Abstract
TAR-DNA binding protein-43 (TDP-43) proteinopathy is seen in multiple brain diseases. A standardized terminology was recommended recently for common age-related TDP-43 proteinopathy: limbic-predominant, age-related TDP-43 encephalopathy (LATE) and the underlying neuropathological changes, LATE-NC. LATE-NC may be co-morbid with Alzheimer’s disease neuropathological changes (ADNC). However, there currently are ill-defined diagnostic classification issues among LATE-NC, ADNC, and frontotemporal lobar degeneration with TDP-43 (FTLD-TDP). A practical challenge is that different autopsy cohorts are composed of disparate groups of research volunteers: hospital- and clinic-based cohorts are enriched for FTLD-TDP cases, whereas community-based cohorts have more LATE-NC cases. Neuropathological methods also differ across laboratories. Here, we combined both cases and neuropathologists’ diagnoses from two research centres—University of Pennsylvania and University of Kentucky. The study was designed to compare neuropathological findings between FTLD-TDP and pathologically severe LATE-NC. First, cases were selected from the University of Pennsylvania with pathological diagnoses of either FTLD-TDP (n = 33) or severe LATE-NC (mostly stage 3) with co-morbid ADNC (n = 30). Sections from these University of Pennsylvania cases were cut from amygdala, anterior cingulate, superior/mid-temporal, and middle frontal gyrus. These sections were stained for phospho-TDP-43 immunohistochemically and evaluated independently by two University of Kentucky neuropathologists blinded to case data. A simple set of criteria hypothesized to differentiate FTLD-TDP from LATE-NC was generated based on density of TDP-43 immunoreactive neuronal cytoplasmic inclusions in the neocortical regions. Criteria-based sensitivity and specificity of differentiating severe LATE-NC from FTLD-TDP cases with blind evaluation was ∼90%. Another proposed neuropathological feature related to TDP-43 proteinopathy in aged individuals is ‘Alpha’ versus ‘Beta’ in amygdala. Alpha and Beta status was diagnosed by neuropathologists from both universities (n = 5 raters). There was poor inter-rater reliability of Alpha/Beta classification (mean κ = 0.31). We next tested a separate cohort of cases from University of Kentucky with either FTLD-TDP (n = 8) or with relatively ‘pure’ severe LATE-NC (lacking intermediate or severe ADNC; n = 14). The simple criteria were applied by neuropathologists blinded to the prior diagnoses at University of Pennsylvania. Again, the criteria for differentiating LATE-NC from FTLD-TDP was effective, with sensitivity and specificity ∼90%. If more representative cases from each cohort (including less severe TDP-43 proteinopathy) had been included, the overall accuracy for identifying LATE-NC was estimated at >98% for both cohorts. Also across both cohorts, cases with FTLD-TDP died younger than those with LATE-NC (P < 0.0001). We conclude that in most cases, severe LATE-NC and FTLD-TDP can be differentiated by applying simple neuropathological criteria.
Keywords: TARDBP, C9ORF72, FTD, GCI, NCI
Age-related TDP-43 pathology occurs in multiple brain diseases. Robinson et al. compare the neuropathological features of LATE and FTLD-TDP, and show that the large majority of LATE and FTLD-TDP cases can be differentiated using simple neuropathological criteria applied to a relatively small number of immunostained slides.
Introduction
TAR-DNA binding protein-43 (TDP-43) proteinopathy is characterized by aberrantly phosphorylated and/or mislocalized TDP-43 protein. There is general agreement that millions of individuals worldwide are affected by age-related TDP-43 proteinopathy (Nelson et al., 2019a). Disease-specific diagnoses are important because some future therapeutic strategies may work for particular subsets of patients but not necessarily for others. However, important questions remain about classification systems for these neurodegenerative disorders.
TDP-43 proteinopathy was discovered as a pathological biomarker among individuals with several seemingly different neurological disorders including amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD-TDP), but a number of overlapping features including TDP-43 proteinopathy suggest the diseases are related pathogenetically (Neumann et al., 2006). A small percentage of individuals on the ALS/FTLD clinical-pathological spectrum harbour mutations in the TDP-43 encoding gene, TARDBP (Sreedharan et al., 2008; Van Deerlin et al., 2008). However, outside of the ALS/FTLD spectrum, there are many additional genetic and environmental factors that drive TDP-43 proteinopathy with neurological impairment (Chornenkyy et al., 2019).
By far the most prevalent known subset(s) of TDP-43 proteinopathy occur in advanced old age; TDP-43 proteinopathy is observed in 30–50% of individuals who die beyond 85 years of age (Nelson et al., 2019a). In this context, the TDP-43 proteinopathy is associated with substantial cognitive impairment, especially in the cognitive domain of episodic memory (Nag et al., 2017; Robinson et al., 2018a). Usually, affected individuals lack clinical features of frontotemporal dementia (FTD), e.g. aphasia and severe disinhibition (Nelson et al., 2013 , 2019a; Jung et al., 2014).
Multiple classification systems for TDP-43 proteinopathy have been proposed. An international group of experts recently conferred about common (>1 in 10 lifetime risk) age-related TDP-43 proteinopathy (Nelson et al., 2019a). New terminology was recommended: limbic-predominant, age-related TDP-43 encephalopathy (LATE) and its underlying substrate of LATE neuropathological changes (LATE-NC). LATE-NC may occur with or without co-morbid Alzheimer’s disease neuropathological changes (ADNC). However, it has been proposed that the suggested classification of age-related TDP-43 proteinopathy as LATE-NC is problematic (Josephs et al., 2019a), partly because of currently ill-defined diagnostic boundary zone issues pertaining to ADNC and FTLD-TDP. Another proposed neuropathological feature related to TDP-43 proteinopathy in aged individuals is an ‘Alpha’ (similar to FTLD-TDP pathological type A) versus ‘Beta’ (occurring in and/or near ADNC-type tau tangle structures) pattern of TDP-43 immunoreactivity in the amygdala (Josephs et al., 2019b).
This is a complex subject area. There are differences in epidemiological, genetic, and neuroimaging features when comparing LATE-NC with FTLD-TDP (Nelson et al., 2019a). However, particular neuropathological features of LATE-NC resemble those seen in FTLD-TDP type A (Aoki et al., 2015). Furthermore, LATE-NC affects both the superior temporal cortex and mid-frontal cortex in 8–20% of cases, prompting speculation that LATE-NC represents a mild or early stage in the development of FTLD-TDP pathology rather than a distinct clinical-pathological entity (Josephs et al., 2016). There are shared elements of genetic risk between FTLD-TDP and LATE-NC (Murray et al., 2014; Dickson et al., 2015; Nelson et al., 2016), as there are between ADNC and LATE-NC and between tauopathies and Parkinson disease (Robinson et al., 2018b; Wennberg et al., 2018; Yang et al., 2018; Chornenkyy et al., 2019). From a neuropathological standpoint, ADNC, hippocampal sclerosis, and brain arteriolosclerosis are all relatively frequent in individuals with LATE-NC (Amador-Ortiz et al., 2007; Neltner et al., 2014; Smith et al., 2017). Unfortunately, LATE-NC and FTLD-TDP have not been systematically compared, partly because research cohorts that have many FTLD-TDP cases may lack ‘pure’ LATE-NC cases, and vice versa.
In the present study, we tested whether or not LATE-NC and FTLD-TDP are neuropathologically differentiable. We obtained cases from the Center for Neurodegenerative Disease Research at the University of Pennsylvania (CNDR at UPENN) and the University of Kentucky Alzheimer’s Disease Center (UK-ADC). We found that the large majority of LATE-NC and FTLD-TDP cases had differentiating pathological features.
