Abstract
Although blood flow in the placental vasculature is governed by the same physiological forces of shear, pressure and resistance as in other organs, it is also uniquely specialized on the maternal and fetal sides. At the materno-fetal interface, the independent uteroplacental and umbilicoplacental circulations must coordinate sufficiently to supply the fetus with the nutrients and substrates it needs to grow and develop. Uterine arterial flow must increase dramatically to accommodate the growing fetus. Recent evidence delineates the hormonal and endothelial mechanisms by which maternal vessels dilate and remodel during pregnancy. The umbilical circulation is established de novo during embryonic development but blood does not flow through the placenta until late in the first trimester. The umbilical circulation operates in the interest of maintaining fetal oxygenation over the course of pregnancy, and is affected differently by mechanical and chemical regulators of vascular tone compared to other organs. The processes that match placental vascular growth and fetal tissue growth are not understood, but studies of compromised pregnancies provide clues. The subtle changes that cause the failure of the normally regulated vascular processes during pregnancy have not been thoroughly identified. Likewise, practical and effective therapeutic strategies to reverse detrimental placental perfusion patterns have yet to be investigated.
Keywords: Blood flow regulation, vasculature, placental insufficiency, compromise, pregnancy
INTRODUCTION
By the time a baby is born, it will have transformed from a 1 cell diploid zygote to a trillion-cell individual capable of existence outside the womb. This enormous feat requires the de novo formation and expansion of a cardiovascular system that circulates blood in the embryo and the fetus. The nutrient flow for the growing fetal body is delivered to the placenta via a newly remodeled maternal cardiovascular system. The growth, development and function of the nascent vascular system in the embryo/fetus is a fascinating field of study that has become specialized by anatomical region. However, there are a few basic physiological principles that are foundational to all vascular beds.
The physical principles that regulate blood flow through an organ are simple enough in their basic form and are explained in any basic physiology text or website [1]. The difference between arterial (Pa) and venous (Pv) pressures drives blood through an organ. The magnitude of the resulting flow (Qo) depends on the energy required to overcome the vascular resistance within the organ (Ro) and can be expressed in a form analogous to Ohm’s Law:
This convective flow equation holds true under the following conditions: 1) steady flow and 2) Newtonian fluid. While these two requirements that are not technically met for blood in the mammalian circulatory system, the equation, nevertheless, reasonably approximates most normal blood flow conditions. The resistance to the flow of blood is determined by well described physical principles (Poiseuille’s law) where the resistance Ro is defined as
where η is viscosity, L is length and r is radius. When a more sophisticated understanding of flow in an organ is needed, there are well conceived mathematical equations that describe pulsatile pressure-flow relationships with corrections for changing viscosity with vessel diameter and equations that estimate organ impedance have been derived. Most all considerations of pulsatile hemodynamics are derived from McDonald’s original treatise [2] on the topic.
It has become increasingly clear that shear and wall forces provide remodeling signals that regulate vessel endothelial function as well as the structural integrity of the vessel wall. These forces are especially important in pregnancy. In a vessel, the frictional shear force that is sensed by the endothelium lining the vessel wall can also be expressed in the form of the Poiseuille’s relationship,
Thus the shear stress, τs, is related to the ratio of viscous flow and shear rate, where η is the viscosity and Q the flow, to vessel radius to the 3rd power [3, 4].
A successful pregnancy requires adaptations of the maternal cardiovascular system, the establishment of a placenta with its two sided vasculature and a well constructed fetal vascular tree. While in most organs the resistance to flow is found at the level of the arteriole which is innervated, the placenta is not innervated. The vascular smooth muscle tone, however, is sensitive to local transmural pressure conditions (myogenic tone) and to vasoactive substances. The local substances that regulate arteriolar function include those that are carried to the organ in the blood (e.g. angiotensin II, arginine vasopressin, atrial natriuretic peptide), those released from nerve endings (e.g. norepinephrine, acetylcholine), those derived from endothelium (e.g. nitric oxide, NO, prostaglandins), those released from endocrine tissue (e.g. steroid hormones such as estrogens, progestins and glucocorticoids) and those released from various cells in the vessel milieu. The maternal hormonal environment changes dramatically over the course of pregnancy and many maternal vascular structures are sensitive to these changes. For example, human chorionic gonadotropin peaks early in pregnancy and plateaus at about 24 weeks [5, 6] and estrogens and progestins rise over the course of pregnancy [5, 6].
MATERNAL ADAPTATIONS TO PREGNANCY
In mammals, the normal adaptation to pregnancy requires enormous changes in the structure and function of the maternal circulatory system [7, 8], largely under the influence of sex steroid hormones [9]. Uterine blood flow increases by 2 to 3 times over the last half of pregnancy in women and ewes (Reviewed by [10]). During pregnancy, maternal oxygen consumption increases about 33%, body weight normally increases by 20% [11] while blood volume increases by 40%. Red cell mass increases by only 30%; thus, the concentration of red blood cells is reduced in the maternal circulation. The structure of the maternal heart also is remodeled. The term “cardiac remodeling” often refers to a pathological process that leads to cardiac restructuring and consequent dysfunction. In the case of pregnancy, non-pathological cardiac structural changes lead to an increase in end diastolic volume over the first half of pregnancy so that stroke volume is augmented about 30%. This increase in end diastolic volume is profound because the chambers of the ventricles actually enlarge- the whole heart gets bigger [11]. Because ejection fraction is maintained during pregnancy, the larger heart ejects a larger volume each beat. This is accomplished without an undue load on the myocardial wall because vascular impedance is simultaneously decreased through the remodeling of the arterial tree, making it possible to eject a stroke volume without large increases in wall stress. Aortic diameter and aortic compliance are increased as are venous capacitance and venous blood volume.
There are racial differences in uterine arterial adaptation to pregnancy. In comparing uterine arterial Doppler flow in Andean and European residents of La Paz, Bolivia (3600 m) at weeks 20, 30, 36 of pregnancy, Andean women had greater uterine cross sectional areas and blood flows by 36 weeks and 1.6 times greater uteroplacental oxygen delivery [12]. With adjustments for gestational age, maternal height, and parity, Andean babies were consistently heavier than European babies. While there are undoubtedly genetic underpinnings explaining these differences, it is becoming increasingly clear that there are transgenerational influences of maternal diet, fetal nutrition and structural features of reproductive organs that are passed on via epigenetic and nonepigenetic mechanisms. Which of these is most important across races in the adaptation to altitude is not known.
Recent studies in non-human primates show that maternal diet affects uteroplacental blood flow. In this model, pregnant macaques are fed a high fat diet – some become obese (high fat diet sensitive) whereas a subset does not become obese (high fat diet resistant). Using Doppler ultrasonography in early third trimester pregnancies (gestational day 120), Frias et al. [13] showed that calculated volume flow in the uterine artery, normalized to maternal weight, was decreased significantly even in maternal animals that were resistant to gaining weight on the high fat diet and in monkeys that were sensitive and gained weight (Fig. 1). Uterine arterial flow was reduced by an average of 38% in the former group and 56% in the latter compared to controls. Both of the experimental groups had a significant increase in the pulsatility index of the uterine artery (Fig. 1). In contrast, umbilical vein flow was decreased by 32% but only in the high fat diet sensitive group (Fig. 1). The pulsatility index in the umbilical artery was not changed in either diet group. The role of diet in regulating utero-placental blood flow in the human has not been explored in depth and is ripe for mechanistic investigation.
Fig. 1.
