Abstract
Objective: Keloid is an abnormal scar that often develops in high-tension skin. It is caused by excessive fibroblast proliferation and collagen deposition. Nonmuscle myosin IIA (NM-IIA) is an important motor protein that regulates the mechanical transduction of cells. However, the role of NM-IIA in keloid pathogenesis remains unclear.
Approach: NM-IIA expression was examined and compared in keloid skin and normal skin by immunofluorescence. The organization of smooth muscle actin (SMA)-mediated stress fibers in normal and keloid fibroblasts (NFs and KFs, respectively) were determined. Cell proliferation and cell contractility were measured in fibroblasts derived from normal and keloids. The NM-II pharmacological inhibitor (blebbistatin) and RNA interference were applied to block NM-IIA and investigate its regulatory role in SMA-mediated stress fibers, cell contractility, and cell proliferation after NM-IIA inhibition.
Results: NM-IIA expression is increased in keloid tissue. Inhibition of NM-II by blebbistatin or targeting NM-IIA by RNA interference reduced transforming growth factor beta (TGF-β)-mediated SMA-mediated stress fiber formation, cell proliferation, and cell contractility of NFs and KFs. Although TGF-β failed to mediate phosphorylation of myosin light chain (pMLC, the activator of NM-II), pMLC can interact with SMA-mediated stress fiber. Finally, inhibition of NM-II by blebbistatin also reduced NF and KF proliferation after TGF-β stimulation.
Innovation: NM-IIA synergizes with TGF-β to regulate fibroblast proliferation, contraction activity, and myofibroblasts differentiation.
Conclusion: NM-IIA might be one of the therapeutic targets in keloids.
Keywords: TGF-β, nonmuscle myosin II, keloids, myofibroblast

Kee-Lung Chang, PhD

Chih-Hung Lee, MD, PhD
Introduction
The keloid is a fibroproliferative skin scar resulting from an abnormal and uncontrolled wound healing process. It extends beyond the edge of the original wound and invades adjacent normal skin. Keloid most commonly develops in areas where the skin is under high tension, such as those in the chest, arms, and back.1 Microscopically, keloid is characterized by excess collagen accumulation, excess fibroblast proliferation, and moderate immune cells infiltrate.1 Surgical removal of keloids is possible, but the recurrence rate is high, and a recurrent keloid always extends beyond the area of surgical scarring.2 Nonsurgical treatment modalities and prevention measures often have limited effectiveness with common recurrence. Therefore, improved treatments for keloid are warranted.3
Potential causes of keloid development include altered growth factor regulation, aberrant collagen turnover, and altered mechanical properties.4 Transforming growth factor beta (TGF-β) is one of the most important cytokines in the pathophysiology of fibrosis.5 Our previous study showed TGF-β enhances cell rigidity by increasing smooth muscle actin (SMA) expression in keloid fibroblasts (KFs).6 The mechanical properties of cells play a critical role in many cellular processes, such as locomotion, division, and differentiation.7 SMA-mediated cell contraction is important in myofibroblast differentiation.8,9 These contractile myofibroblasts participate in the reparative response by secreting large amounts of extracellular matrix proteins and contraction of healing wounds.10,11 However, dysregulated or excessive myofibroblast activity may often result in fibrosis formation and organ dysfunction. Therefore, the regulatory mechanism of fibroblast activity is important for keloid pathogenesis.
Nonmuscle myosin II (NM-II) is the actin-binding motor protein functions in cell migration response to promigratory stimuli.12–14 Three isoforms of NM II heavy chains are identified in mammalians, including NM-IIA, NM-IIB, and NM-IIC, encoded from myosin heavy chain 9 (MYH9), myosin heavy chain 10 (MYH10), and myosin heavy chain 14 (MYH14), respectively. NM-II abnormality contributes to lots of diseases, including neuronal disorders, cancer, and cardiovascular disease.15,16 The activity of NM-II depends on phosphorylation of myosin light chain (pMLC), which initiates myosin ATPase activation.13 In the early scar-remodeling phase of wound healing, NM-II expression is upregulated and then returns to normal after scar maturation.17 Furthermore, the expression of NM-II is reported to be increased in keloid and regulates fibroblast migration.18 However, how NM-II regulates cell proliferation, contraction, and stress fiber formation in keloid pathogenesis remains elusive.
Clinical Problem Addressed
This study focuses on biological function of NM-IIA on fibroblast contraction and proliferation. Myofibroblast is a specialized cell that is differentiated from fibroblast after TGF-β stimulation.19 However, to avoid dysregulated or excessive myofibroblast activity is a crucial point during wound healing process and keloid pathogenesis. Our study indicated the increased NM-IIA expression in keloid tissue. Blocking NM-IIA expression inhibited α-SMA expression, organization of stress fiber, cell contractility, and fibroblast proliferation. Targeting NM-IIA may provide one of the therapeutic options for keloids.
Materials and Methods
Subjects involved
All subjects were Taiwanese of Han Chinese origin >20 years and visited the department of dermatology at Kaohsiung Veterans General Hospital during 2016 and 2018. In this study, the subjects included those with keloids (n = 6, age 44–67 years; four men and two women) and normal controls (n = 6, age 33–51 years; three men and three women) (Table 1). Keloid subjects included patients with keloids exhibiting continuous growth beyond the margin 6 months after surgery or trauma and those with invasive cancers or treatment with liquid nitrogen therapy or intralesional corticosteroid injection within 3 months were excluded. Keloid tissues were sampled from the center of the keloid by the 3 mm of punched biopsy. Control skin was obtained from perilesional normal skin after surgical excision of epidermoid cysts or melanocytic nevus. The biopsy tissues were applied for pathological examination and primary culture. The protocol for this study was approved by the Institutional Review Board (IRB) of Kaohsiung Veterans General Hospital (VGHKS16-CT5–10).
