Abstract
Introduction
Heart failure (HF) has always been an important issue in global public health. The research and development of traditional Chinese medicine (TCM) provide more possibilities for improving the prognosis of HF patients. Because multiple TCM injections (TCMIs) are being widely applied in clinical work, it is important to choose the right TCMIs for HF patients. The purpose of this study is to assess and compare the effect of different TCMIs for HF using network meta-analysis (NMA) and further provide references for clinical decision-making.
Methods and analysis
The clinical randomised controlled trials (RCTs) and meta-analyses of TCMIs for treating HF will be searched in the relevant database, including PubMed, EMBASE, Cochrane Library (No. 2 of 2020), Chinese BioMedical Literature Database, China National Knowledge Infrastructure, Wan Fang Database and Chinese Scientific Journal Database from inception to 29 February 2020. The outcomes of interest include all-cause mortality, rehospitalisation rate, left ventricular ejection fraction, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, brain natriuretic peptide (BNP), N-terminal pro-BNP, cardiac output, stroke volume, 6 min walking distance and adverse events. The risk of bias assessment of the included RCTs will be conducted according to the Cochrane Collaboration’s tool for assessing the risk of bias. NMA will be performed in a Bayesian hierarchical framework using R V.3.6.1 with the gemtc package. Finally, we will rank the efficacy of these treatment programmes according to the surface under the cumulative ranking curve, and perform quality assessment and recommendation grading of the evidence according to the Grading of Recommendations Assessment, Development and Evaluation system.
Ethics and dissemination
This study will extract data from the published literature and will not involve private information from individuals or compromise their rights. Therefore, the study does not require ethical approval. The results will eventually be published in a peer-reviewed journal and disseminated at relevant conferences.
PROSPERO registration number
CRD42020166900.
Keywords: heart failure, complementary medicine, clinical trials, herbal medicine, adverse events
Strengths and limitations of this study.
Compared with traditional pairwise meta-analysis, network meta-analysis (NMA) can comprehensively analyse direct and indirect comparison results of different traditional Chinese medicine injections (TCMIs) for heart failure (HF) to obtain more reliable conclusions.
Compared with traditional pairwise meta-analysis, NMA can compare and rank the efficacy of different TCMIs for HF.
This study can provide more comprehensive suggestions and references for clinical decision-making and guideline development.
Since most TCMIs and clinical trials will come from China, the conclusion may have certain limitations.
This study did not explore the economic benefits of these drugs, and further exploration can be done based on the results of this study.
Introduction
Heart failure (HF) is a complex set of clinical syndromes caused by abnormal changes in the structure and/or function of the heart that impair ventricular contraction and/or diastolic function.1 HF is a severe end stage of heart disease. Due to the high mortality rate, HF has become an important issue in global public health.2 According to the 2016 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic HF, current treatment options for HF are diverse, generally, including cardiotonic, diuretic, vasodilator, ACE inhibitor (ACEI), angiotensin receptor blocker (ARB), β-blocker and so on. Modern medicine has made great progress in the field of HF, but the prognosis of HF patients is still not satisfactory, resulting in a heavy global burden.3 4 The development of new therapeutic drugs is an inevitable trend of future medical development. The research and development of traditional Chinese medicine (TCM) provide more possibilities for improving the prognosis of HF patients. TCM has the advantages of multi-target effect and bidirectional regulation, so there has been increasing attention in the global medical field.5–7 With the development of modernisation of TCM, more and more TCM injections (TCMIs) for the treatment of HF have been developed and widely used in the clinical practice. Many studies have shown that loading TCMIs based on conventional pharmacotherapy (CPT) can effectively improve the clinical symptoms and reduce the incidence of cardiovascular events and adverse reactions in HF patients.8–17 However, due to the lack of direct comparison studies between TCMIs, the comparative results between TCMIs are unclear. Therefore, although the increasing variety of drugs has provided doctors and patients with more choices, meanwhile, it is also a new challenge to choose the best treatment scheme at the same time.
