Abstract
Costunolide, a natural sesquiterpene lactone, has multiple pharmacological activities such as neuroprotection or induction of apoptosis and eryptosis. However, the effects of costunolide on pro-survival factors and enzymes in human erythrocytes, e.g. glutathione and glucose-6-phosphate dehydrogenase (G6PDH) respectively, have not been studied yet. Our aim was to determine the mechanisms underlying costunolide-induced eryptosis and to reverse this process. Phosphatidylserine exposure was estimated from annexin-V-binding, cell volume from forward scatter in flow cytometry, and intracellular glutathione [GSH]i from high performance liquid chromatography. The oxidized status of intracellular glutathione and enzyme activities were measured by spectrophotometry. Treatment of erythrocytes with costunolide dose-dependently enhanced the percentage of annexin-V-binding cells, decreased the cell volume, depleted [GSH]i and completely inhibited G6PDH activity. The effects of costunolide on annexin-V-binding and cell volume were significantly reversed by pre-treatment of erythrocytes with the specific PKC-α inhibitor chelerythrine. The latter, however, had no effect on costunolide-induced GSH depletion. Costunolide induces eryptosis, depletes [GSH]i and inactivates G6PDH activity. Furthermore, our study reveals an inhibitory effect of chelerythrine on costunolide-induced eryptosis, indicating a relationship between costunolide and PKC-α. In addition, chelerythrine acts independently of the GSH depletion. Understanding the mechanisms of G6PDH inhibition accompanied by GSH depletion should be useful for development of anti-malarial therapeutic strategies or for synthetic lethality-based approaches to escalate oxidative stress in cancer cells for their sensitization to chemotherapy and radiotherapy.
Keywords: Eryptosis, Costunolide, Chelerythrine, Glutathione, Glucose-6-phosphate dehydrogenase (G6PDH), Phosphatidylserine exposure
Introduction
Costunolide, a sesquiterpene lactone and natural product of plant origin, counteracts tumor growth and metastasis via suppression of STAT3 [1, 2] and NFκB [3] activities (for review see [4]). Costunolide also inhibits differentiation of pro-inflammatory CD4+ T cells [5], reduces the activity of the pro-survival enzyme Akt [6] and exhibits anti-bacterial activity [7]. The pharmacokinetic profile of costunolide has been reported [8] and its therapeutic potential is supported by numerous animal studies. Costunolide possesses anti-angiogenic [9, 10] and -osteoarthritic effects [11]. It inhibits pulmonary [12] and hepatic fibrosis [13], induces hair growth [14] and shows strong larvicidal activity [15]. Furthermore, costunolide induces apoptosis, an evolutionary conserved cellular programmed cell death by conjugating with sulfhydryl groups and intracellular thiols, e.g. the reduced form of the tripeptide glutathione (GSH / L-γ-glutamyl-L-cysteineglycine) [16]. This type of non-enzymatic interaction with thiols ultimately leads to complete depletion of both intracellular GSH and its oxidized form (GSSG). This principle was recently demonstrated after treatment of human erythrocytes with parthenolide, dimethyl fumarate and Bay 11–7082, respectively [17, 18]. There is an inverse correlation between intracellular glutathione concentration [GSH]i and the activity of allosterically regulated protein kinase C alpha (PKC-α). The phosphatidylserine (PS)- and calcium (Ca2+)-dependent PKC-α, a member of conventional PKCs (cPKCs) family [19, 20] is directly involved in the control of major cellular functions such as neuronal differentiation [21], protein [22] and DNA synthesis [23]. PKC-α enhances survival and proliferation of cancer cells e.g. glioblastoma [24], acute myeloid leukemia cells [25], favours multi-drug resistance [26, 27], cell motility and anti-apoptotic processes [28]. Furthermore, PKC-α-induced Akt activation and their synergistic cross talk acts as a bulwark against stress-induced apoptosis [29]. Interestingly, inhibition of PKC-α commonly triggers apoptosis in nucleated cells [30, 31], while its inhibition protects anucleated human erythrocytes from stress-induced cell death [32, 33], the so-called eryptosis [34]. Eryptosis is triggered by a plenty of xenobiotics [35] and inhibited by several other xenobiotics or by endogenous molecules, including nitric oxide [36], GSH [37] and erythropoietin [38, 39]. Due to a wide range of biological activities, costunolide has been extensively studied over the past 20 years. Our laboratory previously published that costunolide induces eryptosis [40]. The present study explored the effects of costunolide on pro-survival factors and enzymes in human erythrocytes, e.g. GSH and glucose-6-phosphate dehydrogenase (G6PDH), and analysed the effects of the specific conventional PKC-α and -β inhibitor chelerythrine [41–44] on costunolide-induced eryptosis and the GSH synthesis machinery. We have provided new insights into the mechanism of costunolide action that broaden its therapeutic potential. Inhibition of G6PDH and GSH metabolism using costunolide may offer a promising therapeutic strategy to eliminate malaria-infected erythrocytes [45]. Moreover, increase cancer cells’ oxidative stress renders them vulnerable to therapeutic interventions using chemotherapy and radiotherapy [46]. Therefore, understanding the mechanisms of G6PDH inhibition accompanied by GSH depletion should be also useful for exploiting synthetic lethal interactions for targeted cancer therapy.
Results
Impact of costunolide on eryptosis, cell shrinkage and hemolysis
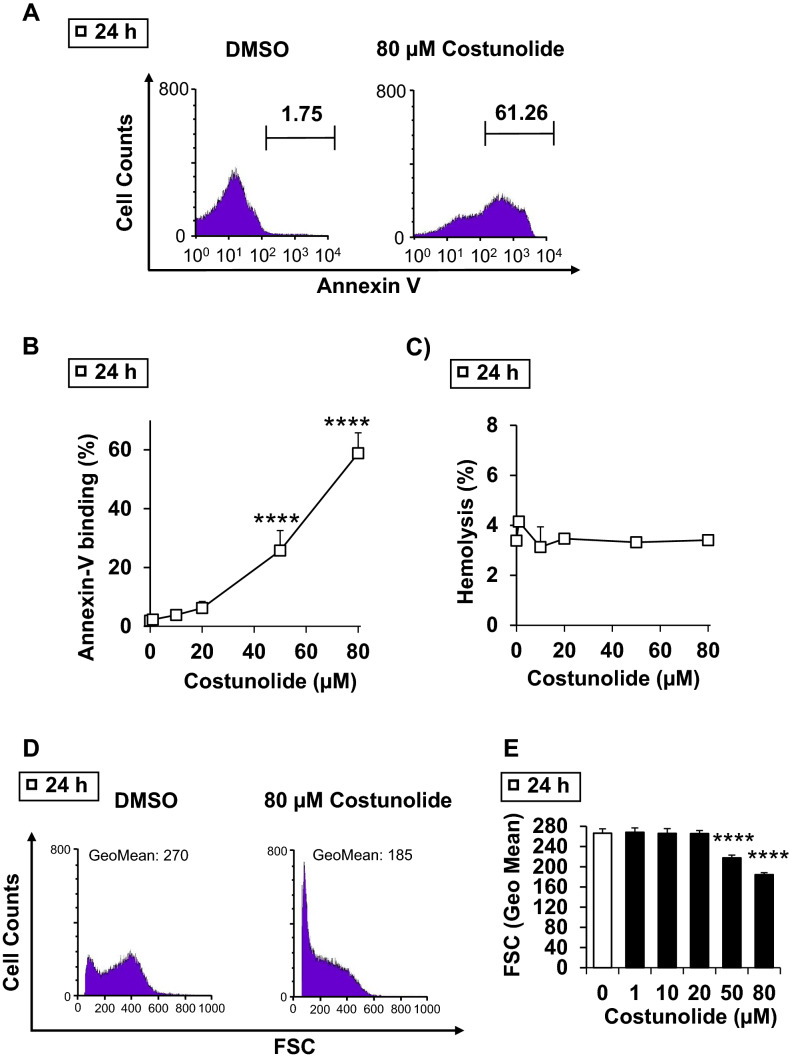
Human erythrocytes were treated with various physiological concentrations of costunolide. After 24 h, its influence on phosphatidylserine (PS) exposure, cell shrinkage and hemolysis was examined. Increasing concentrations of costunolide paralleled the rates of PS exposure (Fig. 1a, b) and cell shrinkage (Fig. 1e, d) without affecting the hemolysis (Fig. 1c). Fluorescence microscopy confirmed the appearance of shrunken, PS-positive erythrocytes after costunolide treatment (Fig. 2).
