Abstract
Introduction
Hemorrhagic shock is a primary injury amongst combat casualties. Hemorrhagic shock can lead to acute lung injury, which has a high mortality rate. Based on studies showing the role of intense light for organ-protection, we sought to evaluate if intense light pretreatment would be protective in a murine model of hemorrhagic shock lung.
Materials and Methods
After exposure to standard room light or to intense light (10 000 LUX), mice were hemorrhaged for 90 minutes to maintain a mean arterial pressure (MAP) of 30–35 mmHg. Mice were then resuscitated with their blood and a NaCl infusion at a rate of 0.2 ml/h over a 3-hour period. During resuscitation, blood pressure was recorded. At the end of resuscitation, bronchoalveolar lavage was analyzed for alveolar epithelial barrier function and inflammation. To get insight into the relevance of intense light for humans, we performed a proteomics screen for lung injury biomarkers in plasma from healthy volunteers following intense light therapy.
Results
We found that intense light pretreated mice had improved hemodynamics and significantly lower albumin, IL-6, and IL-8 levels in their bronchoalveolar lavage than controls. We further discovered that intense light therapy in humans significantly downregulated proinflammatory plasma proteins that are known to cause acute lung injury.
Conclusions
Our data demonstrate that mice exposed to intense light before hemorrhagic shock lung have less lung inflammation and improved alveolar epithelial barrier function. We further show that intense light therapy downregulates lung injury promoting proteins in human plasma. Together, these data suggest intense light as a possible strategy to ameliorate the consequences of a hemorrhagic shock on lung injury.
INTRODUCTION
Presently, there is no Food and Drug Administration (FDA)-approved treatment for acute lung injury (ALI), and despite the relatively large number of patients with the diagnosis, ALI care remains supportive.1 ALI is associated with many conditions, including severe trauma, hemorrhagic shock, and burn.2 ALI affects ~200 000 individuals annually, resulting in 74 500 deaths in the United States.3 In the context of Combat Casualty Care, patients with ALI require additional medical resources and have a 2-fold increased risk of mortality.4 Long-term disabilities in ALI survivors include exercise intolerance, physical and psychological sequelae, decreased quality of life, as well as increased costs and use of health care services leading to reduced rates of return-to-duty in service members.5 Hemorrhage, a common cause of ALI, is the leading cause of trauma-related death in the military setting.6,7 Morbidity and mortality associated with hemorrhage-induced shock is a composite result of a systemic inflammatory response, with subsequently triggered ALI occurring in 9% of combat and 16% of civilian casualties after severe hemorrhage.4,8–12 Overall, ALI mortality in the military or civilian setting ranges from 12–50%.4,10
Following major trauma, a local release of mediators such as cytokines, acid metabolites, and histamine increases the capillary permeability resulting in tissue edema and local infiltration of immunocompetent cells. Intrinsic leukocytes and affected endothelial cells produce and release pro- and anti-inflammatory cytokines acting locally as well as on remote cells in different organs like the lung. Indeed, genome-wide expression pattern studies show that severe trauma alters the expression of >80% of the leukocyte transcriptome during the first 28 days after injury.13
We recently discovered that intense light protects from myocardial ischemia and reperfusion injury (10 000 LUX, broad-spectrum, UV-light filter).14–18 Intense light is the hallmark of circadian rhythm regulation,19,20 and circadian rhythms control fundamental functions of individual cells and organ systems.18,21 In humans, the most potent regulator of circadian rhythms, is intense light (>10 000 LUX intensity [sun: 2000 LUX–120 000 LUX; established bright light therapy to treat depression in humans: 10 000 LUX22]).18
However, intense light as a lung-protective strategy has not been explored yet. Since a hemorrhagic shock causes systemic ischemia and reperfusion injury, we hypothesized that intense light pretreatment would be lung-protective in a murine model of hemorrhagic shock lung.
