ABSTRACT
Fecal microbiota transplantation is now recommended for treating recurrent forms of Clostridioides difficile infection. Recent studies have reported protocols using capsules of either frozen or freeze-dried stool allowing oral administration in in- and out-patient settings. However, a central question remains the viability, engraftment, and efficacy of the microbiome over time during storage life. This study shows that both the freeze-drying and freezing procedures for fecal samples allowed preserving viability, short-chain fatty acids concentration, and anti-Clostridioides difficile properties of microbiota without significant alteration after storage for 12 months. Fecal transplantation with freeze-dried microbiota allowed engraftment of microbiota leading to clearance of Clostridioides difficile infection in a preclinical murine model with a survival rate of 70% versus 53-60% in mice treated with frozen inocula, and 20% in the untreated group. Moreover, the freeze-dried powder can be used to fill oral hard capsules using a very low amount (0.5%) of glidant excipient, allowing oral formulation. Altogether, this study showed that freeze-dried inocula can be used for the treatment of Clostridioides difficile infection with long-lasting stability of the fecal microbiota. This formulation facilitates biobanking and allows the use of hard capsules, an essential step to simplify patient access to treatment.
KEYWORDS: Fecal microbiota transplant, Clostridioides difficile, freeze-dried microbiota, frozen microbiota, pre-clinical model
Introduction
Clostridioides difficile (CD, formerly Clostridium difficile), are one of the most common health care-associated infections, representing a major burden in terms of mortality, morbidity, and cost.1,2 The increasing prevalence, severity, and mortality related to CD infections in the last decade have led to a growing interest in new therapeutics based on the manipulation of the microbiota. Many concordant studies, including randomized controlled trials, have shown that fecal microbiota transplantation (FMT) is highly effective for treating recurrent forms of CD infection relative to conventional antibiotic treatment, with cure rates reaching 85% to 95%,3–6 and a recent report suggests it may be life-saving in severe infections.7 Based on these trials, FMT is now strongly recommended by the European and US infectious disease societies.8,9 Beneficial effects of FMT have also been described in case series and small prospective trials for several other conditions associated with gastrointestinal microbial dysbiosis, including inflammatory bowel diseases,10 metabolic disorders,11 neuropsychiatric conditions,12 and the eradication of multi-resistant bacteria, i.e. extended-spectrum β-lactamase and/or carbapenem-resistant Enterobacteriaceae.13 However, no evidence-based recommendations have yet emerged for such conditions, except for clinical research.
The initial methods for FMT proposed the use of fresh material re-administered through retention enema, colonoscopy, or naso-duodenal delivery within a maximum period of 6 h between donor stool emission and recipient administration. Now, frozen fecal material is widely used and several randomized controlled trials have confirmed that the use of frozen-and-thawed material is non-inferior (versus fresh) in terms of cure rates for recurrent CD infection.4,6 A few studies have reported protocols using encapsulated frozen stool allowing oral administration in in- and out-patient settings with high cure rates in patients with recurrent infections.14–16 Pharmaceutical grade glycerol with final concentration ranging from 10% (G10) to 80% (G80) is currently used as microbial cryoprotectant for the preparation of fecal microbiota transplants.14–17 Costello et al. tested bacterial viability after 6 months of storage at −80°C using culture on plate media and showed that the microbiome remained largely unchanged when stored in G10.18 Recently, protocols using freeze-dried (FD) encapsulated fecal microbiota have been reported with similar cure rates.19–21 These new formulations for FMT represent important advances toward improving access to this therapy. However, apart from the health screening, no standard microbial and biological characterization of fecal transplant does exist and current practice remains mainly empirical. Minimal guidelines exist for the preparation and preservation of fecal transplant17,22 but a central question remains the consequence of long-term storage. In a model of CD infection, Jiang et al. showed that mice treated with frozen or FD samples stored for ≤ 7-months were protected from post-CD challenge diarrhea.23 Thus, the freeze-drying procedure seems to be adapted to FMT, but we lack scientific rationale establishing the biological activity over time of fecal samples after freeze-drying.
In the present study, we followed over 12 months of storage, the bacterial viability, metabolite production, engraftment in a recipient germ-free model, and CD inhibition of new FD versus classical frozen (G10 and G80) fecal transplants. The anti-CD activity was evaluated, both in vitro and in a murine preclinical model of CD infection. We show that the freeze-drying procedure preserves the biological activity of fecal samples after storage for 12 months at 4°C. This formulation is also compatible with the filling of hard oral capsules allowing oral formulation, and represents an easier solution for biobanking.
Results
Microbiota viability in freeze-dried and frozen fecal samples
We compared the viability of representative cultivable microbes from six human stool samples (donors #S1 to #S6) either fresh or preserved for a twelve-month period by either freeze-drying (samples FD, patent application WO2017103225A1) or freezing in 10% or 80% glycerol (samples G10 and G80). Total number of cultivable bacteria was 10.9 ± 0.6 log CFU/g of feces in fresh stool samples. No significant decrease in either total culturable bacteria or Extremely Oxygen-Sensitive bacteria (EOS) was observed until 12 months of storage regardless the procedure of preservation (Figure 1(a) and Table S1 supplementary data). Concerning anaerobic genera, there was no effect on the viability of Bifidobacterium spp. for up to 12 months, regardless of the procedure. In contrast, there was significantly less Bacteroides immediately after processing in the FD and G80 samples than in fresh stools (p < .001) and a subsequent decrease in the FD samples over time (p < .01 at M6 and M12) (Figure 1(a), Table S1 supplementary data). Concerning facultative aero-anaerobic genera, there was less Enterobacteriaceae after M3 in the FD samples (p = .04 at M3 and p < .01 at M6 and M12) and less Enterococcus spp. after 6 months of storage (p < .01 at M6 and p = .04 at M12) than in fresh stools. No other significant difference was observed regardless of the procedure (Figure 1(b), Table S1 supplementary data). No variation in Lactobacilli was observed over time whatever the procedure. Cultivable Clostridia (i.e. cluster I–II) were at very low levels within the microbiota whatever the sample, making it impossible to count. CD was only detected in one out of the six samples. Finally, the different profiles observed within the six donors at inclusion were preserved during storage whatever the process of preservation. No further significant modification was observed in two samples until 18 months of storage (Table S1, supplementary data).
Figure 1.
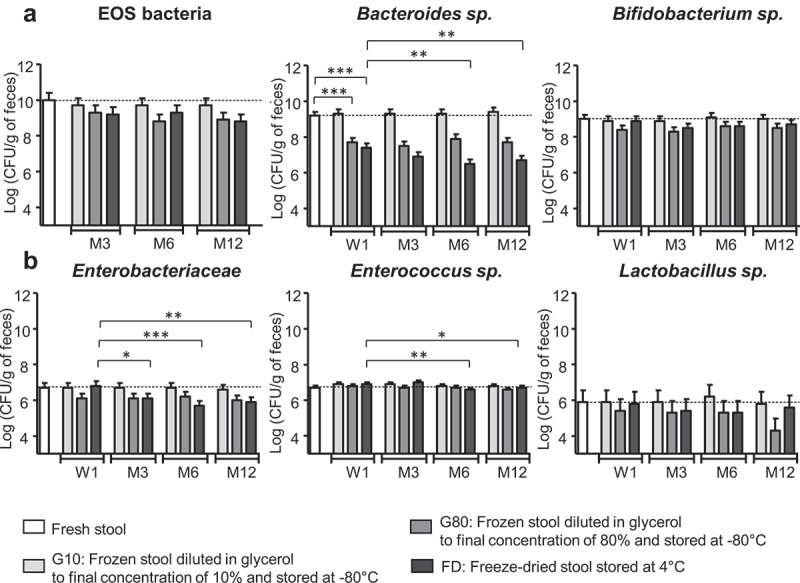
Microbiota viability of freeze-dried and frozen human fecal samples over 12 months of storage. (a) Dominant microbiota: EOS bacteria (n = 6), Bacteroides sp. (n = 6), and Bifidobacterium sp. (n = 6) (b) Subdominant microbiota: Enterobacteriaceae (n = 6), Enterococcus sp. (n = 5), and Lactobacillus sp. (n = 3). Time study: Week 1, W1; Month 3,6 and 12: M3, M6, M12. Results are expressed as the mean ± SEM. Statistical significance: *<0.05, **<0.01, **<0.001.
