron S, Hould FS, Marceau S, Simard S, Thung SN, Kral JG. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999;84:1513–1517. doi: 10.1210/jcem.84.5.5661. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 3.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 4.Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, Shah SR, Rathi PM, Joshi AS, Thakkar H, Menon PS, Shah NS. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–210. [PubMed] [Google Scholar]
- 5.Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol. 2006;40:68–76. doi: 10.1097/01.mcg.0000190774.91875.d2. [DOI] [PubMed] [Google Scholar]
- 6.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S34–S45. doi: 10.2337/dc19-S004. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, Paradis V, Vidaud M, Valla D, Bedossa P, Marcellin P. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, Jonsson JR. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39:1042–1048. doi: 10.1016/s0168-8278(03)00463-x. [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Munteanu M, Charlotte F, Bonyhay L, Poynard T LIDO Study Group. Fibrogenic impact of high serum glucose in chronic hepatitis C. J Hepatol. 2003;39:1049–1055. doi: 10.1016/s0168-8278(03)00456-2. [DOI] [PubMed] [Google Scholar]
- 13.Huang YW, Wang TC, Lin SC, Chang HY, Chen DS, Hu JT, Yang SS, Kao JH. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin Infect Dis. 2013;57:1695–1702. doi: 10.1093/cid/cit603. [DOI] [PubMed] [Google Scholar]
- 14.Huang YW, Yang SS, Fu SC, Wang TC, Hsu CK, Chen DS, Hu JT, Kao JH. Increased risk of cirrhosis and its decompensation in chronic hepatitis C patients with new-onset diabetes: a nationwide cohort study. Hepatology. 2014;60:807–814. doi: 10.1002/hep.27212. [DOI] [PubMed] [Google Scholar]
- 15.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 16.Guo X, Jin M, Yang M, Liu K, Li JW. Type 2 diabetes mellitus and the risk of hepatitis C virus infection: a systematic review. Sci Rep. 2013;3:2981. doi: 10.1038/srep02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599–1608. doi: 10.1053/j.gastro.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 19.Masini M, Campani D, Boggi U, Menicagli M, Funel N, Pollera M, Lupi R, Del Guerra S, Bugliani M, Torri S, Del Prato S, Mosca F, Filipponi F, Marchetti P. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28:940–941. doi: 10.2337/diacare.28.4.940. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Chen J, Wang Y, Han X, Chen X. Hepatitis C virus induced a novel apoptosis-like death of pancreatic beta cells through a caspase 3-dependent pathway. PLoS One. 2012;7:e38522. doi: 10.1371/journal.pone.0038522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, Baba S, Koga H, Kumashiro R, Ueno T, Ogata H, Yoshimura A, Sata M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 23.Harada T, Kim DW, Sagawa K, Suzuki T, Takahashi K, Saito I, Matsuura Y, Miyamura T. Characterization of an established human hepatoma cell line constitutively expressing non-structural proteins of hepatitis C virus by transfection of viral cDNA. J Gen Virol. 1995;76(Pt 5):1215–1221. doi: 10.1099/0022-1317-76-5-1215. [DOI] [PubMed] [Google Scholar]
- 24.Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Meltzer H. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 25.Itou M, Kawaguchi T, Taniguchi E, Sumie S, Oriishi T, Mitsuyama K, Tsuruta O, Ueno T, Sata M. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23:244–251. doi: 10.1111/j.1440-1746.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 26.Del Vecchio Blanco C, Gentile S, Marmo R, Carbone L, Coltorti M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res Clin Pract. 1990;8:29–36. doi: 10.1016/0168-8227(90)90093-9. [DOI] [PubMed] [Google Scholar]
- 27.Grancini V, Trombetta M, Lunati ME, Zimbalatti D, Boselli ML, Gatti S, Donato MF, Resi V, D'Ambrosio R, Aghemo A, Pugliese G, Bonadonna RC, Orsi E. Contribution of β-cell dysfunction and insulin resistance to cirrhosis-associated diabetes: Role of severity of liver disease. J Hepatol. 2015;63:1484–1490. doi: 10.1016/j.jhep.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Moreau R, Delègue P, Pessione F, Hillaire S, Durand F, Lebrec D, Valla DC. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int. 2004;24:457–464. doi: 10.1111/j.1478-3231.2004.0991.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishida T, Tsuji S, Tsujii M, Arimitsu S, Haruna Y, Imano E, Suzuki M, Kanda T, Kawano S, Hiramatsu N, Hayashi N, Hori M. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70–75. doi: 10.1111/j.1572-0241.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 30.Quintana JO, García-Compean D, González JA, Pérez JZ, González FJ, Espinosa LE, Hernández PL, Cabello ER, Villarreal ER, Rendón RF, Garza HM. The impact of diabetes mellitus in mortality of patients with compensated liver cirrhosis-a prospective study. Ann Hepatol. 2011;10:56–62. [PubMed] [Google Scholar]
- 31.García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, González-González JA, Muñoz-Espinosa LE, Villarreal-Pérez JZ, Maldonado-Garza HJ. Subclinical abnormal glucose tolerance is a predictor of death in liver cirrhosis. World J Gastroenterol. 2014;20:7011–7018. doi: 10.3748/wjg.v20.i22.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagel S, Bruns T, Herrmann A, Stallmach A, Schmidt C. Abnormal glucose tolerance: a predictor of 30-day mortality in patients with decompensated liver cirrhosis. Z Gastroenterol. 2011;49:331–334. doi: 10.1055/s-0029-1245933. [DOI] [PubMed] [Google Scholar]
- 33.Moscatiello S, Manini R, Marchesini G. Diabetes and liver disease: an ominous association. Nutr Metab Cardiovasc Dis. 2007;17:63–70. doi: 10.1016/j.numecd.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Elkrief L, Chouinard P, Bendersky N, Hajage D, Larroque B, Babany G, Kutala B, Francoz C, Boyer N, Moreau R, Durand F, Marcellin P, Rautou PE, Valla D. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823–831. doi: 10.1002/hep.27228. [DOI] [PubMed] [Google Scholar]
