ABSTRACT
The human gut microbiota develops soon after birth and can acquire inter-individual variation upon exposure to intrinsic and environmental cues. However, inter-individual variation has not been comprehensively assessed in a multi-ethnic study. We studied a longitudinal birth cohort of 106 infants of three Asian ethnicities (Chinese, Malay, and Indian) that resided in the same geographical location (Singapore). Specific and temporal influences of ethnicity, mode of delivery, breastfeeding status, gestational age, birthweight, gender, and maternal education on the development of the gut microbiota in the first 2 years of life were studied. Mode of delivery, breastfeeding status, and ethnicity were identified as the main factors influencing the compositional development of the gut microbiota. Effects of delivery mode and breastfeeding status lasted until 6M and 3M, respectively, with the primary impact on the diversity and temporal colonization of the genera Bacteroides and Bifidobacterium. The effect of ethnicity was apparent at 3M post-birth, even before the introduction of weaning (complementary) foods, and remained significant after adjusting for delivery mode and breastfeeding status. Ethnic influences remained significant until 12M in the Indian and Chinese infants. The microbiota of Indian infants was characterized by higher abundances of Bifidobacterium and Lactobacillus, while Chinese infants had higher abundances of Bacteroides and Akkermansia. These findings provide a detailed insight into the specific and temporal influences of early life factors and ethnicity in the development of the human gut microbiota.
Trial Registration: Clinicaltrials.gov registration no. NCT01174875.
KEYWORDS: Early gut microbiota, ethnicity, delivery mode, breastfeeding, birth cohort
Introduction
The colonization of the infant gut by a microbial community is likely to play an important role in developmental processes as it serves as a source of biochemical signals that can trigger and drive immunological,1–3 metabolic,4 and neural pathways.5,6 Hence, deviations from the norm with regard to microbiota development might have a long-term bearing on future health, thereby warranting a thorough understanding of factors that influence the acquisition and developmental dynamics of the microbiota. Previous work has shown that mode of delivery7,8 and breastfeeding status9 play important roles. For example, infants born by cesarean section have delayed colonization of Bacteroides and Bifidobacterium spp. compared to infants delivered vaginally.7,10,11 Likewise, the relative abundance of Bacteroides varies between breastfed vs. formula-fed infants.12,13 However, previous studies have primarily focused on individual neonatal exposures that did not negate the effects of concurrent and confounding exposures. The composition of the microbiota has usually been assessed at a single time point, so it is not yet clear how long the effects of particular infant exposures last.
Current developments in adult gut microbiota research have indicated that factors such as ethnicity, geographical location, socioeconomic status, and genome-environment interactions can influence gut microbiota composition.14,15 For example, a recent study of 1,673 healthy adults showed gut microbiota diversity across different ethnicities in the United States.16 Interestingly, the ethnicity-specific microbial taxa also included Christensenellaceae, a heritable taxon identified by Goodrich et al. in a large-scale host-genome interaction study of twins.17 These observations also make it intuitive to test the role of ethnicity in the acquisition and temporal development of the gut microbiota in early life. However, the existing knowledge in this context is limited because the reported studies have either focused on specific population subtypes (mainly Caucasians in Western countries) or lacked a longitudinal design, or have been unable to segregate the ethnicity-specific effects from other infant exposures.16,18,19 Hence, a comprehensive analysis to parse the specific and dynamic effects of infant exposures and ethnicity on the establishment and inter-individual variability of the early gut microbiota is highly warranted.
To comprehensively monitor how ethnicity and early life exposures influence the acquisition and developmental dynamics of the infant gut microbiota, we longitudinally assessed the composition of the gut microbiota of 106 infants from the ‘Growing Up in Singapore Towards healthy Outcomes’ (GUSTO) birth cohort20 at four time points (3, 6, 12, and 24M) spanning the first 2 years of life. We interrogated the effect of seven factors (ethnicity, mode of delivery, breastfeeding status, maternal education, birthweight, gender, and gestational age) and covered three ethnic groups, i.e., Chinese, Malay, and Indian, that represents 80% of Asian and 40% of the world population. Our findings parse the independent and longitudinal effects of early life exposures and ethnicity in the critical developmental window of the first 2 years of life.
Results
Developmental trajectory of the infant gut microbiota
Fecal samples from 106 infants were collected longitudinally covering four time points (3, 6, 12 and 24M) post-birth. A detailed summary of the demographic and growth characteristics of these infants is provided in Table S1. The developmental trajectories of gut microbiota of these infants were profiled by sequencing the V4 region of the 16S rRNA gene. Overall, 11,564,295 high-quality reads were obtained covering a total of 424 fecal samples, with an average of 27,342 ± 7,312 reads per sample. At 97% similarity, these reads yielded 1,286 operational taxonomic units (OTUs). Rare OTUs (relative abundance within each sample <0.1% and existing in less than 5% samples) accounting for 0.67% of the total reads were excluded leaving 404 OTUs for subsequent gut microbiota analysis.