MATERIAL AND METHODS
Mouse experiments
Experimental protocols (#00231) were approved by the Institutional Review Board (Institutional Animal Care and Use Committee [IACUC]) at the University of Colorado Denver, USA. They were following the AAALAC regulations, the US Department of Agriculture Animal Welfare Act, and the Guide for the Care and Use of Laboratory Animals of the NIH. Mice were housed in a 14/10-hour light-dark cycle, and all mouse experiments were conducted at the same time point (ZT3, Zeitgeber Time 3 corresponding to 9 a.m. based on “light ON” at 6 a.m.). To eliminate gender- and age-related variations, we routinely used 12- to 16-week-old, 24 g male mice. Mice were bred in the vivarium at Denver for optimal acclimatization and housed in cages of 5 at 21°C with food (Harlan diets, formulation 2920x, soy-free) and water ad libitum.
Intense light exposure in mice
Mice were exposed to intense light (10 000 LUX, Lightbox, Uplift Technologies DL930, full-spectrum, UV filter) for 5 days and compared to mice maintained at room light (200 LUX) for 5 days.16
Blood pressure and heart rate measurements
A polyethylene catheter was inserted in the left carotid artery, as described previously23. The catheter was connected to a Deltran® pressure transducer (Utah Medical Products Inc., Salt Lake City, UT, USA) located at the same hydrostatic level as the mouse, which was connected to the CyQ BMP02 system (CyberSense, Inc., Nicholasville, KY, USA) designed to measure invasively systolic, diastolic, pulse pressure, mean arterial blood pressure (MAP) and heart rate. Due to a sampling rate of 1000 Hz, the device automatically calculates HR from the amplitude of the pressure signal.
Mouse model of hemorrhagic shock and resuscitation
Based on findings that housing mice under “intense light conditions” (10 000 LUX, full-spectrum, UV-filter, L(light):D(dark) phase 14:10 hours) robustly reduced infarct sizes in a murine model of myocardial ischemia and reperfusion injury,24 we housed wildtype mice for 5 days under intense light conditions before a hemorrhagic shock (Fig. 1A). Control mice were housed under standard housing conditions (200 LUX, L:D 14:10 hours). Before hemorrhagic shock, mice were anesthetized, intubated, and mechanically ventilated (FiO2 0.4). Mice were anesthetized with pentobarbital at a dose of 70 mg/kg, i.p. for induction and 20 mg/kg for maintenance. Once pain reflexes were absent, mice were placed on a temperature-controlled surgical table. The trachea was surgically exposed, and tracheal intubation was performed. A blunt polyethylene cannula (Insyte 22 g, Beckton Dickinson, USA) was inserted into the trachea. The tracheal tube was connected to a mechanical ventilator (Servo 900C, Siemens, Germany with pediatric tubing) and the animals were ventilated using a pressure-controlled ventilation mode (peak inspiratory pressure of 12 mbar, frequency 120 breaths/min, positive end-expiratory pressure of 3 mbar, FiO2 = 0.4). All mice in the hemorrhage group underwent 10 minutes of equilibration after cannulation of the carotid artery followed by 1.5 hours of hemorrhagic shock and 3 hours of resuscitation (Fig. 1B and C). Mice were hemorrhaged for 1.5 hours to maintain a MAP of 30–35 mmHg. Mice were then resuscitated with transfusion of the shed blood and a NaCl infusion at a rate of 0.2 ml/h over 3 hours. Mice in the sham group were cannulated and connected to the pressure monitoring apparatus but did not undergo intense light therapy or hemorrhage/resuscitation (Fig 2A). Before carotid artery catheterization, the mice received 50 IU heparin i.p. The carotid artery was catheterized for continuous recording of blood pressure. The catheter was also used for saline infusion, hemorrhage, and resuscitation. Hemorrhagic shock was initiated by blood withdrawal and a reduction of the mean arterial blood pressure (MAP) to 30–35 mmHg over 15 minutes. The blood was harvested into a 1-ml syringe with heparin to prevent coagulation.
FIGURE 1.