- 35.Butt Z, Jadoon NA, Salaria ON, Mushtaq K, Riaz IB, Shahzad A, Hashmi AM, Sarwar S. Diabetes mellitus and decompensated cirrhosis: risk of hepatic encephalopathy in different age groups. J Diabetes. 2013;5:449–455. doi: 10.1111/1753-0407.12067. [DOI] [PubMed] [Google Scholar]
- 36.Yang WS, Va P, Bray F, Gao S, Gao J, Li HL, Xiang YB. The role of pre-existing diabetes mellitus on hepatocellular carcinoma occurrence and prognosis: a meta-analysis of prospective cohort studies. PLoS One. 2011;6:e27326. doi: 10.1371/journal.pone.0027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 38.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 39.Falleti E, Cussigh A, Cmet S, Fabris C, Toniutto P. PNPLA3 rs738409 and TM6SF2 rs58542926 variants increase the risk of hepatocellular carcinoma in alcoholic cirrhosis. Dig Liver Dis. 2016;48:69–75. doi: 10.1016/j.dld.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Hassan MM, Kaseb A, Etzel CJ, El-Serag H, Spitz MR, Chang P, Hale KS, Liu M, Rashid A, Shama M, Abbruzzese JL, Loyer EM, Kaur H, Hassabo HM, Vauthey JN, Wray CJ, Hassan BS, Patt YZ, Hawk E, Soliman KM, Li D. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: risk and prognosis prediction. Mol Carcinog. 2013;52 Suppl 1:E139–E147. doi: 10.1002/mc.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 42.Ish-Shalom D, Christoffersen CT, Vorwerk P, Sacerdoti-Sierra N, Shymko RM, Naor D, De Meyts P. Mitogenic properties of insulin and insulin analogues mediated by the insulin receptor. Diabetologia. 1997;40 Suppl 2:S25–S31. doi: 10.1007/s001250051393. [DOI] [PubMed] [Google Scholar]
- 43.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 44.Bohinc BN, Diehl AM. Mechanisms of disease progression in NASH: new paradigms. Clin Liver Dis. 2012;16:549–565. doi: 10.1016/j.cld.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Petreaca M, Martins-Green M. Cell and molecular mechanisms of insulin-induced angiogenesis. J Cell Mol Med. 2009;13:4492–4504. doi: 10.1111/j.1582-4934.2008.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 47.Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2014;9:e95485. doi: 10.1371/journal.pone.0095485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanaga K, Matsumata T, Hayashi H, Shimada M, Urata K, Suehiro T, Sugimachi K. Effect of diabetes mellitus on hepatic resection. Arch Surg. 1993;128:445–448. doi: 10.1001/archsurg.1993.01420160087014. [DOI] [PubMed] [Google Scholar]
- 49.Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, Sugimachi K. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–198. doi: 10.1046/j.1365-2168.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 50.Huo TI, Wu JC, Huang YH, Chiang JH, Lee PC, Chang FY, Lee SD. Acute renal failure after transarterial chemoembolization for hepatocellular carcinoma: a retrospective study of the incidence, risk factors, clinical course and long-term outcome. Aliment Pharmacol Ther. 2004;19:999–1007. doi: 10.1111/j.1365-2036.2004.01936.x. [DOI] [PubMed] [Google Scholar]
- 51.Shin JU, Kim KM, Shin SW, Min SY, Park SU, Sinn DH, Gwak GY, Choi MS, Lee JH, Paik SW, Yoo BC, Koh KC. A prediction model for liver abscess developing after transarterial chemoembolization in patients with hepatocellular carcinoma. Dig Liver Dis. 2014;46:813–817. doi: 10.1016/j.dld.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Deschênes M, Somberg KA. Effect of transjugular intrahepatic portosystemic shunt (TIPS) on glycemic control in cirrhotic patients with diabetes mellitus. Am J Gastroenterol. 1998;93:483. doi: 10.1111/j.1572-0241.1998.481_4.x. [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa T, Shiratsuki S, Matsuda T, Iwamoto T, Takami T, Uchida K, Terai S, Yamasaki T, Sakaida I. Occlusion of portosystemic shunts improves hyperinsulinemia due to insulin resistance in cirrhotic patients with portal hypertension. J Gastroenterol. 2014;49:1333–1341. doi: 10.1007/s00535-013-0893-z. [DOI] [PubMed] [Google Scholar]
- 54.Greco AV, Mingrone G, Mari A, Capristo E, Manco M, Gasbarrini G. Mechanisms of hyperinsulinaemia in Child's disease grade B liver cirrhosis investigated in free living conditions. Gut. 2002;51:870–875. doi: 10.1136/gut.51.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Meyts P, Roth J, Neville DM, Jr, Gavin JR, 3rd, Lesniak MA. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973;55:154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]
- 56.Gavin JR, 3rd, Roth J, Neville DM, Jr, de Meyts P, Buell DN. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci USA. 1974;71:84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catalano KJ, Maddux BA, Szary J, Youngren JF, Goldfine ID, Schaufele F. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS One. 2014;9:e108693. doi: 10.1371/journal.pone.0108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shmueli E, Walker M, Alberti G, Record CO. Normal splanchnic but impaired peripheral insulin-stimulated glucose uptake in cirrhosis. Hepatology. 1993;18:86–95. [PubMed] [Google Scholar]
- 59.Kruszynska YT, Harry DS, Bergman RN, McIntyre N. Insulin sensitivity, insulin secretion and glucose effectiveness in diabetic and non-diabetic cirrhotic patients. Diabetologia. 1993;36:121–128. doi: 10.1007/BF00400692. [DOI] [PubMed] [Google Scholar]
- 60.Chen YM, Zhao JF, Liu YL, Chen J, Jiang RL. Chronic ethanol treatment of human hepatocytes inhibits the activation of the insulin signaling pathway by increasing cytosolic free calcium levels. Int J Mol Med. 2015;36:739–746. doi: 10.3892/ijmm.2015.2282. [DOI] [PubMed] [Google Scholar]
- 61.Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res. 2007;31:1581–1588. doi: 10.1111/j.1530-0277.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 62.Bernsmeier C, Meyer-Gerspach AC, Blaser LS, Jeker L, Steinert RE, Heim MH, Beglinger C. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS One. 2014;9:e87488. doi: 10.1371/journal.pone.0087488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baumeier C, Schlüter L, Saussenthaler S, Laeger T, Rödiger M, Alaze SA, Fritsche L, Häring HU, Stefan N, Fritsche A, Schwenk RW, Schürmann A. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol Metab. 2017;6:1254–1263. doi: 10.1016/j.molmet.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, McClain DA. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145:5305–5312. doi: 10.1210/en.2004-0392. [DOI] [PubMed] [Google Scholar]
