Key Points
Question
What is the likelihood of signet ring cell cancer in patients with a germline CDH1 variant who undergo total gastrectomy according to family history?
Findings
In this cohort study of 101 patients who underwent total gastrectomy, signet ring cell cancer was identified in the surgical specimens of 89% of patients with a family history of gastric cancer and 67% of patients lacking family history.
Meaning
Total gastrectomy may be warranted for patients with CDH1 variants and a family history of gastric cancer and may be appropriate for those without a family history.
Abstract
Importance
CDH1 variants are increasingly identified on commercially available multigene panel tests, calling for data to inform counseling of individuals without a family history of gastric cancer.
Objectives
To assess association between CDH1 variant pathogenicity or family history of gastric or lobular breast cancer and identification of signet ring cell cancer and to describe outcomes of risk-reducing minimally invasive and open total gastrectomy.
Design, Setting, and Participants
This cohort study was performed from January 1, 2006, to January 1, 2020, in 181 patients with CDH1 germline variants from a single institution.
Interventions
Genetic counseling, esophagogastroduodenoscopy, and possible total gastrectomy.
Main Outcomes and Measures
CDH1 variant classification, family cancer history, findings of signet ring cell carcinoma at esophagogastroduodenoscopy and surgery, postoperative events and weight changes, and follow-up.
Results
Of 181 individuals with CDH1 germline variants (mean [SD] age at time of testing, 44 [15] years; 126 [70%] female), 165 harbored a pathogenic or likely pathogenic variant. Of these patients, 101 underwent open (n = 58) or minimally invasive (n = 43) total gastrectomy. Anastomotic leaks that required drainage were infrequent (n = 3), and median long-term weight loss was 20% (interquartile range [IQR], 10%-23%). In those undergoing minimally invasive operations, more lymph nodes were retrieved (median, 28 [IQR, 20-34] vs 15 [IQR, 9-19]; P < .001) and the hospital stay was 1 day shorter (median, 6 [IQR, 5-7] vs 7 [IQR, 6-7] days; P = .04). Signet ring cell cancer was identified in the surgical specimens of 85 of 95 patients (89%) with a family history of gastric cancer and 4 of 6 patients (67%) who lacked a family history. Among the latter 6 patients, 4 had a personal or family history of lobular breast cancer, including 2 with signet ring cell cancer. Of the 16 patients with pathogenic or likely pathogenic CDH1 variants who presented with locally advanced or metastatic gastric cancer, 3 (19%) had no family history of gastric cancer or personal or family history of lobular breast cancer.
Conclusions and Relevance
Total gastrectomy may be warranted for patients with pathogenic or likely pathogenic CDH1 variants and a family history of gastric or lobular breast cancer and may be appropriate for those without a family history. A minimally invasive approach is feasible and may be preferred for selected patients.
This cohort study assesses the association between CDH1 variant pathogenicity or family history of cancer and signet ring cell cancer and describes outcomes of minimally invasive and open total gastrectomy.
Introduction
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant cancer predisposition syndrome predominantly caused by loss-of-function germline variants in the tumor suppressor CDH1 (OMIM 192090) gene.1,2 CDH1 encodes for E-cadherin, a transmembrane glycoprotein that mediates cell-cell adhesion and influences epithelial architecture and cell invasion.3,4 Individuals with HDGC harboring a CDH1 variant have an estimated cumulative lifetime risk of diffuse gastric cancer of 70% for men and 56% for women, as well as a 42% lifetime risk for lobular breast cancer in women.4
Diffuse gastric cancer is characterized by poorly cohesive cells with intracytoplasmic mucin, called signet ring cells. Signet ring cell carcinoma (SRCC) can be detected in 0% to 64% of patients with CDH1 mutations by surveillance esophagogastroduodenoscopy (EGD).5,6,7,8,9,10,11,12 Esophagogastroduodenoscopy appears to remain insufficient for surveillance because SRCC is detected in the surgical specimens of 77% to 100% of patients who undergo risk-reducing total gastrectomy.5,6,7,9,10,11,13,14,15,16 Because of the lack of effective surveillance options and the multifocal nature of SRCC in HDGC, risk-reducing total gastrectomy is recommended for individuals with pathogenic or likely pathogenic germline CDH1 variants.17 However, the decision to undergo risk-reducing surgery requires careful discussion of potential benefits, including preventing possible invasive diffuse gastric cancer, against the drawbacks of potential postoperative morbidity and recovery, which affect eating habits and weight in the long term. Because CDH1 variants are increasingly identified on commercially available multigene panel tests, individuals without a family history of gastric cancer must also be counseled regarding the potential risks and benefits of surgery. This counseling is complicated by the lack of conclusive data on the risk of developing gastric cancer among this population.
We describe, to our knowledge, the largest reported single-institution series of patients harboring germline CDH1 variants and evaluate associations between variant pathogenicity or family history of gastric or lobular breast cancer and diagnosis of diffuse gastric cancer or identification of SRCC at EGD or in the surgical specimen. We also assess the pathological and surgical outcomes of patients undergoing open or minimally invasive risk-reducing total gastrectomies.
