Abstract
Animal models of asthma have shown that limonene, a naturally occurring terpene in citrus fruits, can reduce inflammation and airway reactivity. However, the mechanism of these effects is unknown. We first performed computational and molecular docking analyses that showed limonene could bind to both A2A and A2B receptors. The pharmacological studies were carried out with A2A adenosine receptor knock-out (A2AKO) and wild-type (WT) mice using ovalbumin (OVA) to generate the asthma phenotype. We investigated the effects of limonene on lung inflammation and airway responsiveness to methacholine (MCh) and NECA (nonselective adenosine analog) by administering limonene as an inhalation prior to OVA aerosol challenges in one group of allergic mice for both WT and KO. In whole-body plethysmography studies, we observed that airway responsiveness to MCh in WT SEN group was significantly lowered upon limonene treatment but no effect was observed in A2AKO. Limonene also attenuated NECA-induced airway responsiveness in WT allergic mice with no effect being observed in A2AKO groups. Differential BAL analysis showed that limonene reduced levels of eosinophils in allergic WT mice but not in A2AKO. However, limonene reduced neutrophils in sensitized A2AKO mice, suggesting that it may activate A2B receptors as well. These data indicate that limonene-induced reduction in airway inflammation and airway reactivity occurs mainly via activation of A2AAR but A2B receptors may also play a supporting role.
Electronic supplementary material
The online version of this article (10.1007/s11302-020-09697-z) contains supplementary material, which is available to authorized users.
Keywords: A2A adenosine receptors, Asthma, Airway inflammation, Airway responsiveness, Limonene
Introduction
There are various drugs available for the management of asthma and its symptoms, such as corticosteroids, leukotriene modifiers, and β2 agonists. Research is also ongoing with respect to the use of natural products with minimal side effects [1]. Flavonoids have emerged as the major naturally derived chemicals for the treatment of asthma. These compounds have anti-inflammatory properties, reduce levels of reactive oxygen species (ROS), and inhibit the release of various cytokines and cytotoxic materials [2].
The flavonoid of our interest is limonene, which is commercially available as D-(+)-limonene. Limonene has been used for the treatment of asthma, colic and heart problems in Chinese medicine [3]. It is the major component of essential oils obtained from the skin (peel) of citrus fruits like lemon, orange, yuzu, and grapefruit among others. These essential oils are used in aromatherapy for treating various disorders such as heartburn, stress relief, gastroesophageal reflux disease (GERD), and asthma [4]. Chemically, it is a monocyclic monoterpene with a characteristic lemon-like odor. Earlier studies have shown that limonene helps in reducing the levels of ROS, platelet aggregation and exhibits anti-tubercular properties [5, 6]. It has also been shown to inhibit the inflammatory response by inhibiting the IF-kB inflammatory pathway [7].
Asthma is chronic lung disease characterized by increased inflammation and bronchoconstriction [8]. Various systemic and airway inflammatory conditions like eosinophilia and neutrophilia are observed in the asthma as a result of chemotactic movement [9, 10].Though the detailed pathway of the asthma development is not fully known, several studies have shown a role for adenosine as an activator of inflammation and bronchoconstriction in asthma [11]. Asthmatic patients have increased levels of exhaled adenosine and higher bronchoresponsiveness to inhaled adenosine compared to healthy individuals, suggesting adenosine can act as a bronchoprovocant [12]. Studies also observed elevated level of adenosine in chronic asthmatic lungs [11, 13]. Adenosine signals through four main G protein coupled receptor subtypes namely A1, A2A, A2B, and A3. These receptors play different roles in asthma. A1 and A3 adenosine receptors (AR) are known to be involved in bronchoconstriction [14, 15] whereas A2AAR and A2BAR may mediate bronchodilation [16, 17]. Comparative studies have revealed that limonene may be a ligand for different adenosine receptor subtypes and could act like an agonist at the A2AAR which plays a major role in anti-inflammatory processes [7, 18]. Selective A2A agonist CGS 21680 and limonene show competitive binding to the A2AAR with limonene specifically binding to the same site as that of CGS 21680, thereby increasing the levels of unbound CGS 21680 by 91.2% over that of controls [18]. This study confirmed that limonene has a high affinity towards A2AAR and can act as agonistic ligand. Recent studies in mice also show that inhaled limonene attenuated the development of airway hyperresponsiveness and reduced the levels of Th2 cytokines involved in the pathophysiology of airway inflammation [4]. Thus, our study focused on assessing the effects of limonene in asthma-induced and healthy wild-type (WT) as well as A2A receptor deficient (A2A KO) mice to identify the mechanism by which limonene may induce changes in asthma.
Materials and methods
Animals
Experiments were conducted with 6-week-old C57BL/6 wild-type (WT) and A2AKO mice. Each group consisted of equal number of males and females. All the animals were inbred. A2AKO mice (originally from C. Ledent, Universite Libre de Bruxelles, Brussels, Belgium) were obtained from Dr. Stephen Tilley, University of North Carolina Chapel Hill, on C57BL/6J background. C57BL/6J (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). A2AKO mice were backcrossed 12 generations to the C57BL/6J background. A2AKO mice were generated and genotyped by polymerase chain reaction. All the animals were housed under conventional animal room conditions at Long Island University wet lab facility in individually ventilated cages (IVCs) containing corn cob bedding, temperature of 23 °C ± 5 °C, 50–60% relative humidity, 12-h light/dark cycle and received standard rodent chow and water ad libitum. Animals were maintained at above conditions until they were 6 weeks old. The entire study adhered to the animal facility guidelines of Arnold and Marie Schwartz College of Pharmacy, Long Island University, Brooklyn, NY 11201.