Meta-analysis is one of the highest levels of evidence in evidence-based research. However, it is difficult to compare the effects of multiple drugs at the same time by traditional pairwise meta-analysis. Network meta-analysis (NMA) is a further development based on the traditional pairwise meta-analysis. Based on the current clinical research data, NMA can complete direct and indirect comparisons among different TCMIs at the same time, and further comprehensively analyse the results of the direct and indirect comparison, to obtain the efficacy ranking of multiple drugs. At present, some researchers have performed the NMA on randomised controlled trials (RCTs) of TCMIs for HF.18 19 However, there are some shortcomings in the published literature. (1) The types of TCMIs included are not comprehensive. Only a few commonly used drugs have been studied, which severely limits the development and utilisation of other potentially effective drugs. (2) Results of the most important clinical outcomes have not been reported, such as all-cause mortality and rehospitalisation rate. (3) The research data has not been updated in the past 2 years. Therefore, we conceived and designed this study to make up for the above shortcomings. We will comprehensively retrieve relevant data to assess and compare the effectiveness and safety of different TCMIs for the treatment of HF using NMA. The results of this study will provide more updated comprehensive evidence for clinical decision-making.
Objectives
We will systematically search all clinical RCTs on TCMIs for HF and perform a Bayesian NMA.20 21 The purpose is to explore the efficacy and safety of TCMIs in the treatment of HF, and to rank the clinical efficacy of drugs.
Methods and analysis
Patient and public involvement
Patients and the public were not involved in the design or conduct of the study.
Inclusion and exclusion criteria for clinical RCTs
Type of participants
The included studies must indicate that participants meet the diagnostic criteria for HF in the ‘Guidelines for diagnosis and treatment of heart failure in China 2018’ or ‘2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure’.1 2 Primary diseases include coronary heart disease, hypertension, dilated cardiomyopathy, and rheumatic heart disease. There are no restrictions on gender, age, race, duration of disease, source of the case and follow-up time.
Type of interventions and comparisons
The following forms of intervention will be included: CPT+TCMI versus CPT alone, CPT+TCMI versus CPT+placebo, CPT+TCMI A versus CPT+TCMI B. CPTs include cardiotonic, diuretic, vasodilator, ACEI, ARB, β-blocker and so on. And CPTs in the two groups should be the same. TCMIs must have been included in the Pharmacopoeia of the People’s Republic of China or approved by the China Food and Drug Administration. All retrieved eligible TCMIs may be included in the study, but TCMIs without literature support will not be compared and ranked.
Outcomes
Only studies using at least one of the following outcomes may be included.
Primary outcomes
All-cause mortality during different follow-up periods—for example, 3 months, 6 months, 1 year or other periods.
Rehospitalisation rate during different follow-up periods—for example, 3 months, 6 months, 1 year or other periods.
Secondary outcomes
Left ventricular ejection fraction.
Left ventricular end-diastolic diameter.
Left ventricular end-systolic diameter.
Brain natriuretic peptide (BNP).
N-terminal pro-BNP.
Cardiac output.
Stroke volume.
Six-minute walking test.
Adverse events
The adverse events that occurred during the study period include allergic reactions, bleeding events, gastrointestinal discomfort, liver and kidney damage and others.
Type of study
RCTs that investigated the effectiveness and safety of TCMI for HF will be included.
Exclusion criteria
Participants are any of the following: the primary disease is congenital heart disease, pulmonary heart disease, hypertrophic cardiomyopathy, restrictive cardiomyopathy, constrictive pericarditis, systemic invasive disease, hyperthyroid heart disease, alcoholic myocardium disease, perinatal cardiomyopathy, drug-induced cardiomyopathy and Keshan disease.
Participants are any of the following: HF with malignant arrhythmias, malignant tumours, hypothyroidism, severe liver and kidney dysfunction or severe infections.
Studies on the mixed efficacy of TCMIs combined with other TCM treatments will be excluded. For example, interventions have combined TCM decoctions, oral Chinese patent medicines, acupuncture, etc.
None of the outcome indicators for this study.
The full text cannot be obtained after seeking help online or contacting the corresponding author via email.
The data are incomplete or incorrect, and the data cannot be used for synthesis.
Studies with imbalanced or incomparable baseline data between the two groups.
For duplicate literature, choose the one published earlier.
Unfinished protocol.
