Abstract
Aims:
Increased adiposity is a risk factor for suboptimal diabetes control and cardiovascular disease (CVD) complications.
Our goal was to identify modifiable behavioral characteristics of overweight and obese pediatric patients with type 1 diabetes mellitus (T1DM) who achieve optimal glycemic control and to evaluate their CVD risk compared to lean patients. Our hypothesis was that optimally controlled obese and overweight participants require more total daily insulin and are at higher CVD risk compared to optimally controlled lean participants.
Methods:
We analyzed a cohort of 9,263 participants with T1DM aged<21 years in the T1D Exchange Registry. Optimal diabetes control was defined as HbA1c ≤7.5% (58mmol/mol). We compared factors that influence glycemic control in lean, overweight and obese participants with optimal vs. suboptimal control, using logistic regression.
Results:
Age, race, overweight status, continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM) use were important variables influencing glycemic control. In the optimally controlled cohort, 27% of participants were overweight or obese versus 30% in the suboptimally controlled cohort (P<0.001). Overweight and obese participants with optimal control were not significantly different from lean participants in terms of CSII use, total daily insulin dosage per kg of bodyweight, glucose checks per day, boluses with bedtime snack, use of CGM, but had higher LDL cholesterol and triglycerides, and lower HDL cholesterol (P<0.05).
Conclusions:
There were no differences in modifiable behavioral characteristics between the obese, overweight and lean optimally controlled participants. However, predictors of cardiovascular disease were higher in the overweight and obese group.
Keywords: Type 1 diabetes, glycemic control, HbA1c, adolescent, child
1.1. Introduction
In the US, approximately 200,000 children live with type 1 diabetes mellitus (T1DM).1 The American Diabetes Association (ADA) has recently updated its recommendation of a target HbA1c ≤7.5% (58mmol/mol) for all children with T1DM; however, the majority of them don’t achieve this goal.2,3 An analysis of the T1D Exchange Registry data showed that less than 21% of the 13-20 year old group achieve optimal control 2. Poor glycemic control is associated with increased risk of developing micro- and macrovascular complications and higher mortality rates. 4,5
The alarming increase in global obesity also affects children with T1DM, with an estimated prevalence of 12.5% to 33% 6–8. Obesity is a risk factor for cardiovascular disease by itself, and potentially makes optimal diabetes control more challenging.9,10
Our goal was to identify modifiable behavioral characteristics of overweight and obese pediatric participants of the T1D Exchange Registry who achieve optimal glycemic control so that future intervention trials could target these behaviors in order to improve outcomes. We were also interested in the cardiovascular risk of the overweight and obese participants compared to lean individuals. Our hypothesis was that among optimally controlled participants with T1DM, those that are overweight or obese require more intensive insulin and glucose monitoring and are at higher CVD risk compared to those that are lean.
2.1. Subjects, Materials and Methods
Data were collected from the public dataset of the T1D Exchange Registry for the years 2010-2012.Clinical data are obtained from medical chart extraction and self-reported statements. Inclusion criteria were: i) diagnosis of T1DM( as defined in the registry’s criteria of eligibility)11 and ii) age of less than 21 years at consent. Participants were excluded if they had no HbA1c measurement within three months of enrollment, had other missing information (see below) and/or had a body mass index (BMI) >40 kg/m2 or a BMI below the 5th percentile for age and sex12, in order to avoid potential inclusion of participants with combined type 1 and type 2 diabetes, and to avoid potentially inaccurate values of BMI. Participants were also excluded if they were transgender in order to avoid misclassification of sex variable.
Data were extracted from a total of 17,047 participants (see figure 1). Participants with missing data for bolus and basal insulin or short-acting insulin and long-acting insulin respectively (n=4,009), those who had interchangeably used both multiple daily injections (MDIs) and continuous subcutaneous insulin infusion (CSII) (n=145), participants on fixed dosages of insulin (n= 494) were excluded. Participants with a % total daily bolus dose under 10% or over 90% of total daily insulin dose were also excluded to eliminate the effect of outliers (n=191). Lastly, participants were excluded if information on race was missing (n = 3) and if duration of their diabetes was less than 1 year (n = 886). The remaining 9,263 participants who met inclusion criteria were then separated into distinct groups according to their BMI. Participants with a BMI < 85th percentile for their age/sex [as defined by the Centers for Disease Control and Prevention (CDC)] were classified as normal weight, 85-94.9th percentile as overweight and above the 95th percentile as obese. We also categorized participants into three age groups to capture the majority of prepubertal children (<12 years old), pubertal children (12-17 years old) and children who have transitioned to young adulthood (18-21 years old).
Figure 1: Flow Diagram of cohort selection.
