Abstract
This study aims to suggest a simple migratory cell monitoring method in the Transwell system by utilizing retroreflective Janus microparticles (RJPs) as an optical probe. The RJP could be internalized on cells without compromising the cell viability and can be registered as bright spots within the cell body by inducing retroreflection from nonspectroscopic light sources. Conventional optical probes (e.g., fluorophores, chromogens, and nanoparticles) have been extensively studied and applied across diverse platforms (e.g., Boyden chamber, wound closing, and microfluidic chips) for understanding in vitro kinetic cell behavior. However, the complexities of running such platforms and setting up analytical instruments are limiting. In this regard, we aimed to demonstrate a modified Transwell migration assay by introducing the retroreflection principle to the cell quantification procedures that ensure a simplified optical setup, assure easy signal acquisition, and are compatible with conventional platforms. To demonstrate retroreflection as a signaling principle, a half-metal-coated silica particle that can induce interior retroreflection was synthesized. Because the RJPs can concentrate incident light and reflect it back to the light source, retroreflection was distinctively recognizable and enabled sensitive visualization. To verify the applicability of the developed migration assay, cell quantification during the incremental progress of macrophage migration, and cell quantification under gradients of chemoattractant monocyte protein-1, was accomplished by obtaining phagocytosed RJP-mediated retroreflection signals. Considering that conventional assays are designed as endpoint measurements, we anticipate the proposed retroreflection-based cell quantification technique to be a promising solution, bypassing current limitations.
1. Introduction
In the field of cell biology, cell imaging techniques have been extensively investigated to develop an intuitive understanding of cellular behavior and environments using various optical probes (e.g., enzymes, chromogens, nanoparticles, fluoroproteins, and fluorophores) that can be analyzed under complicated spectral configurations. Notably, the diversity of optical probes and analytical instrumentation has led to the empowerment of researchers to confirm experimental results and whether they could find a desired biological phenomenon by acquiring signals from such optical probes. However, surplus complexity in experimental design should be avoided for simple analytical applications. From this point of view, a combination of probes, analyzers, and execution procedures should be considered for effective implementation to reach desired performances. In cases of cell quantification or monitoring cellular kinetic behavior, the analysis strategy should be a simplified procedure with the registration of intuitive results. Evaluation of migration and invasion properties of target cells has been significant in understanding cell behavior during normal proliferation, immune responses, disease development, and cancer progression.1−3 Using this approach, researchers have been able to test the regulatory and stimulatory factors involved in the migration capacity of cells with genome reconstitution or transformation. Traditionally, modified Boyden chamber assays, Transwell insert migration assays, and wound closure assays have been frequently conducted to demonstrate the migratory behavior derived from regulatory signals or other stimuli.4−6 In the case of utilizing a Transwell insert, the membrane with micropores attached to the bottom of the insert structure is integrated on a plate well and cells plated in the upper chamber migrate to the opposite side of the membrane, or settle in the lower chamber, attracted by the chemoattractant and chemokines. After an appropriate migration duration, the cells are carefully removed from the upper chamber using cotton swabs, and the remaining migrated cells are fixed with paraformaldehyde. For evaluating migratory cell populations, dye staining steps using colorimetric or fluorescent dyes have been applied. From this, conventional steps for migratory assays demand skillful and trained users, involve time-consuming processes, and cannot identify the progress of migration status until cell fixation and dye staining steps are conducted. Of course, current modifications to the Transwell migration assay offer alternative options,7−9 such as the use of fluorescent dyes, which enable live-cell imaging with the fluorescence-blocking material coated on Transwell. However, we expect that there is an opportunity for improvements to involve more simplified procedures using other signaling strategies. Therefore, to achieve a simplified method for analyzing the migratory cell property, it is more desirable to omit the cell fixation and staining process. Additionally, a new analytical process would enable real-time monitoring while maintaining conventional Transwell inserts for easy adaptation. In this regard, we introduced a retroreflective Janus microparticle (RJP) as a new optical probe for analyzing the migratory macrophages using the principle of retroreflection. Retroreflection is a reflected light phenomenon that returns in the direction of the light source. RJPs are particles that are able to induce retroreflection via multiple internal reflections. RJPs can be synthesized by a hemispheric coating of metals such as aluminum and gold on transparent particles. Because the size of RJPs is more than 700 nm, the particles can simply reflect the full range of visible light wavelengths, such as polychromatic white light, compared to fluorescence and luminescence analyses, which require sophisticated monochromatic light sources and filter systems.10−15 When acquiring retroreflection using a microscope, the white light-emitting diode (LED) optical component can obtain a retroreflected signal from the sample simply by placing it at a specific angle to the objective lens. Based on the working principles of retroreflection and application of RJPs, a cell assay was designed and tested for applicability in migration assays.
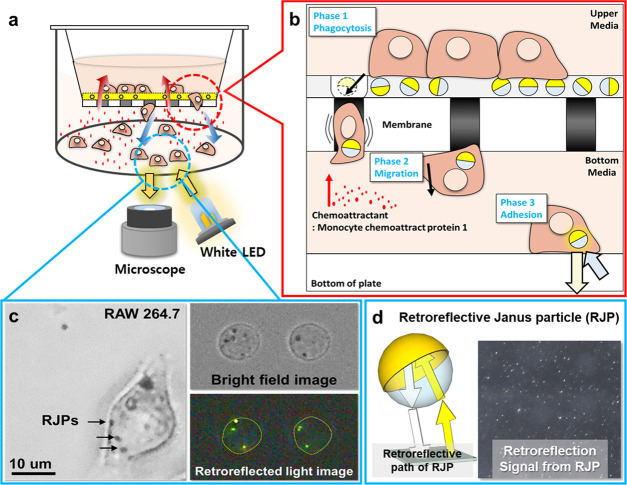
The proposed migration assay uses nearly the same components as the conventional Transwell system except for an extracellular matrix (ECM) coating on micropore membranes that deposited the RJPs (Figure 1a). The ECM was coated with two types of Matrigel mixtures that make two concentric circular areas to confine the cell migration area. The central circular area was coated with the RJP-deposited Matrigel matrix that allowed RJPs to be engulfed by macrophages, which then migrated cells to the lower chamber. The outer circular area was composed of highly concentrated Matrigel that formed a thick layer to impede the migration of cells. By limiting the migration area, the migrated cells were also present in the limited area, saving time for measuring the retroreflection signals. The cellular uptake of RJP was performed before introducing the chemokines or the chemoattractant. At the commencement of the assay, RJP-bearing macrophages migrated toward the lower chamber through the micropores (Figure 1b); the migrated cells could be observed under a retroreflective optical setup on a microscope. Unlike bright-field images, the images acquired under a retroreflective setting did not fully reveal cellular shapes or morphologies and the only signal present in the cell body was that of the RJPs, shining in the form of bright spots (Figure 1c,d). The acquired images were then processed to elicit the number of RJPs using background subtraction and signal counting plugin of ImageJ S/W. Subsequently, the obtained RJP counts were converted into cell counts using a conversion constant that estimated the population distribution of the engulfed RJP counts from single macrophages. Through the above design, we have developed a method to overcome the limitations of endpoint analysis in traditional migration assays, and which is able to produce results in real time. To verify applicability, a migration model of RAW264.7 responses to the chemokine monocyte chemoattractant protein-1 (MCP-1) was implemented.16−19 Details are reported herein.
