Abstract
Background
Intervertebral disc degeneration (IDD) is a common spinal disease affected by environmental and lifestyle factors that has a significant pathological cascade toward inflammation and partial disability. There is currently no therapy that can completely restore the cellular derangement in IDD. Hence, in this study, the therapeutic effects of apigenin on IDD were evaluated using a rat model.
Material/Methods
Animals were separated into 4 groups: Grp 1, sham-operated control; Grp 2, IDD-induced; Grp 3, IDD-induced+apigenin treatment; Grp 4, apigenin control. The animals were assessed for inflammatory cytokines, chemokines, and prostaglandin signaling.
Results
There were significant increases in the inflammatory cytokines IL-1β, IL-2, IL-6, IL-8 and IL-17 in the IDD-induced group compared to that of control. Moreover, with increased levels of MMP-3, MMP-9, ADAMTS-4, and syndecan-4, the levels of TNF-α, IFN-γ, prostaglandin E2, and cyclooxygenase 2 were directly increased in the IDD-induced group. In contrast, apigenin protectively restored levels of prostaglandin signaling and reduced cytokine levels. In addition, nucleus pulposus cells cultured separately with either TNF-α inhibitor or apigenin significantly attenuated the levels of extracellular matrix proteins.
Conclusions
The reduction of cytokine levels under apigenin treatment suggests it may be a promising target drug therapy for the treatment of deleterious IDD conditions.
MeSH Keywords: Cytokine-Induced Killer Cells; Matrilin Proteins; Photoreceptor Cells, Invertebrate
Background
Back pain caused by stress and changes in working practices on the job is the most common disability affecting populations in industrialized societies worldwide [1]. Back pain affects not only the health and relationships of individuals but also puts a strain on the working environment [2]. Overall, it results in a decrease in economic productivity and an increase in medical costs, which include the direct and indirect effects of absence from work [3]. Health departments worldwide are striving to ensure proper working facilities to avoid the rising medical insurance costs, which burden nations [4]. Developed nations have shown an increase in employee-friendly measures to tackle this problem, but developing nations leave this onus on the individuals at work. Hence, we must be aware of the complications arising from back pain and its manifestation from intervertebral disc degeneration (IDD).
The intervertebral disc contains an outer annulus fibrosus (AF) and an inner matrix-rich nucleus pulposus (NP) [5]. The collagen-rich fibrocartilaginous AF is responsible for withstanding the hoop stresses from compressed NP [6]. The NP cells are rich in aggrecan and reside in a hyperosmotic niche [7]. The extracellular matrix (ECM) components of normal discs, such as collagen-type molecules, aid in the smooth functioning of the discs by providing anchoring support of the tissue to the bone and tensile strength to the discs [8]. The chondroitin and keratin sulphate chains of aggrecan hydrate the disc tissues and, along with the ECM, keep the posture upright [9,10].
When the NP structure is under stress from the breakdown of the ECM by proteases and cell senescence and death, tissue degeneration progresses and results in low back pain [11]. The loss of proteoglycans results in decreased hydration of the tissues in the disc, causing a drop in osmotic pressure which then results in disc degeneration [8]. Collagens denature and get ruptured, which is another factor in disc degeneration [2]. IDD cases show an increased loss of aggrecan and increased disc permeability, which allow, for example, the inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) to penetrate the disc, resulting in inflammation and associated chronic back pain [12–14]. Therefore, inflammatory cytokines play a significant role in disc degeneration [15]. Chronic low back pain is always related to sciatica, disc herniation, and stenosis, a condition occurring from a loss in disc height owing to changes in the associated structures of muscles and ligaments.
Apigenin is the most widely available flavonoid found in plants, including parsley, onions, celery, fruits, herbs, tea, and wine [16], and has antioxidant, anti-inflammatory, and anti-mutagenic properties [17,18]. The antioxidative and neuroprotective role apigenin plays in conditions like Alzheimer’s and Parkinson’s disease [19] and depression has given us the impetus to test it against IDD, a condition related to the spinal cord, a neurologically vital organ. Also, its use against spinal cord injury [20] gave us supporting evidence for testing it against IDD in animals. Thus, we hypothesized that apigenin would control the action of inflammatory cytokines by acting against TNF-α, ameliorate the complications arising from IDD, significantly improve the disc regeneration, and improve movement.
Material and Methods
Animals and clinical technique
Wistar male rats (weight, 110 g) were acquired from the Animal Center of Hubei Province, Wuhan, China. The experimental protocol was accepted by the Institutional Animal Care and Use Committee of the First People’s Hospital of Jingmen, Jingmen, Hubei, China. All animal experiments were performed in agreement with committee approval and regulations. To induce IDD, the needle-stab injury model was used, and the protocol followed as per previous publications [21]. Briefly, rats were sedated with ketamine (75 mg/kg body weight; Sigma Aldrich, MO, USA). Using radiography, the intervertebral disc space between the Co7/Co8 or Co8/Co9 caudal vertebral discs was first located, and then a 20-gauge aseptic needle controlled by electronic stopper was inserted to a distance of approximately 5 mm from the dorsal toward the ventral side. The injury was created by rotating the needle twice, and the needle was removed from the site of injury in the direction it was inserted. After the induction, the rats were kept alone in cages and exposed to a 12:12 h photoperiod (light-dark cycle) with unrestricted access to tap water and food.