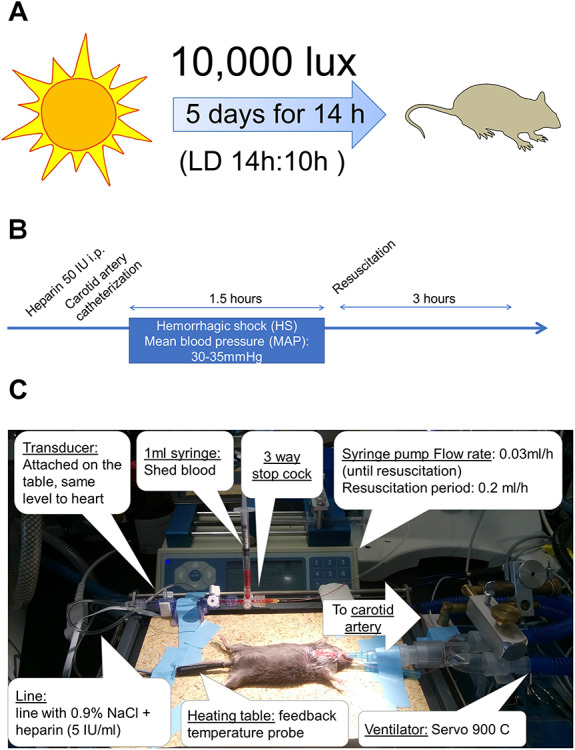
Intense light exposure and experimental setup of a murine hemorrhagic shock lung model. (A) Mice housed under a light (L) dark (D) cycle of 14:10 hours were exposed to intense light instead of room light during the light phase. (B, C) Setup of a murine hemorrhagic shock lung model.
FIGURE 2.
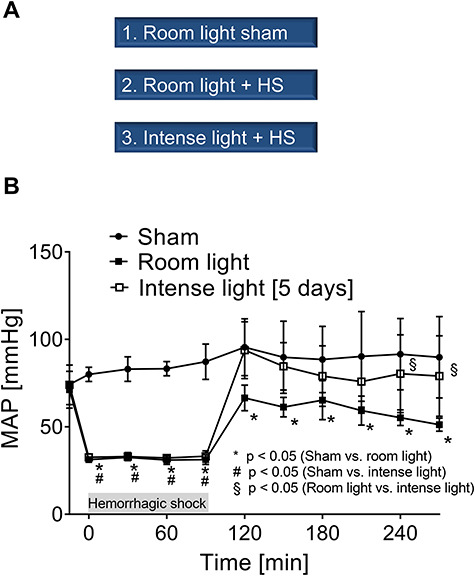
Experimental groups and MAP measurements. (A) Experimental groups. (B) MAP of mice undergoing hemorrhagic shock and resuscitation with and without intense light therapy versus sham mice (room light only). Data are mean ± SD with n = 4–5 mice for each group.
BAL lavage and albumin/protein analysis
To obtain BAL fluid, the tracheal tube was disconnected from the mechanical ventilator and the lungs were lavaged 3 times with 0.5 ml of PBS. All removed fluid was centrifuged immediately, and the supernatant was aliquoted for measurement of the albumin concentration via ELISA (mouse albumin ELISA kit, Bethyl Laboratories). Protein levels in BAL were measured using a BCA protein assay (Thermo Fisher Scientific).
BAL IL-6 and IL-8
IL-6 (R&D Systems) and KC (R&D Systems) levels were evaluated in BAL using a mouse ELISA kit according to the user’s manual.
Human light exposure
Healthy human volunteers were exposed to intense light (10 000 LUX) for 30 minutes every morning for 5 days from 8:30 a.m.–9:00 a.m.. Five milliliter blood was drawn on day 1 at 8:30 a.m. and 9:00 a.m. (before and after light exposure). Although light exposure was repeated every morning for the 5 days, the next blood draws were on days 3 and 5 at 9:00 a.m. as indicated. Blood was collected in EDTA-plasma tubes and spun at 3000 rpm for 8 minutes to separate plasma. We obtained approval from the Institutional Review Board (COMIRB #13–1607) for our human studies before written informed consent from everyone was obtained. A total of 4 healthy volunteers were enrolled (2 females and 2 males).
Proteomics Screen
We analyzed plasma samples on days 1, 3, and 5 from healthy human volunteers exposed to 30 minutes of intense light in the morning on 5 consecutive days using the Slow Off-rate Aptamer (SOMAmer)-based capture array called SOMAscan25,26 (SomaLogic, Inc., CO, USA). The SOMAscan uses a protein signal present in the human plasma and transforms it into a nucleotide signal that can be quantified using fluorescence on microarrays. The SOMAscan assay is one of the most comprehensive protein discovery tools available and measures 1319 plasma proteins.