- 65.Kim JY, Song EH, Lee HJ, Oh YK, Park YS, Park JW, Kim BJ, Kim DJ, Lee I, Song J, Kim WH. Chronic ethanol consumption-induced pancreatic {beta}-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J Biol Chem. 2010;285:37251–37262. doi: 10.1074/jbc.M110.142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim JW, Yoon KH. Glucolipotoxicity in Pancreatic β-Cells. Diabetes Metab J. 2011;35:444–450. doi: 10.4093/dmj.2011.35.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antonelli A, Ferrari SM, Ruffilli I, Fallahi P. Cytokines and HCV-related autoimmune disorders. Immunol Res. 2014;60:311–319. doi: 10.1007/s12026-014-8569-1. [DOI] [PubMed] [Google Scholar]
- 68.Sebeková K, Kupcová V, Schinzel R, Heidland A. Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis - amelioration by liver transplantation. J Hepatol. 2002;36:66–71. doi: 10.1016/s0168-8278(01)00232-x. [DOI] [PubMed] [Google Scholar]
- 69.Lim M, Park L, Shin G, Hong H, Kang I, Park Y. Induction of apoptosis of Beta cells of the pancreas by advanced glycation end-products, important mediators of chronic complications of diabetes mellitus. Ann N Y Acad Sci. 2008;1150:311–315. doi: 10.1196/annals.1447.011. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Z, Zhao C, Zhang XH, Zheng F, Cai W, Vlassara H, Ma ZA. Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology. 2009;150:2569–2576. doi: 10.1210/en.2008-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.John PR, Thuluvath PJ. Outcome of liver transplantation in patients with diabetes mellitus: a case-control study. Hepatology. 2001;34:889–895. doi: 10.1053/jhep.2001.29134. [DOI] [PubMed] [Google Scholar]
- 72.Samuelson AL, Lee M, Kamal A, Keeffe EB, Ahmed A. Diabetes mellitus increases the risk of mortality following liver transplantation independent of MELD score. Dig Dis Sci. 2010;55:2089–2094. doi: 10.1007/s10620-010-1267-5. [DOI] [PubMed] [Google Scholar]
- 73.Younossi ZM, Stepanova M, Saab S, Kalwaney S, Clement S, Henry L, Frost S, Hunt S. The impact of type 2 diabetes and obesity on the long-term outcomes of more than 85 000 liver transplant recipients in the US. Aliment Pharmacol Ther. 2014;40:686–694. doi: 10.1111/apt.12881. [DOI] [PubMed] [Google Scholar]
- 74.Driscoll CJ, Cashion AK, Hathaway DK, Thompson C, Conley Y, Gaber O, Vera S, Shokouh-Amiri H. Posttransplant diabetes mellitus in liver transplant recipients. Prog Transplant. 2006;16:110–116. doi: 10.1177/152692480601600204. [DOI] [PubMed] [Google Scholar]
- 75.John PR, Thuluvath PJ. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl. 2002;8:708–713. doi: 10.1053/jlts.2002.34638. [DOI] [PubMed] [Google Scholar]
- 76.Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, Zein NN. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18:370–375. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 77.Steinmüller TH, Stockmann M, Bechstein WO, Settmacher U, Jonas S, Neuhaus P. Liver transplantation and diabetes mellitus. Exp Clin Endocrinol Diabetes. 2000;108:401–405. doi: 10.1055/s-2000-8136. [DOI] [PubMed] [Google Scholar]
- 78.Bigam DL, Pennington JJ, Carpentier A, Wanless IR, Hemming AW, Croxford R, Greig PD, Lilly LB, Heathcote JE, Levy GA, Cattral MS. Hepatitis C-related cirrhosis: a predictor of diabetes after liver transplantation. Hepatology. 2000;32:87–90. doi: 10.1053/jhep.2000.8270. [DOI] [PubMed] [Google Scholar]
- 79.Tietge UJ, Selberg O, Kreter A, Bahr MJ, Pirlich M, Burchert W, Müller MJ, Manns MP, Böker KH. Alterations in glucose metabolism associated with liver cirrhosis persist in the clinically stable long-term course after liver transplantation. Liver Transpl. 2004;10:1030–1040. doi: 10.1002/lt.20147. [DOI] [PubMed] [Google Scholar]
- 80.Tueche SG. Diabetes mellitus after liver transplant new etiologic clues and cornerstones for understanding. Transplant Proc. 2003;35:1466–1468. doi: 10.1016/s0041-1345(03)00528-1. [DOI] [PubMed] [Google Scholar]
- 81.Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004;10:349–355. doi: 10.1002/lt.20092. [DOI] [PubMed] [Google Scholar]
- 82.Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662–1670. doi: 10.1002/lt.21952. [DOI] [PubMed] [Google Scholar]
- 83.Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 84.Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, McVicar J, Zern M, Torok N. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844–847. doi: 10.1002/lt.20932. [DOI] [PubMed] [Google Scholar]
- 85.Pirenne J, Deloose K, Coosemans W, Aerts R, Van Gelder F, Kuypers D, Maes B, Verslype C, Yap P, Van Steenbergen W, Roskams T, Mathieu C, Fevery J, Nevens F. Combined 'en bloc' liver and pancreas transplantation in patients with liver disease and type 1 diabetes mellitus. Am J Transplant. 2004;4:1921–1927. doi: 10.1111/j.1600-6143.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 86.Infante M, Padilla N, Madiraju S, Alvarez A, Baidal D, Ricordi C, Alejandro R. Chapter 37 - Combined liver and islet transplantation in hepatogenous diabetes, cluster exenteration, and cirrhosis with type 1 diabetes. In: Orlando G, Piemonti L, Ricordi C, Stratta RJ, Gruessner RWG, editors. Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas: Academic Press; 2020: 439-453. [Google Scholar]
- 87.García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, Reyes-Cabello E, González-González JA, Muñoz-Espinosa LE, Vázquez-Elizondo G, Villarreal-Pérez JZ, Maldonado-Garza HJ. The prevalence and clinical characteristics of glucose metabolism disorders in patients with liver cirrhosis. A prospective study. Ann Hepatol. 2012;11:240–248. [PubMed] [Google Scholar]