A significant increase in the Shannon diversity index was observed between 3 and 24M (Figure 1a and Figure S1), with the most prominent shift seen between 6 and 12M, a phase during which infants typically switch to weaning (complementary) foods. Bray-Curtis distance-based PCoA indicated a dynamic trend in the acquisition of infant gut microbiota over time (Figure 1b), and a MANOVA clustering showed similar results, but also indicated a relatively stable gut microbiota composition between 12M and 24M (Figure 1c).
Figure 1.
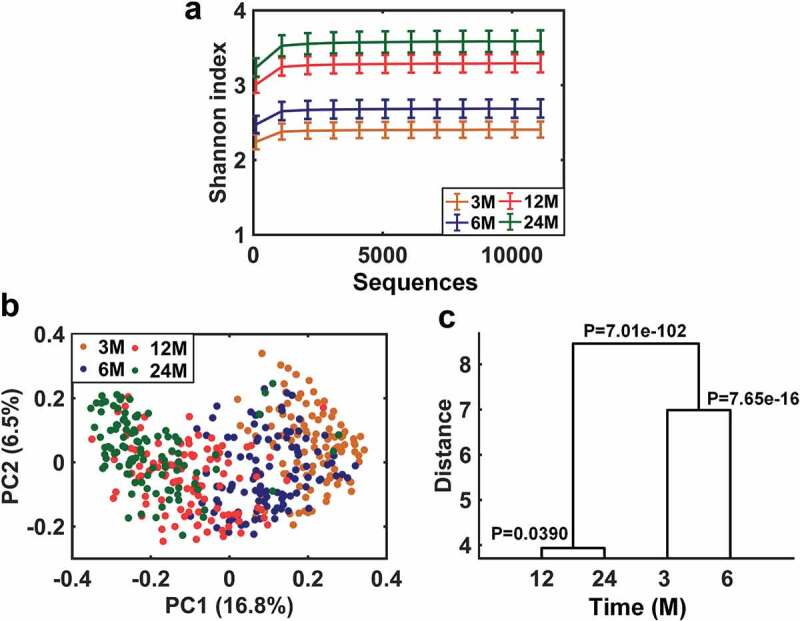
Developmental trajectory of infant gut microbiota from 3 to 24M. A. Change in α-diversity of gut microbiota over time as indicated by the Shannon diversity index. Data are shown as mean ± 95% confidence interval. B. Principal coordinate analysis (PCoA) of gut microbiota based on the Bray-Curtis dissimilarity distance over time. C. Clustering of gut microbiota based on distances between different time points using MANOVA test with Bray-Curtis distance-based PCoA scores (accounting for 80% of total variations).
Three significant factors influencing the gut microbiota in early life
Canonical correspondence analysis (CCA) was performed to assess the association of seven different factors (ethnicity, mode of delivery, feeding type, maternal education, gestational age, birthweight, and gender) with the OTUs detected at each time point. Four of these factors, i.e., ethnicity, delivery mode, breastfeeding status, and maternal education were significantly associated with gut microbiota composition (Figure 2a). However, in the partial CCA analysis used to parse the independent effects of these four factors, maternal education was no longer significant, indicating greater effects of the other three factors (Figure 2b-e and Table S2). Longitudinally, the independent effects of breastfeeding status, mode of delivery and ethnicity on gut microbiota composition lasted for 3, 6 and 12 months, respectively (Table S2).
Figure 2.