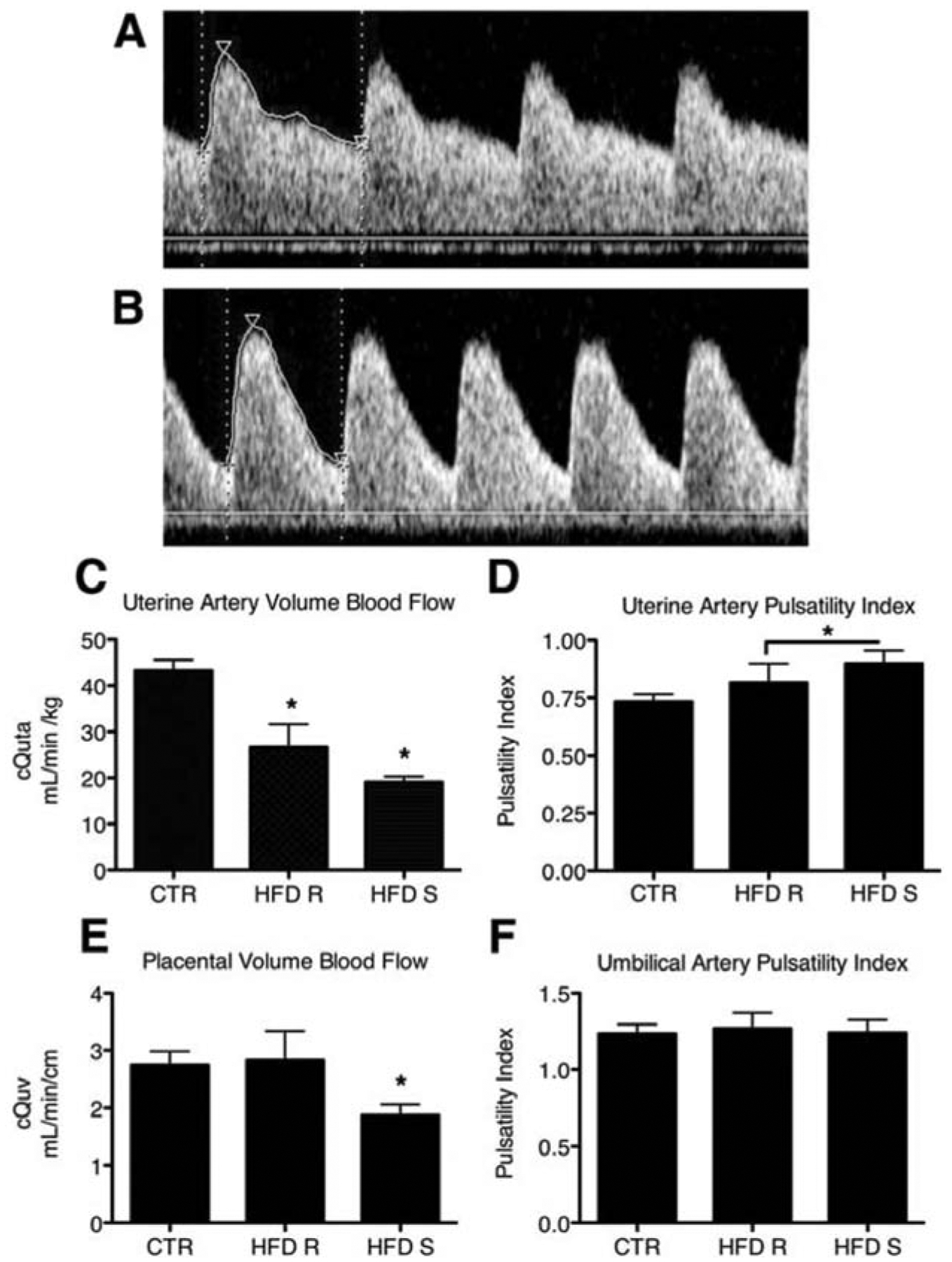
Decreased uteroplacental perfusion in Japanese macaques fed a high fat diet (HFD). Maternal HFD leads to increased uterine artery pulsitility index (PI). A: Uterine artery (Uta) PI is 0.74 in a representative control (CTR) animal. B: The Uta PI is 1.17 in a representative HFD-Sensitive (HFD-S) animal with a Doppler waveform that demonstrates decreased diastolic flow consistent with increased vascular impedance when compared with A. C: The cQUta normalized to maternal weight was significantly reduced in HFD-Resistant (HFD-R) and HFD-S animals when compared with CTR. D: Uta PI is increased in HFD-S animals when compared with CTR. As a group, HFD (HFD-R + HFD-S) had a significant increase in Uta PI when compared with CTR. E: The cQUV normalized to fetal abdominal circumference was reduced in HFD-S animals when compared with controls. There was no difference in HFD-R animals when compared with controls. F: The umbilical artery (UA) PI was unaffected by diet group. * P < 0.05; CTR, n = 9; HFD-R, n = 6; HFD-S, n = 9. Reproduced, with permission [13].
As mentioned above, the rate at which oxygen is delivered to the uteroplacental bed depends upon the driving pressure across the uterine and umbilical beds, the resistance to flow in these beds, as well as the maternal hemoglobin concentration and oxyhemoglobin saturation. Failure of maternal adaptations can lead to maternal hypertension and subsequent stunting of fetal growth. The reasons that fetal well being suffers from this maternal condition are not entirely clear. However, it is clear that the increase in uterine arterial flow capacity must increase dramatically to accommodate the large increases in flow. There are really only two mechanisms by which vessel dimension could be increased enough to accommodate flow adequate to care for fetal growth needs- dilation and structural remodeling. If the vessel could dilate adequately, then simple dilation would be the only mechanism required. If, on the other hand, flow is needed beyond maximal vasodilatory capacity, then one must also postulate vessel structural remodeling. Thus, in a manner similar to the maternal heart, the structural capacity and material properties of the vessel are likely to be profoundly altered.
UTERINE BLOOD FLOW AND ITS REGULATION
The remodeling of the human cardiovascular system accommodates needed increases in blood flow to the breast, kidney and uterus such that breast flow increases by 2.5×, renal flow by 1.7× and uterine blood flow by 10× or greater (Fig. 2). Uterine blood flow has been measured during pregnancy in a number of experimental animals including guinea pig [14, 15], rat [16, 17], pig [18, 19], cow [20], sheep [21, 22] and rabbits [23]. Among these animals there is considerable variability in the degree of increase in uterine blood flow in pregnancy, ranging from 10- to 100-fold [24]. However, in no mammal thus far studied is the resting level of uterine blood delivery adequate to accommodate the increases in oxygen and nutrient flow required for a successful pregnancy. Thus, there must be profound local changes in the delivery system to accommodate the pregnancy in all mammals even though different species may use a variety of mechanisms to accomplish the adaptation.
Fig. 2.
