Abstract
Background & Aims
Functional bowel disorders (FBDs) are the most common gastrointestinal problems managed by physicians. We aimed to assess the burden of chronic symptomatic FBDs on ambulatory care delivery in the United States and evaluate patterns of treatment.
Methods
Data from the National Ambulatory Medical Care Survey were used to estimate annual rates and associated costs of ambulatory visits for symptomatic irritable bowel syndrome, chronic functional abdominal pain, constipation, or diarrhea. The weighted proportion of visits associated with pharmacologic and nonpharmacologic (stress/mental health, exercise, diet counseling) interventions were calculated, and predictors of treatment strategy were evaluated in multivariable multinomial logistic regression.
Results
From 2007–2015, approximately 36.9 million (95% CI, 31.4–42.4) weighted visits in patients of non-federally employed physicians for chronic symptomatic FBDs were sampled. There was an annual weighted average of 2.7 million (95% CI, 2.3–3.2) visits for symptomatic irritable bowel syndrome/chronic abdominal pain, 1.0 million (95% CI, 0.8–1.2) visits for chronic constipation, and 0.7 million (95% CI, 0.5–0.8) visits for chronic diarrhea. Pharmacologic therapies were prescribed in 49.7% (95% CI, 44.7–54.8) of visits compared to nonpharmacologic interventions in 19.8% (95% CI, 16.0–24.2) of visits (P < .001). Combination treatment strategies were more likely to be implemented by primary care physicians and in patients with depression or obesity. The direct annual cost of ambulatory clinic visits alone for chronic symptomatic FBDs is approximately US$358 million (95% CI, 233–482 million).
Conclusions
The management of chronic symptomatic FBDs is associated with considerable health care resource use and cost. There may be an opportunity to improve comprehensive FBD management because fewer than 1 in 5 ambulatory visits include nonpharmacologic treatment strategies.
Keywords: Abdominal Pain, Constipation, Cost, Diarrhea
Abbreviations used in this paper: aOR, adjusted odds ratio; CAM, complementary and alternative medicine; CI, confidence interval; E/M, evaluation and management; FBD, functional bowel disorder; GI, gastrointestinal; IBS, irritable bowel syndrome; ICD, International Classification of Diseases; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10, International Classification of Diseases, 10th Revision; NAMCS, National Ambulatory Medical Care Survey; NCHS, National Center for Health Statistics; OR, odds ratio
What You Need to Know.
Background and Context
Functional bowel disorders (FBDs) including irritable bowel syndrome, chronic abdominal pain, and functional constipation or diarrhea are the most common GI conditions in the United States. Treatment for chronic symptomatic FBDs has changed over time and optimal strategies include both pharmacologic and non-pharmacologic interventions.
New Findings
We analyzed ∼36 million weighted visits from the National Ambulatory Care Survey: over 4 million visits for chronic symptomatic FBDs occur annually in the US, accounting for at least $350 million in clinic visit costs alone. ∼50% of patients received pharmacotherapy, but <20% of visits were associated with non-pharmacologic interventions.
Limitations
This is an analysis of cross-sectional, administrative data but is nationally representative. Longitudinal evaluation of treatment outcomes and downstream healthcare utilization cannot be assessed.
Impact
These findings highlight an opportunity to improve comprehensive FBD care in the US. We identify specific factors associated with combined pharmacologic and non-pharmacologic therapy, including care provided by primary care practitioners and among patients with comorbidities.
The functional bowel disorders (FBDs) represent a spectrum of chronic gastrointestinal (GI) conditions stemming from dysregulation of the gut-brain axis and are mediated by a complex interplay of abnormalities in gut motility, mucosal and immune function, intestinal microbiota diversity, central nervous system processing, and visceral hypersensitivity.1 The term FBD captures several conditions, and the Rome IV criteria recognize 5 categories of FBDs: irritable bowel syndrome (IBS), functional constipation, functional diarrhea, abdominal bloating/distention, and unspecified FBD.2 Importantly, all FBDs are characterized by chronic symptoms and absence of other explanatory anatomic or physiologic abnormalities. Over the past few decades, a conceptual framework for FBDs has evolved within an inclusive biopsychosocial model: this has resulted in advancements in both our understanding of the underlying pathophysiology and improved treatment options.3, 4, 5 Nonetheless, FBDs remain the most common and often most challenging GI conditions to treat.6, 7, 8
Although the management of FBDs should be individualized for each patient, key tenets of treatment include establishing a strong therapeutic physician-patient relationship and comprehensively addressing the underlying cause of symptoms through both pharmacologic and nonpharmacologic approaches. For many patients, lifestyle changes represent a cornerstone in their treatment strategy. Several interventions have been studied in patients with chronic symptomatic FBDs, including increasing dietary fiber intake; limiting fermentable oligo-, di-, monosaccharides and polyols; improving sleep hygiene; treating concomitant depression or anxiety; and increasing physical activity. Psychological interventions such as formal counseling regarding stress reduction, biofeedback techniques, cognitive behavioral or dynamic psychotherapy, and mind-body-breath interventions such as integrated yoga have also been studied.9, 10, 11, 12 For patients with moderate to severe symptoms that impair quality of life, pharmacologic agents are often used adjunctively. Recently, several classes of advanced treatments have been shown to be effective and obtained approval for FBD indications in the United States, including selective chloride channel activators, guanylate cyclase C agonists, nonabsorbable antibiotics, peripheral μ-opioid receptor agonists, and high-affinity 5-hydroxytryptamine receptor-4 agonists.13 , 14
As the number of treatment options in the therapeutic armamentarium for FBDs increases, so does the complexity of managing these conditions in day-to-day practice. Most patients with FBDs are seen in the ambulatory outpatient setting, where tight time constraints and pressures to increase high-throughput efficiency are potential barriers to treating complex, multifactorial diseases. A detailed evaluation of current treatment practices and patterns for FBDs is therefore needed to identify potential therapeutic gaps and areas where the delivery and quality of comprehensive care can be improved. Furthermore, the impact of changes in treatment on the epidemiologic burden of FBDs has not been well studied. Although functional disorders are known to be the most common GI problem presenting for medical attention, estimates of the prevalence and cost associated with managing FBDs vary widely, depending on the study design, cohort definitions, geographic region, and sampling timeframe.15, 16, 17, 18 Understanding trends in health care resource use for FBDs will be critical for informing future resource allocation.
Therefore, we aimed to determine nationally representative and generalizable rates of ambulatory care use, estimate costs for outpatient clinic visits, and evaluate patterns of pharmacologic and nonpharmacologic treatment for FBDs in the United States.
Methods
Study Design and Data Source
We analyzed data collected in the National Ambulatory Medical Care Survey (NAMCS). NAMCS is a national, cross-sectional survey of non–federally employed office-based physicians primarily engaged in patient care, administered by the National Center for Health Statistics (NCHS). Visits are sampled using a 3-stage clustered probability sampling design, based on geographic region, physician specialty, and visits within individual physician practices. Data collection from a systematic random sample of visits to each clinic occurs over a 1-week reporting period and is completed by using a standardized patient record form. Patient record forms may be completed by physicians, medical office personnel, or by trained Census Bureau staff based on the medical chart. NAMCS collects data on patient demographics, reasons for the visit (up to 3), physician diagnoses coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), investigations ordered, and both pharmacologic and nonpharmacologic interventions. Medications for each patient include those that were ordered, supplied, administered, or continued during the visit. Data from NAMCS between 2007 and 2015 were pooled to add power to our analyses.
Study Population
The study population of interest was adult patients (≥18 years) presenting to ambulatory outpatient clinics with (1) a provider diagnosis of a chronic FBD and (2) active, chronic GI symptoms. To identify relevant encounters, we applied (1) provider diagnostic codes used previously in analyses of NAMCS data and (2) patient-reported symptom codes.15 , 19 Visits with a provider diagnosis of IBS or chronic abdominal pain (ICD-9-CM: 789.0, 564.1), constipation (564.0), or diarrhea (564.5, 787.91) were selected. Subsequently, we identified chronically symptomatic patients using a corresponding GI complaint as the reason for visit (abdominal pain: 15450, 15451, 15452, 15453; abdominal distention/bloating: 15651; constipation: 15900; diarrhea: 15950). Patients were categorized as having either (1) IBS/chronic abdominal pain (2) chronic constipation or (3) chronic diarrhea. If multiple diagnoses were coded (for example, a patient with IBS as the primary diagnosis and chronic constipation as a secondary diagnosis), we assigned the category by the highest-ranking diagnostic position (in this example, IBS). Recognizing that the ICD-9-CM diagnostic coding for FBDs lacks specificity, we excluded patients with established GI pathology (abdominal hernias, colorectal cancer, diverticular disease, Crohn’s disease, ulcerative colitis, cholelithiasis, pancreatitis, appendicitis, and celiac disease). Both new consultations and follow-up visits for chronic GI symptoms were included. However, to further improve the specificity of our study population for chronic FBDs, we excluded all visits for patients presenting with a new GI complaint (<3 months) because some of these patients may be diagnosed with nonfunctional pathology on future investigations that would not be captured in a cross-sectional study design. Furthermore, the diagnostic criteria for FBD requires chronicity in symptoms. To ensure the consistency of our findings, we conducted a sensitivity analysis including patients with a provider diagnosis of FBD but without active coded GI symptomatology at the time of the visit.
