Abstract
Metastasis arises when cancer cells disseminate from their site of origin and invade distant organs. While cancer cells rarely colonize muscle, they often induce a debilitating muscle-wasting condition known as cachexia that compromises feeding, breathing, and cardiac function in metastatic cancer patients. In fact, nearly 80% of metastatic cancer patients experience a spectrum of muscle-wasting states, which deteriorates the quality of life and overall survival of cancer patients. Muscle wasting in cancer results from increased muscle catabolism induced by circulating tumor factors and a systemic metabolic dysfunction. In addition, muscle loss can be exacerbated by the exposure to antineoplastic therapies and the process of aging. With no approved therapies to alleviate cachexia, muscle health, therefore, becomes a key determinant of prognosis, treatment response, and survival in metastatic cancer patients. This review will discuss the current understanding of cancer-associated cachexia and highlight promising therapeutic strategies to treat muscle wasting in the context of metastatic cancers.
Muscles make up nearly 50% of our total body mass and play a key role in normal physiological activities such as breathing, chewing and swallowing food, and locomotion. Yet, often unrecognized, these basic functions are severely compromised in 80% of advanced cancer patients due to a muscle-wasting syndrome known as cachexia (Fearon et al. 2012; Baracos et al. 2018). Cachexia involves a dramatic loss of skeletal muscle mass and function that drastically decline the rate of survival and quality of life of metastatic cancer patients. Importantly, cachexia is not caused by the metastasis of cancer cells to muscle. Instead, tumors secrete factors into the circulation that perturb normal physiological and metabolic programs in the host and negatively impact the functions of skeletal and cardiac muscle. Moreover, since tumors compete with their host for available nutrients, a limited nutrient supply can also cause energy deprivation and trigger muscle catabolism (Fearon et al. 2012). Unlike starvation, however, dietary supplementation and parenteral nutrition fail to reverse cachexia, which underscore that cachexia does not emerge merely due to failure of a nutritional supply-and-demand balance, but perhaps an inability of host tissues to utilize available nutrients to fulfill their energy and metabolic needs.
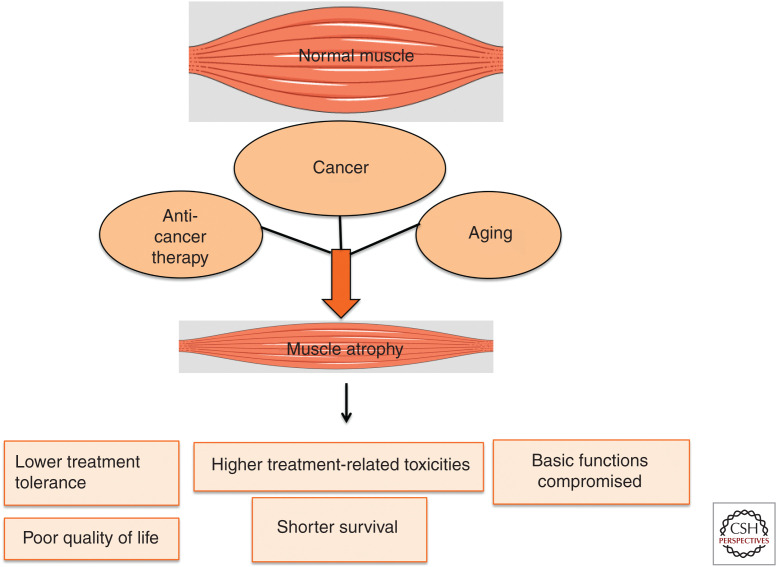
Muscle wasting has dire consequences on vital physiological activities of the cancer patients (Kalantar-Zadeh et al. 2013). For example, when jaw muscles deteriorate, chewing, swallowing, and feeding responses are compromised. With declining muscle mass and function in limb muscles, patients experience restricted movement and lose their functional independence. Due to the dysfunction of the diaphragm and cardiac muscles, cancer patients die prematurely from respiratory distress and cardiac failure, respectively (Baracos et al. 2018). Cachexia also reduces tolerance to chemotherapy and increases the risk of postsurgical complications, which in turn compromise anticancer treatment outcomes and negatively impacts survival (Fearon et al. 2013). The discovery of effective treatment options for cachexia is therefore expected to restore tolerance to anticancer drugs, improve quality of life and prolong survival in advanced cancer patients.
SPECTRUM OF MUSCLE-WASTING STATES IN CANCER PATIENTS
Multiple factors contribute to muscle catabolism during cancer progression and treatment ( Figs. 1 and 2). Chemotherapy-induced muscle damage can exacerbate cachexia. (Dewys et al. 1980; Barreto et al. 2016a). Sarcopenia, an aging-associated decline in muscle mass, is often observed in elderly patients (Fielding et al. 2011; Bowen et al. 2015) and results in the reduction of tolerance to chemotherapy, latency before cancer progression, and life expectancy compared to nonsarcopenic cancer patients (Prado et al. 2009a). Not surprisingly, the aggravation of existing muscle-wasting conditions by anticancer therapy leads to a further decline in cancer patient survival (Prado et al. 2007, 2011a). Moreover, muscle wasting can often be masked by obesity and thereby remain undiagnosed for extended periods of time. For instance, breast cancer patients rarely show overt cachectic symptoms such as body weight loss and even gain weight during cancer treatment. However, body composition analysis by computerized tomography (CT) often reveals muscle mass loss in breast cancer patients can be compensated by increased adipose tissue mass (Martin et al. 2013). It is important to note that muscle depletion or lean tissue loss is associated with higher dose-limiting toxicities from antineoplastic therapies (Mourtzakis et al. 2008; Prado et al. 2009a, 2011a). Knowledge of the underlying mechanisms that drive muscle wasting, therefore, has the potential to eliminate the myriad complications that cancer patients endure with debilitating muscle-wasting conditions during both active treatment and subsequent recovery.
Figure 1.
Spectrum of muscle-wasting states in metastatic cancer patients.
Figure 2.
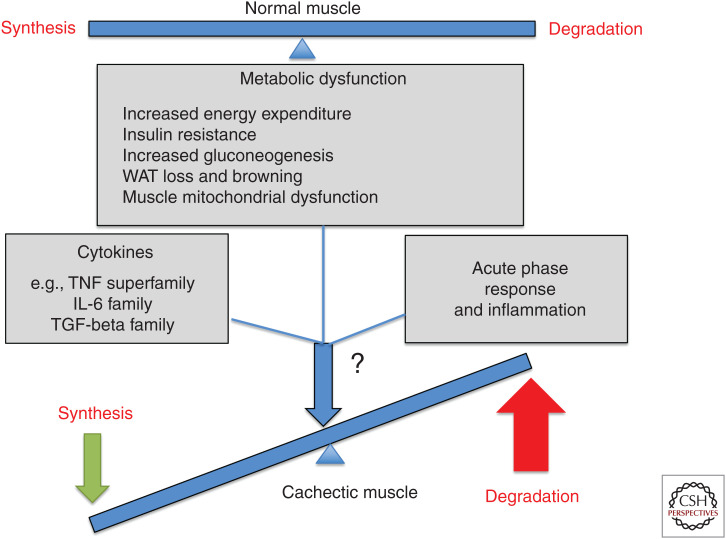
An outline of mediators of muscle atrophy highlighting the multifactorial regulation of muscle wasting in cancer.
CANCER-INDUCED MUSCLE WASTING
Cachexia is frequently associated with cancers that originate in the pancreas, gastrointestinal tract, and lung and manifests as an energy-wasting syndrome that disrupts muscle protein homeostasis (Baracos et al. 2018). The efficient cellular protein homeostasis depends on tight regulation of protein biosynthesis and breakdown pathways. It is estimated that 280 g of protein is synthesized and degraded each day in a 70-kg adult human (Mitch and Goldberg 1996). Through the ubiquitin-mediated proteasomal degradation pathway, the covalent attachment of ubiquitin molecules by E1, E2, and E3 ligases to proteins leads to their degradation by the 26S proteasome. However, in cancer patients, this normal process is often disrupted, which induces the breakdown of muscle proteins, sometimes coupled with reduced synthesis (Mitch and Goldberg 1996; Cohen et al. 2015; Zhao and Goldberg 2016). The increase in muscle protein degradation occurs primarily through the ubiquitin-proteasome and autophagy pathways (Mitch and Goldberg 1996) and, coupled with the additional loss of organelles and cytoplasm from muscle cells, results in muscle atrophy (Cohen et al. 2015; Sandri 2016). In particular, the E3 ligases MuRF1, MAFbx/atrogin-1, MUSA1, TRAF6, and Fbx031 are transcriptionally up-regulated in atrophic muscles under multiple catabolic conditions including denervation, immobilization, sepsis, glucocorticoid exposure, and cancer, leading to the increased turnover of sarcomeric and other muscle proteins (Bodine et al. 2001; Gomes et al. 2001; Paul et al. 2010; Sartori et al. 2013; Milan et al. 2015; Wang et al. 2018). MuRF1 ubiquitinates sarcomeric proteins, myosin heavy chain and actin (Polge et al. 2011) while atrogin-1 degrades the myogenic regulatory factor MyoD in muscle cells (Tintignac et al. 2005), and importantly, loss of Murf1, Mafbx, and Musa1 in mice protects muscles from denervation-induced atrophy (Bodine et al. 2001; Paul et al. 2010; Sartori et al. 2013). However, there is a paucity of loss-of-function genetic studies designed to specifically analyze the role of these E3 ligases in cancer-induced muscle wasting.