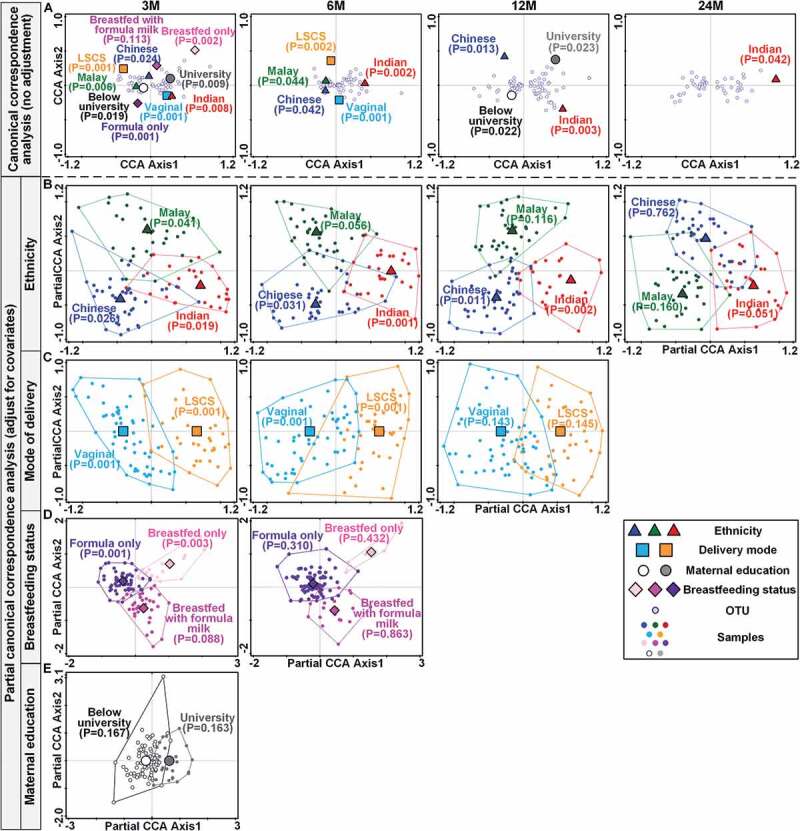
Effects of ethnicity, delivery mode, breastfeeding status and maternal education on the infant gut microbiota over time. A. Canonical correspondence analysis (CCA) ordination biplots illustrating the individual effects of ethnicity, delivery mode, breastfeeding status and maternal education on the variation of infant gut microbiota at four time points (3M, 6M, 12M, and 24M) without adjustment for covariates. B-E. Partial CCA ordination biplots illustrating the independent effects of ethnicity (b), delivery mode (c), breastfeeding status (d) and maternal education (e) on infant gut microbiota after adjusting for the covariates. B-E: At 3M, ethnicity, delivery mode, and breastfeeding status were all used as covariates and adjusted mutually; From 6 M to 24 M, only ethnicity and delivery mode were regarded as covariates and adjusted mutually. The significance of the effects of the environmental variable was tested using the Monte-Carlo Permutation Procedure (MCPP) on each environmental variable.
In alpha-diversity analysis, only the mode of delivery showed a significant effect on the Shannon diversity index at 3M, indicating that infants delivered by lower segment cesarean section (LSCS) had a microbiota that was significantly more diverse. This difference in diversity stayed significant even after adjusting for ethnicity and breastfeeding status (Table S3).
Delivery mode and infant gut microbiota
Among the 106 infants studied for mode of delivery analysis, 66 were delivered vaginally and the remaining 40 were delivered by LSCS. After adjusting for the effects of ethnicity and breastfeeding status, infants delivered by LSCS harbored a gut microbiota distinct to that of vaginally delivered infants. These differences were primarily observed at 3 and 6M of age (Figure 2c). Using partial CCA at these two-time points, we identified 86 and 80 OTUs differentiating the gut microbiota of LSCS vs. the vaginally delivered infants (Figure S2). Among them, 17 OTUs showed a differential longitudinal trend (Figure 3, Table S4). Twelve of these OTUs had higher abundance, while the remaining five had a lower abundance in the vaginally delivered infants (Figure 3). Among the 12 higher abundance OTUs, 8 belonged to Bacteroides, 2 to Parabacteroides, and 1 each to Bifidobacterium and the Bacteroidales S24-7 group. Notably, 8 OTUs from Bacteroides were consecutively higher in the gut of vaginally delivered infants at 3M and 6M. The five OTUs with higher abundances in the LSCS group belonged to Hungatella, Clostridium sensu stricto 1, Erysipelotrichaceae Incertae Sedis, Erysipelatoclostridium, and Ruminiclostridium 5, respectively.
Figure 3.
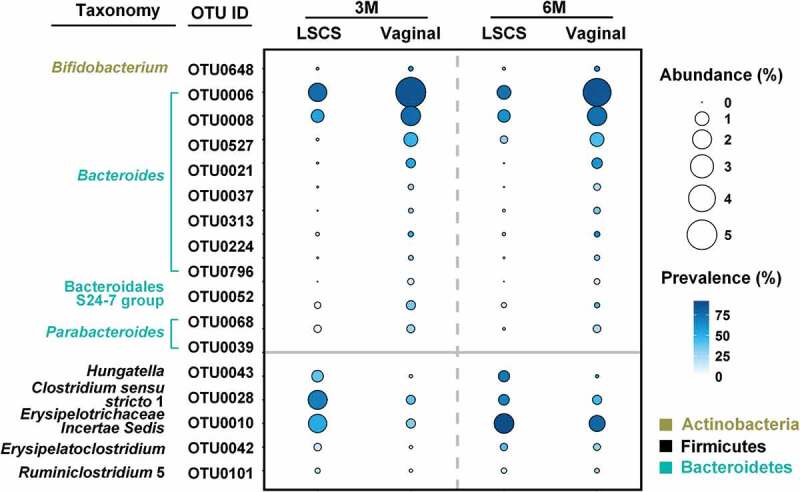
Comparison of changes in the abundances of key OTUs associated with LSCS and vaginally delivered infants over time identified by partial CCA. Only OTUs with mean relative abundance higher than 0.1% in any group and at any time point were shown in the plot. LSCS, lower segment cesarean section. OTUs with higher abundance in the gut of vagina-delivered infants were plotted in the upper panel, and lower abundance in the lower panel.