Materials and methods
Case selection
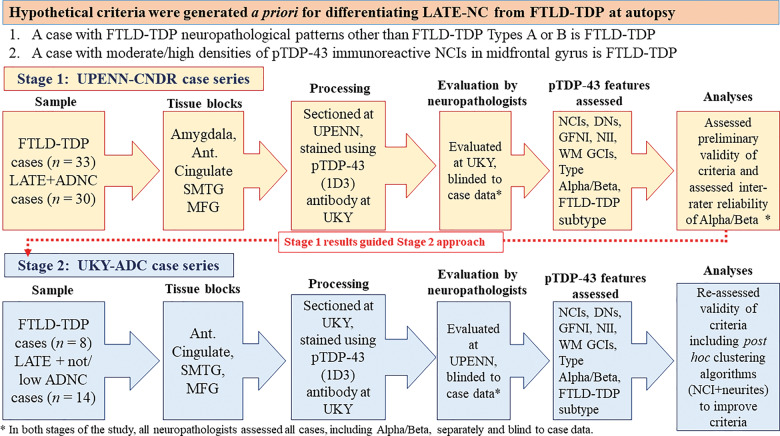
Overall study design and workflow are depicted in Fig. 1. The a priori goal was to select for evaluation in this study pathologically severe LATE-NC cases to compare with FTLD-TDP cases. The reason for this was to focus on the cases that would be most likely to represent diagnostic challenges relative to the distinction between LATE-NC and FTLD-TDP. A two-stage study design was implemented wherein, first, cases originally diagnosed at UPENN were evaluated blindly by UK-ADC neuropathologists. Next, cases originally diagnosed at UK-ADC were evaluated blindly by UPENN neuropathologists. Age at death was not factored into inclusion or exclusion criteria. In terms of FTD clinical features, the behavioural variant of FTD (bvFTD) is signalled by behavioural disinhibition, apathy, loss of empathy, compulsive behaviours, and executive dysfunction (Rascovsky et al., 2007), and can occur with or without motor neuron disease characterized by weakness and spasticity (Strong et al., 2017). Primary progressive aphasia (PPA) is a language disorder with impairment of either naming and understanding of words and objects (semantic variant of PPA), or slowing of speech, or grammar difficulties and muteness (non-fluent/agrammatic PPA) (Gorno-Tempini et al., 2011).
Figure 1.
Overall study design. Before grading the cases, hypothetical criteria were developed for differentiating LATE-NC from FTLD-TDP. These criteria were based on two assumptions: (i) a pattern of TDP-43 immunohistochemical staining interpreted to represent FTLD-TDP types C, D, or E was indicative of FTLD-TDP, not compatible with LATE-NC; and (ii) if the density of TDP-43 immunoreactive neuronal cytoplasmic inclusions (NCIs) was moderate or high in frontal cortex, it also was indicative of FTLD-TDP rather than LATE-NC. Next, we followed a two-stage study design. The first cases series, from University of Pennsylvania (UPENN), consisted of n = 33 cases with FTLD-TDP, and n = 30 cases with relatively severe LATE-NC, the large majority with co-morbid ADNC. Paraffin sections from amygdala, anterior cingulate, superior and middle temporal gyrus, and middle frontal gyrus were selected for the study. The sections were stained for phospho-TDP-43 at University of Kentucky (UKY) and TDP-43 pathology was evaluated by two UKY neuropathologists, applying the ad hoc criteria for differential diagnosis of LATE-NC or FTLD-TDP. Next, a separate cohort from UKY was selected, comprising n = 8 cases with FTLD-TDP and n = 14 cases with relatively severe LATE-NC, lacking co-morbid ADNC. These sections were immunostained for phospho-TDP-43 at UKY and TDP-43 pathology was evaluated by two UPENN neuropathologists, applying the same ad hoc criteria for differential diagnosis of LATE-NC or FTLD-TDP except also including ropy dystrophic neurites in the analyses. Ant Cingulate = anterior cingulate; DN = various types of TDP-43 immunoreactive dystrophic neurites; GFNI = granulofilamentous neuronal inclusions; NII = neuronal intranuclear inclusions; MFG = middle frontal gyrus; SMTG = superior and middle temporal gyri; WM GCI = white matter glial cytoplasmic inclusions.
A series of FTLD-TDP and LATE cases was assembled from patients who participated in the UPENN CNDR brain donation program (Table 1) (Mackenzie et al., 2011; Toledo et al., 2014; Lee et al., 2017). The FTLD-TDP cohort was a subset of 125 patients from the CNDR cohort with a primary neuropathological diagnosis of FTLD-TDP. Forty-four patients had a primary or secondary diagnosis of clinical frontotemporal dementia, behavioural variant (bvFTD). Of these, 35 were well-characterized research participants with additional clinical testing [Mini-Mental State Examination (MMSE) and/or Boston Naming scores], and 33 cases had available mid-frontal cortex, superior temporal cortex, anterior cingulate and amygdala tissue; these 33 cases were included. This cohort included FTLD-TDP type A, B, C and E as diagnosed by neuropathologists at UPENN (Mackenzie and Rademakers, 2007). There was a mix of sporadic and genetic cases, and 19 were known to harbour C9orf72, GRN, or TBK1 mutations. The LATE-NC cohort from UPENN was a subset of 533 patients with a primary neuropathological diagnosis of unremarkable adult brain, primary age-related tauopathy (PART), argyrophilic grain disease, pathological ageing, predominantly cerebrovascular pathology, or ADNC, and with final clinical diagnoses that included unimpaired, cognitively impaired, vascular dementia, or Probable Alzheimer’s disease. From this cohort, 205 patients had a neuropathological diagnosis of LATE-NC. Each of these 205 cases was assigned a TDP-43 proteinopathy severity stage (range 0–6), similar to the staging scheme of Josephs et al. (2016), as well as a LATE-NC stage (range 0–3) (Nelson et al., 2019a). The 30 LATE-NC cases with the highest TDP-43 severity stages and available mid-frontal cortex, superior temporal cortex, anterior cingulate and amygdala tissue were selected for inclusion into the study (n = 30).
Table 1.
Cohort demographics from UPENN CNDR and UK-ADC
| UPENN CNDR |
UK-ADC |
|||
|---|---|---|---|---|
| FTLD-TDP | LATE-NC | FTLD-TDP | LATE-NC | |
| n | 33 | 30 | 8 | 14 |
| Age at death (mean ± SD) | 66.6 ± 9.3 | 88.6 ± 6.4 | 62.9 ± 9.8 | 90.9 ± 8.5 |
| Clinical diagnosis at last exama | ||||
| No documented impairment | 0 | 0 | 2 | 0 |
| Mild cognitive impairment | 0 | 3 | 0 | 0 |
| Vascular dementia | 0 | 1 | 0 | 2 |
| Probable Alzheimer’s disease | 2 | 26 | 0 | 12 |
| bvFTD-FTLD | 2 | 0 | 2 | 0 |
| bvFTD-FTLD/MND | 5 | 0 | 2 | 0 |
| bvFTD-FTLD/PPA | 5 | 0 | 2 | 0 |
| Genetics | ||||
| C9orf72 expansion | 11 | 0 | N/A | N/A |
| GRN variant | 7 | 0 | N/A | N/A |
| TBK1 variant | 1 | 0 | N/A | N/A |
| VCP mutation | 0 | 0 | 1 | N/A |
| FTLD-TDP type | ||||
| Type A or B | 23 | N/A | 4 | N/A |
| Type C | 6 | N/A | 2 | N/A |
| Type D | 0 | N/A | 1 | N/A |
| Type E | 4 | N/A | 1 | N/A |
| LATE-NC stage | ||||
| Stage 0 | N/A | 0 | N/A | 0 |
| Stage 1 | N/A | 0 | N/A | 0 |
| Stage 2 | N/A | 2 | N/A | 9 |
| Stage 3 | N/A | 28 | N/A | 5 |
| Intermediate/high ADNCb | 4 | 28 | 0 | 0 |
bvFTD = behavioural variant FTD; MND = motor neuron disease; N/A = not assessed; PPA = primary progressive aphasia.
For clinical diagnostic criteria and definitions, see ‘Materials and methods’ section.
According to NIA-AA neuropathological criteria (Hyman and Trojanowski, 1997).