After 12 weeks, the animals were sedated briefly and placed in a prone position in a 3.0 T MRI scanner in which the caudal vertebral discs were imaged in the sagittal plane. The obtained results were processed independently by 3 blinded observers. For the experimentation, the rats were separated into 4 groups: Group (Grp) 1: control (sham-operated); Grp 2: IDD was induced; Grp 3: IDD+apigenin treatment (15 mg/kg) (rats underwent IDD procedures, with drug injected into NP cells at surgery and administered orally thereafter); Grp 4: apigenin control. At the end of the experimental period, the animals were killed by an overdose of carbon dioxide; the tissues were removed, fixed in formalin, and stained with hematoxylin and eosin to assess the cellularity and morphology as per the previously described grading scale [22,23].
In vitro culture, isolation, and treatment of rat NP cells
For the analysis of the TNF-α-mediated signaling mechanism, the in vitro cultures of NP cells were cultured and exposed to a TNF-α inhibitor. Later, the cells were assayed for the expression of inflammatory cytokines. Briefly, rat NP cells were isolated according to previously published methods [24]. The cells were cultured in DMEM/F12 medium supplemented with 10% FBS and antibiotics, and the cells were grown till confluence. Once 70% confluence was reached, the cells were separated into groups and exposed to apigenin or TNF-α inhibitor (LMP-420, 50 μM). After 24 h, the cells were scraped off and analyzed for expression of cytokines and matrix metalloproteinases (MMPs) using commercial ELISA kits following the manufacturer’s instructions (Fine Biotech, Wuhan, China).
Biochemical and cytokine analysis
The estimations of glycosaminoglycan, proteoglycan 4 (PRG4), thromboxane B2, prostaglandin E2, cysteinyl leukotriene, and leukotriene B4 were done in the serum samples using commercial ELISA kits following the manufacturer’s instructions (Fine Biotech, Wuhan, China). Furthermore, the proinflammatory and anti-inflammatory cytokines IL-2, IL-6, IL-8, IL-17, IFN-γ, IL-1β, MMP-2, and MMP-9 in the serum samples were estimated using commercial ELISA kits as per the manufacturer’s instructions (Abcam, Inc, USA; Biosource, Inc, USA).
Reverse transcription polymerase chain reaction
For the elucidation of the marker genes and matrix genes associated with IDD, total RNA was isolated from the NP cells isolated from the control and experimental groups using TRIzol reagent. The total RNA extracted was quantified using a NanoDrop spectrophotometer. For the cDNA synthesis, an equal amount of RNA was transcribed to cDNA and the real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was done for specific genes using SYBY green/ROX master mix, and amplified by the Bio-Rad real-time PCR system (Qiagen, Shanghai, China). The gene-specific primers used in the study are shown in Table 1. The gene expression was calculated from the CT values and the fold increase was determined by the comparative CT method (ΔΔCT) with expression values of GAPDH as the endogenous control [25].
Table 1.
Primer details of the study.
| Gene | Primer | Sequence | Annealing |
|---|---|---|---|
| Asporin | F | CCTGGTAGGAGGGCTGGATT | 59 |
| R | CCTTCATGCTGTCCCGTGTA | ||
| Syndecan-4 | F | TGTTTTTGACCCTGGCCCTT | 59 |
| R | CTCGATCTCGAGAAGGCACC | ||
| TNF-α | F | AAGCTGTCTTCAGGCCAACA | 57 |
| R | CCCGTAGGGCGATTACAGTC | ||
| TNF-R1 | F | TTTTCTCCAGGCAGCAACCA | 56 |
| R | GCTAGCAGGACAGTCAAGGG | ||
| COX-2 | F | AAGGCGTTCAACTGAGCTGT | 57 |
| R | ACACAGGAATCTTCACAAATGGAAC | ||
| Periostin | F | CATTCAAGGCAGTCTTCAGCC | 56 |
| R | TTTGCAGGTGTGTCTTTTTGC | ||
| MMP-1 | F | TCAGCATGCTTAGCCTTCCT | 58 |
| R | TAGCTTGGACGTCTTCACCC | ||
| MMP-2 | F | GTTATGAGACCCTGAGCCCG | 57 |
| R | CCTTGGGGCAGCCATAGAAA | ||
| MMP-3 | F | TCATGAACTTGGCCACTCCC | 56 |
| R | AACAAGACTTCTCCCCGCAG | ||
| MMP-9 | F | GATCCCCAGAGCGTTACTCG | 58 |
| R | GTTGTGGAAACTCACACGCC | ||
| ADAMTS-4 | F | CCTCGAGACCAGTGCAAACT | 57 |
| R | TGACCACATCGCTGTATCCG | ||
| ADAMTS-5 | F | ATGCACTTCAGCCACGATCA | 56 |
| R | CACACATTTCCCTTGCAGGC | ||
| GAPDH | F | AGTGCCAGCCTCGTCTCATA | 58 |
| R | GATGGTGATGGGTTTCCCGT |
Statistical examination
The results are expressed as mean±standard error. Statistical significance was evaluated by student t test for comparisons between groups. A P-value of less than 0.05 was considered statistically significant.
