Abstract
Background
After stroke, kinematic measures obtained with non-robotic and robotic devices are highly recommended to precisely quantify the sensorimotor impairments of the upper-extremity and select the most relevant therapeutic strategies. Although the ArmeoSpring exoskeleton has demonstrated its effectiveness in stroke motor rehabilitation, its interest as an assessment tool has not been sufficiently documented. The aim of this study was to investigate the psychometric properties of selected kinematic parameters obtained with the ArmeoSpring in post-stroke patients.
Methods
This study involved 30 post-stroke patients (mean age = 54.5 ± 16.4 years; time post-stroke = 14.7 ± 26.7 weeks; Upper-Extremity Fugl-Meyer Score (UE-FMS) = 40.7 ± 14.5/66) who participated in 3 assessment sessions, each consisting of 10 repetitions of the ‘horizontal catch’ exercise. Five kinematic parameters (task and movement time, hand path ratio, peak velocity, number of peak velocity) and a global Score were computed from raw ArmeoSpring’ data. Learning effect and retention were analyzed using a 2-way repeated-measures ANOVA, and reliability was investigated using the intra-class correlation coefficient (ICC) and minimal detectable change (MDC).
Results
We observed significant inter- and intra-session learning effects for most parameters except peak velocity. The measures performed in sessions 2 and 3 were significantly different from those of session 1. No additional significant difference was observed after the first 6 trials of each session and successful retention was also highlighted for all the parameters. Relative reliability was moderate to excellent for all the parameters, and MDC values expressed in percentage ranged from 42.6 to 102.8%.
Conclusions
After a familiarization session, the ArmeoSpring can be used to reliably and sensitively assess motor impairment and intervention effects on motor learning processes after a stroke.
Trial registration The study was approved by the local hospital ethics committee in September 2016 and was registered under number 05-0916.
Keywords: Learning, Hemiplegia, Exoskeleton device, Psychometrics, ArmeoSpring
Background
More than 40% of post-stroke patients display residual and permanent neurological upper extremity (UE) impairments [1]. It is essential to quantify these impairments in order to assess functional loss and develop more effective therapeutic interventions.
The effectiveness of motor rehabilitation is traditionally appraised using validated and standardized clinical scales [2], such as the upper extremity Fugl-Meyer subscale (UE-FMS) [3]. However, clinical scales are not always appropriate to assess motor strategies during movements, and they are not sensitive enough to capture the quality of sensorimotor performance or the effectiveness of therapeutic interventions [4]. They do not effectively distinguish between restitution and compensation [5, 6]. Some authors therefore recommend using kinematic parameters provided by optokinetic, robotic or gravity-supporting devices to assess movements [5–10]. These parameters are thought to be more sensitive and provide more information on movement performance and quality in the context of health and disease, helping to fill the gap related to the use of clinical scales.
Many robotic and non-robotic devices have been developed for UE rehabilitation after neurological disorders such as stroke [11, 12], with the goal of increasing the intensity and control of therapies. The ArmeoSpring (developed by Hocoma, Inc) is a passive orthosis that assists the movements of patients’ joints, using a structure parallel to the mobilized UE. It also provides kinematic parameters that inform about movement speed, duration and trajectory [9, 13], and thus could be used to assess movement efficacy and smoothness [7, 14]. Based on clinical criteria for impairments and function, the effectiveness of the ArmeoSpring was demonstrated in the rehabilitation of patients with motor deficits related to cerebral palsy, multiple sclerosis and stroke [8, 15, 16].
Given the increasing use of such devices as assessment tools, it is imperative to obtain better knowledge of the psychometric properties of the parameters provided [17, 18]. Indeed, these parameters must be sensitive enough to detect subclinical changes, and the variations observed must reflect a decrease in the motor deficit and not be due to a learning effect of the task. Some studies have addressed these questions [19–22]. Up to now, only one study has investigated the reliability of kinematic parameters provided by the ArmeoSpring [13]. Rudhe et al. demonstrated fair to good reliability of the movement workspace obtained with the ArmeoSpring in healthy participants and in patients with spinal cord injury [13]. Using mostly robotic devices, some authors have shown no or little learning effect [19–21] and advocated a single practice session to shorten the learning process. Other authors have demonstrated the existence of learning processes during mechanized training with the ArmeoSpring in post-stroke patients [23], and in children with cerebral palsy [16]. These latter studies used the vertical catch exercise, with only one or very few kinematic parameters used to assess motor learning and performance with the ArmeoSpring. Furthermore, motor learning is a fundamental process in rehabilitation and recovery post-stroke [6]. An increasing number of authors have suggested the use of kinematic parameters obtained with robotics to also assess motor learning and control in the contexts of health and disease. However, besides skill acquisition, motor learning also implies persistence of the changes brought about (i.e. retention) [24]. It is essential to at least demonstrate that the skills acquired are still present and measurable at a later time point. The majority of studies did not, however, address this question appropriately [24].
There is no consensus on the kinematic parameters to be used for UE assessment and little is known about their ability to identify learning during the post-stroke recovery phase. As far as we know, no study has investigated the extent of learning and its successful retention, together with the reliability of the parameters provided by the ArmeoSpring during the performance of a 2D-horizontal catch assessment exercise after a stroke. Thus, our main objective was to assess the learning effect and the reliability of the repeated measures of selected parameters obtained with the ArmeoSpring in post-stroke patients during their routine clinical care.
Methods
Participants
Thirty hemiparetic post-stroke patients were consecutively recruited during the course of their routine care in the Neurorehabilitation department of the Toulouse University Hospital. The routine care is standardized in accordance with the most recent guidelines for adult stroke rehabilitation and recovery [25] and with the French health authority [26]. Given the preliminary nature of this study for stroke, the sample size seemed appropriate and consistent with other studies [13]. All the patients included were naïve to the use of the ArmeoSpring and gave their written consents in accordance with the Declaration of Helsinki. The study was approved by the local hospital ethics committee in September 2016 (n°05-0916).
The inclusion criteria were: (i) a first ischemic or hemorrhagic stroke as diagnosed by a CT scan or MRI that occurred (ii) more than 3 weeks ago, (iii) an UE-FMS score between 10 and 44/66, and (iv) the presence of at least 10° voluntary movement at the shoulder and elbow. The exclusion criteria were: (i) the presence of apraxia, severe unilateral spatial neglect, (ii) UE pain limiting movement, and (iii) lack of stability of the trunk while seated or sitting position not recommended.
Study design
Each patient made 4 visits over 2 days with the same unique rater who was an advanced user of the ArmeoSpring. During the pre-inclusion visit, the patients were informed by the rater about the protocol details, and the inclusion/exclusion criteria meeting was verified. If included, each patient made 3 visits on 3 consecutive half-days. During the first visit, the patient was comfortably seated on the ArmeoSpring, which was adjusted to allow movements of the UE in a large tridimensional workspace required to perform the assessment exercises (Fig. 1). During the second and third visits, the patient was placed on the device in the same way and performed the same series of exercises as during the first visit.
