Abstract
Sleep maintains the function of the entire body through homeostasis. Chronic sleep deprivation (CSD) is a prime health concern in the modern world. Previous reports have shown that CSD has profound negative effects on brain vasculature at both the cellular and molecular levels, and that this is a major cause of cognitive dysfunction and early vascular ageing. However, correlations among sleep deprivation (SD), brain vascular changes and ageing have barely been looked into. This review attempts to correlate the alterations in the levels of major neurotransmitters (acetylcholine, adrenaline, GABA and glutamate) and signalling molecules (Sirt1, PGC1α, FOXO, P66shc, PARP1) in SD and changes in brain vasculature, cognitive dysfunction and early ageing. It also aims to connect SD-induced loss in the number of dendritic spines and their effects on alterations in synaptic plasticity, cognitive disabilities and early vascular ageing based on data available in scientific literature. To the best of our knowledge, this is the first article providing a pathophysiological basis to link SD to brain vascular ageing.
Keywords: Vascular ageing, Sleep deprivation, Cognition, Synaptic plasticity, Neurochemicals
Sleep in evolution
Sleep is the “reversible behavioural state of perceptual disengagement from, and unresponsiveness to, the environment” (Carskadon and Dement 2005). It involves an interplay of physiological factors and behavioural processes. Great apes by virtue of their inherent skills built sleeping platform which not only helped them to escape from predators (about 18 million years ago) but also provided deep and long hour of sleep. The improved quality of sleep is linked to enhanced cognition and refined engineering skills of the great apes (Nunn et al. 2016). In animals, sleep also serves its functions outside the nervous system, as it can influence nonneural tissues without the involvement of the cephalic nervous system (Anafi et al. 2019). Sleep has also been reported as a means of energy conservation, growth and repair, and increasing efficiency of metabolic processes (Schmidt et al. 2017). Interestingly, humans are the shortest sleeping primates and show flexibility in sleep based on behavioural, psychological, social, and environmental factors (Grandner 2017). For example, nonindustrial indigenous populations show less variability in sleep patterns compared with postindustrial societies (Capellini et al. 2008). Sociocultural, technological, and lifestyle trends are the major factors contributing to disturbed sleep patterns in postindustrial societies. These changes in behaviour are the causative factors leading to an imbalance between the endogenous circadian rhythms and light/dark cycle, which affects sleep duration and quality.
Normal human sleep is comprised of two stages/states: rapid eye movement (REM) sleep and nonrapid eye movement (NREM) sleep. The regular sleep pattern typically begins with the first stage of NREM, progressing to the deeper stages of NREM (stages 2, 3, and 4), followed by an episode of REM sleep. The cycle of NREM and REM continues with each cycle lasting for about 90 min. NREM sleep is characterised by low voltage signals with high amplitude brain waves, whereas REM sleep exhibits high voltage but low amplitude brain waves (Edwards et al. 2010; Siegel 2017). REM sleep is more prone to the risk of predation, and hence mammals living in dangerous environments show less REM sleep (Hartse 2017; Lesku et al. 2006). The quantitative characteristics of human sleep, such as the total sleep and the percentage of REM sleep, have clearly evolved with time along the human lineage (Nunn et al. 2016).
Sleep deprivation, sleep restriction, and sleep disruption
Sleep deprivation (SD), which refers to an insufficient amount of sleep, can be acute or chronic based on the time interval of sleep elimination. Sleep restriction SR is a reduction in sleep time below an individual’s baseline or reduction in the amount of sleep needed (on a regular basis) to maintain an optimal performance. Sleep disruption is the fragmented sleep such as in the case of sleep disorders like sleep apnoea, where frequent awakening weakens dynamics of sleep (Chatburn et al. 2017; Reynolds and Banks 2010).
Clinical implications of SD/SR
SR alters the circadian rhythm, resulting in dysregulation of sleep patterns, metabolic, endocrinal, and immune homeostasis. SD results in a number of pathophysiologic changes, such as reduced glucose intolerance, increased blood pressure, activation of the sympathetic nervous system, reduced leptin levels, increased inflammatory markers, and changes in body fluids (Aggarwal et al. 2018; Banks and Dinges 2007; Hagen et al. 2015; Jha et al. 2016; Priya et al. 2020). Long-term SD can cause various metabolic and cognitive disorders.
Chronic sleep restriction (CSR) has negative effects on metabolism-related derangements like central obesity, hypertension, insulin resistance, increased cortisol levels, systemic inflammation, decreased immune response, and dyslipidaemia (Lam and Ip 2010). Hormonal levels during and after sleep depend on the duration and quality of sleep. Sleep duration and quality regulate leptin and ghrelin levels that modulate feeding intake and energy expenditure. Individuals with less than 7 h of sleep have been shown to have higher body mass indices and obesity rates (Cooper et al. 2018). CSR in healthy young adults increases cortisol levels and causes changes in the secretory profile of growth hormone and signals for hunger and appetite, which in turn promotes weight gain and obesity (Banks and Dinges 2007). Less than 6 h of sleep is reported to increase waist circumference in both men and women associated with metabolic syndrome (Kim et al. 2018). CSR also has detrimental effects on immune response. It is reported to activate the nonspecific host defence mechanisms, which would increase inflammatory cytokines such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumour necrosis factor (TNF) (Banks and Dinges 2007; Irwin et al. 2016). Besides, SD affects cognition which is linked to reduced short-term memory, decreased learning ability, and decreased motor performance and motor skills (Adams et al. 2013).
Important cellular pathways like protein synthesis, stress defence, and autophagy are involved in longevity. The signalling molecules including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), nuclear factor erythroid 2-related factor 2 (Nrf-2), sirtuin-1 (Sirt1), peroxisome proliferator-activated receptor γ coactivator 1 (PGC1), forkhead box protein O1 (FOXO), and p66 isoform of SHC1 (p66shc) (de Almeida et al. 2017; Barzilai et al. 2012) are well studied in ageing. In addition, proteins like Sirt1, PGC1, FOXO, p66shc, and poly (ADP-ribose) polymerase 1 (PARP1) (Eros et al. 2017) are shown to participate in early vascular ageing (Harvey et al. 2015). Scientific evidences strongly indicate that SD adversely affects the above-mentioned signalling molecules (Chang et al. 2009; Sugiyama et al. 1995; Vecsey et al. 2012; Wang et al. 2019). This spurt us to propose and interlink the impact of SD on brain vascular ageing. We performed an exhaustive literature search using various abstracting and indexing databases such as Web of Science, PubMed, and ScienceDirect, and appropriate findings were compiled.
Ageing
The human life expectancy has been increasing due to better-quality health care, sufficient amounts of food, and reduced child mortality (Samarakoon et al. 2011). The World Health Organization (WHO) has projected a drastic increase from 12 to 22% in the population aged above 60 years by 2050 across the globe (World Health Organization 2015). However, the impact of this significant transform is debatable. The rate of ageing is determined by biological, social, lifestyle, environmental, genetic, and psychological conditions. Abnormal conditions lead to accelerated ageing (Fratiglioni et al. 2010). Systematic reviews have discussed the effects of a sedentary lifestyle, smoking, alcohol, unhealthy diet, and irregular sleep patterns on ageing (Daskalopoulou et al. 2018). Individuals with good lifestyle habits are reported to have less sleep problems (Monk et al. 2003).
Ageing is influenced by several variables, including genetic, epigenetic, and environmental factors (Benayoun et al. 2015). Metabolic activities and tissues functions decline with the progression of ageing. One key factor that drives cellular function and health is optimal blood flow (Barzilai et al. 2012). The alterations in peripheral (Donato et al. 2018; La et al. 2010) and central blood flow and the consequent vascular dysfunction that occur in various neurodegenerative diseases and ageing have been discussed in several isolated reports (Donato et al. 2018; Morris et al. 2014; Tarumi and Zhang 2018; Yang et al. 2016). However, reports on the physiological and functional changes specific to vascular ageing in the brain are limited. With ageing, the impaired blood flow to the brain affects region specific functions and vice versa, the same blood vessels’ health (Shaw et al. 1984). These functional and anatomical changes require recurrent clinical monitoring (Purkayastha and Sorond 2012). Alterations in neurovascular coupling (neural activity influencing cerebral blood flow and metabolic needs of surrounding cells) were shown to play crucial role in vascular cognitive impairment (Lourenço et al. 2018). Chronic SD causes dilation of brain blood vessels and limits the blood flow (Phillips et al. 2013), which then creates a deficiency of metabolites/nutrients in the local endothelial (Chen et al. 2014), glial (Attwell et al. 2010), and neuronal cells causing decreased neurovascular coupling. This, in turn, becomes one of the risk factors for vascular dementia (Petzold and Murthy 2011; Tarantini et al. 2017). Retinal blood vessels serve as an alternate model for investigating neurovascular health, as they share common anatomical and physiological features with neuronal vessels (Gopinath et al. 2009). Neurovascular coupling responses were shown to decrease with ageing in retinal arterioles (Lipecz et al. 2019). In line with this, various preclinical and clinical evidences demonstrate that ageing and neurodegenerative diseases impair neurovascular coupling responses which were also shown to advertently affect healthy ageing (Lipecz et al. 2019; Lourenço et al. 2018; Stefanidis et al. 2019; Tarantini et al. 2017). In addition to structural changes in cells, various biochemical changes remain plastic with ageing. Such changes may alter the blood flow and metabolic demands which could be corroborated for impaired neurovascular coupling responses (Sonntag et al. 2007).
Vascular ageing
Vascular ageing refers to the loss in mechanical and structural characteristics of the vascular walls. It leads to a loss in vascular elasticity and reduction in vascular arterial compliance (Jani and Rajkumar 2006). Peripheral vascular resistance and increased arterial stiffening (arteriosclerosis) are the key hallmarks of “normal” vascular ageing and are influenced by age-dependent structural and biochemical changes on elastin and collagen content (Lacolley et al. 2018). These changes are further complicated by pathological arterial vascular ageing that results in characteristic lesions and plaque formation ending in premature vascular problems (Iurciuc et al. 2017; Nilsson 2008). Increased vascular calcification is another pathophysiological characteristic associated with arteriosclerosis. The role of gamma-carboxyglutamic acid (Gla) proteins in calcification has been defined in knockout mice (Shanahan et al. 1998). Posttranslational γ-carboxylation of matrix Gla proteins requires vitamin K. Thus, vitamin K deficiency causes the accumulation of impaired γ-carboxylation of matrix Gla proteins, leading to vascular calcification (Tesfamariam 2019). Dietary intake of vitamin K is uncommon in adults and older people, and this has been correlated to vascular calcification and early vascular ageing (Booth 2007). The progression of vascular calcification is also associated with aberrant regulation of noncoding RNAs including micro, long noncoding, and circular RNAs (Kim and Kook 2019). Furthermore, vascular calcification is influenced by chronic inflammation. TNF-α and monocyte chemotactic protein-1 (MCP-1) levels are elevated in the radial arteries of subjects with chronic inflammation (Hu et al. 2016). Substantial increase in the expression of bone morphogenic proteins-2 (BMP-2) (Li et al. 2008), osteocalcin (Millar et al. 2017), and collagen-1 (Watson et al. 1998) indicates inflammation-triggered osteogenic cell differentiation in vascular tissues, which explains the role of osteogenic cells in vascular calcification. Fos-related antigen 1 (Fra-1) promotes vascular ageing by directly binding and activating p21 and p16. This suggests that Fra-1 might be a potential target in preventing ageing-related vascular disorders (Yang et al. 2019). Microvascular endothelial structures are remodelled by reducing Ca2+ influx in severe oxidative stress during ageing (Socha et al. 2015). Resilience to hydrogen peroxide-induced oxidative stress is developed by preventing intracellular calcium overload and triggering apoptosis in the vascular cells of mouse skeletal muscles (Norton et al. 2019). The dysregulation of Ca2+-activated K+ channels retards the electrical conductivity in arterial endothelial cells, leading to vasodilation which causes hypoperfusion to the organs and tissues in ageing (Behringer et al. 2013). About a 40% decrease in the number of holes per square millimetre of internal elastic lamina (IEL) was observed in aged mice (24–26 months) in comparison with young mice (3–6 months), suggesting a diminished signalling between endothelial and smooth muscle cells, which contributes to age related dysfunction in resistance arteries (Boerman et al. 2016).Other major factors like oxidative stress and inflammation also influence vascular ageing (Wu et al. 2014). Mice with their superoxide dismutase 2 gene (SOD2+/-) knocked out showed decreased elastic lamellae integrity and elastin expressions, and increased collagen-I, and medial smooth muscle cell (SMC) apoptosis in aortic walls over a lifetime. This study reveals altered ROS metabolism in the mitochondria as a causative factor for the increased collagen secretion, SMC apoptosis contributing to aortic stiffening in ageing (Zhou et al. 2012). Increase in DNA damage, premature senescence, apoptosis, and proinflammatory protein expression have been reported in transgenic young mice with high NADPH oxidase 4 (NOX4) expressions. These are the phenotypic features of vascular ageing. Since SOD2 is primarily expressed in mitochondria, these findings suggest that mitochondrial oxidative stress plays an important role in aortic stiffness during ageing (Canugovi et al. 2019). Mitochondrial specific antioxidants like MitoQ have been shown to decrease aortic stiffening by reducing aortic elastin degradation in aged rats, revealing the role of antioxidants on vascular health (Gioscia-Ryan et al. 2018).
Csipo et al. (Csipo et al. 2019) recorded a spike in arterial stiffness along with microvascular and macrovascular endothelial dysfunctions in aged subjects. The study implicated that impaired vascular health is a predictor for cognitive dysfunction evidenced using vascular health index and cognitive impairment index scores (Csipo et al. 2019). Balbi et al. (Balbi et al. 2015) examined the functional status of microcirculation in FVB/N mice, wherein vascular dysfunction was reported to precede the changes detected in cerebral blood flow and this was stated to start in the Pial microvessels. Furthermore, reduced cerebral blood flow can decrease arterial dilation (Bálint et al. 2019).
Vascular endothelial dysfunction is one of the important negative consequences of cellular senescence. Aged subjects with sedentary lifestyles exhibited increased expressions of p53, p21, and p16 proteins, indicating accelerated endothelial senescence (Rossman et al. 2017). Endothelial senescence affects the monolayer barrier function of nonsenescent endothelial cells, which is reflected in downregulated protein expressions of occludin and claudin-5 and resultant disruption in tight junction morphology with surrounding young cells (Krouwer et al. 2012). In addition, interleukin-1α (IL-1α), which is one of the biomarkers for endothelial senescence, is overexpressed and triggers cytokine surge during ageing (Mariotti et al. 2006).
Ageing and brain vascular pathology
Arterial stiffening is one of the major vascular hallmarks of ageing and a potential target in preventing brain microvascular diseases and cognitive impairment (van Sloten et al. 2015). Microvascular endothelial dysfunction and decreased bioavailability of nitric oxide are observed in aged cerebral microcirculation. Vascular endothelial dysfunction with ageing also impairs blood brain barrier (BBB) integrity and affects cerebral blood flow and cognition (Peters 2006). Decreased β-amyloid clearance by glymphatic system aggravates the development of Alzheimer’s disease and is associated with dementia in aged animals (Deak et al. 2016). Ageing and acute SD can disturb episodic memory by altering expression of various genes in young and aged mice (Vecsey et al. 2015). Lacunar infarcts and brain microbleeds increase with ageing. Higher cognitive functions like processing speed and executive functions are impaired with the progression of lacunar infarcts and microbleeds in population aged 75–80 years (Nylander et al. 2018). Young population with long-term prevalence of small vessel diseases after stroke shows 10–20 years of preponement in brain ageing with increased vulnerability to vascular risks (Arntz et al. 2016).