Animal sensitization
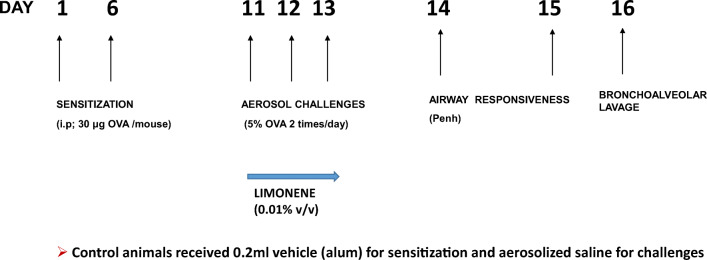
The sensitization protocol used to develop the asthma phenotype is shown in Fig. 1, based on an established protocol [19, 20]. Forty-eight animals (24 each of WT and A2A KO) were randomly chosen and were separated into 4 groups (n = 6). Albumin from chicken egg white (ovalbumin; OVA) was obtained from Sigma-Aldrich (St. Louis, MO). A suspension containing 250 μg/ml of OVA was prepared in Imject® Alum (Thermo Scientific) solution for intraperitoneal (i.p.) sensitization. Five percent OVA solution was prepared in normal saline (0.85% w/v sodium chloride) for aerosol challenge. (R)-(+)-Limonene was obtained from Sigma-Aldrich. It was first dissolved in DMSO (1:9) and then diluted with normal saline (0.9 w/v sodium chloride) to make the desired concentration of 0.01% (v/v) limonene solution [4]. The mice were sensitized on days 1 and 6 by i.p. injection of 25–30 μg of OVA suspended in 200 μl of Imject alum solution. Control mice were injected with a matching volume of Imject alum solution alone on the same days. On days 11, 12, and 13, the sensitized mice were exposed to aerosolized 5% OVA (allergen) in 0.9% saline (vehicle) while the controls were exposed to vehicle only for 20 min twice a day and 6 h apart (morning and evening) using an ultrasonic nebulizer (De Vilbiss Healthcare, Somerset, PA). For limonene-treated groups, the mice were first exposed to aerosolized 0.01% (v/v) limonene solution (7.5 ml) for 20 min and then the OVA challenges were performed 90 min later. The flow rate of the aerosolized allergen was performed at 2 ml/min and the median aerodynamic diameter of the aerosol particles < 4 μm (De Vilbiss Healthcare). In addition, based on some of our initial data, we tested the effects of A2BAR activation in A2AKO mice. For this purpose, one group of sensitized A2AKO received selective A2B agonist BAY 60-6583 (Millipore Sigma; 10 mg/kg) on days 11–13 prior to the OVA challenges.
Fig. 1.
Protocol used to develop allergic model and treatment groups. Mice were divided into control (CON) and allergen sensitized (SEN) groups. Mice were sensitized with ovalbumin (OVA) on day 1 and 6 (25–30 μg; i.p.), followed by 5% OVA aerosol challenge (days 11–13) to obtain the SEN group. For limonene-treated groups, the mice were first exposed to aerosolized 0.01% (v/v) limonene solution (7.5 ml) for 20 min followed by OVA aerosol challenges
Study groups
Wild-type and A2AKO mice were divided into the following study groups, respectively:
Non-asthmatic mice: control (CON).
Non-asthmatic mice receiving limonene: CON+LIM.
Asthmatic mice: allergen sensitized-challenged (SEN).
Asthmatic mice receiving limonene: allergen sensitized-challenged + LIM (SEN + LIM).
Computational analysis
Model selection
A previously published crystal structure for the human A2AAR in complex with agonist UK-432097 was available in the Protein Data Bank (PDB; Code: 3QAK) [21]. We selected this structure for molecular docking based on the resolution (2.71 Å), geometric, and refinement statistics. In addition, this structure has the fewest mutations (for crystal formation) compared to other higher resolution structures.
Homology modeling
Currently, there is no available crystal structure for the A2BAR for computational analysis. A homology model for A2BAR was constructed using the A2AAR structure (3QAK) as a starting template. The program used for homology modeling was Prime (Schrödinger) [22]. The amino acid sequence for the A2B AR was obtained using protein search in NCBI. This sequence (AAA51598) was used to build the homology model. A BLAST search in Prime identified models with high sequence identity and homology. The 3QAK structure was predicted to have high homology and 61.3% sequence identity in the BLAST search. Using 3QAK as the template, PRIME (Schrodinger) was used to construct the homology model for A2B AR. Model validity was assessed based on the following parameters: score; % positives (match) % gaps and (mismatch).
Molecular docking
A docking model for A2AAR (PDB: 3QAK) and limonene was prepared using the molecular docking program Glide [23]. The A2AAR structure was prepared using the Protein Preparation tool; this program added hydrogens to the structure and assigned proper ionization states. Using Grid Generation, we generated a docking grid that occupied all residues interacting within the ligand UK-432097 binding site (~ 6 Å radius around UK-432097 and interacting residues) [21]. The UK-432097 molecule in the structure was deleted, and limonene was docked into the receptor model. Three poses for limonene were generated and the best pose was selected for further analysis. To check the accuracy of our docking method, we “docked in place” UK-432097 and CGS21680 (Supporting Information Table 1) into the receptor grid and found that the best pose matched with original crystal structures.
Airway responsiveness to MCh and NECA
The airway hyperresponsiveness was assessed by using unrestrained whole body plethysmography (WBP) assembly manufactured by Buxco Electronics Inc. (Sharon, CT). This system uses a dimensionless parameter known as enhanced pause (Penh) to estimate the total pulmonary airflow. Animals were placed in individual Plexiglas chambers and allowed to adjust to their surroundings for 10 min. Baseline and vehicle readings were recorded for 5 min each, and the animals were then exposed to methacholine (MCh) via the Buxco aerosol delivery system (version 1.5; Buxco, Sharon, CT). Increasing concentrations of MCh (1.5, 3, 6, 12, 24, and 48 mg/ml) dissolved in normal saline was aerosolized for 2 min to establish a dose-response relationship. Each consecutive dose was administered only after the mice returned to baseline Penh levels. Penh values were normalized to the vehicle values and the increase in response was calculated as the percentage difference between the dose and vehicle values of Penh. On day 15, the entire procedure was repeated to determine the airway hyperresponsiveness to NECA (nonselective adenosine agonist).
Bronchoalveolar lavage (BAL)
On day 16, the animals were sacrificed by i.p. injecting 65 mg/kg of sodium pentobarbital. Bronchoalveolar lavage (BAL) was performed according to an established protocol [19, 20, 24]. BAL involved tracheotomy wherein the trachea was exposed and a small incision was made in the upper region of the trachea and was cannulated. This was followed with 3 lung lavages with normal saline (0.8 ml saline/lavage) and the fluid was withdrawn for cell collection. The collected BAL fluid (BALF) was stored on ice during collection and was centrifuged at 1800 rpm for 7 min at 4 °C (model GS-6R centrifuge; Beckman, Fullerton, CA). The cell pellet was suspended in 1 ml normal saline.
