Abstract
Cardiorespiratory fitness (CRF) refers to the capacity of the circulatory and respiratory systems to supply oxygen to skeletal muscle mitochondria for energy production needed during physical activity. CRF is an important marker of physical and mental health and academic achievement in youth. However, only 40 percent of United States youth are currently believed to have healthy CRF. In this statement, we review the physiologic principles that determine CRF, tools that are available to assess CRF, modifiable and non-modifiable factors influencing CRF, the association of CRF with markers of health in otherwise healthy youth, and the temporal trends in CRF, both in the United States and internationally. Development of a cost-effective CRF measurement process that could readily be incoproated into office visits and in field settings to screen all youth periodically could help identify those at increased risk.
Keywords: cardiorespiratory fitness, physical activity, cardiovascular disease, cognition, mental health
Introduction
Cardiorespiratory fitness (CRF) refers to the capacity of the circulatory and respiratory systems to supply oxygen to skeletal muscle mitochondria for energy production needed during physical activity.1, 2 Low or unhealthy CRF is a strong, independent predictor of cardiovascular disease (CVD) and all-cause mortality in adults.1 In youth, CRF is a predictor of a number of health indicators including cardiometabolic health,3, 4 premature cardiovascular disease,5 academic achievement,6 and mental health.4, 7 Unfortunately, currently only 40 percent of 12–15 year olds in United States (US) are believed to have healthy CRF.8 In addition, over the past six decades, CRF has declined, both in the US and internationally.9–11 Although the reasons for this decline are not well-understood, an increase in obesity, increased sedentary time, decreased levels of moderate-to-vigorous physical activity, and social and economic changes may have contributed.9, 11
Although CRF is at times assessed in certain youth, such as those with congenital heart disease, asthma, and cystic fibrosis, assessment of CRF has a broader range of applications. CRF is an objective measure of health that can be tracked over time and compared across populations.1, 9 Whereas self-reported physical activity levels can be unreliable12 and only provide a snapshot of behavior, assessments of CRF provides a more robust measure of cardiovascular health. Consistent with this sentiment, a recent American Heart Association Statement suggests that CRF be assigned as a “vital sign” as it has the power to predict mortality in adults similar to traditionally assessed risk factors, such as tobacco use, type 2 diabetes mellitus, hypertension, and hypercholesterolemia.1
The central focus of this statement is to raise awareness of clinicians regarding the importance of CRF in predicting current and future health in otherwise healthy youth, knowing that CRF measurements provide an objective measure of health as opposed to physical activity recall which is the current practice. An explicit purpose of this statement is to explore valid, low-cost alternatives to traditional Cardiopulmonary Exercise Tests (CPET) to assess CRF in otherwise healthy youth in office settings with limited space, that can be performed by personnel not formally trained in exercise physiology. This statement will review current knowledge related to the association between CRF and health outcomes in youth, describe the added value of CRF to improve risk prediction, and highlight gaps for future research with the following areas addressed:
Physiologic considerations
Various tests that can be used for assessing CRF in the field and office settings
Key modifiable and non-modifiable factors influencing CRF including effect of interventions
CRF’s impact on cardiovascular, cerebrovascular, cognitive and mental health
Temporal trends in CRF in youth nationally and internationally
Knowledge gaps and suggestions for future research
This statement will not discuss special risk groups of youth such as those with unpalliated/palliated congenital heart disease.13 Physical activity guidelines for youth are covered in detail in other documents14 and its discussion will be limited. The focus of this statement is to primarily examine CRF in otherwise able and healthy, disease free youth.
Health-Related Fitness And Associated Physiological Changes
Although this Statement focuses on CRF, this is only one of four distinct health-related fitness components. CRF, also known as cardiorespiratory endurance, cardiovascular fitness, aerobic capacity, and aerobic fitness among others, refers to the capacity of the body’s circulatory and respiratory systems to supply oxygen to skeletal muscle mitochondria for energy production during physical activity.15 A second component, muscular fitness, is the body’s ability to exert maximal force against an external resistance (i.e., muscular strength), or repeatedly under sub-maximal loads (i.e., local muscular endurance). Third, flexibility refers to an individual’s range of motion around a joint, or group of joints. Flexibility is important for preventing musculoskeletal injury, maintaining functional independence, and for performing sports and activities of daily living. The fourth component, body composition, is the relative proportion of total body mass composed of fat, fat-free tissue, and total-body water.
Table 1 includes a summary of physical activity intensity categories for youth aged 8–18 years based on heart rate, maximum oxygen uptake, perceived exertion and metabolic equivalent (MET). Energy expenditure often is quantified as MET, with one MET equal to 3.5 mL O2/kg/min (oxygen consumed).16 Energy expenditure ranges from low levels used during sedentary activities (1 to 2 METs) to the considerable levels required during sprint interval training (9 to 20 METs).16 Compared to adults, energy expended is typically higher in youth, leading to the underestimation of energy expenditure if adult reference values are used. As such, Table 1 includes age- and sex-appropriate MET values associated with activity of varying intensity.17, 18
Table 1.
Categories of Physical Activity For Youth Aged 8–18 Years
| Intensity category | Description | Example activities | Measures (mean values for age 8–18 years) |
|---|---|---|---|
| Sedentary | Waking behavior typically performed in a sitting, reclining or lying posture. | Sitting or reclining while watching television, playing video games, driving, reading and fishing. | < 1.5 METs |
| <40% HRmax | |||
| <20% HRR | |||
| <20% V̇O2max | |||
| RPE: < 8 | |||
| Light | Light aerobic activity that does not cause a noticeable increase in breathing and can be sustained for atleast 60 minutes. | Domestic or occupational tasks such as washing dishes, ironing, working at a desk or performing office duties | 1.5 to 4 METs |
| 40 to 63% HRmax | |||
| 20 to 39% HRR | |||
| 20 to 45% V̇O2max | |||
| RPE: 8 to 11 | |||
| Moderate | Aerobic activity that can be sustained while maintaining a conversation uninterrupted. | Gentle swimming, social tennis and golf. | 4–6 METs |
| 64 to 76% HRmax | |||
| 40 to 59% HRR | |||
| 46 to 63% V̇O2max | |||
| RPE: 12 to 13 | |||
| Vigorous | Aerobic activity during which a conversation cannot be maintained. An intensity that may last up to 30 minutes. | Jogging, aerobics, fast bicycling, resistance training, competitive sports | 6–9 METs |
| 77 to 95% HRmax | |||
| 60 to 89% HRR | |||
| 64 to 90% V̇O2max | |||
| RPE: 14 to 17 | |||
| Near-maximal to maximal | Activity that typically cannot be sustained for longer than 10 minutes. | Sprinting, periods of competitive team sport activity. | ≥ 9 METs |
| ≥ 96% HRmax | |||
| ≥ 90% HRR | |||
| ≥ 91% V̇O2max | |||
| RPE: ≥ 18 |
Notes: Table adapted from Norton and colleagues,16 ACSM,163 Butte and colleagues,17 and Eather and colleagues.18 The reported MET values in this table were derived from the Youth Compendium of Physical Activities for specific activities and adapted by Eather and colleagues.18 Children undergo systematic changes in body composition as a result of growth and maturation, which has implications for activity intensity classifications. As such, MET cut-points should be adjusted for differences in resting energy expenditure. Youth METs have been adjusted to account for the unique physiological characteristics of children and adolescents; % HRmax, percentage of heart rate maximum (heart rate maximum = 220 – age); %HRR, percentage of heart rate reserve (heart rate reserve = HRmax − resting HR); %V̇O2max, percentage of maximum oxygen uptake. Borg’s Rating of Perceived Exertion (RPE) scale, ranging from 6–20.45, 164–166
Physiologic Changes
At any given baseline level, CRF potentially may increase or decrease, depending on one’s ability to be physically active. Physical activity-induced improvements in CRF may be explained by structural and functional adaptations leading to a better oxygen transport system,19 such as increased blood volume, myocardial contractility, ventricular compliance, and angiogenesis,20 which all lead to an increased cardiac output.21, 22 This was illustrated by Rowland and colleagues22 who found that the cardiac index (cardiac output ÷ body surface area) was significantly greater in trained youth cyclists compared to their non-trained peers. On the other hand, there appears to be little difference in maximal oxygen extraction between trained and untrained youth23, 24 and findings have been equivocal as to whether exercise-induced improvements in stroke volume are due to increases in cardiac dimensions.25
Although the two are often conflated, physical activity and CRF are distinct but related concepts. Physical activity is voluntary movement produced by skeletal muscles that results in energy expenditure.2 “Exercise” and “training” refer to a subset of physical activity where the goal is to improve performance, health or both.2 CRF can reflect an individual’s past physical activity as well as reflect the ability to be physically active (an individual with greater CRF has more capacity for physical activity), forming a virtuous cycle of an active-fit lifestyle. Thus, physical activity is a behavior (“will do”), while CRF represents an individual’s capacity (“can do”) to perform certain types of physical activity.
Key points
Exercise induced improvements in CRF are due to structural and functional adaptations in the oxygen transport system.
Physical activity, exercise and CRF are associated but distinct concepts.
How to Measure Cardiorespiratory Fitness in Youth
CRF can be measured, or estimated, using a variety of tests and protocols. The tests used to measure CRF that require maximal effort are referred to as maximal exercise tests. Maximal exercise tests often but not always are performed in the office setting and usually measure cardiometabolic parameters such as inspiratory and expiratory gases, blood pressure, heart rate, and the heart’s electrical activity. Tests that do not require maximal effort are referred to as submaximal exercise tests. Submaximal exercise tests often estimate CRF using equations or nomograms that have been validated against CRF measurements obtained directly during a maximal exercise test. Submaximal tests can be used when a maximal test cannot be performed due to safety, setting or cost. Although submaximal tests are easier to perform, there are often large measurement errors and thus estimated CRF comparisons are fraught with inaccuracies. However, these tests may be useful for identifying and following those with low/unhealthy CRF. Table 2 summarizes key information regarding some of the commonly used tests to measure or estimate CRF.
Table 2:
Comparison Of Selected Tests Used To Measure Cardiorespiratory Fitness1
| Description | Ability to Assess CRF2 | Limitations | Suggestions for Clinical Practice | |
|---|---|---|---|---|
| Cardiopulmonary Exercise Test (Gas-analyzed) | Participants exercise with incrementally increasing difficulty/workload with V̇O2 measured via respiratory gases | +++ | Sophisticated equipment needed | Gold standard for measurement of V̇O2 |
| 20mSRT3 (Non-gas-analyzed) (Field based) | Participants run/walk between two points on a floor in sync with audio signals with incrementally increasing frequency | ++ | Need 20 meters open space | Modified protocols are available for office populations |
| Run tests (e.g., 1.5 mile / 2400 meters) (Field based) | Participants run a given distance as quickly as possible | ++ | Very dependent on motivation and body size. | Often used in school settings |
| Step Test (Office or Field based) | Participants step up and down on a block of a given height. Each stage is associated with an increased step rate. | + | Validity not well-established | Portable, test can be performed in small spaces |
| Walk tests (Office based) (e.g. 6-minute walk test) | Participants instructed to walk as far as possible in 6 minutes | +/− | Poor validity in healthy populations | Useful for populations with low exercise capacity |
| Questionnaires | Questionnaire to assess fitness level | +/− | Large error in estimation of V̇O2 | Used for population research mainly |
Tests presented were collated to give examples of various testing categories or explanations of protocols and is not meant to be exhaustive.
Scale ranges from +/− (least) to +++ (most) and reflects writing groups’ overall assessment of test’s usefulness in reflecting CRF.
20mSRT – 20-meter Shuttle Run Test
The measurement and reporting of CRF depends on various factors such as: 1) the test used and protocol; 2) whether CRF is measured or estimated; 3) whether CRF measures are reported as absolute values versus indexed to body size; and 4) participant motivation.26 The reader is referred to CRF normative measures that are test and protocol-specific.27–29
For each test described below, it is assumed that participants are able-bodied youth without impairment and that maximal effort is given. Although modifications can certainly be made for many of these tests for youth with physical and/or cognitive impairments, we will not be discussing them in that context as the primary purpose of this statement is to address CRF in healthy youth.