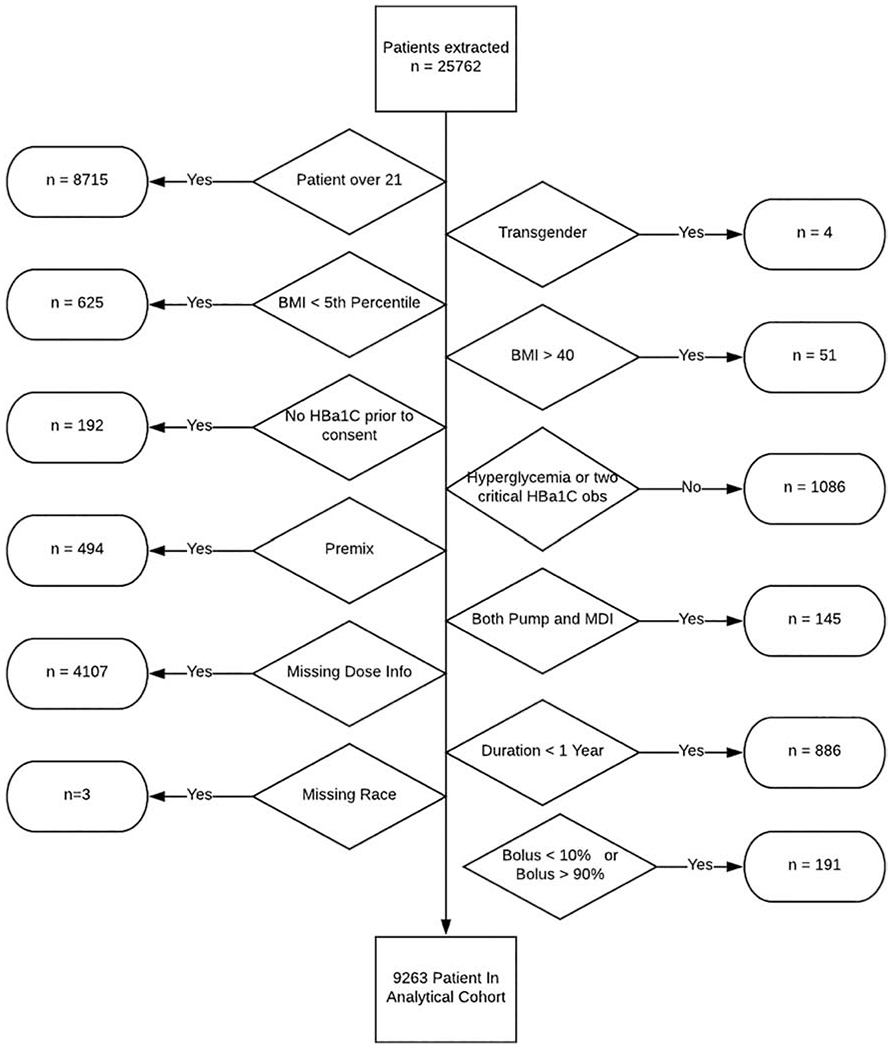
BMI: Body Mass Index, MDI: Multiple Daily Injections
The mean HbA1c was calculated using the most recent available HbA1c measurement (from 6 months prior to 1 month after consent). The analysis was treated cross-sectionally and participants were considered as optimally controlled if the mean most recent HbA1c was less or equal to 7.5% (58mmol/mol). To identify associations between behavioral factors and glycemic control, we extracted data on the following parameters: timing of bolus dose administration, frequency of missing doses, frequency of bolus for day and bedtime snacks, coverage for snacks, frequency of blood glucose monitoring, as well as whether continuous glucose monitoring (CGM) was used. We also collected data on T1DM-related events in the previous 12 months, namely diabetic ketoacidosis (DKA) hospital admissions and frequency of severe hypoglycemic episodes resulting in seizure or loss of consciousness. These were chosen for their high clinical significance and as markers of possible poor compliance with the regimen as well as for their association with CVD risk. We also extracted demographic data on household income, insurance status; we classified households as low income if earned $50,000 and below, mid income between 50,000 to 100,000 and high income if they earned $100,000 and above. For the analysis on lipid measurements, all available lipids measurements were included.
For the descriptive analysis, means and standard deviations were calculated for continuous variables and for the frequency and proportion of categorical variables, respectively. The optimally controlled and suboptimally controlled cohorts were compared using two-sided t-tests and chi-square tests. Further analyses were performed to explore differences associated with daily insulin doses. The total daily dose of insulin was analyzed against method of delivery and glycemic control status within the same insulin delivery method group. One-way ANOVAs and logistic regressions were performed to assess differences after controlling for demographic factors including race, sex, BMI category, and age group. We also adjusted for multiple comparisons using the Holm-Bonferroni correction. Finally, we designed a regression model to identify factors that are important for optimal control in the whole group. P values <0.05 were considered significant. SAS version 9.4 (SAS Institute, Cary, NC) was used for the cohort construction and R 3.3 (. R Foundation for Statistical Computing, Vienna, Austria) for the data analysis.
3.1. Results
Demographic characteristics of all optimally and suboptimally controlled 9,263 participants are presented in the additional files. There were significantly fewer optimally controlled participants in the 12-17-year-old subgroup, among Black and Hispanic individuals, in the overweight and obese subgroups, in females and in MDI users (Table A.1). In the optimally controlled cohort, there were more pump users (58.7% vs 46.3%, P<0.001) and they used significantly less insulin (0.85±0.4 vs 0.97±0.46 units/kg/day), P<0.001), even after adjusting for age, sex, race, duration of diabetes and insurance status. Also, participants in the optimally controlled cohort checked their blood glucose more frequently (6.5± 2. 6 vs 5.6± 2.3 times per day, P<0.001) and a higher proportion of the them used CGM (5.5% vs 2.3%, P<0.001). The optimally controlled cohort had fewer severe hypoglycemic episodes (11.6% vs 17.7%, P<0.001) and hospitalizations for DKA (0.03% vs 0.15%, P<0.001) in the previous 12 months. These results also remained significant after adjusting for age, BMI, race, sex, T1DM duration, income, insurance and multiple comparisons, except for the severe hypoglycemia events (Additional File 2). Finally, the suboptimally controlled cohort had higher mean low density lipoprotein (LDL) cholesterol and total cholesterol and triglycerides levels, but no difference was found in the mean high density lipoprotein (HDL) cholesterol compared to the optimally controlled cohort (Table B.1).
The descriptive statistics of optimally controlled participants according to their BMI status is shown in Table 1. Adolescents (12-17 years old) were more frequently overweight or obese compared younger study participants (P<0.01). Also, obesity rates are higher among Black (23.3%) and Hispanic children (15%), compared to white children (8.1%), (P<0.001).
Table 1:
Descriptive characteristics of the optimally controlled participants according to their BMI
| Variable | Normal n=1720 | Overweight n=430 | Obese n=217 | P |
|---|---|---|---|---|
| HbA1c % | 6.98 (0.44) | 7.04 (0.4) | 6.99 (0.5) | 0.077 |
| HbA1c (mmol/mol) | 53 (0) | 53 (0) | 53 (0) | 0.077 |
| Age n (%) | 0.010 | |||
| < 12 years | 719 (73.7) | 152 (15.6) | 104 (10.7) | |
| 12 – 17 years | 733 (71.6) | 200 (19.5) | 91 (8.9) | |
| 18 – 20 years | 268 (72.8) | 78 (21.2) | 22 (6) | |
| Race n (%) | <0.001 | |||
| 1.White Non-Hispanic | 1484 (73.8) | 364 (18.1) | 162 (8.1) | |
| 2.Black Non-Hispanic | 24 (55.8) | 9 (20.9) | 10 (23.3) | |
| 3.Hispanic or Latino | 138 (66.7) | 38 (18.4) | 31 (15) | |
| 4.Native Hawaiian/Other Pacific Islander | 0 (0) | 1 (33.3) | 2 (66.7) | |
| 5.Asian | 36 (78.3) | 6 (13) | 4 (8.7) | |
| 6. American Indian/Alaskan Native | 1 (33.3) | 0 (0) | 2 (66.7) | |
| 7.More than one race | 37 (67.3) | 12 (21.8) | 6 (10.9) | |
| Sex n (%) | 0.042 | |||
| Female | 789 (74.2) | 195 (18.3) | 80 (7.5) | |
| Male | 931 (71.5) | 235 (18) | 137 (10.5) | |
| BMI (kg/m2) | 19.34 (2.99) | 23.71 (3.47) | 27.33 (4.84) | <0.001 |
Values are represented as mean +/− SD. Values in parentheses represent percent % or the standard deviation SD. Adjusted p values based on logistic regression of overweight/obese controlling for age, race, sex, DM duration, income, insurance, mean Hba1C and multiple comparisons.
