ABSTRACT
This study aimed to investigate the effects of glycated milk casein (Gc) fermented with Lactobacillus rhamnosus 4B15 (FGc) on the intestinal microbiota and physiological and behavioral properties in mice under chronic stress. Mice were administered Gc or FGc for 10 weeks and then exposed to unpredictable chronic mild stress (UCMS) for 7 weeks. FGc administration restored alterations of gut microbiota induced by UCMS. Moreover, FGc significantly reduced the stress-induced increase in serum corticosterone and decrease in serotonin levels. Anxiety-like behaviors induced by UCMS were also significantly decreased in the FGc group. UCMS-induced dysregulation of gene and protein expression related to neuroendocrine function, neuronal development, and inflammation, and gut-blood-brain barrier function was controlled by FGc pre-treatment. These results strongly suggest the protective effects of FGc targeting of intestinal microbiota for abnormal brain activity, which is consistent with the view that FGc plays an important role in regulating stress-related gut-brain axis disorders.
KEYWORDS: Chronic mild stress, brain-gut-microbiome axis, casein, glycation, Lactobacillus rhamnosus 4B15
Introduction
External chronic stress is a risk factor for psychiatric disorders, especially in vulnerable individuals. As psychiatric disorders such as anxiety disorder and depression induced by chronic stress are complex and symptomatically heterogeneous, their etiologies and mechanisms are largely unknown. Chronic stress promotes reduced sucrose consumption and increased serum glucocorticoid levels, severe neuronal cell damage, immune and neuroendocrine system impairments, anxiety-like behaviors, and neurocognitive deficits.1 Moreover, stress is a key risk factor for irritable bowel syndrome (IBS), the most common gastrointestinal disorder, which reflects pathologically altered gut-brain axis homeostasis.2 Several clinical trials testing the probiotics Lactobacillus and Bifidobacteria in IBS and other lower gastrointestinal disorders showed decreased symptoms such as anxiety and depression as well as abdominal pain and bloating and improvement of the intestinal microbiota,3 which plays a critical role in neuro-immuno-endocrine pathways through the gut-brain axis. Accumulating data indicate the bi-directional interaction between brain-gut-microbiome in response to external stress, with the intestinal microbiome likely acting as a key regulator of the immune response, facilitates adaptation to these conditions and protects against the development of stress-related neuronal dysfunction.2 The exact underlying mechanisms that mediate the inhibitory effect of stress are not clearly investigated. However, stress response relies on the neuroendocrine system and structural synaptic plasticity in both the central nervous system (CNS) and the periphery, and results in the changes of corticosterone and serotonergic system through the hypothalamic-pituitary-adrenal (HPA) axis which are the key factors in the brain function involved with a stress reaction.4 Furthermore, the brain circuitry in response to stress influences various organs and tissues including the gut, the heart, smooth muscle of the vasculature, kidney, fat, etc.5
The effects of milk to promote sleep and reduce stress have been attributed to psychological associations (i.e., the memory of mother giving milk)6 and tryptophan, abundantly found in milk and converted to serotonin and melatonin, which induces sleepiness and relaxation, respectively.7 According to a previous study, a diet based on α-lactalbumin, which is one of the whey proteins in milk, increased the plasma tryptophan-to-large neutral amino acid ratio associated with the brain serotonin function and cognitive performance.8 Moreover, αs1-casein hydrolyzates improved sleep disorders by regulating tryptophan metabolism and the serotonergic and gamma-aminobutyric acid (GABA) system and maintained homeostasis with respect to blood pressure and cortisol levels during mental and physical stress in humans.9 Taken together, it is hypothesized that treatment with microbial hydrolyzates of glycated milk casein (Gc) that included specific probiotics (i.e., Lactobacillus rhamnosus 4B15) may reduce stress-related disturbances by modulating the HPA axis. Glycation of milk casein improves their functional and nutritional properties with structural modification.10 The previous study revealed that glycation, oxidation, phosphorylation, and deamidation occurred in the specific sites during glycation reaction of milk casein, simultaneously with the improvement of antioxidative and anti-inflammatory activities, and intestinal epithelial function.10 The probiotics for fermentation of Gc, Lactobacillus rhamnosus 4B15 (4B15), was preliminarily evaluated for its probiotic potential using various tests (e.g., acid and bile tolerance, bacterial adhesion capacity, and anti-oxidative and anti-inflammatory activities).11 Furthermore, in a cell-based in vitro intestinal inflammation model, fermentation of the glycoproteins by 4B15 enhanced the anti-oxidative and anti-inflammatory activities and intestinal barrier function relative to those of non-fermented glycoproteins.10 Therefore, this study aimed to investigate the preventive effects of Gc and Gc fermented with novel probiotics of 4B15 (FGc) on dysbiosis of the intestinal microbiota; abnormal brain function; and anxiety-like markers such as neuroendocrine dysregulation, neurodegeneration, and neuroinflammation; and anxiety-related behaviors in mice under chronic stress.
Materials and methods
Manufacture of probiotic fermented milk protein
Manufacture of glycoprotein from milk protein
Casein (Erie Foods International, Inc., IL, USA) was dissolved together with glucose in deionized water at a 2:1 (w/w) ratio of casein (50 mg/g) and glucose (25 mg/g). The reaction was allowed to proceed with shaking at 60 rpm in a pilot-scale instrument controlled by a pilot-scale pasteurization unit (Powerpoint International, Tokyo, Japan) at 75°C for 24 h. The reaction products were dialyzed twice in a 10,000 molecular weight cutoff ultra-filtration system (Sam Yeon Engineering, Seoul, Korea) to remove the remaining glucose after the reaction with casein, and the dialyzed products were lyophilized.
Probiotic fermentation
A probiotic strain, Lactobacillus rhamnosus 4B15 (KCCM11983P, Korean Culture Center of Microorganisms), was activated three times in de Man, Rogosa, and Sharpe (Difco Laboratories, MI, USA) broth at 37°C for 18 h before use. Fermentation of Gc with 4B15 was performed continuously to manufacture glycoprotein. The glycoprotein manufactured from milk casein and glucose was pasteurized at 85°C for 15 min immediately after 24 h of glycation reaction. Then, bacterial cells (107 CFU/mL) were inoculated into the pasteurized glycoprotein and incubated at 37°C for 48 h. After fermentation, fermented glycoprotein was freeze-dried, and the lyophilized powder was used.
Animals and sample administration
Forty C57BL/6 male mice (Samtako Bio Korea Co. Ltd., Gyeonggi-do, South Korea) were obtained at 5 weeks of age (initial average body weight, 21 g) for determining anxiolytic effects as presented in Figure 1A. Male mice were chosen for this study to avoid any potential effects of the estrous cycle of female mice on the behavioral phenotype in response to stress.12,13 Mice were housed under standard conditions (12-h light-dark cycle). Mice were housed four per cage and classified into five groups (n = 8): non-stressed on normal diet (normal control [N-Con]), UCMS on normal diet (stress control [S-Con]), UCMS plus fluoxetine (15 mg/kg/day; positive control [S-Flu]), UCMS plus Gc (1,500 mg/kg/day; [S-Gc]), and UCMS plus FGc (1,500 mg/kg/day; [S-FGc]). All diet including normal diet and the Gc or FGc diet were administered to the mice as pellet form. The Gc or FGc powder was incorporated into the normal diet pellet. The regular diet composition is addressed in the Supplementary Table 1. We made the Gc or FGc containing pellet based on the previous report.14 Groups of mice were housed in 22 cm wide x 28 cm long x 13 cm tall polycarbonate cages covered filter top with stainless steel lid. The S-Gc and S-FGc groups were administered Gc and FGc ad libitum for 10 weeks after a week of adaptation, and the S-Flu group was administered fluoxetine dissolved in saline via oral gavage (15 mg/kg/day in 100 μL) 30 min prior to behavior testing during the final 5 weeks. All other groups of mice were administered saline via oral gavage as vehicle control. UCMS procedure was performed as previously described15,16 with slight modifications (Supplementary Table 2). The UCMS group was exposed to different stressors per day such as sleep cycle changes, wet bedding, tilted cage, illumination, water deprivation, restraint, and cold-water baths in random order for 7 weeks after 3 weeks of sample pre-treatment. Non-stress group was undisturbed except for housekeeping procedures. Behavioral tests were performed during weeks 7–10 and killed at week 10 for physiological studies. Body weight and food intake were checked every week during the experimental period. Animals had ad libitum access to water. The mice were euthanized by CO2 inhalation and blood was collected by cardiac puncture. The brain (whole brain) and colon tissues were dissected and kept at −20°C until physiological analysis. Moreover, in order to determine the changes of the gut microbiome by UCMS and sample treatment, fecal samples were collected freshly from distal colon at 10 weeks. All experiments involving mice were approved by the Animal Care and Use Committee of Chonbuk National University (2015–05) and conducted in accordance with the guidelines of the Care and Use of Laboratory Animals.
