Abstract
To (1) investigate the efficacy of multiple doses of an orally delivered probiotic bacteria Lactobacillus paracasei (LP) modified to express angiotensin (1–7) (LP-A) in altering physiologic parameters relevant to the gut-brain axis in older rats and to (2) compare this strategy with subcutaneous delivery of synthetic Ang(1–7) peptide on circulating Ang(1–7) concentrations and these gut-brain axis parameters. Male 24-month-old F344BN rats received oral gavage of LP-A, or subcutaneous injection of Ang(1–7) for 0×, 1×, 3×, or 7×/week over 4 weeks. Circulating RAS analytes, inflammatory cytokines, and tryptophan and its downstream metabolites were measured by ELISA, electrochemiluminescence, and LC-MS respectively. Microbiome taxonomic analysis of fecal samples was performed via 16S-based PCR. Inflammatory and tryptophan-related mRNA expression was measured in colon and pre-frontal cortex. All dosing regimens of LP-A induced beneficial changes in fecal microbiome including overall microbiota community structure and α-diversity, while the 3×/week also significantly increased expression of the anti-inflammatory species Akkermansia muciniphila. The 3×/week also increased serum serotonin and the neuroprotective analyte 2-picolinic acid. In the colon, LP-A increased quinolinate phosphoribosyltransferase expression (1×/week) and increased kynurenine aminotransferase II (1× and 3×/week) mRNA expression. LP-A also significantly reduced neuro-inflammatory gene expression in the pre-frontal cortex (3×/week: COX2, IL-1β, and TNFα; 7×/week: COX2 and IL-1β). Subcutaneous delivery of Ang(1–7) increased circulating Ang(1–7) and reduced angiotensin II, but most gut-brain parameters were unchanged in response. Oral—but not subcutaneous—Ang(1–7) altered physiologic parameters related to gut-brain axis, with the most effects observed in 3×/week oral dosing regimen in older rats.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00196-y) contains supplementary material, which is available to authorized users.
Keywords: Aging, RAS, Microbiome, Genetically modified probiotic, Kynurenine
Introduction
Advanced age is associated with a progressive decline in various physiologic functions which predispose older adults to a host of clinical disorders and diseases. Late-life cognitive decline—whether mild cognitive impairment or that attributable to Alzheimer’s disease or related dementias—represents one of the most substantial aging-related public health concerns. Despite extensive exploration into underlying mechanisms and potential treatments for age-related cognitive declines, efficacious therapy remains elusive. Thus, novel thinking and new strategies are needed to preserve cognitive function among a large segment of the rapidly expanding older adult population.
In recent years, the role of the intestinal tract (i.e., the “gut”) has received increased attention for its role in heath and various physiologic processes—including neural communication and cognitive function via an established “gut-brain axis” (Collins et al. 2012; Kennedy et al. 2017; Strandwitz 2018). Notably, advanced age is associated with changes in the gut microbiome that affect both the density and composition of microbiota (i.e., dysbiosis),(Biagi et al. 2010; Jeffery et al. 2016) and these changes have been associated with the development of cognitive impairment (Cattaneo et al. 2017; Lawrence and Hyde 2017). Thus, the gut may be a promising target for intervention to preserve cognitive functions in late life.
One method of addressing age-related dysbiosis is through dietary supplementation with probiotics. Probiotics provide a variety of beneficial effects including antimicrobial activity, enhancement of intestinal barrier function, and immunomodulation via actions on a wide variety of immune cells (Ng et al. 2009). Consistent with these established mechanisms, several studies among older adults have demonstrated beneficial effects of probiotic preparations on gut microflora composition (Ahmed et al. 2007; Rampelli et al. 2013; Liu et al. 2016) and systemic immunity (Gill et al. 2001; Moro-Garcia et al. 2013; Liu et al. 2016). For example, probiotics have been proposed as a safe and efficacious method to deliver other therapeutic compounds as genetically modified probiotics (GMPs) offer a potentially efficacious method to deliver drugs or other therapeutic proteins with precision and a higher degree of site specificity than convention drug regimens (Steidler 2003; Syvanen 2003; Paton et al. 2012; M. Kumar et al. 2016).
The primary objective of the work presented here was to evaluate the efficacy of differing doses of a GMP designed to deliver the bioactive peptide angiotensin (1–7) (Ang[1–7]) on physiologic parameters related to the gut-brain axis. Ang(1–7) is a key vasoprotective effector of the renin-angiotensin system (RAS) that opposes the fibrotic, vasoconstrictive, proliferative, and inflammatory effects of angiotensin 2 (AngII). Components of the Ang(1–7)/Mas axis are found throughout the central nervous system (Young et al. 1988; Chappell et al. 1989; Hamming et al. 2004; Gallagher et al. 2006; Doobay et al. 2007; Becker et al. 2007; Guo et al. 2010) and several studies indicate that Ang(1–7) benefits various aspects of cognition in rodents (Xie et al. 2014; X. L. Wang et al. 2016a; L. Wang et al. 2016b; Lazaroni et al. 2016; Kangussu et al. 2017). More recent evidence also suggests a specific benefit on AD pathobiology, including mitigation of tau deposition (Jiang et al. 2016a; Jiang et al. 2016b; Kehoe et al. 2016).
We previously reported that oral delivery Lactobacillus paracasei expressing Ang(1–7) (LP-A) significantly increased circulating concentrations of Ang(1–7) in older Fischer 344/Brown-Norway (F344/BN) rats (Carter et al. 2019)—demonstrating efficacy of the compound in surviving the gut. Here, we investigate the efficacy of multiple doses of LP-A in altering several physiologic parameters relevant to the gut-brain axis including gut microbiome composition, systemic metabolites of tryptophan involved in neurotransmission, and neuro-inflammatory gene expression within the pre-frontal cortex, a brain region underlying multiple cognitive functions that decline with age. Moreover, a secondary objective of this work was to evaluate the impact of varying doses of subcutaneous, in contrast to oral Ang-(1–7) expressed from probiotic bacteria, delivery of Ang(1–7) on circulating Ang(1–7) concentrations and these gut-brain axis parameters.
Methods
Experimental overview
This work outlines findings from two complimentary 4-week dosing studies designed to (1) identify the optimal dose of orally delivered LP-A for inducing changes in several physiologic indices relevant to the gut-brain axis and to (2) evaluate the efficacy of subcutaneous Ang(1–7) administration for increasing systemic concentrations of relevant RAS analytes, including Ang(1–7), and in altering the same indices of gut-brain physiology. Studies were completed at the University of Florida and the University of Alabama at Birmingham, with all experimental protocols approved by the respective Animal Care and Use Committees in accordance with the “Guide for the Care and Use of Laboratory Animals.” All animals were obtained from the National Institute on Aging (NIA) Colony at Harlan Laboratories (Indianapolis, IN). Due to ongoing shortages of female F334/BN rats in the colony, all experiments were completed in male rats with subsequent experiments planned once females become available.