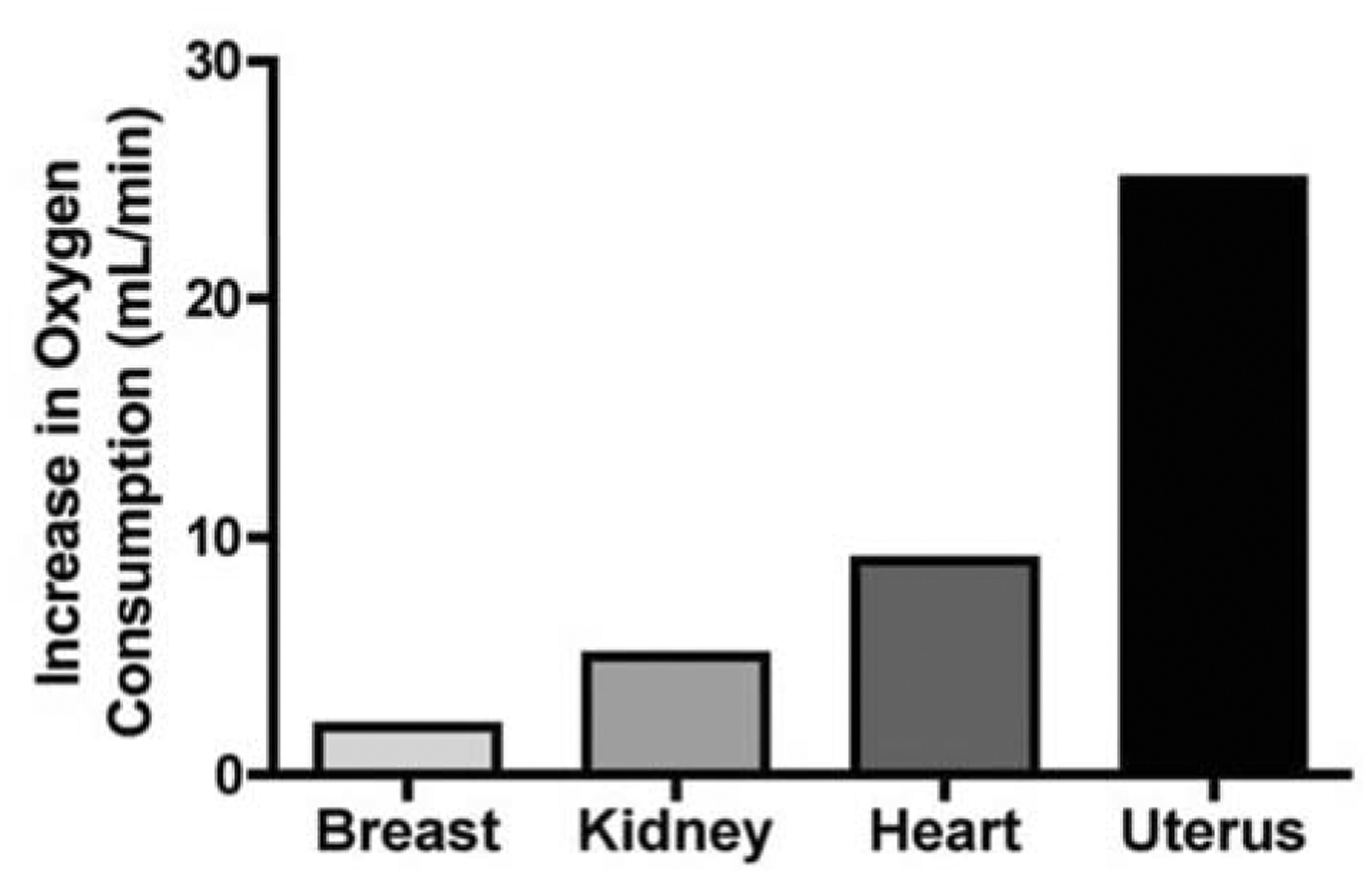
Increases in peak resting oxygen consumption in pregnancy compared to pre-pregnancy values. Data from Metcalfe et al. [11].
Presumably the primary physiological changes that accompany pregnancy are under the control of changing hormone levels. The roles of hormones in regulating uterine blood flow have been recently reviewed [25]. While estradiol 17β (E2β) is believed to be the most potent and physiologically dominant in regulating maternal adaptations [26, 27], other important hormones include other estrogens, progesterone, human chorionic gonadotropin, cortisol, and androgens. Infused E2β leads to increased cardiac output and heart rate in ovariectomized nonpregnant ewes and decreases in their systemic vascular resistance [27]. E2β also causes an increase in uterine blood flow. It is becoming increasingly clear, however, that pharmacological levels of hormone may have different effects than those within the physiological range; thus experimental data need to be judged according to the concentration ranges used. The question remains: how does E2β increase uterine arterial flow? As described above, blood flow increases will necessarily be a result of a combination of increased driving pressure and decreased uterine vascular resistance through dilation and/or remodeling.
Estradiol ordinarily signals through its two primary soluble receptors, estrogen receptor alpha and beta (ERα & ERβ). These receptors are ligand-activated enhancer proteins that are members of the steroid/nuclear receptor superfamily. Once bound to ligand these receptors become transcription factors that bind with high affinity to estrogen response sequences found in the regulatory regions of specific genes and activate gene expression [28]. In addition, there are so called “non-genomic” often membrane associated signaling pathways that are stimulated by estrogens, one of which is a G-protein receptor mediated process via GPR30 [29]. ER associations with Gi proteins in the plasma membrane have been reported to mediate NO production [30] and cAMP inhibition [31]. The roles of these differing signaling pathways need to be investigated in the uterine circulation.
A role for endothelium derived nitric oxide (eNO) in regulating changes in muscular tone in the uterine artery has been appreciated for some time, and it has also been known that endothelial NO production is influenced by circulating estrogen levels [32]. E2β is a powerful regulator of uterine blood flow and its effect is reduced by some 70% if the catalytic enzyme, nitric oxide synthase (NOS) is inhibited [33]. E2β is known to activate endothelial NO synthase (eNOS) through the phosphoinositol-3 kinase cascade [34]. The rate of NO production by eNOS is determined by several features including the capacity of the cell (eNOS expression levels), the phosphorylation state of eNOS, and the intracellular [Ca2+]i concentration [35]. While many scientists have assumed that pregnancy-associated changes in eNOS regulation are responsible for NO-induced increases in the uterine artery during pregnancy, evidence over the past decade suggests an adaptation of sustained [Ca2+]i signaling responses may supersede in importance any changes in eNOS expression and phosphorylation. Thus, the regulation of local NO output in a vessel may occur at the level of the ‘capacitative entry’ [Ca2+]i response which is in turn regulated by gap junction function [36]. Likewise NO output may be reduced by any inhibitor of gap junction function or capacitive entry of Ca2+ via transient receptor potential cation (TRPC) channels. Thus the degree to which estrogens affect these NO regulating processes is complex and not yet thoroughly clarified.
Many other endothelium specific mechanisms are likely to hold high importance in regulating vascular tone in the uterine artery [24, 37]. Some are important in the elevated resistance to vasoconstrictors during pregnancy [38], some are important for vessel remodeling [39], and others may participate as but one of a host of redundant mechanisms that underlie the changes in the chemical regulation of vasodilatory capacity. There is keen interest in the roles of large conductance Ca2+-activated K+ channels [40], release of vasodilator prostaglandins [41], endothelial hyperpolarizing factor [42], atrial natriuretic factor [43] among others. The coordinated roles of these factors have yet to be determined.
THE FETAL PLACENTA
The circulatory system in the fetus is characterized by four shunts, the ductus venosus, the foramen ovale, the ductus arteriosus and the placenta. During the transition from fetus to neonate, these shunts begin to close, and under normal conditions are anatomically sealed within weeks. Each of these shunts is physiologically unique in both its importance in the fetal circulation, but also in the regulation of its closure at birth [44–46]. Increasing evidence suggests that these shunts influence the fetal circulation and organ growth in ways that affect the long-term health of the offspring.
The umbilical circulation derives from the allantois in early embryonic development and is the obvious life line for delivering oxygen and nutrients for the developing fetus. Special features of the fetal placental circulation ensure an uninterrupted blood flow of oxygen during fetal life and a secure cessation of flow after birth to prevent post-partum hemorrhage. Umbilical blood flow increases to keep pace with fetal growth [10]. There is a delicate balance between fetal growth rate and nutrient acquisition and in cases of placental insufficiency, whether natural or experimental, fetal growth is restricted. Roberts et al. [47] showed in Rhesus macaques that disruption of the growth of the placenta and the fetus was dependent upon the timing of experimental reduction in placental exchange area following the ligation of the vessels that bridge the two placental lobes. The growth of the fetus was more depressed when placental tissue loss occurred at 0.7 gestation (110d GA) compared to 0.5 gestation (80d GA) because the earlier placenta as able to gain more mass and thickness than those placentas ligated at a later gestational age. Following the 80 dGA ligation the primary placental lobe increased by 2.2 g/day, a 30% increase above normal, whereas growth at the later gestational age decreased to 0.8 g/day, about half normal. These findings suggest that primate placentas are highly plastic, but have a diminished capacity for adaptive growth as gestation proceeds.