Commonly used terms to describe CRF measures are listed here:
| Unit | |
| V̇O2peak (peak oxygen uptake) | L/min |
| V̇O2max (maximal oxygen uptake) | L/min |
| V̇O2peak (scaled to body weight) | mL/kg/min |
| V̇O2max (scaled to body weight) | mL/kg/min |
| # of 20 meter Shuttle Run Test laps or stages completed | N |
| Work | Watts (absolute or scaled) |
Gas-Analyzed Testing
Graded Cardiopulmonary Exercise Test:
Based on the Fick principle, oxygen uptake (V̇O2) is the product of cardiac output (heart rate and stroke volume) and the arterio-venous oxygen difference.30 Thus, V̇O2 is dependent on cardiac function, the ability of the lungs to act as gas exchange organs, the binding of oxygen to the blood that is primarily dependent on hemoglobin content, and the ability of the muscles to extract oxygen from the circulation for energy transfer. The gold standard for determining V̇O2 is by measuring O2 and CO2 partial pressures in expired air at regular intervals during graded exercise to exhaustion, typically on a treadmill or cycle ergometer. Testing CRF in this way is known by various terms, such as cardiopulmonary exercise test (CPET), cardiorespiratory exercise test, or a graded exercise test.
The highest oxygen uptake attained during graded exercise to volitional exhaustion (V̇O2max) is considered the best indicator of CRF by the World Health Organization.31 V̇O2max is the reflection of the maximal oxygen flux through the lungs, transported by the circulation to the mitochondria of the exercising muscle. V̇O2max remains the only index that integrates pulmonary, circulatory, and muscular function into a single number. However, the utility of V̇O2max measurements in youth has been questioned. Traditionally, for V̇O2max to be determined, there must be a plateau in the oxygen uptake curve. Even the earliest pioneers appreciated that youth do not often demonstrate a plateau during incremental exercise32 and that the greatest V̇O2 measured in youth, termed V̇O2peak, is likely analogous to V̇O2max measured in adults.33, 34 We will use both terms (V̇O2peak and V̇O2max), reflecting as closely as possible the measures used in the cited studies.
Reporting norms for V̇O2peak or V̇O2max in youth is further complicated by the wide range of body sizes even at a given age. Although CRF values often are indexed to body size, it is not clear that this is always appropriate as it may not fully account for the residual effects of body size. In a systematic review and meta-analysis, it was found that adolescent participants with obesity had comparable CRF to participants without obesity when expressed in absolute values (V̇O2peak L/min), but lower values when scaled for weight (V̇O2peak mL/kg/min), and different still when scaled to lean mass.35 On the other hand, if allometric scaling is undertaken, it remains sample-specific and cannot necessarily be extrapolated to all populations.36 At this time, there is no accepted standard in regard to scaling in reporting CRF; hence attention should be paid to units when comparing CRF between participants and studies.
Even as CPET provides a wealth of data, clinicians should be aware of limitations including the limited ability to perform this test in settings other than the office or hospital. The test requires expensive equipment and well-trained staff, which are not always available. The metabolic cart requires meticulous maintenance and calibration. Another limitation is that most CPET parameters are measured breath-by-breath, with a range of options to analyze the data and filter the “noise” in the data. This can introduce differences between laboratories and equipment, which makes comparisons among participants and studies difficult. Finally, the pattern of activity performed during CPET may not reflect the types of physical activities youth are commonly engaged in.
Non-Gas-Analyzed Tests
Field-Based Tests:
1. 20 Meter Shuttle Run Test
For reasons stated above, alternative tests for measuring CRF in youth have been developed.37, 38 One such test is the 20-meter shuttle run test (20mSRT) developed by Leger et al.39, 40 The 20mSRT, and its variants, is now the most widely used test to estimate CRF in youth in the world.41
There are several different names used for the 20mSRT - Beep test, PACER (Progressive Aerobic Cardiovascular Endurance Run) test, and Multi-stage fitness test; however, the protocols are very similar. Typically, youth are instructed to run at an increasing standardized pace (starting around 5 miles/hour [8 kilometers/hour], increasing in 0.3 miles/hour [0.5 kilometers/hour] increments each minute), noting the number of laps or stages they can keep up with the pace as the result, which can then be compared to a reference population.9
The 20mSRT has been studied in both sexes and in a range of ethnicities and ages.27, 42 According to a systematic review, the 20mSRT is a valid estimate of CRF when compared to CPET measured CRF.43 In this review of 73 studies addressing the criterion-related validity of field-based fitness tests in children and adolescents, there was strong evidence that the 20mSRT had moderate to high validity against CPET to estimate CRF. As the 20mSRT can be administered in group settings such as in schools, it is efficient for testing large cohorts of youth simultaneously, and is thus feasible for population-based CRF surveillance. However, as is true for all CRF tests, it is influenced by motivation and performance. If estimated V̇O2peak is used as an endpoint for comparison, large prediction errors can influence results.44 Thus number of laps completed or stages reached may be better endpoints to report. Clinicians should be aware of the specific 20mSRT protocol used when comparing to reference values.
In the U.S., the 20mSRT is commonly used as a component in the “FitnessGram.”45 The FitnessGram is a group of tests used to assess various forms of fitness in school-aged youth. In addition to 20mSRT, the FitnessGram measures body mass index (BMI), abdominal strength, trunk extensor strength, upper-body strength, and flexibility. The results are classified into various “Fitness Zones.”45 All 50 states in the US currently use the FitnessGram to assess over 22 million students each year.29
2. Run Tests
In run tests the participant is given a set distance (e.g., 1.5 miles or 2400 meters) or time (e.g., 12 minutes) and instructed to complete the run in as short amount of time as possible or cover the greatest possible distance, respectively. Mayorga-Vega et al46 recently performed a meta-analysis to determine which distance and/or time was most appropriate to use in youth. Of the various distances and times used, they found that the highest correlation to CPET measured V̇O2max, was with the 1.5 mile (2400 meters) distance (r =0.79) and 12 minutes time (r=0.78), showing moderate-to-high correlation.46 Regarding the validity of the 1.5 mile and 12-minute run tests when compared to the similarly reliable 20mSRT, data from two large meta-analyses42, 44 indicate that run tests are equally valid compared to the 20mSRT.
Office-Based Tests:
The text below describes some of the commonly used tests that are suitable for use in office settings, but is by no means exhaustive.
1. Ebbeling test (single stage treadmill walking test)
This test is performed on a treadmill with a 5 percent grade incline. The heart rate is measured after 4 minutes and is combined with speed, age, and sex to estimate CRF.47 Nemeth et al evaluated the Ebbeling test in 130, 11–14 year-olds who were overweight and concluded that the CRF estimate was within 10 percent of the V̇O2max (mL/min) measured from CPET.48
2. Åstrand-Rhyming test
This test is performed using a cycle ergometer and is often used in Europe. This test is typically performed over six minutes with a constant load (or single stage) aimed at producing a heart rate between 125–170 beats per minute. The heart rate and the workload is used to estimate V̇O2max from a nomogram.49 The Åstrand-Rhyming test has been evaluated in 11–12-years-olds and found to have a strong correlation of 0.82 in girls and moderate correlation of 0.52 in boys when compared to CPET-measured V̇O2peak (L/min).50 The authors did not explore the reasons for the differences in correlation coefficients between boys and girls.
3. PWC170 (Physical Work Capacity Corresponding to a Heart Rate of 170 beats per minute)
The PWC170 test has been used since the 1960’s. It is administered using a cycle ergometer and typically conducted with 3 × 3-minute stages or 3 × 4-minute stages of increasing workload. Work (Watts) is measured once the heart rate reaches 170 beats per minute. PWC170 was moderately well-correlated with measured V̇O2peak (mL/kg/min) in 11–16 years-olds, with the correlation depending on the stage length (i.e., 0.70 for two minutes, 0.56 for three minutes, and 0.61 for six minutes).51
4. 6-minute walk test (6MWT)
This is the most commonly administered walk test and measures the distance walked in 6 minutes.52 The 6MWT is easy to administer and international guidelines have been established,53, 54 along with test-specific reference standards.55 However, the use of the 6MWT is less useful in healthy youth to estimate CRF. The 6-minute walk test shows a relatively poor correlation with V̇O2max, except in populations with moderate-to-severe limitations in CRF54 or reduced walking capacity of less than 300 meters.56 Therefore, their use should be considered only when there is reason to suspect low CRF.54, 57
5. Step tests (Queen’s College or Harvard step tests)
Step tests are another category of tests, using stepping up and down a bench in an effort to engage larger muscle mass. One of the first such protocols described in children (Harvard Step Test) involved stepping up to a 12-inch bench at a rate of 24 steps per minute for a duration of 3 minutes with heart rates collected post-exercise.58 Recently, Hayes et al reported the validity of a step test in elementary school children and showed that the step test along with sex, and BMI significantly predicted V̇o2max (R2 = 0.51).59 Heart rates in youth during step tests have been reported to be strongly associated with V̇O2max (r=0.8, p<0.01)60 regarldess of stepping frequency.60 Step tests require minimal equipment, are easy to administer in limited indoor spaces and can be administered by personnel with little or no formal training in exercise physiology, which make them a suitable alternative to CPET to estimate CRF in office settings. The step test can also be performed on the bleachers at schools and is suitable for testing in group settings simultaneously. It is important to monitor consistency with step cadence and foot strike pattern as repeated breaches may affect results.
Questionnaires:
Some youth are unable to complete fitness testing for various reasons (body size, maturity limitations, etc.) so methods to estimate CRF without objective testing have been evaluated. Questionnaires may offer the least burdensome method for examining CRF in youth. However, questionnaires are currently used for epidemiologic studies and not for estimating CRF in individuals.
The International Fitness Scale (IFIS) is one option.61, 62 It consists of five questions using a five-point Likert scale on general physical fitness, CRF, muscular strength, speed/agility, and flexibility. IFIS is designed to measure CRF in populations and can be completed in about 5 minutes. Ortega et al reported that in 3,059 youth aged 12–18 years, the IFIS was linearly related to CRF (mL/kg/min) as estimated by the 20mSRT with an odds for having a healthy CRF based on FitnessGram thresholds of 7.3 (95% CI 4.0–13.5) for those reporting very good CRF on the IFIS questionnaire.61 However, its usefulness at the individual level is not established.61 It should also be noted that correlation between IFIS and the FitnessGram compares surrogates to surrogates and does not use measured V̇O2 as a reference.
Key points
The most accurate measure of CRF in youth is gas-analyzed (measured) V̇O2peak obtained during a graded CPET but this testing cannot be universally performed.
Graded tests such as the 20mSRT provide the best alternative to CPET in a field setting.
Step tests may be a good alternative to CPET when space and resources are limited.
In general, tests that require more effort are preferred to tests that primarily measure function, such as walk tests.
Estimated V̇O2peak can be misleading and needs to be reconciled with other factors such as the protocol and testing used and participant motivation/effort.
Questionnaires may provide insightful information for epidemiological purposes but are considered the least accurate method for assessing CRF.
Factors Affecting CRF in Youth
Studies have investigated the relationship between CRF and various non-modifiable and modifiable factors including genetics,63 age, sex,64 race/ethnicity,65 physical activity and dietary patterns,66, 67 obesity,68, 69 sedentary time,70 built environment,71 and socioeconomics.72, 73
Below is a discussion of these topics.
Non-Modifiable:
Genetics
In adults, it has been noted that an individual’s response to physical training varies widely, with some people markedly increasing their CRF (“responders”), while others only have a minimal increase in CRF (“non-responders”).74, 75 One study suggested that nearly 50 percent of an individual’s response to training is inherited.63, 76 Further, the variance in response to aerobic training was 2.5 times higher between families than within families.76 But, not one of the nearly 300,000 single nucleotide polymorphisms studied have been found to be associated with exercise-induced changes in V̇O2max (mL/min).77 Thus, evidence supporting specific genetic polymorphisms influencing CRF remains weak78 and the mechanisms by which genes affect CRF are still unclear.75 There is no evidence for genetic variations impacting CRF (mL/kg/min) among elite athletes.79 Studies in youth examining genetic differences in CRF are lacking.