Methods
Study Population
In this cohort study, all individuals (n = 181) with germline CDH1 variants seen at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1, 2006, and January 1, 2020, were identified in the databases of the departments of surgery, pathology, and medicine. All patients underwent formal genetic counseling at our hospital or an outside institution. Most patients’ genetic testing was performed before their referral to MSKCC. Indications for genetic testing included personal gastric cancer diagnosis and family history of gastric or breast cancer. The CDH1 variant classifications were reviewed by a member of the Clinical Genetics Service. This study was approved by the MSKCC Institutional Review Board. Informed consent was waived because this was a retrospective study for which only MSKCC medical professionals had access to identifying information.
Germline CDH1 Analysis
Clinical CDH1 germline analysis was performed in Clinical Laboratory Improvement Amendments–approved laboratories between January 1, 2006, and January 1, 2020. Technologies used to analyze CDH1 included Sanger sequencing, multiplex ligation–dependent probe amplification, and next-generation sequencing. A board-certified molecular geneticist (Z.K.S.) at MSKCC classified variants using American College of Medical Genetics and Genomics criteria.18
Treatment
Individuals with CDH1 germline variants were treated by a multidisciplinary team. Important factors in treatment decisions were the specific CDH1 variant and its classification, family history of cancer, age, comorbidities, social infrastructure and support, and EGD findings and biopsy results. Surveillance EGDs were performed by 4 board-certified gastroenterologists, including 3 of us (M.S., H.G., and A.J.M.) experienced in hereditary gastric cancer. All EGDs used high-definition white light endoscopy and narrow-band imaging. Approximately 25 biopsies were collected throughout all anatomical segments of the stomach. Total gastrectomy was discouraged in patients younger than 21 years and in patients with substantial comorbidities that precluded operation. All patients underwent computed tomography (CT) preoperatively.
All total gastrectomies were performed with Roux-en-Y reconstruction by 1 of 3 dedicated gastric cancer surgeons (S.S.Y., D.G.C., or V.E.S.) at our institution. The operative approach (ie, open, laparoscopic, or robotic) and extent of lymphadenectomy (ie, D1 or D2) was selected at the surgeon’s discretion; the latter decision was informed by preoperative EGD results. Intraoperative frozen sections of the proximal and distal margin (now standard but not performed by 1 surgeon early in the series) were evaluated by a pathologist for esophageal squamous and duodenal mucosa, respectively, to confirm complete resection of gastric mucosa. Postoperative pathological evaluation19 by a dedicated gastrointestinal pathologist (L.H.T.) included mapping of the entire gastric mucosa and annotation of anatomical sites. Pathologists first examined a representative one-third of sections (mean, 68; range, 38-134) from each area of gastric mucosa according to a grid map, and if no intramucosal SRCC was identified, all collected sections were examined (mean, 236; range, 78-480). Patients were closely monitored postoperatively, and a radiologic water-soluble swallow study was performed only if clinically indicated.
Data Collection
All data were collected from the medical record. Family history of gastric and lobular breast cancer was considered in first-, second-, and third-degree relatives. Minimally invasive procedures that converted to open were classified as open. Postoperative events were graded according to the Clavien-Dindo system,20 with grades 1 and 2 considered minor and grades 3 to 5 considered major. Early events were defined as occurring within 30 days of surgery and late events between 30 and 90 days. Up to 3 early and 3 late postoperative events were reported per patient; for patients with more postoperative events, the most severe were reported.
Statistical Analysis
Data were compared between groups using the Kruskal-Wallis test for categorical variables and the Mann-Whitney test for continuous variables. Weight loss was calculated as the percentage of preoperative weight in patients for whom at least 1 postoperative weight was reported. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Inc). Two-sided P < .05 was considered to indicate statistical significance.
Results
Variants, Family History, and Presentation With Diffuse Gastric Cancer
Database review identified 181 individuals (mean [SD] age at the time of testing, 44 [15] years; 126 [70%] female) with germline CDH1 variants seen at MSKCC during the study period. Variant classification as pathogenic (P), likely pathogenic (LP), or of uncertain significance (VUS), family history, and EGD and surgical findings are summarized in Figure 1. Among the 20 patients presenting with metastatic (n = 16) or locally advanced (n = 4) diffuse gastric cancer (Figure 1), mean (SD) age at diagnosis was 46 (17) years and ranged from 18 to 69 years. Of the 16 with a P/LP variant, 3 did not have a family history of gastric cancer or personal or family history of lobular breast cancer; 3 of the 4 patients with a VUS also lacked such history. Five of the 20 patients, of whom 4 had locally advanced and 1 had metastatic cancer (cytologic test result converted to negative), underwent curative-intent gastrectomy. The remaining 15 patients with metastatic cancer received palliative chemotherapy. Of the 35 patients with a P/LP variant who had an EGD and did not undergo surgery at MSKCC, 7 were immediately unavailable for follow-up, 2 were scheduled for surgery, and 26 continued with surveillance and had at least 1 additional EGD; 23 were still being monitored at our institution.
Figure 1. Patient Flowchart.