Total cell count
The total cell count analysis was performed using 400 μl of 0.4% Trypan Blue added to 100 μl of cell suspension. Using a pipette, 100 μl of this suspension was applied to the hemocytometer and the cells were counted under 10× magnification.
Differential cell analysis
The remaining cell suspension was spun on glass slides. Briefly, the glass slides were labeled with respective animal IDs. Cytofunnel was placed on the slide and firmly assembled with the cytoclip. Cell suspension (450 μl) was aliquoted into each well of the cytofunnel and slides were placed in the Cytospin 3® (Shandon Scientific Ltd., MA) and spun at room temperature for 5 min at 800 rpm. After cytospin and air drying for 24–36 h, cells were stained with Kwik-Diff staining solutions (Shandon, Fisher Scientific) as shown in the protocol provided by the manufacturer and were finally washed gently with distilled water. The slides were air dried and different types of cells were counted under 40X magnification. A total of 300 cells were counted on each slide. ELISA analysis was performed on the BAL supernatant using kits for Th2 cytokine IL-5 (Invitrogen, Carlsbard CA catalog # BMS610), OVA-specific IgE (Cayman Chemicals, Ann Arbor MI catalog # 500840) and OVA-specific IgG (Chondrex Inc., Redwood WA catalog #3011). The plates were prepared as per the manufacturers’ instructions and absorbances were measured in a Synergy microplate reader at 450 nm.
Drugs and chemicals
Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma Chemicals (St. Louis, MO).
Statistical analysis
The data are expressed as mean ±SEM. Comparison between different groups of WT and A2AKO were performed using two-way ANOVA followed by Tukey’s multiple comparison test. For IgG analysis, 3-way ANOVA followed by Tukey’s multiple analysis was used. A “p” value less than 0.05 (p < 0.05) was considered as significant. All statistical analyses were performed using GraphPad Prism software (GraphPad Software, San Diego, CA; Software Version 8.2.1).
Results
Computational analysis of docking models for limonene and A2AAR/A2BAR
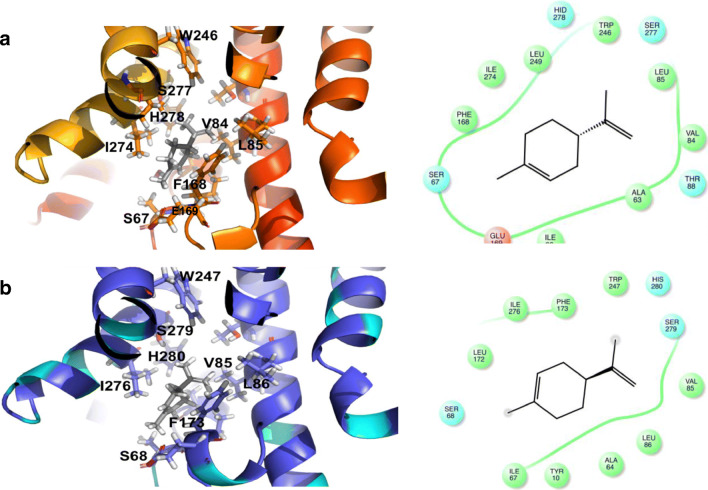
Our selected A2BAR homology model has the following parameters: highest model score; 74% positives, and 12% gaps. The docking scores and receptor interactions suggest that limonene can bind in a similar region of A2AAR as CGS21680 (Supporting Information, Table 1).Based on structural analysis, limonene appears to occupy a region at the top of the receptor. Receptor interactions captured in the docking model include van der Waals contacts with S67, V84, L85, F168, E169, W246, H278, and I274 (Fig. 2a). In comparing interactions with the receptor, it appears that these same residues (Phe168, Leu249, Asn253, and Ile 274) interact with the diphenyl group of UK-432097 [21]. Based on the molecular analysis, we hypothesize that limonene has affinity for A2AAR and may act as an agonist or partial agonist. We also looked at A2BAR-Limonene interactions, as functional data suggested another adenosine receptor may be involved. We found that limonene may also have similar binding interactions with the A2BAR based on the docking analysis with the homology model. Several residues at the top of the binding site of A2BAR are homologous to A2AAR. Thus, limonene interacts with similar residues S68, V85, L86, F173, W247, H280, and I 276 of A2BAR (Fig. 2b).
Fig. 2.
Docking model for limonene binding to A2A (Fig. 2a) and A2B (Fig. 2b) receptors
Airway responsiveness to MCh in WT and A2AKO
WT
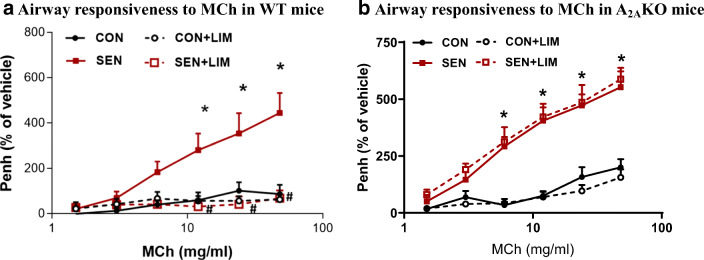
There was no significant difference in airway responsiveness to MCh (48 mg/ml) in control (CON; 87.2 ± 40.7%) and control mice treated with limonene (CON+LIM; 63.1 ± 26.7%). Allergen sensitized and challenged mice or allergic mice (SEN) showed the highest response to MCh (444.5 ± 87.5%; p < 0.05 compared to CON). However, treatment with limonene (SEN + LIM) significantly reduced the airway hyperresponsiveness in allergic mice (SEN 444.5 ± 87. 5% vs. SEN + LIM 69.1 ± 34.2%, p < 0.05) compared to the untreated sensitized groups (SEN). This showed that limonene effectively reduced the airway hyperresponsiveness in the asthmatic mice (Fig. 3a).
Fig. 3.
