To the Editor:
The COVID-19 pandemic has caused over 33 million confirmed cases and over 1 million deaths by the end of September, 2020 (WHO. Coronavirus disease (COVID-19) pandemic, https://www.who.int/emergencies/diseases/novel-coronavirus-2019). A lack of effective therapies has stimulated interest in repurposing established drugs for the treatment of SARS-CoV-2 viral infection as well as symptoms of COVID-19.
Imatinib, a BCR-ABL1 tyrosine kinase inhibitor (TKI) used to treat chronic myeloid leukemia (CML), was reported to inhibit infection of SARS-CoV, the coronavirus responsible for the 2003 SARS outbreak, and MERS-CoV, the cause of Middle East Respiratory Syndrome, in cell culture assays [1–3]. Mechanistic studies suggested that SARS-CoV relies on ABL2 kinase activity to infect host cells and that inhibiting ABL2 with imatinib blocks coronavirus entry via preventing viral fusion with the cell membrane [1, 2]. Since the SARS-CoV-2 genome is ~80% homologous to that of SARS-CoV [4] and both viruses use host cell ACE2 protein as receptors [5], it is plausible that imatinib also has anti-SARS-CoV-2 activity. Moreover, imatinib was identified as a leading hit from a large-scale drug screening for SARS-CoV-2 protease inhibitors using the Reframe library of clinical and near-clinical compounds [6]. These data suggest that imatinib may have anti-SARS-CoV-2 activity, either on-target through inhibition of ABL1/2 or off-target through a previously unrecognized protease-inhibiting effect. Although the precise mechanism remains unclear, these data provide a rationale for testing imatinib as an antiviral against COVID-19 in clinical trials. Three prospective randomized clinical trials are underway to study the therapeutic efficacy of imatinib vs. standard of care or placebo in patients with COVID-19, including NCT04357613 (France), NCT04394416 (USA), and EudraCT2020-001236-10 (The Netherlands). However, the anti-SARS-CoV-2 efficacy of imatinib in a standard viral replication assay has not been demonstrated.
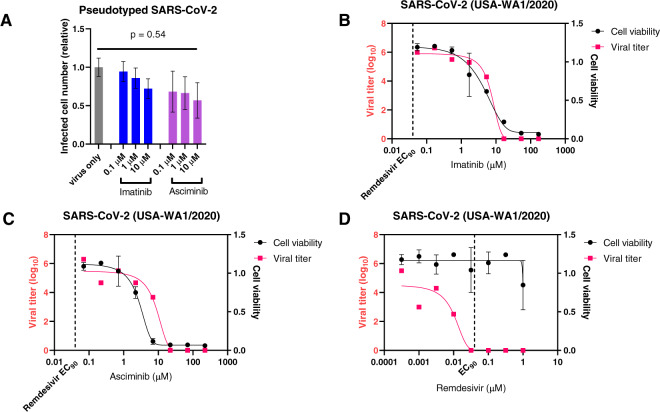
We tested the effects of imatinib and asciminib, a highly specific and potent ABL inhibitor binding to the myristate pocket of the kinase domain [7], on SARS-CoV-2 infection and replication in the naturally susceptible ACE2+ human Caco-2 cells [5]. Using pseudotyped virus with SARS-CoV-2 S protein as the envelope proteins, we first analyzed single-round viral entry/infection. Imatinib did not show an inhibitory effect on SARS-CoV-2 entry/infection at concentrations up to 10 μM, while asciminib showed a mild effect that is not statistically significant by ANOVA analysis (Fig. 1a). We then performed the standard viral replication assay using the USA-WA1/2020 strain of SARS-CoV-2 to test imatinib and asciminib in a blinded fashion, including remdesivir as a positive control. Neither imatinib nor asciminib demonstrated in vitro activity toward SARS-CoV-2 replication (Fig. 1b, c), while remdesivir exhibited potent inhibition of SARS-CoV-2 replication within the non-toxic concentration range (Fig. 1d). Therefore, our data indicate that, within clinically achievable dose ranges, imatinib and asciminib have no significant effect on SARS-CoV-2 infection and replication. This is in accordance with the reported low potency of imatinib against SARS-CoV, with EC50 at 9.82 μM in Vero E6 cell cultures [3], which is also unachievable with standard imatinib dosage (400 or 800 mg/day).
Fig. 1. SARS-CoV-2 entry/infection and replication assays on imatinib and asciminib.
a Single-round virus entry/infection assay was performed in Caco-2 cells using pseudotyped SARS-Cov-2 virus with S proteins as the envelope. Cells were pre-treated with drugs for 1 h before adding the virus. Infected cells (GFP+) were quantified by flow cytometry after 48 h. b–d Virus replication assay were performed in Caco-2 cells with USA-WA1/2020 strain of SARS-CoV-2. Drugs were added to 5 wells of a 96-well plate with 60–80% confluency. Three wells of each dilution were infected with virus, and two wells remained uninfected as toxicity controls. SARS-CoV-2 was added to achieve a multiplicity of infection (MOI) of ~0.002, and supernatant viral titer was quantified after 3 days by a standard endpoint dilution CCID50 assay. Remdesivir was tested in parallel as a positive control.