Figure 1.
Schematic illustration of the working principles of the retroreflection-based migration assay. (a) Transwell system for the migration of macrophages toward a chemoattractant in the RJP-Matrigel coating layer. (b) Schematic illustration of the magnified view of migratory cell behavior on Transwell membranes with the RJP-Matrigel coating. (c) Microscopic images from RJP-engulfed macrophages that had migrated from the upper to the bottom of the lower chamber. (d) Optical properties of retroreflective Janus microparticles.
2. Materials and Methods
2.1. Materials and Apparatus
MCP-1/CCL2 mouse recombinant protein was acquired from Sigma-Aldrich (MO). Bovine serum albumin (BSA) fraction V was acquired from Bioworld (OH). Murine leukemia virus-transformed RAW264.7 cell line was acquired from ATCC (VA). Growth factor-free, phenol red-free Matrigel was purchased from Corning (NY). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin (PS) were purchased from Gibco (NY). For the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reactions, a cytotoxicity test solution was purchased from Promega (WI). A sodium phosphate-buffered saline (PBS) solution (50 mM) containing 0.15 M NaCl (PBS, pH 7.2) was prepared using double-distilled and deionized water (DDW, specific resistance >18 M cm). For the BSA solution, ultrapure water was acquired from Biosesang (Seongnam, South Korea). The RJPs were synthesized by the sequential deposition of aluminum and gold metal layers on 700 nm diameter SiO2 microparticles. For constructing the microscope-attachable retroreflective optical setup, a white light-emitting diode (white, 5500 K) was purchased from Mightex Systems (ON, Canada).
2.2. Preparation of Retroreflective Janus Microparticles
In this study, RJPs were utilized as optical probes for cell monitoring. Previous studies introduced the preparation and application of RJPs as affinity reaction-based optical probes conjugated to recognition components such as antibodies, DNA, and proteins.10−15 Unlike previous reports, in this research, RJPs were prepared with a minimized surface passivation procedure to generate a pristine particle. The detailed methodology of synthesizing RJPs is described by Han et al.10 However, here, we briefly present details of synthesizing RJPs as cell monitoring probes. RJPs were based on transparent and monodispersed silica microparticles, but specific procedures can change RJP optical properties with Au and Al deposition. To this end, the Langmuir–Blodgett method and electron beam physical vapor deposition of metal layers on the hemispheric 700 nm silica microparticles tightly packed on the glass substrate were employed. After the sequential vapor deposition of aluminum and gold layers on the hemispheric side of silica microparticles, the RJP-packed glass substrate was immersed in ultrapure water and sonicated for 1 min. Sonication detached the RJPs from the glass substrate, and dispersed particles were easily collected by centrifugation. Fully dispersed RJPs were washed twice with ultrapure water and stored in 0.2% BSA solution until use.
2.3. Cell Culture
Murine leukemia virus-transformed macrophages (RAW264.7, ATCC) were maintained in DMEM supplemented with 10% FBS and 1% PS (termed complete medium). RAW264.7 cells were cultured in a humidified 5% CO2 atmosphere incubator at 37 °C.
2.4. Cytotoxicity Test
To verify the cytotoxicity of RJPs to RAW264.7 cells, MTS reagent20 was reacted with a range of concentrations of the RJP-containing DMEM medium (0, 0.1, 1, 5, 10, 25, 50, 100 μg/mL). First, 100 μL of RAW264.7 cell suspension was seeded into 96-well plates at a density of 5 × 105 cells/mL and incubated for 2 h to settle onto the well surfaces. After the cells settled, various concentrations of RJPs suspended in the complete medium were used to replace the previous culture medium. The incubation lasted 24 h; this being equal to the incubation time for Transwell migration assays. Finally, 20 μL of MTS solution was added to react for 2 h, and the optical absorbance at 490 nm was measured using a plate reader. Each test was performed in triplicate, and the relative absorbance of samples was calculated as percentages based on the RJP-negative (control) medium.
