Abstract
Background
Diffuse midline gliomas (DMG), including brainstem diffuse intrinsic pontine glioma (DIPG), are incurable pediatric high-grade gliomas (pHGG). Mutations in the H3 histone tail (H3.1/3.3-K27M) are a feature of DIPG, rendering them therapeutically sensitive to small-molecule inhibition of chromatin modifiers. Pharmacological inhibition of lysine-specific demethylase 1 (LSD1) is clinically relevant but has not been carefully investigated in pHGG or DIPG.
Methods
Patient-derived DIPG cell lines, orthotopic mouse models, and pHGG datasets were used to evaluate effects of LSD1 inhibitors on cytotoxicity and immune gene expression. Immune cell cytotoxicity was assessed in DIPG cells pretreated with LSD1 inhibitors, and informatics platforms were used to determine immune infiltration of pHGG.
Results
Selective cytotoxicity and an immunogenic gene signature were established in DIPG cell lines using clinically relevant LSD1 inhibitors. Pediatric HGG patient sequencing data demonstrated survival benefit of this LSD1-dependent gene signature. Pretreatment of DIPG with these inhibitors increased lysis by natural killer (NK) cells. Catalytic LSD1 inhibitors induced tumor regression and augmented NK cell infusion in vivo to reduce tumor burden. CIBERSORT analysis of patient data confirmed NK infiltration is beneficial to patient survival, while CD8 T cells are negatively prognostic. Catalytic LSD1 inhibitors are nonperturbing to NK cells, while scaffolding LSD1 inhibitors are toxic to NK cells and do not induce the gene signature in DIPG cells.
Conclusions
LSD1 inhibition using catalytic inhibitors is selectively cytotoxic and promotes an immune gene signature that increases NK cell killing in vitro and in vivo, representing a therapeutic opportunity for pHGG.
Key Points
1. LSD1 inhibition using several clinically relevant compounds is selectively cytotoxic in DIPG and shows in vivo efficacy as a single agent.
2. An LSD1-controlled gene signature predicts survival in pHGG patients and is seen in neural tissue from LSD1 inhibitor–treated mice.
3. LSD1 inhibition enhances NK cell cytotoxicity against DIPG in vivo and in vitro with correlative genetic biomarkers.
Keywords: DIPG, epigenetics, immunotherapy, LSD1, NK cell
Importance of the Study.
Herein we demonstrate selective cytotoxicity of several clinically relevant LSD1 inhibitors against patient-derived DIPG cells and efficacy in 3 separate orthotopic mouse models. We define an immune gene signature that is upregulated in DIPG cells by catalytic inhibitors of LSD1. This immune gene signature is predictive of prognosis in pHGG patient datasets, consistent with the concept of LSD1 inhibition potentially improving outcomes. NK cell killing of DIPG is enhanced by LSD1 inhibition in vitro and in vivo, providing functional confirmation of this gene signature, and represents the first report of LSD1 inhibition promoting NK cell cytotoxicity of cancer cells. Given the poor prognosis of pHGGs and lack of effective treatments, our results suggest that use of LSD1 inhibition as a single agent or in combination with NK cell therapy may be a safe and efficacious strategy.
Pediatric high-grade gliomas (pHGGs) are pathologically diverse yet uniformly highly malignant central nervous system (CNS) cancers, with 5-year survival rates of <10% postdiagnosis. Surgery is often not possible due to tumor diffusion and the sensitive midline brain structure, which control crucial motor functions such as breathing and heartbeat. Radiotherapy is the standard of care, but survival benefits are slim, with high risks of side effects and decreased quality of life during and after treatment.1 Immunotherapeutic approaches have had limited success due to the low mutational burden and immunosuppressive microenvironment of pediatric brain tumors, such that adaptive immune interventions including checkpoint blockade are ineffective.2 Recent efforts to molecularly profile pHGGs have discovered conserved genomic mutations unique to the pediatric age range and anatomic locations.3 In particular, mutations in histone encoding genes (H3F3A, HIST1H3B) resulting in amino acid substitution of the epigenetically critical lysine residue (H3-K27M) are thought to drive early development of these tumors in multipotent CNS cells.4 As such, the World Health Organization now recognizes these K27M tumors as separate entities in the glioma classification.5
The K27M histone mutations present a therapeutic opportunity for the use of epigenetic regulating drugs, in particular those that target chromatin-modifying proteins. Multiple publications have explored this idea, using inhibitors of histone deacetylases (HDACs),6 demethylases (JMJD3 [Jumonji domain]/UTX [ubiquitously transcribed tetratricopeptide repeat, X chromosome]),7 methyltransferases (enhancer of zeste homolog 2),8 and chromatin readers (bromodomain and extra terminal)9 to demonstrate tumor regression in preclinical models. Clinically translatable compounds exist to target all of these and indeed an ongoing clinical trial is testing the HDAC inhibitor panobinostat as a monotherapy (NCT02717455).10 However, other chromatin modifiers have yet to be explored as therapeutic targets, and there is limited investigation into how the gene expression changes generated by these drugs can be used to augment preexisting therapies.
The histone demethylase LSD1 removes mono- and dimethyl marks from H3K4 and H3K9 and shares structural homology with monoamine oxidases (MAOs). LSD1 is targeted by several drugs11 and has thus far been therapeutically investigated in cancers, including acute myeloid leukemia,12 sarcoma,13 and neuroblastoma.14 LSD1 inhibition has been shown to have an enticing therapeutic window that is selective for cancer cells, in part through its disruption of oncogenic and onco-maintenance transcriptional programs.15,16 Furthermore, the H3K4me1 histone mark regulated by LSD1 was seen to be enriched in intergenic regions of pHGG cells,9 suggesting that LSD1 may control access to enhancers of genes important in pHGG pathology. LSD1 inhibitors can functionally target either the catalytic domain that mediates demethylation17 or the scaffolding tower domain that interfaces with other proteins in epigenetic complexes,18 and it is currently unknown what phenotype these disparate inhibitors would produce in pHGG. Given the highly disrupted yet therapeutically sensitive epigenome of pHGGs, we sought to explore in this study whether LSD1 inhibition could both be cytotoxic to pHGG and generate transcriptional changes that would inform combination therapies.
Our group previously published a report on use of a combination therapy of LSD1 and HDAC inhibition to synergistically induce cell death in adult glioblastoma cell lines and patient-derived glial stem cells.19 In a follow-up study, we used RNA sequencing (RNA-Seq) to explore how the HDAC/LSD1 inhibitor combination therapy produced gene changes in the p53 family members p63 and p73.20 In our current study, we identify an LSD1-induced immunogenic gene signature conserved in pHGG patients21 that predicts longer survival. We further show that LSD1 inhibition is selectively cytotoxic to DIPG cells, and inhibitor-based induction of this gene signature augments innate immune reactivity against DIPG by boosting natural killer (NK) cell immunotherapy response in vitro and in vivo.
Materials and Methods
Detailed experimental procedures are provided in the Supplementary Material.
