Abstract
Icare® HOME allows intraocular pressure (IOP) sampling by the patient. This technology addresses the barrier of limited IOP data from clinic visits and provides a telemedicine workflow for glaucoma care.
Based on knowledge that intraocular pressure (IOP) is a glaucoma risk factor, clinical practice includes setting a target IOP and assessing treatment efficacy during clinic visits. There is growing appreciation that IOP fluctuation may contribute to progression.1 Although many unanswered questions remain to define IOP fluctuation,2 IOP measurements are limited to clinic hours and incompletely characterizes diurnal variations.
Technologies, such as the SENSIMED Triggerfish® (Sensimed AG, Lausanne, Switzerland) and rebound tonometry (Icare® HOME, Icare USA, Raleigh, NC), acquire real-world data.3 This technology can assess fluctuation and treatment efficacy outside of the office. Given the current health environment, such technologies provide IOP data collected outside of the clinic and facilitates data-driven based telemedicine.
The feasibility to acquire real-world IOP with Icare® HOME showed that 84% (n = 144/171) of patients use this technology.4 The precision of measurements by intraclass correlation coefficient was 0.92 comparing Icare® HOME and Goldman applanation tonometry (GAT). However, the caveat was that 1 in 6 patients failed to certify because of large IOP differences comparing GAT and Icare® HOME. Huang et al. showed that 70% of patients could perform self-tonometry and studied diurnal and nocturnal curves in patients with newly diagnosed glaucoma and treatment effects of initial therapy.5
We present two cases that support using such technology to obtain IOP data collected in the real-world. Both patients were trained, certified, and motivated to use Icare® HOME to characterize IOP data under treatment and after changing treatment. With the current instrument design, all IOP measurements were obtained in the upright position, and not supine. Both gave informed consent as part of an IRBMED protocol following the tenets of the Declaration of Helsinki.
Case 1:
A 72-year-old Caucasian male, who is an active general surgeon, transferred care for pseudoexfoliation glaucoma. He had a family history of glaucoma (mother and maternal uncle). His left eye (OS) was progressing with IOP range of 10-16 mmHg. Ocular medications included fixed combination dorzolamide 2%-timolol 0.5% BID both eyes (OU) and travoprost 0.004% QHS OU. Corrected acuities were 20/30 OD and 20/20 OS, and IOPs were 11 mmHg OD and 12 mmHg OS. Central corneal thickness (CCT) was 545 μm OD and 551 μm OS. Iridocorneal angles were open on gonioscopy. Global retinal nerve fiber layer (RNFL) was 91 μm OD and 75 μm OS (supplement 1), which corresponded to vertical cup-to-disc ratio of 0.85 OD and 0.95 OS. Humphrey visual fields (24–2 SITA standard) were reliable with full field OD, and superior and inferior arcuate defects OS (supplement 1).
During the shortage of dorzolamide 2%-timolol 0.5%, he was placed on individual medications. Timolol 0.5% was stopped due to bradycardia. Brimonidine 0.2% was added, but stopped due to orthostatic hypotension and blepharoconjunctivitis. His office-based IOPs were 12.7 ± 2.25 mmHg OD and 12.1 ± 2.11 mmHg OS with a peak IOP of 16 mmHg OU on treatment. A left disc hemorrhage was noted, so selective laser trabeculoplasty (SLT) OS was performed, but did not lower his IOP. Therapy was escalated with the addition of latanoprostene bunod QHS OU, stopping travaprost, and continuing brinzolamide 1.0% TID OU.
He was motivated to capture real-world IOP variability because he was symptomatic from vision loss OS and was hesitant for surgical intervention given his hobbies of scuba diving and basketball. He was instructed to measure six times throughout the day, and add measurements during the night if waking up, over a 1-week period. However, he was committed to gather more data and captured IOP peaks of 28 mmHg OD and 43 mmHg OS that occurred outside of clinic hours while on latanoprostene bunod qhs OU and brinzolamide TID OU (Fig. 1 top). After this 1-week period, he was instructed to stop latanoprostene bunod, start netarsudil qhs 0.02% OU, and continue brinzolamide 1% TID OU. His peak IOPs were 19 mmHg OD and 17 mmHg OS.
Figure 1.