A separate series of FTLD-TDP and LATE-NC cases was assembled from the UK-ADC biobank (Table 1). The FTLD-TDP cohort included each non-tau FTD/FTLD case from the entire UK-ADC Brain Bank (n = 8). Notably, whereas the large majority of cases in the UK-ADC Brain Bank were recruited from the community (with normal cognitive status at recruitment) for extensive longitudinal follow-up, all of the FTLD-TDP cases represented in the UK-ADC were recruited from an associated memory disorders clinic. One of the FTLD-TDP cases was found to harbour a VCP mutation (c.464G>A, p.R155H), genotyped as part of the current study. The UK-ADC LATE-NC series was selected to fulfil the following criteria: (i) documented dementia (because we wanted to compare the LATE-NC cases with FTLD-TDP cases which had substantial disease burden); (ii) lacking ‘intermediate’ or ‘severe’ ADNC according to the National Institute on Aging Alzheimer Association (NIA-AA) consensus criteria (Montine et al., 2012); (iii) lacking other severe pathology such as neocortical Lewy body pathology; and (iv) they represent the most severe TDP-43 proteinopathy possible. Based on the above criteria, cases with brain tumours, neocortical Lewy body disease, or a rare (e.g. prion) brain disease were excluded. There were 189 cases with retrospectively diagnosed LATE-NC in the UK-ADC cohort. Of these, 46 had low/minimal ADNC. However, some of these 46 cases had final documented clinical states other than dementia (n = 21), substantial Lewy body pathology (n = 12), and/or met some other exclusion criterion (e.g. brain cancer). A subset of the cases were assessed for LATE-NC genetic risk alleles as described in detail previously (Nelson et al., 2019c). For the present study, among the cases with LATE-NC but lacking significant ADNC, 14 cases were selected for inclusion, all of which had documented dementia diagnosis. All LATE-NC cases were at least LATE-NC stage 2, i.e. TDP-43 proteinopathy was observed in the hippocampus (Nelson et al., 2019a). For the number of cases in each cohort that were included and excluded for further analyses, stratified by LATE-NC stage status, see Supplementary Table 1.
Immunohistochemistry
Brain tissues were fixed in 10% formaldehyde, processed in paraffin blocks, and sections were cut at 8-µm thickness. Immunohistochemical staining for phospho-TDP-43 (1D3 clone, 1:500 dilution, purchased from EMD Millipore) was performed as described previously (Smith et al., 2017; Gal et al., 2018).
Neuropathological evaluations
Individual TDP-43 proteinopathy subtype scores were assigned on a 4-tier semi-quantitative scale (range 0–3) for eight individual subtypes of brain changes diagnosable using phospho-TDP-43 immunohistochemistry. The TDP-43 neuropathological features analysed included: (i) neuronal cytoplasmic inclusions; (ii) neuronal intranuclear inclusions; (iii) glial cytoplasmic inclusions; (iv) granulofilamentous neuronal cytoplasmic inclusions (Lee et al., 2017); and (v) perivascular compact inclusions [compact perivascular compact inclusions as described by Lin et al., 2009)]. Various types of TDP-43-immunoreactive dystrophic neurites were scored including ropy, long type C and punctate dystrophic neurites (Fig. 2). The rubric for this semi-quantitative operationalization was: no pathological phospho-TDP-43 immunoreactivity = 0; 1–2 structures per ×40 magnification high power microscope field (hpf) = 1; 3–15 structures/hpf = 2; and >15 structures/hpf = 3. We also evaluated whether differentiation of LATE-NC from FTLD-TDP could be improved by assessing for the presence of type Beta inclusions in the amygdala. The presence or absence of type Beta inclusions was noted for the 63 cases from UPENN (Josephs et al., 2019b). The overall FTLD-TDP subtype was assigned based on prior published recommendations (Cairns et al., 2007; Lee et al., 2017; Mackenzie and Neumann, 2017). Briefly, FTLD-TDP subtyping was evaluated through examination of neocortical sections stained for pTDP-43 (1D3 clone), using the morphological criteria described in detail in Supplementary Table 2. For pathological assessment, each rater was blinded to the clinical diagnosis, any other information about the case, and any other pathological data or slides for review. Cases were analysed in a randomized order with anonymized slide labels. For the UPENN case series, pathology was independently assessed by P.N. (UK-1) and F.G. (UK-2). For the UK-ADC cohort, pathology was independently assessed by S.P. (UP-1) and J.R. (UP-2). Type Beta assignment was performed by F.G., P.N., J.R., S.P. and E.L. (UP-3).
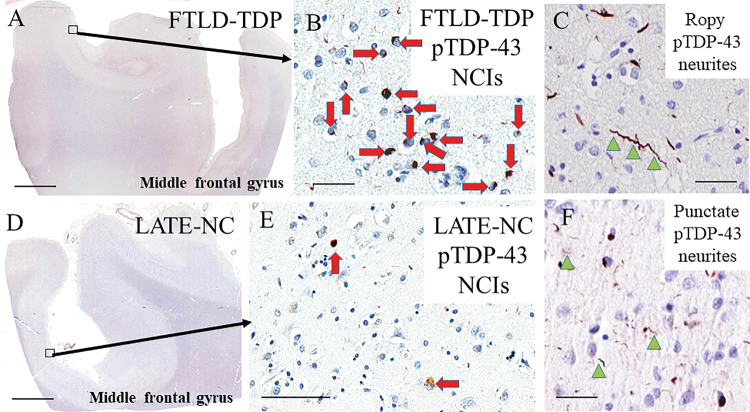
Figure 2.
Representative photomicrographs showing TDP-43 immunoreactive features in LATE-NC and FTLD-TDP. TDP-43 neuropathological features in FTLD-TDP type A/B (A–C) and LATE-NC (D–F) are similar in morphology but frequently differs by severity. Dense neuronal cytoplasmic inclusions (NCIs, red arrows; B) and ropy dystrophic neurites (dystrophic neurites shown with green arrowheads; C) are seen the superficial neocortical layers in FTLD-TDP type A middle frontal gyrus. LATE-NC middle frontal gyrus has milder pathology (E) and punctate dystrophic neurites (F) can be seen in both FTLD-TDP and LATE-NC. Scale bars = 3 mm in A and D; 50 µm in B, C, and F; 100 µm in E.
Statistics
Statistical analyses used the R software, version 3.3.2. Ages at death between FTLD-TDP and LATE were compared using Welch's t-test after running the Shapiro-Wilk test for checking the normality assumption. Rater agreement for individual TDP-43 morphologies between the UK-1 and UK-2 datasets were calculated by weighted Cohen’s κ. Type Beta correlations were calculated by standard, non-weighted Cohen’s κ, unsupervised. TDP-43 histopathological severity scores for the FTLD-TDP and LATE-NC cases were compared by Mann-Whitney-Wilcoxon tests to determine P-values with the introduction of false discovery rate (FDR) corrections for handling multiple testing. Clustering analysis was performed with the k-means clustering method for k = 2, 3 and 4. All statistical tests were two-sided. Statistical significance was set at the 0.05 level.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article, especially in the supplementary tables.
Raw data were generated at the University of Kentucky and the University of Pennsylvania. Although the large majority of data are presented in the paper as stated above, any derived data supporting the findings of this study are available from the corresponding author (P.T.N.) on request.
Results
We tested whether LATE-NC could be pathologically distinguished from FTLD-TDP by the severity of cortical TDP-43 inclusions and/or the presence of type C–E pathology. We also assessed if differentiating LATE-NC could be helped by assessing for the presence of type Beta inclusions in the amygdala. To test these hypotheses, we assembled a selection of FTLD-TDP cases and pathologically severe LATE-NC cases (Table 1).
For the UPENN case series, in groupwise comparisons, FTLD-TDP and LATE-NC cases differed by age of death and clinical symptoms. The average age at death was substantially lower in the FTLD-TDP cases, (66.6 ± 9.3 years), compared to the LATE-NC cases (88.6 ± 6.4 years). Clinically, the FTLD-TDP subjects usually presented with bvFTD compared to a predominantly probable Alzheimer’s disease diagnosis in the LATE-NC group. ADNC was limited in the FTLD-TDP group, with intermediate or high ADNC rarely present (12%, n = 4/33). However, among the LATE-NC group, the majority had an intermediate or high ADNC (93%, n = 28/30). Genetically, 43% of the FTLD-TDP cases were considered sporadic (n = 14/33), and the C9orf72 expansion and GRN mutation carriers were the most common genetic association among those with known pathogenetic alleles, 33% and 21%, respectively.
