Abstract
The periodontal ligament (PDL) is an essential fibrous tissue for tooth retention in the alveolar bone socket. PDL tissue further functions to cushion occlusal force, maintain alveolar bone height, allow orthodontic tooth movement, and connect tooth roots with bone. Severe periodontitis, deep caries, and trauma cause irreversible damage to this tissue, eventually leading to tooth loss through the destruction of tooth retention. Many patients suffer from these diseases worldwide, and its prevalence increases with age. To address this issue, regenerative medicine for damaged PDL tissue as well as the surrounding tissues has been extensively investigated regarding the potential and effectiveness of stem cells, scaffolds, and cytokines as well as their combined applications. In particular, PDL stem cells (PDLSCs) have been well studied. In this review, I discuss comprehensive studies on PDLSCs performed in vivo and contemporary reports focusing on the acquisition of large numbers of PDLSCs for therapeutic applications because of the very small number of PDLSCs available in vivo.
Keywords: Induced pluripotent stem cells, Mesoderm specific transcript, Periodontal ligament stem cells, Periodontal tissue, Regenerative medicine, Semaphorin 3A
Core tip: For patients with severe periodontitis, deep caries, and trauma, which can lead to tooth loss, the development of highly effective regenerative therapies for severely damaged periodontal tissue is an urgent concern. As one possible method to address this issue, cell-based therapy using periodontal ligament stem cells (PDLSCs) shows great promise. However, the number of PDLSCs present in vivo is too small for implementation of this method, and PDLSC isolation requires patients to undergo invasive surgery. In this review, ways to acquire large numbers of PDLSCs and advances in periodontal regenerative therapy during the past two decades are summarized.
HOW ARE REGENERATIVE TREATMENTS OF PERIODONTAL DEFECTS PERFORMED USING CELL-RELATED THERAPY?
Stem cell-based therapy (Figure 1)
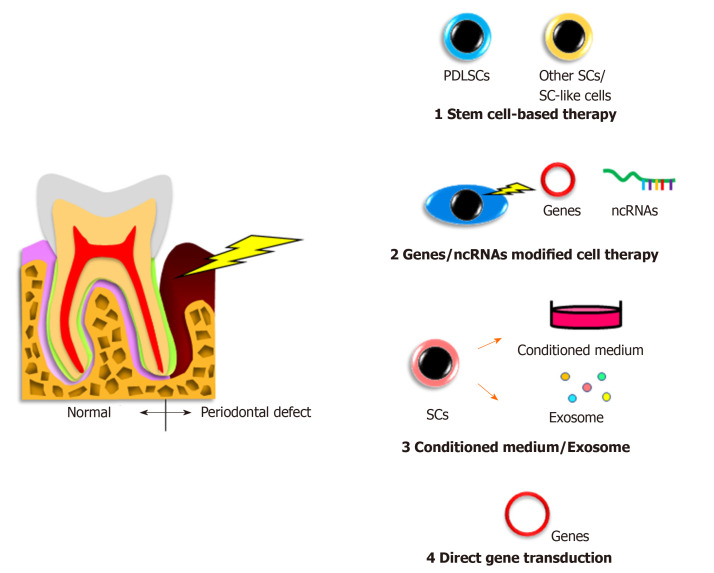
Figure 1.
Cell-related therapies for periodontal regeneration. The regenerative treatments of periodontal defects performed using cell-related therapy are illustrated, which include the transplantation of stem cells, gene or noncoding RNA modified cells, application of conditioned medium or exosome from cell culture, and direct gene transfection to the lesion. PDLSC: Periodontal ligament stem cell; SC: Stem cell; ncRNA: noncoding RNA.
In humans, autologous transplantation of cultured periosteum sheets[1], periodontal ligament (PDL) progenitor cells[2], PDL cell sheets[3], dental pulp stem cells[4,5], gingiva-derived cells[6], and tissue-engineered bone constituted by bone marrow mesenchymal stem cells (BMMSCs)[7] into patients with periodontal defects have been reported. All of the cited studies clinically verified the potency of stem cells for periodontal regeneration. In contrast, Chen et al[8] transplanted PDL stem cell (PDLSC) sheets and found no significant improvement compared with the control group. However, these authors targeted very small periodontal defects and applied scaffolding materials as controls, possibly making it difficult to detect significant differences.