Cells and Human Samples
Human pHGG cells (DIPG IV, IV-luc, VI, and XIII) were cultured in TSM Base medium. DIPG IV and IV-luc possess a H3.1-K27M mutation, DIPG VI and DIPG XIII are H3.3-K27M mutated. LN18, U87, and immortalized normal human astrocyte (NHA) cells were cultured in fetal bovine serum–supplemented DMEM/F12 medium. Pediatric K27M cells–hemagglutinin (PKC-HA) and PKC-luciferase (luc) cells were cultured in NeuroCult medium. Human T cells were cultured in ImmunoCult-XF T-cell Expansion Medium. Human NK cells were cultured as previously described.22
Clinical Datasets and Bioinformatics
Gene expression in pHGGs was analyzed from the dataset published by Mackay et al21 using the cBioPortal interface. CIBERSORT analysis, an in silico method to determine immune cell infiltrate in bulk sequenced tissue, was performed as previously described23 using the standard LM22 leukocyte signature matrix in the same patient dataset.21
Mouse Models of Hemispheric pHGG and Brainstem DIPG
Nonobese diabetic severe combined immunodeficient gamma (NSG) mice were orthotopically implanted with either 500 000 DIPG IV-luc or 300 000 PKC-luc cells as a model of hemispheric pHGG. C57BL/6 mice were injected in the brainstem with 500 000 PKC-HA cells as a model of brainstem DIPG.
Cellular Thermal Shift Assay
Cells were treated for 1 h with LSD1 inhibitors, harvested and subjected to a 3 min melt curve in a thermocycler, then lysed for analysis via western blot.
NK and T-Cell Cytotoxicity Co-Culture
Target cells were labeled with calcein acetoxymethyl (AM), incubated at effector-to-target ratios of 10:1 and below, then co-incubated with effector cells (NK or T cells) for 4 h. Lysed cells release calcein AM into the supernatant, which was read on a plate reader.
Seahorse Metabolic Assays
NK and T cells were subjected to the XF Mito Stress Test on a Seahorse XFe96 using the default protocol, and 300 000 live cells per well adhered with CellTak.
Chemicals and Antibodies
Small-molecule LSD1 inhibitors were suspended and stored at −20°C in dimethyl sulfoxide (DMSO) or phosphate buffered saline (PBS) as appropriate. Antibodies were stored at 4°C or −20°C as appropriate.
Drug Screening
DIPG and NHA cells were plated in 96-well plates and dosed at indicated amounts with LSD1 inhibitors. Plates were incubated for 5 days and alamarBlue readout was measured fluorometrically on a plate reader.
Cell Viability and Apoptosis Assays
Cells were treated with LSD1 inhibitors for 72 or 96 h and harvested for analysis via trypan blue exclusion assay. Cells were also fixed with ice-cold 100% ethanol for later analysis with propidium iodide via flow cytometry.
RNA Isolation and Real-Time Quantitative PCR
RNA was extracted from cells after 24 h LSD1 inhibitor treatment and converted to cDNA, which was used directly in quantitative (q)PCR experiments to measure gene expression relative to DMSO control. Primer sequences are provided in the Supplementary Material.
Flow Cytometry
After LSD1 inhibitor treatment, cells were harvested and stained with a viability dye and antibodies for analysis via flow cytometry.
Cell Transfections
NHA and DIPG IV cells were transfected with LSD1 small interfering (si)RNA using Lipofectamine and harvested after 48 h for analysis via western blot and qPCR.
Western Blotting
Cells were lysed in radioimmunoprecipitation assay buffer and loaded onto polyacrylamide gels and subjected to electrophoresis. Proteins were transferred to polyvinylidene difluoride membranes for detection via horseradish peroxidase‒conjugated antibodies which were detected with a ChemiDoc Touch.
Statistical Analysis
All statistical analysis was performed in GraphPad Prism 8.
Results
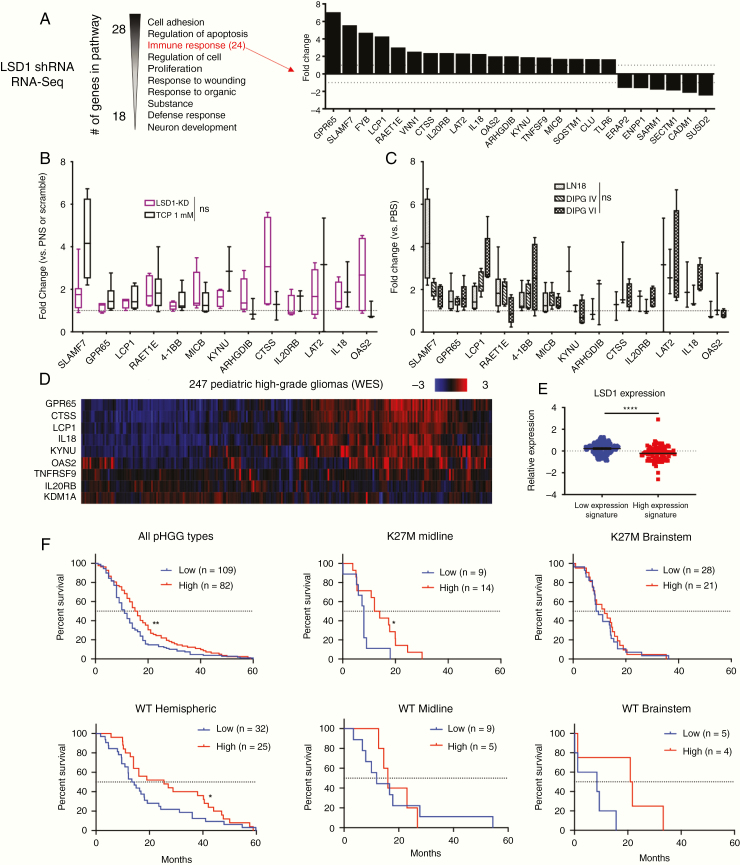
We previously performed RNA-Seq20 on LN18 adult glioblastoma cells when LSD1 was knocked down with short hairpin (sh)RNA in order to explore the mechanism of their sensitivity to dual LSD1 and HDAC inhibition. In the LSD1 shRNA group alone, we applied a 1.5-fold change filter and analyzed the remaining genes with pathway analysis by DAVID (Database for Annotation, Visualization and Integrated Discovery) (Figure 1A). The third most significantly changed pathway was “immune response,” with 24 genes upregulated and downregulated by LSD1 knockdown compared with a scramble control. We sought to validate these gene changes in LN18 cells, and replicated LSD1 knockdown in the cells and confirmed knockdown with western blot. Expression of the 13 most upregulated genes was measured with real time (RT)-qPCR and we observed a significant increase (ANOVA, P < 0.0001) in the gene expression signature with LSD1 knockdown (Figure 1B). This confirmed our RNA-Seq data that LSD1 controls expression of these genes in a glioblastoma cell line. Furthermore, this gene signature matches treatment of LN18 with the established LSD1 inhibitor tranylcypromine (TCP) (Figure 1B), and TCP treatment of LN18 compared with DIPG cells was nonsignificant (Figure 1C), indicating concordance of these upregulated immune genes between pediatric and adult glioma in vitro models. To determine the significance of this signature to patient treatment, we next proceeded to probe a dataset of 247 pHGG patients (Figure 1D). Expression of LSD1 was significantly lower in patients with high expression of our identified gene signature panel, suggesting that LSD1 may influence expression of these genes in pHGG patients (Figure 1E). We found our gene signature of immune response genes could predict significantly improved 5-year survival in all tumors (Figure 1F). The overall benefit was driven by K27M midline (thalamus, cerebellum, spinal cord, ventricles; n = 23) and wild-type (WT) hemispheric (cerebral hemispheres; n = 57) tumors; notably, this survival benefit did not extend to K27M brainstem (pons, midbrain, medulla; n = 49) tumors, and we lacked statistical power in WT brainstem (n = 9) and WT midline (n = 14) tumor samples to make strong conclusions (Figure 1F).