Data analysis
An a priori sample size analysis for blood pressure, IL-6, IL-8, protein, and albumin BAL levels revealed a biologically relevant difference of at least 20 mmHg, 400 pg/ml, 100 pg/ml, 0.5 mg/ml, and 100 μg/ml between control and experimental groups, respectively. Thus, a minimum of four animals per group was necessary to obtain statistically significant results with an alpha error of 0.05 and beta error of 0.1. Based on this analysis we minimized the number (n = 4–5 per group, sham, and treatment) of animals used and their suffering. All data were tested for normality using the Shapiro Wilk test, which confirmed normality. For multiple comparisons, one-way analysis of variance with Tukey’s post hoc test was performed, and for a single comparison, the unpaired or paired Student t-test was applied. Values are expressed as mean (±SD). P < .05 was considered statistically significant. For all statistical analysis, GraphPad Prism 7.0 and BiAS 8.6.3 for windows was used.
RESULTS
Mice in the hemorrhage group had significantly different MAPs compared to sham mice during the 1.5 hours of hemorrhagic shock (Fig. 2B). During 3 hours of resuscitation, mice that were housed under intense light conditions had significantly higher MAPs than the room light housed mice. Moreover, MAPs of room light housed mice had significantly lower MAPs during resuscitation than sham-operated mice. However, MAPs from intense light-exposed mice were not significantly different from sham-operated mice during resuscitation (Fig. 2B).
After 3 hours of resuscitation, the mice were euthanized, the BAL was collected and analyzed for inflammation and albumin or protein leakage (Fig. 3). Analysis of IL-6 and IL-8 cytokine levels in the BAL found significantly increased levels in hemorrhaged room light housed mice when compared to sham-operated controls. Intense light housed mice, however, did not show a significant increase of BAL IL-6 or IL-8 when compared to sham-treated mice (Fig. 3A–C). Like findings on inflammation, mice that were housed under standard lightening (200 LUX) conditions showed significantly increased BAL protein or albumin levels when compared to sham-operated mice. Mice that were pretreated with intense light, however, did not show a significant protein or albumin leakage in their BALs when compared to sham-operated mice. Intense light treated mice had significantly less albumin or protein BAL leakage than room light mice exposed to hemorrhagic shock (Fig. 3 D–F).
FIGURE 3.
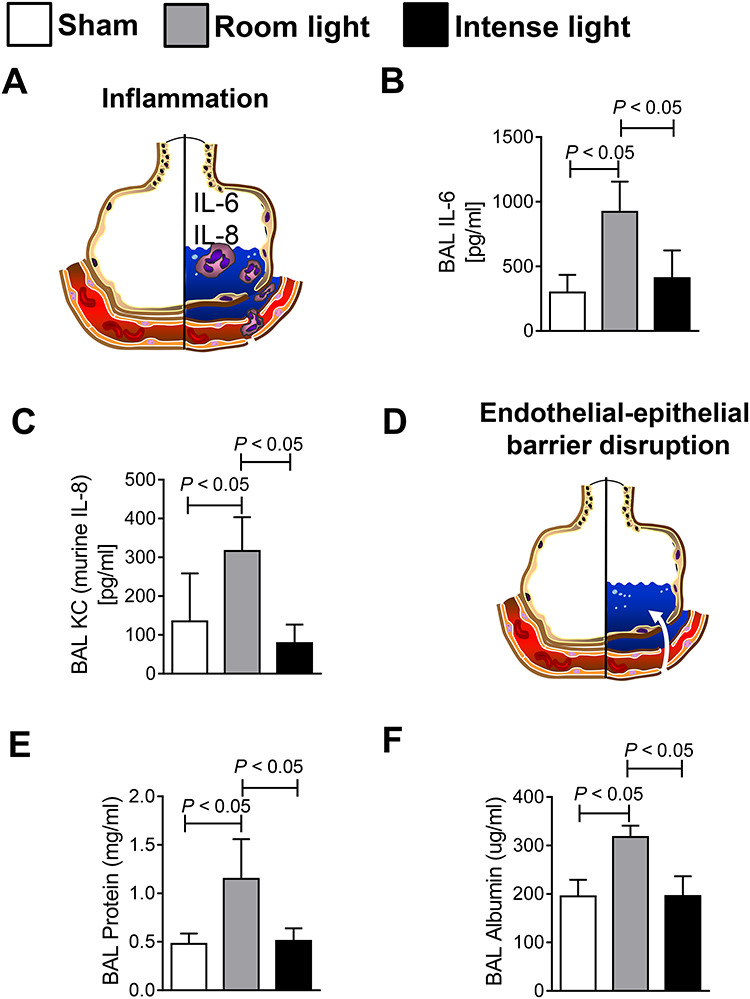
Analysis of BAL of mice undergoing hemorrhagic shock and resuscitation with and without intense light therapy versus sham mice. (A–C) BAL IL-6 and IL-8 concentrations. (D–F) BAL protein and albumin concentrations. Data are mean ± SD with n = 4–5 mice for each group.