Methacholine-induced airway hypersensitivity (enhanced pause, i.e., Penh) in a) wild-type study groups. Values for Fig. 3a are expressed as mean ± SEM, *p < 0.05 compared to WT CON; #p < 0.05 compared to WT SEN, n = 6 mice per group, and b) A2AKO study groups. Values for Fig. 3b are expressed as mean ± SEM, *p < 0.05 compared to A2AKO CON, n = 6 mice per group
A2AKO
There was significant difference in the airway responsiveness to MCh (48 mg/ml) between control and sensitized groups (104.8 ± 15.698% in CON vs 553 ± 85% in SEN, p < 0.05). However, the limonene-treated sensitized mice did not show any significant reduction as compared to the untreated sensitized mice (588 ± 34% in SEN + LIM). This confirmed that the absence of A2AAR renders the treatment with limonene ineffective (Fig. 3b).
Airway responsiveness to NECA in WT and A2AKO mice
WT
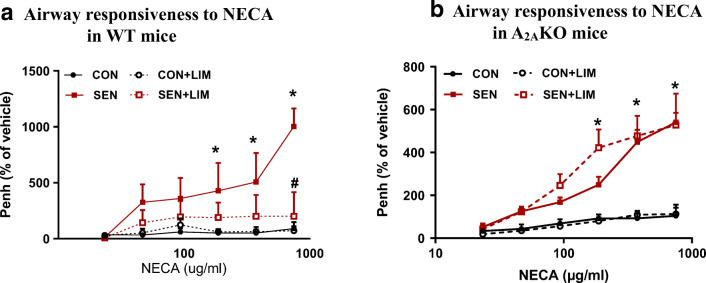
Similar to the MCh responses, exposure to NECA (750 μg/ml) showed significant increase in the airway responsiveness in the sensitized group compared to the control group (90.6 ± 57.7% in CON vs. 1002.8 ± 161.7% in SEN, p < 0.05). On the other hand, sensitized animals treated with limonene showed significant reduction in the airway hyperresponsiveness as compared to the sensitized untreated mice (201.1 ± 89.1% in SEN + LIM vs. 664.1 ± 327.9% in SEN, p < 0.05). NECA being a nonselective adenosine agonist and limonene being an agonist of the A2AAR suggest that limonene acts through the A2AAR to reduce the airway hyperresponsiveness (Fig. 4a).
Fig. 4.
NECA-induced airway responsiveness in a) wild-type study groups. Values for Fig. 4a are expressed as mean ± SEM, *p < 0.05 compared to WT CON; #p < 0.05 compared to WT SEN, n = 6 mice per group, and b) A2AKO study groups. Values for Fig. 4b are expressed as mean ± SEM, *p < 0.05 compared to A2AKO CON, n = 6 mice per group
KO
Airway responsiveness to NECA (750 μg/ml) was significantly higher in the sensitized group compared to the control group (104 ± 52% in CON vs. 541 ± 44% in SEN, p < 0.05). Treatment with limonene in the SEN + LIM group had no significant effect in reducing the airway responsiveness of KO SEN mice (528 ± 147% in SEN + LIM; Fig. 4b).
Total cell count of BAL fluid in WT and A2AKO
WT
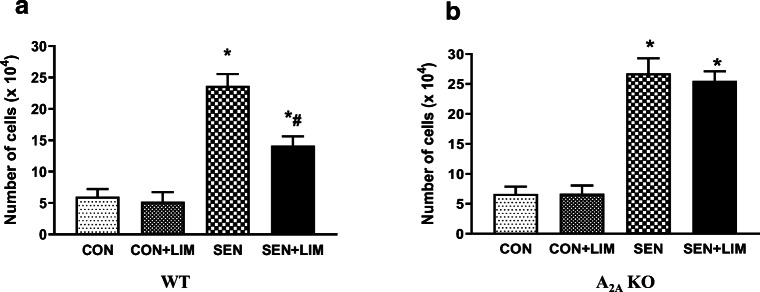
Significant increase was observed in the total cell counts of sensitized WT mice compared to controls (6 × 104 ± 1 in CON vs. 23.6 × 104 ± 1.9in SEN, p < 0.05). Limonene treatment effectively reduced the number of cells significantly in the limonene treated sensitized mice (14.1 × 104 ± 1.5; Fig. 5a).
Fig. 5.
Total cell count in BAL from a) wild-type study groups. Values for Fig. 5a are expressed as mean ± SEM, *p < 0.05 compared to WT CON; #p < 0.05 compared to WT SEN, n = 6 mice per group, and b) A2AKO study groups. Values for Fig. 5b are expressed as mean ± SEM, *p < 0.05 compared to A2AKO CON, n = 6 mice per group
A2AKO
Similarly, we observed that sensitized groups of A2AKO mice had significantly elevated total cell count compared to control (6.6 × 104 ± 1.2 in CON vs. 26.8 × 104 ± 2.5 in SEN, p < 0.05). However, limonene treatment did not lower the cell count in sensitized KO mice (25.5 × 104 ± 1.6 in SEN + LIM; Fig. 5b).
ELISA analysis of BALF supernatant for IL-5
The supernatant of the BALF was analyzed for IL-5 in WT (Fig. 6a) and A2AKO (Fig. 6b). IL-5 was significantly elevated in WT SEN (286.2 ± 45.2 pg/ml compared to 74.2 ± 18.6 pg/ml in CON; p < 0.05). Limonene significantly reduced IL-5 in treated WT asthmatic mice (81.4 ± 25.4 pg/ml in SEN + LIM; p < 0.05) compared to the nontreated SEN mice. A2AKO SEN also had elevated IL-5 compared to the KO control (231.2 ± 30.5 pg/ml vs. 78.5 ± 6.8 pg/ml in CON; p < 0.05), but limonene treatment had no effect on IL-5 levels in asthmatic KO mice (224 ± 45.3 pg/ml). These data suggest that limonene reduced IL-5 only in the presence of A2AAR.
Fig. 6.