Short-chain fatty acids concentrations in fecal samples after 12 months of storage
Total short-chain fatty acids (SCFA) concentrations ranged from 114.8 ± 31 µmol per g of feces in FD samples to 52.7 ± 30.5 µmol per g of feces in samples frozen without any cryoprotectant at M12. SCFA concentrations were significantly higher in stool samples preserved in G10, G80, and FD than in the corresponding samples stored without cryoprotectant (p < .001). This was related to higher concentrations of acetate in the G10 (p = .028), G80 (p = .049), and FD (p < .001) samples (Figure 2(a), Table S2 supplementary data).
Figure 2.
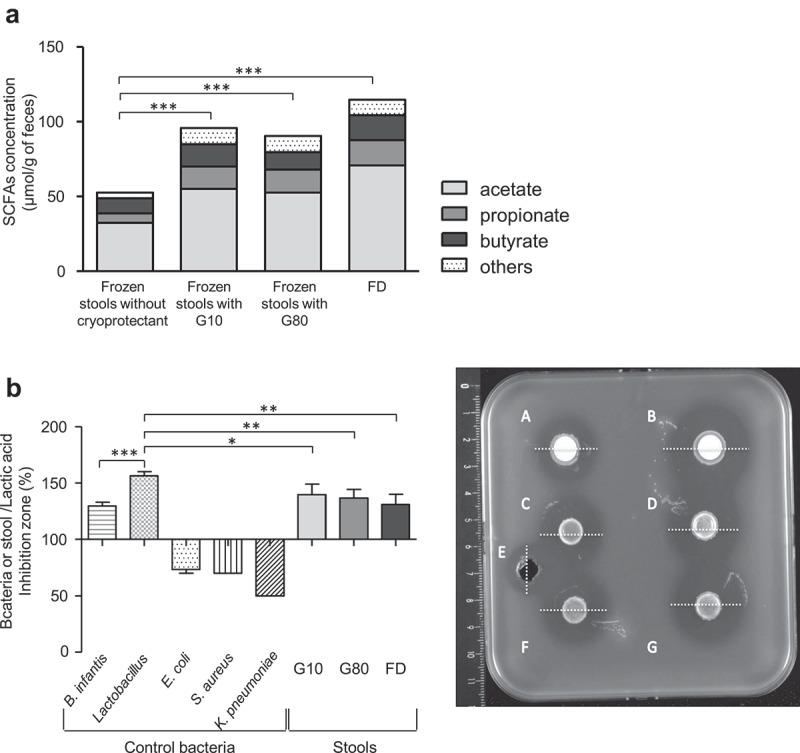
Fecal metabolic activity of frozen and freeze-dried human fecal microbiota after 12 months of storage. (a) Amount of Short-Chain Fatty Acids at M12. Results are expressed as the mean ± SEM. (b) Anti-CD activity of stool samples at M12. Left: results are given as the ratio between inhibition diameters of pure bacteria or stool samples and chemical control, and expressed as the mean ± SEM. Right: representative image of an anti-CD agar spot test: A: Bifidobacterium infantis longum CUETM 89–215, B: Lactobacillus spp., C: stool frozen in G10, D: stool frozen in G80, E: chemical control (lactic acid, 500 mM), F: stool frozen in G10, G: stool lyophilized FD. White lines represent inhibition diameter. Statistical significance: *<0.05, **<0.01, ***<0.001.
In vitro anti-Clostridioides difficile activity in freeze-dried and frozen samples after 12 months of storage
A comparison of the effect of the freeze-drying and freezing procedures on anti-CD activity was carried out after 12 months of storage in comparison to pure bacterial culture used as controls. Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae reference strains (negative controls) did not inhibit the growth of CD, whereas both Lactobacillus spp. and Bifidobacterium infantis reference strains were able to inhibit the growth of CD with an antagonistic effect significantly higher for Lactobacillus (inhibition ratio normalized to lactic acid 500 mM: 161 ± 3% versus 129 ± 3%, p < .001). All fecal samples were antagonistic toward CD R20291 (027 type strain), with results similar for the three conditions and results similar to those observed with the Bifidobacterium reference strain. The inhibition ratio normalized to lactic acid (500 mM) was 131 ± 6% for FD samples; 141 ± 6% for G10 samples and 138 ± 6% for G80 samples (Figure 2(b)).
Colonization of germ-free mice with rehydrated freeze-dried microbiota
We studied the ability of rehydrated FD human fecal microbiota, to colonize the digestive tract of germ-free mice. Two donors were randomly selected (#S4-FD and #S5-FD). After storage for 12 months at 4°C, the rehydrated FD microbiota was inoculated by one oral gavage in recipient germ-free mice (n = 4 per stool sample) and fecal samples were collected for microbial analysis at day 1 (D1), 4 (D4), 11 (D11), 18 (D18) and 28 (D28) after gavage (Figure 3(a)). Engraftment of fecal microbiota was rapid and stable over time for the two stool samples (Figure 3(b)). The total bacterial load and colonization by major groups of commensals like the Clostridium coccoides cluster were high as soon as D1. Maximal colonization by Clostridium leptum and Bacteroides groups was achieved by D4, reaching 109 and 1010 CFU/g of feces, respectively. Colonization by EOS bacteria, such as Faecali-bacterium prausnitzii and Akkermansia muciniphilia was also high as soon as D1, without any significant evolution over the survey period (Figure 3(b)). Engraftment of the microbiota led to the morphological remodeling of epithelium with a significant increase in crypt depth for #S5-FD inoculated mice compared to that of germ-free mice: 188.3 µm [(25-75% percentile: 181.5–194.4) versus 166 µm (25-75% percentile: 124–174), p < .05] (Figure 3(b)). A similar trend was observed for #S4-FD [172.4 µm, 25-75% percentile: 145–186.6)], but the difference relative to germ-free mice did not reach significance. There was no significant difference in the percentage of Ki67-positive cells between groups: germ-free mice: 20.1% (25-75%: 16.5–23.0); #S4-FD: 21.8% (25-75%: 20.7–29.0), and #S5-FD: 18.5% (25-75%: 17–19.8) (Figure 3(c)). The 2 FD fecal samples were thus revivable after 12 months of storage and were able to colonize the digestive tract of recipient germ-free mice without hyper-proliferation of the intestinal epithelium.
Figure 3.
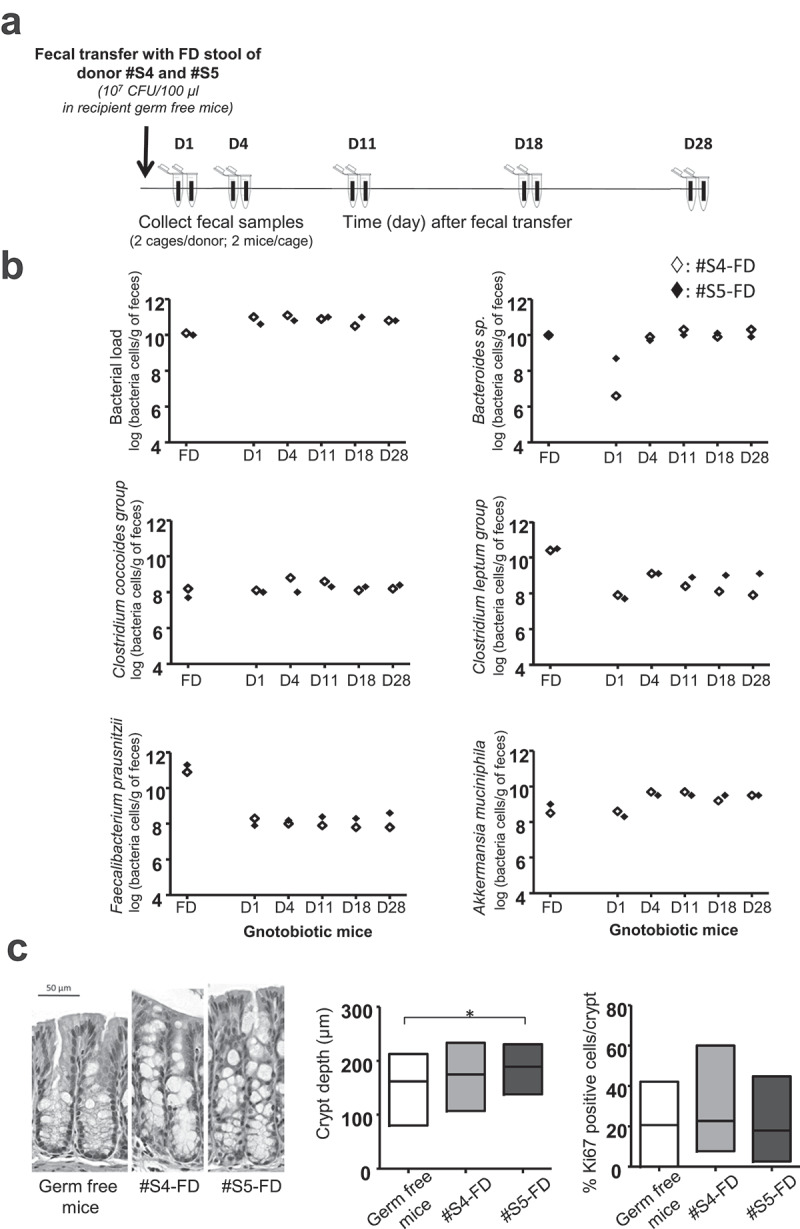
Ability of freeze-dried human fecal microbiota to colonize the digestive tract of germ-free mice. (a) Scheme of the experiment. (b) Follow-up of main bacterial groups per gram of feces after colonization with rehydrated FD microbiota [: #S4-FD (n = 4),: #S5-FD (n = 4), feces sampled per cage]. Results are expressed as the mean for each of the 2 FD samples. (c) Representative image of a colon section from germ-free mice colonized with rehydrated FD microbiota (D30). Scale bar, 50 µm; crypt depth in the colon of germ free and mice colonized with rehydrated FD microbiota (30 crypts per mouse were measured) and Ki67-positive cells expressed as the percentage of total cell number in colon crypts from germ-free mice colonized with rehydrated FD microbiota (60 crypts per mouse were analyzed). Statistical significance: *<0.05.