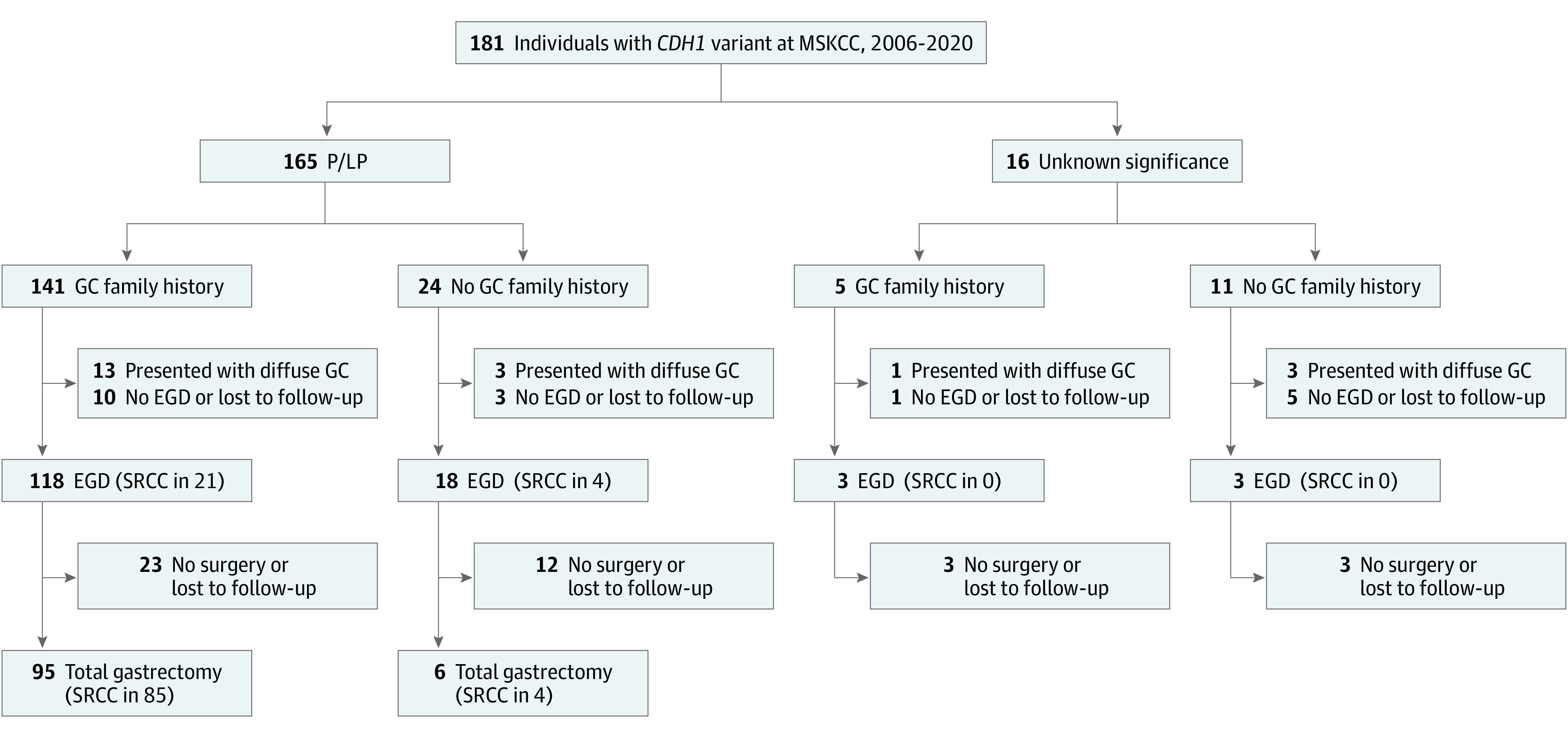
Family history includes first-, second-, and third-degree relatives. EGD indicates esophagogastroduodenoscopy; GC, gastric cancer; MSKCC, Memorial Sloan Kettering Cancer Center; P/LP, pathogenic or likely pathogenic; SRCC, signet ring cell carcinoma.
Asymptomatic Patients Presenting for Total Gastrectomy
A total of 101 asymptomatic patients with P/LP germline CDH1 variants underwent minimally invasive (n = 43) or open (n = 58) total gastrectomy at our institution (Figure 1). Six of these 101 patients had no first-, second-, or third-degree relative family history of gastric cancer; 4 of these patients had a personal or family history of invasive lobular breast cancer, and 2 had findings of SRCC at preoperative EGD with gastric biopsy.
SRCC at EGD and Surgery
In 25 of 142 asymptomatic patients (18%) who underwent surveillance EGD, SRCC was found (Figure 1). Among the 101 asymptomatic patients undergoing surgery, SRCC was found on preoperative EGD in 23 (24%) (Figure 1). Of these 23 patients, 7 had only gross examination of margins and no intraoperative frozen section. In addition, SRCC was detected in the surgical specimen in 88 patients (87%), 86 of whom had a stage 1a tumor (pT1a); the other 2 had SRCC invading the submucosa (pT1b). The number of sections examined was similar between patients with positive and negative SRCC findings. In all cases, lymph nodes tested negative. Among the 78 patients with negative preoperative EGD, 12 (15%) had no SRCC in their surgical specimen and 66 (85%) did; all positive preoperative EGD findings were confirmed on surgical pathological analysis. The sensitivity of EGD for identification of gastric cancer in the surgical specimen was therefore 26%, and the specificity was 100%. In the surgical specimen, SRCC was more common among patients with a family history of gastric cancer compared with those without (85 of 95 [89%] vs 4 of 6 [67%]), but given the limited sample size, this finding did not reach statistical significance (P = .10). Among patients with a family history of gastric cancer, SRCC was more commonly found in patients with a P variant compared with those with an LP variant (72 of 78 [92%] vs 8 of 12 [67%]; P = .009) (eFigure in the Supplement). The SRCC findings at surgery are presented according to family history in Figure 2.