Age and Sex
As youth age, there is an increase in CRF as measured by V̇O2max (mL/min) for both boys and girls.80 While CRF increases in both boys and girls as they age, the increase in girls occurs at a slower rate.81, 82 Regardless of age, boys have a higher V̇O2max than girls across,9, 83 even after controlling for lean body mass and cardiac size.83 Potential explanations for this difference include sex-related differences in muscle fiber type, oxygen extraction, or the lipid content of myofibrils.83, 84
Race/Ethnicity
In adults, V̇O2max has been noted to be higher in Caucasians compared to African Americans85 and to those of Chinese ethnicity.86 However, the relationship between race/ethnicity and CRF (mL/kg/min) in adults weakens after adjustment for BMI, lifestyle factors, socioeconomic status, and other CVD risk factors.87 Similarly, racial/ethnic differences in CRF in youth are unclear. Studies using data from the 1999–2004 and 2012 cohorts from the National Health and Nutrition Examination Survey did not find differences in CRF in youth across race/ethnicity groups (V̇O2 max - mL/kg/min was measured from a submaximal, gas-analyzed test).8 But, Shaibi et al found that Hispanic youth had lower V̇O2peak (mL/kg/min) than non-Hispanic white and non-Hispanic black youth.88 This is consistent with international comparisons in which youth in South America had lower CRF compared to youth from Europe and Africa.89 Similarly, Bansal et al, found that African-American children have lower CRF versus Caucasian children (V̇O2max - mL/kg/min). However, these differences in CRF were not adjusted for environmental and psychosocial factors or habitual physical activity.90
Prematurity
Using data from Northern Ireland Young Hearts Study, investigators found that compared to those born at full-term, even those born slightly early, between 37 and 38 weeks gestation, had a 57 percent higher risk of having low CRF (mL/kg/min) at ages 12, 15 and 22 years.91 These effects were not related to decreased physical activity.91, 92 In a meta-analysis, participants born prematurely had approximately 13 percent lower CRF than those born at term.93 The mechanism is not clear, but may be related to smaller lung volumes.
Modifiable:
Habitual Physical Activity And Exercise Training
It is generally assumed that physically active youth have higher CRF. However, the strength of the association between habitual physical activity and CRF in youth is small-to-moderate,94 with most of the benefits accruing only with sustained vigorous physical activity.94–96 A number of factors may explain the lack of a strong association between physical activity and CRF in youth. First, CRF has an incompletely defined, but clear hereditary component. Second, habitual physical activity levels in youth rarely achieve the vigorous intensity or duration necessary for the improvement of CRF. Finally, challenges in the accurate assessment of both physical activity and CRF may mask the relationship.
Using an objective measure of physical activity, Gutin et al found that CRF (mL/kg/min) in youth had a stronger relationship with the time spent in vigorous physical activity than with the time spent in moderate- or light-intensity physical activities.97 In general, training programs of various intensities can improve V̇O2max or V̇O2peak in pre-pubertal youth, but engaging in increased amounts of intense physical activity can lead to up to a 10 percent improvement in these parameters.98, 99
The importance of high levels of moderate-to-vigorous physical activity is illustrated best by studies of high intensity interval training (HIIT). Evidence is growing that HIIT may be effective in improving youths’ CRF. HIIT is typically considered to be exercise that is characterized by alternating intermittent bursts of vigorous activity with periods of rest or low-intensity activity. Studies have demonstrated that small amounts of vigorous, maximal-to-near-maximal activity can induce improvements in youths’ V̇O2peak. For example, Costigan and colleagues100 conducted a systematic review of the effects of HIIT on youth’s CRF. In this review, the adjusted difference between groups in V̇O2max, was 2.6 mL/kg/min (95% CI 1.8 to 3.3, p<0.001) in favour of adolescents participating in HIIT. Interventions ranged from 4 weeks to 8 months in duration and the majority of studies involved three sessions per week of maximal sprint running. These studies however provide less evidence for the exact dose (i.e., frequency, intensity, time, and type) of physical activity that is needed to improve CRF.
Although the impact of physical activity on CRF is variable, even small improvements in CRF with increases in physical activity resulted in major health benefits in adults.1 In fact, it is well established that moving from the lowest quintile CRF to the next-lowest quintile group is associated with the most striking health benefits in adults.1 No studies to date have measured the impact of physical activity in youth with low baseline CRF, but this is a critical health question to answer as they potentially stand to benefit most from intervention.
Sedentary Time
The amount of time spent sedentary comprises up to 75 percent of a 15 year old’s waking hours and has increased from 7 hours to 8.2 hours per day from 2003 to 2016 in adolescents in England and the US.101, 102 A recent American Heart Association Statement on sedentary time in adults103 noted several meta-analyses suggesting a strong relationship between sedentary time and all-cause death. In a recent meta-analysis in adults, the negative effects of high levels of sedentary time were reduced with high levels of moderate-to-vigorous physical activity, but not eliminated.104
The relationship between sedentary time and CRF in youth is unclear. Studies have demonstrated both the presence70, 105, 106 and absence107, 108 of a relationship. In a large study of 11–13 year-old females, objectively-measured physical activity improved CRF (mL/fat-free mass/min), but there was no relationship between CRF and objectively-measured sedentary time.109 In another study, CRF was associated with objectively-measured sedentary time, independent of time spent in moderate-to-vigorous physical activity.70 The authors of a recent meta-analysis examining the cross-sectional association between total sedentary time and CRF in children and adolescents (n = 4499 participants) found conflicting results. In children, there was a significant association (r = −0.06, p = 0.037), whereas there was no association was found in adolescents (r = 0.02, p = 0.7).110
Obesity
Youth with obesity who are less physically active exhibit lower V̇O2max (mL/kg/min) than their normal weight peers.111 Byrd-Williams et al, in a longitudinal study evaluating risk factors for the development of type 2 diabetes among Hispanic youth, found that high CRF (mL/min) is associated with less subsequent weight gain over time in boys, but not in girls. Specifically, this study found that for each 15 percent increase in V̇O2max from baseline there was an associated 1.4 kg lower fat mass over 4 years.112 Therefore, optimal CRF could modify BMI suggesting a bidirectional relationship between obesity and CRF. There are reports evaluating the relationship between genes associated with obesity and V̇O2max /trainability. Such studies have suggested that there is a shared genetic thread between obesity and CRF, regardless of whether V̇O2max is indexed to fat-free mass or total body weight.113 Lifestyle interventions, regardless of whether youth gain or lose weight, may have a beneficial effect on CRF. In a study of 11–18 year-old females enrolled in a six-month program of dietary counseling combined with supervised aerobic and resistance exercise training, CRF improved in those who lost weight more than in those who did not, but V̇O2max improved with intervention in both groups as a function of the increase in fat free mass.114 The authors however do not report on potential interplay between the dietary and exercise training aspects of this lifestyle intervention.
Diet
An overall healthy dietary quality score was associated with better CRF in the “Coronary Artery Risk Development in Young Adults” study in all race-sex groups of youth studied, except African Americans.67 A dietary pattern specifically rich in fruits and vegetables was associated with healthy CRF in New Zealand and European youth.115, 116 The nutritional contributions to CRF is rooted in mitochondrial energetics that are fundamental to skeletal muscle oxidative capacity and efficiency, and therefore to CRF.117
As defined at the opening of this statement, CRF reflects the integrated ability to transport oxygen from the atmosphere to the mitochondria to perform physical work. A signature feature of mitochondria is their ability to proliferate, or conversely to be degraded in response to nutritional and extracellular environmental stimuli. Exercise training and dietary patterns rich in omega 3 fatty acids and polyphenols are the principle external influences known to promote mitochondrial bioenergetic pathways.118 And several specific essential fatty acids and polyphenolics, including from cocoa, apples, beets, pomegranates, grapes, olives and cruciferous vegetables have been shown to increase mitochondrial biogenesis, and improve mitochondrial function.119 Nitrate, an inorganic ion abundant in fruits and vegetables, can also be converted in the mammalian mouth and gut to bioactive nitric oxide, further reducing the oxygen cost of exercise.120
Social, Economic, and Environmental Factors
Disparities in CRF may be socioeconomically driven with both rates of poor nutrition and physical inactivity greatest among urban youth.121 Also, the environment’s effects on lifestyle and CRF may be mediated through varying levels of physical activity due to the built environment. Gahche et al8 did not find a difference in socioeconomic status and CRF (submaximal, gas-analyzed, measured CRF - mL/kg/min), but other studies have found that poor socioeconomic status is associated with low CRF (measured using the 20mSRT) in youth.73
A recent study identified a strong negative association between country-level CRF and income inequality. In countries with a wide income gap between rich and poor residents, youth had poorer CRF.89 In a review, the same authors reported that countries with a widening economic gap between rich and poor residents had less favorable CRF trends (i.e., large declines).9 While these assessments of income inequality may not be stringent, these data provide some proof of concept that there are social and economic determinants of CRF.122
Figure 1 summarizes key influencers of CRF and outcomes influenced by CRF.
Figure 1 -.
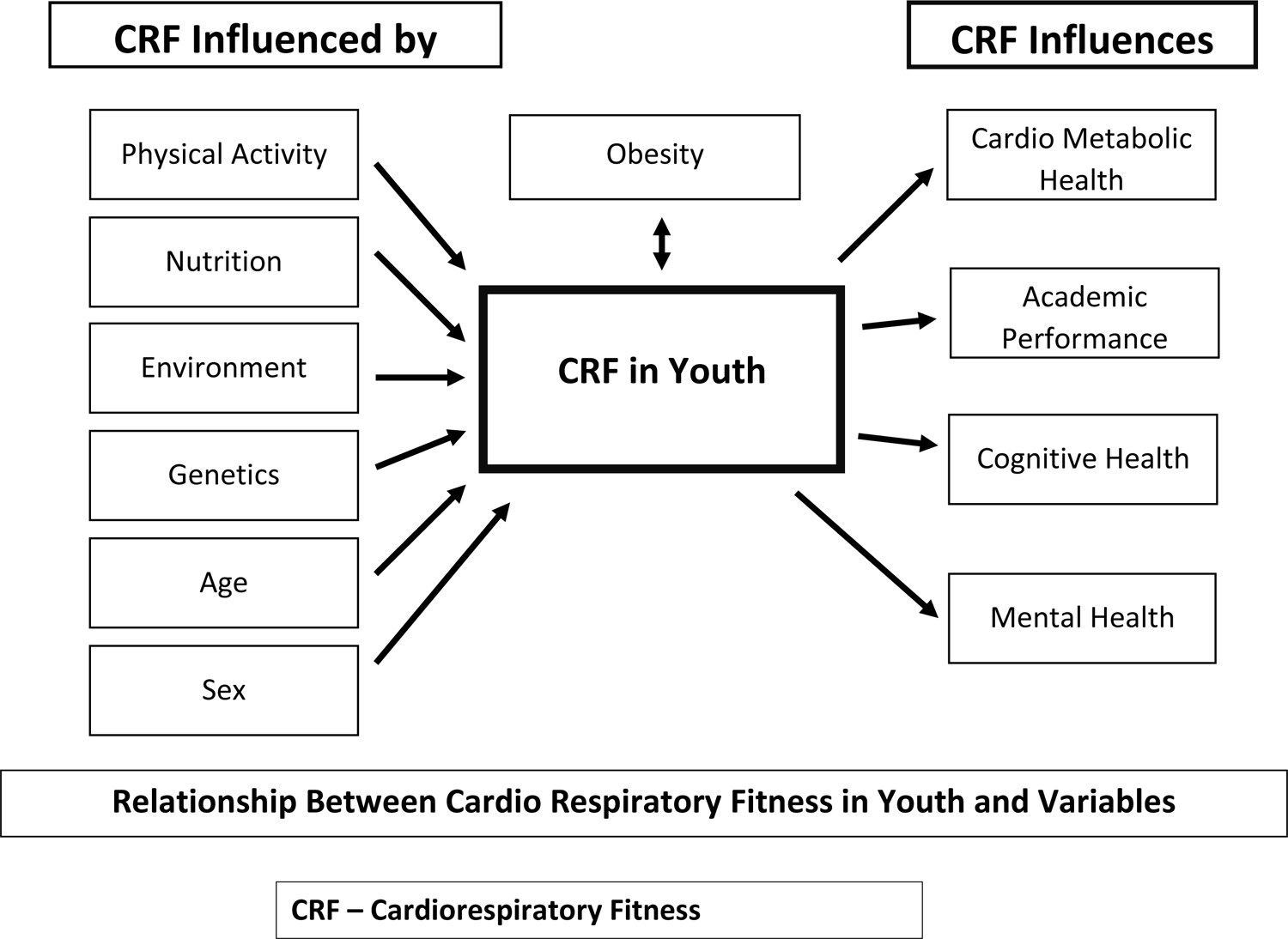
Cardiorespiratory Fitness In Youth – Key Influencers And Effects
Key points
Hereditary factors are known to influence CRF but specific genes that explain these differences have not yet been elucidated.
