Abstract
Rationale: A subpopulation of B cells (age-associated B cells [ABCs]) is increased in mice and humans with infections or autoimmune diseases. Because depletion of these cells might be valuable in patients with certain lung diseases, the goal was to find out if ABC-like cells were at elevated levels in such patients.
Objectives: To measure ABC-like cell percentages in patients with lung granulomatous diseases.
Methods: Peripheral blood and BAL cells from patients with sarcoidosis, beryllium sensitivity, or hypersensitivity pneumonitis and healthy subjects were analyzed for the percentage of B cells that were ABC-like, defined by expression of CD11c, low levels of CD21, FcRL 1–5 (Fc receptor–like protein 1–5) expression, and, in some cases, T-bet.
Measurements and Main Results: ABC-like cells in blood were at low percentages in healthy subjects and higher percentages in patients with sarcoidosis as well as at high percentages among BAL cells of patients with sarcoidosis, beryllium disease, and hypersensitivity pneumonitis. Treatment of patients with sarcoidosis led to reduced percentages of ABC-like cells in blood.
Conclusions: Increased levels of ABC-like cells in patients with sarcoidosis may be useful in diagnosis. The increase in percentage of ABC-like cells in patients with lung granulomatous diseases and decrease in treated patients suggests that depletion of these cells may be valuable.
Keywords: sarcoidosis, beryllium sensitivity, chronic beryllium disease, hypersensitivity pneumonitis, age-associated B cells (ABCs)
At a Glance Commentary
Scientific Knowledge on the Subject
A population of B cells, called age-associated B cells (ABCs), is at increased percentages in mice and humans suffering from autoimmune diseases and various infections. ABC-like cells are defined by their expression of CD11c and T-bet, low levels of the B-cell marker CD21, and an unusual distribution of Fc receptor–like proteins. These B cells might participate in disease processes, not just because they are precursors for antibody production but also because they present antigen very efficiently to T cells.
What This Study Adds to the Field
Because B cells are thought to play a role in some lung granulomatous diseases, we examined the peripheral blood and BAL of patients suffering from these diseases for ABC-like cells. Results were compared with ABC-like cells in healthy individuals. By comparison with other B cells, ABCs had similar phenotypes in healthy people and patients but were increased in percentage in the peripheral blood of patients with sarcoidosis and in the BAL of patients with sarcoidosis, beryllium sensitivity, and hypersensitivity pneumonitis. Moreover, ABC-like percentages decreased in the peripheral blood of treated sarcoidosis patients. These findings suggest that ABC-like cells might contribute to the morbidity of these illnesses, and hence processes to remove these B cells might be useful therapeutically.
We and others have identified a subpopulation of B cells in mice and humans we called age-associated B cells (ABCs) (1–12). These cells differ from other B cells by their low level of CD21 and expression of CD11b, CD11c, and, for one subpopulation, T-bet (13–20). Mouse follicular B cells are converted to ABCs by signals through their B-cell receptor, their IFNγ receptor, their TLR7 and, sometimes, their IL-21 receptor (13, 21). Together, these signals induce T-bet. T-bet, in turn, induces production of many of the other proteins that characterize these cells (14).
ABC-like cells are present in healthy mice but increase in percentage in virus-infected mice; they are major precursors for antiviral antibody production (22, 23) and have been associated with nonviral infections such as Ehrlichia (12, 24). ABCs also appear in autoimmune-prone mice and are the major producers of autoantibodies in these animals (10, 25–28). The appearance of autoantibodies and symptoms of autoimmunity are significantly delayed in animals that cannot express T-bet in B cells and that therefore lack ABCs (14).
B cells expressing low levels of CD21 and other markers characteristic of ABCs have been found in increased numbers in patients suffering from various autoimmune diseases (6, 21, 29–32) and are major precursors for autoantibody secreting cells (6). As far as infectious agents are concerned, various agents and vaccines have also been found to induce ABC-like cells in humans (18, 20, 33–36).
T cells are thought to drive granuloma formation in sarcoidosis, chronic beryllium disease (CBD), and hypersensitivity pneumonitis (HP). Nevertheless, B cells have been implicated in some granulomatous lung diseases in humans. Indeed, rituximab may lead to pulmonary function improvement in chronic HP and sarcoidosis (37–39). Hypergammaglobulinemia, autoantibody production, and circulating immune complexes are frequently observed in sarcoidosis (40, 41). Also, B cells are a component of sarcoidosis granulomas, located on the periphery of, and occasionally within, the granuloma (42).
Knowing these points, we checked the appearance of ABC-like cells in peripheral blood mononuclear cells (PBMCs) and BALs of patients with various granulomatous lung diseases, focusing on sarcoidosis. However, ABC-like cells have been defined in several ways by their low levels of CD21 with or without expression of CD11c and/or T-bet. Therefore, to define the cells more carefully, we tested B cells for expression of various combinations of these markers and also another set of proteins, the FcRLs (Fc receptor–like proteins; alias CD307a-e), which have also, in a few studies, been associated with ABC-similar cells (17, 19, 43–48). FcRLs might affect the functions of the cells bearing them, as some express ITAMs (immunoreceptor tyrosine-based activation motifs, expressed by FcRLs 1, 2, 3, and 5) and some express also or instead ITIMs (immunoreceptor tyrosine-based inhibition motifs, expressed by FcRLs 2, 3, 4, and 5). The goal of our experiments was to identify a set of proteins that could be used to identify various subpopulations, were they to exist, of ABC-like cells and to predict to some extent their functions in subjects with different granulomatous lung diseases.
Methods
Study Cohorts
Patients suffering from sarcoidosis, beryllium sensitivity (BeS), CBD, or HP were recruited during routine clinic visits. Disease status was diagnosed based on the American Thoracic Society/European Respiratory Society guidelines and multidisciplinary consensus discussion for sarcoidosis (49), CBD and BeS (50), and HP (51). Sarcoidosis BAL cells and PBMCs were obtained from the National Jewish Health granuloma sarcoidosis biorepository and subjects undergoing clinically indicated diagnostic bronchoscopies. Healthy donors were volunteers working at National Jewish Health. Table 1 describes the age, sex, race, and treatment, if any, of the donors. Samples were collected after individuals had signed an informed consent and Health Insurance Portability and Accountability Act forms in accordance with National Jewish Health Institutional Board–approved protocols, HS-2984, University of Iowa 201801833 and HS-2750, and the Declaration of Helsinki.
Table 1.
Patient Data
| Experiment | Group | Number | Age (yr) |
Sex |
Race (W/AA/H) | Treatment (Number) | Smoking Status (C/F/N/U) | ||
|---|---|---|---|---|---|---|---|---|---|
| Age [Mean (Range)] | P Value* | M/F | P Value† | ||||||
| Sarcoidosis | 0.14 | 0.19 | |||||||
| HSs‡ | 24 | 44 (23–75) | Ref | 12/12 | Ref | 24/0/0 | None | 0/0/24/0 | |
| SarcPTs | 22 | 53 (33–74) | 0.032 | 16/6 | 0.13 | 18/4/0 | Methotrexate, hydroxychloroquine (1) | — | |
| Methotrexate, prednisone (3) | |||||||||
| Methotrexate, infliximab (1) | |||||||||
| Mycophenolate (1) | |||||||||
| Hydroxychloroquine (3) | |||||||||
| Hydroxychloroquine, prednisone (1) | |||||||||
| Hydroxychloroquine, infliximab (1) | |||||||||
| Prednisone (5) | |||||||||
| SarcPs | 15 | 52 (27–68) | 0.075 | 9/6 | 0.78 | 13/2/0 | None | 0/4/10/1 | |
| Beryllium disease | 0.02 | — | |||||||
| HSs‡ | 6 | 33 (25–41) | Ref | 4/0 | 6/0/0 | None | 0/0/6/0 | ||
| BeS§ | 10 | 62 (43–77) | 0.01 | 9/1 | 7/1/2 | ICS (1)ǁ | 0/0/10/0 | ||
| Chronic beryllium disease | 10 | 64 (53–78) | 0.0037 | 9/1 | 8/1/1 | ICS (4) | 0/0/10/0 | ||
| Adalimumab, hydroxychloroquine (1) | |||||||||
| ICS, IVIG, Hydroxychloroquine (1) | |||||||||
| Hypersensitivity pneumonitis | <0.0001 | 0.75 | |||||||
| HSs‡ | 13 | 39 (23–69) | Ref | 7/6 | Ref | 13/0/0 | None | 0/0/13/0 | |
| Hypersensitivity pneumonitis | 17 | 67 (53–83) | <0.0001 | 7/10 | 0.75 | Not known | Azathioprine, mycophenolate mofetil, mycophenolate mofetil with prednisone, or prednisone alone | 0/0/0/17 | |
Definition of abbreviations: AA = African American; BeS = beryllium sensitivity; C = continuous; F = former; H = Hispanic; HSs = healthy subjects; ICS = inhaled corticosteroids; IVIG = intravenous immunoglobulin; N = never; Ref = HSs used to compare with patients’ data; SarcPs = sarcoidosis patients not on treatment; SarcPTs = sarcoidosis patients on treatment; U = unknown; W = white.