Figure 2. Signet Ring Cell Carcinoma (SRCC) Findings Based on Family History of Gastric Cancer (GC) and Invasive Lobular Breast Cancer (ILBC) Among 101 Asymptomatic Patients With CDH1 Variants Who Underwent Total Gastrectomy at Memorial Sloan Kettering Cancer Center (MSKCC).
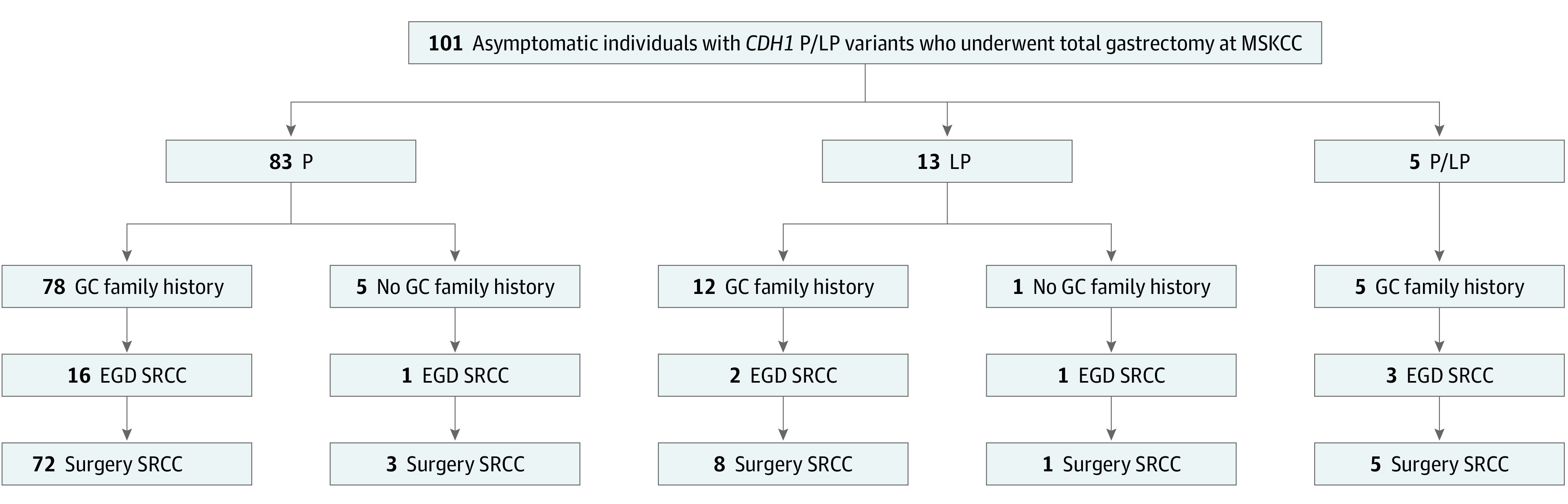
EGD indicates esophagogastroduodenoscopy.
Follow-up and Management
At a median follow-up of 16 months (IQR, 6-43 months), none of the 101 asymptomatic patients developed recurrent or invasive gastric cancer. In contrast, all 5 patients who presented with invasive diffuse gastric cancer experienced recurrence at a median of 18 months (IQR, 14-29 months) after gastrectomy. Four of the 5 died of gastric cancer, with a median time to death of 32 months (IQR, 23-42 months); the remaining patient, who had locally advanced ypT3N2 gastric cancer, remained alive more than 6 years after gastrectomy.
Among the 5 patients who presented with locally advanced or metastatic gastric cancer and underwent gastrectomy, 1 patient was treated in 2006, did not receive neoadjuvant treatment, and had a pT3N3a diffuse gastric cancer. The 4 patients who underwent neoadjuvant chemotherapy had pathological treatment responses of 50%, 80%, 90%, and 100% resulting in ypT4aN3b, ypT2N0, ypT3N2, and ypT0N0 diffuse gastric cancer, respectively. The patient with a 50% response initially had a positive cytologic test result at laparoscopy that converted to a negative result after chemotherapy.
Surgical Outcomes
Of 101 total gastrectomy specimens from asymptomatic patients, 57 had frozen section of the proximal margin, all of which ultimately contained squamous mucosa. Of the remaining 44 patients who had only a gross examination of the proximal margin without intraoperative frozen section confirmation, 3 (6.8%) ultimately had gastric mucosa (n = 2) or SRCC (n = 1) in the final proximal margin. Patient demographics, including body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), were comparable between the open and minimally invasive surgery (MIS) groups (Table 1). Although operative time was significantly longer for MIS, length of hospital stay was significantly shorter, and the number of lymph nodes retrieved was significantly greater.