Breastfeeding status and infant gut microbiota
Data for breastfeeding status at 3 months were available for 105 of the 106 infants. Of these, 16 infants were breastfed only, 33 were mixed fed with breastmilk and formula milk, and the remaining 56 were fed with formula only. After adjusting for ethnicity and delivery mode, breastfeeding status had a significant impact on the gut microbiota at 3M (Figure 2d and Figure S3). Among the 16 OTUs associated with breastfeeding status at 3M (Figure 4, Table S5), 7 OTUs had higher abundance in the breastmilk only group, 3 in the mixed fed group and 6 in the formula fed only group. Infants fed only with breastmilk showed higher abundance of three OTUs from Bacteroides. Mixed feeding was associated with higher abundances of 1 OTU each from Bifidobacterium and Lactobacillus, respectively. Finally, the infants fed with formula milk only were characterized by higher abundances of OTUs from Staphylococcus, Erysipelatoclostridium, and Intestinibacter.
Figure 4.
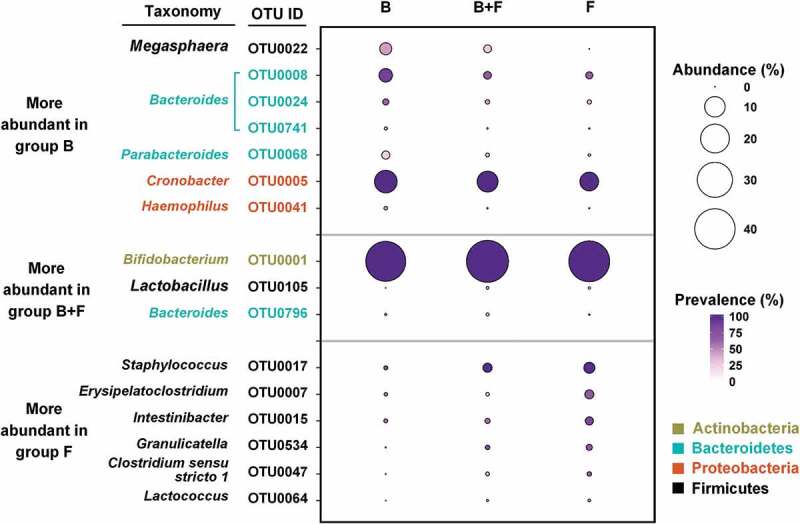
Comparison in the abundances of key OTUs associated with different breastfeeding status identified by partial CCA. Only OTUs with mean relative abundance higher than 0.1% in any group are shown in the plot. B – breastfed only; B + F – breastfed with formula milk; F – formula milk only.
Ethnicity and infant gut microbiota
Ethnicity-specific analysis of the gut microbiota was performed on 106 infants comprising 41 Chinese, 35 Malay, and 30 Indian. The gut microbiota of Indian infants aged between 3 and 12M of age was significantly different (Figure S4) from that of Chinese and Malay infants. Seventeen OTUs were identified as the key OTUs differentiating Indian infants from the other two ethnicities, among which eight OTUs showed longitudinally higher, and nine OTUs lower abundance in the Indian infants (Figure 5, Table S6). Among the eight OTUs with higher abundance, three OTUs were abundant from 3 to 12M, and included one OTU each from Collinsella, Bifidobacterium, and Catenibacterium, respectively. In addition, two OTUs from Lactobacillus, one OTU each from Bifidobacterium, and Lachnoclostridium were also longitudinally higher in abundance from 3 to 6M in the Indian subjects. As for the nine OTUs showing lower abundances in the Indian infants, the relative abundances of three OTUs were longitudinally lower from 3 to 12M, and were from Pseudobutyrivibrio, Ruminococcaceae, and Erysipelotrichaceae Incertae Sedis.
Figure 5.
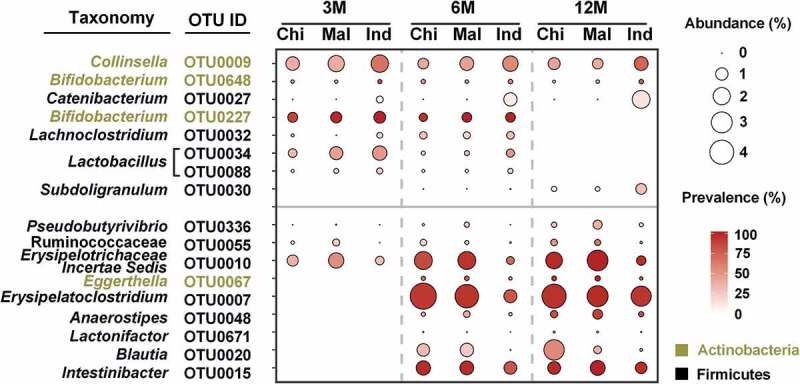
Key bacteria differentiating Indians from Chinese and Malay identified by partial CCA. Only OTUs with mean relative abundance higher than 0.1% in any ethnicity and at any time point are shown in the plot. OTUs with higher abundance in the microbiota of Indian infants were plotted in the upper panel, and lower abundance in the lower panel.