In terms of clinical management, we found frequent pump use in the optimally controlled participants across all BMI groups, with slightly lower rates of use among obese children [58.9% among participants with normal weight, 64% with overweight,47% with obesity (P<0.001)]. However, after adjustment for age, race, sex, T1DM duration, income, and insurance and multiple comparisons, this difference lost statistical significance. The total daily insulin dose expressed as units per bodyweight per day was similar across all BMI categories (P=0.096) within the optimally controlled cohort. The use of CGM was not very common among optimally controlled children [5.6% among normal weight children 6.5% of overweight and 2.8% of obese children (P=0.233)]. Hypoglycemic episodes were more frequent among overweight (17.4%) compared to normal weight (10.3%) and obese children (11.3%) (P=0.013), but this difference did not persist after adjustment for demographic and socio-economic factors and multiple comparisons (P=0.864). Despite their optimal control, overweight and obese children had higher concentrations of LDL cholesterol (P=0.021) and triglycerides (P<0.001) and lower levels of HDL cholesterol (P<0.001) compared to normal weight children (Table 2). This relationship persisted for HDL and triglycerides after adjustment for socioeconomic and demographic factors, but not for LDL. No differences were seen in the number of DKA admissions, the frequency of boluses for bedtime snack, consumption of a bedtime snack or the level of exercise. The behavioral and CVD risk characteristics of participants according to BMI are shown in Table 2.
Table 2:
Behavioral and CVD risk characteristics of the optimally controlled participants according to their BMI
| Variable | Normal n=1720 | Overweight n=430 | Obese n=217 | P | Adjusted P |
|---|---|---|---|---|---|
| Insulin Method (%) | <0.001 | 1.000 | |||
| CSII | 1013 (58.9) | 275 (64) | 102 (47) | ||
| MDI | 707 (41.1) | 155 (36) | 115 (53) | ||
| Total daily insulin dose, (units/kg/day) | 0.84 (0.4) | 0.89 (0.37) | 0.86 (0.43) | 0.096 | 1.000 |
| Number Meter Check | 6.62 (2.57) | 6.46 (2.4) | 6.12 (2.77) | 0.020 | 1.000 |
| CGM Used (%) | 0.233 | 1.000 | |||
| 1.Yes | 97 (5.6) | 28 (6.5) | 6 (2.8) | ||
| 2.No | 1588 (92.3) | 395 (91.9) | 209 (96.3) | ||
| 3.Unknown | 35 (2) | 7 (1.6) | 2 (0.9) | ||
| Severe Hypoglycemic Events (%) | 0.013 | 0.864 | |||
| 1.Yes | 130 (10.3) | 54 (17.4) | 18 (11.3) | ||
| 2.No | 1131 (89.5) | 256 (82.3) | 141 (88.7) | ||
| 3.Don’t know | 3 (0.2) | 1 (0.3) | 0 (0) | ||
| Number of Hospitalizations for DKA | 0.03 (0.29) | 0.04 (0.2) | 0.06 (0.48) | 0.192 | 1.000 |
| Number of Bolus per Day | 6.65 (3.97) | 6.56 (5.13) | 6.48 (3.94) | 0.817 | 1.000 |
| Frequency of Missing Dose | 0.265 | ||||
| 1.Never | 1064 (61.9) | 245 (57) | 146 (67.3) | ||
| 2.Rarely | 431 (25.1) | 130 (30.2) | 47 (21.7) | 1.000 | |
| 3.Sometimes | 179 (10.4) | 44 (10.2) | 20 (9.2) | 1.000 | |
| 4.Often | 36 (2.1) | 8 (1.9) | 3 (1.4) | 1.000 | |
| 5.Very often | 6 (0.3) | 0 (0) | 0 (0) | 1.000 | |
| 6.At least once a day | 3 (0.2) | 3 (0.7) | 1 (0.5) | 1.000 | |
| Frequency of Bolus Bed Time Snack (%) | 0.884 | ||||
| 1.Never | 119 (11.2) | 30 (11.9) | 19 (15.8) | ||
| 2.Rarely | 81 (7.7) | 22 (8.7) | 10 (8.3) | 1.000 | |
| 3.Sometimes | 173 (16.4) | 44 (17.4) | 21 (17.5) | 1.000 | |
| 4.Most of the time | 252 (23.8) | 54 (21.3) | 24 (20) | 1.000 | |
| 5.Always | 433 (40.9) | 103 (40.7) | 46 (38.3) | 1.000 | |
| Eat Bed Time Snack (%) | 0.280 | 1.000 | |||
| 1.Yes | 1059 (61.9) | 252 (59.2) | 120 (55.8) | ||
| 2.No | 638 (37.3) | 172 (40.4) | 92 (42.8) | ||
| 3.Don’t know | 13 (0.8) | 2 (0.5) | 3 (1.4) | ||
| LDL (mg/dl) | 84.32 (23.94) | 86.37 (23.13) | 90.06 (28.42) | 0.021 | 0.182 |
| HDL (mg/dl) | 58.23 (14.53) | 53.99 (12.85) | 51.4 (14.06) | <0.001 | <0.001 |
| Total Cholesterol (mg/dl) | 157.64 (29.51) | 157.26 (28.09) | 162.04 (35.61) | 0.355 | 1.000 |
| Triglycerides (mg/dl) | 80.27 (47.83) | 89.62 (72.09) | 97.42 (58.96) | <0.001 | 0.005 |
| Days of Exercise per week | 5.28 (1.68) | 5.12 (1.64) | 5 (1.86) | 0.081 | 0.987 |
Values are represented as mean +/− SD. Values in parentheses represent percent % or the standard deviation SD. Adjusted p values based on logistic regression of overweight/obese controlling for age, race, sex, DM duration, income, insurance and multiple comparisons.