The severity of eight TDP-43 pathology subtypes was scored in the amygdala and three cortical areas for each case from UPENN. Examples of some of these histopathologies are depicted in Fig. 2. Scores were assigned for the severity of typical neuronal cytoplasmic inclusions (Fig. 2B and E), ropy dystrophic neurites (Fig. 2C), punctate dystrophic neurites (Fig. 2F), compact perivascular inclusions (Lin et al., 2009), and white matter glial cytoplasmic inclusions. Morphologies associated with specific FTLD-TDP subtypes included type C long dystrophic neurites, type D neuronal intranuclear inclusions, and type E granulofilamentous neuronal cytoplasmic inclusions (Lee et al., 2017). See Supplementary Table 2 for detailed description of how FTLD-TDP types were operationalized. Finally, the presence or absence of type Beta inclusions in the amygdala (Josephs et al., 2019b) was noted for each case.
Assessing the UPENN cases, two UK-ADC neuropathologists, blinded to the original neuropathological diagnoses and demographics of each case, independently assigned each case a pathological diagnosis of either LATE-NC or FTLD-TDP (Table 2). Only the four TDP-43 immunostained slides were available to these neuropathologists. However, the diagnostic accuracy of both neuropathologists in terms of predicting the prior diagnoses was quite high. Specificity for LATE-NC and FTLD-TDP was 91–94% and 80–97%, respectively. Sensitivity for LATE-NC and FTLD-TDP was similarly high at 80–97% and 91–94%, respectively.
Table 2.
UPENN cases: diagnostic accuracy of UK-ADC neuropathologists
| Pathologista | UK-ADC blinded diagnosis | UPENN LATE-NC diagnosis | UPENN FTLD-TDP diagnosis | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|
| UK-1 | LATE-NC (n = 32) | 29 | 3 | 91 | 97 |
| FTLD-TDP (n = 31) | 1 | 30 | 97 | 91 | |
| UK-2 | LATE-NC (n = 26) | 24 | 2 | 94 | 80 |
| FTLD-TDP (n = 37) | 6 | 31 | 80 | 94 |
UK-1 is an attending neuropathologist (P.N.); UK-2 is a neuropathology fellow in training (F.G.).
To understand which subset of neuropathological TDP-43 features best distinguish LATE-NC from FTLD-TDP, we asked if any of the scored TDP-43 proteinopathy subtypes were reliably diagnosed between neuropathologists. FTLD-TDP is a rare neurodegenerative disease (Knopman and Roberts, 2011; Coyle-Gilchrist et al., 2016) and many of the pathological morphologies assessed here—especially the type-specific morphologies—are infrequently observed by most practicing neuropathologist. Further, the experience of each neuropathologist can influence the assessment of the severity of each morphology. Here, one of the UK-ADC neuropathologists was an attending neuropathologist (P.N.), whereas the other was a neuropathology fellow in training (F.G.). A weighted Cohen’s κ-test measured the agreement between the two UK-ADC neuropathologists’ scores for each morphology. Ropy dystrophic neurites were the most reliably scored (0.77), followed by granulofilamentous neuronal cytoplasmic inclusions (0.69), type C dystrophic neurites (0.65) and typical neuronal cytoplasmic inclusions (0.64). Less reliably scored between neuropathologists were glial cytoplasmic inclusions (0.49), punctate dystrophic neurites (0.45), neuronal intranuclear inclusions (0.36) and compact perivascular compact inclusions (0.19) (Supplementary Table 3). We emphasize that we used a relatively conservative approach for assessing reliability (e.g. scores of 2 and 3 would be considered disagreement), rather than a presence/absence criteria. These results provided support for the second stage of the study where cases from the UK-ADC were evaluated at UPENN, focusing on the neuronal cytoplasmic inclusions and ropy dystrophic neurites.
Type Beta morphology, as defined previously (Josephs et al., 2019b), was assessed as the presence or absence of the pathology in the amygdala. Our a priori hypothesis was that type Beta would be diagnosed in ∼50% of LATE-NC cases and would be absent in FTLD-TDP cases (Josephs et al., 2019b). Instead, type Beta was variably recorded in 50–80% of LATE-NC cases, and in 18–33% of FTLD-TDP cases (Supplementary Table 4). The same slides were then evaluated blindly by three additional researchers from UPENN. Type Beta was again variably observed in 30–67% of LATE-NC and 6–19% of FTLD-TDP. Overall, there was poor agreement between researchers with κ ranging from −0.02 to 0.59 (Supplementary Table 5). Applying an ad hoc criterion for consensus (three or more of five raters agreed), type Beta was observed in 57% of LATE-NC cases and 12% of FTLD-TDP cases. We conclude that type Beta appears to be enriched in LATE-NC versus FTLD-TDP cases, but low Cohen’s κ makes it an unreliable indicator.
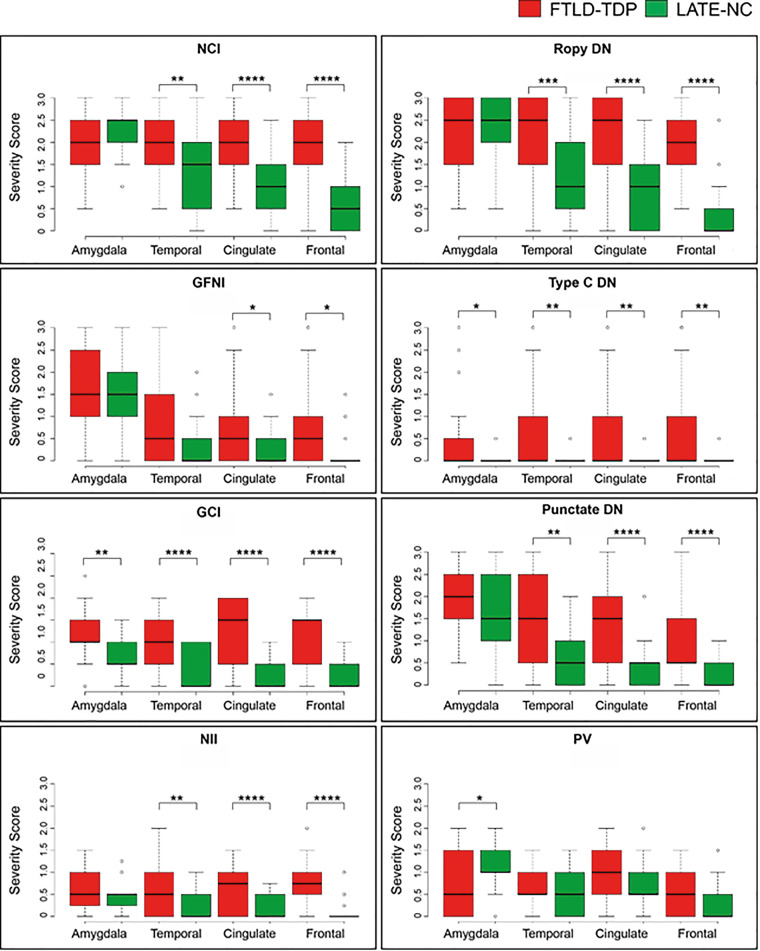
To understand if specific TDP-43 pathological features distinguished FTLD-TDP from LATE-NC, we examined the average pathological severity scores by brain region (Fig. 3). The primary case-level data related to TDP-43 proteinopathy subtypes are provided in Supplementary Table 6. We performed additional p-TDP-43 immunohistochemical stains on the hippocampal formation (cornu ammonis, subiculum, and entorhinal cortex) and these data are reported in Supplementary Table 7. While both FTLD-TDP and LATE-NC groups had similar burdens of pathology in the amygdala (the sample was highly enriched for severe LATE-NC), cortical pathology was typically more severe in FTLD-TDP than in LATE-NC. Moderate to severe neuronal cytoplasmic inclusions and ropy dystrophic neurites were observed in cortical regions in FTLD-TDP and these were mostly rare to mild in LATE-NC. In addition to neuronal cytoplasmic inclusions and ropy dystrophic neurites, white matter glial cytoplasmic inclusions, punctate dystrophic neurites and neuronal intranuclear inclusions were also more severe in FTLD-TDP than LATE-NC in the anterior cingulate and mid-frontal gyri (P-values ≤ 0.0001). The FTLD-TDP type E associated granulofilamentous neuronal cytoplasmic inclusions and the FTLD-TDP type C dystrophic neurites affected only a minority of cases, and were very rarely observed in cortical areas in LATE-NC. Compact perivascular inclusions were variably found across all regions and only in the amygdala was different between FTLD-TDP and LATE-NC (P-value <0.05), with the relevant caveat related to the modest sample sizes.