Statistical differences between the ages of subjects by diagnosis group were assessed by a Kruskal-Wallis test with Dunn’s post hoc test.
Statistical differences between the sex of subjects by diagnosis group were assessed by a chi-squared test.
HS numbers differ for each experiment because the number and identity of the HS controls was different for each analysis. Some HSs were used as control subjects in more than one experiment.
In the beryllium disease experiment, two HSs did not have recorded age or sex.
ǁThis treatment was not used for treatment of BeS but rather for non-BeS airway disease.
Study Procedures
Patients with sarcoidosis undergoing a diagnostic bronchoscopic procedure were consented for use of their excess BAL cells. Bronchoscopy was performed per standard clinical protocols (52), and lavage was performed by instilling sequential aliquots of 0.9% saline in 1–2 segments of the lung and aspirated manually. Blood samples were obtained from an established intravenous catheter immediately before the bronchoscopy procedure.
Staining and Analysis of PBMCs and BAL Samples
PBMCs from human heparinized blood samples were purified by density gradient as a buffy coat. The buffy coat was collected and washed three times with Hanks’ balanced salt solution. Cells were first stained with antibodies against surface proteins and then fixed and lysed using the FoxP3 fixation/permeabilization kit (eBioscience), followed by intracellular staining with anti–T-bet (23). The origins and attached fluorophores for the antibodies are shown in Table E1 in the online supplement. Dead cells were gated out based on their staining with Zombie Violet (BioLegend). Analyses were performed on CYAN (Beckman Coulter) or LSRII (Becton Dickinson) flow cytometers, and the data were analyzed using FlowJo software (FlowJo LLC). The gating strategy is shown in Figure E1. For each day’s set of samples, the gating strategy was identical. However, because of small changes in the sensitivity of the flow cytometer from day to day, the gates were changed somewhat from one day’s samples to another. Labels accompanied by arrows on the flow cytometric figures indicate the mean fluorescent intensity of staining by the labeled antibody.
Statistical Analyses
Associations of diagnosis and treatment group with subject age were estimated nonparametrically using the Kruskal-Wallis test with Dunn’s post hoc test, whereas associations with self-reported sex were estimated using the chi-square test. Differences in mean percentage ABC content between diagnosis/treatment groups were estimated using robust regression methods to minimize sensitivity to the small number of relatively high outlying percentage ABC content values. The impact of adjusting these robust models for subject age was explored. All analyses were performed in R version 3.6.3 (https://ww.R-project.org/). Kruskal-Wallis and Dunn tests were implemented in the Dunn.test package. Robust regression was implemented in the MASS package (53). The statistical significances between groups are indicated on the figures, except when the differences were not significant, in which case no notation was added. The healthy control subjects versus patients with BeS and CBD or healthy subjects (HSs) versus those with HP were significantly different, with the healthy control subjects being younger. However, it has been reported that the percentage of ABC-like cells does not change with age (31), so we do not believe a difference in age between the control subjects and patients is a confounding factor.
Results
B Cells Resembling ABCs Are Found at Increased Frequency in the Peripheral Blood and BAL of Patients with Pulmonary Sarcoidosis
The percentages of B cells in PBMCs from HSs and patients with pulmonary sarcoidosis not on therapy (SarcPs) and patients who were on therapy (SarcPTs) were about the same (Figures E2A and E2B). There were two populations of ABC-like cells (CD11c, CD21lo) (10, 13, 15, 19, 23, 28), one expressing high levels of T-bet (ABC-like Tbethi) and another that was low to negative for T-bet expression (ABC-like Tbetneg-lo) (20) (Figure E1). CD19 levels on ABC-like T-betneg-lo cells were the same as those on CD11c-negative, CD21-moderate to CD21-high B cells (other B cells) (Figures E2C and E2D). However, as previously shown (12), ABC-like T-bethi cells from both HSs and SarcPs bore higher levels of CD19 than the other two B-cell populations (Figure E2E). Higher CD19 levels might increase the B cells’ reactivity and ability to help T cells (54).
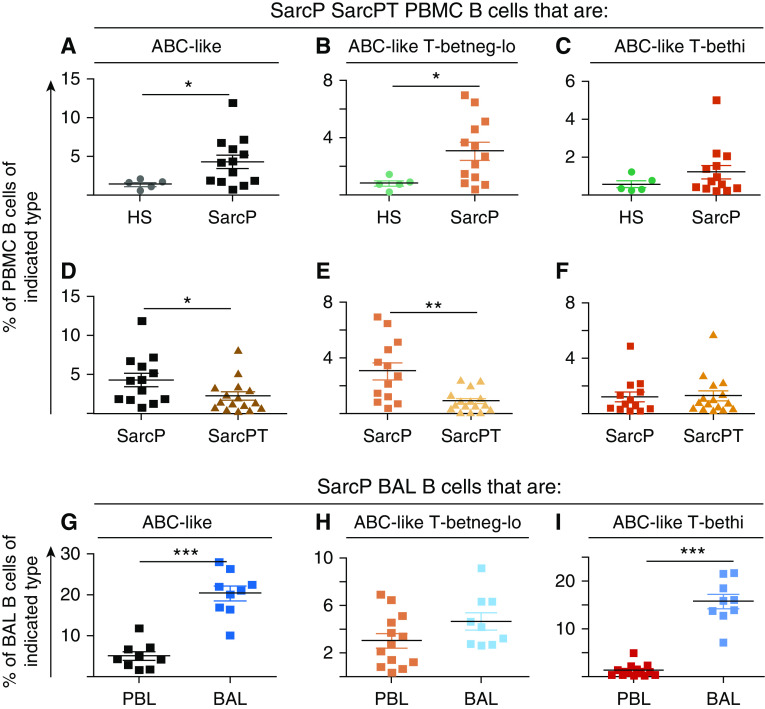
ABC-like cells were significantly increased in percentage among PBMC B cells in SarcPs versus HSs (Figure 1A) but were significantly reduced in patients undergoing therapy for their disease (Figure 1D). However, this result should be viewed with caution given that the SarcPTs were a different cohort than the SarcPs. The same observations apply to the ABC-like Tbetneg-lo subpopulation, which were significantly higher in SarcPs than HSs but considerably reduced in SarcPTs (Figures 1B and 1E). On the other hand, the percentage of ABC-like Tbethi cells was not statistically different from HSs in SarcPs and was not significantly reduced in SarcPTs (Figures 1C and 1F). ABC-like cells were also at high levels in SarcP BAL (Figure 1G), and the increase in percentage over normal applied particularly to the ABC-like T-bethi cells (Figure 1I).
Figure 1.