- 88.McIntyre S, Crowther CA, Hiller JE, McPhee AJ. Lag times between blood sampling, spinning and plasma glucose estimation. Aust N Z J Obstet Gynaecol. 1997;37:286–288. doi: 10.1111/j.1479-828x.1997.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 89.Barr RG, Nathan DM, Meigs JB, Singer DE. Tests of glycemia for the diagnosis of type 2 diabetes mellitus. Ann Intern Med. 2002;137:263–272. doi: 10.7326/0003-4819-137-4-200208200-00011. [DOI] [PubMed] [Google Scholar]
- 90.Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism. 2006;55:434–438. doi: 10.1016/j.metabol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Kim KS, Kim SK, Lee YK, Park SW, Cho YW. Diagnostic value of glycated haemoglobin HbA(1c) for the early detection of diabetes in high-risk subjects. Diabet Med. 2008;25:997–1000. doi: 10.1111/j.1464-5491.2008.02489.x. [DOI] [PubMed] [Google Scholar]
- 92.Imano E, Nishida T, Shibata M, Kanda T. Significance of oral glucose tolerance test for the diagnosis of diabetes mellitus in patients with liver cirrhosis. Intern Med. 1999;38:918. doi: 10.2169/internalmedicine.38.918. [DOI] [PubMed] [Google Scholar]
- 93.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 94.Ko GT, Chan JC, Woo J, Lau E, Yeung VT, Chow CC, Cockram CS. The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem. 1998;35(Pt 1):62–67. doi: 10.1177/000456329803500107. [DOI] [PubMed] [Google Scholar]
- 95.Koenig RJ, Peterson CM, Kilo C, Cerami A, Williamson JR. Hemoglobin AIc as an indicator of the degree of glucose intolerance in diabetes. Diabetes. 1976;25:230–232. doi: 10.2337/diab.25.3.230. [DOI] [PubMed] [Google Scholar]
- 96.Lahousen T, Hegenbarth K, Ille R, Lipp RW, Krause R, Little RR, Schnedl WJ. Determination of glycated hemoglobin in patients with advanced liver disease. World J Gastroenterol. 2004;10:2284–2286. doi: 10.3748/wjg.v10.i15.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nadelson J, Satapathy SK, Nair S. Glycated Hemoglobin Levels in Patients with Decompensated Cirrhosis. Int J Endocrinol. 2016;2016:8390210. doi: 10.1155/2016/8390210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982;59:1348–1350. [PubMed] [Google Scholar]
- 99.Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15:4653–4658. doi: 10.3748/wjg.15.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weinblatt ME, Kochen JA, Scimeca PG. Chronically transfused patients with increased hemoglobin Alc secondary to donor blood. Ann Clin Lab Sci. 1986;16:34–37. [PubMed] [Google Scholar]
- 101.Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem. 2011;57:344–346. doi: 10.1373/clinchem.2010.157321. [DOI] [PubMed] [Google Scholar]
- 102.Addepally NS, George N, Martinez-Macias R, Garcia-Saenz-de-Sicilia M, Kim WR, Duarte-Rojo A. Hemoglobin A1c Has Suboptimal Performance to Diagnose and Monitor Diabetes Mellitus in Patients with Cirrhosis. Dig Dis Sci. 2018;63:3498–3508. doi: 10.1007/s10620-018-5265-3. [DOI] [PubMed] [Google Scholar]
- 103.Hashiba M, Ono M, Hyogo H, Ikeda Y, Masuda K, Yoshioka R, Ishikawa Y, Nagata Y, Munekage K, Ochi T, Hirose A, Nozaki-Fujimura Y, Noguchi S, Okamoto N, Chayama K, Suganuma N, Saibara T. Glycemic variability is an independent predictive factor for development of hepatic fibrosis in nonalcoholic fatty liver disease. PLoS One. 2013;8:e76161. doi: 10.1371/journal.pone.0076161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang A, Liu X, Xu J, Han X, Su Z, Chen S, Zhang N, Wu S, Wang Y, Wang Y. Visit-to-Visit Variability of Fasting Plasma Glucose and the Risk of Cardiovascular Disease and All-Cause Mortality in the General Population. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wakabayashi I. Obesity-independent inverse association between regular alcohol consumption and hemoglobin A(1C) Obes Facts. 2012;5:60–67. doi: 10.1159/000336067. [DOI] [PubMed] [Google Scholar]
- 106.Hong JW, Noh JH, Kim DJ. Association between Alcohol Intake and Hemoglobin A1c in the Korean Adults: The 2011-2013 Korea National Health and Nutrition Examination Survey. PLoS One. 2016;11:e0167210. doi: 10.1371/journal.pone.0167210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inada S, Koga M. Alcohol consumption reduces HbA1c and glycated albumin concentrations but not 1,5-anhydroglucitol. Ann Clin Biochem. 2017;54:631–635. doi: 10.1177/0004563216675646. [DOI] [PubMed] [Google Scholar]
- 108.Hoberman HD, Chiodo SM. Elevation of the hemoglobin A1 fraction in alcoholism. Alcohol Clin Exp Res. 1982;6:260–266. doi: 10.1111/j.1530-0277.1982.tb04972.x. [DOI] [PubMed] [Google Scholar]
- 109.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–2163. [PubMed] [Google Scholar]
- 110.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751–762. doi: 10.1507/endocrj.k10e-138. [DOI] [PubMed] [Google Scholar]
- 111.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 112.Freedman BI, Shenoy RN, Planer JA, Clay KD, Shihabi ZK, Burkart JM, Cardona CY, Andries L, Peacock TP, Sabio H, Byers JR, Russell GB, Bleyer AJ. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int. 2010;30:72–79. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 113.Constanti C, Simo JM, Joven J, Camps J. Serum fructosamine concentration in patients with nephrotic syndrome and with cirrhosis of the liver: the influence of hypoalbuminaemia and hypergammaglobulinaemia. Ann Clin Biochem. 1992;29(Pt 4):437–442. doi: 10.1177/000456329202900412. [DOI] [PubMed] [Google Scholar]
- 114.Koga M, Kasayama S, Kanehara H, Bando Y. CLD (chronic liver diseases)-HbA1C as a suitable indicator for estimation of mean plasma glucose in patients with chronic liver diseases. Diabetes Res Clin Pract. 2008;81:258–262. doi: 10.1016/j.diabres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 115.Rodríguez-Segade S, Rodríguez J, Camiña F. Corrected Fructosamine improves both correlation with HbA1C and diagnostic performance. Clin Biochem. 2017;50:110–115. doi: 10.1016/j.clinbiochem.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 116.Yamanouchi T, Akanuma Y. Serum 1,5-anhydroglucitol (1,5 AG): new clinical marker for glycemic control. Diabetes Res Clin Pract. 1994;24 Suppl:S261–S268. doi: 10.1016/0168-8227(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Yuan Y, Zhang Y, Lei C, Zhou Y, He J, Sun Z. Serum 1,5-anhydroglucitol level as a screening tool for diabetes mellitus in a community-based population at high risk of diabetes. Acta Diabetol. 2017;54:425–431. doi: 10.1007/s00592-016-0944-z. [DOI] [PubMed] [Google Scholar]