Table 1.
Clinical demographic data of patients with keloids and normal controls
| Specimen | Age, years | Gender | Race | Location | Inciting trauma | Duration of keloid, years | Treatments |
|---|---|---|---|---|---|---|---|
| Case 1 | 64 | Male | Han | Chest | Trauma | 5 | SI |
| Case 2 | 50 | Male | Han | Chest | Trauma | 2 | SI |
| Case 3 | 58 | Male | Han | Chest | Chest trauma | 3 | SI |
| Case 4 | 65 | Male | Han | Chest | Sternotomy at cardiac surgery | 4 | SI |
| Case 5 | 44 | Female | Han | Chest | Trauma | 2 | SI |
| Case 6 | 67 | Female | Han | Chest | Trauma | 2 | SI |
| Control 1 | 41 | Female | Han | Forearm | Normal skin | — | — |
| Control 2 | 51 | Female | Han | Inguinal area | Normal skin | — | — |
| Control 3 | 45 | Male | Han | Cheek | Normal skin | — | — |
| Control 4 | 45 | Male | Han | Chest | Normal skin | — | — |
| Control 5 | 33 | Female | Han | Back | Normal skin | — | — |
| Control 6 | 37 | Male | Han | Elbow | Normal skin | — | — |
SI, steroid injection.
Cell culture and treatment
Human fibroblasts were obtained from dermal tissues of normal or keloid skin. Tissues were cut to 1–2 mm3 and incubated in a culture dish with Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, Inc.), 2 mM l-glutamine and 100 U/mL of penicillin and 100 μg/mL of streptomycin (Gibco, Thermo Fisher Scientific, Inc.). Human fibroblasts within the three to sixth passage were used for the experiments in this study. Three strains of KFs (patients 2, 3, and 5 in Table 1) and three strains of normal fibroblasts (NFs) (control 1, 4, and 5 in Table 1) derived from punched tissue samples were used in the study.
For NM-IIA knockdown, dermis fibroblasts were seeded at 70–80% confluence in complete DMEM medium and were either transfected with 40 nM of SMARTpool siRNAs to NM-IIA knockdown (Dharmacon) or transfected with a mock control siRNA (Dharmacon) using Lipofectamine® 3000 Reagent (Thermo Fisher Scientific, Inc.). We found that the siRNAs reduced NM-IIA expression by ∼80% and used these siRNAs for all following in vitro experiments to block NM-IIA expressions.
For TGF-β treatment, fibroblasts were starved in serum-free DMEM for at least 8 h before treatment using TGF-β at indicated concentrations diluted in 2% FBS DMEM for the following in vitro experiments.
Immunofluorescence microscopy
For immunofluorescence assay of tissue, 5 μm of sections from formalin-fixed biopsy tissue were mounted on adhesive glass slides. After deparaffinization, slides were placed in retrieval buffer (10 mM citrate acid, 0.05% Tween-20), autoclaved for 5 min (121°C), washed in phosphate-buffered saline containing 0.05% Tween-20 (PBST), and permeabilized in PBS with 0.1% Triton X-100. Slides were incubated with blocking solution (2% bovine serum albumin in PBS) for 60 min and then incubated with primary antihuman NM-IIA antibody (1:100 dilution; Sigma-Aldrich M8064) and anti-vimentin antibody (1:500 dilution; eBioscience™ 14-9897-80) at 4°C overnight. The next day we incubated the sections with goat anti-rabbit-FITC (1:2,000; Invitrogen, Carlsbad, CA) and goat anti-mouse-IgG-Alexa568 (1:2,000; Sigma, St Louis, MO) for 1 h at room temperature. After DAPI staining (Invitrogen), the slides were mounted and visualized.
For cell staining, fibroblasts that adhered on noncoated micro cover glass (MATSUNAMI) were fixed with 4% paraformaldehyde for 15 min, then permeabilized in PBS with 0.1% Triton X-100 for 5 min. The fixed cells were incubated for 60 min with the blocking solution then incubated overnight with primary anti-phospho-MLC2-Ser19 (pMLC, 1:100 dilution, rabbit polyclonal, CST3671; Cell Signaling Technology, Inc.), anti-alpha-SMA (1:100 dilution, ab5694; Abcam, San Francisco, CA) or primary antihuman NM-IIA antibody (1:200 dilution; Sigma-Aldrich M8064) at 4°C. Then, coverslips were incubated for 60 min with fluorescent secondary antibodies at room temperature (1:1,000 dilution; Thermo Fisher Scientific, Inc.). After DAPI staining (Invitrogen), the slides were mounted and visualized.
Proliferation assay
Proliferation was measured using a Cell-Counting-Kit-8 Cell Proliferation Assay (CCK-8, 96992; Sigma-Aldrich). Fibroblasts or siRNA-treated fibroblasts were plated at 3–5 × 103 cells/well (200 μL) on a 96-well plate in DMEM with 10% FBS overnight. Fibroblasts were treated with blebbistatin or TGF-β in <2% FBS DMEM and determined at indicated time points. Cell medium was replaced with CCK-8 solution (10 μL of CCK-8 in 100 μL of growth medium) and then incubated at 37°C for 2 h. The absorbance at 450-nm wavelength was recorded.