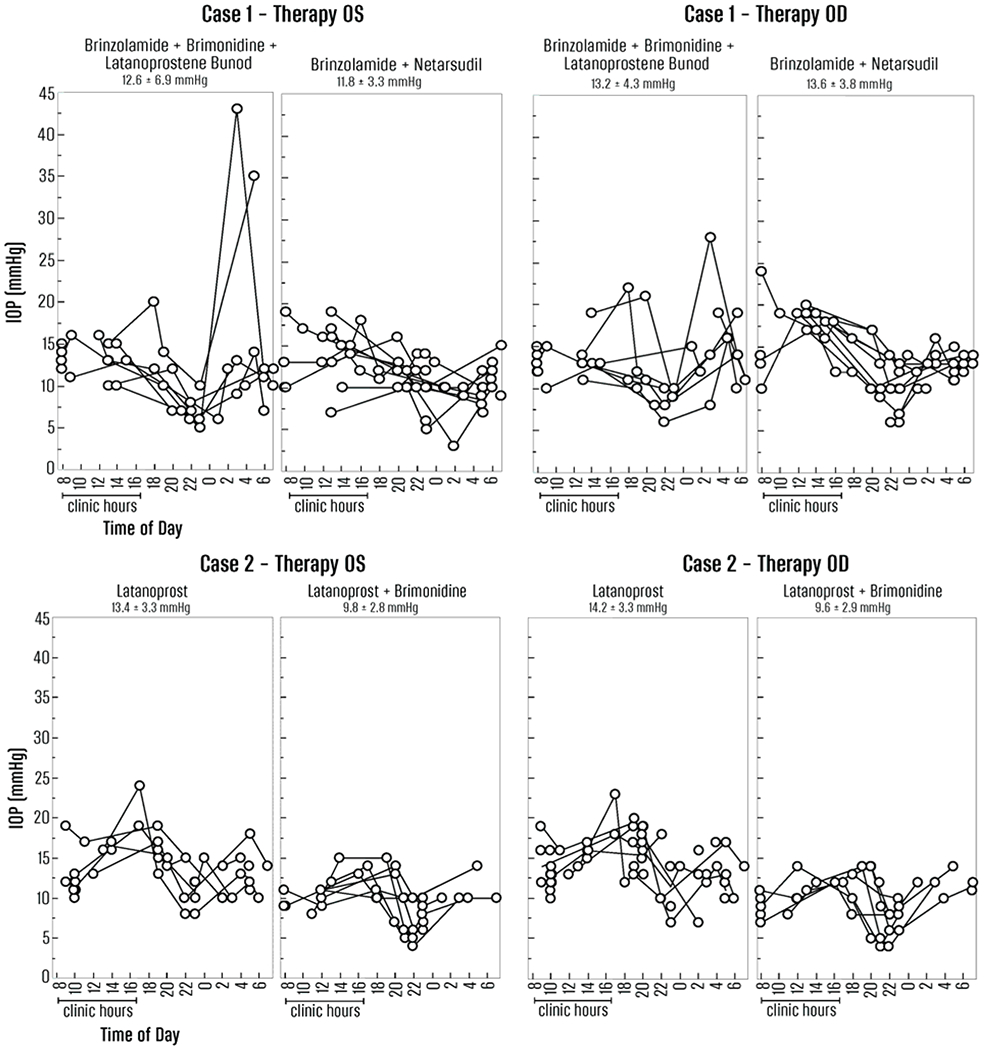
Top panel, Case 1. Left (OS) and right (OD) graphs show IOPs (y-axis, mmHg) measured by the individual throughout the day relative to time of day. The real-world hours and homology to clinic hours (8 am – 5 pm) is on the left side of each graph. The real-world IOPs and homology to outside of clinic hours is on the right and shaded in light gray. The glaucoma medications are described at the top of each graph. The mean ± standard deviation of the IOP measurements are at the top of each graph over that measurement period.
Bottom panel, Case 2.
