Abstract
In oncology, “immunotherapy” is a broad term encompassing multiple means of utilizing the patient’s immune system to combat malignancy. Prominent among these are immune checkpoint inhibitors, cellular therapies including chimeric antigen receptor T-cell therapy, vaccines, and oncolytic viruses. Immunotherapy for glioblastoma (GBM) has had mixed results in early trials. In this context, the past, present, and future of immune oncology for the treatment of GBM was discussed by clinical, research, and thought leaders as well as patient advocates at the first annual Remission Summit in 2019. The goal was to use current knowledge (published and unpublished) to identify possible causes of treatment failures and the best strategies to advance immunotherapy as a treatment modality for patients with GBM. The discussion focuses on past failures, current limitations, failure analyses, and proposed best practices moving forward.
Keywords: brain cancer, failure analysis, glioma, glioblastoma, immunology, immunotherapy
For decades cancer therapy has been predicated on the three classic pillars of surgery, radiation, and cytotoxic chemotherapy, with the addition of targeted therapy including anti-angiogenic agents at the end of the twentieth century. More recently, immunotherapy has been incorporated as a fifth modality of cancer care (Fig. 1). Immunotherapy, in oncology, is a broad term encompassing multiple means of utilizing the patient’s immune system to combat malignancy. Prominent among these are immune checkpoint inhibitors (ICIs); cellular approaches, including chimeric antigen receptor (CAR) T-cells, vaccines, and oncolytic viruses. Each of these modalities has demonstrated success in certain hematologic and solid tissue malignancies and therefore is being tested in challenging tumors such as glioblastoma (GBM).
Fig. 1.
Treatment modalities for cancer.
Immunotherapy for GBM was initially met with great enthusiasm but is now being tempered by some high-profile failures.1,2 These failures came despite efforts to thoughtfully design clinical trials to ensure the ability to measure a clinically relevant treatment response. Potential pitfalls for clinically successful immunotherapy regimens in the treatment of GBM include the lack of penetration through the blood–brain barrier (BBB), overall host immunosuppression, and robust mechanisms of resistance within the tumor and its microenvironment. Better understanding these hurdles and learning from prior failures of promising treatment modalities will inform the design of future treatment approaches.
In this context, the past, present, and future of immuno-oncology for the treatment of GBM was discussed by clinical, research, and thought leaders as well as patient advocates at the first annual Remission Summit in 2019. The goal was to use current knowledge (published and unpublished) to identify possible causes of treatment failures and the best strategies to advance immunotherapy as a treatment modality for patients with GBM. This work is a summary of the Remission review.
Immunotherapy for Brain Cancer: Advances and Failures
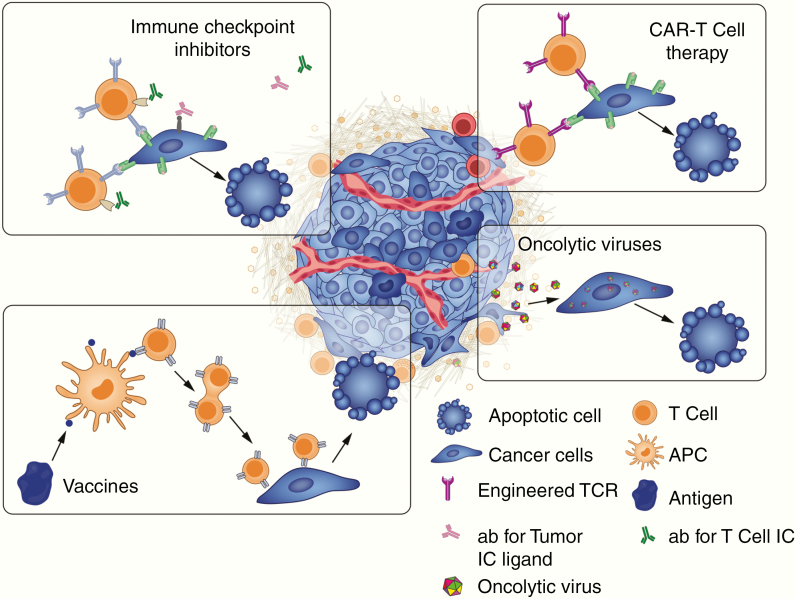
Use of immunotherapy in the brain presents unique challenges compared with its use elsewhere in the body as immune responses in the brain are tightly regulated.3 Both the adaptive immune system in the form of T cells and the innate immune system in the form of macrophages and microglia play a role in an inflammatory immune response in the brain. However, these cells also contribute to the immune-surveillance failures which may allow brain metastases and primary brain tumors to thrive.4 Here we will discuss immunotherapy strategies (Fig. 2) that have been tested in brain cancer and limitations that may inform future strategies.
Fig. 2.
Immunotherapeutic treatment options for cancer. Ab = antibody, IC = immune checkpoint, TCR = T-cell receptor.
Immune Checkpoint Inhibitors
In the healthy host, immune checkpoint pathways maintain constant balance of immune regulation and function to minimize tissue damage incurred by inflammation and prevent autoimmunity. Multiple checkpoints have been discovered, including cytotoxic T-lymphocyte antigen 4,5 programmed cell death 1 (PD1),6 lymphocyte-activation gene 3,7 T-cell immunoglobulin and mucin-domain containing 3 (TIM-3),8 cluster of differentiation (CD)137 (4-1BB),9 glucocorticoid-induced tumor necrosis factor receptor,10 and CD134 (OX40).11
Given the positive results of these agents in non-CNS cancer, they have been investigated for patients with GBM as single agents and in general without the requirement for tumor expression of certain relevant ligands. The results of the first large randomized clinical trial of PD1 inhibition in recurrent GBM have been submitted for publication (CheckMate 143, NCT02017717). This study compared nivolumab monotherapy with bevacizumab. Unfortunately, no improved benefit either in overall survival (OS) or progression-free survival (PFS) was observed with the checkpoint inhibitor (nivolumab) compared with bevacizumab. Even though a durable response was observed in some cases, the percentage of patients who responded was very small (8%). However, a high percentage (>40%) of patients on both treatment arms were on corticosteroids, which have been shown to markedly inhibit response in ICIs.12 The lack of response in GBM may be related to intrinsic mechanisms of resistance or lack of mutations resulting in neoantigens. Patients with other brain tumor types such as melanoma metastases have up to 50% response rates with immune checkpoint blockade.13 Therefore, the BBB cannot explain the lack of response in GBM.
This disappointing result was followed by 2 independent reports of measurable intratumoral immune responses when PD1 blockade was given to patients with recurrent GBM prior to surgery (neoadjuvant).14,15 These studies took advantage of a “window of opportunity” trial design in which patients received the first dose of treatment prior to surgery to evaluate tumor tissue changes induced by treatment. Two independent groups demonstrated an increase in intratumoral inflammation characterized by a rise in interferon (IFN)-gamma when PD1 blockade was given prior to surgery, which correlated with a statistically significant survival benefit. These results have significant implications for the future of trial design in patients with GBM by highlighting the importance of timing of treatment and measuring intratumoral immune signatures rather than relying on peripheral blood immune changes which may not reflect tumor microenvironment (TME) alterations.