Contraction assay
Collagen solution was prepared as 780 μL of 3 mg/mL bovine collagen I (Advanced BioMatrix, Inc., San Diego, CA) with 200 μL of 5 × DMEM and 24 μL of 1 M NaOH and placed on ice. The TGF-β- or siRNA-treated fibroblasts were counted and populated in the collagen solution by quickly mixing four volumes of collagen solution with one volume of cell solution. The 0.5 mL of cell–collagen mixture was carefully loaded into the wells of a 24-well culture plate and incubated at 37°C for 60 min for matrix polymerization. After polymerization of the matrix, the collagen lattices were free and incubated in 1 mL of culture medium containing blebbistatin or TGF-β for following treatment. The area of the collagen lattices was imaged and measured at indicated time points.
Image analysis of stress fibers
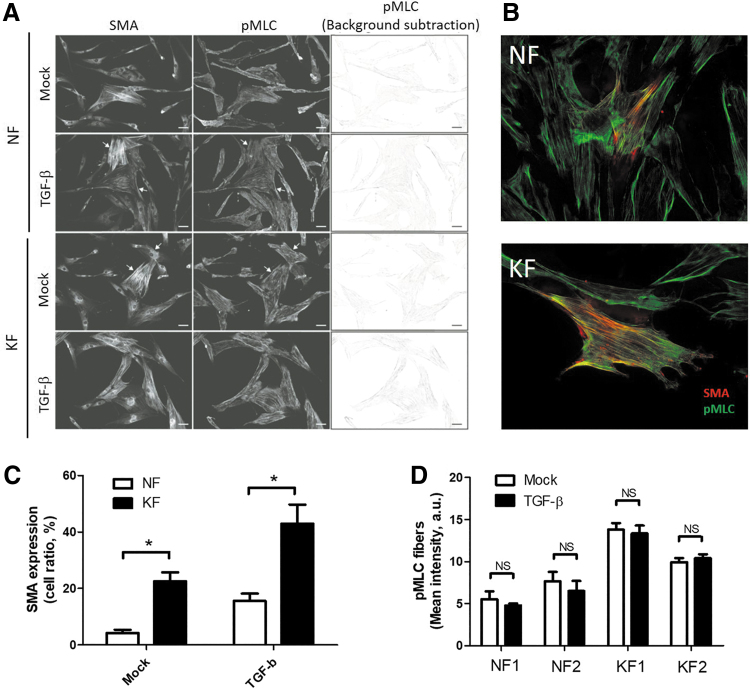
Immunofluorescence images of SMA-mediated stress fibers and pMLC-mediated stress fibers were processed by Image J software to quantify intensity and number as previously described.20 In brief, fluorescence images of pMLC were processed using a median filter with a 13-pixel-square kernel as the background image. The background images were used to create binary images of pMLC (dividing the original image with the background image) and to define the cell outline. The intensity of pMLC-mediated stress fibers was evaluated by subtracting the background of the original image. The SMA-mediated stress fibers were processed by background subtraction and counted with the Cell Counter plug-in. Figure 4A (right panel) shows the processed images.
Figure 4.
TGF-β effect on pMLC-mediated stress fibers. (A) Fluorescence images of KFs and NFs were taken in those stained with anti-SMA and anti-pMLC antibodies. Fibroblasts were treated with 20 ng/mL of TGF-β for 48 h. Right panels show the background-subtracted images of pMLC-mediated stress fibers. Arrows indicate the raw image showing the SMA- and pMLC-mediated stress fibers. Bar = 20 μm. (B) Fluorescence images of NFs and KFs were taken in those stained with anti-SMA antibody (Red) and anti-pMLC antibody. Bar = 20 μm. (C) The ratio of fibroblasts expressing SMA-mediated stress fibers was measured through images analysis in the left panel from (A) (n = 50–100 cells from three randomly selected fields, mean ± standard error of the mean, *p < 0.05, two-tailed t-test). (D) Quantification of the mean intensity of pMLC-mediated stress fibers was determined through analysis of staining images in the right panel of (A). The experiments were repeated for at least three times to reach consistency and each experiment was done in triplicates. pMLC, phosphorylation of myosin light chain. Color images are available online.
Western blotting
Cellular proteins from fibroblasts were extracted by lysing the cells with Cell Lysis Buffer (ab152163; Abcam). After 12,000 g centrifugation, supernatants were collected, and protein concentrations were measured with a protein quantification kit (Bio-Rad, Hercules, CA). Total protein (20 μg) was subjected to NuPAGE4-12% Bis-Tris Protein Gels (Thermo Fisher Scientific, Inc.) and then transferred onto a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% skim milk in PBST for 60 min at room temperature. Primary antibodies of anti-SMA and anti-pMLC were used at a dilution of 1:500. Specific proteins in the nitrocellulose membranes were visualized using a chemiluminescence subtraction kit (Pierce, Rockford, IL).
