Abstract
Introduction:
Pharmacotherapy is a useful adjunct when patients with obesity are unable to achieve adequate benefit from lifestyle interventions.
Areas covered:
This review covers the history of antiobesity drugs, efficacy and risks of currently approved drugs, limits of their usefulness in clinical practice, gaps in knowledge, methodological limitations of clinical trials, and reasons for underutilization.
Expert Opinion:
In randomized controlled trials, currently approved antiobesity drugs have yielded an average weight loss ranging from approximately 3% to 9% relative to placebo at 1 year. Inadequate inclusion of racial and ethnic minorities and men, and high dropout rates in clinical trials limit generalizability of these findings to clinical practice. Weight loss achieved with antiobesity drugs is generally associated with lowered glycemia, but improvements in blood pressure and lipid measures tend to be marginal. There is limited evidence for sustained weight loss beyond 1 year and for safety and efficacy of antiobesity drugs in children and adolescents, and in post-bariatric surgery patients. None have demonstrated reduction in major adverse cardiovascular events or other significant disease outcomes. Limited health insurance coverage and negative perceptions of physicians have hindered the utilization of antiobesity drugs.
Keywords: obesity, antiobesity drugs, weight loss, pharmacotherapy, phentermine, topiramate, lorcaserin, liraglutide, bupropion, naltrexone
1. Introduction
Obesity remains a highly prevalent major health concern globally, accounting for numerous chronic diseases including type 2 diabetes (T2D), hypertension (HTN), dyslipidemia, cardiovascular disease (CVD), nonalcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH), and obstructive sleep apnea, leading to impaired quality of life and increased mortality [1, 2]. Weight loss of 5–10% is associated with prevention and amelioration of many obesity-related comorbidities, but long-term benefits are contingent upon sustainability of the initial weight loss [2, 3]. Intensive lifestyle interventions that combine diet, exercise, and behavior therapy are generally effective in the short-term, but not all patients lose weight, and the majority of patients who achieve initial success are unable to maintain the weight loss during long-term follow-up, even with continued intervention [4, 5]. Since obesity can result from a myriad of causes including genetic, epigenetic, physiological, medical, psychological, and environmental factors, not all patients benefit from any given therapeutic modality. Furthermore, patients who are severely obese and have made numerous prior unsuccessful weight loss attempts with dieting tend to believe that they have a biological illness and may show preference for medical or surgical interventions [6]. Currently, bariatric surgery is the most effective treatment for patients with severe obesity to achieve substantial long-term weight loss [7]. However, bariatric surgery is expensive, irreversible in most cases, carries a small risk of serious complications, and thus is recommended only for patients with severe and complicated obesity. Therefore, for patients with obesity who have not achieved adequate benefit from lifestyle interventions, are unable to maintain the initial weight loss, and/or have other medical conditions that make it difficult for them to comply with lifestyle interventions, pharmacotherapy is the next logical step in clinical care before considering bariatric surgery as the tertiary option [8].
2. History of antiobesity drugs
In 1947, the US Food and Drug Administration (FDA) approved two branded products of desoxyephedrine (Desoxyn, Hydrin), an amphetamine, as “adjuncts to the dietary management of obesity” based on an article that reported no blood pressure (BP) elevations or evidence of addiction among 110 obese patients treated with the drug [9]. Between 1956–1960, five amphetamine congeners were also approved [Table 1] based on safety evaluation only. In 1970, the FDA required that the manufacturers of these already marketed drugs conduct well-controlled studies and submit evidence of efficacy within a year. This ‘Amphetamine Anorectic Drug Project’ involved clinical trials that ranged in duration from 3 weeks to 6 months, mostly around 12 weeks. In the mid-70s, lacking expert consensus on what constituted clinically significant weight loss and how to determine benefit-to-risk balance, the FDA reaffirmed the approval of the previously approved amphetamines and amphetamine congeners based on a crude meta-analysis that revealed “trivial”, but statistically superior weight loss relative to placebo. However, lacking adequate data on addiction potential, the FDA arbitrarily restricted their use for short-term (a few weeks) [9]. This restriction led to a fall in the use of anorectic drugs until publication of a single placebo-controlled study of 121 obese patients (less than one-third completed the study) treated with phentermine plus fenfluramine (Phen-Fen) for up to 4 years [10] led to a steep rise in their prescriptions in the mid-1990s [11].
Table 1.
History of antiobesity drug approvals in the United States
| Year approved | Generic name (Brand names) |
|---|---|
| 1947 | Desoxyephedrine (Desoxyn, Hydrin)* |
| 1956 | Phenmetrazine (Preludin)* |
| 1959 | Phentermine (Ionamin*) |
| 1959 | Diethylpropion (Tenuate) |
| 1959 | Phendimetrazine (Bontril, Plegine) |
| 1960 | Benzphetamine (Didrex) |
| 1973 | Mazindol (Sanorex)* |
| 1973 | Fenfluramine (Pondimin)* |
| 1973 | Chlorphentermine (Pre-Sate)* |
| 1996 | Dexfenfluramine (Redux)* |
| 1997 | Sibutramine (Meridia)* |
| 1999 | Orlistat (Xenical) |
| 2012 | Phentermine/topiramate (Qsymia) |
| 2012 | Lorcaserin (Belviq)* |
| 2014 | Liraglutide 3.0 mg (Saxenda) |
| 2014 | Naltrexone/bupropion (Contrave) |
When they are several brand names, only the originally approved name is shown. For phentermine, Ionamin, a resin product, is no longer marketed, but the drug is available under different brand names such as Adipex-P, Suprenza, and Lomaira.
Withdrawn due to risks or poor sales. Some of the drugs withdrawn in the US continue to be available in other countries.
Not listed above: Aminorex was approved in Germany, Switzerland, and Austria in 1965, but was withdrawn in 1972. Rimonabant was approved in the European Union and a few other countries in 2006, but was removed from markets in 2008.
In 1995, the FDA held an advisory committee meeting which led to the recognition that obesity is a chronic disease and hence short-term treatment would not confer long-term benefits [12]. This view was reflected in the first FDA guidance document for development of antiobesity drugs in 1996, which required demonstration of weight loss efficacy over a minimum duration of 1 year [13]. In 1996, dexfenfluramine became the first antiobesity drug approved for long-term use. Just over a year later, it was removed from the market, along with fenfluramine which was available since 1973, after data emerged that linked use of these two drugs with cardiac valvulopathy [14].
In 2007, the FDA issued an updated guidance which also recommended that new antiobesity drugs be tested in a dedicated phase 3 trial of overweight/obese patients with T2D [15]. Between 1997 and 1999, sibutramine and orlistat were approved for long-term obesity treatment. In 2006, rimonabant, a cannabinoid receptor-1 (CB-1) antagonist, failed to win FDA advisory committee recommendation due to high incidence of psychiatric adverse events [16], although it was already approved by the European Medicines Agency (EMA). In 2008, rimonabant was withdrawn from Europe and other markets as more reports confirmed the increased risk of depression and suicidal ideation with the drug [17]. Sibutramine was withdrawn in the US and several countries in 2010 following review of the results of a cardiovascular outcomes trial (CVOT) among patients with pre-existing CVD or those at high risk. The results of this trial revealed a slightly increased risk of major adverse cardiovascular events (MACE) in the drug group compared with the placebo group (11.4% vs 10.0%; hazard ratio, 1.16; 95% CI, 10.3–1.31; P=0.02), corresponding to approximately 4 excess events per 1,000 patient years [18]. Nevertheless, sibutramine remains legally marketed in a few countries, e, g., Russia [19]. In 2010, three new antiobesity drugs – phentermine plus topiramate, lorcaserin, and naltrexone plus bupropion – failed to win FDA approval, but were subsequently approved between 2012 and 2014 along with liraglutide 3.0 mg (higher than the 1.8 mg dose approved for T2D).