While the umbilical artery has gained notoriety among placentologists for being inert to the usual mechanical and chemical forces that regulate systemic arterial vasomotor activity, this pristine reputation is not wholly deserved. In sheep, umbilical blood flow is generally determined by its driving pressure, estimated by the difference between mean pressures in the umbilical artery and umbilical vein without an intervening surrounding pressure as found in the lung [48]. Placental vascular resistance is increased in response to chronic increases in fetal arterial pressure [49] an indication of autoregulatory capability. However this capacity is limited because placental vascular resistance is not decreased in response to hypotension [50, 51]. The lack of a relaxation response when driving pressure is decreased suggests that the placental resistance bed is normally in a state of maximal relaxation, at least from an autoregulatory point of view. This explanation is reasonable, but further experiments could test whether further relaxation under chemical control would be possible.
The development of the placental circulation is too complex to present in detail here. However, it is nevertheless important to note that the complexities of the inner workings of the vascular tree within the mature placenta proper are the result of early chemical interactions between maternal and fetoplacental tissues. The subject was summarized by Burton et al. [52]. The development of a proper vascular tree in the early placenta is important not only because of the need for optimal placental transport function but because the vascular elements of the placenta offer a continuous loading resistance to the developing heart. In the embryo, pressure loading leads to heart defects [53, 54] while in the fetus it leads to abnormal cardiomyocyte development [55, 56]. Hemangioblastic cords arise from mesenchymal cells deep within the extracellular matrix of the primitive villous cores of the nascent placenta. Under the guidance of vascular endothelial growth factors (VEGFA in particular) primitive vascular elements connect the larger placental stem vessels forming up- and down-stream [57, 58]. VEGF signals through its FLT-1 and KDR receptors, but does not act alone. Placental growth factor, which also signals via the FLT-1 receptor, is a key player in the development of the placental vascular system. Angiopoietin-1 and −2 are both ligands for the tyrosine kinase receptor, TIE2. Activation of TIE2 promotes endothelial cell survival and stabilization of newly formed capillaries while ANG2 may inhibit ANG1 rendering the developing capillaries to be more sensitive to the angiogenic stimulus of other growth factors [52, 59].
The maternal chemical environment is part of the chemical “conversation” with the developing placenta. The trophoblast releases a number of soluble receptors into the maternal circulation [52]. These molecules bind maternal growth factors and affect their availability to act locally in the placenta. In addition, there are a host of molecules including cytokines and chemokines that can interfere with proper placental vasculogenesis and result in abnormal placental function. These molecules may be the basis for diseases ranging from preeclampsia to obesity-related placental inflammation [60, 61]. Maternal estrogen is also a regulator of placental growth in the early period and is thus important in degree of implantation and the establishment of the placental vascular tree. For example, in the baboon exogenous estrogen given at 6 weeks gestation inhibits spiral artery invasion [62].
In addition to the factors mentioned above, oxygen is a powerful regulator of vascular growth and development. The once controversial idea that the placenta develops in a very low oxygen environment during most of the first trimester is now mainstream thinking among placentologists. The relative hypoxia is the result of a lack of maternal blood flow through blocked spiral arteries during early pregnancy. Current evidence suggests that this hypoxic environment is crucial to the proper development of the placenta through the appropriate expression of VEGF, PlGF, ANG1, ANG2 and their receptors each of which is adversely affected by higher levels of oxygen [63]. Eventually, the spiral arteries open up beginning at the periphery and moving in a central direction [64]. Consequently, higher oxygen concentrations and increased levels of oxidative stress are found in the periphery compared to the central areas, as shown in villi sampled from these sites [64].
Several substances are known to affect placental vascular resistance and/or the contractile properties of umbilical vessels. Table 1 shows chemicals that are known or suspected to alter placental vascular resistance in sheep and in humans. The take home message is clear: the placental vascular bed is not inert but interestingly affected by several chemical agents. However, some agents that would alter the resistance of other fetal organs have little effect on the placenta at least in most experimental settings. The powerful vasodilator, adenosine, is an example.
Table 1.
In vivo studies in sheep and in vitro studies of isolated human placental and umbilical blood vessels demonstrate the umbilical-placental circulation is responsive to a variety of vasoactive substances. However, it should be noted that some vasodilators (*) exert their effects in vitro only when vessels are pre-constricted with another substance. Compiled from several sources [90–100].
| Substance | Umbilical artery and placental resistance (sheep) | Isolated placental or umbilical artery or vein (human) |
|---|---|---|
| Acetylcholine | constrict | |
| Adenosine | ↔ | |
| Angiotensin II | ↑ | constrict |
| Bradykinin | constrict | |
| Endothelin 1 | ↑ | |
| Epinephrine | ↑ | constrict |
| Forskolin | ↓ | dilate |
| Histamine | constrict | |
| Isoproteranol | ↔ | |
| Neurokinin B* | dilate | |
| Nitrogycerin* | ↔ | dilate |
| Norepinephrine | ↔ | constrict |
| Oxytocin | constrict | |
| Potassium | constrict | |
| Prostacyclin* | ↔ | dilate |
| Prostaglandin A1 | constrict | |
| Prostaglandin F2α | constrict | |
| Prostaglandin I2 | ↔ | |
| Serotonin | constrict | |
| U-46619 (thromboxane mimetic) | constrict | |
| Vasopressin | ↑ | constrict |
MODELS OF COMPROMISED PREGNANCY
A number of models of compromised pregnancy have been developed in several species. These models have been an enormous benefit to the current understanding of the biology of pregnancy and the mechanisms that regulate fetal growth. The effects on uterine and umbilical flow have been nicely reviewed by Reynolds et al. [10] for many of these models in the sheep. In addition to those reviewed, there are studies of the umbilical circulation in fetuses with inadequate placental gas exchange. These include carunclectomy [65–67] and umbilicoplacental embolization [68–70]. The latter model has been used widely and has brought new insight to the adaptations of specific organs that respond to low oxygen and nutrient transport.
In sheep, placental embolization allows the study of changes in placental blood flow that can occur in late gestation placental insufficiency. Repeated injections of insoluble microspheres (15–50 μm diameter) into the umbilicoplacental circulation increases placental vascular resistance and decreases umbilical flow [68–70], and in severe cases can acutely modify the umbilical artery flow velocity waveform to demonstrate zero or reversed diastolic flow (Fig. 3) [71]. These changes are characteristic of placental insufficiency and intrauterine growth restriction in human fetuses [72]. In humans, the increase in blood flow resistance is correlated more closely with loss of small arteries (<90μm) in the placenta rather than other complications of pregnancy such as maternal hypertension [73]. This is supported by studies in sheep that demonstrate that in non-embolizing models of fetal stress, there are no changes in placental resistance despite decreased placental weight and size, fetal hypoxia, acidemia, blood hyperviscosity, or maternal hypertension [65, 74, 75].
Fig. 3.