2.5. Preparation of Transwell Inserts for the Migration Assay
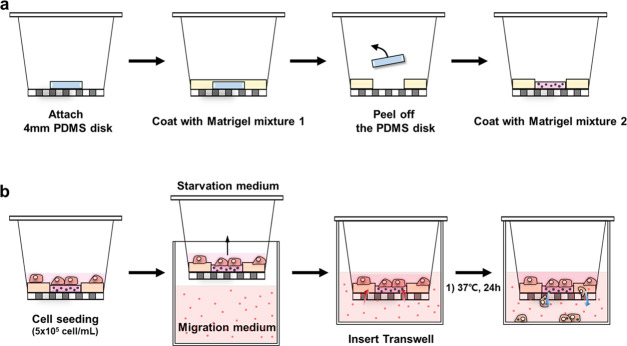
To prepare the RJP-deposited Transwell inserts, RJP suspension added to the Matrigel solution was simply drop-cast on the surface of the Transwell membrane. The Transwell inserts selected were a commercial 12-well format with an 8 μm pore size polyethylene terephthalate (PET) membrane. However, in the experiments described here, the micropore membrane was modified not only to deposit the RJPs onto the Matrigel but also to limit the region for migration on the Transwell membrane to increase convenience during the analysis process. To limit the migration region on the membrane of commercial Transwell inserts, two different density Matrigel matrixes were sequentially coated. To achieve this, we conducted a sequential coating procedure using a poly(dimethylsiloxane) (PDMS) disk (width 0.7 mm, diameter 4 mm) as a template for the coating area. First, the PDMS disk was attached to the center of the membrane, and the remaining area was blocked by coating with a highly concentrated Matrigel mixture termed Matrigel mixture I. Matrigel mixture I was composed of 2 mg/mL Matrigel in a 0.2% BSA solution, resulting in the blockage of membrane surface micropores. After blockage, the confined migration area on the membrane was then coated with another Matrigel mixture referred to as Matrigel mixture II, which was composed of less than 1 mg/mL Matrigel solution in DMEM, with 10 μg/mL RJPs. Matrigel mixtures I and II were freshly prepared under ice-cold conditions, and all equipment used was also prepared under ice-cold conditions. With these two types of Matrigel coatings on the Transwell membrane, the physically restricted compartmentalized migration area was defined and the remaining area was blocked. Within the central area, the Matrigel matrix contained RJPs to be phagocytosed by macrophages. In detail, first, the PDMS disk was placed at the center of the membrane and then Matrigel mixture I was used for coating for 2 h in an oven at 37 °C. The residues were gently washed with 50 mM PBS and DDW twice and dried with continuous airflow. Before the next coating step, the PDMS disk was removed to expose the pristine surface of the membrane. Matrigel mixture II was then coated on the center of the pristine surface. The coating step lasted 1 h at 37 °C. The residues were gently immersed in 50 mM PBS and DDW, and then, the Transwell inserts were dried in the same manner as before the drying step (Figure 2a). From this, the preparation of the RJP-deposited Transwell inserts to be used for retroreflection-based migration assays was completed. Before executing the migration assay for macrophages in our modified Transwell system, murine leukemia virus-transformed RAW264.7 macrophages were prestarved for 12 h in a starvation medium (0.2% BSA in DMEM).16 After starvation, RAW264.7 cells were plated at 5 × 105 cell/mL in the upper chamber of the Transwell insert and incubated at 37 °C for another 4 h. After that, the migration medium containing MCP-1 was prepared by serial dilution (0, 1, 10, 100 ng/mL) of the complete medium. Migration commenced with the immersion of cell-plated Transwell inserts in migration media containing MCP-1 (Figure 2b). In this study, migration toward chemokine MCP-1 by the RAW264.7 cells lasted 24 h, but the migration status at 6, 12, and 24 h was also monitored.
Figure 2.
Schematic illustration of procedures involved in Transwell migration assays. (a) Preparation of Transwell inserts with the Matrigel mixture containing RJPs. In this research, the migration chamber was modified by compartmentation of two different Matrigel mixture coatings to confine the available migration area on Transwell inserts. First, Matrigel mixture I was coated to form the blocked area. Second, Matrigel mixture II, which contained RJPs, was coated to form an RJP-deposited Matrigel matrix. (b) Migration assay procedure with modified Transwell inserts. Cell starvation prior to migration was undertaken with the migration medium containing MCP-1 and incorporating 10% FBS and 1% PS-supplemented DMEM. Migration of macrophages was implemented for 24 h.
2.6. Construction of the Retroreflection-Based Optical Setup and Retroreflection Signal Quantification
RJPs engulfed by macrophages could be obtained with the retroreflective setup installed on a conventional optical microscope, and this was easily achieved by attaching a white LED next to the objective lens. In this research, we used 700 nm of the cat’s eye-type retroreflector that is able to cover the whole visible light wavelength range. Thus, polychromatic white light could be applicable for retroreflection signal acquisition. The white LED (5500 K) for retroreflection induction was attached to the side of the object lens of an inverted optical microscope. The white LED module was prepared on a bracket that was adjusted to the illumination angle. According to the empirical model of retroreflection signaling, the incidence angle of white LED light to the focusing surface was 40°.10 To achieve this angle, the white LED module should be installed adjacent to the objective lens of the microscope to face the sample stage. To acquire retroreflection signals, the microscope was set to the bright-field acquisition mode with the light source turned off. After the sample was placed on the sample stage, retroreflection images were acquired with an exposure time of 500 ms using the external white LED module. Because the white LED module was the only light source during the image acquisition step, the retroreflect light signal could be registered to the object lens. During signal registration, a light-tight condition was not required because the imaging was not affected by ambient light. After image acquisition, ImageJ S/W was used for further image processing, including background subtraction and signal enumeration. As a result, the image processing step could provide a numerical count of the number of retroreflected signals interpreted as RJP counts in magnified image fields.
3. Results and Discussion
3.1. Overall Strategy of the Retroreflection-Based Migratory Cell Quantification and Monitoring Method
This research proposed a method of monitoring and quantifying migratory cells that differs from conventional Transwell migration assays by employing RJPs as signaling probes engulfed by macrophages. The retroreflecting particles could easily be counted using a conventional inverted-type optical microscope, which only requires an extra white LED light as a retroreflective light source because a key element in the induction of retroreflection is the incident angle, not the wavelength. The developed method offers a simplified procedure compared to the traditional migratory cell counting procedure that included cell fixation and staining steps. For signaling probes, retroreflective signal acquisition from the cat’s eye-type retroreflector was applied; these RJPs were constructed using silica microparticles with hemispheric metal coatings.10 Previously, our group confirmed the applicability of RJPs as intense optical probes in various biosensing applications, such as immunoassays, molecular diagnostics, and environmental sensing of metal ions in drinking water.10−15 In particular, based on the virtue of registration in retroreflection, the RJPs were sensitively detected in nondiluted human serum samples, which consist of numerous proteins and lipids.
