Abstract
Respiratory infections from influenza A virus (IAV) cause substantial morbidity and mortality in children relative to adults. T cells play a critical role in the host response to IAV by supporting the innate and humoral responses, mediating cytotoxic activity, and promoting recovery. There are age-dependent differences in the number, subsets, and localization of T cells, which impact the host response to pathogens. In this article, we first review how T cells recognize IAV and examine differences in the resting T-cell populations between juveniles and adults. Next, we describe how the juvenile CD4+, CD8+, and regulatory T-cell responses compare with those in adults and discuss the potential physiologic and clinical consequences of the differences. Finally, we explore the roles of two unconventional T-cell types in the juvenile response to influenza, natural-killer T cells and γδ T cells. A clear understanding of age-dependent differences in the T-cell response is essential to developing therapies to prevent or reverse the deleterious effects of IAV in children.
Keywords: influenza, T cell, viral pneumonia, juvenile, age-dependent
Influenza A virus (IAV) is a common pathogen that causes a spectrum of disease ranging from a mild upper-respiratory illness to a severe, life-threatening lower-respiratory illness. Worldwide, influenza is responsible for 290,000–650,000 deaths annually (1, 2). Children, the elderly, and individuals with chronic medical conditions are at greater risk of developing severe disease requiring hospitalization. Each year, there are estimated to be 90 million new cases of influenza in children under the age of 5 years. Of those cases, 20 million are lower-respiratory-tract infections and 1 million are severe (3). This substantial burden of disease in young children is only partially explained by chronic medical conditions, as half of influenza deaths in the United States from 2016 to 2019 were in previously healthy children (4).
Mouse models of IAV pneumonia support a paradigm in which the increased propensity for healthy children to have severe IAV disease is linked to age-dependent differences in the host response to and recovery from infection. Juvenile mice exhibit increased inflammation, lung injury, and mortality from IAV infection when compared with adult mice (5–7). T cells make up a vital component of the immune response to IAV. In both human and murine adults, the role of T cells in response to IAV has been well described; however, there are key differences between adult and juvenile T cells that change over time in both species (8–10). Understanding how these differences alter the juvenile T-cell response to IAV is critical to developing therapies to prevent or reverse the deleterious effects of IAV pneumonia. In this review, we will examine differences in how several distinct T-cell subsets respond to IAV in juveniles and discuss how those alterations in T-cell biology contribute to the differences in clinical outcomes between juveniles and adults.
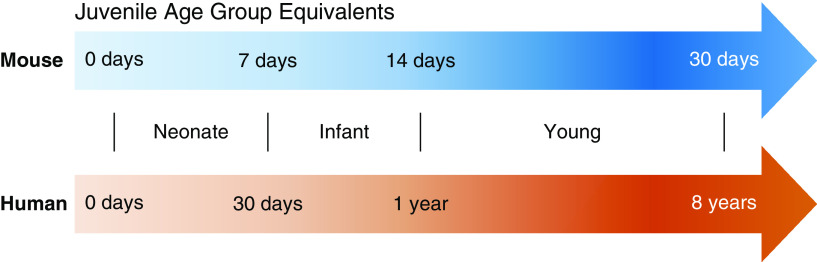
Although mice provide a practical model for human disease, given their similar genome and physiology, it is difficult to equate ages between humans and mice (11). In this review, we defined ages on the basis of lung development and refer to the age on the first day of infection (12). The term “neonate” will refer to humans ages 0–30 days and mice ages 0–7 days. “Infant” will be used for humans ages 31 days to 1 year and mice ages 8–14 days. “Young” will describe humans ages 1–8 years and mice 15–30 days. Finally, “juvenile” will be used as a collective term for any nonadult (Figure 1).
Figure 1.
Equivalent age groups of juvenile mice and humans. Approximate age ranges based on stages of lung development as described in Reference 12.
T-Cell Recognition of IAV
IAV is a single-stranded, enveloped RNA virus of the Orthomyxoviridae family. It contains two surface proteins, HA (hemagglutinin) and NA (neuraminidase). Mutations in these surface proteins allow for evasion of the immune system through antigenic drift, resulting in seasonal influenza epidemics. Exchange of RNA between human and nonhuman strains of IAV creates antigenic shift and is the cause of pandemics (13). The first point of contact between IAV and the host is the respiratory epithelium (for review, see Reference 14). After traversing the mucus layer, IAV attaches to sialic-acid receptors on respiratory epithelial cells via HA, resulting in endocytosis of the virus (15). Acidification of the virus in the endosome allows for release of viral nucleic acid, which is transported to the cell nucleus to initiate viral replication (16). Once viral proteins are synthesized, new virions are assembled and released by NA-mediated cleavage of HA–sialic acid interactions (17). During this process, detection of IAV by the innate immune systems initiates antiviral signaling and a proinflammatory response (for review, see References 18, 19).
Activation of the innate immune system is the first step in engagement of the adaptive immune response (for review, see Reference 20). Dendritic cells (DCs) residing in the pulmonary interstitium express pattern-recognition receptors that bind pathogen-associated molecular patterns. In addition, DCs can be activated through binding of damage-associated molecular patterns, which are cell components released from stressed or dying cells. Once activated, DCs capture viral antigens and migrate to draining lymph nodes through expression of the chemokine receptor CCR7 (21). B cells residing in lymph nodes can bind a viral antigen that has diffused into the lymph node through the afferent lymphatics, internalize the antigen, and process it for presentation to T cells (22). Macrophages are also able to migrate from the lungs and present antigens to T cells in draining lymph nodes; however, their contribution to the initial activation of the adaptive immune system remains unclear (21, 23, 24).
DCs reach maximal numbers on Days 2–4 after infection in draining lymph nodes, where they interact with T cells in high numbers, increasing the likelihood that they will encounter a viral-specific T cell (21). Viral antigens are presented on MHC (major histocompatibility complex) proteins I and II for recognition by CD8+ and CD4+ T cells, respectively (25). When a TCR recognizes a viral antigen in an MHC context, an immune synapse forms, allowing for activation and subsequent proliferation of the cognate T cell (26). Activation via the immune synapse is contingent on a second signal, the binding of CD28 on T cells to B7 (CD80/CD86) on DCs (27). This second signal is an important checkpoint on T-cell activation. Once activated, T cells migrate to the lungs using integrins and a chemokine gradient (24, 28).