3. Currently approved antiobesity drugs
3.1. Drugs approved for short-term weight loss
Four amphetamine congener drugs – phentermine, diethylpropion, phendimetrazine, and benzphetamine - continue to be marketed in the US for short-term treatment of obesity. Mazindol, another drug in this class was discontinued in the US, but remains available in other markets, such as, Japan and Mexico.
3.2. Phentermine
Despite the availability of 5 drugs approved for long-term weight management, phentermine remains highly popular in the US, accounting for 3 of 4 prescriptions among all antiobesity drugs, and although approved for short-term use only, is commonly prescribed for longer durations [20–22]. In a 28-week randomized controlled trial (RCT), phentermine 15 mg (half of the maximum approved dose) group (n=108) achieved an average weight loss of 6.1% compared to 1.7% in the placebo group (n=109) [23]. In the absence of RCTs of at least 1-year duration, it remains unknown whether phentermine-induced weight loss is durable in the long-term. In a 2004 presentation to the FDA scientific advisory committee, late Dr. David Orloff, then Director of the FDA Division of Metabolic and Endocrine Drug Products, stated that lack of long-term studies of phentermine represented a “therapeutic gap” [24]. Sixteen years later, this therapeutic gap has grown bigger, considering that phentermine accounts for approximately 80% of all weight loss drug prescriptions in the recent years. A recent publication [25] that analyzed electronic health record (EHR) data reported that patients who were prescribed phentermine were found to have lost clinically significant weight, but the study had numerous serious limitations, as is the case with EHR-based observational studies. Of the 13,972 patients who had their first prescription filled, only 98 continued phentermine for 2 years and 68% had weight data missing [26]. Most likely, only those who benefitted from phentermine had chosen to continue, thus leaving a bias of overestimation of efficacy. Data pertaining to use of other weight loss medications was not included in the analysis. The control group was short-term phentermine users, rather than a group not taking phentermine. While acknowledging the methodological limitations, the authors concluded that “a clinical trial with regular standardized outcome assessments at predetermined intervals would be important.”
Phentermine is generally well tolerated with the most common adverse effects being insomnia, dry mouth, and constipation [27]. It may be associated with increases in heart rate and blood pressure. FDA-approved phentermine prescribing information reads: “use caution in patients with even mild hypertension (risk of increase in blood pressure).” However, this language was not based on evidence from well-controlled trials. In one RCT, average weight loss of 6.1% with phentermine at 28 weeks was associated with systolic BP (SBP) and diastolic BP (DBP) decreases of 3.5 mmHg and 0.9 mmHg, respectively [23]. The highest dose used in this study was 15 mg, whereas the most commonly prescribed dose in clinical practice is 37.5 mg (or 30 mg base). No published RCTs have examined ambulatory blood pressure (ABP) measurements, which are a stronger predictor of all-cause and cardiovascular mortality than clinic measurements [28]. No data exist with regard to the effects of phentermine on daytime, nighttime, and 24-hour ABP, and whether the effects are dose-dependent, especially at the beginning of treatment when increases are more likely to occur. Such data would be useful to guide physicians on the dose- and time-dependent effects of phentermine on BP, and whether any safety monitoring should be included in the care of patients newly started on phentermine.
3.3. Antiobesity drugs approved for long-term use
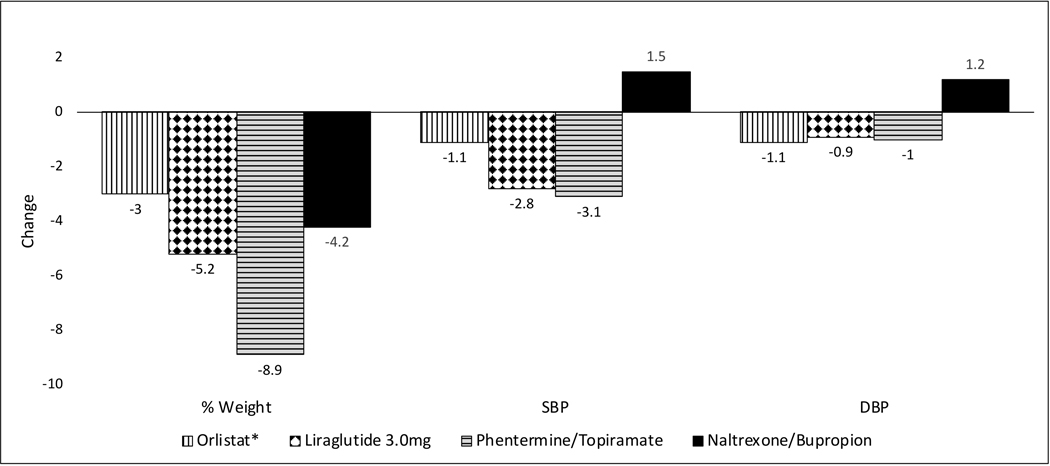
Four drug therapies – orlistat, liraglutide, phentermine/topiramate, and naltrexone/bupropion - are currently approved in the US for long-term weight management in patients with a body mass index (BMI) of ≥30 kg/m2 or those with a BMI of at least 27 kg/m2 in the presence of obesity-related diseases or risk factors as an add-on to lifestyle modification. Compared to lifestyle intervention alone, the addition of these drugs can enhance average weight loss by 3% to 9% over one year [{29}Figure 1]. Among these, only phentermine/topiramate combination and liraglutide 3.0 mg have met both of the FDA benchmark criteria, the first being 5% placebo-subtracted weight loss and the second being a greater proportion of patients achieving at least 5% weight loss [8]. The others fulfilled only the less-stringent second criterion using categorical data.
Figure 1. Changes in weight (%) and BP (mmHg) relative to placebo at 1 year.
*Orlistat associated weight change is based on various meta-analyses and systematic reviews that included different RCTs of various durations. Orlistat-associated change in blood pressure is based on a recent meta-analysis [99]. For all other drugs, the results are based on pooled data of phase 3 RCTs in patients with obesity without diabetes as drawn from the FDA advisory committee briefing documents. When more than one dose was studied, results shown are the ones for the most effective dose. All data are from ITT analyses. [100].
Lorcaserin, approved in the U.S in 2012, was withdrawn in February 2020 based on the finding that 7.7% of the subjects in the lorcaserin group were diagnosed with cancer compared to 7.1% of those in the placebo group in a 5-year RCT of 12,000 patients [30]. The FDA noted that pancreatic, colorectal, and lung cancers occurred more frequently in the lorcaserin group.
Table 2 lists the most frequent adverse events (AEs) and related dropout rates in phase 3 RCTs of 1-year duration. The percentage of patients discontinuing drug due to AEs was the highest for naltrexone/bupropion (24.0%) [31].
Table 2.