Animals
Male F344BN rats (oral study N = 30; subcutaneous study N = 40) were obtained from the NIA colony at 24 months of age and individually housed on a 12-h light and 12-h dark cycle in a specific pathogen-free facility accredited by the American Association for Accreditation of Laboratory Animal Care. Animals were fed a standard rodent chow (18% kcal from fat, no sucrose, 3.1 kcal/g, diet 2018; Harlan Teklad, Madison, WI). Animals were allotted 1 week to acclimate to their housing conditions and to establish baseline rates of food intake and body weight. Health status, body weight, and food intake were monitored daily. Health assessments included checking for a sudden decline in body weight, redness around the eyes and nostrils, ruffled coat, open tail sores, and haunched posture.
Experimental design
Oral study
Animals (n = 6–8/group) were randomized at 24 months of age to the following treatment groups for 4 weeks: vehicle (trehalose, ascorbic acid, and skim milk) or Lactobacillus paracasei expressing Ang-1-7 (LP-A) 1×, 3×, or 7×/week as described (Carter et al. 2019). The concentration of the LP-A did not change, but rather the number of days/week the LP-A was delivered. On days when rats in the 1× and 3×/week group did not receive the LP-A, they were gavaged with vehicle to control for the stress of being handling and gavaged to a similar degree as the 7×/week group. Animals were weighed and their food intake measured daily to ensure there were no anorectic or other adverse effects of the drug administration.
Subcutaneous study
Animals (n = 10/group) were randomized at 24 months of age to the following treatment groups for 4 weeks: PBS vehicle or Ang(1–7) for 1×, 3×, or 7×/week. Again, the concentration of the Ang(1–7) did not change but rather the number of days/week the Ang(1–7) was delivered via subcutaneous injection. On days when rats in the 1× and 3×/week group did not receive the Ang(1–7), they were injected with PBS to control for the stress of being handling and injected to a similar degree as the 7×/week group. PBS group received injection 7×/week. Animals were weighed daily to ensure there were no anorectic or other adverse effects of the drug administration. One animal died of unknown causes at day 22 in the Ang(1–7) 3× group; therefore, this group had n = 9.
Probiotic administration
For the oral study, LP-A were cultured in MRS (deMan Rogosa Sharpe) broth (BD Difco, Houston, TX, USA) supplemented with 5 μg/ml erythromycin at 37 °C for 18 h. The bacteria were harvested by centrifugation at 5000×g for 20 min and resuspended in sterile PBS for oral gavage. For extended storage, harvested bacteria were washed once with PBS and then suspended in TAS buffer (4% trehalose, 4% sodium ascorbate, and 6% skim milk) and frozen in small aliquots at − 20 °C. Colony counting was conducted before the animal experiments to ensure the numbers of surviving bacteria. Animals were orally gavaged with 2 × 1011 CFU/kg body weight or equal volume of buffer. An 18-gauge gavage/feeding needle (3-in. length/3-mm ball diameter) was inserted into the esophagus, ensuring that there was no resistance to its advancement. The fluid was injected slowly and when complete, the needle was pulled straight out. The rats were tolerant of this procedure and no averse outcomes were observed over the 4-week study.
Subcutaneous Ang(1–7) administration
For the subcutaneous study, animals were subcutaneously injected with 1 mg/kg body weight of Ang(1–7) (TXA 127, Constant Therapeutics, Boston, MA) or an equal total injection volume of PBS, ranging between 1.8 and 2.2 ml. The rat was manually restrained and then placed on a solid surface. The skin of the back area was tented by the thumb and forefinger. A 23-gauge needle (BD, Franklin Lakes, NJ, USA) connected to a 3-ml syringe (BD, Franklin Lakes, NJ, USA) was inserted under the lifted skin. The fluid was injected slowly and when complete, the needle was pulled straight out. The rats were tolerant of this procedure and no averse outcomes were observed over the 4-week study.
Tissue collection and euthanasia
On day 8, blood was collected from the tongue of an anesthetized (5% isoflurane) rat that was grasped with tweezers, the sublingual vein punctured with a ¾-in., 26-gauge needle, and blood allowed to drip into a serum tube (BD, Franklin Lakes, NJ, USA). A cotton swab was used to stop bleeding. Rats recover, within 5 min, in their home cages with a warming disk placed underneath the cage. On day 29, rats were euthanized by rapid decapitation with a guillotine (Harvard Apparatus, Holliston, MA), and trunk blood was collected directly into a serum collection tube (BD, Franklin Lakes, NJ, USA). Both on day 8 and day 29, blood collected in the serum collection tube was left at room temperature for 30 min and then spun in a centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) at 4 °C at 2000 rpm for 10 min. Supernatant was collected after centrifugation and stored in − 80 °C until analysis.
At the time of euthanasia on day 29, brain (pre-frontal cortex) and intestinal (colon) tissues were also collected and immediately removed on ice, snap-frozen in liquid nitrogen, and stored at − 80 °C until analysis. Fecal samples were collected directly from the colon, stored in commercial available preservative Para-Pak (Meridian Bioscience Inc., Cincinnati, OH), flash frozen, and stored at − 80 °C until analysis.
Outcomes
Circulating RAS analytes
Circulating concentrations of RAS analytes Ang(1–7), Ang II, angiotensin-converting enzyme (ACE), and ACE2 were measured for the subcutaneous study using commercial assays (Ang[1–7], Ang II) and assays using fluorescent substrates (ACE, ACE2) as previously reported for the oral study (Carter et al. 2019).
Serum cytokines
Circulating concentrations (day 29) of interleukins (ILs) 4, 6, and 10 as well as interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) were determined using a Meso Scale Discovery (MSD; Rockville, MD) Rat Proinflammatory Panel 2 and a Quick Plex SQ 120 imager using electrochemiluminescence technology. Minimum sensitivity (pg/ml) and intra-assay coefficients of variation (%) for each assay were as follows: IL-4 (0.44, 9.12), IL-6 (25.10, 7.06), IL-10 (3.41, 5.65), IFNγ (6.88, 8.92), TNFα (1.17, 7.21).
Serum tryptophan metabolites
Serum concentrations of circulating metabolites reflective of the kynurenine, serotonin, and tryptamine/indole pathways of tryptophan metabolism were determined. Fifty microliters of serum was mixed with 25 ng/ml methanolic tryptophan-d5 internal standard, and 500 μl of acetonitrile 1.0% FA was added to Phree cartridges (Phenomenex, Torrance, CA) on a SPE vacuum manifold. Serum samples were forcefully expelled into acetonitrile contained in Phree cartridge to ensure proper mixing. The mixture was incubated at RT for 5 min. Vacuum was applied to draw the mixture through the sorbent into a borosilicate collection tube. Samples were dried under N2 gas and then reconstituted in 100 μl 0.1% FA.
The LC-MS method used was previously described by Zhu et al. with minor alterations (Zhu et al. 2011). Tryptophan-d5 was the only internal standard employed during this analysis. A shorter Atlantis T3 3 μm 100 × 2.1 mm column (Waters, Milford, MA) was used and a diversion valve was employed to divert column eluent to waste for the first minute of the gradient separation. MultiQuant 3.0.3 was employed for post-acquisition data analysis. All standard curves were linear with 1/x2 weighting.