TCR antigen specificity develops after somatic recombination during T-cell development. However, there is cross-reactivity of antigens between strains of influenza (29). This cross-reactivity plays a role in subsequent influenza infections via memory activation and is known as heterosubtypic immunity. In nonhuman primates, infection with cross-reactive IAV led to early peak activation of T cells and earlier clearance of virus (30). The possibility of childhood encounters with influenza fundamentally biasing future responses is known as imprinting. In influenza research, imprinting has focused on the B-cell response, even though T cells likely contribute (31). Although imprinting may play a role in the juvenile response, this review will primarily focus on the initial T-cell response to IAV.
T-Cell Population and Localization
The juvenile T-cell response to IAV must be understood in the context of how the juvenile adaptive immune systems differs from that of adults. Neonates are transitioning from a period of tolerance of maternal antigens to an environment where they are exposed to numerous innocuous and pathogenic antigens. Evidence of this transition exists in the number, subsets, and localization of juvenile T cells. Both human infant and neonatal murine T cells are predominantly naive, and a large proportion express CD31, a marker of recent thymic emigration (32). One key difference between human and murine newborns is their peripheral T-cell population at birth. Humans are born with an abundant population of T cells in the spleen and lymph nodes (9, 33). Neonatal mice, however, are born with lymphopenia, with few T cells in the spleen and lymph nodes. These populations increase during the first week of life, meaning that the peripheral T-cell population in newborn mice more closely reflects early stages of human fetal development (9, 33, 34).
After the neonatal period, the number, subsets, and localization of T cells continues to differ between juveniles and adults. In lymphoid tissues, human infants have a reduced T-cell:B-cell ratio; however, their CD4+:CD8+ composition is similar to adults (35). Human infant CD4+ cells display a higher frequency of Foxp3 expression (35). Foxp3 is the lineage-defining transcription factor for regulatory T cells (Tregs), a subtype of CD4+ T cells that is essential for mediating self-tolerance and immune homeostasis (36). Differences in memory T-cell populations exist as well. Memory T cells are present in human infant tissues in lower proportions when compared with proportions of adults. This difference is more pronounced in the blood and lymphoid tissues than in nonlymphoid tissues (35). Similar to human infants, infant mice produce tissue-resident memory T cells at lower frequencies than their adult counterparts (37). These differences in T-cell populations and localization reveal that even before exposure to IAV, the juvenile adaptive immune response is poised to respond differently from that of adults.
CD4+ T-Helper Cells
T-helper cells are a subset of CD4+ T cells that facilitate and enhance the activity of both the innate and adaptive immune system. Once activated, T-helper cells exert their effector function through both cytokines and surface proteins. For example, T-helper cells secrete IFN-γ, which increases viral processing in infected cells for targeting by CD8+ cytotoxic cells, and IL-4, which is essential for immunoglobulin class-switching in B cells (38, 39). In addition, they express CD40 ligand, which binds to CD40 on both macrophages and DCs to augment their activation (40). Not all T-helper cells have the same effector profile. There are several subtypes that have distinct functions within the immune response. The cytokine milieu present at the time of activation influences the regulation of gene expression and determines the CD4+ T-cell subtype specification (41).
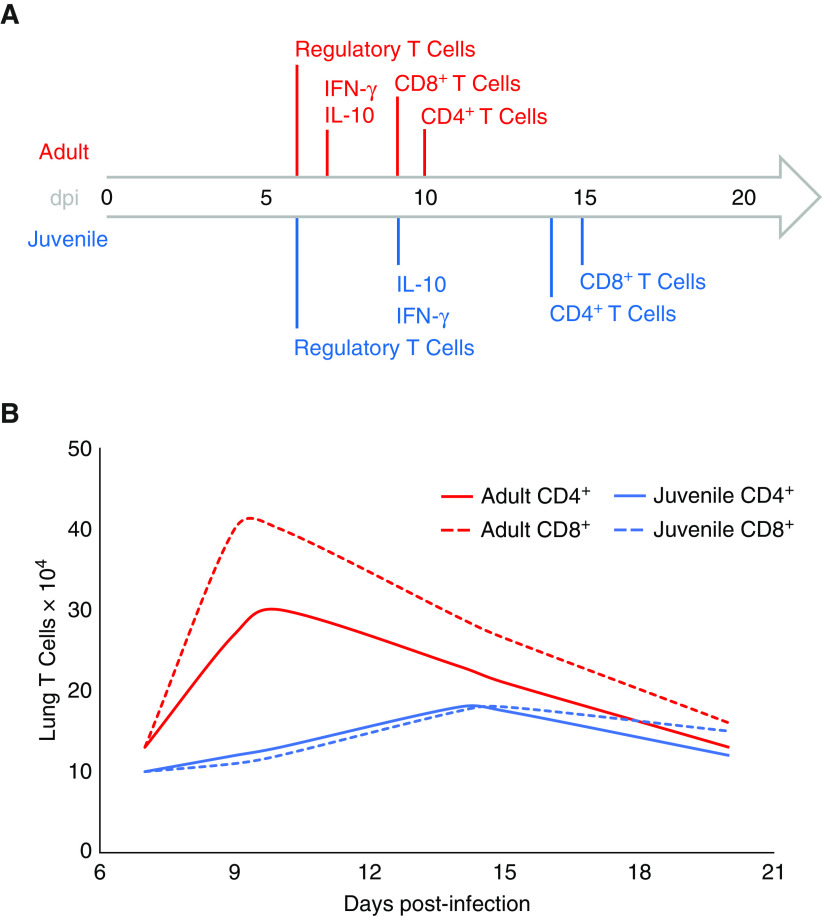
In response to IAV, juveniles exhibit a delayed CD4+ T-cell response as compared with adults (Table 1 and Figure 2). The role of CD4+ T cells during IAV infection is to support immune function. Adult mice without CD4+ T cells infected with IAV demonstrate a diminished CD8+ T-cell response and have delayed viral clearance (42, 43). This finding suggests that delayed recruitment of CD4+ T cells to the lungs impairs the juvenile immune response to IAV. Neonatal mice reach peak counts of CD4+ T cells in the lungs at 14 days after infection with IAV, as opposed to adults, which exhibit peak values at ∼10 days (44). A similar response is seen in young mice, with lower CD4+ T-cell counts in the lungs at 7 days after infection when compared with adults (45, 46). One difference between neonatal and young mice is the recruitment of CD4+ T cells into the airways. Although young mice exhibit CD4+ T cells in the airways during IAV infection, neonatal mice display near-absence of CD4+ T cells in the airways (44–46). The diminished CD4+ T-cell response appears to occur in humans as well. In fatal cases of lower-respiratory-tract infections in infants with influenza, there were very low numbers of lung-tissue CD4+ T cells at autopsy (47). In neonatal mice, the proportion of CD4+ T cells in the lungs with an effector phenotype, CD44hiCD62Llo, is lower when compared with adult mice early in infection; however, these cells equilibrate around Day 14 (37, 44). Thus, there is a delay not only in peak counts of absolute CD4+ T cells in the lungs but also in effector CD4+ T cells. Interestingly, early activation does not seem to be impaired in neonatal mice, as they express the activation marker CD69 on CD4+ T cells throughout infection and at levels that are similar to those of adults (44). Altogether, although early activation may be intact, there is a delay in effector CD4+ T-cell recruitment to the lungs in juveniles. This relative deficiency early in infection may impair the juvenile’s ability to optimally support innate immune-cell and CD8+ T-cell activity in the lungs, leading to prolonged inflammation.