In animal periodontal defect models, Iwasaki et al[9] reported no significant advantage of spheroid formation by human PDLSCs in rats, despite increased expression of genes related to angiogenesis and anti-inflammation[9]. Nevertheless, a recent study demonstrated that coculture of human PDLSC spheroids with vascular endothelial cells promoted rat periodontal regeneration[10]. In addition, a previous study revealed that pellets of cultured human PDLSCs showed periodontal regeneration capacity in mice[11]. Meanwhile, application of other immature cells, including adipose-derived stem cells[12], stem cells from human exfoliated deciduous teeth[13], dental pulp stem cells[14], dental follicle cells[15], induced pluripotent stem (iPS) cells[16], and iPS-derived mesenchymal stem cells (MSCs)[17] was shown to induce periodontal regeneration in vivo. These reports suggest that the indicated cell sources may have potential for clinical use.
Gene/noncoding RNA modified cell therapy
No clinical studies on gene or noncoding RNA modified cell therapy for the treatment of patients with periodontal disease have been reported because of the associated safety issues. However, there have been some reports involving animal models with experimentally produced periodontal defects. Osteoprotegerin gene-transferred rabbit PDLSCs and platelet-derived growth factor-BB-transduced human PDLSCs exhibited increased bone formation in periodontal defects[18,19].
The development and characterization of other tissue-derived cells with gene transduction have been reported. Specifically, bone morphogenetic protein 2-transfected canine BMMSCs[20], fibroblast growth factor 2-transduced canine BMMSCs[21], hepatocyte growth factor-transduced human dental pulp cells[22], and leptin-transduced rat BMMSCs[23] were able to restore periodontal defects in vivo.
Modification of PDLSCs using noncoding RNA, including microRNA, long noncoding RNA, and circular RNA have been reported to induce their osteogenic differentiation, suggesting the application of these cells to bone defective in periodontitis[24].
Although the above studies indicated the potential of novel therapies for repair of severe periodontal defects, further basic studies are indispensable for future clinical trials.
Cell culture conditioned medium and exosomes
Conditioned medium from cultured cells and extracellular vesicles secreted from stem cells have various effects, including tissue regeneration, cell proliferation, chemotactic and metabolic activities, anti-inflammation, and cell-cell communication[25]. Because these cells and vesicles possess great potential, researchers have examined their roles in periodontal regeneration studies, but related clinical trials have not been reported.
There have been some studies on the effects of conditioned medium or exosomes from PDLSCs as well as other tissue-derived stem cells in animal models. Recently, Nagata et al[26] demonstrated periodontal regeneration activity of conditioned medium from cultured human PDLSCs injected into rat periodontal defects. Another study showed the capability of conditioned medium from human gingival stem cells as well as human cultured human PDLSCs for periodontal regeneration[27]. Furthermore, conditioned medium from human BMMSCs was clearly able to repair canine and rat periodontal defects[28,29]. A recent report applying exosomes from human BMMSCs to rat periodontal defects showed induction of newly-formed bone and PDL tissue[30].
Although the above effects do not reflect direct contributions of stem cells to treatment, these indirect effects of stem cells may deserve further consideration as treatment options.
Gene therapy
Direct in vivo gene transfer of the bone morphogenetic protein 2/7[31] or platelet-derived growth factor[32] genes to rat periodontal tissue promoted bone growth or bone regeneration and cementum formation, respectively. Meanwhile, another group directly transferred the bone morphogenetic protein 4 gene to rat PDL tissue by electroporation but did not detect any obvious bone augmentation[33]. Similarly, embedding of a platelet-derived growth factor-B plasmid with collagen gel into alveolar bone defects in rats had no significant effects[34].
The gene therapy method has not been investigated in human patients, and its effectiveness needs to be fully verified before it can be used as a relatively easy therapeutic modality.
WHAT ARE PDLSCs?
Periodontal tissue (periodontium) is a complex tissue mainly composed of two hard tissues (alveolar bone and cementum coating tooth root surfaces) and two soft tissues (PDL tissue and gingival tissue)[35]. In particular, PDL tissue has crucial roles in supporting the tooth and integrating the tissues.
