Abstract
Postpartum depression (PPD) is a significant mental health concern, especially for women in vulnerable populations. Oxytocin (OT), a hormone essential for a variety of maternal tasks, including labor, lactation, and infant bonding, has also been hypothesized to have a role in postpartum depression. Women are routinely given synthetic oxytocin to induce or augment labor and to prevent postpartum hemorrhage. The aim of this study was to review the quality and reliability of literature that examines potential relationships between OT and PPD to determine if there is sufficient data to reliably assess the strength of these relationships. We conducted a literature search in December of 2018 using five databases (PubMed, Web of Science, Embase, Psyclnfo, and CINAE1L). Eligible studies were identified, selected, and appraised using the Newcastle-Ottawa quality assessment scale and Cochrane Collaboration’s tool for assessing risk of bias, as appropriate. Sixteen studies were included in the analysis and broken into two categories: correlations of endogenous OT with PPD and administration of synthetic OT with PPD. Depressive symptoms were largely measured using the Edinburgh Postnatal Depression Scale. OT levels were predominately measured in plasma, though there were differences in laboratory methodology and control of confounders (primarily breast feeding). Of the twelve studies focused on endogenous oxytocin, eight studies suggested an inverse relationship between plasma OT levels and depressive symptoms. We are not able to draw any conclusions regarding the relationship between intravenous synthetic oxytocin and postpartum depression based on current evidence due to the heterogeneity and small number of studies (n=4). Considering limitations of the current literature and the current clinical prevalence of synthetic OT administration, we strongly recommend that rigorous studies examining the effects of synthetic OT exposure on PPD should be performed as well as continued work in defining the relationship between endogenous OT and PPD.
Keywords: Oxytocin, postpartum depression, review, Pitocin
1. Introduction:
Postpartum depression (PPD) is a serious mental health concern affecting between 10-20% of new mothers (Beck, 2001; Ko, Rockhill, Tong, Morrow, & Farr, 2017) with an even higher prevalence in vulnerable populations including, but not limited to, African American women (Cannon & Nasrallah, 2019), Hispanic women (Cannon & Nasrallah, 2019; Lucero, Beckstrand, Callister, & Sanchez Birkhead, 2012) and teenage mothers (Logsdon, Birkimer, Simpson, & Looney, 2005; Phipps, Raker, Ware, & Zlotnick, 2013). PPD has significant consequences for women, including higher healthcare costs, loss of productivity both in employed work and domestically, lack of attachment to their infant, decline of overall mental health, lower rates of breastfeeding, and lower adherence to infant safety behaviors (American Psychological Association, 2015; Dagher, McGovern, Dowd, & Gjerdingen, 2012; Leahy-Warren & McCarthy, 2007). Additionally, maternal depression can have a lasting negative effect on her infant’s cognitive and social development (Tronick & Reck, 2009; Weinberg & Tronick, 1998).
PPD is a complicated, multifactorial disease with both psychologic and physiologic risk factors playing roles in symptom development. Previous meta-analyses have identified many maternal risk factors for PPD, including a history of depression at any time and depression or anxiety during pregnancy (Beck, 2002; Bloch, Rotenberg, Koren, & Klein, 2006; Robertson, Grace, Wallington, & Stewart, 2004). Environmental factors such as low social support, being unmarried, and low socioeconomic status (SES) increase a woman’s risk for PPD, as does increased perceived stress and difficulty breast feeding (Bhati & Richards, 2015; Dias & Figueiredo, 2015; Robertson et al., 2004). Biological risk factors have also been recently identified as significant during the postpartum period, including high levels of inflammatory cytokines and dysregulated interactions between the hypothalamic-pituitary axis and the innate immune system (Brann et al., 2018; Corwin et al., 2013; Corwin & Pajer, 2008; Glynn, Davis, & Sandman, 2013). Reproductive hormonal changes, primarily estrogen and progesterone, are also under study as potential pathophysiology (Schiller, Meltzer-Brody, & Rubinow, 2015; Stewart & Vigod, 2019).
During pregnancy, the placenta helps create a hormonal milieu that is unique compared to any other time in a woman’s life. Circulating levels of placental hormones including estradiol, estrone and progesterone increase significantly throughout pregnancy (Schock et al., 2016), drop sharply after birth, and then stabilize within normal limits by day five postpartum in most women (Hendrick, Altshuler, & Suri, 1998). Similarly, levels of cortisol, human chorionic gonadotrophin, and beta-endorphin also increase across the prenatal period, peak with birth, and quickly decline postpartum (Hendrick et al., 1998). This shifting of multiple hormones has been suggested to influence some women’s mood during the postpartum period (Schiller et al., 2015).
Though not a placental hormone, oxytocin (OT) has a similar shift of concentration in the perinatal and postpartum period. In the majority of women there appears to be an increase in endogenous OT across pregnancy (Prevost et al., 2014) with a peak at and immediately following birth to support uterine contractions (de Geest, Thiery, Piron-Possuyt, & Vanden Driessche, 1985; Leake, Weitzman, Glatz, & Fisher, 1981). Heightened OT is integral to labor and, as such, synthetic OT (synOT) (brand name Syntocinon, Duratocin, and Pitocin) is administered in some women to start (induce) or quicken (augment) labor and to prevent postpartum hemorrhage. Though there are research guidelines from professional organizations regarding synOT administration, little is known about actual dose and duration of exposure during labor and delivery. In 2017, 25.7% of deliveries in the United States were induced (Martin, Hamilton, Osterman, Driscoll, & Drake, 2018) and it is largely unknown how many women receive synOT for augmentation.
Intravenous synOT is given continuously (contrasted to the pulsatile release of endogenous OT) for anywhere from 20 minutes to over 48 hours. Dosing during the first and second stages of labor typically ranges from 2 to 20 mU/minute (Marshall, 1985), though women may receive more if an adequate contraction pattern is not achieved. While synOT is recommended for active management of the third stage of labor (delivery of the placenta) for all births, ideal dose and infusion rates have yet to be established. (“Guidelines for oxytocin administration after birth: AWHONN practice brief number 2,” 2015). Studies have investigated doses between 10 and 40 units (“Guidelines for oxytocin administration after birth: AWHONN practice brief number 2,” 2015) and many protocols are in this range. Overall, administration in all stages of labor is largely variable based on provider and institution.
Synthetic OT has a very short half-life and has only small penetration across the blood brain barrier, thus limiting access to central nervous system. However, OT studies demonstrating central nervous system penetration in animal studies suggest that peripherally administered OT may reach brain receptors (Lee et al., 2018; Yamamoto et al., 2019). Postpartum endogenous OT levels and trajectories are variable by individual but are theoretically higher than during non-peripartum times in order to support lactation, bonding, and development of maternal behaviors (Bell, Erickson, & Carter, 2014; Carter, 2014; Galbally, Lewis, Ijzendoom, & Permezel, 2011; Mehta et al., 2016; Rich, deCardenas, Lee, & Caldwell, 2014; Rilling & Young, 2014). It is unknown exactly what effect exposure to synthetic oxytocin during labor has on postpartum endogenous levels, but preliminary studies suggest there is a link in humans (Gu et al., 2016; Prevost et al., 2014). As such, and in light of the large number of women receiving synthetic oxytocin both during and following labor, investigating the long-term effects of intravenous synthetic OT exposure in the peripartum period, especially in mood regulation, is of great clinical significance.
Moura et al. completed a systematic review in 2016 focused on the relationship of endogenous OT concentrations, concluding that in the perinatal period overall higher levels of OT were associated with less depressive symptomatology (Moura, Canavarro, & Ligueiredo-Braga, 2016). However, they identified only six studies—all of which were focused on general perinatal depression and OT receptor function rather than depression in the postpartum period. In addition, Moura and colleagues did not comment in their review on OT measurement methodologies, which can lead to large variations in OT estimations. Mah also published a review the same year looking at endogenous OT and PPD in conjunction with parenting behaviors (Mah, 2016). She, likewise, concluded that higher plasma OT was associated with lower postpartum depressive symptoms. Neither review considered the role of synOT in PPD, which is of great clinical significance. Additionally, since the Moura and Mah reviews, multiple studies have been published on PPD and OT (Gu et al., 2016; Guintivano et al., 2017; Kroll-Desrosiers et al., 2017; Lara-Cinisomo, D’Anna-Hemandez, Fujimoto, & Pedersen, 2018; Sandraluz Lara-Cinisomo et al., 2018; Lara-Cinisomo, McKenney, Di Llorio, & Meltzer-Brody, 2017; Takács, Seidlerová, Štěrbová, Čepický, & Havlíček, 2018).
Given the importance of PPD, the critical role of OT in the postpartum period, the high frequency of synOT administration in the peripartum period, the limitations of existing reviews, and the number of new publications in this field, an updated systematic review of OT and PPD is needed. The aim of this study was, therefore, to review the quality and reliability of literature that examines potential relationships between OT and PPD to determine if there is sufficient data to assess the strength of these relationships.