Outcomes and Covariables
The primary outcome of interest in our study population was the treatment intervention, categorized as medications alone, nonpharmacologic intervention alone, combination pharmacologic and nonpharmacologic treatment, and no therapy. Medications (up to 8) are recorded and classified in NAMCS by using the Lexicon Plus Cerner Multum, Inc, database (North Kansas City, MO) (Supplementary Table 1). We considered the following classes of relevant therapies for chronic symptomatic FBDs: laxatives, antidiarrheal medications, tricyclic antidepressants, selective serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, benzodiazepines, anticholinergic antispasmodics, probiotics, rifaximin, nonsteroidal anti-inflammatory drugs, and opioid analgesics. Bile acid binders were considered as antidiarrheal medications; guanylate cyclase C agonists and chloride channel activators were categorized as laxatives. Rifaximin, a semisynthetic rifamycin-based nonsystemic antibiotic, is classified in the Multum database as a miscellaneous antibiotic; therefore, we used the NCHS-assigned 5-digit drug code to identify rifaximin use. Treatments for FBDs approved for use after 2015 (eg, eluxadoline, plecanatide, and prucalopride) were not included.
Nonpharmacologic interventions of interest included (1) dietary and nutrition counseling, (2) exercise (including physical therapy) or weight reduction counseling, (3) stress reduction and mental health counseling (including psychotherapy), and (4) complementary and alternative medicine (CAM). Detailed descriptions of each intervention are provided in Supplementary Table 1. Briefly, diet/nutrition counseling included providing patient education relating to consumed foods and beverages, dietary restrictions/guidelines, or a referral to a dietician or nutritionist. Exercise and weight reduction counseling included covering topics relating to the patient’s physical condition or fitness and included referrals to health and fitness professionals. Mental health counseling included provision of advice about psychological issues, stress reduction, biofeedback, or yoga. CAM described interventions such as acupuncture, chiropractic, homeopathy, massage, or herbal therapies. Given that weight reduction counseling alone may not be considered an appropriate treatment for FBDs, we conducted a sensitivity analysis excluding weight reduction as a nonpharmacologic intervention. If the patient was seen by a specialist by referral, recommendations provided specifically for the primary care provider may not be captured if they were not also discussed with the patient.
Health care resource use was evaluated as a secondary endpoint. We determined the visit duration for each encounter and requirement for additional investigations, including cross-sectional imaging by computed tomography or magnetic resonance imaging, ultrasonography, and sigmoidoscopy or colonoscopy. Direct costs associated with ambulatory care visits were estimated based on the type of visit (new consult vs follow-up) and length of time coded for each encounter to assign an evaluation and management (E/M) Current Procedural Code. The cost per encounter was calculated by using the Medicare National Payment Amount for physician services in 2015 using the assigned E/M codes. Total costs were converted to 2020 US dollars using the physician’s services component of the consumer price index.
Other covariables of interest included patient demographic characteristics such as age, sex, race (white, black, and other), number of past medical visits in the preceding 12 months to the same provider, and comorbidities (including depression, obesity, cancer, and diabetes). Visit-related characteristics were also captured, including primary method of payment (private insurance, Medicare/Medicaid, and other [no pay, worker’s compensation, and self-pay]), geographic US census region (Northeast, Midwest, South, and West), specialty of the physician seen (primary care vs medical specialist (primarily internal medicine specialties), metropolitan vs nonmetropolitan area (as defined by the US Bureau of the Census and the US Office of Management and Budget), and practice setting (private practice vs other).
Statistical Analysis
NAMCS data are derived from a complex clustered probability–stratified sample, with deliberate oversampling of certain subgroups. Therefore, to ensure that estimates are representative of the national population, sampling weights were applied in a multistage estimation procedure as recommended by the NCHS. These sampling weights adjust for sampling probability, survey nonresponse, ratio adjustment within specialty groups, and weight smoothing to ensure that an individual provider does not overcontribute to the total sample. Sampling weights were applied for all analyses and to extrapolate unweighted direct observations to weighted estimates reflective of the national population. Baseline demographic characteristics are presented by using summary statistics with 95% confidence intervals (CIs). Results based on fewer than 30 unweighted sample visits per subgroup or with a relative standard error of >30% were suppressed to ensure adequate precision of national-level estimates, in accordance with NCHS standards. Variance estimates were made by using the Taylor linearization method to reflect the survey design.
The estimated annual number of weighted ambulatory visits for symptomatic, chronic FBDs was determined, and the rate of visits per 100,000 US population was calculated by using adult population denominators from the US Census Bureau age and sex tables. To test temporal trends in ambulatory clinic visits, we divided the data set into 3 time periods (2007–2009, 2010–2012, and 2013–2015) and used logistic regression with the time period treated as a linear predictor. The proportions of visits resulting in pharmacologic vs nonpharmacologic interventions and changes in demographic characteristics over time were assessed by using the design-adjusted Pearson chi-square test. Predictors of each treatment strategy were evaluated by using multivariable multinomial logistic regression. The base outcome reference category was set as no treatment; all point estimates for relative risk compared to the base outcome were expressed as adjusted odds ratios (ORs) with 95% CI, controlling for patient demographic, practice, and clinical characteristics that were chosen a priori. P values less than .05 were considered statistically significant.
All analyses were performed with Stata, version 14.2 (StataCorp, College Station, TX). Institutional review board ethics approval was not required because all data used in this analysis are deidentified and publicly available.
Results
Study Population
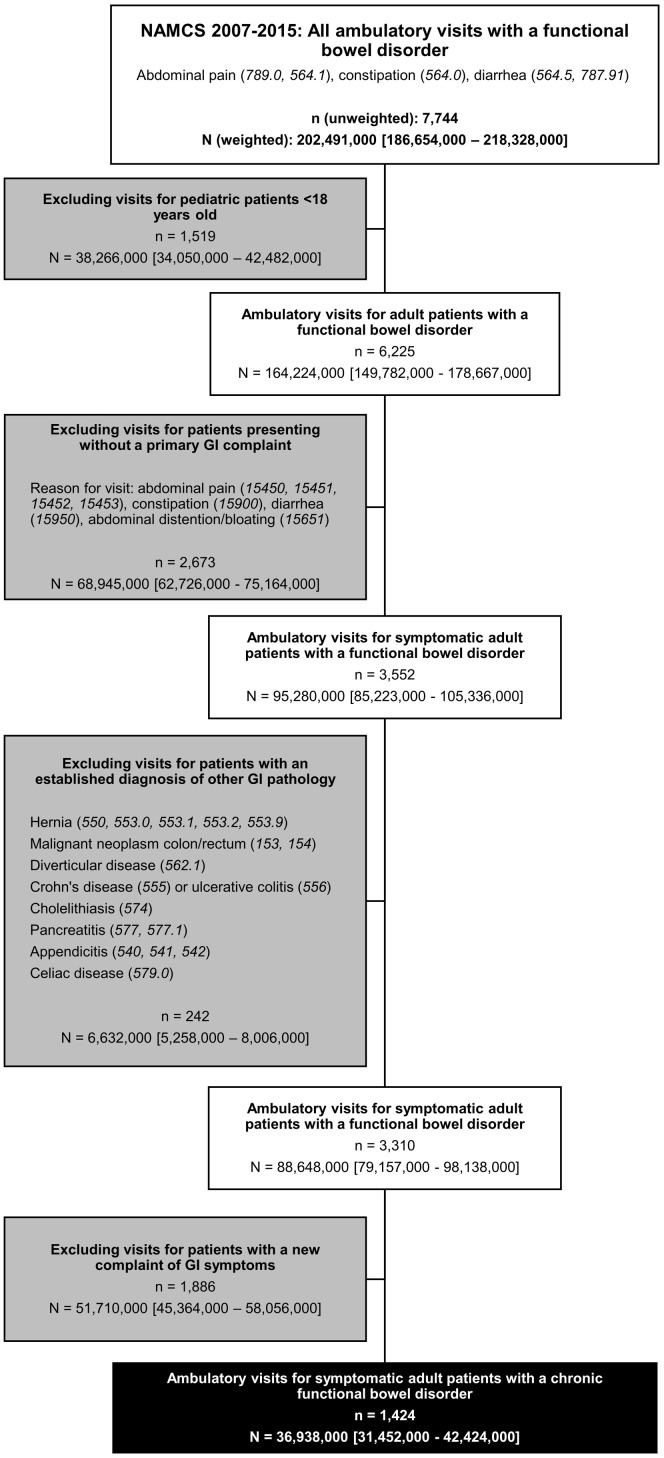
From 2007 to 2015, a total of 361,146 unweighted outpatient visits were sampled, representing approximately 8.7 billion weighted office visits. Of these, a total of 7744 unweighted observations representing an estimated 202 million (95% CI, 187–218 million) weighted ambulatory visits were for IBS/abdominal pain, constipation, or diarrhea. After excluding pediatric visits, encounters without an active GI complaint as the reason for visit, visits for patients with established GI pathology, and visits for new GI symptoms, our study population included 1424 unweighted observations, representing approximately 36.9 million (95% CI, 31.5–42.4 million) weighted ambulatory visits (Figure 1 ).