PDLSCs are somatic stem cells localized in PDL tissue[36] and derived from cranial neural crest cells[37,38]. PDLSCs have similar features to BMMSCs and exhibit self-renewal capacity and multipotency[39]. These cells have the potential to undergo triploblastic differentiation with the ability to differentiate into not only osteoblasts, adipocytes, chondrocytes, cementoblasts, and tendon/ligament fibroblasts[40,41] but also myocytes[42], neural cells[43], retinal cells[44], endothelial cells[45], pancreatic islet cells[46], and hepatic cells[47]. In addition, PDLSCs express cell surface markers such as STRO-1, CD146/MUC18[36], CD44, and CD90 (markers associated with stromal cells), CD105 and CD166 (markers associated with stromal cells and endothelial cells)[48], and CD10, CD26, CD29, CD73, and CD349/FZD9[49] but do not express hematopoietic cell surface markers such as CD31 and CD45, similar to BMMSCs[50].
PDLSCs also express embryonic stem cell-related transcription factors like NANOG and OCT-4 and embryonic stem cell antigens like stage-specific embryonic antigen-1 (SSEA-1)/CD15, SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, alkaline phosphatase, and REX1/ZFP42[49,51]. However, PDLSCs from aged people exhibit decreased capacities for proliferation, migration, and multiple differentiation with reduced SSEA-4 expression[52].
Finally, PDLSCs possess immunomodulatory properties[48], among which reactive oxygen species production may be interestingly regulated by dual mechanisms depending on the degree of inflammation[53].
WHERE ARE PDLSCs REQUIRED?
Severe periodontitis, deep caries, and trauma cause irreversible damage to PDL tissue as well as the surrounding tissues such as alveolar bone, gingiva, and cementum, eventually resulting in tooth loss. The 8020 Promotion Foundation survey for causes of tooth loss in Japan (https://www.8020zaidan.or.jp/english/) performed in 2018 reported that periodontitis, caries, and tooth fracture accounted for 84.0% of all causes of tooth loss. The Global Burden of Disease 2015 study suggested that 7.4% of people worldwide suffered from severe periodontitis[54]. Meanwhile, the National Survey of Dental Diseases in Japan performed in 2016 (https://www.mhlw.go.jp/toukei/list/62-28.html) reported that Japanese people with periodontal health (< 4 mm periodontal pocket depth) comprised less than 37.0% of people aged ≥ 50 years.
To date, transplantation of PDLSCs has led to successful periodontal regeneration in experimentally produced periodontal defects in dogs[55,56], rats[57], and pigs[58]. Furthermore, Yan et al[59] performed a systematic review and meta-analysis and decisively stated that cell-based therapy is an effective therapy for regeneration of lost periodontal tissue.
PDLSCs have been proposed as the most promising cells for regeneration of severely damaged PDL tissue, among other stem cells such as dental pulp stem cells, stem cells from human exfoliated deciduous, dental follicle stem cells, stem cells from apical papilla[60], BMMSCs, and alveolar periosteal cells[61].
Interestingly, the fate of transplanted PDLSCs was examined in a rat periodontal defect model[62]. The findings revealed that PDLSCs contributed to periodontal repair but did not become markedly engrafted, suggesting a supportive role of PDLSCs for activating the regenerative capability in the damaged periodontium. Considering the critical role of PDLSCs in periodontal regenerative therapy, PDLSCs themselves may be difficult to engraft into defect sites, but the use of 2D or 3D construction methods combined with extracellular matrices may be effective.
WHY ARE LARGE AMOUNTS OF PDLSCs NEEDED?
In human PDL tissue, STRO1+/CD146+ cells, regarded as candidate PDLSCs[36,63], were reported to comprise only about 0.07% of the total cells[64]. Another study described that PDLSCs comprised 2.4% of the total cells[63]. Regardless of the actual numbers, both studies indicated that very few PDLSCs are present in PDL tissue.
While the defect volumes in cases reported in human clinical studies have been very limited as described above, the defect areas in severe cases leading to tooth loss can vary across a wide range. Therefore, clinical application of PDLSCs to regenerative therapy of periodontal defects in humans will require the acquisition of large numbers of PDLSCs. Meanwhile, delivery of autologous PDLSCs to patients will necessitate the patients to undergo surgically invasive procedures. In addition, the subsequent expansion of small numbers of PDLSCs in vitro could lead to loss of their stemness. In this regard, it is of concern that large amounts of PDLSCs are needed for regenerative treatment. To address this issue, methods to acquire large numbers of PDLSCs have been explored.
HOW TO SOLVE THE INSUFFICIENCY OF PDLSCs?