Fecal microbiome taxonomy
Taxonomic analysis of the fecal microbiome (day 29) was performed via 16S-based polymerase chain reaction (PCR) procedures as previously described (Buford et al. 2018). Briefly, DNA was extracted from samples and PCR was used with unique bar-coded primers to amplify the V4 region of the 16S rRNA gene to create an “amplicon library” from individual samples as described by Kumar et al. (R. Kumar et al. 2014). The entire PCR reaction was electrophoresed, the PCR product was visualized by UV illumination, and the band was excised and purified from the agarose.
The PCR products were sequenced and paired-end reads of approximately 250 bp from the V4 region of 16S rDNA were analyzed. The samples were first quantitated using Pico Green, adjusted to a concentration of 4 nM then used for sequencing. Fastq conversion of the raw data files was performed following de-multiplexing, and quality control of the fastq files was performed which was then subject to quality assessment and filtering. One sample was removed from analysis due to failing quality control procedures.
Following quality control procedures, sequences were grouped into operational taxonomic units (OTUs) and taxonomic identification and abundance information was obtained. OTUs were then grouped together to summarize taxon abundance at different hierarchical levels of classification (e.g., phylum, class). Alpha diversity was calculated using Shannon’s diversity matrix and beta diversity was measured using unweighted Unifrac analysis. Principal coordinates analysis (PCoA) was performed to visualize the dissimilarity matrix between all samples.
mRNA expression—colon and pre-frontal cortex
Colon and pre-frontal cortex samples (striatal tissue dissected from a 3-mm coronal section) of brain tissue were collected, snap-frozen, and kept at − 80 °C until processing for evaluation of gene expression related to tryptophan metabolism (colon) or neuro-inflammation (pre-frontal cortex). Total RNA was isolated from with RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions or via Trizol (Invitrogen, Carlsbad, CA)/alcohol purification (Buford et al. 2009a; Buford et al. 2009b) with further purification via the RNeasy Plus kit (Qiagen). RNA concentration and purity was assessed photometrically using A260/A280 and A260/A230 ratios. RNA was reverse-transcribed to cDNA using the SuperScript VILO cDNA Synthesis kit (Invitrogen, Carlsbad, CA) or High Capacity cDNA Reverse Transcriptase kit (Applied Biosystems), according to manufacturer instructions. The cDNA was stored at − 20 °C until gene expression analysis. Gene expression analysis was performed using TaqMan Fast Advanced Master Mix (Applied Biosystems, Carlsbad, CA) following the manufacturer’s instructions using the StepOne Real-Time PCR System (Applied Biosystems) or via the SYBRgreen master mix using the Rotor-Gene Q PCR (Qiagen). The threshold cycle (Ct) values were determined and analyzed via respective manufacturer software. All samples were examined in triplicate, and genes of interest (Table 1) were expressed relative to the housekeeping gene (β-actin) as fold change from the reference group by the 2−ΔΔCt method. The housekeeping gene expression was not different among groups (data not shown).
Table 1.
Primers for mRNA expression analyses
| Gene | Primer |
|---|---|
| Housekeeping | |
| Actb (beta-actin) | Rn00667869_m1 |
| Pre-frontal cortex | |
| Il1a | Rn00566700_m1 |
| Il1b | Rn00580432_m1 |
| Il6 | Rn01410330_m1 |
| Ptgs2 (COX2) | Rn01483828_m1 |
| LOC103694380 (TNFα) | Rn99999017_m1 |
| Colon | |
| Ccbl1 (KAT1) | Rn01439192_m1 |
| Aadat (KAT2) | Rn00567882_m1 |
| Ccbl2 (KAT3) | Rn01522586_m1 |
| Got2 (KAT4) | Rn00820736_g1 |
| Naprt1 | Rn01506019_g1 |
| Qprt | Rn01506918_g1 |
| Haao (3-HAO) | Rn01469327_m1 |
All primers obtained from Applied Biosystems (Carlsbad, CA)
Statistics
All data were evaluated for normality and homogeneity of variance prior to determination of descriptive statistics and comparative analyses. When assumptions were met, dependent outcomes other than the 16S microbiome analysis were analyzed using univariate analysis of variance (ANOVA) with pre-planned contrasts comparing each dosing condition (1×, 3×, and 7×) with the 0×/week “control” condition. When assumptions were violated (e.g., non-normal distributions), non-parametric tests were performed. A p value < 0.05 was considered statistically significant. For microbiome data, measures of α-diversity were evaluated as described for other outcomes, while β-diversity differences were determined via PERMANOVA. Differences between groups were evaluated at each level of phylogeny for OTUs with ≥ 0.5% abundance. A p value of < 0.05 was utilized to initially identify OTUs of interest with final determination of significance established after correcting for false discovery rate (FDR) according to the method of Benjamini and Hochberg (1995).
Results
Circulating RAS analytes–subcutaneous administration
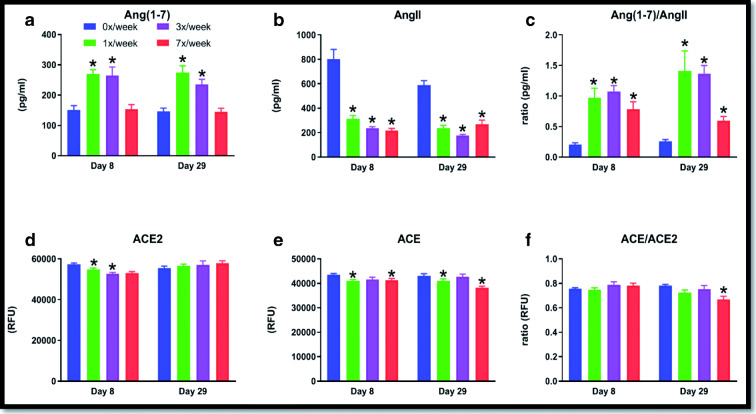
Subcutaneous administration of Ang(1–7) in varying dosing frequencies altered systemic concentrations of various RAS analytes (Fig. 1).
Fig. 1.
Circulating RAS analytes were measured in 24-month-old male F344/BN rats after 8 days (acute) and 29 days (chronic) administration of 1 mg/kg of subcutaneous Ang(1–7) in 4 dosing conditions: 0×, 1×, 3×, or 7×/week. The graphs describe results for (A) Ang(1–7); (B) AngII; (C) Ang(1–7)/Ang (II) ratio; (D) ACE2; (E) ACE; and (F) ACE2/ACE ratio. Data are presented as mean ± SD. Asterisks denote p < 0.05 from the 0×/week condition at respective time point
We have previously reported similar changes in the oral study, including increases after 8 days of delivery that persisted after 29 days of delivery (Carter et al. 2019). Most notably, both the 1×/week (p = 0.001) and 3×/week (p = 0.001) dosing conditions in the systemic study increased circulating Ang(1–7) at day 29 compared with the control condition, while the Ang(1–7)/AngII ratio was increased in all three dosing conditions compared with control (all p’s ≤ 0.001 at 29).