Fig. 1.
Costunolide-induced eryptosis and cell shrinkage in mature human erythrocytes. Original histograms of annexin V-binding (a), concentration-dependent increase of annexin V-binding cells (b), concentration-dependent effect on hemolysis (c), original histograms of forward scatter (FCS) (d), and concentration-dependent decrease of forward scatter (FCS) (e) after treatment of human erythrocytes for 24 h with costunolide are shown. Number of independent experiments: n = 3. Differences of the means were considered to be statistically significant when the calculated p value was less than 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Fig. 2.
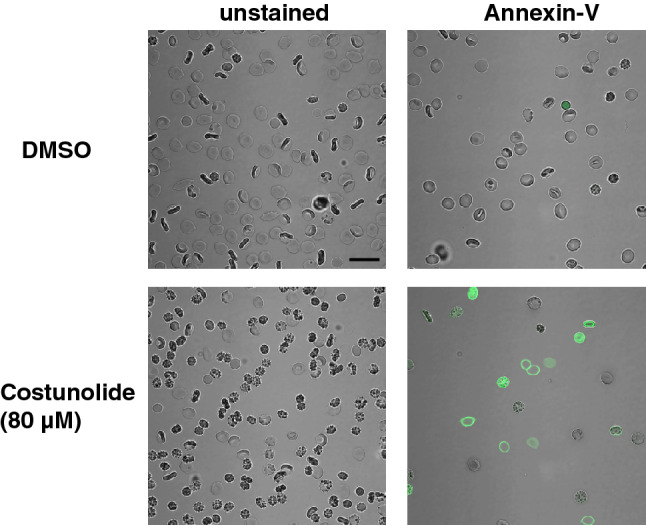
Costunolide-induced annexin V-binding. Human erythrocytes were either treated with DMSO (upper panel) or 80 µM costunolide (lower panel) for 24 h. Fluorescence images of annexin V-stained (right panel) or unstained erythrocytes (left panel) are shown. Scalebar: 20 µm
Impact of costunolide on glucose-6-phosphate dehydrogenase (G6PDH) activity and glutathione level
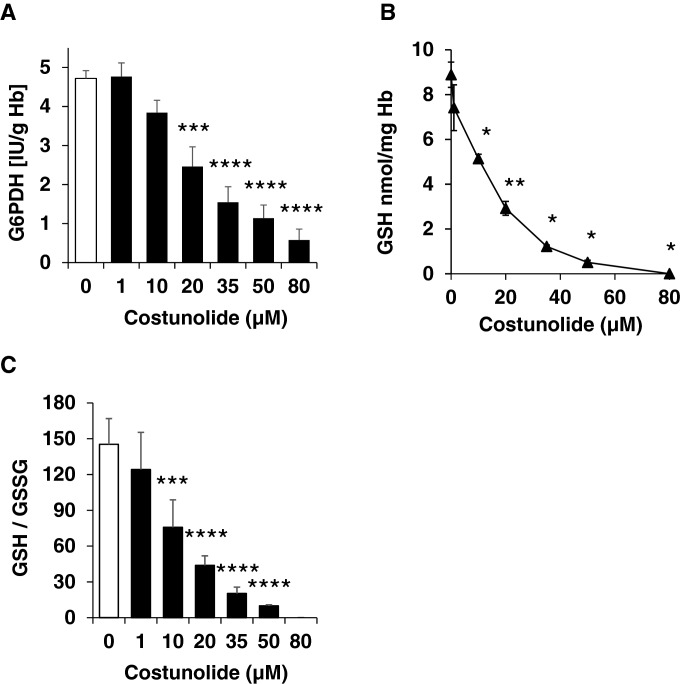
The next step was to investigate if costunolide-induced eryptosis is caused by impairment of the redox balance of human erythrocytes. For this, the influence of costunolide on the activity of the pro-survival enzyme G6PDH as well as on glutathione levels (GSH, GSSG) was studied. Indeed, costunolide was able to completely inhibit G6PDH activity (Fig. 3a) and to deplete the reduced form of glutathion (GSH) thereby decreasing the GSH/GSSG ratio in a concentration-dependent manner (Fig. 3b, c). The physiological concentration of GSSG in erythrocytes is very low compared to the GSH level. During costunolide treatment, the GSSG level remained nearly constant, only a slight decrease was observed at a concentration of 50 µM (Table 1). The NADPH-producing enzymes G6PDH and 6-phosphogluconate dehydrogenase (6-PGD) belong to the pentose phosphate pathway [47]. Interestingly, costunolide did not affect the activities of 6-PGD and glutathione reductase (data not shown).
Fig. 3.
Effects of costunolide on glucose-6-phosphate dehydrogenase (G6PDH) activity, GSH and GSSG levels. Concentration-dependent inhibition of G6PDH activity (a), depletion of GSH (b), reduction of the GSH/GSSG ratio (c) after 24 h treatment of human erythrocytes with costunolide are shown. Number of independent experiments: n = 6. Differences of the means were considered to be statistically significant when the calculated p value was less than 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Table 1.