Methods of obtaining data and analysing data
Search strategy
The clinical RCTs and meta-analyses of TCMIs for treating HF will be searched in the relevant database, including PubMed, EMBASE, Cochrane Library (No. 2 of 2020), Chinese BioMedical Literature Database, China National Knowledge Infrastructure, Wan Fang Database and Chinese Scientific Journal Database without language restriction. The retrieval time is from inception to 29 February 2020. Search terms include HF, TCMI, names of TCMIs that have been used in the clinic, RCT, systemic review, meta-analysis, and their synonyms. The search strategy adopts a combination of Medical Subject Heading and free-text terms, and adopts different search strategies according to the characteristics of each database. The synonyms in the group are connected by ‘or’, and the search terms between the groups are connected by ‘and’. At the same time, we will also search conference papers, papers and meta-analysis references, as well as Google Scholar, to avoid missing certain studies. The development of the search strategy has been completed by the researcher SL with clinical work experience and the researcher QS with evidence-based work experience, and has been modified according to the Cochrane Handbook for Systematic Reviews.22 Take PubMed as an example. The detailed search strategy is shown in online supplemental annex 1.
bmjopen-2020-037331supp001.pdf (76.7KB, pdf)
Literature screening
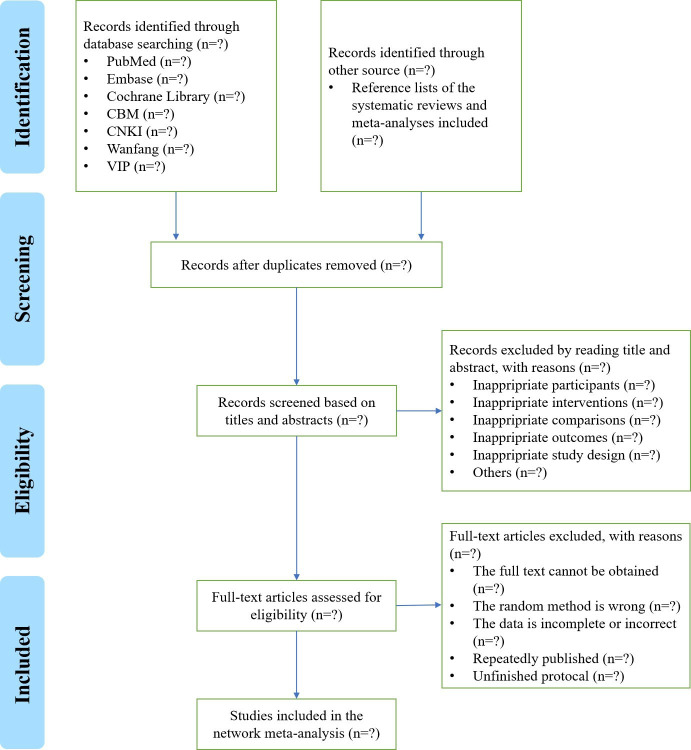
Records from databases will be managed by NoteExpress (V.3.2.0) software. First, we will import all retrieved records into NoteExpress and exclude duplicate records. Second, by reading the title and abstract of each record, we will exclude records that do not meet the inclusion and exclusion criteria. Finally, we will download and read the full texts of potentially relevant studies to perform the second screening. At the same time, the reasons for excluding records after reading the full text will be reported in detail. Literature screening will be done independently and cross-checked by two researchers (SL and QS). Disagreement will be determined through discussion between the two investigators. When consensus cannot be reached, a third investigator (FY) will assist in the judgement. The literature screening based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) is shown in figure 1.23 In the early stage of the study, we will train the evaluators and conduct pretests to ensure a standardised screening process.
Figure 1.
Proposed flowchart of the literature search process. CBM, Chinese BioMedical Literature Database; CNKI, China National Knowledge Infrastructure; VIP, Chinese Scientific Journal Database.
Data extraction and management
Data extraction will be performed independently by two investigators (SL and QS) and cross-checked. Disagreement will be determined through discussion between the two investigators. When consensus cannot be reached, a third investigator (FY) will assist in the judgement. The preset information extraction items are listed in table 1.
Table 1.