Figure 1.
Identification of the study population of symptomatic adult patients with a chronic functional bowel disorder. n represents unweighted observations based on direct visit sampling; N represents weighted estimates after applying survey sample weights.
Epidemiologic Burden of Chronic Symptomatic Functional Bowel Disorders
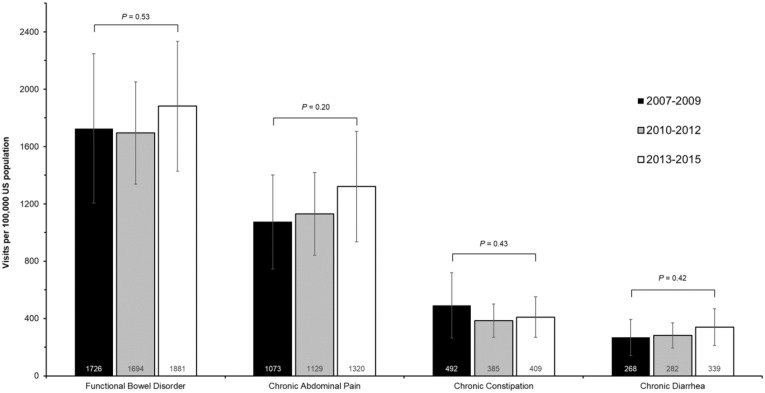
On average, there were approximately 4.0 million (95% CI, 3.5–4.7 million) annual visits for chronic symptomatic FBDs; most of these visits were for IBS or chronic abdominal pain (annual average, 2.7 million visits ; 95% CI, 2.3–3.2 million). Rates of ambulatory visits per 100,000 US population were 1181 (95% CI, 983–1379) visits for chronic abdominal pain, 429 (95% CI, 332–526) for chronic constipation, and 298 (95% CI, 231–364) for chronic diarrhea. Overall, ambulatory visits for FBDs were stable over time (test for trend P values, .20–.53) (Supplementary Figure 1).
Supplementary Figure 1.
Average annual rates per 100,000 US population of adult ambulatory clinic visits for chronic, symptomatic FBDs over time (2007–2015). Error bars represent 95% CIs.
The demographic characteristics of patients presenting to ambulatory care for chronic symptomatic FBDs are summarized in Table 1 . Approximately two thirds of visits involved women (66.8% ; 95% CI, 62.1–71.1%), and 40.7% (95% CI, 35.8–45.8) of patients had more than 2 other comorbid conditions, including 1 in 7 patients with depression (14.2%; 95% CI, 11.7–17.1). Both primary care (47.0%; 95% CI, 39.8–54.4) and medical care (45.4%; 95% CI, 37.5–53.5) specialists were heavily involved in the management of patients with chronic symptomatic FBDs. The demographic distribution of patients presenting for ambulatory care was stable over time, with no significant changes in patient age, sex, race, primary method of payment, or comorbidity burden.
Table 1.
Survey-Weighted Baseline Patient Demographic Characteristics From Sampled Ambulatory Visits for Chronic, Symptomatic FBDs in the NAMCS, 2007–2015
| Characteristics | All Chronic Functional Bowel Disorders (95% CI) | IBS or Chronic Abdominal Pain (95% CI) | Chronic Constipation (95% CI) | Chronic Diarrhea (95% CI) |
|---|---|---|---|---|
| Weighted sample visits, N | 36,938,000 (31,452,000–42,424,000) | 24,381,000 (20,304,000–28,458,000) | 8,930,000 (6,809,000–10,948,000) | 6,197,000 (4,824,000–7,570,000) |
| Average annual weighted visits, N | 4,104,000 (3,495,000–4,714,000) | 2,730,000 (2,273,000–3,187,000) | 992,000 (768,000–1,216,000) | 689,000 (536,000–841,000) |
| Age, y, % | ||||
| 18–39 | 18.3 (15.6–21.4) | 21.0 (17.3–25.3) | 17.9 (12.7–24.6) | 17.7 (11.6–25.9) |
| 40–59 | 35.5 (31.1–40.1) | 37.2 (31.7–43.1) | 26.6 (19.8–34.8) | 39.7 (30.4–49.8) |
| 60–79 | 35.6 (30.8–40.7) | 34.7 (28.4–41.5) | 40.4 (32.4–49.0) | 28.7 (21.4–37.3) |
| ≥80 | 10.6 (8.0–14.0) | 7.2 (4.6–11.0) | 15.1 (10.0–22.1) | a |
| Female sex, % | 66.8 (62.1–71.1) | 66.9 (60.6–72.7) | 70.0 (61.6–77.2) | 64.5 (54.7–73.2) |
| Race, % | ||||
| White | 84.4 (79.2–88.4) | 83.2 (76.3–88.4) | 85.5 (78.7–90.4) | 90.7 (83.0–95.1) |
| Black | 9.8 (7.3–13.1) | 9.6 (6.7–13.4) | 11.0 (6.8–17.2) | a |
| Other | 5.7 (3.0–10.2) | a | a | a |
| Current tobacco use, % | 15.0 (12.0–18.7) | 14.5 (10.8–19.2) | 12.8 (7.7–20.4) | 22.2 (14.3–32.8) |
| Primary payment method, % | ||||
| Private insurance | 52.0 (46.4–57.4) | 57.4 (51.1–63.4) | 44.5 (36.1–53.3) | 47.7 (37.8–57.7) |
| Medicare/Medicaid | 44.7 (39.5–49.9) | 39.7 (34.0–45.8) | 53.6 (44.9–62.1) | 44.3 (35.4–53.6) |
| Other | 3.3 (2.2–4.8) | 2.9 (1.9–4.5) | a | a |
| US region, % | ||||
| Northeast | 18.3 (13.7–24) | 20.1 (14.7–26.8) | 14.0 (8.7–21.8) | 14.8 (8.5–24.7) |
| Midwest | 19.0 (14.5–24.5) | 17.4 (12.6–23.6) | 22.8 (15.4–32.4) | 19.5 (12.5–29.2) |
| South | 40.1 (32.7–47.9) | 39.3 (31.3–47.9) | 47.5 (36.4–58.9) | 38.6 (28.8–49.3) |
| West | 22.5 (16.9–29.2) | 23.2 (16.5–31.6) | 15.7 (9.5–24.8) | 27.1 (17.7–39.1) |
| Physician specialty, % | ||||
| Primary care | 47.0 (39.8–54.4) | 47.9 (39.5–56.4) | 44.2 (33.8–55.0) | 41.6 (31.3–52.7) |
| Medical care | 45.4 (37.5–53.5) | 44.3 (35.5–53.4) | 49.3 (37.9–60.8) | 52.2 (41.2–63.0) |
| Health care visits in past year, % | ||||
| 0 | 27.2 (22.2–32.8) | 27.1 (21.8–33.2) | 26.9 (18.7–37.0) | 27.7 (20.1–36.9) |
| 1–2 | 33.3 (28.1 – 39.0) | 36.7 (30.1–43.8) | 31.9 (25.2–39.5) | 23.7 (16.7–32.5) |
| 3–6 | 26.1 (22.2–30.4) | 23.4 (19.2–28.2) | 29.1 (21.8–37.6) | 33.1 (24.6–43.0) |
| ≥7 | 13.3 (10.1–17.4) | 12.8 (8.8–18.1) | 12.2 (8.0–18.1) | 15.1 (8.5–26.4) |
| Comorbidity burden, % | ||||
| 0 comorbidities | 35.4 (30.8–40.1) | 35.9 (30.4–41.7) | 36.4 (28.5–45.2) | 35.8 (27.4–45.3) |
| 1 comorbidity | 23.8 (19.5–28.7) | 25.8 (20.1–32.5) | 22.7 (16.3–30.5) | 20.7 (14.7–28.3) |
| ≥2 comorbidities | 40.7 (35.8–45.8) | 38.3 (32.3–44.8) | 40.9 (32.5–50.0) | 43.5 (33.5 – 54.0) |
| Selected comorbidities, % | ||||
| Depression | 14.2 (11.7–17.1) | 15.5 (12.2–19.5) | 12.2 (7.8–18.6) | 15.8 (9.5–25.0) |
| Obesity | 9.4 (7.2–12.1) | 9.0 (6.6–12.1) | 9.9 (5.8–16.3) | 12.4 (7.0 – 21.1) |
| Cancer | 7.5 (4.3–12.8) | a | a | a |
| Diabetes | 16.2 (12.5–20.9) | 13.1 (8.4 – 19.9) | 16.7 (11.1–24.2) | 24.7 (16.7–35.0) |
Weighted estimate suppressed because of <30 unweighted observations or relative standard error of >30% (unreliable estimate).