Therapeutic efficacy of freeze-dried and frozen human fecal microbiota in Clostridioides difficile infected mice
We then evaluated the protective effect of FMT performed with stools from two donors randomly selected (#S1 and #S6) either frozen in G10 (FMT-G10 group, n = 10 per stool sample) or G80 (FMT-G80 group, n = 10 per stool sample) or FD (FMT-FD group, n = 10 per stool sample), in adult C57/BL6 mice orally challenged with 8.103 CFU of the vegetative form of CD R20291 strain 027 after antibiotic pretreatment (Figure 4(a)). Four uninfected mice were used as negative controls (CD- group). CD challenge led to 80% of the mice becoming moribund by day 2 or 3 in untreated animals (CD+ group, n = 10), leading to euthanasia. CD+ surviving mice displayed a major loss in body weight compared to day 0 (−16.0 ± 2.4%) and a high clinical score (3.0 ± 0.4) at day 3 post-infection (Figure 4(b), Table 1).
Figure 4.
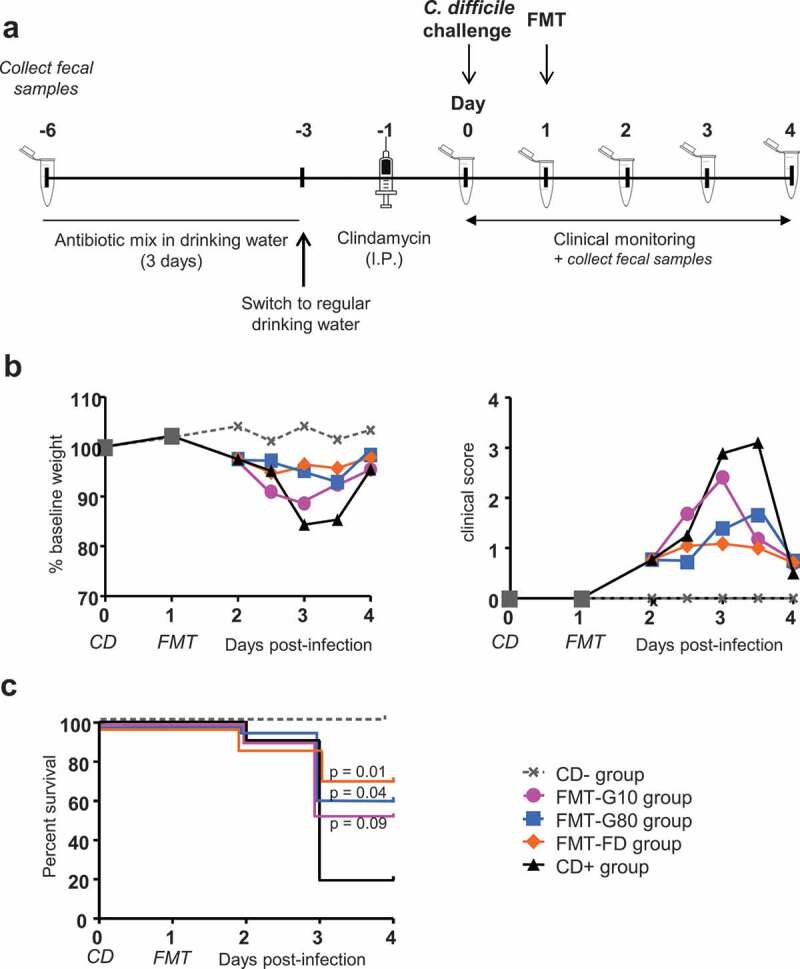
Evolution of clinical parameters after frozen and freeze-dried FMT in Clostridioides difficile-infected mice. (a) Experimental design of the CD-infected-FMT mouse model. Two stools samples were evaluated #S1 and #S6. (b) Monitoring of body weight and a clinical score of all survival mice (up to the day of death). Daily measurements were made starting from Day 0 (day of infection). Grey squares show the total number of mice before randomization into the various FMT groups. Results are expressed as the mean (c). Survival curves of CD-infected mice receiving FMT with FD or frozen microbiota. CD-: control group receiving PBS (n = 4), CD+: CD-infected mice (n = 10); FMT-G10: CD-infected mice receiving FMT with frozen microbiota diluted in G10; FMT-G80: CD-infected mice receiving FMT with frozen microbiota diluted in G80 and FMT-FD: CD-infected mice receiving FMT with freeze-dried microbiota. For each CD group receiving FMT, n = 20, i.e. 10 mice per stool sample.
Table 1.
Clinical score performed daily on animals after Clostridioides difficile infection. The score includes the variables “weight” and “behavior,” with a score from 0 to 3. The final score was obtained by adding the two scores. Mice with a total score > 3 were euthanized within 2 h.
| score | Behaviour | Weight |
|---|---|---|
| 0 | Normal | No weight loss |
| 1 | Decrease in grooming | Weight loss < 10% |
| 2 | Matted fur and/or slitted eyes Decrease in mobility |
10% ≤ weight loss ≤20% |
| 3 | Prostration associated with matted fur and slitted eyes | Weight loss > 20% |
The survival rate of mice at day 4 post-infection was significantly higher in those receiving FMT, reaching 70%, 53%, and 60% for the FMT-FD, FMT-G10 and FMT-G80 group, respectively (p = .01, 0.09 and 0.04 compared to the CD+ group, Figure 4(c)). This was associated with a reduced loss in body weight in the FMT-FD and FMT-80 groups (−4.5 ± 1.7%, p < .001, and −5.1 ± 1.7%, p = .002, compared to CD+ mice) (Figure 4(b), Table 2). The clinical score and cecal load were also significantly reduced for the FMT-FD and FMT-G80 groups (p < .01) (Figures 4(b) and 5(a), Table 2).
Table 2.
Main characteristics of the effect of FMT in the preclinical mouse model of Clostridioides difficile infection. Clinical data were measured at day 3 post-infection and biological results were measured at the time of necropsy. Clinical data are given as mean ± SEM and biological and histological data as median, 25-75% percentile. FMT-FD: CD-infected mice receiving FMT with freeze-dried microbiota; FMT-G10: CD-infected mice receiving FMT with frozen microbiota diluted in G10; FMT-G80: CD-infected mice receiving FMT with frozen microbiota diluted in G80. For each CD group treated with FMT, n = 20, i.e. 10 mice per stool sample. CD-infected mice (n = 10). All results are compared to those of untreated CD+ mice.
| FMT-FD | FMT-G10 | FMT-G80 | CD-infected mice | |
|---|---|---|---|---|
| Body weight loss vs day 0 | −4.5 ± 1.7% (p < .001) |
−11.8% ± 1.8 (NS) |
−5.1 ± 1.7 (p = .002) |
−16.0 ± 3.0 |
| Clinical score | 1.2 ± 0.3 (p < .01) |
2.5 ± 0.3 (NS) |
1.4 ± 0.3 (p < .01) |
3.0 ± 0.4 |
| CD load (log CFU/g of cecal content) |
4.9 (3.3–6.3) (p = .007) |
5.8 (4.8–6.7) (p = .06) |
4.9 (3.6–6.4) (p = .005) |
6.8 (6.3–9.9) |
| MPO (ng/g of colonic tissue) |
300 (209.3–1054) (p = .0005) |
384 ng (286.2–1529) (p = .002) |
446 (290.3–890.8) (p = .0002) |
2465 (1512–8928) |
Histopathological score
|
1.5 0–2.75 (p = .003) 1.5 0.75–4.5 (p = .005) |
4.5 3–5.75 (p = .03) 3 1.75–4.25 (p = .004) |
4.5 2.75–6.75 4.5 2–7.5 |
7.5 5.5–9 7 5.75–7.25 |
Figure 5.