This research suggested the utilization of a retroreflection-based signaling principle, replacing the traditional dye staining step and/or cell fixation process, to enable real-time monitoring of cell migration. Although most live-cell migration assay techniques relied on complex optical instruments (e.g., time-lapse microscope system, environment-controlled microscope, fluorescence scanner) by employing fluorescent dyes (Table 1), we have shown that an essentially similar result could be achieved using RJPs inside of the living cells with the conventional optical microscope. With these simplified processes, apparatus, along with our signal evaluation strategy, we anticipate that such modifications could improve traditional migration assays to be more informative and provide an improved understanding of the migratory behavior of cells. For the applicability of our developed test to quantify the migratory behavior of cells, murine macrophage cell line RAW264.7 was chosen due to its active phagocytic characteristics.25,26 For migration assays, the macrophage plating step involving the Transwell insert was introduced after 12 h of starvation.16 After this, starved cells were plated on the RJP-deposited Matrigel matrix. The Transwell insert was then integrated with 12-well plates containing a chemoattractant in the medium, which induced cell activation and migration to the lower chamber. Notably, the RJP-deposited Matrigel matrix was coated on the inner side of the Transwell to present RJPs to peripheral macrophages. Although the Matrigel matrix is frequently used in invasion assays as a steric cell barrier, in this research, we intentionally used a Matrigel matrix of low integrity to create 3D structures for enabling cell growth and for preventing immobilization of cells on the opposite side of the membrane.27 After migration, the retroreflection optical setup-installed microscope was able to acquire retroreflected signals from the RJPs engulfed by migrated cells. The signaling process for counting RJPs from cells consisted of two steps: background subtraction and signal counting. Both measures can be processed by ImageJ (from NIH), and individual RJPs in each image acquired could be counted as a numerical integer. However, the phagocytic behavior of macrophages involving RJP varied on a cell-by-cell basis. In accordance with the variable counts of RJPs in single cells, the statistical count of RJPs in single cells was calculated as the conversion constant between RJP counts and actual cell counts. Consequently, the RJP counts were divided by this constant to elicit the number of cells in each microscope field.
Table 1. Examples of Optical Methods Applied to Live-Cell Migration Analysis.
| signal transduction strategy | type of optical probe | aim of cell monitoring | optical apparatus | labeling method | ref | |
|---|---|---|---|---|---|---|
| 1 | fluorescence | fluorescent dye | spheroid formation | time-lapse microscope system | dye staining | Jiang et al.21 |
| 2 | fluorescence | fluorescent dye | chemotaxis | microplate reader | dye staining | Iseoka et al.9 |
| 3 | bright field | none | cell migration | time-lapse microscope system | none | Mahmood et al.22 |
| 4 | bright field | none | wound healing assay | motorized environment-controlled microscope | none | Jonkman et al.23 |
| 5 | fluorescence | fluorescent dye | wound healing assay | infrared fluorescence scanner | dye staining | Menon et al.24 |
| 6 | nonspectroscopic retroreflection | microretroreflector | cell migration | optical microscope | none | this work |
3.2. Validation of Cytotoxicity and Phagocytosis by Macrophages at Different RJP Concentrations
RJPs have served as optical probes in in vitro diagnostic applications, employing various conjugated biomaterials (e.g., enzymes, antibodies, DNA); however, there have been no reports identifying their potential cytotoxicity in cell culture applications. Thus, MTS determination-based cytotoxicity testing using RAW264.7 cells exposed to different concentrations of RJPs was conducted.20 Briefly, RAW264.7 cells were incubated with various concentrations (0, 0.1, 1, 5, 10, 25, 50, 100 μg/mL) of RJPs in the complete medium for 24 h (n = 3). As shown in Figure 3, cell viability decreased by 5.1% with 25 μg/mL RJPs in the medium, whereas cells remained viable below 10 μg/mL RJPs. In addition to this, bright-field microscopy images of the phagocytic behavior of RAW264.7 cells to RJPs were recorded after 24 h of incubation. Figure S1a,b illustrates the positive and negative presence of RJPs in the culture medium. In the presence of RJPs, macrophages presented a random number of black dots in the cell body (Figure S1a): Such cellular morphological changes were not observed in the control group (Figure S1b). However, above 25 μg/mL RJPs shows decreasing cell viability. Therefore, the RJP-deposited Matrigel matrix was constructed using 10 μg/mL RJPs to maintain cell viability for migration assays.
Figure 3.
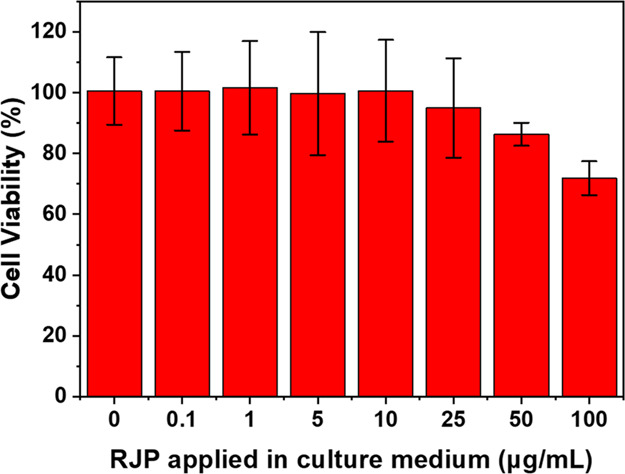
Cytotoxicity test for the RJP-containing growth medium. MTS reactions were used to evaluate cell viability with different concentrations of the RJP-containing medium. Each assay was triplicated. Error bars represented the mean ± standard deviation.