2. Methods
2.1. Criteria for Considering Studies in this Review
Articles were eligible for inclusion if they presented peer reviewed original quantitative findings from human studies detailing any relationship between the OT system and postpartum depression (either alone or in concert with prenatal depression). Postpartum was defined as up to one-year post birth. The following exclusion criteria were applied: abstracts without full peer reviewed publication, theoretical articles, review or meta-analysis articles, exclusively prenatal studies, depressive measurements outside one year postpartum, case studies, those exploring OXTR genotype as the sole oxytocin variable. Post-hoc exclusion criteria included those studies that focused on intranasal OT as a treatment for PPD (n=4) as a full review was recently published on this topic (De Cagna et al., 2019).
2.2. Quality Assessment
Non randomized studies included in this review were critically assessed, using an adapted version of the Newcastle-Ottawa quality assessment scale (NOQ) (Wells et al.). We chose to utilize the same assessment scale and quality indicator (NOQ) as previous systematic reviews in the field to maintain consistency in evaluation method. (Moura et al., 2016). Under the NOQ, each study was evaluated as high, low, or moderate quality based on a “star system” wherein stars are awarded based on the quality of various study facets. These include: representativeness of the cohort (degree sample represents postpartum women in the community); assessment of exposure (measurement of OT through validated laboratory measures and/or secured record); control for confounders (appropriateness of confounders based on PPD and OT literature); assessment of outcome (validated measure of PPD or clinical diagnosis); and adequacy of follow up (measurement within one year postpartum and no evidence that subjects lost to follow up introduced bias). Assessment of exposure was given an additional star from the original NOQ as laboratory methods are essential to study of plasma OT levels. Each study was eligible for a total of eight stars. A low-quality study is indicated by 0-2 stars, moderate quality by 3-5 stars, and 6-8 indicate a high-quality study. Randomized studies were assessed based on a modified Cochrane Collaboration’s tool for assessing risk of bias in randomized trials (Higgins et al., 2011) and no studies were excluded on the basis of poor quality.
2.3. Literature Search Strategy
Utilizing the assistance of a health sciences librarian in December of 2018, five large databases (PubMed, Web of Science, Embase, Psyclnfo, and CINAL) were searched for articles relevant to our central question. The following keywords were searched individually and in combination: postpartum depression, postnatal depression, oxytocin, synthetic oxytocin, Svntocinon, Duratocin, andPitocin (i.e. “postpartum depression OR postnatal depression” AND “oxytocin, synthetic oxytocin, Synotocinon, Duratocin OR Pitocin”). The initial search yielded 618 records. Duplicates resulting from utilizing multiple search engines were removed, yielding 355 citations for title and abstract review. These articles were then screened for appropriateness of inclusion. Any review articles identified in the initial search were manually searched for additional pertinent studies that may have been missed initially, but none were identified. After exclusion criteria were applied, 60 articles remained for full text review. After applying exclusion criteria during full text review, 16 publications remained for inclusion in the final review. The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) (Moher, Liberati, Tetzlaff, & Altman, 2009) flow diagram was used to illustrate the study selection (see Figure 1).
Figure 1.
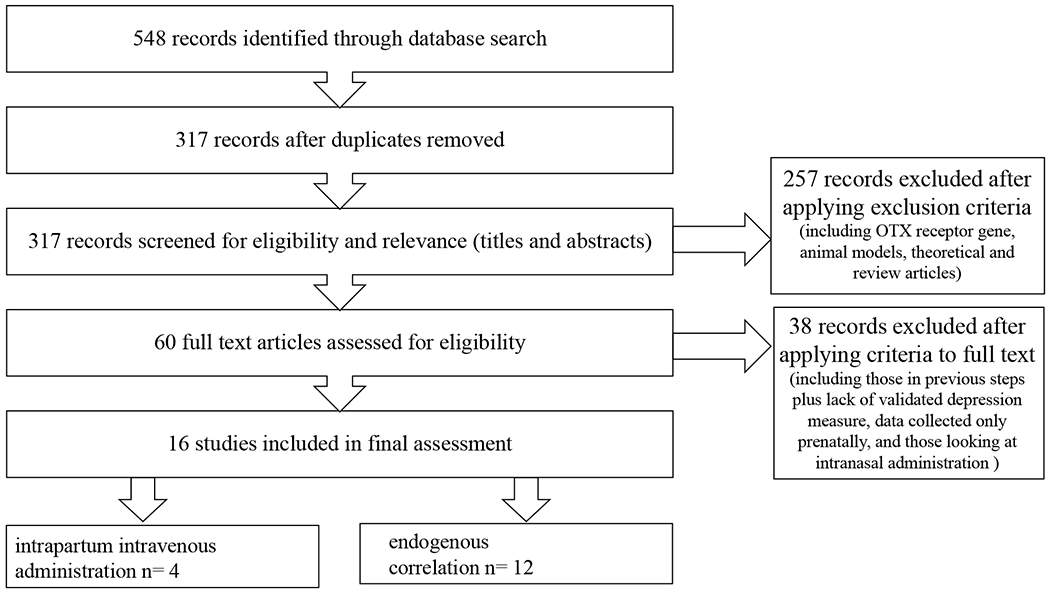
PRISMA flow diagram showing study selection
2.4. Data Abstraction and Synthesis
Included articles were assessed by the first author (T.T.) and reviewed with the author team. Each record eligible for this study was entered into a data extraction table which included: author name(s), publication date, sample size, article title, aim(s), participant characteristics and recruitment, design, measurement tools, and main results (see Tables 1 and 2). Figure 1 illustrates a summary of our literature search. Effect sizes are noted, when available. These were gathered from either the publications themselves or calculated based on reported data (if available), utilizing an online effect size calculation tool (Wilson, 2020).
Table 1.
Studies correlations between endogenous levels and postpartum depressive symptoms
| Results |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Purpose# | Setting & Sample | Methods | Endogenous Oxytocin Measurement | Mood Measurement | Prenatal | Early Postpartum (≤ 6 weeks) | Late Postpartum (> 6 weeks) | Newcastle-Ottawa quality score* |
| Cox et al. 2015 | To test associations between OT levels during breastfeeding and stress reactivity and quantify the acute effects of lactation on stress response | n= 52 pregnant community women with intention to breast-feed, mixed history of depression, well-educated majority (83%) white same cohort as Stuebe et al. (2013) | Mothers were evaluated for depression and anxiety at 2- and 8-weeks postpartum. They underwent the Trier Social Stress test at 8 weeks postpartum after they either breastfeed or held their infants. Plasma samples were collected during feeding (at baseline, at 1, 3, 7, and 10 minutes of feeding, and postfeed) and at minutes 2 and 4 of the stress test and 10 minutes poststressor. | ELISA (Enzo) Extracted plasma, visits at 1 pm | Clinical interview (SCID-NP) EPDS STAI | Symptomatic breastfeeding mothers (EPDS ≥ 10 and/or STAI ≥34)had lower levels of OT during feedings (p<0.05). In this group, higher OT AUC was associated with higher stress during testing (p < 0.05). | 8 | ||
| Eapen et al. 2014 | To explore the associations between pregnancy and early PP OT plasma levels and early maternal attachment experiences, symptoms of separation anxiety, and depression. | n= 57 52% Caucasian, purposefully sampled into “separation anxiety” and “without separation anxiety” groups based on ASA-27 | At either 28 or 32 weeks gestation women completed mood measures and gave blood for plasma OT. This was repeated at 3 months postpartum with some additional questionnaires. | Radioimmunoassay Extracted plasma | EPDS, STAI, Adult Separation Anxiety Questionnaire (ASA-27) | No association between plasma OT and EPDS scores | At 3 months lower levels of OT were associated with higher depressive symptoms (p<001) | 7 | |
| Guintivano et al. 2017 | To examine the contributions of genetic ancestry, adverse life events, psychological, and biological factors in the development of PPD | n=1517 Black, Latina, and white women (549 PPD, 968 controls) | Both cases and controls recruitedn from outpatient OB clinics at 6 weeks postpartum where they were screened using EPDS. Participants were assessed for psychiatric disorders and gave plasma and serum for biological assays and genotyping. Depressed defined as EPDS ≥ 11, v not depressed defined as EPDS ≤ 7 | ELISA, Extracted plasma, | EPDS, Mini International Neuropsychiatric Interview (MINI-Plus) | OT levels were not significantly associated with PPD between the two groups after controlling for factors that may influence hormone levels such as breastfeeding status, genetic ancestry, maternal age, etc. | 7 | ||
| Jobst et al. 2016 | To evaluate the relationship between oxytocin plasma levels during pregnancy and postpartum and presence of depressive symptoms | n=89 healthy community sample | Women were recruited at 34 weeks gestation. Plasma and mood measures were collected at 5 timepoints (35 and 38 weeks gestation, 2 days, 7 weeks and 6 months postpartum. | ELISA (Enzo) Unextracted plasma diluted 1:3 Collected between 8-10 AM | Montgomery-Åsberg Depression Rating Scale (MADRS) | The change in trajectory of OT plasma levels was significantly different between women with and without depressive symptoms. Women in the ‘non-depressed’ group showed continuous increase in OT levels at all timepoints while those in the ‘depressed’ group dropped between late pregnancy and 2 days postpartum; OR .989 | 6 | ||
| Lara-Cinisomo et al. 2017 | To obtain pilot data exploring associations between PPD, BF practices, and OT response to infant feeding | n=28 Latina women | Mothers assessed at three times: 3rd trimester for demographics and complete psychological assessment, 4 weeks PP by phone-EPDS, bonding and BF; 8 weeks PP in person EPDS, BF status and plasma OT (via IV catheter 10 minutes before, at minutes 3, 7, and 10 during feeding, and 10 minutes after feeding) | EIA (Enzo) Extracted plasma, visits all beginning at 9 AM See above Lara-Cinisomo et al. (2018). | EPDS Postpartum Bonding Questionnaire (PDQ) Clinical interview | At 8 weeks depressed women (EPDS ≥ 10) that were not BF had lower OT AUC than nondepressed, non-BF women (p=0.044). | 8 | ||