Table 1.
Day of Peak Cell Recruitment and Cytokine Levels in Influenza A–infected Lungs
| Cell/Cytokine | Juvenile Age | Juvenile Peak (dpi) | Adult Peak* (dpi) | Citation |
|---|---|---|---|---|
| CD4+ T cells | 2 d | 14 | 10 | 44 |
| 2 d | 21 | 14 | 54 | |
| 3 wk | 7 | 7 | 45 | |
| CD8+ T cells | 2 d | 14 | 10 | 44 |
| 2 d | 21 | 10 | 54 | |
| 3 wk | 10† | 7 | 45 | |
| Regulatory T cells | 2 d | 6‡ | 6‡ | 54 |
| IFN-γ | 2 d | 14 | 7‡ | 44 |
| 7 d | 4 | — | 75 | |
| IL-10 | 3 wk | 9 | 7 | 46 |
Definition of abbreviation: dpi = days post-infection.
Adult mice 8–10 weeks old.
Last time point assessed.
First time point assessed.
Figure 2.
Differences between juvenile and adult T-cell responses to influenza A virus. (A) Timeline depicting peak cell recruitment and cytokine levels in the lungs of juvenile and adult mice. (B) In addition to being delayed, the juvenile CD4+ and CD8+ T cell responses to influenza A virus are diminished compared with those of adults. Curves and time points approximated from References 44–46, 54, 75. dpi = days post-infection.
An important distinction between the juvenile and adult response to IAV is the type of T-helper cell response mounted. It has been well described using noninfluenza antigens that neonatal mice have a bias toward a T-helper cell type 2 (Th2) response (48–50). One theory for why this bias exists is that it promotes perinatal adaption and protects developing tissues (51, 52). Type 2 immune responses, which are important for immunity against extracellular organisms, generate lower levels of inflammation and are involved in tissue repair. In contrast, type 1 immune responses, which are important for immunity against intracellular organisms, are proinflammatory. Thus, a type 2–biased immune response in neonates may have evolved to limit exposure to damaging inflammation during the perinatal period. In the context of IAV infection, this bias may be detrimental to the neonatal T-cell response. In studies of adult mice, Th2 cells are less effective against IAV than are Th1 cells, as a primarily Th2-driven response is associated with increased morbidity, mortality, and delayed viral clearance (53). During IAV infection, adult mice display a predominantly Th1-skewed response, whereas neonatal mice exhibit a mixed response without clear skewing (44, 54, 55). As juveniles age, there is a shift to a Th1-skewed response. Young mice display a decrease in the absolute number of Th1, Th2, and Th17 T cells in the lungs during IAV infection relative to adults; however, their proportions are similar (45). Although these observations show that the bias of the T-helper cell response changes as juveniles age, there is a persistent deficiency in lung Th1 cells among juveniles. The lack of a robust Th1 cell response likely contributes to an inadequate type 1 immune response that is insufficient to support cytotoxic and phagocytic cells in the lungs.
Consistent with differences between the juvenile and adult T-helper cell response, there are differences in the levels and production of IFN-γ between juveniles and adults during IAV infection (Table 1 and Figure 2). IFN-γ is the sole type II IFN and is primarily secreted by T cells (56). It is involved in several biologic processes, including adaptive immunity, regulation of inflammation, apoptosis, and the cell cycle. During IAV infection, IFN-γ has many functions. This is evident when examining mice deficient in IFN-γ production or signaling. Although there is no change in mortality or viral clearance, absence of IFN-γ during IAV infection results in delayed CD8+ T-cell recruitment, increased inflammation, and more severe impairment of lung function (57–59). This IFN-γ–deficient phenotype is similar to that of juveniles. Neonatal mice exhibit a delayed and lower peak level of IFN-γ in their lungs when compared with adults (44). In addition, there is a near-absence of IFN-γ in the airways of neonatal mice (44). Similarly, young mice display lower levels of IFN-γ in their lungs during IAV infection (45). Differences are seen in humans as well. Peripheral CD4+ cells from young children produce less IFN-γ in response to stimulation with influenza HA antigens when compared with adult cells (60). One consequence of lower levels of IFN-γ in juveniles is decreased T-cell recruitment, as administration of recombinant IFN-γ during IAV infection in mice improves lung migration (44, 45). In addition, in young mice, recombinant IFN-γ increases B-cell populations in the spleen and IAV-specific antibody titers; however, histopathology scores remain unchanged (45). These findings suggest that the decreased production of IFN-γ in juveniles impairs both cell-mediated and humoral immunity during IAV infection.
Transcriptional regulation of the Ifng gene partially explains the decrease of IFN-γ production observed in juvenile mice. During T-cell development and in response to antigen recognition, there are changes in transcription orchestrated by transcription factors and epigenetic phenomena that determine T-cell activity (61–63). Methylation of DNA near gene promoters and transcription start sites is an epigenetic change that generally represses gene expression (64). CD4+ T cells isolated from human cord blood are hypermethylated within and adjacent to the IFNG promoter (65). This hypermethylation likely contributes to the decreased production of IFN-γ observed in neonates. In young mice, the mechanism behind decreased IFN-γ may be different. Binding of IL-12 to its receptor on Th1 cells activates STAT4 (signal transducer and activator of transcription 4), which in turn induces expression of ERM, an Ets family transcription factor, to promote Ifng gene expression (66). Young mouse CD4+ T cells have decreased expression of ERM and its downstream transcripts during activation when compared with adult CD4+ cells (45). This implies that although the effects of decreased IFN-γ are similar across juveniles of different ages, the mechanisms regulating the deficiency change over time.