Reprogrammed cells (Figure 2)
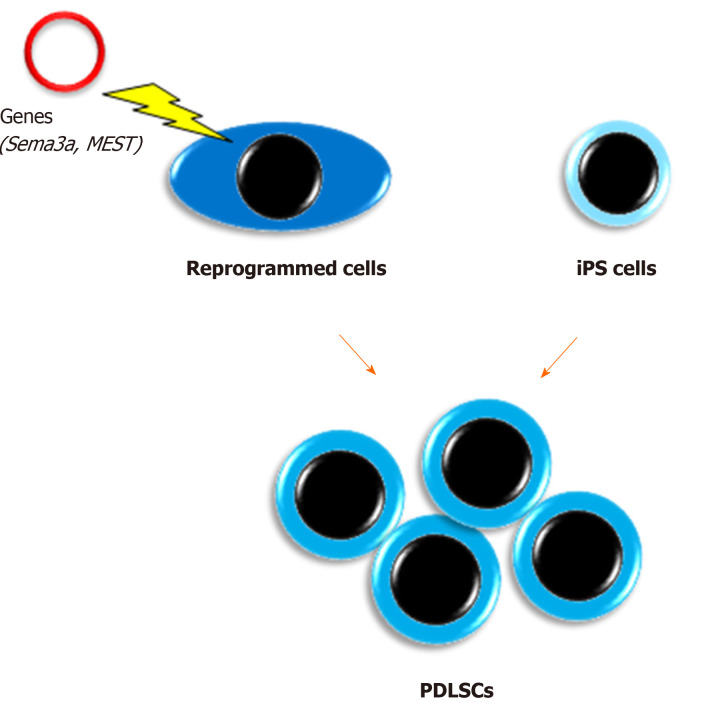
Figure 2.
Acquisition of a large number of periodontal ligament stem cells. The illustration shows that the reprogramming of periodontal ligament cells with semaphorin 3A or mesoderm-specific transcript, or differentiation induction of induced pluripotent stem cells to human periodontal ligament stem cell lineage are promising to acquire a large number of periodontal ligament stem cells. iPS cells: Induced pluripotent stem cells; PDLSCs: Periodontal ligament stem cells. Sema3a: Semaphorin 3A; MEST: Mesoderm-specific transcript.
We have reported unique methods for conversion of PDL cells to PDLSCs by gene transduction[65,66]. In a previous study, semaphorin 3A-transduced human PDL cells were converted into stem-like cells that showed multipotency and expressed both embryonic stem cell and MSC markers[65]. Furthermore, we recently demonstrated that an unexplored gene, mesoderm-specific transcript, was expressed in PDLSCs and that human PDL cells transduced with the mesoderm-specific transcript gene acquired PDLSC properties similar to semaphorin 3A-transduced cells[66]. In addition, the transduction changed the spindle shape of PDL cells to a stem cell-like round shape. Therefore, although the safety of these cells in vivo needs to be confirmed for clinical use, cell transformation with these genes is a potential method for mass acquisition of PDLSCs.
iPS cells
Our group was the first to report the development of PDLSC-like cells from human skin fibroblast-derived iPS cells[67]. Our study indicated that iPS cells themselves were unable to directly differentiate into PDLSCs, whereas neural crest-like cells developed from iPS cells attained PDLSC properties when cultured on extracellular matrix secreted from human primary PDL cells. We believe that this method may have great potential to solve the issue of insufficient numbers of PDLSCs. In addition, a recent study produced human leukocyte antigen homozygous iPS cells by gene modification, which have immune compatibility[68]. This development will enable the clinical use of iPS cell-derived PDLSCs benefiting many patients with severe periodontal defects. However, the issue of cost needs to be solved.
CONCLUSION
Many researchers have attempted to develop innovative and critical methods for periodontal therapy from various angles to support people’s health and life and address the aging society. PDLSC-based therapy is one of these methods, and we believe that it has the potential to deliver sustainable oral health to people around the world.
ACKNOWLEDGEMENTS
The author thanks Drs. Tomokiyo, Hamano, Hasegawa, Sugii, Yoshida, and Itoyama for their great support in the preparation of this review.