Racial/ethnic differences in CRF seem to be related to extrinsic factors such as lifestyle, other CVD risk factors, and socioeconomic status.
Age, sex, and vigorous physical activity are the most influential determinants of CRF in youth. Influence of modifiable factors on CRF is likely mediated by duration, frequency, and intensity of physical activity.
There is little evidence to suggest that sedentary behavior is related to CRF in youth, once adjusted for objectively measured physical activity.
Nutrient modulation of CRF may be mediated by mitochondria number and function.
Implications Of CRF For Health Outcomes
CRF And Health Outcomes In Adults
Numerous large studies have established that in adults, low CRF is associated with greater risk for all-cause mortality, CVD events, and cancer mortality, independent of, and perhaps more strongly than, traditional risk factors.1, 123, 124 A nonlinear pattern, whereby the largest benefit occurs between the least fit and next-least fit groups, underscores the potential benefits of even modestly increasing CRF in the most sedentary individuals,1 but there are no studies in youth in this regard. Apart from mortality, low CRF in adults is also associated with greater risks for congestive heart failure, stroke, type 2 diabetes mellitus, some cancers, and neuropsychological disturbances (e.g., dementia, anxiety, and depression).1, 123, 125 Most importantly, improvements in CRF over time are associated with reduced mortality and morbidity.125, 126
CRF Tracking
In light of these well-documented benefits of optimal CRF in adults, the degree of CRF tracking from childhood to adulthood is of interest. Several studies have found that the degree to which CRF tracks into adulthood varies by methodology (e.g., measured or estimated V̇O2), sex, and length of follow up. In general, studies found that tracking was low-to-moderate for spans up to 40 years.127–130
Childhood CRF And Health Outcomes
Longitudinal data on the relationship between CRF in youth and CVD endpoints have primarily come from studies following male military recruits. These studies have collectively demonstrated inverse associations between CRF (Watts/kg) in youth and all-cause mortality (hazard ratio [HR] 0.49, 95% CI, 0.47–0.51 for highest versus lowest quintile of CRF);131 CRF and myocardial infarction (HR 0.82, 95% CI 0.80–0.85 per 1 SD higher CRF);5 CRF (Watts) and stroke (HR 0.84, 95% CI 0.81–0.88 per 1 SD higher CRF);132 CRF (Watts/kg) and heart failure (HR 1.60, 95% CI 1.44–1.77 for low vs high CRF);133 and CRF (Watts) and disability (HR 1.85, 95% CI 1.71–2.00 for low vs high CRF).134
Childhood CRF has also been associated with cardiometabolic risks and a variety of more proximal health outcomes.135 In a study of 154 youth followed for 24 years, improvement in CRF was associated with lower arterial stiffness (for each unit increase in measured CRF adjusted for body weight, carotid compliance was higher [p=0.04], even after adjustment for several risk factors).136 Cross-sectional and short-term longitudinal studies have also shown an inverse relationship of childhood CRF with adiposity,112, 137, 138 waist circumference,139 blood pressure,140 insulin resistance, nonalcoholic fatty liver disease,141, 142 and a clustered cardiometabolic risk score.135 Further, in a systematic review and meta-analysis, low CRF was significantly associated with the development of pediatric metabolic syndrome.143 In the only prospective study included in this meta-analysis, youth with metabolic syndrome had an odds ratio of 6.1 (95% CI, 1.2–60.3) for having had low CRF (mL/kg/min) seven years earlier.144
Given these associations, several studies have developed criterion-referenced CRF cut points to help identify youth with high cardiometabolic risk.145 These studies attempt to define CRF thresholds in youth to help providers identify those with the highest risk of cardiometabolic disease. In a meta-analysis combining seven published criterion-referenced standards on 9280 youth aged 8–19 years from 14 countries, CRF below 35 mL/kg/min for girls and 42 mL/kg/min for boys identified youth with higher likelihood of adverse cardiometabolic risk factors (e.g., insulin resistance, dyslipidemia, adiposity, high blood pressure) with odds ratios of 5.7 (95% CI, 4.8–6.7) for girls and 3.6 (3.0–4.3) for boys.146 For ease of interpretation, 20mSRT stages that achieve these CRF cut points for boys and girls of different ages have also been published.146
CRF and Lung Function
In a population-based study with cross-sectional and longitudinal components, each standard deviation higher CRF was associated with 2–3 percent greater predicted value of both forced expiratory volume in the first second and forced vital capacity among individuals aged 9 through 38 years. Moreover, improvements in CRF during youth were associated with better lung volumes.147 However, these improvements were not necessarily related to any measures of change in physical activity or interventions undertaken during the course of the longitudinal follow up.
Childhood CRF – Cognitive And Mental Health Outcomes
CRF has been associated with a range of cognitive and academic outcomes in youth. Academic achievement generally has been found to be positively associated with CRF, though most studies have used a cross-sectional design.3, 6, 148 Among longitudinal studies, maintaining a healthy CRF, or improving CRF over time, has been associated with better academic achievement.148–150 For example, in a recent large longitudinal study of ~400,000 Taiwanese junior high school students followed for three years, there was a dose-dependent, positive association between number of years with high CRF (top age- and sex-specific quartile vs. bottom three quartiles of CRF for all three years) and standardized test scores in the third year with between-group differences up to 0.3 standard deviations for math and science after adjustment for sex, BMI, and urbanization.151 Although effect sizes have varied across studies, even small effect sizes could be impactful at the population level.
High CRF may improve school achievement through improving cognitive abilities or psychological factors.6 Higher CRF has been associated with better attention allocation, cognition modulation (as assessed by task performance and event-related brain potentials), as well as more efficient neural activation in the prefrontal and parietal cortices (as assessed by functional magnetic resonance imaging).152 In a randomized trial involving a physical activity intervention in 8-year-olds, neural efficiency increased in direct proportion to the increase in CRF.153 In another intervention, youth receiving structured physical activity had both an increase in performance on cognitive tests and in V̇O2max (mL/kg/min), although the relationship between the change in V̇O2max and cognitive performance was not assessed.154 Higher CRF has also been associated with better relational memory (learning about the relationship between two stimuli), potentially mediated by larger bilateral hippocampal volume.152 Indeed, a variety of structural brain changes (e.g., altered cortical grey matter thickness and integrity of white matter tracts) have been observed in association with CRF, potentially related to effects of CRF on angiogenesis, neurogenesis, and neuroplasticity via increases in brain-derived neurotrophic factor.6, 7, 148
Further, better childhood CRF has also been associated with a lower incidence of mental disorders (mood disorders, psychosis, or suicidality)155 as well as improved self-worth156, 157 and life satisfaction.158, 159 In fact, in an exercise intervention study in children, effects on mental health outcomes were more strongly related to improvements in CRF than to changes in body composition.157 These mental health effects are thought to be related to structural brain changes and changes in brain signalling (e.g., serotonin).
Key points
A linear inverse relationship exists between CRF during youth years and all-cause mortality, as well as cardiovascular disease across the lifespan.
In youth, a protective inverse association has been demonstrated between CRF and multiple conditions that compound cardiovascular risk, including but not limited to metabolic syndrome, type 2 diabetes-mellitus, nonalcoholic fatty liver disease, and mental health disorders.
CRF is also positively associated with cognitive function, self-worth, and life satisfaction in youth.
Epidemiology Of CRF In Youth - Temporal Trends
Both in the US and internationally, CRF in youth is thought to have declined over the past 40 years.8–10 Globally, a decline in CRF in youth has been noted since the 1960s.11 Armstrong et al. reported a small but downward trend in the gas-analyzed V̇O2peak (mL/kg/min) in approximately 4000 youth from five countries between 1962 and 1994. While this represents the best available data on trends in gas-analyzed V̇O2peak, the study is dated.99 No study has examined trends in allometrically-scaled V̇O2peak for youth.
United States:
Using a nationally representative sample in the US, only 42 percent of 12–15 year olds had healthy CRF (mL/k/min) in 2012 (Figure 2).8 The percentage of boys who had healthy CRF decreased significantly from 65 percent in 1999–2000 to 50 percent in 2012. For girls, the percentage decreased over the same time period, though not as substantially, from 41 to 34 percent.8 Additionally, 54 percent of normal-weight youth had healthy CRF, whereas only 30 percent of youth who were overweight (BMI ≥85th percentile for age and sex) and 20 percent of youth with obesity (BMI ≥95th percentile for age and sex) had healthy CRF. This percentage did not differ by race and Hispanic origin or family income-to-poverty ratio.8 Others have reported declines in mean CRF of 0.9 mL/kg/min, per decade, between 1995 and 2013 in 166,900 US youth aged 9–17 years.9
Figure 2 -.
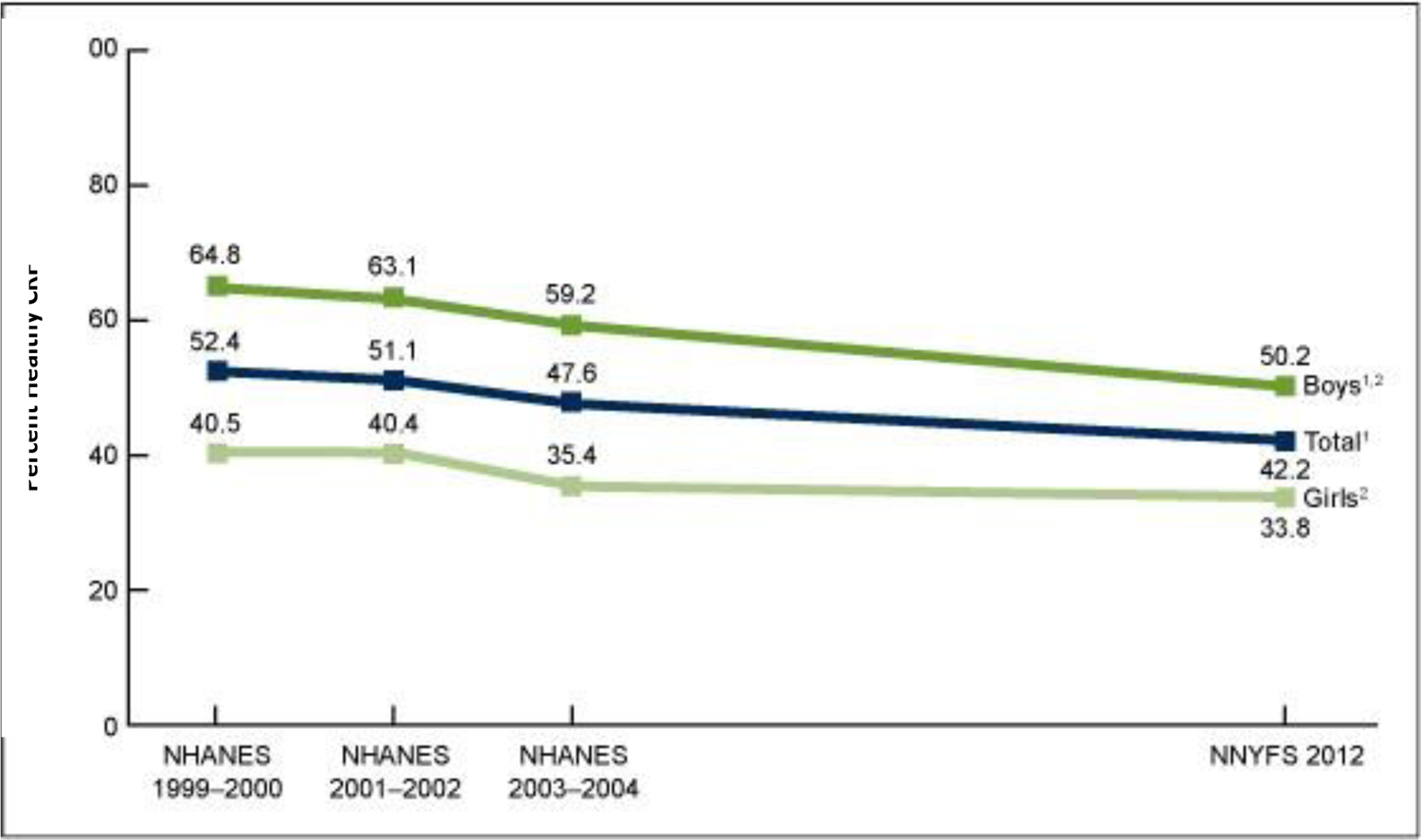
Percentage Of Youth Aged 12–15 Years Who Had Healthy Cardiorespiratory Fitness, By Sex And Survey Period: United States, 1999–2004 and 2012.8
International:
CRF declined by over seven percent from 1981 to 2014 in a recent analysis of 137 studies that reported 20mSRT data on youth aged 9–17 years.9 Temporal trends were estimated at the country-sex-age level for 19 high-income and upper middle-income countries. CRF (mL/kg/min) trends varied over time and across countries. Moderate CRF declines were seen in earlier years, these declines have then slowed and stabilized since 2000.9 However, not all data suggest that there has been a decrease. In Greece, using a measure of CRF based on the 20mSRT, there was an increase in CRF in both genders from the cohorts evaluated in 1992–93 and 2006–07.160 It should be noted that these CRF estimates based on the 20mSRT, which are only moderately correlated with V̇O2peak, remain imperfect.