T cell–generated cytokines differ during the adult and juvenile response to IAV. IL-4 is a cytokine associated with type 2 immune responses (67). In addition to being essential for B-cell immunoglobulin class-switching, it is critical for differentiation of Th2 cells (39). Consistent with their mixed T-helper cell response, neonatal mice have higher levels of IL-4 in their lungs during IAV infection when compared with adults (44). These higher levels of IL-4 are not seen during IAV infections in young mice, which parallels their shift to a Th1-skewed response (45). TNF-α is a proinflammatory cytokine that is produced by many cell types, including macrophages, endothelial cells, B cells, and T cells (68). Although there is no difference in the levels of TNF-α seen between either neonatal or juvenile mice and adults, the peak of TNF-α is delayed in neonates and correlates with the delayed recruitment of CD4+ T cells to the lungs (44, 45). IL-10 is an immunosuppressive cytokine that targets both innate and adaptive immune cells to reduce inflammation and tissue damage; it is decreased in the lungs of young mice infected with IAV when compared with adults (Table 1) (46, 69). This observation of low IL-10 levels together with low IFN-γ levels in the lungs of young mice during IAV infection is consistent with the observation that IL-10 and IFN-γ levels are closely correlated during IAV infection in adult mice (70). Although many immune cells can produce IL-10, the predominant source of IL-10 in adult mice during IAV infection is effector CD4+ and CD8+ T cells (69, 70). In young mice, the expression of IL-10 was found to be downregulated in both CD4+ and CD8+ T cells (46). When IL-10 is blocked in adult mice there is uncontrolled inflammation during IAV infection (70). Administration of IL-10 to juvenile mice during IAV infection improves histopathology scores (46). Thus, the diminished production of IL-10 in juveniles likely allows for severe inflammation after IAV infection, resulting in increased tissue damaged and delayed recovery.
Altogether, the juvenile CD4+ T-cell and T cell–generated cytokine responses to IAV pneumonia are delayed and diminished when compared with those of adults (Figure 3). Transcriptional regulation partially explains the decreased production of IFN-γ observed in juveniles. The relative deficiency of the CD4+ T-cell response may result in insufficient support of the innate, cytotoxic, humoral, and antiinflammatory immune responses, causing severe, prolonged inflammation. This results in significant lung injury and the severe clinical phenotype from IAV pneumonia observed in juveniles.
Figure 3.
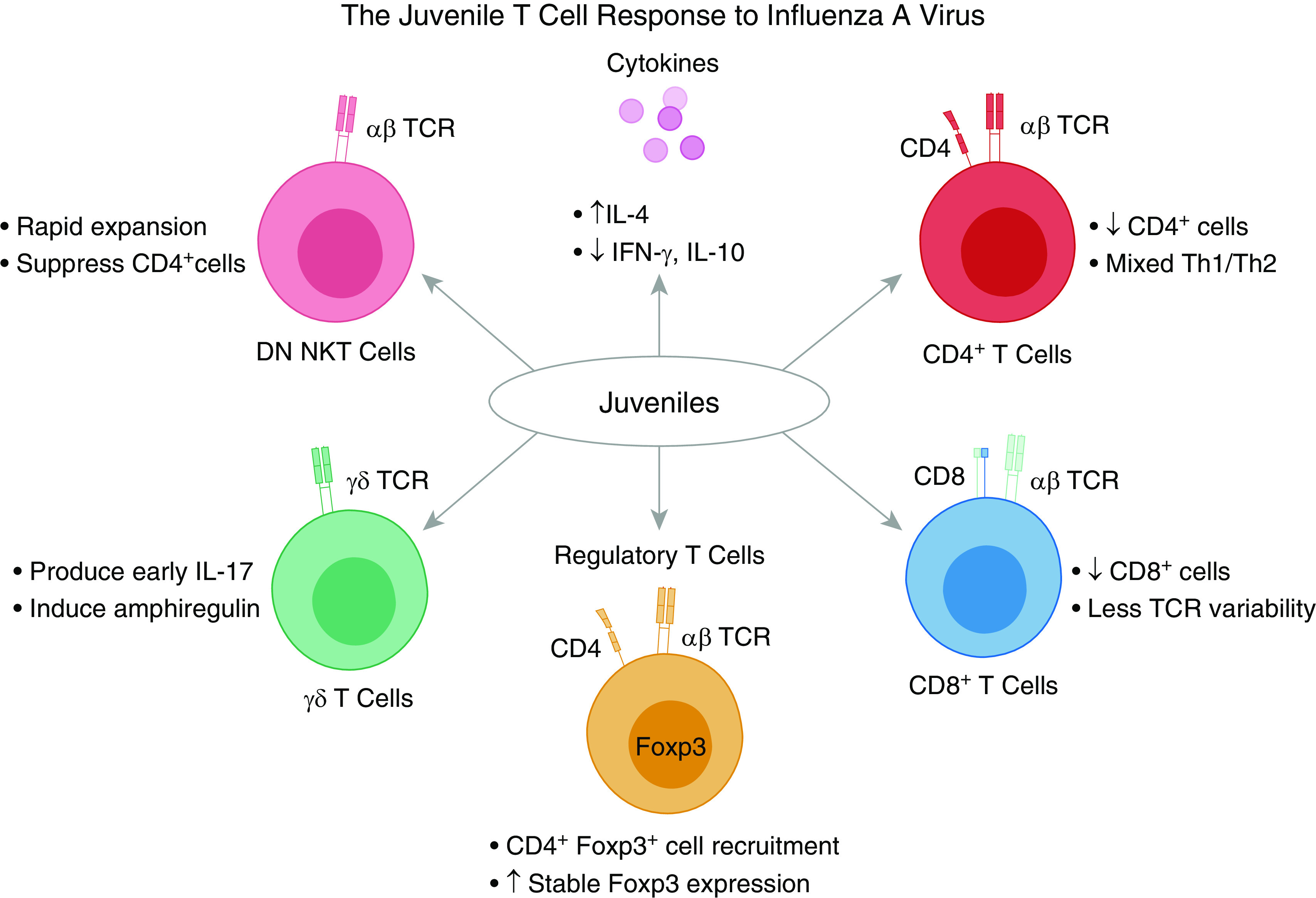
The juvenile T-cell response to influenza A virus. Recruitment of CD4+ and CD8+ T cells to the lungs is delayed and diminished relative to that of adults. Regulatory T cells are recruited in similar numbers. Production of inflammatory and suppressive cytokines IFN-γ and IL-10 is reduced. In neonates, there is a mixed T-helper cell type 1 (Th1) and Th2 response that contributes to increased levels of IL-4. As juveniles age, there is a shift to a Th1 response. In addition, neonates have less TCR variability, which increases as they age, as well as an increased propensity to induce stable Foxp3 expression, which decreases as they age. γδ T cells are a source of IL-17 in neonates and promote tissue recovery by inducing amphiregulin. DN NKT cells rapidly expand and exhibit the ability to suppress CD4+ T cells. DN NKT = double-negative natural-killer T cell.