Fig. 1.
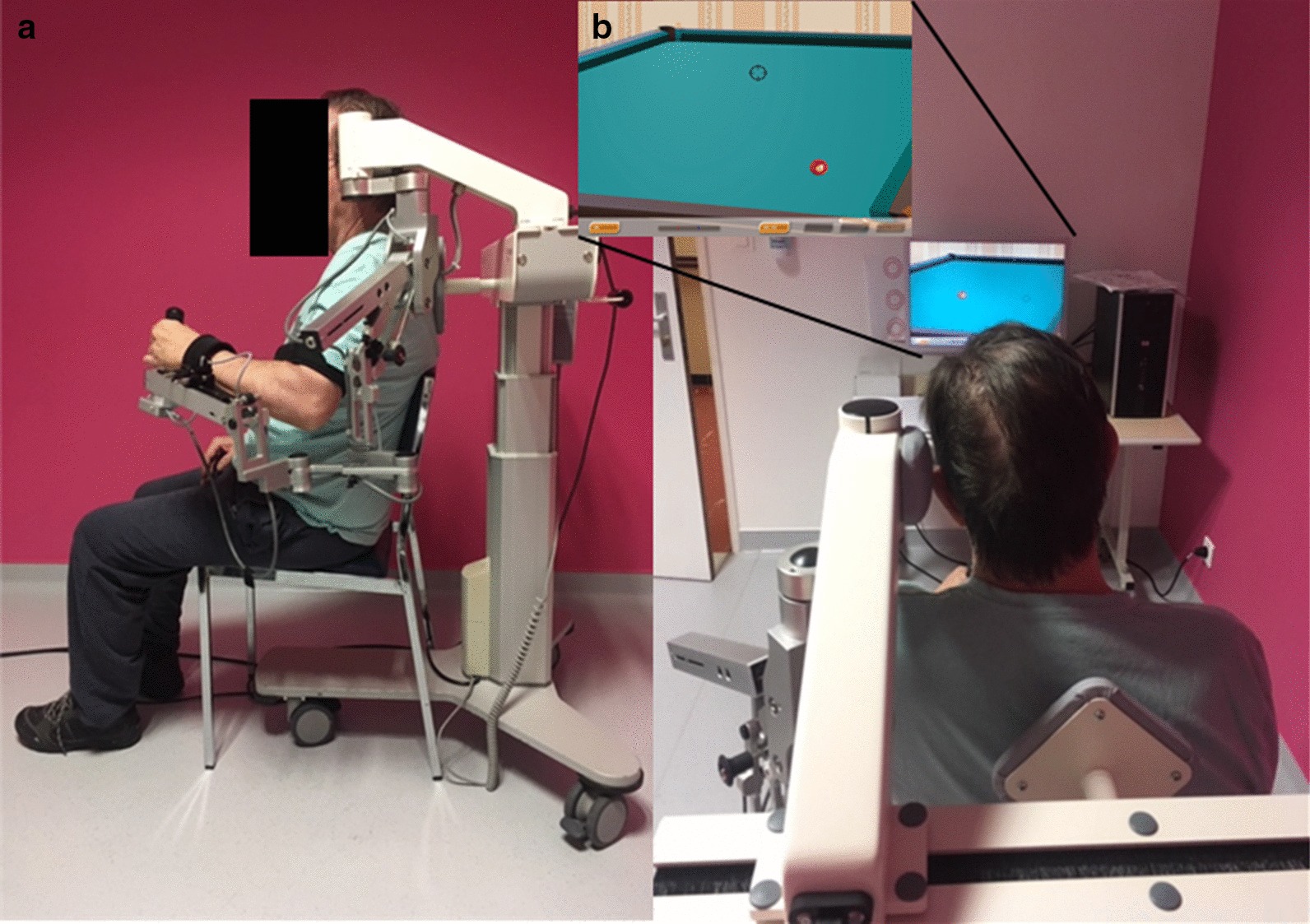
Experimental setup. a Installation of the patient performing a training exercise of the impaired upper limb with the ArmeoSpring. b Screenshot of the 2D-horizontal catch assessment exercise used in this study
The ArmeoSpring device
The ArmeoSpring (Hocoma, Switzerland), is a passive exoskeleton which provides UE weight support and allows early training of motor skills [27]. It has six degrees of freedom and can be attached to the UE at the level of the arm, forearm and wrist. It thus allows self-initiated arm movements in a large tridimensional workspace. Support against gravity is provided by adjustable springs for the upper arm (9 levels, from A, no tension exerted and minimum support, to I, maximum tension and support) and the forearm (5 levels from A to E). It is supplied with the Armeocontrol 1.22 software, which provides many functional exercises, simulated in a virtual environment with auditory and visual feedback. The software also allows recording kinematic parameters via seven sensors positioned on the different exoskeleton joints, and provides all the exoskeleton joint angles, the effector location in a tridimensional workspace (used to control a cursor on a screen) and the grip pressure. Several assessment exercises are available, with different levels of difficulty. Difficulty can be modulated by the workspace size and the number of targets to be caught.
Experimental procedures
All the patients were seated in the same standardized and ergonomic central position, in front of the computer screen (Fig. 1a). The exoskeleton was adjusted to the length of the arm and forearm, but the same level of weight support was set for all the patients (medium support, level E for the arm, and level C for the forearm). Initially, the patient's shoulder was placed between 0 and 20° elevation and elbow at 90° flexion. Patient-specific settings on the ArmeoSpring were retained between the three consecutive sessions.
Each testing session lasted between 20 and 30 min, depending on the patient’s motor impairment. Each patient performed with their paretic upper limb 10 repetitions (trials) of the same assessment exercise (the 2D-horizontal catch, Fig. 1b) separated by 30 s of rest. The 2D-horizontal pointing task required moving the cursor (corresponding to the patient’s hand) in order to catch the targets (represented by red billiard balls) that appeared sequentially on the screen. Depending on the position of the target in the workspace, the patients had to perform shoulder movements or a combination of shoulder and elbow movements in order to reach the most distant targets. Each patient was instructed to move as accurately as possible and at a self-selected speed, while being aware that they had a time limit of 10 s to catch each ball. When a ball was caught, it disappeared and another appeared at a new fixed location. During a trial, 12 balls had to be caught and the time to catch a ball was limited to 10 s; if this period was exceeded, the ball disappeared and another ball appeared at the new location. For this study, the difficulty of the 2D-horizontal catch exercise was set to the easiest level (level 1) for all the patients, with a predefined number of targets (12 targets) and a horizontal workspace size of 40 30 cm.
Each patient was subjected to three repetitions of the testing session, resulting in 30 trials per patient. The target positions and sequence order remained fixed throughout the 3 visits. The between-patient standardization of the protocol settings (compensation level, exercise difficulty and rest period) allowed attributing the potential differences between patients to performance changes rather than changes related to different settings. The sessions were controlled independently by the rater.