Most frequent AEs and AE-related dropout rates
| Drug | Adverse events (AEs) | AE-related dropout rate (%) | |
|---|---|---|---|
| Drug | Placebo | ||
| Orlistat* | Fecal urgency, fecal incontinence, flatus with discharge, oily spotting | 8.2 | 4.8 |
| Liraglutide | Nausea, vomiting, diarrhea | 9.8 | 4.3 |
| Phentermine/topiramate | Paresthesia, insomnia, dizziness | 17.5 | 8.5 |
| Naltrexone/bupropion | Nausea, vomiting, dizziness, headache | 24.0 | 11.9 |
3.4. Effects of antiobesity drugs on glycemia, blood pressure, lipids, and NASH
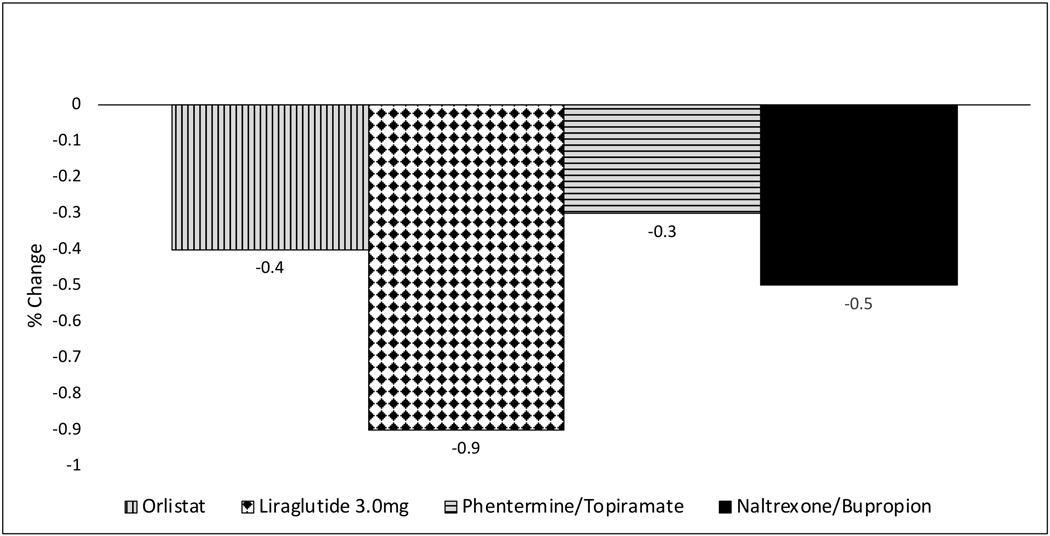
The prevalence rates of T2D, HTN, and dyslipidemia tend to rise linearly with increasing BMI and are very high among individuals with obesity [32]. In clinical practice, the primary purpose in prescribing therapeutic weight loss interventions is to prevent or ameliorate the burden of these weight-related chronic diseases. In patients with T2D, for each kg of mean weight loss, hemoglobin A1c (HbA1c) is expected to decrease by approximately 0.1 percentage points [33]. Thus, 5% weight loss in a 100 kg individual with T2D is expected to reduce HbA1c by 0.5 percentage points. All currently marketed drugs for long-term weight management have demonstrated significant improvement of glycemic control in 1-year RCTs among adults with overweight/obesity and T2D [Figure 2].
Figure 2. Change in HbA1C at 1 year in patients with overweight/obesity and T2D.
Shown is placebo-subtracted HbA1C change in percentage points. Data are from ITT analyses in dedicated RCTs in patients with overweight/obesity and type 2 diabetes (T2D). No dedicated phase 3 RCT was conducted for phentermine/topiramate in T2D patients; the data shown represent the subgroup of patients with T2D in the CONQUER trial [86].
Antiobesity drug therapies have not been consistently associated with clinically significant BP reductions [Figure 1]. Orlistat has been well studied in patients with overweight/obesity and HTN in 4 RCTs with average placebo-adjusted SBP and DBP reductions of 2.5 mmHg and 1.9 mmHg, respectively [34]. These reductions were less pronounced when RCTs involving all subjects regardless of their hypertensive status were included in the meta-analysis. Other antiobesity drugs have not been tested in RCTs dedicated to patients with hypertension. Phentermine/topiramate and liraglutide were associated with significant reductions in BP. In contrast to all other approved antiobesity drugs, naltrexone/bupropion, despite inducing greater weight loss than placebo, was associated with significant increases in placebo-adjusted SBP and DBP at 1 year. The BP elevations were more pronounced at 2 months after initiation of treatment. An ambulatory blood pressure monitoring (ABPM) substudy revealed placebo-adjusted 24-hour average SBP and DBP increases of 2.9 mmHg and 3.0 mmHg, respectively, with naltrexone/bupropion causing concern to the FDA to require a CVOT prior to approval of the drug [35]. While approval was granted on the basis of an interim analysis, the required CVOT was never completed. After one CVOT was prematurely terminated [36] due to inappropriate disclosure of data by the manufacturer, the FDA stated that the company must conduct another CVOT [37], which was started and abandoned again [38].
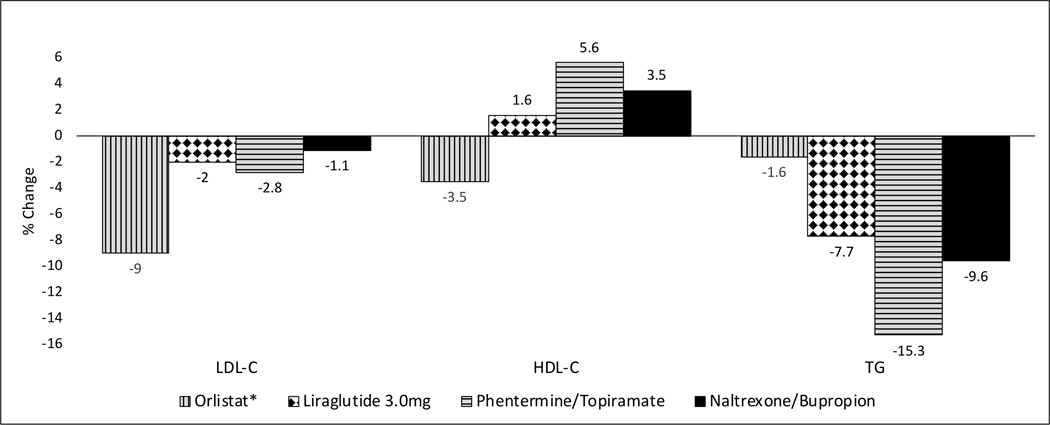
Weight loss achieved through antiobesity drugs has generally resulted in clinically significant reduction in serum triglycerides, small and clinically insignificant reduction in low-density lipoprotein cholesterol (LDL-C), and small increase in high-density lipoprotein cholesterol (HDL-C) [Figure 3]. Orlistat is an exception in that it is associated with significant reduction in LDL-C, lowered HDL-C and minimal change in triglycerides.
Figure 3. Percent changes in serum lipids relative to placebo at 1 year.
*Orlistat-associated changes in lipids are based on 1-year data shown in prescribing information [83]. For all other drugs, the results are based on ITT analyses of pooled data of phase 3 RCTs in patients with obesity without diabetes as drawn from the FDA advisory committee briefing documents. When pooled data are not available, data from the largest study are shown. When more than one dose was studied, results shown are the ones for the most effective dose.
In a 48-week RCT in overweight/obese patients with NASH, 9 of 23 patients who received liraglutide and 2 of 22 patients given placebo demonstrated resolution of NASH based on repeated biopsy [39]. There are no published RCTs of histopathologic improvement of NASH with other antiobesity drugs.