The gut microbiota of Chinese infants was significantly different from that of the Indian and Malay infants from 3 to 12M (Figure S5A). They had higher abundances of two OTUs from Bacteroides and one OTU from Akkermansia, and lower abundance of one OTU each from Lactobacillus and Collinsella (Figure S5B, Table S6). However, the temporal differences between the gut microbiota of Malay and the other two ethnicities were shorter as they lasted only until 6M (Figure S6A). They were characterized by higher abundances of one OTU each from Eggerthella, Lachnoclostridium, Pseudobutyrivibrio, and Faecalitalea, and lower abundances of one OTU each from Bacteroides and Bifidobacterium (Figure S6B, Table S6).
Discussion
The study of 106 Asian infants residing in the same geographical location, spanning the first 2 years of life, revealed differences in the compositional development of the gut microbiota. In all infants, the microbiota developed in complexity between 3 and 24 months of age, with most changes occurring during the first year of life. This kind of progression in infant microbiota complexity has been recorded in several studies.8,21-28 Effects of delivery mode7,8,10,29,30 and infant nutrition (duration of breastfeeding)28,31-38 have also been reported by others, and are confirmed in our study. The predominance of bifidobacteria (OTU0001) in our study at 3M regardless of the type of nutrition (breast milk, formula milk, or combined) could be due to the use of fructo- and/or galacto-oligosaccharides containing formula milks in Singapore. These supplements are reported to promote the abundance of bifidobacterial populations in the gut.39,40
The notable effect of ethnicity on the composition of the gut microbiota of Asian infants was a novel outcome of our study. The gut microbiotas of Indian, Chinese, and Malay infants were different and these differences were apparent even at 3 months of age when feeding of weaning (complementary) foods has usually not yet commenced. Therefore, these ethnic differences in microbiota composition point to the potential impact of human genetics, fecal microbiotas of family members, and general household environment on the development of the gut microbiota. Recent published evaluations of the relative importance of environment versus genetics on the development of the microbiota are relevant to the discussion of our results.41,42
In general, the measurement of the contribution of human genetics in shaping the microbiota is considered elusive. According to Rothschild and colleagues, data from 1,046 individuals of different ancestral origins did not show a genetic association with microbiota composition.41 Nevertheless, ‘heritable taxa,’ notably members of the family Christensenellaceae, have been recognized in studies of Canadian, Europeans, and Koreans, as well as an association between the higher relative abundance of bifidobacteria in the gut microbiota and the human lactase ‘non-persister’ genotype of Europeans.40 Overall, the genotype of the host probably has little impact on the composition of the adult microbiota,43 but the situation in infants has not been investigated comprehensively. The outcomes of our study suggest that host genetics could be important in delineating the development of the gut microbiota in very early life, and so should be investigated further.
Differences in gut microbiota compositions between ethnic groups at 3 months of age might also be due to environmental factors such as dietary preferences of the mother and other household members that could select for particular bacterial strains that would be dispersed to the infants.44 Humans sharing the same environment tend to have microbiotas that are more similar in composition, indicating greater dispersal/acquisition of gut bacteria.45–48 We also tested if the first generation status (maternal migration) of the 41 infants (Table S7) born in Singapore had any influence on the 3M gut microbiota composition, but no significant associations were observed in either the univariate (P = .066), or the adjusted (for ethnicity, delivery mode, and breastfeeding status, P = .332) analysis. Differences in the breast milk chemistry might also influence the strains of bacteria that colonize the infant gut. The composition of breast milk has been reported to differ between mothers in different countries and even between mothers in different locations in the same country.49–55 Therefore, biochemical analysis of breast milk composition of Indian, Chinese, and Malay mothers could be helpful in understanding the variations in microbiota composition. However, due to the limitation of the current study of not having collected maternal stool samples, absence of breast milk composition and the availability of limited maternal dietary intake data, we cannot reflect much on this aspect. Future studies that take into account all of these features is clearly warranted, and would potentially lead to a deeper understanding of the phenomenon of heritable taxa and its importance in the subsequent health and well-being of infants.