Figure 1.
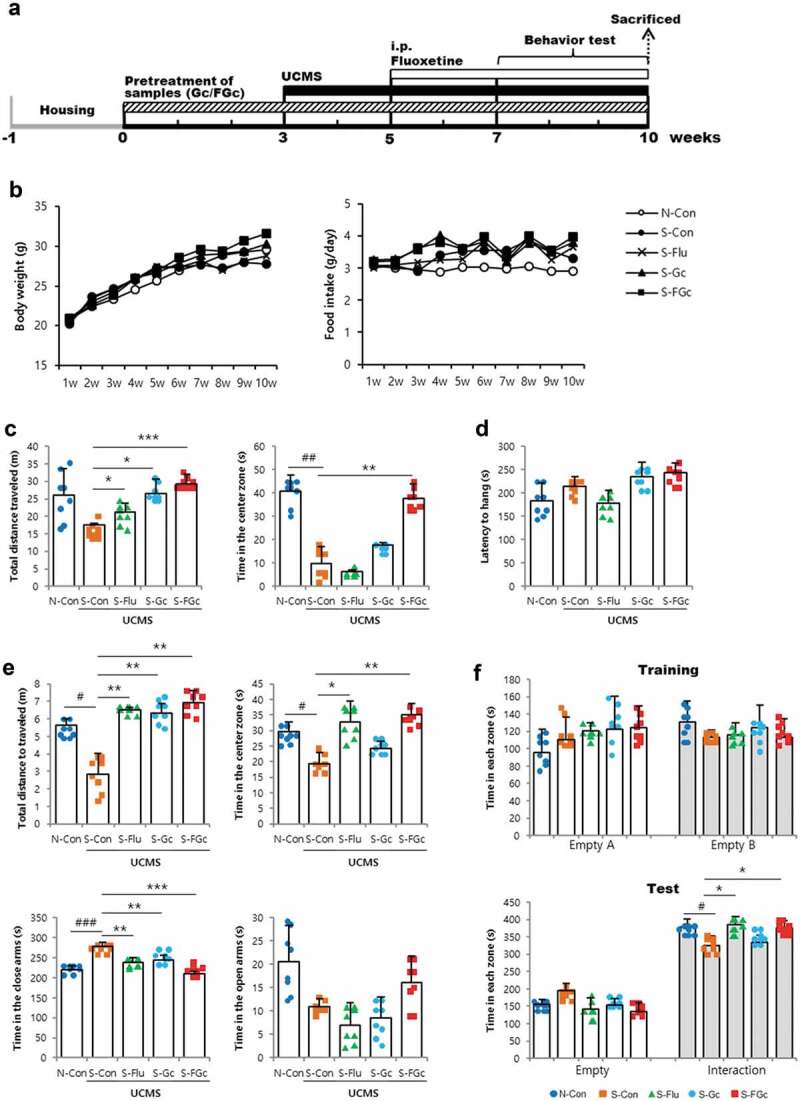
(A) Schematic overview of the in vivo experiment, (B) changes of body weight, food intake, and depressive-like behaviors of mice under UCMS. (C) open-field test, (D) rotarod test, (E) elevated-plus maze test, and (F) social-interaction test. Data are expressed as mean ± SD (n = 8). #Significant difference between N-Con and S-Con (#P < .05, ##P < .005, ###P < .001). *Significant difference with S-Con (*P < .05, **P < .005, ***P < .001).
Behavioral tests
Open-field test
The open-field apparatus consisted of a square arena (40 cm long) with 30-cm-high walls. Open-field behaviors of the mice were recorded and analyzed using the ANY-maze software (Stoelting, Wood Dale, IL, USA). Animals were exposed to the open field for 10 min. The animals were placed in one of the corner areas, and the experiment was started immediately afterward. Between subjects, the box was thoroughly cleaned with 70% ethyl alcohol and completely dried.
Rotarod test
The rotarod test was performed to evaluate basic motor activity. Mice were trained on the rotarod apparatus for four consecutive trials. During each training session, mice were placed for 1 min on the rotating rod (4 rpm, constant speed) and returned to their cages for 1 min before the next trial. Once the animals were able to stay on the rod rotating at 4 rpm for at least 60 s, they were subjected to the rotarod test. Tests were performed 24 h after the training session. On test day, mice were tested on the rotarod (4–40 rpm, gradually increasing the pace). Their latency to fall was recorded with a maximum cutoff of 300 s. Between subjects, the rotarod apparatus was thoroughly cleaned with 70% ethyl alcohol and completely dried. Mice were tested for eight consecutive trials with at least 5-min intervals. The data from the last four trials were averaged as the latency to fall.
Elevated-plus maze test
The apparatus consisted of two open arms and two closed arms arranged in a plus-sign orientation. Each arm was 5 cm wide × 30 cm long × 40 cm tall. The walls on the closed arms were 15 cm high. At the start of the test, each subject was placed in the center of the EPM facing a closed arm. Mice explored the maze for 5 min, and exploratory activities in both open and closed arms were recorded and analyzed using the ANYmaze system. Between subjects, the apparatus was thoroughly cleaned with 70% ethyl alcohol and completely dried. Because rodents naturally prefer dark and enclosed compartments, a greater willingness to explore open arms indicates less anxiety behavior, while more time spent in the closed arms is indicative of increased anxiety.17
Social-interaction test
Mice were tested in a Plexiglas cage divided into three chambers: two equal-size ends (31.5 × 25.5 cm) and a smaller neutral section between them (10.5 × 25.5 cm). During the habituation phase, the end areas contained an empty “holding cell” (10.16 cm in diameter and 13.97 cm tall). Each mouse was placed in the center of the box and allowed to explore the entire box for 10 min. The subject was then returned to the cage while another adult male was placed under the holding cell (on one randomly selected side) and an empty cell was placed on the opposite side. During the test phase, the subjects were placed in the center and allowed to explore the entire box for 5 min. The ANYmaze system scored the number of entrances into each side and the time spent investigating the novel mouse. Investigation was defined as the test mouse’s nose touching the novel mouse through the bars or sniffing within 1 cm. The test arena was carefully cleaned with 70% ethanol between subjects and completely dried.
Physiological tests
Serum analysis
Blood samples were immediately collected in BD Vacutainer® SST™ II Advance (Becton Dickinson, NJ, USA) by cardiac puncture after mice were euthanized, and the samples were stored at −20°C for 30 mins. After centrifugation at 2,000 rpm for 15 mins, the serum was transferred to a new tube for further analysis. Serum corticosterone and serotonin levels were measured using the corticosterone (Abnova, Taipei, Taiwan) and serotonin (Abcam, Cambridge, MA, USA) enzyme-linked immunosorbent assay kits, respectively, according to the manufacturer’s instructions. All measurements were made in triplicate.
mRNA expression
Total RNA was extracted from the frozen brain (whole brain) and colon tissues using Oh et al.’s method.18 cDNA was generated using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). cDNA samples were stored at −20°C until further use. qRT-PCR (CFX Connect Real-Time PCR Detection System; Bio-Rad, Hercules, CA, USA) was used to detect mRNA expression using KAPA SYBR FAST qPCR kit universal master mix (2×) (Kapa Biosystems, Boston, MA, USA). The reaction protocol was as follows: denaturation at 95°C for 10 min, annealing at 95°C for 15 s, and extension at the primer melting temperature for 30 s. Relative gene expression was calculated using the comparative threshold cycle method, and values were normalized with those of glyceraldehyde-3-phosphate dehydrogenase. The primer sequences used for qRT-PCR are listed in Supplementary Table 3. Expression of 5HT1AR, GABAAR, GPR43, and TPH-1 was determined for neuroendocrine functions; caspase-3, bcl-2, bax, and BDNF for neurodegeneration; TLR4, iNOS, COX-2, TNF-α, and IL-6 for neuroinflammation; and zo-1, occludin, claudin-5, and MUC-2 for the tight junction. All samples were run in triplicate.