None of the currently approved antiobesity drugs have yet been demonstrated to be effective for primary prevention of CVD or in reducing MACE or mortality among patients with obesity. Liraglutide 1.8 mg has been shown to reduce MACE, but that was in patients with T2D and high CVD risk [40]. As noted earlier, the FDA stipulated a CVOT requirement for naltrexone/bupropion, but this has not been completed yet. At the time of phentermine/topiramate approval in 2012, the FDA mandated that a post-approval CVOT be conducted and final report submitted to the FDA by December 2018 [41]. To date, no phentermine/topiramate CVOT has been registered.
3.5. Pros and cons of fixed-dose combination drugs
Drugs with different mechanisms of action, when used in combination, could yield greater efficacy via additive or synergistic effects, and possibly via overcoming the human body’s natural homeostatic mechanisms that defend body weight [42]. In a 24-week RCT, phentermine/topiramate 15/100 mg led to an average weight loss of 10.7% compared with 4.6% with phentermine 15 mg and 6.3% with topiramate 100 mg [43]. In this study, which used commercially available drugs, phentermine was dosed in the morning and topiramate in the evening. In a subsequent 28-week RCT in which a fixed-dose combination once-daily product was used, phentermine/topiramate 15/92 mg resulted in an average weight loss of 9.2% compared with 6.1% with phentermine 15 mg and 6.4% with topiramate 92 mg [23].
In a 16-week RCT, naltrexone/bupropion 50/300 mg failed (4.0% vs 3.6%) to achieve greater weight loss than bupropion 300 mg alone [44]. In a subsequent 24-week RCT, naltrexone/bupropion 32/400 mg led to an average weight loss of 5.4% compared to 2.7% with bupropion monotherapy and 1.2% with naltrexone monotherapy. The higher-dose combination therapy (naltrexone 48 mg/bupropion 360 mg) was not superior to bupropion alone with 63% dropout rate [45]. The average placebo-subtracted weight loss of 1.9% with bupropion 400 mg was much lower than the placebo-subtracted difference of 3.7% and 2.7% demonstrated with the drug in two prior RCTs of similar duration in adults with obesity [46, 47]. In an End-of-Phase 2 meeting with the sponsor in 2007, the FDA raised concern that the results of this study were not sufficient to satisfy the requirements for a fixed-dose combination product, but subsequently accepted the data in support of efficacy based on a mechanistic explanation of possible synergy [35].
Combination drugs for obesity have generally been associated with worse tolerability than monotherapies. A major disadvantage of fixed-dose combination drug products is that the doses of all drug components in the combination are increased or decreased when having to change dose rather than having the flexibility of changing the doses of the individual components based on tolerability [48]. In clinical practice, physicians generally begin treatment with a single drug, and then increase the dose or add another drug, or switch to another drug based on assessment of therapeutic response and tolerability rather than starting with a fixed-dose combination.
4. Therapeutic gaps
4.1. Very few treatment options for pediatric obesity
In combination with lifestyle interventions, pharmacotherapy is considered appropriate for adolescents with BMI at ≥95th percentile for age and sex with at least one obesity-related comorbidity [15]. Some have recommended that pharmacotherapy is also appropriate for adolescents with BMI ≥120% of 95th percentile with or without comorbidities [49]. The FDA recommends that a drug’s safety be first examined in adults, and a pharmacokinetic (PK) study be conducted in pediatric subjects before embarking on larger long-term trials [15]. Because linear growth over time needs to be taken into account while assessing change in body weight in pediatric subjects, the primary efficacy measure in the pivotal trials should be change in BMI rather than change in weight. Currently, orlistat is the only approved drug for long-term weight management in adolescents (≥12 years). In a 1-year RCT, orlistat decreased BMI by 0.55 kg/m2 whereas BMI increased by 0.31 kg/m2 with placebo, a net difference of 0.86 kg/m2 relative to placebo [50]. The currently available amphetamine congeners were first approved before 1960, at which time minimal data were collected for pediatric patients. Prior to 1979, the FDA did not have any specific labeling regulations or rule; thus, there was no definition of pediatric patients for the purpose of labeling. Current FDA labeling regulations define adult patients as those 17 years or older. Therefore, drugs approved for adults are approved for patients who are 17 years and older (FDA personal communication with the corresponding author, 17 Jan 2020). In an 8-week RCT of phentermine/topiramate among 42 adolescents with obesity, average weight changes in the placebo, 7.5/46 mg, and 15/92 mg groups were 1.1%, −3.8%, and −5.0% [51]. However, SBP decreased more with placebo than with either of the phentermine/topiramate doses, and DBP (mmHg) increased with both doses of the drug (placebo-adjusted DBP changes of +5.7 and +4.3). A phase 4 RCT of 1-year duration among 200 obese adolescents is currently underway with an estimated completion in September 2020 [52]. In a recently reported RCT among 251 adolescents [53], treatment with liraglutide 3.0 mg daily s.c. injections led to placebo-adjusted reduction of 0.22 in BMI standard-deviation score (SDS) after 56 weeks; however, 10.4% of subjects discontinued liraglutide due to AEs vs none in the placebo group, and 26 weeks after treatment discontinuation, there was significantly greater increase in BMI SDS in the liraglutide group than in the placebo group (0.22 vs 0.07). There are no published clinical trials of naltrexone/bupropion in adolescents and none are registered to date.
4.2. Medication-induced weight gain
Several commonly used medications have been documented to be associated with weight gain as an adverse effect. Most notable are psychotropic medications, several of which have been implicated in inducing significant weight gain and new-onset T2D. The 2007 FDA guidance defined significant medication-induced weight gain as increase of at least 5% body weight within 6 months after starting a medication known to cause weight gain [15]. Since most antiobesity drugs are also centrally-acting, it is critical that these drugs do not adversely affect the efficacy or safety of the medication causing the weight gain. No antiobesity drug has yet been approved specifically to treat medication-induced weight gain.
4.3. Suboptimal weight loss and weight regain after bariatric surgery
While it is widely acknowledged that bariatric surgery is superior to non-surgical weight loss interventions, there is a growing recognition that a large proportion of patients achieve suboptimal weight loss, reach a weight loss plateau sooner than predicted, or regain significant weight after initial weight loss [54–59]. Lifestyle interventions have demonstrated marginal or no benefit in promoting additional weight loss among patients with suboptimal weight loss, and in attenuating the regained weight [60–63]. During our review of the literature on the use of pharmacotherapy to treat suboptimal weight loss or weight regain after bariatric surgery, we found 11 published reports, of which 7 were retrospective chart reviews [64, 58, 65–69], two were open-label studies [70, 71], and two were publications reporting data for younger and older patients from a previously published paper [72, 73]. To date, there are no published RCTs of drug therapies to treat suboptimal weight loss or weight regain following bariatric surgery. Given the increasing utilization of bariatric surgery, rigorously tested pharmacotherapy could be a valuable tool in enhancing long-term outcomes for patients after bariatric surgery.