GSH and GSSG levels after 24 h of costunolide treatment
| DMSO [v/v] | Costunolide [µM] | ||||||
|---|---|---|---|---|---|---|---|
| 0.2% | 1 | 10 | 20 | 35 | 50 | 80 | |
| GSH [nmol/mg Hb] | 8.89 | 7.41 | 5.14 | 2.92 | 1.22 | 0.50 | 0.00 |
| GSSG [nmol/mg Hb] | 0.07 | 0.06 | 0.08 | 0.07 | 0.07 | 0.05 | 0.07 |
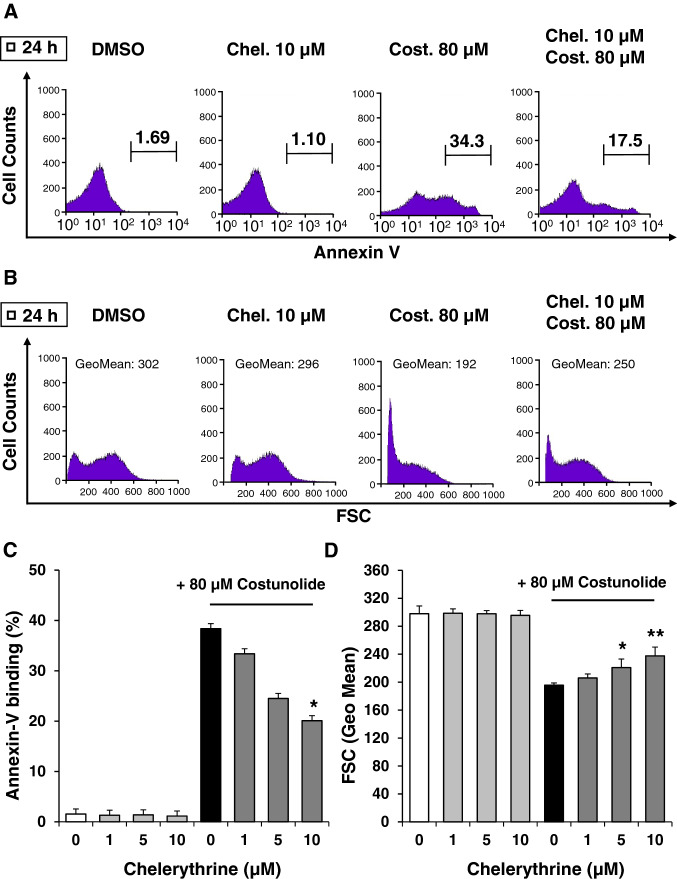
Inhibitory effect of chelerythrine on costunolide-induced eryptosis and cell shrinkage
We then examined whether chelerythrine, a natural benzophenanthridine alkaloid, has the potential to inhibit costunolide-induced eryptosis and cell shrinkage. To achieve this aim, human erythrocytes were first treated for 2 h with various concentrations of chelerythrine (1 to 10 µM) followed by addition of the highest costunolide concentration (80 µM). In fact, chelerythrine was able to partially inhibit costunolide-induced phosphatidylserine exposure (Fig. 4a, c) and cell shrinkage (Fig. 4b, d). In comparision to DMSO-treated erythrocytes, a combination of the highest costunolide and chelerythrine concentrations induced slight hemolysis (4.45% vs. 6.93%) (Fig. 5).
Fig. 4.
Chelerythrine blunts costunolide-induced annexin V-binding and erythrocyte shrinkage. Human erythrocytes were preincubated with the PKC-α inhibitor chelerythrine and then treated with 80 µM costunolide for 24 h. Original histograms of annexin V-binding (a), original histograms of forward scatter (FCS) (b), concentration-dependent decrease of costunolide-induced annexin V-binding (c), and concentration-dependent increase of forward scatter (FCS) of costunolide-shrunken erythrocytes (d) after treatment of human erythrocytes as described above are shown. Number of independent experiments: n = 3. Differences of the means were considered to be statistically significant when the calculated p value was less than 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Chel: chelerythrine; Cos: costunolide
Fig. 5.
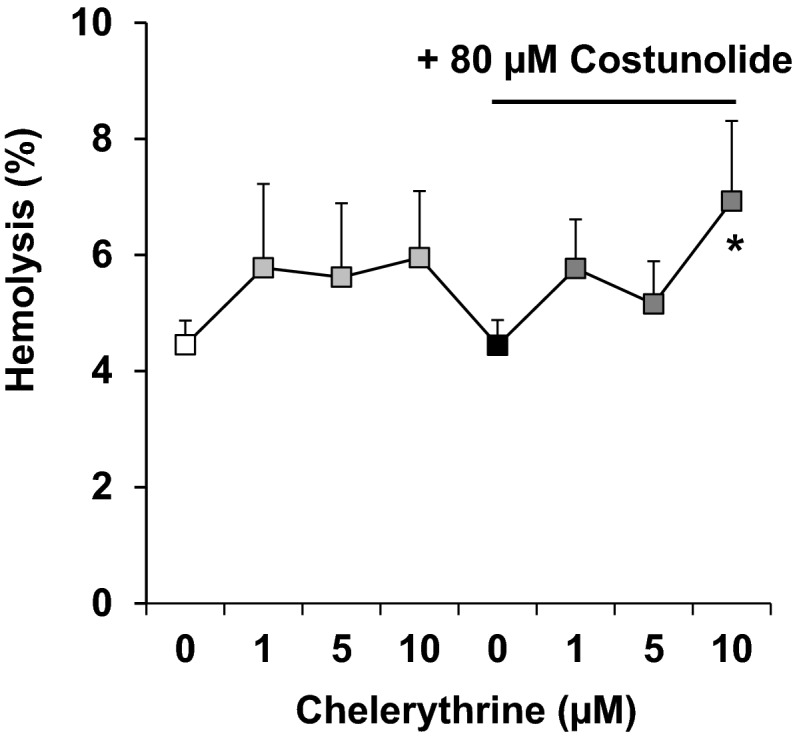
Effect of costunolide and chelerythrine on hemolysis. Human erythrocytes were preincubated with the PKC-α inhibitor chelerythrine and then treated with 80 µM costunolide for 24 h. The concentration-dependent effect of chelerythrine on hemolysis in the absence (left) or presence of costunolide (right) is shown. Number of independent experiments: n = 3. Differences of the means were considered to be statistically significant when the calculated p value was less than 0.05 (*p < 0.05)
Chelerythrine does not impair the glutathione synthesis machinery of mature human erythrocytes
Finally, we investigated whether chelerythrine interferes with costunolide-induced GSH depletion. As shown in Fig. 6a, b, chelerythrine did neither reverse the costunolide-induced GSH depletion nor the costunolide-induced slight decrease of the GSSG level. Thus, in our experimental setup chelerythrine seems to act downstream of the glutathione cascade and has no influence on GSH levels.
Fig. 6.