In conclusion, the comprehensive analysis conducted in this study identified three key factors with specific and dynamic effects on the development of the early gut microbiota. Changes in the abundances of particular bacteria were associated with mode of delivery and infant feeding practices. This is particularly important in the context of the increasing rates of elective cesarian section, and the decline in breastfeeding duration. Notably, the results show that ethnic diversity among Asians is an important consideration when studying pediatric gut microbiota. It also necessitates future research to identify the role of ethnic diversity in acquiring heritable taxa and their potential role in predisposition to population-specific health adversities.
Materials and methods
Study cohort
Infants included in this study were part of the GUSTO mother-offspring birth cohort study, designed to investigate the developmental origins of health and disease (DOHaD). Pregnant women of at least 18 years of age and in their first trimester of pregnancy were recruited from the two major public hospitals in Singapore with obstetric services (KK Women’s and Children’s Hospital (KKH) and the National University Hospital (NUH)) between 2009 and 2010. All participants were of Chinese, Malay, or Indian ethnicity, with homogeneous parental ethnic background. A detailed description of the GUSTO cohort can be accessed through the previous publication by Soh et al. (Clinicaltrials.gov registration no. NCT01174875).20
One hundred and six infants within the normal range of BMI were selected from the GUSTO cohort with characteristics shown in Table S1. We investigated the impact of seven factors on the acquisition and developmental dynamics of gut microbiota in the first 2-years post-birth, including ethnicity, mode of delivery, breastfeeding status, maternal education, gestational age, birthweight, and gender. Breastfeeding status was classified as breastfed only, breastfed with formula milk, and formula milk only. Breastfed only meant infants received breast milk and certain liquids (water or water-based drinks) without non-human milk or solids. Infants included in the study did not receive any antibiotics at birth. Data for administration of antibiotics to mother during labor were available for 89 of the 106 infants. Of these, 45 mothers received antibiotics, but this did not have a significant effect on the fecal microbiota of infants at 3M (P = .099; adjusted for ethnicity, delivery mode, and breastfeeding status). Stool samples of 106 infants were collected at 3, 6, 12 and 24 months, and stored at −80°C. Altogether, 106 samples per time point, making it a total of 424 stool samples, were used for 16S rRNA gene sequencing and subsequent gut microbiota analysis.
DNA extraction and 16S rRNA gene sequencing
DNA was extracted from 250 mg feces according to the kit protocol provided by the manufacturer (PowerSoil DNA isolation kit, catalog number 12855–100; Mo Bio). Amplification of the 16S rRNA gene V4 region, library preparation, and sequencing were carried out at Argonne National Laboratories (University of Chicago) using 250-bp paired-end reads on an Illumina MiSeq instrument.
Quality filtration and bioinformatics analysis of gut microbiota data
Raw sequencing data was processed using QIIME 1.9.0 and USEARCH v8.1.186.56,57 Both ends of the forward and reverse reads were truncated at the base where Q-value was no more than 2. If the forward and reverse reads had a minimum of 50 bp-length overlap, they were merged into a complete read. The reads with length between 252 bp and 254 bp and the expected errors less than 1 were retained for subsequent analysis.57,58
OTUs were delineated at the cutoff of 97% using USEARCH v8.1.186. Sequences for each sample were rarefied to 12,000 for 1000 times to remove the differences in the sequencing depth. Rare OTUs (<0.1% relative abundance within each sample and <5% prevalence at four time points) were removed. Alpha-diversity indices based on the rarefied OTU abundance table were calculated in QIIME 1.9.0.56 The Shannon diversity index was normally distributed. The longitudinal change of the Shannon diversity index over time was evaluated by one-way ANOVA, followed by Tukey post hoc test under the same sequencing depth of 11,100 reads. Multiple linear regression was used to evaluate the effects of various factors on the Shannon diversity index after adjusting for the covariates in MATLAB 2016b (The MathWorks, MA, USA). At 3M, ethnicity, delivery mode, and breastfeeding status were used as the covariates. From 6 to 24 months, ethnicity and delivery mode were used as the covariates. The Shannon diversity index, gestational age, and birth weight were used as continuous variables and z-score transformed. Others measures were used as categorical variables.
CCA was used in CANOCO 5.0 (Microcomputer Power, Ithaca, USA) to assess the impact of individual factors on the infant gut microbiota without adjusting for covariates. Partial CCA was used to account for the effects of covariates and to identify key OTUs corresponding to the specific effects of three main factors (ethnicity, delivery mode, and breastfeeding status). Monte-Carlo permutation test was performed to evaluate the significance of individual factor’s effect on the infant gut microbiota during the CCA and partial CCA. We performed 999 permutations under the unrestricted model. The taxonomy of each OTU was assigned against the SILVA SSU database 123. The weighted scatter plots for the key OTUs were plotted in R (v3.3.1).
Supplementary Material
Acknowledgments
We would like to thank the participants and the GUSTO study group which includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, Fabian Yap, George Seow Heong Yeo, Helen Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette P Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter D. Gluckman, Pratibha Agarwal, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Chin-Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan, and Yung Seng Lee.