Kinematic assessment
A unitary movement was defined between two consecutive targets and considered only if both balls, the previous and the next, were successfully caught. During a trial, a maximum of 12 balls had to be caught, thus representative of 12 consecutive unitary movements.
The Armeocontrol software records raw data, specific to the assessment exercise, at a frequency of 64 Hz, corresponding in this case to the hand position in the horizontal plane (XY), and the time when the target appeared, was caught and disappeared. From the raw data file, we computed kinematic parameters with a custom code implemented on Matlab software (see Additional file 1: S1), freely downloadable at https://github.com/davidgasq/Armeo_2DHorizCatch.git. These parameters were chosen because, based on the recommendations by Schwarz et al. [5], they are relevant to explore different dimensions of the movement performed.
The task time (TaskTime in seconds, s) was the duration needed to complete the exercise (the maximal duration was 120 s). The movement time (MovementTime, s) was the duration given to catch one ball (10 s maximum per ball) and reflected the efficiency of movement. The peak velocity (PeakVel, cm/s) was the maximal absolute velocity recorded during each movement. The hand path ratio (HPR, dimensionless) was the ratio between the real path in the horizontal plane and the shortest possible one (a value ranging between 1 and infinity) and reflected movement efficiency. The number of velocity peaks (nPeak) was the number of peaks, defined as the number of times the derivative of velocity changes sign from positive to negative, and which reflected the smoothness of the movement. The Score (%) corresponded to the game score, computed as the number of balls reached divided by the total number of balls that could be reached, and summarized the efficiency of the movement.
The Armeocontrol software systematically provided a summary report where 3 parameters among those described above were given: HPR, TaskTime and Score.
Data analysis
The statistical analyses were performed using Statistica software (StatSoft. Inc. Version 10). The significance threshold of the p-value was set at 0.05. For each trial and each patient, the parameter data were averaged from all the successful unitary movements (a maximum of 12 balls). The data were also averaged for each session (10 consecutives trials).
We first ensured that the kinematic parameters of the summary report and those calculated with the custom code were consistent (paired t-tests not statistically significant, see Additional file 1: Figure S2). Although we tried to standardize the starting position at the beginning of the exercise, we observed that not all the patients started from the same position. Some patients had their hands already almost placed over the ball. Accordingly, the first trajectory (corresponding to the movement which starts from the 1st ball caught) was excluded from the analysis. The number of failed attempts was also significantly decreased for the target 1, which supports our observation (see Additional file 1: Figure S3). Consequently, only the last 11 unitary movements were considered to compute the parameters. We detected outliers using the Tukey method [28] and removed them from the statistical analysis.
Secondly, the learning effect was studied using a 2-way repeated measures ANOVA (rm-ANOVA, 10 trials * 3 sessions) to determine if differences existed between the ten trials of each of the three sessions. The dependent variables were tested for non-sphericity using Mauchly’s test and those not meeting the sphericity assumption were adjusted using the Greenhouse–Geisser correction and corrected p-values were reported instead. If significant, a Tukey post-hoc analysis was applied to analyze significant main effects and interactions. If no trial * session interaction was found, we considered the same trial effect across sessions. The retention of the kinematic parameters was inferred from the rm-ANOVA results with the between-session comparisons. Indeed, the data obtained from the last day of training (S3) were compared to those obtained at the end of the previous day, during S2.
Thirdly, reliability was studied specifically on the averaged data of the sessions and trials for which we considered there was no longer an obvious learning effect (the last four trials of S2 and S3, see “Results” section for details). The relative reliability was evaluated using the intraclass correlation coefficients (ICC) that provide information on inter- and intra-session reliability. We used the ICC2,k because we analyzed averaged data which were independent from the rater [29]. An ICC ≥ 0.75 was considered excellent, moderate if between 0.40 and 0.75 and weak if < 0.40 [30].
The MDC95 (minimal detectable change) represents the magnitude of change necessary to exceed the measurement error of 2 repeated measures at a confidence interval of 95% (CI95%) [31]. It integrates the variability of the measurement related to the patient, the tool and also possible systematic biases between test–retest sessions, such as a learning effect. A low MDC corresponds to a better theoretical capability of the parameter to detect a real change. First, the standard error measurement (SEM) was computed, considering the systematic differences between the test and retest, with the following formula:
where represented the variance of individual differences between the test–retest measurements and (residual)2; the residual variance of the interaction between intra- and inter-individual differences obtained from a repeated ANOVA [31]. Then, the MDC95 was computed as follows [32]:
MDC95 was also expressed as a percentage (MDC%) so that it could be independent of the measurement unit and comparable across the kinematic parameters, thus:
where the mean is the parameter averaged for all the observations across the selected trials of two sessions. Finally, the CI95 of the mean difference was computed between the test and retest measures to identify any systematic trends or outliers, and no residual systematic bias was considered if it included the zero [33].
Results
All the 30 patients performed the 3 assessment sessions under the rater’s control. Only one patient (#27) performed 6 trials instead of 10 in each of the 3 sessions, due to fatigue. The mean age was 54.5 ± 16.4 years; the post-stroke time was 14.7 ± 26.7 weeks. The average UE-FMS was 40.7 ± 14.5 [from 15 to 65]. The detailed data for each patient are presented in Table 1.
Table 1.
Patient characteristics
| Patients | Gender | Age (years) | Dominant hand | Post-stroke time (weeks) | Paretic side | UE-FMS (/66) |
|---|---|---|---|---|---|---|
| 1 | M | 74 | R | 4 | L | 29 |
| 2 | M | 69 | R | 3 | L | 30 |
| 3 | M | 54 | R | 22 | L | 24 |
| 4 | F | 76 | R | 8 | L | 42 |
| 5 | M | 49 | R | 16 | L | 48 |
| 6 | M | 23 | L | 18 | R | 57 |
| 7 | F | 70 | R | 10 | L | 64 |
| 8 | M | 59 | R | 6 | R | 15 |
| 9 | M | 61 | R | 5 | L | 39 |
| 10 | M | 62 | R | 8 | L | 38 |
| 11 | F | 34 | R | 11 | R | 15 |
| 12 | M | 48 | R | 7 | R | 33 |
| 13 | F | 76 | R | 20 | L | 36 |
| 14 | F | 72 | R | 7 | R | 51 |
| 15 | F | 33 | R | 9 | R | 22 |
| 16 | M | 46 | R | 3 | R | 54 |
| 17 | M | 76 | R | 14 | R | 36 |
| 18 | M | 53 | R | 7 | R | 28 |
| 19 | F | 47 | R | 10 | L | 31 |
| 20 | M | 65 | R | 153 | L | 28 |
| 21 | M | 35 | R | 3 | R | 48 |
| 22 | F | 38 | R | 10 | R | 48 |
| 23 | M | 33 | R | 11 | R | 65 |
| 24 | M | 69 | L | 15 | L | 53 |
| 25 | M | 67 | L | 4 | L | 42 |
| 26 | M | 43 | R | 5 | L | 44 |
| 27 | M | 52 | R | 20 | L | 23 |
| 28 | M | 59 | R | 5 | L | 55 |
| 29 | F | 70 | R | 8 | R | 58 |
| 30 | M | 22 | R | 66 | R | 64 |
F female, L left, M male, R right, UE-FMS upper extremity Fugl Meyer scale
Learning effect
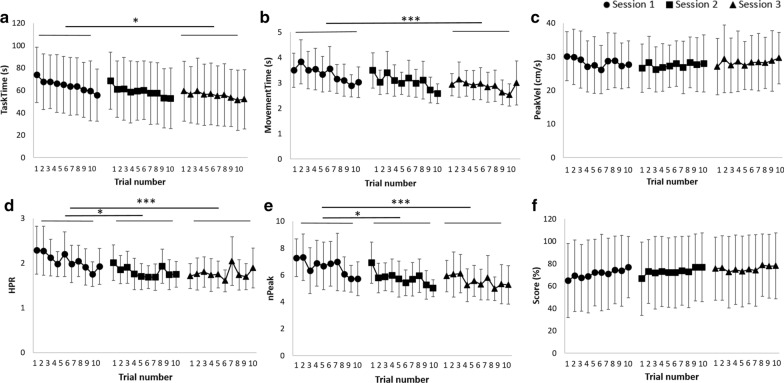
Most of the parameters showed an intra- and/or inter-session learning effect, independent from each patient’s initial performance (results not shown), corresponding to a significant improvement of the parameters across trials and/or sessions, respectively. The ANOVA values and the significant differences between sessions and/or trials are reported in Table 2. The learning curves are shown in Fig. 2.