Fig. 1.
LSD1 immunogenic signature is predictive of survival benefit in pediatric high-grade glioma patients. (A) RNA-Seq pathway analysis performed in LSD1 shRNA transduced LN18 cells. Immune response genes and associated fold changes are shown. (B) RT-qPCR of immune gene signature in LN18 cells with LSD1 shRNA or 1 mM TCP treatment for 24 h analyzed by one-way ANOVA with FDR correction. (C) RT-qPCR of immune gene signature in LN18, DIPG IV, and DIPG VI after 1 mM TCP treatment for 24 h analyzed by one-way ANOVA with FDR correction. (D) Heat map of pHGG patient exome data probed for LSD1 immune gene signature. (E) LSD1 expression of patients expressing high and low levels of gene signature analyzed by unpaired t-test. (F) Survival curves of pHGG patient data subdivided by histone mutation and tumor location and analyzed by log-rank or Wilcoxon tests. *P < 0.05, **P < 0.01, ****P < 0.0001, ns = not significant. At least 3 biological replicates were used for RT-qPCR experiments.
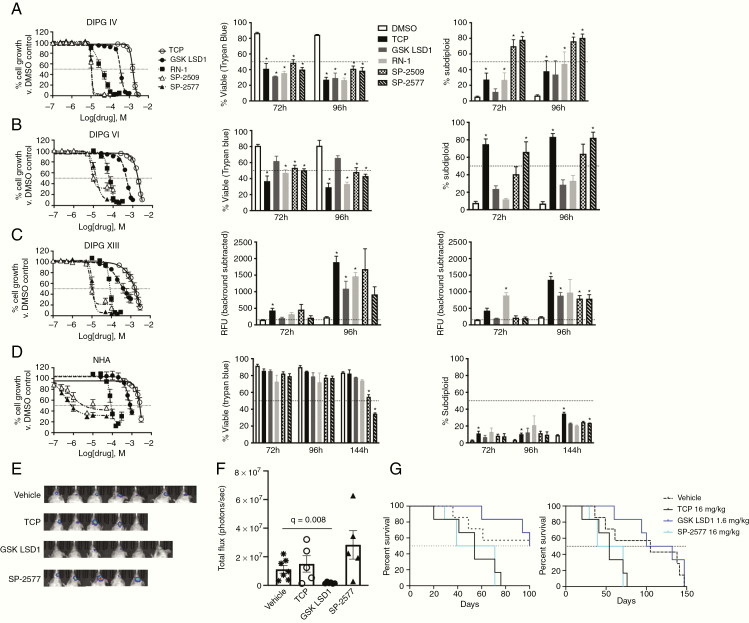
In order to explore the potential of therapeutically triggering this gene signature, we profiled the potency of 3 irreversible catalytic LSD1 inhibitors (tranylcypromine [TCP], GSK LSD1, and RN-1) and 2 reversible scaffolding LSD1 inhibitors (SP-2509 and SP-2577). As we have previously published, LSD1 inhibition alone in adult glioblastoma cells does not potently reduce viability.19,20 In pHGG cells, the same inhibitors display much greater potency that correlates with their specificity and sensitivity for inhibition of LSD1 over the related proteins LSD2, MAO-A, and MAO-B. We observed highly similar half-maximal inhibitory concentration (IC50) values between the unique DIPG cell types for each LSD1 inhibitor tested (Figure 2A–C). While alamarBlue screening is a sensitive assay for cell proliferation, it cannot determine if drugs are cytostatic or cytotoxic due to its reliance on metabolic activity. Therefore, we used trypan blue (membrane integrity) and propidium iodide stain (DNA fragmentation) assays to quantify cell death at the IC50 observed with alamarBlue (TCP: ~1.5 mM, GSK LSD1: ~400 µM, RN-1: ~60 µM, SP-2509/2577: ~13 µM). Cell death was selectively induced in DIPG cells over NHA beginning at 3 days posttreatment (Figure 2D). For neurosphere-forming DIPG XIII cells, we adapted another high-throughput technique to quantify cell death by use of the DNA-binding dye GelGreen and observed the same effects. In order to ascertain in vivo efficacy, luciferase labeled murine pHGG PKC-luc cells were implanted intracranially into NSG mice which were treated intraperitoneally 4 times weekly with vehicle, LSD1 catalytic (TCP and GSK LSD1), or LSD1 scaffolding (SP-2577) inhibitors. Non-invasive imaging showed reduction of tumor burden in mice treated with GSK LSD1 (Figure 2E, F) but not TCP or SP-2577. GSK LSD1 provides an initial survival benefit over vehicle control but this is not maintained (Figure 2G), likely due to adaptive resistance of the tumor to continued targeted therapy.
Fig. 2.
LSD1 inhibitors are growth inhibitory in vitro and in vivo and induce selective cell death in DIPG cells. (A) Dose response curves of LSD1 inhibitors in DIPG IV, (B) DIPG VI, (C) DIPG XIII, and (D) NHA measured using alamarBlue after 120 h treatment. Cell viability after 72 and 96 h measured using trypan blue cell exclusion and analyzed by t-test comparing with DMSO control using FDR correction. DNA fragmentation measured using propidium iodide on flow cytometry analyzed by T-test comparing with DMSO control using false discovery rate (FDR) correction. Cell death of DIPG XIII (C) measured using GelGreen fluorescent intensity in 96-well plate reader and analyzed by t-test comparing with DMSO control using FDR correction. (E) Images of orthotopic tumor luminescence in an NSG pHGG hemispheric mouse model. Mice are shown after 2 weeks of treatment and 4 weeks after tumor implantation. (F) Quantification of tumor burden shown in (E). Vehicle group compared with GSK LSD1 group via t-test with FDR correction. (G) Survival curves of NSG pHGG mice at 100 days and 150 days. *P < 0.05. At least 3 biological replicates were used for all experiments. Error bars represent mean ± SEM.