The delicate balance between protein synthesis and degradation is maintained by a cross-talk between their regulatory pathways (Fig. 2). For instance, the IGF1/PI3K/AKT pathway induces hypertrophy in muscle cells via activation of protein synthesis while simultaneously blocking induction of the catabolic atrophy mediators MuRF1 and MAFbx by forkhead box O (FOXO) transcription factors (Sandri et al. 2004; Stitt et al. 2004). AKT phosphorylates all three mammalian FOXO family members (FOXO1, FOXO3, and FOXO4), which blocks their nuclear function by sequestering them in the cytoplasm. Nonphosphorylated FOXO factors, on the other hand, are free to enter the nucleus and induce the expression of MuRF1 and MAFbx. Under atrophy-inducing conditions, PI3K/AKT pathway activity diminishes in differentiated muscle cells (myotubes) leading to FOXO3-mediated induction of MAFbx (Sandri et al. 2004). Consistently, the expression of IGF-1, activated PI3K or activated AKT is sufficient to inhibit the up-regulation of MuRF1 and MAFbx during dexamethasone-induced muscle atrophy in a FOXO-dependent manner (Stitt et al. 2004). Moreover, the transcriptional activity of FOXO1 and FOXO3a increases under muscle-atrophy-inducing conditions (Reed et al. 2012), and transgenic overexpression of FOXO1 in otherwise-normal skeletal muscle results in decreased muscle mass and reduced muscle function (Kamei et al. 2004). The inhibition of FOXO activity in muscles has also been shown to prevent cancer-induced muscle atrophy as well as reduce the expression of atrophy signature genes such as Murf1, Mafbx, and Bnip3 (Reed et al. 2012). The IGF1/PI3K/AKT and FOXO pathways, therefore, play a pivotal role in muscle fiber size regulation.
It is unclear whether cancer-induced muscle wasting exists to benefit tumors. Skeletal muscle comprises 75% of the protein of the human body (Wolfe 2006) and thereby serves as the primary reservoir for amino acids that sustain both protein synthesis and energy production if other energy sources become depleted (Parry-Billings et al. 1991; Chen et al. 1993; Souba 1993; Luo et al. 2014; Argilés et al. 2016). As such, skeletal muscle proteolysis represents a vast energy resource for a developing tumor (DeBerardinis and Cheng 2010), which may initiate muscle catabolism to meet its high energy requirements. Yet, human tumors comprising <1% of the total body mass can trigger muscle catabolism (Fearon et al. 2012), and while increased nutritional intake or intravenous feeding partially reverses fat loss in cancer patients, neither is able to reverse muscle wasting (Nixon et al. 1981; Popp et al. 1981; Cohn et al. 1982; Nixon 1982). Since muscle catabolism also persists in the presence of hypercaloric feeding (Kotler 2000), it may be driven by mechanisms that are independent of the energy needs of a growing tumor. An alternative hypothesis to explain cancer-associated muscle wasting is that muscle catabolism is merely an unintended consequence of a cancer-induced disruption in muscle energy utilization. The possibility of ineffective energy utilization in the muscle is supported by the frequent development of insulin resistance in the muscles of cachectic cancer patients as well as an increase in energy-inefficient processes driven by the liver, such as gluconeogenesis and the acute phase response (APR) (Fig. 2; Argilés et al. 2014). Additional experiments are still needed to clarify whether cancer-induced muscle wasting evolved as a means to support the growth and survival of cancer cells.
New insights from Drosophila models have shed light on the connection between mediators of insulin resistance and muscle atrophy (Figueroa-Clarevega and Bilder 2015; Kwon et al. 2015). In a Drosophila cancer cachexia model generated by the Bilder group, secretion of the insulin growth factor binding protein homolog ImpL2, an antagonist of insulin signaling, leads to insulin resistance in peripheral tissues as well as muscle wasting (Figueroa-Clarevega and Bilder 2015). ImpL2 also causes systemic metabolic changes and muscle wasting when secreted from an overproliferating gut (Kwon et al. 2015). Whether an analogous pathway drives human cancer cachexia remains to be elucidated, yet insulin resistance is indeed common in cancer patients and animal models with cachexia (Asp et al. 2010, 2011; Honors and Kinzig 2012). It can accelerate muscle proteolysis through suppression of the PI3K/AKT pathway and activation of the ubiquitin-mediated proteasome pathway (Wang et al. 2006). Interestingly, an insulin tolerance test revealed that the C26 cancer cachexia model exhibits a blunted blood glucose response to insulin before the onset of weight loss (Asp et al. 2010). In this mouse model, insulin resistance also correlates with reduced AKT phosphorylation and increased expression of genes involved in proteolysis and autophagy, such as Murf1, Mafbx, and Bnip3I, in the muscle (Asp et al. 2010). Furthermore, insulin resistance leads to an increase in hepatic gluconeogenesis, an energy-wasteful process in which glucose is synthesized from substrates such as lactate, alanine, glycerol, and glutamine in the liver (Hatting et al. 2018). Amino acids released from muscle protein breakdown during cancer cachexia are used as substrates for this process (Argilés et al. 2016). Insulin resistance, therefore, contributes to the negative energy balance observed in cachectic cancer patients (Honors and Kinzig 2012; Hatting et al. 2018) and may be an early event during the development of cachexia.
The liver initiates an APR where large quantities of tissue injury response proteins are synthesized at the expense of structural proteins (Kushner 1993; Kotler 2000; Argilés et al. 2018). This is similar to the pathological response involving the innate immune system that is normally triggered during infection. The APR is defined by a change in the concentration of certain plasma proteins that reflect either a 25% increase (termed “positive APR proteins”) or 25% decrease (termed “negative APR proteins”) (Gabay and Kushner 1999) in their levels (Fearon et al. 1999). Examples of positive APR proteins are C-reactive protein, serum amyloid A (SAA) and fibrinogen while negative APR proteins include albumin and transferrin. C-reactive proteins are opsonins that bind to denatured lipopolysaccharides/proteins and nucleic acids and lead to complement activation and macrophage-mediated phagocytosis (Kushner 1993; Cray et al. 2009) whereas APR SAA proteins are involved in immune cell chemotaxis (Cray et al. 2009; Lee et al. 2019). Under inflammatory conditions triggered by exposure to cytokines such as interleukin-6 (IL-6) and IL-1, by glucocorticoids (Baumann et al. 1987; Mackiewicz et al. 1992) or in the context of cancer, structural protein synthesis gives way to increased production of APR proteins (Falconer et al. 1995; Fearon et al. 1999). Synthesis of many of the plasma proteins not involved in host defense, such as albumin, is down-regulated (Kushner 1993; Cray et al. 2009). In fact, clinical studies in pancreatic cancer patients show that the plasma concentration of APR proteins can be altered by 40% at the time of diagnosis and up to 80% at the time of death (Falconer et al. 1995; Fearon et al. 1999). Since the APR alters host metabolism and reduces appetite, it is thought to contribute to the negative energy balance in cachectic patients by both reducing energy intake and increasing energy expenditure. The APR, therefore, represents yet another factor that contributes to increased resting energy expenditure (REE) and hypermetabolism in cancer cachexia (Falconer et al. 1994). Whether the APR actually benefits the host or represents a futile, energy-intensive response to cancer remains debatable; however, it is clear that the chronic activation of the APR that occurs in the context of cancer leads to a continuous subversion of amino acid resources and has adverse effects on cancer patients.
Increased energy expenditure is a common feature of cachexia across many cancer types (Cao et al. 2010; Xu et al. 2012). White adipose tissue (WAT) loss and adipose tissue browning contribute to this phenomenon and thereby promote a negative energy balance in patients with cancer cachexia. Genetic studies show that ablation of the adipose tissue triglyceride lipase gene (Atgl) prevents loss of both white adipose cells and muscle protein, suggesting an important cross-talk between adipose tissue and muscle during cachexia development (Das et al. 2011). An increase in energy expenditure also arises from the browning of WAT to form beige adipose cells. WAT specializes in storing energy in the form of lipids while brown adipose tissue participates in energy expenditure (Cypess et al. 2009). WAT browning can be triggered by exposure to a number of stimuli including cold temperatures (Sidossis et al. 2015), the tumor-derived polypeptide parathyroid hormone-related protein (PTHrP) (Kir et al. 2014), lactate (Carriere et al. 2014), chronic inflammation and IL-6 (Petruzzelli et al. 2014), or the muscle-derived cytokine irisin (Boström et al. 2012). As such, each of these events has the potential to exacerbate the negative energy balance observed in cachectic cancer patients.
The oxidative capacity of a muscle cell is largely determined by its mitochondrial function (Vitorino et al. 2015). During cancer-induced muscle wasting, mitochondrial function is compromised (Brown et al. 2017). For example, cachectic muscles from multiple cancer models exhibit distorted mitochondria with swollen and fragmented cristae and sarcomeric disintegration (Shum et al. 2012; Tzika et al. 2013; Brown et al. 2017). Moreover, the ratio of mitochondrial DNA to nuclear DNA is reduced in the Apc(Min/+) cancer cachexia model along with decreases in the protein levels of cytochrome c and cytochrome c oxidase complex subunit IV (White et al. 2011a). A similar reduction in complex IV activity and mitochondrial oxidative capacity was observed in cachectic rats with peritoneal carcinosis (Julienne et al. 2012). This occurred without either mitochondrial uncoupling or changes to the efficiency of ATP synthesis. In contrast, a separate study in cachectic muscles from mice bearing Lewis lung carcinomas showed a reduction in both ATP synthesis rate and TCA cycle flux, which is indicative of mitochondrial uncoupling (Tzika et al. 2013). While it remains unclear whether the difference in outcome between these two studies is due to the specific model, cancer type or degree of cachexia, it is likely that mitochondrial dysfunction in muscle cells also contributes to the negative energy balance observed in patients with cancer cachexia.