Table 1. Characteristics of Asymptomatic Individuals With CDH1 Germline Variants Who Underwent Total Gastrectomy a.
| Characteristic | Total (N = 101) | Open gastrectomy (n = 58)b | MIS (n = 43)c | P value |
|---|---|---|---|---|
| Age, mean (SD), y | 42 (13) | 42 (13) | 43 (13) | .42 |
| Female | 68 (67) | 40 (69) | 28 (65) | .69 |
| BMI, median (IQR) | 26 (24-32) | 27 (30-52) | 25 (24-29) | .06 |
| History of other cancer | ||||
| None | 83 (82) | 47 (83) | 36 (84) | .90 |
| Invasive lobular breast cancer | 10 (10) | 5 (9) | 5 (12) | |
| Invasive ductal breast cancer | 1 (1) | 0 | 1 (2) | |
| Breast LCIS | 3 (3) | 2 (3) | 1 (2) | |
| Melanoma | 3 (3) | 2 (3) | 1 (2) | |
| Rectal cancer | 2 (2) | 2 (3) | 0 | |
| Family history of gastric cancer | ||||
| First or second degree | 89 (88) | 56 (97) | 33 (77) | .002 |
| Third degree only | 6 (6) | 2 (3) | 4 (9) | |
| None | 6 (6) | 0 | 6 (14) | |
| First- or second-degree history of lobular breast cancer | 17 (17) | 8 (14) | 9 (21) | .36 |
| Positive preoperative EGD | 23 (24) | 11 (20) | 12 (28) | .99 |
| ASA physical status | ||||
| I | 19 (19) | 9 (16) | 10 (23) | .49 |
| II | 55 (55) | 33 (57) | 22 (51) | |
| III | 27 (27) | 16 (28) | 11 (26) | |
| Operative time, median (IQR), min | 193 (163-218) | 169 (142-194) | 218 (203-233) | <.001 |
| SRCC detected | 88 (87) | 52 (90) | 37 (86) | .58 |
| No. of foci | ||||
| None | 13 (13) | 7 (12) | 6 (14) | .55 |
| ≤2 | 46 (46) | 29 (50) | 17 (40) | |
| ≥3 | 42 (42) | 22 (38) | 20 (47) | |
| pT | ||||
| 0 | 12 (12) | 6 (10) | 6 (14) | .23 |
| In situ | 1 (1) | 0 | 1 (2) | |
| 1a | 86 (85) | 50 (86) | 36 (84) | |
| 1b | 2 (2) | 2 (3) | 0 | |
| No. lymph nodes dissected, median (IQR) | 18 (13-26) | 15 (9-19) | 28 (20-34) | <.001 |
| Length of hospital stay, median (IQR), d | 7 (6-7) | 7 (6-7) | 6 (5-7) | .04 |
| Follow-up duration, median (IQR), mo | 14 (6-45) | 12 (6-44) | 17 (10-24) | .79 |
| Deceased | 4 (4) | 3 | 1 | |
| From other cause | 3 (3) | 2 | 1 | .56 |
| From gastrectomy | 1 (1) | 1 | 0 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LCIS, lobular carcinoma in situ; MIS, minimally invasive surgery; SRCC, signet ring cell carcinoma.
Data are presented as number (percentage) of patients unless otherwise indicated.
Includes 4 conversions from robotic to open.
Includes 35 complete robotic procedures and 8 complete laparoscopic procedures.
Rates of postoperative events are reported in Table 2. One patient with multiple comorbidities whose treatment was carefully optimized during a 3-month period and was cleared by treating physicians before the operation, died after a complicated postoperative course. This patient had a history of kidney transplant and hepatitis, developed pneumonia and sepsis, and died secondary to multiorgan failure, although a radiologic water-soluble swallow study result was negative for an esophagojejunal leak. In patients who underwent open surgery, 1 anastomotic leakage required radiologic drainage or endoscopic stent placement and 1 was treated with temporary total parenteral nutrition. Twelve patients (12%) were readmitted within 30 days of surgery. Three patients were readmitted after 30 days and required a percutaneous endoscopic jejunostomy for 2, 9, and 15 months.
Table 2. Postoperative Eventsa.
| Postoperative event | Patients, No. (%) (N = 101) |
|---|---|
| Early (<30 d) | |
| Major | 16 (16) |
| Any | 28 (28) |
| Death | 1 (1) |
| Anastomotic leakage requiring intervention | 3 (3) |
| Anastomotic leakage not requiring intervention | 3 (3) |
| Duodenal stump leakage | 1 (1) |
| Hemorrhage requiring surgery | 2 (2) |
| Small-bowel necrosis requiring surgery | 1 (1) |
| Anastomotic stricture requiring dilatation | 5 (5) |
| Pleural effusion requiring drainage | 4 (4) |
| Pneumothorax requiring chest tubes | 4 (4) |
| Intubation | 1 (1) |
| Wound infection requiring antibiotics or incision and drainage | 8 (8) |
| Deep vein thrombosis or pulmonary embolism | 2 (2) |
| Pneumonia | 2 (2) |
| Urinary tract infection | 1 (1) |
| Late (1-3 mo) | |
| Major | 18 (18) |
| Any | 20 (20) |
| Surgical hernia repair | 4 (4) |
| Revision surgery | 1 (1) |
| Anastomotic stricture requiring dilatation(s) | 15 (15) |
| Readmission for feeding tube | 3 (3) |
| Abscess requiring drainage | 1 (1) |
| Wound infection requiring antibiotics or incision and drainage | 1 (1) |
Up to 3 of the most severe early and late events were collected.