Analysis of IL-5 levels in BAL from a) wild-type study groups. Values for Fig. 6a are expressed as mean ± SEM, *p < 0.05 compared to WT CON; #p < 0.05 compared to WT SEN, n = 6 mice per group, and b) A2AKO study groups. Values for Fig. 6b are expressed as mean ± SEM, *p < 0.05 compared to A2AKO CON, n = 6 mice per group
ELISA analysis of BALF supernatant for OVA-specific IgE and OVA-specific IgG
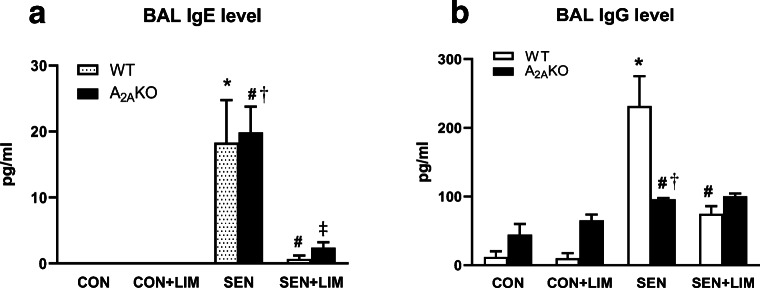
Analyses for OVA-specific IgE (Fig. 7a) and IgG (Fig. 7b) were performed using the BALF supernatant in all groups. IgE was significantly elevated in both sensitized WT (18.3 ± 6.4 pg/ml in SEN vs. undetected in CON; p < 0.001) and A2AKO (19.9 ± 3.9 pg/ml vs. undetected in CON; p < 0.001) groups compared to respective controls. Limonene significantly reduced IgE levels in SEN WT (0.7 ± 0.4 pg/ml in SEN + LIM; p < 0.0001) and A2AKO (2.4 ± 0.8 pg/ml in SEN + LIM; p < 0.001). There was no difference between WT and A2AKO in the BALF levels of OVA-specific IgE.
Fig. 7.
Analysis of IgE (a) and IgG (b) levels in BAL from WT and A2AKO mice. Values are expressed as mean ± SEM, *p < 0.05 compared to WT CON; #p < 0.05 compared to WT SEN; †p < 0.05 compared to A2AKO CON; ‡p < 0.05 compared to A2AKO SEN; n = 4 mice per group
Elevated levels of OVA-specific IgG were observed in SEN WT (231.7 ± 43.4 pg/ml vs. 12.2 ± 8.2 pg/ml in CON; p < 0.0001) and SEN A2AKO (96 ± 1.7 pg/ml in SEN vs. 44.2 ± 15.8 in CON; p < 0.05). The level of IgG in WT SEN was significantly higher than sensitized A2AKO mice (p < 0.01). While limonene reduced the IgG levels in SEN WT (74.8 ± 11.4, p < 0.001), it did not reduce the IgG level in treated SEN A2AKO (100.1 ± 4.2 pg/ml; p > 0.05).
Differential cell studies of bronchoalveolar lavage
The differential cell count for eosinophils, macrophages, neutrophils, and lymphocytes was obtained for all study groups.
Effects of limonene on eosinophils, neutrophils, macrophages, and lymphocytes in WT
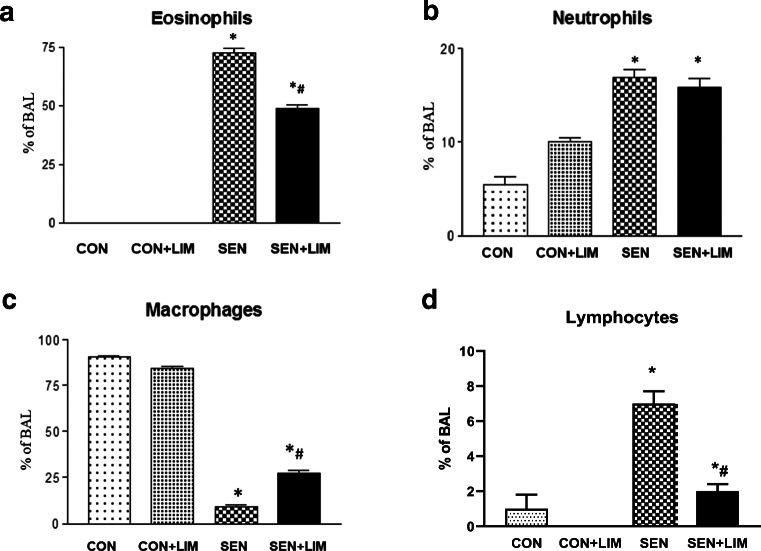
Sensitized WT mice (nontreated asthmatic group) had significantly higher number of eosinophils compared to control group (0 ± 0% in CON vs. 70.7 ± 2.6% in SEN; p < 0.0001; Fig. 8a). Limonene significantly reduced number of eosinophils in asthmatic mice (48.7 ± 1.4% in SEN + LIM, p < 0.05) compared to nontreated SEN groups (Fig. 7a). Neutrophils were significantly increased in SEN group compared to CON (5 ± 2.5% in CON vs 15.9 ± 1.1% of cells in SEN; p < 0.05; Fig. 8b). However, limonene-treatment had no effect on the increase in neutrophils (16.8 + 1% in SEN + LIM). In control groups, macrophages had the highest contribution to the cellular infiltrate while in SEN group, macrophages were reduced significantly (90.6 ± 1.8% in CON vs. 9.2 ± 2.8% in SEN; Fig. 8c). In the limonene-treated sensitized group, the decrease in macrophages was significantly attenutated compared to the SEN group (27.2 ± 4.4%; p < 0.05). Lymphocytes were significantly elevated in asthmatic WT mice (7 ± 1% in SEN vs. 1 ± 1% in CON; p < 0.05; Fig. 8d) and limonene reduced the elevated lymphocytes observed (2 ± 0.04% in SEN + LIM; p < 0.05).
Fig. 8.