Information extraction items
| Categories | Specific items |
| Study characteristics | Title, first author, journal name, publication year and type of study |
| Participants | Diagnostic criteria, sample size, gender, age, ethnicity, case source and baseline status |
| Intervention | Drug name, medication route, drug dose, course of treatment and patient compliance |
| Control | Drug name, medication route, drug dose, course of treatment and patient compliance |
| Outcomes | Whether there is an intention-to-treat, loss to follow-up and withdrawal, and outcomes |
| Risk of bias | Random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting and other bias |
| Others | Author’s main conclusions, funding and others |
Dealing with missing data
When data are missing, we will contact the original authors for complete data. If the missing value of outcomes cannot be obtained from the original author, we will delete the comparison results related to the missing data and fully consider the risk of bias. Besides, sensitivity analyses will be performed by repeating the main analysis with an imputed dataset using multiple imputation by chained equations.24
Assessment of risk of bias
According to the Cochrane Collaboration’s tool for assessing the risk of bias in randomised trials,25 we will assess the risk of bias in the included literature from the following seven items: (1) random sequence generation; (2) allocation concealment; (3) participant and personnel blinding; (4) outcome assessment blinding; (5) incomplete outcome data; (6) selective reporting and (7) other bias. The results of the risk of bias assessment include the low risk of bias, the high risk of bias and the unclear risk of bias. This process will be done independently by two investigators (SL and QS) and cross-checked. Disagreement will be determined through discussion between the two investigators. When consensus cannot be reached, a third investigator (FY) will assist in the judgement. When there is a difference in the risk of bias between studies, we will try to analyse the impact of risk of bias. The risk of bias graph and the risk of bias summary will be generated by RevMan V.5.3.
Data analysis
Pairwise meta-analysis and NMA
A Bayesian approach will be used to conduct pairwise meta-analyses and NMAs according to the Markov chain Monte Carlo method.21 In a Bayesian hierarchical framework, we will assume the vague prior distribution parameters for the between-study heterogeneity with uniform distribution in advance. The convergence of the model will be assessed using the Brooks-Gelman-Rubin plot.26 Dichotomous variables will be presented as the relative risk or OR with a 95% credible interval (CrI). Continuous variables will be presented as the weight mean difference with a 95% CrI. The χ2 test and Ι2 test will be conducted to assess the potential heterogeneity. P<0.05 is considered statistically significant. To achieve the highest generalisability in the pooled treatment effects, a random-effects model will be used to synthesise the data for pairwise meta-analysis and NMA.27 A pairwise meta-analysis will be conducted when at least two studies compared the same intervention and comparator. When the treatment nodes formed a network of evidence, we will indirectly compare different treatment plans using the common comparator or placebo. A network diagram of each outcome will be generated to visualise the connections between different treatment programmes included. If direct evidence exists, NMA will conduct a comprehensive evaluation of direct and indirect comparative evidence. If direct comparison evidence is lacking, we will only make adjusted indirect comparisons. For each outcome, a contribution matrix will be performed to demonstrate the percentage contribution of each direct comparison to the whole evidence body. The efficacy of different treatment programmes will be ranked according to the surface under the cumulative ranking curve (SUCRA).28 The SUCRA is a value range from 0 to 1 and can be re-expressed as a percentage. The larger the SUCRA, the better the treatment regimen.
Examination of assumptions in NMA
Heterogeneity
The Cochran’s Q statistics will be employed to assess heterogeneity.29 If there is significant clinical heterogeneity or methodological heterogeneity (p<0.1, I2 >50%), the subgroup analysis will be performed to explore sources of heterogeneity. To assess potential bias resulting from baseline risk, we will perform meta-regression with regressors which included age of participants, sample size, duration of disease, course of treatment and so on. Besides, sensitivity analyses will be performed by excluding studies with a high risk of bias or poor quality to judge the stability of the results.
Transitivity
We will verify the transitivity of this network by plotting the central trends (eg, mean and median) of patient characteristics in each treatment comparison.
Consistency
Node-splitting analysis will be used to split mixed evidence into direct evidence and indirect evidence to evaluate the inconsistency of the model. And then, we will compare the direct and indirect evidence. If there is no statistically significant difference between direct and indirect evidence, the study fits the consistency model. If the 95% CrI of the result does not include the invalid value, the inconsistency will be considered to exist.
Assessment of publication bias
The comparison-adjusted funnel plots will be obtained with the specific ranking order to detect small sample size study effects and publication bias.
All analyses will be conducted using R V.3.6.1 with the gemtc package.