| Lara-Cinisomo et al. 2018a | To explore associations between PPD, traumatic life events and maternal/ infant bonding | n=28 Latina women, community sample [same sample as Lara-Cinisomo et al. (2017)] | See above Lara-Cinisomo et al. (2017). | See above Lara-Cinisomo et al. (2017). | See above Lara-Cinisomo et al. (2017). | At 8 weeks women with PPD exhibited non-significantly lower mean plasma OT AUC than those without symptoms | 8 | ||
| Lara-Cinisomo et al. 2018b | To develop a descriptive picture of Latinas’ postnatal depression and OT profiles and to explore associations between maternal mood and OT levels | n=108 sub study (Pedersen et al., 2016) of women that identify as either Hispanic or Latina, community sample from public health prenatal clinic | Women completed home visits at 35-36 weeks of pregnancy and 6 weeks postpartum. Depression and anxiety data and 24-hour participant collected urine samples collected at both visits | ELISA (Enzo) 24-hour urine samples. Extracted samples | EPDS STAI | Women with PPD showed persistently higher mean OT over time. Non-PPD had a significant decrease in OT levels over time (p=.002). | 8 | ||
| Massey et al. 2016 | To examine plasma oxytocin concentration and PPD symptom severity and if this might differ by lifetime history of major depressive disorder (MDD) | n= 66 low risk, community sample without prenatal depression, majority (73%) Caucasian | Women in the third trimester of pregnancy were assessed for current mood disorders and gave plasma OT samples. 6 weeks PP participants were assed (primarily via telephone) for birth outcomes and depressive symptoms/ severity | ELISA (Enzo) Unextracted plasma diluted 1:8 | MINI Mood Disorders Questionnaire Inventory of Depressive Symptomatology (IDS-SR30) EPDS | Positive relationship between third trimester OT and PPD symptom severity (p=.019) only in women with a history of MDD | 8 | ||
| Samuel et al. 2015 | To investigate if levels of OT in mothers with mood or anxiety disorders differ from mentally healthy controls and if maternal mental health moderates the relationship between OT levels and interactive behavior. | n= 110 20 in “clinical sample” with a clinically diagnosed mood or anxiety disorder, 90 postpartum in comparison group scoring low for depressive symptoms | At 2 months PP every mother/ infant dyad was assessed in home. Blood was drawn for serum OT and mothers were filmed (and later coded) interacting with infant. | ELISA (Enzo) Unextracted plasma diluted 1:2 or 1:4 | EPDS Global Rating Scale of Depression (GRS) | No group differences in OT levels However, in the clinical sample, higher OT levels were associated with less depressive behavior (p≤.01). | 7 | ||
| Skrundz et al. 2011 | To explore the relationship between plasma OT levels prenatally and the development of PPD symptoms. | n=74 healthy women | Plasma OT and depressive symptoms were assessed between 30-34 weeks gestation. At 2 weeks PP, the EPDS was repeated. Other birth variables were collected by chart review. | Radioimmunoassay Plasma | EPDS | No significant associations between prenatal EPDS and OT levels. | Lower OT level mid pregnancy predicted higher EPDS at 2 weeks postpartum (p<0.05). | 6 | |
| Stuebe et al. 2013 | To explore the relationship between maternal mood and neuroendocrine response to breast feeding | n=47 pregnant community women with intention to breast-feed, mixed history of depression, welleducated majority (83%) white same cohort as Cox et al. | EPDS and STAI as well as assessment by a psychiatrist and blood sample for plasma OT in the 3rd trimester. At 2 and 8 weeks postpartum the EPDS and STAI were repeated and plasma OT was measured at baseline, 1, 4, 7, and 10 minutes of feeding, and postfeed. | EIA (Enzo) Extracted plasma | Clinical interview EPDS STAI | Third-trimester plasma OT were inversely correlated with EPDS scores (p=0.03) | A two weeks baseline OT was inversely correlated with EPDS scores (p=0.03). | At eight weeks EPDS were inversely correlated with OT AUC during feedings (p<0.01) | 8 |
| Zelkowitz et al. 2014 | To investigate the relationship of psychosocial stress, PPD symptoms and maternal sensitive behavior to endogenous OT levels | n=287 well educated, community sample | Women were assessed at three time points (12–14 weeks and 32–34 weeks gestation, and 7–9 weeks PP) At each point, participants completed psychosocial measures and gave blood. | ELISA (Enzo) Unextracted plasma diluted 1:2 or 1:3 | EPDS Antenatal Risk Questionnaire (ANRQ) | At 12-14 weeks in mothers with high stress, higher OT was associated with lower depressive symptoms (p<0.05). | At 7-9 weeks in mothers with high stress, higher levels of OT were associated with lower depressive symptoms (p<0.001). | 6 | |
0-2: low quality, 3-5: moderate quality, 6-8: high quality
purpose as defined in primary study
PPD= postpartum depression; OT= oxytocin; BF= breastfeeding; EPDS= Edinburgh Postnatal Depression Scale; SCID-NP= Structured Clinical Interview for the DSMIV (Diagnostic and Statistical Manual of Mental Disorders); STAI= State Trait Anxiety Inventory; ELISA= enzyme-linked immunosorbent assay; AUC= area under the curve
Table 2.
Studies describing correlations between intrapartum intravenous synOT exposure and postpartum depressive symptoms
| Results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Purpose# | Setting & Sample | Methods | Endogenous Oxytocin Measurement | Mood Measurement | synOT Measurement | Early Postpartum (≤ 6 weeks) | Late Postpartum (> 6 weeks) | Other | Newcastle-Ottawa quality score* |
| Gu et al. 2016 | To examine the long-term effects of synOT administration on the endogenous oxytocin system and concomitant indices of functional status, specifically breastfeeding and emotional well being | n= 386 primarily community with some clinical in Quebec subsample of two larger studies (Zelkowitz et al. 2014 above) | Data collected at recruitment and in home at 2 months postpartum including demographics and breastfeeding status | ELISA (Enzo) Unextracted plasma diluted 1:2 or 1:4 | EPDS GAD-8 Perinatal Posttraumatic Stress Questionnaire | Total intra and postpartum intravenous and intramuscular dose calculated from hospital records | 29 women without any OT exposure (7.5% of sample) | At 2 months synOT was positively correlated with endogenous OT levels (p<.001). SynOT was significant predictor of EPDS score. Higher synOT was associated with greater PPD symptoms | 7 | |
| Hinshaw et al., 2008 | To test the hypothesis that early use of oxytocin reduces the need for caesarean delivery | n= 412 low risk nulliparous women with spontaneous term labor, diagnosis of primary dysfunctional labor | Randomized control trial. Active managementstarted synOT immediately after primary labor diagnosed, conservative management synOT withheld for 8 hours psychological assessment within 48 hours of delivery | None | EPDS | 100% exposure to synOT. Dose and time of exposure differ on each arm | No significant difference in median EPDS scores or women scoring > 12 (depression cutoff in this study) between arms within 48 hours postpartum | n/a | ||
| Kroll-Desrosiers et al. 2017 | To examine the relationship between peripartum synOT administration and the development of depressive and anxiety disorders within the first year PP | n= 46732 from Massachusetts Integrated Clinical Academic Research Database | Retrospective analysis from clinical data repository over nine years. Collected from birth to 1 year postpartum | None | Psychiatric diagnosis ICD-9 codes Psychotropic medications | Yes/no synOT exposure from hospital record | 20% of deliveries with synOT exposure | Within women without any history of anxiety or depression, exposure increased risk by 32%; increased by 36% in women with a history of depression | 50% of patients received PPD diagnosis within just over 2 months postpartum | 7 |
| Takacs et al., 2018 | To explore both short- and long-term effects of intrapartum synOT on maternal mood | n= 260 community sample in Czech Republic | prospective longitudinal design data collection at the third trimester of pregnancy, 1-7 days, 6 weeks, and 9 months postpartum | None | EPDS Maternity Blues Questionnaire | Yes/no synOT exposure from hospital record | No effect of synOT on postpartum blues. Receiving synOT predicted a lower risk of positive PPD screen at 6 weeks PP (p=.014) | Receiving synOT significantly predicted a lower risk of positive PPD screen (EPDS > 12) at 9 months PP | 8 | |
0-2: low quality, 3-5: moderate quality, 6-8: high quality
purpose as defined in primary study
synOT= synthetic oxytocin (Pitocin); PPD= postpartum depression; EPDS= Edinburgh Postnatal Depression Scale; ELISA= enzyme-linked immunosorbent assay; ICD-9= International Classification of Diseases, 9th revision
3. Results
After reviewing all final documents, two distinct areas of study emerged: 1) correlation of maternal endogenous OT levels with depressive symptomatology, 2) association of peripartum intravenous synthetic OT exposure with PPD (see Figure 2 for overview of results).