Footnotes
Conflict-of-interest statement: There is no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: May 19, 2020
First decision: June 5, 2020
Article in press: August 1, 2020
Specialty type: Cell and tissue engineering
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fang FC, Yang RL S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
References
- 1.Yamamiya K, Okuda K, Kawase T, Hata K, Wolff LF, Yoshie H. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects. J Periodontol. 2008;79:811–818. doi: 10.1902/jop.2008.070518. [DOI] [PubMed] [Google Scholar]
- 2.Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16:20–28. doi: 10.1111/j.1601-0825.2009.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwata T, Yamato M, Washio K, Yoshida T, Tsumanuma Y, Yamada A, Onizuka S, Izumi Y, Ando T, Okano T, Ishikawa I. Periodontal regeneration with autologous periodontal ligament-derived cell sheets - A safety and efficacy study in ten patients. Regen Ther. 2018;9:38–44. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aimetti M, Ferrarotti F, Cricenti L, Mariani GM, Romano F. Autologous dental pulp stem cells in periodontal regeneration: a case report. Int J Periodontics Restorative Dent. 2014;34 Suppl 3:s27–s33. doi: 10.11607/prd.1635. [DOI] [PubMed] [Google Scholar]
- 5.d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 6.Hou LT, Tsai AY, Liu CM, Feng F. Autologous transplantation of gingival fibroblast-like cells and a hydroxylapatite complex graft in the treatment of periodontal osseous defects: cell cultivation and long-term report of cases. Cell Transplant. 2003;12:787–797. doi: 10.3727/000000003108747262. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Nakamura S, Ito K, Umemura E, Hara K, Nagasaka T, Abe A, Baba S, Furuichi Y, Izumi Y, Klein OD, Wakabayashi T. Injectable bone tissue engineering using expanded mesenchymal stem cells. Stem Cells. 2013;31:572–580. doi: 10.1002/stem.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, Lu H, Chu Q, Xu J, Yu Y, Wu RX, Yin Y, Shi S, Jin Y. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki K, Nagata M, Akazawa K, Watabe T, Morita I. Changes in characteristics of periodontal ligament stem cells in spheroid culture. J Periodontal Res. 2019;54:364–373. doi: 10.1111/jre.12637. [DOI] [PubMed] [Google Scholar]
- 10.Sano K, Usui M, Moritani Y, Nakazawa K, Hanatani T, Kondo H, Nakatomi M, Onizuka S, Iwata T, Sato T, Togari A, Ariyoshi W, Nishihara T, Nakashima K. Co-cultured spheroids of human periodontal ligament mesenchymal stem cells and vascular endothelial cells enhance periodontal tissue regeneration. Regen Ther. 2020;14:59–71. doi: 10.1016/j.reth.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Jin F, Zhang X, Ma D, Han C, Huo N, Wang Y, Zhang Y, Lin Z, Jin Y. Tissue engineering of cementum/periodontal-ligament complex using a novel three-dimensional pellet cultivation system for human periodontal ligament stem cells. Tissue Eng Part C Methods. 2009;15:571–581. doi: 10.1089/ten.tec.2008.0561. [DOI] [PubMed] [Google Scholar]
- 12.Tobita M, Uysal CA, Guo X, Hyakusoku H, Mizuno H. Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy. 2013;15:1517–1526. doi: 10.1016/j.jcyt.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Fu X, Jin L, Ma P, Fan Z, Wang S. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J Periodontol. 2014;85:845–851. doi: 10.1902/jop.2013.130254. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Cao Y, Xie Y, Wang H, Fan Z, Wang J, Zhang C, Wang J, Wu CT, Wang S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 2016;7:130. doi: 10.1186/s13287-016-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshima M, Inoue K, Nakajima K, Tachikawa T, Yamazaki H, Isobe T, Sugawara A, Ogawa M, Tanaka C, Saito M, Kasugai S, Takano-Yamamoto T, Inoue T, Tezuka K, Kuboki T, Yamaguchi A, Tsuji T. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep. 2014;4:6044. doi: 10.1038/srep06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G, Kaplan D, Yang P, Chen J. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011;226:150–157. doi: 10.1002/jcp.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes K, Menicanin D, Han J, Marino V, Mrozik K, Gronthos S, Bartold PM. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92:833–839. doi: 10.1177/0022034513498258. [DOI] [PubMed] [Google Scholar]