Age-associated B cell (ABC)-like cells are enriched in the peripheral blood mononuclear cells (PBMCs) and BAL of patients with sarcoidosis and are reduced in percentage in patients on therapy. PBMCs and BAL cells were obtained from healthy subjects (HSs), sarcoidosis patients not on treatment (SarcPs), and sarcoidosis patients on treatment (SarcPTs) as described in Methods. The treatment protocols and data for the patients are listed in Table 1. Cells were stained as described in Methods, and staining was detected on a CyAn (Beckman Coulter) instrument. Data were analyzed using FlowJo software (Tree Star). The percentage of PBMC B cells from HSs and SarcPs that were (A) ABC-like, (B) ABC-like T-betneg-lo, and (C) ABC-like T-bethi are shown. The results in each panel were analyzed using Mann-Whitney tests. (D–F) Data are as described for A–C, except each panel compares data for each cell type for SarcPs and SarcPTs. (G–I) Data are as described for A–C, except each panel contains data for the indicated cell types from the B cells in PBMCs of SarcPs and B cells in BAL from the same patients. *P < 0.05, **P < 0.01, and ***P < 0.001. PBL = peripheral blood lymphocytes.
Several results suggest that the increase in ABC-like cells in SarcPs is associated with the severity of disease. First, the percentage of ABC-like cells in PBMCs is lower in SarcPTs than in SarcPs (Figures 1D and 1E). Second, the percentage of ABC-like cells is significantly higher in BAL than in PBMCs (Figure 1G and 1I). Lastly, in untreated patients, the severity of the disease, as defined by numbers of extrapulmonary affected organs, is related to the percentage of ABC-like cells in peripheral blood. Thus, when patients were divided into two groups, with >8% or <3% of their PBMC B cells as ABC-like cells, ∼50% or more of the patients with >8% ABC-like cells had active disease and extrapulmonary organ involvement, whereas <17% of the patients in the group with low percentages of ABC-like cells had active disease and extrapulmonary organ involvement (data not shown).
Levels of FcRLs Differ between ABC-like and Other B Cells
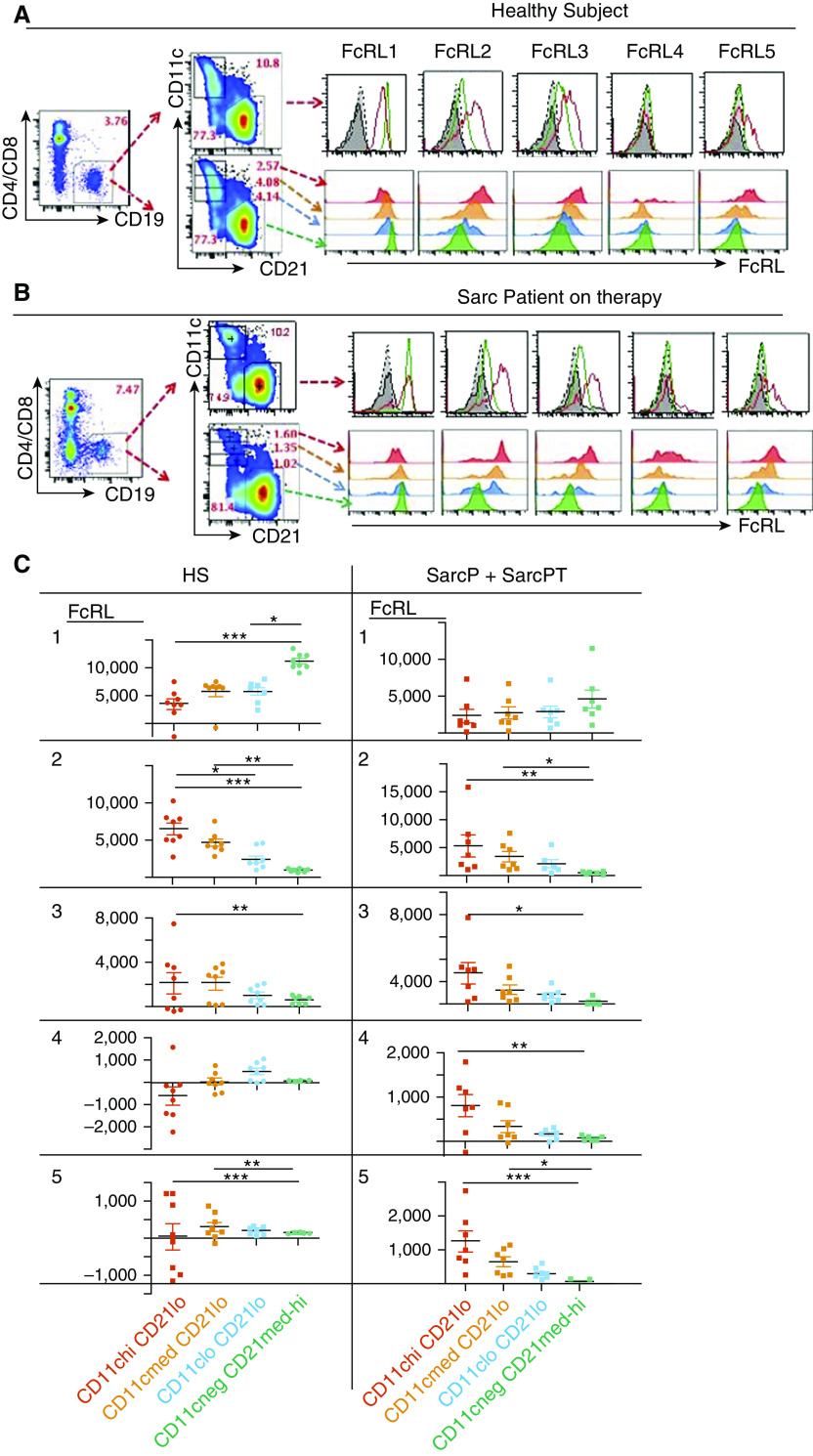
ABC-like cells express a distribution of FcRLs (17, 19, 21, 34, 55–59). To investigate this for ABC-like B cells, we screened other B cells and ABC-like cells from HSs and SarcPs, six of eight of whom were on therapy (SarcP + SarcPT), for expression of FcRLs 1–5.
Representative results are shown Figures 2A and 2B (fluorescence minus one [FMO] = staining of samples with all antibodies without the anti-FcRL antibodies), and data for all HSs and SarcP + SarcPT are in Figures E3A and E3B. The average percentage of ABC-like cells in PBMCs was similar in HSs (4.38 ± 1.02) and SarcP + SarcPT (4.74 ± 1.35), as expected from the results in Figure 1B.
Figure 2.