RNA extraction and analysis
Total RNA was extracted from fibroblasts by using the RNeasy Plus Universal Mini Kit (QIAGEN) according to the manufacturer instructions. Total RNA (1 μg) was reverse transcribed to cDNA and then amplified with Luna Universal qPCR Master Mix (New England Biolabs). Each sample was assayed in triplicate on the Roche Light Cycler Real-Time PCR System (Roche Molecular Systems, Inc.). The housekeeping gene GAPDH was used to normalize mRNA concentration and PCR amplified with the primer pairs (forward: 5′-CCATCACCATCTTCCAG-3′ and reverse: 5′-CCTGCTTCACCACCTTCT-3′). The NM-IIA (myh9) was PCR amplified with the primer pairs (forward: 5′-CTCCCTAAAGAACAAGCTCAGG-3′ and reverse: 5′-GCTTTGCCATCTACCTCTTCG-3′).
Statistical analysis
Numeric values were compared between the two groups by two-tailed t-test. A p-value <0.05 was considered statistically significant. The experiments were repeated for at least three times to reach consistency and each experiment was done in triplicates.
Results
NM-IIA expressing cells are increased in the hypercellular center of keloids
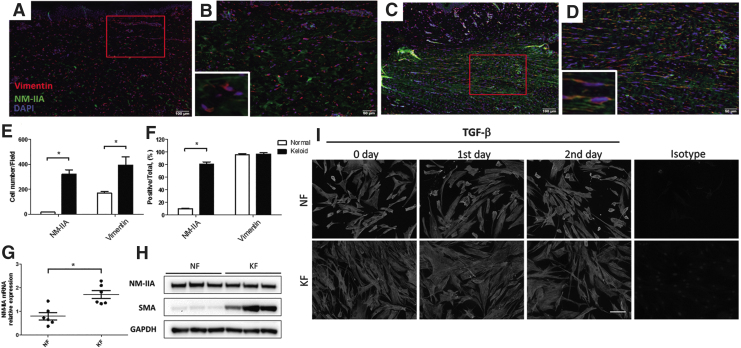
We first examined the NM-IIA expression in normal skin and keloid specimens by immunofluorescence staining. Vimentin, an intermediate filament protein in the mesenchymal cells, was measured too. The data showed that NM-IIA expression were significantly higher in the densely hypercellular region of keloid than those in dermis of normal skin (Fig. 1A, C). Both number and percentage of cells expressing NM-IIA were increased significantly in the hypercellular region of keloid as compared with those in normal skin (Fig. 1B, D–F). Vimentin-expressing cells were observed in the dermis of normal skin (Fig. 1B), but they are increasing in numbers in the densely hypercellular region of keloid (Fig. 1B, D, E). The vimentin-positive cells in keloid displayed higher NM-IIA expression than those cells in normal skin (Fig. 1B, C). These data suggested that NM-IIA expression in cells, mostly fibroblasts, of keloid tissue is higher than those of normal skin.
Figure 1.
NM-IIA expression is increased in keloid tissue and keloid-derived fibroblasts. Five-micrometer paraffin sections of normal skin (n = 6) and keloids (n = 6) were deparaffinized and incubated with the following antibodies against NM-IIA and vimentin. NM-IIA (green) and vimentin (red) expression is shown in normal skin (A) and in keloid (C). High-power view of the skin showed colocalization of NM-IIA (green) and vimentin (red) in a normal skin (B) and in a keloid (D). Red rectangle in (A) and (C) indicates the enlarged area for high magnification in (B) and (D), respectively. The numbers of NM-IIA-expressing and vimentin-expressing cells were quantified from five randomly selected high-power fields (E). The percentage of NM-IIA-expressing or vimentin-expressing cells normalized to total cells (DAPI-stained cells) in the hypercellular areas was shown (F). (G) Real-time PCR analyses of NM-IIA in KFs and NFs. The expression levels are presented as scatter plots; the middle line represents the mean value (six NF and six KF, *p < 0.05). (H) Western blot showed the NM-IIA and SMA protein expression in NFs and KFs. The GAPDH levels were used as a loading control. (I) The fibroblasts were treated with TGF-β (20 ng/mL) at indicated times to examine the NM-IIA expression by immunofluorescence examination. The experiments were repeated for at least three times to reach consistency and each experiment was done in triplicates. KF, keloid fibroblast; NF, normal fibroblast; NM-IIA, nonmuscle myosin IIA; SMA, smooth muscle actin; TGF-β, transforming growth factor beta. Color images are available online.
To investigate whether NM-IIA expression was also increased in KFs, we measured the NM-IIA expression in fibroblasts derived from normal skin and keloid tissue (NF and KF, respectively). The mRNA levels of NM-IIA were higher significantly in KF as compared with those in NF (Fig. 1G). However, the protein level of NM-IIA was similar between NF and KF (Fig. 1H). TGF-β is one of the key cytokines in keloid pathogenesis.21 To investigate whether NM-IIA expression was regulated by TGF-β, we examined the NM-IIA expression in NF and KF treated with TGF-β by immunofluorescence assay. The result showed similar expression of NM-IIA in KF and NF (Fig. 1I). The expression of NM-IIA in NF and KF remained unchanged in the first and secondary day after TGF-β stimulation (Fig. 1I). These data suggested that NM-IIA expression may be independent of the TGF-β signal pathway.