Results
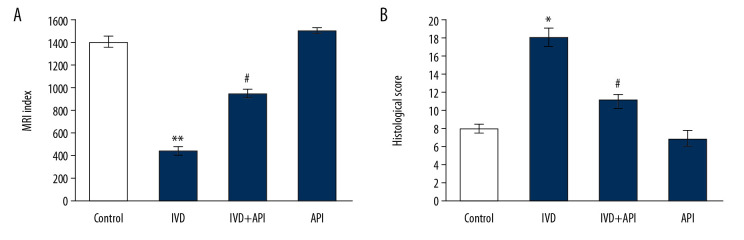
Results on the apigenin-mediated protective mechanism on IDD and the various analyses made are herein presented. Initially, the onset of injury was analyzed using MRI and histological scores. MRI data were corroborated with the histological results and demonstrated a significantly lower MRI signal intensity in the IDD-induced group compared to that of the sham-operated control group at 12 weeks after the initial stab injury. However, rats in the apigenin-treated group displayed higher MRI signal intensity compared with that of the IDD-induced group. In addition, the histological structure of the NP tissue displayed a well-characterized and organized network of cells and ECM in the apigenin-treated rats, while poor cellular structure was found in the IDD-induced rats (Figure 1).
Figure 1.
(A) MRI index to revealing water content and nucleus pulposus (NP) tissue structure. (B) The histological score of control and experimental groups of rats. Statistical significance shown as * P<0.05, ** P<0.01 compared to sham-controls, # P<0.05, IDD+apigenin-treated compared to IDD-induced rats.
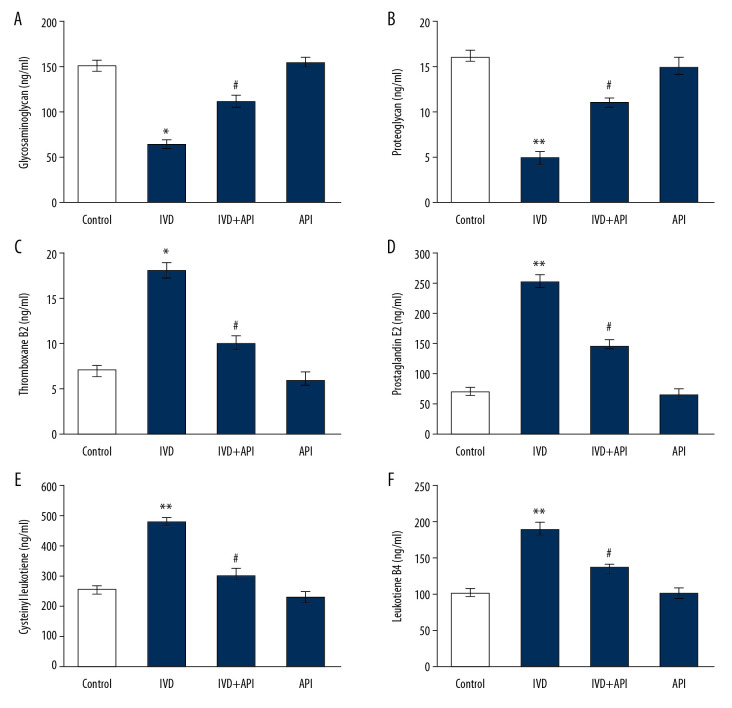
The levels of proteoglycans and prostacyclin molecules evaluated in the control and the experimental groups are presented in Figure 2. The results demonstrated a significant decrease in glycosaminoglycan and proteoglycan content, and a significant increase in the inflammatory mediators thromboxane B2, prostaglandin E2, cysteinyl leukotriene, and leukotriene B4 in the IDD-induced group compared to those in the sham-operated control group (P<0.01). On the other hand, the levels of glycoproteins and prostacyclin were significantly restored in the apigenin-treated group (P<0.01), suggesting a protective event had been initiated in the IDD-induced animals by apigenin treatment (Figure 2).
Figure 2.
(A–F) represents the proteoglycans and prostacyclin in the control and experimental group of rats. The details of the assay were given in the methodology section. Statistical significance shown as * P<0.05, ** P<0.01, compared to sham-controls, # P<0.05, IDD+apigenin-treated compared to IDD-induced rats.