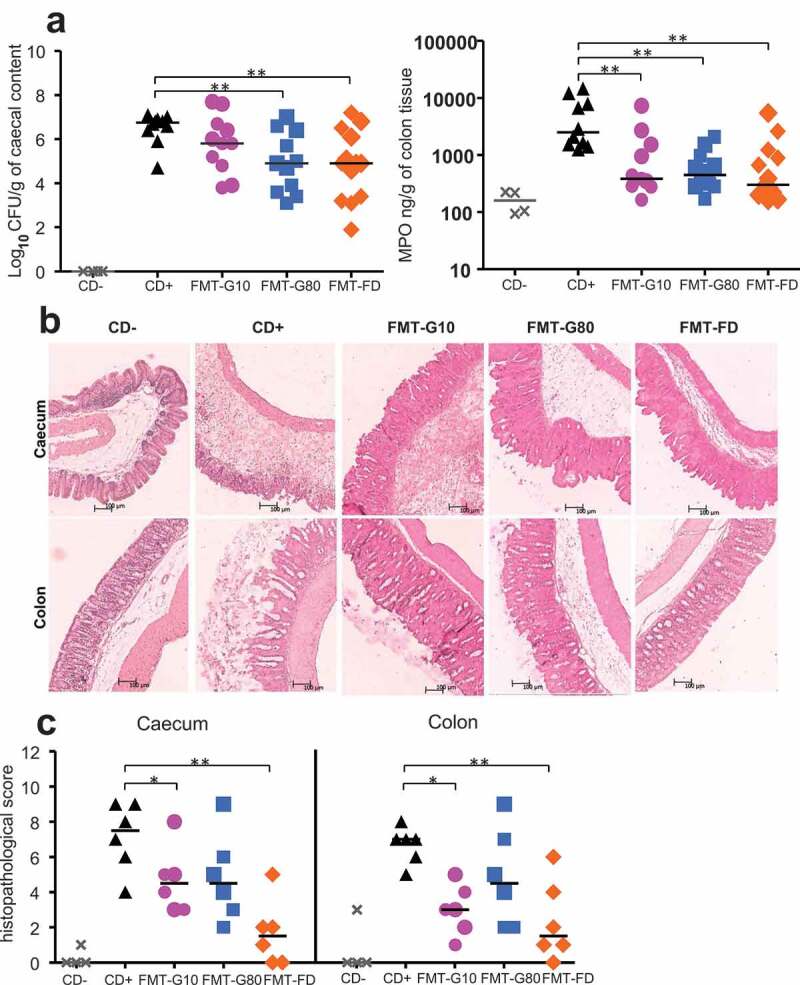
Evolution of the biological parameters of Clostridioides difficile-infected mice following frozen or freeze-dried FMT (a) Level of CD in the cecal content and level of MPO in colonic tissue at the time of necropsy. (b) Representative images of colonic and cecal sections from control (CD- and CD+) mice and mice receiving FMT with frozen or FD microbiota (day 4 post-infection). Scale bar, 100 μm. (c) Histopathological score measured from CD-infected mice receiving FMT with frozen or FD microbiota. Six mice randomly selected for each point. Statistical significance: *<0.05, **<0.01.
At the time of necropsy, colonic myeloperoxidase (MPO) was measured as a marker of inflammation. Median MPO levels reached 2465 ng/g of colon tissue in CD+ untreated mice. In contrast, animals that received FMT, performed either with rehydrated FD or frozen and thawed stools showed significantly lower colonic inflammation, with median MPO levels at least five times lower (<500 ng/g, p < .01) (Figure 5(a), Table 2). Histopathological analyses were performed at day 4 post-infection for mice receiving FMT and for CD-group, and at the time of necropsy for mice from the CD+ group. Six randomly selected animals were analyzed per group except for the CD-group (n = 4). There were histopathological changes in the cecal and colonic tissues of all CD-infected mice, but of varying severity, depending on the type of treatment (Figure 5(b)). We observed the maximal histopathological score in CD+ mice: 7.5 (25-75% percentile: 5.5–9) in the cecum and 7 (25-75% percentile: 5.75–7.25) in the colon. Treatment with FD-FMT was the most efficient to reduce histological damages (p < .01) (Figure 5(c), Table 2).
Finally, the infection was associated with a marked decrease in α-diversity of microbiota compared to CD-mice, as shown by the OTU number and Shannon indices (Figure 6(a,c). FMT performed at day 1 post-infection restored the α-diversity only for #S1 (p < .05), regardless of the procedure of conservation of fecal samples (Figure 6(a)), whereas there was no major impact on β-diversity (Figure 6(b)). However, microbiota composition was clearly modified following FMT.
Figure 6.
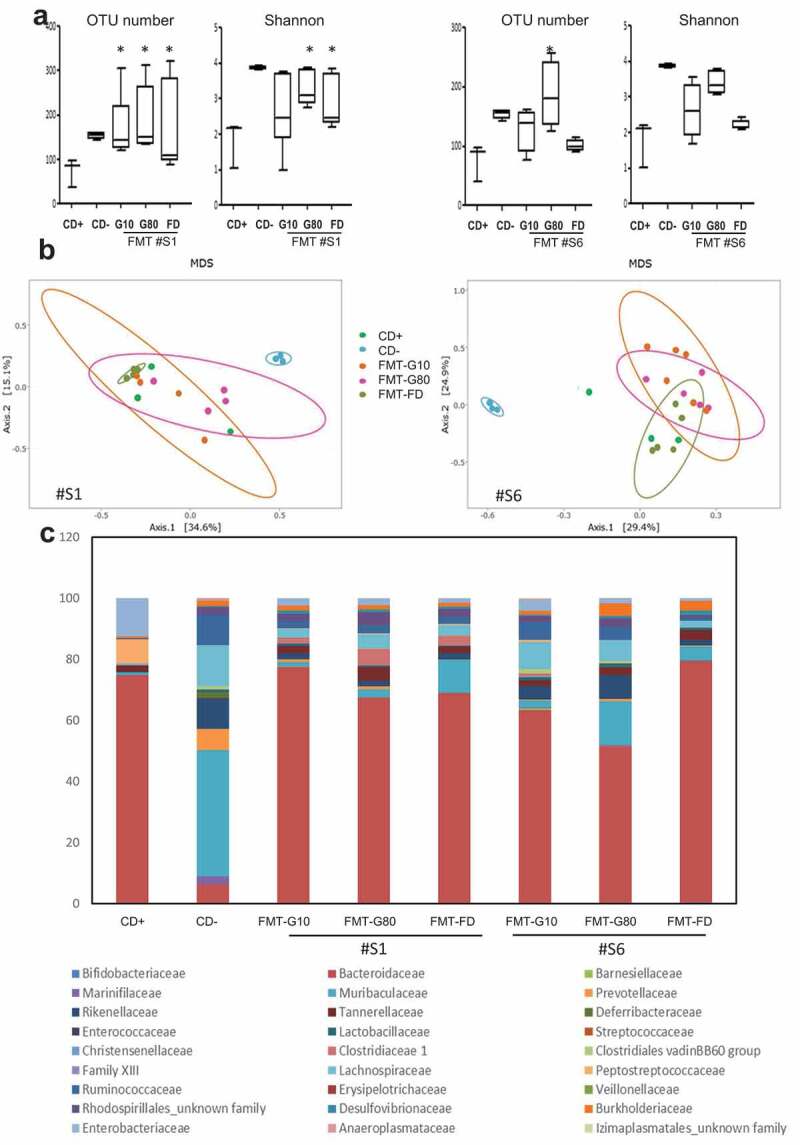
Comparison of the microbiota at day 4 between control mice and Clostridioides difficile-infected mice that received, or not, frozen or freeze-dried FMT. (a) α-diversity. (b) β-diversity using Bray–Curtis distance. (c) Composition of the microbiota at the family level. The two stool samples, #S1 and #S6, used for FMT are represented. Statistical significance: *<0.05.