Table 2.
Learning effect analysis with a two-way repeated measures ANOVA
| Inter-session effect (among 3 sessions) | Intra-session effect (among 10 trials) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | F-value; p-value | F-value; p-value | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | |
| TaskTime (s) | S3 | 4.23; p < 0.05 | 7.59; p < 0.0001 | T2–T10 | – | T9 | – | – | – | – | – | – | – |
| MovementTime(s) | S3 | 9.71; p < 0.001 | 9.96; p < 0.0001 | T7–T10 | T7–T10 | T9–T10 | T9–T10 | T9–T10 | T9–T10 | – | – | – | – |
| PeakVel (cm/s) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| HPR | S2/S3 | 10.69; p < 0.001 | 5.74; p < 0.0001 | T9–T10 | T6, T9–T10 | T9 | – | T9 | – | – | – | – | – |
| nPeak | S2/S3 | 11.16; p < 0.001 | 10.76; p < 0.0001 | T4, T6–T10 | T3–T4, T6–T10 | T10 | – | – | – | – | – | – | – |
| Score (%) | – | – | 3.59; p < 0.01 | T2, T5–T10 | – | – | – | – | – | – | – | – | – |
HPR, hand path ratio (dimensionless); MovementTime, movement time; nPeak, number of velocity peaks; PeakVel, peak velocity; Score, the game score corresponding to the number of balls reached divided by the total number of balls that could be reached; S1, session 1; S3, session 3; TaskTime, task time; T1 to T10, trials 1 to 10. The second column report the session(s) significantly different from session 1 (S1). The columns of the intra-session effect report the trial(s) significantly different from each other
Fig. 2.
Learning curves of the averaged parameters (± SD) showing the evolution of a specific parameter over the trials (1 to 10) for the 3 sessions. a Task time (TaskTime, s). b Movement time (MovementTime, s). c Peak velocity (PeakVel, cm/s). d Hand path ratio (HPR, dimensionless). e Number of peak velocity (nPeak). f Game score (Score, %). Between-session significances are represented with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) and within-session significances are reported in Table 2
A session effect was observed for most parameters, except for PeakVel and Score (Table 2). Tuckey post-hoc tests revealed that the session effect occurred mainly between S3 and S1 for TaskTime (p = 0.02) and MovementTime (p = 0.0006) and between S2/S3 and S1 for HPR (p = 0.026 and p = 0.0005 respectively) and nPeak (p = 0.037 and p = 0.0004 respectively). A trial effect was also observed for nPeak (p < 0.05, Table 2) and the learning effect was no longer observed after the 6th trial (four last columns of the Table 2). For Score, only a trial effect was highlighted between the first trial and the second on one part, and the 5th to the 10th on the other part (p < 0.05). No session or trial effect was shown for PeakVel.
Learning occurred mainly between S1 and S2/S3 (Fig. 2), which made us consider retention between S2 and S3. Indeed, no significant difference (p > 0.05, Table 2, Fig. 2) was observed between the kinematic parameters measured in S3 and those measured at the end of the previous day, in S2, thus revealing the successful retention of the skills acquired for all the kinematic parameters.
Additionally, the Additional file 2: Figure S4 represents the individual learning curves obtained from two patients’ data (patient #21 and #29). These patients had mild residual motor deficits (as reflected by their UE-FMS > 47/66 [34], Table 1). Their performance in the task are similar when looking at the TaskTime or MovementTime (Additional file 2: Fig. S4.A and B) and Score (reaching a score of 100/100, Additional file 2: Fig. S4.F) but not so much when looking at the PeakVel, HPR and nPeak parameters (Additional file 2: Fig. S4.C–E respectively).
Reliability
Considering the previous ANOVA results and the graphical observation of the evolution of reliability (see Additional file 2: Figure S5 for details), data from trials 7 to 10 (the last 4 trials) of sessions 2 and 3 were selected for the reliability analysis. The reliability data are reported in Table 3. All the parameters, except MovementTime, HPR and nPeak, had excellent relative reliability, as expressed by the CI95 lower bound of the ICC ≥ 0.75.
Table 3.
Reliability data for the 30 patients computed from the last 4 trials of sessions 2 and 3
| Mean S2 (SD) | Mean S3 (SD) | ICC (CI95%) | MDC95 | MDC% | mDiff (CI95%) | |
|---|---|---|---|---|---|---|
| TaskTime (s) | 58.95 (31.73) | 53.82 (31.16) | 0.97 (0.91 to 0.99) | 57.94 | 102.77 | − 5.13 (− 9.13 to − 1.13) |
| MovementTime (s) | 3.20 (0.74) | 2.97 (0.77) | 0.77 (0.47 to 0.90) | 2.58 | 83.78 | − 0.24 (− 0.50 to 0.03) |
| PeakVel (cm/s) | 28.32 (9.97) | 27.61 (9.29) | 0.93 (0.83 to 0.97) | 12.06 | 43.11 | − 0.71 (− 2.85 to 1.43) |
| HPR | 1.91 (0.39) | 1.79 (0.36) | 0.77 (0.47 to 0.90) | 1.28 | 68.92 | − 0.12 (− 0.25 to 0.02) |
| nPeak | 6.25 (1.58) | 5.61 (1.34) | 0.78 (0.45 to 0.91) | 6.51 | 109.77 | − 0.64 (− 1.12 to − 0.16) |
| Score (%) | 70.66 (36.81) | 73.46 (36.90) | 0.99 (0.97 to 0.99) | 32.84 | 45.57 | 2.80 (− 0.003 to 5.59) |
ICC, intraclass correlation coefficient (lower and upper-bound of the 95% confidence interval [CI95%]); MDC95, minimal detectable change values computed with a 95% confidence interval; MDC%, minimal detectable change values expressed as a percentage of the mean; mDiff, mean difference computed between test and retest measures (lower and upper-bound of the CI95%); Mean S2/S3, mean (standard deviation [SD]) value of parameters for session 2 (S2) and 3 (S3)
The MDC% values were heterogeneous from one parameter to another, ranging from 43.1 to 109.8%, with only PeakVel and Score showing a MDC% < 50%. For TaskTime and nPeak, a residual systematic bias (i.e. CI95 of mDiff not including zero) can be seen, reflecting an improvement between sessions 2 and 3.