5. Methodological deficiencies in clinical trials of antiobesity drugs
Dropout rates in phase 3 trials of approved antiobesity drugs were in the range of 40–50% over 1 year [74–76]. In a trial of naltrexone/bupropion or placebo in combination with intensive lifestyle intervention, 42% dropped out in the lifestyle intervention plus placebo group, with 12% citing their early withdrawal to an adverse event [77]. High attrition introduces a serious bias and the results can be uninterpretable regardless of how sophisticated the imputation procedures are, when primary outcome data are missing for half of the study participants [78, 79]. The FDA encourages companies developing antiobesity drugs to make their best efforts to retain subjects in clinical trials, and also recommends that for subjects who withdraw early, the primary outcome measure (body weight) be collected near the calendar date at which the subject is scheduled to complete the trial [15]. This could easily be accomplished by explaining to the study participants that if they withdrew early, they would be requested to return for their final visit assessments, which would be valuable for the study’s success, as demonstrated in a 1-year RCT of pharmacotherapy in patients with obesity [80]. While the FDA has not specified maximum tolerated dropout rate in trials included in new drug applications, this should be a consideration in future.
The majority of participants in phase 3 trials of antiobesity drugs have been white women. Although the prevalence of obesity is slightly higher among women, men carry a higher risk of CVD, which could be related to their propensity to accumulate visceral fat [81, 82]. Many racial and ethnic minorities have a higher prevalence of obesity and carry greater susceptibility for T2D, CVD, and other cardiometabolic illnesses. Thus, the inadequate representation of men and ethnic and racial minorities compromises the generalizability of the findings as well as translation to clinical practice.
Long-term efficacy and safety data for antiobesity drugs are inadequate. Orlistat is an exception with several 2-year RCTs [83] and a 4-year study [84]. Liraglutide has been studied for up to 3 years [85] in one RCT. Naltrexone/bupropion has not been studied beyond 1 year for weight loss as the primary outcome. However, in a CVOT that was terminated early, only 27% were still taking naltrexone/bupropion at 2 years and placebo-subtracted weight loss was only 2.5% [36]. At the end of the largest phase 3 trial [86] of phentermine/topiramate, a second-year extension was offered for patients who completed 1 year while taking the study drug at selected sites only [87]. Consequently, only a total of 27% of the originally randomized cohort continued in the second year. Due to significant selection bias, 2-year results of this trial cannot be compared to those of orlistat, and liraglutide.
6. Challenges faced by antiobesity drugs
6.1. Difficulties in securing marketing authorization, and marketing restrictions
Although phentermine/topiramate is FDA approved, its marketing authorization application (MAA) was rejected by EMA in October 2012 and in February 2013 [88]. Although naltrexone/bupropion received EMA approval in 2015, National Institute for Health and Care Excellence (NICE) recommended against its coverage by the National Health Service (NHS) in the UK [89]. Use of phentermine/topiramate in the US is complicated by a risk evaluation and mitigation strategy (REMS) requiring clinician and patient education and the requirement for a negative pregnancy test prior to starting treatment and monthly thereafter to minimize the risk of potential teratogenicity associated with topiramate [90].
6.2. Underutilization
A recent report by the US Government Accountability Office (GAO) found that among the approximate 71.6 million U.S. adults with obesity, antiobesity drug utilization is quite low [Table 3] at an estimated 660,000 people per year [22]. Among those who reported trying to lose weight from 2013–2016, only about 3% reported using prescription medication for weight loss [22]. One study analyzing the use of antiobesity drugs in the Veterans Health Administration’s MOVE! Weight Management Program from 2013 through 2016 found that only 1% of veterans enrolled in the program were prescribed an antiobesity drug [91]. When patients are prescribed antiobesity drugs, adherence seems to drop after the first month of treatment [92]. In the FDA’s analysis of Sentinel System data of antiobesity drugs dispensed in 2008 through 2017, it was identified that 58% of patients who used these drugs did so for 90 days or less with an average duration of 69 days [22]. The number of prescriptions for antidiabetes drugs is about 15 times the number for antiobesity drugs [21].
Table 3.
Barriers to utilization of antiobesity drugs
| Health Care Providers |
| • Negative perceptions of safety and efficacy |
| • Negative USPSTF clinical recommendations |
| • Desire to use least invasive options first (i.e. behavioral interventions) |
| • Patient costs and ability to afford the drugs |
| • Gaps in knowledge and limited experience |
| Patients |
| • Lack of insurance coverage |
| • Out of pocket costs |
| • Potential for adverse effects |
| • Unrealistic expectations for efficacy and rapidity of benefit |
Among various reasons for underutilization of antiobesity drugs, GAO analysis report found that physicians and health care providers may not feel comfortable prescribing obesity drugs due to their perception of the drugs not being safe or effective. Some of this concern may be related to the removal of fenfluramine and dexfenfluramine from the market in 1997 following reports of their association with heart valve abnormalities [22]. Another reason for the reluctance of health care providers to prescribe antiobesity drugs is an influential U.S. Preventive Services Task Force (USPSTF) report which concluded that antiobesity drugs, but not behavior-based interventions, were associated with higher rates of harm [93]. It was also noted in the GAO report that physicians may have gaps in knowledge about obesity drugs, including lack of knowledge that FDA-approved drugs for obesity are available. In a study of 111 primary care providers, many identified limited experience with obesity drugs as a barrier to prescribing them [94]. Lack of insurance coverage, high out-of-pocket costs, and patients’ inability to afford antiobesity drugs are other factors that contribute to the reluctance of health care providers to prescribe antiobesity drugs [22].
In the GAO analysis, it was discovered that insurance coverage for obesity drugs is limited. Some prescription drug plans may choose to cover antiobesity drugs, however they are often considered supplemental drugs under an alternative coverage plan which requires patients to pay full premium cost for those additional benefits. Many plans cover supplemental drugs based on consumer demand and obesity drugs typically do not meet the threshold for offering coverage [22]. Coverage for obesity drugs in the US varies by each private health insurance plan and by each state. One large insurer reported that about 90% of members had coverage for obesity drugs, whereas another large insurer reported that 4 out of 6 employer-sponsored plans and 3 out of 6 individually purchased plans covered obesity drugs [22]. Even when coverage is offered by insurers, there are often requirements to determine eligibility including prior authorization, determination of medical necessity, and a review of the drug’s effectiveness. Further, when coverage is offered but a patient fails to achieve clinical benefit within a certain time frame, plans may require re-approval of coverage every 6 to 12 months and a prior authorization for continuing coverage [22].
7. Conclusion
Antiobesity drugs currently approved for long-term weight management, when added to lifestyle intervention, are more effective than lifestyle intervention alone, yielding an additional 3% to 9% weight loss over 1 year. In RCTs, weight loss achieved with these drugs is associated with significant improvements in glycemic measures and small improvements in blood pressure and lipids. There is limited evidence for sustained weight loss beyond 1 year and for safety and efficacy of antiobesity drugs in children and adolescents, and in post-bariatric surgery patients. None have demonstrated reduction in major adverse cardiovascular events or other significant disease outcomes. Limited health insurance coverage, challenges with long term adherence, perceptions of risk/benefit ratio, and comfort level of physicians have hindered the utilization of antiobesity drugs.
8. Expert opinion
In the primary care setting, if implemented skillfully, antiobesity medications can provide significant benefit above and beyond behavioral weight loss interventions [95]. Antiobesity drugs assist patients with obesity to reduce their energy intake and thereby promote greater compliance with lifestyle modification plans.
Although it is well recognized that weight loss among overweight/obese patients is critical for prevention of T2D and for improving outcomes among those with established T2D, few clinicians seem to give importance to weight management. Prescriptions for antidiabetes medications have soared in the recent years, but physicians remain reluctant to prescribe medications to assist their patients in losing weight.