Development of a formulation adapted for the filling of capsules with freeze-dried fecal microbiota for oral administration
To propose a formulation adapted for the filling of hard capsules, we determined the bulk density, particle size and aspect, and the flowability of the FD samples. We observed variable macroscopic and microscopic aspects, depending on the sample (Figure 7(a,b)). It was not possible to qualify the particle-size distribution of the FD samples due to the extreme intra- and inter-sample diversity of particle nature, shape, and size. Scanning electron microscopy showed a microbiota embedded in its fecal environment (Figure 7(c)). Filling of hard capsules was not possible without excipients. Addition of up to 50% fillers failed to improve flowability, but 75% allowed the FD samples to flow vertically. Addition of glidants G3 (0.5%) was highly effective in improving the flowability of the FD samples and the bulk density of the free-flowing mixture was 0.30–0.47 g/mL allowing the filling of hard capsules without dilution of the FD samples (Figure 7, lower panel). In such a condition, 1 g of powder corresponds approximately to 1 g of native stool.
Figure 7.
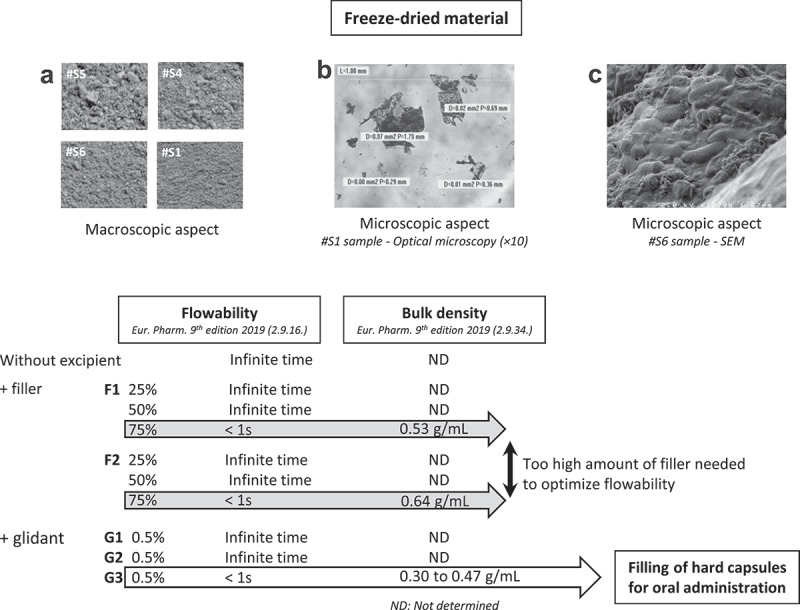
Development of hard capsules for oral administration of freeze-dried fecal microbiota. Upper panel: (a) examples of macroscopic aspects (n = 4). (b) microscopic aspects Optical Microscopy (x10) and (c) SEM. Lowe panel: flowability and bulk density of FD samples without excipient (n = 5), with 25% to 75% filler (n = 2) or with glidant (G1 n = 1, G2 n = 1, G3 n = 6), ND: not determined.
Discussion
In the present study, we showed that bacterial viability and metabolic production were preserved in FD, maltodextrin-trehalose-loaded microbiota stored for at least 12 months at 4°C without significant variation compared to microbiota preserved frozen at −80°C in the presence of glycerol. Using this new FD preparation of fecal microbiota, engraftment was observed to levels usually observed in gnotobiotic rodent models and the therapeutic effectiveness of FMT was confirmed in a preclinical mice model of CD infection with a clear improvement in survival, clinical score, and histopathological lesions in the hindgut. Finally, it was possible to use the FD powder to fill oral hard capsules after the addition of a very small amount of a glidant excipient. Altogether, this work showed that the bacterial viability and clinical efficacy of the fecal microbiota were maintained in this new FD fecal preparation, allowing refinement of the FMT procedure toward oral administration of a dry material that was stable for over 1 year at 4°C.
In recent years, several important advances have been made in an effort to improve the accessibility of FMT through the administration of frozen4,6,16,24 or FD stool.19–21 However, these formulations remained to be better standardized and characterized to guarantee the availability and efficacy of fecal transplants.
Here, we assessed the performance of a new freeze-drying process using non-penetrating cryoprotectants, and compared over time results with those obtained with the corresponding samples stored frozen at −80°C after dilution in glycerol, a penetrating cryoprotective agent at both concentrations (G10 and G80) currently used for the preservation of frozen fecal microbiota in clinical assays of FMT.14,17,22 We showed the good preservation until 12 months of storage, and even 18 months for two samples regardless the process of preservation, of both viability and diversity of the main targeted bacterial groups, i.e. the dominant (EOS and Bifidobacteria) or subdominant populations (Enterobacteria, Enterococcus spp., and Lactobacillus spp.) in the inocula. The main variation was observed for Bacteroides spp. This could be due to the aerobic preparation of fecal transplant for samples preserved FD or frozen in G80, leading to damaged cells which would affect the culture and delay colonization which however remain efficient in recipient germ-free mice. As most anaerobic bacteria are not easily cultivable (i.e. Faecalibacterium or Akkermansia) and even culturability do not imply engraftment, we evaluated the engraftment of those FD microbiota. For F. prausnitzii, engraftment was coherent with our previous observations in two gnotobiotic models.25,26 Moreover, personal preliminary data suggest that FMT performed with either fresh or FD stools have a similar effect on the epithelium of recipient germ-free mice. Recently, it was suggested that the anaerobic treatment of stool samples lead to a better efficiency of FMT in patients with ulcerative colitis.27 For others, precautions to ensure anaerobiosis during transplant manufacturing was not essential to preserve bacterial viability.28 Here, using germ-free mice, we further showed that the aerobic preparation of transplants did allow a prompt and stable engraftment of the microbiota participating to gut homeostasis. This included major butyrate producers bacteria that are particularly sensitive to growth conditions, such as F. prausnitzii29-31 which is known for its potential anti-CD activity32 and for its importance in human health.33 Concomitant with bacterial engraftment, we observed deepening of the epithelium, especially for one donor, without hyperproliferation, thus reflecting the establishment of a host-bacteria relationship.34–36
We evaluated the protective effect of two fecal FD samples and the same two samples simply frozen in G10 or G80 at −80°C (all samples stored for 3 months) in a preclinical murine model of CD infection. The clinical parameters (low survival rate and major weight loss) observed in the CD-infected mice were consistent with those reported in the literature.37–39 FMT performed with rehydrated FD microbiota resulted in a significant decrease in symptomatology, i.e. a significant reduction in body weight loss, clinical score, and mortality relative to untreated CD-infected mice. These results are consistent with those of Jiang et al. who showed that frozen and FD transplants stored up to 7 months were both as effective as a fresh transplant in a mouse model of CD infection.23 The beneficial effects observed for the clinical symptoms were consistent with the biological parameters as animals receiving FD-FMT showed a reduced CD load in the cecum, limited colonic inflammation, and limited histological damages. That CD was still detectable in the cecum at the time of sacrifice (i.e. D4 post-infection) coincides with previous results where complete clearance of the pathogen was observed only by D10 after FMT with fresh stool in a murine model of relapsing disease.40 Based on our in vitro studies showing no significant evolution of microbiota profile between M3 and M12, we can expect that samples stored for up to 12 months should be as efficient for the FMT procedure.
Besides the reconstitution of a diverse microbiota allowing the reinforcement of the barrier effect, various components of the fecal matter can provide health benefits and may work synergistically to restore homeostasis. Among them, SCFA (mainly acetate, propionate, and butyrate) are of major importance in the host-bacteria relationship.41–43 Indeed, a decrease in Ruminococcaceae, Lachnospiraceae, and butyrate-producing bacteria that belong to Clostridium clusters IV and XIVa have been reported for patients with CD infection.44 Here, we showed that freeze-drying in maltodextrin-trehalose or freezing in G10 or G80, better preserved SCFA during storage compared to samples frozen without any cryoprotectant. This included butyrate, which has a well-established role as an energetic source for the colonocytes, in promoting the intestinal barrier, and as an anti-inflammatory substrate.45 Moreover, high concentrations of acetate, a key element for the development of butyrate-producing microbes through cross-feeding,46 was detected in the cryoprotected stool samples.