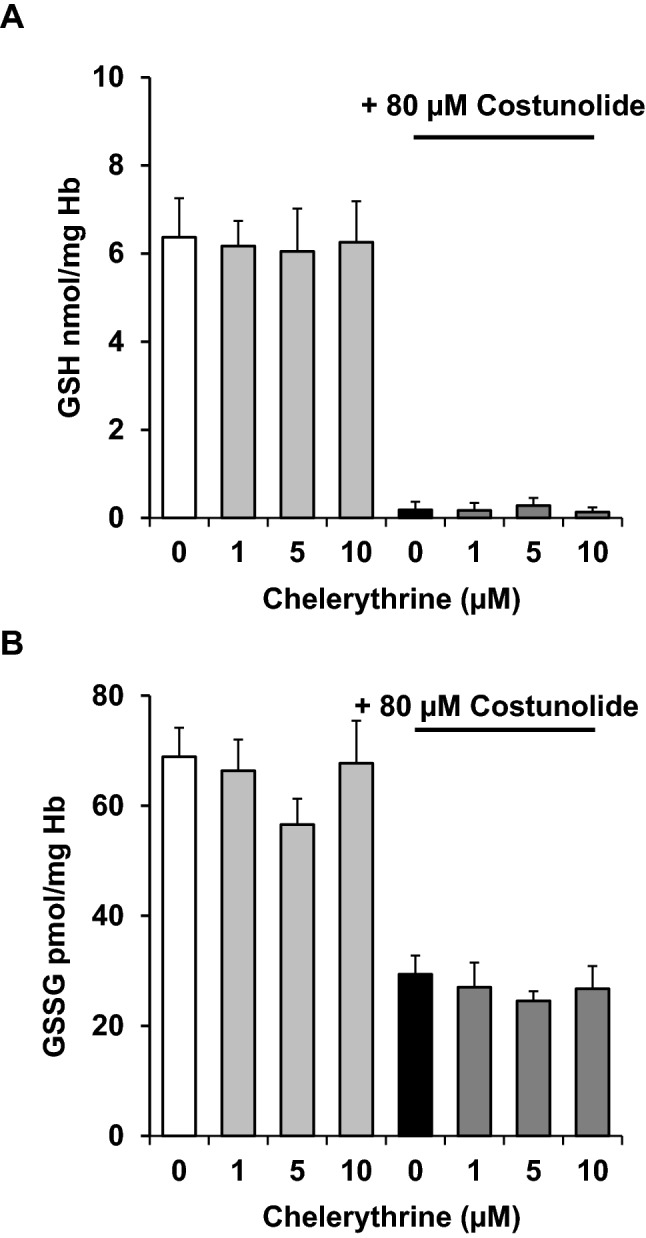
Chelerythrine does not affect costunolide-induced GSH- or GSSG-depletion. Human erythrocytes were preincubated with the PKC-α inhibitor chelerythrine and then treated with 80 µM costunolide for 24 h. Concentration-dependent effects of chelerythrine on GSH (a) or GSSG levels (b) after treatment of human erythrocytes in the absence (left) or presence of costunolide (right) are shown. Number of independent tests: n = 5. Note that chelerythrine did not significantly affect the GSH (a) or GSSG (b) levels
Discussion
The present study shows that costunolide triggers eryptosis with cell shrinkage (Figs. 1 and 2). Eryptosis-inducing substances may dose-dependently cause both cell swelling and cell shrinkage [36]. Costunolide induces a sustained influx of Ca2+ ions [40], which activates the Gardos channel, a Ca2+-sensitive and K+-selective channel, by which cellular K+ ions leave the cell followed by chloride ions and water, thus leading to erythrocyte shrinkage [48]. Volume-sensitive transport pathways are regulated by kinase(s) and phosphatase(s) activities [49, 50].
We further investigated if costunolide-induced eryptosis was caused by impairment of the redox balance of human erythrocytes. Costunolide was indeed able to inhibit the activity of the pro-survival enzyme G6PDH completely (Fig. 3a) and deplete GSH in a concentration-dependent manner (Fig. 3b and c). The GSSG concentration, however, remained at a very low level independent of the costunolide concentration used (Fig. 3d). This type of GSH depletion that is completely decoupled from GSSG formation has already been demonstrated with other compounds, such as Bay 11-7082, parthenolide and dimethyl fumarate [17].
The linear tripeptide glutathione (GSH), a major non-protein thiol plays a vital role in both prokaryotic [51] and eukaryotic cells [52, 53]. Here, we focus on the vital functions of GSH in mature human erythrocytes. GSH with its turnover time of about 4–6 days and its total intracellular concentration of 3 mM [54, 55], is a linchpin of cellular defences protecting cells from biotic and abiotic stresses. GSH is involved in detoxification mechanisms [56, 57], destruction of free radicals [58, 59], protection of hemoglobin [60, 61], post-translational modification of thiol proteins, the so-called glutathionylation [62], ascorbate recycling and defences against oxidant damage of cell membranes [63, 64]. In addition, GSH depletion is associated with the induction of eryptosis [17, 45]. It is to note that (a) ATP-dependent GSH synthesis is the result of two concerted enzymatic step [47, 65, 66], (b) the capacity of erythrocytes to synthesize GSH exceeds the rate of GSH turnover by 150-fold [67], (c) erythrocytes are permeable to oxidized glutathione (GSSG) whose efflux is an active unidirectional process [68] and (d) GSSG is rapidly recycled to GSH by the NADPH-dependent enzyme glutathione reductase [69–71]. NADPH, as an electron donor reductant, is mainly provided via the irreversible dehydrogenase/decarboxylase system of the oxidative branch of the pentose phosphate pathway by the enzymes G6PDH and 6-phosphogluconolactonase [47]. Thus, it is plausible that G6PDH inhibition should lead to a depletion of NADPH, diminishing the activity of glutathione reductase. This would deplete GSH while excess GSSG is transported outside the cell. Numerous works have been published on NADPH production and its absolute necessity for fatty acid biosynthesis [47, 72, 73] and folate metabolism [74, 75] in mammals. Based on the clinical relevance of GSH, NADPH and G6PDH in the regulation of several human diseases, the modulation of GSH levels and G6PDH acitivity by costunolide is a therapeutic approach to influence the course of various diseases.
In the next step, we examined whether chelerythrine, a natural benzophenanthridine alkaloid and specific inhibitor of conventional protein kinase C (cPKC) can inhibit costunolide-induced eryptosis and cell shrinkage. To achieve this aim, human erythrocytes were first treated for two hours with various concentrations of chelerythrine (1 to 10 µM) and then the highest concentration of costunolide (80 µM) was added. In fact, chelerythrine was able to inhibit costunolide-induced eryptosis and cell shrinkage (Fig. 4). Human erythrocytes possess four isoforms of PKCs: alpha, zeta, mu and iota, of which only the subtype PKC-α translocates to the plasma membrane in order to perform biological activities there [76]; i.e. induction of eryptosis [32, 33]. Furthermore, PMA-stimulated PKC-α activation and the resulting Ca2+ influx and cell shrinkage are significantly inhibited by chelerythrine [42]. It is to note that in comparision to DMSO-treated erythrocytes, a combination of the highest costunolide and chelerythrine concentrations induced slight hemolysis (4.45% vs. 6.93%) (Fig. 5).
Finally, we investigated whether chelerythrine can influence the costunolide-caused GSH depletion in any way. This was not the case, showing that chelerythrine likely acts downstream of GSH depletion in mature human erythrocytes (Fig. 6). The overall results of this study are summarized in Fig. 7.
Fig. 7.