Western blot analysis
Dissected brain (whole brain) and colon tissues were homogenized and lysed in RIPA buffer (Thermo Scientific, Rockford, IL, USA) supplemented with protease inhibitor cocktail (Roche, Basel, Switzerland). After sonic dismembranation, the supernatant was collected using centrifugation and the total protein concentration was determined in the cleared lysates with the BCA protein assay kit (Thermo Scientific). Samples were normalized to contain 1 mg/mL of protein, and 15 μg of sample was run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 12% gel (Bio-Rad). The gel was transferred to a polyvinylidene difluoride membrane, using the Trans-Blot Turbo™ Transfer System (Bio-Rad). The membrane was blocked with Tris-buffered saline buffer supplemented with 0.1% Tween-20 and 5% skim milk for an hour and then incubated with primary antibody overnight at 4°C. The blots were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for an hour at room temperature. Protein bands were visualized using enhanced chemiluminescence substrate (Bio-Rad) and imaged using the Bio-Rad ChemiDoc MP imaging system. Protein expression was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The antibodies used are listed in Supplementary Table 4. All values were normalized against those of β-actin.
Measuring intestinal permeability
Intestinal mucosa-to-blood permeability was measured using fluorescein FD4 as previously described.19,20 Briefly, the liquid FD4 penetrating from the colon segment into the incubation buffer was spectrophotometrically measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Histopathological analysis
Pathological histology was evaluated according to previously described methods with modifications.21 After removal of the colon, tissues were fixed in formaldehyde. The formaldehyde-fixed colon tissue samples were processed for histological study. Paraffin-embedded tissue sections were stained with hematoxylin and eosin and then examined and photographed under a light microscope for histopathological changes.
Fecal microbiome analysis
DNA isolation
Whole genomic DNA was isolated from fecal samples using FastDNA® Spin Kit for soil (MPbio, Santa Ana, CA, USA) according to the manufacturer’s instructions. Briefly, following lysis of fecal pellets, samples were centrifuged to pellet debris and lysing matrix. DNA was purified from the supernatant with a silica-based GENECLEAN® procedure using SPIN filters. The eluted DNA was used for PCR and sequencing.
Microbiome sequencing
PCR amplification was performed using primers targeting the V3 to V4 regions of the 16 S rRNA gene with extracted DNA. Bacterial amplification was conducted using primers 341 F (5ʹ-TCGTCGGCAGCGTC-AGATGTGTATAAGAGACAG-CCTACGGGNGGCWGCAG-3ʹ) and 805 R (5ʹ-GTCTCGTGGGCTCGG-AGATGTGTATAAGAGACAG-GACTACHVGGGTATCTAATCC-3ʹ). The PCR conditions were as follows: initial denaturation at 95°C for 3 min followed by 25 cycles of denaturation at 95°C for 30 s, primer annealing at 55°C for 30 s, and extension at 72°C for 30 s, with a final elongation at 72°C for 5 min. Secondary amplification for attaching the Illumina NexTera barcode was performed with i5 forward primer (5ʹ-AATGATACGGCGACCACCGAGATCTACAC-XXXXXXXX-TCGTCGGCAGCGTC-3ʹ; X indicates the barcode region) and i7 reverse primer (5ʹ-CAAGCAGAAGACGGCATACGAGAT-XXXXXXXX-GTCTCGTGGGCTCGG-3ʹ). The conditions for secondary amplification were the same as described above, except the amplification step, was set to eight cycles. The amplified products were purified, pooled in equal quantities, and then sequenced with the Illumina MiSeq Sequencing system (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. Processing raw reads started with a quality check (QC) and filtering of low-quality (<Q25) reads using Trimmomatic 0.32. After passing the QC, paired-end reads were merged using PANDAseq. Primers were trimmed with ChunLab’s (Seoul, South Korea) in-house program at a similarity cutoff of 0.8. The EzBioCloud database was used for a taxonomic assignment using USEARCH (8.1.1861_linux32) followed by more precise pairwise alignment. UCHIME and the non-chimeric 16 S rRNA database from EzBioCloud were used to detect chimera on reads that contained less than 97% best-hit similarity rate. Sequence data were then clustered using CD-HIT and UCLUST.
Correlation analysis
Spearman’s rank correlation of relative abundance of fecal microbiota with stress-induced dysregulated markers was visualized as a clustered heatmap using Ward’s method. The analysis was performed with MATLAB version 2013a (MathWorks, Natick, MA, USA).
Statistical analysis
All data are expressed as means ± standard deviations. Statistical significance for between-group differences was assessed using an independent-sample t-test. SPSS version 22.0 (IBM, Chicago, IL, USA) was used for statistical analyses.
Results
Body weight and anxiety-like behavioral phenotype
Mice were pretreated Gc or FGc for 3 weeks before exposure to 7 weeks of stress to investigate their preventive effects against brain dysfunction induced by chronic stress. Changes in body weight or food intake showed no significant difference between groups (Figure 1B). Several behavioral tests were performed to investigate the effects of Gc and FGc on anxiety-like behaviors induced by unpredictable chronic mild stress (UCMS). The stress-induced open-field test, rotarod test, elevated-plus maze (EPM) test, and social-interaction test are widely used for anxiety-like behavior and cognitive function measurement.22 Under UCMS conditions, mice administered FGc traveled a significantly greater distance in the center of the open-field box, although total distance traveled in the entire field was similar to that of mice administered Gc (Figure 1C). In the EPM test, FGc-administered mice spent significantly less time in the closed arms and more time in the open arms than S-Con (Figure 1E). The total distance traveled and time spent in the center zone during the EPM test were higher in the FGc-administered mice than in the S-Con group (Figure 1E). To explore the effects of Gc and FGc on basal motor activity, the same cohort of mice was subjected to rotarod tests. There were no statistically significant differences in the latency to fall in the rotarod test between groups (Figure 1D). Thus, changes in anxiety-like behaviors in mice are unlikely due to altered motor activity because of Gc or FGc administration. In the social-interaction test, Gc- or FGc-administered mice showed interactions similar to other mice groups (figure 1F). Collectively, these data suggest that FGc attenuates or prevents anxiety-like behavioral changes in mice experiencing chronic stress.
FGc ameliorates impairment of neuroendocrine functions in stressed mice via regulation of HPA pathway
To determine the response to chronic stress and treatment with Gc or FGc on the activation of the hypothalamic-pituitary-adrenal (HPA) axis and central nervous system (CNS), serum levels of corticosterone and serotonin and mRNA and protein expression of corticotropin-releasing factor receptor (CRFR), N-methyl-D-aspartate receptor (NMDAR), and neurotransmitter receptors were evaluated (Figure 2A, B and Supplementary Figure 1A). Serum levels of corticosterone and serotonin were significantly altered by UCMS and treatment of samples as shown in Figure 2A. Corticosterone, the major glucocorticoid stress hormone, and serotonin play an important role in regulating neuronal function and leading anxiety/depressive-like behaviors. Particularly, dysfunction of the serotonergic system induced by chronic stress dysregulates the HPA axis and disturbed neuronal function.22 Serum corticosterone was significantly increased (P < .001), while serum serotonin was sharply decreased (P < .05) in response to UCMS. However, Gc and FGc treatment reduced the UCMS-induced changes in serum hormone levels as did fluoxetine. Especially, FGc showed the highest activity for reducing serotonin in stressed mice (P < .001). Moreover, treatment with FGc significantly suppressed the overexpression of CRFR1 and NMDA2 R due to UCMS (P < .001 and P < .05, respectively), which is consistent with the fluoxetine results. CRFR1 is also a major regulator of the HPA pathway in the CNS, mediating endocrine, autonomic, immune, and behavioral responses to stress.23 NMDAR is a glutamate receptor and ion channel protein found in nerve cells. Glutamate is an important neurotransmitter regulating the neural portion of HPA axis activity.24 Overexpression of NMDAR causes an excessive influx of Ca2+ leading to the excitotoxicity involved in neurodegenerative disorders. Moreover, the expression levels of neurotransmitter receptors, such as serotonin and dopamine, in the FGc treatment group were similar to those of the non-stressed and fluoxetine groups, whereas the stressed group showed 0.6-fold (P < .005) and 0.2-fold (P < .001) lower expression, respectively, than those in the non-stressed group. The mRNA expression levels of serotonin 1A receptor, 5-hydroxytryptamine 1A receptor (5HT1AR) and gamma-aminobutyric acid receptor (GABAR) showed a similar pattern with the protein level. The treatment with FGc altered the neuroendocrine-related protein expression in the colon as well as in the brain (Figure 2C). Expression of TPH-1 protein, which plays an important role in serotonin synthesis, was higher in the S-Flu (P < .001) and S-FGc (P < .05) groups than in the S-Con group. Additionally, G-protein-coupled receptor 43 (GPR43), also known as free fatty acid receptor 2 (FFA2), was down-regulated by UCMS, which was attenuated by Gc or FGc treatment (P < .05) in colon tissue. GPR43 binds to short-chain fatty acids and has promising therapeutic potential for treating inflammatory and metabolic diseases.25 These findings suggest that treatment with FGc ameliorates UCMS-induced impairment of neuroendocrine functions via the HPA pathway by regulating neurotransmitter synthesis and uptake in both brain and colon.