Figure 2. Overall Results of all Included Studies.
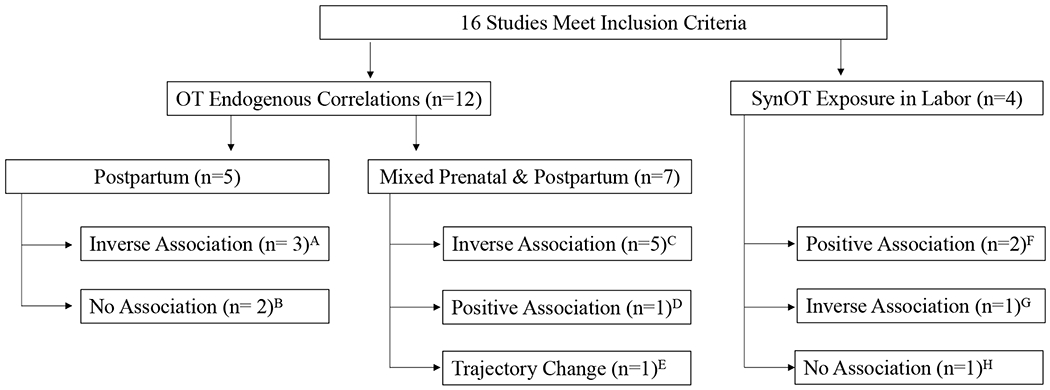
The chart shows the breakdown of the 16 studies meeting the inclusion criteria and a summary of the findings. A: Cox (2015), Lara-Cinisomo (2017), Samuel (2015); : Laura-Cinisomo (2018a), Guintivano (2017); C: Eapen (2014), Massey (2016), Skrundz (2011), Stuebe (2013), Zelkowitz (2014); D: Laura-Cinisomo (2018b); E: Jobst (2016); F: Gu (2016), Kroll-Desrosiers (2017); G: Takacs (2018); H: Hinshaw (2008)
3.1. Correlation of Maternal Endogenous OT Levels with Depressive Symptomatology
We identified twelve studies with the generalized goal of correlating endogenous OT concentration with depressive symptomatology in the perinatal period (Table 1). The publications identified were split between those that looked exclusively postpartum (n=5) and those that examined OT measurements both prenatally and postpartum (n=7). Eight found an inverse association between plasma OT and PPD symptoms (i.e. lower OT associated with higher depressive symptoms), two did not find any significant relationships and one found a change of OT trajectory over pregnancy and postpartum (rather than relative value) predictive of depressive symptoms. Only one study found a positive association, however it was the only to use urine rather than plasma OT measurement, making it difficult to compare to the remainder of the literature.
3.1.1. Early and Late Postpartum
Five publications were identified looking at the relationship between maternal endogenous OT in the early (≤ 6 weeks) and late (> 6 weeks) postpartum period with depressive symptomatology using a screening tool or PPD diagnosis. Four publications, from three studies (Cox et al., 2015; Lara-Cinisomo et al., 2018a; Lara-Cinisomo et al., 2017; Stuebe, Grewen, & Meltzer-Brody, 2013), reported outcomes specifically around breastfeeding (BF) by measuring plasma levels serially before, during, and after a feeding. As OT release is necessary for successful breastfeeding, controlling for BF status is essential when examining the relationship between PPD and OT. Changes in plasma OT levels during feeding were noted in all cases, as was to be expected. Most reported an inverse relationship between plasma OT levels and PPD symptoms, with varying levels of significance.
Cox et al. (2015) found overall that women who breastfed during their study visit had higher levels of OT, which is to be expected. Breastfeeding mothers with a positive depression and/or anxiety screening (n=11) had overall lower plasma OT levels during feeding than breastfeeding mothers without symptoms (n=28) (Cohen’s D= .73, p<0.05). Furthermore, they found women with anxiety and depression symptoms had an altered association between OT and the cortisol stress response. In asymptomatic women the OT surge from breastfeeding (plasma was drawn within context of feeding) was protective against cortisol response to stress. Interestingly, in women with depressive symptoms, the opposite was true indicating PPD may modify the relationship between OT and stress (Cox et al., 2015).
In a slightly smaller study (n=28) of exclusively Latina women, Laura-Cinisomo et al., found that in nonbreastfeeding mothers, those with depression showed lower plasma OT levels than those without depressive symptoms (p=0.04) (Lara-Cinisomo et al., 2017). However, this subsample of nonbreastfeeding mothers was quite limited (n=5). In breastfeeding women (n= 23) there was no significant relationship. In a different paper from the same study, overall women with depressive symptoms exhibited a non-statistically significant lower average plasma OT area under the curve during feeding (Lara-Cinisomo et al., 2018a). Blood samples were carefully controlled around and during breastfeeding intervals.
Samuel and colleagues (2015) compared cases of women with clinically diagnosed mood or anxiety disorders (n=20) with mothers from the general population (n=90) who screened negative for depression by EPDS. Blood was drawn at least 30 minutes after breastfeeding for consistency. They found, only in women with diagnosed PPD, higher OT levels were strongly positively (correlation coefficient= .560) associated with less depressive interactive behavior (p≤.01). However, there was no significant difference in plasma OT levels between depressed vs non-depressed groups at two months postpartum (Samuel et al., 2015). The comparison groups were uneven, but otherwise similar in baseline demographics.
Using the same general premise, but on a much larger scale (n=1517), Guintivano et al. (2017) compared women diagnosed PPD with a community sample sans diagnosis. Women in recruitment were screened with EPDS, then depression was confirmed by structured clinical interview. They found no significant difference in plasma OT levels between the two groups after controlling for covariates including adverse life events and breastfeeding status (Guintivano et al., 2017). However, they did not tightly control blood draws around breastfeeding as others did.
3.1.2. Mixed Prenatal and Postpartum
In a study with the main purpose of examining the relationship between separation anxiety, depression and oxytocin levels, Eapen et al (2014), found three-month OT levels postpartum were inversely correlated with depressive symptoms both prenatally (p<0.001) and three months postpartum (p<0.001) in a bivariate analysis (n=57). Prenatal OT levels were not associated with postpartum depression. Their mediation analysis showed depression to be a significant mediator between separation anxiety and plasma OT levels (Eapen et al., 2014). There was a weak negative impact of depression on OT levels (correlation coefficient= −.22). Breastfeeding status was not controlled for in these analyses.
In Jobst’s 2016 study, a change in prenatal OT trajectory, rather than absolute value, was associated with PPD development. In all women (n=89) OT plasma levels increased significantly from late prenatal to 6 months postpartum. Their non-depressed cohort had a continual elevation of plasma OT levels, however, while the depressed group experienced a decrease in hormone concentration between the 38th week of gestation and 2 days postpartum before continuing to increase. This difference between the groups was small (OR=.989) but significant (p=.046) after controlling for breastfeeding problems and prenatal depression (Jobst et al., 2016). Participant’s blood samples were collected during the same two-hour window during the day (8-10am) to minimize diurnal fluctuations, however, no consideration was given to potential recent feeding during postpartum visits. Breastfeeding problems were included as a covariate in analysis. Of note, this study also considered synthetic oxytocin exposure, but only 5.6% of the sample received the drug. SynOT did not correlate with postpartum OT plasma level and therefore it was not included in analysis. They also used the Montgomery-Åsberg Depression Rating Scale rather than the more common EPDS, reducing comparability to other’s results.