Serum cytokines
Circulating cytokine concentrations at day 29 of the oral LP-A delivery experiment are shown in Fig. 2. Modest, but statistically significant differences from the 0×/week dosing group were observed for IL-6 in the 7×/week group (p = 0.026) and for TNFα in the 1×/week (p = 0.037) and 7×/week (p = 0.037) dosing groups. No significant differences were observed for these circulating cytokines in the subcutaneous delivery experiment (Supplemental Fig. 1).
Fig. 2.
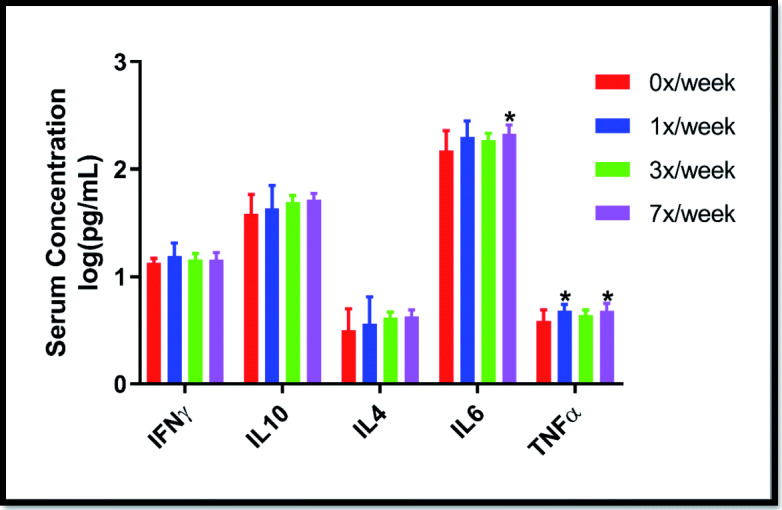
Serum cytokine concentrations among 24-month-old F344/BN rats following 4 weeks of supplementation with oral Ang(1–7) expressing Lactobacillus paracasei either 0×, 1×, 3×, or 7×/week. Data are presented as mean ± SD. *p < 0.05 from the 0×/week condition
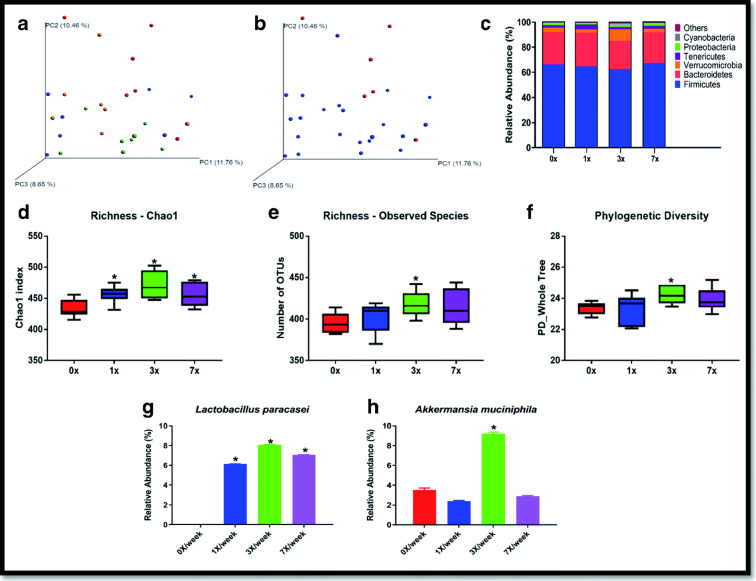
Fecal microbiome analyses
Oral LP-A delivery induced several changes in the fecal microbiome of the rats, particularly in the 3×/week group (Fig. 3). Principal coordinates analysis (PCoA) revealed that groups differed in the overall microbiome community structure as determined by unweighted UniFrac (p = 0.004) (Fig. 3A), with the most striking difference observed when comparing control (0×/week) with experimental conditions (Fig. 3B). Figure 3 C depicts the overall composition of the fecal microbiomes at the phylum level of taxonomy across dosing groups. Across all groups, the composition of the phylum-level fecal microbiome was 64.9% Firmicutes, 25.1% Bacteroidetes, 4.5% Verrucomicrobia, 2.5% Tenericutes, 1.3% Proteobacteria, 1% Cyanobacteria, and 0.7% from remaining phyla. Key measures of α-diversity, including richness (chao1 and observed species) and phylogenetic diversity, displayed significant group differences—with the most dramatic effects observed in the 3×/week group (Fig. 3D, E, F). Overall sample diversity, measured according to the Shannon and Simpson metrics, did not significantly differ among dosing regimens.
Fig. 3.
Key indices of microbiome composition in response to oral LP-A delivery measured at day 29. (A) Comparison of fecal microbiome β-diversity (unweighted UniFrac) by experimental group (0×, 1×, 3×, 7×/week) and (B) by experimental (1×, 3×, 7×/week) vs. control (0×/week). (C) Taxonomic distribution of fecal microbiome of experimental groups at the phylum level. (D, E, F) Comparison of α-diversity of the fecal microbiome among experimental groups including (D) chao1, (E) observed species, and (F) phylogenetic diversity. (G, H) Species-level fecal microbial DNA populations differentially expressed between groups including (G) Lactobacillus paracasei and (H) Akkermansia muciniphila. Asterisks indicate statistically significant difference from the 0×/week dosing group after correcting for multiple comparisons via false discovery rate
The phyla Verrucomicrobia was significantly enriched by the 3×/week dosing regimen (p = 0.008). The 1×/week dosing group had an initial p value < 0.05 for a decline in Proteobacteria (p = 0.042) compared with the 0×/week regimen, but this value did not remain significant after FDR correction. At the species level, two bacteria displayed differences in DNA abundance among treatment groups. The bacteria species used for LP-A, Lactobacillus paracasei, was significantly enriched among all treatment groups (Fig. 3G) and Akkermansia muciniphila (Verrucomicrobia) was enriched in the 3×/week treatment group.
For the subcutaneous study, there were no statistical differences at any level of taxonomy following correction for multiple comparisons (Supplemental Figure 2). Indices of beta and alpha diversity were also not significant, though a trend toward significance was observed for Simpson diversity (p = 0.068) for the 7×/week delivery (0.85 ± 0.12) compared with the control condition (0.910 ± 0.05).
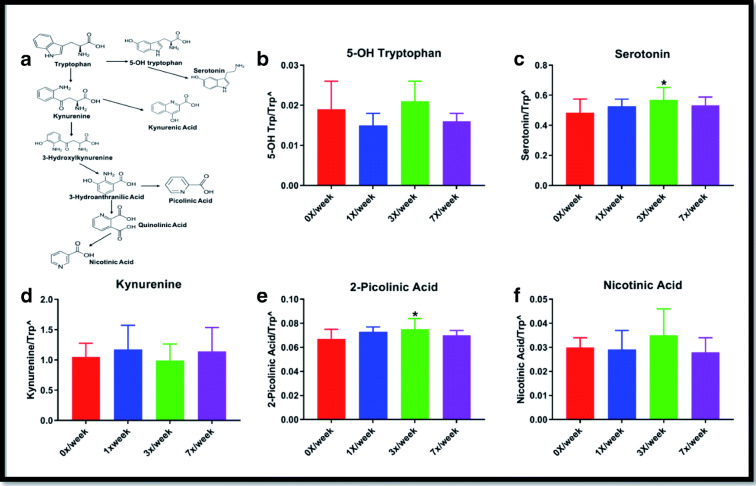
Serum tryptophan metabolism
Changes observed in the oral study for circulating concentrations of serotonin and kynurenine-related tryptophan metabolites are shown in Fig. 4. Statistically significant differences from the 0×/week group were observed in the 3×/week cohort for both serotonin (p = 0.034) and 2-picolinic acid (p = 0.045). No significant differences were observed for tryptamine, indole-3 lactate, or indole-3 acetate (not shown).