3.3. Characterization of the RJP-Phagocyted Macrophages
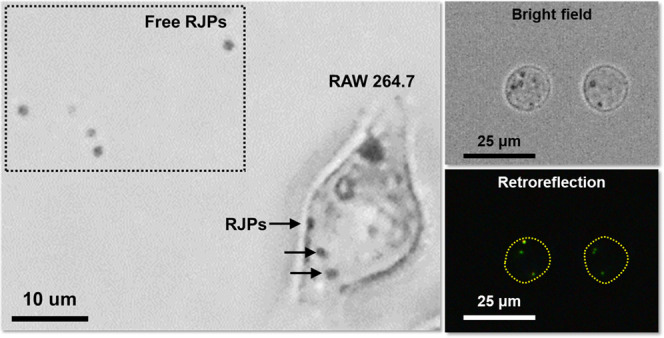
The developed assay for RAW264.7 cell migration toward chemoattractant MCP-1 was tested on Transwell inserts with RJPs deposited in the Matrigel matrix. Prior to the introduction of the chemoattraction, starved RAW264.7 cells were settled and spread on the RJP-deposited Matrigel matrix. During settlement and adhesion of cells to the membrane, the cells began to engulf nearby RJPs in the Matrigel matrix. Induced by the chemoattractant diffused from the lower chamber of the Transwell system, RAW264.7 cells gradually migrated to the lower chamber. Most conventional procedures for Transwell migration assays use collagen-coated surfaces and even the pristine membrane surface to induce migration of target cells. However, in this case, the Matrigel matrix coated on the inner side of the membrane did not make the cells migrate to the opposite side of the membrane surface but, rather, settled at the bottom of the lower chamber. To evaluate the migrated cells, we used a conventional optical microscope (×100 objective lens) focused on the bottom of the lower chamber to take five random bright-field images and retroreflection images. Schematic illustration of Figure 4a showed that the incident light of bright field was illuminated from the upper side of the objective lens (Figure 4a, blue arrow (b)) and the incident light of retroreflection was illuminated from the side of the objective lens (Figure 4a, red arrow (c)). Figure 4 exhibits the representative examples of bright-field microscopic images (Figure 4b) and retroreflection images (Figure 4c) of cells that were chemoattracted to the bottom surface for 24 h by 10 ng/mL MCP-1. Microscopic images showed that RAW264.7 cells that engulfed RJPs had successfully migrated and adhered to the lower chamber well walls. Most of the RJPs appeared as black dots in the cell body under bright-field illumination; in contrast, they became bright dots under the retroreflection setup. This phenomenon could be interpreted as the RJP-deposited matrix being optimized in a way that an appropriate quantity of signaling probes was provided to the cells while maintaining cell viability. Therefore, retroreflection signal acquisition from migrated RAW264.7 cells was a proof-of-concept demonstration of real-time monitoring and quantification of migratory cells, accomplished using modified Transwell inserts, and enabling omission of the fixation and staining processes.
Figure 4.
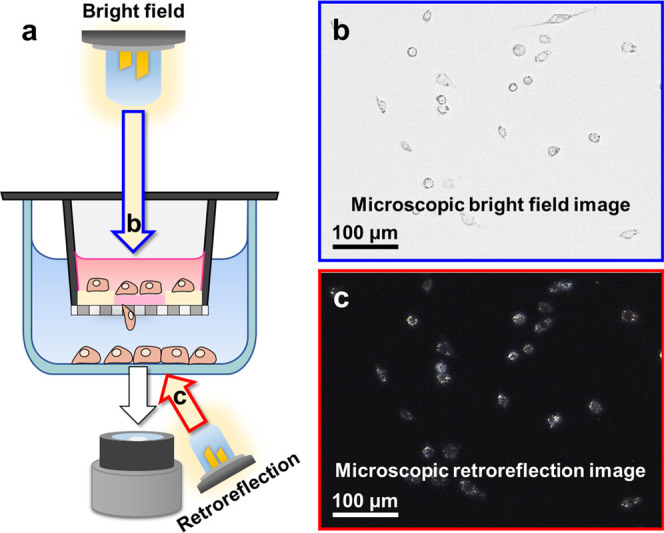
Evaluation of retroreflection signals from migratory macrophages by comparing different microscopic image fields. (a) Schematic illustration of microscopic image acquisition under bright field (blue arrow, b) and retroreflection (red arrow, c) illumination. (b) Bright-field microscopic images of cells on the surface of the lower chamber. (c) Retroreflection signals from the RJPs inside of cells at the same focus as (b).
3.4. Identification of Phagocyted RJPs in Macrophage for Cell Quantification
Among the quantified results of retroreflection signal detection, there was a constant discrepancy between migrated cell counts under bright-field illumination and RJP counts under the retroreflection setup. This phenomenon was derived from the phagocytic behavior of RAW264.7 cells. In particular, the numbers of cells and RJPs were not equivalent due to the random numbers of RJPs found in RAW264.7 cells. Although the quantity of RJPs deposited was controlled in the Matrigel matrix, phagocytosed RJP counts in RAW264.7 cells were not steady. However, there was a correlated tendency involving RJP and cell counts, in which cell counts were found to be approximately 5.0–6.2 times higher than RJP counts. To identify the correlation between RJP and cell numbers, we sorted 120 individual cells from 5 sets of migration assays to statistically validate the average number of engulfed RJPs in individual macrophages (Figure S2). Figure 5a illustrates the distribution of RJPs in individual cells based on the RJP counts from individual cells depicted in Figure 5b. Approximately 50% of the cell population presented 4–8 RJPs per single cell. To calculate the average RJP number in single RAW264.7 cells, we used a simple statistical method to elicit the expected value of RJP counts per individual cell by establishing a lump-sum amount of the multiplying group’s percentage share by the number of RJPs. From this, the expected RJP counts per single cell were calculated to be 5.059, referred to here as a conversion constant between RJP counts and cell counts. This constant allowed us to divide the RJP count by the conversion constant in a subsequent cell quantification procedure to obtain estimates of migrated cell numbers.
Figure 5.