Inhibition of NM-IIA prevents the induction SMA by TGF-β
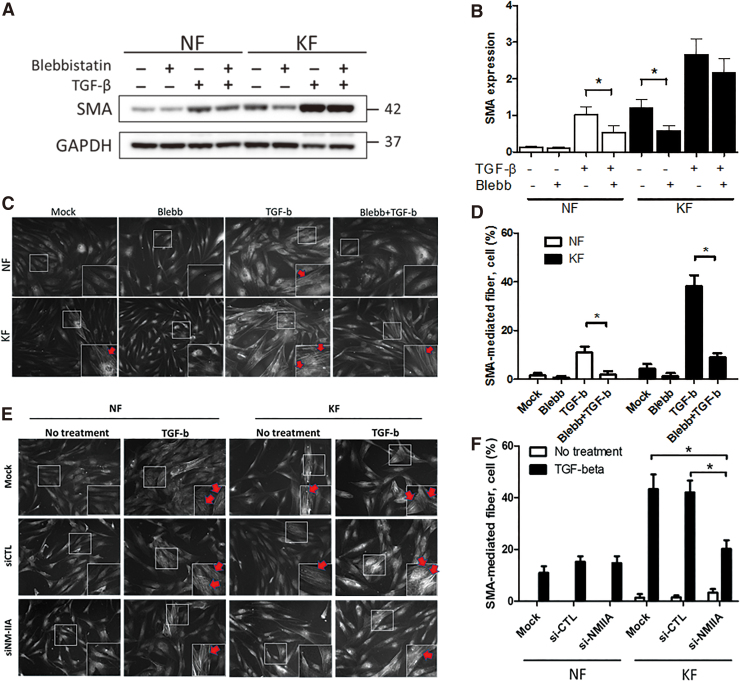
To investigate the role of NM-IIA in myofibroblast differentiation, SMA expression was determined by Western blot in NF and KF treated with blebbistatin, a specific NM-II inhibitor. These data showed that inhibition of NM-II activity by blebbistatin decreased SMA expression in both NF and KF (Fig. 2A, B). However, blebbistatin treatment significantly decreased TGF-β-induced SMA expression in NF, although not significantly in KF (Fig. 2A, B). The results indicate that SMA expression could be regulated by the NM-II.
Figure 2.
NM-IIA blocking partially reduced the TGF-β-mediated SMA expression and SMA stress fiber formation in fibroblasts. (A) Western blot examination of SMA in NFs and KFs. Fibroblasts were pretreated with 50 μM of blebbistatin (an inhibitor for NM-II) for 24 h followed by cotreatment with 20 ng/mL of TGF-β for 48 h. The GAPDH levels were used as loading controls. (B) is the quantification of Western blotting from (A). (C–F) KFs and NFs were treated with blebbistatin (C, D) or transfected with si-NM-IIA (E, F) for 24 h followed by treatment with TGF-β (20 ng/mL) for 48 h. The fibroblasts were stained with anti-SMA antibody using immunofluorescence examination. White rectangle in each image indicates the enlarged area for high magnification in right-down panel, respectively. The percentage of fibroblasts expressing SMA-mediated stress fibers (C, E) was quantified in (D, F), respectively. The experiments were repeated for at least three times to reach consistency and each experiment was done in triplicates. Color images are available online.
Next, immunofluorescence staining was performed to determine whether NM-II activity regulated the formation of SMA-mediated stress fibers, we again treated fibroblasts with blebbistatin and calculated the percentage of fibroblast expressing SMA-mediated fibers. This result indicated that blebbistatin reduced the percentage of fibroblasts expressing SMA-mediated stress fibers in both NF and KF after TGF-β treatment (Fig. 2C, D). In this part, we also knocked down NM-IIA expression by RNA interference to confirm whether NM-IIA contributes to the formation of SMA-mediated stress fibers. The immunofluorescence result showed that NM-IIA knockdown also decreased percentage of fibroblasts expressing SMA-mediated stress fibers in KF (Fig. 2E, F). These data showed that inhibition of NM-IIA prevents the induction of SMA by TGF-β.
Inhibition of NM-II activity partially suppresses TGF-β-induced cell contraction
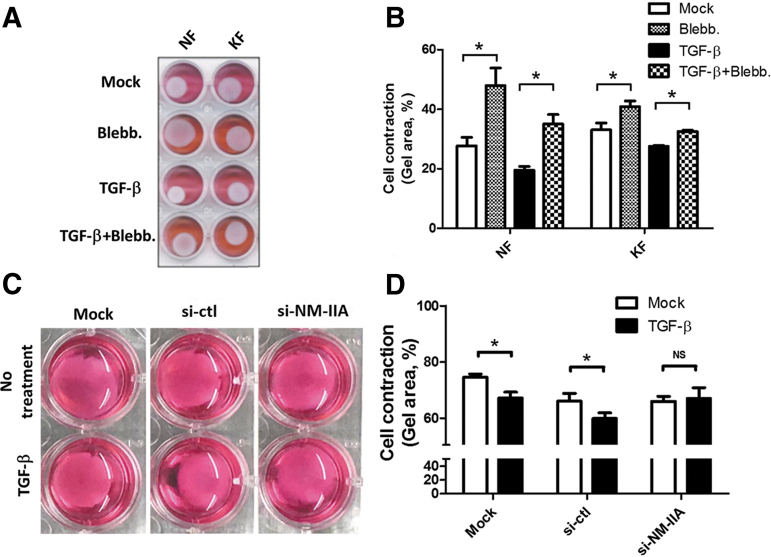
Treatment with TGF-β is known to induce cell contraction in both NF and KF.8 To investigate whether TGF-mediated cell contraction depends on NM-IIA, the contraction ability of fibroblasts was evaluated by the collagen contraction assay after treatment of blebbistatin and/or TGF-β. The result showed that blebbistatin markedly increased the area of collagen lattice populated by NF and KF with or without TGF-β stimulation (Fig. 3A, B). These data suggest that cell contraction induced by TGF-β treatment is dependent on NM-II activity, at least in part. We also knock down NM-IIA expression by RNA interference to confirm whether NM-IIA affects KF contraction. The result indicated that knockdown of NM-IIA can increase the area of collagen lattice populated by KF after TGF-β stimulation (Fig. 3C, D). These evidences suggest the expression and activity of NM-IIA regulate TGF-β-mediated cell contraction in KF.