Fig. 4.
Serum concentrations of downstream metabolites of tryptophan metabolism genes following 29 days of oral Ang(1–7) expressing Lactobacillus paracasei delivery. (A) Simplified schematic of serotonin and kynurenine pathways of tryptophan metabolism and (B) serum 5-OH tryptophan, (C) serotonin, (D) kynurenine, (E) 2-picolinic acid, and (F) nicotinic acid. Carets indicate data expressed as metabolite concentration (mean ± SD) relative to total tryptophan normalized via the square root function (due to non-normally distribution of raw data). Asterisks indicate a significant difference from the 0×/week condition
In the subcutaneous study, significant differences were observed for serum kynurenine, with concentrations (normalized as ratio to total tryptophan) increased in the 1× (0.70 ± 0.10; p = 0.009) and 7×/week (0.67 ± 0.15; 0.034) condition compared with the 0×/week control (0.56 ± 0.11) (Supplemental Fig. 3). Serum serotonin was also significantly increased in the 7×/week condition (0.22 ± 0.15 vs. 0.20 ± 0.16 in 0×/week; p = 0.035). No other statistically significant differences were observed in the subcutaneous study.
Colon tryptophan-related mRNA expression
In the oral study, we observed significantly lower mRNA expression of quinolinate phosphoribosyltransferase (QPRT; p = 0.040) in the 1×/week condition (2−ΔΔCT = 0.17 ± 0.20) compared with 0×/week (1.00 ± 0.66). We also observed significantly greater expression of AADAT (kynurenine aminotransferase II) in the 1× (p = 0.046), and 3×/week conditions (p = 0.037) and a trend toward significance (p = 0.053) for the 7×/week group (Fig. 5). No statistically significant differences were observed for any of the target mRNA for the subcutaneous study.
Fig. 5.
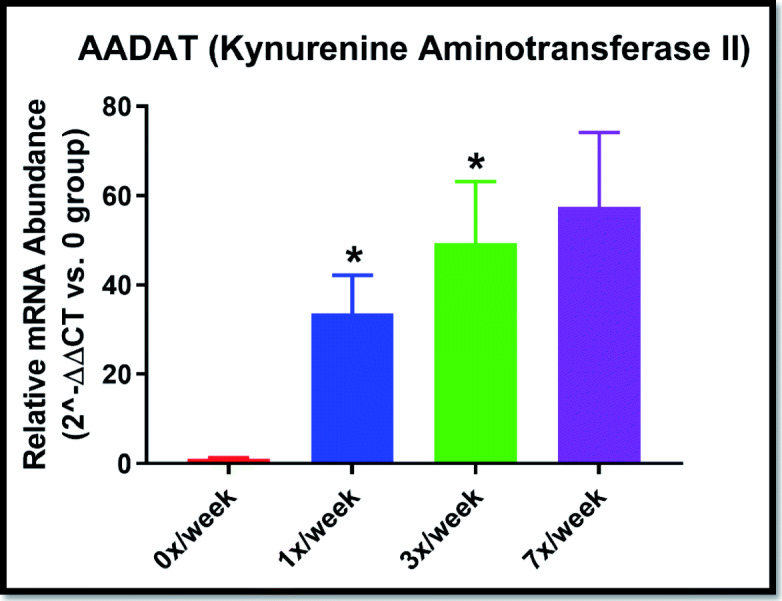
Colon mRNA expression of AADAT (kynurenine aminotransferase II) gene following 29 days of oral Ang(1–7) expressing Lactobacillus paracasei delivery. Data expressed as relative expression (2^−ΔΔ CT) vs. the 0×/week group (mean ± SEM). *p < 0.05; p = 0.053 for the 7×/week group
Pre-frontal cortex inflammatory gene expression
Statistically significant differences in expression of inflammation-related cytokines were observed in the pre-frontal cortex in the oral study (Fig. 6). The 1×/week regimen significantly reduced COX2 expression (p = 0.003), 3×/week reduced COX2 (p = 0.003), IL-1β (p = 0.040), and TNFα (p = 0.013) expression, and 7×/week reduced COX2 (p = 0.003) and IL-1β (p = 0.040) expression. No statistically significant differences were observed in response to subcutaneous delivery of Ang(1–7) (Supplemental Fig. 4).
Fig. 6.
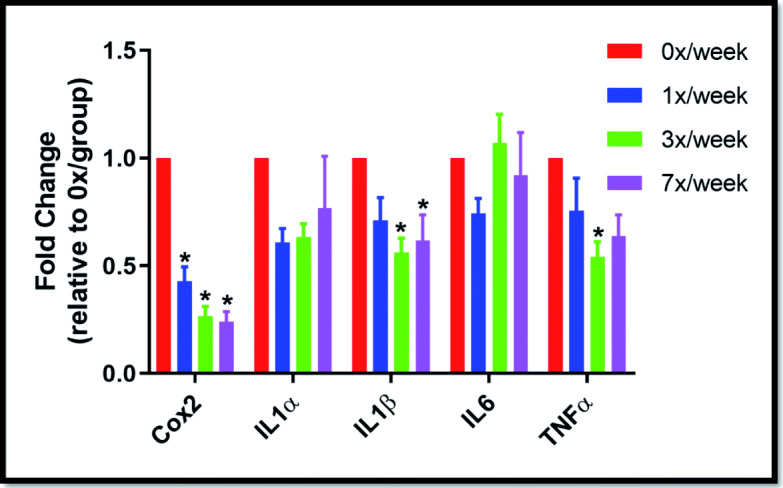
Pre-frontal cortex mRNA expression of inflammation-related genes following 29 days of oral Ang(1–7) expressing Lactobacillus paracasei delivery. Data expressed as relative expression (2−ΔΔ CT) vs. the 0×/week group (mean ± SEM). *p < 0.05
Discussion
This manuscript outlined findings from two 4-week dosing studies designed to identify any dose-dependent effects of oral (in form of a genetically modified probiotic) and subcutaneous administration of angiotensin (1–7) and to explore potential differences in indices of gut-brain axis physiology between the two routes of administration. Data from these experiments will be used to inform procedures regarding the optimal delivery method and dose for more comprehensive, longer-term studies in this area. To our knowledge, this is the first study to show potential benefits of a genetically modified probiotic on the gut-brain axis in older animals.