- 18.Su F, Liu SS, Ma JL, Wang DS, E LL, Liu HC. Enhancement of periodontal tissue regeneration by transplantation of osteoprotegerin-engineered periodontal ligament stem cells. Stem Cell Res Ther. 2015;6:22. doi: 10.1186/s13287-015-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan J, Deng J, Luo Y, Yu L, Zhang W, Han X, You Z, Liu Y. Thermosensitive Hydrogel Delivery of Human Periodontal Stem Cells Overexpressing Platelet-Derived Growth Factor-BB Enhances Alveolar Bone Defect Repair. Stem Cells Dev. 2019;28:1620–1631. doi: 10.1089/scd.2019.0184. [DOI] [PubMed] [Google Scholar]
- 20.Chung VH, Chen AY, Kwan CC, Chen PK, Chang SC. Mandibular alveolar bony defect repair using bone morphogenetic protein 2-expressing autologous mesenchymal stem cells. J Craniofac Surg. 2011;22:450–454. doi: 10.1097/SCS.0b013e3182077de9. [DOI] [PubMed] [Google Scholar]
- 21.Tan Z, Zhao Q, Gong P, Wu Y, Wei N, Yuan Q, Wang C, Liao D, Tang H. Research on promoting periodontal regeneration with human basic fibroblast growth factor-modified bone marrow mesenchymal stromal cell gene therapy. Cytotherapy. 2009;11:317–325. doi: 10.1080/14653240902824757. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Liu Z, Xie Y, Hu J, Wang H, Fan Z, Zhang C, Wang J, Wu CT, Wang S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res Ther. 2015;6:249. doi: 10.1186/s13287-015-0244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng B, Jiang J, Chen Y, Lin M, Du Z, Xiao Y, Luo K, Yan F. Leptin Overexpression in Bone Marrow Stromal Cells Promotes Periodontal Regeneration in a Rat Model of Osteoporosis. J Periodontol. 2017;88:808–818. doi: 10.1902/jop.2017.170042. [DOI] [PubMed] [Google Scholar]
- 24.Qiu W, Wu BL, Fang FC. Overview of noncoding RNAs involved in the osteogenic differentiation of periodontal ligament stem cells. World J Stem Cells. 2020;12:251–265. doi: 10.4252/wjsc.v12.i4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muhammad SA, Nordin N, Fakurazi S. Regenerative potential of secretome from dental stem cells: a systematic review of preclinical studies. Rev Neurosci. 2018;29:321–332. doi: 10.1515/revneuro-2017-0069. [DOI] [PubMed] [Google Scholar]
- 26.Nagata M, Iwasaki K, Akazawa K, Komaki M, Yokoyama N, Izumi Y, Morita I. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng Part A. 2017;23:367–377. doi: 10.1089/ten.tea.2016.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J, Wang X, Zhou H, Zhang C, Wang Y, Huang J, Liu M, Yang P, Song A. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res Ther. 2020;11:42. doi: 10.1186/s13287-019-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inukai T, Katagiri W, Yoshimi R, Osugi M, Kawai T, Hibi H, Ueda M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem Biophys Res Commun. 2013;430:763–768. doi: 10.1016/j.bbrc.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 29.Kawai T, Katagiri W, Osugi M, Sugimura Y, Hibi H, Ueda M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy. 2015;17:369–381. doi: 10.1016/j.jcyt.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH, Lim LP, Lim SK, Toh WS. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019;89:252–264. doi: 10.1016/j.actbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Kawai M, Kataoka YH, Sonobe J, Yamamoto H, Inubushi M, Ishimoto T, Nakano T, Maruyama H, Miyazaki JI, Yamamoto T, Bessho K, Ohura K. Non-surgical model for alveolar bone regeneration by bone morphogenetic protein-2/7 gene therapy. J Periodontol. 2018;89:85–92. doi: 10.1902/jop.2017.170328. [DOI] [PubMed] [Google Scholar]