Figure 3.
Scores of TDP-43 proteinopathic features by brain region, stratified by diagnosis of LATE-NC or FTLD. Cortical pathology is more severe in FTLD-TDP (red) compared to LATE-NC cases (green). TDP-43 pathology was scored separately for individual morphologies for each case and region. The burden of TDP-43 pathology in amygdala was similar between severe LATE-NC and FTLD-TDP and was typically characterized by the presence of neuronal cytoplasmic inclusions (NCI), ropy dystrophic neurites (DN) and punctate dystrophic neurites. Cortical pathology in anterior cingulate, superior and middle temporal, and middle frontal gyrus was more limited in LATE-NC. In FTLD-TDP cases, there was typically moderate to severe neuronal cytoplasmic inclusions and ropy dystrophic neurites, which were usually mild to rare to LATE-NC, although these cases were selected to represent the more severe portion of the LATE-NC pathological spectrum. Box and whisker plots show the median (solid line) and whiskers indicate variability outside the upper and lower quartiles (average of datasets from two neuropathologists, UK-1 and UK-2). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 Mann-Whitney-Wilcoxon analysis. DN = various types of TDP-43 immunoreactive dystrophic neurites, including DN characteristic of FTLD type C (Type C DN), ropy DN and punctate DN; GFNI = granulofilamentous neuronal inclusions; NII = neuronal intranuclear inclusion; PV = perivascular TDP-43 proteinopathy; WM GCI = white matter glial cytoplasmic inclusion.
To verify that our data support the existence of different clusters of pathological features, we performed unsupervised k-means cluster analysis for k = 2, 3, 4 using all eight subtypes of TDP-43 proteinopathy features across four different brain regions (Supplementary Tables 8 and 9). With k = 2, one cluster had accuracy for LATE-NC of 79% while the other cluster had accuracy for FTLD-TDP of 83%. With k = 3, one cluster had a LATE-NC accuracy of 79% and the two other clusters were primarily FTLD-TDP cases: a large cluster with 83% accuracy and small cluster of n = 7 with 100% accuracy for FTLD-TDP which also contained all the type E cases. With k = 4, a more accurate LATE-NC cluster was defined with 97% accuracy and this accounted for the majority of LATE-NC cases. The remaining clusters were primarily FTLD-TDP cases: a large cluster with 83% accuracy, a small cluster of n = 7 with 100% accuracy for FTLD-TDP and containing all the type E cases and a small cluster of n = 6 containing all the type C cases with 100% accuracy for FTLD-TDP. A priori, our hypothesis was that FTLD-TDP types C–E neuropathological patterns would only be seen in cases with FTLD-TDP, but not LATE-NC. The k = 4 clustering analysis supports the existence of FTLD-TDP type C, type E, type A/B and LATE-NC pathological groups with high specificity.
Since the presence of FTLD-TDP types C–E define pathological features that allowed for the clear differentiation of FTLD-TDP from LATE-NC, we next asked which regional morphologies distinguished FTLD-TDP types A/B from LATE-NC. Mann-Whitney-Wilcoxon analysis revealed that neuronal cytoplasmic inclusions and ropy dystrophic neurites in the anterior cingulate, superior temporal and middle frontal cortices were more severe in FTLD-TDP types A/B cases than LATE-NC cases (P-values < 0.001, Table 3). Similar results were obtained as expected if LATE-NC was compared to all FTLD-TDP types A–E (Supplementary Table 10). In these same regions, mild to moderate punctate dystrophic neurites, mild neuronal intranuclear inclusions and a mild burden of white matter glial cytoplasmic inclusions also were different comparing FTLD-TDP and LATE-NC cases (P-values < 0.01). We conclude that the presence of moderate to severe neuronal cytoplasmic inclusions and ropy dystrophic neurites in multiple cortical regions are sufficient criteria to distinguish reliably between FTLD-TDP types A/B and severe LATE-NC.
Table 3.
FTLD-TDP type A/B versus LATE-NC pathological severity differences
| TDP-43 pathological feature | FTLD-TDP type A/B cases only versus LATE-NC comparison by pathological feature: P-values |
|||
|---|---|---|---|---|
| Amygdala | Anterior cingulate | Superior temporal | Middle frontal | |
| Ropy DN | 0.17 | <0.001 | <0.001 | <0.001 |
| NCI | 0.57 | <0.001 | <0.001 | <0.001 |
| WM GCI | 0.001 | <0.001 | <0.001 | <0.001 |
| Punctate DN | 0.03 | <0.001 | 0.003 | <0.001 |
| NII | 0.05 | <0.001 | <0.001 | <0.001 |
| Compact PV | 0.16 | 0.17 | 0.45 | 0.04 |
P-values for Mann-Whitney-Wilcoxon tests for comparison of the LATE-NC cases (n = 30) and the FTLD-TDP Type A/B cases (n = 24), i.e. FTLD-TDP types C–E cases were excluded in this analysis. Shown are results from UPENN cases as read blind by UK-ADC neuropathologists. Compact PV = compact perivascular lesions as described by Lin et al. (2009). DN = various types of TDP-43 immunoreactive dystrophic neurites; NCI = neuronal cytoplasmic inclusion; NII = neuronal intranuclear inclusion; WM GCI = white matter glial cytoplasmic inclusion.
To test the hypothesis that either the presence of FTLD-TDP type C–E pathology or a moderate to severe burden of cortical pathology would distinguish FTLD-TDP versus LATE-NC, we assembled an additional case series from the UK-ADC biobank (Table 1). The a priori goal was to include all cases from this biobank that could be diagnostic dilemmas between severe LATE-NC and FTLD-TDP. We also wanted to assess cases where the TDP-43 proteinopathy occurred without substantial ADNC. To accomplish these goals, the convenience sample included LATE-NC cases that had substantial TDP-43 proteinopathy, with documented clinical dementia, but without intermediate or severe levels of ADNC. The FTLD-TDP cohort included all of the FTLD-TDP cases from the entire UK-ADC biobank (n = 8). All of these cases were referred to the UK-ADC from a University of Kentucky-affiliated dementia clinic, most presented clinically with bvFTD, and the average age at death was 62.9 years (±9.8 years standard deviation, SD). By contrast, the LATE-NC group was derived from a community-based cohort, most were diagnosed clinically as probable Alzheimer’s disease, and they were older at death (90.9 years ± 8.5 years SD). As with the UPENN sample, there was minimal overlap in the FTLD-TDP and LATE-NC cohorts’ age ranges (Supplementary Fig 1).
For the four cases in the UPENN dataset that were mis-called by the University of Kentucky (UK-1) rater, three were FTLD-TDP cases called LATE-NC, and one case was the reverse. Notably, each of the FTLD-TDP cases mis-diagnosed as LATE-NC had a genetic mutation. Two were positive for C9orf72 expansion, one had GRN mutation. This shows that genetic analysis would help to reduce the number of misdiagnoses; further, C9orf72 cases can be accurately diagnosed by adding p62 immunohistochemical stains (Mackenzie et al., 2014). All four of the cases mis-diagnosed were scored as type Alpha according to the consensus of the five readers (Supplementary Table 7), which may indicate that integrating Alpha/Beta assessment in the future could help with challenging cases. We did not find evidence that the hippocampal formation phosphorylated TDP-43 immunohistochemical staining results provided additional helpful information with the caveat that the number of cases with misdiagnoses was only four (Supplementary Table 7).
After the brain tissues from the UK-ADC cohort were cut and stained, two independent UPENN researchers, blinded to each case’s demographics or any other information, assigned each case a pathological diagnosis of LATE-NC or FTLD-TDP using the simple ad hoc criteria (Table 4). For this analysis, only anterior cingulate, temporal and mid-frontal cortex slides were analysed. After assigning all FTLD-TDP type C, D and E cases a FTLD-TDP diagnosis, the remaining cases with moderate neuronal cytoplasmic inclusions and ropy dystrophic neurites scores were diagnosed as FTLD-TDP. These diagnostic criteria made by UPENN readers on cases from the UK-ADC cohort were quite accurate. Specificity for LATE-NC and FTLD-TDP was 88–93% and 79–88%, respectively. Sensitivity for diagnosing LATE-NC and FTLD-TDP was similarly high at 88–93% and 88%, respectively.