Figure 2.
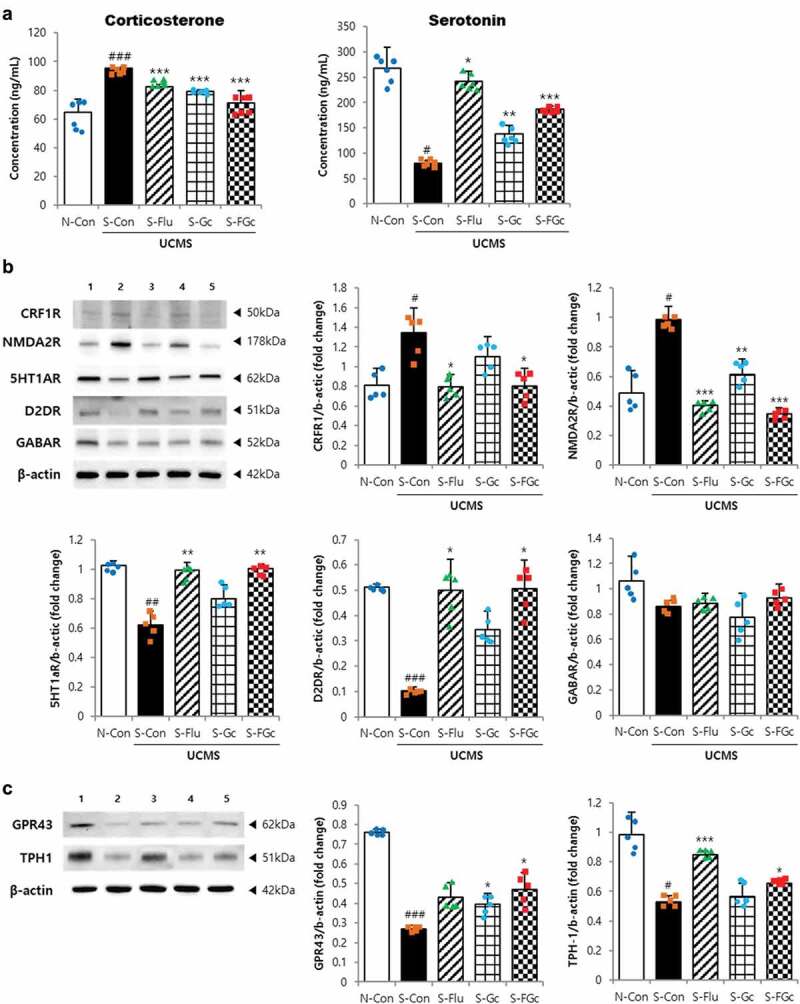
Effects of Gc and FGc on neuroendocrine in mice brain and colon under UCMS. (A) serum corticosterone and serotonin levels (n = 6). (B) Protein expression of NMDA2 R, CRF1 R, 5HT1AR, D2DR, and GABAAR by western blot. Lane 1 = N-Con; lane 2 = S-Con; lane 3 = S-Flu; lane 4 = S-Gc; lane 5 = S-FGc. (C) Protein expression of GPR43 and TPH-1 in mice colon by western blot. Lane 1 = N-Con; lane 2 = S-Con; lane 3 = S-Flu; lane 4 = S-Gc; lane 5 = S-FGc. Data are expressed as mean ± SD (n = 5). #Significant difference between N-Con and S-Con (#P < .05, ##P < .005, ###P < .001). *Significant difference with S-Con (*P < .05, **P < .005, ***P < .001).
FGc attenuates UCMS-induced neurodegeneration
To investigate the underlying mechanisms of Gc and FGc neuroprotection, quantitative real-time polymerase chain reaction (qRT-PCR) and western blotting were performed to quantify related mRNA and protein expression in dissected brains (Figure 3 and Supplementary Figure 1b). For all factors (i.e., brain-derived neurotrophic factor [BDNF], bcl-2, bax, cytochrome c, and caspase-3), both mRNA and protein expression was significantly affected by exposure to UCMS. The neuroprotective molecule BDNF was most highly expressed in the S-FGc group despite UCMS exposure (P < .001). In addition, FGc treatment had greater suppressive effects on the UCMS-induced changes in bcl-2, bax, and caspase-3 expression, but no effect on cytochrome c expression, all of which are associated with the cellular apoptosis pathway, than in the S-Con group (P < .05). Bax is a pro-apoptotic molecule that induces apoptosis via cytochrome c release preceding caspase activation and subsequent proteolysis, which can be inhibited by anti-apoptotic bcl-2.26 Additionally, these results indicate the neuroprotective mechanisms of FGc occurred through regulating apoptosis in the brain.27
Figure 3.
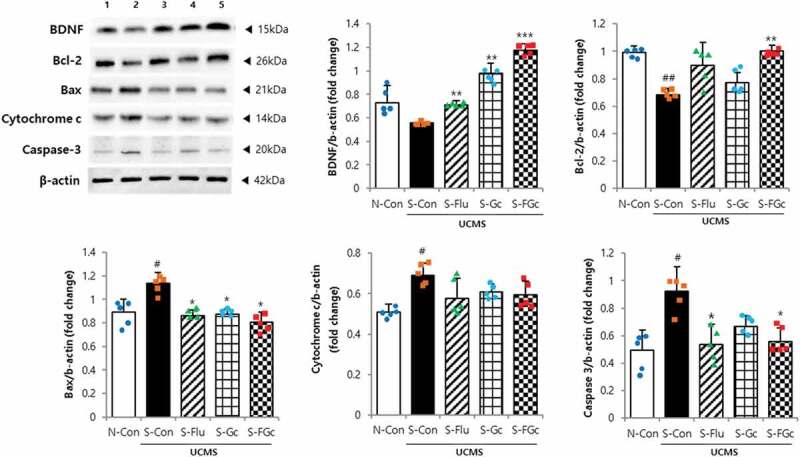
Effects of Gc and FGc on neurodegeneration in mice under UCMS. Expression of proteins related to neurogenesis by western blot. Lane 1 = N-Con; lane 2 = S-Con; lane 3 = S-Flu; lane 4 = S-Gc; lane 5 = S-FGc. Data are expressed as mean ± SD (n = 5). #Significant difference between N-Con and S-Con (#P < .05, ##P < .005, ###P < .001). *Significant difference with S-Con (*P < .05, **P < .005, ***P < .001).
Effects of FGc on immune response and barrier function in stressed mice
Further investigation of Gc and FGc effects on chronic stress-induced inflammation in the brain and colon revealed a significant suppression of inflammatory markers (Figure 4A). Toll-like receptor 4 (TLR4) plays an important role in the inflammatory response, and the signals from TLR activate the NF-κB signaling pathway, which causes a subsequent increase in pro-inflammatory mediators such as iNOS, COX-2, and pro-inflammatory cytokines.10 The increase in brain tissue of TLR4, iNOS, COX-2, TNF-α, and IL-6 mRNA expression due to UCMS was attenuated in mice administered FGc (P < .05) (Supplementary Figure 1C). An increase in iNOS and COX-2 proteins due to UCMS was also attenuated in the brain of FGc-treated mice (P < .005). Pre-treatment with FGc affected the inflammatory response to UCMS in the colon in the same manner. The suppressive effect was observed in those treated with Gc (P < .05), but to a lesser extent than in those treated with FGc. The blood-brain barrier (BBB) and the gut-blood barrier effectively protect the brain from circulating pathogens.28 The tight junction transmembrane proteins, which are critical for the barrier function, restrict paracellular diffusion of pathogens from the blood to the brain and from the gut to the blood.28 As presented in Figure 4B and Supplementary Figure 1D, the expression of tight junction proteins and genes such as zo-1, occludin, and claudin-5 were significantly decreased by UCMS in both the brain and colon (P < .05). On the contrary, FGc treatment remarkably prevented the stress-induced down-regulation of tight junction-related proteins and genes in the brain and colon (P < .05), which was higher than for the effect of Gc treatment. Moreover, 4-kDa fluorescein isothiocyanate-dextran (FD4), an indicator of intestinal epithelial permeability, was significantly increased in the colon of S-Con mice, but not in S-Flu or S-FGc mice, compared with N-Con mice (Figure 4C). In agreement with these results, histological analysis of colon tissues showed that chronic stress seemingly disturbed the firmness of epithelial cells, but pre-treatment with FGc protected the colon epithelium from the stress-induced disturbance (Figure 4D). These findings indicate that FGc treatment prevented the chronic stress-induced neuronal and intestinal inflammatory response and suppressed disruption of barrier functions in the brain and colon as well as the increased intestinal permeability, as did fluoxetine.