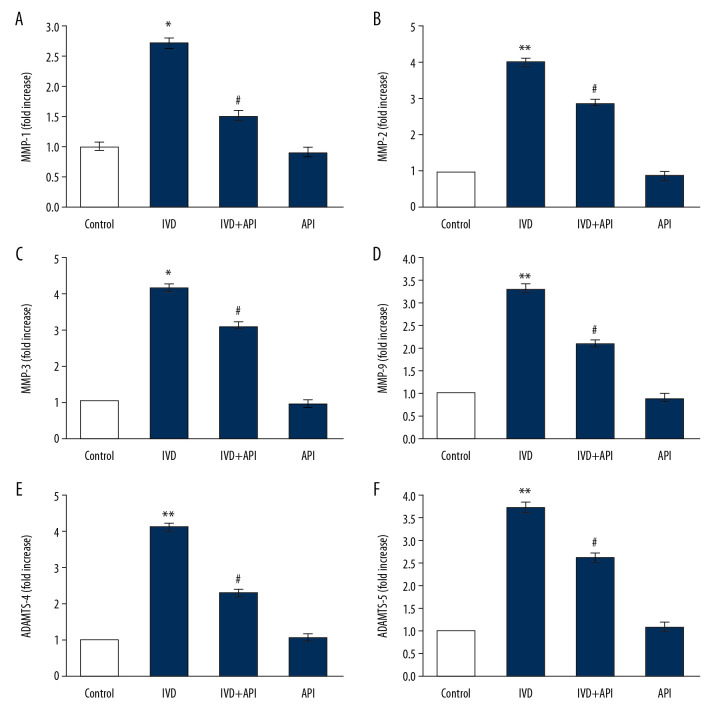
Because of the observed decrease in proteoglycans in the IDD-induced group, we evaluated the levels of MMPs to corroborate the signaling that had been activated in the disc degeneration. The results showed a substantial upsurge in the mRNA levels of MMP-1 (2.1-fold), MMP-2 (4-fold), MMP-3 (4.2-fold), MMP-9 (3.3-fold), A disintegrin and thrombospondin motifs (ADAMTS)-4 (4.1-fold), and ADAMTS-5 (3.7-fold) in the IDD-induced group compared to those in the sham control group. Because the rats in the apigenin-treated group elicited a significant (P<0.01) decline in the levels of these matrix protein markers, it suggested that a restorative mechanism had been initiated in these IDD-induced apigenin-treated rats (Figure 3).
Figure 3.
(A–F) represents qRT-PCR mRNA expression analysis of MMP-1, MMP-2, MMP-3, MMP-9, ADAMTS-4 and ADAMTS-5 of control and experimental groups of rats. The fold increase of gene expression is compared with the housekeeping gene GAPDH. Statistical significance shown as * P<0.05, ** P<0.01, compared to sham controls, # P<0.05, IDD+apigenin-treated compared to IDD-induced rats.
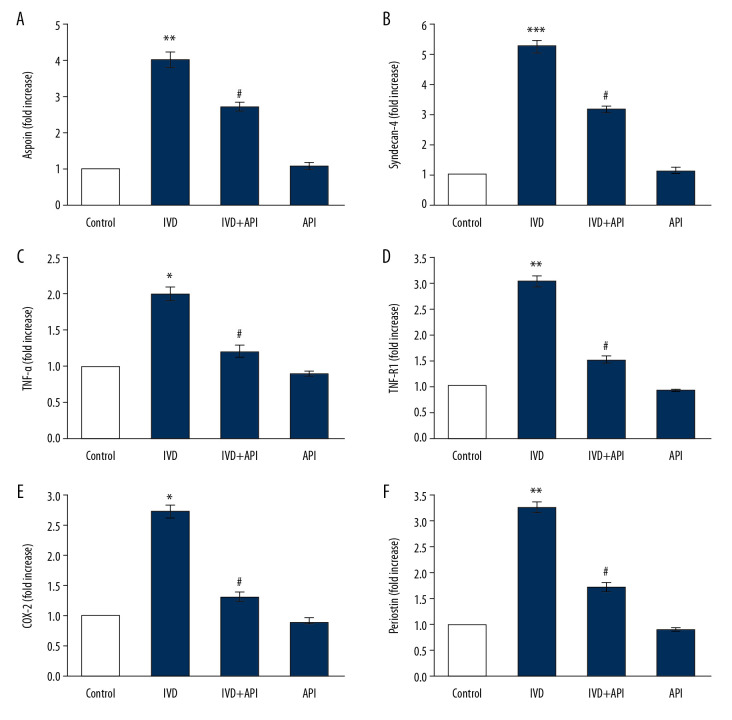
We documented the mRNA expressions in the current study to validate the role of apigenin on the modulation of IDD markers. The expression levels of the control genes demonstrated by RT-qPCR are shown in Figure 4. The results showed the mRNA expressions of asporin (4-fold), syndecan-4 (5.2-fold), TNF-α (2-fold), TNF-R1 (3-fold), COX-2 (2.7-fold), and periostin (3.2 fold) were significantly increased (P<0.01) in the IDD-induced group compared to those in the sham control group. However, the enhanced levels of these genes were decreased in the apigenin-treated group, indicating that the drug initiated the restorative mechanism to return normal functioning (Figure 4).
Figure 4.
(A–F) represents qRT-PCR mRNA expression analysis of asporin, syndecan-4, TNF-α, TNF-R1, COX-2 and periostin of control and experimental group of rats. The fold increase of gene expression is compared with the housekeeping gene GAPDH. Statistical significance shown as * P<0.05, ** P<0.01, *** P<0.001 compared to sham controls, # P<0.05, IDD+apigenin-treated compared to IDD-induced rats.