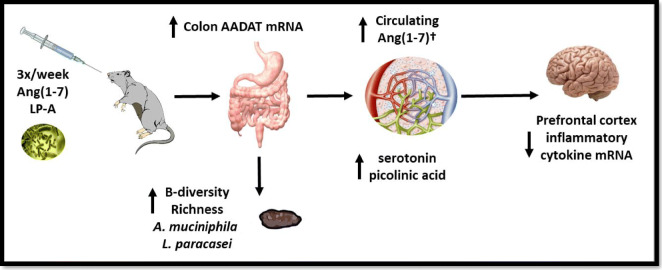
In summary, oral delivery of Ang(1–7) expressing Lactobacillus paracasei (LP-A) demonstrated significant alterations in the gut microbiome with concomitant increases in beneficial circulating neurotransmitters and decreased neuro-inflammatory gene expression in the pre-frontal cortex. Notably, these changes were most robust in the 3×/week dosing regimen (Fig. 7)—in line with our prior findings on the influence of LP-A on circulating Ang(1–7) (Carter et al. 2019). In contrast, despite significantly increasing circulating Ang(1–7) and related analytes, subcutaneous administration of Ang(1–7) resulted in few changes in gut-brain axis parameters.
Fig. 7.
Summary of changes observed with 3x/week dosing of oral Ang(1–7) expressing Lactobacillus paracasei (LP-A) delivery. The dagger indicates changes in circulating Ang(1–7) were published previously (Carter et al. 2019)
A primary premise for the use of LP-A is the concomitant actions of both a probiotic bacteria and Ang(1–7)—each which has independent effects on the central nervous system. Indeed, others have purported the potential benefits and therapeutic applications of genetically modified probiotics (GMPs), particularly those from lactic acid bacteria (LeBlanc et al. 2013; Bermudez-Humaran et al. 2013; Cano-Garrido et al. 2015; Plavec and Berlec 2019; Borner et al. 2019). Major benefits of this approach to drug delivery include (1) typical inherent benefits of the bacteria itself, (2) ease of production, (3) ability for oral administration due to the ability of the bacteria to survive gut digestive processes, and (4) ability to influence both systemic and mucosal immune responses. Thus, GMPs are considered a major opportunity in biotherapeutic development (Bron and Kleerebezem 2018; Mays and Nair 2018). Interestingly, the 3×/week delivery of LP-A induced significant increases in fecal expression of Akkermansia muciniphila, an anti-inflammatory bacterial species known to improve gut barrier integrity and associated with reduced prevalence of several age- and inflammation-related conditions (Everard et al. 2013; Reunanen et al. 2015; Schneeberger et al. 2015; Dao et al. 2016; Fransen et al. 2017; Plovier et al. 2017; Hanninen et al. 2018; Chelakkot et al. 2018; Grander et al. 2018; Anhe et al. 2019). Given the consistently beneficial outcomes ascribed to increases in this bacterial species, our finding of an increase through LP-A administration may represent one mechanism involved in the observed decrease in neuro-inflammatory gene expression—though further follow-up is needed to confirm.
Components of the Ang(1–7) axis are found throughout the central nervous system (CNS) including within neurons, astrocytes, cerebral arteries, and various brain regulatory centers (Young et al. 1988; Chappell et al. 1989; Hamming et al. 2004; Gallagher et al. 2006; Doobay et al. 2007; Becker et al. 2007; Guo et al. 2010). Notably, Ang(1–7) stimulates numerous molecular pathways responsible for beneficial actions which could contribute to improved cognitive function including increased (1) endothelial nitric oxide synthase (eNOS), (2) brain-derived neurotrophic factor (BDNF), and (3) vascular endothelial growth factor (VEGF) as via reducing oxidative stress and inflammation (Passos-Silva et al. 2013; Zheng et al. 2014; Jiang et al. 2014; X. L. Wang et al. 2016a; Kamel et al. 2018). Similarly, probiotics are thought to influence the CNS via modulation of the gut microbiota which can communicate with the CNS in several different ways including (1) release of pro-inflammatory cytokines to activate the hypothalamic-pituitary-adrenal (HPA) axis or directly impact CNS immune activity, (2) production of short-chain fatty acids, (3) release of neurotransmitters, or (4) by modulating tryptophan metabolism and downstream metabolites (Sharon et al. 2016; Hampton 2017; Kennedy et al. 2017; Strandwitz 2018; Fulling et al. 2019).
Interestingly, components of the Ang(1–7) axis are also present in significant amounts within the gut including the small intestinal brush border and muscularis mucosa and propria, as well as microvascular endothelium and vascular smooth muscle cells (Hamming et al. 2004). In fact, the highest tissue concentrations of mRNA for ACE2—the enzyme responsible for producing Ang(1–7) endogenously—are found in the terminal ileum, duodenum, and colon (Tipnis et al. 2000; Harmer et al. 2002). ACE2 is also essential to intestinal absorption of tryptophan (Hashimoto et al. 2012; Singer et al. 2012) as well as in regulating intestinal immune function, ecology of the gut microbiota, and attenuating intestinal inflammation suffered in response to epithelial damage (Kowalczuk et al. 2008; Camargo et al. 2009; Singer et al. 2012; Hashimoto et al. 2012). Thus, both the Lactobacillus strain and the Ang(1–7) in our GMP hold promise for altering these neuro-regulatory pathways.
The changes in the systemic neurotransmitters related to tryptophan metabolism are one particularly relevant link between LP-A and the gut-brain axis. The vast majority (~ 95%) tryptophan is absorbed in the intestines for transport to the liver and release into the circulation. Once released into the circulation, a minority of this tryptophan is broken down to support protein synthesis and the production of serotonin while the vast majority is metabolized to the neuro-regulatory kynurenine pathway. This pathway is proposed as a key link between gut dysbiosis and various neurologic conditions including AD, with elevated kynurenine proposed as a risk factor for neurodegeneration while picolinic acid—which was increased in response to 3×/week LP-A administration—proposed as a neuroprotective agent (Widner et al. 2000; Gulaj et al. 2010; Agudelo et al. 2014; Curto et al. 2015; Parrott et al. 2016; Lovelace et al. 2017). Thus, this pathway may contribute to the reductions in neuro-inflammatory gene expression, though further work remains to confirm this hypothesis.
An interesting finding from these studies is that both oral and subcutaneous delivery methods increased circulating Ang(1–7) concentrations, but only the oral method induced major physiologic changes in the indices of the gut-brain axis evaluated. Notably, this outcome should not be taken to limit the potential utility of subcutaneous administration on overall CNS function or cognition as other evidence indicates that Ang(1–7) axis modulation benefits multiple aspects of cognition in several models of cognitive dysfunction (Xie et al. 2014; Lazaroni et al. 2016; L. Wang et al. 2016b; Kangussu et al. 2017; Hay et al. 2017; Hay et al. 2019). Thus, it is likely that subcutaneous administration had other effects on CNS function which we did not measure here. However, subcutaneous delivery of Ang(1–7) requires burdensome infusions and/or repeated injections as well as the expensive and cumbersome production process for the drug. Moreover, unfavorable pharmacologic properties (i.e., extreme short half-life and rapid clearance in circulation and tissues) may limit its clinical applications. Thus, we were interested in finding a delivery method which could influence CNS function while delivered orally. Thus, the utility of LP-A may be in specifically modulating gut-mediated pathways of CNS function.