Differential cell count of a) eosinophils, b) neutrophils, c) macrophages, and d) lymphocytes in BAL from WT mice. Values are described as mean ± SEM, *p < 0.05 compared to WT CON; #p < 0.05 compared to WT SEN; n = 6 mice per group
Effects of limonene on eosinophils, neutrophils, macrophages, and lymphocytes in A2AKO
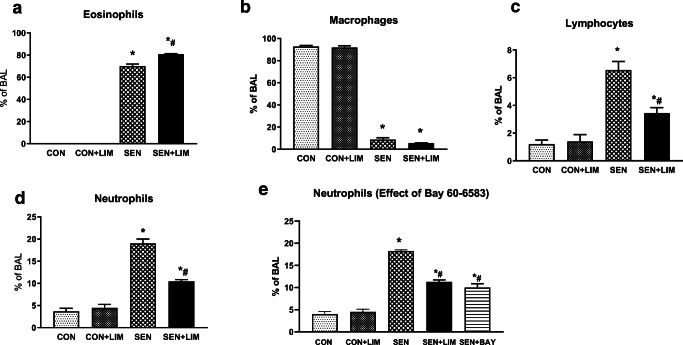
Sensitized A2AKO mice (nontreated asthmatic group) had significantly higher number of eosinophils compared to control group (0 ± 0% in CON vs. 76.3 ± 1.4% in SEN; p < 0.0001; Fig. 9a). Limonene failed to reduce the eosinophils in treated KO asthmatic mice (79 ± 0.69% in SEN + LIM). In A2AKO mice, macrophages were significantly reduced in SEN mice with limonene treatment having no effect on the macrophage count (Fig. 9b). Lymphocytes were also significantly elevated in asthmatic A2AKO mice (7.5 ± 0.6% in SEN vs. 1.6 ± 0.5% in CON; p < 0.05; Fig. 9c) and limonene reduced the elevated lymphocytes observed (3.4 ± 0.4% in SEN + LIM; p < 0.05).
Fig. 9.
Differential cell count of a) eosinophils, b) macrophages, c) lymphocytes, d) neutrophils, and e) neutrophils in groups treated with BAY 60-6583 from A2AKO mice. Values are described as mean ± SEM, *p < 0.05 compared to A2AKO CON; #p < 0.05 compared to A2AKO SEN; n = 6 mice per group
Neutrophils were significantly increased in SEN group compared to CON (4 ± 0.5% in vs 18.2 ± 0.7% in SEN; p < 0.05; Fig. 9d). However, unlike the WT group, limonene-treatment significantly reduced neutrophils in treated KO mice (11.2 ± 1.1% in SEN + LIM, p < 0.05). This suggested that in the absence of A2AAR, limonene could activate another receptor that affected neutrophilic infiltration. Based on the docking model studies, it appeared A2BAR could also be activated by limonene. To confirm the function of the A2BAR, we treated sensitized A2AKO mice with selective A2B agonist BAY 60–6583 [25]. These data showed that BAY 60–6583 also significantly reduced the neutrophilic infiltration in sensitized A2AKO mice (18.2 ± 0.68% in SEN vs 10 ± 2% in SEN + BAY; p < 0.05 Fig. 9e).
Discussion
In the current study, we investigated the effects of limonene using A2AKO and WT allergic mice to identify whether limonene attenuated inflammation and airway hyperresponsiveness by activating A2A receptors. The primary finding of this study is that allergic WT mice had increased bronchoconstriction and eosinophilic inflammation, which were lowered by limonene. Allergic A2AKO mice also had elevated bronchoconstriction and eosinophilic inflammation but treatment with limonene failed to lower these outcomes, suggesting that limonene mediated its effects partly through the A2AAR. Limonene, however, can also bind to A2BAR and may have an effect on neutrophil infiltration via activation of these receptors.
Our computational analysis and molecular docking data confirmed that limonene binds to the A2AAR as an agonist and to A2BAR as well. The effects of limonene on other adenosine receptors (A1, A3) remain unclear, and more studies are required to characterize if limonene acts as an agonist, partial agonist or antagonist at these receptors. From our studies, it appears that limonene acts as a nonselective agonist at the A2A and A2B receptors.
Next, we measured airway responsiveness using whole-body plethysmography with methacholine as a bronchoprovocant as previously described [24, 26]. While the use of whole body plethysmography is controversial, it continues to be used to assess airway responsiveness [24, 26–29]. We measured effect of limonene on airway responsiveness as enhanced pause (Penh) using increasing doses of methacholine and nonselective adenosine analog NECA in A2AKO and WT mice. Significantly increased airway responsiveness to both MCh and NECA was observed in sensitized WT and A2AKO mice compared to their respective controls. These responses were significantly lowered with limonene treatment in WT sensitized mice. However, limonene had no effect on increased airway responsiveness in A2AKO group. This data suggested that limonene attenuated the airway responsiveness through activation of the A2AAR and the absence of A2AAR renders the treatment with limonene ineffective.
Increased level of adenosine is seen in BAL fluid of asthma patients and studies show that adenosine contributes to increased inflammation in asthma [11]. We carried out total cell count of BAL fluid to assess the cellular infiltration in the lungs in each experimental group. Sensitized mice had the highest number of inflammatory cells compared to nonsensitized group in both WT and A2AKO groups, suggesting infiltration of inflammatory cells in the lungs. Treatment with limonene significantly attenuated the number of cells in allergic WT mice whereas the elevated total cell count noted in sensitized A2AKO mice remained unchanged with limonene treatment. These results indicate that limonene attenuated the inflammatory infiltration via the A2AAR, as in the absence of the A2AAR, there was no change in the increased cell counts in the allergic mice. Thus, limonene may have an anti-inflammatory role by acting as an agonist of A2AAR. This supports previous findings that activation of A2A AR has anti-inflammatory effects in a mouse model of asthma [30]. Previous studies have examined the effects of limonene and ozone exposures on inflammation in asthma [31]. The findings of these studies suggest that while limonene has an effect on lowering inflammation, it did not affect cellular infiltration to a great extent. The differences in this study and ours are possibly due to the differences in exposure duration of limonene, the dose selected, and the model used.