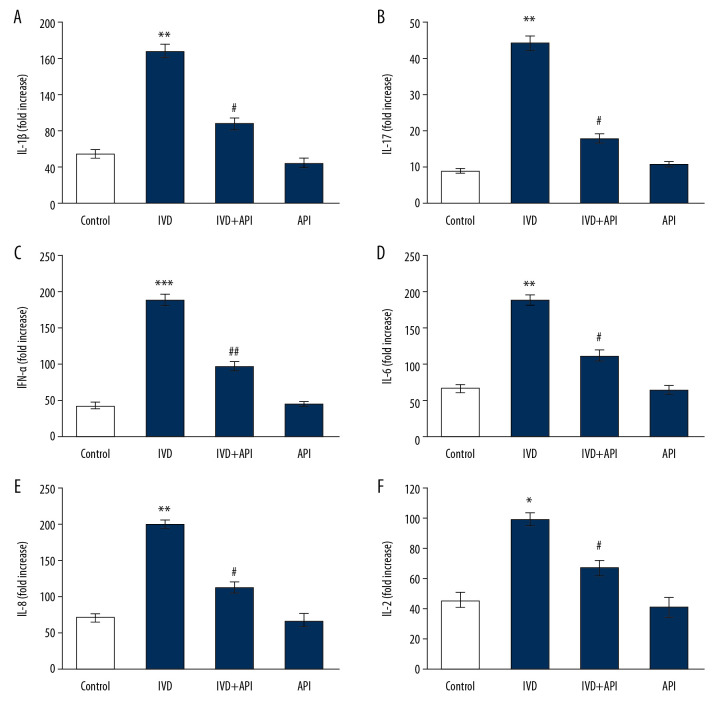
Further experiments to measure cytokines levels were done (Figure 5). Rats in the IDD-induced group showed a substantial increase in the levels of cytokines, including IL-1β (P<0.01), IL-17 (P<0.05), IFN-γ (P<0.01), IL-6 (P<0.001), IL-8 (P<0.05), and IL-2 (P<0.05) over those in the sham control group. Although, these inflammatory cytokines were reduced in the apigenin-treated group, indicating that the signaling of IDD progression was reduced (Figure 5).
Figure 5.
(A–F) represents cytokine expression analysis of control and experimental groups of rats. The detail of the experiment is given in the methodology section. Statistical significance shown as * P<0.05, ** P<0.01, *** P<0.001 compared to sham controls, # P<0.05, ## P<0.01, IDD+apigenin-treated compared to IDD-induced rats.
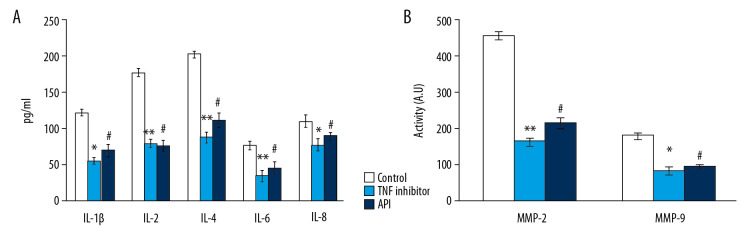
Conversely, cultured NP cells from the IDD-induced group exposed to the TNF-α inhibitor also showed a reduction in cytokine levels by the reduced expression of MMPs in the NP cells. Therefore, the results of the current investigation confirm that the protective role of apigenin might be mediated via TNF-α inhibition and can contribute to the development of novel treatment approaches in IDD (Figure 6).
Figure 6.
(A, B) represents cytokines such as IL-1β, IL-17, IFN-γ, TNF-α and MMP-2 and MMP -9 activity of nucleus pulposus (NP) cells. The detail of the experiment is given in the methodology section. Statistical significance shown as * P<0.05, ** P<0.01 TNF-α inhibitor compared to untreated cells, # P<0.05, apigenin compared to untreated cells.
Discussion
We have evaluated the importance of using apigenin in IDD-induced animals to assess its effects on disc degeneration reduction, and hence the back pain associated with it. Disc degeneration occurs with aging, decreased nutrient supply, diminished matrix resuscitation, and other mechanical stressors. In IDD, the gelatinous NP undergoes a change in texture to a fibrous fissure-like structure over time [26]. In our IDD model, we have shown that the needle-stab injury affected the NP of the IDD-induced animals by degrading the ECM proteins, thereby making the structure lose its water content and hence the gelatinous nature that absorbs mechanical stress. Clinically, MRI has been an essential method for assessing the extent of IDD [26]. Since the disc structure was weakened in the IDD-induced animals, the MRIs were weak, indicating the abnormal anatomy in the effected animals [27]. However, the treatment with apigenin improved the signals and, hence, showed the ameliorating or therapeutic nature of apigenin on the ECM of the discs and its ability to restructure the affected discs. The MRI signals could be correlated with the extent of fibrotic lesions in the affected regions of the discs [28] by the increased histological score in the IDD-induced animals; the fibrosis was controlled by the reduced inflammation in the apigenin-treated group.
Degenerated discs spontaneously synthesize the proinflammatory metabolites of arachidonic acid to prostaglandin E2 (PGE2), thromboxane B2, cysteinyl leukotriene, and leukotriene B4, which are associated with disc herniation and were released in the IDD-induced rats. Such molecules cause sensitization of nociceptors and increase the pain in the spine [29]. This increasing trend is due to the degeneration of the tissues and subsequent complications of pain due to the degradation of the ECM. Such increases were controlled in the apigenin-treated group where the pain was curbed by the control of PGE2 production, for example. This was made possible by a decrease in the expression of COX-2, which is responsible for PGE2 production and upregulation, and is involved in the pathogenesis of disc herniation [29–32].