- 32.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchiya S, Chiba M, Kishimoto KN, Nakamura H, Tsuchiya M, Hayashi H. Transfer of the bone morphogenetic protein 4 gene into rat periodontal ligament by in vivo electroporation. Arch Oral Biol. 2017;74:123–132. doi: 10.1016/j.archoralbio.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Plonka AB, Khorsand B, Yu N, Sugai JV, Salem AK, Giannobile WV, Elangovan S. Effect of sustained PDGF nonviral gene delivery on repair of tooth-supporting bone defects. Gene Ther. 2017;24:31–39. doi: 10.1038/gt.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda H, Tomokiyo A, Fujii S, Wada N, Akamine A. Promise of periodontal ligament stem cells in regeneration of periodontium. Stem Cell Res Ther. 2011;2:33. doi: 10.1186/scrt74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 37.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 38.Mitsiadis TA, Graf D. Cell fate determination during tooth development and regeneration. Birth Defects Res C Embryo Today. 2009;87:199–211. doi: 10.1002/bdrc.20160. [DOI] [PubMed] [Google Scholar]
- 39.Tomokiyo A, Wada N, Maeda H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019;28:974–985. doi: 10.1089/scd.2019.0031. [DOI] [PubMed] [Google Scholar]
- 40.Huang CY, Pelaez D, Dominguez-Bendala J, Garcia-Godoy F, Cheung HS. Plasticity of stem cells derived from adult periodontal ligament. Regen Med. 2009;4:809–821. doi: 10.2217/rme.09.55. [DOI] [PubMed] [Google Scholar]
- 41.Maeda H, Wada N, Tomokiyo A, Monnouchi S, Akamine A. Prospective potency of TGF-β1 on maintenance and regeneration of periodontal tissue. Int Rev Cell Mol Biol. 2013;304:283–367. doi: 10.1016/B978-0-12-407696-9.00006-3. [DOI] [PubMed] [Google Scholar]
- 42.Song M, Kim H, Choi Y, Kim K, Chung C. Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars. Korean J Orthod. 2012;42:249–254. doi: 10.4041/kjod.2012.42.5.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomokiyo A, Maeda H, Fujii S, Wada N, Shima K, Akamine A. Development of a multipotent clonal human periodontal ligament cell line. Differentiation. 2008;76:337–347. doi: 10.1111/j.1432-0436.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 44.Huang L, Liang J, Geng Y, Tsang WM, Yao X, Jhanji V, Zhang M, Cheung HS, Pang CP, Yam GH. Directing adult human periodontal ligament-derived stem cells to retinal fate. Invest Ophthalmol Vis Sci. 2013;54:3965–3974. doi: 10.1167/iovs.13-11910. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi M, Okubo N, Chosa N, Takahashi N, Ibi M, Kamo M, Mizuki H, Ishisaki A, Kyakumoto S. Fibroblast growth factor-1-induced ERK1/2 signaling reciprocally regulates proliferation and smooth muscle cell differentiation of ligament-derived endothelial progenitor cell-like cells. Int J Mol Med. 2012;29:357–364. doi: 10.3892/ijmm.2011.847. [DOI] [PubMed] [Google Scholar]
- 46.Lee JS, An SY, Kwon IK, Heo JS. Transdifferentiation of human periodontal ligament stem cells into pancreatic cell lineage. Cell Biochem Funct. 2014;32:605–611. doi: 10.1002/cbf.3057. [DOI] [PubMed] [Google Scholar]
- 47.Vasanthan P, Jayaraman P, Kunasekaran W, Lawrence A, Gnanasegaran N, Govindasamy V, Musa S, Kasim NH. Generation of functional hepatocyte-like cells from human deciduous periodontal ligament stem cells. Naturwissenschaften. 2016;103:62. doi: 10.1007/s00114-016-1387-7. [DOI] [PubMed] [Google Scholar]
- 48.Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667–676. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 49.Trubiani O, Zalzal SF, Paganelli R, Marchisio M, Giancola R, Pizzicannella J, Bühring HJ, Piattelli M, Caputi S, Nanci A. Expression profile of the embryonic markers nanog, OCT-4, SSEA-1, SSEA-4, and frizzled-9 receptor in human periodontal ligament mesenchymal stem cells. J Cell Physiol. 2010;225:123–131. doi: 10.1002/jcp.22203. [DOI] [PubMed] [Google Scholar]
- 50.Tomokiyo A, Yoshida S, Hamano S, Hasegawa D, Sugii H, Maeda H. Detection, Characterization, and Clinical Application of Mesenchymal Stem Cells in Periodontal Ligament Tissue. Stem Cells Int. 2018;2018:5450768. doi: 10.1155/2018/5450768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawanabe N, Murata S, Murakami K, Ishihara Y, Hayano S, Kurosaka H, Kamioka H, Takano-Yamamoto T, Yamashiro T. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation. 2010;79:74–83. doi: 10.1016/j.diff.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Ng TK, Chen CB, Xu C, Xu Y, Yao X, Huang L, Liang JJ, Cheung HS, Pang CP, Huang Y. Attenuated regenerative properties in human periodontal ligament-derived stem cells of older donor ages with shorter telomere length and lower SSEA4 expression. Cell Tissue Res. 2020;381:71–81. doi: 10.1007/s00441-020-03176-y. [DOI] [PubMed] [Google Scholar]