MDI: Multiple Daily Injections,, CGM: Continuous glucose monitoring , , CSII: continuous subcutaneous insulin infusion
Finally, in the logistic regression model we found that age, sex, race, overweight status, duration of diabetes, family income, insurance type, method of insulin delivery (MDI vs CSII), number of glucose checks, use of CGM, history of DKA, significantly affected glycemic control (See table 3 and figure 2).
Table 3:
Logistic regression model for factors that are important for optimal control
| Variable | OR | CI | P |
|---|---|---|---|
| Sex | 0.011 | ||
| Male | 1.14 | [1.04,1.27] | |
| Female | -ref | ||
| BMI | |||
| Normal (5-84.9th %) | -ref | ||
| Overweight (85th-94.9th %) | 0.87 | [0.77,0.99] | 0.039 |
| Obese (≥95th %) | 0.87 | [0.73,1.04] | 0.134 |
| Age | 1.09 | [1.07,1.11] | <0.001 |
| Race | |||
| White | -ref | ||
| Black | 0.46 | [0.33,0.65] | <0.001 |
| Hispanic | 1.13 | [0.94,1.35] | 0.201 |
| Asian | 1.51 | [1.07,2.36] | 0.020 |
| Other | 0.68 | [0.50,0.93] | 0.011 |
| Diabetes Duration (per 2 years) | 0.84 | [0.81,0.87] | <0.001 |
| Annual Income | |||
| High | -ref | ||
| Low | 0.61 | [0.52,074] | <0.001 |
| Mid | 0.70 | [0.63,0.80] | <0.001 |
| Not Present | 0.81 | [0.70,0.94] | 0.003 |
| Insurance | |||
| Medicaid | -ref | ||
| Medicare | 0.89 | [0.63,1.24] | 0.476 |
| No Coverage | 0.862 | [0.34,1.85] | 0.688 |
| Other | 1.28 | [1.02,1.61] | 0.036 |
| Private | 1.50 | [1.25,1.79] | <0.001 |
| Unknown | 1.20 | [0.93,1.55] | 0.134 |
| Total daily insulin dose (units/kg/day) | 0.54 | [0.47,0.62] | <0.001 |
| Method | |||
| CSII | -ref | ||
| MDI | 0.77 | [0.68,0.86] | <0.001 |
| Bed Time Snack | |||
| Yes | -ref | ||
| No | 1.10 | [0.99,1.23] | 0.065 |
| Number of Hospital DKA’s | 0.51 | [0.41,0.63] | <0.001 |
| Number of Times Meter Checked | 1.18 | [1.16,1.21] | <0.001 |
| CGM Not Used | 0.51 | [0.39,0.66] | <0.001 |
OR=Odds ratio, CI=Confidence Interval, Diabetes duration refers to participants that had diabetes for at least one year. Model regresses the odds of controlled Hba1C < 7.5%(58mmol/mol] against sex, BMI, age, race, income of the family, insurance, insulin method and insulin amount used, number of hospital DKA’s, number of meter checks, CGM use, and if bedtime snack was taken.
BMI: Body Mass Index, CGM: Continuous glucose monitoring, DKA: diabetic Ketoacidosis , CSII: continuous subcutaneous insulin infusion, MDI: Multiple Daily Injections
Figure 2: Forest plot of the multiple linear regression with controlled HgA1c<7.5% (58 mmol/mol) as dependent variable.
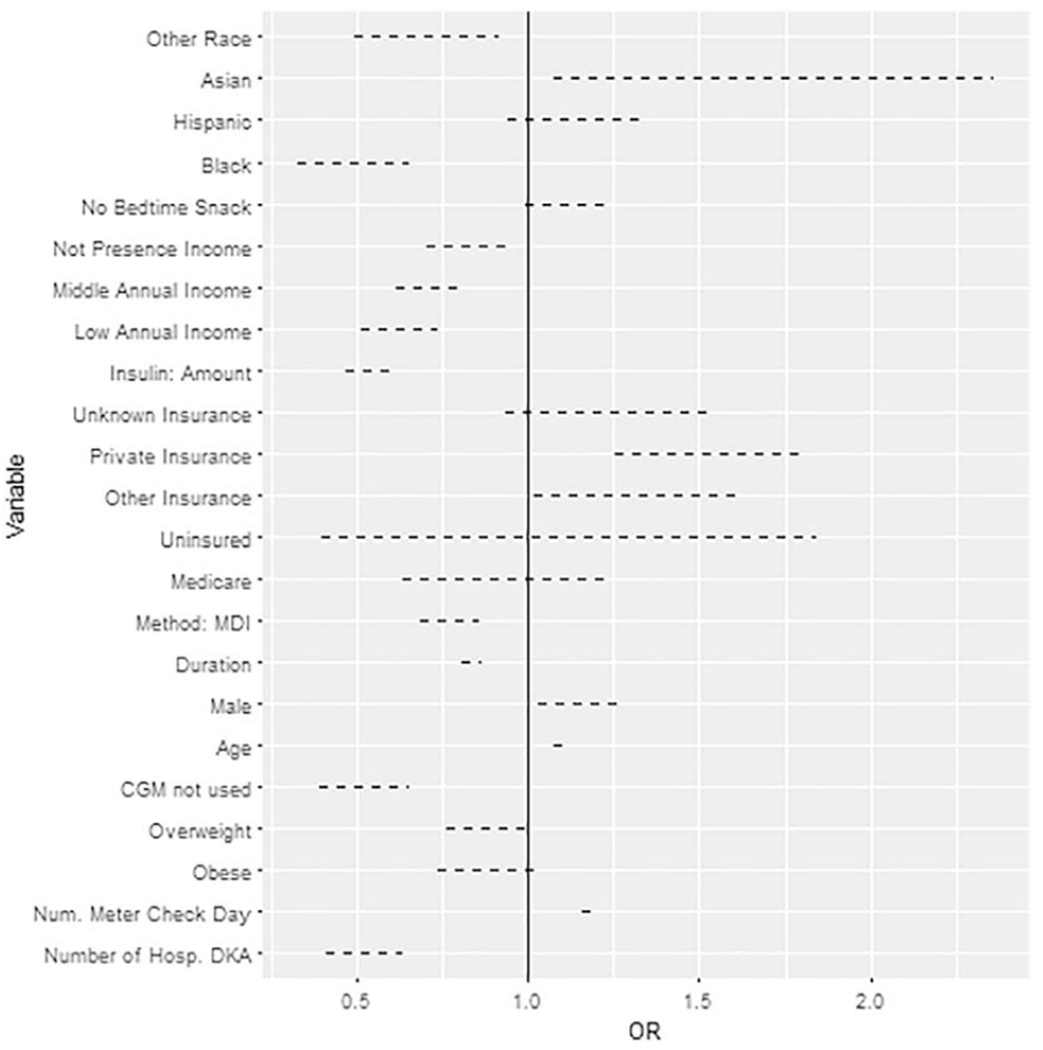
MDI: Multiple Daily Injections, CGM: Continuous glucose monitoring, DKA: diabetic ketoacidosis
4,1. Discussion
Poor glycemic control is associated with a higher risk of diabetes complications, such as retinopathy, cardiovascular disease and diabetic kidney disease13 Children with T1DM who are obese or overweight have an additional risk factor for diabetes complications 9,10 and identifying ways to improve their glycemic control is important in order to minimize this risk. To our knowledge, the effects of demographic and behavioral factors on optimal glycemic control optimal glycemic control defined as HbA1c ≤7.5% (58mmol/mol) has not been previously studied. In this analysis, we confirmed that overweight status was associated with sub optimal control. Our study additionally highlights that within the optimally controlled cohort, whether of normal weight, overweight or obese, use of CSII, CGM and frequency of blood glucose checks were not different. Likewise, optimally controlled participants had similar daily insulin requirements per kg body weight across all BMI categories. In contrast, the optimally controlled obese youth with T1DM had lipid abnormalities that are predictive of higher cardiovascular risk compared to the lean participants.