Discussion
In this pilot study, kinematic parameters computed from data provided by the ArmeoSpring exoskeleton were analyzed to investigate the relevance of these parameters in the assessment of post-stroke hemiplegic patients during a 2D-horizontal catching exercise. The results highlighted an intra- and inter-session learning effect for all the parameters except PeakVel. The reliability analysis, applied to data without a priori learning, showed that PeakVel and Score had the lowest margin of error.
Learning effect
We observed an inter- and intra-session learning effect for the parameters MovementTime, TaskTime, HPR and nPeak and an intra-session effect only for the Score. This result highlights the importance of the learning effect, even most studies reported little or no learning effect for the kinematic parameters obtained with robotic and non-robotic devices [19–21, 35]. This difference may be explained by the fact that, unlike us, the authors of the latter studies used robotic devices. Such devices provide some assistance during movements, thus maybe limiting the learning process during the performance of the task. It has already been described that physical assistance hinders motor learning of a simple walking balance task in healthy subjects [36]. Furthermore, in order to shorten the learning process, the authors preconized a single practice session before the real training sessions, which may have also limited the learning effect observed during the latter. MovementTime was the parameter most sensitive to the learning effect, showing a significant decrease of the time needed to catch a ball across trials and sessions. This parameter is used to globally assess the patient's ability to perform the movement [7], reflecting movement efficiency [5], and is classified in the "activity" domain of the ICF [4]. Given the importance of this learning effect, it seems necessary to repeat the exercise at least ten times per session, and consider only the last four trials of the second session to obtain a consistent result. The entire first session and the first six trials of the following sessions should not be considered because MovementTime continues to decrease, independently of any intervention or recovery. nPeak, used to characterize the smoothness of the movement [4, 5] and which has been shown to decrease following robotic training [37], is also sensitive to a persistent inter- and intra-session learning effect. This learning effect was already described in post-stroke patients during a frontal plane reaching task with the ArmeoSpring [23]. In this latter study, the fast and early improvement of this parameter was considered to reflect the improvement of performance due to learning processes, while its late and slower improvement was considered to reflect a reduction of UE motor impairments. However, in view of the design of our study, which took place over only 2 days, we cannot extrapolate this latter result. The parameters that showed a persistent learning effect over sessions may be used to assess the effect of a specific therapeutic intervention on learning processes that are known to occur in post-stroke settings [38, 39].
The PeakVel and Score parameters were less sensitive to the learning effect. PeakVel, which evolves with time post-stroke to match healthy patient values [12], showed a concurrent validity with the UE-FMS score [9] and moderate quality of evidence regarding its reliability [5]. However, within a session, the Score stabilized quickly after 1 trial. It was shown to correlate with wrist function [40] and reflects movement efficacy [5]. Consequently, these two parameters should be used to assess patient performance/impairment and motor recovery at a given time or over time. Although considered similar, MovementTime and PeakVel showed different sensitivity to the learning effect. This may be due to the fact that these parameters reflect different aspects of movement properties. As already mentioned, MovementTime reflects a global dimension of the temporal efficiency of a movement [5, 17]. This parameter is correlated and predicts well the residual motor deficits of stroke patients as assessed with the UE-FMS [41], thus it is recommended for the evaluation of motor recovery and robot-assisted rehabilitation after stroke [4, 42]. PeakVel is a speed metric that reflects the first (i.e. ballistic) phase of a movement, its strategy and ease [17]. Contrary to movement duration, PeakVel showed weak correlation with clinical scales [21, 42] and less sensitivity to changes [37, 43]. These arguments may explain their different sensitivities to the learning effect. Whereas patients continued to perform the movements in an increasingly shorter time, PeakVel remain unchanged and the time of occurrence of the peak velocity during the movement increased across session (see Additional file 2: Figure S6), thus revealing a right-shift of the velocity profile. This result is in favor of the improvement of the corrective and controlled phase of the movement across sessions [44, 45]. Additional file 2: Figure S4 also highlights the importance of a kinematic assessment with different parameters from those computed by the Armeocontrol to highlight subtle differences between subjects, not shown by UE-FMS, or due to recovery and/or therapeutic intervention.
Retention refers to the persistence of the performance acquired during the training period. This phenomenon is an important part of motor learning [24]. The gains in all the kinematic parameters chosen were retained for at least 24 h (as revealed by the absence of significant differences between S2 and S3). These results revealed the successful inter-session retention of the 2D-horizontal exercise with this paradigm in our stroke population and are in line with previous studies [16].
Reliability
All the parameters selected showed overall an excellent (TaskTime, PeakVel and Score) or moderate (MovementTime, HPR and nPeak) relative reliability [30]. These results are consistent with those of other studies investigating this type of task in stroke populations [19, 46, 47]. Thus, they may be appropriate for intra-individual comparisons [35].
MDC95 and MDC% are useful in determining whether a change of a parameter is metrically real or if it is due to a measurement error. Thus, the lower the measurement error, the greater the reliability [47–49]. For a patient, a significant improvement may therefore be suggested when the improvement of the parameter exceeds the MDC95 values reported in Table 3. MDC% values ranged from 42.6 to 109.8%, indicating that some parameters require larger variations than others to highlight real changes. For TaskTime, its variation must exceed 102% to indicate a real change, which is congruent with the literature [17, 19, 47, 50]. For example, in a study assessing stroke patients performing a simple forward-reaching task measured with an optical tracking system, MDC% ranged from 7.4 to 98%, depending on the kinematic parameter, the task instructions and the analysis method used [47]. For the HPR, the MDC% ranged between 7.4 and 28.9%, whereas the values ranged between 24.4 and 67.6% for the nPeak [47]. The higher values found in our study for these parameters could be explained by the method of MDC computation we used which, unlike [47], incorporated the presence of a systematic bias between tests and retests [31]. Although we have shown that there are still systematic bias residues (i.e. learning effect) when calculating the reliability between sessions 2 and 3, our MDC values are higher but may better reflect the reality of clinical practice.
Important considerations and limitations
Since we wanted unrestricted arm movements, the exoskeleton was unlocked at the level of shoulder and elbow. Sometimes, the hand was directly located at the first ball position and therefore the movement observed did not reflect the real one. Consequently, in our study, the data were averaged over 11 consecutive movements and not 12 as designed in the horizontal catching task, and as the Armeocontrol software computes kinematic parameters.