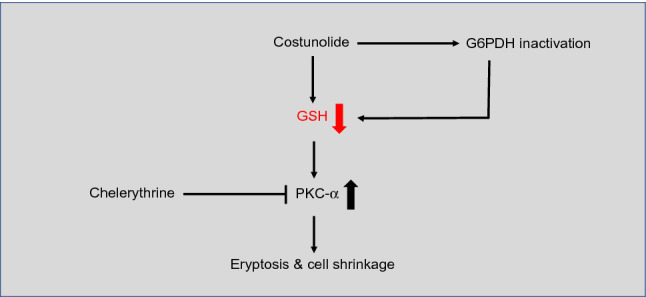
Schematic illustration of inhibition of costunolide-induced eryptosis and cell shrinkage by PKC-α inhibitor chelerythrine
In conclusion, costunolide-induced eryptosis is due to its ability to deplete GSH and inhibit the activity of the pro-survival enzyme G6PDH. Chelerythrine as a specific inhibitor of conventional PKC-α and -β isoforms is able to inhibit costunolide-induced eryptosis and cell shrinkage. This work therefore functionally links costunolide and PKC-α. Furthermore, we show that chelerythrine does not influence GSH depletion indicating that the effect of PKC-α in human erythrocytes may occur downstream of GSH depletion. However, a direct effect of costunolide on PKC-α can not be excluded; the exact mechanism how chelerythrine reverses costunolide-induced eryptosis and cell shrinkage requires further studies. Both the induction of eryptosis and its inhibition are of major clinical relevance. The former can be used to combat malaria or to eliminate cancerous erythroid cells through synthetic lethal interactions and the latter to treat anemia-related diseases.
Materials and methods
Study design
Single treatment: Erythrocytes were treated with costunolide for 24 h. Double treatments: Erythrocytes were pre-incubated with chelerythrine for 2 h, followed by addition of costunolide for further 24 h. Thus, the incubation time for chelerythrine and costunolide was 26 and 24 h, respectively. In single (0.2% v/v DMSO) and double treatments (0.4% v/v DMSO), DMSO-treated cells served as negative controls. The treatment procedures and incubation were performed under aseptic conditions and all standardized requirements were met. Erythrocyte samples were incubated at 37 °C and gently mixed by inverting or vortexing the tubes (50 ml or 5 ml) several times during the incubation period.
Erythrocytes
Highly purified erythrocyte suspensions from healthy volunteers with white blood cell or thrombocyte contaminations below 0.1% [77] were provided by the blood bank of the University of Tübingen. Aliquots of the individual erythrocyte concentrates were either used directly at 0.6% hematocrit (Hct) or stored at 4 °C for up to one week. The study was approved by the ethics committee of the University of Tübingen (184/2003 V), the study was performed in agreement with the declaration of Helsinki, and volunteers gave written consent.
Incubation time
In our experimental setup the decisive factor was the concentration and incubation time at which costunolide was able to achieve complete G6PDH inhibition and GSH depletion. It turned out that a 24-h incubation of the erythrocytes was the most suitable.
Solutions
Experiments analysing annexing V binding and cell volume (0.6% Hct) were carried out in Ringer solution. Staining of erythrocytes with annexin-V-FLUOS was performed in annexin binding buffer. Ringer solution was composed of (in mM): 125 NaCl, 5 KCl, 1.2 MgSO4, 32 N-2-hydroxyethyl-piperazine-N´-ethanesulfonic acid (HEPES)/NaOH (pH 7.4), 5 glucose, and 1 CaCl2. Annexin-binding buffer contained (in mM): 125 NaCl, 10 HEPES/NaOH (pH 7.4), and 5 CaCl2. Ringer solution and annexin binding buffer were sterile filtered. For this purpose the Setrile-Vaccum-Filtration-System of Millipore was used (pore size of the filter: 0.22 µm).
Chemicals
1 mg chelerythrine was dissolved in 520 µl and 5 mg costunolide in 538 µl DMSO to achieve 5 mM and 40 mM stock solutions, respectively. These stocks were subsequently aliquoted and stored at − 20 °C for up to one month. Annexin-V-FLUOS was also aliquoted and stored at − 20 °C for several months. Chelerythrine, costunolide, DMSO, annexin-V-FLUOS and N-Ethylmaleimide (NEM) were purchased from Sigma (Taufkirchen, Germany).
Annexin-V-FLUOS working concentration
On the day of the measurements, the required annexin-V-FLUOS was diluted 1:33 in annexin binding buffer. 48 µl of this solution were taken for staining one sample containing 3 × 106 erythrocytes (for more details see flow cytometry).
Flow cytometry
At the end of the incubation period, 0.1 ml erythrocytes (3 × 106) were added to 500 µl annexin wash buffer, mixed thoroughly and pelleted by centrifuging. Erythrocyte pellets were gently vortexed to obtain a homogeneous cell suspension. To detect the exposure of phosphatidylserine (PS) on the outer leaflet of the plasma membrane (a measure of the percentage of eryptotic cells), erythrocytes were stained with 48 µl of diluted annexin-V-FLUOS and carefully vortexed. After an incubation period of 20 min in the dark at room temperature, 200 µl annexin binding buffer was added to each sample, thoroughly vortexed to obtain single cell suspensions, and analyzed by flow cytometry on a FACS Calibur (Becton Dickinson, Heidelberg, Germany) as described. Binding of annexin-V-FLUOS (eryptosis) was measured in the FL1-channel. Erythrocyte volume was determined by analyzing the forward scatter (FSC). To this end, corresponding erythrocytes suspensions were immediately analysed by flow cytometry.
Hemolysis measurement
After 24 h treatment of erythrocytes (0.6% Hct) with costunolide and/or chelerythrine, hemolysis was determined. 600 µl cell suspension from each condition containing 3.6 × 106 erythrocytes were centrifuged for 8 min at 420×g, 4 °C, and the supernatants were harvested. As a measure of hemolysis, the hemoglobin (Hb) concentration of the supernatants was determined photometrically at 405 nm. The absorption of the supernatant of DMSO-treated erythrocytes lysed in 600 µl distilled water was defined as 100%, and a standard curve was established by a serial dilution.
Fluorescence microscopy
After incubation with costunolide, erythrocytes were stained with Annexin-FLUOS as described above. 10 µl of the suspension were applied to a slide and covered with a coverglass. Finally, the cells were analyzed on a LSM 800 microscope (Zeiss Oberkochen, Germany) operated under the Zen software (Version 2.3).
Intracellular GSH and GSSG analysis
For single or double treatments, pure erythrocytes (0.6% Hct) were suspended in 30 ml Ringer solution and treated with varying concentrations of costunolide (1–80 µM] or different concentrations of chelerythrine (1–10 µM) in combination with the highest costunolide concentration (80 µM). DMSO-treated erythrocytes served as a negative control. After the incubation time (24 h), 58 µl from a 310 mM NEM stock was given to each sample, gently mixed for 1 min and then centrifuged at 10 °C, 228×g. Supernatants were removed and the cell pellets were stored at − 20 °C until analyses. GSH and GSSG were measured on the clear supernatant obtained by treatment of 0.1 ml erythrocytes with 0.12 ml 15% (w/v) trichloroacetic acid. For GSH analysis one aliquot (0.05 ml) of supernatant was loaded onto HPLC and the GS-NEM conjugate was revealed by a diode-array detector at 265 nm wavelength [78]. GSSG was measured at the spectrophotometer by the GSH recycling method with slight modifications [79]. One aliquot of erythrocytes (10 μl) was hemolyzed by a 1:200 dilution with H2O for hemoglobin determination [80]. The HPLC analyses were carried out by an Agilent series 1100 instrument. The spectrophotometric analyses were performed by a Jasco V-530 instrument.