The quality of RCTs of antiobesity drugs needs a major facelift to bring them on par with clinical trials in other major chronic diseases. The majority of participants in clinical trials of antiobesity drugs are women. Improved enrollment of men and minorities can enhance the generalizability of the clinical trial findings. High attrition rates could be reduced by improving the engagement of study participants. It is important to demonstrate that antiobesity drugs can significantly ameliorate weight-related comorbidities to convince physicians of their utility in clinical practice. Trials dedicated to overweight/obese patients with hypertension and dyslipidemia can add value in gauging the clinically meaningful efficacy of these drugs beyond weight loss. It is also important to generate the type of data that provide the demonstration that antiobesity drugs can modify comorbid chronic diseases and thereby save future costs. More research is needed to demonstrate the efficacy and safety of antiobesity drugs in adolescents and in patients who have regained weight after initial weight loss following bariatric surgery.
Regulatory agencies have been inconsistent in evaluating new antiobesity drugs. They seem to hold the antiobesity drugs to higher standards that are difficult to meet, especially in the context of poor financial returns from marketing these drugs. Risk evaluations for the same drug by different divisions at the FDA’s Center for Drug Evaluation and Research can vary. For example, the Division of Neurology has not mandated REMS to mitigate teratogenic risk with topiramate when it is used to prevent migraine, but the Division of Metabolism and Endocrinology Products (DMEP) has required REMS for using phentermine/topiramate to treat obesity, although the topiramate dose typically used is the same (100 mg vs 92 mg) for both indications. The argument that has been made to justify this difference in regulatory policy that antiobesity drugs are most frequently used by women during their reproductive years is rather weak because migraine is 2–3 times more frequent among women aged 18–44 years [96], hence the risk for teratogenicity in the latter case is no less. Despite reduction in BP, an average increase in heart rate of <2 bpm with phentermine/topiramate has concerned the DMEP to require a CVOT. However, the Division of Psychiatry has approved lisdexamfetamine for treatment of binge eating disorder (BED) in adults without requiring a CVOT although the drug is associated with increases in both BP (2–4 mmHg) and heart rate (3–6 bpm) [97]. Because patients seeking treatment for BED commonly present with overweight and obesity [98], risks related to increases in heart rate and BP would not be less with lisdexamfetamine use by BED patients than with phentermine use by patients with obesity. The DMEP advisory committees that reviewed lorcaserin, phentermine/topiramate, and naltrexone/bupropion did not have adequate representation of panelists with expertise and clinical knowledge in obesity medicine. It was unclear whether the panelists had the understanding that 5–10% weight loss can confer significant health benefits. Finally, the two major regulatory agencies – FDA and EMA – must try to implement consistent policies for approval of drugs to treat obesity.
Article highlights.
1-year treatment with currently available antiobesity drugs leads to 3% to 9% average weight loss above and beyond lifestyle intervention
Weight loss achieved with antiobesity drugs leads to improved glycemia
Improvements in BP and lipid profile are less consistent and small.
Naltrexone/bupropion is associated with adverse effects on BP
No antiobesity drug is yet to have a demonstration of reduction in MACE
Barring orlistat, minimal knowledge of efficacy and safety in adolescents
No RCTs to treat suboptimal weight loss or weight regain after bariatric surgery
There is currently a cumbersome and inconsistent regulatory approval process in addition to post-approval restrictions. There are also numerous barriers to utilization
This box summarizes the key points contained in the article
Acknowledgments
Funding:
This work is supported by National Institutes of Health grant U01DK048377 to KM Gadde.
List of Abbreviations
- ABP
Ambulatory blood pressure
- ABPM
Ambulatory blood pressure monitoring
- AEs
Adverse events
- BED
Binge eating disorder
- BMI
Body mass index
- BP
Blood pressure
- CB-1
Cannabinoid receptor-1
- CVD
Cardiovascular disease
- CVOT
Cardiovascular outcomes trial
- DBP
Diastolic blood pressure
- DMEP
Division of Metabolism and Endocrinology Products
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- GAO
Government Accountability Office
- HbA1c
Hemoglobin A1c
- HDL-C
High-density lipoprotein cholesterol
- HTN
Hypertension
- LDL-C
Low-density lipoprotein cholesterol
- MAA
Marketing authorization application
- MACE
Major adverse cardiovascular events
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- NHS
National Health Service
- NICE
National Institute for Health and Care Excellence
- PK
Pharmacokinetic
- REMS
Risk evaluation and mitigation strategy
- RCT
Randomized controlled trial
- SBP
Systolic blood pressure
- T2D
Type 2 diabetes
- USPSTF
U.S. Preventive Services Task Force
Footnotes
Declaration of Interest:
KM Gadde has received research grants from AstraZeneca, BioKier and the National Institutes of Health. He is also an advisor to AstraZeneca, with payments made to his employer, Pennington Biomedical Research Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377(1):13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadde KM, Martin CK, Berthoud HR, et al. Obesity: pathophysiology and management. J Am Coll Cardiol 2018;71:69–84• Recent comprehensive review of the pathophysiology and management of obesity.
- 3.Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an Endocrine Society scientific statement. Endocrine Rev 2018;39:79–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dombrowski SU, Knittle K, Avenell A, et al. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ 2014;348:g2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlin JW, Brantley PJ, Champagne CM, et al. The impact of continued intervention on weight: Five‐year results from the weight loss maintenance trial. Obesity 2016;24:1046–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daigle KM, Gang CH, Kopping MF, et al. Relationship between perceptions of obesity causes and weight loss expectations among adults. J Nutr Educ Behav 2019;51:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pareek M, Schauer PR, Kaplan LM, et al. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol 2018;71:670–87 [DOI] [PubMed] [Google Scholar]
- 8.Gadde KM, Apolzan JW, Berthoud H-R. Pharmacotherapy for patients with obesity. Clin Chem 2018;64:118–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 2005;143:380–5• Paper that details history of antiobesity drugs in the US and evolution of the FDA’s perspective over the years.
- 10.Weintraub M. Long‐term weight control study: conclusions. Clin Pharmacol Ther 1992;51:642–6 [DOI] [PubMed] [Google Scholar]
- 11.Stafford RS, Radley DC. National trends in antiobesity medication use. Arch Intern Med 2003;163:1046–50 [DOI] [PubMed] [Google Scholar]
- 12.Colman E. Food and Drug Administration’s obesity drug guidance document: a short history. Circulation 2012;125:2156–64 [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. Draft guidance clinical evaluation of weight control drug. Rockville, MD: FDA; 1996. [Google Scholar]
- 14.Jick H, Vasilakis C, Weinrauch LA, et al. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med 1998;339:719–24. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Guidance for industry: developing products for weight management, draft guidance, revision 1. Center for Drug Evaluation and Research (CDER), FDA, Rockville, MD: 2007. Available at: https://www.fda.gov/media/71252/download [Accessed 18 March 2020] [Google Scholar]
- 16.Gadde KM, Allison DB. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation 2006;114(9):974–84 [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency. Press release: The European Medicines Agency recommends the suspension of the marketing authorisation of Acomplia. Doc. Ref. EMEA/CHMP537777/2008; London, 23 October 2008. Available at: https://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-recommends-suspension-marketing-authorisation-acomplia_en.pdf [Accessed 18 March 2020] [Google Scholar]
- 18.James WPT, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010;363:905–17 [DOI] [PubMed] [Google Scholar]
- 19.Dedov II, Melnichenko GA, Troshina EA, et al. Body weight reduction associated with the sibutramine treatment: overall results of the PRIMAVERA Primary Health Care Trial. Obes Facts 2018;11:335–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Kelton CM, Guo JJ, et al. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity 2015;23:1721–8 [DOI] [PubMed] [Google Scholar]
- 21.Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: A comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity 2016;24:1955–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Government Accountability Office. Obesity drugs: few adults used prescription drugs for weight loss and insurance coverage varied GAO-19-577, August 2019. Available at: https://www.gao.gov/products/GAO-19-577 [Accessed 18 March 2020]• A report of the US Government Accountability office presented to the Congressional Committees in 2019.