Treatment Patterns for Chronic Symptomatic Functional Bowel Disorders
Treatment patterns for chronic symptomatic FBDs are described in Table 2 . A significantly higher proportion of patients were managed with pharmacologic strategies compared to nonpharmacologic strategies (49.7% vs 19.8%; P < .001). These findings were consistent in a sensitivity analysis including patients with a provider diagnosis of FBD but without active symptoms recorded at the time of the clinic visit (48.8% vs 21.9%; P < .001). Pharmacologic treatments were more likely to be used in patients with chronic symptomatic FBDs who were seen in follow-up compared to new consultations (42.5% vs 26.7%; P = .01). There was no significant difference in nonpharmacologic management use according to private vs nonprivate practices (18.2% vs 21.3%; P = .32) or by metropolitan vs nonmetropolitan location (19.0% vs 25.3%; P = .46).
Table 2.
Treatment Patterns for Chronic Symptomatic FBDs in the United States
| Disorder, n (95% CI) | Weighted percentage of visits (95% CI) |
|||
|---|---|---|---|---|
| Medication only | Nonpharmacologic therapya only | Combined medication and nonpharmacologic therapy | No treatment | |
| All chronic functional bowel disorders 36,694,000 (31,233,000–42,156,000) |
39.3 (35.6–46.5) | 9.3 (7.0–12.4) | 10.5 (7.9–13.7) | 40.9 (35.6–46.5) |
| IBS or chronic abdominal pain 24,381,000 (20,304,000–28,458,000) |
36.7 (31.5–42.1) | 9.7 (7.1–13.3) | 9.2 (6.1–13.5) | 44.4 (37.8–51.3) |
| Chronic constipation 8,820,000 (6,807,000–10,832,000) |
47.4 (39.2–55.8) | b | 12.8 (8.2–19.4) | 29.4 (22.0–38.1) |
| Chronic diarrhea 6,165,000 (4,793,000–7,538,000) |
44.1 (33.4–55.3) | b | 12.1 (6.5–21.2) | 37.5 (27.8–48.3) |
Nonpharmacologic therapy includes dietary/nutrition counseling, exercise (including physical therapy) or weight reduction counseling, stress reduction and mental health counseling (including psychotherapy), and complementary and alternative medicine.
Weighted estimate suppressed because of <30 unweighted observations or relative standard error of >30% (unreliable estimate).
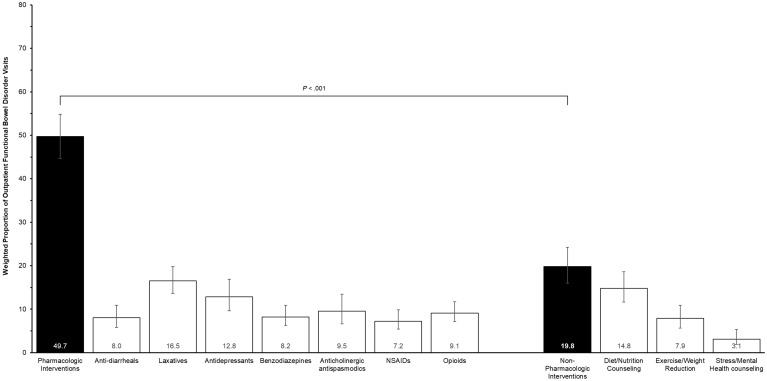
A total of 39.3% (95% CI, 35.6–46.5) of visits for chronic symptomatic FBDs were managed with pharmacologic treatment alone. The most commonly prescribed classes of pharmacologic therapy included laxatives (16.5%; 95% CI, 13.6–19.8], antidepressants (12.8%; 95% CI, 9.6–16.9), and anticholinergic antispasmodics (9.5%; 95% CI, 6.6–13.4) (Figure 2 ). Opioids were prescribed in 9.2% (95% CI, 7.1–11.7) of visits for analgesia. An insufficient number of unweighted observations for prescriptions of chloride channel activators, guanylate cyclase-C agonists, or rifaximin were available to produce reliable national estimates (<2.5%).
Figure 2.
Weighted proportions of pharmacologic and nonpharmacologic interventions in ambulatory outpatient visits for chronic, symptomatic functional bowel disorders. There were insufficient observations to provide reliable estimates of CAM use. NSAID, nonsteroidal anti-inflammatory drug.
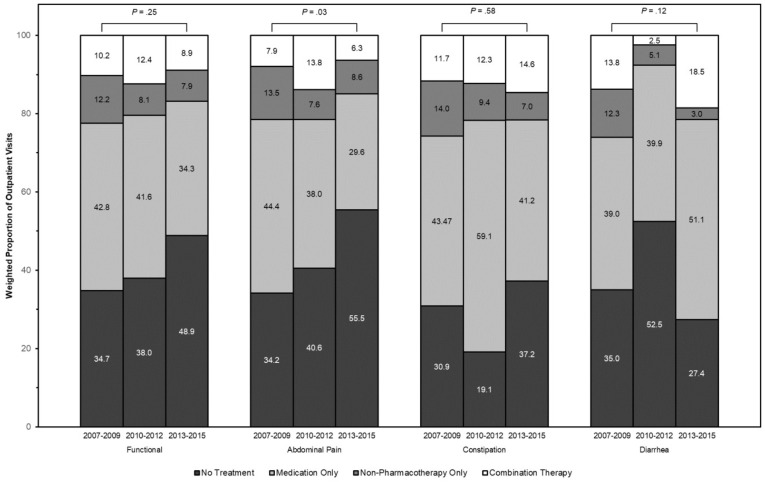
A total of 19.8% (95% CI, 16.0–24.2) of visits were managed with nonpharmacologic interventions, most commonly dietary and nutrition counseling (14.7%; 95% CI, 11.6–18.6]). In sensitivity analysis, 18.6% (95% CI, 14.9–23.1) of visits were managed with nonpharmacologic strategies excluding weight reduction. Only 3.2% of visits (95% CI, 1.9–5.3) involved formal stress reduction education or mental health counseling, and an insufficient number of visits for CAM were available to produce reliable estimates. Overall, rates of pharmacologic (P = .12) and nonpharmacologic (P = .27) interventions were stable over time, although a smaller proportion of patients with abdominal pain received medications alone in 2013–2015 (29.6%; 95% CI, 21.8–38.9) compared to 2007–2009 (44.4%; 95% CI, 34.7–54.6) (P = .03) (Supplementary Figure 2).
Supplementary Figure 2.
Weighted proportions of pharmacologic and nonpharmacologic interventions in ambulatory outpatient visits for chronic, symptomatic FBDs over time.