Chimeric Antigen Receptor T Cells
CAR T-cell therapy is another therapeutic platform being investigated for the treatment of brain tumors. The first generation of CAR T cells contained only the CD3 zeta domain, while following generations also include CD28 and 4-1BB costimulatory signaling to enhance their potency. Fourth-generation CAR constructs incorporate the controllable suicide caspase-9 gene or interleukin (IL)-12 secreting genes. While CAR T-cell therapy has shown paramount successful recovery in end-stage hematologic malignancies, efficacy is restricted in solid tumors because of the limited expansion, trafficking, and persistence of the CAR T cells into the tumors.
Anti-CD19-specific CAR T-cell therapy is FDA approved for hematologic malignancies.16–18 However, such success has yet to be reproduced in the context of brain tumors. Intracranial administration of engineered T cells targeting IL-13Ra2 was the first CAR T strategy published in the treatment of GBM.19 This first-generation construct demonstrated radiological responses in 2 out of 3 GBM patients with a significant reduction of IL-13Ra2 expression. The study established the feasibility and safety of this approach in high-grade glioma and set the framework to optimize the platform. The same group reported a successful clinical response sustained for 7.5 months after initiating local and intraventricular CAR T-cell therapy in a patient with recurrent and O6-methylguanine-DNA methyltransferase non-methylated GBM, using their second-generation anti–IL-13Ra2 CAR T cells.20 Unfortunately, tumor relapse was observed after several months but was notable for loss of IL-13R2a expression.
Human epidermal growth factor receptor 2 (HER2)/cytomegalovirus (CMV) CAR T cells encoding FRP5 (anti-HER2) scFv is a second-generation CAR T-cell construct generated from virus-specific T cells. These CAR T cells were administered i.v, in 17 adult and pediatric patients with recurrent HER2+ GBM demonstrating an overall PFS of 3.5 months and median OS of 11.1 months. Five of these patients showed long-term stabilized disease for 24 months. Contrary to expectation, i.v. infusion of the CAR T cells did not promote peripheral T-cell expansion, but cells in the tumor persisted for 12 months after the first infusion.21 O’Rourke and colleagues tested CAR T-cell therapy to target epidermal growth factor receptor (EGFR) variant III (vIII) in GBM.22 A single dose of EGFRvIII CAR T cells was infused intravenously into 10 patients with recurrent GBM and benefited 1 patient with stabilized disease for up to 18 months. Results showed the expansion of EGFRvIII CAR T cells within 3–10 days posttreatment infusion, but the cells did not persist. All patients had various levels of CAR T- and non-CAR T-cell infiltrates in the tumor.22 These clinical trials established feasibility and safety, and there were no reports of cytokine release syndrome (CRS) after treatment. Tumor editing (loss of the target antigen in tumor cells) reflects treatment effect, supporting the conclusion that the cells targeted the cancer, and suggests a potential greater curative effect when targeting multiple antigens to overcome antigen heterogeneity.
CAR T cells targeting H3 K27M gliomas have been developed and have the potential to avoid pitfalls related to heterogeneous target expression or loss of target, since all of the tumor cells express the mutation. Mount et al described a CAR T cell targeting the disialoganglioside GD2, which is expressed by H3 K27M diffuse intrinsic pontine gliomas (DIPGs).23 Their CAR T cell also incorporated a 4-1BB costimulatory domain and resulted in clearance of most of the tumor cells in 5 patient-derived orthotopic xenograft models. They also reported that a fraction of the animals died from peritumoral edema and hydrocephalus related to inflammation. Other groups have also developed T-cell therapies targeting H327M DIPGs. Chheda et al described a novel approach establishing a CD8+ T-cell clone by stimulating human leukocyte antigen (HLA)-A2+ T cells with a synthetic peptide of the H3.3K27M mutation.24 The T-cell receptor (TCR) chains were then cloned into a retroviral vector that was used for transduction into HLA-A2+ T cells. These TCR-transduced T cells could efficiently kill A2+ H3.3K27M glioma cells and suppress tumor growth in xenografts.
CAR T-cell products are currently derived from autologous T cells, which may represent a limiting factor for large-scale clinical application caused by high cost and lengthy production procedures. Novel gene editing tools—including ZFN (zinc finger nuclease), TALEN (transcription activator-like effector nuclease), and CRISPR-Cas9 (clustered regularly interspaced short palindromic repeat/CRISPR-associated nuclease 9)—can potentially be used to generate allogeneic T cells needed for the preparation of universal CAR T cells that can serve as ready-to-use off-the-shelf therapeutic agents enabling large-scale clinical application.25 These technologies provide opportunities to knock out unwanted properties or knock in desired functions to enhance the cells’ intrinsic properties regulating their fitness, trafficking, survival, persistence, and activity.
Vaccines
Tumor cells can present specific antigens that are absent from normal tissue. These tumor-specific antigens are proteins that are encoded by mutant genes and can serve as target for immunotherapy such as vaccines. Such vaccines involve the injection of the mutated tumor-specific peptides to engender an immune response toward cells expressing the antigens. Vaccines manipulate immune memory following the initial encounter with the cancer associated or specific antigen to activate T lymphocytes and induce a response targeted against the tumor.
Peptide vaccines represent off-the-shelf therapies that have the advantage of being centrally and universally produced. The receptor for EGFR was considered an ideal immunogenic target in GBM which is frequently mutated. The resulting EGFRvIII truncated mutant is specific to GBM and is constitutively active, therefore driving tumorigenesis. EGFRvIII is the most common gain-of-function mutation in GBM and is present in up to 30% of GBM patients.26 Preclinical studies testing EGFRvIII peptide vaccine in murine models demonstrated the efficacy and specific immunity against EGFRvIII.27,28 These studies eventually led to the double-blind phase III ACT-IV trial, which randomized 745 patients with newly diagnosed EGFRvIII+ GBM to receive rindopepimut vaccine or placebo concurrent with TMZ.1 Unfortunately, the study was negative and the trial was suspended in 2016 as the rindopepimut group showed a median overall survival of 20.1 months versus 20.0 months for the control group. The lack of efficacy may be explained by the fact that only a fraction of tumor cells expresses EGFRvIII, hence targeting only a subpopulation and enabling the outgrowth of EGFRvIII negative cells or that placebo vaccine was composed of keyhole limpet hemocyanin, which is a highly immunogenic peptide that may have engendered a nonspecific immune response potentially affecting disease outcome. Spontaneous loss of EGFRvIII expression may also partially explain these negative results.29 With the data available, it is difficult to assess the intratumoral immunoreactivity and determine if the treatment efficiently targeted EGFRvIII+ cells or if the cells downregulated the receptor and other vaccine strategies or adjuvants are needed to produce an effective response. The disappointing results of these studies revealed the potential limitation of targeting a single tumor antigen such as EGFRvIII. The spontaneous loss of EGFRvIII observed in more than 50% of recurrent GBM without EGFRvIII targeting therapy also highlights the challenge for recurrent GBM trials that enroll based on evaluation of the initially resected, primary tumor.30
Heat shock protein (HSP) vaccines have also been developed for GBM. Heat shock proteins act as chaperones and can bind tumor-associated antigens. Therefore, HSP and peptide antigen complexes have been developed to serve as vaccines for patients. The most notable is HSP peptide complex 96. This vaccine has been tested in phase I and II studies in patients with newly diagnosed31 and recurrent GBM.32,33 These were single arm studies and the survival rates were in the range expected for patients who are candidates for surgical resection and are selected in a clinical trial setting. In a separate analysis of one of the phase II studies, patients with high expression of PD1 ligand (PD-L1) on circulating myeloid cells had reduced survival compared with patients with low expression.34 However, this may be an independent factor related to prognosis and may not be related to vaccine response.