- 23.Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in abese Adults. Obesity 2013;21:2163–71 [DOI] [PubMed] [Google Scholar]
- 24.Orloff D Toward new therapeutics for obesity. In: Presentation before the Science Board to the Food and Drug Administration April 2004 Available at: https://slideplayer.com/slide/3568934/ [Accessed 18 March 2020] [Google Scholar]
- 25.Lewis KH, Fischer H, Ard J, et al. Safety and effectiveness of longer‐term phentermine use: clinical outcomes from an electronic health record cohort. Obesity 2019;27:591–602 [DOI] [PubMed] [Google Scholar]
- 26.Murali S. Knowledge gaps in long-term phentermine use: making the case for maintenance. Obesity 2019;27:1219. [DOI] [PubMed] [Google Scholar]
- 27.Gadde KM. Current pharmacotherapy for obesity: extrapolation of clinical trials data to practice. Expert Opin Pharmacother 2014;15:809–22 [DOI] [PubMed] [Google Scholar]
- 28.Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 2018;378:1509–20 [DOI] [PubMed] [Google Scholar]
- 29.Khera R, Murad MS, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events. A systematic review and meta-analysis. JAMA 2016;315:2424–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration. Drug Safety Communications. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. Potential risk of cancer outweighs the benefits. Available at: https://www.fda.gov/media/135189/download. [Accessed 18 March 2020] [Google Scholar]
- 31.Gadde KM, Raj YP. Pharmacotherapy of obesity: clinical trials to clinical practice. Curr Diab Rep 2017;17:34. [DOI] [PubMed] [Google Scholar]
- 32.Saydah S, Bullard KM, Cheng Y, et al. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999‐2010. Obesity 2014;22:1888–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gummesson A, Nyman E, Knutsson M, et al. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab 2017;19:1295–305 [DOI] [PubMed] [Google Scholar]
- 34.Siebenhofer A, Jeitler K, Horvath K, et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev 2016;3:CD007654 [DOI] [PubMed] [Google Scholar]
- 35.Food and Drug Administration. FDA briefing document: NDA 200063: Contrave (Naltrexone 4mg, 8 mg/Bupropion HCL 90 mg extended release tablet). Advisory committee; - December 7, 2010. Available at: https://wayback.archive-it.org/7993/20170405220555/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM235671.pdf. [Accessed 18 March 2020] [Google Scholar]
- 36.Nissen SE, Wolski KE, Prcela L, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA 2016;315:990–1004 [DOI] [PubMed] [Google Scholar]
- 37.Herper M The FDA is forcing Orexigen to do a second safety study because of Contrave disclosures. Forbes Business News, 3 March 2015. Available at: https://www.forbes.com/sites/matthewherper/2015/03/03/the-fda-will-force-orexigen-to-do-a-second-safety-study-because-of-contrave-disclosures/#7505ad1b4e98 [Accessed 18 March 2020] [Google Scholar]
- 38.Husten L. Orexigen terminates another cardiovascular outcomes trial. CardioBrief, 13 April 2016. Available at: http://www.cardiobrief.org/2016/04/13/orexigen-terminates-another-cardiovascular-outcomes-trial/ [Accessed 18 March 2020] [Google Scholar]
- 39.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–90 [DOI] [PubMed] [Google Scholar]
- 40.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Food and Drug Administration. NDA022580 approval letter to Vivus 17 July 2012. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/022580Origs000ltr.pdf [Accessed 18 March 2020] [Google Scholar]
- 42.Gadde KM, Allison DB. Combination pharmaceutical therapies for obesity. Expert Opin Pharmacother 2009;10:921–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gadde KM, Yonish G, Foust M, et al. A 24-week randomized controlled trial of VI-0521, a combination weight loss therapy, in obese adults. Obes Res 2006;14:A17. [Google Scholar]
- 44.Greenway FL, Whitehouse M, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity 2009;17:30–9 [DOI] [PubMed] [Google Scholar]
- 45.Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab 2009;94:4898–906 [DOI] [PubMed] [Google Scholar]
- 46.Anderson J, Greenway F, Fujioka K, et al. Bupropion SR enhances weight loss: A 48-week double-blind, placebo-controlled trial. Obes Res 2002;10:633–41 [DOI] [PubMed] [Google Scholar]
- 47.Jain AK, Kaplan RA, Gadde KM, et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obes Res 2002;10:1049–56 [DOI] [PubMed] [Google Scholar]
- 48.Gadde KM, Allison DB. Combination therapy for obesity and metabolic disease. Curr Opin Endocrinol Diabetes Obes 2009;16:353–8 [DOI] [PubMed] [Google Scholar]
- 49.Cardel MI, Jastreboff AM, Kelly AS. Treatment of adolescent obesity in 2020. JAMA 2019;322:1707–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chanoine J-P, Hampl S, Jensen C, et al. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 2005;293:2873–83• The largest RCT of antiobesity drug in adolescents.