Studies performed over the past several decades have identified the proinflammatory cytokines tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and IL-6 as important mediators of cancer cachexia. A comprehensive review on the role of these cytokines function during cancer cachexia is available elsewhere (Fearon et al. 2012). For example, cachexia can be induced in nude mice when inoculated with Chinese hamster ovary tumor cells overexpressing either TNF-α or IFN-γ (Oliff et al. 1987; Matthys et al. 1991). Blockade of TNF-α signaling in mice reduces tumor-induced muscle wasting (Llovera et al. 1998a,b), and loss of IL-6 in the Apc(Min/+)-intestinal and C26 colon-cancer cachexia models (McCart et al. 2008) protects against muscle atrophy (Strassmann et al. 1992; Fujita et al. 1996; Tsujinaka et al. 1996; White et al. 2011b). However, clinical trials have shown that blocking these cytokines does not effectively reverse cachexia, which could be due to their varying expression levels in cancer patients or the existence of multiple mediators of cachexia. Indeed, TNF-α acts in concert with IFN-γ to inhibit myosin heavy chain transcription in muscle cells through reduction of MyoD expression (Acharyya et al. 2004), and the TNF/TNFR superfamily exhibits functional redundancy among its family members (Dogra et al. 2007; Bhatnagar et al. 2012; Johnston et al. 2015). IL-6 family members also exhibit redundancy with respect to their ability to induce cachexia (Bonetto et al. 2011); ciliary neurotrophic factor (CNTF) and leukemia inhibitor factor (LIF) can induce muscle wasting by activating the same downstream STAT3-mediated signaling pathway as IL-6 (Henderson et al. 1994; Espat et al. 1996; Bonetto et al. 2011; Kandarian et al. 2018). Thus, blocking STAT3 might represent an effective alternative strategy for treating cachexia (Bonetto et al. 2012; Zimmers et al. 2016). The existence of functional redundancy among these different cytokine families ultimately highlights the importance of targeting multiple mediators to effectively reverse cachexia.
Several members of the transforming growth factor-beta (TGF-β) superfamily, including myostatin, have been implicated in cancer cachexia. Myostatin plays a key role in regulating muscle size. Mice lacking myostatin or growth and differentiation factor 8 (GDF8) develop profound muscle hypertrophy (McPherron et al. 1997), while systemic overexpression of myostatin induces severe cachexia (Zimmers et al. 2002). Myostatin binds to the activin type II receptors ACVR2 and ACVR2B and thereby triggers the phosphorylation of activin type I receptors and activation of the downstream SMAD2/3 signaling pathway (Benny Klimek et al. 2010). Importantly, systemic administration of soluble forms of ACVR2B, either ACVR2B-Fc secreted from implanted CHO cells (Benny Klimek et al. 2010) or the decoy receptor sActRIIB (Zhou et al. 2010), inhibits cachexia and prolongs survival in mouse models (Zhou et al. 2010), thus providing promising translational opportunities.
The TGF-β signaling pathway has another important function in mediating cachexia specifically in the context of metastatic cancers (Waning and Guise 2014; Waning et al. 2015; Regan et al. 2017). The release of TGF-β from bone initiates a vicious cycle of bone destruction and metastatic tumor growth as well as muscle weakness in mice with bone metastases (Waning et al. 2015). In this model, TGF-β up-regulates NAPDH oxidase 4 (NOX4) in muscle cells, which increases the oxidation of ryanodine receptor/calcium release channel (RYR1) on the sarcoplasmic reticulum (Waning et al. 2015). Excess RYR1 oxidation promotes leakiness of calcium channels and thereby compromises muscle force generation. As such, muscle weakness can be reversed by inhibiting either TGF-β, TGF-β receptor I kinase, NOX1/4, or the RYR1 Ca2+ release channel stabilizer RYCAL, providing new avenues of intervention for cancer cachexia. Recent studies from our laboratory showed that TGF-β up-regulates the metal-ion transporter ZRT- and IRT-like protein 14 (ZIP14) in muscle cells, which functions as a mediator of cachexia in metastatic cancers (Wang et al. 2018). ZIP14 up-regulation causes an increase in muscle-cell zinc uptake that induces myosin heavy chain loss in mature muscle cells and blocks muscle differentiation in muscle progenitors (Wang et al. 2018). Therefore, blocking TGF-β signaling, inhibiting the function of ZIP14, and modulating dietary zinc intake represent additional potentially effective strategies for preventing or treating cachexia in metastatic cancer patients.
THERAPY-INDUCED MUSCLE WASTING
Many antineoplastic agents give rise to adverse side effects such as reduced appetite and premature satiety, which can impact food intake and body weight in cancer patients (Kayl and Meyers 2006). Skeletal muscle catabolism and loss of lean muscle mass (Gilliam and St Clair 2011; Prado et al. 2011a; Gilliam et al. 2013; Barreto et al. 2016a; Pin et al. 2018) are further consequences of antineoplastic therapy and can be readily measured by body composition analysis. Longitudinal CT scans provide precision, clarity, and specificity with respect to the type, location and quantity of adipose and skeletal muscle tissue loss in cancer patients (Mourtzakis et al. 2008). Consequently, studies have shown that cancer patients with reduced lean muscle mass develop greater toxicities from a number of drugs, such as 5-fluorouracil, capecitabine, and sorafenib (Prado et al. 2011a). In fact, the loss of lean muscle serves as an independent predictor of toxicity in cancer patients exposed to antineoplastic therapy (Prado et al. 2007, 2009a, 2011b, 2014; Antoun et al. 2010a,b). Patients with reduced lean muscle tissue are also more likely to require a dose reduction of, delays in, or early termination of their anticancer treatment and to be excluded from participating in clinical trials (Prado et al. 2011a). This has a significant negative impact on cancer patient survival. Therefore, it is critically important to identify the underlying mechanisms that drive therapy-induced muscle loss and to design effective strategies to prevent or reverse these devastating side effects of anticancer therapy.
Chemotherapy is the mainstay of treatment for metastatic cancers (Schakman et al. 2009), and while it effectively kills cancer cells, it also causes extensive damage to normal tissues such as the gut, bone marrow, kidney, and nervous system (Kayl and Meyers 2006). Fatigue and weakness represent yet another prominent side effect of most chemotherapy agents that continues even after cessation of treatment (Tavio et al. 2002). Fatigue is largely due to loss of muscle mass and function, and is responsible for the morbidity and poor quality of life of chemotherapy-treated patients (Tavio et al. 2002). Indeed, cancer patients with low muscle mass have significantly lower rates of survival and increased treatment-induced toxicities (Prado et al. 2009a; Blauwhoff-Buskermolen et al. 2016; Guigni et al. 2018). For instance, when adjusted for variables such as sex, age, and scope of metastases, colon-cancer patients with chemotherapy-induced muscle loss of ≥9% had a 7% 1-yr survival rate compared to patients with <9% muscle loss showing 49% survival (Blauwhoff-Buskermolen et al. 2016). Still, it remains unclear whether chemotherapy-induced muscle wasting is similar to or distinct from cancer-induced muscle wasting, which pathways mediate chemotherapy-induced muscle wasting and whether different chemotherapy agents cause unique muscle-damaging effects. Over the past decade, new studies have begun to address these questions and are briefly discussed below.
The TNF-α signaling pathway and its effector NF-κB are commonly activated in muscle cells in response to both the presence of cancer and treatment with chemotherapy (Li et al. 1998; Guttridge et al. 2000; Judge et al. 2007; Damrauer et al. 2018). Muscle-specific activation of NF-κB was originally shown to promote muscle catabolism and muscle mass loss (in the absence of cancer and chemotherapy) through up-regulation of the E3 ligase MuRF1 and activation of the ubiquitin-dependent proteasome pathway (Li and Reid 2000; Cai et al. 2004). In one study, Cisplatin treatment (3 doses of 5 mg/kg body weight given intravenously over 2 wk) was shown to activate NF-κB signaling in the limb muscles of healthy mice and to elicit features of cachexia without inducing MuRF1 or activating the ubiquitin-proteasome pathway (Damrauer et al. 2018). In contrast, a different study showed that cisplatin treatment of healthy mice (2.5 mg/kg body weight given intraperitoneally daily over 10 d) leads to the muscle-specific up-regulation of E3 ligases, atrogin-1 and to a lesser extent MuRF1, and activation of the ubiquitin-proteasome pathway (Chen et al. 2015). Cisplatin treatment also triggered a number of additional events in this study that may contribute to muscle loss, including the increased expression of p38 and myostatin and decreased expression of Akt. Disruption of the Akt pathway, as well as hyperactivation of the proteasome and autophagy pathways, may also mediate cisplatin-induced muscle wasting of differentiated muscle cells (Fanzani et al. 2011). Importantly, the administration of ghrelin, an endogenous ligand of the growth hormone secretagogue receptor (GHSR), inhibits cisplatin-induced muscle toxicity and improves muscle function (Chen et al. 2015). The mechanisms driving cisplatin-induced muscle loss, as well as the influence of cisplatin dosage and duration on the severity of the associated phenotypes, remain to be further investigated.
Diaphragm weakness and dyspnea (shortness of breath) are common in chemotherapy-treated cancer patients (Morrow et al. 2002; Gilliam and St Clair 2011). Treatment with doxorubicin induces diaphragm and limb muscle weakness in healthy mice (Gilliam et al. 2011a) and leads to an increase in the expression of TNF receptor 1 (TNFR1) and its localization to the membrane (Gilliam et al. 2009, 2011a). This causes a depression of diaphragm-specific force, which can be rescued by etanercept, a soluble form of the TNF receptor that blocks the activity of circulating TNF, or by the genetic loss of TNFR1 (Gilliam et al. 2009, 2011b). Similar to cisplatin (Fanzani et al. 2011; Chen et al. 2015), doxorubicin treatment in healthy mice (a single dose of 15 mg/kg body weight given intraperitoneally) up-regulates the expression of atrogin-1 and activates the proteasome in skeletal and heart muscles (Yamamoto et al. 2008) suggesting common pathways driving muscle wasting between these two anticancer agents.