Predictors of treatment pattern for chronic symptomatic FBDs in multivariable multinomial logistic regression are summarized in Table 3 . Compared to visits managed without pharmacologic or nonpharmacologic treatment, medications were more likely to be used in those patients with depression (adjusted OR [aOR], 1.82; 95% CI, 1.06–3.11; P = .03) and those with Medicare/Medicaid (aOR, 1.70; 95% CI, 1.05–2.78; P = .03). Patients who had repeated health care visits in the preceding year also were more likely to be prescribed pharmacotherapy. A combined pharmacologic and nonpharmacologic approach was almost twice as likely to be used by primary care physicians compared to medical care specialists (aOR, 1.88; 95% CI, 1.00–3.55; P = .05), and there was evidence suggesting geographic variability in using a combination treatment approach (South region: aOR, 0.38; 95% CI, 0.17–0.87; P = .02 and Midwest region: aOR, 0.34; 95% CI, 0.15–0.78; P = .01, compared to the Northeast). Patients with obesity (aOR, 7.84; 95% CI, 3.14–19.61; P < .001) and depression (aOR, 4.33; 95% CI, 2.19–8.56; P < .001), but not diabetes, were also more likely to receive combined pharmacologic and nonpharmacologic therapy for chronic FBDs. This trend continued to be observed in sensitivity analyses excluding weight reduction as a nonpharmacologic intervention (aOR for obesity, 6.26; 95% CI, 2.52–15.57; P < .001).
Table 3.
Predictors of Treatment Pattern for Chronic, Symptomatic FBDs in Multivariable Multinomial Logistic Regression
| Predictor | Pharmacologic treatment only |
Nonpharmacologic treatment only |
Combined treatment |
|||
|---|---|---|---|---|---|---|
| aOR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Age, per decade | 0.99 (0.88–1.13) | .91 | 0.90 (0.73–1.10) | .30 | 0.94 (0.79–1.12) | .52 |
| Male sex | 0.72 (0.45–1.13) | .15 | 1.37 (0.73–2.57) | .32 | 1.21 (0.65–2.28) | .55 |
| Nonwhite race | 0.63 (0.33–1.24) | .18 | 0.73 (0.29–1.87) | .52 | 1.10 (0.43–2.86) | .84 |
| Payment method | ||||||
| Private insurance | — | — | — | — | — | — |
| Medicare/Medicaid | 1.70(1.05–2.78) | .03 | 1.99 (0.86–4.58) | .11 | 1.85 (0.94–3.62) | .07 |
| Other | 1.22 (0.41–3.64) | .72 | 1.50 (0.42–5.31) | .53 | 1.68 (0.54–5.24) | .37 |
| US geographic region | ||||||
| Northeast | — | — | — | — | — | — |
| Midwest | 1.37 (0.75–2.51) | .30 | 1.32 (0.47–3.67) | .60 | 0.34 (0.15–0.78) | .01 |
| South | 1.27 (0.75–2.16) | .37 | 0.82 (0.35–1.93) | .65 | 0.38 (0.17–0.87) | .02 |
| West | 0.94 (0.47–1.88) | .86 | 1.30 (0.46–3.64) | .62 | 0.52 (0.19–1.37) | .18 |
| Primary care physician | 0.86 (0.51–1.45) | .57 | 1.57 (0.68–3.61) | .29 | 1.88 (1.00–3.55) | .05 |
| Health care visits in past year | ||||||
| 0 | — | — | — | — | — | — |
| 1–2 | 1.05 (0.58–1.92) | .87 | 0.62 (0.24–1.60) | .32 | 0.76 (0.32–1.82) | .54 |
| 3–6 | 2.15 (1.04–4.42) | .04 | 1.11 (0.48–2.55) | .80 | 1.47 (0.60–3.58) | .40 |
| ≥7 | 3.82 (1.70–8.58) | .001 | 0.88 (0.26–2.95) | .84 | 2.25 (0.79–6.42) | .13 |
| Depression | 1.82 (1.06–3.11) | .03 | 3.04 (1.19–7.75) | .02 | 4.33 (2.19–8.56) | <.001 |
| Obesity | 1.37 (0.68–2.76) | .37 | 2.36 (0.79–7.01) | .12 | 7.84 (3.14–19.61) | <.001 |
| Diabetes | 0.85 (0.45–1.64) | .64 | 0.75 (0.25–2.23) | .61 | 0.94 (0.39–2.26) | .89 |
NOTE. All ORs represent effect sizes compared to the base outcome reference category (no treatment). Bold values are statistically significant (P<.05).
Health Care Resource Use
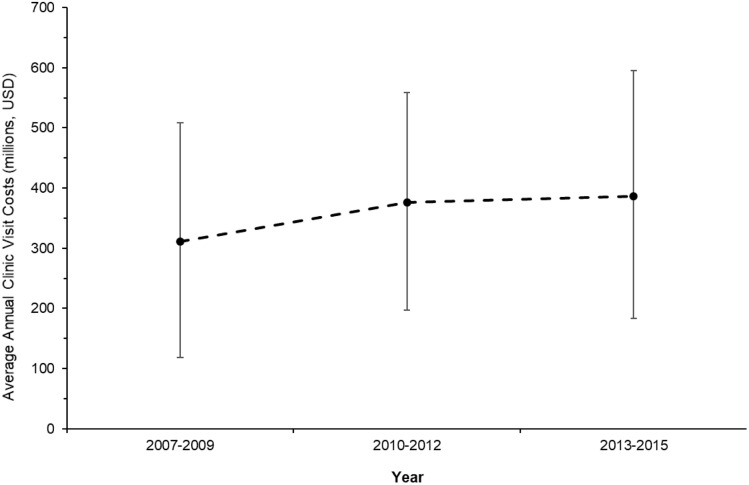
A total of 10.8% (95% CI, 8.0–14.3) of visits for chronic symptomatic FBDs generated subsequent cross-sectional imaging (computed tomography/magnetic resonance imaging), 5.8% (95% CI, 4.1–8.2) in ultrasonographic imaging, and 11.9% (95% CI, 9.6–14.7) in sigmoidoscopy or colonoscopy. The mean visit duration was 22.6 minutes (95% CI, 21.3–23.8), with approximately 1 in 7 visits lasting >30 minutes. Approximately 1 in 5 visits were new consultations for chronic GI symptoms. The total estimated average annual cost for ambulatory visits alone for chronic symptomatic FBDs is US$358 million (95% CI, 233–482 million) (approximately $84 million for new consultation visits and approximately $274 million for follow-up visits in established patients). The average annual costs over time are summarized in Supplementary Figure 3.
Supplementary Figure 3.
Average annual costs for adult ambulatory clinic visits for chronic, symptomatic FBDs over time (2007–2015). Error bars represent 95% CIs. USD, US dollars.
Discussion
Although FBDs are the most prevalent gastrointestinal condition diagnosed in the United States, they are frequently challenging to treat and negatively affect both patients and the health care system. In this analysis of 36 million weighted ambulatory visits among patients of non-federally employed physicians spanning nearly a decade, we determined contemporary, nationally representative estimates for the burden of FBDs on health care resource use in the United States. Conservatively, we estimate that more than 4 million visits occur each year for chronic symptomatic FBDs, and this is associated with a direct cost of approximately US$350 million per year for outpatient clinic visits alone (notwithstanding costs associated with other investigations, referrals, or medication and nonpharmacologic treatment). Despite this substantial economic burden and the high rate of repeated visits among symptomatic patients, we identify a potential gap in comprehensive FBD care because most patients in this study population did not receive nonpharmacologic treatment advice on diet, exercise, stress reduction, mental health counseling, or mind-body interventions. Furthermore, the likelihood of receiving a combined medication and nonpharmacologic approach for managing chronic symptomatic FBDs varies by provider, geographic region, and patient profile. Taken together, these findings highlight an opportunity to improve the quality of care for FBDs in the United States.
Our data show that patients with chronic symptomatic FBDs are predominantly treated with pharmacologic therapies. However, several considerations should be factored into the interpretation of these findings. Importantly, this was a cross-sectional analysis rather than a longitudinal cohort study. Therefore, it is possible that patients may have previously already tried and failed nonpharmacologic therapies, particularly among patients being referred to specialist care. However, we did not find a significant difference in treatment strategy use when we compared patients who were evaluated in new consultations vs follow-up visits. Second, we were unable adjust for patient preference for pharmacologic vs nonpharmacologic interventions in this type of survey study, and treatment decisions may have been dictated by complex patient-provider conversations in a shared decision-making model that is not easily captured with binary data points.