A significant percent of the reported decline in CRF (mL/kg/min) may be attributable to the increasing prevalence of obesity.9 Caution should be used while interpreting associations between V̇O2peak when indexed to body weight, as indexed values may systematically underestimate V̇O2peak in youth with obesity. Thus, a weight scaled CRF may underestimate fitness in this population. For example, in a study of Norwegian military volunteers over a 22-year period, CRF declined by 8 percent, but body weight increased by 7 percent, suggesting only a minimal change in absolute V̇O2max (mL/min) over this period.161 Similarly, Andersen and colleagues162 found that there was no difference in absolute V̇O2max (mL/min) between cohorts tested in 1983, 1997, and 2003 in both boys and girls. The authors noted that there were changes in BMI and that maximal performance decreased with time, suggesting that these trends need to be validated in rigorous studies before determining if there have been secular decreases in CRF over the past several decades.162 While it is difficult to know whether declines in field tested CRF reflects a true decline in underlying cardiovascular function, or an increase in body size, or both, tests such as the 20mSRT, 1.5 mile run, and 12-minute run tests are suggestive of a decline in underlying V̇O2peak (mL/kg/min). Trends in these weight-bearing CRF tests better reflect trends in typical youth aerobic activities of daily living.
Key points
One-half of boys and two-thirds of girls aged 12–15 years do not have healthy CRF.
Only 1 in 5 youth with obesity have healthy CRF.
Gaps and Limitations
Although several tests beyond CPET are currently available to measure CRF in office and field settings in youth, there is a pressing need for standardization of testing protocols, uniform interpretation of tests and, data harmonization. Tests such as the step test may be a suitable alternative to CPET in the office setting but needs further study.
Stronger clinic-community partnerships to share results or easily access CRF assessments performed at different settings would be meaningful in providing a customized counseling and intervention.
Research is needed to further determine which interventions improve CRF in youth including youth with obesity and or low CRF. We need more research to determine thresholds at which intervention is needed.
There is need for continued collection of data to assess impact of CRF in youth on CVD outcomes as currently longitudinal data are limited.
Furthermore, research should aim to determine the reasons for the reported decline in CRF in youth in order to develop strategies to reverse this trend.
Conclusion
Healthy CRF is positively associated with cardiovascular health, academic achievement and mental wellbeing in youth. Accurate and reliably measured CRF may identify youth who would benefit from lifestyle interventions but may be missed by subjective physical activity recall, anthropometric measures, or CVD risk factor testing which are current standards of care.
Although accurate assessment of CRF in youth has traditionally relied on CPET, less resource-intensive tests, in particular, the 20mSRT in the field setting, are useful. Office-based CRF testing that can be performed by providers with little or no formal training in exercise physiology and using low-cost equipment is also superior to physical activity recall. With future research, a practical, widely applicable test to estimate CRF in office settings may become a reality and an essential part of health assessment in all youth during office visits.
Every child will benefit from a CRF estimate as part of a yearly physical. Repeated bursts of vigorous physical activity, including HIIT, improve youth CRF. Public health measures and school policies that support lifestyle improvements to improve CRF in individuals and populations are expected to result in substantial health and cognitive benefits.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the American Heart Association or the Centers for Disease Control and Prevention.
References
- 1.Ross R, Blair SN, Arena R, Church TS, Després J-P, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U, American Heart Association Physical Activity Committee of the Council on L, Cardiometabolic H, Council on Clinical C, Council on E, Prevention, Council on C, Stroke N, Council on Functional G, Translational B and Stroke C. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 2.Caspersen CJ, Powell KE and Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports (Washington, DC: 1974). 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 3.Lang JJ, Belanger K, Poitras V, Janssen I, Tomkinson GR and Tremblay MS. Systematic review of the relationship between 20m shuttle run performance and health indicators among children and youth. J Sci Med Sport. 2018;21:383–397. [DOI] [PubMed] [Google Scholar]
- 4.Ortega FB, Ruiz JR, Castillo MJ and Sjostrom M. Physical fitness in childhood and adolescence: a powerful marker of health. International Journal of Obesity (London). 2008;32:1–11. [DOI] [PubMed] [Google Scholar]
- 5.Hogstrom G, Nordstrom A and Nordstrom P. High aerobic fitness in late adolescence is associated with a reduced risk of myocardial infarction later in life: a nationwide cohort study in men. Eur Heart J. 2014;35:3133–40. [DOI] [PubMed] [Google Scholar]
- 6.Santana CCA, Azevedo LB, Cattuzzo MT, Hill JO, Andrade LP and Prado WL. Physical fitness and academic performance in youth: A systematic review. Scand J Med Sci Sports. 2017;27:579–603. [DOI] [PubMed] [Google Scholar]
- 7.Lubans D, Richards J, Hillman C, Faulkner G, Beauchamp M, Nilsson M, Kelly P, Smith J, Raine L and Biddle S. Physical Activity for Cognitive and Mental Health in Youth: A Systematic Review of Mechanisms. Pediatrics. 2016;138. [DOI] [PubMed] [Google Scholar]
- 8.Gahche J, Fakhouri T, Carroll DD, Burt VL, Wang CY and Fulton JE. Cardiorespiratory fitness levels among U.S. youth aged 12–15 years: United States, 1999–2004 and 2012. NCHS Data Brief. 2014:1–8. [PubMed] [Google Scholar]
- 9.Tomkinson GR, Lang JJ and Tremblay MS. Temporal trends in the cardiorespiratory fitness of children and adolescents representing 19 high-income and upper middle-income countries between 1981 and 2014. British Journal of Sports Medicine. 2019;53:478–486. [DOI] [PubMed] [Google Scholar]
- 10.Moraes Ferrari GL, Bracco MM, Matsudo VK and Fisberg M. Cardiorespiratory fitness and nutritional status of schoolchildren: 30-year evolution. J Pediatr (Rio J). 2013;89:366–73. [DOI] [PubMed] [Google Scholar]
- 11.Tomkinson GR and Olds TS. Secular changes in pediatric aerobic fitness test performance: the global picture. Med Sport Sci. 2007;50:46–66. [DOI] [PubMed] [Google Scholar]
- 12.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S and Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. International Journal of Behavioral Nutrition and Physical Activity 2008;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longmuir PE, Brothers JA, de Ferranti SD, Hayman LL, Van Hare GF, Matherne GP, Davis CK, Joy EA and McCrindle BW. Promotion of physical activity for children and adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;127:2147–59. [DOI] [PubMed] [Google Scholar]
- 14.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 15.Committee on Fitness Measures and Health Outcomes in Youth; Food and Nutrition Board, Fitness Measures and Health Outcomes in Youth. 2012.