CD8+ Cytotoxic T Cells
The primary function of CD8+ cytotoxic T cells is to eliminate infected cells. Cytotoxic T cells are activated through their TCR and via other costimulatory signals (71). Once activated, CD8+ T cells deploy several mechanisms to kill target cells. They release perforins and proteases that form channels in the target cell membrane and degrade cellular proteins, respectively. In addition, they can induce apoptosis of the target cell through the Fas–Fas ligand (72). Cytotoxic T-cell effector function is not limited to cell lysis, as they contribute to production of immunomodulatory cytokines such as IFN-γ, TNF, and IL-10 (70, 72).
During IAV infection, juveniles display a delayed and diminished CD8+ T-cell response relative to adults (Figure 2, Table 1). The involvement of CD8+ T cells during IAV infection is crucial, as their absence in adult mice results in delayed viral clearance with sublethal doses and increased mortality with lethal doses of IAV when compared with wild-type mice (73). This critical role of CD8+ T cells is observed in humans as well. Adults with an early, prominent peak of CD8+ T cells in peripheral blood recover more quickly from IAV than those adults with a delayed peak of CD8+ T cells (74). Although this early peak likely represents a heterosubtypic memory response, it illustrates the importance of a timely CD8+ T-cell response, which juveniles are lacking. Neonatal and infant mice have decreased IAV-specific CD8+ T cells in the lungs relative to older juveniles and adults during infection (32, 44, 75–77). The peak of CD8+ T cells in the lungs is not only lower but also delayed (77). Young mice similarly exhibit a lower and delayed peak of CD8+ T cells in the lungs during infection with IAV (46). One difference between neonatal and young mice is that neonates have few to no CD4+ or CD8+ T cells in their airways during IAV infection, whereas young mice display these lymphocyte populations in their airways during IAV infection (44, 46). Alterations in CD8+ T-cell migration to the lungs is also seen in humans. In fatal cases of lower-respiratory-tract infections from influenza, infants have a near-absence of CD8+ T cells in the lungs (47). The consequence of the delayed and diminished CD8+ T-cell response in juveniles during IAV infection is likely prolonged immune activation.
There are cell-autonomous differences between adult and juvenile CD8+ T-cell responses to IAV. Adoptive transfer of adult CD8+ T cells into neonatal mice results in improved CD8+ T-cell expansion, enhanced pulmonary function, and reduced inflammation during IAV infection (75, 77). Conversely, when neonatal CD8+ T cells are transferred into an adult mouse, no improvement in T-cell expansion is observed (77). One potential source of this difference is the TCR. Neonatal mice have less TCR variability, and the antigen-specific response of neonatal CD8+ cells is less diverse because of differences in usage of TCR gene segments as well as decreased nucleotide addition to complementarity-determining regions (77). When CD8+ T cells with a high-affinity TCR are selectively transferred from a neonatal mouse to an adult, CD8+ T-cell expansion is similar to that of adult CD8+ T cells (77). It is important to note that this likely only affects neonates, as there is rapid evolution of the TCR repertoire in mice during the first week after birth (77). Another potential mechanism for differences between juvenile and adult CD8+ T-cell responses is transcriptional regulation. The transcriptional profile of peripheral-blood CD8+ T cells isolated from young children (<7 yr old) with influenza revealed decreased upregulation of IFN-stimulated genes relative to older children (>7 yr old) (78). This difference could only partially be accounted for by evidence of previous influenza exposure, suggesting that T-cell memory alone does not account for age-dependent differences (78). Altogether, these studies suggest that age-related differences in transcriptional regulation and TCR diversity influence the CD8+ T-cell response to IAV.
Collectively, the juvenile CD8+ T-cell response to IAV pneumonia is delayed and diminished when compared with that of adults (Figure 3). Age-dependent differences in TCR somatic recombination and transcriptional regulation may contribute to this observation. The consequence of an inadequate CD8+ T-cell response is prolonged immune activation. This state likely delays the initiation of prorecovery functions and allows for ongoing lung injury from inflammation, resulting in the severe disease from IAV pneumonia observed in juveniles.
Tregs
Tregs are a subset of CD4+ lymphocytes that mediate self-tolerance and maintain immune homeostasis (for review, see Reference 36). They are defined by expression of the transcription factor Foxp3. Tregs can develop in the thymus (natural Tregs) or differentiate in the periphery from CD4+ T cells (induced Tregs) (79). They have multiple mechanisms to suppress the immune response, including expression of cell-surface proteins CTLA4, lymphocyte activation gene 3, CD39, CD73, and CD25 as well as production of the cytokines IL-10, IL-35, and transforming growth factor β (79–81). During lung injury, Tregs play an essential role in recovery and produce the epidermal growth factor receptor ligand amphiregulin (82–84). Their absence results in delayed recovery, decreased epithelial proliferation, and increased fibroproliferation (82, 83, 85).
Few studies have examined the role of Tregs in the juvenile response to IAV. In neonatal mice, there are higher proportions of Tregs in the lungs at 6 days after infection when compared with adults; however, the absolute number is similar throughout infection (54). In another model of lung injury using intrapharyngeal LPS, adoptive transfer of adult Tregs into neonatal mice attenuated inflammation and decreased weight loss, suggesting cell-autonomous differences between juveniles and adults (86). There is also a difference in the propensity to generate Tregs. When exposed to antigens, murine neonatal CD4+ T cells are more likely to induce stable expression of Foxp3 and acquire a regulatory phenotype when compared with adult CD4+ T cells (87). This propensity dramatically decreases in the first 2 weeks of life, so it likely does not play a role in older juveniles. The specific role of Tregs during juvenile IAV infection is unclear. Loss of Tregs in neonatal mice increases the number of CD4+ and CD8+ T cells in the lungs, increases activation of T cells, and increases IFN-γ production (54). Even with this increase in T-cell recruitment and IFN-γ, neonatal mice without Tregs have delayed viral clearance (54). This suggests a role for Tregs in mediating viral elimination. It is interesting that despite equal-to-increased numbers of Tregs in the lungs, juveniles develop increased inflammation and tissue damage after IAV infection because Tregs have an essential role in the recovery from lung injury (Figure 3). Altogether, these findings raise questions about the ability of juvenile Tregs to promote tissue recovery and repair during IAV infection.