Funding Statement
This work was supported by Singapore-New Zealand Food for Health joint funding (Agency for Science, Technology and Research (A*STAR), Singapore; Ministry for Business, Innovation, and Employment, New Zealand) under grant [1411624007]; the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Program on Developmental Pathways to Metabolic Disease and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC) under grant [Singapore-NMRC/TCR/004-NUS/2008 and NMRC/TCR/012-NUHS/2014]; and Strategic Positioning Fund (SPF) by the Agency for Science, Technology and Research (A*STAR) under grant [SPF 002/2013 GUSTO].
Disclosure of Potential Conflicts of Interest
YSC and PDG have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. NK, YSC, and PDG are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. The other authors declare no competing interests.
Data availability
The 16S rRNA gene sequence data are available through the Sequence Read Archive (SRA) database, under the accession number PRJNA598280.
Ethics approval and consent to participate
Written informed consent was obtained from all women who participated in the study. Approval for the study was granted by the ethics boards of both KK Women’s and Children’s Hospital (KKH) and National University Hospital (NUH), which are the Centralized Institute Review Board (CIRB) and the Domain Specific Review Board (DSRB), respectively.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Gensollen T, Iyer SS, Kasper DL, Blumberg RS.. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honda K, Littman DR.. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 4.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 7.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Bjorksten B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 8.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:852. doi: 10.1016/j.chom.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ: Canadian Medical Association Journal = Journal De l’Association Medicale Canadienne. 2013;185:385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Li M, Wu S, Lebrilla CB, Chapkin RS, Ivanov I, Donovan SM. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr. 2015;60:825–833. doi: 10.1097/MPG.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaulke CA, Sharpton TJ. The influence of ethnicity and geography on human gut microbiome composition. Nat Med. 2018;24:1495–1496. doi: 10.1038/s41591-018-0210-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Guo Z, Xue Z, Sun Z, Zhang M, Wang L, Wang G, Wang F, Xu J, Cao H, et al. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. Isme J. 2015;9:1979–1990. doi: 10.1038/ismej.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018;16:e2006842. doi: 10.1371/journal.pbio.2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stearns JC, Zulyniak MA, de Souza RJ, Campbell NC, Fontes M, Shaikh M, Sears MR, Becker AB, Mandhane PJ, Subbarao P, et al. Ethnic and diet-related differences in the healthy infant microbiome. Genome Med. 2017;9:32. doi: 10.1186/s13073-017-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, Savage JR, Carey V, O’Connor G, Sandel M, et al. Factors influencing the infant gut microbiome at age 3-6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol. 2017;139:482–91 e14. doi: 10.1016/j.jaci.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stunkel W, Holbrook JD, Kwek K, Chong YS, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 21.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology. 2011;157:1385–1392. doi: 10.1099/mic.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 22.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, Molgaard C, Michaelsen KF, Licht TR. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol. 2014;80:2889–2900. doi: 10.1128/AEM.00342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laursen MF, Andersen LB, Michaelsen KF, Molgaard C, Trolle E, Bahl MI, Licht TR. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere. 2016;1. doi: 10.1128/mSphere.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laursen MF, Bahl MI, Michaelsen KF, Licht TR. First foods and gut microbes. Front Microbiol. 2017;8:356. doi: 10.3389/fmicb.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qasem W, Azad MB, Hossain Z, Azad E, Jorgensen S, Castillo San Juan S, Cai C, Khafipour E, Beta T, Roberts LJ 2nd, et al. Assessment of complementary feeding of Canadian infants: effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr. 2017;17:54. doi: 10.1186/s12887-017-0805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leong C, Haszard JJ, Lawley B, Otal A, Taylor RW, Szymlek-Gay EA, Fleming EA, Daniels L, Fangupo LJ, Tannock GW, et al. Mediation analysis as a means of identifying dietary components that differentially affect the fecal microbiota of infants weaned by modified baby-led and traditional approaches. Appl Environ Microbiol. 2018;84. doi: 10.1128/AEM.00914-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawley B, Otal A, Moloney-Geany K, Diana A, Houghton L, Heath AM, Taylor RW, Tannock GW. Fecal microbiotas of Indonesian and New Zealand children differ in complexity and bifidobacterial taxa during the first year of life. Appl Environ Microbiol. 2019;85. doi: 10.1128/AEM.01105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22:250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokholm J, Thorsen J, Chawes BL, Schjorring S, Krogfelt KA, Bonnelykke K, Bisgaard H. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016;138:881–9 e2. doi: 10.1016/j.jaci.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Murphy R, Morgan XC, Wang XY, Wickens K, Purdie G, Fitzharris P, Otal A, Lawley B, Stanley T, Barthow C, et al. Eczema-protective probiotic alters infant gut microbiome functional capacity but not composition: sub-sample analysis from a RCT. Benef Microbes. 2019;10:5–17. doi: 10.3920/BM2017.0191. [DOI] [PubMed] [Google Scholar]