In the same year, in a moderately sized sample (n=66), Massey et al. (2016) assessed plasma OT and PPD symptoms severity stratified by a history of major depressive disorder. No difference was found in OT levels between those with and without a history of depression. Nor was plasma OT related to PPD symptom severity. However, they did find plasma OT levels interacted with depression history to predict PPD symptom severity (β=.328, p=.003) after controlling for third trimester depressive symptom severity, history of depression, and third trimester plasma OT (adjusted R2=.450). Higher levels of OT predicted higher PPD symptom burden only in women with a history of depression (p=.019) but not in women without a depressive history (Massey, Schuette, Poumajafi-Nazarloo, Wisner, & Carter, 2016). Similar to Jobst et al. (2016), prenatal samples were collected during the same two-hour window during the day (11a-1p) for consistency. Nearly all (98.5%) of the sample intended to breastfeed, but feeding status was not reassessed in postpartum data collection.
Skrundz et al. (2011) found in a sample of healthy women (n=74), lower mid-pregnancy plasma OT levels were predictive of higher PPD symptoms at 2 weeks postpartum (OR=.290, p<0.05). Prenatal blood was routinely collected between 1-3 pm. OT levels were not measured postpartum and in their sample 90% of women were breastfeeding (Skrundz, Bolten, Nast, Hellhammer, & Meinlschmidt, 2011).
Similarly, Stuebe et al. (2013), found a weak negative relationship between OT and depressive symptoms, i.e. lower plasma OT concentrations in depressed mothers (n=17) compared to non-depressed (n=29). In their cohort this held true prenatally, at the third trimester mark (Spearman R=−.30, p=0.03), at two weeks postpartum at baseline (Spearman R=−.33, p=0.03), and at eight weeks postpartum around infant feedings (Spearman R=−.36, p<0.01). The postpartum plasma measurements were procured around BF occurrences (Stuebe et al., 2013).
Finally, in a large sample (n=287), Zelkowitz et al. (2014) found significance between plasma OT and PPD symptoms, where higher levels of plasma OT weakly correlated with less depressive symptomatology in early pregnancy (Pearson’s r= −.208 to −.229, p<0.05) and at 7-9 weeks (Pearson’s r= −.227 to −.367, p<0.001) postpartum, but only in a subpopulation of ‘high stress’ women (as defined by The Antenatal Risk Questionnaire) (Ruyak & Qeadan, 2018). Their regression analysis supported psychosocial stress as moderating the relationship between OT and depressive behavior. Postpartum blood sample was controlled around breastfeeding occurrences. The team quickly notes Pitocin during labor was associated with postpartum OT levels; however, Pitocin was not controlled for in their moderation analysis nor expanded upon (Zelkowitz et al., 2014).
In the only study to measure OT in urine, Laura-Cinisomo et al. (2018b) followed women from the third trimester to six weeks postpartum. Urinary OT levels decreased in all participants from the third trimester of pregnancy to six weeks postpartum. Women with probable prenatal depression (n=30) did not display significant changes in OT overtime, whereas women without depression (n=78) demonstrated a significant decrease in OT overtime (p=.002). Additionally, in a subsample of only depressed women, those who reported any breastfeeding after birth showed significantly lower prenatal (p = .012), and postpartum (p = .015) OT levels compared to those that did not breastfeed (S. Lara-Cinisomo et al., 2018b). This was the only study identified fitting the inclusion criteria utilizing urinary OT measurement, making it unclear whether its contradictory finding might be due to difference in methodologies.
3.2. Intrapartum Intravenous Synthetic Oxytocin
We identified only four studies exploring the relationship between intrapartum intravenous synOT exposure and postpartum depression (see Table 2).
Gu et al., found higher synOT doses weakly correlated with depressive symptoms (Pearson’s r= 0.15, p<0.01) in a large community sample (n=386) before adjusting for covariates. SynOT also significantly predicted higher levels of depressive symptomatology at two months postpartum (p<0.05) in their full regression model after adjusting for years of education and relationship status. Furthermore, they found a significant (p<0.001), positive relationship between synthetic oxytocin dose and endogenous plasma OT levels at two months postpartum, indicating synthetic OT administration in labor might have long lasting effects on endogenous maternal OT production (Gu et al., 2016). This was only the study in this category to measure postpartum endogenous OT, a significant strength, however no standardization of the timing of blood collection was reported. Infant feeding status was considered in analysis.
Those results were supported by a large population-based study (n=46,732) showing peripartum synOT exposure was associated with a significant 32% increased risk (CI: 1.23–1.42) of postpartum depression (measured by either clinical diagnosis or antidepressant prescription) in women without any history of depressive or anxiety disorders (Kroll-Desrosiers et al., 2017). It is generally agreed upon that PPD is underdiagnosed and thus undertreated (Wemer, Miller, Osborne, Kuzava, & Monk, 2015), thus the sole use of clinical diagnosis in this study likely lowers the PPD detection rate. Due to the retrospective design, synOT exposure was measured dichotomously in this study and feeding was not considered.
Conversely, in a longitudinal study of 260 women, Takacs et al. (2019) found that receiving synthetic oxytocin lowered risk of a positive PPD screen postpartum (HR = 0.66, p=.014). Interestingly, however, synOT exposure had no effect on “baby blues,” measured in hospital after delivery (and therefore closest to synOT exposure) when assessed as a single timepoint (Takacs, Seidlerova, Sterbova, Cepicky, & Havlicek, 2019). This team also utilized a dichotomous measurement of synOT exposure, rather than total dose or length of time exposed.
Finally, a randomized control trial administered synthetic OT immediately in labor or delayed for eight hours (Hinshaw, Simpson, Cummings, Hildreth, & Thornton, 2008). In the delayed group, positive PPD screens within the first 48 hours postpartum occurred 15% of the time as compared to 20% in the group with longer exposure, however this difference was not statistically significant.
4. Discussion
The aim of this systematic review was to assess the state of the literature on the relationship between OT and PPD in both healthy community and clinical populations in order to make recommendations for further studies if needed. In this review, 16 studies were identified that addressed the association between OT and PPD during the postpartum period. The landscape of the literature in this area is quite varied with diversity in both methods and outcomes. The link between OT and PPD is clearly a subject of interest across a variety of disciplines and teams.
Of the twelve studies focused on endogenous oxytocin, eight studies suggested an inverse relationship between plasma OT levels and depressive symptoms, two found no significant relationship, one found a change of OT trajectory significant and one found a positive relationship between plasma OT and PPD. Discrepancies in results across studies are possibly due to individual study limitations and inconsistency of methodology including sample sizes, OT laboratory measurement procedures, consideration of covariates and interval of follow up. Some publications presented absolute values of OT concentration, however, comparing numeric values between studies is unwise as different lab methods may yield vastly different results.
There is currently a debate within the overall field of oxytocin research as different common lab methods often produce concentrations that vary wildly and are largely uncorrelated (Leng & Sabatier, 2016; MacLean et al., 2019). One possible reason forthe discrepancy is due to variable amounts of additional factors present in plasma that can impact assay results, such as OT degrading enzymes. Plasma extraction procedures can mitigate this problem in both RIAs and ELISAs and yield values closer to that of bioassays (Leng & Sabatier, 2016). Indeed, extraction of plasma samples is ‘strongly recommended’ by Enzo, a manufacture of a widely used ELISA. Dilution of the sample to at least eight-fold may also be sufficient to minimize interference but each lab must verify the dilution amount is appropriate for their sample (Assay Designs, 2007). Comparison of OT levels in extracted vs unextracted plasma vary widely, often by hundreds of pg/mLs. A recent systematic review has concluded that studies using ELISA on unextracted samples have produced no convincing evidence that peripheral oxytocin might be reliable biomarkers in psychiatric disorders (Rutigliano et al., 2016). However, plasma extraction is not foolproof and may also remove OT in other states or breakdown products that are biologically meaningful (MacLean et al., 2019).
A great amount of high-quality research would be necessary to establish reference ranges of OT in postpartum women in order for it to be used as a depression biomarker. As such, it is difficult and inadvisable to compare absolute values between studies or develop any reference range for ‘normal’ postpartum OT levels. In this review the most useful comparisons came from relative OT differences from woman to woman within the same sample rather than absolute value. In the majority of studies presented above, either relative comparative values (i.e. ‘higher’ or ‘lower’ relative to the sample or comparison group) or hormone trajectory is reported rather than absolute values of OT. Even considering limitations and the further research that must be done, it is useful to know that in some cases, given a consistently reliable laboratory, relative higher concentrations of OT are associated with fewer depressive symptoms. There are a number of low-cost interventions, such as skin to skin contact with her infant, that can trigger release of maternal endogenous OT and these should be encouraged.