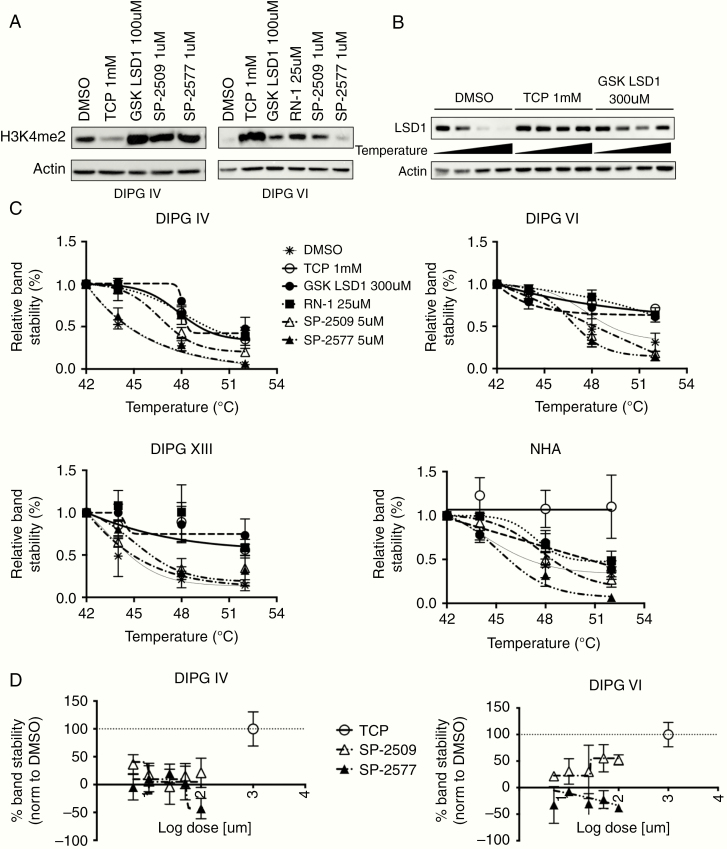
We further profiled the on-target binding of our LSD1 inhibitor suite through assessment of the H3K4me2 mark and by use of the cellular thermal shift assay (CETSA). Western blotting in DIPG IV and VI lines treated with LSD1 inhibitors showed increased expression of the H3K4me2 mark consistently by GSK LSD1 in both lines (Figure 3A). Using CETSA, we could determine if LSD1 is bound by various LSD1 inhibitors in DIPG and NHA cells by heating live cells under treatment with candidate compounds and interrogating the thermostability of the target protein via western blot (Figure 3B). It was observed that all catalytic LSD1 inhibitors could bind LSD1 in all cell types, while results were less consistent with the scaffolding LSD1 inhibitor compounds (Figure 3C). We hypothesized that the dose of SP-2509 and SP-2577 may be too low to thermostabilize LSD1, so we conducted dose response CETSAs with TCP as a positive control. We found a ~50% increase in binding in DIPG VI by raising doses of SP-2509, but no increase in binding above DMSO control with higher doses of SP-2577 in either DIPG cell type (Figure 3D). Given that we dosed up to 100 µM for the dose response CETSA, which is almost 10 times the IC50 of the scaffolding inhibitors in DIPG cells, either the CETSA assay cannot capture the protein complex-disruption properties of the scaffolding compounds or there exists off-target effects, of which there is published data for rationale of the latter.24,25
Fig. 3.
LSD1 inhibitors alter histone methylation levels and thermostabilize LSD1 in DIPG and NHA cells. (A) Western blots of DIPG IV and VI cells treated with LSD1 inhibitors for 24 h and probed for H3K4me2 expression. (B) Representative western blot of CETSA probing LSD1 thermostability in DIPG VI cells. (C) Protein melt curves for LSD1 in different cell types. Each data point was normalized to beta-actin level and further normalized to 42°C data point for each experimental condition. (D) Dose response CETSA for scaffolding inhibitors SP-2509/2577. LSD1 was destabilized at 48°C for all doses and DMSO control was set as 0% stability and 1mM TCP was set as 100% stability. At least 3 biological replicates were used for all experiments. Error bars represent mean ± SEM.
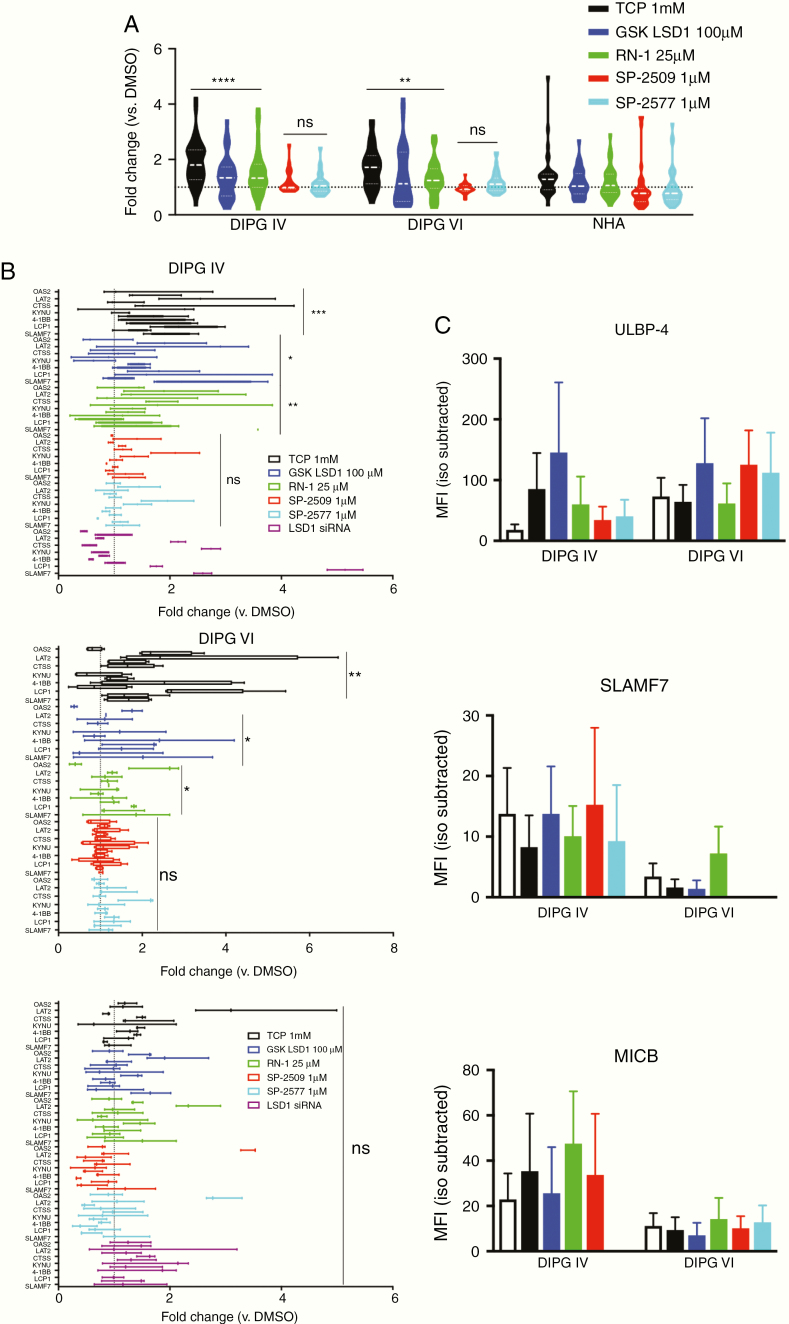
With sensitivity and on-target activity of LSD1 inhibition in DIPG established, we next treated cells with subcytotoxic doses of LSD1 inhibitors for 24 h and isolated RNA to measure expression of our immune gene signature. DIPG cells display a significant upregulation of the signature under treatment with irreversible catalytic LSD1 inhibitors but no significant changes when treated with reversible scaffolding LSD1 inhibitors SP-2509 and its clinical successor SP-2577 (Seclidemstat). This gene signature was also selective for DIPG, as the same treatment did not induce upregulation of NHA cells (Figure 4A, B). We confirmed this selectivity by using LSD1 siRNA in DIPG IV and NHA cells, where we observed upregulation in DIPG but not NHA, at comparable levels of LSD1 knockdown (Supplementary Material). Several genes in the signature correspond to immune signaling receptors, so we next profiled protein expression of 3 innate immune receptors known to play roles in NK cell signaling (SLAMF7, MICB, and ULBP-4). Using flow cytometry, we found that DIPG cells display differing baseline levels of these receptors, perhaps due to their mutational differences in histone alleles (H3.1 versus H3.3). Overall, however, we could detect increased expression on live cells after LSD1 inhibitor treatment for 48 h (Figure 4C).