Dendritic cell (DC) vaccines have also been developed for GBM, and initial studies have demonstrated feasibility of targeting various tumor antigens.35–37 This platform has yet to be tested in a large phase III study, but earlier-phase studies have demonstrated measurable immune responses correlating with clinical response in select patients. A small randomized controlled trial of patients with GBM treated with a CMV RNA DC vaccine demonstrated a significant survival increase in patients who also received a tetanus toxoid covaccination due to improved DC migration after vaccination.38 This suggests the potential for enhanced clinical efficacy of a vaccine platform in the setting of efficient “presentation” of the antigen to the relevant T cells. Additionally, this study demonstrated clinical benefit of immunotherapy even when targeting an antigen with low expression levels within the tumor (CMV RNA). This phenomenon may be a result of induction of other immune mechanisms once tumor antigens are released or due to a robust “bystander” effect after cytolysis. This is especially relevant as CMV may not be expressed in all gliomas.39
Newer efforts in the vaccine arena include targeting isocitrate dehydrogenase (IDH1[R132H]) mutations in grade II and grade III gliomas. Researchers have found that the IDH1 mutation includes an immunogenic epitope for which a peptide vaccine has been developed. Interestingly, use of this peptide vaccine leads to expression via major histocompatibility complex class II and a CD4 T-helper 1 response.40 This work has led to ongoing studies in patients with low and high grade gliomas with IDH1 mutations in China, Europe, and the US. As the IDH1 mutation is tumor specific and is present in all tumor cells, some of the pitfalls of the EGFRvIII vaccine may be avoided.
DC and peptide vaccines continued to be studied for GBM in combination with other modalities such as tumor-treating fields or leveraging a personalized medicine approach by targeting tumor-specific neoepitopes.41–43 The results of ongoing trials will help address fundamental questions in determining how to maximize the success of immunotherapeutic strategies. Future vaccine trials will need to be designed to allow the understanding of the nature and level of intratumoral immune response as a result of the therapy and to monitor treatment response and resistance in correlation to conventional imaging, peripheral immune biomarkers, and outcome.
Overcoming Limitations
Tumor Biology
Tumor heterogeneity is a hallmark of GBM and presents a major obstacle to development of successful therapies due to the “robustness” that allows for preserved growth despite external perturbations.44 Both inter- and intratumoral heterogeneities, as well as temporal variation in expression of known driver mutations, underlie many of the barriers to the development of novel GBM treatment modalities. While GBM has a moderately low mutational tumor burden,45 there is still a wide range of variation in the expression of specific mutations known to drive tumor formation and growth. As one of the initial focuses of The Cancer Genome Atlas (TCGA), genomic analysis of GBM has clarified key alterations in the biology of this complex tumor, revealing a great deal about the fundamental driver/passenger mutations common in GBM.46,47 Information gleaned from TCGA led to the division of GBM into 4 subtypes: classical, proneural, mesenchymal, and neural. These subtypes were classified according to mutations in EGFR (classical), platelet derived growth factor receptor alpha/IDH1, and high expression of sex determining region Y–box 2 (proneural), mutation of neurofibromatosis type 1 (mesenchymal), and broad expression of neuronal genes (neural).47,48 However, the development of these classifications has done little to influence clinical outcomes. At present, no correlation has been found between subtype and response to treatment in prospective studies. In fact, Sottoriva et al demonstrated the presence of multiple molecular subtypes within the same tumor in human samples,49 underscoring the variation observed even between patients of similar subtypes and within a patient’s own tumor.
TCGA data revealed a diversity of driver mutations common in GBM. These included growth factor signaling aberrations due to either amplification of specific receptors or mutations that lead to constitutive activation of tyrosine kinase activity, phosphatidylinositol-3 kinase activation, and/or p53 or retinoblastoma inactivation.50,51 No single mutation is solely responsible for tumor development and there is variation in prevalence between primary and secondary GBM as well as among subtypes. Attempts have been made to target the most common of these mutations, such as EGFRvIII, which occurs in 19–40% of GBMs.26,51 However, as previously noted, targeting the EGFRvIII mutation with vaccination has failed for the treatment of GBM.1 Furthermore, recent research has shown that genetic heterogeneity also exists temporally within GBMs. Schafer et al reported that comparison of paired patient primary versus recurrent GBM samples showed variation in expression of 90% of druggable targets.52 These results are not surprising as treatment-resistant glioma cell populations are enriched in recurrent tumors, a further product of tumor heterogeneity. Taken together, inter-, intra-, and temporal heterogeneities significantly impede the employment of traditional cancer therapies, necessitating the development of alternative approaches.
The highly heterogeneous nature of GBM is a major deterrent to successful immunotherapy approaches if the goal is to recognize all malignant cells with a monomodal approach. Loss of antigen in progressive disease and evolving heterogeneity from diagnosis throughout disease progression, coupled with additional acquired mutations that drive recurrence, create tumors that are resistant and/or insensitive to immunotherapy approaches targeting single antigens. Advances in sequencing techniques designed to map tumor heterogeneity53–55 combined with targeted immunotherapies, such as DC vaccines or CAR T cells, may allow us to better leverage the plasticity of immune-based approaches against GBM in a patient-specific manner. Additionally, proof of concept studies derived from preclinical models of GBM mimicking disease heterogeneity at both early stages of tumor development and in response to pressures from treatment will be valuable in testing new approaches. Ideally, insights from these studies may have value for informing clinical trial design and targeted patient selection.