- 53.Zhou LL, Liu W, Wu YM, Sun WL, Dörfer CE, Fawzy El-Sayed KM. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020;2020:1327405. doi: 10.1155/2020/1327405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kassebaum NJ, Smith AGC, Bernabé E, Fleming TD, Reynolds AE, Vos T, Murray CJL, Marcenes W GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990-2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J Dent Res. 2017;96:380–387. doi: 10.1177/0022034517693566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwata T, Yamato M, Tsuchioka H, Takagi R, Mukobata S, Washio K, Okano T, Ishikawa I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30:2716–2723. doi: 10.1016/j.biomaterials.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 56.Park JY, Jeon SH, Choung PH. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011;20:271–285. doi: 10.3727/096368910X519292. [DOI] [PubMed] [Google Scholar]
- 57.Yu N, Oortgiesen DA, Bronckers AL, Yang F, Walboomers XF, Jansen JA. Enhanced periodontal tissue regeneration by periodontal cell implantation. J Clin Periodontol. 2013;40:698–706. doi: 10.1111/jcpe.12113. [DOI] [PubMed] [Google Scholar]
- 58.Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829–1838. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan XZ, Yang F, Jansen JA, de Vries RB, van den Beucken JJ. Cell-Based Approaches in Periodontal Regeneration: A Systematic Review and Meta-Analysis of Periodontal Defect Models in Animal Experimental Work. Tissue Eng Part B Rev. 2015;21:411–426. doi: 10.1089/ten.TEB.2015.0049. [DOI] [PubMed] [Google Scholar]
- 60.Bassir SH, Wisitrasameewong W, Raanan J, Ghaffarigarakani S, Chung J, Freire M, Andrada LC, Intini G. Potential for Stem Cell-Based Periodontal Therapy. J Cell Physiol. 2016;231:50–61. doi: 10.1002/jcp.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R, Ohno T, Lin K, Yamato M, Ishikawa I, Okano T, Izumi Y. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32:5819–5825. doi: 10.1016/j.biomaterials.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 62.Iwasaki K, Akazawa K, Nagata M, Komaki M, Honda I, Morioka C, Yokoyama N, Ayame H, Yamaki K, Tanaka Y, Kimura T, Kishida A, Watabe T, Morita I. The Fate of Transplanted Periodontal Ligament Stem Cells in Surgically Created Periodontal Defects in Rats. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J, Wang W, Kapila Y, Lotz J, Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18:487–496. doi: 10.1089/scd.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hidaka T, Nagasawa T, Shirai K, Kado T, Furuichi Y. FGF-2 induces proliferation of human periodontal ligament cells and maintains differentiation potentials of STRO-1(+)/CD146(+) periodontal ligament cells. Arch Oral Biol. 2012;57:830–840. doi: 10.1016/j.archoralbio.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Wada N, Maeda H, Hasegawa D, Gronthos S, Bartold PM, Menicanin D, Fujii S, Yoshida S, Tomokiyo A, Monnouchi S, Akamine A. Semaphorin 3A induces mesenchymal-stem-like properties in human periodontal ligament cells. Stem Cells Dev. 2014;23:2225–2236. doi: 10.1089/scd.2013.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hasegawa D, Hasegawa K, Kaneko H, Yoshida S, Mitarai H, Arima M, Tomokiyo A, Hamano S, Sugii H, Wada N, Kiyoshima T, Maeda H. MEST regulates the stemness of human periodontal ligament stem cells. Stem Cells International. 2020;2020:15. doi: 10.1155/2020/9672673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamano S, Tomokiyo A, Hasegawa D, Yoshida S, Sugii H, Mitarai H, Fujino S, Wada N, Maeda H. Extracellular Matrix from Periodontal Ligament Cells Could Induce the Differentiation of Induced Pluripotent Stem Cells to Periodontal Ligament Stem Cell-Like Cells. Stem Cells Dev. 2018;27:100–111. doi: 10.1089/scd.2017.0077. [DOI] [PubMed] [Google Scholar]
- 68.Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, Ueda T, Gee P, Nishikawa M, Nomura M, Kitaoka F, Takahashi T, Okita K, Yoshida Y, Kaneko S, Hotta A. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell. 2019;24:566–578.e7. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]