Across the field, the majority of plasma studies supported an inverse relationship between OT levels and depressive symptoms. However, a number of questions remain regarding causality. Primarily, it is unclear whether low OT impacts depression or if depression leads to emotions and behaviors that result in lower OT. Especially considering, as was the case in Massey et al, a relationship was identified only in a subset of women with specific traits such as a lifetime history of depression or high stress. More research is needed to elucidate the mechanisms of this relationship and if OT reduces depressive symptoms or if OT concentrations are the result of depression. The nuance of the relationship is especially of interest as OT is investigated as a treatment modality in PPD (De Cagna et al., 2019).
Of note, a strength of this body of literature is the almost universal utilization of the Edinburgh Postnatal Depression Scale (EPDS) (Cox, Holden, & Sagovsky, 1987) in screening. This is a well validated measure in the postpartum period in a variety of populations. PPD is quite often not diagnosed, so while clinical interviews are useful in research, screening tools often capture women who might not receive a clinical diagnosis (Evins, Theofrastous, & Galvin, 2000). Future researchers should continue to use this tool as it allows for easier comparison between study outcomes, leading to an overall richer understanding of the phenomenon.
Conclusions regarding the relationship between intravenous synthetic oxytocin and postpartum depression cannot be made based on current evidence. Of the four studies identified in this arena, two found a negative association (Gu et al., 2016; Kroll-Desrosiers et al., 2017), Hinshaw et al. (2008) found no relationship, and Takacs et al. (2019) noted a positive relationship. The four studies utilized vastly different designs and methodology which is a potential source of discrepancy. Indeed, the designs are so heterogenous it is difficult even to compare between studies. However, we do know women are receiving synOT quite often before, during, and immediately after labor (“Guidelines for oxytocin administration after birth: AWHONN practice brief number 2,” 2015; Salati, Leathersich, Williams, Cuthbert, & Tolosa, 2019). Given the relationship between oxytocin, parturition, and maternal bonding, this is an important question that is understudied at this point. Clearly additional research is needed focused on the long-term impact of synOT exposure during labor on mood.
5. Limitations
Due to the small number of studies that met inclusion criteria we chose not to conduct a meta-analysis, which would have quantitatively combined the data from multiple studies. As such our results and recommendations are derived from an assessment of study quality and synthesis across the field rather than aggregate quantitative values.
6. Conclusions
In conclusion, the state of the literature is not of sufficient quality to make any conclusions with regard to an association between endogenous OT concentrations or synOT administration, and PPD. Based on the limitations of the current literature, and the current clinical prevalence of synOT administration, we strongly recommend that rigorous studies examining synOT exposure on PPD should be performed. The studies should take into account a variety of exposure variables including route of exposure, total dosage, and total time of exposure. When conducting that work, we recommend continuing to utilize consistent, reliable and validated methods including the EPDS as a depression measure, controlling for breastfeeding status and history of depression and, if quantifying OT blood levels, utilizing extracted plasma and ensuring sample collection is standardized around breastfeeding occurrences.
Highlights:
Oxytocin is hypothesized to have a role in postpartum depression.
Synthetic oxytocin (Pitocin) is regularly administered to women during labor and immediately postpartum.
Literature studying the relationship between both endogenous and synthetic oxytocin and postpartum depression are reviewed.
Acknowledgements:
This work was support by a National Institute of Nursing Research (NINR) of the National Institutes of Health (NIH) NRSA F31NR0183669 to TAT. LJY’s contribution was supported by NIH grants P50MH100023 to LJY and P51OD011132 to Yerkes National Primate Research Center. As well as NIH grant 5R01MD009746 to EC and PB. NSC was supported by the NIH NINR under Award Number K01NR016984 during research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to acknowledge Sharon Leslie, Nursing Informationist at the Woodruff Health Sciences Center Library for her assistance with the literature search.
Appendix
Table A1.
Newcastle - Ottawa Quality Assessment Scale for studies describing correlations between endogenous oxytocin and postpartum depressive symptoms
| Selection (score 0-3) | Comparability (score 0-2) | Outcome (0-3) | |||||
|---|---|---|---|---|---|---|---|
| Author, year | Representativeness of exposed cohort (score 0-1) | Assessment of exposure (score 0-2) | Control for confounders (0-2) | Assessment of outcome (0-1) | Follow- up period after delivery (0-1) | Adequacy of follow up (0-1) | Total Score (0-8) |
| Cox et al. 2015 | *Somewhat representative of the average healthy pregnant women in the community | **Secure record: extracted blood samples analyzed by ELISA | **Controlled for BF status, maternal age, ethnicity, education level, BMI, parity, and mode of delivery | *EPDS | *8 weeks | *Subjects lost to follow up unlikely to introduce bias. | 8 |
| Eapen et al. 2014 | *Somewhat representative of the average healthy pregnant women in the community | **Secured record: extracted blood samples analyzed by RIA | *Did not control for BF status. Controlled for maternal age, marital status, and parity | *EPDS | *3 months | *Subjects lost to follow up unlikely to introduce bias. | 7 |
| Guintivano et al. 2017 | *Cases and controls well defined and representative of community | **Secure record: extracted blood samples analyzed by ELISA | ** Controlled for BF status, maternal age, ethnicity, marital status, education, BMI, parity, genetic information | *EPDS | *6 weeks | One time point | 7 |
| Jobst et al. 2016 | *Truly representative of the healthy pregnant women in the community | *Secure record: unextracted blood samples diluted 1:3 analyzed by ELISA | **Controlled for BF status, maternal age, education, and parity | MADRS | *6 months | *Subjects lost to follow up unlikely to introduce bias. Provided description of lost subjects | 6 |
| Lara-Cinisomo et al. 2017 | *Somewhat representative of the healthy pregnant Latino women in the community | **Secure record: extracted blood samples analyzed by ELISA | **controlled for BF status, maternal marital status, education, income, and history of depression | *EPDS | *8 weeks | *Subjects lost to follow up unlikely to introduce bias. Provided description of lost subjects | 8 |
| Lara-Cinisomo et al. 2018A | *Somewhat representative of the healthy pregnant Latino women in the community | **Secure record: extracted blood samples analyzed by ELISA | **Controlled for BF status, maternal marital status, education, and parity | *EPDS | *8 weeks | *Subjects lost to follow up unlikely to introduce bias. | 8 |
| Lara-Cinisomo et al. 2018B | *Somewhat representative of the healthy pregnant Latino women in the community | **Secure record: extracted urine samples analyzed by ELISA | **controlled for BF status, maternal marital status, education, income, and history of depression | *EPDS | *6 weeks | *Subjects lost to follow up unlikely to introduce bias. Secondary analysis of only Latino women. | 8 |
| Massey et al. 2016 | *Cases and controls well defined and somewhat representative of community | **Secure record: blood samples (diluted 1:8) analyzed by ELISA | **Controlled for BF status, maternal age, marital status, BMI, history of depression, mode of delivery, and infant gestational age, weight and gender | *EPDS | *6 weeks | *Subjects lost to follow up unlikely to introduce bias. Provided description of lost subjects | 8 |
| Samuel et al. 2015 | *Cases and controls well defined and somewhat representative of community | *Secure record: blood samples (diluted 1:2 or 1:4) analyzed by ELISA | *Controlled for BF in timing of blood draw, clinical and community samples were matched for demographic variables (parity, age, educational level, infant gender) and breast- feeding status | *Mixed, clinical interview and EPDS | *2 months | *Subjects lost to follow up unlikely to introduce bias. Provided description of lost subjects | 7 |
| Skrundz et al. 2011 | *Somewhat representative of the average healthy pregnant women in the community | *Secure record: blood samples analyzed by RIA, extraction not reported | **Controlled for BF status, maternal age, income, infant gestational age, birth weight, and gender | *EPDS | 2 weeks | *Subjects lost to follow up unlikely to introduce bias. Provided description of lost subjects | 6 |
| Stuebe et al. 2013 | *Somewhat representative of the average healthy pregnant women in the community | **Secure record: extracted blood samples analyzed by ELISA | **Controlled for BF status, maternal age, ethnicity, education level, BMI, parity, and mode of delivery | *EPDS | *8 weeks | *Subjects lost to follow up unlikely to introduce bias | 8 |
| Zelkowi tz et al. 2014 | *Truly representative of the average healthy pregnant women in the community | *Secured record: unextracted blood samples analyzed by ELISA | *Did not control for BF status. Controlled for maternal age, education, marital status, parity and infant gender | *EPDS | *9 weeks | *Subjects lost to follow up unlikely to introduce bias. Provided description of lost subjects | 6 |
Total Score: 0-8; 0-2: low quality; 3-5: moderate quality; 6-8: high quality
Table A2.