We investigated the psychometric properties of certain carefully chosen kinematic parameters based on a recent review [5], that represent all the dimensions of a movement. The kinematic parameters were slightly different (although not significantly) from those provided in the ArmeoSpring report (see Additional file 1: Figure S2), but computed with a stricter and more rigorous methodology (removal of the first target, trajectories considered only if the departure and arrival targets are reached). This may be used to administer a short assessment protocol to post-stroke patients with the ArmeoSpring, but could also limit its ease of use in routine care by a clinician. To exceed these limits, we have made available to the community the script used to calculate the parameters (see Additional file 1: S1).
Depending on the research questions and hypothesis, some parameters may be more appropriate than others to capture movement patterns. As demonstrated, we must be careful in the interpretation since the initial parameter value may also depend on learning processes that are relatively independent from the impairment reductions [23]. In our study, learning occurred mainly between session 1 and sessions 2 and 3, and until the sixth repetition for some parameters. Consequently, in similar conditions and particularly for the parameters MovementTime, TaskTime, HPR and nPeaks, we suggest considering the first session (consisting of 10 repeated trials) as a session of familiarization with the device and the task to avoid data corruption by learning processes. For the learning effect to be minimized, the actual assessment session should include a minimum of 1 to 6 trials, depending on the parameter used (see Table 2). The measurement error data computed in our study are applicable for judging a change over time (e.g., pre-post treatment) only if 10 trials are performed and the last 4 averaged. However, some MDC values were still high and variable across parameters, with a systematic bias for some of them. It may therefore be more relevant to identify for each parameter a specific number of trials per session to overcome the learning effect observed.
We cannot exclude the influence of the exoskeleton support on the results since some devices are known to affect the validity of kinematic data [9, 51]. However, since the ArmeoSpring is a passive orthosis, we can assume that it was limited. A comparison with the kinematic parameters obtained during the same task but without weight support and with a free UE may appropriately address this question.
Unfortunately, we were not able to assess the successful transfer or generalization of the task, which is another important aspect of motor learning. A transfer test is usually administered after the training period and assesses the skill with another effector or a skill that was not practiced [24], thus revealing the effects of learning on untrained effectors/contexts/tasks. It would be interesting to carry out further studies to evaluate retention over a much longer period of time and generalization to other functionally relevant tasks [52, 53].
Conclusions
This study demonstrated that the ArmeoSpring may be effectively used for a reliable, objective and quantitative assessment of upper-extremity motor and functional impairments, and to assess therapeutic effects on motor learning in post-stroke patients. The results provided greater precision for structuring an assessment session with the device, depending on the research question. An initial session with a specific number of trials (depending on the parameter) must be performed to allow the patient to familiarize themselves with the procedure, before carrying out the actual assessment sessions. Certain parameters such as PeakVel and Score may be used to assess performance at a specific time whereas TaskTime, MovementTime, HPR and nPeak may be used to assess the effect of specific interventions on learning processes. This preliminary study confirms the importance of such studies aimed at standardizing the use of kinematic assessment, and emphasizes the relevance of using such devices to track and highlight subtle changes and progress due to learning, recovery and the administration of therapeutic interventions.
Supplementary information
Additional file 1. Additional methodological file. Additional material S1. Custom Matlab code. The Matlab code used for the calculation of kinematic parameters, available on GitHub website. Figure S2. Box plot comparison between kinematics calculation methods. Comparison between the averaged parameter values provided in the summary report of the ArmeoSpring (Armeocontrol Software) and those calculated with the Matlab code (custom code). Statistical results of the paired t-tests for the hand path ratio (A. HPR), Task Time (B. TaskTime in seconds) and the Score (C. Score in percentage) are reported on the figure. Figure S3. Graphical representation of the average number of failed attempts to catch the consecutive balls (targets 1 to 12). Statistical parameters of the ANOVA are shown below the graph. The number of failed attempts to catch target 1 is significantly lower compared to the other targets (*p < 0.0001).
Additional file 2. Additional results file. Figure S4. Learning curves of two example patients’ data (#21 and #29) showing the evolution of a specific parameter over the trials (1 to 10) for the 3 sessions. A. Task time (TaskTime, s). B. Movement time (MovementTime, s). C. Peak velocity (PeakVel, cm/s). D. Hand path ratio (HPR, dimensionless). E. Number of peak velocity (nPeak). F. Game score (Score, %). Figure S5. Evolutions of Minimal Detectable Change (MDC, dashed lines) and Intraclass Correlation Coefficient (ICC, solid lines) according to the number of trials (of sessions 2 and 3), taking into account the calculation of reliability (1: only the 10th trial, to 10: the 10th to the 1st trial). Different parameters are represented by different colors and shapes (black (■): peak velocity, gray (♦): score, orange (●): number of peaks, blue (▲): task time, green (⁃): movement time, yellow (▬): HPR). Figure S6. Learning curves of the averaged time of occurrence of the peak velocity during the movement expressed in percentage (PercPeakVel (%) ±SD), showing the evolution of this parameter over the trials (1 to 10) of the 3 sessions. Between-session significance is represented with asterisks (* p < 0.05). No significant trial effect was observed.
Acknowledgements
The authors thank all the patients who accepted to participate in the study and all the physiotherapists and occupational therapist teams of the Department of Physical and Rehabilitation Medicine of the Toulouse University Hospital.
Abbreviations
- UE
Upper extremity
- UE-FMS
Upper extremity Fugl-Meyer subscale
- TaskTime
Task time
- MovementTime
Movement time
- PeakVel
Peak velocity
- HPR
Hand path ratio
- nPeak
Number of velocity peaks
- ICC
Intraclass correlation coefficient
- MDC95
Minimal detectable change at a confidence interval of 95%
- SEM
Standard error measurement
- MDC%
Minimal detectable change expressed as a percentage
Authors’ contributions
NB; patient recruitment, study design, analysis and interpretation of the results, preparation and writing of the manuscript; IL: help in the interpretation of the results; ECL: patient recruitment; PM: concept and regulatory aspects of the study; DG: study design, analysis and interpretation of the results, preparation and writing of the manuscript. All authors read and approved the final manuscript.
Funding
The author(s) received no specific funding for this work.
Availability of data and materials
The dataset used and analyzed during the current study is available upon reasonable request from the corresponding author.
Ethics approval and consent to participate
The present study was approved by the local hospital ethics committee in September 2016 (n° 05-0916). All the participants provided written informed consent in accordance with the ethical guidelines.
Consent for publication
All the authors have approved the manuscript for publication.
Competing interests
The author(s) declare no potential conflicts of interest concerning this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12984-020-00759-2.