Figure 3.
Blocking of NM-IIA decreases the TGF-β-mediated fibroblast contraction. (A) NF- or KF-populated collagen lattice was treated with blebbistatin (40 μM) for 24 h followed by treatment with TGF-β (20 ng/mL) for 24 h. The image of collagen lattice was taken to visualize the shrinkage of the collagen. (C) The si-NM-IIA-transfected KF-populated collagen lattice was treated with TGF-β (20 ng/mL) for 24 h. The contraction ratio of blebbistatin-treated (B) or si-NM-IIA-transfected KF (D) was shown. The contraction ratio indicated that area of collagen lattice normalized to area of each well. The experiments were repeated for at least three times to reach consistency and each experiment was done in triplicates. Color images are available online.
Association of pMLC and SMA-mediated fiber formation is independent of TGF-β
Our previous experiment showed that the NM-IIA expression is independent of TGF-β signaling (Fig. 1G). However, the NM-II activity is also important in cell contraction. Previous article reported that NM-II activity can be regulated by pMLC through Rho signaling.13 To investigate the activity of NM-IIA after TGF-β stimulation, pMLC in fibroblasts was analyzed by immunofluorescence assay. The result showed that pMLC was colocalized with SMA-mediated stress fiber, and is higher in KF (Fig. 4B). KF also had increased numbers of pMLC-mediated stress fibers (Fig. 4B) and SMA-mediated fibers (Fig. 4C) than NF did. These data suggested that pMLC-NM-IIA axis associates with formation of SMA-mediated fiber in KF. However, TGF-β itself did not increase formation of pMLC-mediated stress fibers neither in KF nor in NF (Fig. 4A, D). These data suggested that the regulation of pMLC-mediated NM-II activity in KF was independent of TGF-β signaling.
NM-II enhanced fibroblast proliferation
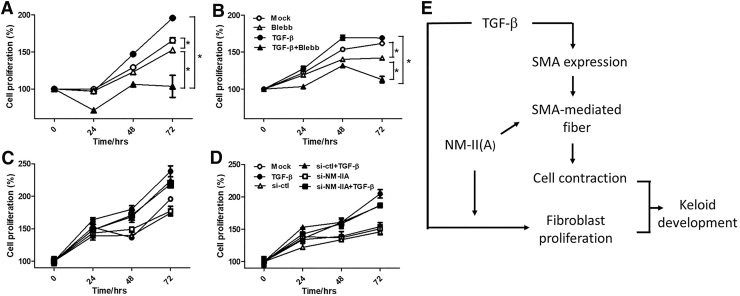
Our previous experiment showed that there are more fibroblasts in the densely hypercellular region of keloid (Fig. 1C). To demonstrate whether NM-IIA could regulate fibroblast proliferation, the CCK8 proliferation assay of NFs and KFs was performed after TGF-β stimulation with or without blebbistatin treatment. The data showed blebbistatin inhibited fibroblast proliferation by TGF-β (Fig. 5A, B). Surprisingly, more proliferation of fibroblasts was inhibited under combination of TGF-β and blebbistatin treatment than blebbistatin treatment alone without TGF-β. Knockdown of NM-IIA by RNA interference was also applied to study the role of NM-IIA in fibroblast proliferation. However, the data showed that knockdown of NM-IIA failed to inhibit fibroblast proliferation by TGF-β in KF and NF (Fig. 5C, D). These evidences suggested that NM-IIA contributed to cell proliferation of fibroblasts and knockdown of NM-IIA may not be enough to block fibroblast proliferation. Other members of NM-II might be important in fibroblast proliferation.
Figure 5.
The effect on blocking NM-IIA expression on fibroblast proliferation. NFs and KFs were pretreated with 40 μM of blebbistatin (A, B) or transfected with si-NM-IIA (C, D), followed by treatment with TGF-β for 24, 48, and 72 h. The CCK-8 assay was used to determine cell proliferation. (E) The scheme of how NM-IIA contributes to the pathogenesis of keloid. Color images are available online.
Discussion
In this study, the results reported here that NM-IIA expression was increased in keloid scar, especially in area of pathological collagen accumulation. NM-IIA promotes TGF-β-mediated SMA expression and SMA-mediated fiber formation. Moreover, NM-IIA enhanced cell contraction. The pMLC was found to interact with SMA and associated with SMA-mediated fiber formation.