Weight Loss
At least 1 postdischarge weight was available for 79 patients (78%). One patient was underweight preoperatively, and she had lost 17% of her weight by 3 months after gastrectomy. Median long-term weight loss was 20% (interquartile range [IQR], 10%-23%). Weight loss varied with preoperative BMI, with the greatest loss, a median of 28% at 2 years, among obese patients, followed by 18% among overweight patients and 14% among those of normal weight (Figure 3A). Despite substantial weight loss, only 1 patient (4%) was underweight (Figure 3B).
Figure 3. Weight Change After Total Gastrectomy.
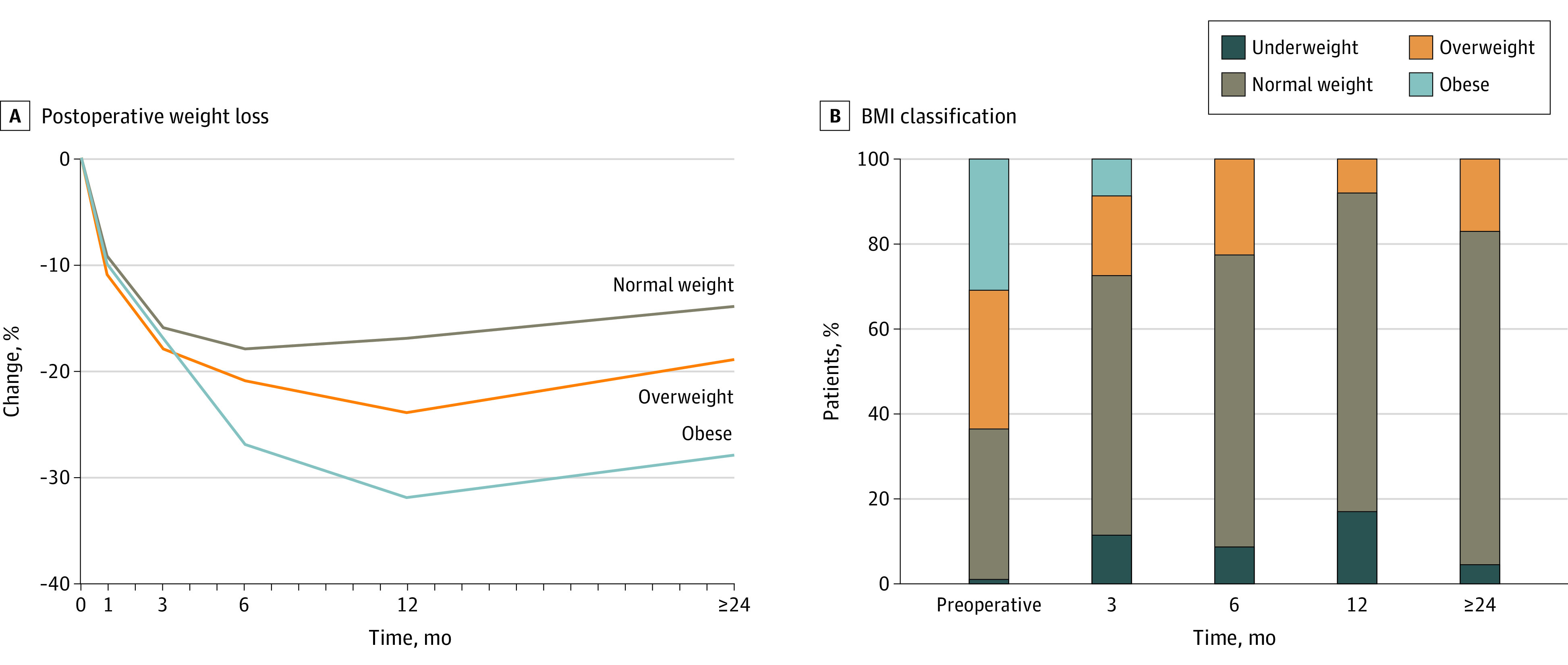
A, Postoperative weight loss as a percentage of preoperative weight, according to preoperative body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). B, Postoperative change in BMI. Weight at postoperative months 3, 6, 12, and 24 or later represents the mean from 2 to 4, 5 to 8, 6 to 17, and 18 months onward, respectively.