Elevated levels of inflammatory cells such as eosinophils and neutrophils are typically observed in the BAL fluid in mouse models of asthma [19, 20, 30, 32]. We assessed the lung inflammation in all study groups by differential cell analysis of the BAL to identify the cell types. Presence of highly elevated eosinophil counts in the BAL indicates the presence of allergic inflammation and exacerbates airway hyper-reactivity [9, 33]. We found that both WT and A2AKO sensitized mice had highest number of eosinophils compared to their respective nonsensitized controls. This eosinophilic infiltration was lowered significantly with the treatment with limonene in WT mice. However, treatment with limonene did not affect eosinophil number in A2AKO sensitized mice, suggesting again that limonene was reducing the eosinophilia via A2A receptor activation. The predominant cells in BAL in healthy individuals are macrophages [34]. In the present study, macrophages were highest in BAL of control groups of both WT and A2AKO mice and were lowered in sensitized mice. Limonene attenuated the decrease in macrophage number in sensitized WT mice but did not have any effect in A2AKO. Neutrophils are also elevated in asthma [10]. Differential cell analysis showed that neutrophils were increased in the sensitized mice in both WT and A2AKO groups. Interestingly, limonene did not lower the neutrophilia in sensitized WT mice but significantly lowered the neutrophil count in sensitized A2AKO mice. This led us to surmise that limonene could possibly activate other pathways as well to reduce inflammation. Based on our docking studies and previously published work that showed A2BAR activation lowered neutrophil infiltration [35], it was possible that the A2BAR was being activated by limonene in our model. In order to confirm the effect of A2B activation on neutrophil infiltration, we treated sensitized A2AKO mice with selective A2B agonist BAY 60-6583. Our data showed that BAY 60-6583 also lowered the neutrophil count in the sensitized A2AKO mice to the same degree as limonene. This led us to conclude that limonene lowered eosinophilia via A2A AR activation and could lower neutrophilia by activation of the A2BAR. However, we did not observe lowered neutrophils in the WT mice, even though the A2B receptors were present in these mice. It has been established that among the adenosine receptors, the A2B receptor is considered as the low affinity receptor [35]. Thus, high amounts of agonist are required before activation of A2BAR can occur. It is possible that the dose of limonene given to WT mice was not enough to activate both A2A and A2B receptors, with the likelihood that A2AAR activation dominated in the WT. On the other hand, in the absence of the A2AAR in the A2AKO, the limonene administered could now solely target the A2BAR. Taken together with the BAY 60-6583 compound effects, it appears A2BAR activation may be responsible for the lowered neutrophil counts observed in A2AKO. This is a novel finding where limonene may have differential effects in asthma by activating A2A and A2B receptors.
We measured OVA-specific IgE and IgG in the BAL and found elevations for both immunoglobulins in the sensitized WT and A2AKO mice compared to controls. The role of IgE in allergic asthma is well-established [36] and there also appears to be a role for IgG in driving airway hyperreactivity and inflammation, as evidenced by IgE knockout mice still developing some of the features of allergic asthma [37, 38]. Limonene reduced IgE in both WT and A2AKO sensitized mice. For IgG, we observed that sensitized WT had higher IgG levels compared to A2AKO sensitized mice. While limonene reduced the level of IgG significantly in allergic WT mice, it did not affect the elevated IgG level in sensitized A2AKO mice. We also found that IL-5 was significantly elevated in the BAL in sensitized mice from both WT and A2AKO groups. Limonene reduced the IL-5 level in the WT sensitized group but not the A2AKO sensitized mice. IL-5 is a key cytokine for the increased infiltration of eosinophils in allergic asthma [39]. Our finding on IL-5 corresponded with the exacerbated eosinophilia in sensitized A2AKO mice that was unaffected by limonene. IL-5 is also known to increase airway responsiveness [35]. Our findings on IL-5 and eosinophils correlated with the Penh measurements of airway hyperresponsiveness in sensitized A2AKO mice treated with limonene. Studies have also shown that neutrophil numbers may not be associated with higher responsiveness to methacholine [40]. Thus, even though there were lowered neutrophils in treated sensitized A2AKO mice, the unabated eosinophilia and elevated IL-5 could possibly be responsible for the higher airway responsiveness in this group. IgE and IgG may both be elevated on account of initial allergen sensitization.
In summary, our data shows that limonene decreases airway inflammation and airway responsiveness in allergic mice by the activation of A2AAR. In the absence of the A2A receptor, limonene fails to have an effect on eosinophilia and airway reactivity. However, limonene can also lower the neutrophilia observed in asthma by activation of A2B receptors. These novel findings support a role for limonene as an adenosine receptor agonist, targeting both A2A and A2B receptors for beneficial effects in asthma. To our knowledge, this is the first study to show that limonene may have therapeutic benefits in asthma by acting on adenosine-mediated pathways through both A2A and A2B receptor activation.
Electronic supplementary material
(PDF 8.41 kb)
Acknowledgments
This work was supported by LIU startup funds (DSP) and National Institutes of Health grant HL027339 (SJM).
Funding information
Long Island University start-up funds (DSP), HL027339 (SJM).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional guidelines for the care and use of animals were followed. All procedures performed were in accordance with the ethical standards of Long Island University under an approved IACUC protocol DP-AA-2018.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehaben Patel, Email: mehaben.patel@my.liu.edu.
Deven Narke, Email: deven.narke@my.liu.edu.
Mangesh Kurade, Email: Mangesh.kurade@my.liu.edu.
Kathleen M. Frey, Email: kmfrey@fdu.edu
Sahith Rajalingam, Email: sahith.rajalingam@my.liu.edu.
Armaan Siddiquee, Email: armaan.siddiquee@my.liu.edu.
S Jamal Mustafa, Email: sjmustafa@hsc.wvu.edu.
Catherine Ledent, Email: Catherine.Ledent@ulb.ac.be.