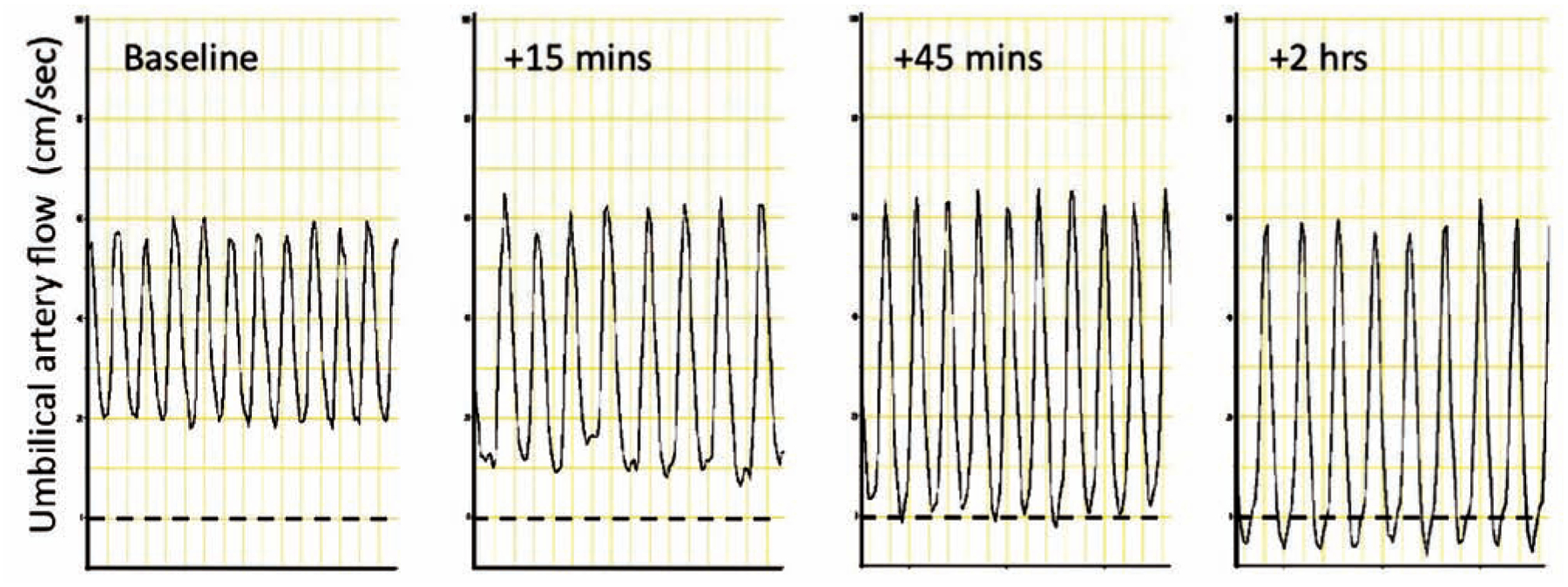
Umbilical artery flow (cm/sec) changes in response to severe umbilicoplacental embolization in sheep. Rapid, repeated injections of microspheres into the umbilicoplacental circulation will induce fetal distress and umbilical artery flow patterns that parallel those seen in human IUGR. In this experiment, diastolic flow became absent 45 minutes after embolization, and reversed flow was evident 2 hours after embolization. Louey & Thornburg, unpublished data.
Thus it is possible to isolate experimentally the effects of placental insufficiency stemming from umbilicoplacental embolization where uterine artery or uteroplacental flows are not altered [68]. In contrast, a subset of small-for-gestational age cases in humans include a maternal component in which uterine artery blood flow is impaired [76]. Similarly, utero-placental embolization in sheep can be used to limit nutrient availability to the fetus by injecting microspheres into the maternal uterine artery. Resistance in the uterine vascular bed is increased [77], but in contrast to umbilicoplacental embolization, fetal placental vascular resistance as estimated by umbilical artery pulsatility index is not altered, even under conditions of severe fetal distress [78].
Additionally, umbilicoplacental embolization (UPE) in sheep has been used to study the effects of placental insufficiency on growth and development, and mimics many clinical signs of intrauterine growth retardation (IUGR) including fetal hypoxemia, hypoglycemia and hypercortisolemia. The severity of the embolization is controllable and thus can be used to study different degrees of stress on fetoplacental physiological parameters, as well as the effects on specific tissues. In its most severe form, frequent injections of microspheres in a relatively brief period of time will induce severe fetal hypoxemia and acidemia, hypertension (+15mmHg), bradycardia, a 50% increase umbilical perfusion pressure, a 70% reduction in umbilical blood flow, a three- to five-fold increase in umbilical vascular resistance and umbilical artery pulsatility index, reversed umbilical artery diastolic flow and fetal demise within hours of embolization [71, 79].
More moderate and controlled UPE that allows for the study of fetoplacental adaptations to placental insufficiency can induce a 30–50% decrease in fetal oxygenation and blood glucose, as well as premature activation of the hypothalamic-pituitary-adrenal axis [69, 80, 81]. Consequent to the occlusion of the villous arteries [82] and increased placental resistance, fetal arterial pressure is transiently elevated [80, 83]. Given some placental vascular reserve, fetal oxygen levels can return to normal within a day of embolization, thus requiring daily microsphere administration. Prolonged embolization (days to weeks) diminishes this reserve, and the need for daily injections is reduced (Fig. 4) [69, 80, 83].
Fig. 4.
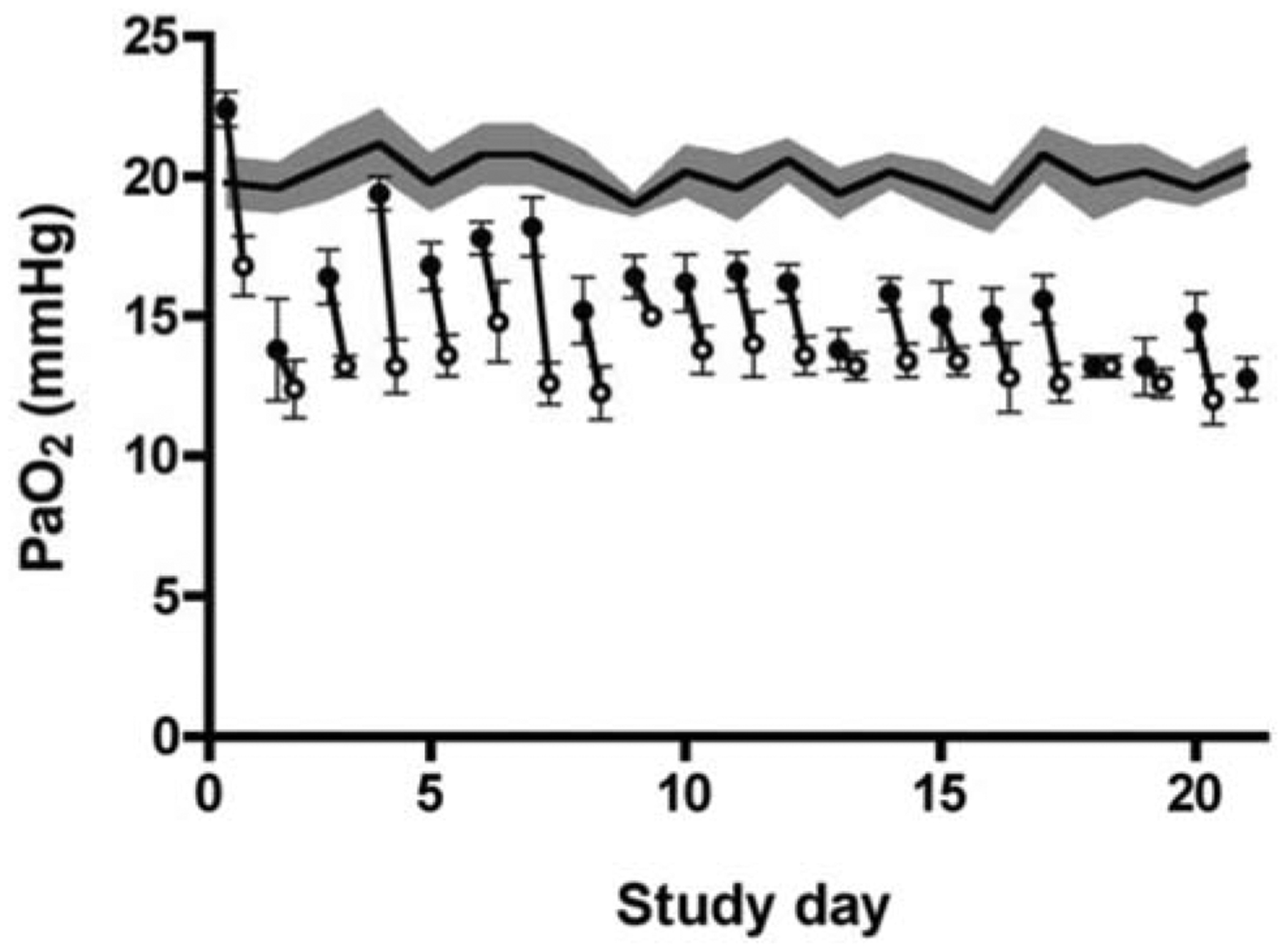
Fetal partial pressure of arterial oxygen during umbilicoplacental embolization (UPE) in sheep. Mean data for the control group (n=5) are shown by the continuous line ± SEM (shaded area). Mean ± SEM data for the UPE fetuses (n=5) are shown for the daily pre- (black circle, ●) and post-UPE periods (open circle, ○). Data modified from Louey et al. [80].