Discussion
We assessed outcomes of, to our knowledge, the largest reported single-institution series of patients with germline CDH1 variants. Our findings confirm the appropriateness of offering prophylactic gastrectomy to patients with P/LP CDH1 variants and a family history of gastric cancer as advised by the International Gastric Cancer Linkage Consortium; 89% of such patients had SRCC on total gastrectomy. However, we also found that patients with CDH1 variants but no family history of gastric cancer had a quantifiable risk of SRCC, and several such patients, including some with no personal or family history of lobular breast cancer, presented with metastatic or locally advanced SRCC. We also found that the sensitivity of surveillance EGD remained low at 26%. The 100% specificity of surveillance EGD suggests that gastrectomy in patients with positive EGD findings is needed. The poor outcomes in patients who presented with locally advanced or metastatic gastric cancer underscore the potential risks of failing to intervene. Finally, our initial data on the surgical outcomes of open and minimally invasive gastrectomy indicate that such an approach may be feasible in this population.
Our results may inform the management of patients with a P/LP CDH1 variant in the absence of a family history of gastric cancer. Of 6 such patients in our study, 4 (67%) had SRCC on final pathologic analysis, 2 of whom had no family or personal history of gastric cancer or lobular breast cancer. Moreover, among the total cohort of 24 patients with a P/LP variant and no family history of gastric cancer, 3 presented with metastatic or locally advanced diffuse gastric cancer and had no personal or family history of lobular breast cancer. Our study suggests that family history is not a reliable determinant associated with SRCC risk, as concluded by Jacobs et al,12 who found that 3 of 12 patients with P/LP CDH1 variants and SRCC identified at EGD with gastric biopsy did not have a family history of gastric cancer in their 3-generation pedigree. These data indicate that these patients may not currently be adequately counseled regarding their potential risk and emphasize the need for referral centers that focus on the multidisciplinary management of CDH1 variants.
Of the 20 patients who presented with metastatic or locally advanced gastric cancer, 4 had a VUS. However, the pathogenicity of these VUSs remained unproven, and it remains unclear whether any of the VUSs contributed to the development of diffuse gastric cancer or, much more likely, were incidental. We do not recommend total gastrectomy to patients who harbor VUSs but encourage patients to continue follow-up with a genetics service for potential updates in variant pathogenicity classification.
The current data also provide information on the penetrance of variants classified by American College of Medical Genetics and Genomics as P/LP. We found that 9 of 13 patients (69%) with an LP CDH1 variant had SRCC on total gastrectomy compared with 75 of 83 patients (90%) with a P variant. Although the number of patients was small, this finding may support nonpathogenicity of a subset of variants or less penetrant, likely pathogenic variants that retain some level of protein function. However, microscopic foci of SRCC may never grow into an invasive diffuse gastric cancer because a previous study21 suggested that early CDH1-associated lesions were transient or remained indolent. The reported 33% to 70% lifetime risk of clinical gastric cancer4,22 is therefore lower than the reported 77% to 100% incidence of SRCC in surgical specimens.5,6,7,9,10,11,13,14,15,16 Further investigation into predictors of cancer development among patients with P/LP CDH1 variants is warranted.
The early postoperative event rate of 28% in our study was within the range previously reported from other institutions (10%-48%) in case series of 10 and 23 individuals with CDH1 variants treated with total gastrectomy.5,7 Compared with these studies,5,7 in the present study, the rate of anastomotic leaks requiring drainage was lower at 3% vs 9%, late postoperative event rates were lower at 20% vs 35%, and median weight loss was similar at 20% vs 19%. Other series of total gastrectomy in individuals with CDH1 variants, including 6 to 18 patients, did not describe postoperative events or stated that there were none.6,9,10,11,13,14,15
Although we found that MIS allows extended lymphadenectomy, which is performed at our center in patients with SRCC-positive EGD biopsy results, this is not essential for an asymptomatic patient undergoing prophylactic gastrectomy.17 Lymph node metastasis has not been reported in asymptomatic patients with CDH1 variants, and the risk of lymph node metastasis in patients with pT1a gastric cancer without a CDH1 variant is low (2%-6%). In addition, laparoscopic or robotic gastrectomy offers the well-established advantages of fewer potential long-term sequelae, such as incisional hernia or intra-abdominal adhesions, and quicker recovery.
Limitations
This study has several limitations. First, some patients were genetically counseled elsewhere, obscuring the reasons for their genetic testing. The use of family history for risk calculations also has disadvantages; for example, family history could be unknown to the patient or limited in situations, such as adoptions or small families. In addition, a significant number of patients were unavailable for follow-up, leaving their outcomes unknown, and the numbers of patients with an LP variant or without a family history of gastric cancer were too small to provide conclusive data regarding their risk. In addition, the lack of randomization between the open and minimally invasive approach, as well as inconsistent follow-up, limited our ability to compare outcomes. However, to our knowledge, this is the only study to date that describes a large cohort in whom the MIS approach was used for risk-reducing total gastrectomy in patients with germline CDH1 variants.