Under both ageing and SD conditions, the expressions of genes PrKab2 (energy metabolism and regulation of sleep), Arc (synaptic plasticity), Tsc22d3, Hspa5, and Hspb1 (stress response) are increased, and the expressions of genes Rbm3, Hnrpdl (RNA metabolism and trafficking), and Usp2 (deubiquitination and control of circadian rhythms) are decreased (Vecsey et al. 2015). The secretion of complement C1q increases with age and induces proliferation of vascular smooth muscle cells leading to arterial stiffness. Serum C1q, TNFα, and IL-6 and carotid-femoral pulse wave velocity are also increased in middle and aged population as compared with young subjects, indicating increased risk of CVS with age (Hasegawa et al. 2019). PDGF-AB/BB, VEGF-A, and GDF11 are found to be increased in plasma of healthy individuals above 45 years of age compared to healthy individuals below 45 years (Tian et al. 2019). Small vessel diseases cause an increase of the perivascular spaces in basal ganglia and periventricular white matter hypersensitivities, thereby contributing to cognitive impairment in elderly subjects (Shibata et al. 2019).
Thorn shaped astrocytes in subpial, subependymal, and perivascular areas indicate astrocytic tau pathologies in aged human brain (Okamoto et al. 2019). Extravascular fibrinogen in the cerebrospinal fluid of elderly population is indicative of cerebral atherosclerotic small vessel diseases (McAleese et al. 2019).
Studies on the vascular changes in the brain are limited due to complexities of the brain vasculature and interpersonal age-related anomalies (Baruah and Vasudevan 2019). Nonetheless, there are several investigations on the association of functional changes with brain vascular ageing. Cardiovascular risks like hypertension can cause cognitive decline and dementia (Iadecola and Gottesman 2019; Tzourio 2007). In summary, the existing data strongly suggest that brain vascular pathologies contribute to ageing and structural changes in white matter and associated functional changes in brain contribute to preponement of biological ageing.
SD and brain vascular ageing
Sleep disorders including difficulty in falling asleep, sleep fragmentation, and poor sleep quality affects not only emotional and physical health but also the cognitive performance (Dauvilliers 2007; Moran et al. 2005). Sleep loss accelerates neurodegeneration in dementia, affecting neural circuits including suprachiasmatic nucleus (Swaab et al. 1985; Van Erum et al. 2018). Hughes et al. (Hughes et al. 2014) showed that arterial stiffness is strongly associated with progressive brain deposition of amyloid-β in nondemented aged subjects. A recent clinical report revealed that overnight SD causes accumulation of amyloid-β in hippocampus, which is a causative factor of impaired brain function (Shokri-Kojori et al. 2018). These data indicate the effects of sleep on brain and vascular functions.
Longer sleep duration was reported to decrease the risk of calcification of coronary arteries (King et al. 2008). This is because SD activates the molecular pathogenetic mechanisms of biological ageing and causes an imbalance of collagen, elastin, MMPs, oxidative stress, and cytokines. In long-term SD, downregulation of transcription factors which modulate the expression of procollagen type I causes dysregulation of collagen, and thus, collagen serves as an indicator of vascular ageing (Cirelli et al. 2006).
Sleep fragmentation increases Ly-6Chigh monocytes, which induces atherosclerotic lesions and decreases production of hypocretin in lateral hypothalamus in mice. On the other hand, colony stimulating factor 1 (CSF1) deficiency or hypocretin supplementation is reported to reduce circulating monocyte numbers and atherosclerotic lesions in sleep fragmented mice (McAlpine et al. 2019). Inefficient drainage from perivascular region leads to enlarged basal ganglia perivascular spaces (PVS) in subjects having poor sleep efficiency (Del Brutto et al. 2019). Elevated grey matter resting cerebral blood flow (rCBF) was seen in medial, lateral occipital cortices, in anterior cingulate gyrus and insula of both the hemispheres in individuals with a day of wakefulness followed by a night of SD (Elvsåshagen et al. 2019). CSR prolongs neural activity and restricts vascular compliance, promoting vascular deficits and neural trauma (Schei and Rector 2011). SR decreases survival time and triggers neurological dysfunctions in rats subjected to cerebral hypoperfusion (Kim et al. 2016).
SD of 40 h upregulates the levels of E-selectin, i.e. CD62 antigen-like family member E (CD62E), intracellular adhesion molecule-1 (ICAM-1), IL-1b, and IL-1ra, and downregulates CRP and IL-6. No significant alterations are observed with vascular adhesion molecule-1 (VCAM-1). These results indicate that pro- and antiinflammatory markers and cell adhesion markers are altered in SD (Frey et al. 2007). Chronic sleep disturbance increases the expression of vascular endothelial growth factor (VEGF), which activates ERK1/2 and causes an increase of DII4, thereby causing an osteoarthritis like condition by promoting angiogenesis (Dong et al. 2017). Participants in military missions have shown upregulated VEGF and brain derived neurotrophic factor (BDNF) under SD and stress (Suzuki et al. 2014). Results from the above studies indicate that SD alters cellular adhesion molecules, inflammatory mediators, and vascular growth factors, implicating that pathological vascular changes are characteristic features of ageing.
SD and neurotransmitter systems
Glutamatergic system
Glutamate levels increase progressively during waking and REM sleep and decrease progressively in nonREM sleep (Dash et al. 2009). The expression of glutamate receptor (mGLU1) in regions of the hippocampus is increased significantly in total SD, REM-SD, and SR (Saygin et al. 2017). The effects of age on acute and chronic SD on the expression of n-methyl-D-aspartate (NMDA) receptor subunits and nitric oxide synthase isoforms were evaluated. It was shown that ageing decreases expression of NMDA receptors and downregulates NOS isoforms in some SD groups. This also corroborates with cognitive decline in the elderly (Kristofikova et al. 2019). SD activates oxidoinflammation in the prefrontal cortex, decreasing glutamate receptor subunits and PSD95 in PND33 (postnatal day 33) in rats. It can cause the disruption of synapse formation and maturation, leading to the development of initial anxiety like behaviour leading to depression in the later stages (Atrooz et al. 2019). REM-SD rats on chronic caffeine consumption upregulate Grin2a, a subunit of NMDA receptors, to maintain the arousal time without altering learning and memory performance (Sahin et al. 2019). Higher levels of glutamate, along with other metabolomes such as homovanillic acid, lactate, and pyruvate are found in awake mice when compared with sleeping mice. This implies that there is a wake-dependent need to promote synthesis of essential biochemical components (Bourdon et al. 2018). Sleep-wake regulation by the basal forebrain requires a balance between excitatory—wake promoting effects mediated by glutamate receptors and inhibitory—sleep promoting effects mediated by adenosine degradation (Yang et al. 2018). Mice with their glutamate receptors of subtype 5 (mGluR5) knocked down exhibit dysregulated sleep-wake homeostasis, lack of recovery sleep, and impaired behavioural adjustment to novel tasks after SD (Holst et al. 2017). The expressions of glutamine synthetase and innexin2 are increased following SD, indicating that they are dynamically linked to wake state (Farca Luna et al. 2017).
Glutamate transporter 1 (GLT1) is increased around melanin-concentrating hormone (MCH) neurons and almost absent around the somata of orexin neurons following SD. Both MCH and orexin neurons show varied forms of synaptic plasticity on SD (Briggs et al. 2018). AMPA receptors (GluA1Rs) are internalized from the membranes of neurons by endocytosis via calcium/calmodulin-dependent protein kinase IIα upon waking. They return to the membrane by recycling endosomes during SR (del Cid-Pellitero et al. 2017). In contrast to this, the sublaterodorsal tegmental nucleus (SLD) neurons, which trigger paradoxical (REM) sleep, are glutamatergic in nature. Nearly 84% of Fos+ neurons are localized in SLD after paradoxical sleep hypersomnia and express vGLUT2 mRNA, which strongly suggests that SLD glutamatergic neurons play an important role in the generation of paradoxical sleep (Clément et al. 2011).
GABAergic system
SD increases the expression of GABA-B and mGlu1α receptors in the Cornu Ammonis 1 (CA1) region of hippocampus. GABA-B and mGlu1α receptors are implicated in pathogenesis of psychiatric disorders. Colocalization and heterodimerization of these receptors are also increased in SD rats (Tadavarty et al. 2011). Rats exposed to total SD for 5 days and treated with carbamazepine and fluoxetine show significantly increased levels of GABA in both prefrontal cortex and thalamus. However, rats treated with desipramine show no alteration in GABA levels. These results suggest the role of GABA in SD and beneficial effects of carbamazepine and fluoxetine in total SD (Kamal 2010). A significant decrease in GABA level was recorded in locus coeruleus (LC), dorsal raphe, pedunculopontine tegmentum (PPT), frontal lobe, cortex, and hippocampus of rats subjected to REM SD after 96 h. However, most of these altered neurotransmitter levels return to normal in postREM SD recovery rats (Mehta et al. 2017).
In oxGKO mice (mice lacking the GABAB1 gene in orexin neurons), the absence of GABAB receptors decreases the sensitivity of orexin neurons. This suggests that GABAB receptors in orexin neurons are important in the control of orexinergic tone through sleep-wake states, as they stabilize the state switching mechanisms (Matsuki et al. 2009). The frequency and amplitude of mEPSCs, NMDAR, GluR1, and pCaMKII (α, β) increase and CREB levels decrease in APP/PS1 mice subjected to SD, indicating the adverse effects of SD in young APP/PS1 transgenic mice through the enhancement of neuronal excitation. Jujuboside A enhances GABAergic inhibition and reduces SD-enhanced excitatory transmission in APP/PS1 mice (Tabassum et al. 2019). Early life sleep disruption (ELSD) for a week increases GABAergic activity in primary somatosensory cortex in adulthood in prairie voles (Microtus ochrogaster), suggesting that sleep is required to tune inhibitory neural circuits and develop species-typical affiliative social behaviour like social bonding (Jones et al. 2019). Tryptic hydrolysate of αS1-casein (αS1-CH), a milk protein, increases hypothalamic GABAA expression in total SD rats, suggesting the beneficial role of αS1-CH in sleep disorders (Yayeh et al. 2018).
Mutations in the Rodgi locus lead to a genetic disorder namely Kohlschutter-Tonz syndrome (KTS). The Rogdi mutant Drosophila exhibits insomnia-like behaviour, sleep fragmentation, and delay in sleep initiation due to suppression in a specific subset of GABA transmission (Kim et al. 2017). Melatonin prevents anxiety-like behaviour induced by SD via establishing a balance between GABAergic and glutamatergic transmissions and reducing oxidative stress (Zhang et al. 2017). Orexin neurons in the mice brain show increased immunostaining of the alpha 1 subunit of the GABA A receptor and neuroligin 2, which are involved in inhibitory synapse specialization on 6 h of SD (Matsuki et al. 2015). This implicates the GABAergic property of orexin neurons. Thus, the data proves that the GABAergic system and its modulators play a crucial role in SD.
Cholinergic system
The cholinergic system is one of the major neurochemical players in sleep, specifically in REM sleep along with its role in learning and memory. SD inhibits cholinergic nuclei, leading to reduced cortical acetylcholine levels (Brown et al. 2012). SD for 96 h showed decreased acetylcholine content in the telencephalon (Tsuchiya et al. 1969). Amnesia is observed in both total and REM-SD rats treated with cholinesterase inhibitor physostigmine and muscarinic receptor antagonist scopolamine through CA1 cholinergic muscarinic receptors, hinting at the important role of acetylcholine in sleep and learning (Javad-Moosavi et al. 2017). Enforced wakening via prolonged activity results in a homeostatic downscaling in the excitability of Mo5 trigeminal motor neurons (Toossi et al. 2017). Optical stimulation of cholinergic neurons (locally) increases both acetylcholine and wakefulness in mice. The process is reversed by simultaneous reverse microdialysis of cholinergic receptor antagonists, suggesting that the wake-promoting effect of cholinergic stimulation requires the local release of acetylcholine in the basal forebrain (Zant et al. 2016). Basal forebrain cholinergic neurons enhance adenosine and nitric oxide levels along with increase in low frequency theta waves in EEG upon SD. Activation of iNOS with SD in forebrain cholinergic neurons appears to be the key mechanism driving both biochemical and EEG changes (Kalinchuk et al. 2015). CSR in NMDA intoxicated animals causes a substantial loss of cholinergic nucleus basalis magnocellularis (NBM) cells and cortical fibres. This indicates that SR makes cells more sensitive to subsequent excitotoxic insult (Novati et al. 2012). Neural serine metabolism regulates sleep during starvation via cholinergic signalling (Sonn et al. 2018). Slight increase in kynurenic acid (nicotinic receptor antagonist) level formed by tryptophan metabolism induced wakefulness and reduced REM duration, thereby disrupting sleep (Pocivavsek et al. 2017). Redeye (rye), one of the proteins which encodes nicotinic acetylcholine receptors, is involved in sleep regulation in Drosophila. The upregulation of rye is seen with homeostatic drive to sleep (Shi et al. 2014). Sleep fragmentation and cognitive impairment are observed in vesicular acetylcholine transporter (VAChT) deficient mice, suggesting the key role of cholinergic plasticity in sleep homeostasis (Queiroz et al. 2013). Type 2- and type 4-muscarinic receptor knockout mice show differential response in the modulation of sleep-wake cycle under different homeostatic insults like influenza and candida infection (Turner et al. 2010). c-Fos activation is shown to increase in basal forebrain cholinergic neurons after SD (McKenna et al. 2009).
In an experiment to explore the role of nucleus accumbens in flavour neophobia and its attenuation during ageing, adult rats exhibited a delayed attenuation of flavour, neophobia, and increased nucleus accumbens shell (NAcbSh) c-Fos activity (Grau-Perales et al. 2019). Differences in the gene expressions of cholinergic basal forebrain cells between sleeping and SD mice were assessed. The SD group showed the upregulation of 55 transcripts, including 25 from protein folding pathways such as chaperones (Nikonova et al. 2017). Thus, SD significantly alters the protein folding pathways.
Adrenergic system
Chronic REM-SR animals exhibit impaired physical development and higher corticosterone levels along with increased noradrenaline and serotonin levels in the amygdala and ventral hippocampus regions, without changes in the β1 adrenergic receptors (da Silva et al. 2018). α2 receptors in the CA1 region of the hippocampus play an important role in regulating sleep and memory retention processes. The activation and deactivation of CA1 α2 adrenergic receptors by clonidine and yohimbine, respectively, followed by total SD, shows that total SD and reversal of circadian rhythm impair memory functions (Norozpour et al. 2016). This suggests the important role of the adrenergic system in sleep and memory. SD reduces adenosine A2a receptor density in olfactory tubercle and decreases β-adrenergic receptor density in substantia innominata and ventral pallidum. The condition is retained even at the end of recovery sleep for 3 days (Kim et al. 2015). SD also leads to stress and increases the level of adrenaline, which regulates the innate immune systems, like the natural killer cells and natural killer T cells. Alterations in the brain’s norepinephrine content in the basal forebrain and cingulate cortex mediate the allosteric changes in sleep time and intensity in CSR (Kim et al. 2013). The infusion of dexmedetomidine, an α2-adrenergic agonist, in critically ill patients improves sleep through increasing stage 2 sleep and sleep efficiency. It also modifies the 24-h sleep pattern by shifting sleep to the night (Alexopoulou et al. 2014). Anaesthesia and the abolition of adrenergic signals activate the glymphatic system. The rate of clearance is increased by twofold during sleep, suggesting decreased adrenergic content (Mendelsohn and Larrick 2013). SD disrupts the consolidation of fearful memories through facilitation of interactions between glucocorticoid and adrenergic systems (Cohen et al. 2012). Following 2 h of SD, clonidine, an α2-adrenergic receptor agonist, hyperpolarizes the hypocretin/orexin neurons by activating G protein gated inwardly rectifying potassium (GIRK) channels (Uschakov et al. 2011). Thus, the adrenergic system plays an essential role in regulating memory consolidation and activation of the glymphatic system during sleep.