To evaluate the impact of intense light in humans, we sought to perform a proteomics screen for lung injury biomarkers using plasma samples from healthy human volunteers that were exposed to intense light for 5 days (Fig. 4A–B). Based on strategies using intense light therapy (10 000 LUX) to treat seasonal mood disorders in humans,22 we adopted a similar protocol. We exposed 4 healthy human volunteers to 30 minute of intense light in the morning on 5 consecutive days and performed serial blood draws on days 0, 3, and 5. SOMAscan analysis revealed the significant regulation of 66 plasma proteins out of 1319 (Supplementary Table S1). Ingenuity pathway analysis found that light inhibited the chemotactic recruitment of leukocytes (Fig. 4C). Reactome pathway analysis of those proteins indicated that intense light dominantly changed pathways of the immune system (Fig. 4D, Supplementary Figure S1). Moreover, many light-downregulated plasma-proteins have been found to cause ALI (Supplementary Figure S2, Supplementary Table S1).
FIGURE 4.
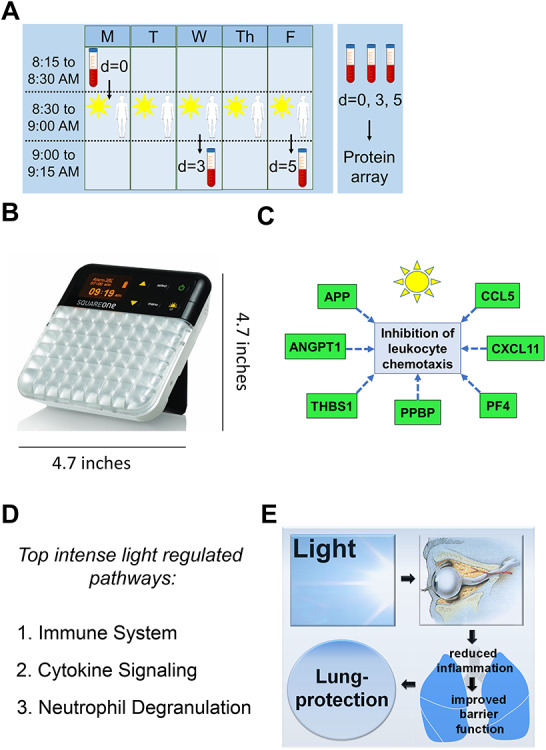
Plasma proteomics from light-exposed human subjects. (A) 4 healthy human volunteers were exposed to 30 minutes of intense light (10 000 Lux) at 8:30 a.m. on 5 consecutive days. A blood draw was performed before light exposure on the first day (8:30 a.m.) and 3 or 5 days after light exposure (9.00 a.m.). Plasma samples were analyzed using the SOMAscan platform (see Supplementary Figure S2, Supplementary Table S1 for significantly regulated proteins). (B) Image of the lightbox used with dimensions. (C) Ingenuity pathway analysis of intense light-regulated proteins. (D) Reactome pathway analysis of intense light-regulated proteins (see Supplementary Figure S1). (E) Proposed model of intense light therapy.
DISCUSSION
In the current murine studies, we found that intense light pretreatment protects from systemic ischemia and reperfusion injury, as seen with a hemorrhagic shock model. We demonstrated improved hemodynamics, reduced lung inflammation, and improved alveolar-epithelial barrier function in mice pretreated with intense light before a hemorrhagic shock-induced lung injury. The role of intense light for lung protection has never been evaluated yet. Interestingly, our human proteomic study indicates that intense light significantly inhibits pro-inflammatory plasma-proteins. As systemic inflammation is critical in causing alveolar-epithelial barrier disruption in the lungs, ultimately leading to ALI, intense light might be a possible strategy to ameliorate the severity of a hemorrhagic shock in combat casualties (Fig. 4D).