- 51.Vivus, Inc. ClinicalTrials.gov Identifier: NCT02714062. A pharmacokinetic study comparing VI-0521 with placebo in obese adolescents. Available at: https://clinicaltrials.gov/ct2/show/results/NCT02714062 [Accessed 18 March 2020]
- 52.Vivus, Inc. ClinicalTrials.gov Identifier: NCT03922945. A phase IV safety and efficacy of VI-0521 in obese adolescents. Available at: https://clinicaltrials.gov/ct2/show/NCT03922945 [Accessed 18 March 2020]
- 53.Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med 2020. March 31. doi: 10.1056/NEJMoa1916038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Hsu LK, Benotti PN, Dwyer J, et al. Nonsurgical factors that influence the outcome of bariatric surgery: a review. Psychosom Med 1998;60:338–46 [DOI] [PubMed] [Google Scholar]
- 55.Sjöström CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477–84 [DOI] [PubMed] [Google Scholar]
- 56.Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH. Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg 2008;12:250–5 [DOI] [PubMed] [Google Scholar]
- 57.Perugini RA, Mason R, Czerniach DR, et al. Predictors of complication and suboptimal weight loss after laparoscopic Roux-en-Y gastric bypass: a series of 188 patients. Arch Surg 2003;138:541–6 [DOI] [PubMed] [Google Scholar]
- 58.Schwartz J, Suzo A, Wehr AM, et al. Pharmacotherapy in conjunction with a diet and exercise program for the treatment of weight recidivism or weight loss plateau post-bariatric surgery: a retrospective review. Obes Surg 2016;26:452–8 [DOI] [PubMed] [Google Scholar]
- 59.Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93 [DOI] [PubMed] [Google Scholar]
- 60.Kalarchian MA, Marcus MD, Courcoulas AP, et al. Optimizing long-term weight control after bariatric surgery: a pilot study. Surg Obes Relat Dis 2012;8:710–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Himes SM, Grothe KB, Clark MM, et al. Stop regain: a pilot psychological intervention for bariatric patients experiencing weight regain. Obes Surg 2015;25:922–7 [DOI] [PubMed] [Google Scholar]
- 62.Bradley LE, Forman EM, Kerrigan SG, et al. A pilot study of an acceptance-based behavioral intervention for weight regain after bariatric surgery. Obes Surg 2016;26:2433–41 [DOI] [PubMed] [Google Scholar]
- 63.Bradley LE, Forman EM, Kerrigan SG, et al. Project HELP: a remotely delivered behavioral intervention for weight regain after bariatric surgery. Obes Surg 2017;27:586–98 [DOI] [PubMed] [Google Scholar]
- 64.Pajecki D, Halpern A, Cercato C, et al. Short-term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir 2013;40:191–5 [DOI] [PubMed] [Google Scholar]
- 65.Stanford FC, Alfaris N, Gomez G, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Surg Obes Relat Dis 2017;13:491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanipah ZN, Nasr EC, Bucak E, et al. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg Obes Relat Dis 2018;14:93–8 [DOI] [PubMed] [Google Scholar]
- 67.Srivastava G, Buffington C. A specialized medical management program to address post-operative weight regain in bariatric patients. Obes Surg 2018;28:2241–6 [DOI] [PubMed] [Google Scholar]
- 68.Rye P, Modi R, Cawsey S, et al. Efficacy of High-Dose Liraglutide as an adjunct for weight loss in patients with prior bariatric surgery. Obes Surg 2018;28:3553–8 [DOI] [PubMed] [Google Scholar]
- 69.Wharton S, Kuk JL, Luszczynski M, et al. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes 20199:e12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jester L, Wittgrove AC, Clark GW. Adjunctive use of appetite suppressant medications for improved weight management in bariatric surgical patients. Obes Surg 1996;6:412–5 [DOI] [PubMed] [Google Scholar]
- 71.Zilberstein B, Pajecki D, De Brito ACG, et al. Topiramate after adjustable gastric banding in patients with binge eating and difficulty losing weight. Obes Surg 2004;14:802–5 [DOI] [PubMed] [Google Scholar]
- 72.Stanford FC, Toth AT, Shukla AP, et al. Weight loss medications in older adults after bariatric surgery for weight regain or inadequate weight loss: a multicenter study. Bariatr Surg Pract Patient Care 2018;13:171–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toth AT, Gomez G, Shukla AP, et al. Weight loss medications in young adults after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Children 2018;5:E116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010;363:245–56 [DOI] [PubMed] [Google Scholar]
- 75.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376:595–605 [DOI] [PubMed] [Google Scholar]
- 76.Allison DB, Gadde KM, Garvey WT, et al. Controlled‐release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity 2012;20:330–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wadden TA, Foreyt JP, Foster GD, et al. Weight Loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR-BMOD Trial. Obesity 2011;19:110–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simons-Morton DG, Obarzanek E, Cutler JA. Obesity research—limitations of methods, measurements, and medications. JAMA 2006;295:826–8 [DOI] [PubMed] [Google Scholar]
- 79.Elobeid MA, Padilla MA, McVie T, et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS One 2009;4(8):e6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gadde KM, Kopping MF, Wagner HR, et al. Zonisamide for weight reduction in obese adults: a 1-Year randomized controlled trial. Arch Intern Med 2012;172:1557–64• A 1-year RCT that demonstrated that it could be possible to contain high attrition rate in clinical trials of antiobesity drugs.
- 81.Freedman DS, Jacobsen SJ, Barboriak JJ, et al. Body fat distribution and male/female differences in lipids and lipoproteins. Circulation 1990;81:1498–506 [DOI] [PubMed] [Google Scholar]
- 82.Larsson B, Bengtsson C, Björntorp P, et al. Is abdominal body fat distribution a major explanation for the sex difference in the incidence of myocardial infarction? The study of men born in 1913 and the study of women, Göteborg, Sweden. Am J Epidemiol 1992;135:266–73 [DOI] [PubMed] [Google Scholar]
- 83.Prescribing information for Xenical (orlistat) capsules. H2-Pharma, LLC, Montgomery, AL, 2017 [Google Scholar]
- 84.Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–61• Longest study of orlistat for weight loss and preventio of type 2 diabetes.
- 85.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–1409• Longest and largest study of liraglutide for weight management.
- 86.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–52• Largest RCT of phentemine/topiramate for weight loss.
- 87.ClinicalTrials.gov Identifier NCT00796367. A safety and efficacy study of VI-0521 to evaluate the long-term treatment of obesity in adults with obesity-related comorbid conditions. An extension of protocol OB-303; (NCT00553787). Available at: https://clinicaltrials.gov/ct2/show/results/NCT00796367?term=vivus [Accessed 18 March 2020] [Google Scholar]
- 88.European Medicines Agency. Refusal of the marketing authorisation for Qsiva (phentermine/topiramate): outcome of re-examination. European Medicines Agency; London, UK: 21 February 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002350/WC500139215.pdf [Accessed 18 March 2020] [Google Scholar]
- 89.National Institute for Health and Care Excellence. Naltrexone-Bupropion for managing overweight and obesity. Technology appraisal guidance [TA494] 12 December 2017. Available at: https://www.nice.org.uk/guidance/ta494/resources/naltrexonebupropion-for-managing-overweight-and-obesity-pdf-82605086955205 [Accessed 18 March 2020]
- 90.Vivus, Inc. Qsymia (phentermine and topiramate extended-release) Capsules: risk evaluation and mitigation strategy (REMS). Available at: https://www.qsymiarems.com/ [Accessed 18 March 2020]
- 91.Thomas DD, Waring ME, Ameli O, et al. Patient characteristics associated with receipt of prescription weight‐management medications among veterans participating in MOVE! Obesity. 2019;27:1168–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ganguly R, Tian Y, Kong SX, et al. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Res Clin Pract 2018;143:348–56 [DOI] [PubMed] [Google Scholar]
- 93.LeBlanc ES, Patnode CD, Webber EM, et al. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;320:1172–91 [DOI] [PubMed] [Google Scholar]
- 94.Simon R, Lahiri SW. Provider practice habits and barriers to care in obesity management in a large multicenter health system. Endocr Pract 2018;24:321–8 [DOI] [PubMed] [Google Scholar]
- 95.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache 2018;58:496–505 [DOI] [PubMed] [Google Scholar]
- 97.Prescribing information for Vyvanse (lisdexamfetamine dimesylate) capsules, CII. Shire US, Inc, Lexington, MA, 2018 [Google Scholar]
- 98.de Zwaan M. Binge eating disorder and obesity. Int J Obes 2001;25(S1):S51–5 [DOI] [PubMed] [Google Scholar]
- 99.Sahebkar A, Simental-Mendia LE, Kovanen PT, et al. Effects of orlistat on blood pressure: a systematic review and meta-analysis of 27 randomized controlled clinical trials. J Am Soc Hypertens. 2018;12:80–96 [DOI] [PubMed] [Google Scholar]
- 100.Cohen JB, Gadde KM. Weight loss medications in the treatment of obesity and hypertension. Curr Hypertens Rep 2019;21:16. [DOI] [PMC free article] [PubMed] [Google Scholar]