Vascular ageing is a biological process which starts early in life (Nilsson 2015). The associated structural and functional changes in vasculature are wide. Several studies have reported the association of neurochemicals to peripheral vascular changes (Alborch et al. 1984; Connelly et al. 2019). However, the profound effects of SR leading to major modulations in neurochemicals and vascular ageing are not elucidated.
Molecular basis of vascular homeostasis in ageing and SD
Sirtuins
Seven sirtuin family proteins, Sirt1, Sirt2, Sirt3, Sirt4, Sirt5, Sirt6, and Sirt7, are present in mammals. They are nicotinamide adenine dinucleotide (NAD)-dependent deacetylases. Sirt3, Sirt4, and Sirt5 are localized within mitochondria, whereas Sirt1, Sirt6, and Sirt7 are nuclear proteins. Sirt1 is specific to cytosol, and Sirt2 is expressed in both nucleus and cytosol. Initial studies on sirtuins were mainly on longevity of mammals. However, divergent functions of sirtuins have emerged, ranging from metabolic sensing properties to mitochondrial biogenesis (Sack and Finkel 2012).
Sleep loss causes injury to Locus coeruleus neurons (LCns) and accelerates neurodegeneration in individuals experiencing AD and PD. Sirt1 shows a neuroprotective role in young animals, which is lost upon ageing. This implicates that the neurons are exposed to metabolic insults. Sirt1-deficient mice and aged mice have shown similar patterns of sleep/wake cycles. This suggests that wake impairments in ageing may occur partially due to age-related Sirt1 loss (Panossian et al. 2011). Upon SD, Sirt3 pathway gets impaired through ROS or NAD+ biosynthesis as a result of DNA damage (Nogueiras et al. 2012). SD mice treated with melatonin preserve the number of Sox2/BrdU+ cells in the dentate gyrus, promoting the overexpression of Let-7b, mir-124-2 transcripts. Sirt1 level is reduced while MECP2 expression increases (Hinojosa-Godinez et al. 2019). In addition, treatment with melatonin increases COX and Sirt1 activities in SD rats along with improved behavioural function (Chang et al. 2009). Sirt3 is localized in the inner mitochondrial membrane and regulates cellular energy homeostasis (Fifel 2014). SD is also shown to alter mitochondrial specific proteins expression (Ren et al. 2016) (Fig. 1). However, the link between Sirt1 and these protein changes need to be studied.
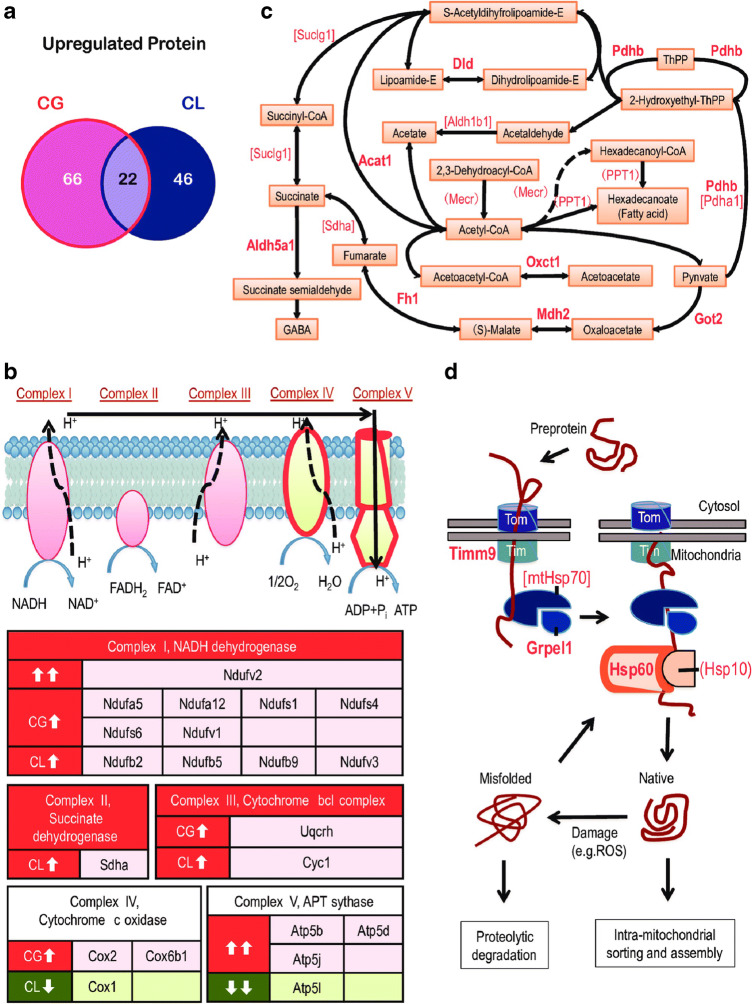
Fig. 1.
Sleep deprivation causes cellular respiration proteins upregulation (Ren et al. 2016) (reused as per the PLOS ONE journal’s copyright permission policy). Proteins belonging to TCA cycle and mitochondrial complexes are upregulated in sleep deprived C57BL/6 N mice brain. CG represents sleep deprivation induced by gentle handling; CL represents locomotion induced sleep deprivation. a Venn diagram showing distribution of 66 upregulated proteins in the CG group and 46 upregulated proteins in CL groups. Twenty-two shared proteins are common in both CG and CL groups. b Influence of sleep deprivation on complexes of the electron transport chain, where in complexes I–III had upregulated subunits while complexes IV and V had both up- and downregulated subunits. Upregulated proteins are shown in red boxes and downregulated proteins are shown in green. c Proteins involved in small molecule metabolism. The proteins upregulated in CG and CL groups are shown in parentheses and square brackets, respectively and presented in bold text if upregulated in both the CG and CL groups. d Preproteins synthesized in the cytosol enter mitochondria via translocases of outer membrane (TOM) and translocase of the inner membrane (TIM). Chaperones upregulated in CG or CL groups are presented in parentheses and square brackets, respectively. Chaperones upregulated in both the groups are shown in bold
Mitochondrial energy production and redox homeostasis are integral functions of Sirt3. LCns are active and show increased rates of firing during sustained wakefulness. Wakefulness, as a metabolic stressor, causes mitochondria to undergo adaptive metabolic responses, which may fail and result in injury. Brief wakefulness activates Sirt3 and antioxidant systems in LCns, which plays a protective role. However, brief wakefulness in mice lacking Sirt3 shows no adaptive antioxidant response, resulting in oxidative injury in LCns. Extended wakefulness reduces Sirt3 activity, causing degeneration of LCns (Zhang et al. 2014).
Sirt1 regulates proteins and genes involved in antioxidant response (FOXO3), antiinflammatory response (NF-kB), antiapoptotic response (p53 and FOXO3), insulin response (IGF-1), and gene transcription (PGC-1). Sirt1 also regulates mitochondrial biogenesis and structural integrity, as well as synaptic plasticity, learning, and memory. SD alters mitochondrial structure (Fig. 2) through downregulation of Sirt1 (Somarajan et al. 2016).
Fig. 2.
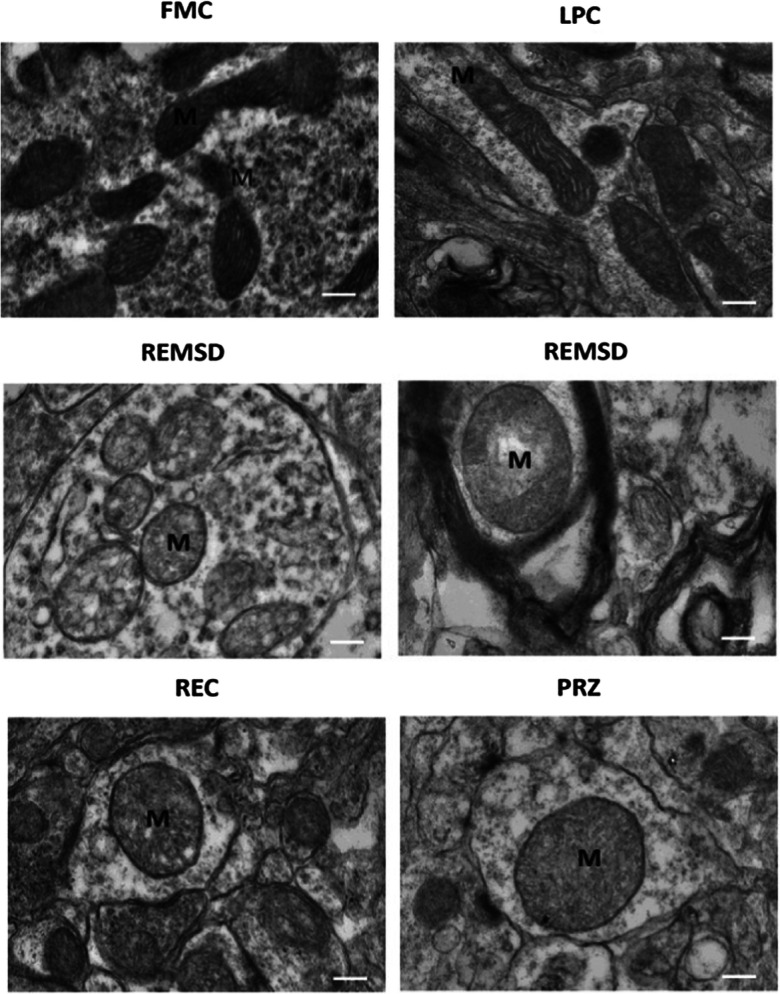
Representative electron microscopy images show structural changes in mitochondria in locus coeruleus (Somarajan et al. 2016) (reused as per the Frontiers in Neurology journal’s copyright permission policy). One-millimetre thick brain tissue blocks of Locus Coeruleus were prepared after intracardial perfusion of rats for transmission electron microscopy to observe structural changes in mitochondria in free moving control (FMC), large platform control (LPC), REM sleep deprivation (REMSD), recovery (REC), and Prazosin treated (PRZ) groups. Control groups retain normal dumbbell-shaped mitochondria with intact cristae. Swollen mitochondria with disintegrated cristae appear in REMSD, and the REC group shows less distorted cristae. PRZ group shows marginal swelling in mitochondria with intact cristae
The sirtuin pathway is thus important in cell survival under stressful conditions such as calorie restriction. Laboratory investigations support the role of sirtuin1 in the proliferative, antiageing effects of calorie restriction in mammals. Loss of Sirt1 is also linked to preponement of ageing.
PGC1α
Vascular ageing and atherosclerosis with telomere shortening and DNA damage are preponed in the absence or removal of PGC1α. A decreased expression of telomerase reverse transcriptase (TERT) and amplified p53 level are observed upon the deletion of PGC1α-lipoic acid upregulates PGC1α-dependent TERT and Nrf-2-mediated antioxidant-responsive element signalling cascades, offsetting high-fat-diet induced age-dependent arteriopathy. This confirms the role of PGC1α in ameliorating senescence, ageing, and age-associated chronic diseases (Xiong et al. 2015).
PGC1α-knockout mice have shown decreased expression of autophagy adaptor p62 and reduced conversion of LC3-I to LC3-II, which, in turn, causes reduced autophagosome numbers. PGC1α deficiency induces P62 downregulation, and siRNA-induced p62 downregulation induces senescence and inhibits autophagy in vascular endothelial and smooth muscle cells (VSMCs). The expression of p62 is upregulated by PGC1α, resulting in the clearance of the damaged mitochondria (mitophagy) and the prevention of vascular senescence (Salazar et al. 2016). SD rats treated with functional food chitooligosaccharides (COS) show a remarkable increase in the expression of PGC1α and cytochrome cmRNA and an improvement in mitochondrial biogenesis (Cho et al. 2010). This suggests that the decrease in PGC1α in SD may have adverse effects on vascular ageing including brain vascular structures.
FOXO transcription factors
FOXO transcription factor has FOXO 1, 3, 4, and 6 in its family with nearly similar anatomy and physiology between each variant (Tia et al. 2018). In C. elegans DAF-16, the FOXO transcription factor plays a key role in providing homeostatic response to SD. The study also showed a reduced rate of survival when sleep bout deprivation is combined with stress (Bennett et al. 2018). The downregulation of nutrient and stress sensing insulin, i.e. IIS/TOR, showed dFOXO-mediated amelioration of daytime sleep fragmentation in Drosophila. As IIS/TOR also plays an important role in delaying ageing, it could be of therapeutic interest to treat fragmented sleep during ageing (Metaxakis et al. 2014). Flies lacking FOXO genes exhibited sensitivity to the circadian clock. Increased oxidative stress along with significant alterations in circadian rhythms was observed in FOXO mutant flies, indicating the importance of FOXO in circadian rhythm (Zheng et al. 2007). A 2–3-h quiescence period in C. elegans lethargus is considered as sleep-like behaviour. Activation of a stress marker DAF-16/ FOXO, a transcription factor, was observed upon deprivation of lethargus quiescence. In addition, the formation of dauer larvae, which requires DAF-16 as promoter, confirms the role of FOXO in sleep homeostasis (Driver et al. 2013). Ageing is affected by genetic alterations. FOXO-signalling pathway and other pathways such as the peroxisome, mTOR, and AGE-RAGE-signalling pathway are key players in longevity (Li et al. 2019). The antiageing property of Glochidion zeylanicum, an antioxidant-rich plant found in Thailand, is attributed to DAF-16/FOXO and SKN-1/Nrf-2 transcription factors (Duangjan et al. 2019). The livers of aged rats and obese mice with insulin resistance showed elevated FOXO 6 levels (Kim et al. 2019). Ginkgo biloba extract has shown a preservative effect on mesenteric arterioles of old rats through vascular elasticity and AKt/FOXO3a signalling pathway (Chen et al. 2019). Angiogenesis in the fracture repair process is regulated by chondrocytes through FOXO 1-dependent upregulation of VEGFA mRNA, while silencing FOXO 1 reduces angiogenesis (Zhang et al. 2019). Thus, a close relation between FOXO and ageing process is a key factor in maintaining vascular properties. However, the direct role of FOXO in SD is still not understood.
PARP1
PARP-1 catalyses the covalent attachment of polymers of ADP-ribose moieties to its target proteins (Ray Chaudhuri and Nussenzweig 2017). PARP-1 activation promotes DNA repair. However, high levels of PARP-1 cause cell death. Increased protein levels of orexin-A, OX1R, OX2R and PARP-1 and decreased protein level of ERK1/2 are observed in the hippocampus of SD rats (Wang et al. 2019). PARP1-1, the sensor of DNA damage, is a new melatonin-dependent regulator of senescence-associated secretory phenotype (SASP) gene induction on oncogene-induced senescence (OIS). Melatonin, a novel anti-SASP molecule, regulates SASP through PARP1 and interrupts the interaction of PARP-1 with telomeric long noncoding RNA (IncRNA) or chromatin (Yu et al. 2017).