The NM-IIA is a pivotal motor protein in cell contraction and tissue remodeling during the wound healing. The fibroblasts express NM-IIA through the repair phase but diminished or cleared in the end.17 In the study, we showed that NM-IIA expression was increased in area of pathological collagen accumulation but was similar between NF and KF although NM-IIA mRNA level remains higher in KF than that in NF. This area of keloid often displays a rigid environment that contributed to more activation and differentiation of fibroblast. The result that NM-IIA expression was not regulated by TGF-β suggested that NM-IIA expression may be regulated by extracellular matrix of environment. Therefore, when fibroblasts lost the pathogenic environment of the keloid in culture system, the increased NM-IIA protein expression may not be observed in KF in vitro. Alternatively, that KF still displayed higher NM-IIA mRNA than NF in vitro indicated that NM-IIA protein expression may be regulated by post-transcriptional, protein degradation, or protein stability.
SMA is a myofibroblast marker. Its presence in keloid tissue indicates its potential role in pathogenesis.6,22 The TGF-β is one of the most powerful stimulators of SMA expression.23 Studies indicate that TGF-β-induced SMA expression in human tenon's fibroblasts causes cell contractility.8 NM-II A is considered a major regulator of cell contraction.13 However, few studies have investigated whether NM-IIA activity has a direct regulatory role in TGF-β-induced myofibroblast differentiation. This study found that a direct blockade of NM-II ATPase activity in NFs reduced SMA expression induced by TGF-β, which indicates that NM-IIA-mediated cell tension could mediate TGF-β-mediated myofibroblast differentiation.
Recruitment of SMA to stress fibers is controlled by tension-mediated focal adhesion size.24 The maturation and growth of focal adhesion are dependent on NM-II activity.25 NM-II activity is often regulated by pMLC from signaling of mechanotransduction stimulation through rigid environment.26 In this study, pMLC was not regulated by TGF-β signal in both NF and KF. However, pMLC was found to interact with SMA-mediated fiber in this study. These data supported that pMLC-NM-II (A) enhanced the organization of SMA into stress fiber through interacting with SMA. Moreover, the inhibition of NM-II activity by blebbistatin or knockdown of NM-IIA was found to decrease SMA-mediated fiber formation and cell contraction. This suggested that NM-IIA promoted the SMA-mediated cell contraction after TGF-β stimulation in keloid development.
TGF-β was reported to enhance fibroblast proliferation in fibrotic diseases.27 In this study, we found that blocking NM-IIA activity can inhibit fibroblast proliferation and TGF-β-mediated fibroblast proliferation. Interestingly blocking NM-IIA activity causes more inhibitory effect on fibroblast proliferation stimulated with TGF-β. This indicated that NM-IIA inhibition is a potential strategy to treat keloid scar. TGF-β is a multiple functional cytokine not only for SMA expression in fibroblast differentiation and cell proliferation but also for cell death. NM-IIA activity may be an important factor to interact with TGF-β signaling. However, knockdown of NM-IIA failed to inhibit fibroblast proliferation in this study. This effect may result from incomplete knockdown of NM-IIA or the presence of other NM-II isoforms.
The limitation of this study includes (1) the limited numbers of the samples may pose difficulties to fully matching to gender, age, or other confounders; (2) this study was done in vitro and it may not be extrapolated to the in vivo findings. More subjects and in vivo examination may be required in the future for result consistency and reproducibility.
In this study, we found upregulation of NM-IIA expression in fibroblasts of keloid tissue. NM-IIA inhibition can inhibit TGF-β-mediate SMA expression, organization in stress fiber, cell contraction, and cell proliferation. Indeed, NM-IIA regulates expression of SMA both in the presence and absence of TGF-β, indicating the TGF-β-independent effects of NM-IIA in the pathogenesis of fibrosis. The study indicated that blocking NM-IIA might be one of the therapeutic targets in keloids.
Innovation
Our study has clinical relevance because NM-II expressions are increased in keloids and it promotes TGF-β-induced-SMA expression, fiber formation, cell contraction, and also proliferation. Blebbistatin can inhibit TGF-β-induced-SMA expression, fiber formation, cell contraction, and proliferation. NM-IIA may become a therapeutic target in keloid therapy.
Key Findings
Our study demonstrates that NM-II role in TGF-β-mediated effect on keloid development.
NM-IIA expression increased at the pathological region of keloids.
NM-IIA promoted TGF-β-induced SMA expression during myofibroblast differentiation.
NM-IIA regulates SMA-mediated fiber formation, cell contraction, and fibroblast proliferation.
Acknowledgments and Funding Sources
We thank Institute for Translational Research in Biomedicine of Kaohsiung Chang Gung Memorial Hospital of Taiwan for technical assistance and reagents. This study was supported by grants to C-H.L. and Y-Y.L. from the Ministry of Science and Technology (MOST-107-2314-B-182A-082-MY3, MOST-107-2314-B-075B-009, and MOST-108-2314-B-075B-001-MY3), from the Kaohsiung Veterans General Hospital (VGHKS 106-68, VGHKS 107-70, VGHKS 108-85 and VGHKS 109-124) and to Y-H.L. and C-H.L. from Chang Gung Medical Foundation(CMRPG8K0291).