4.1.1. BMI and glycemic control
The role of BMI in predicting glycemic control is controversial and overall BMI has been shown to have a negative or no effect in glycemic control 14–20. In our sample, more overweight and obese participants (27%) were in the cohort with suboptimal blood glucose control than in the well controlled cohort (30 %). In addition, we found that overweight status is an important factor in the logistic regression model for control status. It is possible that the smaller sample size of obese youth compared to overweight youth was not enough to reach statistical significance in the logistic regression model; we suggest that this relationship is further explored by future large scale studies. The relationship between adiposity and higher insulin requirements as a result of insulin resistance may negatively impact diabetes management.21 In a Finnish cohort of 105 children and adolescents with T1DM, higher BMI correlated with worse glycemic control.22 No apparent link of BMI with HbA1c was found in the longitudinal and cross-sectional analyses of 340 children aged 9.0 to 14.9 years at four pediatric endocrinology clinics in the US.19 In contrast, evidence from the Search for Diabetes in Youth study for the years 2001-2005 showed worse glycemic control in underweight or normal youth with T1D.17 Authors speculated that poorer residual beta cell function in children with low BMI may play a role. Based on our own data, we recommend that physicians should promote healthy weight maintenance in light of the short and long-term complications associated with increased adiposity.3
4.1.2. BMI and insulin delivery method, use of CGM and blood glucose checks
When comparing the two insulin delivery methods across the BMI subgroups in the optimally controlled cohort we found high rates of use of CSII in all subgroups, although CSII was used less frequently among obese participants. This finding suggests that increased use of CSII, along with the use of CGM and frequent blood glucose checks, can be as effective in overweight as normal-weight youths. The logistic regression model highlighted that the method of insulin delivery was also an important factor for optimal control and the use of MDI was associated with poorer control compared to CSII. Several studies have previously reported lower HbA1c levels in CSII users compared to MDI users.23–25 In 2008, a meta-analysis of randomized control trials compared the short-term metabolic control of children with T1DM managed with CSII and MDI and concluded that the use of CSII had a favorable effect on HbA1c. Additionally, in that meta-analysis insulin requirements were lower by 0.22 units/kg/day 26. Similar results were reported when data from three large pediatric diabetes registries were compared.24 Among 54,410 children with T1DM, HbA1c levels were on average 0.5% lower in children receiving insulin via insulin pumps25. Data from the T1D Exchange Registry for children <6 years old demonstrated a statistically significant reduction of HbA1c by 0.2% after initiation of CSII regimen among previous MDI users that was independent of anthropometric and socioeconomic factors.23
CGM use was not very popular among children in our cohort (roughly 6%) , which could be due to older versions of CGMs (used in 2010) that were perhaps not as user friendly as the newer versions of CGM that are available today. Overall, CGM use was similar among all BMI subgroups. Inconsistent results have been published about the use of CGM in improving glycemic control in pediatric patients27. In a large randomized study by the Juvenile Diabetes Research Foundation (JDRF), the use of CGM was not associated with improved HbA1c among the pediatric population.28 However, this could be partially explained by inconsistent daily use, which remains a challenge for many patients.29,30
4.1.3. BMI and total daily dose of insulin
Our analysis revealed that optimally controlled participants across all BMI subgroups used the same amount of total daily insulin per kg even after adjusting for age, race, duration of diabetes, income, sex, insurance and multiple comparisons Although this constitutes a borderline statistical trend (p=0.096), it failed to reach statistical significance despite the large number of participants. This suggests that perhaps BMI is not the best marker to characterize the metabolic aspects of obesity and estimate insulin sensitivity in youth with T1DM. Another explanation could be similar insulin sensitivity between the three groups, despite their differences in BMI. Decreased insulin sensitivity has been documented among participants with T1DM, although the precise mechanisms behind it are not fully elucidated.31 Two recent studies showed the beneficial effects of metformin as insulin sensitizer in youth with T1DM. Both studies showed that metformin improved vascular health in children with T1DM and decreased their insulin requirements, indicating that addressing insulin sensitivity could be beneficial for cardiovascular health of youth with T1DM 32,33. We were unable to further address the question of insulin sensitivity in this study, because neither anthropometric measures such as waist circumference nor serial testing (frequently samples oral or venous glucose tolerance tests or clamp studies) are available in the T1D Registry, and merits further studying by large scale cohorts.
4.1.4. BMI and dyslipidemia
We found that overweight and obese children with optimal blood glucose control had worse lipid profiles than those with normal weight. It is known that children with T1DM have a higher cardiovascular disease risk compared to healthy children and that poor glycemic control and obesity are important contributors to this risk.10,34 Participants who followed an intensive insulin regimen during the DCCT trial gained more weight than the participants who had a less aggressive regimen. This could be attributed to increased lipogenesis with intensive therapy.35 However, in our analysis, the similar insulin requirements among different BMI groups of optimally controlled participants indicate that other factors are important, rather than insulin itself. For example, poor diet, decreased exercise, napping, increased screen time, not having regular breakfast and dinner or genetic factors may play a role.8 Therefore, counselling children with T1DM to maintain a healthy lifestyle is clearly important to achieve optimal glycemic control and reduce cardiovascular risk.