Reductions in lifespan and the acceleration of biological ageing in PARP-1 deficient mice have been reported by Piskunova et al. (2008). Acceleration in biological ageing was measured using characteristics like body weight gain, body temperature, oestrous cycle, behaviour, and other biochemical indices. While the incidence of tumours was similar in both PARP-1 deficient and wild type groups, the inactivation of PARP-1 accelerates ageing, shortens lifespan and increases spontaneous carcinogenesis in mice (Piskunova et al. 2008). DNA strand breaks (DSB) in flies and mice showed delayed DNA repair on extended awake. In both species, the repair of DNA strands was faster in sleep. In addition, genes responsible for DNA repair such as PARP-1, chek2, Brcc3 and Tipin were upregulated during sleep. Cognitive performance is affected by acute SD radiation induced DNA strand breaks (Bellesi et al. 2016). Carotid artery remodelling and damage to neuronal tissue are studied in spontaneous hypertensive and normotensive rats with and without the inhibition of PARP-1 by L-2286, a poly (ADP-ribose) polymerase inhibitor. Increased oxidative stress, thickening of vascular walls with fibrotic tissue and increased blood pressure are observed. The inhibition of PARP-1 interrupts these processes, reduces apoptosis and cell death, and produces an improvement in the structural and functional features of carotid arteries. An assessment of dorsal hippocampus using nitrotyrosine, 4-hydroxinonenal and 8-oxoguanosine immunohistochemistry in Cornu Ammonis 1 (CA1) of hypertensive rats (Eros et al. 2017) shows that chronic inhibition of PARP-1 protects neuronal tissue against oxidative damage, suggesting that PARP-1 is involved in ageing and blood vessel functions.
p66Shc
p66Shc is present in Xenophus, Botia Dario and mammals but absent in Drosophila and Caenorhabditis. The SHC1 gene is located on chromosome 1 and encodes 3 main protein isoforms, p66Shc, p52Shc and p46Shc, differing in their molecular weight. p66Shc is a longevity protein with functions including nutrient metabolism via tyrosine kinase signal transduction (Martins 2016). It is the first protein identified whose deletion in mice prolongs life span and protects from a variety of ageing-associated diseases without apparent negative effects (Trinei et al. 2009).
Being an adaptor protein, p66Shc plays a vital role as a redox enzyme involved in mitochondrial reactive oxygen species generation and translates oxidative signals to apoptosis. Mice lacking p66 gene reduce the production of intracellular oxidants and have a 30% prolonged life span. p66-deficient mice exhibit protection against age-dependent endothelial dysfunction along with age-related risk factors such as diabetes and hypercholesterolemia (Camici et al. 2008). p66Shc phosphorylation at Ser36 occurs through the JNK/ERK or PKC pathway and Rac1 binding to non-phosphorylated p66Shc in endothelial cells is promoted by VEGF. Reduction in endogenous p66Shc inhibits VEGF-induced Rac1 activity and ROS production, which in turn stimulates VEGF-induced EC migration, proliferation and capillary-tube formation. Thus, p66Shc acts as a positive regulator of ROS-dependent VEGFR2 signalling in endothelial cells, suggesting a potential role of p66Shc in angiogenesis-dependent diseases (Oshikawa et al. 2011). Deletion of p66Shc has protective effects in mice from ischemia/reperfusion brain injury caused by reduction in the activation of pro-oxidant enzyme NADPH oxidase, a downstream target of p66Shc, thus reducing the production of free radicals (Spescha et al. 2013). p66Shc deficient mice show reduced oxidative stress and longer life span. Increased levels of brain-derived neurotrophic factor (BDNF) and oxidative markers are observed in hippocampus of p66Shc-negative adult mice (Berry et al. 2008). From the above reports, it is clear that p66Shc has a role in vascular health during ageing. However, its role in sleep deprived status is yet to be established.
SD and learning and memory
Vascular dementia
The risk of dementia in sleep disturbances was assessed in a multicentric longitudinal study. Midlife insomnia or long sleep duration (> 9 h per night) were revealed to be associated with higher late-life dementia risks (Sindi et al. 2018). A study on the influence of lifestyle factors on cognitive functions in an elderly community revealed that sleep is the most closely associated with cognitive functions. This links the importance of sleep to vascular dementia (Kimura et al. 2019). The relation between daily sleep duration and risk of dementia and death was investigated in Japanese elderly population aged 60 and older. The study revealed that changes in daily sleep duration and hypnotic use are the key risk factors for dementia and death in Japanese elderly population (Ohara et al. 2018). A systematic review and meta-analysis of predictive roles of overall sleep disturbances and their subtypes in dementia, Alzheimer’s disease and vascular dementia subtypes showed that sleep disturbances may help predict the incidence of dementia (Shi et al. 2018).
SD and dendritic spine
Dendritic spines are prominent sites for synaptic transmission. Any change in the strength of synaptic connections directly alters the neuronal communications required for information storage and processing (Raven et al. 2018). As little as 5 h of SD is shown to profoundly affect the dendritic structure in dentate gyrus of dorsal hippocampus. The inferior blade of the dentate gyrus is more vulnerable to spine loss than superior blade, explaining the cognitive deficits on SD. The results suggest the requirement for structural reorganization of the synaptic networks for cognition (Raven et al. 2019). Parallelly, an increase in cortical spines on waking and spine loss during sleep were observed through two-photon microscopy in adolescent mice (Maret et al. 2011). Formation and elimination of spines or filopodia of layer 5 pyramidal neurons in the barrel cortex of mice was performed in sleep and wake states. The initial 2 hours of sleep and wake had a significant impact on the formation of spines. It was observed that the rate of elimination was more in wakefulness than during sleep. The observations are consistent with dendritic protrusion dynamics and confirm the role of sleep in establishing plasticity of synaptic connections in the mouse cortex (Yang and Gan 2012). The induction of Long-term depression (LTD) through low frequency stimulation causes shrinkage of spines (Fig. 3), which is inverse of Long-term potentiation (LTP). Both spine shrinkage and LTD require NMDA receptor activation and calcineurin. Spine shrinkage is mediated through cofilin, but not protein phosphatase 1 (PP1), which is required for LTD (Chidambaram et al. 2019; Zhou et al. 2004). In line with this, several studies have revealed the induction of LTD on sleep loss, which in turn may cause loss of spines (Fig. 4) (de Vivo et al. 2019).
Fig. 3.
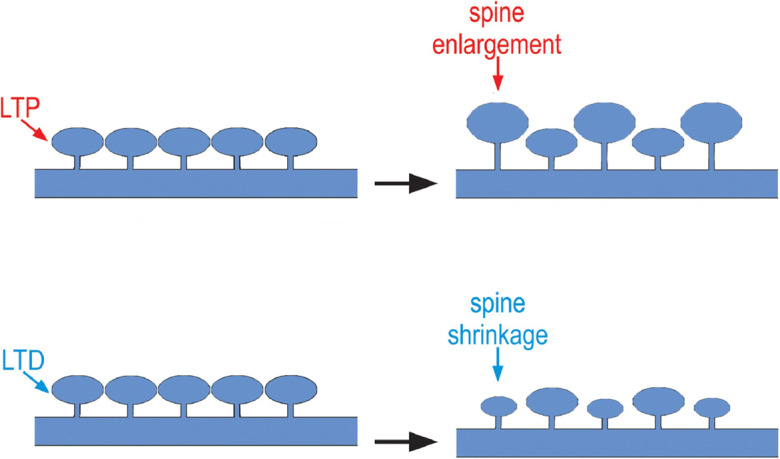
Effect of SD on structural changes in spines during LTP and LTD (Adopted with minor modifications from Chidambaram et al. 2019). SD attenuates LTP and enhances LTD, while LTP causes spine enlargement and LTD causes spine shrinkage
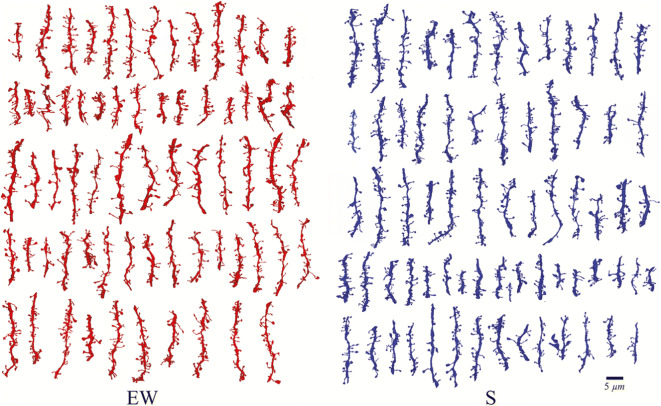
Fig. 4.
Reconstruction of dendritic segments during forced wake (EW) and sleep (S). Each row depicts the spines of a single animal from each group (de Vivo et al. 2019) (reused as per the Sleep journal’s copyright permission policy). Blocks of 1 mm2 brain tissue encompassing the primary motor cortex were prepared from mice pups. Serial images were obtained through ΣIGMA VP field emission scanning electron microscope. Spiny dendritic segments between the diameter range of 0.47–1.01 μm were selected. The results of enforced wakefulness group showed induction of shrinkage in dendritic spines compared to sleep group. Note: The red spines depict enforced wakefulness while the blue spines depict sleep state
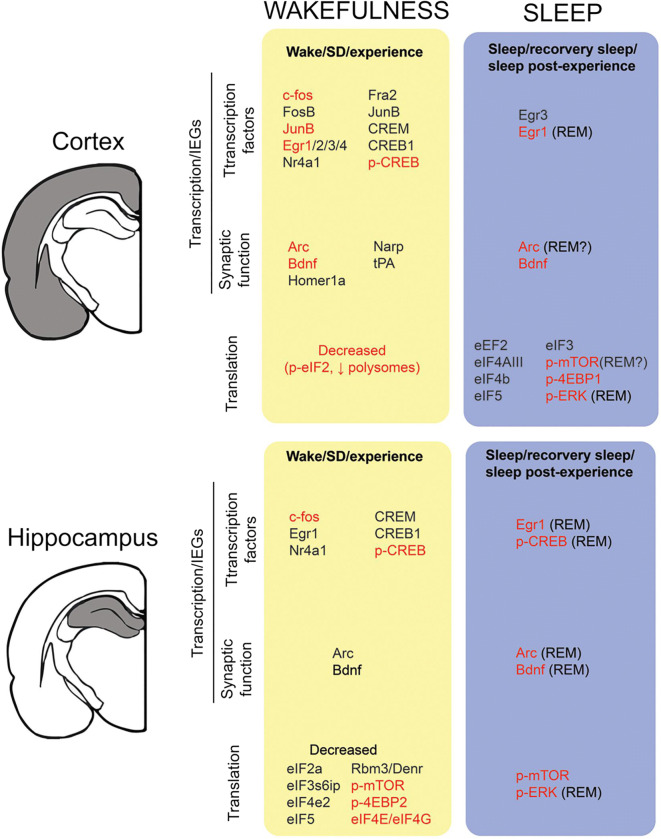
It is hypothesised that the cognitive benefits of sleep include renormalization of the total synaptic strength, after disruption caused by ongoing learning during wake, lead to net synaptic potentiation. This was confirmed in cerebral cortex where cortical synapses are found to be larger in size and length after wake and smaller after sleep (de Vivo et al. 2019). Similarly, CA1 of the hippocampus also shows sleep/wake synaptic changes, which is consistent with sleep-dependent renormalization (Spano et al. 2019). Differences in transcription and translation of various genes between wake and sleep were evidenced in the cortex and hippocampus of the brain (Fig. 5) (Seibt and Frank 2019).
Fig. 5.
Alteration in transcription and translation of various genes and proteins in sleep and wakefulness in the cortex and hippocampus of mammals (Seibt and Frank 2019) (Reused as per the Frontiers in System Neuroscience journal’s copyright permission policy). Waking data includes periods of sleep deprivation <8 h. wakefulness upregulates transcription of c-fos, Egr1, p-CREB, Arc, and BDNF while sleep deprivation has shown decreased translation of elF2a, elF4e2, elF5, Rbm3, Denr, p-mtorc1, and p-4EBP2. The methodologies employed include microarray, quantitative PCR, immunohistochemistry, Western blot, in-situ-hybridization and Rnase protection assay. The genes highlighted in black colour are active during transcription, while those highlighted in red are active during the translational phase
SD, LTD, and LTP
SD is reported to inhibit spatial learning and LTP (Nasehi et al. 2018). The ability to modulate either the strength of neural connections or synaptic plasticity is called metaplasticity. Five hours of SD in mice impairs hippocampal synaptic tagging and capture (STC). Following SD, STC in the stratum radiatum of CA1 area is impaired in controlling a mouse which is normal prior to SD. Potentiation decays to baseline at the weakly stimulated synapses. Thus, it is clear that SD disrupts metaplasticity, where two independent inputs interact to generate long-lasting LTP (Vecsey et al. 2018). Furthermore, maternal SD (MSD) is associated with increased neurodevelopmental disorders in the offspring. MSD at various stages of pregnancy suppresses CA1 LTP of hippocampus, which in turn causes an increase in LTD. MSD also reduces the expression of postsynaptic GluA2-containing alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionicacid receptors (AMPARs). The inhibition of AMPAR endocytosis by a synthetic peptide Tat-GluA2 (3Y) upregulates the expression of AMPARs, reduces LTD, and restores LTP (Yu et al. 2018). MSD at different stages of pregnancy leads to suppression of hippocampal LTP and basal synaptic transmission. Electrophysiological recordings show impaired hippocampal CA1 LTP, supplemented by a decrease in the miniature excitatory postsynaptic current in the hippocampal CA1 pyramidal neurons (Peng et al. 2016). Another interesting study shows that regular exercise can prevent SD-induced inhibition of late phase LTP and downregulation of phosphorylated cAMP response element-binding protein (P-CREB) and BDNF (Zagaar et al. 2018). Sleep reverses the increased synaptic strength and decreased LTP-like plasticity induced by SD (Kuhn et al. 2016). Acute REM SD for 4 h disturbs the contextual fear conditioning and LTP and causes a reduction in the density of Egr1/Zif268-expressing neurons in the CA1 region of dorsal hippocampus. Restoration of REM sleep facilitates induction of both CFC consolidation and LTP (Ravassard et al. 2016).
SD causes an increased expression of transcription factors Zif268, c-Fos, Arc, and BDNF in the CA1 region of the hippocampus. These factors are involved in dendritic protein synthesis and long-term structural changes of synapses, leading to long lasting LTP (Ravassard et al. 2015). Another study elucidated the effects of SD on long-term memory consolidation and synaptic plasticity. Impaired LTP is observed in 3 h SD mice 1 h after training. However, SD immediately after training does not impair spatial memory and does not affect LTP. This suggests that 3 h is the critical window period after training during which SD impairs hippocampal functions (Prince et al. 2014).