However, these in vitro measurements are only one clue to, not a proof of high SCFA production in the recipient animals.
Overall, our results validate both the freezing and freeze-drying processes for the conservation of an efficient fecal transplant for up to 12 months. Moreover, we showed that the new FD fecal preparation for FMT lead to a significant decrease in symptoms together with an increase in survival rate in mice infected with CD. Such preservation methods would simplify clinical works by facilitating the fecal biobanking from carefully selected and screened donors. In addition, the FD process allowed producing a powdery material with good stability over 12 months, offering the possibility to prepare hard capsules for more convenient oral administration to the patient.
Methods
Preparation of stool samples
Fecal samples were collected from 6 healthy volunteers (none of whom suffering from gastrointestinal disorders or taking antibiotics in the previous 3 months), placed immediately in anaerobic conditions using a catalyst (GENbag Anaert; Biomérieux) according to the Human Microbiome Standards47 and sent to the laboratory within 2 h of collection. Written informed consent was provided by all volunteers. For the freeze-drying procedure, fresh stool were immediately diluted 1/6 (v/v) in the maltodextrin-trehalose cocktail (patent application WO2017103225A1), homogenized in aerobic condition using a dedicated blender, filtrated with sterile gauze and frozen at −80°C for a minimum of 8 h, and subjected to the freeze-drying process. Vials were sealed under vacuum at the end of the process and stored at +4°C until analysis. At the same time, aliquots of the same stool samples were prepared as described above with dilution 1/6 (v/v) in G10 or G80, used as a cryoprotectant, and stored at −80°C.
Evaluation of microbiota viability in human fecal samples preserved by freeze-drying or freezing
Viability of the fecal microbiota in FD or frozen samples was evaluated after 1 week (W1) and 3, 6, or 12 months (M3, M6, M12) of storage and compared to that of the corresponding fresh stool samples, using culture methods for aerobic and anaerobic bacteria. Two samples were studied until 18 months (M18) of storage. Briefly, samples were serially diluted in sterile peptone water before spreading on several culture media with an automatic spiral system (Chemunex-AES Laboratory, France), allowing the enumeration of total bacteria, Bifidobacterium spp., Bacteroides spp.; Enterobacteriaceae; Enterococcus spp., and Lactobacillus spp. Plates were incubated at 37°C under aerobic or anaerobic conditions for 2 to 5 days as previously described.48 Bacterial counts were expressed as log CFU/g of feces. The threshold of detection was 3 log CFU/g of feces. EOS bacteria were cultured on a rich nonselective culture medium (YBHI supplemented with cellobiose, maltose, and cysteine) under anaerobic conditions (N2/CO2/H2:80/10/10) for enumeration. Each dilution was spread on two plates, one being placed under anaerobic conditions, whereas the other was left out of the anaerobic chamber for 1 h, to eliminate EOS strains, before proceeding to incubation under anaerobic conditions for 48 h. The difference between the number of colonies on the two plates corresponded to the level of EOS strains.29
Evaluation of the fermentative activity of human fecal microbiota
The concentrations of total SCFAs, acetate, propionate, and butyrate were measured by gas-liquid chromatography (Nelson 1020; Perkin-Elmer, France) after a 12-month period of storage in frozen and FD samples in comparison to control frozen samples stored at −80°C without cryoprotectants, as described previously.49 Results are presented as µmol/g of feces.
In vitro evaluation of the anti-Clostridioides difficile activity of human fecal microbiota
The agar spot test adapted from Tejero-Sarinena et al.50 was used for the detection of activity against CD R20291 (027 type strain). Lactic acid (500 mM), Lactobacillus sp., and Bifidobacterium infantis subsp. longum CUETM 89–215 were used as positive chemical and biological controls, whereas Escherichia. coli CIP 54.8, Klebsiella pneumonia CIP104771, and Staphylococcus aureus ATCC 25923 were used as negative controls. An overnight culture of each bacterial suspension (20 μl) was used as control and an equal volume of each fecal suspension of thawed G10 and G80 and rehydrated FD samples were deposited on cellulose disks and placed on WCB agar for overnight incubation at 37°C in an anaerobic atmosphere. Agar plates were then covered with Brucella agar containing a CD R20291 culture (OD = 1). The chemical control, lactic acid, was added to an open well (50 µl). Plates were incubated for another overnight period under anaerobic conditions at 37°C and the zones of inhibition of the CD culture around each spot were measured. Results (from three independent experiments) are expressed as the ratio between inhibition diameters of pure bacteria or stool samples and chemical control.
Preparation of hard oral capsules containing freeze-dried fecal microbiota
The bulk density (2.9.34. Eur. Pharm. 9th edition 2019),51 particle size (Optika Vision Pro, Optika SRL, Italy), and aspect (Scanning Electron Microscopy) of the FD samples were assessed. Flow properties were measured (2.9.16. Eur. Pharm. 9th edition 2019) with and without the addition of 25% to 75% directly compressible fillers with good flow properties, designated as F1 and F2, or 0.5% glidant excipients, designated as G1, G2, and G3. Gastro-resistant hydroxypropyl methylcellulose (HPMC) capsules (DRcaps® V43-700, size 00, Capsugel, Belgium) were manually filled with the FD samples with optimized flow properties.
Animal experiments
Two experimental models, performed in accordance with the European guidelines for the care and use of laboratory animals, were used for this study.
Evaluation of intestinal colonization by rehydrated freeze-dried microbiota in germ-free mice
Thirteen-week-old female C3 H/HeN germ-free mice (n = 8) (INRAE, Anaxem, Jouy-en-Josas, France), bred under axenic conditions (two/cage), were orally inoculated with 100 µL (107 bacteria) of 2 randomly selected rehydrated FD samples (#S4-FD and #S5-FD, n = 4 for each donor) that had been stored for 12 months at +4°C. Before fecal transfer, the absence of microbes was verified in germ-free mice by microscopic observation of fresh feces and culturing of fecal material on various bacterial culture media. All mice were maintained in Trexler type isolators (one isolator per donor, two cages of two mice/isolator) and received the same standard diet (ad libitum, R03-40 SAFE sterilized by gamma irradiation at 45 kGy). Feces were collected per cage at days 1, 4, 11, 18, and 28 post-inoculation. At D28 post-inoculation, mice were euthanized. Total fecal DNA was extracted as previously described.52 Total bacterial counts and specific bacterial profiles were assessed by real-time PCR (qPCR) of the bacterial 16 S rRNA gene using previously described primers and conditions for qPCR amplifications.30,53 Histological examination of the transverse colon was performed on 4-µm sections stained with hematoxylin, eosin, and saffron, or immuno-labeled with rat monoclonal anti-Ki67 antibody (Dako, France). Thirty and sixty crypts per mouse were analyzed to determine the average crypt length and percentage of Ki67-positive cells, respectively. Slides were scanned using Panoramic Scan (3DHistech) and CaseViewer software (3DHistech) was used to count Ki67-positive cells and colon crypt depth. Experimentation was approved by the Regional Council of Ethics for animal experimentation C2EA-45 with authorization APAFIS#3441-2016010614307552 v1.
Evaluation of the inhibitory effect of FMT in a Clostridioides difficile infection mouse model
The CD model of infection was developed according to the procedure of Chen et al.37 Six- to seven-week-old female C57BL/6 mice (Charles River, France) was housed in groups of 4 to 5 per cage and maintained under a 12-h light-dark cycle. The food (standard chow), water, bedding, and cages were autoclaved. Cage changes and daily assessment of the physical condition and behavior of the animals were performed under a laminar flow hood.
All animals were treated for 3 days with a mixture of antibiotics dissolved in drinking water consisting of kanamycin (0.4 mg/mL), gentamicin (0.035 mg/mL), colistin (850 U/mL), metronidazole (0.215 mg/mL), and vancomycin (0.045 mg/mL) (Sigma Aldrich, France). After 1 day of wash-out, mice received a single intra-peritoneal injection of clindamycin (10 mg/kg) (Sigma Aldrich). The day after, mice were orally infected with 8.103 CFU of PBS-washed vegetative CD (strain R20291, exponential phase).