Determination of G6PDH activity
Pure erythrocytes (0.6% Hct) were incubated in 20 ml Ringer solution and treated with DMSO or different concentrations of costunolide. 24 h later, cell suspensions were centrifuged at 10 °C, 228×g. Supernatants were removed and the cell pellets were stored at − 80 °C until analyses. G6PDH activity was measured in erythrocytes lysates according to methods recommended by the International Committee for Standardization in Hematology [81]. The activity was expressed in units per grams of hemoglobin (IU/g Hb).
Statistical analysis
Data are presented as the mean values ± SEM of at least 3 independent experiments with different blood samples. A total of 18 different blood samples were used in this study. One-way ANOVA with Dunnet’s post test was used for statistical comparisons of treated samples with controls. Differences of the means were considered to be statistically significant when the calculated p value was less than 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Acknowledgements
Open Access funding provided by Projekt DEAL.
Author contributions
MG designed the project. MG and FL wrote mainly the manuscript. PK and DG partly wrote the section material and methods. MG performed eryptosis and FACS experiments. MG, DG and RR performed glutathione measurements. MG, PK, RM and VD conducted the enzyme experiments. MG, BF and MS performed fluorescence microscopy. MG and MK made the statistics, figures and submitted the manuscript. MG, FL, PK, DG, RM, VD, PD, UM, AMR, MK and TW analysed and discussed the data.
Funding
The project costs were mainly financed by M.G. The enzymatic measurements were supported by Czech Health Research Council, project NV19-07-00412.
Data availability
Raw data can be provided upon request.
Compliance with ethical standards
Conflicts of interest
The authors declare that no competing financial interests or otherwise exist.
Ethics approval
The study was approved by the ethics committee of the University of Tübingen (184/2003V).
Informed consent
All authors read and reviewed the manuscript and approved the final version.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Florian Lang and Martin Köberle share senior authorship.
References
- 1.Jin X, Wang C, Wang L. Costunolide inhibits osteosarcoma growth and metastasis via suppressing STAT3 signal pathway. Biomed Pharmacother. 2020;121:109659. doi: 10.1016/j.biopha.2019.109659. [DOI] [PubMed] [Google Scholar]
- 2.Butturini E, Cavalieri E, de Prati AC, et al. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS ONE. 2011;6:e20174. doi: 10.1371/journal.pone.0020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YK, Cho SG, Woo SM, et al. Saussurea lappa clarke-derived costunolide prevents TNF alpha -induced breast cancer cell migration and invasion by inhibiting NF- kappa B activity. Evid Based Complement Alternat Med. 2013;2013:936257. doi: 10.1155/2013/936257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Wang Z, Xie Y, Hu H. Antitumor activity and mechanism of costunolide and dehydrocostus lactone: two natural sesquiterpene lactones from the Asteraceae family. Biomed Pharmacother. 2020;125:109955. doi: 10.1016/j.biopha.2020.109955. [DOI] [PubMed] [Google Scholar]
- 5.Park E, Song JH, Kim MS, Park SH, Kim TS. Costunolide, a sesquiterpene lactone, inhibits the differentiation of pro-inflammatory CD4(+) T cells through the modulation of mitogen-activated protein kinases. Int Immunopharmacol. 2016;40:508–516. doi: 10.1016/j.intimp.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Yang YI, Lee KT, Park HJ, Choi JH. Costunolide induces apoptosis in human endometriotic cells through inhibition of the prosurvival Akt and nuclear factor kappa B signaling pathway. Biol Pharm Bull. 2011;34:580–585. doi: 10.1248/bpb.34.580. [DOI] [PubMed] [Google Scholar]
- 7.Park JB, Lee CK, Park HJ. Anti-Helicobacter pylori effect of costunolide isolated from the stem bark of Magnolia sieboldii. Arch Pharm Res. 1997;20:275–279. doi: 10.1007/BF02976157. [DOI] [PubMed] [Google Scholar]
- 8.Peng Z, Wang Y, Gu X, Guo X, Yan C. Study on the pharmacokinetics and metabolism of costunolide and dehydrocostus lactone in rats by HPLC-UV and UPLC-Q-TOF/MS. Biomed Chromatogr. 2014;28:1325–1334. doi: 10.1002/bmc.3167. [DOI] [PubMed] [Google Scholar]
- 9.Jeong SJ, Itokawa T, Shibuya M, et al. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002;187:129–133. doi: 10.1016/s0304-3835(02)00361-0. [DOI] [PubMed] [Google Scholar]
- 10.Saraswati S, Alhaider AA, Abdelgadir AM. Costunolide suppresses an inflammatory angiogenic response in a subcutaneous murine sponge model. APMIS. 2018;126:257–266. doi: 10.1111/apm.12808. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Moqbel S, Xu L, et al. Costunolide inhibits matrix metalloproteinases expression and osteoarthritis via the NF-κB and Wnt/β-catenin signaling pathways. Mol Med Rep. 2019;20:312–322. doi: 10.3892/mmr.2019.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B, Rong Y, Sun D, et al. Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-beta1/Smad2/Nrf2-NOX4 signaling pathways. Biochem Biophys Res Commun. 2019;510:329–333. doi: 10.1016/j.bbrc.2019.01.104. [DOI] [PubMed] [Google Scholar]
- 13.Ge MX, Liu HT, Zhang N, et al. Costunolide represses hepatic fibrosis through WW domain-containing protein 2-mediated Notch3 degradation. Br J Pharmacol. 2020;177:372–387. doi: 10.1111/bph.14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YE, Choi HC, Nam G, Choi BY. Costunolide promotes the proliferation of human hair follicle dermal papilla cells and induces hair growth in C57BL/6 mice. J Cosmet Dermatol. 2019;18:414–421. doi: 10.1111/jocd.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu ZL, He Q, Chu SS, Wang CF, Du SS, Deng ZW. Essential oil composition and larvicidal activity of Saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae) Parasitol Res. 2012;110:2125–2130. doi: 10.1007/s00436-011-2738-0. [DOI] [PubMed] [Google Scholar]
- 16.Choi JH, Ha J, Park JH, et al. Costunolide triggers apoptosis in human leukemia U937 cells by depleting intracellular thiols. Jpn J Cancer Res. 2002;93:1327–1333. doi: 10.1111/j.1349-7006.2002.tb01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghashghaeinia M, Giustarini D, Koralkova P, et al. Pharmacological targeting of glucose-6-phosphate dehydrogenase in human erythrocytes by Bay 11–7082, parthenolide and dimethyl fumarate. Sci Rep. 2016;6:28754. doi: 10.1038/srep28754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rostami-Yazdi M, Clement B, Schmidt TJ, Schinor D, Mrowietz U. Detection of metabolites of fumaric acid esters in human urine: implications for their mode of action. J Invest Dermatol. 2009;129:231–234. doi: 10.1038/jid.2008.197. [DOI] [PubMed] [Google Scholar]