Synaptic plasticity in sleep-deprived dendritic spines
Memory consolidation and storage is related to structural changes in synaptic connectivity. Decrease in the dendritic spine number in the CA1 region of the hippocampus is observed following 5 h of SD. Cofilin activity is upregulated due to CAMP-PKA-LIMK signalling affected by cAMP-degrading phosphodiesterase-4A5 (PDE4A5) during SD. However, recovery in sleep suppresses cofilin functions, normalizing structural alterations in spines, spine loss, and deficits in hippocampal synaptic plasticity. cAMP-PDE4-PKA-LIMK-cofilin activation signalling pathway plays a cardinal role in restoring SD-induced memory disruption (Havekes et al. 2016). In the rat cortex and hippocampus, GluR1-containing AMPA receptors (AMPAR) levels are found to be high during wake and low during sleep. Modifications in the phosphorylation states of AMPARs, CamKII, and GSK3β were reported, which is in line with synaptic potentiation on wake and depression on sleep and explains the preservation of overall balance of synaptic strength (Vyazovskiy et al. 2008).
Hippocampal neurogenesis
In a recent study by Hinojosa-Godinez. (2019), the effect of long-term SD on hippocampal neurogenesis was studied and epigenetic factors were involved in modulating the responses. After an induction of 96 h, SD was treated with melatonin and epigenetic factors were assessed by computational text mining and keyword clustering. The result shows increased MECP2 expression and reduced Sirt1 expression, specifically in dentate gyrus. Increased expression of let-7b, mir-132, and mir-124 was also observed in dentate gyrus after the administration of melatonin. The treated groups have more BrdU cells compared with the untreated group. This suggests that melatonin mediates the expression of epigenetic factors, which in turn control the proliferation of neural progenitor cells in adult dentate gyrus under long-term SD conditions (Hinojosa-Godinez et al. 2019). SD is associated with increased production of proinflammatory mediators. Acute REM SD for 3 days leads to increased IL-17A, IL-17F, and activation of p38 MAPK in the mouse hippocampus followed by suppression of cell proliferation in dentate gyrus. The administration of recombinant IL-17 in mice without SD activates MAPK and suppresses neural progenitor cell proliferation in the dentate gyrus (Cui et al. 2019). An inverse relationship between SD and neurogenesis was noticed. The aged brain possesses a lower rate of neurogenesis. Young mice treated with cytosine-beta-d-arabinofuranoside (AraC), an antimitotic agent, show sleep-wake architecture like that of aged mice. This suggests that a decrease in hypothalamic cell proliferation is detrimental to the sleep-wake cycle, which is also observed in the ageing brain (Kostin et al. 2019). Mounting evidence proposes that the hippocampus is segregated anatomically and functionally into two regions, dorsolateral controlling cognitive functions and ventromedial regulating mood and stress response. Regional differences in the adverse effects of SD on hippocampal neurogenesis show a significant decrease in the densities of Ki-67 and doublecortin (DCX)—immunopositive cells in both dorsal and ventral hippocampus. The results implicate the negative effects of SD on hippocampal neurogenesis in both the dorsal and ventral dentate gyrus (Murata et al. 2018). To establish the role of sleep in the earliest periods of nervous system development, a sleep state in Drosophila larvae that coincides with a major wave of neurogenesis was identified. Using real-time behavioural monitoring in a closed loop SD, sleep loss in larvae impairs cell division of neural progenitors. This suggests that sleep, in early life, regulates neural stem cell proliferation (Szuperak et al. 2018). SD also upsurges intracellular adenosine levels and impairs neural cell proliferation and cognition. Selective antagonistic effects of adenosine A1R on adult neural cell proliferation were examined using 8-cyclopentyl-1,3-dimethylxanthine (8-CPT. The 8-CPT treated group shows an increase in BrdU, Ki-67, and DCX positive cells. BDNF was decreased in DG and CA1 regions after SD, whereas BDNF level was increased after 8-CPT treatment (Chauhan et al. 2016). SD induces initial decrease and subsequent increase in adult neurogenesis (Fig. 6) (Mirescu et al. 2006).
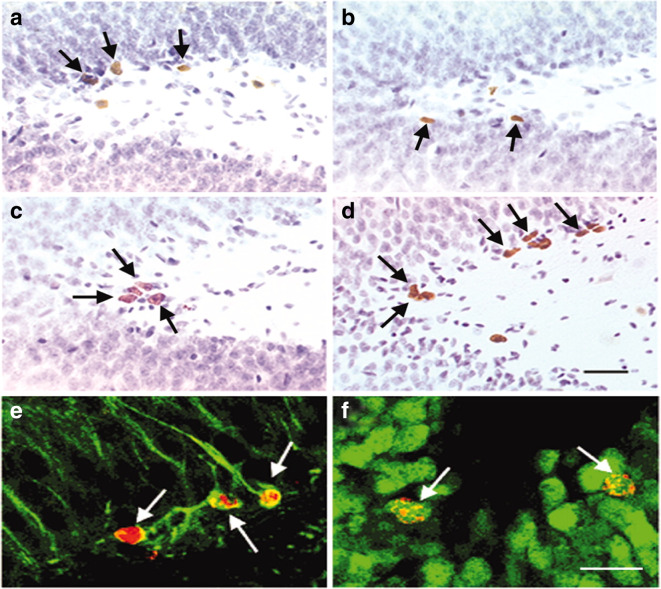
Fig. 6.
Effects of SD on neurogenesis analysed using BrdU staining. Adult male Sprague Dawley rats subjected to SD by small platform (SP) method. a, b An initial decrease in BrdU immunopositive cells in dentate gyrus of hippocampal region of SD rats. c, d Subsequent increase in granular cell proliferation and neurogenesis after 1 week of unrestricted recovery of sleep. e, f Immunofluorescence analysis indicating maturation of neuronal cells after unrestricted sleep recovery. e After 1 week of BrdU administration, BrdU positive cells showed morphological characteristics of granule cells colabelled with Tuj1, a marker for immature neurons. F indicates the BrdU positive cells colabelled with NeuN, a marker for mature neurons after 3 weeks of BrdU administration (Mirescu et al. 2006) (reused as per the “Copyright (1993-2008) National Academy of Sciences”)
Dendritic spine aberrations and learning and memory
Formation of postsynaptic dendritic spines on layer V neurons of the mouse motor cortex is promoted in sleep after motor learning. With respect to different learning tasks, new spines are formed on different sets of dendritic branches and protected from elimination on learning new tasks. The activated neurons during the learning process are reactivated following NREM sleep, indicating the key role of sleep in ageing and encouraging learning-dependent synapse formation and maintenance that contribute to memory storage (Yang et al. 2014). Memory consolidation involves synaptic consolidation and system consolidation. SD attenuates cAMP signalling, CREB-mediated gene transcription, and translation process through mTOR signalling, while structural plasticity is inhibited through PKA-LIMK-cofilin pathway (Havekes and Abel 2017). CSR of 18 h per day for a period of 21 days causes a decrease in length of dendritic tree in the CA1 region of the hippocampus. This shortening in the dendritic length is protected by treatment with curcumin, which also prevents the loss of stubby and mushroom spines (Noorafshan et al. 2018). The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons (Zagrebelsky et al. 2005). Mutant male mice with a deletion of the p75 neurotrophin receptor (p75(NTR)) gene show no loss in the structural and functional plasticity of the hippocampus. The detrimental effects of SD on hippocampal cAMP-CREB-BDNF, cAMP-PKA-LIMK1-cofilin, and RhoA-ROCK2 pathways are also prevented in mice lacking p75 (NTR). Hence, p75 (NTR) is a potential novel target to protect from the detrimental effects of SD (Wong et al. 2019). The negative effects of SD are also linked to dysregulation in cofilin’s phosphorylation, which is a downstream signalling pathway in the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease (Shaw and Bamburg 2017).
Synaptic tagging and capture (STC) is a type of heterosynaptic metaplasticity, where combining strong stimulation in one synaptic output with weak stimulation at another input leads to long-lasting synaptic strengthening induced by weak input. SD impairs STC in stratum radiatum of CA1 area in hippocampus (Vecsey et al. 2018). Aged mice show increased zinc levels, tetani-induced late LTP and STC. These changes in synaptic plasticity are reversed upon zinc chelation, which is increased with age (Shetty et al. 2017). Twenty-four hours of SD in mice reduces the expression of NMDA receptor subunit NR1 and excitatory postsynaptic currents mediated by NMDAR in hippocampal perforate path-dentate granule cell synapses. SD-induced decrease in hippocampus-dependent contextual memory and long-term synaptic plasticity is related to the underlying decrease in the NMDAR expression (Chen et al. 2006). A blockade of A1 receptor signalling using an adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dimethylxanthine (8-CPT), is shown to prevent SD-induced deficit in late phase LTP. The results indicate the role of A1R activity in SD-induced deficits in hippocampal synaptic plasticity and its potential as a novel therapeutic target to reverse SD-induced impairment in hippocampal synaptic plasticity (Florian et al. 2011).
Conclusion
Sleep forms an indispensable component of overall health and well-being. The loss of sleep exerts adverse effects on not only physical health but also mental health, particularly the cognitive functions, by triggering various pathological factors. SD affects neurogenesis, dendritic spines structure and functions, LTD and LTP, synaptic plasticity and produces cognitive decline. These phenomena are also noted in ageing and vascular dementia. Alteration in neurotransmitter levels (e.g. glutamate, GABA, acetyl choline, and adrenaline) that facilitate synaptic communication, is well established in SD and ageing. Furthermore, a similar alteration in the levels of various signalling molecules like Sirt-1, PGC1α, FOXO, PARP1, and P66shc was shown to impose pathologically detrimental roles on vascular structure and functions as reported in ageing.
Interpreting the data gathered from abstracting and indexing databases suggest a striking pathological overlapping between SD and vascular ageing, which spurts to propose the possible impact of SD on “early” brain-vascular ageing (Fig. 7). Clinically, these observations indicate that optimal sleep is required for the maintenance of vascular structure and functions (including brain vasculature) and cognitive performance. To the best of our knowledge, this is the first manuscript providing a molecular pathological basis that links SD to brain vascular ageing. However, further direct experiments are warranted to check the validity of our inferences and provide data on the exact pathological mechanisms that link SD to preponement of brain-vascular ageing. One possible limitation of this review is the limited number of articles in this area, and this may have affected the scope of our inference.
Fig. 7.
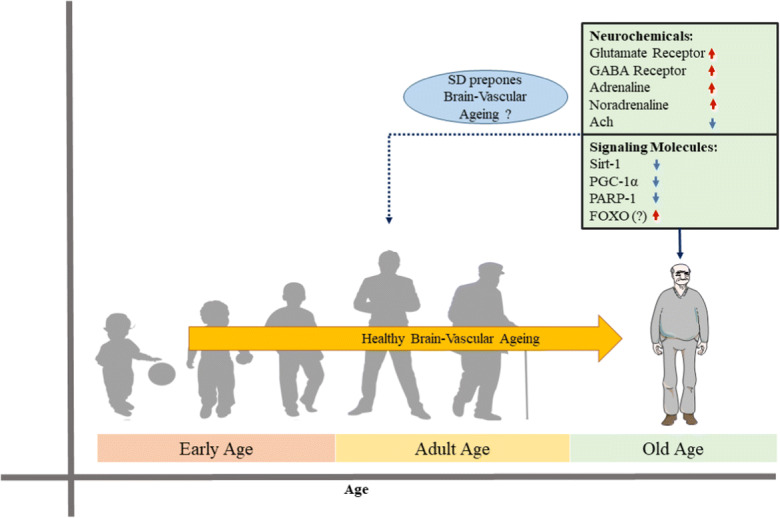
Sketch outlining the effects of SD on the proposed accelerated brain vascular ageing. We propose that the pathological alterations in neurochemicals and signalling molecules in SD might be a risk factor for early brain-vascular ageing. These effects are not reported in healthy ageing with adequate sleep. Orange coloured arrow indicates healthy brain-vascular ageing. Red and blue colour arrows indicate the up-regulation and down-regulation of neurochemicals and signalling molecules in SD condition which could possibly contribute for the brain early vascular ageing (proposed)
Funding information
AMM is thankful to JSS AHER, Mysuru, for providing the seed grant. GJG is supported by the NMHRC, the ARC and Macquarie University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Saravana Babu Chidambaram, Email: babupublications@gmail.com.
Meena Kishore Sakharkar, Email: meena.sakharkar@usask.ca.
References
- Adams SK, Daly JF, Williford DN. Adolescent sleep and cellular phone use: recent trends and implications for research. Health Serv Insights. 2013;6:99–103. doi: 10.4137/HSI.S11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal B, Nour M, Riddhi S, Memet E, Ying W, Marie-Pierre S-O, Sanja J. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association go red for women strategically focused research network. J Am Heart Assoc. 2018;7:e008590. doi: 10.1161/JAHA.118.008590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alborch E, Torregrosa G, Terrasa JC, Estrada C. GABA receptors mediate cerebral vasodilation in the unanesthetized goat. Brain Res. 1984;321:103–110. doi: 10.1016/0006-8993(84)90685-1. [DOI] [PubMed] [Google Scholar]
- Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, Bolaki M, Georgopoulos D. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121:801–807. doi: 10.1097/ALN.0000000000000361. [DOI] [PubMed] [Google Scholar]
- Anafi RC, Kayser MS, Raizen DM. Exploring phylogeny to find the function of sleep. Nat Rev Neurosci. 2019;20:109. doi: 10.1038/s41583-018-0098-9. [DOI] [PubMed] [Google Scholar]
- Arntz RM, van den Broek SMA, van Uden IWM, Ghafoorian M, Platel B, Rutten-Jacobs LCA, Maaijwee NAM, Schaapsmeerders P, Schoonderwaldt HC, van Dijk EJ, de Leeuw F-E. Accelerated development of cerebral small vessel disease in young stroke patients. Neurology. 2016;87:1212–1219. doi: 10.1212/WNL.0000000000003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrooz F, Liu H, Kochi C, Salim S. Early life sleep deprivation: role of oxido-inflammatory processes. Neuroscience. 2019;406:22–37. doi: 10.1016/j.neuroscience.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi M, Ghosh M, Longden TA, Vega MJ, Gesierich B, Hellal F, Lourbopoulos A, Nelson MT, Plesnila N. Dysfunction of mouse cerebral arteries during early aging. J Cereb Blood Flow Metab. 2015;35:1445–1453. doi: 10.1038/jcbfm.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint AR, Puskás T, Menyhárt Á, Kozák G, Szenti I, Kónya Z, et al. Aging impairs cerebrovascular reactivity at preserved resting cerebral arteriolar tone and vascular density in the laboratory rat. Front Aging Neurosci. 2019;11. [DOI] [PMC free article] [PubMed]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Baruah J, Vasudevan A. The vessels shaping mental health or illness. Open Neurol J. 2019;13:1. doi: 10.2174/1874205X01913010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+−activated K+ channel activation. Arterioscler Thromb Vasc Biol. 2013;33:1892–1901. doi: 10.1161/ATVBAHA.113.301514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, Bushey D, Chini M, Tononi G, Cirelli C. Contribution of sleep to the repair of neuronal DNA double-strand breaks: evidence from flies and mice. Sci Rep. 2016;6:36804. doi: 10.1038/srep36804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;16:593–610. doi: 10.1038/nrm4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HL, Khoruzhik Y, Hayden D, Huang H, Sanders J, Walsh MB, Biron D, Hart AC. Normal sleep bouts are not essential for C. elegans survival and FoxO is important for compensatory changes in sleep. BMC Neurosci. 2018;19:10. doi: 10.1186/s12868-018-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A, Greco A, Giorgio M, Pelicci PG, de Kloet R, Alleva E, Minghetti L, Cirulli F. Deletion of the lifespan determinant p66(Shc) improves performance in a spatial memory task, decreases levels of oxidative stress markers in the hippocampus and increases levels of the neurotrophin BDNF in adult mice. Exp Gerontol. 2008;43:200–208. doi: 10.1016/j.exger.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Boerman EM, Everhart JE, Segal SS. Advanced age decreases local calcium signaling in endothelium of mouse mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol. 2016;310:H1091–H1096. doi: 10.1152/ajpheart.00038.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth SL. Vitamin K status in the elderly. Curr Opin Clin Nutr Metab Care. 2007;10:20–23. doi: 10.1097/MCO.0b013e328011ab5f. [DOI] [PubMed] [Google Scholar]
- Bourdon AK, Spano GM, Marshall W, Bellesi M, Tononi G, Serra PA, Baghdoyan HA, Lydic R, Campagna SR, Cirelli C. Metabolomic analysis of mouse prefrontal cortex reveals upregulated analytes during wakefulness compared to sleep. Sci Rep. 2018;8:1–17. doi: 10.1038/s41598-018-29511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C, Hirasawa M, Semba K. Sleep deprivation distinctly alters glutamate transporter 1 apposition and excitatory transmission to orexin and MCH neurons. J Neurosci. 2018;38:2505–2518. doi: 10.1523/JNEUROSCI.2179-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camici GG, Cosentino F, Tanner FC, Lüscher TF. The role of p66Shc deletion in age-associated arterial dysfunction and disease states. J Appl Physiol. 2008;105:1628–1631. doi: 10.1152/japplphysiol.90579.2008. [DOI] [PubMed] [Google Scholar]
- Canugovi C, Stevenson MD, Vendrov AE, Hayami T, Robidoux J, Xiao H, et al. Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening. Redox Biol. 2019:101288. 10.1016/j.redox.2019.101288. [DOI] [PMC free article] [PubMed]
- Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–1776. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Chapter 2 - normal human sleep: an overview. 2005.