- 16.Norton K, Norton L and Sadgrove D. Position statement on physical activity and exercise intensity terminology. Journal of Science and Medicine in Sport. 2010;13:496–502. [DOI] [PubMed] [Google Scholar]
- 17.Butte NF, Watson KB, Ridley K, Zakeri IF, McMurray RG, Pfeiffer KA, Crouter SE, Herrmann SD, Bassett DR, Long A, Berhane Z, Trost SG, Ainsworth BE, Berrigan D and Fulton JE. A Youth Compendium of Physical Activities: Activity Codes and Metabolic Intensities. Med Sci Sports Exerc. 2018;50:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eather N, Ridley K and Leahy A. Physiological health benefits of physical activity for young people In: Brusseau T, Fairclough S and Lubans DR, eds. The Routledge Handbook of Physical Activity for Youth Oxon, UK: Routledge; 2020: 103–120. [Google Scholar]
- 19.Malina RM, Bouchard C and Bar-Or O. Growth, Maturation, and Physical Activity: Human kinetics; 2004. [Google Scholar]
- 20.McArdle WD, Katch FI and Katch VI. Exercise physiology: energy, nutrition and human performance. Baltimore, MA: Lipponcott Williams & Wilkins; 2015. [Google Scholar]
- 21.Armstrong N and Barker AR. Endurance training and elite young athletes. Medicine and Sport Sciences. 2011;56:59–83. [DOI] [PubMed] [Google Scholar]
- 22.Rowland T, Unnithan V, Fernhall B, Baynard T and Lange C. Left ventricular response to dynamic exercise in young cyclists. Med Sci Sports Exerc. 2002;34:637–642. [DOI] [PubMed] [Google Scholar]
- 23.Nottin S, Vinet A, Stecken F, N’Guyen LD, Ounissi F, Lecoq AM and Obert P. Central and peripheral cardiovascular adaptations to exercise in endurance-trained children. Acta Physiol Scand. 2002;175:85–92. [DOI] [PubMed] [Google Scholar]
- 24.Rowland T, Wehnert M and Miller K. Cardiac responses to exercise in competitive child cyclists. Medicine and Science in Sports and Exercise. 2000;32:747–752. [DOI] [PubMed] [Google Scholar]
- 25.Rowland TW, Unnithan VB, MacFarlane NG, Gibson NG and Paton JY. Clinical manifestations of the ‘athlete’s heart’ in prepubertal male runners. Int J Sports Med. 1994;15:515–519. [DOI] [PubMed] [Google Scholar]
- 26.Tomkinson GR, Leger LA, Olds TS and Cazorla G. Secular trends in the performance of children and adolescents (1980–2000): an analysis of 55 studies of the 20m shuttle run test in 11 countries. Sports Medicine (Auckland, NZ). 2003;33:285–300. [DOI] [PubMed] [Google Scholar]
- 27.Tomkinson GR, Lang JJ, Tremblay MS, Dale M, LeBlanc AG, Belanger K, Ortega FB and Leger L. International normative 20 m shuttle run values from 1 142 026 children and youth representing 50 countries. Br J Sports Med. 2017;51:1545–1554. [DOI] [PubMed] [Google Scholar]
- 28.Takken T, Mylius CF, Paap D, Broeders W, Hulzebos HJ, Van Brussel M and Bongers BC. Reference values for cardiopulmonary exercise testing in healthy subjects - an updated systematic review. Expert Rev Cardiovasc Ther. 2019;17:413–426. [DOI] [PubMed] [Google Scholar]
- 29.Plowman SA and Meredith MD. Fitnessgram/Activitygram Reference Guide (4th Edition). Dallas, TX: The Cooper Institute; 2013. [Google Scholar]
- 30.Fick A Ueber die Messung des Blutquantums in den Herzventrikeln. Wurzburg: Sitx. der Physik-Med. Ges.; 1870. [Google Scholar]
- 31.Shephard RJ, Allen C, Benade AJ, Davies CT, Di Prampero PE, Hedman R, Merriman JE, Myhre K and Simmons R. The maximum oxygen intake. An international reference standard of cardiorespiratory fitness. Bull World Health Organ. 1968;38:757–764. [PMC free article] [PubMed] [Google Scholar]
- 32.Wold B, and Hendry L. Social and environmental factors associated with physical activity in young people In: Biddle S, Sallis J, and Cavill N, ed. Young People and Health-Enhancing Physical Activity: Evidence and Implications London: Health Education Authority; 1998: 119–132. [Google Scholar]
- 33.Rowland TW. Does peak VO2 reflect VO2max in children?: evidence from supramaximal testing. Medicine and Science in Sports and Exercise. 1993;25:689–693. [PubMed] [Google Scholar]
- 34.Armstrong N, Welsman J and Winsley R. Is peak VO2 a maximal index of children’s aerobic fitness? Int J Sports Med. 1996;17:356–9. [DOI] [PubMed] [Google Scholar]
- 35.Hansen AW, Marinus N, Remans M, Courtois I, Cools F, Calsius J, Massa G and Takken T. Exercise tolerance in obese vs. lean adolescents: a systematic review and meta-analysis. Obes Rev. 2014;15:894–904. [DOI] [PubMed] [Google Scholar]
- 36.Werneck AO, Conde J, Coelho ESMJ, Pereira A, Costa DC, Martinho D, Duarte JP, Valente-Dos-Santos J, Fernandes RA, Batista MB, Ohara D, Cyrino ES and Ronque ERV. Allometric scaling of aerobic fitness outputs in school-aged pubertal girls. BMC Pediatr. 2019;19:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper DM. Rethinking exercise testing in children: a challenge. Am J Respir Crit Care Med. 1995;152:1154–7. [DOI] [PubMed] [Google Scholar]
- 38.Batista MB, Romanzini CLP, Castro-Pinero J and Ronque ERV. Validity of Field Tests to Estimate Cardiorespiratory Fitness in Children and Adolescents: A Systematic Review. Rev Paul Pediatr. 2017;35:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Léger LA, Mercier D, Gadoury C and Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci. 1988;6:93–101. [DOI] [PubMed] [Google Scholar]
- 40.Léger L, Lambert J, Goulet A, Rowan C and Dinelle Y. Aerobic capacity of 6 to 17-year-old Quebecois--20 meter shuttle run test with 1 minute stages. Can J Appl Sport Sci. 1984;9:64–69. [PubMed] [Google Scholar]
- 41.Tomkinson GR, Lang JJ, Blanchard J, Leger LA and Tremblay MS. The 20-m Shuttle Run: Assessment and Interpretation of Data in Relation to Youth Aerobic Fitness and Health. Pediatr Exerc Sci. 2019;31:1–12. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz JR, Silva G, Oliveira N, Ribeiro JC, Oliveira JF and Mota J. Criterion-related validity of the 20-m shuttle run test in youths aged 13–19 years. J Sports Sci. 2009;27:899–906. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Pinero J, Espana-Romero V, Ortega FB, Sjostrom M, Suni J and Ruiz JR. Criterion-related validity of field-based fitness tests in youth: a systematic review. Br J Sports Med. 2010;44:934–43. [DOI] [PubMed] [Google Scholar]
- 44.Mayorga-Vega D, Bocanegra-Parrilla R, Ornelas M and Viciana J. Criterion-Related Validity of the Distance- and Time-Based Walk/Run Field Tests for Estimating Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. PLoS One. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welk GJ, De Saint-Maurice Maduro PF, Laurson KR and Brown DD. Field evaluation of the new FITNESSGRAM(R) criterion-referenced standards. Am J Prev Med. 2011;41:S131–42. [DOI] [PubMed] [Google Scholar]
- 46.Mayorga-Vega D, Aguilar-Soto P and Viciana J. Criterion-Related Validity of the 20-M Shuttle Run Test for Estimating Cardiorespiratory Fitness: A Meta-Analysis. J Sports Sci Med. 2015;14:536–47. [PMC free article] [PubMed] [Google Scholar]
- 47.Ebbeling CB, Ward A, Puleo EM, Widrick J and Rippe JM. Development of a single-stage submaximal treadmill walking test. Med Sci Sports Exerc. 1991;23:966–73. [PubMed] [Google Scholar]
- 48.Nemeth BA, Carrel AL, Eickhoff J, Clark RR, Peterson SE and Allen DB. Submaximal treadmill test predicts VO2max in overweight children. J Pediatr. 2009;154:677–81. [DOI] [PubMed] [Google Scholar]
- 49.Åstrand P-O and Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during submaximal work. J Appl Physiol. 1954;7:218–221. [DOI] [PubMed] [Google Scholar]
- 50.Woynarowska B The validity of indirect estimations of maximal oxygen uptake in children 11–12 years of age. European Journal of Applied Physio Occupied Physiology. 1980;43:19–23. [DOI] [PubMed] [Google Scholar]
- 51.Bland J, Pfeiffer K and Eisenmann JC. The PWC170: comparison of different stage lengths in 11–16 year olds. Eur J Appl Physiol. 2012;112:1955–61. [DOI] [PubMed] [Google Scholar]
- 52.Bartels B, de Groot JF and Terwee CB. The six-minute walk test in chronic pediatric conditions: a systematic review of measurement properties. Phys Ther. 2013;93:529–41. [DOI] [PubMed] [Google Scholar]
- 53.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, Wanger J, MacIntyre N, Kaminsky DA, Culver BH, Revill SM, Hernandes NA, Andrianopoulos V, Camillo CA, Mitchell KE, Lee AL, Hill CJ and Singh SJ. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. The European Respiratory Journal. 2014;44:1428–46. [DOI] [PubMed] [Google Scholar]
- 54.Committee ATS on Proficiency Standards for Clinical Pulmonary Function Laboratories: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 55.Mylius CF, Paap D and Takken T. Reference value for the 6-minute walk test in children and adolescents: a systematic review. Expert Rev Respir Med. 2016;10:1335–1352. [DOI] [PubMed] [Google Scholar]
- 56.Lammers AE, Diller GP, Odendaal D, Tailor S, Derrick G and Haworth SG. Comparison of 6-min walk test distance and cardiopulmonary exercise test performnce in children with pulmonary hypertension. Arch Dis Child. 2011;96:141–147. [DOI] [PubMed] [Google Scholar]
- 57.Takken T Six-minute walk test is a poor predictor of maximum oxygen uptake in children. Acta Paediatr. 2010;99:958; author reply 958–9. [DOI] [PubMed] [Google Scholar]
- 58.Kasch FW. A comparison of the exercise tolerance of post-rheumatic and normal boys. J Assoc Phys Mental Rehabil. 1961;15:35–40. [Google Scholar]
- 59.Hayes RM, Maldonado D, Gossett T, Shepherd T, Mehta SP and Flesher SL. Developing and Validating a Step Test of Aerobic Fitness among Elementary School Children. Physiother Can. 2019;71:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Francis K and Feinstein R. A simple height-specific and rate-specific step test for children. South Med J. 1991;84:169–74. [DOI] [PubMed] [Google Scholar]
- 61.Ortega FB, Ruiz JR, Espana-Romero V, Vicente-Rodriguez G, Martinez-Gomez D, Manios Y, Beghin L, Molnar D, Widhalm K, Moreno LA, Sjostrom M and Castillo MJ. The International Fitness Scale (IFIS): usefulness of self-reported fitness in youth. Int J Epidemiol. 2011;40:701–711. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez-Velez R, Cruz-Salazar SM, Martinez M, Cadore EL, Alonso-Martinez AM, Correa-Bautista JE, Izquierdo M, Ortega FB and Garcia-Hermoso A. Construct validity and test-retest reliability of the International Fitness Scale (IFIS) in Colombian children and adolescents aged 9–17.9 years: the FUPRECOL study. PeerJ. 2017;5:e3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarzynski MA, Ghosh S and Bouchard C. Genomic and transcriptomic predictors of response levels to endurance exercise training. The Journal of Physiology. 2017;595:2931–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfeiffer KA, Dowda M, Dishman RK, Sirard JR and Pate RR. Physical fitness and performance. Cardiorespiratory fitness in girls-change from middle to high school. Med Sci Sports Exerc. 2007;39:2234–41. [DOI] [PubMed] [Google Scholar]
- 65.Howard EN, Frierson GM, Willis BL, Haskell WL, Powell-Wiley TM and Defina LF. The impact of race and higher socioeconomic status on cardiorespiratory fitness. Med Sci Sports Exerc. 2013;45:2286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Judice PB, Silva AM, Berria J, Petroski EL, Ekelund U and Sardinha LB. Sedentary patterns, physical activity and health-related physical fitness in youth: a cross-sectional study. Int J Behav Nutr Phys Act. 2017;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shikany JM, Jacobs DR Jr., Lewis CE, Steffen LM, Sternfeld B, Carnethon MR and Richman JS. Associations between food groups, dietary patterns, and cardiorespiratory fitness in the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr. 2013;98:1402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendelson M, Michallet AS, Tonini J, Favre-Juvin A, Guinot M, Wuyam B and Flore P. Low Cardiorespiratory Fitness is Partially Linked to Ventilatory Factors in Obese Adolescents. Pediatr Exerc Sci. 2016;28:87–97. [DOI] [PubMed] [Google Scholar]