Abbreviations and Acronyms
- CCK-8
Cell-Counting-Kit-8
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- IRB
Institutional Review Board
- KF
keloid fibroblast
- MYH9
myosin heavy chain 9
- NF
normal fibroblast
- NM-II
nonmuscle myosin II
- PBST
phosphate-buffered saline with Tween-20
- pMLC
phosphorylation of myosin light chain
- SI
steroid injection
- SMA
smooth muscle actin
- TGF-β
transforming growth factor beta
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Authors
Ying-Yi Lu, MD, is an assistant professor of dermatologist and pediatrician in Kaohsiung Veterans General Hospital, a research expert in melanoma and wound healing. Her specialty is Pediatric Dermatology. Cheng-Chieh Fang, PhD, is a basic scientist in Kaohsiung Chang Gung Memorial Hospital, studying signaling mechanisms and immunological regulation of fibrosis-related diseases and carcinogenesis. Chien-Hui Hong, MD, PhD, the chair of Dermatology in Kaohsiung Veterans General Hospital, Associate Professor of Dermatology in National Yang Ming University, a research expert in the pathophysiology of tuberous sclerosis and atopic dermatitis. Chieh-Hsin Wu, MD, PhD, is a neurosurgeon and assistant professor of surgery in Kaohsiung Medical University Hospital, a research expert in brain and spinal tumor and chronic neuropathic pain. His specialty is neurovascular intervention. Yu-Hung Lin, PhD, is a basic scientist in Kaohsiung Chang Gung Memorial Hospital, studying signaling mechanisms of cell contractility. Kee-Lung Chang, PhD, is a professor of biochemistry in Kaohsiung Medical University, studying areas combined basic and clinical medicine in immunology about tumor and allergy. Chih-Hung Lee, MD, PhD, the chair and the professor of dermatology in Kaohsiung Chang Gung Memorial Hospital, is an internationally renowned expert in the immunological regulations of the dynamic wound-healing process and chemical carcinogenesis.
References
- 1. Robles DT, Berg D. Abnormal wound healing: keloids. Clin Dermatol 2007;25:26–32 [DOI] [PubMed] [Google Scholar]
- 2. Trisliana Perdanasari A, Lazzeri D, Su W, et al. . Recent developments in the use of intralesional injections keloid treatment. Arch Plast Surg 2014;41:620–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec 2015;7:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shih B, Garside E, McGrouther DA, Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen 2010;18:139–153 [DOI] [PubMed] [Google Scholar]
- 5. Faler BJ, Macsata RA, Plummer D, Mishra L, Sidawy AN. Transforming growth factor-beta and wound healing. Perspect Vasc Surg Endovasc Ther 2006;18:55–62 [DOI] [PubMed] [Google Scholar]
- 6. Lee CH, Hong CH, Chen YT, Chen YC, Shen MR. TGF-beta1 increases cell rigidity by enhancing expression of smooth muscle actin: keloid-derived fibroblasts as a model for cellular mechanics. J Dermatol Sci 2012;67:173–180 [DOI] [PubMed] [Google Scholar]
- 7. Barnes LA, Marshall CD, Leavitt T, et al. . Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv Wound Care (New Rochelle) 2018;7:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyer-ter-Vehn T, Sieprath S, Katzenberger B, Gebhardt S, Grehn F, Schlunck G. Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci 2006;47:4895–4904 [DOI] [PubMed] [Google Scholar]
- 9. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001;12:2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 1994;124:401–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darby IA, Zakuan N, Billet F, et al. . The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci 2016;73:1145–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ridley AJ, Schwartz MA, Burridge K, et al. . Cell migration: integrating signals from front to back. Science 2003;302:1704–1709 [DOI] [PubMed] [Google Scholar]
- 13. Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009;10:778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Even-Ram S, Doyle AD, Conti MA, et al. . Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol 2007;9:299–309 [DOI] [PubMed] [Google Scholar]
- 15. Ma X, Adelstein RS. The role of vertebrate nonmuscle myosin II in development and human disease. Bioarchitecture 2014;4:88–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newell-Litwa KA, Horwitz R, Lamers ML. Non-muscle myosin II in disease: mechanisms and therapeutic opportunities. Dis Model Mech 2015;8:1495–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bond JE, Ho TQ, Selim MA, Hunter CL, Bowers EV, Levinson H. Temporal spatial expression and function of non-muscle myosin II isoforms IIA and IIB in scar remodeling. Lab Invest 2011;91:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bond JE, Bergeron A, Thurlow P, et al. . Angiotensin-II mediates nonmuscle myosin II activation and expression and contributes to human keloid disease progression. Mol Med 2011;17:1196–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eddy RJ, Petro JA, Tomasek JJ. Evidence for the nonmuscle nature of the “myofibroblast” of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol 1988;130:252–260 [PMC free article] [PubMed] [Google Scholar]
- 20. Lin YH, Zhen YY, Chien KY, et al. . LIMCH1 regulates nonmuscle myosin-II activity and suppresses cell migration. Mol Biol Cell 2017;28:1054–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujiwara M, Muragaki Y, Ooshima A. Upregulation of transforming growth factor-beta1 and vascular endothelial growth factor in cultured keloid fibroblasts: relevance to angiogenic activity. Arch Dermatol Res 2005;297:161–169 [DOI] [PubMed] [Google Scholar]
- 22. Luo LF, Shi Y, Zhou Q, Xu SZ, Lei TC. Insufficient expression of the melanocortin-1 receptor by human dermal fibroblasts contributes to excess collagen synthesis in keloid scars. Exp Dermatol 2013;22:764–766 [DOI] [PubMed] [Google Scholar]
- 23. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 2006;172:259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 2010;188:877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol 2000;150:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caja L, Dituri F, Mancarella S, et al. . TGF-β and the tissue microenvironment: Relevance in fibrosis and cancer. Int J Mol Sci 2018;19:e1294. [DOI] [PMC free article] [PubMed] [Google Scholar]