Tumor Microenvironment
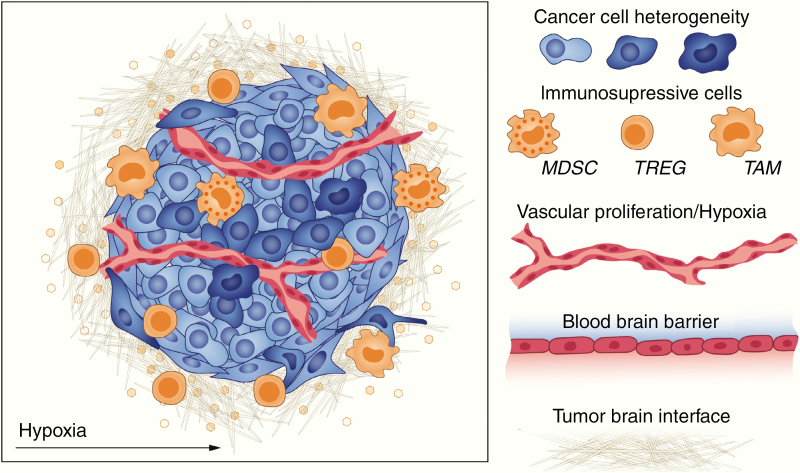
The highly immune-suppressed GBM TME substantially limits the efficacy of established immunotherapy approaches (Fig. 3). Numerous factors contribute to the immune-suppressed TME, primarily driven by infiltrating immune suppressive cells and environmental factors combining, often synergistically, to produce the characteristic GBM TME.
Fig. 3.
Factors that limit immunotherapy efficacy include heterogeneity of cancer cells, immunosuppressive cells such as myeloid derived cells and macrophages, hypoxia and vascular proliferation, and the blood–brain barrier.
Considered an immune privileged compartment, infiltration of peripheral immune cells into brain parenchyma is tightly regulated by the BBB. Under non-pathological conditions, microglia (resident macrophages arising from embryonic progenitor cells) represent the lone functional immune cell present within this tissue.56 However, tumor development increases permeability of the BBB, resulting in tumor infiltration of macrophages and, to a lesser extent, lymphocytes. Recruitment/activation of immune-suppressive cells is driven by enhanced secretion of pro-inflammatory cytokines noted in GBM tumors, including IL-10,57 transforming growth factor beta (TGF-β),58 and chemokine ligand 2.59
Tumor infiltrating lymphocytes (TILs), representing less than 1% of the total tumor immune cell infiltrate,60 include both pro- and antitumor populations. Classically, CD8+, cytotoxic lymphocytes (CTLs), and CD4+ T cells exhibit antitumor activity. However, the TIL population in GBM generally lacks substantial CTL infiltration, and CD4+ populations have increased coexpression of CD25 and/or the transcription factor forkhead box P3, distinguishing them as immune-suppressive regulatory T cells (Tregs). The presence of Tregs in human GBM patients has been reported as a negative prognostic factor,61 and their secretions of IL-10 and TGF-β act to polarize macrophages toward the immune-suppressive M2 phenotype.
Tumor-associated macrophages (TAMs), including myeloid-derived suppressor cells (MDSCs) and M2 polarized macrophages, make up the bulk of the tumor immune cell infiltrate and are a hallmark of high-grade gliomas.62 Hypoxia induced expression of programmed death ligand 1 (PD-L1)63 on TAMs directly impedes proper CTL function upon binding to its cognate receptor, PD1. Expression of arginase 1 (ARG1) in MDSCs indirectly impairs CTL function via depletion of key nutrients,64 as described below, as well as via production of nitric oxide due to enhanced expression of induced nitric oxide synthase.65
Various environmental factors common to the GBM TME, including hypoxia and nutrient depletion, limit CTL function and contribute to the immune-suppressed TME. Hypoxia, a distinguishing characteristic of GBM, contributes to GBM morphology as well as immunosuppression. Pseudopalisading, a histologic signature of GBM, arises as an adaptation to hypoxia via overexpression of hypoxia inducible factor 1 alpha (HIF-1α).66,67 Overexpression of HIF-1α has also been shown to enhance recruitment of MDSCs to the TME,68 which can further influence immune-suppressive environmental factors through nutrient depletion. As a hallmark of MDSC suppressive function, ARG1-dependent depletion of L-arginine has a profound negative effect on CTL function.64 Glioma cell expression of indoleamine-2,3-dioxygenase (IDO) further shapes the nutrient-depleted TME as tryptophan catabolism drives production of kynurenine.69 Reduced tryptophan levels, as well as presence of kynurenine catabolites, impede CTL function and promote T-cell anergy.70–72 Interestingly, IDO has been found to “protect” GBM from chemoradiation by preventing vascular activation and complement-dependent tumor destruction. Li et al demonstrated that IDO blockade synergized with chemotherapy and radiation in murine glioma models by upregulating vascular cell adhesion molecule 1 on vascular endothelium within the tumor and widespread deposition of complement.73
Immunomodulating drugs targeting these immune-suppressive mechanisms may augment existing immunotherapies. Recent failures of single intervention strategies, likely influenced by the GBM TME, signal the need for novel treatment modalities relying on combinatorial therapies. These approaches aim to either reprogram the TME or reshape its constituents and may have broad influence due to the noted interrelation of factors contributing to immunosuppression in GBM. At present, efforts directed against immune-suppressive factors such as IDO, IL-10, TGF-β, and chemokines, as well as approaches targeting infiltrating immune cells, are ongoing.74 However, the safety, tolerability, and pharmacologic properties of immunomodulatory agents in combination with immunotherapy will likely determine the chance that an enhanced beneficial effect over monotherapy is observed. Synergy in efficacy through combinatorial approaches will need to be balanced with increased risk of toxicities arising from multi-agent therapies. Finally, heterogeneous immune-suppressive mechanisms within the TME likely vary tumor to tumor, which implies that a tailored approach may be required for maximum efficacy.
Mutational Burden, Immunogenicity, and Heterogeneity
Tumors harboring higher mutational burden are associated with tumor neoantigens driving cytotoxic responses against tumor cells. The mutation loads of patients with metastatic melanoma or non-small-cell lung cancer, for example, were shown to be significantly associated with response to checkpoint blockade,75,76 whereas poorly immunogenic tumors such as pancreatic and prostate cancers with low mutation load show greater resistance to checkpoint inhibition.77 The threshold for what is high or low tumor mutation burden is subjective and researchers have found that within histology types, the tumors in the top 10% of tumor mutation burden had improved survival when treated with immune checkpoint inhibitors.78 Of note, this study excluded glial tumors. Therefore, the total mutation burden can vary but more is still associated with potentially better response to certain immunotherapy platforms. Similarly, GBMs exhibit a relatively low mutational rate and are considered “cold” tumors with reduced immunogenicity. Immunoediting by tumor cells to reduce mutational immunogenicity can also be a mechanism of acquired treatment resistance.79,80 The one exception to this rule is the rare glioma with a mismatch repair deficiency (dMMR) or microsatellite instability (MSI).81 These tumors, like other dMMR/MSI tumors (as commonly noted in colon or lung carcinoma), have a high mutational burden and show better response to immune checkpoint blockade.