Muscle-wasting phenotypes vary with the type of chemotherapy agent, mode of action and metabolism. For instance, a single dose of the alkylating agent cystemustine (20 mg/kg body weight) leads to a reduction in body weight and loss of muscle mass within 5 d of treatment in healthy mice, which recover gradually but never back to the levels observed in untreated mice (Samuels et al. 2001; Tilignac et al. 2002). In mice bearing C26 colon adenocarcinomas that develop cachexia, the same dosing schedule of cystemustine showed an additive effect with the cachexia-induced muscle wasting and completely eliminated the tumors. Interestingly, body and muscle mass in these mice were restored to levels observed in cystemustine-treated, nontumor-bearing mice, possibly as a direct result of treatment with this particular type of chemotherapy agent. Moreover, cystemustine treatment eventually led to a reduction in ubiquitin-dependent proteolysis and protein breakdown in both control and tumor-bearing mice to levels below those observed in untreated healthy mice, an effect that was mimicked by the combination of the alkylating agent ifosfamide with cisplatin (Tilignac et al. 2002) and associated with the down-regulation of proteasome subunit expression. Thus, while treatment with alkylating agents initially leads to muscle wasting, it also appears to support a compensatory muscle-rebuilding response after complete tumor elimination in the C26 model that has not been observed with other anticancer agents (Barreto et al. 2016b). Some chemotherapeutic drugs disrupt muscle function through mechanisms that are independent of proteasome pathway activation. Folfiri (5-fluorouracil, leucovorin and CPT-11), a drug combination frequently used in treating metastatic colon cancers, significantly depletes skeletal muscle mass and strength (Barreto et al. 2016b) through a combination of decreased muscle anabolism (via reduced Akt phosphorylation) and activation of catabolic pathways (ERK1/2 and p38-MAPK) (Barreto et al. 2016b). Notably, these folfiri-induced effects could be rescued by blocking MAPK signaling in differentiated muscle-cell cultures. Muscle deterioration, therefore, represents a common side effect of chemotherapies in general, and a better understanding of the various mechanisms that drive this process is likely to improve the quality of life of cancer patients with muscle wasting.
Mitochondrial dysfunction has emerged as another feature associated with cancer- and chemotherapy-induced muscle wasting, although the phenotypes vary with tumor type, therapy regimen, and animal model. In the muscles of patients with cancer cachexia, bioenergetic insufficiency likely arises due to an altered mitochondrial structure characterized by swollen vesicles and fragmented cristae (Shum et al. 2012), a reduced oxidative capacity arising from a decrease in complex IV activity (Julienne et al. 2012), and/or a decreased rate of ATP synthesis (Tzika et al. 2013). Treatment with chemotherapies such as doxorubicin has been shown to inhibit mitochondrial respiration and disrupt mitochondrial energy metabolism (Gilliam and St Clair 2011; Gilliam et al. 2013) and redox balance (Gilliam et al. 2013). Moreover, a single dose of doxorubicin administered to rats increases mitochondrial hydrogen peroxide emission and impairs mitochondrial calcium retention in single muscle fibers (Gilliam et al. 2013). In response to folfiri, mice show decreased mitochondrial size and number along with a significant reduction in the expression of genes that regulate mitochondrial metabolism in muscles (Barreto et al. 2016b). Moreover, cyclical treatment of doxorubicin and dexamethasone every 3 wk for four cycles (doxorubicin intraperitoneally at 10 mg/kg body weight and dexamethasone subcutaneously at 2.5 mg/kg body weight) significantly impairs mitochondrial function in muscles and degrades muscle mass and function (Gouspillou et al. 2015). In contrast to the folfiri regimen (Barreto et al. 2016b), the doxorubicin–dexamethasone treatment fails to change the expression of most key mitochondrial metabolism genes. Instead, it induces a significant increase in reactive oxygen species in muscle-cell mitochondria accompanied by a sustained impairment of mitochondrial respiratory capacity (Gouspillou et al. 2015). Interestingly, mitochondrial dysfunction and long-term muscle damage are also observed in cardiac muscles in response to chemotherapy (Ewer and Ewer 2010), but the underlying mechanisms are thought to be distinct. Long-term, chemotherapy-induced cardiotoxicity is driven by oxidative-stress-induced loss of mitochondrial DNA and reduced mitochondrial volume (Yamada et al. 1995; Palmeira et al. 1997; Serrano et al. 1999; Lebrecht et al. 2003, 2007, 2010), neither of which are observed in skeletal muscles (Gouspillou et al. 2015). The disruption of muscle-cell mitochondria, therefore, appears to be a key mediator of the many debilitating features of chemotherapy- and cancer-induced cachexia.
Experimental and clinical studies indicate that cancer- and chemotherapy-induced muscle damage and compromised physical function persist as a chronic problem in cancer survivors (Scheede-Bergdahl and Jagoe 2013; Kurk et al. 2019). Recent studies have also begun to address whether there are distinct mechanisms that drive muscle wasting in response to either cancer or chemotherapy and whether other anticancer therapies induce muscle damage. Bonetto and colleagues revealed unique alterations in metabolic flux through the tricarboxylic (TCA) cycle and β-oxidation pathways between cancer- and chemotherapy-induced muscle wasting. They found that low-density lipoprotein particles (LDL-Ps) increased in cancer-induced, but not chemotherapy-induced, muscle wasting (Pin et al. 2019) and that plasma levels of branched-chain amino acids, which normally comprise 14%–18% of total amino acids and stimulate protein synthesis, decrease during cancer-induced muscle wasting but increase in response to chemotherapy (Pin et al. 2019). However, whether these metabolic alterations have a causative role in the development of muscle wasting in response to either cancer or chemotherapy is unclear. Muscle damage also occurs with targeted anticancer agents in both animal models and patients (Blumenschein et al. 2009; Chen and Wang 2018; Huot et al. 2019). For example, treatment of mice with kinase inhibitors such as sorafenib or regorafenib reduces cardiac and skeletal muscle mass, and induces muscle weakness, through the ERK1/2 and GSK3 pathways (Huot et al. 2019). Clinical studies show that 2.1 kg of skeletal muscle is lost every 6 mo in patients treated with sorafenib compared to placebo-control-treated patients who show stable body weight during the same time interval (Antoun et al. 2010a). Whether additional targeted inhibitors or immunotherapies induce muscle wasting has yet to be explored. Interestingly, a targeted MEK1/2 inhibitor fails to elicit a strong antitumor response but surprisingly, increases skeletal muscle mass in biliary tract cancer patients (Bekaii-Saab et al. 2011; Prado et al. 2012a; Talbert et al. 2017). The analysis of these findings in cancer cachexia mouse models showed that MEK inhibition prevents muscle wasting even in mice bearing a MEK-inhibitor-resistant tumor clone in which tumor growth is unaffected by MEK inhibition (Quan-Jun et al. 2017; Talbert et al. 2017). These experiments indicate that the ability of MEK inhibition to preserve muscle is independent of its antitumor functions. Furthermore, the combination of both a MEK inhibitor and a PI3K/AKT inhibitor significantly reduces tumor burden and preserves skeletal muscle mass (Talbert et al. 2017), providing a viable means of simultaneously treating both cancer and associated cachexia. A comprehensive analysis of anticancer therapies for their ability to inhibit or promote muscle loss, and for their associated long-term sequelae, is therefore expected to better inform treatment strategies to improve drug tolerance, prognosis, and quality of life in cancer patients.
AGING-RELATED MUSCLE WASTING
Sarcopenia is a muscle-wasting state that develops as part of the natural aging process and involves a degenerative loss of skeletal muscle mass, quality, and strength that often leads to functional impairment and disability (Argilés et al. 2015). Muscle mass loss typically begins around 30 yr of age and continues gradually such that by 80 yr of age, 30% of the original muscle mass is lost (Hughes et al. 2002). Interestingly, 40%–50% of newly diagnosed cancer patients exhibit sarcopenia (median age of 65 yr) compared to 15% of healthy individuals of similar age (von Haehling et al. 2010; Prado et al. 2012b). The consequences of sarcopenia are severe for cancer patients, who experience a higher incidence of toxicity from chemotherapy, poor surgical outcomes, a shorter time to cancer progression and a lower rate of survival (Prado et al. 2009a, 2016; Barret et al. 2014; Joglekar et al. 2015). There is a misconception that sarcopenia is inversely associated with body mass index (BMI), but body composition studies have shown that overweight or obese patients with a high BMI can have severe muscle depletion, which is termed “sarcopenic obesity” (Prado et al. 2016). Moreover, since the calculation for chemotherapy drug dosage is based on body surface area (determined by height and weight), lean tissue content and muscle mass are rarely considered despite a large body of clinical evidence demonstrating that lower muscle mass correlates with an increased likelihood of dose-limiting drug toxicities (Prado et al. 2007, 2009a, 2013; Antoun et al. 2010a). A comprehensive review of the link between body composition analysis and outcome in cancer patients is available elsewhere (Mourtzakis et al. 2008; Prado et al. 2009b). Since many aspects of aging-related muscle loss can be counteracted by regulating dietary intake and exercise (Penna et al. 2011; Ballarò et al. 2019), these findings make a compelling case for body composition analysis before administering cancer treatment.