- 69.Ortega FB, Ruiz JR, Labayen I, Martinez-Gomez D, Vicente-Rodriguez G, Cuenca-Garcia M, Gracia-Marco L, Manios Y, Beghin L, Molnar D, Polito A, Widhalm K, Marcos A, Gonzalez-Gross M, Kafatos A, Breidenassel C, Moreno LA, Sjostrom M and Castillo MJ. Health inequalities in urban adolescents: role of physical activity, diet, and genetics. Pediatrics. 2014;133:e884–95. [DOI] [PubMed] [Google Scholar]
- 70.Santos R, Mota J, Okely AD, Pratt M, Moreira C, Coelho-e-Silva MJ, Vale S and Sardinha LB. The independent associations of sedentary behaviour and physical activity on cardiorespiratory fitness. Br J Sports Med. 2014;48:1508–1512. [DOI] [PubMed] [Google Scholar]
- 71.Hoehner CM, Handy SL, Yan Y, Blair SN and Berrigan D. Association between neighborhood walkability, cardiorespiratory fitness and body-mass index. Soc Sci Med. 2011;73:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ombrellaro KJ, Perumal N, Zeiher J, Hoebel J, Ittermann T, Ewert R, Dorr M, Keil T, Mensink GBM and Finger JD. Socioeconomic Correlates and Determinants of Cardiorespiratory Fitness in the General Adult Population: a Systematic Review and Meta-Analysis. Sports Med Open. 2018;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai Y, Saint-Maurice PF, Welk GJ, Allums-Featherston K and Candelaria N. Explaining Disparities in Youth Aerobic Fitness and Body Mass Index: Relative Impact of Socioeconomic and Minority Status. The Journal of School Health. 2016;86:787–793. [DOI] [PubMed] [Google Scholar]
- 74.Bouchard C, Rankinen T and Timmons JA. Genomics and genetics in the biology of adaptation to exercise. Compr Physiol. 2011;1:1603–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams CJ, Williams MG, Eynon N, Ashton KJ, Little JP, Wisloff U and Coombes JS. Genes to predict VO2max trainability: a systematic review. BMC Genomics. 2017;18:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS and Rao DC. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. Journal of Applied Physiology (Bethesda, Md: 1985). 1999;87:1003–8. [DOI] [PubMed] [Google Scholar]
- 77.Bouchard C, Sarzynski MA, Rice TK, Krause WE, Church TS, Sung YJ, Rao DC and Rankinen T. Genomic predictors of the maximal O(2) uptake response to standardized exercise training programs. J Appl Physiol. 1985;110:1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venezia AC and Roth SM. Recent Research in the Genetics of Exercise Training Adaptation. Med Sport Sci. 2016;61:29–40. [DOI] [PubMed] [Google Scholar]
- 79.Rankinen T, Fuku N, Wolfarth B, Wang G, Sarzynski MA, Alexeev DG, Ahmetov II, Boulay MR, Cieszczyk P, Eynon N, Filipenko ML, Garton FC, Generozov EV, Govorun VM, Houweling PJ, Kawahara T, Kostryukova ES, Kulemin NA, Larin AK, Maciejewska-Karlowska A, Miyachi M, Muniesa CA, Murakami H, Ospanova EA, Padmanabhan S, Pavlenko AV, Pyankova ON, Santiago C, Sawczuk M, Scott RA, Uyba VV, Yvert T, Perusse L, Ghosh S, Rauramaa R, North KN, Lucia A, Pitsiladis Y and Bouchard C. No Evidence of a Common DNA Variant Profile Specific to World Class Endurance Athletes. PLoS One. 2016;11:e0147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Armstrong N and Welsman J. Development of peak oxygen uptake from 11–16 years determined using both treadmill and cycle ergometry. Eur J Appl Physiol. 2019;119:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catley MJ and Tomkinson GR. Normative health-related fitness values for children: analysis of 85347 test results on 9–17-year-old Australians since 1985. Br J Sports Med. 2013;47:98–108. [DOI] [PubMed] [Google Scholar]
- 82.Ortega FB, Artero EG, Ruiz JR, Espana-Romero V, Jimenez-Pavon D, Vicente-Rodriguez G, Moreno LA, Manios Y, Beghin L, Ottevaere C, Ciarapica D, Sarri K, Dietrich S, Blair SN, Kersting M, Molnar D, Gonzalez-Gross M, Gutierrez A, Sjostrom M and Castillo MJ. Physical fitness levels among European adolescents: the HELENA study. Br J Sports Med. 2011;45:20–9. [DOI] [PubMed] [Google Scholar]
- 83.Winsley RJ, Fulford J, Roberts AC, Welsman JR and Armstrong N. Sex difference in peak oxygen uptake in prepubertal children. J Sci Med Sport. 2009;12:647–51. [DOI] [PubMed] [Google Scholar]
- 84.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC and Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2007;292:R1271–8. [DOI] [PubMed] [Google Scholar]
- 85.Swift DL, Johannsen NM, Earnest CP, Newton RL Jr., McGee JE and Church TS. Cardiorespiratory Fitness and Exercise Training in African Americans. Prog Cardiovasc Dis. 2017;60:96–102. [DOI] [PubMed] [Google Scholar]
- 86.Ong KC, Loo CM, Ong YY, Chan SP, Earnest A and Saw SM. Predictive values for cardiopulmonary exercise testing in sedentary Chinese adults. Respirology. 2002;7:225–231. [DOI] [PubMed] [Google Scholar]
- 87.Pandey A, Park BD, Ayers C, Das SR, Lakoski S, Matulevicius S, de Lemos JA and Berry JD. Determinants of Racial/Ethnic Differences in Cardiorespiratory Fitness (from the Dallas Heart Study). The American Journal of Cardiology. 2016;118:499–503. [DOI] [PubMed] [Google Scholar]
- 88.Shaibi GQ, Ball GD and Goran MI. Aerobic fitness among Caucasian, African-American, and Latino youth. Ethn Dis. 2006;16:120–125. [PubMed] [Google Scholar]
- 89.Lang JJ, Tremblay MS, Leger L, Olds T and Tomkinson GR. International variability in 20 m shuttle run performance in children and youth: who are the fittest from a 50-country comparison? A systematic literature review with pooling of aggregate results. Br J Sports Med. 2018;52:276. [DOI] [PubMed] [Google Scholar]
- 90.Bansal N, Mahadin DR, Smith R, French M, Karpawich PP and Aggarwal S. Comparative Cardiorespiratory Fitness in Children: Racial Disparity May Begin Early in Childhood. Pediatric Cardiology. 2019;40:1183–1189. [DOI] [PubMed] [Google Scholar]
- 91.Ferreira I, Gbatu PT and Boreham CA. Gestational Age and Cardiorespiratory Fitness in Individuals Born At Term: A Life Course Study. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Welsh L, Kirkby J, Lum S, Odendaal D, Marlow N, Derrick G and Stocks J. The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax. 2010;65:165–72. [DOI] [PubMed] [Google Scholar]
- 93.Edwards MO, Kotecha SJ, Lowe J, Watkins WJ, Henderson AJ and Kotecha S. Effect of preterm birth on exercise capacity: A systematic review and meta-analysis. Pediatr Pulmonol. 2015;50:293–301. [DOI] [PubMed] [Google Scholar]
- 94.Ruiz JR, Rizzo NS, Hurtig-Wennlöf A, Ortega FB, W àrnberg J and Sjöström M. Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. American Journal of Clinical Nutrition. 2006;84:299–303. [DOI] [PubMed] [Google Scholar]
- 95.Gralla MH, McDonald SM, Breneman C, Beets MW and Moore JB. Associations of objectively measured vigorous physical activity with body composition, cardiorespiratory fitness, and cardiometabolic health in youth: a review. Am J Lifestyle Med. 2019;13:61–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hussey J, Bell C, Bennett K, O’Dwyer J and Gormley J. Relationship between the intensity of physical activity, inactivity, cardiorespiratory fitness and body composition in 7–10-year-old Dublin children. British Journal of Sports Medicine. 2007;41:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gutin B, Yin Z, Humphries MC and Barbeau P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. The American Journal Of Clinical Nutrition. 2005;81:746–50. [DOI] [PubMed] [Google Scholar]
- 98.Baquet G, van Praagh E and Berthoin S. Endurance training and aerobic fitness in young people. Sports Medicine (Auckland, NZ). 2003;33:1127–43. [DOI] [PubMed] [Google Scholar]
- 99.Armstrong N, Tomkinson G and Ekelund U. Aerobic fitness and its relationship to sport, exercise training and habitual physical activity during youth. Br J Sports Med. 2011;45:849–858. [DOI] [PubMed] [Google Scholar]
- 100.Costigan SA, Eather N, Plotnikoff RC, Taaffe DR and Lubans DR. High intensity interval training for improving health-related fitness in adolescents: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1253–1261. [DOI] [PubMed] [Google Scholar]
- 101.Janssen X, Mann KD, Basterfield L, Parkinson KN, Pearce MS, Reilly JK, Adamson AJ and Reilly JJ. Development of sedentary behavior across childhood and adolescence: longitudinal analysis of the Gateshead Millennium Study. Int J Behav Nutr Phys Act. 2016;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang L, Cao C, Kantor ED, Nguyen LH, Zheng X, Park Y, Giovannucci EL, Matthews CE, Colditz GA and Cao Y. Trends in Sedentary Behavior Among the US Population, 2001–2016. JAMA. 2019;321:1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, Lewis CE, Owen N, Perry CK, Siddique J and Yong CM. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation. 2016;134:e262–79. [DOI] [PubMed] [Google Scholar]
- 104.Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N and Powell KE. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. The Lancet. 2016;388:1302–1310. [DOI] [PubMed] [Google Scholar]
- 105.Aggio D, Ogunleye AA, Voss C and Sandercock GR. Temporal relationships between screen-time and physical activity with cardiorespiratory fitness in English schoolchildren: a 2-year longitudinal study. Prev Med. 2012;55:37–9. [DOI] [PubMed] [Google Scholar]
- 106.Martinez-Gomez D, Ortega FB, Ruiz JR, Vicente-Rodriguez G, Veiga OL, Widhalm K, Manios Y, Beghin L, Valtuena J, Kafatos A, Molnar D, Moreno LA, Marcos A, Castillo MJ and Sjostrom M. Excessive sedentary time and low cardiorespiratory fitness in European adolescents: the HELENA study. Arch Dis Child. 2011;96:240–6. [DOI] [PubMed] [Google Scholar]
- 107.Grund A, Krause H, Siewers M, Rieckert H and Muller MJ. Is TV viewing an index of physical activity and fitness in overweight and normal weight children? Public Health Nutr. 2001;4:1245–51. [DOI] [PubMed] [Google Scholar]
- 108.Hardy LL, Dobbins TA, Denney-Wilson EA, Okely AD and Booth ML. Sedentariness, small-screen recreation, and fitness in youth. Am J Prev Med. 2009;36:120–5. [DOI] [PubMed] [Google Scholar]
- 109.Denton SJ, Trenell MI, Plotz T, Savory LA, Bailey DP and Kerr CJ. Cardiorespiratory fitness is associated with hard and light intensity physical activity but not time spent sedentary in 10–14 year old schoolchildren: the HAPPY study. PLoS One. 2013;8:e61073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cliff DP, Hesketh KD, Vella SA, Hinkley T, Tsiros MD, Ridgers ND, Carver A, Veitch J, Parrish AM, Hardy LL, Plotnikoff RC, Okely AD, Salmon J and Lubans DR. Objectively measured sedentary behaviour and health and development in children and adolescents: systematic review and meta-analysis Obes Rev. 2016;17:330–344. [DOI] [PubMed] [Google Scholar]
- 111.Pate RR, Wang CY, Dowda M, Farrell SW and O’Neill JR. Cardiorespiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999–2002 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2006;160:1005–12. [DOI] [PubMed] [Google Scholar]
- 112.Byrd-Williams CE, Shaibi GQ, Sun P, Lane CJ, Ventura EE, Davis JN, Kelly LA and Goran MI. Cardiorespiratory fitness predicts changes in adiposity in overweight Hispanic boys. Obesity (Silver Spring). 2008;16:1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schnurr TM, Gjesing AP, Sandholt CH, Jonsson A, Mahendran Y, Have CT, Ekstrom CT, Bjerregaard AL, Brage S, Witte DR, Jorgensen ME, Aadahl M, Thuesen BH, Linneberg A, Eiberg H, Pedersen O, Grarup N, Kilpelainen TO and Hansen T. Genetic Correlation between Body Fat Percentage and Cardiorespiratory Fitness Suggests Common Genetic Etiology. PLoS One. 2016;11:e0166738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Browning MG, Bean MK, Wickham EP, Stern M and Evans RK. Cardiometabolic and Fitness Improvements in Obese Girls Who Either Gained or Lost Weight during Treatment. The Journal of Pediatrics. 2015;166:1364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Howe AS, Skidmore PM, Parnell WR, Wong JE, Lubransky AC and Black KE. Cardiorespiratory fitness is positively associated with a healthy dietary pattern in New Zealand adolescents. Public Health Nutr. 2016;19:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zaqout M, Vyncke K, Moreno LA, De Miguel-Etayo P, Lauria F, Molnar D, Lissner L, Hunsberger M, Veidebaum T, Tornaritis M, Reisch LA, Bammann K, Sprengeler O, Ahrens W and Michels N. Determinant factors of physical fitness in European children. International journal of public health. 2016;61:573–82. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalez-Freire M, Scalzo P, D’Agostino J, Moore ZA, Diaz-Ruiz A, Fabbri E, Zane A, Chen B, Becker KG, Lehrmann E, Zukley L, Chia CW, Tanaka T, Coen PM, Bernier M, de Cabo R and Ferrucci L. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell. 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Delille HK, Alves R and Schrader M. Biogenesis of peroxisomes and mitochondria: linked by division. Histochem Cell Biol. 2009;131:441–6. [DOI] [PubMed] [Google Scholar]