Conflicting data have been published regarding the incidence of COVID-19 in CML patients. A retrospective Chinese study reported a higher incidence of infection in CML patients than in the general public. In contrast, a prospective study from the Netherlands and a survey by the Italian CML network showed very low incidences, and suggested a potential protective effect of TKIs [8–10]. Population-based studies and comprehensive viral testing will be required to resolve these discrepancies.
Even though we find no evidence that imatinib is an antiviral drug, it may still hold promise for the treatment of COVID-19. Similar to SARS and septic acute lung injury, lethal COVID-19 is mainly manifested as acute respiratory distress syndrome caused by overt immune activation and inflammatory cytokine storm [11]. Imatinib has immune-modulatory effects [12, 13], and on-target inhibition of ABL1/2 was shown to mitigate acute lung injuries in various pre-clinical models [14]. Imatinib mediated inhibition of cytokine receptor signaling (PDGFR, c-Kit and CSF1R) may also reduce cytokine-induced inflammatory response and tissue injury. Therefore, it is possible that imatinib will show clinical benefit for COVID-19 because of its immune-modulatory effects. Indeed, rapid improvement of pulmonary infiltrates was reported in a patient with COVID-19 treated with imatinib [15]. These data support additional prospective investigations into the potential beneficial effect of imatinib and possibly other ABL inhibitors on COVID-19, but provide insufficient evidence for the off-label use of imatinib in patients with COVID-19. Our data indicate that any beneficial effects should not be attributed to direct antiviral activity.
Acknowledgements
The author would like to thank Thomas O’Hare for valuable feedback on the manuscript. This work was supported in part by NCI CCSG grant P30CA42014-31 to the Huntsman Cancer Institute and NIH grant R01CA178397 to Thomas O’Hare and MWD.
Compliance with ethical standards
Conflict of interest
MWD is an advisory board member of Blueprint, Takeda, Incyte, and Sangamo, is a consultant for Blueprint, Fusion Pharma, Medscape, Novartis, Sangamo and DisperSol, and receives research funding from Blueprint, Takeda, Novartis, Incyte, SPARC, Leukemia & Lymphoma Society, and Pfizer.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sisk JM, Frieman MB, Machamer CE. Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J Gen Virol. 2018;99:619–30. doi: 10.1099/jgv.0.001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman CM, Sisk JM, Mingo RM, Nelson EA, White JM, Frieman MB. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and middle east respiratory syndrome coronavirus fusion. J Virol. 2016;90:8924–33. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–93. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janes J, Young ME, Chen E, Rogers NH, Burgstaller-Muehlbacher S, Hughes LD, et al. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc Natl Acad Sci USA. 2018;115:10750–5. doi: 10.1073/pnas.1810137115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. 2018;61:8120–35. doi: 10.1021/acs.jmedchem.8b01040. [DOI] [PubMed] [Google Scholar]
- 8.Ector G, Huijskens EGW, Blijlevens NMA, Westerweel PE. Prevalence of COVID-19 diagnosis in Dutch CML patients during the 2020 SARS-CoV2 pandemic. A prospective cohort study. Leukemia. 2020;34:2533–5. doi: 10.1038/s41375-020-0964-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breccia M, Abruzzese E, Bocchia M, Bonifacio M, Castagnetti F, Fava C, et al. Chronic myeloid leukemia management at the time of the COVID-19 pandemic in Italy. A campus CML survey. Leukemia. 2020;34:2260–1. doi: 10.1038/s41375-020-0904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Wang D, Guo J, Yuan G, Yang Z, Gale RP, et al. COVID-19 in persons with chronic myeloid leukaemia. Leukemia. 2020;34:1799–804. doi: 10.1038/s41375-020-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov. 2010;9:956–70. doi: 10.1038/nrd3297. [DOI] [PubMed] [Google Scholar]
- 13.Mohty M, Blaise D, Olive D, Gaugler B. Imatinib: the narrow line between immune tolerance and activation. Trends Mol Med. 2005;11:397–402. doi: 10.1016/j.molmed.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo AN, Aman J, van Nieuw Amerongen GP, Dudek SM. Targeting Abl kinases to regulate vascular leak during sepsis and acute respiratory distress syndrome. Arterioscler Thromb Vasc Biol. 2015;35:1071–9. doi: 10.1161/ATVBAHA.115.305085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales-Ortega A, Bernal-Bello D, Llarena-Barroso C, Frutos-Perez B, Duarte-Millan MA, Garcia de Viedma-Garcia V, et al. Imatinib for COVID-19: a case report. Clin Immunol. 2020;218:108518. doi: 10.1016/j.clim.2020.108518. [DOI] [PMC free article] [PubMed] [Google Scholar]