- 118.Shibayama M, Oba N, Senda Y, Nishibu M, Hashimoto T. [Clinical evaluation of serum 1,5AG levels in patients with liver cirrhosis] Rinsho Byori. 1997;45:1187–1190. [PubMed] [Google Scholar]
- 119.Yamagishi S, Ohta M. Serum 1,5-anhydro-D-glucitol levels in liver cirrhosis. Acta Diabetol. 1998;35:65–66. doi: 10.1007/s005920050104. [DOI] [PubMed] [Google Scholar]
- 120.Juraschek SP, Miller ER, 3rd, Appel LJ, Christenson RH, Sacks FM, Selvin E. Effects of dietary carbohydrate on 1,5-anhydroglucitol in a population without diabetes: results from the OmniCarb trial. Diabet Med. 2017;34:1407–1413. doi: 10.1111/dme.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 122.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 123.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 124.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 125.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 126.Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ, Hoofnagle JH. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157–163. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, Akarca U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 129.Doycheva I, Loomba R. Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis (NASH): when to use metformin in nonalcoholic fatty liver disease (NAFLD) Adv Ther. 2014;31:30–43. doi: 10.1007/s12325-013-0084-6. [DOI] [PubMed] [Google Scholar]
- 130.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 131.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, Vauthey JN. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 133.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 134.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, Ganne-Carrie N, Grando-Lemaire V, Vicaut E, Trinchet JC, Beaugrand M. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601–2608. doi: 10.1210/jc.2010-2415. [DOI] [PubMed] [Google Scholar]
- 135.Ampuero J, Ranchal I, Nuñez D, Díaz-Herrero Mdel M, Maraver M, del Campo JA, Rojas Á, Camacho I, Figueruela B, Bautista JD, Romero-Gómez M. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS One. 2012;7:e49279. doi: 10.1371/journal.pone.0049279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008–2016. doi: 10.1002/hep.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brackett CC. Clarifying metformin's role and risks in liver dysfunction. J Am Pharm Assoc (2003) 2010;50:407–410. doi: 10.1331/JAPhA.2010.08090. [DOI] [PubMed] [Google Scholar]
- 138.Kim YH, Hwang JH, Kim KS, Noh JR, Choi DH, Kim DK, Tadi S, Yim YH, Choi HS, Lee CH. Metformin ameliorates acetaminophen hepatotoxicity via Gadd45β-dependent regulation of JNK signaling in mice. J Hepatol. 2015;63:75–82. doi: 10.1016/j.jhep.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 139.Saeedi Saravi SS, Hasanvand A, Shahkarami K, Dehpour AR. The protective potential of metformin against acetaminophen-induced hepatotoxicity in BALB/C mice. Pharm Biol. 2016;54:2830–2837. doi: 10.1080/13880209.2016.1185633. [DOI] [PubMed] [Google Scholar]
- 140.Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care. 2014;37:2291–2295. doi: 10.2337/dc14-0464. [DOI] [PubMed] [Google Scholar]
- 141.Trinkley KE, Anderson HD, Nair KV, Malone DC, Saseen JJ. Assessing the incidence of acidosis in patients receiving metformin with and without risk factors for lactic acidosis. Ther Adv Chronic Dis. 2018;9:179–190. doi: 10.1177/2040622318779760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Conlay LA, Karam JH, Matin SB, Loewenstein JE. Serum phenformin concentrations in patients with phenformin-associated lactic acidosis. Diabetes. 1977;26:628–631. doi: 10.2337/diab.26.7.628. [DOI] [PubMed] [Google Scholar]
- 143.Bailey CJ, Wilcock C, Day C. Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol. 1992;105:1009–1013. doi: 10.1111/j.1476-5381.1992.tb09093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Radziuk J, Zhang Z, Wiernsperger N, Pye S. Effects of metformin on lactate uptake and gluconeogenesis in the perfused rat liver. Diabetes. 1997;46:1406–1413. doi: 10.2337/diab.46.9.1406. [DOI] [PubMed] [Google Scholar]
- 145.Zilva JF. The origin of the acidosis in hyperlactataemia. Ann Clin Biochem. 1978;15:40–43. doi: 10.1177/000456327801500111. [DOI] [PubMed] [Google Scholar]
- 146.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 147.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 148.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 150.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 151.Bril F, Kalavalapalli S, Clark VC, Lomonaco R, Soldevila-Pico C, Liu IC, Orsak B, Tio F, Cusi K. Response to Pioglitazone in Patients With Nonalcoholic Steatohepatitis With vs Without Type 2 Diabetes. Clin Gastroenterol Hepatol. 2018;16:558–566.e2. doi: 10.1016/j.cgh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 152.Yang Y, Zhao LH, Huang B, Wang RY, Yuan SX, Tao QF, Xu Y, Sun HY, Lin C, Zhou WP. Pioglitazone, a PPARγ agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol Carcinog. 2015;54:1584–1595. doi: 10.1002/mc.22231. [DOI] [PubMed] [Google Scholar]
- 153.Li S, Ghoshal S, Sojoodi M, Arora G, Masia R, Erstad DJ, Lanuti M, Hoshida Y, Baumert TF, Tanabe KK, Fuchs BC. Pioglitazone Reduces Hepatocellular Carcinoma Development in Two Rodent Models of Cirrhosis. J Gastrointest Surg. 2019;23:101–111. doi: 10.1007/s11605-018-4004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sumie S, Kawaguchi T, Kawaguchi A, Kuromatsu R, Nakano M, Satani M, Yamada S, Okamura S, Yonezawa Y, Kakuma T, Torimura T, Sata M. Effect of pioglitazone on outcome following curative treatment for hepatocellular carcinoma in patients with hepatitis C virus infection: A prospective study. Mol Clin Oncol. 2015;3:115–120. doi: 10.3892/mco.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med. 1998;338:916–917. doi: 10.1056/NEJM199803263381314. [DOI] [PubMed] [Google Scholar]