Table 4.
UK-ADC cases: diagnostic accuracy of UPENN neuropathologists
| Observer | UPENN blinded diagnosis | UK-ADC LATE-NC diagnosis | UK-ADC FTLD-TDP diagnosis | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|
| UP-1 | LATE-NC (n = 14) | 13 | 1 | 88 | 93 |
| FTLD-TDP (n = 8) | 1 | 7 | 93 | 88 | |
| UP-2 | LATE-NC (n = 12) | 11 | 1 | 88 | 79 |
| FTLD-TDP (n = 10) | 3 | 7 | 79 | 88 |
None of the subjects in this table had intermediate or high Alzheimer’s disease neuropathological changes, all had dementia.
Based on the estimated sensitivity for correctly diagnosing LATE-NC provided in Tables 2 and 4, we considered how including all cases at our respective brain banks that were LATE-NC may affect the results. Because moderate-to-high TDP-43 proteinopathy in the middle temporal gyri is required for diagnosis of FTLD-TDP, and given Stage 1 and 2 LATE-NC have no TDP-43 in the middle temporal gyri, we assumed that no Stage 1 or Stage 2 cases would be misclassified as FLTD-TDP. Assuming that the sensitivity on the included cases would be comparable to the excluded cases, the overall sensitivity for identifying LATE-NC versus FLTD-TDP was 99% for both UK-ADC and UPENN. Thus, given the rather larger number of LATE-NC cases that were Stage 1 or 2 relative to Stage 3, our sensitivity to discriminate LATE-NC from FTLD-TDP would increase.
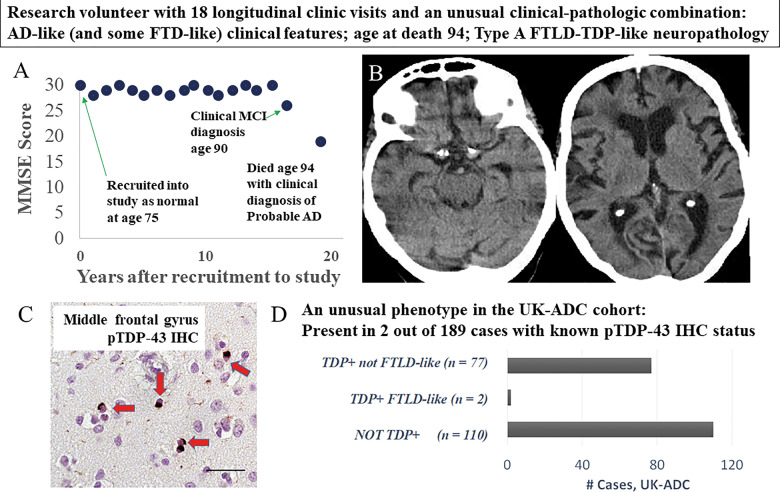
Among 189 UK-ADC cases stained for TDP-43 proteinopathy in the frontal cortex, 79 brains had pathological TDP-43 immunoreactivity and 46 brains with TDP-43 proteinopathy lacked severe ADNC. Of these 46 TDP[+]ADNC[−] individuals, one case showed substantial TDP-43 proteinopathy that was diagnosed as FTLD-TDP by both UPENN readers. Detailed information on this subject is shown in Fig. 4. Note that there were features of the clinical history that were suggestive of ‘overlap’ with clinical FTD—language disturbance (although not frank progressive aphasia), disinhibition, and appetite problems. Still, the overall clinical picture prior to death was considered to represent probable Alzheimer’s disease dementia. Other findings including radiographical and genetics results related to this individual are described in Fig. 4 and its legend. This small minority of cases (1% of all cases and 2.5% of cases with LATE-NC in the UK-ADC cohort) demonstrated overlap between the features of LATE-NC and FTLD-TDP.
Figure 4.
Detailed information on a presumed LATE-NC research subject with an unusual clinical-pathological combination of clinical features. Clinical-pathological features included Alzheimer’s disease- and FTD-associated signs and symptoms, and neuropathology that was similar to FTLD-TDP type A. Changes in global cognitive status over the course of 20 years on study, operationalized by longitudinal MMSE scores at 18 successive clinic visits, are presented in A. Mild cognitive impairment (MCI) was diagnosed at age 90 and a final probable Alzheimer’s disease (AD) diagnosis was made at age 94, within a year of death. Although the overall clinical picture was compatible with Alzheimer’s disease, FTD-like symptoms were noted (including disinhibition and disorders in appetite and language), particularly in the final 5 years of life (Supplementary Table 11). A CT scan at age 90 when MCI was diagnosed (B) revealed frontal and temporal atrophy. In terms of genetic findings, this subject’s APOE allele status was ε2/ε3, homozygous for the TMEM106B risk allele (rs1990622 status TT), homozygous for the ABCC9 risk allele (rs704180 status AA), and homozygous for the non-risk GRN allele (rs5848 status CC). At autopsy, neuropathology (C) revealed a moderate number of neuronal cytoplasmic inclusions (red arrows) and dystrophic neurites across multiple cortical areas including the middle frontal gyrus. Scale bar = 50 µm. There was also hippocampal sclerosis pathology, but virtually no brain amyloid-β (not shown). Notably, whereas a quarter of cases from this community-based cohort had LATE-NC but lacked severe ADNC, only 2/79 of the UK-ADC cases with TDP-43 pathology had moderately severe frontal cortex TDP-43 proteinopathy (D).
Discussion
TDP-43 proteinopathy was assessed blindly by multiple neuropathologists, in order to compare pathological features in severe LATE-NC with FTLD-TDP. Neuropathologists from two institutions assessed over 300 slides each, including cases with FTLD-TDP, severe LATE-NC with co-morbid ADNC, and also severe LATE-NC lacking ADNC. In the UPENN CNDR cohort (a dementia clinic-based sample), we compared findings in FTLD-TDP to cases with severe LATE-NC and co-morbid ADNC. From the UK-ADC cohort, we compared FTLD-TDP cases to severe LATE-NC cases lacking substantial co-morbid ADNC. The UK-ADC LATE-NC cases were recruited from a community-based autopsy cohort with most cases followed from a baseline of normal cognitive status. We found that the neuropathological features of LATE-NC and FTLD-TDP were not identical; the density of TDP-43 proteinopathy in neocortical regions was more severe in FTLD-TDP cases than in severe LATE-NC cases (with or without co-morbid ADNC). We generated criteria that would differentiate between FTLD-TDP and LATE-NC with ∼90% confidence, even when the inclusion criteria focus on advanced LATE-NC cases. Yet we also found that a small group of subjects had pathological features that did not discriminate between subjects with LATE-type clinical features and FTD/FTLD. In evaluating cases with amygdala TDP-43 proteinopathy, we found poor inter-rater reliability between neuropathologists in applying the Alpha/Beta schema.
Age-related TDP-43 proteinopathy is a fast-moving and controversial research area (Josephs et al., 2019a; Nelson et al., 2019b). If and when success is achieved in the domain of therapeutic strategies, it may become all the more important to have useful criteria for differentiating disease entities. Neuropathology is considered the gold standard of determining neurodegenerative disease presence and severity (Jack et al., 2018). Among pathologists in general, autopsies are performed taking into account at least some clinical information. However, in recent years, there has been an emphasis—in the area of neurodegenerative disease pathological studies—on creating criteria for neuropathological diagnoses that would not require integration of clinical information (Jack et al., 2018). The benefit of this approach is that it acknowledges that a disease (as defined by pathological features) can be present in a prodromic state, as one does not need clinical manifestations to identify that a disease process has begun (analogous, for example, to prostate cancer in advanced ageing). The application of specific criteria for operationalizing the identification of disease presence and severity also provides testable hypotheses for clinical-pathological correlations.