The load-bearing inability of the disc changes with the change in the architecture and biochemical components of the disc, and is the significant indicator of IDD. This is owing to the compression which makes the disc too rigid to accept the load and leads to degeneration. Aggrecan loses its proteoglycans and hydration to transform from a gelatinous structure to fibrous tissues [2]. The resultant loss of glycosaminoglycans makes the osmotic pressure decrease in the disc matrix, leading to a decrease in load-bearing capacity [8]. This is followed by an increase in the synthesis and activity of MMPs and ADAMTS.
It has been reported that the onset of IDD displays an increase in IL-1β, TNF-α, IL-6 [5], and IL-8, which are expressed as a result of the initiation of the innate immunity in animals and are known to be pain intensity quotients [8]. Activated macrophages, eosinophils, neutrophils, and CD4+ lymphocytes produce TNF-α and regulate the activity of immune cells [33]. TNF-α is proinflammatory and mediated the pain in the herniated discs of the IDD-induced animals. The nociceptive nerve fibers in the disc largely mediate the pain in the lower disc with the infiltration of TNF-α into it. Many researchers have shown that TNF-α and IL-1β induce the expression of MMPs, which would accelerate the catabolism of ECM proteins such as aggrecan and collagen [34]. Disc degeneration occurs with ECM disruption and hence induction of catabolic enzymes such as collagenase (MMP-1), stromelysin (MMP-3), gelatinases (MMP-2), and MMP-9 are broadly expressed in ECM turnover and disc degeneration. Aggrecanases such as ADAMTS-4 and ADAMTS-5, which degrade the aggrecan, are also highly expressed in IDD. An imbalance in the expression and activation of MMPs and their inhibitors leads to IDD, as evident in the increase in MMP-1, MMP-2, MMP-3, and MMP-9, and ADAMTS-4 and ADAMTS-5 in our IDD-induced group. They work together with other inflammatory cytokines to activate apoptosis of the cells in the disc and contribute to IDD [35,36]. This creates an environment that is suitable for the synthesis of inflammatory cytokines by T cells.
Activated proinflammatory Th17 cells produce IL-17, in particular IL-17A, which is implicated in the pathogenesis of lumbar disc herniation [37–39]. The evidence of the role of Th17 lymphocytes in the pathology of IDD and existence of low back pain was shown in our study with the increased IL-17 expression in the IDD-induced group. In agreement with previous research [40], we observed that increased IL-17 expression was associated with increased IFN-gamma expression, which could be a synergistic action in IDD herniation.
There is crosstalk between various ECM proteins and MMPs in the pathology of IDD. Proteins such as asporins, COX-2, periostin, and syndecans interact with MMPs in disc degenerative complications to exhibit as pain, tissue fibrosis, and herniation. COX-2 is an essential regulator in the expression of PGE2, which in turn acts as a catabolic factor by tilting the balance between matrix synthesis and expression of MMPs toward inducing MMPs in osteoarthritis [41–43]. The expression of periostin in rats [44] with degenerated discs shows that it is participating in the process of IDD. The overprotective reaction of periostin has accelerated the fibrillogenesis in the degenerated discs. The level of periostin expressed is correlated with the amount of fibrosis on the NP cells and the degree of disc degeneration [45]. The increased expression of periostin in our animal model has satisfactorily explained the degree of disc degeneration that has occurred.
Periostin upregulates MMP-2 expression via the α5β3 integrin/extracellular signal-regulated kinase signaling pathway [46]. The effect of increased expression of periostin on the level of MMP-2 expression was demonstrated in this study. The increased expression of periostin together with MMP-2 upregulation and disc degeneration has been positively correlated in many earlier studies [47,48]. We observed an increase in IDD severity with the increased expression of both periostin and MMP-2 in our IDD-induced group as compared to that in the healthy group. The IDD-induced groups were under the stress of matrix degradation and, as in humans, IDD could be due to stress on the spinal cord from the lifting of heavy weight or aging-related issues. Apigenin treatment reversed and reduced the effects of IDD induction in the rats by lowering periostin expression, thereby lessening MMP-2’s action on the matrix proteins.
Another interesting correlation was observed between increases in MMP-3 and syndecan 4, which involve TNF-α and IL-1β in the NP in IDD. TNF-α regulated the MMP-3 expression (as well as ADAMTS-4 and ADAMTS-5) in the degradation of ECM components. However, recent findings show that syndecan 4 is a mediator of TNF-α-mediated MMP-3 expression [49]. These results are supported by our findings whereby MMP3 increased with increased syndecan 4 and TNF-α expression in the IDD-induced group. The result of which was the observed increase in ADAMTS-5, which initiates aggrecan degradation. This indicates that MMP-3 expression was the initiator of ECM degradation in those animals with the upstream activators and mediators actively pursuing it through NP cell stimulation. MMP-2 and MMP-3 expressions are crucial as they are vital to activating other MMPs like MMP-1 and MMP-9 [50]. Apigenin treatment acted to inhibit TNF-α expression and hence the expression of syndecan 4 and MMP-3 regulation in rescuing the pretreated animals from matrix degradation, allowing for restoration. Therefore, a more in-depth analysis is required to understand the signaling pathways of the inflammatory cytokines affecting IDD.