Our recent studies established a critical role for intense light in regulating critical biological processes.15,16,24,27–29 In vivo murine studies using 14 hour-intense light exposure ~1 week revealed robust and time-dependent protection from myocardial ischemia and reperfusion injury.24 Mechanistic studies on intense light elicited cardioprotection uncovered strengthening of the circadian amplitude as an underlying mechanism.24 Enhancement of the circadian amplitude has been implicated as a protective mechanism in different settings30,31 and is currently under intense investigation.32–34 However, only a few studies have shown that strengthening of the circadian amplitude could be organ-protective.35,36 As we used the same light protocol in our current studies on hemorrhagic shock lung as done in our previous studies on myocardial ischemia and reperfusion injury, our findings suggest that strengthening of the circadian amplitude is also protective in global ischemia-reperfusion injury.
Trauma induced hemorrhagic shock creates a global ischemia-reperfusion injury leading to dysfunctional systemic inflammatory response and organ injury. Therapeutic approaches for severe trauma, one of the leading causes of morbidity and mortality worldwide, have not changed during the past 30 years despite a better understanding of the pathophysiology of trauma.37 Although the recent introduction of the concept of damage control resuscitation may provide new avenues for decreasing the intensity of the inflammatory response associated with severe trauma, this approach remains a therapy to treat the symptoms only and might have limitations in a situation where blood products are not readily available or treatment is delayed.37 Thus, intense light therapy might be, in particular, useful in those limited resource scenarios.
Combat casualties with ALI consume significantly more health care resources compared with other mechanically ventilated patients.4 Although advances in military medicine and transport have expedited delivery of early38 and coordinated trauma care to the critically injured combat casualty, multiorgan failure remains a significant factor in potentially preventable died-of-wounds cases.6 Moreover, these numbers will likely increase with prolonged evacuation times projected for the future overseas contingency operations (eg, A2AD). Thus, studies are warranted to refine our understanding of ALI in combat casualty care to identify strategies to prevent ALI, and ultimately to decrease ALI-associated mortality.4
Our findings are of high relevance for combat-injured in enroute care transports (especially longer distances by CCATT or TCCET teams, and others such as GHOST and SOST/SOCCET39), or in prolonged field care settings.40 Further research on intense light therapy could lead to a paradigm shift in the treatment of combat-injured. The concept is simple and readily available. No FDA approval for a novel medical device is necessary as the FDA does not regulate intense light therapy. There is no harm from an intense lightbox, as it is not a medical device and is a commercially available, also in the form of glasses. Moreover, it has been long used effectively to treat bipolar depression with no or minimal side effects.22 This intervention has a high potential to achieve several combat casualty care goals: to optimize prolonged field care for multiple combat injuries in resource-limited far-forward settings and improve the size, weight, and portability of medical equipment required to support operations and improve patient outcomes.
Despite our robust data in mice and first promising results from healthy human subjects, further research will be necessary to understand the mechanisms of intense light in lung protection fully. Many questions will need to be addressed before such a therapy could be effectively used in humans. As we used broad-spectrum white light, it is possible, if using only one wavelength, that the lung-protective effect becomes even more pronounced. Furthermore, as we exposed mice to 14 hours of intense light daily, it would be crucial to evaluate the impact of longer exposure times in humans as well. We only used 30 minutes of exposure in our healthy subjects, as this is currently the recommended strategy for the treatment of seasonal disorders but also was most feasible. Future research will also have to test if light treatment after a hemorrhagic shock could have similar beneficial effects. Currently, possible benefits of treatment after an injury are unknown and our data only support the use of light as a preventative strategy. Finally, elucidating the molecular mechanisms of intense light in lung protection could lead to the development of novel pharmacological compounds that would allow replacing light therapy one day and might be even more effective than light alone.