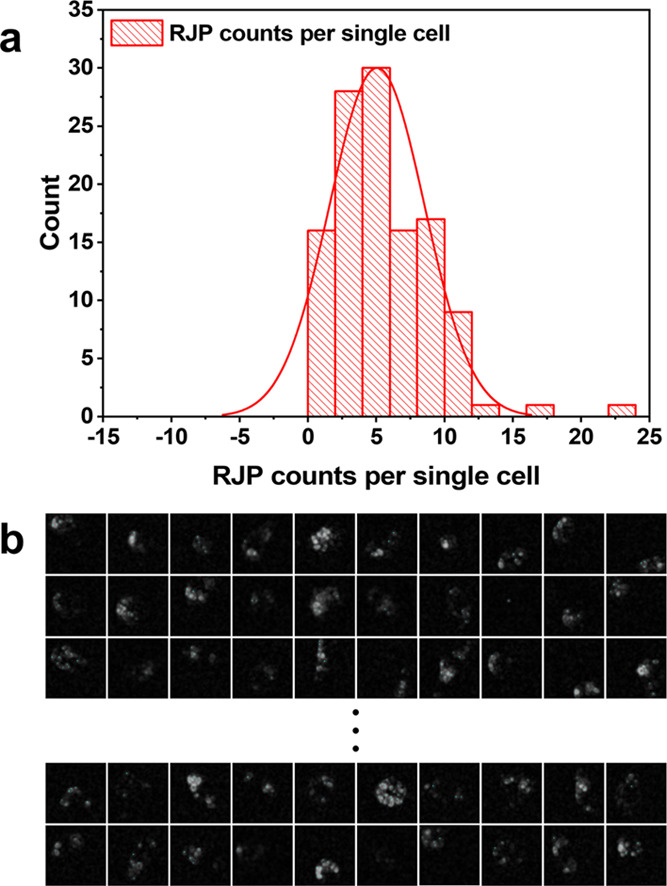
Verification of quantitative RJP phagocytic properties of RAW264.7 cells to (a) distribution of cell counts versus phagocytosed RJP counts in the cell body. (b) Microscopic retroreflection images of single cells having engulfed various numbers of RJPs. (Each grid is 55 × 55 μm2 in size).
3.5. Retroreflection-Based Migratory Macrophage Quantification
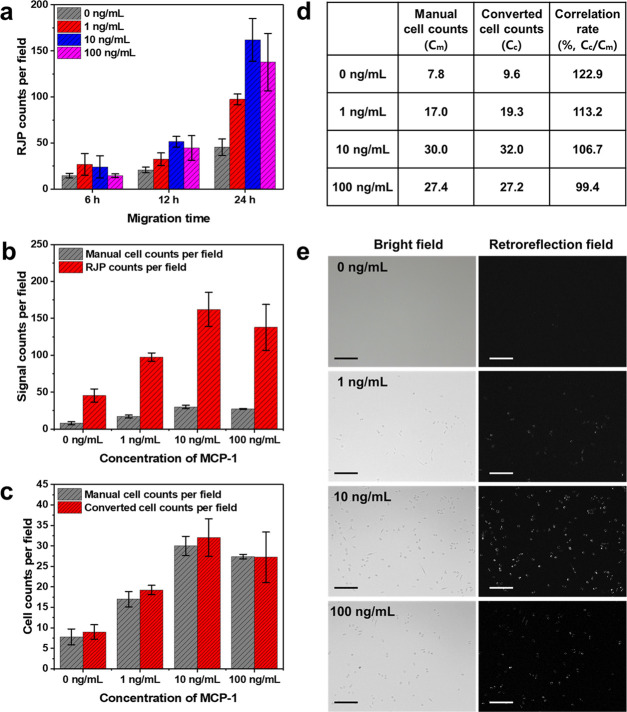
One of the significant features of this study was the introduction of RJP-based cell counting to replace the cell fixation and staining steps from conventional migration assays. To validate the applicability of retroreflection-based migratory cell quantification, we designed a model migration system for RAW264.7 cell enumeration utilizing MCP-1 as a chemoattractant. MCP-1 is a well-known chemokine that has the ability to induce cell migratory activity by binding to CC chemokine receptor 2 (CCR2), which initiates the activation of signaling transduction pathways.28 Conventionally, the Transwell migration assay was mandated to determine the duration of migration for further fixation and quantification processes; however, in this method, the incremental progression of migrating cells could be immediately monitored and quantified by registering retroreflection signals from RJPs in the cell body. In the case of verification of the migratory performance of RAW264.7 cells toward MCP-1 chemoattractant, real-time quantification was conducted while the migration assay progressed. As Figure 6a indicates, the migratory cell-engulfed RJP count status was quantified at 6, 12, and 24 h for a total incubation time of 24 h. The 10 ng/mL MCP-1-containing migration medium displayed a sharp increment tendency compared to the MCP-1-negative control group. The 6 h of migration exhibited comparable differences between control and other concentrations of MCP-1, while after 24 h, migrating cell groups exhibited significant differences from the control group. Therefore, with the quantification results for each migratory status, the duration of migration for RAW264.7 cells on modified Transwell toward the MCP-1-containing migration medium was chosen to be 24 h. From the determination of the migration time, a more specific migration rate for the various concentrations of MCP-1 (0, 1, 10, 100 ng/mL) was investigated. Furthermore, the proposed quantification method using the expected RJP counts per single RAW264.7 cell was re-evaluated. As indicated in Figure 6b,e, the results showed that cell counts of the migrated RAW264.7 cells were dependent on MCP-1 concentration. Specifically, cell counts with MCP-1 increasing from 0 to 10 ng/mL exhibited a linear increment. However, at levels above 10 ng/mL, MCP-1 induced decrement in migration. From these results, re-evaluation of the conversion constant was implemented by comparing manual cell counts under bright-field illumination with retroreflection signal counts under retroreflection conditions. Because more than one RJP could be engulfed by macrophages during migration, the RJP counts presented more significant numbers than manual cell counts. With this discrepancy, RJP counts could be converted to provide an estimated number of migrated cells. In the previous section, we elicited the expected RJP counts for individual cells that could be utilized as a constant for converting between RJP and cell counts. As indicated in Figure 6c, the manually determined cell numbers and converted cell counts from RJP counts presented mean values and error ranges. Converted cell counts were found to match approximately 99–123% of the correlation rate with manual cell counts (Figure 6d). In conclusion, migratory cell quantification under retroreflection enables not only the ability to monitor the migratory status during the migration process but also results in comparable accuracy with manual counting.
Figure 6.
Retroreflection-based migration assay result analysis for real-time migratory cell quantification and evaluation of cell numbers from the acquired retroreflection signals. (a) Migratory cell quantification results from various migration times. (b) Comparative study of migratory cell analysis with various concentrations of MCP-1 using manual counting and retroreflection signal counting. (c) Verification of correlation testing involving retroreflection-based cell counting and manual cell counts. Error bars were calculated as mean ± S.D. (d) Correlation rate chart between manual cell counts and retroreflection signaling-based converted cell counts. (e) Microscopic images of migratory cells exposed to various concentrations of MCP-1 under retroreflective optical setup conditions (scale bar = 200 μm).