Quality assessment and recommendation grading of the evidence
Two reviewers (SL and QS) will independently perform quality assessment and recommendation grading of the evidence of the direct, indirect and mixed estimates of all comparisons according to Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.30 31In particular, the GRADE system was used to rank the quality of evidence for direct comparison from four aspects: limitation, inconsistency, indirectness and publication bias, but without imprecision.32 The grading of the evidence quality includes four levels, which are ‘high’, ‘medium’, ‘low’ or ‘very low’, according to the GRADE rating standards.33 34 High indicates that the authors are very confident that the real effect is close to the estimate of the effect. Moderate indicates that the authors are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low indicates that the authors’ confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low indicates that the authors have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.29 Cross-checking will be performed after the classification is completed. In case of disagreement, it will be decided by discussion between the two parties or judged by the third evaluator (FY).
Ethics and dissemination
This study will extract data from the published literature and will not involve private information from individuals or compromise their rights. Therefore, the study does not require ethical approval. The procedures of this systematic review and NMA will be conducted in accordance with the PRISMA guidelines. Details of this study will be submitted to open access. The results will be published in a peer-reviewed journal and disseminated at relevant conferences.
Supplementary Material
Acknowledgments
We would like to thank Luoqian Liu for the language revision.
Footnotes
Contributors: SL, JM and XW conceived and designed the study together. SL, QS and FY developed the search strategy together. SL drafted the protocol manuscript. All the authors have reviewed and approved the final manuscript.
Funding: This work was supported by the 'Innovation team development Plan' of the Ministry of Education-Research on the prevention and treatment of cardiovascular diseases in traditional Chinese medicine (No.IRT_16R54), the Standardisation project of the 'Guidelines for Clinical Application of Traditional Chinese Medicines to Treat Advantageous Diseases' by the State Administration of Traditional Chinese Medicine (No.SATCM-2015-BZ[402]), and the National Administration of Traditional Chinese Medicine: 2019 Project of building evidence based practice capacity for TCM (No.2019XZZX-XXG007).
Disclaimer: The funder has not taken part in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chinese Society of Cardiology Guidelines for diagnosis and treatment of heart failure in China 2018. Zhonghua Xin Xue Guan Bing Za Zhi 2018;46:760. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;2016:2129–200. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation 2019;139:e56–28. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 4.Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368–76. 10.1016/j.ijcard.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Wang AY, Wei ZH. Exploring the thinking and methods of Chinese medicine based on "holistic view", 2019. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2019&filename=SJKX201901003&v=MDM4NjZZUzdEaDFUM3FUcldNMUZyQ1VSN3FmWnVSbkZDdmhXN3pQTmlmQWRyRzRIOWpNcm85Rlo0UjhlWDFMdXg=
- 6.SY Y, Lu Y. Discussion on action mechanisms of traditional Chinese medicine, 2018. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2018&filename=YLBS201805001&v=MzEwOTJxbzlGWllSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVI3cWZadVJuRkN2aFc3dk1QQ0hKZmJHNEg5bk0=
- 7.Bian J, Li Z. Theory and clinical research progress of dual-direction regulation, 2016. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2016&filename=ZCXW201609018&v=MTYxMzRya1VyeklQeTdUZWJHNEg5Zk1wbzlFYklSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVI3cWZadVJuRkM=
- 8.MX G, Feng YL, Zhang XX, et al. Meta-analysis of Salvia miltiorrhiza ligustrazine injection combined with conventional medication in treatment of chronic heart failure. Drug Evaluation Research 2019;42:2084–91. [Google Scholar]
- 9.YW O, Dong YJ, Zhu YL. Meta-analysis on Sofren injection in treatment of heart failure, 2019. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2019&filename=SHZZ201907015&v=MDAwNDFyQ1VSN3FmWnVSbkZDcmtVNzNNTmlYUmRMRzRIOWpNcUk5RVlZUjhlWDFMdXhZUzdEaDFUM3FUcldNMUY=