- Chang H-M, Wu U-I, Lan C-T. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res. 2009;47:211–220. doi: 10.1111/j.1600-079X.2009.00704.x. [DOI] [PubMed] [Google Scholar]
- Chatburn A, Kohler MJ, Payne JD, Drummond SPA. The effects of sleep restriction and sleep deprivation in producing false memories. Neurobiol Learn Mem. 2017;137:107–113. doi: 10.1016/j.nlm.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Chauhan G, Ray K, Sahu S, Roy K, Jain V, Wadhwa M, Panjwani U, Kishore K, Singh SB. Adenosine A1 receptor antagonist mitigates deleterious effects of sleep deprivation on adult neurogenesis and spatial reference memory in rats. Neuroscience. 2016;337:107–116. doi: 10.1016/j.neuroscience.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu K, Hu Y, Chang Y. Ginkgo biloba extract protects mesenteric arterioles of old rats via improving vessel elasticity through Akt/FoxO3a signaling pathway. Ann Vasc Surg. 2019;57:220–228. doi: 10.1016/j.avsg.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Chidambaram SB, Rathipriya AG, Bolla SR, Bhat A, Ray B, Mahalakshmi AM, Manivasagam T, Thenmozhi AJ, Essa MM, Guillemin GJ, Chandra R, Sakharkar MK. Dendritic spines: revisiting the physiological role. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;92:161–193. doi: 10.1016/j.pnpbp.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Cho SY, Lee JH, Song MJ, Park PJ, Shin ES, Sohn JH, Seo D-B, Lim KM, Kim WG, Lee S-J. Effects of chitooligosaccharide lactate salt on sleep deprivation-induced fatigue in mice. Biol Pharm Bull. 2010;33:1128–1132. doi: 10.1248/bpb.33.1128. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. J Neurochem. 2006;98:1632–1645. doi: 10.1111/j.1471-4159.2006.04058.x. [DOI] [PubMed] [Google Scholar]
- Clément O, Sapin E, Bérod A, Fort P, Luppi P-H. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep. 2011;34:419–423. doi: 10.1093/sleep/34.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37:2388–2404. doi: 10.1038/npp.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly PJ, Adams F, Tayar ZI, Khan F. Peripheral vascular responses to acetylcholine as a predictive tool for response to cholinesterase inhibitors in Alzheimer’s disease. BMC Neurol. 2019;19:88. doi: 10.1186/s12883-019-1316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CB, Neufeld EV, Dolezal BA, Martin JL. Sleep deprivation and obesity in adults: a brief narrative review. BMJ Open Sport Exerc Med. 2018;4:e000392. doi: 10.1136/bmjsem-2018-000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo B-TN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z, Yabluchanskiy A. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41:125–136. doi: 10.1007/s11357-019-00063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Xue R, Zhang X, Chen S, Wan Y, Wu W. Sleep deprivation inhibits proliferation of adult hippocampal neural progenitor cells by a mechanism involving IL-17 and p38 MAPK. Brain Res. 2019;1714:81–87. doi: 10.1016/j.brainres.2019.01.024. [DOI] [PubMed] [Google Scholar]
- da Silva R-LJ, Machado RB, Suchecki D. Chronic REM sleep restriction in juvenile male rats induces anxiety-like behavior and alters monoamine systems in the amygdala and hippocampus. Mol Neurobiol. 2018;55:2884–2896. doi: 10.1007/s12035-017-0541-3. [DOI] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalopoulou C, Koukounari A, Ayuso-Mateos JL, Prince M, Prina AM. Associations of lifestyle behaviour and healthy ageing in five Latin American and the Caribbean countries—a 10/66 population-based cohort study. Nutrients. 2018;10. 10.3390/nu10111593. [DOI] [PMC free article] [PubMed]
- Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Med. 2007;8(Suppl 4):S27–S34. doi: 10.1016/S1389-9457(08)70006-6. [DOI] [PubMed] [Google Scholar]
- de Almeida AJPO, Ribeiro TP, de Medeiros IA. Aging: molecular pathways and implications on the cardiovascular system. Oxidative Med Cell Longev. 2017;2017:7941563. doi: 10.1155/2017/7941563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vivo L, Nagai H, De Wispelaere N, Spano GM, Marshall W, Bellesi M, et al. Evidence for sleep-dependent synaptic renormalization in mouse pups. Sleep. 2019;42. 10.1093/sleep/zsz184. [DOI] [PMC free article] [PubMed]
- Deak F, Freeman WM, Ungvari Z, Csiszar A, Sonntag WE. Recent developments in understanding brain aging: implications for Alzheimer’s disease and vascular cognitive impairment. J Gerontol A Biol Sci Med Sci. 2016;71:13–20. doi: 10.1093/gerona/glv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto OH, Mera RM, Del Brutto VJ, Castillo PR. Enlarged basal ganglia perivascular spaces and sleep parameters. A population-based study. Clin Neurol Neurosurg. 2019;182:53–57. doi: 10.1016/j.clineuro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- del Cid-Pellitero E, Plavski A, Mainville L, Jones BE. Homeostatic changes in GABA and glutamate receptors on excitatory cortical neurons during sleep deprivation and recovery. Front Syst Neurosci. 2017;11. 10.3389/fnsys.2017.00017. [DOI] [PMC free article] [PubMed]
- Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Wu G, Zhu T, Chen H, Zhu Y, Zhu G, Han F, Zhao H. VEGF promotes cartilage angiogenesis by phospho-ERK1/2 activation of Dll4 signaling in temporomandibular joint osteoarthritis caused by chronic sleep disturbance in Wistar rats. Oncotarget. 2017;8:17849–17861. doi: 10.18632/oncotarget.14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver RJ, Lamb AL, Wyner AJ, Raizen DM. DAF-16/FOXO regulates homeostasis of essential sleep-like behavior during larval transitions in C. elegans. Curr Biol. 2013;23:501–506. doi: 10.1016/j.cub.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangjan C, Rangsinth P, Gu X, Zhang S, Wink M, Tencomnao T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine. 2019;64:153061. doi: 10.1016/j.phymed.2019.153061. [DOI] [PubMed] [Google Scholar]
- Edwards BA, O’Driscoll DM, Ali A, Jordan AS, Trinder J, Malhotra A. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31:618–633. doi: 10.1055/s-0030-1265902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsåshagen T, Mutsaerts HJ, Zak N, Norbom LB, Quraishi SH, Pedersen PØ, Malt UF, Westlye LT, van Someren EJ, Bjørnerud A, Groote IR. Cerebral blood flow changes after a day of wake, sleep, and sleep deprivation. NeuroImage. 2019;186:497–509. doi: 10.1016/j.neuroimage.2018.11.032. [DOI] [PubMed] [Google Scholar]
- Eros K, Magyar K, Deres L, Skazel A, Riba A, Vamos Z, et al. Chronic PARP-1 inhibition reduces carotid vessel remodeling and oxidative damage of the dorsal hippocampus in spontaneously hypertensive rats. PLoS One. 2017;12. 10.1371/journal.pone.0174401. [DOI] [PMC free article] [PubMed]
- Farca Luna AJ, Perier M, Seugnet L. Amyloid precursor protein in Drosophila glia regulates sleep and genes involved in glutamate recycling. J Neurosci. 2017;37:4289–4300. doi: 10.1523/JNEUROSCI.2826-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifel K. Sirtuin 3: a molecular pathway linking sleep deprivation to neurological diseases. J Neurosci. 2014;34:9179–9181. doi: 10.1523/JNEUROSCI.1848-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31:6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Mangialasche F, Qiu C. Brain aging: lessons from community studies. Nutr Rev. 2010;68(Suppl 2):S119–S127. doi: 10.1111/j.1753-4887.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol. 2018;124:1194. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B, Wang JJ, Flood VM, Burlutsky G, Wong TY, Mitchell P. The associations between blood levels of homocysteine, folate, vitamin B12, and retinal vascular caliber. Am J Ophthalmol. 2009;148:902–909. doi: 10.1016/j.ajo.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Grandner MA. Sleep, health, and society. Sleep Med Clin. 2017;12:1–22. doi: 10.1016/j.jsmc.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau-Perales AB, Gómez-Chacón B, Gallo M. Differential activity pattern of c-Fos in the nucleus accumbens between adult and aged rats during flavor recognition memory. Behav Brain Res. 2019;371:111935. doi: 10.1016/j.bbr.2019.111935. [DOI] [PubMed] [Google Scholar]
- Hagen EW, Starke SJ, Peppard PE. The association between sleep duration and leptin, ghrelin, and adiponectin among children and adolescents. Curr Sleep Med Rep. 2015;1:185–194. [Google Scholar]
- Hartse KM. Phylogeny of sleep. In: Chokroverty S, editor. Sleep disorders medicine: basic science. New York: Technical Considerations and Clinical Aspects. Springer New York; 2017. pp. 127–142. [Google Scholar]
- Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing—implications in hypertension. J Mol Cell Cardiol. 2015;83:112–121. doi: 10.1016/j.yjmcc.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa N, Fujie S, Horii N, Uchida M, Toyama Y, Inoue K, Sanada K, Hamaoka T, Iemitsu M. Aging-induced elevation in circulating complement C1q level is associated with arterial stiffness. Exp Gerontol. 2019;124:110650. doi: 10.1016/j.exger.2019.110650. [DOI] [PubMed] [Google Scholar]
- Havekes R, Abel T. The tired hippocampus: the molecular impact of sleep deprivation on hippocampal function. Curr Opin Neurobiol. 2017;44:13–19. doi: 10.1016/j.conb.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes R, Park AJ, Tudor JC, Luczak VG, Hansen RT, Ferri SL, et al. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. eLife. 2016;5. 10.7554/eLife.13424. [DOI] [PMC free article] [PubMed]
- Hinojosa-Godinez A, Jave-Suarez LF, Flores-Soto M, Gálvez-Contreras AY, Luquín S, Oregon-Romero E, González-Pérez O, González-Castañeda RE. Melatonin modifies SOX2+ cell proliferation in dentate gyrus and modulates SIRT1 and MECP2 in long-term sleep deprivation. Neural Regen Res. 2019;14:1787–1795. doi: 10.4103/1673-5374.257537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst SC, Sousek A, Hefti K, Saberi-Moghadam S, Buck A, Ametamey SM, et al. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprivation. eLife. 2017;6. 10.7554/eLife.28751. [DOI] [PMC free article] [PubMed]
- Hu ZB, Chen Y, Gong YX, Gao M, Zhang Y, Wang GH, Tang RN, Liu H, Liu BC, Ma KL. Activation of the CXCL16/CXCR6 pathway by inflammation contributes to atherosclerosis in patients with end-stage renal disease. Int J Med Sci. 2016;13:858. doi: 10.7150/ijms.16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, Kuller LH, Barinas-Mitchell EJM, McDade EM, Klunk WE, Cohen AD, Mathis CA, DeKosky ST, Price JC, Lopez OL. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–568. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension: epidemiology, pathobiology, and treatment. Circ Res. 2019;124:1025–1044. doi: 10.1161/CIRCRESAHA.118.313260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurciuc S, Cimpean AM, Mitu F, Heredea R, Iurciuc M. Vascular aging and subclinical atherosclerosis: why such a “never ending” and challenging story in cardiology? Clin Interv Aging. 2017;12:1339–1345. doi: 10.2147/CIA.S141265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani B, Rajkumar C. Ageing and vascular ageing. Postgrad Med J. 2006;82:357–362. doi: 10.1136/pgmj.2005.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javad-Moosavi B-Z, Vaezi G, Nasehi M, Haeri-Rouhani S-A, Zarrindast M-R. Critical role of CA1 muscarinic receptors on memory acquisition deficit induced by total (TSD) and REM sleep deprivation (RSD) Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;79:128–135. doi: 10.1016/j.pnpbp.2017.05.024. [DOI] [PubMed] [Google Scholar]
- Jha PK, Foppen E, Kalsbeek A, Challet E. Sleep restriction acutely impairs glucose tolerance in rats. Physiol Rep. 2016;4:e12839. doi: 10.14814/phy2.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Opel RA, Kaiser ME, Chau AQ, Quintana JR, Nipper MA, Finn DA, Hammock EAD, Lim MM. Early-life sleep disruption increases parvalbumin in primary somatosensory cortex and impairs social bonding in prairie voles. Sci Adv. 2019;5:eaav5188. doi: 10.1126/sciadv.aav5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, Porkka-Heiskanen T, McCarley RW, Basheer R. Cholinergic neurons of the basal forebrain mediate biochemical and electrophysiological mechanisms underlying sleep homeostasis. Eur J Neurosci. 2015;41:182–195. doi: 10.1111/ejn.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal SM. Pharmacological modulation of brain levels of glutamate and GABA in rats exposed to total sleep deprivation. J Exp Pharmacol. 2010;2:65–71. doi: 10.2147/jep.s11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-K, Kook H. Diverse roles of noncoding RNAs in vascular calcification. Arch Pharm Res. 2019;42:244–251. doi: 10.1007/s12272-019-01118-z. [DOI] [PubMed] [Google Scholar]
- Kim Y, Chen L, McCarley RW, Strecker RE. Sleep allostasis in chronic sleep restriction: the role of the norepinephrine system. Brain Res. 2013;1531:9–16. doi: 10.1016/j.brainres.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Elmenhorst D, Weisshaupt A, Wedekind F, Kroll T, McCarley RW, Strecker RE, Bauer A. Chronic sleep restriction induces long-lasting changes in adenosine and noradrenaline receptor density in the rat brain. J Sleep Res. 2015;24:549–558. doi: 10.1111/jsr.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LJ, Coelho FM, Araujo P, Tedesco RC, Souza RB, Tufik S, Andersen ML. Sleep restriction reduces the survival time and aggravates the neurological dysfunction and memory impairments in an animal model of cerebral hypoperfusion. Brain Res. 2016;1644:213–221. doi: 10.1016/j.brainres.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Kim M, Jang D, Yoo E, Oh Y, Sonn JY, Lee J, Ki Y, Son HJ, Hwang O, Lee C, Lim C, Choe J. Rogdi defines GABAergic control of a wake-promoting dopaminergic pathway to sustain sleep in Drosophila. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-11941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CE, Shin S, Lee H-W, Lim J, Lee J, Shin A, Kang D. Association between sleep duration and metabolic syndrome: a cross-sectional study. BMC Public Health. 2018;18:720. doi: 10.1186/s12889-018-5557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Lee B, Lee J, Kim ME, Lee JS, Chung JH, et al. FoxO6-mediated IL-1β induces hepatic insulin resistance and age-related inflammation via the TF/PAR2 pathway in aging and diabetic mice. Redox Biol. 2019;24. 10.1016/j.redox.2019.101184. [DOI] [PMC free article] [PubMed]