As the fetus adapts to this placental insufficiency, vascular and tissue remodeling occurs over days to weeks. These adaptations permit fetal survival in a reduced nutrient environment, but with consequences to the developing tissues. Although most organ sizes remain proportionate to the body, even those traditionally thought to be somewhat “protected” from prenatal hypoxic stress (brain, heart) show modifications to their cellular makeup [80, 84, 85]. Most notable is the consistent finding that maturation is delayed: the heart [80], lungs [81], kidney [86], retina [87] all show signs of structural immaturity. Cellular proliferation is also affected with fewer hematopoietic cell clusters in the liver [82] and fewer myocytes in the heart [80]. At least some of these fetal (mal)adaptations have been reported to persist into the postnatal period, notably in the brain and retina [85], adipose [88] and lungs [89], and have implications for long term health.
SUMMARY
Utero- and umbilico-placental bloods flow independently in separate circulatory units that interface at the placental membrane where they are separated by a few micrometers. The entire transplacental flow of nutrients and oxygen into the fetus over its life time is determined by a highly regulated chemical and mechanical regulation of matching vascular elements. Interestingly, the two circuits have enough autonomy and differences in chemical sensitivity that they grow under highly unique environments. What is not known is how the placenta is able to regulate its vascular growth and how tissue growth and vascular growth are matched. In some cases the placenta enlarges in response to apparent nutritional and oxygenation needs. In other cases it seems unable to mount a response and the normal development of the fetus is hindered. At present we do not know how to identify a poorly perfused placenta in its early stages nor do we know what therapeutic strategies might be helpful. These mysteries need to be solved because the lifelong health of the embryo and fetus is determined by the subtleties of the nutritional flow, about which we know little.
ACKNOWLEDGEMENTS
The authors would like to acknowledge funding support from NICHD grant P01 HD034430, NHLBI grant R01 HL102763, the M. Lowell Edwards Endowment, and The Collins Medical Trust.
SOURCES OF FUNDING
Funding comes in part from the NICHD grant P01 HD034430, NHLBI grant R01 HL102763, the M. Lowell Edwards Endowment, and The Collins Medical Trust.
ABBREVIATIONS
- CTR
Control
- dGA
Days gestational age
- eNO
Endothelium derived nitric oxide
- eNOS
Endothelial nitric oxide synthase
- HFD
High fat diet
- HFD-R
High fat diet resistant
- HFD-S
High fat diet sensitive
- IUGR
Intrauterine growth retardation
- L
Length
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- Pa
Arterial pressure
- Pv
Venous pressure
- PI
Pulsatility index
- Q
Blood flow
- Qo
Blood flow within an organ
- Ro
Vascular resistance within an organ
- r
Radius
- TPRC
Transient receptor potential cation
- UA
Umbilical artery
- UPE
Umbilicoplacental embolization
- Uta
Uterine artery
- VEGF
Vascular endothelial growth factor
- η
Viscosity
- τs
Shear stress
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Klabunde RE. Cardiovascular Physiology Concepts, http://www.cvphysiology.com/. [Website] 2012. [updated 2010; cited 2012]; Available from: http://www.cvphysiology.com/.
- [2].McDonald D The relation of pulsatile pressure to flow in arteries. J Physiol 1955;127:533–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999;282:2035–42. [DOI] [PubMed] [Google Scholar]
- [4].Sprague B, Chesler NC, Magness RR. Shear stress regulation of nitric oxide production in uterine and placental artery endothelial cells: experimental studies and hemodynamic models of shear stresses on endothelial cells. Int J Dev Biol 2010;54:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kase NG, Reyniak JV. Endocrinology of pregnancy. Mt Sinai J Med 1985;52:11–34. [PubMed] [Google Scholar]
- [6].Goebelsmann U. Protein and steroid hormones in pregnancy. J Reprod Med 1979;23:166–77. [PubMed] [Google Scholar]
- [7].Thornburg K, Bagby S, Giraud G. Maternal adaptation to pregnancy In: Neill JD, editor. Knobil & Neill’s Physiology of Reproduction. 3rd ed London: Elsevier Adademic Press; 2006. p. 2899–924. [Google Scholar]
- [8].Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol 2000;24:11–4. [DOI] [PubMed] [Google Scholar]
- [9].Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 2001;276:36869–72. [DOI] [PubMed] [Google Scholar]
- [10].Reynolds LP, Caton JS, Redmer DA, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol 2006;572:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Metcalfe J, McAnulty J, Ueland K. The effects of pregnancy on the cardiovascular system and oxygen transport Burwell and Metcalfe’s Heart Disease in Pregnancy: Physiology and Management. Boston: Little & Brown; 1986. p. 11–54. [Google Scholar]
- [12].Wilson MJ, Lopez M, Vargas M, et al. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol 2007;293:R1313–24. [DOI] [PubMed] [Google Scholar]
- [13].Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011;152:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bjellin L, Sjoquist PO, Martensson L, Carter AM. Regional blood flow measurements with 15 micron and 50 micron microspheres in pregnant guinea-pigs. J Reprod Fertil 1979;57:415–8. [DOI] [PubMed] [Google Scholar]
- [15].Hart MV, Hosenpud JD, Hohimer AR, Morton MJ. Hemodynamics during pregnancy and sex steroid administration in guinea pigs. Am J Physiol 1985;249:R179–85. [DOI] [PubMed] [Google Scholar]
- [16].Bruce NW. The distribution of blood flow to the reproductive organs of rats near term. J Reprod Fertil 1976;46:359–62. [DOI] [PubMed] [Google Scholar]
- [17].Dowell RT, Kauer CD. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find Exp Clin Pharmacol 1997;19:613–25. [PubMed] [Google Scholar]
- [18].Ford SP, Christenson RK. Blood flow to uteri of sows during the estrous cycle and early pregnancy: local effect of the conceptus on the uterine blood supply. Biol Reprod 1979;21:617–24. [DOI] [PubMed] [Google Scholar]
- [19].Ford SP, Reynolds LP, Ferrell CL. Blood flow, steroid secretion and nutrient uptake of the gravid uterus during the periparturient period in sows. J Anim Sci 1984;59:1085–91. [DOI] [PubMed] [Google Scholar]
- [20].Ford SP, Chenault JR, Echternkamp SE. Uterine blood flow of cows during the oestrous cycle and early pregnancy: effect of the conceptus on the uterine blood supply. J Reprod Fertil 1979;56:53–62. [DOI] [PubMed] [Google Scholar]
- [21].Greiss FC Jr., Anderson SG. Uterine blood flow during early ovine pregnancy. Am J Obstet Gynecol 1970;106:30–8. [DOI] [PubMed] [Google Scholar]
- [22].Rosenfeld CR, Morriss FH Jr., Makowski EL, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest 1974;5:252–68. [DOI] [PubMed] [Google Scholar]