Results of previous studies have implicated the expressions of different ECM protein sequences in the degenerated disc to be behind the pathophysiology of degeneration. Asporin, an ECM protein of the leucine-rich proteoglycans family [51], is highly expressed in degenerated intervertebral discs [52]. The 14 aspartate residues of asporin make it a risk molecule in osteoarthritis [53], and since IDD is also a degenerative disease and the expression of asporin in IDD is confirmed, we explored its expression by RT-qPCR from NP cells in all the groups. Our observation that asporin expression increased with an increase in disc degeneration in rats in the IDD-induced group coincided with the previous findings that this protein expression is higher in highly degenerated discs. Since IL-1β is known to increase the matrix-degrading enzymes, decrease matrix synthesis, and increase proinflammatory cytokine expression [53], it is also implicated in the expression of asporin in IDD [52].
We have demonstrated that our IDD model satisfactorily induced IDD in rats and that the hallmark of IDD pathogenesis is the imbalance in the expression of catabolic factors by NP cells of intervertebral discs. This is primarily mediated by the proinflammatory cytokines, especially TNF-α, which is implicated in the expression of many of the other inflammatory cytokines and MMPs. Finally, to prove that IDD pathogenesis primarily occurs through TNF-α, and apigenin acts overwhelmingly against the TNF-α receptor, we used a TNF inhibitor along with apigenin in cultured NP cells. The outcome of the experiment provided strong evidence that apigenin works like the TNF-α inhibitor and blocks the TNF-α receptor; hence, we observed a decrease in the expression of the inflammatory cytokines IL-1β, IL-6, and IL-8, and MMP-2 and MMP-9 with apigenin and the TNF-α inhibitor.
Conclusions
The results of our study reconfirm the participation of the immune system in the formation of cytokines and chemokines in the disc region. Apigenin could be used as a therapeutic molecule in the control of IDD by reducing the expression of the molecules involved in the pathophysiology of the disease.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by National Natural Sciences Foundation of China (No: 81401827), and Health Commission of Hubei Province scientific research project (No: WJ2019M075)
References
- 1.Hoy D, March L, Brooks P, et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 2.Khan AN, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann NY Acad Sci. 2017;1410(1):68–84. doi: 10.1111/nyas.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976) 2006;31(23):2724–27. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 5.Johnson ZI, Schoepflin ZR, Choi H, et al. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–16. doi: 10.22203/ecm.v030a08. discussion 116–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guterl CC, See EY, Blanquer SB, et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater. 2013;25:1–21. doi: 10.22203/ecm.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: New perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21(1):29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–30. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban JP, Maroudas A, Bayliss MT, et al. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16(6):447–64. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- 10.Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–70. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Roughley PJ. Biology of intervertebral disc aging and degeneration: Involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29(23):2691–99. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 12.Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178–81. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 13.Johnson WE, Caterson B, Eisenstein SM, et al. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46(10):2658–64. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- 14.Melrose J, Roberts S, Smith S, et al. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976) 2002;27(12):1278–85. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostetler GL, Ralston RA, Schwartz SJ. Flavones: food sources, bioavailability, metabolism, and bioactivity. Adv Nutr. 2017;8(3):423–35. doi: 10.3945/an.116.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla S, Gupta S. Apigenin. A promising molecule for cancer prevention. Pharm Res. 2010;27(6):962–78. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madunic J, Madunic IV, Gajski G, et al. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Nabavi SF, Khan H, D’Onofrio G, et al. Apigenin as neuroprotective agent: Of mice and men. Pharmacol Res. 2018;128:359–65. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Li F, Chen G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol Sci. 2014;35(4):583–88. doi: 10.1007/s10072-013-1566-7. [DOI] [PubMed] [Google Scholar]
- 21.Fang W, Zhou X, Wang J, et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int Immunopharmacol. 2018;65:539–49. doi: 10.1016/j.intimp.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Silveira JW, Issy AC, Castania VA, et al. Protective effects of cannabidiol on lesion-induced intervertebral disc degeneration. PLoS One. 2014;9(12):e113161. doi: 10.1371/journal.pone.0113161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang W, Zhou X, Wang J, et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int Immunopharmacol. 2018;65:539–49. doi: 10.1016/j.intimp.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Chen S, Ma K, et al. Comparison of different methods for the isolation and purification of rat nucleus pulposus-derived mesenchymal stem cells. Connect Tissue Res. 2019;17:1–9. doi: 10.1080/03008207.2019.1611793. [DOI] [PubMed] [Google Scholar]
- 25.Yang Q, Yu D, Zhang Y. β-Sitosterol attenuates the intracranial aneurysm growth by suppressing TNF-α-mediated mechanism. Pharmacology. 2019;104(5–6):303–11. doi: 10.1159/000502221. [DOI] [PubMed] [Google Scholar]