At D1 post-infection, the test groups received FMT by gavage (200 µl) with either frozen and thawed (FMT-G10 and FMT-G80) or rehydrated FD (FMT-FD) microbiota that had been stored for 3 months (n = 10/stool/preservation mode; 2 stools randomly selected, i.e. #S1 and #S6). The positive-control group (CD+) was fed CD alone (n = 10) without any subsequent treatment and the negative control group (CD-) was fed PBS (n = 4). All animals were observed daily from infection until sacrifice for symptoms and mortality, and weight was recorded (clinical scoring, Table 1). At D4 post-infection or before for animals judged to be in a moribund state (scoring > 3), animals were euthanized. Quantification of the CD load was performed on cecal contents on selective agar plates (chromID CDIF, Biomérieux, France). Colonic MPO was measured as a marker of colonic inflammation (Mouse Myeloperoxidase DuoSet kit, R&D Systems, USA). Histological evaluation of the cecum and colon was performed on 4 µm sections stained with hematoxylin-eosin, coded, randomized, and scored by two independent operators in a blind manner.37 Briefly, grading considered the following features: i) edema of the mucosa and hemorrhagic congestion, ii) tissue infiltration by neutrophils, and iii) epithelial damage. A score of 0–3, denoting increasingly severe abnormality was assigned to each parameter and the overall score obtained by summing the component scores. Experimentation was approved by the Regional Council of Ethics for animal experimentation C2EA-34 with authorization APAFlS#7600-20l60620l6336853 v3.
16 S genomic analysis of microbiota following FMT
Total bacterial DNA was extracted from the fecal content of CD-infected mice taken at D4 post-infection using the DNeasy PowerSoil Kit (Qiagen France). The V3-V4 region of the 16 S rRNA gene was amplified for 30 cycles with an annealing temperature of 65°C using Taq polymerase MTP (Sigma Aldrich) and primers PCR1F_460 (CTTTCCCTACACGACGCTCTTCCGATCTACGGRAGGCAGCAG) and PCR1R_460 (GGAGTTCAGACGTGTGCTCTTCCGATCTTACCAGGGTATCTAATCCT) and sequenced on Illumina MiSeq platform. The raw 16 S rRNA sequences were analyzed using the bioinformatics pipeline FROGS (Find Rapidly OTU with Galaxy Solution).54 After sequence processing, affiliations were performed with BLAST using the silva132 16 S database. Data were filtered by retaining only those sequences that were present in at least three samples and contributed 0.005% to the microbial community and multi-affiliation was manually checked with leBiBi (Quick BioInformatic Phylogeny of Prokaryotes). The resulting OTU table (478 OTUs) was used for subsequent statistical analysis using R software and phyloseq package.55 The phylogenetic tree was constructed using Mafft and Fasttree. The samples were standardized to the same depth (7325 sequences). α Diversity was studied by calculating the number of observed OTUs and Shannon indices. β Diversity was studied using Bray–Curtis distance. Differences between groups were evaluated by principal-coordinate analysis (PCoA) and multivariate analysis of permutation variances using distance matrices (PERMANOVA).56
Statistical analysis
The stability of microbiota following conservation procedures was analyzed using R.57 For all paired data, mixed effect models58 were fitted per case, as described below, followed by appropriate contrast calculations, to account for non-independence of the data and avoid increasing the degrees of freedom of error, which could lead to the spurious significance or increased variance, which could mask significant variations. Moreover, we used estimated marginal means59 to correct for the unbalanced design due to missing values. The stability over time was assessed by fitting a mixed effect model to the microbiota data over time for each microbiota group. Time and freezing procedure were modeled as fixed effects while the stool sample was modeled as a random effect. Dunnett’s contrasts were applied to adjust for multiple comparisons of each time measurement versus the first one (W1 or M3), used as a reference.
A mixed-effect model was fitted to the SCFA concentrations, using freezing condition and SCFA as fixed effects and stool sample as a random effect. Dunnett’s contrasts were used to adjust for multiple comparisons of each freezing condition versus the fresh stool used as a reference.
A Mann Whitney test was used to evaluate the anti-CD growth activity of preserved fecal samples versus positive bacterial controls.
For in vivo experiment, a mixed effect model was fitted to % baseline weight and clinical score using time and freezing procedure as fixed effects and animal as a random effect. Contrasts were constructed in order to compare the score and body weight evolutions from D0 to D4 of treated vs untreated animals, adjusting for multiple comparisons. Chi-2 test was used for survival analysis and Kruskal–Wallis (1-way ANOVA) followed by Mann Whitney tests were used for a charge of CD in ceacal content, MPO, and histological score.
Supplementary Material
Acknowledgments
The authors thank the two animal care facilities, i.e. Anaxem facility of the MICALIS Institute (INRAE, Jouy en-Josas, France) and CRP2-UMS 3612 CNRS-US25 INSERM-IRD at the Faculté de Pharmacie de Paris, Université Paris Descartes, France for their help; Cellular and Molecular Imaging facility, INSERM UMS 025 – CNRS UMS 3612 at Faculté de Pharmacie de Paris, Université Paris Descartes, France for their technical assistance in histology with a special thanks to Virginie Mignon; Chantal Labellie for her technical help, and Mingsen Wang who participated in the development of the hard capsules.
Funding Statement
This work was partially funded by the Agence Nationale de Sécurité du Médicament et des Produits de Santé ANSM) (grant number 2015/2019). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Specific author contributions
ChC, FJ, AJW, MT, and NK designed the study; JR was responsible for the study; JR, ChC, JD, CM, CC, AJW, AM, MM, TM, and JA collected data; IN performed statistical analysis; JR and NK drafted the manuscript; NK, AJW, and MT interpreted the data and critically revised the manuscript. All authors discussed the results, data analysis, and preparation of the manuscript. NK and MT should be considered as joint senior authors.
References
- 1.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ.. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165:609–616. doi: 10.7326/M16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, Weese JS, Collins S, Moayyedi P, Crowther M, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315:142–149. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 5.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 6.Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, Alm EJ, Gevers D, Russell GH, Hohmann EL.. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515–1522. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocquart M, Lagier J-C, Cassir N, Saidani N, Eldin C, Kerbaj J, Delord M, Valles C, Brouqui P, Raoult D, et al. Early fecal microbiota transplantation improves survival in severe clostridium difficile infections. Clin Infect Dis. 2018;66:645–650. doi: 10.1093/cid/cix762. [DOI] [PubMed] [Google Scholar]
- 8.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 9.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reygner J, Kapel N. Actuality on fecal microbiota transplantation. In: Joel F., Salomao F. editor.Current status of fecal microbiota transplantation. Microbiome and metabolome in diagnosis, therapy and other strategic applications. Elsevier inc; 2019. p. 155–165. ISBN 9780128152492. 10.1016/B978-0-12-815249-2.00016-6. [DOI] [Google Scholar]
- 11.Aron-Wisnewsky J, Clément K, Nieuwdorp M. Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Curr Diab Rep. 2019;19:51. doi: 10.1007/s11892-019-1180-z. [DOI] [PubMed] [Google Scholar]