One lingering question from this study is whether the impact of LP-A was due solely to the bacteria itself or due to the combination. Thus, subsequent studies are still needed to compare the effects of LP-A with unmodified Lactobacillus paracasei. Moreover, given that a primary objective of these studies was to inform regarding dosing and delivery for larger-scale studies, there remains much to learn beyond these studies. For instance, the LP-A compound should be evaluated for a longer time period with behavioral measurements and more comprehensive physiology to more fully identify therapeutic potential. Secondly, studies will need to be performed with both sexes as differences between males and females have been observed in both circulating and tissue levels Ang(1–7) (Pendergrass et al. 2008; Sullivan et al. 2010). Third, evaluation of the impact of potential effect modulators such as diet and exercise may hold critical importance for ultimately translating to humans. Finally, studies in more formal models of Alzheimer’s disease or other dementias may also hold meaningful insight for ultimately aiding in therapeutic development.
In summary, administration of LP-A—but not subcutaneous—Ang(1–7) to 24-month-old F344/BN rats altered several physiologic parameters relevant to gut-brain communication. Strongest effects were observed with 3×/week oral dosing; thus, we will utilize this dosing regimen for subsequent studies in this area.
Electronic supplementary material
(DOCX 14 kb)
(PNG 74 kb)
(PNG 382 kb)
(PNG 382 kb)
(PNG 77 kb)
Acknowledgments
We would like to thank Brian Bouverat and Liana Juarez for assistance with specific technical aspects of the projects. We would also like to thank Richard Franklin, MD, PhD of Constant Therapeutics for the donation of TXA 127 (Ang[1-7]) for the study involving subcutaneous administration of Ang(1-7).
Funding information
This work was funded by a grant from the National Institutes of Health/National Institute on Aging (R01AG054538). The work was also supported by the following: The UAB Nathan Shock Center (P30AG50886), Microbiome Resource (supported by NIH grants P30CA013148 and UL1TR001417), Metabolism Core Laboratory (supported by P30DK56336, P30DK079626, and U4TR001368), and O’Brien Center Bioanalytical Core (supported by P30DK079337).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thomas W. Buford and Yi Sun contributed equally to this work.
Contributor Information
Thomas W. Buford, Email: twbuford@uabmc.edu
Christy S. Carter, Email: cartercs@uabmc.edu
References
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Prasad J, Gill H, Stevenson L, Gopal P. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J Nutr Health Aging. 2007;11:26–31. [PubMed] [Google Scholar]
- Anhe FF, Nachbar RT, Varin TV, Trottier J, Dudonne S, Le Barz M, et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut. 2019;68(3):453–64. 10.1136/gutjnl-2017-315565. [DOI] [PubMed]
- Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol. 2007;293:H1416–H1424. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc:289-290-300.
- Bermudez-Humaran LG, Aubry C, Motta JP, Deraison C, Steidler L, Vergnolle N, Chatel JM, Langella P. Engineering lactococci and lactobacilli for human health. Curr Opin Microbiol. 2013;16:278–283. doi: 10.1016/j.mib.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner RA, Kandasamy V, Axelsen AM, Nielsen AT, Bosma EF. Genome editing of lactic acid bacteria: opportunities for food, feed, pharma and biotech. FEMS Microbiol Lett. 2019;366. 10.1093/femsle/fny291. [DOI] [PMC free article] [PubMed]
- Bron PA, Kleerebezem M. Lactic acid bacteria for delivery of endogenous or engineered therapeutic molecules. Front Microbiol. 2018;9:1821. doi: 10.3389/fmicb.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab. 2009;34:745–753. doi: 10.1139/h09-067. [DOI] [PubMed] [Google Scholar]
- Buford TW, Cooke MB, Willoughby DS. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physiol. 2009;107:463–471. doi: 10.1007/s00421-009-1145-z. [DOI] [PubMed] [Google Scholar]
- Buford TW, Carter CS, VanDerPol WJ, Chen D, Lefkowitz EJ, Eipers P, Morrow CD, Bamman MM. Composition and richness of the serum microbiome differ by age and link to systemic inflammation. Geroscience. 2018;40:257–268. doi: 10.1007/s11357-018-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA, Kuba K, Danilczyk U, Skovby F, Kleta R, et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitos E. Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb Cell Fact. 2015;14. 10.1186/s12934-015-0313-6. [DOI] [PMC free article] [PubMed]
- Carter CS, Morgan D, Verma A, Lobaton G, Aquino V, Sumners E, et al. Therapeutic delivery of Ang(1-7) via genetically modified probiotic: a dosing study. J Gerontol A Biol Sci Med Sci. 2019;glz222. 10.1093/gerona/glz222. [DOI] [PMC free article] [PubMed]
- Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB, INDIA-FBP Group Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–16523. [PubMed] [Google Scholar]
- Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Curto M, Lionetto L, Negro A, Capi M, Perugino F, Fazio F, et al. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J Headache Pain. 2015;17. 10.1186/s10194-016-0620-2. [DOI] [PMC free article] [PubMed]
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. doi: 10.3389/fimmu.2017.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101:998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- Gulaj E, Pawlak K, Bien B, Pawlak D. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv Med Sci. 2010;55:204–211. doi: 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- Guo F, Liu B, Tang F, Lane S, Souslova EA, Chudakov DM, Paton JF, Kasparov S. Astroglia are a possible cellular substrate of angiotensin(1-7) effects in the rostral ventrolateral medulla. Cardiovasc Res. 2010;87:578–584. doi: 10.1093/cvr/cvq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton T. Organoids reveal clues to gut-brain communication. JAMA. 2017;318:787–788. doi: 10.1001/jama.2017.11545. [DOI] [PubMed] [Google Scholar]
- Hanninen A, Toivonen R, Poysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67:1445–1453. doi: 10.1136/gutjnl-2017-314508. [DOI] [PubMed] [Google Scholar]
- Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/S0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Vanderah TW, Samareh-Jahani F, Constantopoulos E, Uprety AR, Barnes CA, Konhilas J. Cognitive impairment in heart failure: a protective role for angiotensin-(1-7) Behav Neurosci. 2017;131:99–114. doi: 10.1037/bne0000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Polt R, Heien ML, Vanderah TW, Largent-Milnes TM, Rodgers K, Falk T, Bartlett MJ, Doyle KP, Konhilas JP. A novel angiotensin-(1-7) glycosylated Mas receptor agonist for treating vascular cognitive impairment and inflammation-related memory dysfunction. J Pharmacol Exp Ther. 2019;369:9–25. doi: 10.1124/jpet.118.254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10:170–182. doi: 10.1038/ismej.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Lu J, Gao Q, Zhang YD, et al. Angiotensin-(1-7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br J Pharmacol. 2014;171:4222–4232. doi: 10.1111/bph.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Tan L, Gao Q, Lu H, Zhu XC, Zhou JS, Zhang YD. Plasma angiotensin-(1-7) is a potential biomarker for Alzheimer’s disease. Curr Neurovasc Res. 2016;13:96–99. doi: 10.2174/1567202613666160224124739. [DOI] [PubMed] [Google Scholar]