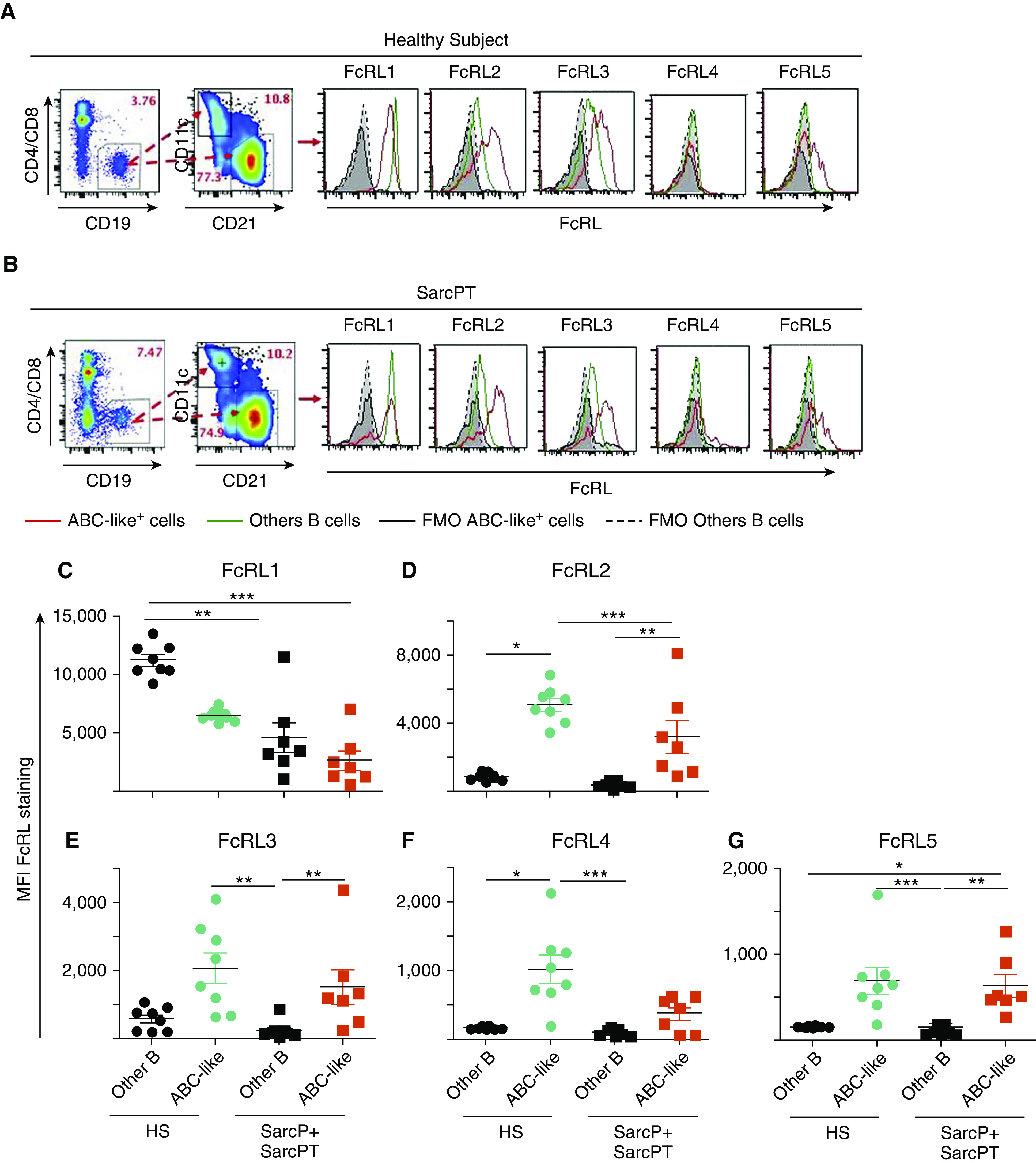
FcRL 1 (Fc receptor–like protein 1) is expressed at lower levels, and FcRLs 2 and 5 are expressed at higher levels on age-associated B cell (ABC)-like cells than on B cells that are not ABC-like regardless of whether the cells were obtained from healthy subjects (HSs), sarcoidosis patients not on treatment (SarcPs), or sarcoidosis patients on treatment (SarcPTs). Patient abbreviations are as described in the legend to Figure 1. Peripheral blood mononuclear cells from an HS and SarcPT were stained to define B cells that were not ABC-like (CD19+, CD11cneg CD21med-hi,) and ABC-like cells (CD19+ CD11c+ CD21lo). The different types of B cells were gated, and their levels of expression of FcRLs1–5, using phycoerythrin-labeled antibodies, are shown. (A and B) For each sample, the percentages of peripheral blood mononuclear cells that were B cells and the percentages of B cells that were or were not ABC-like are shown by numbers in red on the figures. Staining of the different types of B cells with the various anti-FcRL antibodies are shown in the histograms. The origins of the cells (HS or SarcP + SarcPT) are shown. (A) Sample from an HS. (B) Sample from a SarcPT. (C–G) The levels of intensity of staining with anti-FcRL antibodies of B cells that were not ABC-like and were ABC-like are shown. Staining was with antibody against: (C) FcRL1, (D) FcRL2, (E) FcRL3, (F) FcRL4, and (G) FcRL5. Data were analyzed with Kruskal-Wallis tests with Dunn’s post hoc tests. *P < 0.05, **P < 0.01, and ***P < 0.001. FMO = fluorescence minus one; MFI = mean fluorescent intensity.
FcRL1 appeared on other B cells at higher levels than on ABC-like cells in both HSs and SarcP + SarcPT, although its expression trended higher on other B cells in HSs than on other B cells in SarcP + SarcPT (Figure 2C). Likewise, FcRL1 levels on ABC-like cells were higher in HSs than in SarcP + SarcPT.
The other FcRLs were expressed at low levels on other B cells, regardless of their source. For HSs, FcRLs 2–5 were all expressed at significantly higher levels on ABC-like cells than on other B cells (Figures 2D–2G). Although a similar trend appeared for ABC-like cells versus other B cells in SarcP + SarcPT, the difference reached statistical significance only for FcRL2 and FcRL5 (Figures 2D and 2G).
Overall, the distribution of levels of expression of FcRLs 2–5 is different from that of FcRL1, with the B-cell stimulatory FcRL1 (60, 61) expressed at higher levels on other B cells than on ABC-like cells and the other, potentially inhibitory FcRLs (43–45) expressed more generously on ABC-like cells.
To find out whether the amount of FcRLs was related to the amount of CD11c on each cell, we divided the ABC-like cells into three groups depending on their expression of CD11c. The staining intensity of each FcRL was then measured for each group and the CD11cneg CD21med-hi (other B) cells. Examples of these analyses for an HS and a SarcP + SarcPT are shown in Figures 3A and 3B and for all subjects examined in Figures E4A and E4B.
Figure 3.
In sarcoidosis patients not on treatment (SarcPs) plus sarcoidosis patients on treatment (SarcPTs), levels of expression of FcRLs 2–5 (Fc receptor–like proteins 2–5) on age-associated B cell (ABC)-like cells correlate with those of CD11c. Patient abbreviations are as described in the legend to Figure 1. Cells were stained as described in the legend to Figure 2 and gated according to the levels of CD11c they bore. (A) Example of cells from a healthy subject. (B) Example of cells from a SarcPT. Staining of B cells that were not ABC-like and ABC-like cells and fluorescence minus one (staining levels in the channel that would be detecting the anti-FcRL antibodies in the absence of anti-FcRL antibodies in the sample) were as described in the legend to Figure 2. (C) The intensities of staining with antibodies against different FcRLs of ABC-like cells expressing different levels of CD11c and of B cells that were not ABC-like are shown. Staining levels with phycoerythrin isotype controls were subtracted from those with phycoerythrin-labeled anti-FcRLs. All but one of the SarcPs analyzed were on therapy. Data were analyzed with Kruskal-Wallis tests with Dunn’s post hoc tests. *P < 0.05, **P < 0.01, and ***P < 0.001. HS = healthy subject.
For HS B cells, amounts of FcRL1 were significantly higher on other B cells than on ABC-like cells, regardless of the amount of CD11c on the ABC-like cells. FcRL1 expression on other B cells from SarcP + SarcPT was not significantly different from that on ABC-like cells regardless of CD11c amounts, although a trend toward lower levels was observed as CD11c levels increased.
In HSs, FcRL2 was most highly expressed on ABC-like cells with the highest levels of CD11c and was progressively and significantly less present on the cells as their CD11c amounts fell (Figure 3C). Although a similar trend was present on B cells in SarcP + SarcPT, the difference reached significance only between the ABC-like cells bearing the highest amounts of CD11c and the other B cells (CD11cneg CD21med-hi, in green).
Similar observations apply to the expression of FcRLs 3–5 in SarcP + SarcPT. On the whole, significant increases in levels of these proteins applied only to those cells that expressed the highest levels of CD11c (Figure 3C). In HSs, on the other hand, no statistically different levels of FcRLs 3–5 were seen regardless of CD11c amounts on the same cells (Figure 3C).