- [23].Laird MR, Faber JJ, Binder ND. Maternal placental blood flow is reduced in proportion to reduction in uterine driving pressure. Pediatr Res 1994;36:102–10. [DOI] [PubMed] [Google Scholar]
- [24].Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chang K, Zhang L. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci 2008;15:336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Siiteri PK, MacDonald PC. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab 1966;26:751–61. [DOI] [PubMed] [Google Scholar]
- [27].Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol 1989;256:E536–42. [DOI] [PubMed] [Google Scholar]
- [28].Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J Mol Endocrinol 2004;33:387–410. [DOI] [PubMed] [Google Scholar]
- [29].Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol 2007;265–266:138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wyckoff MH, Chambliss KL, Mineo C, et al. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i). J Biol Chem 2001;276:27071–6. [DOI] [PubMed] [Google Scholar]
- [31].Navarro CE, Saeed SA, Murdock C, et al. Regulation of cyclic adenosine 3’,5’- monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol 2003;17:1792–804. [PubMed] [Google Scholar]
- [32].Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol 2001;280:H1699–705. [DOI] [PubMed] [Google Scholar]
- [33].Gibson TC, Phernetton TM, Wiltbank MC, Magness RR. Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycles in sheep. Biol Reprod 2004;70:1886–94. [DOI] [PubMed] [Google Scholar]
- [34].Haynes MP, Sinha D, Russell KS, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 2000;87:677–82. [DOI] [PubMed] [Google Scholar]
- [35].Boeldt DS, Yi FX, Bird IM. eNOS activation and NO function: pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium--new insights into eNOS regulation through adaptive cell signaling. J Endocrinol 2011;210:243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yi FX, Boeldt DS, Gifford SM, et al. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod 2010;82:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol 2003;284:R245–58. [DOI] [PubMed] [Google Scholar]
- [38].Rosenfeld CR, Naden RP. Uterine and nonuterine vascular responses to angiotensin II in ovine pregnancy. Am J Physiol 1989;257:H17–24. [DOI] [PubMed] [Google Scholar]
- [39].Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta 2004;25:103–13. [DOI] [PubMed] [Google Scholar]
- [40].Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab 2010;298:E222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Magness RR, Rosenfeld CR, Faucher DJ, Mitchell MD. Uterine prostaglandin production in ovine pregnancy: effects of angiotensin II and indomethacin. Am J Physiol 1992;263:H188–97. [DOI] [PubMed] [Google Scholar]
- [42].Mandala M, Gokina N, Barron C, Osol G. Endothelial-derived hyperpolarization factor (EDHF) contributes to PlGF-induced dilation of mesenteric resistance arteries from pregnant rats. J Vasc Res 2012;49:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sala C, Campise M, Ambroso G, Motta T, Zanchetti A, Morganti A. Atrial natriuretic peptide and hemodynamic changes during normal human pregnancy. Hypertension 1995;25:631–6. [DOI] [PubMed] [Google Scholar]
- [44].Schneider DJ. The patent ductus arteriosus in term infants, children, and adults. Semin Perinatol 2012;36:146–53. [DOI] [PubMed] [Google Scholar]
- [45].Calvert PA, Rana BS, Kydd AC, Shapiro LM. Patent foramen ovale: anatomy, outcomes, and closure. Nat Rev Cardiol 2011;8:148–60. [DOI] [PubMed] [Google Scholar]
- [46].Tchirikov M, Schroder HJ, Hecher K. Ductus venosus shunting in the fetal venous circulation: regulatory mechanisms, diagnostic methods and medical importance. Ultrasound Obstet Gynecol 2006;27:452–61. [DOI] [PubMed] [Google Scholar]
- [47].Roberts VH, Rasanen JP, Novy MJ, et al. Restriction of placental vasculature in a non-human primate: a unique model to study placental plasticity. Placenta 2012;33:73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Thornburg KL, Bissonnette JM, Faber JJ. Absence of fetal placental waterfall phenomenon in chronically prepared fetal lambs. Am J Physiol 1976;230:886–92. [DOI] [PubMed] [Google Scholar]
- [49].Giraud GD, Faber JJ, Jonker SS, Davis L, Anderson DF. Effects of intravascular infusions of plasma on placental and systemic blood flow in fetal sheep. Am J Physiol Heart Circ Physiol 2006;291:H2884–8. [DOI] [PubMed] [Google Scholar]
- [50].Anderson DF, Faber JJ. Regulation of fetal placental blood flow in the lamb. Am J Physiol 1984;247:R567–74. [DOI] [PubMed] [Google Scholar]
- [51].Faber JJ, Anderson DF, Louey S, Thornburg KL, Giraud GD. Insignificant response of the fetal placental circulation to arterial hypotension in sheep. J Appl Physiol 2011; 111(4): 1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction 2009;138:895–902. [DOI] [PubMed] [Google Scholar]
- [53].Groenendijk BC, Stekelenburg-de Vos S, Vennemann P, Wladimiroff JW, Nieuwstadt FT, Lindken R, et al. The endothelin-1 pathway and the development of cardiovascular defects in the haemodynamically challenged chicken embryo. J Vasc Res 2008;45:54–68. [DOI] [PubMed] [Google Scholar]
- [54].Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res 1997;80:473–81. [DOI] [PubMed] [Google Scholar]
- [55].Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 2000;279:R1157–64. [DOI] [PubMed] [Google Scholar]
- [56].Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, Giraud GD. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol 2007;292:R913–9. [DOI] [PubMed] [Google Scholar]
- [57].Demir R, Kayisli UA, Seval Y, et al. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta 2004;25:560–72. [DOI] [PubMed] [Google Scholar]
- [58].Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta 2006;27:535–9. [DOI] [PubMed] [Google Scholar]
- [59].Charnock-Jones DS. Soluble flt-1 and the angiopoietins in the development and regulation of placental vasculature. J Anat 2002;200:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc Res 2011;89:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008;29:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta 2006;27:483–90. [DOI] [PubMed] [Google Scholar]
- [63].Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta 2000;21 Suppl A:S16–24. [DOI] [PubMed] [Google Scholar]
- [64].Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol 2003;162:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Giles WB, Trudinger BJ, Stevens D, Alexander G, Bradley L. Umbilical artery flow velocity waveform analysis in normal ovine pregnancy and after carunculectomy. J Dev Physiol 1989;11:135–8. [PubMed] [Google Scholar]
- [66].Owens JA, Falconer J, Robinson JS. Effect of restriction of placental growth on umbilical and uterine blood flows. Am J Physiol 1986;250:R427–34. [DOI] [PubMed] [Google Scholar]
- [67].Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J Dev Physiol 1979;1:379–98. [PubMed] [Google Scholar]