- Jiang T, Zhang YD, Zhou JS, Zhu XC, Tian YY, Zhao HD, Lu H, Gao Q, Tan L, Yu JT. Angiotensin-(1-7) is reduced and inversely correlates with tau hyperphosphorylation in animal models of Alzheimer’s disease. Mol Neurobiol. 2016;53:2489–2497. doi: 10.1007/s12035-015-9260-9. [DOI] [PubMed] [Google Scholar]
- Kamel AS, Abdelkader NF, Abd El-Rahman SS, Emara M, Zaki HF, Khattab MM. Stimulation of ACE2/ANG(1-7)/Mas axis by diminazene ameliorates Alzheimer’s disease in the D-galactose-ovariectomized rat model: role of PI3K/Akt pathway. Mol Neurobiol. 2018;55:8188–8202. doi: 10.1007/s12035-018-0966-3. [DOI] [PubMed] [Google Scholar]
- Kangussu LM, Almeida-Santos AF, Moreira FA, Fontes MAP, Santos RAS, Aguiar DC, Campagnole-Santos MJ. Reduced anxiety-like behavior in transgenic rats with chronically overproduction of angiotensin-(1-7): role of the Mas receptor. Behav Brain Res. 2017;331:193–198. doi: 10.1016/j.bbr.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-beta and tau pathology. Alzheimers Res Ther. 2016;8. 10.1186/s13195-016-0217-7. [DOI] [PMC free article] [PubMed]
- Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112:399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Kowalczuk S, Broer A, Tietze N, Vanslambrouck JM, Rasko JE, Broer S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J. 2008;22:2880–2887. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- Kumar R, Eipers P, Little RB, Crowley M, Crossman DK, Lefkowitz EJ, Morrow CD. Getting started with microbiome analysis: sample acquisition to bioinformatics. Curr Protoc Hum Genet. 2014;82:18.8.1–18.829. doi: 10.1002/0471142905.hg1808s82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Yadav AK, Verma V, Singh B, Mal G, Nagpal R, Hemalatha R. Bioengineered probiotics as a new hope for health and diseases: an overview of potential and prospects. Future Microbiol. 2016;11:585–600. doi: 10.2217/fmb.16.4. [DOI] [PubMed] [Google Scholar]
- Lawrence K, Hyde J. Microbiome restoration diet improves digestion, cognition and physical and emotional wellbeing. PLoS One. 2017;12:e0179017. doi: 10.1371/journal.pone.0179017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lazaroni TL, Bastos CP, Moraes MF, Santos RS, Pereira GS. Angiotensin-(1-7)/Mas axis modulates fear memory and extinction in mice. Neurobiol Learn Mem. 2016;127:27–33. doi: 10.1016/j.nlm.2015.11.012. [DOI] [PubMed] [Google Scholar]
- LeBlanc JG, Aubry C, Cortes-Perez NG, de Moreno de LeBlanc A, Vergnolle N, Langella P, Azevedo V, Chatel JM, Miyoshi A, Bermudez-Humaran LG. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: an update. FEMS Microbiol Lett. 2013;344:1–9. doi: 10.1111/1574-6968.12159. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gibson GR, Walton GE. An in vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS One. 2016;11:e0162604. doi: 10.1371/journal.pone.0162604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K, Guillemin GJ, Brew BJ. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373–388. doi: 10.1016/j.neuropharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Mays ZJ, Nair NU. Synthetic biology in probiotic lactic acid bacteria: at the frontier of living therapeutics. Curr Opin Biotechnol. 2018;53:224–231. doi: 10.1016/j.copbio.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro-Garcia MA, Alonso-Arias R, Baltadjieva M, Fernandez Benitez C, Fernandez Barrial MA, Diaz Ruisanchez E, Alonso Santos R, Alvarez Sanchez M, Saavedra Mijan J, Lopez-Larrea C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age (Dordr) 2013;35:1311–1326. doi: 10.1007/s11357-012-9434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6:e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci (Lond) 2013;124:443–456. doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- Paton AW, Morona R, Paton JC. Bioengineered microbes in disease therapy. Trends Mol Med. 2012;18:417–425. doi: 10.1016/j.molmed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–H20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavec TV, Berlec A. Engineering of lactic acid bacteria for delivery of therapeutic proteins and peptides. Appl Microbiol Biotechnol. 2019;103:2053–2066. doi: 10.1007/s00253-019-09628-y. [DOI] [PubMed] [Google Scholar]
- Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- Rampelli S, Candela M, Severgnini M, Biagi E, Turroni S, Roselli M, Carnevali P, Donini L, Brigidi P. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J Nutr Health Aging. 2013;17:166–172. doi: 10.1007/s12603-012-0372-x. [DOI] [PubMed] [Google Scholar]
- Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D, Camargo SM, Ramadan T, Schafer M, Mariotta L, Herzog B, Huggel K, Wolfer D, Werner S, Penninger JM, et al. Defective intestinal amino acid absorption in Ace2 null mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G686–G695. doi: 10.1152/ajpgi.00140.2012. [DOI] [PubMed] [Google Scholar]
- Steidler L. Genetically engineered probiotics. Best Pract Res Clin Gastroenterol. 2003;17:861–876. doi: 10.1016/S1521-6918(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1-7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension. 2010;56:658–666. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvanen M. Churning out safer microbes for drug delivery. Nat Biotechnol. 2003;21:758–759. doi: 10.1038/nbt0703-758. [DOI] [PubMed] [Google Scholar]
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, Ludin JA, Oh SP, Katovich MJ, Frazier CJ, et al. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology. 2016;105:114–123. doi: 10.1016/j.neuropharm.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Iwanami J, Min LJ, Tsukuda K, Nakoka H, Bai HY, Shan BS, Kan-no H, Kukida M, Chisaka T et al (2016b) Deficiency of angiotensin-converting enzyme 2 causes deterioration of cognitive function. NPJ Aging Mech Dis 2. doi: 10.1038/npjamd.2016.24. [DOI] [PMC free article] [PubMed]
- Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm (Vienna) 2000;107:343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- Xie W, Zhu D, Ji L, Tian M, Xu C, Shi J. Angiotensin-(1-7) improves cognitive function in rats with chronic cerebral hypoperfusion. Brain Res. 2014;1573:44–53. doi: 10.1016/j.brainres.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Young D, O’Neill K, Jessell T, Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci U S A. 1988;85:5339–5342. doi: 10.1073/pnas.85.14.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Li G, Chen S, Bihl J, Buck J, Zhu Y, Xia H, Lazartigues E, Chen Y, Olson JE. Activation of the ACE2/Ang-(1-7)/Mas pathway reduces oxygen-glucose deprivation-induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience. 2014;273:39–51. doi: 10.1016/j.neuroscience.2014.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Stevens AP, Dettmer K, Gottfried E, Hoves S, Kreutz M, Holler E, Canelas AB, Kema I, Oefner PJ. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:3249–3261. doi: 10.1007/s00216-011-5436-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)
(PNG 74 kb)
(PNG 382 kb)
(PNG 382 kb)
(PNG 77 kb)