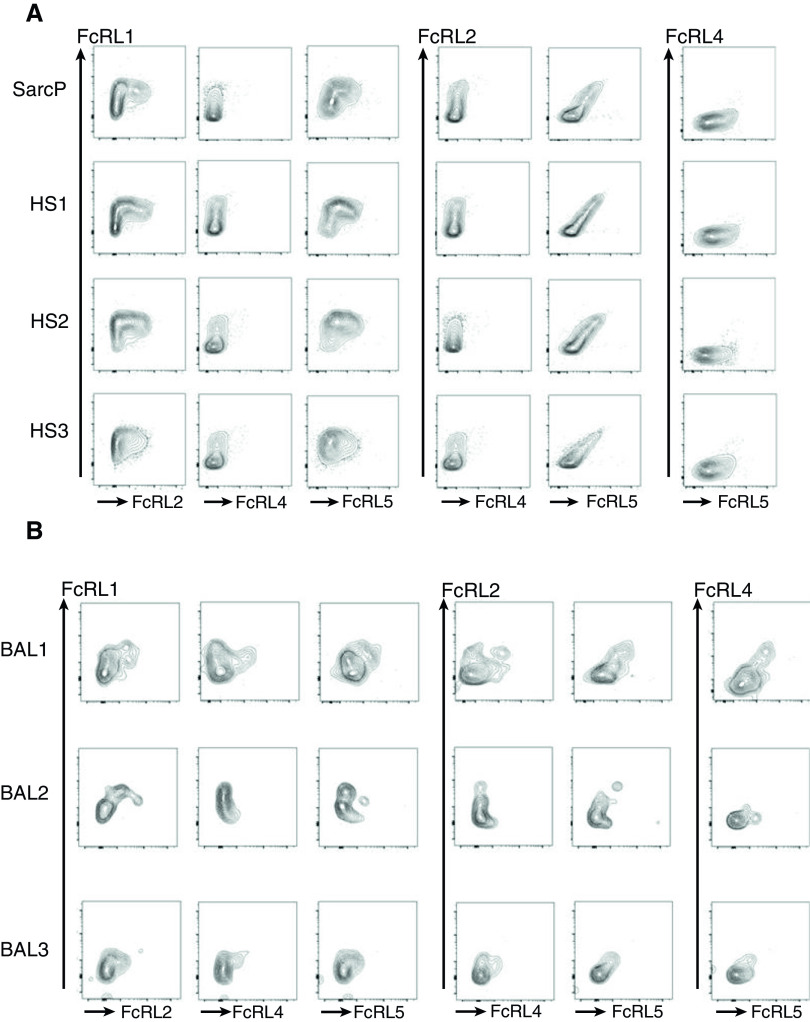
To find out whether the levels of FcRLs were related to each other, we developed a stain set to identify ABC-like cells and simultaneously measure the levels of FcRL 1–5 on each cell (Figure 4). With this stain set, no signal was obtained for FcRL3 for any sample, so data for FcRL3 are not shown, and FcRL4 was detected only in BAL.
Figure 4.
Correlation of levels of FcRLs (Fc receptor–like proteins) on peripheral blood mononuclear cells and BAL. Patient abbreviations are as described in the legend to Figure 1. (A) Peripheral blood mononuclear cells from three healthy subjects and one sarcoidosis patient not on treatment (SarcP) were stained as described in Methods, such that age-associated B cells could be identified and their levels of all five of the FcRLs analyzed with a single stain set. Data show the levels of expression of each FcRL with respect to that of each of the other FcRLs. Data for FcRL3 are not shown because, with this stain set, FcRL3 could not be detected. (B) Data shown are for BAL isolated from three different SarcPs. HS = healthy subject.
For ABC-like proteins in PBMCs, the staining patterns of ABC-like cells from all three HSs and the single SarcP analyzed were very similar. FcRL2 and FcRL5 appeared only on the cells that bore the highest levels of FcRL1. On the other hand, the levels of FcRL2 and FcRL5 were positively related, with the gradient of FcRL2 expression mirroring with that of FcRL5 (Figure 4A). The staining patterns of ABC-like B cells were less uniform among the BAL samples from three SarcPs (Figure 4B). The relationship between FcRL2, FcRL5, and FcRL1 was similar to that seen among the PBMC samples (Figures 4A and 4B), and, again, the levels of FcRL2 and FcRL5 were correlated. FcRL4 was detected on BAL sample 1 and 3 and had an expression pattern versus FcRL1 that was similar to that of FcRL2 and FcRL5. In the BAL1 sample, FcRL4 correlated with FcRL5.
Overall, these data indicate that ABC-like cells occupy a gradient in which different cells in the subpopulation express different levels of CD11c, and these correlate positively with T-bet levels as well as those of FcRLs 2, 5, and, perhaps in BAL, 4. These results suggest that ABC-like cells are not a discrete population as far as their levels of FcRLs and CD11c are concerned but rather occupy a gradient in which they progressively mature toward a completely ABC-like phenotype.
B Cells Resembling ABCs Appear at Elevated Levels in the BAL of Patients Suffering from Beryllium-related Disease or HP
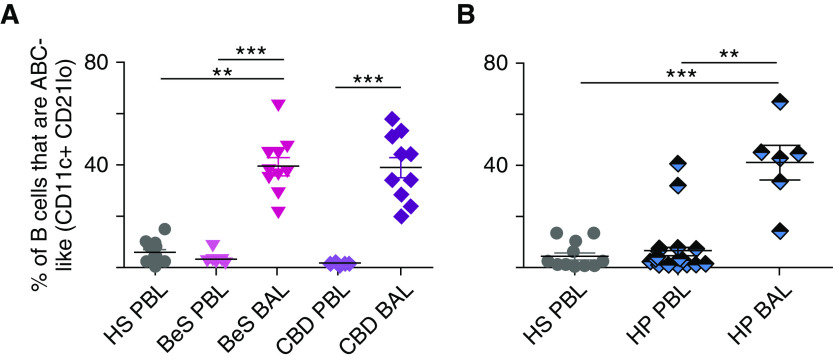
People with BeS may not have lung granulomas at the time of diagnosis, but they often acquire such as time passes (50). Once pulmonary granulomas occur, the patient develops symptoms of disease and are identified as having CBD (62, 63). Given that the symptoms of patients with CBD are similar to those of SarcPs (64–67), we investigated whether the percentages of ABC-like cells were elevated in the PBMCs from patients with BeS and CBD by comparison with HSs. ABC-like cells were not elevated in the patients’ PBMCs (Figure 5A); this is not because 6 of the 10 patients with CBD were on therapy (Table 1), as there was no significant difference between those who were or were not on therapy. In contrast to the PBMC results, the percentages of ABC-like cells in BAL of patients with disease were elevated. There was no statistical difference between the percentages of ABC-like cells in the BAL of individuals with BeS versus CBD (Figure 5A). This is surprising because those with BeS are not thought to have detectable granulomas, although they might develop such later. These results suggest that, as previously reported (64–67), the disease processes in SarcPs and patients with BeS or CBD are similar.
Figure 5.
A high percentage of B cells in the BAL of patients with beryllium sensitivity (BeS), chronic beryllium disease (CBD), or hypersensitivity pneumonitis (HP) are age-associated B cell (ABC)-like cells. (A) ABC-like cells in the peripheral blood mononuclear cells or BAL of patients with BeS or CBD were stained as described in the legend to Figure 1. (B) ABC-like cells in the peripheral blood mononuclear cells or BAL of HP were stained as described in the legend to Figure 1. Data were analyzed using Kruskal-Wallis tests with Dunn’s post hoc tests. **P < 0.01 and ***P < 0.001. HS = healthy subject; PBL = peripheral blood lymphocytes.
Granulomas appear in the lungs of patients suffering from HP (68). Although these granulomas are sometimes different in structure than those that appear in SarcPs or patients with CBD (69), we wondered whether the patients with HP would also be enriched in ABC-like cells. The percentages of B cells in PBMCs and BAL that were ABC-like in such patients were therefore assessed. As shown in Figure 5B, ABC-like B cells averaged at about the same level in PBMCs from HP and HSs. However, ABC-like cells were at very high levels in BAL from the patients with HP (Figure 5B).
Discussion
The data presented here show that ABC-like cells, defined by expression of CD11c and low levels of CD21, are enriched in the peripheral blood of patients suffering from sarcoidosis who are not currently being treated. Where BAL could be checked, such cells were found to be even more enriched in BAL. The BAL results also applied to patients suffering from other lung granulomatous diseases, CBD, or HP as well as patients with BeS, even though such individuals are not thought to have lung granulomas at the time of diagnosis.