Figure 4.
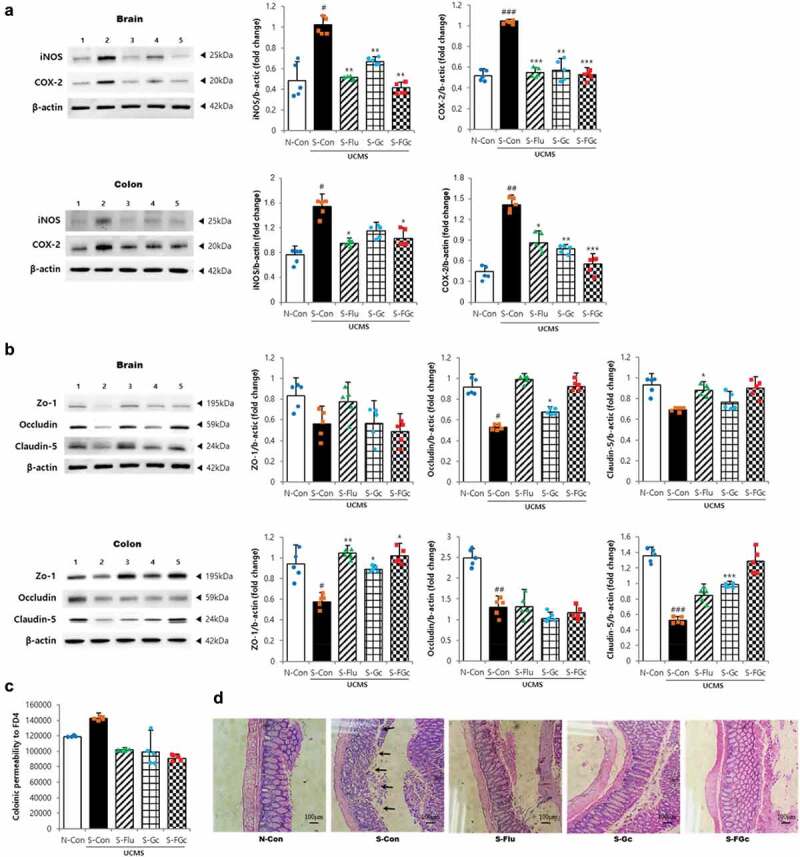
Effects of Gc and FGc on inflammation and barrier function of mice brain and colon under UCMS. (A) Expression of proteins related to inflammation by western blot. Lane 1 = N-Con; lane 2 = S-Con; lane 3 = S-Flu; lane 4 = S-Gc; lane 5 = S-FGc. (B) Expression of proteins related to tight junction by western blot. Lane 1 = N-Con; lane 2 = S-Con; lane 3 = S-Flu; lane 4 = S-Gc; lane 5 = S-FGc. (C) Intestinal epithelial permeability to FD4. (D) Representative H&E stained colon sections. Arrows indicate depleted epithelial cells. Data are expressed as mean ± SD (n = 5). #Significant difference between N-Con and S-Con (#P < .05, ##P < .005, ###P < .001). *Significant difference with S-Con (*P < .05, **P < .005, ***P < .001).
FGc normalized UCMS-induced alteration of the microbiota
To assess whether Gc and FGc, as well as UCMS, alter intestinal microbiota, 16S rRNA sequencing on genomic DNA isolated from fecal samples was performed. Alpha-diversity assessed by richness (Chao1, ACE) and diversity (Shannon) was decreased in all five mice groups (Supplementary Table 5). In the previous study, the microbial diversity (alpha-diversity) was significantly decreased by exposure to stress or psychiatric disorders, such as ADHD.29,30 However, the behavior tests were likely to act as physical stressors to mice (stressed mice as well as non-stressed mice) in this study.31 In terms of microbiota composition, principal-coordinate analysis and UniFrac unweighted pair-group method with arithmetic mean cluster analysis using phylogenetic distance, for beta-diversity, showed distinct clustering of samples with the non-stressed control (N-Con), stressed and Gc-treated (S-Gc), and stressed and FGc-treated (S-FGc) groups clustered more closely and separated from the stressed control (S-Con) and stressed and fluoxetine-treated (S-Flu) groups (Figure 5A, B). A more in-depth taxonomic analysis indicated several significant changes in the microbiota composition (Figure 5C, D and Supplementary Figure 2). At the phylum level, investigation of taxonomic shifts revealed that the ratio of the two most dominant phyla, Firmicutes and Bacteriodetes, tended to increase after exposure to UCMS but was normalized by treatment of Gc and FGc. Moreover, pre-treatment with Gc and FGc prevented the increase of the relative abundance of Proteobacteria, as a possible microbial signature involved with metabolic disorders and inflammatory bowel disease,32 induced by UCMS. At the family level, the microbiota was dominated by S24-7_f and Lachnospiraceae, and the relative abundance of S24-7_f, Lactobacillaceae, and Ruminococcaceae was reduced, whereas the relative abundance of Lachnospiraceae, Staphylococcaceae, Clostridiaceae, and Bacteroidaceae increased in stressed mice. However, FGc treatment significantly ameliorated the microbiota alteration induced by UCMS. Accordingly, at the genus level, S24-7_f_uc and Lactobacillus were dominant microbes. The relative abundance of Bacteroides, Caproiciproducens, Clostridium, Desulfovibrio, Turicibacter, Enterococcus, and Helicobacter was significantly increased by UCMS; moreover, the relative abundance of Akkermansia, Adlercreutzia, Psychrobacillus, and Blautia showed a stress-induced increasing trend. More interestingly, FGc treatment effectively normalized the increased proportion by UCMS for Bacteroides, Caproiciproducens, Clostridium, Desulfovibrio, Turicibacter, Enterococcus, and Helicobacter. However, there was a significantly lower abundance of Lactobacillus and Enterohabdus in S-Con than in N-Con, but these stress-induced changes tended to be prevented by the administration of FGc. These results suggested that chronic stress disturbed the homeostasis of the intestinal microbiota, but pre-treatment with FGc restored the stress-induced alteration of the intestinal microbiota.
Figure 5.