- 156.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 157.Hampp C, Pippins J. Pioglitazone and bladder cancer: FDA's assessment. Pharmacoepidemiol Drug Saf. 2017;26:117–118. doi: 10.1002/pds.4154. [DOI] [PubMed] [Google Scholar]
- 158.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T LIDO Study Group. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 159.Athyros VG, Alexandrides TK, Bilianou H, Cholongitas E, Doumas M, Ganotakis ES, Goudevenos J, Elisaf MS, Germanidis G, Giouleme O, Karagiannis A, Karvounis C, Katsiki N, Kotsis V, Kountouras J, Liberopoulos E, Pitsavos C, Polyzos S, Rallidis LS, Richter D, Tsapas AG, Tselepis AD, Tsioufis K, Tziomalos K, Tzotzas T, Vasiliadis TG, Vlachopoulos C, Mikhailidis DP, Mantzoros C. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism. 2017;71:17–32. doi: 10.1016/j.metabol.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 160.Panikar V, Kale NJ, Hoskote SS, Deogaonkar N, Joshi SR. Effect of Low (7.5 mg/day), Standard (15 mg/ day) and High (30 mg/day) Dose Pioglitazone Therapy on Glycemic Control and Weight Gain in Recently-Diagnosed Type 2 Diabetes Patients. J Assoc Physicians India. 2015;63:36–39. [PubMed] [Google Scholar]
- 161.Chilcott J, Tappenden P, Jones ML, Wight JP. A systematic review of the clinical effectiveness of pioglitazone in the treatment of type 2 diabetes mellitus. Clin Ther. 2001;23:1792–823; discussion 1791. doi: 10.1016/s0149-2918(00)80078-8. [DOI] [PubMed] [Google Scholar]
- 162.Filipova E, Uzunova K, Kalinov K, Vekov T. Effects of pioglitazone therapy on blood parameters, weight and BMI: a meta-analysis. Diabetol Metab Syndr. 2017;9:90. doi: 10.1186/s13098-017-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Matthews L, Kleiner DE, Chairez C, McManus M, Nettles MJ, Zemanick K, Morse CG, Benator D, Kovacs JA, Hadigan C. Pioglitazone for Hepatic Steatosis in HIV/Hepatitis C Virus Coinfection. AIDS Res Hum Retroviruses. 2015;31:961–966. doi: 10.1089/aid.2015.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Imano E, Kanda T, Nakatani Y, Motomura M, Arai K, Matsuhisa M, Yamasaki Y, Hori M. Impaired splanchnic and peripheral glucose uptake in liver cirrhosis. J Hepatol. 1999;31:469–473. doi: 10.1016/s0168-8278(99)80039-7. [DOI] [PubMed] [Google Scholar]
- 165.Gentile S, Turco S, Guarino G, Oliviero B, Annunziata S, Cozzolino D, Sasso FC, Turco A, Salvatore T, Torella R. Effect of treatment with acarbose and insulin in patients with non-insulin-dependent diabetes mellitus associated with non-alcoholic liver cirrhosis. Diabetes Obes Metab. 2001;3:33–40. doi: 10.1046/j.1463-1326.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- 166.Gentile S, Guarino G, Romano M, Alagia IA, Fierro M, Annunziata S, Magliano PL, Gravina AG, Torella R. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3:184–191. doi: 10.1016/s1542-3565(04)00667-6. [DOI] [PubMed] [Google Scholar]
- 167.Komatsu M, Tanaka N, Kimura T, Fujimori N, Sano K, Horiuchi A, Sugiura A, Yamazaki T, Shibata S, Joshita S, Umemura T, Matsumoto A, Tanaka E. Miglitol attenuates non-alcoholic steatohepatitis in diabetic patients. Hepatol Res. 2018;48:1092–1098. doi: 10.1111/hepr.13223. [DOI] [PubMed] [Google Scholar]
- 168.Negishi M, Shimomura K, Proks P, Shimomura Y, Mori M. Alpha glucosidase inhibitor voglibose can prevent pioglitazone-induced body weight gain in Type 2 diabetic patients. Br J Clin Pharmacol. 2008;66:318–319. doi: 10.1111/j.1365-2125.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Diaz-Gutierrez FL, Ladero JM, Diaz-Rubio M. Acarbose-induced acute hepatitis. Am J Gastroenterol. 1998;93:481. doi: 10.1111/j.1572-0241.1998.481_1.x. [DOI] [PubMed] [Google Scholar]
- 170.Kihara Y, Ogami Y, Tabaru A, Unoki H, Otsuki M. Safe and effective treatment of diabetes mellitus associated with chronic liver diseases with an alpha-glucosidase inhibitor, acarbose. J Gastroenterol. 1997;32:777–782. doi: 10.1007/BF02936954. [DOI] [PubMed] [Google Scholar]
- 171.Chao CT, Wang J, Huang JW, Chien KL. Acarbose Use and Liver Injury in Diabetic Patients With Severe Renal Insufficiency and Hepatic Diseases: A Propensity Score-Matched Cohort Study. Front Pharmacol. 2018;9:860. doi: 10.3389/fphar.2018.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 174.Cuthbertson DJ, Irwin A, Gardner CJ, Daousi C, Purewal T, Furlong N, Goenka N, Thomas EL, Adams VL, Pushpakom SP, Pirmohamed M, Kemp GJ. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One. 2012;7:e50117. doi: 10.1371/journal.pone.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, Chen W, Yin T, Zhu D. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9:800–809. doi: 10.1111/1753-0407.12555. [DOI] [PubMed] [Google Scholar]
- 176.Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, Li Y, Dou J, Yang W, Qin G, Yuan H, Xiao X, Luo S, Shan Z, Deng H, Tan Y, Xu F, Xu W, Zeng L, Kang Z, Weng J. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:2414–2426. doi: 10.1002/hep.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, Araki N, Tanaka K, Yamaguchi M, Matsuda Y, Ide Y, Otsuka T, Ozaki I, Ono N, Eguchi T, Anzai K Japan Study Group for NAFLD (JSG-NAFLD) Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J) Hepatol Res. 2015;45:269–278. doi: 10.1111/hepr.12351. [DOI] [PubMed] [Google Scholar]
- 178.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 179.Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, Ronsin O, Pradel V, Lesavre N, Martin JC, Jacquier A, Lefur Y, Bernard M, Gaborit B. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18:882–891. doi: 10.1111/dom.12680. [DOI] [PubMed] [Google Scholar]
- 180.Thomsen M, Dencker D, Wörtwein G, Weikop P, Egecioglu E, Jerlhag E, Fink-Jensen A, Molander A. The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacol Biochem Behav. 2017;160:14–20. doi: 10.1016/j.pbb.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 181.Thomsen M, Holst JJ, Molander A, Linnet K, Ptito M, Fink-Jensen A. Effects of glucagon-like peptide 1 analogs on alcohol intake in alcohol-preferring vervet monkeys. Psychopharmacology (Berl) 2019;236:603–611. doi: 10.1007/s00213-018-5089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, Tomlinson JW, Newsome PN. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234–242. doi: 10.1111/apt.12149. [DOI] [PubMed] [Google Scholar]