The underlying causes of sarcopenia, which is characterized by loss of both muscle size and number, are multifactorial (Fielding et al. 2011) and range from an age-related decline in neurons, hormones and growth factors to nutritional deficiencies and reduced physical activity (Fig. 2; Campbell et al. 2001; Visser et al. 2003). Unlike tumor-induced muscle wasting in which REE and systemic inflammation are high, sarcopenia involves a low REE and a low level of chronic inflammation. Whether decreased muscle protein synthesis and/or increased protein degradation drives sarcopenia remains controversial at present. For instance, the IGF1/AKT1/mTOR signaling pathway has been shown to induce muscle protein synthesis and suppress protein breakdown through FoxO regulation (Milan et al. 2015). While muscle-expressed IGF1 declines with age in rodents and its reactivation can reduce sarcopenia (Musaro et al. 2001), this phenomenon was not observed in humans (Sandri et al. 2013). Instead, a compensatory but futile attempt at maintaining muscle mass, involving increased activation of biosynthetic pathways such as those driven by mTOR, occurs with aging in both rodents and humans (Kimball et al. 2004). During cachexia, muscle protein breakdown is increased through hyperactivation of the ubiquitin-proteasome and autophagy pathways; however, it either remains unchanged or is sometimes suppressed during sarcopenia (Welle et al. 2004). For instance, E3 ligases such as MuRF1 and MAFbx are up-regulated in many muscle-wasting states, including cancer cachexia, but not in aged muscles (Whitman et al. 2005; Sandri et al. 2013). Thus, the nature and degree of muscle protein breakdown represent a major difference between sarcopenia and cachexia. It has been postulated that autophagy may be an important mechanism driving sarcopenia (Carnio et al. 2014). Autophagy is required for the clearance of dysfunctional organelles and aberrant protein in cells, and its inhibition by muscle-specific deletion of Atg7 results in a premature aging phenotype in mice in which the development of sarcopenia is accelerated by mitochondrial dysfunction, increased oxidative stress and inhibition of neuromuscular structure and function (Carnio et al. 2014). Interestingly, alterations in neuromuscular junction genes were reported in a global muscle transcriptomic analysis in aged rats with sarcopenia that indicated a functional denervation of the neuromuscular junction (Ibebunjo et al. 2013). Autophagy-related genes are suppressed in the muscles of older, frail women (Drummond et al. 2014), suggesting a potential link between an autophagy defect and sarcopenia development. A comprehensive review of the mechanisms driving sarcopenia is available elsewhere (Larsson et al. 2019).
Sarcopenia is driven in part by a disruption of mitochondrial energy metabolism, which involves a decrease in mitochondrial mass, DNA content, oxygen consumption, and oxidative phosphorylation, as well as changes to mitochondrial morphology (Welle et al. 2003; Short et al. 2005; Gouspillou et al. 2014a; Leduc-Gaudet et al. 2015; del Campo et al. 2018). Muscles from sarcopenic rats exhibit repression of genes that regulate mitochondrial fission and fusion, oxidative phosphorylation, and the TCA cycle, along with a depletion of myofibrillar protein, increased apoptosis and declining muscle mass (Ibebunjo et al. 2013). Aged muscles were shown in a different study to be associated with dysfunctional mitochondria, impaired mitophagy, increased apoptosis, and lower mitochondrial ADP affinity (Gouspillou et al. 2014a,b). The increase in muscle-cell apoptosis appears to be triggered by the generation of reactive oxygen species from damaged mitochondria (Dirks et al. 2006; Barbieri and Sestili 2012), and since fusion and fission regulate mitochondrial morphology (Farmer et al. 2018), the reduced expression of the mitochondrial-fusion regulator optic atrophy protein 1 (OPA1) observed in sarcopenic patients may account for the morphological defects (Tezze et al. 2017). Indeed, Opa1 deletion in adult muscle results in smaller mitochondria with dilated cristae and increased oxidative stress, which culminates in muscle atrophy (Tezze et al. 2017).
Exercise and orally administered therapies show promise with respect to reversing sarcopenia. Clinical studies have demonstrated that regular endurance exercise can partially reduce aging-related mitochondrial dysfunction (Lanza et al. 2008) and that high-intensity resistance training counteracts physical frailty and improves muscle strength and size in elderly individuals (mean age of 87 yr) (Fiatarone et al. 1994). Moreover, long-term oral administration of nicotinamide mononucleotide, a key NAD+ intermediate, in aging mice has been shown to enhance mitochondrial respiratory capacity in skeletal muscle, to improve insulin sensitivity and to act as an overall antiaging intervention (Mills et al. 2016). Interestingly, the aging-related decline of the bone-derived hormone osteocalcin has been shown to correlate with reduced muscle mass (Mera et al. 2016a). Karsenty and colleagues showed that administration of exogenous osteocalcin is sufficient to increase muscle mass in aged mice and that deletion of osteocalcin, or the gene encoding its only known receptor GPRC6A, inhibits muscle protein synthesis and decreases muscle mass (Mera et al. 2016b). Osteocalcin signaling in muscle fibers is also necessary for adaptation to exercise by promoting uptake and catabolism of glucose and fatty acids, and exogenous osteocalcin is sufficient to restore the exercise capacity of 15-mo-old (aged) mice to that of 3-mo-old (young) mice (Mera et al. 2017). This bone-to-muscle endocrine axis could potentially be exploited to improve muscle function and exercise capacity in patients with sarcopenia.
CONCLUDING REMARKS
The importance of preserving lean muscle mass and function in cancer patients is often overlooked in the study of metastatic disease. Skeletal muscle wasting can be driven by aging, tumor, and/or anticancer therapy. Metastatic cancer patients can indeed experience a spectrum of these muscle-wasting states during the course of their disease and subsequent lifetime. The loss of lean muscle mass is almost invariably detrimental to patient outcome, treatment tolerance, quality of life, and survival and therefore represents a powerful predictor of morbidity and mortality (Baracos et al. 2018). It is important to acknowledge that a thin and emaciated physique cannot be relied upon as an identifying feature of cachexia or muscle wasting in obese cancer patients, where cachexia is often masked by fat gain accompanied by minimal changes to total body weight or BMI (Mourtzakis et al. 2008; Prado et al. 2016). Indeed, CT-based imaging analysis has significantly improved the diagnosis of lean body mass changes over time (Prado et al. 2016); however, this is not routinely practiced in oncology clinics. The challenge following the diagnosis of muscle wasting is devising strategies to reverse it. There is a significant gap in our understanding mechanisms of cachexia and as such, there are currently no approved treatments for cachexia. Several roadblocks are likely responsible (Penna et al. 2016). First, cachexia appears to be driven by multiple mediators, so single therapies targeting pathways individually may not be effective. Second, cachexia results from a systemic metabolic dysfunction, which is poorly understood and difficult to target therapeutically without adversely affecting normal physiology. Third, the limited success of prospective cachexia therapies in clinical trials is likely due to an insufficient understanding of the mechanisms that drive human cancer cachexia, the lack of clinical validation of experimental studies using patient samples, and the paucity of preclinical models of cachexia in the context of advanced cancer. However, as highlighted in this review, advances are being made in each of these areas with the development of promising targeted agents. It is important to generate awareness among the medical community that cachexia is not simply a problem of reduced appetite and it cannot be solved by excess dietary supplementation. It is also crucial to identify the antineoplastic agents that induce muscle wasting so that cancer treatment strategies can be devised to enhance success rate and reduce muscle toxicities and fatigue. As such, therapies that combine strong antitumor activity with the preservation of lean muscle mass currently hold the most promise for improving quality of life and survival in patients with metastatic cancer.
COMPETING INTEREST STATEMENT
Both authors declare no conflicts of interest and are not aware of any affiliations, funding, memberships, or financial holding that might be affecting the objectivity of this review.
ACKNOWLEDGMENTS
We would like to acknowledge our funding sources: National Cancer Institute (NCI) RO1 (CA231239), Pershing Square Sohn Prize, Irving Scholar Award, Interdisciplinary Research Initiatives Seed (IRIS) Program, developmental funds from National Institutes of Health (NIH)/NCI Cancer Center support grant P30CA013696 and The Irma T. Hirschl Monique Weill-Caulier Trust Award to S.A.