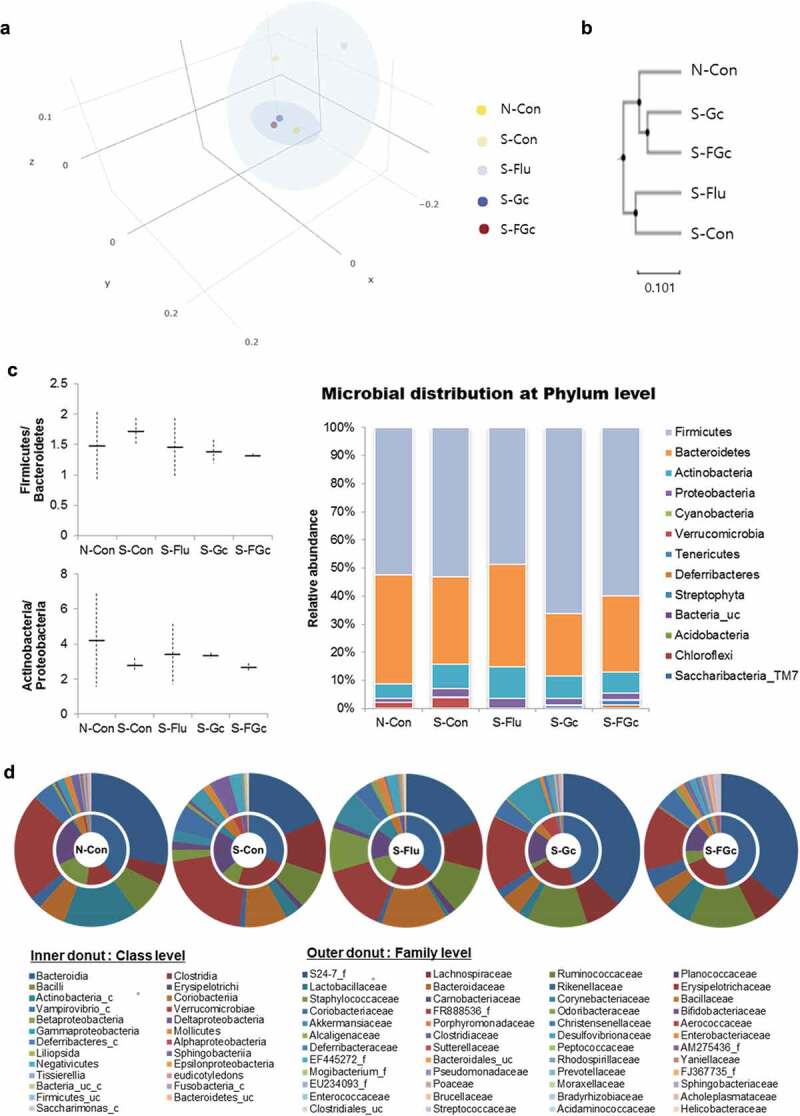
(A) Principal coordinate analysis (PCoA), (B) UniFrac unweighted pair-group method with arithmetic mean (UPGMA) cluster analysis, and microbial distribution at (C) phylum level and (D) class and family level.
Correlation analysis of intestinal microbiota with stress-induced dysregulated markers
After determining the microbiota composition and stress-induced dysregulated, the next step was to generate a heatmap of pairwise correlations between these two parameters (Figure 6). The genera Lactobacillus, Ruminococcus, Olsenella, Enterohabdus, Sphingobacterium, Pseudomonas, Alistipes, Ochrobactrum, S24-7_F_uc, Bradyrhizobium, Mucispiruillum, Acetatifactor, and Bacillus were all close relatives on the tree, which exhibited a positive correlation with the markers of the brain and gastrointestinal health. On the other hand, Clostridium, Adlercreutzia, Turicibacter, Enterococcus, Caproiciproducens, and Desulfovibrio exhibited a high positive correlation with the markers of chronic stress such as serum corticosterone concentration, anxiety-like behaviors, pro-apoptotic molecules, and inflammatory mediators. These results agree with the taxonomic analysis indicating that the intestinal microbiota composition was significantly affected by external chronic stress and statistically related to brain function and behavioral properties.
Figure 6.
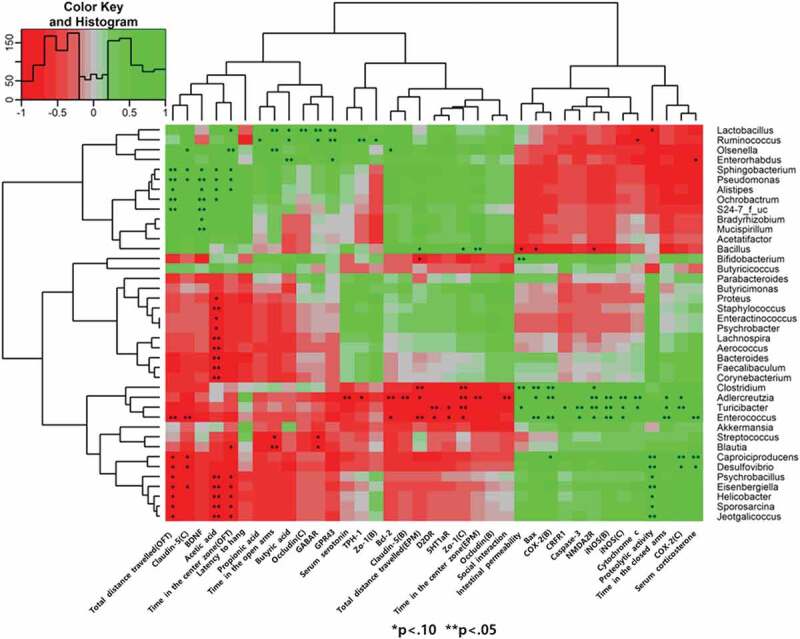
Heat map showing the correlation coefficients between the selected genera and stress-induced dysregulated markers. Red and green colors indicate positive and negative correlations, respectively. An asterisk indicates *P < .1, **P < .05.
Discussion
Repetitive exposure to unpredictable stressors activates the HPA system as an adaptive response, which is initiated by the release of glucocorticoids, altering numerous physiological (e.g., metabolic and immune) and behavioral (e.g., emotion, cognition, and motor) processes.33,34 A number of studies have attempted to prevent chronic stress-induced effects through diets consisting of various food sources and/or probiotics as well as medicines.35–37 In particular, several researchers found that intestinal microbiota alterations induced by therapeutic administration of probiotics influenced brain function and behavior through the brain-gut-microbiome axis.38 In this study, FGc pre-treatment affected stress-induced alterations of the intestinal microbiota, which could play a role in attenuating the dysregulation of corticosterone and neurotransmitters related to the HPA axis, neurodegeneration, neuroinflammation, and the barrier dysfunction response to UCMS. Additionally, chronic stress-induced anxiety-like behaviors were significantly decreased by the administration of FGc. Similarly, Gc also showed preventive effects against chronic stress-induced changes, but to a significantly lesser extent than FGc. Preliminarily, the structural modification through the glycation of milk casein was determined, for example, glycation occurred mainly in lysine residue; oxidation, phosphorylation, and deamidation were detected in methionine, serine, glutamine, and asparagine residues which might result the strong antioxidative and anti-inflammatory properties.10 Furthermore, Lactobacillus rhamnosus 4B15 and bioactive peptides derived through microbial proteolysis of glycated casein could have an important role in these protective effects, based on previous studies on introducing bacteria or gut contents and their consequences on brain structure and functions including behavior.39 The milk-derived opioid peptide casoxin C (κ-casein f(25–34), Tyr-Ile-Pro-Ile-Gln-Tyr-Val-Leu-Ser-Arg) was reported to possess psychoactive properties with hormonal and neurotransmitter activities.40 In our previous study, several peptides that composed in FGc were demonstrated their biological functions such as antioxidative, antimicrobial, immunomodulatory, anti- hypertensive, and ACE inhibitory activities.10 However, KHPIKHQGLPQEVLN, FSDIPNPIGSENSEKTTMP, FSDIP NPIGSENSEKTTMPLW, and IPNPIGSENSEKTTM derived from αs1-casein, PGEIVESLSSSEESI, FSDIPNPIGSENSEKTTM, and MPIQAFLLYQEPVLGPVRGPFPIIV from β-casein, and SPEVIESPPEINTVQVTSTAV (amino acid underlined indicates post-translated site) from κ-casein were newly detected and needed to assess their contribution to psychological function.10
Administration of FGc showed a marked effect on reducing stress-induced pathological changes of intestinal microbiota composition, anxiety-like behaviors, and physiological properties in brain and colon. Consistent with a recent study, the abundance of Lachnospiraceae, Clostridium, Adlercreutzia, Turicibacter, Helicobacter, and Bacteroides, which were positively correlated with the markers of chronic stress, increased, while the abundance of Lactobacillus, Ruminococcus, and S24-7 decreased in the stressed group compared with the normal group.41 Especially, the increase in Lachnospiraceae is related to human diseases such as ulcerative colitis and Crohn’s and celiac diseases.41 On the other hand, the reduced relative abundance of Lactobacillus and Ruminococcus, which showed a high negative correlation with stress, were normalized with FGc treatment. Lactobacillus has received a lot of attention for its beneficial role in protection against inflammation, colitis pathology, and cognitive impairment. Moreover, some members of the Ruminococcaceae family are responsible for producing short-chain fatty acids in the gut and believed to be protective against inflammation. Evidence supports the idea that the intestinal microflora of stressed mice was significantly depleted of butyrate-producing bacteria (i.e., Butyricicoccus, Clostridium, and Ruminococcus);41 indeed, a decreased abundance of related microbes was observed in S-Con mice and the concentration of butyrate in fecal samples of the stressed groups was also significantly reduced (Supplementary Figure 3). These results agree with recent studies demonstrating that the composition of the intestinal microbiota is modified by chronic stress.42 The modulation of the intestinal microbiota composition by the administration of FGc could be an additional way to reduce the effects of chronic stress, indicating that intestinal microbes significantly affect neurochemical, immunological, and behavioral changes related to stress-induced psychiatric disorders.43