Fig. 4.
Irreversible catalytic LSD1 inhibitors selectively generate immunogenic signature in DIPG cells. (A) RT-qPCR for immune gene signature performed on cells after treatment with indicated LSD1 inhibitors for 24 h. Catalytic inhibitors (TCP, GSK LSD1, and RN-1) and scaffolding inhibitors (SP-2509/2577) are compared with matched NHA controls using one-way ANOVA with FDR correction. (B) RT-qPCR data replotted with individual genes and including siRNA treatment for 48 h. Fold change compared with DMSO control analyzed via one-way ANOVA with FDR correction. (C) Median fluorescent intensity of indicated receptors after 48 h of LSD1 inhibitor treatment. Matched species and fluorophore isotype controls used to measure background fluorescence. (D) RT-qPCR data with individual genes from PKC-HA and PHC-HA murine cells. Fold change compared with DMSO control analyzed via one-way ANOVA with FDR correction. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant. At least 3 biological replicates were used for all experiments. Error bars represent mean ± SEM.
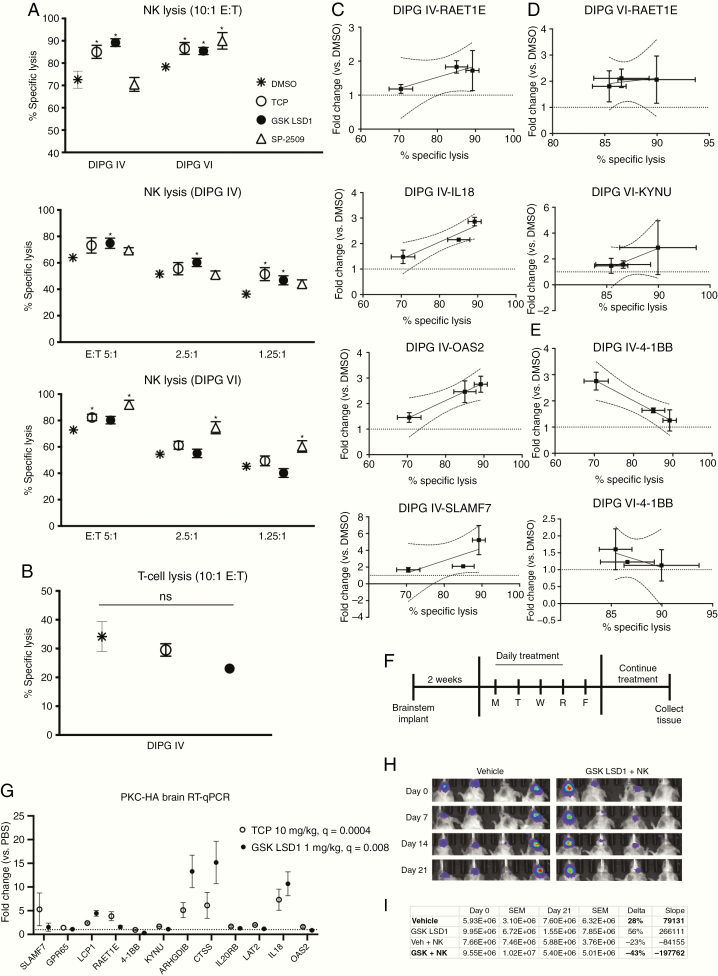
The above receptors function either as ligands of natural killer group 2 member D (NKG2D) or as self-ligating receptors, and stimulation through these receptors increases NK cell activity and lysis of target cells. Given our observed upregulation of these receptors under LSD1 inhibition, we hypothesized that NK cells would lyse target DIPG cells more readily upon LSD1 inhibition. Fluorescently labeled DIPG IV and VI cells were incubated with effector human NK cells at various effector to target (E:T) ratios. Across 3 unique healthy blood donors from whom we expanded NK cells, we could observe increases in lysis in 2 DIPG lines when treated with catalytic LSD1 inhibitors TCP and GSK LSD1, but inconsistently under scaffolding LSD1 inhibition by SP-2509 (Figure 5A). We hypothesize discrepancies between DIPG IV and VI to be due to higher basal levels of ULBP-4 in DIPG VI and greater upregulation of ULBP-4 in DIPG VI after pre-treatment with SP-2509 (Figure 4C). Notably, the lysis efficacy of expanded healthy human donor T cells was much lower than NK cells, and could not be augmented by LSD1 inhibitor pretreatment (Figure 5B). We aimed to correlate genetic biomarkers of NK lysis by probing our gene signature from matched co-culture samples, and observed strong positive trends for 4 genes in DIPG IV (Figure 5C) and 2 genes in DIPG VI (Figure 5D). Unexpectedly, a negative correlation could be found for 4–1BB (Figure 5E), traditionally a T-cell stimulatory factor, which could indicate alternative function during NK cell engagement. Mice implanted with PKC-HA cells in the brainstem of syngeneic C57BL/6 mice and treated with catalytic LSD1 inhibitors (Figure 5F) showed increased expression of the gene signature in neural tissue harvested when mice were moribund (Figure 5G). Given that adaptive resistance to GSK LSD1 was seen in our mouse model (Figure 2G), we combined GSK LSD1 with NK cell infusion to model enhancement of innate immunity after LSD1 inhibition in vivo. Mice treated with intraperitoneal GSK LSD1 and intracranial human ex vivo expanded NK cells had the greatest reduction (43%) in tumor burden from baseline compared with vehicle control, GSK LSD1 alone, or NK cells alone (Figures 5H, I). GSK LSD1 alone did not exert single agent anti-tumor efficacy in this human xenograft model, which is in contrast to our results in mouse orthotopic models, likely due to species mismatch. However, this highlights the anti-tumor effect of the combination of GSK LSD1 and NK cells as particularly striking.
Fig. 5.