Dovenia S. Ponnoth, Email: dponnoth@osteo.wvsom.edu
References
- 1.Barnes PJ. Pathophysiology of asthma. Br J Clin Pharmacol. 1996;42(1):3–10. doi: 10.1046/j.1365-2125.1996.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi G, Wei M, Xie X, Soromou LW, Liu F, Zhao S. Suppression of MAPK and NF-kappaB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation. 2013;36(2):501–511. doi: 10.1007/s10753-012-9571-1. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka T, Takahashi R. Flavonoids and asthma. Nutrients. 2013;5(6):2128–2143. doi: 10.3390/nu5062128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota R, et al. Limonene inhalation reduces allergic airway inflammation in Dermatophagoides farinae-treated mice. Inhal Toxicol. 2012;24(6):373–381. doi: 10.3109/08958378.2012.675528. [DOI] [PubMed] [Google Scholar]
- 5.Zuo L, et al. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol Immunol. 2013;56(1–2):57–63. doi: 10.1016/j.molimm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Bibi H, Reany O, Waisman D, Keinan E. Prophylactic treatment of asthma by an ozone scavenger in a mouse model. Bioorg Med Chem Lett. 2015;25(2):342–346. doi: 10.1016/j.bmcl.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Hirota R, Roger NN, Nakamura H, Song HS, Sawamura M, Suganuma N. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J Food Sci. 2010;75(3):H87–H92. doi: 10.1111/j.1750-3841.2010.01541.x. [DOI] [PubMed] [Google Scholar]
- 8.Lambrecht BN, Hammad H. Asthma: the importance of dysregulated barrier immunity. Eur J Immunol. 2013;43(12):3125–3137. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- 9.Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005;11(4):148–152. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel SE, et al. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156(3 Pt 1):737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 11.Driver AG, et al. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148(1):91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 12.Cushley MJ, Tattersfield AE, Holgate ST. Adenosine-induced bronchoconstriction in asthma. Antagonism by inhaled theophylline. Am Rev Respir Dis. 1984;129(3):380–384. doi: 10.1164/arrd.1984.129.3.380. [DOI] [PubMed] [Google Scholar]
- 13.Mann JS, et al. Airway effects of purine nucleosides and nucleotides and release with bronchial provocation in asthma. J Appl Physiol (1985) 1986;61(5):1667–1676. doi: 10.1152/jappl.1986.61.5.1667. [DOI] [PubMed] [Google Scholar]
- 14.Tawfik HE, et al. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288(3):H1411–H1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 15.Talukder MA, et al. Targeted deletion of adenosine A(3) receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol. 2002;282(6):H2183–H2189. doi: 10.1152/ajpheart.00964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abebe W, Makujina SR, Mustafa SJ. Adenosine receptor-mediated relaxation of porcine coronary artery in presence and absence of endothelium. Am J Phys. 1994;266(5 Pt 2):H2018–H2025. doi: 10.1152/ajpheart.1994.266.5.H2018. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa S, Abebe W (1996) Coronary vasodilation by adenosine receptor subtypes and mechanism of action. Drug Dev Res
- 18.Park HM, et al. Limonene, a natural cyclic terpene, is an agonistic ligand for adenosine A(2A) receptors. Biochem Biophys Res Commun. 2011;404(1):345–348. doi: 10.1016/j.bbrc.2010.11.121. [DOI] [PubMed] [Google Scholar]
- 19.Siddiquee A, Patel M, Rajalingam S, Narke D, Kurade M, Ponnoth DS. Effect of omega-3 fatty acid supplementation on resolvin (RvE1)-mediated suppression of inflammation in a mouse model of asthma. Immunopharmacol Immunotoxicol. 2019;41(2):250–257. doi: 10.1080/08923973.2019.1584903. [DOI] [PubMed] [Google Scholar]
- 20.Patel M, et al. Role of angiotensin II type 1 (AT1) and type 2 (AT2) receptors in airway reactivity and inflammation in an allergic mouse model of asthma. Immunopharmacol Immunotoxicol. 2019;41(3):428–437. doi: 10.1080/08923973.2019.1609026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332(6027):322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson MP, Pincus DL, Rapp CS, Day TJ, Honig B, Shaw DE, Friesner RA. A hierarchical approach to all-atom protein loop prediction. Proteins. 2004;55(2):351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- 23.Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004;47(7):1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- 24.Fan M, Mustafa SJ. Adenosine-mediated bronchoconstriction and lung inflammation in an allergic mouse model. Pulm Pharmacol Ther. 2002;15(2):147–155. doi: 10.1006/pupt.2001.0329. [DOI] [PubMed] [Google Scholar]
- 25.Hinz S, et al. BAY60-6583 acts as a partial agonist at adenosine A2B receptors. J Pharmacol Exp Ther. 2014;349(3):427–436. doi: 10.1124/jpet.113.210849. [DOI] [PubMed] [Google Scholar]
- 26.Drazen JM, Finn PW, De Sanctis GT. Mouse models of airway responsiveness: physiological basis of observed outcomes and analysis of selected examples using these outcome indicators. Annu Rev Physiol. 1999;61:593–625. doi: 10.1146/annurev.physiol.61.1.593. [DOI] [PubMed] [Google Scholar]
- 27.El-Hashim AZ, Mathews S, Al-Shamlan F. Central adenosine A1 receptors inhibit cough via suppression of excitatory glutamatergic and tachykininergic neurotransmission. Br J Pharmacol. 2018;175(15):3162–3174. doi: 10.1111/bph.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015;125(5):2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosnjak B, et al. Tiotropium bromide inhibits relapsing allergic asthma in BALB/c mice. Pulm Pharmacol Ther. 2014;27(1):44–51. doi: 10.1016/j.pupt.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Nadeem A, Ponnoth DS, Ansari HR, Batchelor TP, Dey RD, Ledent C, Mustafa SJ. A2A adenosine receptor deficiency leads to impaired tracheal relaxation via NADPH oxidase pathway in allergic mice. J Pharmacol Exp Ther. 2009;330(1):99–108. doi: 10.1124/jpet.109.151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen JS, et al. Limonene and its ozone-initiated reaction products attenuate allergic lung inflammation in mice. J Immunotoxicol. 2016;13(6):793–803. doi: 10.1080/1547691X.2016.1195462. [DOI] [PubMed] [Google Scholar]
- 32.Bousquet J, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 33.Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci. 2007;64(10):1269–1289. doi: 10.1007/s00018-007-6527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eschenbacher WL, Gravelyn TR. A technique for isolated airway segment lavage. Chest. 1987;92(1):105–109. doi: 10.1378/chest.92.1.105. [DOI] [PubMed] [Google Scholar]
- 35.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oettgen HC, et al. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370(6488):367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 38.Mehlhop PD, et al. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci U S A. 1997;94(4):1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi HZ, Xiao CQ, Zhong D, Qin SM, Liu Y, Liang GR, Xu H, Chen YQ, Long XM, Xie ZF. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. Am J Respir Crit Care Med. 1998;157(1):204–209. doi: 10.1164/ajrccm.157.1.9703027. [DOI] [PubMed] [Google Scholar]
- 40.Woodruff PG, et al. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol. 2001;108(5):753–758. doi: 10.1067/mai.2001.119411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 8.41 kb)