Intriguingly, FGc pre-treatment suppressed the increase of serum corticosterone levels due to chronic stress-induced activation of the HPA axis. CRF is considered the primary initiator of stress responses, activating a chain of reactions such as the secretion of adrenocorticotropic hormone from the anterior pituitary.44 CRF primarily binds to CRFR1, then activates the sympathetic nervous system, specifically triggering the activation of the HPA axis, and increases anxiety and depression.45 Elevated corticosterone and hippocampal glutamate under chronic stress activate the glucocorticoid receptor and NMDAR, which naturally results in inhibition of neuronal cell proliferation and suppression of neurogenesis.46 Overactivation of the NMDAR causes an excessive influx of Ca2+ and consequent excitotoxicity, which is involved in neurodegenerative disorders.46 In recent decades, several studies determined that chronic stress affects synaptic plasticity, dendritic morphology, and neurogenesis.47 Fluoxetine developed for the treatment of major depressive disorders has been shown to stimulate neurogenesis, dependent on 5-HT1A receptors, which increase cell proliferation through a postsynaptic effect.48 FGc may act to inhibit chronic stress-induced neurodegeneration through these mechanisms, based on the results of the statistically similar expression of 5HT1A receptor in FGc and fluoxetine groups. In addition, FGc treatment regulated the expression of bcl-2, bax, and caspase-3, which are directly involved in cellular apoptosis. The anti-apoptotic bcl-2 mediates bax-induced cytochrome c release from mitochondria and caspase activation, which in turn leads to degradation of specific protein substrates. Neuroprotection of FGc against chronic stress-induced neurodegeneration is likely mediated in part by up-regulation of bcl-2 and down-regulation of bax and caspase-3. BDNF is another prevalent growth factor in the development and plasticity of the brain. Previous studies have indicated that the regulation of BDNF is closely associated with chronic stress-induced depressive disorders.49 Several studies suggest the involvement of the corticosterone level and monoaminergic system in the regulation of BDNF expression.50 For example, treatment with exogenous corticosterone decreased BDNF expression in the hippocampus and frontal cortex of rats;51 however, increased levels of monoaminergic neurotransmitters, including serotonin and dopamine, and the activation of 5-HT receptors induced BDNF expression. Consistent with these findings, FGc treatment also increased BDNF expression in brain tissue, with a simultaneous decrease in corticosterone level and increase in serotonin and expression of its receptors, when compared with the stress control group. Taken together, these data suggest that the regulation of CRFR- and NMDAR-dependent HPA activation, monoaminergic neurotransmitters, and ultimately neurogenesis-related markers may be involved in the anti-anxiolytic effect of FGc.
In addition, chronic stress caused intestinal as well as neuronal inflammation as reflected by the significant increase of inflammatory mediators such as TLR4, iNOS, COX-2, and pro-inflammatory cytokines in the brain and colon of chronic stressed mice; however, FGc treatment suppressed the stress-induced increases. Continuous exposure to stress affects the immune responses in the brain and colon owing to the interaction between the HPA axis, enteric nervous system, and the immune system. Accumulating data suggest that the infectious microbes in the gut affect depressive- and anxiety-like behaviors through the activation of immune signaling pathways from the gut to the brain.42,52,53 Hence, several specific probiotics have recently been proposed to regulate inflammatory responses that can lead to an increased incidence of stress-related disorders.2 However, information on the regulation of inflammatory mediators in the CNS is limited. A few studies have shown that glucocorticoids and neurotransmitters released under stress induce pro-inflammatory cytokines and their receptors;54 particularly, an excess of pro-inflammatory mediators in the brain may lead to structural damage (i.e., neurodegeneration) and neuronal dysfunction. More interestingly, cytokines can cross the BBB, when compromised by pathological conditions; thus, the CNS can be affected not only by cytokines within the brain but also through the actions of inflammatory mediators produced from macrophages and lymphocytes.55 Increasing evidence suggests that decreased BBB integrity, due to disruption of tight junctions, alters transport of molecules including inflammatory mediators between the blood and brain, inflammatory responses, and brain hypoperfusion, resulting in progressive neuronal dysfunction and loss in neuronal disorders.56 Consistent with this knowledge, this study revealed that exposure to chronic stress resulted in increased serum corticosterone levels, overexpression of pro-inflammatory markers, down-regulation of BBB tight junction proteins, and consequently neuronal damage and loss; however, these stress-induced pathological factors were normalized in the FGc-treated group. Moreover, FGc pre-treatment strengthened the gut-blood barrier by improving the tight junctions and reducing intestinal permeability, additionally regulated by TPH-1, an isoenzyme for the synthesis of gut-derived serotonin, and GPR43 in the colon, which is in contrast with those of the chronic stress control group.
Recent studies suggest the concept that the brain-gut axis is a bidirectional communication system between the CNS and the gastrointestinal tract, which is highly correlated with the BBB and gut-blood barrier functions.57 Furthermore, the gut microbiota is a critical node within the brain-gut axis, because of the growing evidence that it can modulate brain development and function by regulating the immune response, endocrine pathway, and intestinal barrier function through the brain-gut-microbiome axis.58 Indeed, CRF and its receptors induced by chronic stress have been reported to play a key role in intestinal permeability dysfunction,58 and in the FGc-treated mouse group in the present study, CRFR1 was down-regulated and the intestinal barrier was maintained against chronic stress. Moreover, previous pre-clinical and clinical studies reported that Lactobacillus and Bifidobacterium effectively improved CNS functions and psychiatric disorders related to anxiety- and depressive-like behaviors, autism-spectrum disorders, and memory.59 Currently available studies suggest that the mechanisms of action of probiotic effects are highly correlated with the endocrine system, immune system, enteric neuron activities, and gut microbiota and their metabolic activities.60,61 Administration of FGc consisting of Lactobacillus rhamnosus affected not only brain circuitry but also the intestine, including regulation of the intestinal microbiota composition and the tryptophan pathway which is involved with serotonin synthesis in the colon and the prevention of gut leakiness through the brain-gut-microbiome axis.
Combined with the current findings, administration of FGc, the novel functional ingredients, attenuated the chronic stress-induced dysbiosis of intestinal microbiota, neurodegeneration, neuroinflammatory reactions, and anxiety-like behaviors. The manufacture of FGc was a simple and economical process, which was heat treatment of milk casein with glucose and fermentation with probiotic L. rhamnosus 4B15, and the ingredients were easy to be obtained and handled. Treatment of the FGc normalized the dysregulation of the intestinal microflora and reduced neuronal damage and loss by regulating CRFR- and NMDAR-dependent HPA activation, the endocrine pathway involving serotonin and dopamine, and anti- and pro-apoptotic molecules and the neurotrophic factor BDNF. The anti-inflammatory effects were also attributed to the suppressive effects of FGc on pro-inflammatory mediators, such as iNOS, COX-2, and pro-inflammatory cytokines. Moreover, pre-treatment with FGc reduced the increase in BBB and intestinal barrier permeability induced by chronic stress, leading to attenuation of the HPA axis response, which consequently ameliorated anxiety-like behaviors and maintained brain function despite the chronic stress-induced abnormal brain circuitry (Supplementary Figure 4). In particular, these findings suggest that FGc treatment could be a useful therapeutic alternative for the intestinal microbiota and stress-related neuronal disorders such as anxiety and stress-related disorders.