4.1.5. Non-modifiable factors for optimal control
Finally, we found that age, sex and race were important factors in our logistic regression model that determine optimal control, which is in agreement with other studies. Adolescence has previously been reported as a challenging period for diabetes management 3,36–38 which may be attributed to decreased insulin sensitivity due to the effect of growth hormone and other puberty related hormonal changes. Adolescent females have been identified as a high-risk group for poor metabolic control.9,36,39–45 Regarding race and ethnicity, significantly higher mean HbA1c levels have been reported in black children with T1DM compared to white and non-black Hispanic children, even after adjustment for socioeconomic factors, diabetes duration, age and sex.46–49 Limited access to healthcare, lower rates of enrollment to health insurance 50 and lack of prescription coverage 51 may significantly limit successful diabetes management. Notable disparities in the insulin delivery methods have also been reported.46,52 In a US multicenter study involving 2,743 participants <20 years old, 52.7% of the non-Hispanic white children were treated with CSII compared to 19.1% of black children and 27.8% of Native American children.38
4.1.6. Stengths and limitations
Our study’s main strengths are the quality and the large size of available data provided by the T1D Exchange Registry. We analyzed a large cohort of 9,263 well-characterized children and adolescents with T1DM who received care in a wide range of settings throughout the US. Limitations include that black children are underrepresented in this registry (5.9%), as are children aged less than 6 years. Also, data collected in this database are self-reported and participants are self-enrolled, which might have introduced some errors. Moreover, the database provides limited information about the insulin sensitivity and C-peptide measurements of participants and further adjustments for these parameters were not possible. Since information about pubertal status was not available, age groups were created as an approximation to include most children in the prepubertal, pubertal and post-pubertal era. Further breakdown of age groups by sex to account for differences in age of puberty was beyond the scope of this paper. Finally, since 2014 the ADA recommends that a HbA1C target of <7.5% (58 mmol/mol) should be considered for all children and adolescents. Data in this cohort are from participants enrolled from 2010-2012 who were treated by their providers using a more relaxed HbA1c goal of less than 8.5% (69 mmol/mol) for children under 6 years of age, less than 8.0% (64mmol/mol) for children 6-12 years of age and less than 7.5% (58mmol/mol) for adolescents. It is therefore possible that nowadays providers recommend more intensive insulin regimens are used across all ages, and that some of our results might not be representative of the current pediatric practice. However, because we used the newest definition of HbA1c target to define optimal control, we believe our results can guide current management of overweight and obese children with T1DM. Furthermore, although an HbA1C target of <7.0% is recommended in non-pregnant adult populations, the designated target for children and adolescents was used for all participants included to ensure consistency. Finally, although fasting lipid measurements are traditionally used for screening, there were not available fasting measurements for all participants, so all measurements were included in the analysis. Although this is a limitation, there is a recent trend towards interchangeable use of fasting and non-fasting for metabolic screening for patients who are not receiving therapy for hyperlipidemia and who are not being investigated for inherited lipid conditions.53 In a recent nationwide cross-section pediatric study, the comparison of fasting and non-fasting measurements did not yield clinically significant differences.54
5.1. Conclusions
In summary, we found that in the group with HbA1c ≤ 7.5% (58mmol/mol) there were no significant differences in use of CSII, CGM and frequent blood glucose checks between overweight and obese participants as compared to the lean participants. However, predictors of cardiovascular disease were higher in the overweight and obese group. The use of CSII versus MDI, use of CGM and the multiple checks of blood glucose are important modifiable behaviors to achieve optimal glycemic control in lean overweight and obese children with T1DM. Furthermore, overweight and obese children with optimal glycemic control don’t have to use more insulin/kg/day to achieve their glycemic goal. Future studies can further investigate the role of CSII use, insulin sensitivity and increased residual C-peptide levels in maintaining excellent control in overweight/obese participants. Finally, counselling about lifestyle changes to maintain a healthy weight is important for the cardiovascular health of all overweight and obese children with optimal glycemic control.
Highlights.
We analyzed a cohort of 9,263 children and adolescents with T1DM in the US.
Overweight/obese children with optimal GC use the same insulin/kg/day as lean ones
There is increased CVD risk among the overweight/obese group compared to the lean one
The use of CSII, CGM and the multiple checks of BG are important modifiable behaviors
6.1. Acknowledgements:
Funding: National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001432 awarded to Dr. Evgenia Gourgari
Abbreviations
- BG
Blood glucose
- CSII
continuous subcutaneous insulin infusion
- CVD
cardiovascular disease
- CGM
continuous glucose monitoring
- GC
glycemic control
- T1DM
Type I diabetes mellitus
Table A1:
Descriptive characteristics of the optimally and suboptimally controlled participants
| Variable | Optimal control (n=2367) | Suboptimal control (n=6896) | P |
|---|---|---|---|
| HBA1c | 6.99 (0.44) | 8.92 (1.28) | <0.001 |
| Age (%) | 0.009 | ||
| < 12 | 975 (41.2) | 2763 (40.1) | |
| 12 - 17 | 1024 (43.3) | 3208 (46.5) | |
| 18 - 20 | 368 (15.5] | 925 (13.4] | |
| Race (%) | <0.001 | ||
| 1.White Non-Hispanic | 2010 (84.9] | 5469 (79.3] | |
| 2.Black Non-Hispanic | 43 (1.8] | 386 (5.6] | |
| 3.Hispanic or Latino | 207 (8.7] | 661 (9.6] | |
| 4.Native Hawaiian/Other Pacific Islander | 3 (0.1] | 15 (0.2] | |
| 5.Asian | 46 (1.9] | 87(1.3] | |
| 6.American Indian/Alaskan Native | 3 (0.1] | 35 (0.5] | |
| 7.More than one race | 55(2.3] | 243 (3.5] | |
| Sex (%) | 0.009 | ||
| Female | 1064 (45] | 3335 (48.4] | |
| Male | 1303 (55] | 3561 (51.6] | |
| BMI (%) | 20.86 (4.22] | 21.32 (4.47] | <0.001 |
| Normal (5-84.9th %] | 1720 (72.7] | 4617 (67] | |
| Overweight (85th to 94.9th %] | 430 (18.2] | 1464 (21.2] | |
| Obese (≥95th %] | 217 (9.2] | 815 (11.8] |
Values are represented as mean +/− SD. Values in parentheses represent percent % or the standard deviation (SD).