LSD1 inhibition upregulates innate immune receptors and sensitizes DIPG cells to NK cell lysis which correlates with unique genetic identifiers of response. (A) Lysis of target DIPG cells co-cultured with NK cells after 48 h pretreatment of target cells with LSD1 inhibitors (TCP 0.5 mM, GSK LSD1 300 µM, SP-2509 5 µM). Treatments analyzed versus DMSO control using t-test with FDR correction. (B) Lysis of target DIPG cells co-cultured with T-cells after 48 h LSD1 inhibitor pretreatment. (C) DIPG IV RT-qPCR from matched co-culture experiments, genes with positive Pearson’s correlation R2 > 0.80 are shown with 95% CIs. (D) DIPG VI RT-qPCR from matched co-culture experiments. (E) RT-qPCR from matched co-culture experiments, negative correlation with R2 > 0.80 shown with 95% CIs. (F) Schematic of immunocompetent C57BL/6 PKC-HA brainstem mouse model. (G) RT-qPCR was performed on RNA extracted from PKC-HA mouse brains. Fold change is plotted versus PBS control. TCP and GSK LSD1 were compared with PBS via one-way ANOVA with FDR correction. (H) Images of orthotopic tumor luminescence in NSG DIPG IV-luc mice starting from day 0 prior to start of treatment. (I) Tumor burden of NSG DIPG IV-luc mice quantified (photons/sec/cm2) and analyzed for % change (delta) and linear regression (slope) between day 0 and day 21. *P < 0.05 and ns = not significant. At least 3 biological replicates were used for all experiments. Error bars represent mean ± SEM.
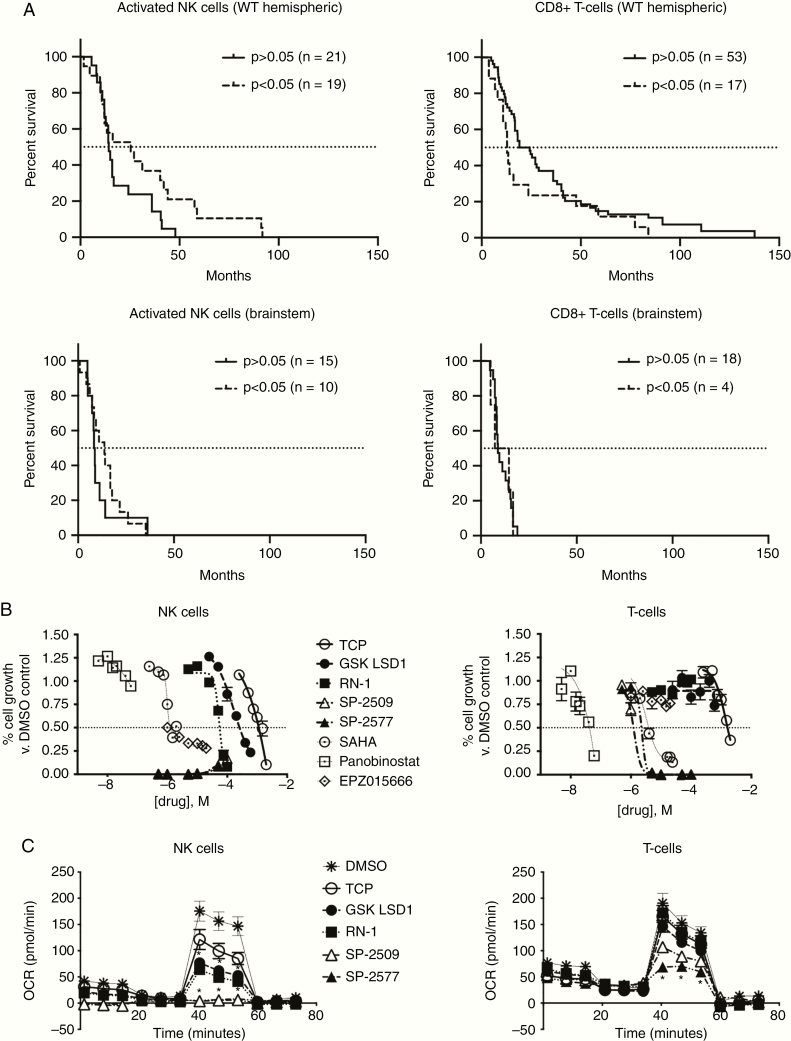
To further validate our finding that catalytic LSD1 inhibition can enhance NK cell lysis of DIPG in vitro and in vivo, we revisited our patient data for analysis using CIBERSORT. We found that significant NK cell infiltration is positively prognostic for H3-WT hemispheric tumors, but CD8 T-cell infiltrate is negatively prognostic. Brainstem tumors benefited less from NK infiltrate, but significant NK presence was still a superior prognostic indicator versus CD8 T cells in the brainstem (Figure 6A). We next investigated how already-present or ex vivo infused immune cells would respond to LSD1 inhibition, and treated expanded NK and T cells with a panel of chromatin-modifier inhibitors including our LSD1 suite. As has been known,26 T-cells are sensitive to HDAC inhibition, but are fairly resistant to LSD1 inhibition except at higher doses of the scaffolding inhibitors. Conversely, NK cells are resistant to HDAC inhibition but highly sensitive to scaffolding LSD1 inhibitors, with no live cells detected even at 500 nM doses of SP-2509/2577 (Figure 6B). Catalytic LSD1 inhibitors are comparatively nonperturbing, with the IC50s against NK cells being 2–10X higher than doses needed to induce our gene signature. Given our data showing the scaffolding LSD1 inhibitors are cytostatic but not cytotoxic to NHA cells, we profiled the metabolism of both NK and T-cells after LSD1 inhibitor treatment, as active metabolism of nutrients has been shown to be crucial to anti-tumor effects of both cell types. Strikingly, the scaffolding LSD1 inhibitors completely suppress the metabolism of NK cells, rendering them metabolically quiescent but still alive at 48 h posttreatment (Figure 6C). Collectively, these data suggest that catalytic LSD1 inhibitors may be used at therapeutic doses to induce increased NK cell reactivity without harming the NK cells directly.
Fig. 6.
NK cell tumor infiltration is predictive of survival benefit in pediatric high-grade glioma patients and catalytic LSD1 inhibitors are non-perturbing to mature NK and T cells. (A) CIBERSORT analysis of pHGG patient data sub-analyzed by tumor location and immune cell type. Survival curves show significant versus nonsignificant presence of indicated immune cell in patient tissue. (B) Purified expanding T and NK cells treated with indicated chromatin-modifier inhibitors for 120 h and measured using alamarBlue. (C) XF Mito Stress Test performed on NK and T cells after 48 h of LSD1 inhibitor treatment (TCP 0.5 mM, GSK LSD1 300 µM, RN-1 25 µM, SP-2509/2577 5 µM) and treatments compared with DMSO control analyzed by t-test with FDR correction. *P < 0.05. At least 3 biological replicates or unique donors were used for all experiments. Error bars represent mean ± SEM.