- Kimura N, Aso Y, Yabuuchi K, Ishibashi M, Hori D, Sasaki Y, et al. Modifiable lifestyle factors and cognitive function in older people: a cross-sectional observational study. Front Neurol. 2019;10. 10.3389/fneur.2019.00401. [DOI] [PMC free article] [PubMed]
- King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostin A, Alam MA, McGinty D, Szymusiak R, Alam MN. Chronic suppression of hypothalamic cell proliferation and neurogenesis induces aging-like changes in sleep-wake organization in young mice. Neuroscience. 2019;404:541–556. doi: 10.1016/j.neuroscience.2019.01.053. [DOI] [PubMed] [Google Scholar]
- Kristofikova Z, Sirova J, Klaschka J, Ovsepian SV. Acute and chronic sleep deprivation-related changes in N-methyl-D-aspartate receptor-nitric oxide signalling in the rat cerebral cortex with reference to aging and brain lateralization. Int J Mol Sci. 2019:20. 10.3390/ijms20133273. [DOI] [PMC free article] [PubMed]
- Krouwer VJ, Hekking LH, Langelaar-Makkinje M, Regan-Klapisz E, Post JA. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc Cell. 2012;4:12. doi: 10.1186/2045-824X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Wolf E, Maier JG, Mainberger F, Feige B, Schmid H, Bürklin J, Maywald S, Mall V, Jung NH, Reis J, Spiegelhalder K, Klöppel S, Sterr A, Eckert A, Riemann D, Normann C, Nissen C. Sleep recalibrates homeostatic and associative synaptic plasticity in the human cortex. Nat Commun. 2016;7:12455. doi: 10.1038/ncomms12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La H, T-T C, Wl K. Aging and the control of human skin blood flow. Front Biosci (Landmark Ed) 2010;15:718–739. doi: 10.2741/3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res. 2018;114:513–528. doi: 10.1093/cvr/cvy009. [DOI] [PubMed] [Google Scholar]
- Lam JCM, Ip MSM. Sleep & the metabolic syndrome. Indian J Med Res. 2010;131:206–216. [PubMed] [Google Scholar]
- Lesku JA, Roth TC, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am Nat. 2006;168:441–453. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- Li X, Yang H-Y, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Duan D, Zhang J, Zhou Y, Qin X, Du G, Gao L. Bioinformatic prediction of critical genes and pathways involved in longevity in Drosophila melanogaster. Mol Gen Genomics. 2019;294:1463–1475. doi: 10.1007/s00438-019-01589-1. [DOI] [PubMed] [Google Scholar]
- Lipecz A, Csipo T, Tarantini S, Hand RA, Ngo B-TN, Conley S, Nemeth G, Tsorbatzoglou A, Courtney DL, Yabluchanska V, Csiszar A, Ungvari ZI, Yabluchanskiy A. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience. 2019;41:341–349. doi: 10.1007/s11357-019-00078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço CF, Ledo A, Caetano M, Barbosa RM, Laranjinha J. Age-dependent impairment of neurovascular and neurometabolic coupling in the hippocampus. Front Physiol. 2018;9:913. doi: 10.3389/fphys.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti M, Castiglioni S, Bernardini D, Maier JA. Iterleukin 1 alpha is a marker of endothelial cellular senescent. Immun Ageing. 2006;3:4. doi: 10.1186/1742-4933-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins IJ. Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Adv Aging Res. 2016;05:9. [Google Scholar]
- Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, Yagami K, Kilduff TS, Bettler B, Yanagisawa M, Sakurai T. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci U S A. 2009;106:4459–4464. doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T, Takasu M, Hirose Y, Murakoshi N, Sinton CM, Motoike T, Yanagisawa M. GABAA receptor-mediated input change on orexin neurons following sleep deprivation in mice. Neuroscience. 2015;284:217–224. doi: 10.1016/j.neuroscience.2014.09.063. [DOI] [PubMed] [Google Scholar]
- McAleese KE, Graham S, Dey M, Walker L, Erskine D, Johnson M, Johnston E, Thomas AJ, McKeith IG, DeCarli C, Attems J. Extravascular fibrinogen in the white matter of Alzheimer’s disease and normal aged brains: implications for fibrinogen as a biomarker for Alzheimer’s disease. Brain Pathol. 2019;29:414–424. doi: 10.1111/bpa.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566:383–387. doi: 10.1038/s41586-019-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Cordeira JW, Jeffrey BA, Ward CP, Winston S, McCarley RW, Strecker RE. c-Fos protein expression is increased in cholinergic neurons of the rodent basal forebrain during spontaneous and induced wakefulness. Brain Res Bull. 2009;80:382–388. doi: 10.1016/j.brainresbull.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Singh S, Khanday MA, Mallick BN. Reciprocal changes in noradrenaline and GABA levels in discrete brain regions upon rapid eye movement sleep deprivation in rats. Neurochem Int. 2017;108:190–198. doi: 10.1016/j.neuint.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Mendelsohn AR, Larrick JW. Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res. 2013;16:518–523. doi: 10.1089/rej.2013.1530. [DOI] [PubMed] [Google Scholar]
- Metaxakis A, Tain LS, Grönke S, Hendrich O, Hinze Y, Birras U, Partridge L. Lowered insulin signalling ameliorates age-related sleep fragmentation in Drosophila. PLoS Biol. 2014;12:e1001824. doi: 10.1371/journal.pbio.1001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SA, Patel H, Anderson SI, England TJ, O’Sullivan SE. Osteocalcin, vascular calcification, and atherosclerosis: a systematic review and meta-analysis. Front Endocrinol. 2017;8:183. doi: 10.3389/fendo.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, III, Buysse DJ, DeGrazia JM, Kupfer DJ. The relationship between lifestyle regularity and subjective sleep quality. Chronobiol Int. 2003;20:97–107. doi: 10.1081/cbi-120017812. [DOI] [PubMed] [Google Scholar]
- Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Morris AWJ, Carare RO, Schreiber S, Hawkes CA. The cerebrovascular basement membrane: role in the clearance of β-amyloid and cerebral amyloid Angiopathy. Front Aging Neurosci. 2014;6. 10.3389/fnagi.2014.00251. [DOI] [PMC free article] [PubMed]
- Murata Y, Oka A, Iseki A, Mori M, Ohe K, Mine K, Enjoji M. Prolonged sleep deprivation decreases cell proliferation and immature newborn neurons in both dorsal and ventral hippocampus of male rats. Neurosci Res. 2018;131:45–51. doi: 10.1016/j.neures.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Nasehi M, Mosavi-Nezhad S-M, Khakpai F, Zarrindast M-R. The role of omega-3 on modulation of cognitive deficiency induced by REM sleep deprivation in rats. Behav Brain Res. 2018;351:152–160. doi: 10.1016/j.bbr.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Nikonova EV, Gilliland JD, Tanis KQ, Podtelezhnikov AA, Rigby AM, Galante RJ, et al. Transcriptional profiling of cholinergic neurons from basal forebrain identifies changes in expression of genes between sleep and wake. Sleep. 2017;40. 10.1093/sleep/zsx059. [DOI] [PMC free article] [PubMed]
- Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag. 2008;4:547–552. doi: 10.2147/vhrm.s1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson PM. Early vascular ageing – a concept in development. Eur Endocrinol. 2015;11:26–31. doi: 10.17925/EE.2015.11.01.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorafshan A, Karimi F, Kamali A-M, Karbalay-Doust S, Nami M. Could curcumin protect the dendritic trees of the CA1 neurons from shortening and shedding induced by chronic sleep restriction in rats? Life Sci. 2018;198:65–70. doi: 10.1016/j.lfs.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Norozpour Y, Nasehi M, Sabouri-Khanghah V, Torabi-Nami M, Zarrindast M-R. The effect of CA1 α2 adrenergic receptors on memory retention deficit induced by total sleep deprivation and the reversal of circadian rhythm in a rat model. Neurobiol Learn Mem. 2016;133:53–60. doi: 10.1016/j.nlm.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Norton CE, Sinkler SY, Jacobsen NL, Segal SS. Advanced age protects resistance arteries of mouse skeletal muscle from oxidative stress through attenuating apoptosis induced by hydrogen peroxide. J Physiol. 2019;597:3801–3816. doi: 10.1113/JP278255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novati A, Hulshof HJ, Granic I, Meerlo P. Chronic partial sleep deprivation reduces brain sensitivity to glutamate N-methyl-D-aspartate receptor-mediated neurotoxicity. J Sleep Res. 2012;21:3–9. doi: 10.1111/j.1365-2869.2011.00932.x. [DOI] [PubMed] [Google Scholar]
- Nunn CL, Samson DR, Krystal AD. Shining evolutionary light on human sleep and sleep disorders. Evol Med Public Health. 2016;2016:227–243. doi: 10.1093/emph/eow018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander R, Kilander L, Ahlström H, Lind L, Larsson E-M. Small vessel disease on neuroimaging in a 75-year-old cohort (PIVUS): comparison with cognitive and executive tests. Front Aging Neurosci. 2018;10. 10.3389/fnagi.2018.00217. [DOI] [PMC free article] [PubMed]
- Ohara T, Honda T, Hata J, Yoshida D, Mukai N, Hirakawa Y, Shibata M, Kishimoto H, Kitazono T, Kanba S, Ninomiya T. Association between daily sleep duration and risk of dementia and mortality in a Japanese community. J Am Geriatr Soc. 2018;66:1911–1918. doi: 10.1111/jgs.15446. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Amari M, Fukuda T, Suzuki K, Takatama M. Astrocytic tau pathologies in aged human brain. Neuropathology. 2019;39:187–193. doi: 10.1111/neup.12544. [DOI] [PubMed] [Google Scholar]
- Oshikawa J, Kim S-J, Furuta E, Caliceti C, Chen G-F, McKinney RD, Kuhr F, Levitan I, Fukai T, Ushio-Fukai M. Novel role of p66Shc in ROS-dependent VEGF signaling and angiogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2011;302:H724–H732. doi: 10.1152/ajpheart.00739.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian L, Fenik P, Zhu Y, Zhan G, McBurney MW, Veasey S. SIRT1 regulation of wakefulness and senescence-like phenotype in wake neurons. J Neurosci. 2011;31:4025–4036. doi: 10.1523/JNEUROSCI.5166-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Wang W, Tan T, He W, Dong Z, Wang YT, et al. Maternal sleep deprivation at different stages of pregnancy impairs the emotional and cognitive functions, and suppresses hippocampal long-term potentiation in the offspring rats. Mol Brain. 2016;9. 10.1186/s13041-016-0197-3. [DOI] [PMC free article] [PubMed]
- Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, Schei JL, Rector DM. Vascular compliance limits during sleep deprivation and recovery sleep. Sleep. 2013;36:1459–1470. doi: 10.5665/sleep.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskunova TS, Yurova MN, Ovsyannikov AI, Semenchenko AV, Zabezhinski MA, Popovich IG, et al. Deficiency in poly(ADP-ribose) polymerase-1 (PARP-1) accelerates aging and spontaneous carcinogenesis in mice. Curr Gerontol Geriatr Res. 2008;2008. 10.1155/2008/754190. [DOI] [PMC free article] [PubMed]
- Pocivavsek A, Baratta AM, Mong JA, Viechweg SS. Acute kynurenine challenge disrupts sleep-wake architecture and impairs contextual memory in adult rats. Sleep. 2017;40. 10.1093/sleep/zsx141. [DOI] [PMC free article] [PubMed]
- Prince T-M, Wimmer M, Choi J, Havekes R, Aton S, Abel T. Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiol Learn Mem. 2014;109:122–130. doi: 10.1016/j.nlm.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya S, Mahalakshmi AM, Tuladhar S, Ray B, Sushmitha BS, Shivashree S, Saravanan B, Bishir M, Bhat A, Saravana Babu C. Sleep and body fluids. Int J Nutr Pharmacol Neurol Dis. 2020;10:65. [Google Scholar]
- Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Thieme Medical Publishers. 2012 pp 411–420. [DOI] [PMC free article] [PubMed]
- Queiroz CM, Tiba PA, Moreira KM, Guidine PAM, Rezende GHS, MFD M, Prado MAM, Prado VF, Tufik S, Mello LE. Sleep pattern and learning in knockdown mice with reduced cholinergic neurotransmission. Braz J Med Biol Res. 2013;46:844–854. doi: 10.1590/1414-431X20133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravassard P, Hamieh AM, Malleret G, Salin P-A. Paradoxical sleep: a vigilance state to gate long-term brain plasticity? Neurobiol Learn Mem. 2015;122:4–10. doi: 10.1016/j.nlm.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Ravassard P, Hamieh AM, Joseph MA, Fraize N, Libourel P-A, Lebarillier L, Arthaud S, Meissirel C, Touret M, Malleret G, Salin P-A. REM sleep-dependent bidirectional regulation of hippocampal-based emotional memory and LTP. Cereb Cortex. 2016;26:1488–1500. doi: 10.1093/cercor/bhu310. [DOI] [PubMed] [Google Scholar]
- Raven F, Van der Zee EA, Meerlo P, Havekes R. The role of sleep in regulating structural plasticity and synaptic strength: implications for memory and cognitive function. Sleep Med Rev. 2018;39:3–11. doi: 10.1016/j.smrv.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Raven F, Meerlo P, Van der Zee EA, Abel T, Havekes R. A brief period of sleep deprivation causes spine loss in the dentate gyrus of mice. Neurobiol Learn Mem. 2019;160:83–90. doi: 10.1016/j.nlm.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Zhang M-J, Li T-M, Zhang J, Lin R, Chen S, et al. Quantitative proteomics of sleep-deprived mouse brains reveals global changes in mitochondrial proteins. PLoS One. 2016:11. 10.1371/journal.pone.0163500. [DOI] [PMC free article] [PubMed]
- Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010;185:91–103. doi: 10.1016/B978-0-444-53702-7.00006-3. [DOI] [PubMed] [Google Scholar]
- Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313:H890–H895. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol. 2012;4:a013102. doi: 10.1101/cshperspect.a013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin L, Cevik OS, Koyuncu DD, Kocahan S. Caffeine as a potential arousal enhancer: altered NMDA subunit gene expression without improving cognitive performance in REM sleep deprived rats. Cell Mol Biol (Noisy-le-grand) 2019;65:63–68. [PubMed] [Google Scholar]
- Salazar G, Huang J, Zhao Y. PGC-1alpha a novel regulator of autophagy. FASEB J. 2016;30:1062.4. [Google Scholar]
- Samarakoon SMS, Chandola HM, Ravishankar B. Effect of dietary, social, and lifestyle determinants of accelerated aging and its common clinical presentation: a survey study. Ayu. 2011;32:315–321. doi: 10.4103/0974-8520.93906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin M, Ozguner MF, Onder O, Doguc DK, Ilhan I, Peker Y. The impact of sleep deprivation on hippocampal-mediated learning and memory in rats. Bratisl Lek Listy. 2017;118:408–416. doi: 10.4149/BLL_2017_080. [DOI] [PubMed] [Google Scholar]