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389(10069):641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 2.Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther. 2013;26(2):104–115. doi: 10.1016/j.jht.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fugl-Meyer A, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 4.Do Tran V, Dario P, Mazzoleni S. Kinematic measures for upper limb robot-assisted therapy following stroke and correlations with clinical outcome measures: a review. Med Eng Phys. 2018;53:13–31. doi: 10.1016/j.medengphy.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz A, Kanzler CM, Lambercy O, Luft AR, Veerbeek JM. Systematic review on kinematic assessments of upper limb movements after stroke. Stroke. 2019;50(3):718–727. doi: 10.1161/STROKEAHA.118.023531. [DOI] [PubMed] [Google Scholar]
- 6.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 7.Frisoli A, Sotgiu E, Procopio C, Bergamasco M, Chisari C, Lamola G, et al. Training and assessment of upper limb motor function with a robotic exoskeleton after stroke. In: 2012 4Th IEEE Ras Embs Int Conf Biomed Robot Biomechatronics. 2012;1782–7. 10.1109/BioRob.2012.6290843.
- 8.Gijbels D, Lamers I, Kerkhofs L, Alders G, Knippenberg E, Feys P. The Armeo Spring as training tool to improve upper limb functionality in multiple sclerosis: a pilot study. J Neuroeng Rehabil. 2011;8(1):5. doi: 10.1186/1743-0003-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordin N, Xie SQ, Wunsche B. Assessment of movement quality in robot- assisted upper limb rehabilitation after stroke: a review. J Neuroeng Rehabil. 2014;11:137. doi: 10.1186/1743-0003-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwakkel G, Van Wegen EEH, Burridge JH, Winstein CJ, van Dokkum LEH, Alt Murphy M, et al. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2019;14(8):783–791. doi: 10.1177/1747493019873519. [DOI] [PubMed] [Google Scholar]
- 11.Laffont I, Bakhti K, Coroian F, van Dokkum L, Mottet D, Schweighofer N, et al. Innovative technologies applied to sensorimotor rehabilitation after stroke. Ann Phys Rehabil Med. 2014;57(8):543–551. doi: 10.1016/j.rehab.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 12.van Dokkum L, Hauret I, Mottet D, Froger J, Métrot J, Laffont I. The contribution of kinematics in the assessment of upper limb motor recovery early after stroke. Neurorehabil Neural Repair. 2014;28(1):4–12. doi: 10.1177/1545968313498514. [DOI] [PubMed] [Google Scholar]
- 13.Rudhe C, Albisser U, Starkey ML, Curt A, Bolliger M. Reliability of movement workspace measurements in a passive arm orthosis used in spinal cord injury rehabilitation. J Neuroeng Rehabil. 2012;9:37. doi: 10.1186/1743-0003-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colomer C, Baldoví A, Torromé S, Navarro MD, Moliner B, Ferri J, et al. Efficacy of Armeo® Spring during the chronic phase of stroke. Study in mild to moderate cases of hemiparesis. Neurologia. 2013;28(5):261–267. doi: 10.1016/j.nrl.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Chan IHL, Fong KNK, Chan DYL, Wang AQL, Cheng EKN, Chau PHY, et al. Effects of arm weight support training to promote recovery of upper limb function for subacute patients after stroke with different levels of arm impairments. Biomed Res Int. 2016;2016:9346374. doi: 10.1155/2016/9346374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller JW, Van Hedel HJA. Weight-supported training of the upper extremity in children with cerebral palsy: a motor learning study. J Neuroeng Rehabil. 2017;14(1):1–13. doi: 10.1186/s12984-017-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivan M, O’Connor RJ, Makower S, Levesley M, Bhakta B. Systematic review of outcome measures used in the evaluation of robot-assisted upper limb exercise in stroke. J Rehabil Med. 2011;43(3):181–189. doi: 10.2340/16501977-0674. [DOI] [PubMed] [Google Scholar]
- 18.Teasell R, Foley N, Salter K, Bhogal S, Jutai J, Speechley M. Evidence-based review of stroke rehabilitation: executive summary, 12th edition. Top Stroke Rehabil. 2009;16(6):463–488. doi: 10.1310/tsr1606-463. [DOI] [PubMed] [Google Scholar]
- 19.Colombo R, Cusmano I, Sterpi I, Mazzone A, Delconte C, Pisano F. Test-retest reliability of robotic assessment measures for the evaluation of upper limb recovery. IEEE Trans Neural Syst Rehabil Eng. 2014;22(5):1020–1029. doi: 10.1109/TNSRE.2014.2306571. [DOI] [PubMed] [Google Scholar]
- 20.Finley MA, Dipietro L, Ohlhoff J, Whitall J, Krebs HI, Bever CT. The effect of repeated measurements using an upper extremity robot on healthy adults. J Appl Biomech. 2009;25(2):103–110. doi: 10.1123/jab.25.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliaux M, Lejeune T, Detrembleur C, Sapin J, Dehez B, Selves C, et al. Using the robotic device REAplan as a valid, reliable, and sensitive tool to quantify upper limb impairments in stroke patients. J Rehabil Med. 2014;46(2):117–125. doi: 10.2340/16501977-1245. [DOI] [PubMed] [Google Scholar]
- 22.Merlo A, Longhi M, Giannotti E, Prati P, Giacobbi M, Ruscelli E, et al. Upper limb evaluation with robotic exoskeleton. Normative values for indices of accuracy, speed and smoothness. NeuroRehabilitation. 2013;33(4):523–530. doi: 10.3233/NRE-130998. [DOI] [PubMed] [Google Scholar]
- 23.Schweighofer N, Wang C, Mottet D, Laffont I, Bakthi K, Reinkensmeyer DJ, et al. Dissociating motor learning from recovery in exoskeleton training post-stroke. J Neuroeng Rehabil. 2018;15(1):1–10. doi: 10.1186/s12984-018-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shishov N, Melzer I, Bar-Haim S. Parameters and measures in assessment of motor learning in neurorehabilitation; a systematic review of the literature. Front Hum Neurosci. 2017;11:82. doi: 10.3389/fnhum.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):98–169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 26.Haute Autorité de Santé (HAS), DAQSS, SIPAQSS. Prise en charge de l’ accident vasculaire cérébral (AVC) en Soins de Suite et Réadaptation (SSR). 2018.
- 27.Sanchez RJ, Jiayin Liu J, Rao S, Shah P, Smith R, Rahman T, et al. Automating arm movement training following severe stroke: functional exercises with quantitative feedback in a gravity-reduced environment. IEEE Trans Neural Syst Rehabil Eng. 2006;14(3):378–389. doi: 10.1109/TNSRE.2006.881553. [DOI] [PubMed] [Google Scholar]
- 28.Tukey JW. Exploratory data analysis. Vol. 23, Exploratory data analysis. 1977. 702 p. 10.1007/978-1-4419-7976-6.
- 29.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 30.Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil. 2000;81(12 Suppl 2):15–20. doi: 10.1053/apmr.2000.20619. [DOI] [PubMed] [Google Scholar]
- 31.de Vet HCWW, Terwee CB, Knol DL, Bouter LM. When to use agreement versus reliability measures. J Clin Epidemiol. 2006;59(10):1033–1039. doi: 10.1016/j.jclinepi.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Pearson. 2007. 892 p. https://www.pearson.com/us/higher-education/program/Portney-Foundations-of-Clinical-Research-Applications-to-Practice-3rd-Edition/PGM274308.html.