Metabolic Derangements
Malignant brain tumors reprogram pathways of nutrient acquisition and metabolism to meet their bioenergetic and biosynthetic demands. The most predominant metabolic reprogramming is increased glycolytic flux. Immune evasion and metabolic reprogramming are now well recognized hallmarks of cancer and are considered to be functionally linked. Similar to cancer cells, T cells initiate metabolic regulation to drive their activation and effector functions.82 This metabolic switch impacts greatly the TME, which in turn acts as a major barrier for successful targeting of cancer by antitumor immune cells. Activated T cells engage aerobic glycolysis, consuming a massive amount of glucose, and blocking glycolysis reduces their activation. Cancer and immune cells share similarities in nutrient utilization, particularly glucose, and engage similar metabolic regulation to sustain survival, proliferation, and other key functions. Tumor glycolytic activity has been associated with poor prognosis and low tumor infiltration and activation of T cells in multiple types of cancer.83 Tumor cell energetics dictate the metabolic landscape of the TME. For example, malignant glioma cells express tryptophan-2,3-dioxygenase (TDO) due to activation of prostaglandin-E (PGE4).84 TDO induces intratumoral PGE2 metabolism resulting in an immunosuppressive environment due to induction of Tregs85 and inhibition of effector T cells.86
Altered metabolic characteristics of GBM lead to intratumoral heterogeneity and immunosuppression that contribute to the failure of immunotherapy. The brain tumor microenvironment provides local restraints via metabolic competition suppressing antitumor immunity, specifically inhibiting infiltration and tumoricidal functions of host and adoptively transferred tumor-reactive T cells. Host or transferred tumor infiltrative T cells not only have to surpass the conventional immune checkpoints but must also face a wide range of metabolic checkpoints that shape their energetic behavior and dampen their function. Strategies to rewire and restore the metabolic fitness and flexibility of immune cells will likely improve efficacy of immunotherapeutic approaches. Modulation of cellular metabolism holds great promise, using combinatorial approaches of metabolic disruptors with immunotherapy. The metabolic status of the patient or the tumor may be predictive of treatment sensitivity and may be used to stratify patients.87
Host Immunosuppression
One of the most important factors that limit immunotherapy efficacy in patients with GBM is the profound immunosuppression that exists at the time of diagnosis that is worsened with standard-of-care treatment. Patients with GBM are found to have T cells sequestered in the bone marrow at the time of diagnosis.88 Circulating T cells are less responsive to activation, and intratumoral T cells have an upregulation of markers of exhaustion (PD1, CD39, TIM-3).89 This immunosuppression is only worsened with standard treatment with temozolomide,90–93 radiation, and steroids.12,94 Therefore, agents that may seem promising in preclinical models or in early-phase studies as monotherapies may fail when given to a larger patient population in which many people are severely immunosuppressed and receiving concomitant treatment with immunosuppressive therapies.
Efforts to fully understand the immune-deficient nature of patients and the role of standard therapies are now emerging. The importance of these factors was well demonstrated in the small phase I trial testing personalized neoantigen vaccines in patients with GBM.41 Patients on corticosteroids did not have the ability to generate polyfunctional T-cell responses to vaccination. These responses were seen in the 2 patients who were vaccinated before being placed on steroids. Being able to fully measure the efficacy of a treatment strategy will likely require evaluation of patients for functional immune defects prior to enrolling,95 as well as thoughtfully designed treatment in combination with chemotherapy and radiation, such as by leveraging the homeostatic lymphoproliferation that follows lymphodepletive treatment.96–98
Clinical Trial Design for Brain Tumor Immunotherapy
Immunotherapy for brain tumors was initially a niche field where studies were mostly limited to evaluating immune interventions in preclinical and translational studies.99–103 However, over the last 10 years, immunotherapy has exploded as a major advance in many previously highly malignant cancers. Reports of efficacy of T cell–based therapy104–106 and ICIs107–109 for traditionally resistant solid tissue malignancies109–117 have invigorated immunotherapy research for brain tumors. Unfortunately, reports from the initial studies have been disappointing.1,2,118 These failures have underscored the need to better understand limitations of the host and the tumor in patients with GBM to better identify how to leverage immune-based therapies in this patient population.
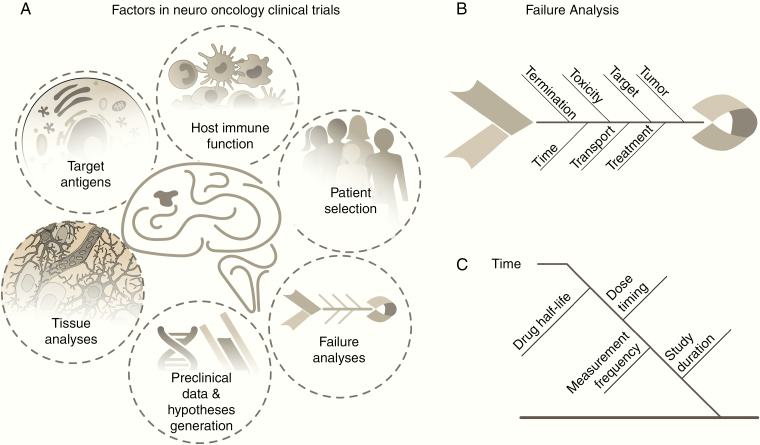
In this section we will discuss possible strategies moving forward that have the potential to reduce treatment and trial failures and will allow the research and clinical communities to learn more from each trial investigating immunotherapy in the brain tumor patient population (Fig. 4A).
Fig. 4.
(A) Strategies to improve the success of clinical immunotherapy trials in adult GBM involve trials based on a biologic understand and effects, preclinical data and hypotheses, careful design and selection of patient enrollment, and cause and effect analysis of factors that affect outcomes. (B, C) Fishbone diagram for failure analysis in clinical trials.
Strategies to Improve Success of Future Trials
The average time from inception of idea to release of a drug to market is 14 years119 and requires $200 million to $2.9 billion.120 Given the expensive and long process of regulatory approval and the limits on patent protection, there has been a sense of urgency in testing these immunotherapy strategies. Therefore, the pressure to reduce the time and cost of bringing therapies to patients remains a high priority. This reality is especially relevant for patients with GBM who continue to suffer from poor response to treatment despite investigations of several novel therapeutic strategies.121–125
However, this time pressure must be balanced with the need to understand the true biologic effects of immune-based strategies on the host and tumor prior to testing in human patients. Based on failures of prior strategies with small-molecule inhibitors and targeted therapies, the key to success of immunotherapy in GBM will largely depend on our ability to design thoughtful clinical trials that are based on robust preclinical data, are hypothesis based, and contain extensive correlative biologic studies. Assessment of target antigen in patients prior to enrollment is one example of a strategy that would increase the likelihood of response to a particular immunotherapeutic strategy. Although this strategy seems intuitive, the literature has several examples of drug failures in trials that did not predicate enrollment based on tumor expression of the treatment target. For example, suppression of the dysregulated tyrosine kinase pathway in GBM has been shown to have no correlation with expression of amplification of EGFR.126 Even when this is performed, other pitfalls can result in a failed trial. The EGFRvIII vaccine trial enrolled patients with expression of the relevant mutation. However, loss of the mutation from follow-up tissue analysis in early-phase trials was used as a proof of efficacy. This approach can miss recognition of other pathways that tumors may exploit and is an important lesson in the significance of tumor editing.