Nevertheless, nonpharmacologic interventions may be better suited for addressing the multifactorial biological, psychological, and social framework in which functional GI symptoms develop.4 We found that stress and mental health counseling were underused in this patient population, despite the established association with mood and somatization disorders.4 , 20 , 21 Central factors such as psychologic distress substantially affect patient-reported outcomes22 , 23 and are more predictive of impaired quality of life in patients with IBS than GI symptoms alone.24 A recent meta-analysis showed that centrally directed therapies such as cognitive-behavioral therapy, relaxation therapy, gut-directed hypnotherapy, and dynamic psychotherapy are safe and effective for the treatment of FBDs with a number needed to treat of 4–5.25 The benefits from these modalities are durable, and home-based/remote delivery of therapy has also been shown to be effective, a consideration that is particularly relevant given the current coronavirus pandemic.26, 27, 28, 29, 30
Although a combined pharmacologic and nonpharmacologic multidisciplinary approach has been suggested as the optimal method for treating chronic symptomatic FBDs,4 , 31 , 32 several factors likely contribute to the limited use of combination therapy. First, not all physicians will be comfortable recommending nonpharmacologic interventions. For example, psychologic therapies require specialized training, and although an experienced physician may have knowledge about effective dietary treatments, a skilled dietitian is likely more adept at identifying and managing nutritional deficiencies and orthorexia.4 , 28 , 30 Second, there may be extra costs associated with psychological counseling, mind-body interventions, and dietary approaches, particularly if these strategies are not covered by insurance plans or require a substantial copayment. Third, in addition to financial costs, lifestyle intervention resources are often not colocated with medical clinics requiring patient time for additional appointments and time away from work. Fourth, there may be less awareness of the effectiveness of nonpharmacologic interventions compared to medications for chronic symptomatic FBDs. This is compounded by the relative ease of either writing a prescription or taking a pill to relieve GI symptoms compared to the substantial time and effort that must be invested by both clinicians and patients to optimize lifestyle therapies.
We identified that primary care providers were more likely than medical specialists (predominantly internal medicine specialists) to prescribe combination nonpharmacologic and pharmacologic interventions, potentially reflecting increased time spent with patients, a more established long-term patient-physician relationship, better awareness of an individual patient’s complex biopsychosocial background, or better access or comfort with a multidisciplinary strategy.33 There is, of course, a selection bias toward more severe cases being referred to gastroenterologists, and it should be acknowledged that not all patients with chronic symptomatic FBDs necessarily require a multidisciplinary approach, particularly if the severity is low. However, using the number of medical visits in the past year to the same provider as a surrogate for disease severity, our findings suggest that nonpharmacologic treatments are underused even on the severe end of the FBD spectrum: although medication use significantly increased among patients with frequent clinic visits, nonpharmacologic interventions were no more likely to be implemented.
Determining the precise epidemiologic and economic burden of chronic symptomatic FBDs is challenging for several reasons. First, because FBDs are clinical diagnoses based predominantly on symptomatic criteria, heterogeneity can be introduced by the study definitions alone. Second, only a subset of symptomatic patients seek out medical care, so the total burden of disease is typically underestimated.34 , 35 Third, patients presenting with GI symptoms at the index visit are often difficult to appropriately classify pending additional investigations. Recognizing these limitations, we purposively sampled a highly restricted study population to maximize our diagnostic specificity, including only patients with both a provider diagnosis of FBD and active, chronic GI symptomatology at the time of the clinic visit. Therefore, although our estimate of approximately 4 million annual visits for chronic symptomatic FBDs in the United States represents a substantial use of health care resources, it is also highly conservative compared to previous studies.15 , 19 , 36 , 37
Accordingly, our cost estimates for chronic symptomatic FBDs in the United States almost certainly underestimate the true economic impact of this disorder. Importantly, we estimated only the direct ambulatory clinic costs associated with the visit encounter and did not account for costs of referrals to other specialists, additional investigations such as cross-sectional imaging or endoscopy, or recurrent follow-up visits for the same patient. Specialized tests that are frequently done in patients with FBDs such as motility testing, celiac serology, or advanced biliary imaging are also not accounted for. Second, we estimated visit costs using Medicare data, and only 44.7% of patients used Medicare/Medicaid as their primary method of payment. The National Payment Amount for Medicare as an estimate of the physician costs per visit may be on the lower end of reimbursement compared to private insurance. Furthermore, Medicare does not reimburse the E/M code series 99241–99245 for new outpatient consultations; these codes are associated with higher relative value units compared to the 99201–99205 code series for new office visits we used in our analysis as a replacement.38 Our estimates do not include the cost of insurance premiums and copayments that represent a substantial out-of-pocket expense that patients pay for their care. Third, clinic costs account for only a small fraction of the total cost of FBDs. The costing analysis does not include the high cost of pharmaceuticals or indirect costs to society, such as decreased productivity and increased workplace absenteeism as well as reduced quality of life for both patients and their partners, which are known to be substantial.34 , 35 , 39, 40, 41, 42 Overall, the total cost of FBDs to society is enormous.
Our study has some important strengths. We used national survey data that are geographically diverse, include all payer types, and capture a broad range of both patients and physicians. Applying survey weights allowed us to report generalizable findings that reflect national-level practice patterns. Although only approximately 2% of the total unweighted sample visits within NAMCS were for FBDs, NAMCS is not GI specific but broadly captures ambulatory care delivery in the United States. For comparison, all hypertension-related visits in the United States accounted for only 1.6% of the NAMCS sample in 2015. Given that we included adult patients from a wide range of ages and ethnicities, seen by different types of care providers, sampled from across the United States and over a 9-year study period, with data analysis by application of appropriate sample weights, we believe the results are nationally representative. Data collection within NAMCS is robust, data quality is routinely monitored, and we used a range of statistical methodologies to capture the scope of both the epidemiologic burden and treatment of FBDs. Our estimates of pharmacotherapy use are aligned with recent studies conducted using other methodologies: for example, in a prospective population-based survey, Oh et al43 estimated that 47.8% of patients used medications to manage chronic constipation, which is almost identical to our estimate of 47.4%. However, there may be important underreporting of behavioral nonpharmacologic counseling in the NAMCS survey, particularly because patient report forms can be completed by medical office personnel or US Census Bureau staff reviewing the medical chart. In these circumstances, complex discussions and shared decision-making processes between providers and patients may not always be extracted. Gilchrist et al44 compared NAMCS reporting with direct visit observations by trained research nurses in primary care, showing a 9.6% difference in coding for dietary counseling and a 7.7% difference in coding for exercise counseling. However, the specificity of NAMCS coding for nonpharmacologic interventions was high (90%–93%). Furthermore, even accounting for a possible 10% underreporting difference, fewer than 1 in 3 patients with chronic symptomatic FBDs receive nonpharmacologic treatment.
We also acknowledge some other important limitations. First, all administrative database studies are susceptible to potential misclassification errors in identifying the patient population, exposures, and outcomes. To mitigate this, we have used previously established ICD-9-CM coding and applied restrictive exclusion criteria to improve the specificity for identifying patients with chronically symptomatic FBDs. Validation of ICD coding for functional GI conditions is an area of research need; some previous work has shown high sensitivity and specificity for IBS, although robust validation of coding for individual FBD subtypes has not been performed.45 There is no specific ICD-9-CM classification for functional abdominal distention/bloating, although this may be evaluated in future studies as US databases migrate to the ICD, 10th Revision (ICD-10). Direct validation of our coding against medical records is impossible because all data within NAMCS are deidentified for public use. The highest risk of misclassification is in patients with nonspecific chronic abdominal pain because there may be other diagnoses associated with pain symptoms. However, we believe the likelihood of this is low because we excluded patients with other causes of nonfunctional pain, recognizing that there is no direct method within NAMCS to determine the timing of concurrent conditions. Additionally, we used the 2015 data set to check if any patients in our study had associated upper GI or genitourinary causes of abdominal pain. No patients from our study sample had concurrent ICD-9-CM coding for dyspepsia, peptic ulcer disease, ovarian cysts, endometriosis, or ovarian/fallopian tube/uterine cancer.