- 26.Yu LP, Qian WW, Yin GY, et al. MRI assessment of lumbar intervertebral disc degeneration with lumbar degenerative disease using the Pfirrmann grading systems. PLoS One. 2012;7(12):e48074. doi: 10.1371/journal.pone.0048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–99. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 28.Yee A, Lam MP, Tam V, et al. Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthritis Cartilage. 2016;24(3):503–13. doi: 10.1016/j.joca.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto H, Saura R, Harada T, et al. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J Med Sci. 2000;46(1–2):13–28. [PubMed] [Google Scholar]
- 30.O’Donnell JL, O’Donnell AL. Prostaglandin E2 content in herniated lumbar disc disease. Spine (Phila Pa 1976) 1996;21(14):1653–55. doi: 10.1097/00007632-199607150-00007. discussion 1655–56. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto H, Saura R, Doita M, et al. The role of cyclooxygenase-2 in lumbar disc herniation. Spine (Phila Pa 1976) 2002;27(22):2477–83. doi: 10.1097/00007632-200211150-00011. [DOI] [PubMed] [Google Scholar]
- 32.Takada T, Nishida K, Maeno K, et al. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 2012;64(8):2601–10. doi: 10.1002/art.34456. [DOI] [PubMed] [Google Scholar]
- 33.Sutovsky J, Benco M, Sutovska M, et al. Cytokine and chemokine profile changes in patients with lower segment lumbar degenerative spondylolisthesis. Int J Surg. 2017;43:163–70. doi: 10.1016/j.ijsu.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFα expression profile. Arthritis Res Ther. 2007;9(4):R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui LY, Liu SL, Ding Y, et al. IL-1β sensitizes rat intervertebral disc cells to Fas ligand mediated apoptosis in vitro. Acta Pharmacol Sin. 2007;28(10):1671–76. doi: 10.1111/j.1745-7254.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhao CQ, Liu D, Li H, et al. Interleukin-1β enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis. 2007;12(12):2155–61. doi: 10.1007/s10495-007-0137-x. [DOI] [PubMed] [Google Scholar]
- 37.Cheng L, Fan W, Liu B, et al. Th17 lymphocyte levels are higher in patients with ruptured than non-ruptured lumbar discs and are correlated with pain intensity. Injury. 2013;44(12):1805–10. doi: 10.1016/j.injury.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Nie L, Wang Y, et al. CCL20 Secretion from the nucleus pulposus improves the recruitment of CCR6-expressing Th17 cells to degenerated IVD tissues. PLoS One. 2013;8(6):e66286. doi: 10.1371/journal.pone.0066286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Nie L, Guo YJ, et al. Th17 cell frequency and IL-17 concentration correlate with pre- and postoperative pain sensation in patients with intervertebral disk degeneration. Orthopedics. 2014;37(7):e685–91. doi: 10.3928/01477447-20140626-62. [DOI] [PubMed] [Google Scholar]
- 40.Miljkovic D, Trajkovic V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004;15(1):21–32. doi: 10.1016/j.cytogfr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Goldring MB, Birkhead J, Sandell LJ, et al. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988;82(6):2026–37. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1998;10(3):263–68. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, McCluskey K, Fujii K, et al. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-α, granulocyte-macrophage CSF, and IL-1 β through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998;161(6):3071–76. [PubMed] [Google Scholar]
- 44.Han B, Zhu K, Li FC, et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine (Phila Pa 1976) 2008;33(18):1925–34. doi: 10.1097/BRS.0b013e31817c64a9. [DOI] [PubMed] [Google Scholar]
- 45.Tsai TT, Lai PL, Liao JC, et al. Increased periostin gene expression in degenerative intervertebral disc cells. Spine J. 2013;13(3):289–98. doi: 10.1016/j.spinee.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T, Yasue A, Fujihara S, et al. PERIOSTIN regulates MMP-2 expression via the αvβ3 integrin/ERK pathway in human periodontal ligament cells. Arch Oral Biol. 2012;57(1):52–59. doi: 10.1016/j.archoralbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Crean JK, Roberts S, Jaffray DC, et al. Matrix metalloproteinases in the human intervertebral disc: Role in disc degeneration and scoliosis. Spine (Phila Pa 1976) 1997;22(24):2877–84. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 48.Rutges JP, Kummer JA, Oner FC, et al. Increased MMP-2 activity during intervertebral disc degeneration is correlated to MMP-14 levels. J Pathol. 2008;214(4):523–30. doi: 10.1002/path.2317. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Wang H, Yang H, et al. Tumor necrosis factor-α- and interleukin-1β-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kappaB axis: Implications in inflammatory disc disease. Am J Pathol. 2014;184(9):2560–72. doi: 10.1016/j.ajpath.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Roughley PJ, Mort JS. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 1991;9(4):568–75. doi: 10.1002/jor.1100090413. [DOI] [PubMed] [Google Scholar]
- 51.Henry SP, Takanosu M, Boyd TC, et al. Expression pattern and gene characterization of asporin. A newly discovered member of the leucine-rich repeat protein family. J Biol Chem. 2001;276(15):12212–21. doi: 10.1074/jbc.M011290200. [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Liu C, Sun Z, et al. IL-1β increases asporin expression via the NF-kappaB p65 pathway in nucleus pulposus cells during intervertebral disc degeneration. Sci Rep. 2017;7(1):4112. doi: 10.1038/s41598-017-04384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song YQ, Cheung KM, Ho DW, et al. Association of the asporin D14 allele with lumbar-disc degeneration in Asians. Am J Hum Genet. 2008;82(3):744–47. doi: 10.1016/j.ajhg.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]