4. Conclusions
In this study, a novel retroreflection-based migration assay was developed and tested to demonstrate its applicability in quantifying migratory macrophages. RJPs were utilized as signaling probes in monitoring and evaluating RAW264.7 cell migration in the modified Transwell system. To materialize this, we deposited RJPs on the migration chamber so that RAW264.7 cells could phagocytose RJPs during migration. RAW264.7 cells exhibited successful RJP phagocytosis and migratory properties on chemokine stimulation. Consequently, we confirmed the successful registration of the retroreflection signals originating from the engulfed RJPs in the cell body. From this, the quantitative analysis of migrating status was also verified. Specifically, the numbers of migrating cells, the time duration, and migration dependency upon MCP-1 were analyzed using the retroreflection signal registration. To verify the correlation between RJP counts and cell counts, the RJP counts were converted into cell counts by eliciting the mean RJP count per single cell. The correlation rate between the converted counts and the manual counts was demonstrated to be 99–123%. The performance of the developed strategy was found to be compatible with the conventional cell migration assays while requiring simple instrumentation and procedure. An advantage of the proposed method is that it is designed to be free from fixation and dye staining steps for live-cell quantification. Meanwhile, cell migration through Matrigel was found to be delayed in time compared to the conventional method, which could be improved by changing the membrane structure and composition. On the whole, we expect that our approach could be applicable in various migratory and/or live-cell assay models to conduct cell behavior analyses.
Acknowledgments
This research was mainly supported by the Creative Materials Discovery Program (NRF-2019M3D1A1078943). We also acknowledge the support from the Priority Research Centers Program (NRF-2019R1A6A1A11051471) funded by the National Research Foundation of Korea.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03454.
Microscopic images of RAW264.7 cells incubated with 10 μg/mL RJP in the complete medium and with the complete medium as control (Figure S1) and microscopic images of individual macrophages engulfing RJPs under retroreflection field (Figure S2) (PDF)
Author Contributions
H.C.Y. conceived, designed, and supervised the research. J.-H.K. and M.S.K. provided conceptual input and engineering insight into this research. K.R.K., K.W.L., and H.C.Y. designed the experiment. D.L. and K.Y.J. carried out cell culturing and performed the experiments. K.R.K. and H.C.Y. analyzed data and wrote the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sarkar P. L.; Lee W.; Williams E. D.; Lubik A. A.; Stylianou N.; Shokoohmand A.; Lehman M. L.; Hollier B. G.; Gunter J. H.; Nelson C. C. Insulin Enhances Migration and Invasion in Prostate Cancer Cells by Up-Regulation of FOXC2. Front. Endocrinol. 2019, 10, 481 10.3389/fendo.2019.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H. T. L.; Kim J.; Park J.; Cho S. A Review of Electrical Impedance Characterization of Cells for Label-Free and Real-Time Assays. Biochip J. 2019, 13, 295–305. 10.1007/s13206-019-3401-6. [DOI] [Google Scholar]
- Spatarelu C. P.; Zhang H.; Nguyen D. T.; Han X.; Liu R.; Guo Q.; Notbohm J.; Fan J.; Liu L.; Chen Z. Biomechanics of Collective Cell Migration in Cancer Progression: Experimental and Computational Methods. ACS Biomater. Sci. Eng. 2019, 5, 3766–3787. 10.1021/acsbiomaterials.8b01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijuan J.; Barceló C.; Moreno D. F.; Maiques O.; Sisó P.; Marti R. M.; Macià A.; Panosa A. In Vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107 10.3389/fcell.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A.; Das A.; Krett N. L.; Guzman G.; Thomas A.; Mancinelli G.; Bauer J.; Ushio-Fukai M.; Fukai T.; Jung B. Nuclear Translocation of Atox1 Potentiates Activin A-Induced Cell Migration and Colony Formation in Colon Cancer. PLoS One 2020, 15, e027916 10.1371/journal.pone.0227916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. T.; Muppala S.; Wu J.; Krukovets I.; Solovjev D.; Verbovetskiy D.; Obiako C.; Plow E. F.; Stenina-Adognravi O. Effects of Thrombospondin-4 on pro-Inflammatory Phenotype Differentiation and Apoptosis in Macrophages. Cell Death Dis. 2020, 11, 53 10.1038/s41419-020-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin R. H.Long-Term Live Cell Imaging of Cell Migration: Effects of Pathogenic Fungi on Human Epithelial Cell Migration. In Methods in Molecular Biology; Humana Press: New York, 2016; Vol. 1365, pp. v–vi. [DOI] [PubMed] [Google Scholar]
- Lee J. P.; Li Y. C.; Chen H. Y.; Lin R. H.; Huang S. S.; Chen H. L.; Kuan P. C.; Liao M. F.; Chen C. J.; Kuan Y. H. Protective Effects of Luteolin against Lipopolysaccharide-Induced Acute Lung Injury Involves Inhibition of MEK/ERK and PI3K/Akt Pathways in Neutrophils. Acta Pharmacol. Sin. 2010, 31, 831–838. 10.1038/aps.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseoka H.; Miyagawa S.; Saito A.; Harada A.; Sawa Y. Role and Therapeutic Effects of Skeletal Muscle-Derived Non-Myogenic Cells in a Rat Myocardial Infarction Model. Stem Cell Res. Ther. 2020, 11, 69 10.1186/s13287-020-1582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. D.; Kim H.-S.; Park Y. M.; Chun H. J.; Kim J.-H.; Yoon H. C. Retroreflective Janus Microparticle as a Nonspectroscopic Optical Immunosensing Probe. ACS Appl. Mater. Interfaces 2016, 8, 10767–10774. 10.1021/acsami.6b02014. [DOI] [PubMed] [Google Scholar]