B cells with surface proteins like those of ABC-like cells have been found at elevated levels in the PBMCs of people and mice with a number of infectious and autoimmune diseases (47). When checked, the antibodies made by such cells are enriched for reactivity against the relevant infection or for production of autoantibodies. Mice lacking ABCs deal with virus infections less effectively and, in one model, suffer less from lupus-like disease. Therefore, ABC-like cells may be important contributors to some human diseases. However, there is confusion over the markers that define ABC-like cells (70), with different publications defining the cells in different ways. In this work, we had three goals with respect to human B cells: first, to define some of the markers that characterize ABC-like cells; second, to investigate via the marker studies whether ABC-like cells are heterogeneous; and third, to find out whether or not ABCs are elevated in patients suffering from granulomatous lung diseases, particularly sarcoidosis.
With respect to these goals, the data here show that ABC-like B cells, defined by expression of CD11c and low levels of CD21, are composed of two subpopulations, one that expresses relatively high levels of the transcription factor, T-bet, and another that does not express significant amounts of T-bet. The ratio of these subpopulations is different in SarcPs versus SarcPTs, as in SarcPTs, the T-betneg-lo cells appear more affected by therapy. It is also different in the BAL of SarcPs, with BAL dramatically enriched in the Tbethi cells. The significance of these two different types of ABC-like cells is currently unknown, but their differences in disease certainly warrants more investigation.
Both subpopulations of ABC-like cells have a gradient of expression of their defining markers. For ABC-like cells as a whole, their levels of CD11c correlate inversely with their levels of CD21 and also correlate in different ways with expression of another set of markers that are characteristic of various types of B cells, the FcRLs (17, 19, 21, 34, 55–59). FcRL1 is inversely expressed with respect to CD11c levels, and FcRLs 2, 3, and 5 are at higher levels on cells expressing higher levels of CD11c. These results suggest two ideas. First, ABC-like cells in humans may be at various stages of differentiation, ranging from expression of low to high levels of CD11c. This effect may reflect maturation of the cells as they differentiate and/or are exposed to different levels of the factors that, at least in mice, drive differentiation of B cells into ABC-like cells (13, 21, 23). Second, the finding that, by comparison with other B cells, ABCs express lower levels of FcRL1 and CD21, both of which might stimulate B cells (60, 61, 71, 72), and higher levels of the potentially inhibitory FcRL2 and 5 (71) may explain why ABC-like cells are relatively unresponsive to triggering. Note that such effects may be even more marked in BAL, in which, for at least one patient, some of the ABCs express FcRL4, a protein bearing no ITAMs and two ITIMs (71). Although there is evidence that the balance between ITAM and ITIM effects on cell responses may depend on other conditions in the cell (discussed in References 73 and 74), these data and previous publications nevertheless suggest that negative signaling in ABC-like cells may be stronger than in other B cells. On the other hand, ABC-like cells bear higher levels of CD19 than other B cells do, a property that might increase the ABC-like cell responses.
ABC-like cells were present, not only in patients but also in the PBMCs of HSs. Although ABC-like cell numbers were lower in HSs versus SarcPs, the phenotypes of the cells were quite similar.
Mouse ABCs are most efficiently induced by signals through the B-cell receptor for antigen (surface immunoglobulin), engagement of B-cell TLR7 and IFNγ receptor, and, under some circumstances, signals through the IL21 receptor (13, 21, 23). Given that ABC-like cells appear in patients’ BAL, we can consider whether such signals could be present in the lungs of these individuals. IFNγ is at higher than normal levels in SarcPs (75, 76) and in patients with CBD or HP (66). A polymorphism in the coding sequence of TLR7 has been linked to the incidence of sarcoidosis in women (77), but no such linkage has been reported for CBD or HP. As far as the engagement of B-cell antigen receptors is concerned, antibodies against Mycobacterium tuberculosis and autoantibodies are apparent in some SarcPs (78), and HP is associated with antibodies to many environmental antigens (reviewed in Reference 79). The presence of antibodies against various targets is less well documented in CBD, although BeS (with or without granulomatous inflammation) clearly involves at least T-cell responses (63, 80). Therefore, as far as parallels to mouse ABCs are concerned, IFNγ is probably a contributor to the induction of ABC-like cells in the diseases examined here, but additional contributions from TLR7 may not be required and may perhaps be replaced by TLR9 (81).
Because ABC-like cell percentages increase in the PBMCs of people with a variety of diseases (6, 9, 11, 19, 21, 31), their presence probably cannot be used for specific diagnoses, except to indicate that some type of immune/inflammatory response is going on. On the other hand, increases in ABC-like cells may provide useful clues about the factors driving disease; for example, induction and assessment of the specificities of the antibodies they bear may help understand the ongoing disease. Such might be important for understanding of diseases such as sarcoidosis, for which the nature of the inciting agent has been, for many years, controversial.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Shirley Sobus and Josh Loomis of the National Jewish Health Cytometry Core.
Footnotes
Supported in part by NIH grants R21 AI128738 (P.M., B.B., L.A.M., and N.H.) and grants from Genentech (E.R.F.-P.) and Boehringer Ingelheim (E.R.F.-P.).
Author Contributions: S.P., K.A., K.R., A.R., L.P., and B.W. performed and analyzed the experiments and helped write the manuscript. B.B., E.R.F.-P., L.A.M., and N.H. designed experiments, provided patient material, and wrote the manuscript. J.L.C. performed statistical analyses. J.W.K. and P.M. designed and analyzed the experiments and wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201911-2151OC on June 5, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Arumugakani G, Wood PM, Carter CR. Frequency of Treg cells is reduced in CVID patients with autoimmunity and splenomegaly and is associated with expanded CD21lo B lymphocytes. J Clin Immunol. 2010;30:292–300. doi: 10.1007/s10875-009-9351-3. [DOI] [PubMed] [Google Scholar]
- 2.Ferre EM, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016;1:e88782. doi: 10.1172/jci.insight.88782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibi S, Zaki-Dizaji M, Rafiemanesh H, Lo B, Jamee M, Gámez-Díaz L, et al. Clinical, immunologic, and molecular spectrum of patients with lps-responsive beige-like anchor protein deficiency: a systematic review. J Allergy Clin Immunol Pract. 2019;7:2379–2386, e5. doi: 10.1016/j.jaip.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho J, Moir S, Malaspina A, Howell ML, Wang W, DiPoto AC, et al. Two overrepresented B cell populations in HIV-infected individuals undergo apoptosis by different mechanisms. Proc Natl Acad Sci USA. 2006;103:19436–19441. doi: 10.1073/pnas.0609515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsley AW, Saal HM, Burrow TA, Hopkin RJ, Shchelochkov O, Khandelwal P, et al. Defects of b-cell terminal differentiation in patients with type-1 kabuki syndrome. J Allergy Clin Immunol. 2016;137:179–187, e10. doi: 10.1016/j.jaci.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauli NT, Henry Dunand CJ, Wilson PC. Exploiting human memory B cell heterogeneity for improved vaccine efficacy. Front Immunol. 2011;2:77. doi: 10.3389/fimmu.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci USA. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c⁺ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnatz K, Wehr C, Dräger R, Schmidt S, Eibel H, Schlesier M, et al. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology. 2002;206:502–513. doi: 10.1078/0171-2985-00198. [DOI] [PubMed] [Google Scholar]