Our data are not without limitations and should be interpreted with caution. Although we found anti-inflammatory effects in mice and humans, differences in size and physiology, as well as variations in the homology of targets between mice and humans, may lead to translational limitations. Moreover, the inflammatory signature observed in human plasma might not reflect the pulmonary status. In addition, low sample size and test limitations (selection of 1319 proteins) of our proteomics platform might make our conclusions on light having an impact on humans appear premature. Nevertheless, we have analyzed 12 plasma samples from 4 healthy volunteers over a week. As the most robustly regulated proteins (Supplementary Figure S2, Supplementary Table S1) over a week dominated the pathway analysis, we believe that our data at least partly support our conclusions of light as an anti-inflammatory strategy for humans. Although intense light therapy has been validated for the treatment of seasonal disorders, studies on the biological effects on intense light are scarce. In fact, to our knowledge, there are no studies that have performed a wide protein screen from plasma samples following intense light therapy in humans. The SOMAscan platform, which we chose, is a highly multiplexed, aptamer-based assay optimized for protein biomarker discovery, which is made possible by the simultaneous measurement of a broad range of protein targets. This assay has been successful in the identification of biomarker signatures in a variety of recent biomedical applications.41 As such, despite the limitations of our analysis, our results will hopefully stimulate future research on the role of intense light therapy in the regulation of inflammatory processes.
CONCLUSION
Given that hemorrhagic shock is a primary injury on the battlefield, this study may provide a novel concept for the treatment of hemorrhagic shock lung or hemorrhagic shock associated organ dysfunction. Our findings underscore the importance of intense light for lung protection in the setting of a hemorrhagic shock but indicate the need for future in-depth studies to explore light as preventive strategy or as therapy, and the underlying mechanisms further.
Funding
Source of financial support for the work: National Heart, Lung, and Blood Institute (NIH-NHLBI) 5R01HL122472 Grant to T.E.; American Heart Association (AHA) Postdoctoral Fellowship 19POST34380105 to Y.O.
Supplementary Material
References
- 1. Levitt JE, Matthay MA: Clinical review: early treatment of acute lung injury–paradigm shift toward prevention and treatment prior to respiratory failure. Crit Care 2012; 16: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laffey JG, Matthay MA: Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med 2017; 196: 266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howell MD, Davis AM: Management of ARDS in adults. JAMA 2018; 319: 711–2. [DOI] [PubMed] [Google Scholar]
- 4. Park PK, et al. : Incidence, risk factors, and mortality associated with acute respiratory distress syndrome in combat casualty care. J Trauma Acute Care Surg 2016; 81: S150–s156. [DOI] [PubMed] [Google Scholar]
- 5. Herridge MS, et al. : Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364: 1293–304. [DOI] [PubMed] [Google Scholar]
- 6. Eastridge BJ, et al. : Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 2012; 73: S431–7. [DOI] [PubMed] [Google Scholar]
- 7. Eastridge BJ, et al. : Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma 2011; 71: S4–8. [DOI] [PubMed] [Google Scholar]
- 8. Nikolian VC, et al. : Lung protective effects of low-volume resuscitation and pharmacologic treatment of swine subjected to Polytrauma and Hemorrhagic shock. Inflammation 2017; 40: 1264–74. [DOI] [PubMed] [Google Scholar]
- 9. Park PK, et al. : Transfusion strategies and development of acute respiratory distress syndrome in combat casualty care. J Trauma Acute Care Surg 2013; 75: S238–46. [DOI] [PubMed] [Google Scholar]
- 10. Robinson BRH, et al. : Risk factors for the development of acute respiratory distress syndrome following Hemorrhage. Shock 2017; 50: 258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edens JW, et al. : Predictors of early acute lung injury at a combat support hospital: a prospective observational study. J Trauma 2010; 69(Suppl 1): S81–6. [DOI] [PubMed] [Google Scholar]
- 12. Chan CM, Shorr AF, Perkins JG: Factors associated with acute lung injury in combat casualties receiving massive blood transfusions: a retrospective analysis. J Crit Care 2012; 27: 419.e417–4. [DOI] [PubMed] [Google Scholar]