Conclusions
The findings suggest that although surveillance EGD can identify SRCC in some patients with CDH1 variants, it is not adequate for long-term surveillance of diffuse gastric cancer in this patient population. For patients with P/LP CDH1 germline variants and a family history of gastric cancer who are acceptable candidates for an operation, risk-reducing total gastrectomy may be an appropriate treatment option. When performed by surgeons experienced in the approach, MIS appears to be feasible and may provide advantages. In this study, patients with P/LP CDH1 variants and no family history of gastric cancer had a quantifiable risk of SRCC. Future studies should evaluate additional patients in this population to further corroborate these findings and stratify more precise risks of SRCC for individuals with a CDH1 variant but without a family history of gastric and/or lobular breast cancer.
eFigure. Signet Ring Cell Carcinoma (SRCC) Findings in 101 Asymptomatic Patients With CDH1 Variants Who Underwent Total Gastrectomy at MSK Based on Variant Pathogenicity and Family History of Gastric Cancer (GC)
References
- 1.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392(6674):402-405. doi: 10.1038/32918 [DOI] [PubMed] [Google Scholar]
- 2.Guilford PJ, Hopkins JB, Grady WM, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14(3):249-255. doi: [DOI] [PubMed] [Google Scholar]
- 3.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4(2):118-132. doi: 10.1038/nrc1276 [DOI] [PubMed] [Google Scholar]
- 4.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23-32. doi: 10.1001/jamaoncol.2014.168 [DOI] [PubMed] [Google Scholar]
- 5.Hebbard PC, Macmillan A, Huntsman D, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol. 2009;16(7):1890-1895. doi: 10.1245/s10434-009-0471-z [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Kingham K, Ford JM, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol. 2011;18(9):2594-2598. doi: 10.1245/s10434-011-1648-9 [DOI] [PubMed] [Google Scholar]
- 7.Pandalai PK, Lauwers GY, Chung DC, Patel D, Yoon SS. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery. 2011;149(3):347-355. doi: 10.1016/j.surg.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Shaw D, Blair V, Framp A, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut. 2005;54(4):461-468. doi: 10.1136/gut.2004.049171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norton JA, Ham CM, Van Dam J, et al. CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann Surg. 2007;245(6):873-879. doi: 10.1097/01.sla.0000254370.29893.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber ME, Save V, Carneiro F, et al. Histopathological and molecular analysis of gastrectomy specimens from hereditary diffuse gastric cancer patients has implications for endoscopic surveillance of individuals at risk. J Pathol. 2008;216(3):286-294. doi: 10.1002/path.2415 [DOI] [PubMed] [Google Scholar]
- 11.Hüneburg R, Marwitz T, van Heteren P, et al. Chromoendoscopy in combination with random biopsies does not improve detection of gastric cancer foci in CDH1 mutation positive patients. Endosc Int Open. 2016;4(12):E1305-E1310. doi: 10.1055/s-0042-112582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs MF, Dust H, Koeppe E, et al. Outcomes of endoscopic surveillance in individuals with genetic predisposition to hereditary diffuse gastric cancer. Gastroenterology. 2019;157(1):87-96. doi: 10.1053/j.gastro.2019.03.047 [DOI] [PubMed] [Google Scholar]
- 13.Carneiro F, Huntsman DG, Smyrk TC, et al. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol. 2004;203(2):681-687. doi: 10.1002/path.1564 [DOI] [PubMed] [Google Scholar]
- 14.Charlton A, Blair V, Shaw D, Parry S, Guilford P, Martin IG. Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut. 2004;53(6):814-820. doi: 10.1136/gut.2002.010447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch HT, Kaurah P, Wirtzfeld D, et al. Hereditary diffuse gastric cancer: diagnosis, genetic counseling, and prophylactic total gastrectomy. Cancer. 2008;112(12):2655-2663. doi: 10.1002/cncr.23501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strong VE, Gholami S, Shah MA, et al. Total gastrectomy for hereditary diffuse gastric cancer at a single center: postsurgical outcomes in 41 patients. Ann Surg. 2017;266(6):1006-1012. doi: 10.1097/SLA.0000000000002030 [DOI] [PubMed] [Google Scholar]
- 17.van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52(6):361-374. doi: 10.1136/jmedgenet-2015-103094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K, Krempely K, Roberts ME, et al. Specifications of the ACMG/AMP variant curation guidelines for the analysis of germline CDH1 sequence variants. Hum Mutat. 2018;39(11):1553-1568. doi: 10.1002/humu.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers WM, Dobo E, Norton JA, et al. Risk-reducing total gastrectomy for germline mutations in E-cadherin (CDH1): pathologic findings with clinical implications. Am J Surg Pathol. 2008;32(6):799-809. doi: 10.1097/PAS.0b013e31815e7f1a [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Post RS, Carneiro F. Emerging concepts in gastric neoplasia: heritable gastric cancers and polyposis disorders. Surg Pathol Clin. 2017;10(4):931-945. doi: 10.1016/j.path.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 22.Roberts ME, Ranola JMO, Marshall ML, et al. Comparison of CDH1 penetrance estimates in clinically ascertained families vs families ascertained for multiple gastric cancers. JAMA Oncol. 2019;5(9):1325-1331. doi: 10.1001/jamaoncol.2019.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Signet Ring Cell Carcinoma (SRCC) Findings in 101 Asymptomatic Patients With CDH1 Variants Who Underwent Total Gastrectomy at MSK Based on Variant Pathogenicity and Family History of Gastric Cancer (GC)