Discussion
Here we have described a novel dual role for the histone demethylase LSD1 in controlling cell death and innate immune responses against pHGG and DIPG cells in vitro and in vivo. Expression of immune response genes accurately categorized pHGG patients’ survival probability, and therapy with catalytic LSD1 inhibitors triggered gene expression changes that enhanced NK cell-mediated lysis. Epigenetic drugs targeting chromatin modifiers or readers have shown promising antitumor effects in preclinical DIPG models, but these agents have not yet been combined with clinically relevant immunotherapy regimens. NK cell administration is effective against malignant gliomas,27 can be grown without autologous sources of leukocytes,28 and has already begun clinical trials using our exact NK-expansion methodology,29 including in pediatric solid tumors and brain tumors (NCT02271711, NCT03420963). Furthermore, combination therapy with LSD1 inhibitors is desirable due to the demonstrated selectivity of LSD1 inhibitor–induced DIPG cell death versus normal astrocytes. These results suggest the clinical potential of a regimen combining irreversible catalytic LSD1 inhibitors and NK cell infusion in pHGG.
LSD1 recently was shown to control the double-stranded RNA stress response and subsequently synergize with anti‒programmed cell death 1 therapy in murine models.30,31 Notably, this phenomenon was described only in epithelial-derived tissue such as breast, skin, and lung cancer cell lines. We could not recapitulate these genetic changes in our LSD1 shRNA RNA-Seq and believe this aspect of LSD1 influence on tumor immunity may be restricted to only certain cell types. Furthermore, these investigations did not thoroughly explore use of LSD1 inhibitors with differential mechanisms of action to induce enhanced adaptive immunity. DIPG and its microenvironment has recently been described as “immune cold” to the adaptive immune system but potentially vulnerable to modulation of innate immunity.32–34 Another consideration is the pharmacokinetic (PK) properties of currently available LSD1 inhibitors, which have recently been shown to be ineffective against intracranial medulloblastoma despite very potent in vitro activity.35 Our data indicate GSK LSD1 can cross the blood–brain barrier in hemispheric pHGG mouse models, suggesting intracranial tumor location plays a major role in the efficacy of GSK LSD1 against brain tumors. Two separate groups in Japan recently described new catalytic LSD1 inhibitors with favorable PK properties for potential use in neuro-oncology.36,37 The transcription factor GFI1B is not disrupted by their compounds, reducing peripheral toxicity and sparing immune cells that would be critical for our combination therapy. Furthermore, they target the LSD1 catalytic site and may be able to induce the NK-boosting gene changes seen in our experiments.
NK cells have been shown to specifically target glioblastoma stem cells38 and can circumvent antigen loss seen with chimeric antigen receptor T cell–based modalities.39 A clinical trial of NK cell locoregional infusion for pediatric patients with posterior fossa malignant tumors is already in progress40 and preliminary results indicate an excellent safety profile and feasibility of NK cell harvest and expansion. Our analyses of pHGG datasets indicating that a survival advantage is associated with hemispheric and midline tumors, but not brainstem tumors, that display the LSD1 gene signature and the CIBERSORT results of NK infiltrate suggests there are differences in vascularity between these tumor sites, which has been confirmed recently by another group.41 Other promising clinically actionable drug candidates in DIPG could also be combined with NK cell therapy, such as the dopamine receptor D2‒antagonist ONC201, which has been shown to recruit NK cells into tumors42 and is currently in clinical trials in K27M+ tumors.43 Oncolytic viruses targeting DIPG have also begun clinical trials,44 and NK cells have been shown to be involved in the immune response to lysed tumor cells in the brain.45 We did not yet test synergy of NK cells with standard-of-care radiotherapy or chemotherapy in our models, which is warranted based on preclinical46,47 and clinical trial48 data of adult gliomas.
The novel findings in our paper set the stage for further investigations into innate immune interactions in DIPG and how they can be therapeutically exploited with LSD1 inhibitors that can induce both cell death and NK cell reactivity in vitro and in vivo. Our extensive use of LSD1 inhibitors with unique binding properties suggests future investigation of combining brain-penetrant catalytic LSD1 inhibitors and NK cell infusions for synergistic killing of DIPG tumor tissue.
Funding
This work was supported by the NIH under award numbers (i) R21 NS093387 (to J.C.); (ii) Brain Tumor SPORE P50 CA127001 (Developmental Research Project to JC); and (iii) P30CA016672 for use of the Characterized Cell Line Core and Research Animal Support Facility. We are also grateful for support from the Cancer Prevention Research Institute of Texas (CPRIT) grant RP150301 (to VG) and core facility grant RP170628, Center for Cancer Epigenetics at MD Anderson (Pilot Project Grant & Sequencing Core Allowance (to JC) and Scholar Award (to CPB)), and to the Schissler Family Foundation Fellowship (to CPB). Funding from the Team Connor Foundation; the Marnie Rose Foundation; and the Thomas Scott Family Foundation (to JC) is also gratefully acknowledged.
Supplementary Material
Acknowledgments
The authors thank Drs Michael Curran, Candelaria Gomez-Manzano, Andrew Gladden, and Min Gyu Lee for their input on experimental design and directions of the study. We thank Drs Ryan G. Kruger and Helai P. Mohammed for experimental suggestions; Drs Ruolan Han, Aundrietta Duncan, and Jeffrey L. Larson for providing SP-2577 and helpful discussions; Amanda C. Young and Jimi Lynn Young for preparation of tissue slides and IHC; Dr Collene R. Jeter and Jennifer J. Terpstra for immunofluorescence staining and imaging; and Dr Xiaoping Su and Clark Andersen for consultations on biostatistics and bioinformatics analysis.
Conflict of interest statement
The authors declare no relevant conflicts of interest.
Authorship statement
Generated hypotheses, performed experimental procedures, designed experiments, interpreted data and wrote manuscript: C.P.B., M.F., A.G., B.A.K., T.C., V.H., L.W., J.N., W.Z., J.C. Generated and provided cells and designed experiments: Y.Y., M.M.R., O.J.B., M.M., S.Y., D.A.L., V.G.