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. 2004. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest 114: 370–378. 10.1172/JCI200420174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. 2010a. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 21: 1594–1598. 10.1093/annonc/mdp605 [DOI] [PubMed] [Google Scholar]
- Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. 2010b. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol 28: 1054–1060. 10.1200/JCO.2009.24.9730 [DOI] [PubMed] [Google Scholar]
- Argilés JM, Fontes-Oliveira CC, Toledo M, López-Soriano FJ, Busquets S. 2014. Cachexia: a problem of energetic inefficiency. J Cachexia Sarcopenia Muscle 5: 279–286. 10.1007/s13539-014-0154-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. 2015. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol 22: 100–106. 10.1016/j.coph.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L. 2016. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc 17: 789–796. 10.1016/j.jamda.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. 2018. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol 15: 9–20. 10.1038/s41574-018-0123-0 [DOI] [PubMed] [Google Scholar]
- Asp ML, Tian M, Wendel AA, Belury MA. 2010. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer 126: 756–763. 10.1002/ijc.24784 [DOI] [PubMed] [Google Scholar]
- Asp ML, Tian M, Kliewer KL, Belury MA. 2011. Rosiglitazone delayed weight loss and anorexia while attenuating adipose depletion in mice with cancer cachexia. Cancer Biol Ther 12: 957–965. 10.4161/cbt.12.11.18134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarò R, Beltrà M, De Lucia S, Pin F, Ranjbar K, Hulmi JJ, Costelli P, Penna F. 2019. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J 33: 5482–5494. 10.1096/fj.201801862R [DOI] [PubMed] [Google Scholar]
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. 2018. Cancer-associated cachexia. Nat Rev Dis Primers 4: 17105 10.1038/nrdp.2017.105 [DOI] [PubMed] [Google Scholar]
- Barbieri E, Sestili P. 2012. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct 2012: 982794 10.1155/2012/982794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. 2014. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 66: 583–589. 10.1080/01635581.2014.894103 [DOI] [PubMed] [Google Scholar]
- Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. 2016a. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol 7: 472 10.3389/fphys.2016.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. 2016b. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 7: 43442–43460. 10.18632/oncotarget.9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Richards C, Gauldie J. 1987. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol 139: 4122–4128. [PubMed] [Google Scholar]
- Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, O'Neil BH, Balsom S, Balint C, Liersemann R, et al. 2011. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 29: 2357–2363. 10.1200/JCO.2010.33.9473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. 2010. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun 391: 1548–1554. 10.1016/j.bbrc.2009.12.123 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Mittal A, Gupta SK, Kumar A. 2012. TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J Cell Physiol 227: 1042–1051. 10.1002/jcp.22821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, Verheul HM. 2016. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34: 1339–1344. 10.1200/JCO.2015.63.6043 [DOI] [PubMed] [Google Scholar]
- Blumenschein GR Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O'Leary J, Reck M. 2009. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol 27: 4274–4280. 10.1200/JCO.2009.22.0541 [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, et al. 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708. 10.1126/science.1065874 [DOI] [PubMed] [Google Scholar]
- Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, Zimmers TA. 2011. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 6: e22538 10.1371/journal.pone.0022538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. 2012. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 303: E410–E421. 10.1152/ajpendo.00039.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. 2012. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468. 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen TS, Schuler G, Adams V. 2015. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle 6: 197–207. 10.1002/jcsm.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, Haynie WS, Hardee JP, Carson JA, Wiggs MP, et al. 2017. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle 8: 926–938. 10.1002/jcsm.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE Jr., Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, et al. 2004. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119: 285–298. 10.1016/j.cell.2004.09.027 [DOI] [PubMed] [Google Scholar]
- Campbell WW, Trappe TA, Wolfe RR, Evans WJ. 2001. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci 56: M373–M380. 10.1093/gerona/56.6.M373 [DOI] [PubMed] [Google Scholar]
- Cao DX, Wu GH, Zhang B, Quan YJ, Wei J, Jin H, Jiang Y, Yang ZA. 2010. Resting energy expenditure and body composition in patients with newly detected cancer. Clin Nutr 29: 72–77. 10.1016/j.clnu.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, et al. 2014. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 8: 1509–1521. 10.1016/j.celrep.2014.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere A, Jeanson Y, Berger-Muller S, Andre M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, et al. 2014. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63: 3253–3265. 10.2337/db13-1885 [DOI] [PubMed] [Google Scholar]
- Chen J, Wang J. 2018. Risk of regorafenib-induced cardiovascular events in patients with solid tumors: a systematic review and meta-analysis. Medicine (Baltimore) 97: e12705 10.1097/MD.0000000000012705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Espat NJ, Bland KI, Copeland EM 3rd, Souba WW. 1993. Influence of progressive tumor growth on glutamine metabolism in skeletal muscle and kidney. Ann Surg 217: 655–666. discussion 666–667 10.1097/00000658-199306000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JA, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, Halder T, Zhang G, Li YP, Garcia JM. 2015. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle 6: 132–143. 10.1002/jcsm.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Nathan JA, Goldberg AL. 2015. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 14: 58–74. 10.1038/nrd4467 [DOI] [PubMed] [Google Scholar]
- Cohn SH, Vartsky D, Vaswani AN, Sawitsky A, Rai K, Gartenhaus W, Yasumura S, Ellis KJ. 1982. Changes in body composition of cancer patients following combined nutritional support. Nutr Cancer 4: 107–119. 10.1080/01635588209513746 [DOI] [PubMed] [Google Scholar]
- Cray C, Zaias J, Altman NH. 2009. Acute phase response in animals: a review. Comp Med 59: 517–526. [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. 2009. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517. 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC. 2018. Chemotherapy-induced muscle wasting: association with NF-κB and cancer cachexia. Eur J Transl Myol 28: 7590 10.4081/ejtm.2018.7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, et al. 2011. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333: 233–238. 10.1126/science.1198973 [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T. 2010. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29: 313–324. 10.1038/onc.2009.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo A, Contreras-Hernández I, Castro-Sepúlveda M, Campos CA, Figueroa R, Tevy MF, Eisner V, Casas M, Jaimovich E. 2018. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging (Albany NY) 10: 34–55. 10.18632/aging.101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr., Engstrom PF, Ezdinli EZ, et al. 1980. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69: 491–497. 10.1016/S0149-2918(05)80001-3 [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. 2006. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 5: 179–195. 10.1016/j.arr.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. 2007. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J 21: 1857–1869. 10.1096/fj.06-7537com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, Marcus RL. 2014. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J Gerontol A Biol Sci Med Sci 69: 1040–1048. 10.1093/gerona/glu004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espat NJ, Auffenberg T, Rosenberg JJ, Rogy M, Martin D, Fang CH, Hasselgren PO, Copeland EM, Moldawer LL. 1996. Ciliary neurotrophic factor is catabolic and shares with IL-6 the capacity to induce an acute phase response. Am J Physiol 271: R185–R190. [DOI] [PubMed] [Google Scholar]
- Ewer MS, Ewer SM. 2010. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol 7: 564–575. 10.1038/nrcardio.2010.121 [DOI] [PubMed] [Google Scholar]
- Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC. 1994. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg 219: 325–331. 10.1097/00000658-199404000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC. 1995. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer 75: 2077–2082. [DOI] [PubMed] [Google Scholar]
- Fanzani A, Zanola A, Rovetta F, Rossi S, Aleo MF. 2011. Cisplatin triggers atrophy of skeletal C2C12 myotubes via impairment of Akt signalling pathway and subsequent increment activity of proteasome and autophagy systems. Toxicol Appl Pharmacol 250: 312–321. 10.1016/j.taap.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Farmer T, Naslavsky N, Caplan S. 2018. Tying trafficking to fusion and fission at the mighty mitochondria. Traffic 19: 569–577. 10.1111/tra.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. 1999. Pancreatic cancer as a model: inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg 23: 584–588. 10.1007/PL00012351 [DOI] [PubMed] [Google Scholar]
- Fearon KC, Glass DJ, Guttridge DC. 2012. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16: 153–166. 10.1016/j.cmet.2012.06.011 [DOI] [PubMed] [Google Scholar]
- Fearon K, Arends J, Baracos V. 2013. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 10: 90–99. 10.1038/nrclinonc.2012.209 [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. 1994. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330: 1769–1775. 10.1056/NEJM199406233302501 [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. 2011. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256. 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Clarevega A, Bilder D. 2015. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell 33: 47–55. 10.1016/j.devcel.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J, Tsujinaka T, Jano M, Ebisui C, Saito H, Katsume A, Akamatsu K, Ohsugi Y, Shiozaki H, Monden M. 1996. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATP-ubiquitin-dependent proteolytic pathways. Int J Cancer 68: 637–643. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- Gilliam LA, St Clair DK. 2011. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal 15: 2543–2563. 10.1089/ars.2011.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St Clair DK, Reid MB. 2009. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol (1985) 107: 1935–1942. 10.1152/japplphysiol.00776.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam LA, Moylan JS, Callahan LA, Sumandea MP, Reid MB. 2011a. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve 43: 94–102. 10.1002/mus.21809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam LA, Moylan JS, Ferreira LF, Reid MB. 2011b. TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm weakness. Am J Physiol Lung Cell Mol Physiol 300: L225–L231. 10.1152/ajplung.00264.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam LAA, Fisher-Wellman KH, Lin CT, Maples JM, Cathey BL, Neufer PD. 2013. The anticancer agent doxorubicin disrupts mitochondrial energy metabolism and redox balance in skeletal muscle. Free Radic Biol Med 65: 988–996. 10.1016/j.freeradbiomed.2013.08.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. 2001. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci 98: 14440–14445. 10.1073/pnas.251541198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Biran M, Deschodt-Arsac V, Miraux S, Thiaudiere E, Pasdois P, Detaille D, et al. 2014a. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 13: 39–48. 10.1111/acel.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouspillou G, Sgarioto N, Kapchinsky S, Purves-Smith F, Norris B, Pion CH, Barbat-Artigas S, Lemieux F, Taivassalo T, Morais JA, et al. 2014b. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J 28: 1621–1633. 10.1096/fj.13-242750 [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Scheede-Bergdahl C, Spendiff S, Vuda M, Meehan B, Mlynarski H, Archer-Lahlou E, Sgarioto N, Purves-Smith FM, Konokhova Y, et al. 2015. Anthracycline-containing chemotherapy causes long-term impairment of mitochondrial respiration and increased reactive oxygen species release in skeletal muscle. Sci Rep 5: 8717 10.1038/srep08717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigni BA, Callahan DM, Tourville TW, Miller MS, Fiske B, Voigt T, Korwin-Mihavics B, Anathy V, Dittus K, Toth MJ. 2018. Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am J Physiol Cell Physiol 315: C744–C756. 10.1152/ajpcell.00002.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. 2000. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289: 2363–2366. 10.1126/science.289.5488.2363 [DOI] [PubMed] [Google Scholar]
- Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. 2018. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci 1411: 21–35. 10.1111/nyas.13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JT, Seniuk NA, Richardson PM, Gauldie J, Roder JC. 1994. Systemic administration of ciliary neurotrophic factor induces cachexia in rodents. J Clin Invest 93: 2632–2638. 10.1172/JCI117276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honors MA, Kinzig KP. 2012. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle 3: 5–11. 10.1007/s13539-011-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. 2002. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76: 473–481. 10.1093/ajcn/76.2.473 [DOI] [PubMed] [Google Scholar]
- Huot JR, Essex AL, Gutierrez M, Barreto R, Wang M, Waning DL, Plotkin LI, Bonetto A. 2019. Chronic treatment with multi-kinase inhibitors causes differential toxicities on skeletal and cardiac muscles. Cancers (Basel) 11: 571 10.3390/cancers11040571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, Vickers C, Wu Z, Clarke BA, Shi J, et al. 2013. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol 33: 194–212. 10.1128/MCB.01036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, Mezhir JJ. 2015. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 111: 771–775. 10.1002/jso.23862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AJ, Murphy KT, Jenkinson L, Laine D, Emmrich K, Faou P, Weston R, Jayatilleke KM, Schloegel J, Talbo G, et al. 2015. Targeting of Fn14 prevents cancer-induced cachexia and prolongs survival. Cell 162: 1365–1378. 10.1016/j.cell.2015.08.031 [DOI] [PubMed] [Google Scholar]
- Judge AR, Koncarevic A, Hunter RB, Liou HC, Jackman RW, Kandarian SC. 2007. Role for IκBα, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol 292: C372–C382. 10.1152/ajpcell.00293.2006 [DOI] [PubMed] [Google Scholar]
- Julienne CM, Dumas JF, Goupille C, Pinault M, Berri C, Collin A, Tesseraud S, Couet C, Servais S. 2012. Cancer cachexia is associated with a decrease in skeletal muscle mitochondrial oxidative capacities without alteration of ATP production efficiency. J Cachexia Sarcopenia Muscle 3: 265–275. 10.1007/s13539-012-0071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. 2013. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle 4: 89–94. 10.1007/s13539-013-0111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, et al. 2004. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279: 41114–41123. 10.1074/jbc.M400674200 [DOI] [PubMed] [Google Scholar]
- Kandarian SC, Nosacka RL, Delitto AE, Judge AR, Judge SM, Ganey JD, Moreira JD, Jackman RW. 2018. Tumour-derived leukaemia inhibitory factor is a major driver of cancer cachexia and morbidity in C26 tumour-bearing mice. J Cachexia Sarcopenia Muscle 9: 1109–1120. 10.1002/jcsm.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayl AE, Meyers CA. 2006. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol 18: 24–28. 10.1097/01.gco.0000192996.20040.24 [DOI] [PubMed] [Google Scholar]
- Kimball SR, O'Malley JP, Anthony JC, Crozier SJ, Jefferson LS. 2004. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am J Physiol Endocrinol Metab 287: E772–E780. 10.1152/ajpendo.00535.2003 [DOI] [PubMed] [Google Scholar]
- Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. 2014. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513: 100–104. 10.1038/nature13528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler DP. 2000. Cachexia. Ann Intern Med 133: 622–634. 10.7326/0003-4819-133-8-200010170-00015 [DOI] [PubMed] [Google Scholar]
- Kurk S, Peeters P, Stellato R, Dorresteijn B, de Jong P, Jourdan M, Creemers GJ, Erdkamp F, de Jongh F, Kint P, et al. 2019. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle 10: 803–813. 10.1002/jcsm.12436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. 1993. Regulation of the acute phase response by cytokines. Perspect Biol Med 36: 611–622. 10.1353/pbm.1993.0004 [DOI] [PubMed] [Google Scholar]
- Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. 2015. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell 33: 36–46. 10.1016/j.devcel.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. 2008. Endurance exercise as a countermeasure for aging. Diabetes 57: 2933–2942. 10.2337/db08-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. 2019. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99: 427–511. 10.1152/physrev.00061.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. 2003. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation 108: 2423–2429. 10.1161/01.CIR.0000093196.59829.DF [DOI] [PubMed] [Google Scholar]
- Lebrecht D, Geist A, Ketelsen UP, Haberstroh J, Setzer B, Walker UA. 2007. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br J Pharmacol 151: 771–778. 10.1038/sj.bjp.0707294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrecht D, Kirschner J, Geist A, Haberstroh J, Walker UA. 2010. Respiratory chain deficiency precedes the disrupted calcium homeostasis in chronic doxorubicin cardiomyopathy. Cardiovasc Pathol 19: e167–e174. 10.1016/j.carpath.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Leduc-Gaudet JP, Picard M, St-Jean Pelletier F, Sgarioto N, Auger MJ, Vallée J, Robitaille R, St-Pierre DH, Gouspillou G. 2015. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6: 17923–17937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, et al. 2019. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567: 249–252. 10.1038/s41586-019-1004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Reid MB. 2000. NF-κB mediates the protein loss induced by TNF-α in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol 279: R1165–R1170. 10.1152/ajpregu.2000.279.4.R1165 [DOI] [PubMed] [Google Scholar]
- Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. 1998. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumor necrosis factor alpha. FASEB J 12: 871–880. 10.1096/fasebj.12.10.871 [DOI] [PubMed] [Google Scholar]
- Llovera M, Garcia-Martinez C, López-Soriano J, Agell N, López-Soriano FJ, Garcia I, Argilés JM. 1998a. Protein turnover in skeletal muscle of tumour-bearing transgenic mice overexpressing the soluble TNF receptor-1. Cancer Lett 130: 19–27. 10.1016/S0304-3835(98)00137-2 [DOI] [PubMed] [Google Scholar]
- Llovera M, Garcia-Martinez C, López-Soriano J, Carbó N, Agell N, López-Soriano FJ, Argiles JM. 1998b. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrinol 142: 183–189. 10.1016/S0303-7207(98)00105-1 [DOI] [PubMed] [Google Scholar]
- Luo Y, Yoneda J, Ohmori H, Sasaki T, Shimbo K, Eto S, Kato Y, Miyano H, Kobayashi T, Sasahira T, et al. 2014. Cancer usurps skeletal muscle as an energy repository. Cancer Res 74: 330–340. 10.1158/0008-5472.CAN-13-1052 [DOI] [PubMed] [Google Scholar]
- Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. 1992. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol 149: 2021–2027. [PubMed] [Google Scholar]
- Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. 2013. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31: 1539–1547. 10.1200/JCO.2012.45.2722 [DOI] [PubMed] [Google Scholar]
- Matthys P, Dukmans R, Proost P, Van Damme J, Heremans H, Sobis H, Billiau A. 1991. Severe cachexia in mice inoculated with interferon-γ-producing tumor cells. Int J Cancer 49: 77–82. 10.1002/ijc.2910490115 [DOI] [PubMed] [Google Scholar]
- McCart AE, Vickaryous NK, Silver A. 2008. Apc mice: models, modifiers and mutants. Pathol Res Pract 204: 479–490. 10.1016/j.prp.2008.03.004 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. 1997. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387: 83–90. 10.1038/387083a0 [DOI] [PubMed] [Google Scholar]
- Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, et al. 2016a. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab 23: 1078–1092. 10.1016/j.cmet.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera P, Laue K, Wei J, Berger JM, Karsenty G. 2016b. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab 5: 1042–1047. 10.1016/j.molmet.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, et al. 2017. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab 25: 218 10.1016/j.cmet.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, et al. 2015. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6: 6670 10.1038/ncomms7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, et al. 2016. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab 24: 795–806. 10.1016/j.cmet.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL. 1996. Mechanisms of muscle wasting—the role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905. 10.1056/NEJM199612193352507 [DOI] [PubMed] [Google Scholar]
- Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. 2002. Fatigue associated with cancer and its treatment. Support Care Cancer 10: 389–398. 10.1007/s005200100293 [DOI] [PubMed] [Google Scholar]
- Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. 2008. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33: 997–1006. 10.1139/H08-075 [DOI] [PubMed] [Google Scholar]
- Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. 2001. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27: 195–200. 10.1038/84839 [DOI] [PubMed] [Google Scholar]
- Nixon DW. 1982. Hyperalimentation in the undernourished cancer patient. Cancer Res 42: 727s–728s. [PubMed] [Google Scholar]
- Nixon DW, Lawson DH, Kutner M, Ansley J, Schwarz M, Heymsfield S, Chawla R, Cartwright TH, Rudman D. 1981. Hyperalimentation of the cancer patient with protein–calorie undernutrition. Cancer Res 41: 2038–2045. [PubMed] [Google Scholar]
- Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. 1987. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell 50: 555–563. 10.1016/0092-8674(87)90028-6 [DOI] [PubMed] [Google Scholar]
- Palmeira CM, Serrano J, Kuehl DW, Wallace KB. 1997. Preferential oxidation of cardiac mitochondrial DNA following acute intoxication with doxorubicin. Biochim Biophys Acta 1321: 101–106. 10.1016/S0005-2728(97)00055-8 [DOI] [PubMed] [Google Scholar]
- Parry-Billings M, Leighton B, Dimitriadis GD, Curi R, Bond J, Bevan S, Colquhoun A, Newsholme EA. 1991. The effect of tumour bearing on skeletal muscle glutamine metabolism. Int J Biochem 23: 933–937. 10.1016/0020-711X(91)90082-X [DOI] [PubMed] [Google Scholar]
- Paul PK, Gupta SK, Bhatnagar S, Panguluri SK, Darnay BG, Choi Y, Kumar A. 2010. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol 191: 1395–1411. 10.1083/jcb.201006098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna F, Busquets S, Pin F, Toledo M, Baccino FM, Lopez-Soriano FJ, Costelli P, Argiles JM. 2011. Combined approach to counteract experimental cancer cachexia: eicosapentaenoic acid and training exercise. J Cachexia Sarcopenia Muscle 2: 95–104. 10.1007/s13539-011-0028-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna F, Busquets S, Argilés JM. 2016. Experimental cancer cachexia: evolving strategies for getting closer to the human scenario. Semin Cell Dev Biol 54: 20–27. 10.1016/j.semcdb.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. 2014. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20: 433–447. [DOI] [PubMed] [Google Scholar]