- 19.Orr JW, Keranen LM, Newton AC. Reversible exposure of the pseudosubstrate domain of protein kinase C by phosphatidylserine and diacylglycerol. J Biol Chem. 1992;267:15263–15266. [PubMed] [Google Scholar]
- 20.Flint AJ, Paladini RD, Koshland DE., Jr Autophosphorylation of protein kinase C at three separated regions of its primary sequence. Science. 1990;249:408–411. doi: 10.1126/science.2377895. [DOI] [PubMed] [Google Scholar]
- 21.Trubiani O, Guarnieri S, Diomede F, et al. Nuclear translocation of PKCalpha isoenzyme is involved in neurogenic commitment of human neural crest-derived periodontal ligament stem cells. Cell Sign. 2016;28:1631–1641. doi: 10.1016/j.cellsig.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Zhu L, Kuokkanen S, Pollard JW. Activation of protein synthesis in mouse uterine epithelial cells by estradiol-17beta is mediated by a PKC-ERK1/2-mTOR signaling pathway. Proc Natl Acad Sci USA. 2015;112:E1382–1391. doi: 10.1073/pnas.1418973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alisi A, Spagnuolo S, Napoletano S, Spaziani A, Leoni S. Thyroid hormones regulate DNA-synthesis and cell-cycle proteins by activation of PKCalpha and p42/44 MAPK in chick embryo hepatocytes. J Cell Physiol. 2004;201:259–265. doi: 10.1002/jcp.20060. [DOI] [PubMed] [Google Scholar]
- 24.Valdes-Rives SA, de la Fuente-Granada M, Velasco-Velazquez MA, Gonzalez-Flores O, Gonzalez-Arenas A. LPA1 receptor activation induces PKCalpha nuclear translocation in glioblastoma cells. Int J Biochem Cell Biol. 2019;110:91–102. doi: 10.1016/j.biocel.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Takami M, Katayama K, Noguchi K, Sugimoto Y. Protein kinase C alpha-mediated phosphorylation of PIM-1L promotes the survival and proliferation of acute myeloid leukemia cells. Biochem Biophys Res Commun. 2018;503:1364–1371. doi: 10.1016/j.bbrc.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Kim CW, Asai D, Kang JH, Kishimura A, Mori T, Katayama Y. Reversal of efflux of an anticancer drug in human drug-resistant breast cancer cells by inhibition of protein kinase Calpha (PKCalpha) activity. Tumour Biol. 2016;37:1901–1908. doi: 10.1007/s13277-015-3963-4. [DOI] [PubMed] [Google Scholar]
- 27.Fine RL, Chambers TC, Sachs CW. P-glycoprotein, multidrug resistance and protein kinase C. Stem Cells. 1996;14:47–55. doi: 10.1002/stem.140047. [DOI] [PubMed] [Google Scholar]
- 28.Morrison MM, Young CD, Wang S, et al. mTOR Directs breast morphogenesis through the PKC-alpha-Rac1 signaling axis. PLoS Genet. 2015;11:e1005291. doi: 10.1371/journal.pgen.1005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Zhang J, Flechner L, et al. Protein kinase C-alpha overexpression stimulates Akt activity and suppresses apoptosis induced by interleukin 3 withdrawal. Oncogene. 1999;18:6564–6572. doi: 10.1038/sj.onc.1203065. [DOI] [PubMed] [Google Scholar]
- 30.Yun BR, Lee MJ, Kim JH, Kim IH, Yu GR, Kim DG. Enhancement of parthenolide-induced apoptosis by a PKC-alpha inhibition through heme oxygenase-1 blockage in cholangiocarcinoma cells. Exp Mol Med. 2010;42:787–797. doi: 10.3858/emm.2010.42.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasinski P, Zwolak P, Terai K, Borja-Cacho D, Dudek AZ. PKC-alpha inhibitor MT477 slows tumor growth with minimal toxicity in in vivo model of non-Ras-mutated cancer via induction of apoptosis. Invest New Drugs. 2011;29:33–40. doi: 10.1007/s10637-009-9330-9. [DOI] [PubMed] [Google Scholar]
- 32.de Jong K, Rettig MP, Low PS, Kuypers FA. Protein kinase C activation induces phosphatidylserine exposure on red blood cells. Biochemistry. 2002;41:12562–12567. doi: 10.1021/bi025882o. [DOI] [PubMed] [Google Scholar]
- 33.Klarl BA, Lang PA, Kempe DS, et al. Protein kinase C mediates erythrocyte "programmed cell death" following glucose depletion. Am J Physiol Cell Physiol. 2006;290:C244–253. doi: 10.1152/ajpcell.00283.2005. [DOI] [PubMed] [Google Scholar]
- 34.Lang KS, Lang PA, Bauer C, et al. Mechanisms of suicidal erythrocyte death. Cell Physiol Biochem. 2005;15:195–202. doi: 10.1159/000086406. [DOI] [PubMed] [Google Scholar]
- 35.Lang E, Lang F. Triggers, inhibitors, mechanisms, and significance of eryptosis: the suicidal erythrocyte death. Biomed Res Int. 2015;2015:513518. doi: 10.1155/2015/513518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghashghaeinia M, Wesseling MC, Ramos E, et al. Trifluoperazine-induced suicidal erythrocyte death and s-nitrosylation inhibition, reversed by the nitric oxide donor sodium nitroprusside. Cell Physiol Biochem. 2017;42:1985–1998. doi: 10.1159/000479838. [DOI] [PubMed] [Google Scholar]
- 37.Ghashghaeinia M, Cluitmans JC, Akel A, et al. The impact of erythrocyte age on eryptosis. Br J Haematol. 2012;157:606–614. doi: 10.1111/j.1365-2141.2012.09100.x. [DOI] [PubMed] [Google Scholar]
- 38.Myssina S, Huber SM, Birka C, et al. Inhibition of erythrocyte cation channels by erythropoietin. J Am Soc Nephrol. 2003;14:2750–2757. doi: 10.1097/01.asn.0000093253.42641.c1. [DOI] [PubMed] [Google Scholar]
- 39.Vota DM, Maltaneri RE, Wenker SD, Nesse AB, Vittori DC. Differential erythropoietin action upon cells induced to eryptosis by different agents. Cell Biochem Biophys. 2013;65:145–157. doi: 10.1007/s12013-012-9408-4. [DOI] [PubMed] [Google Scholar]
- 40.Fink M, Al Mamun Bhuyan A, Zacharopoulou N, Lang F. Stimulation of eryptosis, the suicidal erythrocyte death, by costunolide. Cell Physiol Biochem. 2018;50:2283–2295. doi: 10.1159/000495088. [DOI] [PubMed] [Google Scholar]
- 41.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 42.Andrews DA, Yang L, Low PS. Phorbol ester stimulates a protein kinase C–mediated agatoxin-TK–sensitive calcium permeability pathway in human red blood cells. Blood. 2002;100:3392–3399. doi: 10.1182/blood.V100.9.3392. [DOI] [PubMed] [Google Scholar]
- 43.Chmura SJ, Dolan ME, Cha A, Mauceri HJ, Kufe DW, Weichselbaum RR. In vitro and in vivo activity of protein kinase C inhibitor chelerythrine chloride induces tumor cell toxicity and growth delay in vivo. Clin Cancer Res. 2000;6:737–742. [PubMed] [Google Scholar]
- 44.Shi B, Li S, Ju H, Liu X, Li D, Li Y. Protein kinase C inhibitor chelerythrine attenuates partial unilateral ureteral obstruction induced kidney injury in neonatal rats. Life Sci. 2019;216:85–91. doi: 10.1016/j.lfs.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 45.Ghashghaeinia M, Bobbala D, Wieder T, et al. Targeting glutathione by dimethylfumarate protects against experimental malaria by enhancing erythrocyte cell membrane scrambling. Am J Physiol Cell Physiol. 2010;299:C791–804. doi: 10.1152/ajpcell.00014.2010. [DOI] [PubMed] [Google Scholar]