Table B1:
Behavioral and clinical characteristics of the optimally and suboptimally controlled participants
| Variable | Optimal control (n=2367) | Suboptimal control (n=6896) | P | Adjusted P. |
|---|---|---|---|---|
| Insulin Method (%) | <0.001 | <0.001 | ||
| Pump | 1390 (58.7) | 3190 (46.3) | ||
| MDI | 977 (41.3) | 3706 (53.7) | ||
| Insulin Amount, units/kg/day | 0.85 (0.4) | 0.97 (0.46) | <0.001 | <0.001 |
| Number Meter Check | 6.54 (2.56) | 5.61 (2.29) | <0.001 | <0.001 |
| CGM Used (%) | <0.001 | <0.001 | ||
| 1.Yes | 131 (5.5) | 161 (2.3) | ||
| 2.No | 2192 (92.6) | 6659 (96.6) | ||
| 3.Unknown | 44 (1.9) | 76 (1.1) | ||
| Severe Hypoglycemic Events (%) | <0.001 | 0.138 | ||
| 1.Yes | 202 (11.6) | 920 (17.7) | ||
| 2.No | 1528 (88.1) | 4225 (81.5) | ||
| 3.Don’t know | 4(0.2) | 40 (0.8) | ||
| Number of Hospitalizations for DKA | 0.03 (0.3) | 0.15 (0.65) | <0.001 | <0.001 |
| Number of Bolus per Day | 6.41 (4.39) | 6.61 (4.05) | 0.143 | <0.001 |
| Missing Insulin Dose | <0.001 | |||
| 1.Never | 3001 (43.5) | 1455 (61.5) | ||
| 2.Rarely | 1693 (24.6) | 608 (25.7) | <0.001 | |
| 3.Sometimes | 1299 (18.8) | 243 (10.3) | <0.001 | |
| 4.Often | 631 (9.2) | 47 (2) | <0.001 | |
| 5.Very often | 143 (2.1) | 6 (0.3) | <0.001 | |
| 6.At least once a day | 119 (1.7) | 7 (0.3) | <0.001 | |
| Frequency of Bolus Bed Time Snack (%) | <0.001 | |||
| 1.Never | 168 (11.7) | 564 (12.7) | ||
| 2.Rarely | 113 (7.9) | 389 (8.7) | 1.000 | |
| 3.Sometimes | 238 (16.6) | 1013 (22.8) | 0.463 | |
| 4.Most of the time | 330 (23.1) | 1075 (24.2) | 1.000 | |
| 5.Always | 582 (40.7) | 1409 (31.7) | 0.058 | |
| Bed Time Snack (%) | 0.002 | 0.138 | ||
| 1.Yes | 1431 (60.9) | 4426 (65) | ||
| 2.No | 902 (38.4) | 2345 (34.4) | ||
| 3.Don’t know | 18 (0.8) | 43 (0.6) | ||
| LDL | 85.24 (24.29) | 91.57 (27.89) | <0.001 | <0.001 |
| HDL | 56.79 (14.38) | 57.41 (14.45) | 0.141 | 0.463 |
| Total Cholesterol | 157.99 (29.88) | 165.27 (32.12) | <0.001 | <0.001 |
| Triglycerides | 83.63 (54.62) | 97.53 (85.77) | <0.001 | <0.001 |
| Days of Exercise per week | 5.22 (1.69) | 5.2 (1.72) | 0.645 | 1.000 |
Values are represented as mean +/− SD. Values in parentheses represent percent % or the standard deviation SD. Adjusted p values based on logistic regression controlling for age, BMI race, sex, DM duration, income, insurance and multiple comparisons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
The authors have no conflict of interest to declare.
References
- 1.CDC. National Diabetes Statistics Report. 2014.
- 2.Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial (DCCT);Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research G. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care. 2016;39(5):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research G. Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care. 2016;39(8):1378–1383.27411699 [Google Scholar]
- 6.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minges KE, Whittemore R, Grey M. Overweight and obesity in youth with type 1 diabetes. Annu Rev Nurs Res. 2013;31:47–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11(1):4–11. [DOI] [PubMed] [Google Scholar]
- 9.Akesson K, Hanberger L, Samuelsson U. The influence of age, gender, insulin dose, BMI, and blood pressure on metabolic control in young patients with type 1 diabetes. Pediatr Diabetes. 2015;16(8):581–586. [DOI] [PubMed] [Google Scholar]
- 10.Maahs DM, Daniels SR, de Ferranti SD, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130(17):1532–1558. [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389. [DOI] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000(314):1–27. [PubMed] [Google Scholar]
- 13.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 2017;317(8):825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipsky LM, Gee B, Liu A, Nansel TR. Glycemic control and variability in association with body mass index and body composition over 18months in youth with type 1 diabetes. Diabetes Res Clin Pract. 2016;120:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holl RW, Grabert M, Sorgo W, Heinze E, Debatin KM. Contributions of age, gender and insulin administration to weight gain in subjects with IDDM. Diabetologia. 1998;41(5):542–547. [DOI] [PubMed] [Google Scholar]
- 16.Domargard A, Sarnblad S, Kroon M, Karlsson I, Skeppner G, Aman J. Increased prevalence of overweight in adolescent girls with type 1 diabetes mellitus. Acta Paediatr. 1999;88(11):1223–1228. [DOI] [PubMed] [Google Scholar]
- 17.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155(5):668–672. e661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulten RJ, Piet J, Bruijning PC, de Waal WJ. Lower dose basal insulin infusion has positive effect on glycaemic control for children with type I diabetes on continuous subcutaneous insulin infusion therapy. Pediatr Diabetes. 2017;18(1):45–50. [DOI] [PubMed] [Google Scholar]
- 19.Nansel TR, Lipsky LM, Iannotti RJ. Cross-sectional and longitudinal relationships of body mass index with glycemic control in children and adolescents with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2013;100(1):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietilainen KH, Virtanen SM, Rissanen A, Rita H, Maenpaa J. Diet, obesity, and metabolic control in girls with insulin dependent diabetes mellitus. Arch Dis Child. 1995;73(5):398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krochik AG, Botto M, Bravo M, et al. Association between insulin resistance and risk of complications in children and adolescents with type 1 diabetes. Diabetes Metab Syndr. 2015;9(1):14–18. [DOI] [PubMed] [Google Scholar]
- 22.Virtanen SM. Metabolic control and diet in Finnish diabetic adolescents. Acta Paediatr. 1992;81(3):239–243. [DOI] [PubMed] [Google Scholar]
- 23.Blackman SM, Raghinaru D, Adi S, et al. Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes. 2014;15(8):564–572. [DOI] [PubMed] [Google Scholar]