A second limitation is that data from NAMCS are captured at the level of the clinic visit, rather than the individual patient, so more granular details such as disease duration, severity, and previous treatments are not available. As previously identified, it is likely that some patients may have tried other therapies in the past that cannot be captured in this cross-sectional survey design. However, patients identified in this study who remain chronically symptomatic and seek care for FBDs are likely to still benefit from either trying other nonpharmacologic interventions or reinforcing existing lifestyle measures. Third, the cross-sectional nature of this study precludes direct evaluation of the effectiveness of interventions, although this is outside the scope of this study. Finally, we pooled available data from NAMCS up to 2015, yet there were still insufficient unweighted observations to accurately estimate the use of newer treatments such as linaclotide (approved in the United States in 2012), eluxadoline (2015), plecanatide (2017), or prucalopride (2018). Currently, 2016 is the last year of available data from NAMCS, although this was also the first year that the data structure changed to ICD-10. To avoid potential confounding from this coding change, we excluded the 2016 data set pending further validation of the mapping between ICD-9-CM and ICD-10 codes in this patient population.
In conclusion, FBDs are responsible for a substantial burden of health care resource use in the United States, accounting for at least 4 million outpatient visits each year that cost more than US$350 million for the clinic visits alone. Future initiatives aimed at increasing the uptake of effective diet, exercise, stress reduction, and mental health counseling in symptomatic patients and evaluating the subsequent impact on health care use may improve the overall quality of care delivery for patients with functional GI disorders.
Acknowledgments
CRediT Authorship Contributions
Christopher Ma, MD, MPH: conceptualization (lead), methodology, formal analysis (lead), data curation, writing-original draft (lead), writing-reviewing & editing, visualization, supervision, project administration.
Stephen E. Congly, MD, MSc: conceptualization (support), formal analysis (support), writing-original draft (support), writing-review & editing.
Kerri L. Novak, MD, MSc: writing-review & editing.
Paul J. Belletrutti, MD: writing-review & editing.
Maitreyi Raman, MD, MSc: writing-review & editing.
Matthew Woo, MD: writing-review & editing.
Christopher N. Andrews, MD, MSc: writing-review & editing.
Yasmin Nasser, MD, PhD: conceptualization (support), writing-original draft (support), writing-review & editing.
Footnotes
Conflicts of interest The authors disclose the following: Christopher Ma reports consulting or advisory board fees from AbbVie, AVIR Pharma Inc, Janssen, Takeda, Pfizer, Roche, and Robarts Clinical Trials Inc and speaker's fees from AbbVie, Janssen, Takeda, and Pfizer. Stephen E. Congly reports grants from Gilead Sciences, Boehringer Ingelheim, Genfit, Allergan, and Sequana Medical and personal fees from Intercept Pharmaceuticals and Eisai outside the submitted work. Kerri L. Novak reports advisory board fees from AbbVie, Janssen, Pfizer, and Ferring; speaker fees from AbbVie and Janssen; and research support from AbbVie and Janssen. Paul J. Belletrutti reports speaker’s fees from Pentax Medical and Pendopharm. Maitreyi Raman reports advisory board fees from Takeda, Pfizer, AbbVie and Baxter and speaking fees from Takeda. Matthew Woo reports speaker fees from Proctor and Gamble. Christopher N. Andrews reports advisory board or speaker fees from AbbVie, Allergan, Cipher, Lupin, and Pendopharm and research support from Allergan, Janssen, and Nimble Science. Yasmin Nasser reports speaker fees and grant support from Allergan.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.09.041.
Supplementary Material
Supplementary Table 1.
Coding Definitions for Pharmacologic and Nonpharmacologic Interventions
| Outcome | Coding | Examples |
|---|---|---|
| Antidiarrheal agents | Level 1: 087 (GI agents) Level 2: 090 (Antidiarrheal agents) |
Loperamide Diphenoxylate-atropine Bismuth subsalicylate |
| Laxatives | Level 1: 087 (GI agents) Level 2: 095 (Laxatives) |
Senna/senna-based laxatives Bisacodyl PEG 3350 Docusate Lactulose Magnesium citrate |
| Anticholinergic antispasmodics | Level 1: 087 (GI agents) Level 2: 355 (FBD agents) Level 3: 089 (Anticholinergic antispasmodics) |
Atropine Hycosamine Dicyclomine |
| Serotoninergic neuroenteric modulators | Level 1: 087 (GI agents) Level 2: 355 (FBD agents) Level 3: 356 (Serotoninergic neuroenteric modulators) |
Alosetron Tegaserod |
| Chloride channel activators | Level 1: 087 (GI agents) Level 2: 355 (FBD agents) Level 3: 362 (Chloride channel activators) |
Lubiprostone |
| Guanylate cyclase C agonists | Level 1: 087 (GI agents) Level 2: 355 (FBD agents) Level 4: 455 (Guanylate cyclase C agonists) |
Linaclotide |
| Peripheral opioid receptor antagonists | Level 1: 087 (GI agents) Level 2: 355 (FBD agents) Level 3: 375 (Peripheral opioid receptor antagonists) |
Naloxegol |
| Tricyclic antidepressants | Level 1: 242 (Psychotherapeutic agents) Level 2: 249 (Antidepressants) Level 3: 209 (Tricyclic antidepressant) |
Amitriptyline Nortriptyline Imipramine |
| Selective serotonin reuptake inhibitors | Level 1: 242 (Psychotherapeutic agents) Level 2: 249 (Antidepressants) Level 3: 208 (SSRI) |
Fluoxetine Sertraline Paroxetine Citalopram Escitalopram |
| Selective serotonin and norepinephrine reuptake inhibitors | Level 1: 242 (Psychotherapeutic agents) Level 2: 249 (Antidepressants) Level 3: 308 (SSNRI) |
Duloxetine Venlafaxine |
| Benzodiazepines | Level 1: 057 (CNS agents) Level 2: 067 (Anxiolytics) Level 3: 069 (Benzodiazepines) |
Lorazepam Diazepam Clonazepam Alprazolam |
| Opioid analgesics | Level 1: 057 (CNS agents) Level 2: 058 (Analgesics) Level 3: 060 (Narcotic analgesics), 191 (Narcotic analgesic combinations) |
Morphine Oxycodone Hydromorphone Fentanyl Tramadol |
| Nonsteroidal anti-inflammatories | Level 1: 057 (CNS agents) Level 2: 067 (Anxiolytics) Level 3: 061 (NSAIDs), 278 (COX2 inhibitors) |
Naproxen Ibuprofen Indomethacin Celecoxib |
| Probiotics | Level 1: 218 (Alternative medicines) Level 2: 363 (Probiotics) |
Align BioGaia Colon Health Florastor |
| Bile acid sequestrants | Level 1: 358 (Metabolic agents) Level 2: 019 (Antihyperlipidemic agents) Level 3: 252 (Bile acid sequestrants) |
Cholestyramine Colestid/colestipol |
| Rifaximin | Drug ID: d05294, a11549, 06122 | Rifaximin |
| Diet and nutrition counseling DIETNUTR |
Diet or nutrition education includes any topic related to the foods and/or beverages consumed by the patient. Examples include general dietary guidelines for health promotion and disease prevention, dietary restrictions to treat or control a specific medical problem or condition, and dietary instructions related to medications. Includes referrals to other health professionals, for example, dietitians and nutritionists. | |
| Exercise counseling EXERCISE |
Exercise education includes any topics related to the patient’s physical conditioning or fitness. Examples include information aimed at general health promotion and disease prevention and information given to the patient to treat or control a specific medical condition. It includes referrals to other health and fitness professionals but excludes referrals for physical therapy. | |
| Physical therapy PT |
Physical therapy includes treatments using heat, light, sound, or physical pressure or movement (eg, ultrasonic, ultraviolet, infrared, whirlpool, diathermy, cold, or manipulative therapy). | |
| Weight reduction WTREDUC |
Education on weight reduction refers to information given to the patient to assist in the goal of weight reduction. It includes referrals to other health professionals for the purpose of weight reduction. | |
| Stress management STRESMGT |
Stress management counseling refers to information intended to help patients reduce stress through exercise, biofeedback, yoga, etc. It includes referrals to other health professionals for the purpose of coping with stress. | |
| Mental health counseling MENTAL |
Mental health counseling includes general advice and counseling about mental health issues and education about mental disorders. It includes referrals to other mental health professionals for mental health counseling but excludes psychotherapy. | |
| Psychotherapy PSYCHOTH |
Psychotherapy includes all treatment involving the intentional use of verbal techniques to explore or alter the patient’s emotional life to effect symptom reduction or behavior change. | |
| Complementary and alternative medicine CAM |
CAM includes medical interventions neither widely taught in medical schools nor generally available in physician offices or hospitals (eg, acupuncture, chiropractic, homeopathy, massage, or herbal therapies). | |
CNS, central nervous system; COX2, cyclooxygenase 2; ID, identifier; NSAID, nonsteroidal anti-inflammatory drug.