- 46.Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27:156–157. doi: 10.1016/j.ccell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Ghashghaeinia M, Koberle M, Mrowietz U, Bernhardt I. Proliferating tumor cells mimick glucose metabolism of mature human erythrocytes. Cell Cycle. 2019;18:1316–1334. doi: 10.1080/15384101.2019.1618125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardos G. The role of calcium in the potassium permeability of human erythrocytes. Acta Physiol Acad Sci Hung. 1959;15:121–125. [PubMed] [Google Scholar]
- 49.Grinstein S, Furuya W, Bianchini L. Protein kinases, phosphatases, and the control of cell volume. Physiology. 1992;7:232–237. [Google Scholar]
- 50.Kahle KT, Khanna AR, Alper SL, et al. K-Cl cotransporters, cell volume homeostasis, and neurological disease. Trends Mol Med. 2015;21:513–523. doi: 10.1016/j.molmed.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smirnova GV, Oktyabrsky ON. Glutathione in bacteria. Biochemistry (Moscow) 2005;70:1199–1211. doi: 10.1007/s10541-005-0248-3. [DOI] [PubMed] [Google Scholar]
- 52.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2008;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 53.Rana SV, Allen T, Singh R. Inevitable glutathione, then and now. Indian J Exp Biol. 2002;40:706–716. [PubMed] [Google Scholar]
- 54.Van’t Erve TJ, Wagner BA, Ryckman KK, Raife TJ, Buettner GR. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radical Biol Med. 2013;65:742–749. doi: 10.1016/j.freeradbiomed.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griffith OW. Glutathione turnover in human erythrocytes. Inhibition by buthionine sulfoximine and incorporation of glycine by exchange. J Biol Chem. 1981;256:4900–4904. [PubMed] [Google Scholar]
- 56.Jowett M, Quastel JH. The glyoxalase activity of the red blood cell: the function of glutathione. Biochem J. 1933;27:486–498. [PMC free article] [PubMed] [Google Scholar]
- 57.Ghashghaeinia M, Wieder T, Duszenko M, et al. Common features of oxidative stress and metabolic impairements in human erythrocytes and nucleated cells. In: Dichi I, et al., editors. Role of oxidative stress in chronic diseases. Boca Raton: CRC Press; 2014. pp. 421–478. [Google Scholar]
- 58.Cimen MY. Free radical metabolism in human erythrocytes. Clin Chim Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Łapiński R, Siergiejuk M, Worowska A, Gacko M. Oxidants and antioxidants of erythrocytes. Prog Health Sci. 2014;4:211–219. [Google Scholar]
- 60.Hill AS, Jr, Haut A, Cartwright GE, Wintrobe MM. The role of nonhemoglobin proteins and reduced glutathione in the protection of hemoglobin from oxidation in vitro. J Clin Invest. 1964;43:17–26. doi: 10.1172/JCI104889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simoni J, Villanueva-Meyer J, Simoni G, Moeller JF, Wesson DE. Control of oxidative reactions of hemoglobin in the design of blood substitutes: role of the ascorbate-glutathione antioxidant system. Artif Organs. 2009;33:115–126. doi: 10.1111/j.1525-1594.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 62.Kehr S, Jortzik E, Delahunty C, Yates JR, 3rd, Rahlfs S, Becker K. Protein S-glutathionylation in malaria parasites. Antioxid Redox Signal. 2011;15:2855–2865. doi: 10.1089/ars.2011.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.May JM. Ascorbate function and metabolism in the human erythrocyte. Front Biosci. 1998;3:d1–10. doi: 10.2741/a262. [DOI] [PubMed] [Google Scholar]
- 64.May JM, Qu Z, Morrow JD. Mechanisms of ascorbic acid recycling in human erythrocytes. Biochim Biophys Acta. 2001;1528:159–166. doi: 10.1016/s0304-4165(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 65.Raftos JE, Whillier S, Kuchel PW. Glutathione synthesis and turnover in the human erythrocyte: alignment of a model based on detailed enzyme kinetics with experimental data. J Biol Chem. 2010;285:23557–23567. doi: 10.1074/jbc.M109.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson ME, Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci USA. 1983;80:707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minnich V, Smith MB, Brauner MJ, Majerus PW. Glutathione biosynthesis in human erythrocytes. I. Identification of the enzymes of glutathione synthesis in hemolysates. J Clin Invest. 1971;50:507–513. doi: 10.1172/JCI106519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava SK, Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem. 1969;244:9–16. [PubMed] [Google Scholar]
- 69.Staal GE, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys Acta. 1969;185:39–48. doi: 10.1016/0005-2744(69)90280-0. [DOI] [PubMed] [Google Scholar]
- 70.Worthington DJ, Rosemeyer MA. Human glutathione reductase: purification of the crystalline enzyme from erythrocytes. Eur J Biochem. 1974;48:167–177. doi: 10.1111/j.1432-1033.1974.tb03754.x. [DOI] [PubMed] [Google Scholar]
- 71.Rall TW, Lehninger AL. Glutathione reductase of animal tissues. J Biol Chem. 1952;194:119–130. [PubMed] [Google Scholar]
- 72.Kuhajda FP, Jenner K, Wood FD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA. 1994;91:6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–562. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen L, Zhang Z, Hoshino A, et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat Metab. 2019;1:404–415. [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis CA, Parker SJ, Fiske BP, et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell. 2014;55:253–263. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Govekar R, Zingde S. Protein kinase C isoforms in human erythrocytes. Ann Hematol. 2001;80:531–534. doi: 10.1007/s002770100352. [DOI] [PubMed] [Google Scholar]
- 77.Lang PA, Kempe DS, Tanneur V, et al. Stimulation of erythrocyte ceramide formation by platelet-activating factor. J Cell Sci. 2005;118:1233–1243. doi: 10.1242/jcs.01730. [DOI] [PubMed] [Google Scholar]
- 78.Giustarini D, Dalle-Donne I, Milzani A, Rossi R. Detection of glutathione in whole blood after stabilization with N-ethylmaleimide. Anal Biochem. 2011;415:81–83. doi: 10.1016/j.ab.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat Protoc. 2013;8:1660–1669. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]
- 80.Di Iorio EE. Preparation of derivatives of ferrous and ferric hemoglobin. Methods Enzymol. 1981;76:57–72. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
- 81.Beutler E, Blume KG, Kaplan JC, Lohr GW, Ramot B, Valentine WN. International committee for standardization in haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977;35:331–340. doi: 10.1111/j.1365-2141.1977.tb00589.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data can be provided upon request.