Natural-Killer T Cells and γδ T Cells
Natural-killer T (NKT) cells and γδ T cells are unconventional T cells that have both innate and adaptive properties. Like conventional T cells, NKT cells express an αβ TCR; however, there are limited rearrangements resulting in recognition of conserved antigens on MHC I–like molecules (for review, see Reference 88). γδ T cells express a TCR composed of γ and δ subunits, which recognizes conserved nonpeptide antigens that are upregulated by cells under stress (for review, see References 89, 90). Both NKT cells and γδ T cells have diverse populations with a spectrum of effector functions ranging from proinflammatory to immunosuppression and have been shown to play a role in the response to IAV (88–90).
Infant mice have lower counts of NKT cells at baseline when compared with adults (91). When infant mice are infected with IAV, there is rapid expansion of the NKT-cell population (91). One specific population that increases is CD4− CD8− (double-negative) NKT cells (91, 92). These cells produce IFN-γ and suppress activity of CD4+ T cells in vitro (91). During reinfection in adulthood, these cells are protective against airway hyperreactivity (91, 92).
γδ T cells appear to play a protective role in the neonatal IAV response. Neonatal mice without γδ T cells have increased weight loss, increased inflammation, and decreased survival with IAV infection when compared with wild-type mice (93). In neonatal mice, γδ T cells are early producers of IL-17, which induces epithelial-cell production of IL-33 to cause higher levels of amphiregulin, driving tissue protection and recovery (93, 94). Both types of unconventional T cells described here appear to have protective roles for juveniles during IAV infection; however, additional studies are needed to further delineate the roles of NKT cells and γδ T cells as well as to describe the roles of other unconventional lymphoid cells, such as mucosa-associated invariant T cells and innate lymphoid cells (Figure 3).
Conclusions
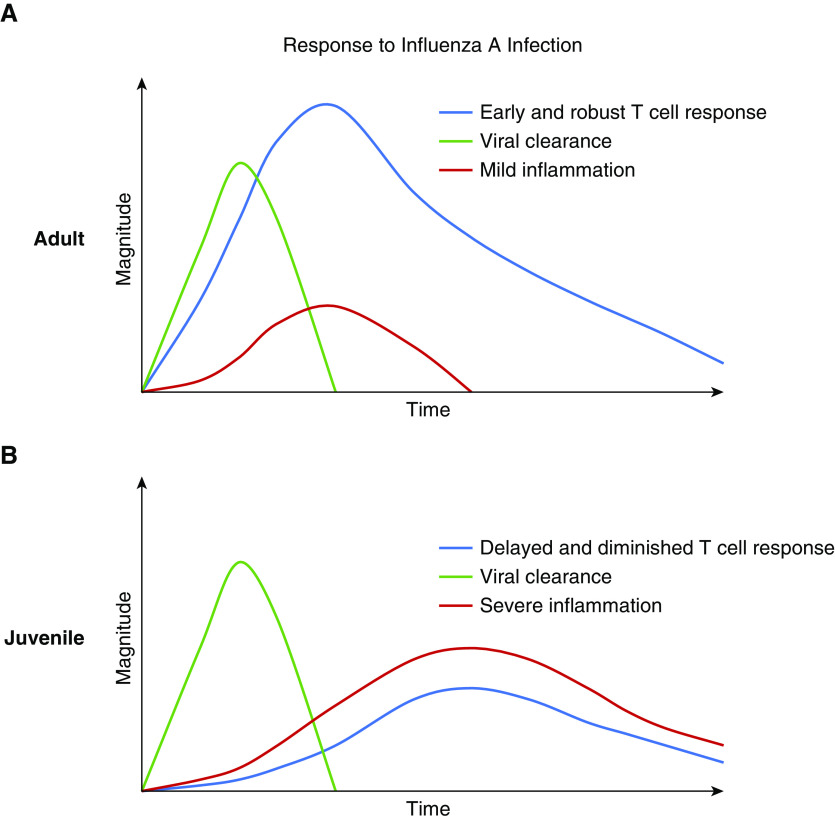
Healthy children are more severely affected by IAV than are adults. This difference in outcomes may be due to age-dependent differences in their T-cell response. The juvenile adaptive immune system is poised for immune tolerance and changes throughout development in the number, subsets, and localization of T cells. This shifting landscape alters the T-cell response to IAV as juveniles age, creating differences among neonates, infants, and young children. Nevertheless, despite these developmental changes, similar themes exist. There is a parallel delayed and diminished peak of the juvenile CD4+ T-cell, CD8+ T-cell, and IFN-γ response to IAV in the lungs. Similarly, other T cell–generated cytokines such as IL-10 are decreased, and despite an equal-to-increased presence of Tregs, there is increased inflammation and tissue damage. This relatively deficient T-cell response in juveniles may be providing insufficient support to the innate, humoral, and prorecovery immune responses, resulting in increased lung injury that manifests as severe clinical disease (Figure 4).
Figure 4.
Comparison of T-cell response, viral clearance, and lung inflammation in adults and juveniles with influenza A infection. (A) In healthy adults, there is an early and robust T-cell response with mild inflammation that manifests as mild clinical disease. (B) In juveniles, there is a delayed and diminished T-cell response, resulting in prolonged, severe inflammation that manifests as severe clinical disease.
Current studies suggest transcriptional and epigenetic programming are at least partially responsible for these differences. Further studies are needed to better under understand the mechanisms behind the delayed and diminished response. In addition, future investigation should explore juvenile repair mechanisms and the regulation of switching from proinflammatory to prorecovery processes. An understanding of the mechanisms underlying why the T-cell response is delayed and diminished and how this influences the balance of inflammation and recovery is vital to developing therapies to prevent and reverse the detrimental effects of IAV in children.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Jennifer Davis for her assistance in figure preparation and copy editing. Figure 3 was created with BioRender.com.
Footnotes
Supported by the Gorter Family Foundation, the Stanley Manne Children’s Research Institute, and the Ann & Robert H. Lurie Children’s Hospital of Chicago (A.D.P.); a David and Christine Cugell Fellowship (R.M.); U.S. National Institutes of Health/National Heart, Lung, and Blood Institute grants K08 HL143127 (B.M.C.), K08HL128867, R01HL149883, R01HL114800, and U19AI135964 (B.D.S.); and National Institutes of Health grants P01HL071643, R01HL128194, P01GM0969971, and P01AG049665 (K.M.R.).