- 33.Giavarina D. Understanding Bland Altman analysis. Biochem Medica. 2015;25(2):141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodbury ML, Velozo CA, Richards LG, Duncan PW. Rasch analysis staging methodology to classify upper extremity movement impairment after stroke. Arch Phys Med Rehabil. 2013;94(8):1527–1533. doi: 10.1016/j.apmr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Germanotta M, Cruciani A, Pecchioli C, Loreti S, Spedicato A, Meotti M, et al. Reliability, validity and discriminant ability of the instrumental indices provided by a novel planar robotic device for upper limb rehabilitation. J Neuroeng Rehabil. 2018;15(1):39. doi: 10.1186/s12984-018-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domingo A, Ferris DP. Effects of physical guidance on short-term learning of walking on a narrow beam. Gait Posture. 2009;30(4):464–468. doi: 10.1016/j.gaitpost.2009.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, et al. Movement smoothness changes during stroke recovery. J Neurosci. 2002;22(18):8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popa T, Velayudhan B, Hubsch C, Pradeep S, Roze E, Vidailhet M, et al. Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb Cortex. 2013;23(2):305–314. doi: 10.1093/cercor/bhs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avanzino L, Gueugneau N, Bisio A, Ruggeri P, Papaxanthis C, Bove M. Motor cortical plasticity induced by motor learning through mental practice. Front Behav Neurosci. 2015;9(105):1–10. doi: 10.3389/fnbeh.2015.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baniña MC, Mullick AA, McFadyen BJ, Levin MF. Upper limb obstacle avoidance behavior in individuals with stroke. Neurorehabil Neural Repair. 2017;31(2):133–146. doi: 10.1177/1545968316662527. [DOI] [PubMed] [Google Scholar]
- 41.Bosecker C, Dipietro L, Volpe B, Igo KH. Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabil Neural Repair. 2010;24(1):62–69. doi: 10.1177/1545968309343214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otaka E, Otaka Y, Kasuga S, Nishimoto A, Yamazaki K, Kawakami M, et al. Clinical usefulness and validity of robotic measures of reaching movement in hemiparetic stroke patients. J Neuroeng Rehabil. 2015;12(1):66. doi: 10.1186/s12984-015-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finley MA, Fasoli SE, Dipietro L, Ohlhoff J, MacClellan L, Meister C, et al. Short-duration robotic therapy in stroke patients with severe upper-limb motor impairment. J Rehabil Res Dev. 2005;42(5):683. doi: 10.1682/JRRD.2004.12.0153. [DOI] [PubMed] [Google Scholar]
- 44.Trombly CA, Wu CY. Effect of rehabilitation tasks on organization of movement after stroke. Am J Occup Ther. 1999;53(4):333–344. doi: 10.5014/ajot.53.4.333. [DOI] [PubMed] [Google Scholar]
- 45.Alt Murphy M, Willén C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. 2011;25(1):71–80. doi: 10.1177/1545968310370748. [DOI] [PubMed] [Google Scholar]
- 46.Patten C, Kothari D, Whitney J, Lexell J, Lum PS. Reliability and responsiveness of elbow trajectory tracking in chronic poststroke hemiparesis. J Rehabil Res Dev. 2003;40(6):487–500. doi: 10.1682/JRRD.2003.11.0487. [DOI] [PubMed] [Google Scholar]
- 47.Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys Ther. 2008;88(5):652–663. doi: 10.2522/ptj.20070255. [DOI] [PubMed] [Google Scholar]
- 48.Lin K, Fu T, Wu C, Wang Y, Liu J, Hsieh C, et al. Minimal detectable change and clinically important difference of the stroke impact scale in stroke patients. Neurorehabil Neural Repair. 2010;24(5):486–492. doi: 10.1177/1545968309356295. [DOI] [PubMed] [Google Scholar]
- 49.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 50.Alt Murphy M, Häger CK. Kinematic analysis of the upper extremity after stroke—how far have we reached and what have we grasped? Phys Ther Rev. 2015;20(3):137–156. doi: 10.1179/1743288X15Y.0000000002. [DOI] [Google Scholar]
- 51.Prange GB, Kottink AIR, Buurke JH, Eckhardt MMEM, Van K-R, Ribbers GM, et al. The effect of arm support combined with rehabilitation games on upper-extremity function in subacute stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2015;29(2):174–182. doi: 10.1177/1545968314535985. [DOI] [PubMed] [Google Scholar]
- 52.Krakauer JW. The applicability of motor learning to neurorehabilitation. In: Dietz V, Ward NS, editors. Oxford textbooks in clinical neurology. Oxford: Oxford University Press; 2015. pp. 55–63. [Google Scholar]
- 53.Schmidt R, Lee T. Motor control and learning: a behavioral emphasis. Human Kinetics Publishers, editor. Champaign; 1999. 592 p.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional methodological file. Additional material S1. Custom Matlab code. The Matlab code used for the calculation of kinematic parameters, available on GitHub website. Figure S2. Box plot comparison between kinematics calculation methods. Comparison between the averaged parameter values provided in the summary report of the ArmeoSpring (Armeocontrol Software) and those calculated with the Matlab code (custom code). Statistical results of the paired t-tests for the hand path ratio (A. HPR), Task Time (B. TaskTime in seconds) and the Score (C. Score in percentage) are reported on the figure. Figure S3. Graphical representation of the average number of failed attempts to catch the consecutive balls (targets 1 to 12). Statistical parameters of the ANOVA are shown below the graph. The number of failed attempts to catch target 1 is significantly lower compared to the other targets (*p < 0.0001).
Additional file 2. Additional results file. Figure S4. Learning curves of two example patients’ data (#21 and #29) showing the evolution of a specific parameter over the trials (1 to 10) for the 3 sessions. A. Task time (TaskTime, s). B. Movement time (MovementTime, s). C. Peak velocity (PeakVel, cm/s). D. Hand path ratio (HPR, dimensionless). E. Number of peak velocity (nPeak). F. Game score (Score, %). Figure S5. Evolutions of Minimal Detectable Change (MDC, dashed lines) and Intraclass Correlation Coefficient (ICC, solid lines) according to the number of trials (of sessions 2 and 3), taking into account the calculation of reliability (1: only the 10th trial, to 10: the 10th to the 1st trial). Different parameters are represented by different colors and shapes (black (■): peak velocity, gray (♦): score, orange (●): number of peaks, blue (▲): task time, green (⁃): movement time, yellow (▬): HPR). Figure S6. Learning curves of the averaged time of occurrence of the peak velocity during the movement expressed in percentage (PercPeakVel (%) ±SD), showing the evolution of this parameter over the trials (1 to 10) of the 3 sessions. Between-session significance is represented with asterisks (* p < 0.05). No significant trial effect was observed.
Data Availability Statement
The dataset used and analyzed during the current study is available upon reasonable request from the corresponding author.