Case 2:
A 63-year-old African American male, who is a computer programmer, with primary open-angle glaucoma (POAG) was managed since 1993 initially with timolol and then switched to latanoprost QHS OU in 1998. Risk factors included family history (mother and maternal aunt), CCT of 538 μm OD and 538 μm OS, and maximum IOP of 36 mmHg OD and 38 mmHg OS. Best acuities were 20/20 OU. He was highly adherent for 20 years with a target IOP of “teens”. His iridocorneal angles were open by gonioscopy. Office-based IOPs were 15.4 ± 5.67 mmHg OD and 16.4 ± 5.90 mmHg OS on treatment. Global RNFL was 86 μm OD and 77 μm OS (supplement 2), which corresponded clinically to vertical cup-to-disc ratio of 0.85 OD and 0.95 OS. Humphrey visual fields (24–2 SITA standard) were reliable with full field OD, and progression OS with superior nasal step and inferior arcuate defects (supplement 2).
Given excellent adherence and clinic-based IOPs at target, there was concern for IOP fluctuation not captured during office hours. His IOP data showed peaks to 23 mmHg OD and 24 mmHg OS while on latanoprost qhs OU (Fig. 1 bottom). After adding brimonidine 0.2% BID OU, his IOP peaks were 14 mmHg OD and 15 mmHg OS.
In summary, these carefully selected cases demonstrate that rebound tonometry can evaluate IOP modulation under treatment and changing treatment for patients with glaucoma.6 The data captured by these individuals identified peak IOPs over a 1-week period, and the effect of added glaucoma therapy to modulate these IOP peaks over a repeat 1-week period. However, not all patients can perform rebound tonometry at home.4, 5 Another limitation is the instrument cost as a barrier for general use, which we addressed by using a library-like model of instrument ‘check out and return’. Future studies are needed to determine both the number of IOP measurements over 24 hours and the number of repeated days sufficient to characterize the IOP curves, capture IOP peaks, and then to understand this individual data and risk for glaucoma progression. Given current health circumstances, such technology provides a telemedicine workflow for glaucoma management to assess the risk factor of IOP.
Supplementary Material
Supplement 1. Case 1. The OCT imaging (A) for the optic nerves show global RNFL of 91 μm OD and 75 μm OS. The corresponding right visual field (B) is full and the left visual field (C) shows superior and inferior arcuate defects.
Supplement 2. Case 2. The global RNFL (A) is 86 μm OD and 77 μm OS. The corresponding right visual field (B) is full and the left visual field (C) shows a superonasal step and inferior arcuate defects.
Acknowledgement:
Jesse Gilbert, BS, trained and certified these patients.
Financial support: Supported in part by P30 EY007003 (University of Michigan), unrestricted grant from Research to Prevent Blindness (University of Michigan), R01 EY022124 (S.E.M.)
Conflict of interest: Moroi (Aerie Pharmaceuticals, Inc [clinical trial grant support], Allergan [clinical trial grant support], Icare USA [clinical trial grant support], Ocuphire [clinical trial grant support], Wolters Kluwer Health [royalty], NSF AWD010114 [grant support], R01 EY022124 [grant support], R21EY03063 [grant support], grateful patients). Reed (none). Rojas (none).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: none
References
- 1.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9: 134–142. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Caprioli J. Intraocular Pressure Fluctuation: Is It Important? J Ophthalmic Vis Res. 2018;13: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sit AJ, Pruet CM. Personalizing Intraocular Pressure: Target Intraocular Pressure in the Setting of 24-Hour Intraocular Pressure Monitoring. Asia Pac J Ophthalmol (Phila). 2016;5: 17–22. [DOI] [PubMed] [Google Scholar]
- 4.Mudie LI, LaBarre S, Varadaraj V, et al. The Icare HOME (TA022) Study: Performance of an Intraocular Pressure Measuring Device for Self-Tonometry by Glaucoma Patients. Ophthalmology. 2016;123: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Katalinic P, Kalloniatis M, Hennessy MP, Zangerl B. Diurnal Intraocular Pressure Fluctuations with Self-tonometry in Glaucoma Patients and Suspects: A Clinical Trial. Optom Vis Sci. 2018;95: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Ophthalmology. Telemedicine for Ophthalmology Information Statement. San Francisco, CA: American Academy of Ophthalmology, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Case 1. The OCT imaging (A) for the optic nerves show global RNFL of 91 μm OD and 75 μm OS. The corresponding right visual field (B) is full and the left visual field (C) shows superior and inferior arcuate defects.
Supplement 2. Case 2. The global RNFL (A) is 86 μm OD and 77 μm OS. The corresponding right visual field (B) is full and the left visual field (C) shows a superonasal step and inferior arcuate defects.