In addition to assessment of the target, the ability of patients to mount an appropriate immune response against antigen is critical for the success of any immunotherapeutic platform. Patients may be profoundly immunosuppressed at the time of diagnosis,89,127 and this is further impaired with the standard radiation and chemotherapy treatment.92,128,129 As previously discussed, steroids have the ability to significantly decrease the ability of patients to mount antitumor immune responses.41 Additionally, patients with GBM have intrinsic defects in T-cell function that prevent response to mitogen stimulation.95,130 However, despite the recognition of the disease and iatrogenic immunocompromise, ability to mount an immune response is not commonly tested prior to enrollment in immunotherapy trials. Studies have found that patients with an immunosuppressed phenotype have poorer outcomes regardless of treatment modality. For example, GBM patients participating in the autologous HSP study had reduced survival if they had PD-L1 expression on circulating myeloid cells.34 Measuring the ability of a patient to mount an immune response can include tests such as cutaneous testing to common bacterial antigens or testing peripheral blood lymphocyte responsiveness to mitogens.131
Stratifying enrollment of patients based on ability to mount a response may be a strategy to identify key factors associated with outcome of clinical trials evaluating immunotherapy agents. Although immune response measured peripherally may not correlate with intratumoral effects, assessment of peripheral blood response may define those with improved or reduced chance of having treatment efficacy.14 Restated, having a peripheral blood response to immunotherapy is likely necessary but may not always be sufficient for an immune response within an intraparenchymal brain tumor. This strategy was elegantly demonstrated in a neoantigen vaccine study where patients first underwent vaccination using a “warehouse” of tumor antigens while mutational analysis of patients’ tumors and HLA binding predictions were performed. Patients then received a second vaccine consisting of personalized neoepitope peptides. To receive the first set of vaccines, the patients peripheral T cells were tested to ensure they were reactive against the chosen antigens.43
Well-designed clinical trials may help to further interrogate the correlation of peripheral with tumor immune responses. For example, the recent neoadjuvant PD1 inhibitor trials demonstrated that patients with recurrent GBM who underwent treatment with neoadjuvant PD1 inhibition prior to surgery demonstrated measurable intratumoral genomic changes consistent with an upregulation of T-cell function and IFN-gamma.14,132 A future study emulating this design but also including parallel measurement in peripheral blood could determine if limiting enrollment based on peripherally measured immune response should exclude patients who would otherwise demonstrate benefit from a treatment strategy that is only measurable from tissue analysis. The “window of opportunity” trial design allows for analysis of important biomarkers of response, drug penetration into the tumor, and early intratumoral changes that lead to the generation of insightful data to guide development of promising treatments even if the study is underpowered for a clinical efficacy.
The potential disconnect between tumor and peripheral immune responses in neuro-oncology patients underscores the importance of obtaining tissue as an integral part of trial design. Tissue analysis is a powerful tool to demonstrate a treatment effect or cause of failure. More importantly, intratumoral changes inform mechanism of action and potential for combinatorial strategies. Measuring tumor response also bypasses overdependence on utilizing imaging to determine a clinical effect. In practice, dependence on MRI changes has inherent limitations due to inability to distinguish treatment effect, pseudoprogression, and true progression.133 This often leads to differential management strategies for various patients that potentially impact outcomes.
Cause-and-Effect Analysis
When analyzing negative clinical trial outcomes, the performance of a cause-and-effect analysis may help improve future trials by clarifying factors that may have contributed to the lack of success. Such failure analysis approaches have been pioneered by engineers in product design and quality control. One of the most established methods to examine and document failures is the “Fishbone Diagram” that was developed by Kaoru Ishikawa (such graphics are often called Ishikawa diagrams). These failure analysis diagrams illustrate the diverse and often disparate inputs to a system that can lead to failure in a single graphical image. The primary advantages to this type of analysis are that they allow a quick visual representation of the major causal contributors, facilitate identification of major contributors that may have been overlooked, and suggest areas where more analysis, control, or precision may improve outcomes.
Development of a robust diagram to analyze clinical trials will be iterative and time-consuming but will provide a formulaic method to compare trials and carry forward lessons learned from one trial into the planning stages of new trials. Fig. 4B, C illustrates a starting point that attempts to capture causal contributors to success and provide a first-order diagram. In these diagrams the various dominant contributors are grouped into individual ribs; here this is shown as time, toxicity, target, tumor, termination, transport, and treatment. Each of the ribs off of the backbone should capture the major contributors as a group, which will have a series of subbranches with increasingly specific contributors. As an example:
Time—dose schedule, drug half-life, tumor measurement frequency, duration of study
Toxicity—liver, kidney, heart, other organs and systems
Target—genes, microenvironment, cell surface proteins, immune cell checkpoints
Tumor—type, characterization, biopsy results, size, location, heterogeneity, complexity
Termination—end criteria, imaging decision points, controls, study arms, adverse events
Transport—drug distribution, drug availability, blood–brain barrier, delivery approach
Treatment—combination schedules, radiation, proton, diet, surgery.
Root cause analysis has an extensive history in engineering, product development, manufacturing, marketing, and process control. Once adopted, this process of methodically capturing and preserving various factors that influence the success or failure of a trial is preserved as a record for future development. Based on past experiences across disparate fields, it is highly likely that building from lessons learned, and developing a robust framework from which to evaluate the success and failures of clinical trials, will improve the quality and outcomes of future trials.
Conclusion
Recent years have witnessed remarkable advances and breakthroughs in cancer immunotherapy. However, immunotherapy approaches to treat brain cancer have yielded modest results at best, emphasizing the need for a more thoughtful approach when translating preclinical ideas to the clinical arena and designing clinical trials. Based on what we have learned from prior and current studies, we advocate the following:
• Robust preclinical testing focused on validating mechanism and effect in more than one model system
• Targeting more than one antigen or induction of multiple parallel immune processes as single antigen targeting has a high chance of failure
• Leveraging “window of opportunity” trial design to allow for in-depth tissue analysis after treatment is delivered to allow for collection of biologic activity of therapy
• Stratification to evaluate patient capability to mount clinically relevant systemic immune response
• Preclinical or clinical rationale to support combinatorial therapy prior to testing in patients
• A priori determination of clinically relevant effect size prior to a clinical trial
• Root cause analysis after every clinical trial to interrogate success and failures to inform future trials
The future of immunotherapy for GBM will depend on our ability as a research and medical community to critically assess the work we have already produced to inform the path forward. Therefore, we should ensure that every trial provides new knowledge about the disease and moves beyond “Does it work?” as the only question that trials answer. Using platforms such as the Remission Summit to bring patient advocates, family survivors of brain cancer, researchers, oncologists, radiation oncologists, pathologists, and neurosurgeons together toward the common goal of finding durable treatment strategies for patients with GBM is a key step in this direction.
Funding
This work was supported by NINDS K08 NS099484 held by Dr Rahman.