Author Contributions: A.D.P. wrote the initial draft. R.M., B.M.C., B.D.S., and K.M.R. further added to, revised, and updated the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0169TR on July 1, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, et al. Global Seasonal Influenza-associated Mortality Collaborator Network. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva, Switzerland: World Health Organization; 2018. Fact sheets: influenza (seasonal) [accessed 2020 Jan 23]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) [Google Scholar]
- 3.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention FluView Interactive: influenza-associated pediatric mortality Atlanta, GA: Centers for Disease Control and Prevention; 2020[accessed 2020 Jan 23]. Available from: https://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html [Google Scholar]
- 5.Sun S, Zhao G, Xiao W, Hu J, Guo Y, Yu H, et al. Age-related sensitivity and pathological differences in infections by 2009 pandemic influenza A (H1N1) virus. Virol J. 2011;8:52. doi: 10.1186/1743-422X-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coates BM, Staricha KL, Koch CM, Cheng Y, Shumaker DK, Budinger GRS, et al. Inflammatory monocytes drive influenza A virus-mediated lung injury in juvenile mice. J Immunol. 2018;200:2391–2404. doi: 10.4049/jimmunol.1701543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates BM, Staricha KL, Ravindran N, Koch CM, Cheng Y, Davis JM, et al. Inhibition of the NOD-like receptor protein 3 inflammasome is protective in juvenile influenza A virus infection. Front Immunol. 2017;8:782. doi: 10.3389/fimmu.2017.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Liu S, Goraya MU, Maarouf M, Huang S, Chen JL. Host immune response to influenza A virus infection. Front Immunol. 2018;9:320. doi: 10.3389/fimmu.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fike AJ, Kumova OK, Carey AJ. Dissecting the defects in the neonatal CD8+ T-cell response. J Leukoc Biol. 2019;106:1051–1061. doi: 10.1002/JLB.5RU0319-105R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Chao CM, El Agha E, Tiozzo C, Minoo P, Bellusci S. A breath of fresh air on the mesenchyme: impact of impaired mesenchymal development on the pathogenesis of bronchopulmonary dysplasia. Front Med (Lausanne) 2015;2:27. doi: 10.3389/fmed.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Sandt CE, Kreijtz JH, Rimmelzwaan GF. Evasion of influenza A viruses from innate and adaptive immune responses. Viruses. 2012;4:1438–1476. doi: 10.3390/v4091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benam KH, Denney L, Ho LP. How the respiratory epithelium senses and reacts to influenza virus. Am J Respir Cell Mol Biol. 2019;60:259–268. doi: 10.1165/rcmb.2018-0247TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rust MJ, Lakadamyali M, Zhang F, Zhuang X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol. 2004;11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samji T. Influenza A: understanding the viral life cycle. Yale J Biol Med. 2009;82:153–159. [PMC free article] [PubMed] [Google Scholar]
- 17.Rossman JS, Lamb RA. Influenza virus assembly and budding. Virology. 2011;411:229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coates BM, Staricha KL, Wiese KM, Ridge KM. Influenza A virus infection, innate immunity, and childhood. JAMA Pediatr. 2015;169:956–963. doi: 10.1001/jamapediatrics.2015.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hufford MM, Kim TS, Sun J, Braciale TJ. The effector T cell response to influenza infection. Curr Top Microbiol Immunol. 2015;386:423–455. doi: 10.1007/82_2014_397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, Hung SC, et al. The other function: class II-restricted antigen presentation by B cells. Front Immunol. 2017;8:319. doi: 10.3389/fimmu.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol. 2009;183:1983–1989. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol. 2015;33:169–200. doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- 26.Courtney AH, Lo WL, Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem Sci. 2018;43:108–123. doi: 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bugeon L, Dallman MJ. Costimulation of T cells. Am J Respir Crit Care Med. 2000;162:S164–S168. doi: 10.1164/ajrccm.162.supplement_3.15tac5. [DOI] [PubMed] [Google Scholar]
- 28.D’Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–1275. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- 29.Koutsakos M, Illing PT, Nguyen THO, Mifsud NA, Crawford JC, Rizzetto S, et al. Human CD8+ T cell cross-reactivity across influenza A, B and C viruses. Nat Immunol. 2019;20:613–625. doi: 10.1038/s41590-019-0320-6. [DOI] [PubMed] [Google Scholar]
- 30.Weinfurter JT, Brunner K, Capuano SV, III, Li C, Broman KW, Kawaoka Y, et al. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog. 2011;7:e1002381. doi: 10.1371/journal.ppat.1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson SA, Sant AJ. Imprinting and editing of the human CD4 T cell response to influenza virus. Front Immunol. 2019;10:932. doi: 10.3389/fimmu.2019.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fike AJ, Nguyen LT, Kumova OK, Carey AJ. Characterization of CD31 expression on murine and human neonatal T lymphocytes during development and activation. Pediatr Res. 2017;82:133–140. doi: 10.1038/pr.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am J reprod Immunol. 2013;69:346–358. doi: 10.1111/aji.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedberg SH, Weissman IL. Lymphoid tissue architecture: II. Ontogeny of peripheral T and B cells in mice. Evidence against Peyer’s patches as the site of generation of B cells. J Immunol. 1974;113:1477–1492. [PubMed] [Google Scholar]
- 35.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22:72–77. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Liao X, Kang Y. Tregs: where we are and what comes next? Front Immunol. 2017;8:1578. doi: 10.3389/fimmu.2017.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zens KD, Chen JK, Guyer RS, Wu FL, Cvetkovski F, Miron M, et al. Reduced generation of lung tissue-resident memory T cells during infancy. J Exp Med. 2017;214:2915–2932. doi: 10.1084/jem.20170521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 39.Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 41.Tripathi SK, Lahesmaa R. Transcriptional and epigenetic regulation of T-helper lineage specification. Immunol Rev. 2014;261:62–83. doi: 10.1111/imr.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76:12388–12393. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+) T-cell responses in CD4-depleted Ig(−/−) mice. J Virol. 2000;74:9762–9765. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol. 2010;185:2980–2988. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhoeven D, Perry S, Pryharski K. Control of influenza infection is impaired by diminished interferon-γ secretion by CD4 T cells in the lungs of toddler mice. J Leukoc Biol. 2016;100:203–212. doi: 10.1189/jlb.4A1014-497RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verhoeven D, Perry S. Differential mucosal IL-10-induced immunoregulation of innate immune responses occurs in influenza infected infants/toddlers and adults. Immunol Cell Biol. 2017;95:252–260. doi: 10.1038/icb.2016.91. [DOI] [PubMed] [Google Scholar]