- 119.Trevino-Saldana N and Garcia-Rivas G. Regulation of Sirtuin-Mediated Protein Deacetylation by Cardioprotective Phytochemicals. Oxid Med Cell Longev. 2017;2017:1750306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO and Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–59. [DOI] [PubMed] [Google Scholar]
- 121.Albarwani S, Al-Hashmi K, Al-Abri M, Jaju D and Hassan MO. Effects of overweight and leisure-time activities on aerobic fitness in urban and rural adolescents. Metab Syndr Relat Disord. 2009;7:369–74. [DOI] [PubMed] [Google Scholar]
- 122.Blackorby C and Donaldson D. A theoretical treatmetn of indices of absolute inequality. International Economic Review. 1980;21. [Google Scholar]
- 123.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N and Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–35. [DOI] [PubMed] [Google Scholar]
- 124.Imboden MT, Harber MP, Whaley MH, Finch WH, Bishop DL and Kaminsky LA. Cardiorespiratory Fitness and Mortality in Healthy Men and Women. J Am Coll Cardiol. 2018;72:2283–2292. [DOI] [PubMed] [Google Scholar]
- 125.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e66. [DOI] [PubMed] [Google Scholar]
- 126.Ehrman JK, Brawner CA, Al-Mallah MH, Qureshi WT, Blaha MJ and Keteyian SJ. Cardiorespiratory Fitness Change and Mortality Risk Among Black and White Patients: Henry Ford Exercise Testing (FIT) Project. Am J Med. 2017;130:1177–1183. [DOI] [PubMed] [Google Scholar]
- 127.Soric M, Jembrek Gostovic M, Gostovic M, Hocevar M and Misigoj-Durakovic M. Tracking of BMI, fatness and cardiorespiratory fitness from adolescence to middle adulthood: the Zagreb Growth and Development Longitudinal Study. Ann Hum Biol. 2014;41:238–43. [DOI] [PubMed] [Google Scholar]
- 128.Van Oort C, Jackowski SA, Eisenmann JC, Sherar LB, Bailey DA, Mirwald R and Baxter-Jones AD. Tracking of aerobic fitness from adolescence to mid-adulthood. Ann Hum Biol. 2013;40:547–53. [DOI] [PubMed] [Google Scholar]
- 129.Trudeau F, Shephard RJ, Arsenault F and Laurencelle L. Tracking of physical fitness from childhood to adulthood. Can J Appl Physiol. 2003;28:257–71. [DOI] [PubMed] [Google Scholar]
- 130.Campbell PT, Katzmarzyk PT, Malina RM, Rao DC, Perusse L and Bouchard C. Prediction of physical activity and physical work capacity (PWC150) in young adulthood from childhood and adolescence with consideration of parental measures. American Journal of Human Biology: The Official Journal of the Human Biology Council. 2001;13:190–6. [DOI] [PubMed] [Google Scholar]
- 131.Hogstrom G, Nordstrom A and Nordstrom P. Aerobic fitness in late adolescence and the risk of early death: a prospective cohort study of 1.3 million Swedish men. Int J Epidemiol. 2016;45:1159–1168. [DOI] [PubMed] [Google Scholar]
- 132.Hogstrom G, Nordstrom A, Eriksson M and Nordstrom P. Risk factors assessed in adolescence and the later risk of stroke in men: a 33-year follow-up study. Cerebrovasc Dis. 2015;39:63–71. [DOI] [PubMed] [Google Scholar]
- 133.Lindgren M, Aberg M, Schaufelberger M, Aberg D, Schioler L, Toren K and Rosengren A. Cardiorespiratory fitness and muscle strength in late adolescence and long-term risk of early heart failure in Swedish men. European Journal of Preventive Cardiology. 2017;24:876–884. [DOI] [PubMed] [Google Scholar]
- 134.Rabiee R, Agardh E, Kjellberg K and Falkstedt D. Low cardiorespiratory fitness in young adulthood and future risk of disability pension: a follow-up study until 59 years of age in Swedish men. J Epidemiol Community Health. 2015;69:266–71. [DOI] [PubMed] [Google Scholar]
- 135.Agbaje AO, Haapala EA, Lintu N, Viitasalo A, Barker AR, Takken T, Tompuri T, Lindi V and Lakka TA. Peak oxygen uptake cut-points to identify children at increased cardiometabolic risk - The PANIC Study. Scand J Med Sci Sports. 2018;29:16–24. [DOI] [PubMed] [Google Scholar]
- 136.Ferreira I, Twisk JW, Stehouwer CD, van Mechelen W and Kemper HC. Longitudinal changes in VO2max: associations with carotid IMT and arterial stiffness. Med Sci Sports Exerc. 2003;35:1670–8. [DOI] [PubMed] [Google Scholar]
- 137.Johnson MS, Figueroa-Colon R, Herd SL, Fields DA, Sun M, Hunter GR and Goran MI. Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics. 2000;106:E50. [DOI] [PubMed] [Google Scholar]
- 138.Koutedakis Y, Bouziotas C, Flouris AD and Nelson PN. Longitudinal modeling of adiposity in periadolescent Greek schoolchildren. Med Sci Sports Exerc. 2005;37:2070–4. [DOI] [PubMed] [Google Scholar]
- 139.Sigal RJ, Alberga AS, Goldfield GS, Prud’homme D, Hadjiyannakis S, Gougeon R, Phillips P, Tulloch H, Malcolm J, Doucette S, Wells GA, Ma J and Kenny GP. Effects of aerobic training, resistance training, or both on percentage body fat and cardiometabolic risk markers in obese adolescents: the healthy eating aerobic and resistance training in youth randomized clinical trial. JAMA pediatrics. 2014;168:1006–14. [DOI] [PubMed] [Google Scholar]
- 140.Agostinis-Sobrinho C, Ruiz JR, Moreira C, Abreu S, Lopes L, Oliveira-Santos J, Mota J and Santos R. Cardiorespiratory Fitness and Blood Pressure: A Longitudinal Analysis. The Journal of Pediatrics. 2018;192:130–135. [DOI] [PubMed] [Google Scholar]
- 141.Kelishadi R, Cook SR, Amra B and Adibi A. Factors associated with insulin resistance and non-alcoholic fatty liver disease among youths. Atherosclerosis. 2009;204:538–43. [DOI] [PubMed] [Google Scholar]
- 142.Schmidt MD, Magnussen CG, Rees E, Dwyer T and Venn AJ. Childhood fitness reduces the long-term cardiometabolic risks associated with childhood obesity. Int J Obes (Lond). 2016;40:1134–40. [DOI] [PubMed] [Google Scholar]
- 143.Oliveira RG and Guedes DP. Physical Activity, Sedentary Behavior, Cardiorespiratory Fitness and Metabolic Syndrome in Adolescents: Systematic Review and Meta-Analysis of Observational Evidence. PLoS One. 2016;11:e0168503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McMurray RG, Bangdiwala SI, Harrell JS and Amorim LD. Adolescents with metabolic syndrome have a history of low aerobic fitness and physical activity levels. Dyn Med. 2008;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lang JJ, Tremblay MS, Ortega FB, Ruiz JR and Tomkinson GR. Review of criterion-referenced standards for cardiorespiratory fitness: what percentage of 1,142,026 international children and youth are apparently healthy? Br J Sports Med. 2019;53:953–958. [DOI] [PubMed] [Google Scholar]
- 146.Ruiz JR, Cavero-Redondo I, Ortega FB, Welk GJ, Andersen LB and Martinez-Vizcaino V. Cardiorespiratory fitness cut points to avoid cardiovascular disease risk in children and adolescents; what level of fitness should raise a red flag? A systematic review and meta-analysis. Br J Sports Med. 2016;50:1451–1458. [DOI] [PubMed] [Google Scholar]
- 147.Hancox RJ and Rasmussen F. Does physical fitness enhance lung function in children and young adults? Eur Respir J. 2018;51:1701374. [DOI] [PubMed] [Google Scholar]
- 148.Marques A, Santos DA, Hillman CH and Sardinha LB. How does academic achievement relate to cardiorespiratory fitness, self-reported physical activity and objectively reported physical activity: a systematic review in children and adolescents aged 6–18 years. Br J Sports Med. 2018;52:1039. [DOI] [PubMed] [Google Scholar]
- 149.Sardinha LB, Marques A, Minderico C, Palmeira A, Martins S, Santos DA and Ekelund U. Longitudinal Relationship between Cardiorespiratory Fitness and Academic Achievement. Med Sci Sports Exerc. 2016;48:839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wittberg RA, Northrup KL and Cottrell LA. Children’s aerobic fitness and academic achievement: a longitudinal examination of students during their fifth and seventh grade years. Am J Public Health. 2012;102:2303–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hsieh SS, Tsai JR, Chang SH, Ho JY, Chen JF, Chen PH, Sung YT and Hung TM. The subject-dependent, cumulative, and recency association of aerobic fitness with academic performance in Taiwanese junior high school students. BMC Pediatr. 2019;19:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haapala EA. Cardiorespiratory fitness and motor skills in relation to cognition and academic performance in children - a review. Journal of Human Kinetics. 2013;36:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Drollette ES, Pontifex MB, Raine LB, Scudder MR, Moore RD, Kao SC, Westfall DR, Wu CT, Kamijo K, Castelli DM, Khan NA, Kramer AF and Hillman CH. Effects of the FITKids physical activity randomized controlled trial on conflict monitoring in youth. Psychophysiology. 2018;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR, Drollette ES, Moore RD, Wu CT and Kamijo K. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics. 2014;134:e1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tacchi MJ, Heggelund J and Scott J. Predictive validity of objective measures of physical fitness for the new onset of mental disorders in adolescents and young adults. Early Intervention in Psychiatry. 2019. [DOI] [PubMed] [Google Scholar]
- 156.Reddon H, Meyre D and Cairney J. Physical Activity and Global Self-worth in a Longitudinal Study of Children. Med Sci Sports Exerc. 2017;49:1606–1613. [DOI] [PubMed] [Google Scholar]
- 157.Goldfield GS, Adamo KB, Rutherford J and Murray M. The effects of aerobic exercise on psychosocial functioning of adolescents who are overweight or obese. J Pediatr Psychol. 2012;37:1136–47. [DOI] [PubMed] [Google Scholar]
- 158.Padilla-Moledo C, Castro-Pinero J, Ortega FB, Mora J, Marquez S, Sjostrom M and Ruiz JR. Positive health, cardiorespiratory fitness and fatness in children and adolescents. Eur J Public Health. 2012;22:52–6. [DOI] [PubMed] [Google Scholar]
- 159.Rodriguez-Ayllon M, Cadenas-Sanchez C, Esteban-Cornejo I, Migueles JH, Mora-Gonzalez J, Henriksson P, Martin-Matillas M, Mena-Molina A, Molina-Garcia P, Estevez-Lopez F, Enriquez GM, Perales JC, Ruiz JR, Catena A and Ortega FB. Physical fitness and psychological health in overweight/obese children: A cross-sectional study from the ActiveBrains project. J Sci Med Sport. 2018;21:179–184. [DOI] [PubMed] [Google Scholar]
- 160.Smpokos EA, Linardakis M, Papadaki A, Lionis C and Kafatos A. Secular trends in fitness, moderate-to-vigorous physical activity, and TV-viewing among first grade school children of Crete, Greece between 1992/93 and 2006/07. J Sci Med Sport. 2012;15:129–35. [DOI] [PubMed] [Google Scholar]
- 161.Dyrstad SM, Aandstad A and Hallen J. Aerobic fitness in young Norwegian men: a comparison between 1980 and 2002. Scand J Med Sci Sports. 2005;15:298–303. [DOI] [PubMed] [Google Scholar]
- 162.Andersen LB, Froberg K, Kristensen PL, Moller NC, Resaland GK and Anderssen SA. Secular trends in physical fitness in Danish adolescents. Scand J Med Sci Sports. 2010;20:757–63. [DOI] [PubMed] [Google Scholar]
- 163.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP and American College of Sports M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. [DOI] [PubMed] [Google Scholar]
- 164.Borg G Borg’s perceived exertion and pain scales. Champaign, IL.: Human Kinetics; 1998. [Google Scholar]
- 165.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SFM, Altenburg TM and Chinapaw MJM. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. International Journal of Behavioral Nutrition and Physical Activity. 2017;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR Jr., Schmitz KH, Emplaincourt PO, Jacobs DR Jr., and Leon AS. Compedium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S516. [DOI] [PubMed] [Google Scholar]