Conflict of interest statement. The authors do not have conflicts of interest related to the information in this manuscript. Details of paid consulting and funding not related to this content are below: Greg Sawyer, funding from Merck. Duane A. Mitchell, licensed patents Celldex therapeutics, Annias, Immunomic Therapeutics, and iOncologi, Research funding Immunomic Therapeutics, consultant Bristol-Myers Squibb, Tocagen, Imvax; co-founder iOncologi Inc. Michael Lim, research support: Arbor, BMS, Accuray, DNAtrix, Tocagen, Biohaven, Kyrin-Kyowa; consultant: Tocagen, SQZ Technologies, VBI, Patent: Focused radiation + checkpoint inhibitors. David Reardon, Abbvie; Advantagene; Agenus; Amgen; Bayer; Bristol-Myers Squibb; Celldex; DelMar; EMD Serono; Genentech/Roche; Inovio; Merck; Merck KGaA; Monteris; Tocagen, SQZ Technologies, VBI.
References
- 1. Weller M, Butowski N, Tran DD, et al. ; ACT IV Trial Investigators Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 2. Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8(53):91779–91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28(1):12–18. [DOI] [PubMed] [Google Scholar]
- 4. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. [DOI] [PubMed] [Google Scholar]
- 5. Walunas TL, Lenschow DJ, Bakker CY, et al. . CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. [DOI] [PubMed] [Google Scholar]
- 6. Freeman GJ, Long AJ, Iwai Y, et al. . Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huard B, Prigent P, Pagès F, Bruniquel D, Triebel F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur J Immunol. 1996;26(5):1180–1186. [DOI] [PubMed] [Google Scholar]
- 8. Su EW, Lin JY, Kane LP. TIM-1 and TIM-3 proteins in immune regulation. Cytokine. 2008;44(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shuford WW, Klussman K, Tritchler DD, et al. . 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol. 2012;24(2):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92(6):475–480. [DOI] [PubMed] [Google Scholar]
- 12. Giles AJ, Hutchinson MND, Sonnemann HM, et al. . Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tawbi HA, Forsyth PA, Algazi A, et al. . Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. . Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. . Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 16. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grupp SA, Kalos M, Barrett D, et al. . Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown CE, Badie B, Barish ME, et al. . Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown CE, Alizadeh D, Starr R, et al. . Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmed N, Brawley V, Hegde M, et al. . HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Rourke DM, Nasrallah MP, Desai A, et al. . A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mount CW, Majzner RG, Sundaresh S, et al. . Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat Med. 2018;24(5):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chheda ZS, Kohanbash G, Okada K, et al. . Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. 2018;215(1):141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi BD, Archer GE, Mitchell DA, et al. . EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19(4):713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moscatello DK, Ramirez G, Wong AJ. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997;57(8):1419–1424. [PubMed] [Google Scholar]
- 29. van den Bent MJ, Gao Y, Kerkhof M, et al. . Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015;17(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weller M, Roth P, Preusser M, et al. . Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017;13(6): 363–374. [DOI] [PubMed] [Google Scholar]
- 31. Ji N, Zhang Y, Liu Y, et al. . Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial. JCI Insight. 2018;3(10):e99145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crane CA, Han SJ, Ahn B, et al. . Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin Cancer Res. 2013;19(1):205–214. [DOI] [PubMed] [Google Scholar]
- 33. Bloch O, Crane CA, Fuks Y, et al. . Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16(2):274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bloch O, Lim M, Sughrue ME, et al. . Autologous heat shock protein peptide vaccination for newly diagnosed glioblastoma: impact of peripheral PD-L1 expression on response to therapy. Clin Cancer Res. 2017;23(14):3575–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liau LM, Prins RM, Kiertscher SM, et al. . Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. [DOI] [PubMed] [Google Scholar]
- 36. Akasaki Y, Kikuchi T, Homma S, et al. . Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Cancer Immunol Immunother. 2016;65(12):1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Batich KA, Reap EA, Archer GE, et al. . Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin Cancer Res. 2017;23(8):1898–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitchell DA, Batich KA, Gunn MD, et al. . Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zapatka M, Borozan I, Brewer DS, et al. ; PCAWG Pathogens; PCAWG Consortium The landscape of viral associations in human cancers. Nat Genet. 2020;52(3):320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schumacher T, Bunse L, Pusch S, et al. . A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. [DOI] [PubMed] [Google Scholar]
- 41. Keskin DB, Anandappa AJ, Sun J, et al. . Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. . Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. [DOI] [PubMed] [Google Scholar]
- 43. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. . Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. [DOI] [PubMed] [Google Scholar]
- 44. Kitano H. Cancer as a robust system: implications for anticancer therapy. Nat Rev Cancer. 2004;4(3):227–235. [DOI] [PubMed] [Google Scholar]
- 45. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. ; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patel AP, Tirosh I, Trombetta JJ, et al. . Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sottoriva A, Spiteri I, Piccirillo SG, et al. . Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Furnari FB, Fenton T, Bachoo RM, et al. . Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. [DOI] [PubMed] [Google Scholar]
- 52. Schäfer N, Gielen GH, Rauschenbach L, et al. . Longitudinal heterogeneity in glioblastoma: moving targets in recurrent versus primary tumors. J Transl Med. 2019;17(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chowdhury S, Sarkar RR. Exploring notch pathway to elucidate phenotypic plasticity and intra-tumor heterogeneity in gliomas. Sci Rep. 2019;9(1):9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stackhouse CT, Rowland JR, Shevin RS, Singh R, Gillespie GY, Willey CD. A novel assay for profiling GBM cancer model heterogeneity and drug screening. Cells. 2019;8(7):702. doi: 10.3390/cells8070702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fasterius E, Uhlén M, Al-Khalili Szigyarto C. Single-cell RNA-seq variant analysis for exploration of genetic heterogeneity in cancer. Sci Rep. 2019;9(1):9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40(2):252–259. [DOI] [PubMed] [Google Scholar]
- 57. Huettner C, Czub S, Kerkau S, Roggendorf W, Tonn JC. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 1997;17(5A):3217–3224. [PubMed] [Google Scholar]
- 58. Maxwell M, Galanopoulos T, Neville-Golden J, Antoniades HN. Effect of the expression of transforming growth factor-beta 2 in primary human glioblastomas on immunosuppression and loss of immune surveillance. J Neurosurg. 1992;76(5):799–804. [DOI] [PubMed] [Google Scholar]
- 59. Chang AL, Miska J, Wainwright DA, et al. . CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5671–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Han S, Ma E, Wang X, et al. . Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncol Lett. 2016;12(4):2924–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han S, Zhang C, Li Q, et al. . Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110(10):2560–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46(4):957–961; discussion 961. [DOI] [PubMed] [Google Scholar]
- 63. Noman MZ, Desantis G, Janji B, et al. . PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bronte V, Serafini P, De Santo C, et al. . IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170(1):270–278. [DOI] [PubMed] [Google Scholar]
- 65. Mazzoni A, Bronte V, Visintin A, et al. . Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168(2):689–695. [DOI] [PubMed] [Google Scholar]
- 66. Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65(6):529–539. [DOI] [PubMed] [Google Scholar]
- 67. Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. . Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. [DOI] [PubMed] [Google Scholar]