References
- 1. Johung TB, Monje M. Diffuse intrinsic pontine glioma: new pathophysiological insights and emerging therapeutic targets. Curr Neuropharmacol. 2017;15(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang SS, Bandopadhayay P, Jenkins MR. Towards immunotherapy for pediatric brain tumors. Trends Immunol. 2019;40(8):748–761. [DOI] [PubMed] [Google Scholar]
- 3. Schwartzentruber J, Korshunov A, Liu XY, et al. . Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 4. Lewis PW, Müller MM, Koletsky MS, et al. . Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 6. Grasso CS, Tang Y, Truffaux N, et al. . Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(6):555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashizume R, Andor N, Ihara Y, et al. . Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med. 2014;20(12):1394–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohammad F, Weissmann S, Leblanc B, et al. . EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat Med. 2017;23(4):483–492. [DOI] [PubMed] [Google Scholar]
- 9. Piunti A, Hashizume R, Morgan MA, et al. . Therapeutic targeting of polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat Med. 2017;23(4):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooney T, Onar-Thomas A, Huang J, et al. . Dipg-22. a phase 1 trial of the histone deacetylase inhibitor panobinostat in pediatric patients with recurrent or refractory diffuse intrinsic pontine glioma: a pediatric brain tumor consortium (Pbtc) study. Neuro Oncol. 2018;20(suppl_2):i53. [Google Scholar]
- 11. Fu X, Zhang P, Yu B. Advances toward LSD1 inhibitors for cancer therapy. Future Med Chem. 2017;9(11):1227–1242. [DOI] [PubMed] [Google Scholar]
- 12. Maes T, Mascaro C, Tirapu I, et al. . ORY-1001, a potent and selective covalent KDM1A inhibitor, for the treatment of acute leukemia. Cancer Cell. 2018;33(3):495–511.e412. [DOI] [PubMed] [Google Scholar]
- 13. Sankar S, Theisen ER, Bearss J, et al. . Reversible LSD1 inhibition interferes with global EWS/ETS transcriptional activity and impedes Ewing sarcoma tumor growth. Clin Cancer Res. 2014;20(17):4584–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta S, Doyle K, Mosbruger TL, et al. . Reversible LSD1 inhibition with HCI-2509 induces the p53 gene expression signature and disrupts the MYCN signature in high-risk neuroblastoma cells. Oncotarget. 2018;9(11):9907–9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cusan M, Cai SF, Mohammad HP, et al. . LSD1 inhibition exerts its antileukemic effect by recommissioning PU.1- and C/EBPα-dependent enhancers in AML. Blood. 2018;131(15):1730–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohammad HP, Smitheman KN, Kamat CD, et al. . A DNA hypomethylation signature predicts antitumor activity of LSD1 inhibitors in SCLC. Cancer Cell. 2015;28(1):57–69. [DOI] [PubMed] [Google Scholar]
- 17. Maiques-Diaz A, Spencer GJ, Lynch JT, et al. . Enhancer activation by pharmacologic displacement of LSD1 from GFI1 induces differentiation in acute Myeloid Leukemia. Cell Rep. 2018;22(13):3641–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pishas KI, Lessnick SL. Ewing sarcoma resistance to SP-2509 is not mediated through KDM1A/LSD1 mutation. Oncotarget. 2018;9(92):36413–36429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh MM, Manton CA, Bhat KP, et al. . Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro Oncol. 2011;13(8):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh MM, Johnson B, Venkatarayan A, et al. . Preclinical activity of combined HDAC and KDM1A inhibition in glioblastoma. Neuro Oncol. 2015;17(11):1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mackay A, Burford A, Carvalho D, et al. . Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic Pontine Glioma. Cancer Cell. 2017;32(4):520–537.e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Somanchi SS, Lee DA. Ex vivo expansion of human NK cells using K562 engineered to express membrane bound IL21. Methods Mol Biol. 2016;1441:175–193. [DOI] [PubMed] [Google Scholar]
- 23. Newman AM, Liu CL, Green MR, et al. . Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sonnemann J, Zimmermann M, Marx C, et al. . LSD1 (KDM1A)-independent effects of the LSD1 inhibitor SP2509 in cancer cells. Br J Haematol. 2018;183(3):494–497. [DOI] [PubMed] [Google Scholar]
- 25. Mascaró C, Ortega A, Carceller E, et al. . Chemoprobe-based assays of histone lysine demethylase 1A target occupation enable in vivo pharmacokinetics and pharmacodynamics studies of KDM1A inhibitors. J Biol Chem. 2019;294(20):8311–8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bensaid D, Blondy T, Deshayes S, et al. . Assessment of new HDAC inhibitors for immunotherapy of malignant pleural mesothelioma. Clin Epigenetics. 2018;10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang C, Burger MC, Jennewein L, et al. . ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst. 2016;108(5):djv375. [DOI] [PubMed] [Google Scholar]
- 28. Somanchi SS, Kennis BA, Gopalakrishnan V, Lee DA, Bankson JA. In vivo (19)F-magnetic resonance imaging of adoptively transferred nk cells. Methods Mol Biol. 2016;1441:317–332. [DOI] [PubMed] [Google Scholar]
- 29. Ciurea SO, Schafer JR, Bassett R, et al. . Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130(16):1857–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheng W, LaFleur MW, Nguyen TH, et al. . LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell. 2018;174(3):549–563.e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qin Y, Vasilatos SN, Chen L, et al. . Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene. 2019;38(3):390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lieberman NAP, DeGolier K, Kovar HM, et al. . Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol. 2019;21(1):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haberthur K, Brennan K, Hoglund V, et al. . NKG2D ligand expression in pediatric brain tumors. Cancer Biol Ther. 2016;17(12):1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mackay A, Burford A, Molinari V, et al. . Molecular, pathological, radiological, and immune profiling of non-brainstem pediatric high-grade glioma from the HERBY phase II randomized trial. Cancer Cell. 2018;33(5):829–842.e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee C, Rudneva VA, Erkek S, et al. . Lsd1 as a therapeutic target in Gfi1-activated medulloblastoma. Nat Commun. 2019;10(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saito S, Kikuchi J, Koyama D, et al. . Eradication of central nervous system leukemia of T-cell origin with a brain-permeable LSD1 inhibitor. Clin Cancer Res. 2019;25(5):1601–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsuda S, Baba R, Oki H, et al. . T-448, a specific inhibitor of LSD1 enzyme activity, improves learning function without causing thrombocytopenia in mice. Neuropsychopharmacology. 2019;44(8):1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haspels HN, Rahman MA, Joseph JV, Gras Navarro A, Chekenya M. Glioblastoma stem-like cells are more susceptible than differentiated cells to natural killer cell lysis mediated through killer immunoglobulin-like receptors-human leukocyte antigen ligand mismatch and activation receptor-ligand interactions. Front Immunol. 2018;9:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O’Rourke DM, Nasrallah MP, Desai A, et al. . A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khatua S, Sandberg D, Ketonen L, et al. . Ept-02phase I study of fourth ventricle infusions of autologous Ex-Vivo expanded Nk cells in children with recurrent/refractory malignant posterior fossa tumors of the central nervous system. Neuro Oncol. 2016;18(suppl_3):iii24. [Google Scholar]
- 41. Wei X, Hartley R, Bear H, Fuller C, Phoenix TN. Hgg-13. Determining regional differences in high-grade glioma vasculature phenotype. Neuro Oncol. 2019;21(Supplement_2):ii89. [Google Scholar]
- 42. Wagner J, Kline CL, Zhou L, et al. . Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. J Clin Invest. 2018;128(6):2325–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chi AS, Tarapore RS, Hall MD, et al. . Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol. 2019;145(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tejada S, Díez-Valle R, Domínguez PD, et al. . DNX-2401, an oncolytic virus, for the treatment of newly diagnosed diffuse intrinsic pontine gliomas: a case report. Front Oncol. 2018;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim Y, Yoo JY, Lee TJ, et al. . Complex role of NK cells in regulation of oncolytic virus-bortezomib therapy. Proc Natl Acad Sci U S A. 2018;115(19):4927–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in Glioblastoma. Cancer Res. 2018;78(4):1031–1043. [DOI] [PubMed] [Google Scholar]
- 47. Suryadevara CM, Desai R, Abel ML, et al. . Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology. 2018;7(6): e1434464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pellegatta S, Eoli M, Cuccarini V, et al. . Survival gain in glioblastoma patients treated with dendritic cell immunotherapy is associated with increased NK but not CD8+ T cell activation in the presence of adjuvant temozolomide. Oncoimmunology. 2018;7(4):e1412901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.