- [68].Trudinger BJ, Stevens D, Connelly A, et al. Umbilical artery flow velocity waveforms and placental resistance: the effects of embolization of the umbilical circulation. Am J Obstet Gynecol 1987;157:1443–8. [DOI] [PubMed] [Google Scholar]
- [69].Gagnon R, Challis J, Johnston L, Fraher L. Fetal endocrine responses to chronic placental embolization in the late- gestation ovine fetus. Am J Obstet Gynecol 1994;170:929–38. [DOI] [PubMed] [Google Scholar]
- [70].Gagnon R, Johnston L, Murotsuki J. Fetal placental embolization in the late-gestation ovine fetus: alterations in umbilical blood flow and fetal heart rate patterns. Am J Obstet Gynecol 1996;175:63–72. [DOI] [PubMed] [Google Scholar]
- [71].Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 1989;161:1055–60. [DOI] [PubMed] [Google Scholar]
- [72].Trudinger BJ, Giles WB, Cook CM, Bombardieri J, Collins L. Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. Br J Obstet Gynaecol 1985;92:23–30. [DOI] [PubMed] [Google Scholar]
- [73].Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol 1985;92:31–8. [DOI] [PubMed] [Google Scholar]
- [74].Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Hypoxic acidemia, hyperviscosity, and maternal hypertension do not affect the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 1990;163:1313–20. [DOI] [PubMed] [Google Scholar]
- [75].Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Acute hypoxemia does not affect the umbilical artery flow velocity waveform in fetal sheep. Obstet Gynecol 1990;75:590–3. [PubMed] [Google Scholar]
- [76].Trudinger BJ, Giles WB, Cook CM. Uteroplacental blood flow velocity-time waveforms in normal and complicated pregnancy. Br J Obstet Gynaecol 1985;92:39–45. [DOI] [PubMed] [Google Scholar]
- [77].Saunders HM, Burns PN, Needleman L, et al. Hemodynamic factors affecting uterine artery Doppler waveform pulsatility in sheep. J Ultrasound Med 1998;17:357–68. [DOI] [PubMed] [Google Scholar]
- [78].Muijsers GJ, Hasaart TH, van Huisseling H, de Haan J. The response of the umbilical artery pulsatility index in fetal sheep to acute and prolonged hypoxaemia and acidaemia induced by embolization of the uterine microcirculation. J Dev Physiol 1990;13:231–6. [PubMed] [Google Scholar]
- [79].Muijsers GJ, van Huisseling H, Hasaart TH. The effect of selective umbilical embolization on the common umbilical artery pulsatility index and umbilical vascular resistance in fetal sheep. J Dev Physiol 1991;15:259–67. [PubMed] [Google Scholar]
- [80].Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol 2007;580:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cock ML, Albuquerque CA, Joyce BJ, Hooper SB, Harding R. Effects of intrauterine growth restriction on lung liquid dynamics and lung development in fetal sheep. Am J Obstet Gynecol 2001;184:209–16. [DOI] [PubMed] [Google Scholar]
- [82].Cheung CY, Bogic L, Gagnon R, Harding R, Brace RA. Morphologic alterations in ovine placenta and fetal liver following induced severe placental insufficiency. J Soc Gynecol Investig 2004;11:521–8. [DOI] [PubMed] [Google Scholar]
- [83].Cock ML, Harding R. Renal and amniotic fluid responses to umbilicoplacental embolization for 20 days in fetal sheep. Am J Physiol 1997;273:R1094–102. [DOI] [PubMed] [Google Scholar]
- [84].Duncan JR, Cock ML, Harding R, Rees SM. Relation between damage to the placenta and the fetal brain after late-gestation placental embolization and fetal growth restriction in sheep. Am J Obstet Gynecol 2000;183:1013–22. [DOI] [PubMed] [Google Scholar]
- [85].Duncan JR, Cock ML, Loeliger M, Louey S, Harding R, Rees SM. Effects of exposure to chronic placental insufficiency on the postnatal brain and retina in sheep. J Neuropathol Exp Neurol 2004;63:1131–43. [DOI] [PubMed] [Google Scholar]
- [86].Mitchell EK, Louey S, Cock ML, Harding R, Black MJ. Nephron endowment and filtration surface area in the kidney after growth restriction of fetal sheep. Pediatr Res 2004;55:769–73. [DOI] [PubMed] [Google Scholar]
- [87].Loeliger M, Duncan J, Louey S, Cock M, Harding R, Rees S. Fetal growth restriction induced by chronic placental insufficiency has long-term effects on the retina but not the optic nerve. Invest Ophthalmol Vis Sci 2005;46:3300–8. [DOI] [PubMed] [Google Scholar]
- [88].Louey S, Cock ML, Harding R. Long term consequences of low birthweight on postnatal growth, adiposity and brain weight at maturity in sheep. J Reprod Dev 2005;51:59–68. [DOI] [PubMed] [Google Scholar]
- [89].Maritz GS, Cock ML, Louey S, Suzuki K, Harding R. Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr Res 2004;55:287–95. [DOI] [PubMed] [Google Scholar]
- [90].Paulick RP, Meyers RL, Rudolph AM. Vascular responses of umbilical-placental circulation to vasodilators in fetal lambs. Am J Physiol 1991;261:H9–14. [DOI] [PubMed] [Google Scholar]
- [91].Paulick RP, Meyers RL, Rudolph CD, Rudolph AM. Umbilical and hepatic venous responses to circulating vasoconstrictive hormones in fetal lamb. Am J Physiol 1991;260:H1205–13. [DOI] [PubMed] [Google Scholar]
- [92].Adamson SL, Morrow RJ, Bull SB, Langille BL. Vasomotor responses of the umbilical circulation in fetal sheep. Am J Physiol 1989;256:R1056–62. [DOI] [PubMed] [Google Scholar]
- [93].Bertrand C, St-Louis J. Reactivities to serotonin and histamine in umbilical and placental vessels during the third trimester after normotensive pregnancies and pregnancies complicated by preeclampsia. Am J Obstet Gynecol 1999;180:650–9. [DOI] [PubMed] [Google Scholar]
- [94].Clark KE, Irion GL, Mack CE. Differential responses of uterine and umbilical vasculatures to angiotensin II and norepinephrine. Am J Physiol 1990;259:H197–203. [DOI] [PubMed] [Google Scholar]
- [95].Kossenjans W, Eis A, Sahay R, Brockman D, Myatt L. Role of peroxynitrite in altered fetal-placental vascular reactivity in diabetes or preeclampsia. Am J Physiol Heart Circ Physiol 2000;278:H1311–9. [DOI] [PubMed] [Google Scholar]
- [96].Mombouli JV, Le SQ, Wasserstrum N, Vanhoutte PM. Endothelins 1 and 3 and big endothelin-1 contract isolated human placental veins. J Cardiovasc Pharmacol 1993;22 (Suppl 8):S278–81. [DOI] [PubMed] [Google Scholar]
- [97].Rankin JH, Phernetton TM, Anderson DF, Berssenbrugge AD. Effect of prostaglandin I2 on ovine placental vasculature. J Dev Physiol 1979;1:151–60. [PubMed] [Google Scholar]
- [98].Adamson SL, Whiteley KJ, Langille BL. Endothelin-1 constricts fetoplacental microcirculation and decreases fetal O2 consumption in sheep. Am J Physiol 1996;270:H16–23. [DOI] [PubMed] [Google Scholar]
- [99].Altura BM, Malaviya D, Reich CF, Orkin LR. Effects of vasoactive agents on isolated human umbilical arteries and veins. Am J Physiol 1972;222:345–55. [DOI] [PubMed] [Google Scholar]
- [100].Santos-Silva AJ, Cairrao E, Marques B, Verde I. Regulation of human umbilical artery contractility by different serotonin and histamine receptors. Reprod Sci 2009;16:1175–85. [DOI] [PubMed] [Google Scholar]