References
- 1.Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Mearin F., Lacy B.E., Chang L. Bowel disorders. Gastroenterology. 2016;150:1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y., Kanazawa M., Fukudo S. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Oudenhove L., Levy R.L., Crowell M.D. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150:1355–1367. doi: 10.1053/j.gastro.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacy B.E., Chey W.D., Lembo A.J. New and emerging treatment options for irritable bowel syndrome. Gastroenterol Hepatol (N Y) 2015;11(4 Suppl 2):1–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Mearin F., Malfertheiner P. Functional gastrointestinal disorders: complex treatments for complex pathophysiological mechanisms. Dig Dis. 2017;35(Suppl 1):1–4. doi: 10.1159/000485407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olden K.W. Approach to the patient with severe, refractory irritable bowel syndrome. Curr Treat Options Gastroenterol. 2003;6:311–317. doi: 10.1007/s11938-003-0023-8. [DOI] [PubMed] [Google Scholar]
- 8.Corazziari E. Definition and epidemiology of functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:613–631. doi: 10.1016/j.bpg.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Simren M., Tornblom H., Palsson O.S. Management of the multiple symptoms of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2017;2:112–122. doi: 10.1016/S2468-1253(16)30116-9. [DOI] [PubMed] [Google Scholar]
- 10.Moayyedi P., Mearin F., Azpiroz F. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5:773–788. doi: 10.1177/2050640617731968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chey W.D., Kurlander J., Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 12.Schumann D., Anheyer D., Lauche R. Effect of yoga in the therapy of irritable bowel syndrome: a systematic review. Clin Gastroenterol Hepatol. 2016;14:1720–1731. doi: 10.1016/j.cgh.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg D.S., Smalley W., Heidelbaugh J.J. American Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1146–1148. doi: 10.1053/j.gastro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M., Ford A.C. Pharmacotherapy for irritable bowel syndrome. J Clin Med. 2017;6(11):101. doi: 10.3390/jcm6110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peery A.F., Crockett S.D., Murphy C.C. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156:254–272. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz I., Palsson O.S., Tornblom H. The prevalence and impact of overlapping Rome IV-diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: a cross-sectional general population study in three countries. Am J Gastroenterol. 2018;113:86–96. doi: 10.1038/ajg.2017.421. [DOI] [PubMed] [Google Scholar]
- 17.Lovell R.M., Ford A.C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Hungin A.P., Whorwell P.J., Tack J. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 19.Peery A.F., Dellon E.S., Lund J. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto-Sanchez M.I., Ford A.C., Avila C.A. Anxiety and depression increase in a stepwise manner in parallel with multiple FGIDs and symptom severity and frequency. Am J Gastroenterol. 2015;110:1038–1048. doi: 10.1038/ajg.2015.128. [DOI] [PubMed] [Google Scholar]
- 21.Zamani M., Alizadeh-Tabari S., Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:132–143. doi: 10.1111/apt.15325. [DOI] [PubMed] [Google Scholar]
- 22.Lackner J.M., Gudleski G.D., Thakur E.R. The impact of physical complaints, social environment, and psychological functioning on IBS patients’ health perceptions: looking beyond GI symptom severity. Am J Gastroenterol. 2014;109:224–233. doi: 10.1038/ajg.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simren M., Tornblom H., Palsson O.S. Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient-reported outcomes in irritable bowel syndrome. Gastroenterology. 2019;157:391–402. doi: 10.1053/j.gastro.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Weerts Z., Vork L., Mujagic Z. Reduction in IBS symptom severity is not paralleled by improvement in quality of life in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2019;31(8) doi: 10.1111/nmo.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford A.C., Lacy B.E., Harris L.A. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21–39. doi: 10.1038/s41395-018-0222-5. [DOI] [PubMed] [Google Scholar]
- 26.Lackner J.M., Jaccard J., Radziwon C.D. Durability and decay of treatment benefit of cognitive behavioral therapy for irritable bowel syndrome: 12-month follow-up. Am J Gastroenterol. 2019;114:330–338. doi: 10.1038/s41395-018-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lackner J.M., Jaccard J., Keefer L. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018;155:47–57. doi: 10.1053/j.gastro.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flik C.E., Laan W., Zuithoff N.P.A. Efficacy of Individual and Group Hypnotherapy in Irritable Bowel Syndrome (IMAGINE): a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:20–31. doi: 10.1016/S2468-1253(18)30310-8. [DOI] [PubMed] [Google Scholar]
- 29.Sampaio F., Bonnert M., Olen O. Cost-effectiveness of internet-delivered cognitive-behavioural therapy for adolescents with irritable bowel syndrome. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everitt H.A., Landau S., O’Reilly G. Assessing telephone-delivered cognitive-behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut. 2019;68:1613–1623. doi: 10.1136/gutjnl-2018-317805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moayyedi P., Andrews C.N., MacQueen G. Canadian Association of Gastroenterology clinical practice guideline for the management of irritable bowel syndrome (IBS) J Can Assoc Gastroenterol. 2019;2:6–29. doi: 10.1093/jcag/gwy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basnayake C., Kamm M.A., Salzberg M.R. Delivery of care for functional gastrointestinal disorders: a systematic review. J Gastroenterol Hepatol. 2020;35:204–210. doi: 10.1111/jgh.14830. [DOI] [PubMed] [Google Scholar]
- 33.Bijkerk C.J., de Wit N.J., Stalman W.A. Irritable bowel syndrome in primary care: the patients’ and doctors’ views on symptoms, etiology and management. Can J Gastroenterol. 2003;17:363–368. doi: 10.1155/2003/532138. [DOI] [PubMed] [Google Scholar]
- 34.Buono J.L., Mathur K., Averitt A.J. Economic burden of irritable bowel syndrome with diarrhea: retrospective analysis of a U.S. commercially insured population. J Manag Care Spec Pharm. 2017;23:453–460. doi: 10.18553/jmcp.2016.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buono J.L., Carson R.T., Flores N.M. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2017;15:35. doi: 10.1186/s12955-017-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinkley K.E., Porter K., Nahata M.C. Prescribing patterns for the outpatient treatment of constipation in the United States. Dig Dis Sci. 2010;55:3514–3520. doi: 10.1007/s10620-010-1196-3. [DOI] [PubMed] [Google Scholar]
- 37.Dorn S.D., Meek P.D., Shah N.D. Increasing frequency of opioid prescriptions for chronic abdominal pain in US outpatient clinics. Clin Gastroenterol Hepatol. 2011;9:1078–1085. doi: 10.1016/j.cgh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Medicare & Medicaid Services Physician fee schedule - April 2020 release. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu20b
- 39.Maxion-Bergemann S., Thielecke F., Abel F. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 40.Bentkover J.D., Field C., Greene E.M. The economic burden of irritable bowel syndrome in Canada. Can J Gastroenterol. 1999;13(Suppl A):89A–96A. doi: 10.1155/1999/569613. [DOI] [PubMed] [Google Scholar]
- 41.Polster A.V., Palsson O.S., Tornblom H. Subgroups of IBS patients are characterized by specific, reproducible profiles of GI and non-GI symptoms and report differences in healthcare utilization: a population-based study. Neurogastroenterol Motil. 2019;31(1) doi: 10.1111/nmo.13483. [DOI] [PubMed] [Google Scholar]
- 42.Wong R.K., Drossman D.A., Weinland S.R. Partner burden in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:151–155. doi: 10.1016/j.cgh.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Oh S.J., Fuller G., Patel D. Chronic constipation in the United States: results from a population-based survey assessing healthcare seeking and use of pharmacotherapy. Am J Gastroenterol. 2020;115:895–905. doi: 10.14309/ajg.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilchrist V.J., Stange K.C., Flocke S.A. A comparison of the National Ambulatory Medical Care Survey (NAMCS) measurement approach with direct observation of outpatient visits. Med Care. 2004;42:276–280. doi: 10.1097/01.mlr.0000114916.95639.af. [DOI] [PubMed] [Google Scholar]
- 45.Sands B.E., Duh M.S., Cali C. Algorithms to identify colonic ischemia, complications of constipation and irritable bowel syndrome in medical claims data: development and validation. Pharmacoepidemiol Drug Saf. 2006;15:47–56. doi: 10.1002/pds.1118. [DOI] [PubMed] [Google Scholar]