Key methodological features of the present work included a relatively open-ended study design given that multiple neuropathologists and researchers from both institutions reviewed each slide blindly and independently. All of the neuropathologists were only able to review a limited subsample of slides, without any information about clinical features of the cases. These slides had been previously diagnosed, and the question we sought to address was whether or not FTLD-TDP and severe LATE-NC cases could be differentiated from each other. If there had been extensive overlap between the findings in FTLD-TDP and LATE-NC, that would have been reported. For the sake of uniformity, the TDP-43 immunohistochemical staining were performed using the 1D3 antibody, which recognizes phospho-Ser409/Ser410 residues of TDP-43 (Neumann et al., 2009). This is the reagent that most (about two-thirds of) American Alzheimer’s disease research centres use for TDP-43 immunohistochemical evaluation (Katsumata et al., 2018).
The clinical and pathological boundaries between LATE-NC and FTLD-TDP have not been fully delineated, as was discussed in the consensus working group report on LATE-NC (Nelson et al., 2019a). However it was previously shown that LATE-NC histopathology resembles that seen in the FTLD-TDP type A (Aoki et al., 2015). Arguments for commonalities between LATE-NC and FTLD-TDP include the neuroimaging finding of frontal and temporal atrophy in cases with autopsy-proven LATE-NC, less dramatic atrophy overall than FTLD-TDP cases, but still in the same regions (Kotrotsou et al., 2015; Nelson et al., 2019a). There are also areas of overlap in terms of genetic risk factors, although neurodegenerative disease genetic risk factors tend to be pleiotropic and the genetic risk factors for LATE-NC and FTLD-TDP are not identical (Chornenkyy et al., 2019).
In the present study, a large majority of LATE-NC cases could be reliably differentiated from FTLD-TDP cases based on evaluating several slides of brain sections immunostained for phosphorylated TDP-43. We generated and applied simple diagnostic criteria: LATE-NC had lower densities of TDP-43 immunoreactive neuronal cytoplasmic inclusions and ropy dystrophic neurites in anterior cingulate and mid-frontal cortical regions. Whereas in our test cases the rate of correct diagnoses using this criteria was ∼90%, that number could have been far higher if we had selected LATE-NC cases blindly, since overall only ∼20% of cases with LATE-NC in community-based cohorts have neocortical TDP-43 pathology (Keage et al., 2014; Nag et al., 2015). This is an important point: in the UK-ADC and UPENN cohorts, well over 95% of LATE-NC cases would probably be differentiated from FTLD-TDP using our criteria. The biggest discrepancies between FTLD-TDP and LATE-NC are in epidemiology and clinical features. LATE-NC is far more common and (as underscored in the present study) affects older individuals, with differing clinical outcomes (Nelson et al., 2019a).
Although neocortical involvement with TDP-43 pathology in FTLD-TDP was significantly higher than in LATE-NC, several individuals (one exceptional case is presented in Fig. 4 in detail) had late-onset clinical features of Alzheimer’s disease dementia, but had pathology that appeared similar to FTLD-TDP. In these cases, there was a substantial amount of TDP-43 neuronal cytoplasmic inclusions in neocortex. We note that there exist similar cases at the border zones for other neuropathological entities so that individual neuropathologists might disagree as to the correct diagnosis. For example, in chronic traumatic encephalopathy, other tauopathies, and, in other disease categories, unusual cases can constitute diagnostic challenges despite the presence of improved diagnostic tools and criteria (Schneider et al., 1997; Wakabayashi and Takahashi, 2004; Boeve et al., 2013; Kovacs et al., 2018; Forrest et al., 2019; Iverson et al., 2019; Malek-Ahmadi et al., 2019). It still is theoretically possible that FTLD-TDP and LATE-NC truly represent a unitary continuum, analogous to younger/rare- and older/common-onset versions of ADNC. However, it is notable that unlike Alzheimer’s disease, the condition that affects older individuals (LATE-NC) usually has a different clinical phenotype and a more distinct distribution of pathology.
Synergistic mechanisms appear to affect both ADNC and LATE-NC, because the two pathologies frequently co-occur in aged brains (Josephs et al., 2008, 2019a; Nag et al., 2017; Smith et al., 2017). However, this tendency needs to be critically assessed. Some ADNC is detectable in ∼80% of all elderly individuals’ brains (with or without LATE-NC or clinical dementia) (Braak et al., 2011), so the common finding of ADNC with LATE-NC needs to be judged in that context: even if the pathological features were independent of each other, 80% of individuals with LATE-NC would have co-morbid ADNC. Further, many aged subjects with severe ADNC lack LATE-NC, which indicates that ADNC does not lead inevitably to LATE-NC. Conversely, as shown in the current study and elsewhere (Nelson et al., 2011; Nag et al., 2015), LATE-NC often occurs in the absence of co-morbid ADNC. It has been suggested that TDP-43 proteinopathy in the context of ADNC is merely an ‘added’ pathology, analogous to Lewy body pathology in the amygdala (Josephs et al., 2019a). However, the presence of LATE-NC in cases with co-morbid ADNC is still highly worthy of diagnostic note since ADNC with LATE-NC has a more severe clinical phenotype than ADNC without LATE-NC (Nelson et al., 2010; Robinson et al., 2013). Recent imaging studies also imply that brains with ADNC and co-morbid LATE-NC have more atrophic hippocampi than those with ADNC alone (Dawe et al., 2011; Josephs et al., 2017; Sahoo et al., 2018; Bejanin et al., 2019; Nelson et al., 2019a). Here, importantly, we found similar accuracy in differentiating FTLD-TDP from LATE-NC, with or without co-morbid ADNC.
We also tried to replicate the finding of a diagnostically helpful stratification of cases by the Alpha and Beta pattern of TDP-43 proteinopathy (Josephs et al., 2019b). We attempted to apply the diagnostic criteria that were published, after discussing them together. Five different researchers judged the same panel of cases. The diagnostic inter-rater reliability was low for distinguishing Alpha and Beta types (Supplementary Table 3). Nor were these diagnostic cues very helpful in differentiating between LATE-NC and FTLD-TDP in the UPENN sample. While Beta type morphology was described by consensus in 57% of LATE-NC versus 12% of FTLD-TDP, the diagnoses lacked practical utility in the present study. Criteria for Alpha and Beta subtypes of LATE-NC may in the future be sharpened to enable better inter-rater reliability which is a prerequisite for any diagnostic criterion.
This study had limitations and potential confounders. For example, the presence of co-morbid pathologies in human brains differs according to age, and also tends to change according to the criteria applied in recruiting research volunteers. Further, the lifetime risk for FTLD-TDP is ∼1:1000 (Knopman and Roberts, 2011; Coyle-Gilchrist et al., 2016; Nelson et al., 2019a), but FTLD-TDP may be highly enriched in autopsy cohorts that draw from dementia clinics or hospitals. By contrast, LATE-NC, including cases lacking co-morbid ADNC, are more common in community-based cohorts (Keage et al., 2014; Nag et al., 2017, 2018). Here we included subjects derived from two institutions, representing one clinic-based cohort the second cohort that was predominantly a community-based sample. However, neither of these cohorts is ethnically diverse and more work is required in more population-representative samples.
In addition, there were case selection biases in these convenience samples. The study design was biased to compare FTLD-TDP and severe LATE-NC since the great majority of cases with presumed incipient or moderately severe LATE-NC were excluded. By contrast, cases more likely to have overlapping neuropathological features, with neocortical TDP-43 pathology and with frank dementia, were preferentially included. It remains an open question how the neuropathologies in the early and mild cognitive impairment (MCI) stage of FTLD-TDP could be compared with LATE-NC. Unfortunately, we did not have enough cases to assess this. We were able to provide preliminary criteria to differentiate between FTLD-TDP and LATE-NC, even in LATE-NC brains enriched for relatively severe neocortical TDP-43 pathology, all without foreknowledge of clinical symptoms, age of onset or genetic information. Future criteria could be more specific by integrating genetic data, and also perhaps other information such as non-phosphorylated TDP-43 proteinopathy and pathological lesions in other brain regions (Hunter et al., 2020).
The tendency is remarkable for TDP-43 proteinopathy in LATE-NC to not progress—even in individuals with advanced old age that lack co-morbid ADNC—to severe levels of frontal and cingulate cortical involvement. It is tempting to speculate that there is a parallel with PART, in which the anatomical progression of tau tangles, in the absence of amyloid-β plaques, does not progress beyond Braak neurofibrillary tangle stage IV (Nelson et al., 2009; Crary et al., 2014). The observed phenomena in LATE-NC and FTLD-TDP may indicate that an additive pathogenetic mechanism or factor is present in FTLD-TDP that is lacking in LATE-NC. More generally, the observations in LATE-NC underscore that individual neurodegenerative diseases tend to have distinct clinical and pathological progression trajectories over time and they do not necessarily have the same disease-defining clinical or pathological end point.