Newcastle - Ottawa Quality Assessment Scale for studies describing correlations between intrapartum intravenous synOT exposure and postpartum depressive symptoms
| Selection (score 0-3) | Comparability (score 0-2) | Outcome (0-1) | |||||
|---|---|---|---|---|---|---|---|
| Author, year | Representativeness of exposed cohort | Assessment of exposure | Control for confounders | Assessment of outcome | Follow-up period after delivery | Adequacy of follow up | Total Score (0-8) |
| Gu et al. 2016 | *Cases and controlled defined, fairly representative of community | *secure record: synOT dose calculated from hospital records; unextracted blood samples analyzed by ELISA | **Controlled for parity, age, marital status, years of education, sex of baby, and BF | *EPDS | *8 weeks | *Secondary analysis. Unlikely to introduce bias | 7 |
| Kroll-Desrosiers et al. 2017 | *Representative of the average women in the community, very large sample size | *synOT exposure (yes/no) from hospital records | *Controlled for basic demographics | *Diagnosis or prescribed psychotropic medication | *1 year | *Database analysis. Unlikely to introduce bias | 7 |
| Hinshaw et al., 2008 | n/a, see Table A3 for RoB | Assessment | |||||
| Takacs et al., 2018 | *Representative of the average healthy pregnant women in the community | **synOT exposure (yes/no) from hospital records | **Controlled for demographics, history of depression and multiple labor and delivery variables | *EPDS | *1-7 days, 6 weeks, 9 months | *Subjects lost to follow up unlikely to introduce bias. Provided description of lost subjects. | 8 |
Total Score: 0-8; 0-2: low quality; 3-5: moderate quality; 6-8: high quality
Table A3.
Cochrane Risk of Bias Tool for assessing randomized control trials of intrapartum synOT exposure and postpartum depressive symptoms
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of Blinding of participants and personnel (performance bias) | Blinding of Blinding of outcome assessment (detection bias) (patient-reported outcomes) | Blinding of outcome assessment (detection bias) (all cause mortality) | Incomplete outcome data (attrition bias) (short-term [2-6 weeks]) | Incomplete outcome data (attrition bias)(long-term [>6 weeks]) | Selective reporting (reporting bias) | |
|---|---|---|---|---|---|---|---|---|
| Hinshaw et al., 2008 | Low | Low | High | Unknown | High | Low | Low | unclear |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- American Psychological Association. (2015). Postpartum Depression. Washington, DC. [Google Scholar]
- Assay Designs. (2007). Oxytocin Enzyme Immunoassy Kit: Manufacturer’s instructions http://www.enzolifesciences.com/fileadmin/redacteur/pdf/adi/ADI-900-153.pdf.
- Beck CT (2001). Predictors of postpartum depression: an update. Nurs Res, 50(5), 275–285. [DOI] [PubMed] [Google Scholar]
- Beck CT (2002). Postpartum depression: a metasynthesis. Qual Health Res, 12(4), 453–472. [DOI] [PubMed] [Google Scholar]
- Bell AF, Erickson EN, & Carter CS (2014). Beyond labor: the role of natural and synthetic oxytocin in the transition to motherhood. J Midwifery Womens Health, 59(1), 35–42: quiz 108. doi: 10.1111/jmwh.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhati S, & Richards K (2015). A Systematic Review of the Relationship Between Postpartum Sleep Disturbance and Postpartum Depression. J Obstet Gynecol Neonatal Nurs. doi: 10.1111/1552-6909.12562 [DOI] [PubMed] [Google Scholar]
- Bloch M, Rotenberg N, Koren D, & Klein E (2006). Risk factors for early postpartum depressive symptoms. Gen Hosp Psychiatry, 28( 1), 3–8. doi: 10.1016/j.genhosppsych.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Brann E, Fransson E, White RA, Papadopoulos FC, Edvinsson A, Kamali-Moghaddam M, … Skalkidou A (2018). Inflammatory markers in women with postpartum depressive symptoms. J Neurosci Res. doi: 10.1002/jnr.24312 [DOI] [PubMed] [Google Scholar]
- Cannon C, & Nasrallah HA (2019). A focus on postpartum depression among African American women: A literature review. Ann Clin Psychiatry, 31(2), 138–143. [PubMed] [Google Scholar]
- Carter CS (2014). Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol, 65, 17–39. doi: 10.1146/annurev-psych-010213-115110 [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Guo Y, Pajer K, Lowe N, McCarthy D, Schmiege S, … Stafford B (2013). Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology, 38(9), 1786–1796. doi: 10.1016/j.psyneuen.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, & Pajer K (2008). The psychoneuroimmunology of postpartum depression. J Womens Health (Larchmt), 17(9), 1529–1534. doi: 10.1089/jwh.2007.0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EQ, Stuebe A, Pearson B, Grewen K, Rubinow D, & Meltzer-Brody S (2015). Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology, 55, 164–172. doi: 10.1016/j.psyneuen.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry, 150, 782–786. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Dagher RK, McGovern PM, Dowd BE, & Gjerdingen DK (2012). Postpartum depression and health services expenditures among employed women. J Occup Environ Med, 54(2), 210–215. doi: 10.1097/JOM.0b013e31823fdf85 [DOI] [PubMed] [Google Scholar]
- De Cagna F, Fusar-Poli L, Damiani S, Rocchetti M, Giovanna G, Mori A, … Brondino N (2019). The Role of Intranasal Oxytocin in Anxiety and Depressive Disorders: A Systematic Review of Randomized Controlled Trials. Clin Psychopharmacol Neurosci, 17(1), 1–11. doi: 10.9758/cpn.2019.17.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geest K, Thiery M, Piron-Possuyt G, & Vanden Driessche R (1985). Plasma oxytocin in human pregnancy and parturition. J Perinat Med, 75(1), 3–13. [DOI] [PubMed] [Google Scholar]
- Dias CC, & Figueiredo B (2015). Breastfeeding and depression: a systematic review of the literature. J Affect Disord, 171, 142–154. doi: 10.1016/j.jad.2014.09.022 [DOI] [PubMed] [Google Scholar]
- Eapen V, Dadds M, Barnett B, Kohlhoff J, Khan F, Radom N, & Silove DM (2014). Separation Anxiety, Attachment and Inter-Personal Representations: Disentangling the Role of Oxytocin in the Perinatal Period. PLoS One, 9(9). doi: 10.1371/joumal.pone.0107745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins GG, Theofrastous JP, & Galvin SL (2000). Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol, 182(5), 1080–1082. doi: 10.1067/mob.2000.105409 [DOI] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, Ijzendoom M, & Permezel M (2011). The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv Rev Psychiatry, 19(1), 1–14. doi: 10.3109/10673229.2011.549771 [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, & Sandman CA (2013). New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides, 47(6), 363–370. doi: 10.1016/j.npep.2013.10.007 [DOI] [PubMed] [Google Scholar]
- Gu V, Feeley N, Gold I, Hayton B, Robins S, Mackinnon A, … Zelkowitz P. (2016). Intrapartum Synthetic Oxytocin and Its Effects on Maternal Well-Being at 2 Months Postpartum. Birth, 43(1), 28–35. doi: 10.1111/birt.12198 [DOI] [PubMed] [Google Scholar]
- Guidelines for oxytocin administration after birth: AWHONN practice brief number 2. (2015). J Obstet Gynecol Neonatal Nurs, 44(1), 161–163. doi: 10.1111/1552-6909.12528 [DOI] [PubMed] [Google Scholar]
- Guintivano J, Sullivan PF, Stuebe AM, Penders T, Thorp J, Rubinow DR, & Meltzer-Brody S (2017). Adverse life events, psychiatric history, and biological predictors of postpartum depression in an ethnically diverse sample of postpartum women. Psychol Med doi: 10.1017/S0033291717002641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick V, Altshuler LL, & Suri R (1998). Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics, 39(2), 93–101. doi: 10.1016/s0033-3182(98)71355-6 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, … Sterne JA. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj, 343, d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw K, Simpson S, Cummings S, Hildreth A, & Thornton J (2008). A randomised controlled trial of early versus delayed oxytocin augmentation to treat primary dysfunctional labour in nulliparous women. Bjog-an International Journal of Obstetrics and Gynaecology, 115(10), 1289–1295. doi: 10.1111/j.1471-0528.2008.01819.x [DOI] [PubMed] [Google Scholar]
- Jobst A, Krause D, Maiwald C, Härtl K, Myint AM, Kästner R, … Müller N (2016). Oxytocin course over pregnancy and postpartum period and the association with postpartum depressive symptoms. Arch Womens MentHealth, 19(4), 571–579. doi: 10.1007/s00737-016-0644-2 [DOI] [PubMed] [Google Scholar]