- 32.Tannock GW, Lawley B, Munro K, Gowri Pathmanathan S, Zhou SJ, Makrides M, Gibson RA, Sullivan T, Prosser CG, Lowry D, et al. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl Environ Microbiol. 2013;79:3040–3048. doi: 10.1128/AEM.03910-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mah KW, Chin VI, Wong WS, Lay C, Tannock GW, Shek LP, Aw MM, Chua KY, Wong HB, Panchalingham A, et al. Effect of a milk formula containing probiotics on the fecal microbiota of asian infants at risk of atopic diseases. Pediatr Res. 2007;62:674–679. doi: 10.1203/PDR.0b013e31815991d5. [DOI] [PubMed] [Google Scholar]
- 35.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji H, Oozeer R, Matsuda K, Matsuki T, Ohta T, Nomoto K, Tanaka R, Kawashima M, Kawashima K, Nagata S, et al. Molecular monitoring of the development of intestinal microbiota in Japanese infants. Benef Microbes. 2012;3:113–125. doi: 10.3920/BM2011.0038. [DOI] [PubMed] [Google Scholar]
- 37.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology. 2010;156:3329–3341. doi: 10.1099/mic.0.043224-0. [DOI] [PubMed] [Google Scholar]
- 38.Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS One. 2012;7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, Klarczyk M, Schopfer H, Bockler HM, Wells J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr. 2005;40:36–42. doi: 10.1097/00005176-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, Tappenden KA. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2012;36:95S–105S. doi: 10.1177/0148607111430087. [DOI] [PubMed] [Google Scholar]
- 41.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 42.Goodrich JK, Davenport ER, Clark AG, Ley RE. The relationship between the human genome and microbiome comes into view. Annu Rev Genet. 2017;51:413–433. doi: 10.1146/annurev-genet-110711-155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nayfach S, Rodriguez-Mueller B, Garud N, Pollard KS. An integrated metagenomics pipeline for strain profiling reveals novel patterns of bacterial transmission and biogeography. Genome Res. 2016;26:1612–1625. doi: 10.1101/gr.201863.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, Lucas SK, Beura LK, Thompson EA, Till LM, et al. US immigration Westernizes the human gut microbiome. Cell. 2018;175:962–72 e10. doi: 10.1016/j.cell.2018.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez I, Stegen JC, Maldonado-Gomez MX, Eren AM, Siba PM, Greenhill AR, Walter J. The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 49.Castanys-Munoz E, Martin MJ, Prieto PA. 2ʹ-fucosyllactose: an abundant, genetically determined soluble glycan present in human milk. Nutr Rev. 2013;71:773–789. doi: 10.1111/nure.12079. [DOI] [PubMed] [Google Scholar]
- 50.Marx C, Bridge R, Wolf AK, Rich W, Kim JH, Bode L. Human milk oligosaccharide composition differs between donor milk and mother’s own milk in the NICU. J Hum Lact. 2014;30:54–61. doi: 10.1177/0890334413513923. [DOI] [PubMed] [Google Scholar]
- 51.McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr. 2017;105:1086–1100. doi: 10.3945/ajcn.116.139980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence ECH, Patel AL, Hou J, Lewis NE, Bode L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut. 2018;67:1064–1070. doi: 10.1136/gutjnl-2016-312819. [DOI] [PubMed] [Google Scholar]
- 53.Gay MCL, Koleva PT, Slupsky CM, Toit ED, Eggesbo M, Johnson CC, Wegienka G, Shimojo N, Campbell DE, Prescott SL, et al. Worldwide variation in human milk metabolome: indicators of breast physiology and maternal lifestyle? Nutrients. 2018;10:1151. doi: 10.3390/nu10091151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Gallego C, Morales JM, Monleon D, Du Toit E, Kumar H, Linderborg KM, Zhang Y, Yang B, Isolauri E, Salminen S, et al. Human breast milk NMR metabolomic profile across specific geographical locations and its association with the milk microbiota. Nutrients. 2018;10:1355. doi: 10.3390/nu10101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Leeuwen SS, Stoutjesdijk E, Ten Kate GA, Schaafsma A, Dijck-Brouwer J, Muskiet FAJ, Dijkhuizen L. Regional variations in human milk oligosaccharides in Vietnam suggest FucTx activity besides FucT2 and FucT3. Sci Rep. 2018;8:16790. doi: 10.1038/s41598-018-34882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 58.Edgar RC, Flyvbjerg H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics. 2015;31:3476–3482. doi: 10.1093/bioinformatics/btv401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA gene sequence data are available through the Sequence Read Archive (SRA) database, under the accession number PRJNA598280.