- 183.Giorda CB, Nada E, Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine. 2014;46:406–419. doi: 10.1007/s12020-014-0179-0. [DOI] [PubMed] [Google Scholar]
- 184.Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis. 2013;226:305–314. doi: 10.1016/j.atherosclerosis.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 185.Pratley RE, Salsali A. Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin. 2007;23:919–931. doi: 10.1185/030079906x162746. [DOI] [PubMed] [Google Scholar]
- 186.Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E, Tatar G. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242–250. [PubMed] [Google Scholar]
- 187.Firneisz G, Varga T, Lengyel G, Fehér J, Ghyczy D, Wichmann B, Selmeci L, Tulassay Z, Rácz K, Somogyi A. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: a novel liver disease biomarker. PLoS One. 2010;5:e12226. doi: 10.1371/journal.pone.0012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miyazaki M, Kato M, Tanaka K, Tanaka M, Kohjima M, Nakamura K, Enjoji M, Nakamuta M, Kotoh K, Takayanagi R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–733. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 189.Ohyama T, Sato K, Yamazaki Y, Hashizume H, Horiguchi N, Kakizaki S, Mori M, Kusano M, Yamada M. MK-0626, a selective DPP-4 inhibitor, attenuates hepatic steatosis in ob/ob mice. World J Gastroenterol. 2014;20:16227–16235. doi: 10.3748/wjg.v20.i43.16227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ideta T, Shirakami Y, Miyazaki T, Kochi T, Sakai H, Moriwaki H, Shimizu M. The Dipeptidyl Peptidase-4 Inhibitor Teneligliptin Attenuates Hepatic Lipogenesis via AMPK Activation in Non-Alcoholic Fatty Liver Disease Model Mice. Int J Mol Sci. 2015;16:29207–29218. doi: 10.3390/ijms161226156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alam S, Ghosh J, Mustafa G, Kamal M, Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepat Med. 2018;10:23–31. doi: 10.2147/HMER.S158053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, Haufe W, Hooker C, Brenner DA, Sirlin CB, Loomba R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Joy TR, McKenzie CA, Tirona RG, Summers K, Seney S, Chakrabarti S, Malhotra N, Beaton MD. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J Gastroenterol. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Müller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, Mikami D, Needleman B, Satoskar AR, Rajagopalan S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62:149–157. doi: 10.2337/db12-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kos K, Baker AR, Jernas M, Harte AL, Clapham JC, O'Hare JP, Carlsson L, Kumar S, McTernan PG. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes Metab. 2009;11:285–292. doi: 10.1111/j.1463-1326.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 197.McCaughan GW, Siah CL, Abbott C, Wickson J, Ballesteros M, Bishop GA. Dipeptidyl peptidase IV is down-regulated in rat hepatoma cells at the mRNA level. J Gastroenterol Hepatol. 1993;8:142–145. doi: 10.1111/j.1440-1746.1993.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 198.Stecca BA, Nardo B, Chieco P, Mazziotti A, Bolondi L, Cavallari A. Aberrant dipeptidyl peptidase IV (DPP IV/CD26) expression in human hepatocellular carcinoma. J Hepatol. 1997;27:337–345. doi: 10.1016/s0168-8278(97)80180-8. [DOI] [PubMed] [Google Scholar]
- 199.Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K, Hokimoto S, Jinnouchi H, Ogawa H. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337–1344. doi: 10.1253/circj.cj-12-1168. [DOI] [PubMed] [Google Scholar]
- 200.Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, Takagi T, Yunoki K, Miyoshi T, Hirata K, Yoshikawa J, Ito H. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. doi: 10.1186/s12933-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, Wong E, Busse JW, Ebrahim S, Malaga G, Rios LP, Wang Y, Chen Q, Guyatt GH, Sun X. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ. 2016;352:i610. doi: 10.1136/bmj.i610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu XY, Zhang N, Chen R, Zhao JG, Yu P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2years. J Diabetes Complications. 2015;29:1295–1303. doi: 10.1016/j.jdiacomp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 203.Honda Y, Imajo K, Kato T, Kessoku T, Ogawa Y, Tomeno W, Kato S, Mawatari H, Fujita K, Yoneda M, Saito S, Nakajima A. The Selective SGLT2 Inhibitor Ipragliflozin Has a Therapeutic Effect on Nonalcoholic Steatohepatitis in Mice. PLoS One. 2016;11:e0146337. doi: 10.1371/journal.pone.0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45. doi: 10.1186/s13098-016-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 206.Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364–1372. doi: 10.2337/dc17-0518. [DOI] [PubMed] [Google Scholar]
- 207.Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A, Farrell K, Sunny NE, Kalavalapalli S, Pettus J, Ciaraldi TP, Mudaliar S, Henry RR. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:812–821. doi: 10.1111/dom.13584. [DOI] [PubMed] [Google Scholar]
- 208.Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923–1934. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 210.Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, Iijima M, Takekawa H, Usui I, Hiraishi H, Aso Y. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 211.Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, Kabisch S, Henkel E, Kopf S, Lagerpusch M, Kantartzis K, Kupriyanova Y, Markgraf D, van Gemert T, Knebel B, Wolkersdorfer MF, Kuss O, Hwang JH, Bornstein SR, Kasperk C, Stefan N, Pfeiffer A, Birkenfeld AL, Roden M. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care. 2020;43:298–305. doi: 10.2337/dc19-0641. [DOI] [PubMed] [Google Scholar]