- 47.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh RR, Hahn BH, Sercarz EE. Neonatal peptide exposure can prime T cells and, upon subsequent immunization, induce their immune deviation: implications for antibody vs. T cell-mediated autoimmunity. J Exp Med. 1996;183:1613–1621. doi: 10.1084/jem.183.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, et al. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 50.Rose S, Lichtenheld M, Foote MR, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J Immunol. 2007;178:2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris NL, Loke P. Recent advances in type-2-cell-mediated immunity: insights from helminth infection. Immunity. 2017;47:1024–1036. doi: 10.1016/j.immuni.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliphant S, Lines JL, Hollifield ML, Garvy BA. Regulatory T cells are critical for clearing influenza A virus in neonatal mice. Viral Immunol. 2015;28:580–589. doi: 10.1089/vim.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Román E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-γ by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol. 2001;158:119–130. doi: 10.1016/s0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner SJ, Olivas E, Gutierrez A, Diaz G, Doherty PC. Disregulated influenza A virus-specific CD8+ T cell homeostasis in the absence of IFN-gamma signaling. J Immunol. 2007;178:7616–7622. doi: 10.4049/jimmunol.178.12.7616. [DOI] [PubMed] [Google Scholar]
- 60.Shannon I, White CL, Murphy A, Qiu X, Treanor JJ, Nayak JL. Differences in the influenza-specific CD4 T cell immunodominance hierarchy and functional potential between children and young adults. Sci Rep. 2019;9:791. doi: 10.1038/s41598-018-37167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Makar KW, Wilson CB. DNA methylation is a nonredundant repressor of the Th2 effector program. J Immunol. 2004;173:4402–4406. doi: 10.4049/jimmunol.173.7.4402. [DOI] [PubMed] [Google Scholar]
- 63.McGrath-Morrow SA, Ndeh R, Helmin KA, Chen SY, Anekalla KR, Abdala-Valencia H, et al. DNA methylation regulates the neonatal CD4+ T-cell response to pneumonia in mice. J Biol Chem. 2018;293:11772–11783. doi: 10.1074/jbc.RA118.003589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morales-Nebreda L, McLafferty FS, Singer BD. DNA methylation as a transcriptional regulator of the immune system. Transl Res. 2019;204:1–18. doi: 10.1016/j.trsl.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO− T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 66.Ouyang W, Jacobson NG, Bhattacharya D, Gorham JD, Fenoglio D, Sha WC, et al. The Ets transcription factor ERM is Th1-specific and induced by IL-12 through a Stat4-dependent pathway. Proc Natl Acad Sci USA. 1999;96:3888–3893. doi: 10.1073/pnas.96.7.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Matikainen S, Sirén J, Tissari J, Veckman V, Pirhonen J, Severa M, et al. Tumor necrosis factor alpha enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J Virol. 2006;80:3515–3522. doi: 10.1128/JVI.80.7.3515-3522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 70.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 73.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Wan Y, Qiu C, Quiñones-Parra S, Zhu Z, Loh L, et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8+ T cells. Nat Commun. 2015;6:6833. doi: 10.1038/ncomms7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.You D, Ripple M, Balakrishna S, Troxclair D, Sandquist D, Ding L, et al. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol. 2008;181:3486–3494. doi: 10.4049/jimmunol.181.5.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fike AJ, Kumova OK, Tardif VJ, Carey AJ. Neonatal influenza-specific effector CTLs retain elevated CD31 levels at the site of infection and have decreased IFN-γ production. J Leukoc Biol. 2019;105:539–549. doi: 10.1002/JLB.4A0518-191R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carey AJ, Gracias DT, Thayer JL, Boesteanu AC, Kumova OK, Mueller YM, et al. Rapid evolution of the CD8+ TCR repertoire in neonatal mice. J Immunol. 2016;196:2602–2613. doi: 10.4049/jimmunol.1502126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henrickson SE, Manne S, Dolfi DV, Mansfield KD, Parkhouse K, Mistry RD, et al. Genomic circuitry underlying immunological response to pediatric acute respiratory infection. Cell Rep. 2018;22:411–426. doi: 10.1016/j.celrep.2017.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Georgiev P, Charbonnier LM, Chatila TA. Regulatory T cells: the many faces of Foxp3. J Clin Immunol. 2019;39:623–640. doi: 10.1007/s10875-019-00684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venet F, Chung CS, Huang X, Lomas-Neira J, Chen Y, Ayala A. Lymphocytes in the development of lung inflammation: a role for regulatory CD4+ T cells in indirect pulmonary lung injury. J Immunol. 2009;183:3472–3480. doi: 10.4049/jimmunol.0804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dowling MR, Kan A, Heinzel S, Marchingo JM, Hodgkin PD, Hawkins ED. Regulatory T cells suppress effector T cell proliferation by limiting division destiny. Front Immunol. 2018;9:2461. doi: 10.3389/fimmu.2018.02461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mock JR, Garibaldi BT, Aggarwal NR, Jenkins J, Limjunyawong N, Singer BD, et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol. 2014;7:1440–1451. doi: 10.1038/mi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garibaldi BT, D’Alessio FR, Mock JR, Files DC, Chau E, Eto Y, et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. 2013;48:35–43. doi: 10.1165/rcmb.2012-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A distinct function of regulatory T cells in tissue protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singer BD, Mock JR, Aggarwal NR, Garibaldi BT, Sidhaye VK, Florez MA, et al. Regulatory T cell DNA methyltransferase inhibition accelerates resolution of lung inflammation. Am J Respir Cell Mol Biol. 2015;52:641–652. doi: 10.1165/rcmb.2014-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGrath-Morrow SA, Lee S, Gibbs K, Lopez A, Collaco JM, Neptune E, et al. Immune response to intrapharyngeal LPS in neonatal and juvenile mice. Am J Respir Cell Mol Biol. 2015;52:323–331. doi: 10.1165/rcmb.2014-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. “Default” generation of neonatal regulatory T cells. J Immunol. 2010;185:71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 88.Trottein F, Paget C. Natural killer T cells and mucosal-associated invariant T cells in lung infections. Front Immunol. 2018;9:1750. doi: 10.3389/fimmu.2018.01750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng M, Hu S. Lung-resident γδ T cells and their roles in lung diseases. Immunology. 2017;151:375–384. doi: 10.1111/imm.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Y, Lin L, Xiao Z, Li M, Wu X, Li W, et al. Protective role of γδ T cells in different pathogen infections and its potential clinical application. J Immunol Res. 2018;2018:5081634. doi: 10.1155/2018/5081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chuang YT, Leung K, Chang YJ, DeKruyff RH, Savage PB, Cruse R, et al. A natural killer T-cell subset that protects against airway hyperreactivity. J Allergy Clin Immunol. 2019;143:565–576, e7. doi: 10.1016/j.jaci.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 93.Guo XJ, Dash P, Crawford JC, Allen EK, Zamora AE, Boyd DF, et al. Lung γδ T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity. 2018;49:531–544, e6. doi: 10.1016/j.immuni.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.