- Chun H. J.; Kim S.; Han Y. D.; Kim D. W.; Kim K. R.; Kim H. S.; Kim J. H.; Yoon H. C. Water-Soluble Mercury Ion Sensing Based on the Thymine-Hg2+-Thymine Base Pair Using Retroreflective Janus Particle as an Optical Signaling Probe. Biosens. Bioelectron. 2018, 104, 138–144. 10.1016/j.bios.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Chun H. J.; Kim S.; Han Y. D.; Kim K. R.; Kim J.-H.; Yoon H.; Yoon H. C. Salmonella Typhimurium Sensing Strategy Based on the Loop-Mediated Isothermal Amplification Using Retroreflective Janus Particle as a Nonspectroscopic Signaling Probe. ACS Sens. 2018, 3, 2261–2268. 10.1021/acssensors.8b00447. [DOI] [PubMed] [Google Scholar]
- Kim D. W.; Chun H. J.; Kim J. H.; Yoon H.; Yoon H. C. A Non-Spectroscopic Optical Biosensor for the Detection of Pathogenic Salmonella Typhimurium Based on a Stem-Loop DNA Probe and Retro-Reflective Signaling. Nano Convergence 2019, 6, 16 10.1186/s40580-019-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. R.; Chun H. J.; Lee K. W.; Jeong K. Y.; Kim J. H.; Yoon H. C. Wash-Free Non-Spectroscopic Optical Immunoassay by Controlling Retroreflective Microparticle Movement in a Microfluidic Chip. Lab Chip 2019, 19, 3931–3942. 10.1039/C9LC00973F. [DOI] [PubMed] [Google Scholar]
- Han Y. D.; Chun H. J.; Yoon H. C. Low-Cost Point-of-Care Biosensors Using Common Electronic Components as Transducers. Biochip J. 2020, 14, 32–47. 10.1007/s13206-020-4104-8. [DOI] [Google Scholar]
- Yang J. X.; Hsiung T. C.; Weng F. C.; Ding S. L.; Wu C. P.; Conti M.; Chuang T. H.; Catherine Jin S. L. Synergistic Effect of Phosphodiesterase 4 Inhibitor and Serum on Migration of Endotoxin-Stimulated Macrophages. Innate Immunity 2018, 24, 501–512. 10.1177/1753425918809155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. A.; Liao C. C.; Cheng Y. C.; Chen Y. P.; Hsu Y. F.; Liang C. M.; Liang S. M. Stimulation of Proliferation and Migration of Mouse Macrophages by Type B CpG-ODNs Is F-Spondin and IL-1Ra Dependent. PLoS One 2015, 10, e0128926 10.1371/journal.pone.0128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Wang Z.; Ren H.; Yue M.; Huang K.; Gu H.; Liu M.; Du B.; Qian M. P2Y 6 Agonist Uridine 5′-Diphosphate Promotes Host Defense against Bacterial Infection via Monocyte Chemoattractant Protein-1–Mediated Monocytes/Macrophages Recruitment. J. Immunol. 2011, 186, 5376–5387. 10.4049/jimmunol.1002946. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Su J.; Yuan B.; Fu D.; Niu Y.; Yue D. The Role of C1QBP in CSF-1-Dependent PKCζ Activation and Macrophage Migration. Exp. Cell Res. 2018, 362, 11–16. 10.1016/j.yexcr.2017.09.038. [DOI] [PubMed] [Google Scholar]
- Sutherland M. W.; Learmonth B. A. The Tetrazolium Dyes MTS and XTT Provide New Quantitative Assays for Superoxide and Superoxide Dismutase. Free Radical Res. 1997, 27, 283–289. 10.3109/10715769709065766. [DOI] [PubMed] [Google Scholar]
- Jiang C. F.; Hsu S. hui.; Sun Y. M.; Tsai M. H. Quantitative Bioimage Analysis of Passaging Effect on the Migratory Behavior of Human Mesenchymal Stem Cells During Spheroid Formation. Cytometry, Part A. 2020, 97, 394–406. 10.1002/cyto.a.23985. [DOI] [PubMed] [Google Scholar]
- Mahmood S.; Nandagopal S.; Sow I.; Lin F.; Kung S. K. P. Microfluidic-Based, Live-Cell Analysis Allows Assessment of NK-Cell Migration in Response to Crosstalk with Dendritic Cells. Eur. J. Immunol. 2014, 44, 2737–2748. 10.1002/eji.201344244. [DOI] [PubMed] [Google Scholar]
- Jonkman J. E. N.; Cathcart J. A.; Xu F.; Bartolini M. E.; Amon J. E.; Stevens K. M.; Colarusso P. An Introduction to the Wound Healing Assay Using Live-Cell Microscopy. Cell Adhes. Migr. 2014, 8, 440–451. 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M. B.; Ronkina N.; Schwermann J.; Kotlyarov A.; Gaestel M. Fluorescence-Based Quantitative Scratch Wound Healing Assay Demonstrating the Role of MAPKAPK-2/3 in Fibroblast Migration. Cell Motil. Cytoskeleton 2009, 66, 1041–1047. 10.1002/cm.20418. [DOI] [PubMed] [Google Scholar]
- Bie X.; Zhang S.; Luo X.; Qi R. Q. Candida Albicans Cell Wall Mannoprotein Synergizes with Lipopolysaccharide to Affect RAW264.7 Proliferation, Phagocytosis and Apoptosis. Microb. Pathog. 2019, 131, 98–105. 10.1016/j.micpath.2019.03.038. [DOI] [PubMed] [Google Scholar]
- Wen L.; Chen Y.; Zhang L.; Yu H.; Xu Z.; You H.; Cheng Y. Rice Protein Hydrolysates (RPHs) Inhibit the LPS-Stimulated Inflammatory Response and Phagocytosis in RAW264.7 Macrophages by Regulating the NF-KB Signaling Pathway. RSC Adv. 2016, 6, 71295–71304. 10.1039/C6RA08927E. [DOI] [Google Scholar]
- Chaw K. C.; Manimaran M.; Tay F. E. H.; Swaminathan S. Matrigel Coated Polydimethylsiloxane Based Microfluidic Devices for Studying Metastatic and Non-Metastatic Cancer Cell Invasion and Migration. Biomed. Microdevices 2007, 9, 597–602. 10.1007/s10544-007-9071-5. [DOI] [PubMed] [Google Scholar]
- Xie J.; Yang L.; Tian L.; Li W.; Yang L.; Li L. Macrophage Migration Inhibitor Factor Upregulates MCP-1 Expression in an Autocrine Manner in Hepatocytes during Acute Mouse Liver Injury. Sci. Rep. 2016, 6, 27665 10.1038/srep27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.