- 68. Du R, Lu KV, Petritsch C, et al. . HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miyazaki T, Moritake K, Yamada K, et al. . Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. Laboratory investigation. J Neurosurg. 2009;111(2):230–237. [DOI] [PubMed] [Google Scholar]
- 70. Fallarino F, Grohmann U, Vacca C, et al. . T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183–190. [DOI] [PubMed] [Google Scholar]
- 71. Fallarino F, Grohmann U, Vacca C, et al. . T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–1077. [DOI] [PubMed] [Google Scholar]
- 72. Opitz CA, Litzenburger UM, Sahm F, et al. . An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. [DOI] [PubMed] [Google Scholar]
- 73. Li M, Bolduc AR, Hoda MN, et al. . The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer. 2014;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wainwright DA, Chang AL, Dey M, et al. . Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Van Allen EM, Miao D, Schilling B, et al. . Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rizvi H, Sanchez-Vega F, La K, et al. . Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 78. Samstein RM, Lee CH, Shoushtari AN, et al. . Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGranahan N, Rosenthal R, Hiley CT, et al. . Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 2017;171(6):1259–1271.e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Matsushita H, Vesely MD, Koboldt DC, et al. . Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site—when a biomarker defines the indication. New Engl J Med. 2017;377(15):1409–1412. [DOI] [PubMed] [Google Scholar]
- 82. MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cascone T, McKenzie JA, Mbofung RM, et al. . Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27(5):977–987 e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ochs K, Ott M, Rauschenbach KJ, et al. . Tryptophan-2,3-dioxygenase is regulated by prostaglandin E2 in malignant glioma via a positive signaling loop involving prostaglandin E receptor-4. J Neurochem. 2016;136(6):1142–1154. [DOI] [PubMed] [Google Scholar]
- 85. Sharma S, Yang SC, Zhu L, et al. . Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65(12):5211–5220. [DOI] [PubMed] [Google Scholar]
- 86. Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Z, Aguilar EG, Luna JI, et al. . Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chongsathidkiet P, Jackson C, Koyama S, et al. . Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mohme M, Schliffke S, Maire CL, et al. . Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin Cancer Res. 2018;24(17):4187–4200. [DOI] [PubMed] [Google Scholar]
- 90. Grossman SA, Ye X, Lesser G, et al. ; NABTT CNS Consortium Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Karachi A, Dastmalchi F, Mitchell DA, Rahman M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018;20(12):1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Karachi A, Yang C, Dastmalchi F, et al. . Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro Oncol. 2019;10;21(6):730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Litterman AJ, Zellmer DM, Grinnen KL, et al. . Profound impairment of adaptive immune responses by alkylating chemotherapy. J Immunol (Baltimore, Md.: 1950). 2013;190(12):6259–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tokunaga A, Sugiyama D, Maeda Y, et al. . Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J Exp Med. 2019;216(12):2701–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100(1-2):216–232. [DOI] [PubMed] [Google Scholar]
- 96. Ridolfi L, Petrini M, Granato AM, et al. . Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med. 2013;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sanchez-Perez LA, Choi BD, Archer GE, et al. . Myeloablative temozolomide enhances CD8+ T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS One. 2013;8(3):e59082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Suryadevara CM, Desai R, Abel ML, et al. . Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology. 2018;7(6):e1434464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Heimberger AB, Archer GE, Crotty LE, et al. . Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002;50(1):158–164; discussion 164–166. [DOI] [PubMed] [Google Scholar]
- 100. Liau LM, Jensen ER, Kremen TJ, et al. . Tumor immunity within the central nervous system stimulated by recombinant Listeria monocytogenes vaccination. Cancer Res. 2002;62(8):2287–2293. [PubMed] [Google Scholar]
- 101. Heimberger AB, Learn CA, Archer GE, et al. . Brain tumors in mice are susceptible to blockade of epidermal growth factor receptor (EGFR) with the oral, specific, EGFR-tyrosine kinase inhibitor ZD1839 (Iressa). Clin Cancer Res. 2002;8(11):3496–3502. [PubMed] [Google Scholar]
- 102. Liebert M, Wahl RL, Lawless G, et al. . Direct stereotactic intracerebral injection of monoclonal antibodies and their fragments: a potential approach to brain tumor immunotherapy. Am J Physiol Imaging. 1990;5(2):55–59. [PubMed] [Google Scholar]
- 103. Curtin JF, King GD, Candolfi M, et al. . Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr Top Med Chem. 2005;5(12):1151–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mazumder A, Rosenberg SA. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984;159(2):495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang L, Morgan RA, Beane JD, et al. . Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21(10):2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ying Z, Huang XF, Xiang X, et al. . A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25(6):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McGranahan N, Furness AJ, Rosenthal R, et al. . Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rizvi NA, Hellmann MD, Snyder A, et al. . Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Snyder A, Makarov V, Merghoub T, et al. . Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 111. Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 112. Hamid O, Robert C, Daud A, et al. . Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hodi FS, O’Day SJ, McDermott DF, et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Le DT, Uram JN, Wang H, et al. . PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Robert C, Thomas L, Bondarenko I, et al. . Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. [DOI] [PubMed] [Google Scholar]
- 116. Topalian SL, Hodi FS, Brahmer JR, et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wolchok JD, Kluger H, Callahan MK, et al. . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Abernathy A. Bristol-Myers Squibb Announces Results from Checkmate-143, a Phase 3 Study of Opdivo (Nivolumab) in Patients with Glioblastoma Multiforme. https://news.bms.com/press-release/bmy/bristol-myers-squibb-announces-results-checkmate-143-phase-3-study-opdivo-nivoluma:Bristol-MyersSquibb. Accessed September 8, 2019. [Google Scholar]
- 119. American Cancer Society. The Basics of Clinical Trials. 2016. https://www.cancer.org/treatment/treatments-and-side-effects/clinical-trials/what-you-need-to-know/clinical-trial-basics.html. Accessed April 29, 2019. [Google Scholar]
- 120.World Health Organization. Technical Report: The Pricing of Cancer Medicines and its Impact. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 121. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 122. Gilbert MR, Dignam JJ, Armstrong TS, et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rich JN, Reardon DA, Peery T, et al. . Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22(1):133–142. [DOI] [PubMed] [Google Scholar]
- 124. Stupp R, Hegi ME, Gorlia T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 125. Dresemann G, Weller M, Rosenthal MA, et al. . Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96(3):393–402. [DOI] [PubMed] [Google Scholar]
- 126. Yung WK, Vredenburgh JJ, Cloughesy TF, et al. . Safety and efficacy of erlotinib in first-relapse glioblastoma: a phase II open-label study. Neuro Oncol. 2010;12(10):1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Woroniecka K, Chongsathidkiet P, Rhodin KE, et al. . T cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Campian JL, Piotrowski AF, Ye X, et al. . Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol. 2017;135(2):343–351. [DOI] [PubMed] [Google Scholar]
- 129. Ellsworth S, Balmanoukian A, Kos F, et al. . Sustained CD4+ T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3(1):e27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol (Baltimore, Md.: 1950). 1997;159(9):4415–4425. [PubMed] [Google Scholar]
- 131. Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136(6):1631–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. . Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 133. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response Assessment in Neuro-Oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]