- 12.Evrensel A, Önen Ünsalver B, Ceylan ME. Therapeutic potential of the microbiome in the treatment of neuropsychiatric disorders. Med Sci (Basel). 2019;7(2) : 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huttner BD, de Lastours V, Wassenberg M, Maharshak N, Mauris A, Galperine T, Zanichelli V, Kapel N, Bellanger A, Olearo F, et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: a randomized clinical trial. Clin Microbiol Infect. 2019;25:830–838. doi: 10.1016/j.cmi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Cheminet G, Kapel N, Bleibtreu A, Sadou-Yaye H, Bellanger A, Duval X, Joly F, Fantin B, de Lastours V. Faecal microbiota transplantation with frozen capsules for relapsing Clostridium difficile infections: the first experience from 15 consecutive patients in France. J Hosp Infect. 2018. doi: 10.1016/j.jhin.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 16.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, Chang H-J, Coward S, Goodman KJ, Xu H, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318:1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello SP, Conlon MA, Vuaran MS, Roberts-Thomson IC, Andrews JM. Faecal microbiota transplant for recurrent Clostridium difficile infection using long-term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther. 2015;42:1011–1018. doi: 10.1111/apt.13366. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z-D, Jenq RR, Ajami NJ, Petrosino JF, Alexander AA, Ke S, Iqbal T, DuPont AW, Muldrew K, Shi Y, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS One. 2018;13:e0205064. doi: 10.1371/journal.pone.0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staley C, Hamilton MJ, Vaughn BP, Graiziger CT, Newman KM, Kabage AJ, Sadowsky MJ, Khoruts A. Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota; pragmatic cohort study. Am J Gastroenterol. 2017;112:940–947. doi: 10.1038/ajg.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecker MT, Obrenovich ME, Cadnum JL, Jencson AL, Jain AK, Ho E, Donskey CJ. Fecal microbiota transplantation by freeze-dried oral capsules for recurrent Clostridium difficile infection. Open Forum Infect Dis. 2016;3:ofw091. doi: 10.1093/ofid/ofw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, Putignani L, Fischer M, Keller JJ, Costello SP, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Z-D, Alexander A, Ke S, Valilis EM, Hu S, Li B, DuPont HL. Stability and efficacy of frozen and lyophilized fecal microbiota transplant (FMT) product in a mouse model of Clostridium difficile infection (CDI). Anaerobe. 2017;48:110–114. doi: 10.1016/j.anaerobe.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 25.Wrzosek L, Miquel S, Noordine M-L, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miquel S, Leclerc M, Martin R, Chain F, Lenoir M, Raguideau S, Hudault S, Bridonneau C, Northen T, Bowen B, et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio. 2015;6. doi: 10.1128/mBio.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burz SD, Abraham A-L, Fonseca F, David O, Chapron A, Béguet-Crespel F, Cénard S, Le Roux K, Patrascu O, Levenez F, et al. A guide for Ex Vivo handling and storage of stool samples intended for fecal microbiota transplantation. Sci Rep. 2019;9:8897. doi: 10.1038/s41598-019-45173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martín R, Miquel S, Benevides L, Bridonneau C, Robert V, Hudault S, Chain F, Berteau O, Azevedo V, Chatel JM, et al. Functional characterization of novel faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of f. prausnitzii as a next-generation probiotic. Front Microbiol. 2017;8:1226. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux -J-J, Blugeon S, Bridonneau C, Furet J-P, Corthier G, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miquel S, Martín R, Bridonneau C, Robert V, Sokol H, Bermúdez-Humarán LG, Thomas M, Langella P. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes. 2014;5:146–151. doi: 10.4161/gmic.27651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roychowdhury S, Cadnum J, Glueck B, Obrenovich M, Donskey C, Cresci GAM. Faecalibacterium prausnitzii and a prebiotic protect intestinal health in a mouse model of antibiotic and Clostridium difficile Exposure. JPEN J Parenter Enteral Nutr. 2018;42:1156–1167. doi: 10.1002/jpen.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuentes S, Rossen NG, van der Spek MJ, Hartman JH, Huuskonen L, Korpela K, Salojärvi J, Aalvink S, de Vos WM, D’Haens GR, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. Isme J. 2017;11:1877–1889. doi: 10.1038/ismej.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherbuy C, Honvo-Houeto E, Bruneau A, Bridonneau C, Mayeur C, Duée P-H, Langella P, Thomas M. Microbiota matures colonic epithelium through a coordinated induction of cell cycle-related proteins in gnotobiotic rat. Am J Physiol Gastrointest Liver Physiol. 2010;299:G348–357. doi: 10.1152/ajpgi.00384.2009. [DOI] [PubMed] [Google Scholar]
- 35.Cherbuy C, Tomas J, Thomas M, Langella P. Interactions of the intestinal microbiota with mucosal epithelial cells [Internet]. In: Brassart D, editor. Intestinal Microbiota in Health and Disease. CRC Press; 2014. [cited 2019 October16]. Available from: http://www.crcnetbase.com/doi/abs/10.1201/b16442-4 [Google Scholar]
- 36.Tomas J, Reygner J, Mayeur C, Ducroc R, Bouet S, Bridonneau C, Cavin J-B, Thomas M, Langella P, Cherbuy C. Early colonizing Escherichia coli elicits remodeling of rat colonic epithelium shifting toward a new homeostatic state. Isme J. 2015;9:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes. 2011;2:145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theriot CM, Koumpouras CC, Carlson PE, Bergin II, Aronoff DM, Young VB. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes. 2011;2:326–334. doi: 10.4161/gmic.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seekatz AM, Theriot CM, Molloy CT, Wozniak KL, Bergin IL, Young VB. Fecal microbiota transplantation eliminates clostridium difficile in a murine model of relapsing disease. Infect Immun. 2015;83:3838–3846. doi: 10.1128/IAI.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baktash A, Terveer EM, Zwittink RD, Hornung BVH, Corver J, Kuijper EJ, Smits WK. Mechanistic insights in the success of fecal microbiota transplants for the treatment of clostridium difficile infections. Front Microbiol. 2018;9:1242. doi: 10.3389/fmicb.2018.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gill PA, van Zelm MC, Muir JG, Gibson PR. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48:15–34. doi: 10.1111/apt.14689. [DOI] [PubMed] [Google Scholar]
- 43.Seekatz AM, Theriot CM, Rao K, Chang Y-M, Freeman AE, Kao JY, Young VB. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe. 2018;53:64–73. doi: 10.1016/j.anaerobe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, Wang GP. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51:2884–2892. doi: 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M, Zhou Q, Dorfman RG, Huang X, Fan T, Zhang H, Zhang J, Yu C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16:84. doi: 10.1186/s12876-016-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HL, Shen H, Hwang IY, Ling H, Yew WS, Lee YS, Chang MW. Targeted approaches for in situ gut microbiome manipulation. Genes (Basel). 2018;9. doi: 10.3390/genes9070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dore J, Ehrlich SD, Levenez F, Pelletier E, Alberti A, Bertrand L, Bork P, Costea PI, Sunagawa S, Guarner F, et al. IHMS-SOP 03V2: standard operating procedure for fecal sample self-collection, laboratory analysis handled within 4 to 24 hours (4 hours< x <24 hours) (2015). International Human Microbiome Standards. http://www.microbiome‐standards.org
- 48.Rougé C, Goldenberg O, Ferraris L, Berger B, Rochat F, Legrand A, Göbel UB, Vodovar M, Voyer M, Rozé J-C, et al. Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe. 2010;16:362–370. doi: 10.1016/j.anaerobe.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Lan A, Bruneau A, Bensaada M, Philippe C, Bellaud P, Rabot S, Jan G. Increased induction of apoptosis by propionibacterium freudenreichii TL133 in colonic mucosal crypts of human microbiota-associated rats treated with 1,2-dimethylhydrazine. Br J Nutr. 2008;100:1251–1259. doi: 10.1017/S0007114508978284. [DOI] [PubMed] [Google Scholar]
- 50.Tejero-Sariñena S, Barlow J, Costabile A, Gibson GR, Rowland I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe. 2012;18:530–538. doi: 10.1016/j.anaerobe.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 51.2.9.34.Eur. Pharma. 9th edition 2019 [Internet]. 2019. [cited 2019 Nov 4].. Available from: https://clicktime.symantec.com/3PcXyyoWliS3Pmdf9AhmwMy6H2?u=https%3A%2F%2Fwww.edqm.eu%2Ffr
- 52.Godon JJ, Zumstein E, Dabert P, Habouzit F, Moletta R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol. 1997;63:2802–2813. doi: 10.1128/AEM.63.7.2802-2813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayeur C, Gratadoux -J-J, Bridonneau C, Chegdani F, Larroque B, Kapel N, Corcos O, Thomas M, Joly F. Faecal D/L lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PLoS One. 2013;8:e54335. doi: 10.1371/journal.pone.0054335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escudié F, Auer L, Bernard M, Mariadassou M, Cauquil L, Vidal K, Maman S, Hernandez-Raquet G, Combes S, Pascal G, et al. Rapidly, OTUs with galaxy solution. Bioinformatics. 2018;34:1287–1294. doi: 10.1093/bioinformatics/btx791. [DOI] [PubMed] [Google Scholar]
- 55.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oksanen J, Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin P, O’Hara R, Simpson G, Solymos P, et al. Vegan: community ecology package. R Package Version. 2018;2:5–2. [Google Scholar]
- 57.R Core Team . R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2017. [Google Scholar]
- 58.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft 2015; 67:1–48. [Google Scholar]
- 59.Lenth R Emmeans: estimated marginal means, aka least-squares means. R package version1.2.4. [Internet]. 2018. [cited 2019 Dec 14]. Available from: https://CRAN.R-project.org/package=emmeans.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 2.9.34.Eur. Pharma. 9th edition 2019 [Internet]. 2019. [cited 2019 Nov 4].. Available from: https://clicktime.symantec.com/3PcXyyoWliS3Pmdf9AhmwMy6H2?u=https%3A%2F%2Fwww.edqm.eu%2Ffr
- Lenth R Emmeans: estimated marginal means, aka least-squares means. R package version1.2.4. [Internet]. 2018. [cited 2019 Dec 14]. Available from: https://CRAN.R-project.org/package=emmeans.