- 10.Xie N, Dai XH. Meta analysis on curative effects of Yiqi Fumai injection (lyophilization) for heart failure, 2019. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2019&filename=ZYYY201910016&v=MDkwMTFOcjQ5RVlvUjhlWDFMdXhZUzdEaDFUM3FUcldNMUZyQ1VSN3FmWnVSbkZDcmtWYnJQUHpUU2Q3RzRIOWo=
- 11.Lin WJ, SS L, Han JD. The hemodynamic effects of Huangqi injection in the treatment of chronic heart failure: a meta-analysis of clinical controlled trials, 2019. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2019&filename=JZZY201901016&v=MTg2NDl1Um5GQ3JtVTd2Skx6ZlJkN0c0SDlqTXJvOUVZb1I4ZVgxTHV4WVM3RGgxVDNxVHJXTTFGckNVUjdxZlo=
- 12.Zhu YH, Shen XX, Han QQ. A meta-analysis of Shenfu injection in myocardial infarction with heart failure, 2018. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2018&filename=PZXX201804005&v=MTg0NjdmWnVSbkZDcm1WTHpOTlRmVGRyRzRIOW5NcTQ5RllZUjhlWDFMdXhZUzdEaDFUM3FUcldNMUZyQ1VSN3E=
- 13.Xu T, Shi XQ, Wang F. Effectiveness and safety of Shenmai injection in the treatment of heart failure, 2018. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2018&filename=SQYX201807022&v=MDYzMzRxVHJXTTFGckNVUjdxZlp1Um5GQ3JtV3JyTk5qelNkckc0SDluTXFJOUhab1I4ZVgxTHV4WVM3RGgxVDM=
- 14.Wang KH, JR W, Duan XJ. Meta-analysis on randomized controlled trials of Shenqi Fuzheng injection in the treatment of chronic heart failure, 2018. Available: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2018&filename=YWLX201801008&v=MjAzMDBlWDFMdXhZUzdEaDFUM3FUcldNMUZyQ1VSN3FmWnVSbkZDcm5VTHJPUERySGRyRzRIOW5Ncm85RmJJUjg=
- 15.Lu X, Zhang L, Wang J, et al. Clinical efficacy and safety of xinmailong injection for the treatment of chronic heart failure: a meta-analysis. Front Pharmacol 2018;9:810. 10.3389/fphar.2018.00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Wu J, Duan X, et al. Huangqi injection in the treatment of chronic heart failure: a systematic review and meta-analysis. Medicine 2017;96:e8167. 10.1097/MD.0000000000008167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai D, Yue G-X, Wang R-H, et al. [Clinical characteristics of five traditional Chinese medicine injections in treating heart failure based on Meta-analysis literature]. Zhongguo Zhong Yao Za Zhi 2018;43:4152–62. 10.19540/j.cnki.cjcmm.20180709.002 [DOI] [PubMed] [Google Scholar]
- 18.Yang F-W, Zou J-H, Wang Y, et al. [Network meta-analysis of Chinese medical injections for heart failure]. Zhongguo Zhong Yao Za Zhi 2018;43:1247–53. 10.19540/j.cnki.cjcmm.2018.0049 [DOI] [PubMed] [Google Scholar]
- 19.Wang K-H, Wu J-R, Zhang D, et al. Comparative efficacy of Chinese herbal injections for treating chronic heart failure: a network meta-analysis. BMC Complement Altern Med 2018;18:41. 10.1186/s12906-018-2090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonas DE, Wilkins TM, Bangdiwala S, et al. Findings of Bayesian mixed treatment comparison meta-analyses: comparison and exploration using real-world trial data and simulation, 2013. Available: https://pubmed.ncbi.nlm.nih.gov/23469378-findings-of-bayesian-mixed-treatment-comparison-meta-analyses-comparison-and-exploration-using-real-world-trial-data-and-simulation-internet/?from_single_result=Findings+of+Bayesian+Mixed+Treatment+Comparison+Meta-Analyses%3A+Comparison+and+Exploration+Using+Real-World+Trial+Data+and+Simulation [PubMed]
- 21.Jansen JP, Crawford B, Bergman G, et al. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health 2008;11:956–64. 10.1111/j.1524-4733.2008.00347.x [DOI] [PubMed] [Google Scholar]
- 22.Higgins J, Thomas J. Cochrane handbook for systematic reviews of interventions version 6, 2019. Available: https://training.cochrane.org/handbook/current [DOI] [PMC free article] [PubMed]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buuren Svan, Groothuis-Oudshoorn K. mice : Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 25.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434–55. [Google Scholar]
- 27.Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607–17. 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015;15:58. 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H, Chen Q, Chen M, et al. Nonpharmacological conservative treatments for chronic functional constipation: a systematic review and network meta-analysis. Neurogastroenterol Motil 2019;31:e13441. 10.1111/nmo.13441 [DOI] [PubMed] [Google Scholar]
- 30.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 31.Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 32.Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36–44. 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of clinical epidemiology. J Clin Epidemiol 2011;64:380–2. 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037331supp001.pdf (76.7KB, pdf)