- 12.Winslow GM, Papillion AM, Kenderes KJ, Levack RC. CD11c+ T-bet+ memory B cells: immune maintenance during chronic infection and inflammation? Cell Immunol. 2017;321:8–17. doi: 10.1016/j.cellimm.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, et al. Cutting edge: il-4, il-21, and ifn-gamma interact to govern t-bet and cd11c expression in tlr-activated b cells. J Immunol. 2016;197:1023–1028. doi: 10.4049/jimmunol.1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest. 2017;127:1392–1404. doi: 10.1172/JCI91250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jöhrens K, Shimizu Y, Anagnostopoulos I, Schiffmann S, Tiacci E, Falini B, et al. T-bet-positive and IRTA1-positive monocytoid B cells differ from marginal zone B cells and epithelial-associated B cells in their antigen profile and topographical distribution. Haematologica. 2005;90:1070–1077. [PubMed] [Google Scholar]
- 16.Lau D, Lan LY, Andrews SF, Henry C, Rojas KT, Neu KE, et al. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol. 2017;2:eaai8153. doi: 10.1126/sciimmunol.aai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Borrego F, Nagata S, Tolnay M. Fc receptor-like 5 expression distinguishes two distinct subsets of human circulating tissue-like memory b cells. J Immunol. 2016;196:4064–4074. doi: 10.4049/jimmunol.1501027. [DOI] [PubMed] [Google Scholar]
- 18.Nipper AJ, Smithey MJ, Shah RC, Canaday DH, Landay AL. Diminished antibody response to influenza vaccination is characterized by expansion of an age-associated B-cell population with low PAX5. Clin Immunol. 2018;193:80–87. doi: 10.1016/j.clim.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK. Atypical memory B cells in human chronic infectious diseases: an interim report. Cell Immunol. 2017;321:18–25. doi: 10.1016/j.cellimm.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JL, Rosenthal RL, Knox JJ, Myles A, Naradikian MS, Madej J, et al. The transcription factor t-bet resolves memory b cell subsets with distinct tissue distributions and antibody specificities in mice and humans. Immunity. 2020;52:842–855, e6. doi: 10.1016/j.immuni.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, et al. IFNγ induces epigenetic programming of human T-bethi B cells and promotes TLR7/8 and IL-21 induced differentiation. eLife. 2019;8:e41641. doi: 10.7554/eLife.41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubtsova K, Rubtsov AV, Halemano K, Li SX, Kappler JW, Santiago ML, et al. T cell production of ifngamma in response to tlr7/il-12 stimulates optimal b cell responses to viruses. PLoS One. 2016;11:e0166322. doi: 10.1371/journal.pone.0166322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci USA. 2013;110:E3216–E3224. doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racine R, Chatterjee M, Winslow GM. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J Immunol. 2008;181:1375–1385. doi: 10.4049/jimmunol.181.2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi K, Kozono Y, Waldschmidt TJ, Berthiaume D, Quigg RJ, Baron A, et al. Mouse complement receptors type 1 (CR1;CD35) and type 2 (CR2;CD21): expression on normal B cell subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J Immunol. 1997;159:1557–1569. [PubMed] [Google Scholar]
- 26.Stepensky P, Rensing-Ehl A, Gather R, Revel-Vilk S, Fischer U, Nabhani S, et al. Early-onset Evans syndrome, immunodeficiency, and premature immunosenescence associated with tripeptidyl-peptidase II deficiency. Blood. 2015;125:753–761. doi: 10.1182/blood-2014-08-593202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aranburu A, Höök N, Gerasimcik N, Corleis B, Ren W, Camponeschi A, et al. Age-associated B cells expanded in autoimmune mice are memory cells sharing H-CDR3-selected repertoires. Eur J Immunol. 2018;48:509–521. doi: 10.1002/eji.201747127. [DOI] [PubMed] [Google Scholar]
- 28.Myles A, Sanz I, Cancro MP. T-bet+ B cells: a common denominator in protective and autoreactive antibody responses? Curr Opin Immunol. 2019;57:40–45. doi: 10.1016/j.coi.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Wang Z, Wang J, Diao Y, Qian X, Zhu N. T-bet-expressing b cells are positively associated with crohn’s disease activity and support th1 inflammation. DNA Cell Biol. 2016;35:628–635. doi: 10.1089/dna.2016.3304. [DOI] [PubMed] [Google Scholar]
- 30.Fleischer SJ, Daridon C, Fleischer V, Lipsky PE, Dörner T. Enhanced tyrosine phosphatase activity underlies dysregulated b cell receptor signaling and promotes survival of human lupus b cells. Arthritis Rheumatol. 2016;68:1210–1221. doi: 10.1002/art.39559. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. Autoimmunity Molecular Medicine Team. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat Commun. 2018;9:1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, et al. Age-associated b cells with proinflammatory characteristics are expanded in a proportion of multiple sclerosis patients. J Immunol. 2016;197:4576–4583. doi: 10.4049/jimmunol.1502448. [DOI] [PubMed] [Google Scholar]
- 33.Comarmond C, Lorin V, Marques C, Maciejewski-Duval A, Joher N, Planchais C, et al. TLR9 signalling in HCV-associated atypical memory B cells triggers Th1 and rheumatoid factor autoantibody responses. J Hepatol. 2019;71:908–919. doi: 10.1016/j.jhep.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Terrier B, Nagata S, Ise T, Rosenzwajg M, Pastan I, Klatzmann D, et al. CD21(-/low) marginal zone B cells highly express Fc receptor-like 5 protein and are killed by anti-Fc receptor-like 5 immunotoxins in hepatitis C virus-associated mixed cryoglobulinemia vasculitis. Arthritis Rheumatol. 2014;66:433–443. doi: 10.1002/art.38222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visentini M, Cagliuso M, Conti V, Carbonari M, Cibati M, Siciliano G, et al. Clonal B cells of HCV-associated mixed cryoglobulinemia patients contain exhausted marginal zone-like and CD21 low cells overexpressing Stra13. Eur J Immunol. 2012;42:1468–1476. doi: 10.1002/eji.201142313. [DOI] [PubMed] [Google Scholar]
- 36.Lin W, Cerny D, Chua E, Duan K, Yi JT, Shadan NB, et al. Human regulatory B cells combine phenotypic and genetic hallmarks with a distinct differentiation fate. J Immunol. 2014;193:2258–2266. doi: 10.4049/jimmunol.1303214. [DOI] [PubMed] [Google Scholar]
- 37.Belkhou A, Younsi R, El Bouchti I, El Hassani S. Rituximab as a treatment alternative in sarcoidosis. Joint Bone Spine. 2008;75:511–512. doi: 10.1016/j.jbspin.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Bomprezzi R, Pati S, Chansakul C, Vollmer T. A case of neurosarcoidosis successfully treated with rituximab. Neurology. 2010;75:568–570. doi: 10.1212/WNL.0b013e3181ec7ff9. [DOI] [PubMed] [Google Scholar]
- 39.Dasilva V, Breuil V, Chevallier P, Euller-Ziegler L. Relapse of severe sarcoidosis with an uncommon peritoneal location after tnfalpha blockade. Efficacy of rituximab, report of a single case. Joint Bone Spine. 2010;77:82–83. doi: 10.1016/j.jbspin.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Häggmark A, Hamsten C, Wiklundh E, Lindskog C, Mattsson C, Andersson E, et al. Proteomic profiling reveals autoimmune targets in sarcoidosis. Am J Respir Crit Care Med. 2015;191:574–583. doi: 10.1164/rccm.201407-1341OC. [DOI] [PubMed] [Google Scholar]
- 41.Saussine A, Tazi A, Feuillet S, Rybojad M, Juillard C, Bergeron A, et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS One. 2012;7:e43588. doi: 10.1371/journal.pone.0043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamphuis LS, van Zelm MC, Lam KH, Rimmelzwaan GF, Baarsma GS, Dik WA, et al. Perigranuloma localization and abnormal maturation of B cells: emerging key players in sarcoidosis? Am J Respir Crit Care Med. 2013;187:406–416. doi: 10.1164/rccm.201206-1024OC. [DOI] [PubMed] [Google Scholar]
- 43.Ehrhardt GR, Leu CM, Zhang S, Aksu G, Jackson T, Haga C, et al. Fc receptor-like proteins (FCRL): immunomodulators of B cell function. Adv Exp Med Biol. 2007;596:155–162. doi: 10.1007/0-387-46530-8_14. [DOI] [PubMed] [Google Scholar]