Supplementary Material
Funding Statement
This research was supported by the High Value-Added Food Technology Development Program of the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (iPET), and the Ministry for Food, Agriculture, Forestry and Fisheries of Republic of Korea [115006-03-3-SB010, 318090-03-1-SB010] and a grant from the Next-Generation BioGreen 21 Program [PJ01322302], Rural Development Administration, Republic of Korea.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Li S, Wang C, Wang W, Dong H, Hou P, Tang Y.. Chronic mild stress impairs cognition in mice: from brain homeostasis to behavior. Life Sci. 2008;82(17–18):934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146(6):1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui F, Morimoto M, Yoshimoto K, Nakatomi Y, Syoji H, Nishimura A, Isoda K, Tanda K, Hosoi H. Effects of stress of postnatal development on corticosterone, serotonin and behavioral changes. Brain Dev. 2010;32(7):517–523. doi: 10.1016/j.braindev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Kyrou I, Tsigos C. Stress mechanisms and metabolic complications. Horm Metab Res. 2007;39(6):430–438. doi: 10.1055/s-2007-981462. [DOI] [PubMed] [Google Scholar]
- 6.Urita Y, Goto M, Watanabe T, Matsuzaki M, Gomi A, Kano M. Continuous consumption of fermented milk containing Bifidobacterium bifidum YIT 10347 improves gastrointestinal and psychological symptoms in patients with functional gastrointestinal disorders. Biosci Microbiota Food Health. 2015;34(2):37–44. doi: 10.12938/bmfh.2014-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dela Pena IJ, Hong E, de la Pena JB, Kim HJ, Botanas CJ, Hong YS, Hwang YS, Moon BS, Cheong JH. Milk collected at night induces sedative and anxiolytic-like effects and augments pentobarbital-induced sleeping behavior in mice. J Med Food. 2015;18(11):1255–1261. doi: 10.1089/jmf.2015.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markus CR, Olivier B, de Haan EH. Whey protein rich in alpha-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am J Clin Nutr. 2002;75(6):1051–1056. doi: 10.1093/ajcn/75.6.1051. [DOI] [PubMed] [Google Scholar]
- 9.Messaoudi M, Lefranc-Millot C, Desor D, Demagny B, Bourdon L. Effects of a tryptic hydrolysate from bovine milk alphaS1-casein on hemodynamic responses in healthy human volunteers facing successive mental and physical stress situations. Eur J Nutr. 2005;44(2):128–132. doi: 10.1007/s00394-004-0534-7. [DOI] [PubMed] [Google Scholar]
- 10.Oh NS, Joung JY, Lee JY, Kim Y, Kim SH. Enhancement of antioxidative and intestinal anti-inflammatory activities of glycated milk casein after fermentation with Lactobacillus rhamnosus 4B15. J Agric Food Chem. 2017;65(23):4744–4754. doi: 10.1021/acs.jafc.7b01339. [DOI] [PubMed] [Google Scholar]
- 11.Oh NS, Joung JY, Lee JY, Kim Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS One. 2018;13(2):e0192021. doi: 10.1371/journal.pone.0192021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6(2):192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 13.Ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS. Relevance of stress and female sex hormones for emotion and cognition. Cell Mol Neurobiol. 2012;32(5):725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32(6):435–443. doi: 10.1023/A:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Zhou SP, Jin YP, Huang XF, Zhou W, Han M, Yu Y, Yan K-J, Li S-M, Ma X-H, et al. Salvianolic acids T and U: a pair of atropisomeric trimeric caffeic acids derivatives from root of Salvia miltiorrhiza. Fitoterapia. 2014;98:248–253. doi: 10.1016/j.fitote.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16(6):965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- 17.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh NS, Park MR, Lee KW, Kim SH, Kim Y. Dietary Maillard reaction products and their fermented products reduce cardiovascular risk in an animal model. J Dairy Sci. 2015;98(8):5102–5112. doi: 10.3168/jds.2015-9308. [DOI] [PubMed] [Google Scholar]
- 19.Kim BK, Lee IO, Tan PL, Eor JY, Hwang JK, Kim SH. Protective effect of Lactobacillus fermentum LA12 in an alcohol-induced rat model of alcoholic steatohepatitis. Korean J Food Sci An. 2017;37:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mateer SW, Cardona J, Marks E, Goggin BJ, Hua S, Keely S. Ex vivo intestinal sacs to assess mucosal permeability in models of gastrointestinal disease. J Vis Exp. 2016;(108):e53250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renju GL, Muraleedhara Kurup G, Bandugula VR. Effect of lycopene isolated from Chlorella marina on proliferation and apoptosis in human prostate cancer cell line PC-3. Tumour Biol. 2014;35(11):10747–10758. doi: 10.1007/s13277-014-2339-5. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan R, Forrester L, Chiaia NL, Yamamoto BK. Chronic-stress-induced behavioral changes associated with subregion-selective serotonin cell death in the dorsal raphe. J Neurosci. 2017;37(26):6214–6223. doi: 10.1523/JNEUROSCI.3781-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm S, Wirth K, Fan Y, Weigand A, Gartner M, Feeser M, Dziobek I, Bajbouj M, Aust S. The interaction of corticotropin-releasing hormone receptor gene and early life stress on emotional empathy. Behav Brain Res. 2017;329:180–185. doi: 10.1016/j.bbr.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 24.Evanson NK, Herman JP. Metabotropic glutamate receptor-mediated signaling dampens the HPA axis response to restraint stress. Physiol Behav. 2015;150:2–7. doi: 10.1016/j.physbeh.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne). 2012;3:111. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DM F, Bossy-Wetzel E, NJ W, TG C, DR G. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999;274(4):2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, Fan JT, Gu XL, Zhang LY, Han J, Du SH, et al. Neuroprotective activity of cerebrosides from Typhonium giganteum by regulating Caspase-3 and Bax/Bcl-2 signaling pathways in PC12 cells. J Nat Prod. 2017;80(6):1734–1741. doi: 10.1021/acs.jnatprod.6b00954. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 29.McGaughey KD, Yilmaz-Swenson T, Elsayed NM, Cruz DA, Rodriguiz RM, Kritzer MD, Peterchev AV, Roach J, Wetsel WC, Williamson DE, et al. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci Rep. 2019;9(1):3281. doi: 10.1038/s41598-019-40140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, Fischer A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS One. 2018;13(7):e0200728. doi: 10.1371/journal.pone.0200728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, et al. Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol. 2018;9:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int. 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 34.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55(11):1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, Baqi Y, Müller CE, Rodrigues ALS, Porciúncula LO, et al. Caffeine acts through neuronal adenosine A 2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112(25):7833–7838. doi: 10.1073/pnas.1423088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadi HS, Goudarzi I, Lashkarbolouki T, Abrari K, Elahdadi Salmani M. Chronic administration of quercetin prevent spatial learning and memory deficits provoked by chronic stress in rats. Behav Brain Res. 2014;270:196–205. doi: 10.1016/j.bbr.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 39.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Xu RJ. Bioactive peptides in milk and their biological and health implications. Food Rev Int. 1998;14(1):1–16. doi: 10.1080/87559129809541147. [DOI] [Google Scholar]
- 41.Li S, Wang Z, Yang Y, Yang S, Yao C, Liu K, Cui S, Zou Q, Sun H, Guo G, et al. Lachnospiraceae shift in the microbial community of mice faecal sample effects on water immersion restraint stress. AMB Express. 2017;7(1):82. doi: 10.1186/s13568-017-0383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82(7):472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Arboleya S, Sanchez B, Milani C, Duranti S, Solis G, Fernandez N, de Los Reyes-gavilán CG, Ventura M, Margolles A, Gueimonde M, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538–544. doi: 10.1016/j.jpeds.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 44.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91(19):8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44(1):525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 46.Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6(3):333–344. doi: 10.1016/0896-6273(91)90243-S. [DOI] [PubMed] [Google Scholar]
- 47.Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21(12):505–509. doi: 10.1016/S0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 48.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29(3):450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 49.Hosang GM, Shiles C, Tansey KE, McGuffin P, Uher R. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med. 2014;12:7. doi: 10.1186/1741-7015-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, et al. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsen JP, Mork A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006;1110(1):221–225. doi: 10.1016/j.brainres.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 52.Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017:43859. doi: 10.1038/srep43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21(6):797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 2008;32(6):1136–1151. doi: 10.1016/j.neubiorev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Brzozowski B, Mazur-Bialy A, Pajdo R, Kwiecien S, Bilski J, Zwolinska-Wcislo M, Mach T, Brzozowski T. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut axis. Curr Neuropharmacol. 2016;14(8):892–900. doi: 10.2174/1570159X14666160404124127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609.e1–3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil. 2016;22(4):589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun B, Yoo JY, Park MR, Ryu S, Lee WJ, Choi HJ, Kang MK, Kim Y, Oh S. Ingestion of Gouda cheese ameliorates the chronic unpredictable mild stress in mice. Food Sci Anim Resour. 2020;40(1):145–153. doi: 10.5851/kosfa.2019.e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