- Schei JL, Rector DM. Evoked electrical and cerebral vascular responses during sleep and following sleep deprivation. Prog Brain Res. 2011;193:233–244. doi: 10.1016/B978-0-444-53839-0.00015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MH, Swang TW, Hamilton IM, Best JA. State-dependent metabolic partitioning and energy conservation: a theoretical framework for understanding the function of sleep. PLoS One. 2017;12. 10.1371/journal.pone.0185746. [DOI] [PMC free article] [PubMed]
- Seibt J, Frank MG. Primed to Sleep: The Dynamics of Synaptic Plasticity Across Brain States. Front Syst Neurosci. 2019:13. 10.3389/fnsys.2019.00002. [DOI] [PMC free article] [PubMed]
- Shanahan CM, Proudfoot D, Farzaneh-Far A, Weissberg PL. The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr. 1998;8:357. doi: 10.1615/critreveukargeneexpr.v8.i3-4.60. [DOI] [PubMed] [Google Scholar]
- Shaw AE, Bamburg JR. Peptide regulation of cofilin activity in the CNS: a novel therapeutic approach for treatment of multiple neurological disorders. Pharmacol Ther. 2017;175:17–27. doi: 10.1016/j.pharmthera.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34:855. doi: 10.1212/wnl.34.7.855. [DOI] [PubMed] [Google Scholar]
- Shetty MS, Sharma M, Sajikumar S. Chelation of hippocampal zinc enhances long-term potentiation and synaptic tagging/capture in CA1 pyramidal neurons of aged rats: implications to aging and memory. Aging Cell. 2017;16:136–148. doi: 10.1111/acel.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Yue Z, Kuryatov A, Lindstrom JM, Sehgal A. Identification of redeye, a new sleep-regulating protein whose expression is modulated by sleep amount. eLife. 2014;3:e01473. doi: 10.7554/eLife.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Chen S-J, Ma M-Y, Bao Y-P, Han Y, Wang Y-M, Shi J, Vitiello MV, Lu L. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Shibata K, Sugiura M, Nishimura Y, Sakura H. The effect of small vessel disease on motor and cognitive function in Parkinson’s disease. Clin Neurol Neurosurg. 2019;182:58–62. doi: 10.1016/j.clineuro.2019.04.029. [DOI] [PubMed] [Google Scholar]
- Shokri-Kojori E, Wang G-J, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, Santi SD, Tomasi D, Benveniste H, Volkow ND. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci. 2018;115:4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Chapter 8 - rapid eye movement sleep. In: Kryger M, Roth T, Dement WC, editors. Principles and practice of sleep medicine. sixth. Amsterdam: Elsevier; 2017. pp. 78–95.e6. [Google Scholar]
- Sindi S, Kåreholt I, Johansson L, Skoog J, Sjöberg L, Wang H-X, Johansson B, Fratiglioni L, Soininen H, Solomon A, Skoog I, Kivipelto M. Sleep disturbances and dementia risk: a multicenter study. Alzheimers Dement. 2018;14:1235–1242. doi: 10.1016/j.jalz.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Socha MJ, Boerman EM, Behringer EJ, Shaw RL, Domeier TL, Segal SS. Advanced age protects microvascular endothelium from aberrant Ca(2+) influx and cell death induced by hydrogen peroxide. J Physiol. 2015;593:2155–2169. doi: 10.1113/JP270169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somarajan BI, Khanday MA, Mallick BN. Rapid eye movement sleep deprivation induces neuronal apoptosis by noradrenaline acting on Alpha1 adrenoceptor and by triggering mitochondrial intrinsic pathway. Front Neurol. 2016;7:25. doi: 10.3389/fneur.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonn JY, Lee J, Sung MK, Ri H, Choi JK, Lim C, Choe J. Serine metabolism in the brain regulates starvation-induced sleep suppression in Drosophila melanogaster. Proc Natl Acad Sci. 2018;115:7129–7134. doi: 10.1073/pnas.1719033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Eckman DM, Ingraham J, Riddle DR. Regulation of cerebrovascular aging. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Boca Raton: CRC Press/Taylor & Francis; 2007. [Google Scholar]
- Spano GM, Banningh SW, Marshall W, de Vivo L, Bellesi M, Loschky SS, et al. Sleep deprivation by exposure to novel objects increases synapse density and axon-spine interface in the hippocampal CA1 region of adolescent mice. J Neurosci. 2019:0380–19. 10.1523/JNEUROSCI.0380-19.2019. [DOI] [PMC free article] [PubMed]
- Spescha RD, Shi Y, Wegener S, Keller S, Weber B, Wyss MM, Lauinger N, Tabatabai G, Paneni F, Cosentino F, Hock C, Weller M, Nitsch RM, Lüscher TF, Camici GG. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J. 2013;34:96–103. doi: 10.1093/eurheartj/ehs331. [DOI] [PubMed] [Google Scholar]
- Stefanidis KB, Askew CD, Klein T, Lagopoulos J, Summers MJ. Healthy aging affects cerebrovascular reactivity and pressure-flow responses, but not neurovascular coupling: a cross-sectional study. PLoS One. 2019;14:e0217082. doi: 10.1371/journal.pone.0217082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Yamada K, Ozawa T. Preservation of mitochondrial respiratory function by coenzyme Q10 in aged rat skeletal muscle. Biochem Mol Biol Int. 1995;37:1111–1120. [PubMed] [Google Scholar]
- Suzuki G, Tokuno S, Nibuya M, Ishida T, Yamamoto T, Mukai Y, Mitani K, Tsumatori G, Scott D, Shimizu K. Decreased plasma brain-derived neurotrophic factor and vascular endothelial growth factor concentrations during military training. PLoS One. 2014;9:e89455. doi: 10.1371/journal.pone.0089455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Szuperak M, Churgin MA, Borja AJ, Raizen DM, Fang-Yen C, Kayser MS. A sleep state in Drosophila larvae required for neural stem cell proliferation. eLife. 2018:7. 10.7554/eLife.33220. [DOI] [PMC free article] [PubMed]
- Tabassum S, Misrani A, Tang B, Chen J, Yang L, Long C. Jujuboside A prevents sleep loss-induced disturbance of hippocampal neuronal excitability and memory impairment in young APP/PS1 mice. Sci Rep. 2019;9. 10.1038/s41598-019-41114-3. [DOI] [PMC free article] [PubMed]
- Tadavarty R, Rajput PS, Wong JM, Kumar U, Sastry BR. Sleep-deprivation induces changes in GABA(B) and mGlu receptor expression and has consequences for synaptic long-term depression. PLoS One. 2011;6:e24933. doi: 10.1371/journal.pone.0024933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem. 2018;144:595–608. doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B. Involvement of vitamin K-dependent proteins in vascular calcification. J Cardiovasc Pharmacol Ther. 2019;24:323–333. doi: 10.1177/1074248419838501. [DOI] [PubMed] [Google Scholar]
- Tia N, Singh AK, Pandey P, Azad CS, Chaudhary P, Gambhir IS. Role of forkhead box O (FOXO) transcription factor in aging and diseases. Gene. 2018;648:97–105. doi: 10.1016/j.gene.2018.01.051. [DOI] [PubMed] [Google Scholar]
- Tian J, Lei XX, Xuan L, Tang JB, Cheng B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti-aging proteins in platelet-rich plasma. Platelets. 2019;30:773–792. doi: 10.1080/09537104.2018.1514110. [DOI] [PubMed] [Google Scholar]
- Toossi H, Del Cid-Pellitero E, Jones BE. Homeostatic regulation through GABA and acetylcholine muscarinic receptors of motor trigeminal neurons following sleep deprivation. Brain Struct Funct. 2017;222:3163–3178. doi: 10.1007/s00429-017-1392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinei M, Berniakovich I, Beltrami E, Migliaccio E, Fassina A, Pelicci P, Giorgio M. P66Shc signals to age. Aging. 2009;1:503–510. doi: 10.18632/aging.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K, Toru M, Kobayashi T. Sleep deprivation: changes of monoamines and acetylcholine in rat brain. Life Sci. 1969;8:827–835. [PubMed] [Google Scholar]
- Turner J, Hughes LF, Toth LA. Sleep, activity, temperature and arousal responses of mice deficient for muscarinic receptor M2 or M4. Life Sci. 2010;86:158–169. doi: 10.1016/j.lfs.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Tzourio C. Hypertension, cognitive decline, and dementia: an epidemiological perspective. Dialogues Clin Neurosci. 2007;9:61. doi: 10.31887/DCNS.2007.9.1/ctzourio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uschakov A, Grivel J, Cvetkovic-Lopes V, Bayer L, Bernheim L, Jones BE, Mühlethaler M, Serafin M. Sleep-deprivation regulates α-2 adrenergic responses of rat hypocretin/orexin neurons. PLoS One. 2011;6:e16672. doi: 10.1371/journal.pone.0016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erum J, Van Dam D, De Deyn PP. Sleep and Alzheimer’s disease: a pivotal role for the suprachiasmatic nucleus. Sleep Med Rev. 2018;40:17–27. doi: 10.1016/j.smrv.2017.07.005. [DOI] [PubMed] [Google Scholar]
- van Sloten TT, Protogerou AD, Henry RMA, Schram MT, Launer LJ, Stehouwer CDA. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;53:121–130. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Peixoto L, Choi JHK, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S, Abel T. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics. 2012;44:981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Park AJ, Khatib N, Abel T. Effects of sleep deprivation and aging on long-term and remote memory in mice. Learn Mem. 2015;22:197–202. doi: 10.1101/lm.036590.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Huang T, Abel T. Sleep deprivation impairs synaptic tagging in mouse hippocampal slices. Neurobiol Learn Mem. 2018;154:136–140. doi: 10.1016/j.nlm.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wang L, Gu Y, Zhang J, Gong L. Effects of sleep deprivation (SD) on rats via ERK1/2 signaling pathway. Med Sci Monit. 2019;25:2886–2895. doi: 10.12659/MSM.913839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KE, Parhami F, Shin V, Demer LL. Fibronectin and collagen I matrixes promote calcification of vascular cells in vitro, whereas collagen IV matrix is inhibitory. Arterioscler Thromb Vasc Biol. 1998;18:1964–1971. doi: 10.1161/01.atv.18.12.1964. [DOI] [PubMed] [Google Scholar]
- Wong L-W, Tann JY, Ibanez CF, Sajikumar S. The p75 neurotrophin receptor is an essential mediator of impairments in hippocampal-dependent associative plasticity and memory induced by sleep deprivation. J Neurosci. 2019;39:5452–5465. doi: 10.1523/JNEUROSCI.2876-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World report on ageing and health: World Health Organization; 2015.
- Wu J, Xia S, Kalionis B, Wan W, Sun T. The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res Int. 2014, 2014. 10.1155/2014/615312. [DOI] [PMC free article] [PubMed]
- Xiong S, Patrushev N, Forouzandeh F, Hilenski L, Alexander RW. PGC-1α modulates telomere function and DNA damage in protecting against aging-related chronic diseases. Cell Rep. 2015;12:1391–1399. doi: 10.1016/j.celrep.2015.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Gan W-B. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev Neurobiol. 2012;72:1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan W-B. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Cabral D, Gaspard EN, Lipton RB, Rundek T, Derby CA. Cerebral hemodynamics in elderly: a transcranial Doppler study in the Einstein aging study (EAS) cohort. J Ultrasound Med. 2016;35:1907–1914. doi: 10.7863/ultra.15.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Larin A, McKenna JT, Jacobson KA, Winston S, Strecker RE, Kalinchuk A, Basheer R, Brown RE. Activation of basal forebrain purinergic P2 receptors promotes wakefulness in mice. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-29103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Xiao C, Long F, Wu W, Huang M, Qu L, Liu X, Zhu Y. Fra-1 plays a critical role in angiotensin II–induced vascular senescence. FASEB J. 2019;33:7603–7614. doi: 10.1096/fj.201801671RRRR. [DOI] [PubMed] [Google Scholar]
- Yayeh T, Leem Y-H, Kim K-M, Jung J-C, Schwarz J, Oh K-W, Oh S. Administration of Alphas1-casein hydrolysate increases sleep and modulates GABAA receptor subunit expression. Biomol Ther. 2018;26:268–273. doi: 10.4062/biomolther.2017.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Wang X, Geng P, Tang X, Xiang L, Lu X, et al. Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J Pineal Res. 2017;63. 10.1111/jpi.12405. [DOI] [PubMed]
- Yu Y, Huang Z, Dai C, Du Y, Han H, Wang YT, Dong Z. Facilitated AMPAR endocytosis causally contributes to the maternal sleep deprivation-induced impairments of synaptic plasticity and cognition in the offspring rats. Neuropharmacology. 2018;133:155–162. doi: 10.1016/j.neuropharm.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Zagaar MA, Dao AT, Alhaider IA, Alkadhi KA. Correction to: prevention by regular exercise of acute sleep deprivation-induced impairment of late phase LTP and related signaling molecules in the dentate gyrus. Mol Neurobiol. 2018;55:902. doi: 10.1007/s12035-017-0789-7. [DOI] [PubMed] [Google Scholar]
- Zagrebelsky M, Holz A, Dechant G, Barde Y-A, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zant JC, Kim T, Prokai L, Szarka S, McNally J, McKenna JT, Shukla C, Yang C, Kalinchuk AV, McCarley RW, Brown RE, Basheer R. Cholinergic neurons in the basal forebrain promote wakefulness by actions on neighboring non-cholinergic neurons: an opto-dialysis study. J Neurosci. 2016;36:2057–2067. doi: 10.1523/JNEUROSCI.3318-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu Y, Zhan G, Fenik P, Panossian L, Wang MM, Reid S, Lai D, Davis JG, Baur JA, Veasey S. Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci. 2014;34:4418–4431. doi: 10.1523/JNEUROSCI.5025-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Guo H-L, Zhang H-Q, Xu T-Q, He B, Wang Z-H, Yang Y-P, Tang X-D, Zhang P, Liu F-E. Melatonin prevents sleep deprivation-associated anxiety-like behavior in rats: role of oxidative stress and balance between GABAergic and glutamatergic transmission. Am J Transl Res. 2017;9:2231–2242. [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Feinberg D, Alharbi M, Ding Z, Lu C, O’Connor JP, Graves DT. Chondrocytes promote vascularization in fracture healing through a FOXO1-dependent mechanism. J Bone Miner Res. 2019;34:547–556. doi: 10.1002/jbmr.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci U S A. 2007;104:15899–15904. doi: 10.1073/pnas.0701599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo M. Shrinkage of dendritic spines associated with Long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhou R-H, Vendrov AE, Tchivilev I, Niu X-L, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR, Runge MS. Mitochondrial oxidative stress in aortic stiffening with age. Arterioscler Thromb Vasc Biol. 2012;32:745–755. doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]