- 24.Sherr JL, Hermann JM, Campbell F, et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia. 2016;59(1):87–91. [DOI] [PubMed] [Google Scholar]
- 25.Enes P, Martin-Frias M, Alvarez MA, Yelmo R, Alonso M, Barrio R. Achievement of metabolic control goals set by the American Diabetes Association and the International Society for Pediatric and Adolescent Diabetes in pediatric patients with type 1 diabetes from Spain. Diabetes Res Clin Pract. 2015;107(2):300–305. [DOI] [PubMed] [Google Scholar]
- 26.Pankowska E, Blazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes. 2009;10(1):52–58. [DOI] [PubMed] [Google Scholar]
- 27.Lal RA, Maahs DM. Clinical Use of Continuous Glucose Monitoring in Pediatrics. Diabetes Technol Ther. 2017;19(S2):S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. [DOI] [PubMed] [Google Scholar]
- 29.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G, Beck RW, Buckingham B, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chase HP, Beck RW, Xing D, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2010;12(7):507–515. [DOI] [PubMed] [Google Scholar]
- 31.Bjornstad P, Snell-Bergeon JK, Nadeau KJ, Maahs DM. Insulin sensitivity and complications in type 1 diabetes: New insights. World J Diabetes. 2015;6(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson JJA, Couper JJ, Giles LC, et al. Effect of Metformin on Vascular Function in Children With Type 1 Diabetes: A 12-Month Randomized Controlled Trial. J Clin Endocrinol Metab. 2017;102(12):4448–4456. [DOI] [PubMed] [Google Scholar]
- 33.Bjornstad P, Schafer M, Truong U, et al. Metformin Improves Insulin Sensitivity and Vascular Health in Youth With Type 1 Diabetes Mellitus. Circulation. 2018;138(25):2895–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gourgari E, Dabelea D, Rother K. Modifiable Risk Factors for Cardiovascular Disease in Children with Type 1 Diabetes: Can Early Intervention Prevent Future Cardiovascular Events? Curr Diab Rep. 2017;17(12):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbauer J, Dost A, Karges B, et al. Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care. 2012;35(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2016;17(5):327–336. [DOI] [PubMed] [Google Scholar]
- 38.Paris CA, Imperatore G, Klingensmith G, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(2):183–189. e181. [DOI] [PubMed] [Google Scholar]
- 39.Samuelsson U, Anderzen J, Gudbjornsdottir S, Steineck I, Akesson K, Hanberger L. Teenage girls with type 1 diabetes have poorer metabolic control than boys and face more complications in early adulthood. J Diabetes Complications. 2016;30(5):917–922. [DOI] [PubMed] [Google Scholar]
- 40.Forsander G, Bogelund M, Haas J, Samuelsson U. Adolescent life with diabetes-Gender matters for level of distress. Experiences from the national TODS study. Pediatr Diabetes. 2016. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman RP, Vicini P, Sivitz WI, Cobelli C. Pubertal adolescent male-female differences in insulin sensitivity and glucose effectiveness determined by the one compartment minimal model. Pediatr Res. 2000;48(3):384–388. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed ML, Ong KK, Watts AP, Morrell DJ, Preece MA, Dunger DB. Elevated leptin levels are associated with excess gains in fat mass in girls, but not boys, with type 1 diabetes: longitudinal study during adolescence. J Clin Endocrinol Metab. 2001;86(3):1188–1193. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan S, Fields DA, Copeland KC, Blackett PR, Anderson MP, Gardner AW. Sex differences in cardiovascular disease risk in adolescents with type 1 diabetes. Gend Med. 2012;9(4):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49(4):626–632. [DOI] [PubMed] [Google Scholar]
- 45.Kabadi UM, Vora A, Kabadi M. Hyperinsulinemia and central adiposity: influence of chronic insulin therapy in type 1 diabetes. Diabetes Care. 2000;23(7):1024–1025. [DOI] [PubMed] [Google Scholar]
- 46.Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delamater AM, Albrecht DR, Postellon DC, Gutai JP. Racial differences in metabolic control of children and adolescents with type I diabetes mellitus. Diabetes Care. 1991;14(1):20–25. [DOI] [PubMed] [Google Scholar]
- 48.Chalew SA, Gomez R, Butler A, et al. Predictors of glycemic control in children with type 1 diabetes: the importance of race. J Diabetes Complications. 2000;14(2):71–77. [DOI] [PubMed] [Google Scholar]
- 49.Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Effect of ethnicity on HbA1c levels in individuals without diabetes: Systematic review and meta-analysis. PLoS One. 2017;12(2):e0171315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wintergerst KA, Hinkle KM, Barnes CN, Omoruyi AO, Foster MB. The impact of health insurance coverage on pediatric diabetes management. Diabetes Res Clin Pract. 2010;90(1):40–44. [DOI] [PubMed] [Google Scholar]
- 51.Briesacher B, Limcangco R, Gaskin D. Racial and ethnic disparities in prescription coverage and medication use. Health Care Financ Rev. 2003;25(2):63–76. [PMC free article] [PubMed] [Google Scholar]
- 52.Valenzuela JM, La Greca AM, Hsin O, Taylor C, Delamater AM. Prescribed regimen intensity in diverse youth with type 1 diabetes: role of family and provider perceptions. Pediatr Diabetes. 2011;12(8):696–703. [DOI] [PubMed] [Google Scholar]
- 53.Driver SL, Martin SS, Gluckman TJ, Clary JM, Blumenthal RS, Stone NJ. Fasting or Nonfasting Lipid Measurements: It Depends on the Question. J Am Coll Cardiol. 2016;67(10):1227–1234. [DOI] [PubMed] [Google Scholar]
- 54.Steiner MJ, Skinner AC, Perrin EM. Fasting might not be necessary before lipid screening: a nationally representative cross-sectional study. Pediatrics. 2011;128(3):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]