- Pin F, Couch ME, Bonetto A. 2018. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care 12: 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. 2019. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 10: 140–154. 10.1002/jcsm.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polge C, Heng AE, Jarzaguet M, Ventadour S, Claustre A, Combaret L, Béchet D, Matondo M, Uttenweiler-Joseph S, Monsarrat B, et al. 2011. Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J 25: 3790–3802. 10.1096/fj.11-180968 [DOI] [PubMed] [Google Scholar]
- Popp MB, Fisher RI, Wesley R, Aamodt R, Brennan MF. 1981. A prospective randomized study of adjuvant parenteral nutrition in the treatment of advanced diffuse lymphoma: influence on survival. Surgery 90: 195–203. [PubMed] [Google Scholar]
- Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG, Sawyer MB. 2007. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13: 3264–3268. 10.1158/1078-0432.CCR-06-3067 [DOI] [PubMed] [Google Scholar]
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. 2009a. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15: 2920–2926. 10.1158/1078-0432.CCR-08-2242 [DOI] [PubMed] [Google Scholar]
- Prado CM, Birdsell LA, Baracos VE. 2009b. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 3: 269–275. 10.1097/SPC.0b013e328331124a [DOI] [PubMed] [Google Scholar]
- Prado CM, Antoun S, Sawyer MB, Baracos VE. 2011a. Two faces of drug therapy in cancer: drug-related lean tissue loss and its adverse consequences to survival and toxicity. Curr Opin Clin Nutr Metab Care 14: 250–254. 10.1097/MCO.0b013e3283455d45 [DOI] [PubMed] [Google Scholar]
- Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, Mackey JR, Kuzma M, Damaraju VL, Sawyer MB. 2011b. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 67: 93–101. 10.1007/s00280-010-1288-y [DOI] [PubMed] [Google Scholar]
- Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, Sawyer MB. 2012a. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 106: 1583–1586. 10.1038/bjc.2012.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. 2012b. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr 31: 583–601. 10.1016/j.clnu.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Prado CM, Maia YL, Ormsbee M, Sawyer MB, Baracos VE. 2013. Assessment of nutritional status in cancer—the relationship between body composition and pharmacokinetics. Anticancer Agents Med Chem 13: 1197–1203. 10.2174/18715206113139990322 [DOI] [PubMed] [Google Scholar]
- Prado CM, Baracos VE, Xiao J, Birdsell L, Stuyckens K, Park YC, Parekh T, Sawyer MB. 2014. The association between body composition and toxicities from the combination of Doxil and trabectedin in patients with advanced relapsed ovarian cancer. Appl Physiol Nutr Metab 39: 693–698. 10.1139/apnm-2013-0403 [DOI] [PubMed] [Google Scholar]
- Prado CM, Cushen SJ, Orsso CE, Ryan AM. 2016. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc 75: 188–198. 10.1017/S0029665115004279 [DOI] [PubMed] [Google Scholar]
- Quan-Jun Y, Yan H, Yong-Long H, Li-Li W, Jie L, Jin-Lu H, Jin L, Peng-Guo C, Run G, Cheng G. 2017. Selumetinib attenuates skeletal muscle wasting in murine cachexia model through ERK inhibition and AKT activation. Mol Cancer Ther 16: 334–343. 10.1158/1535-7163.MCT-16-0324 [DOI] [PubMed] [Google Scholar]
- Reed SA, Sandesara PB, Senf SM, Judge AR. 2012. Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J 26: 987–1000. 10.1096/fj.11-189977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JN, Mikesell C, Reiken S, Xu H, Marks AR, Mohammad KS, Guise TA, Waning DL. 2017. Osteolytic breast cancer causes skeletal muscle weakness in an immunocompetent syngeneic mouse model. Front Endocrinol (Lausanne) 8: 358 10.3389/fendo.2017.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels SE, Knowles AL, Tilignac T, Debiton E, Madelmont JC, Attaix D. 2001. Higher skeletal muscle protein synthesis and lower breakdown after chemotherapy in cachectic mice. Am J Physiol Regul Integr Comp Physiol 281: R133–R139. 10.1152/ajpregu.2001.281.1.R133 [DOI] [PubMed] [Google Scholar]
- Sandri M. 2016. Protein breakdown in cancer cachexia. Semin Cell Dev Biol 54: 11–19. 10.1016/j.semcdb.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. 2004. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412. 10.1016/S0092-8674(04)00400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, et al. 2013. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 14: 303–323. 10.1007/s10522-013-9432-9 [DOI] [PubMed] [Google Scholar]
- Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, Stantzou A, Mouisel E, Toniolo L, Ferry A, et al. 2013. BMP signaling controls muscle mass. Nat Genet 45: 1309–1318. 10.1038/ng.2772 [DOI] [PubMed] [Google Scholar]
- Schakman O, Gilson H, Kalista S, Thissen JP. 2009. Mechanisms of muscle atrophy induced by glucocorticoids. Horm Res 72: 36–41. 10.1159/000229762 [DOI] [PubMed] [Google Scholar]
- Scheede-Bergdahl C, Jagoe RT. 2013. After the chemotherapy: potential mechanisms for chemotherapy-induced delayed skeletal muscle dysfunction in survivors of acute lymphoblastic leukaemia in childhood. Front Pharmacol 4: 49 10.3389/fphar.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano J, Palmeira CM, Kuehl DW, Wallace KB. 1999. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim Biophys Acta 1411: 201–205. 10.1016/S0005-2728(99)00011-0 [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. 2005. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci 102: 5618–5623. 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum AM, Mahendradatta T, Taylor RJ, Painter AB, Moore MM, Tsoli M, Tan TC, Clarke SJ, Robertson GR, Polly P. 2012. Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting. Aging (Albany NY) 4: 133–143. 10.18632/aging.100436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis LS, Porter C, Saraf MK, Børsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, et al. 2015. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 22: 219–227. 10.1016/j.cmet.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souba WW. 1993. Glutamine and cancer. Ann Surg 218: 715–728. 10.1097/00000658-199312000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. 2004. The IGF-1/PI3 K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403. 10.1016/S1097-2765(04)00211-4 [DOI] [PubMed] [Google Scholar]
- Strassmann G, Fong M, Kenney JS, Jacob CO. 1992. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 89: 1681–1684. 10.1172/JCI115767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert EE, Yang J, Mace TA, Farren MR, Farris AB, Young GS, Elnaggar O, Che Z, Timmers CD, Rajasekera P, et al. 2017. Dual inhibition of MEK and PI3K/Akt rescues cancer cachexia through both tumor-extrinsic and -intrinsic activities. Mol Cancer Ther 16: 344–356. 10.1158/1535-7163.MCT-16-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavio M, Milan I, Tirelli U. 2002. Cancer-related fatigue (review). Int J Oncol 21: 1093–1099. [PubMed] [Google Scholar]
- Tezze C, Romanello V, Desbats MA, Fadini GP, Albiero M, Favaro G, Ciciliot S, Soriano ME, Morbidoni V, Cerqua C, et al. 2017. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab 25: 1374–1389.e6. 10.1016/j.cmet.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilignac T, Temparis S, Combaret L, Taillandier D, Pouch MN, Cervek M, Cardenas DM, Le Bricon T, Debiton E, Samuels SE, et al. 2002. Chemotherapy inhibits skeletal muscle ubiquitin-proteasome-dependent proteolysis. Cancer Res 62: 2771–2777. [PubMed] [Google Scholar]
- Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. 2005. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 280: 2847–2856. 10.1074/jbc.M411346200 [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y, Shiozaki H, et al. 1996. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest 97: 244–249. 10.1172/JCI118398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzika AA, Fontes-Oliveira CC, Shestov AA, Constantinou C, Psychogios N, Righi V, Mintzopoulos D, Busquets S, Lopez-Soriano FJ, Milot S, et al. 2013. Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. Int J Oncol 43: 886–894. 10.3892/ijo.2013.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Deeg DJ, Lips P, Longitudinal Aging Study Amsterdam. 2003. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 88: 5766–5772. 10.1210/jc.2003-030604 [DOI] [PubMed] [Google Scholar]
- Vitorino R, Moreira-Gonçalves D, Ferreira R. 2015. Mitochondrial plasticity in cancer-related muscle wasting: potential approaches for its management. Curr Opin Clin Nutr Metab Care 18: 226–233. 10.1097/MCO.0000000000000161 [DOI] [PubMed] [Google Scholar]
- von Haehling S, Morley JE, Anker SD. 2010. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 1: 129–133. 10.1007/s13539-010-0014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu Z, Hu J, Du J, Mitch WE. 2006. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168. 10.1210/en.2006-0251 [DOI] [PubMed] [Google Scholar]
- Wang G, Biswas AK, Ma W, Kandpal M, Coker C, Grandgenett PM, Hollingsworth MA, Jain R, Tanji K, Lόpez-Pintado S, et al. 2018. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med 24: 770–781. 10.1038/s41591-018-0054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waning DL, Guise TA. 2014. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin Cancer Res 20: 3071–3077. 10.1158/1078-0432.CCR-13-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, Chiechi A, Wright LE, Umanskaya A, Niewolna M, et al. 2015. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat Med 21: 1262–1271. 10.1038/nm.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA. 2003. Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol (1985) 94: 1479–1484. 10.1152/japplphysiol.01061.2002 [DOI] [PubMed] [Google Scholar]
- Welle S, Brooks AI, Delehanty JM, Needler N, Bhatt K, Shah B, Thornton CA. 2004. Skeletal muscle gene expression profiles in 20–29 year old and 65–71 year old women. Exp Gerontol 39: 369–377. 10.1016/j.exger.2003.11.011 [DOI] [PubMed] [Google Scholar]
- White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. 2011a. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol 300: R201–R211. 10.1152/ajpregu.00300.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. 2011b. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the ApcMin/+ mouse. PLoS One 6: e24650 10.1371/journal.pone.0024650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SA, Wacker MJ, Richmond SR, Godard MP. 2005. Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflügers Arch 450: 437–446. 10.1007/s00424-005-1473-8 [DOI] [PubMed] [Google Scholar]
- Wolfe RR. 2006. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482. 10.1093/ajcn/84.3.475 [DOI] [PubMed] [Google Scholar]
- Xu WP, Cao DX, Lin ZM, Wu GH, Chen L, Zhang JP, Zhang B, Yang ZA, Jiang Y, Han YS, et al. 2012. Analysis of energy utilization and body composition in kidney, bladder, and adrenal cancer patients. Urol Oncol 30: 711–718. 10.1016/j.urolonc.2010.07.017 [DOI] [PubMed] [Google Scholar]
- Yamada K, Sugiyama S, Kosaka K, Hayakawa M, Ozawa T. 1995. Early appearance of age-associated deterioration in mitochondrial function of diaphragm and heart in rats treated with doxorubicin. Exp Gerontol 30: 581–593. 10.1016/0531-5565(95)00033-X [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, Hayata N, Uozumi Y, Maeda M, Fujio Y, et al. 2008. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res 79: 89–96. 10.1093/cvr/cvn076 [DOI] [PubMed] [Google Scholar]
- Zhao J, Goldberg AL. 2016. Coordinate regulation of autophagy and the ubiquitin proteasome system by MTOR. Autophagy 12: 1967–1970. 10.1080/15548627.2016.1205770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, et al. 2010. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142: 531–543. 10.1016/j.cell.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. 2002. Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488. 10.1126/science.1069525 [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Fishel ML, Bonetto A. 2016. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol 54: 28–41. 10.1016/j.semcdb.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]