- 212.Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, Furukawa M, Kawaratani H, Kitade M, Moriya K, Namisaki T, Yoshiji H. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142:1712–1722. doi: 10.1002/ijc.31193. [DOI] [PubMed] [Google Scholar]
- 213.Devineni D, Curtin CR, Marbury TC, Smith W, Vaccaro N, Wexler D, Vandebosch A, Rusch S, Stieltjes H, Wajs E. Effect of hepatic or renal impairment on the pharmacokinetics of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Ther. 2015;37:610–628.e4. doi: 10.1016/j.clinthera.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 214.Sahasrabudhe V, Terra SG, Hickman A, Saur D, Raje S, Shi H, Matschke K, Zhou S, Cutler DL. Pharmacokinetics of Single-dose Ertugliflozin in Patients With Hepatic Impairment. Clin Ther. 2018;40:1701–1710. doi: 10.1016/j.clinthera.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 215.Macha S, Rose P, Mattheus M, Cinca R, Pinnetti S, Broedl UC, Woerle HJ. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16:118–123. doi: 10.1111/dom.12183. [DOI] [PubMed] [Google Scholar]
- 216.Kasichayanula S, Liu X, Zhang W, Pfister M, LaCreta FP, Boulton DW. Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: an open-label, parallel-group, single-dose study. Clin Ther. 2011;33:1798–1808. doi: 10.1016/j.clinthera.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 217.Hermann LS, Scherstén B, Bitzén PO, Kjellström T, Lindgärde F, Melander A. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care. 1994;17:1100–1109. doi: 10.2337/diacare.17.10.1100. [DOI] [PubMed] [Google Scholar]
- 218.Schopman JE, Simon AC, Hoefnagel SJ, Hoekstra JB, Scholten RJ, Holleman F. The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014;30:11–22. doi: 10.1002/dmrr.2470. [DOI] [PubMed] [Google Scholar]
- 219.Johnson DG, Alberti KG, Faber OK, Binder C. Hyperinsulinism of hepatic cirrhosis: Diminished degradation or hypersecretion? Lancet. 1977;1:10–13. doi: 10.1016/s0140-6736(77)91652-x. [DOI] [PubMed] [Google Scholar]
- 220.van de Wiel A. Diabetes mellitus and alcohol. Diabetes Metab Res Rev. 2004;20:263–267. doi: 10.1002/dmrr.492. [DOI] [PubMed] [Google Scholar]
- 221.Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, Gardenal R, Dal Mas M, Casarin P, Zanette G, Miranda C. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–891; quiz 892. doi: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 223.Lee JY, Jang SY, Nam CM, Kang ES. Incident Hepatocellular Carcinoma Risk in Patients Treated with a Sulfonylurea: A Nationwide, Nested, Case-Control Study. Sci Rep. 2019;9:8532. doi: 10.1038/s41598-019-44447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gangopadhyay KK, Singh P. Consensus Statement on Dose Modifications of Antidiabetic Agents in Patients with Hepatic Impairment. Indian J Endocrinol Metab. 2017;21:341–354. doi: 10.4103/ijem.IJEM_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Holmes G, Galitz L, Hu P, Lyness W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol. 2005;60:469–476. doi: 10.1111/j.1365-2125.2005.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kupčová V, Arold G, Roepstorff C, Højbjerre M, Klim S, Haahr H. Insulin degludec: pharmacokinetic properties in subjects with hepatic impairment. Clin Drug Investig. 2014;34:127–133. doi: 10.1007/s40261-013-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Corrall RJ, Frier BM, Davidson NM, French EB. Hormonal and substrate responses during recovery from hypoglycaemia in man during beta 1-selective and non-selective beta-adrenergic blockade. Eur J Clin Invest. 1981;11:279–283. doi: 10.1111/j.1365-2362.1981.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 229.Hamed AE, Elsahar M, Elwan NM, El-Nakeep S, Naguib M, Soliman HH, Aboubakr AA, AbdelMaqsod A, Sedrak H, Assaad SN, Elwakil R, Esmat G, Salh S, Mostafa T, Mogawer S, Sadek SE, Saber MM, Ezelarab H, Mahmoud AA, Sultan S, El Kassas M, Kamal E, ElSayed NM, Moussa S Jury, Reviewers and Advisory Board: Sherif Abdelfattah, Samir Helmy ASSAAD-KHALIL, Ahmed Ali Elgarem, Rawia Khater, Amr Helmi, Hisham Elborlsy, Abdelhamid Attia (President of the Arab Federation Of Evidence-Based Medicine) Managing diabetes and liver disease association: Practice guidelines from the Egyptian Association for the Study of Liver and Gastrointestinal Disease (EASLGD) Arab J Gastroenterol. 2019;20:61–63. doi: 10.1016/j.ajg.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 230.Brown A, Guess N, Dornhorst A, Taheri S, Frost G. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: What can be done? Diabetes Obes Metab. 2017;19:1655–1668. doi: 10.1111/dom.13009. [DOI] [PubMed] [Google Scholar]
- 231.Jansen HJ, Vervoort GM, de Haan AF, Netten PM, de Grauw WJ, Tack CJ. Diabetes-related distress, insulin dose, and age contribute to insulin-associated weight gain in patients with type 2 diabetes: results of a prospective study. Diabetes Care. 2014;37:2710–2717. doi: 10.2337/dc13-1205. [DOI] [PubMed] [Google Scholar]
- 232.Rigalleau V, Delafaye C, Baillet L, Vergnot V, Brunou P, Gatta B, Gin H. Composition of insulin-induced body weight gain in diabetic patients: a bio-impedance study. Diabetes Metab. 1999;25:321–328. [PubMed] [Google Scholar]
- 233.American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 234.Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 235.ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 236.Khan R, Foster GR, Chowdhury TA. Managing diabetes in patients with chronic liver disease. Postgrad Med. 2012;124:130–137. doi: 10.3810/pgm.2012.07.2574. [DOI] [PubMed] [Google Scholar]
- 237.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 238.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 239.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020:Online ahead of print. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 240.Wang C, He M, Peng J, Li S, Long M, Chen W, Liu D, Yang G, Zhang L. Increased plasma osteopontin levels are associated with nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Cytokine. 2020;125:154837. doi: 10.1016/j.cyto.2019.154837. [DOI] [PubMed] [Google Scholar]