- 13. Xiao W, et al. : A genomic storm in critically injured humans. J Exp Med 2011; 208: 2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonney S, et al. : Cardiac period 2 in myocardial ischemia: clinical implications of a light dependent protein. Int J Biochem Cell Biol 2013; 45: 667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eckle T, et al. : Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 2012; 18: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartman CM, et al. : Intense light-elicited upregulation of miR-21 facilitates glycolysis and cardioprotection through Per2-dependent mechanisms. PLoS One 2017; 12: e0176243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oyama Y, et al. : Intense light-mediated circadian cardioprotection via transcriptional reprogramming of the endothelium. Cell Rep 2019; 28: 1471–84.e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brainard J, Gobel M, Scott B, Koeppen M, Eckle T: Health implications of disrupted circadian rhythms and the potential for daylight as therapy. Anesthesiology 2015; 122: 1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wright KP Jr, et al. : Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol 2013; 23: 1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Remi J: Humans entrain to sunlight—impact of social jet lag on disease and implications for critical illness. Curr Pharm Des 2015; 21: 3431–7. [DOI] [PubMed] [Google Scholar]
- 21. Brainard J, et al. : Circadian rhythms in anesthesia and critical care medicine: potential importance of circadian disruptions. Semin Cardiothorac Vasc Anesth 2015; 19: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yorguner Kupeli N, Bulut NS, Carkaxhiu Bulut G, Kurt E, Kora K: Efficacy of bright light therapy in bipolar depression. Psychiatry Res 2017; 260: 432–8. [DOI] [PubMed] [Google Scholar]
- 23. Koeppen M, Eckle T, Eltzschig HK: Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One 2009; 4: e6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oyama Y, et al. : Intense light-mediated circadian Cardioprotection via transcriptional reprogramming of the endothelium. Cell Rep 2019; 28: 1471–84.e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powell K: New platform for cataloging hundreds of proteins gets test drive. Nat Med 2014; 20: 1082–3. [DOI] [PubMed] [Google Scholar]
- 26. Loffredo FS, et al. : Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013; 153: 828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bartman CM, Oyama Y, Eckle T: Daytime variations in perioperative myocardial injury. Lancet 2018; 391: 2104. [DOI] [PubMed] [Google Scholar]
- 28. Oyama Y, Bartman CM, Gile J, Eckle T: Circadian MicroRNAs in Cardioprotection. Curr Pharm Des 2017; 23: 3723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oyama Y, Bartman CM, Gile J, Sehrt D, Eckle T: The circadian PER2 enhancer Nobiletin reverses the deleterious effects of midazolam in myocardial ischemia and reperfusion injury. Curr Pharm Des 2018;24: 3376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He B, et al. : The small molecule Nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab 2016; 23: 610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatori M, et al. : Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 2012; 15: 848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gloston GF, Yoo SH, Chen ZJ: Clock-enhancing small molecules and potential applications in chronic diseases and aging. Front Neurol 2017; 8: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gile J, Scott B, Eckle T: The period 2 enhancer Nobiletin as novel therapy in murine models of circadian disruption resembling delirium. Crit Care Med 2018; 46: e600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, et al. : Time-restricted feeding shifts the skin circadian clock and alters UVB-induced DNA damage. Cell Rep 2017; 20: 1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martino TA, et al. : Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 2007; 49: 1104–13. [DOI] [PubMed] [Google Scholar]
- 36. Noyan H, et al. : Cardioprotective signature of short-term caloric restriction. PLoS One 2015; 10: e0130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pierce A, Pittet JF: Inflammatory response to trauma: implications for coagulation and resuscitation. Curr Opin Anaesthesiol 2014; 27: 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maddry JK, et al. : Impact of prehospital medical evacuation (MEDEVAC) transport time on combat mortality in patients with non-compressible torso injury and traumatic amputations: a retrospective study. Mil Med Res 2018; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benavides JM, Benavides LC, Hale DF, Lundy JB: The golden hour offset surgical treatment team operational concept: experience of the 102nd forward surgical team in operation Freedom's sentinel 2015-2016. J Spec Oper Med 17: 46–50. [DOI] [PubMed] [Google Scholar]
- 40. Ball JA, Keenan S: Prolonged field care working group position paper: prolonged field care capabilities. J Spec Oper Med 2015; 15: 76–7. [DOI] [PubMed] [Google Scholar]
- 41. Candia J, et al. : Assessment of variability in the SOMAscan assay. Sci Rep 2017; 7: 14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