- 44.Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Natl Acad Sci USA. 2007;104:9770–9775. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson TA, Haga CL, Ehrhardt GR, Davis RS, Cooper MD. FcR-like 2 Inhibition of B cell receptor-mediated activation of B cells. J Immunol. 2010;185:7405–7412. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poonia B, Ayithan N, Nandi M, Masur H, Kottilil S. HBV induces inhibitory FcRL receptor on B cells and dysregulates B cell-T follicular helper cell axis. Sci Rep. 2018;8:15296. doi: 10.1038/s41598-018-33719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karnell JL, Kumar V, Wang J, Wang S, Voynova E, Ettinger R. Role of CD11c+ T-bet+ B cells in human health and disease. Cell Immunol. 2017;321:40–45. doi: 10.1016/j.cellimm.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Pérez-Mazliah D, Gardner PJ, Schweighoffer E, McLaughlin S, Hosking C, Tumwine I, et al. Plasmodium-specific atypical memory B cells are short-lived activated B cells. eLife. 2018;7:e39800. doi: 10.7554/eLife.39800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), The European Respiratory Society (ERS) and The World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 50.Balmes JR, Abraham JL, Dweik RA, Fireman E, Fontenot AP, Maier LA, et al. ATS Ad Hoc Committee on Beryllium Sensitivity and Chronic Beryllium Disease. An official American Thoracic Society statement: diagnosis and management of beryllium sensitivity and chronic beryllium disease. Am J Respir Crit Care Med. 2014;190:e34–e59. doi: 10.1164/rccm.201409-1722ST. [DOI] [PubMed] [Google Scholar]
- 51.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. American Thoracic Society Committee on BAL in Interstitial Lung Disease. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 53.Venables WN, Ripley BD, Venables WN. Modern applied statistics with s. New York: Springer; 2002. [Google Scholar]
- 54.Yanaba K, Kamata M, Asano Y, Tada Y, Sugaya M, Kadono T, et al. CD19 expression in B cells regulates atopic dermatitis in a mouse model. Am J Pathol. 2013;182:2214–2222. doi: 10.1016/j.ajpath.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang LY, Li Y, Kaplan DE. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J Viral Hepat. 2017;24:389–396. doi: 10.1111/jvh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dement-Brown J, Newton CS, Ise T, Damdinsuren B, Nagata S, Tolnay M. Fc receptor-like 5 promotes B cell proliferation and drives the development of cells displaying switched isotypes. J Leukoc Biol. 2012;91:59–67. doi: 10.1189/jlb.0211096. [DOI] [PubMed] [Google Scholar]
- 57.Ehrhardt GR, Cooper MD. Immunoregulatory roles for fc receptor-like molecules. Curr Top Microbiol Immunol. 2011;350:89–104. doi: 10.1007/82_2010_88. [DOI] [PubMed] [Google Scholar]
- 58.Rostamzadeh D, Kazemi T, Amirghofran Z, Shabani M. Update on Fc receptor-like (FCRL) family: new immunoregulatory players in health and diseases. Expert Opin Ther Targets. 2018;22:487–502. doi: 10.1080/14728222.2018.1472768. [DOI] [PubMed] [Google Scholar]
- 59.Simmonds MJ, Brand OJ, Barrett JC, Newby PR, Franklyn JA, Gough SC. Association of Fc receptor-like 5 (FCRL5) with Graves’ disease is secondary to the effect of FCRL3. Clin Endocrinol (Oxf) 2010;73:654–660. doi: 10.1111/j.1365-2265.2010.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leu CM, Davis RS, Gartland LA, Fine WD, Cooper MD. FcRH1: an activation coreceptor on human B cells. Blood. 2005;105:1121–1126. doi: 10.1182/blood-2004-06-2344. [DOI] [PubMed] [Google Scholar]
- 61.Wang K, Pei H, Huang B, Yang RL, Wu HY, Zhu X, et al. Overexpression of fFc receptor-like 1 associated with B-cell activation during hepatitis b virus infection. Braz J Med Biol Res. 2012;45:1112–1118. doi: 10.1590/S0100-879X2012007500130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawyer RT, Maier LA, Kittle LA, Newman LS. Chronic beryllium disease: a model interaction between innate and acquired immunity. Int Immunopharmacol. 2002;2:249–261. doi: 10.1016/s1567-5769(01)00177-1. [DOI] [PubMed] [Google Scholar]
- 63.Palmer BE, Mack DG, Martin AK, Gillespie M, Mroz MM, Maier LA, et al. Up-regulation of programmed death-1 expression on beryllium-specific CD4+ T cells in chronic beryllium disease. J Immunol. 2008;180:2704–2712. doi: 10.4049/jimmunol.180.4.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Gundy K, Sharma OP. Pathogenesis of sarcoidosis. West J Med. 1987;147:168–174. [PMC free article] [PubMed] [Google Scholar]
- 65.Richeldi L. Chronic beryllium disease: a model for pulmonary sarcoidosis? Acta Biomed. 2005;76:11–14. [PubMed] [Google Scholar]
- 66.Li L, Silveira LJ, Hamzeh N, Gillespie M, Mroz PM, Mayer AS, et al. Beryllium-induced lung disease exhibits expression profiles similar to sarcoidosis. Eur Respir J. 2016;47:1797–1808. doi: 10.1183/13993003.01469-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang IV, Konigsberg I, MacPhail K, Li L, Davidson EJ, Mroz PM, et al. DNA methylation changes in lung immune cells are associated with granulomatous lung disease. Am J Respir Cell Mol Biol. 2019;60:96–105. doi: 10.1165/rcmb.2018-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grunes D, Beasley MB. Hypersensitivity pneumonitis: a review and update of histologic findings. J Clin Pathol. 2013;66:888–895. doi: 10.1136/jclinpath-2012-201337. [DOI] [PubMed] [Google Scholar]
- 69.Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: Clues and pitfalls: Number 4 in the series “Pathology for the Clinician” edited by Peter Dorfmuller and Alberto Cavazza. Eur Respir Rev. 2017;26:170012. doi: 10.1183/16000617.0012-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phalke S, Marrack P. Age (autoimmunity) associated B cells (ABCs) and their relatives. Curr Opin Immunol. 2018;55:75–80. doi: 10.1016/j.coi.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Davis RS. Fc receptor-like molecules. Annu Rev Immunol. 2007;25:525–560. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 72.Cherukuri A, Cheng PC, Sohn HW, Pierce SK. The CD19/CD21 complex functions to prolong B cell antigen receptor signaling from lipid rafts. Immunity. 2001;14:169–179. doi: 10.1016/s1074-7613(01)00098-x. [DOI] [PubMed] [Google Scholar]
- 73.Li FJ, Won WJ, Becker EJ, Jr, Easlick JL, Tabengwa EM, Li R, et al. Emerging roles for the FCRL family members in lymphocyte biology and disease. Curr Top Microbiol Immunol. 2014;382:29–50. doi: 10.1007/978-3-319-07911-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Z, Li R, Li H, Zhou T, Davis RS. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc Natl Acad Sci USA. 2013;110:E1282–E1290. doi: 10.1073/pnas.1215156110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saltini C, Amicosante M. Beryllium disease. Am J Med Sci. 2001;321:89–98. doi: 10.1097/00000441-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Schupp JC, Vukmirovic M, Kaminski N, Prasse A. Transcriptome profiles in sarcoidosis and their potential role in disease prediction. Curr Opin Pulm Med. 2017;23:487–492. doi: 10.1097/MCP.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bordignon M, Bargagli E, Agostini C, Cinetto F, Baldo V, Alaibac M, et al. Tlr7 gln11leu single nucleotide polymorphism in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:157–161. [PubMed] [Google Scholar]
- 78.Rook GA, Stanford JL. Slow bacterial infections or autoimmunity? Immunol Today. 1992;13:160–164. doi: 10.1016/0167-5699(92)90119-R. [DOI] [PubMed] [Google Scholar]
- 79.Morell F, Villar A, Ojanguren I, Muñoz X, Cruz MJ. Hypersensitivity pneumonitis: challenges in diagnosis and management, avoiding surgical lung biopsy. Semin Respir Crit Care Med. 2016;37:395–405. doi: 10.1055/s-0036-1580692. [DOI] [PubMed] [Google Scholar]
- 80.Clayton GM, Wang Y, Crawford F, Novikov A, Wimberly BT, Kieft JS, et al. Structural basis of chronic beryllium disease: linking allergic hypersensitivity and autoimmunity. Cell. 2014;158:132–142. doi: 10.1016/j.cell.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wade MF, Collins MK, Richards D, Mack DG, Martin AK, Dinarello CA, et al. Tlr9 and il-1r1 promote mobilization of pulmonary dendritic cells during beryllium sensitization. J Immunol. 2018;201:2232–2243. doi: 10.4049/jimmunol.1800303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.