Supplementary Material
Acknowledgements
We are very grateful to the research volunteers, their families, and clinicians, as well as the other researchers that made this work possible.
Funding
This study was also supported by NIH grants P30 AG028383, P30 AG010124, P01 AG017586, R01 AG057187, R01 AG042475.
Competing interests
The authors report no competing interests.
Glossary
- ADNC =
Alzheimer’s disease neuropathological changes;
- FTD =
frontotemporal dementia;
- FTLD =
frontotemporal lobar degeneration;
- LATE-NC =
limbic-predominant, age-related TDP-43 encephalopathy with underlying neuropathological changes;
- TDP-43 =
TAR-DNA binding protein-43;
- UK-ADC =
University of Kentucky Alzheimer’s Disease Center
References
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol 2007; 61: 435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol 2015; 129: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejanin A, Murray ME, Martin P, Botha H, Tosakulwong N, Schwarz CG, et al. Antemortem volume loss mirrors TDP-43 staging in older adults with non-frontotemporal lobar degeneration. Brain 2019; 142: 3621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 2013; 14: 754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70: 960–9. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007; 114: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chornenkyy Y, Fardo DW, Nelson PT. Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy. Lab Invest 2019; 99: 993–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle-Gilchrist IT, Dick KM, Patterson K, Vazquez Rodriquez P, Wehmann E, Wilcox A, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 2016; 86: 1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014; 128: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One 2011; 6: e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Rademakers R, Nicholson AM, Schneider JA, Yu L, Bennett DA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology 2015; 85: 1354–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest SL, Kril JJ, Wagner S, Honigschnabl S, Reiner A, Fischer P, et al. Chronic Traumatic Encephalopathy (CTE) is absent from a European community-based aging cohort while cortical Aging-Related Tau Astrogliopathy (ARTAG) is highly prevalent. J Neuropathol Exp Neurol 2019; 78: 398–405. [DOI] [PubMed] [Google Scholar]
- Gal J, Chen J, Katsumata Y, Fardo DW, Wang WX, Artiushin S, et al. Detergent insoluble proteins and inclusion body-like structures immunoreactive for PRKDC/DNA-PK/DNA-PKcs, FTL, NNT, and AIFM1 in the amygdala of cognitively impaired elderly persons. J Neuropathol Exp Neurol 2018; 77: 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S, Hokkanen SRK, Keage HAD, Fleming J, Minett T, Polvikoski T, et al. TDP-43 related neuropathologies and phosphorylation state: associations with age and clinical dementia in the Cambridge City over-75s Cohort. J Alzheimers Dis 2020; 75: 337–50. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997; 56: 1095–7. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Gardner AJ, Shultz SR, Solomon GS, McCrory P, Zafonte R, et al. Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain 2019; 142: 3672–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW, Tosakulwong N, Weigand SD, Murray ME, Petrucelli L, et al. Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer's disease: a longitudinal retrospective study. Lancet Neurol 2017; 16: 917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Mackenzie I, Frosch MP, Bigio EH, Neumann M, Arai T, et al. LATE to the PART-y. Brain 2019. a; 142: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Murray ME, Tosakulwong N, Weigand SD, Serie AM, Perkerson RB, et al. Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol 2019. b; 137: 227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, et al. Updated TDP-43 in Alzheimer's disease staging scheme. Acta Neuropathol 2016; 131: 571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 2008; 70 (19 pt 2): 1850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Dickson DW, Murray ME, Whitwell JL, Knopman DS, Boeve BF, et al. TDP-43 in Alzheimer's disease is not associated with clinical FTLD or Parkinsonism. J Neurol 2014; 261: 1344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata Y, Fardo DW, Kukull WA, Nelson PT. Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer's disease and cerebrovascular disease pathologies. Acta Neuropathol Commun 2018; 6: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keage HA, Hunter S, Matthews FE, Ince PG, Hodges J, Hokkanen SR, et al. TDP-43 pathology in the population: prevalence and associations with dementia and age. J Alzheimers Dis 2014; 42: 641–50. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci 2011; 45: 330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrotsou A, Schneider JA, Bennett DA, Leurgans SE, Dawe RJ, Boyle PA, et al. Neuropathologic correlates of regional brain volumes in a community cohort of older adults. Neurobiol Aging 2015; 36: 2798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Kwong LK, Grossman M, Irwin DJ, Lee EB, Robinson JL, et al. Tauopathy with hippocampal 4-repeat tau immunoreactive spherical inclusions: a report of three cases. Brain Pathol 2018; 28: 274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Porta S, Michael Baer G, Xu Y, Suh E, Kwong LK, et al. Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol 2017; 134: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WL, Castanedes-Casey M, Dickson DW. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 2009; 68: 1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Frick P, Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol 2014; 127: 347–57. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M. Reappraisal of TDP-43 pathology in FTLD-U subtypes. Acta Neuropathol 2017; 134: 79–96. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011; 122: 111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R. The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments. Neurogenetics 2007; 8: 237–48. [DOI] [PubMed] [Google Scholar]
- Malek-Ahmadi M, Beach TG, Zamrini E, Adler CH, Sabbagh MN, Shill HA, et al. Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease. PLoS One 2019; 14: e0217566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol 2014; 128: 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer's disease. Acta Neuropathol Commun 2018; 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 2015; 77: 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 2017; 88: 653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol 2009; 68: 774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 2010; 20: 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 2019. a; 142: 1503–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Reply: LATE to the PART-y. Brain 2019. b; 142: e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Gal Z, Wang WX, Niedowicz DM, Artiushin SC, Wycoff S, et al. TDP-43 proteinopathy in aging: associations with risk-associated gene variants and with brain parenchymal thyroid hormone levels. Neurobiol Dis 2019. c; 125: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 2011; 134(Pt 5): 1506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, Neltner JH, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 2013; 126: 161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, et al. “New Old Pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol 2016; 75: 482–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neltner JH, Abner EL, Baker S, Schmitt FA, Kryscio RJ, Jicha GA, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 2014; 137 (Pt 1): 255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 2009; 117: 137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–3. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 2007; 21: S14–8. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX, et al. Non-Alzheimer's contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathol 2018. a; 136: 377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Geser F, Stieber A, Umoh M, Kwong LK, Van Deerlin VM, et al. TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta Neuropathol 2013; 125: 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018. b; 141: 2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo A, Bejanin A, Murray ME, Tosakulwong N, Weigand SD, Serie AM, et al. TDP-43 and Alzheimer's disease pathologic subtype in non-amnestic Alzheimer's disease dementia. J Alzheimers Dis 2018; 64: 1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS. Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 1997; 48: 959–69. [DOI] [PubMed] [Google Scholar]
- Smith VD, Bachstetter AD, Ighodaro E, Roberts K, Abner EL, Fardo DW, et al. Overlapping but distinct TDP-43 and tau pathologic patterns in aged hippocampi. Brain Pathol 2017; 28: 264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008; 319: 1668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Abrahams S, Goldstein LH, Woolley S, McLaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18: 153–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, et al. A platform for discovery: the University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement 2014; 10: 477–84 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol 2008; 7: 409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology 2004; 24: 79–86. [DOI] [PubMed] [Google Scholar]
- Wennberg AM, Tosakulwong N, Lesnick TG, Murray ME, Whitwell JL, Liesinger AM, et al. Association of Apolipoprotein E epsilon4 with transactive response DNA-binding protein 43. JAMA Neurol 2018; 75: 1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Yu L, White CC, Chibnik LB, Chhatwal JP, Sperling RA, et al. Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE epsilon4 haplotype status: a community-based cohort study. Lancet Neurol 2018; 17: 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article, especially in the supplementary tables.
Raw data were generated at the University of Kentucky and the University of Pennsylvania. Although the large majority of data are presented in the paper as stated above, any derived data supporting the findings of this study are available from the corresponding author (P.T.N.) on request.