- Ko JY, Rockhill KM, Tong VT, Morrow B, & Farr SL (2017). Trends in Postpartum Depressive Symptoms - 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep, 66(6), 153–158. doi: 10.15585/mmwr.mm6606a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll-Desrosiers AR, Nephew BC, Babb JA, Guilarte-Walker Y, Moore Simas TA, & Deligiannidis KM (2017). Association of peripartum synthetic oxytocin administration and depressive and anxiety disorders within the first postpartum year. Depress Anxiety, 34(2), 137–146. doi : 10.1002/da.22599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Cinisomo S, D’Anna-Hernandez K, Fujimoto EM, & Pedersen CA (2018). Exploring associations between perinatal depression, anxiety, and urinary oxytocin levels in Latinas. Arch Womens Ment Health. doi: 10.1007/s00737-018-0910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Cinisomo S, Kefu Z, Kexin F, Yumeng B, Weston AP, Ravat U, … Bu Y. (2018). Traumatic events: exploring associations with maternal depression, infant bonding, and oxytocin in Latina mothers. BMC Womens Health, 18, 1–9. doi: 10.1186/s12905-018-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Cinisomo S, McKenney K, Di Florio A, & Meltzer-Brody S (2017). Associations Between Postpartum Depression, Breastfeeding, and Oxytocin Levels in Latina Mothers. Breastfeeding Medicine, 12(7), 436–442. doi: 10.1089/bfm.2016.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy-Warren P, & McCarthy G (2007). Postnatal depression: prevalence, mothers’ perspectives, and treatments. Arch Psychiatr Nurs, 21(2), 91–100. doi: 10.1016/j.apnu.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Leake RD, Weitzman RE, Glatz TH, & Fisher DA (1981). Plasma oxytocin concentrations in men, nonpregnant women, and pregnant women before and during spontaneous labor. J Clin EndocrinolMetab, 53(4), 730–733. doi: 10.1210/jcem-53-4-730 [DOI] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, … Averbeck BB (2018). Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry, 23(1), 115–122. doi: 10.1038/mp.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, & Sabatier N (2016). Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J Neuroendocrinol, 28(10). doi: 10.1111/jne.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon MC, Birkimer JC, Simpson T, & Looney S (2005). Postpartum depression and social support in adolescents. J Obstet Gynecol Neonatal Nurs, 34(1), 46–54. doi: 10.1177/0884217504272802 [DOI] [PubMed] [Google Scholar]
- Lucero NB, Beckstrand RL, Callister LC, & Sanchez Birkhead AC (2012). Prevalence of postpartum depression among Hispanic immigrant women. J Am Acad Nurse Pract, 24(12), 726–734. doi: 10.1111/j.1745-7599.2012.00744.x [DOI] [PubMed] [Google Scholar]
- MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, & Carter CS (2019). Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology, 107, 225–231. doi: 10.1016/j.psyneuen.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah BL (2016). Oxytocin, Postnatal Depression, and Parenting: A Systematic Review. Harv Rev Psychiatry, 24(1), 1–13. doi: 10.1097/hrp.0000000000000093 [DOI] [PubMed] [Google Scholar]
- Marshall C (1985). The art of induction/augmentation of labor. J Obstet Gynecol Neonatal Nurs, 14(1), 22–28. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, & Drake P (2018). Births: Final Data for 2017. Natl Vital Stat Rep, 67(8), 1–50. [PubMed] [Google Scholar]
- Massey SH, Schuette SA, Pournajafi-Nazarloo H, Wisner KL, & Carter CS (2016). Interaction of oxytocin level and past depression may predict postpartum depressive symptom severity. Arch Womens Ment Health, 19(5), 799–808. doi: 10.1007/s00737-016-0616-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Eapen V, Kohlhoff J, Mendoza Diaz A, Barnett B, Silove D, & Dadds MR (2016). Genetic Regulation of Maternal Oxytocin Response and Its Influences on Maternal Behavior. Neural Plast, 2016, 5740365. doi: 10.1155/2016/5740365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura D, Canavarro MC, & Figueiredo-Braga M (2016). Oxytocin and depression in the perinatal period-a systematic review. Arch Womens Ment Health, 19(4), 561–570. doi: 10.1007/s00737-016-0643-3 [DOI] [PubMed] [Google Scholar]
- Pedersen C, Leserman J, Garcia N, Stansbury M, Meltzer-Brody S, & Johnson J (2016). Late pregnancy thyroid-binding globulin predicts perinatal depression. Psychoneuroendocrinology, 65, 84–93. doi: 10.1016/j.psyneuen.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps MG, Raker CA, Ware CF, & Zlotnick C (2013). Randomized controlled trial to prevent postpartum depression in adolescent mothers. Am J Obstet Gynecol, 208(3), 192.e191–196. doi: 10.1016/j.ajog.2012.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost M, Zelkowitz P, Tulandi T, Hayton B, Feeley N, Carter CS, … Gold I (2014). Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front Public Health, 2, 1. doi: 10.3389/fpubh.2014.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich ME, deCardenas EJ, Lee HJ, & Caldwell HK (2014). Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PLoS One, 9(6), e98839. doi: 10.1371/journal.pone.0098839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, & Young LJ (2014). The biology of mammalian parenting and its effect on offspring social development. Science, 345(6198), 771–776. doi: 10.1126/science.1252723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, & Stewart DE (2004). Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry, 26(4), 289–295. doi : 10.1016/j.genhosppsych.2004.02.006 [DOI] [PubMed] [Google Scholar]
- Rutigliano G, Rocchetti M, Paloyelis Y, Gilleen J, Sardella A, Cappucciati M, … Fusar-Poli P. (2016). Peripheral oxytocin and vasopressin: Biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res, 241, 207–220. doi: 10.1016/j.psychres.2016.04.117 [DOI] [PubMed] [Google Scholar]
- Ruyak SL, & Qeadan F (2018). Use of the Antenatal Risk Questionnaire to Assess Psychosocial Risk Factors Associated with Risk for Postpartum Depression: A Pilot Study. JMidwifery! Womens Health, doi: 10.1111/jmwh.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salati JA, Leathersich SJ, Williams MJ, Cuthbert A, & Tolosa JE (2019). Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database SvstRev, 4, Cd001808. doi: 10.1002/14651858.CD001808.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Hayton B, Gold I, Feeley N, Carter CS, & Zelkowitz P (2015). Maternal mental health moderates the relationship between oxytocin and interactive behavior. Infant Ment Health J, 36(4), 415–426. doi: 10.1002/imhj.21521 [DOI] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, & Rubinow DR (2015). The role of reproductive hormones in postpartum depression. CNS Spectr, 20(1), 48–59. doi: 10.1017/s1092852914000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock EL, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso HA, Idahl A, … Fortner RT (2016). Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study. BMC Pregnancy Childbirth, 76(1), 146. doi: 10.1186/s12884-016-0937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, & Meinlschmidt G (2011). Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Nenropsychopharmacology, 36(9), 1886–1893. doi: 10.1038/npp.2011.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DE, & Vigod SN (2019). Postpartum Depression: Pathophysiology, Treatment, and Emerging Therapeutics. Anna Rev Med, 70, 183–196. doi: 10.1146/annurev-med-041217-011106 [DOI] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, & Meltzer-Brody S (2013). Association between maternal mood and oxytocin response to breastfeeding. Journal of Women’s Health, 22(4), 352–361. doi: 10.1089/jwh.2012.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs L, Seidlerova JM, Sterbova Z, Cepicky P, & Havlicek J (2019). The effects of intrapartum synthetic oxytocin on maternal postpartum mood: findings from a prospective observational study. Arch Womens Ment Health, 22(4), 485–491. doi: 10.1007/s00737-018-0913-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács L, Seidlerová JM, Štěrbová Z, Čepický P, & Havlíček J (2018). The effects of intrapartum synthetic oxytocin on maternal postpartum mood: findings from a prospective observational study. Arch Womens Ment Health, doi: 10.1007/s00737-018-0913-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E, & Reck C (2009). Infants of depressed mothers. Hctrv Rev Psychiatry, 17(2), 147–156. doi: 10.1080/10673220902899714 [DOI] [PubMed] [Google Scholar]
- Weinberg ΜK, & Tronick EZ (1998). The impact of maternal psychiatric illness on infant development. J Clin Psychiatry, 59 Snppl 2, 53–61. [PubMed] [Google Scholar]
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, & Tugwell P The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses.
- Wemer E, Miller M, Osborne LM, Kuzava S, & Monk C (2015). Preventing postpartum depression: review and recommendations. Arch Womens Ment Health, 75(1), 41–60. doi: 10.1007/s00737-014-0475-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DB (2020). Practical Meta-Analysis Effect Size Calculator. Retrieved from https://campbellcollaboration.org/escalc/html/EffectSizeCalculator-Home.php
- Yamamoto Y, Liang M, Munesue S, Deguchi K, Harashima A, Furuhara K, … Higashida H. (2019). Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun Biol, 2, 76. doi: 10.1038/s42003-019-0325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, … Levin P (2014). Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm Behav, 66(2), 351–360. doi: 10.1016/j.yhbeh.2014.06.014 [DOI] [PubMed] [Google Scholar]


