Abstract
This work was designed to optimize thermosensitive copolymeric depot-based system for delivering insulin at a controlled rate for a prolonged period following a single subcutaneous injection. Intrinsic ability of insulin to form hexamers in the presence of zinc and electrostatic complexes with chitosan (CS) were explored for improving stability and release characteristics of insulin through the copolymeric depot. CS-zinc-insulin complexes were prepared using CS of different chain lengths (5, 30, 50, 200 kDa). Effect of different chain lengths of CS on the thermal stability, binding constant, and release profile of insulin was determined. Increasing chain length of CS demonstrated increasing thermal stability of insulin. However, higher chain length of CS adversely affected the release profile of insulin. Hydrolytic degradation analysis showed rapid degradation of copolymer in formulation containing higher chain length of CS (200 kDa)-zinc-insulin complexes, implying formation of bigger pores and channels in copolymeric matrix during initial release in this system. However, formulation containing smaller chain length of CS (5 kDa)-zinc-insulin complexes demonstrated slow copolymer degradation and sustained insulin release profile. Additionally, CS-zinc-insulin complexes were effective in preserving stability of insulin during the entire duration of release and storage.
Keywords: thermosensitive copolymer, controlled release, protein/peptide delivery, electrostatic complex, protein stability, chitosan chain length, differential scanning calorimetry
Graphical Abstract
1. Introduction
Diabetes mellitus (DM) is a serious public health concern affecting more than 8.5% of global adult population (422 million) and about 12.2% of the U.S. adult population (30.2 million). It is also the seventh leading cause of death worldwide. DM is rising rapidly with an estimated 1.5 million cases diagnosed each year in U.S. alone. It is classified as a chronic progressive metabolic syndrome characterized by relative or absolute deficiency of insulin. Insulin is a vital peptide hormone which mediates glucose transport from bloodstream into the cells for energy production. Type 1 DM (5 – 10% of total DM cases) occurs due to selective slow and progressive autoimmune destruction of body’s own healthy insulin-producing pancreatic β-cells. It leads to an absolute deficiency of insulin production. Type 2 DM results from reduced action of insulin on insulin-responsive cells (insulin resistance), and inadequate insulin secretion owing to progressive β-cell dysfunction. Exogenous insulin delivery is a necessity for type 1 diabetics, and eventually for type 2 diabetics upon failure of oral antidiabetic therapy.
In a healthy human, other than large amount of insulin secretion following meals (bolus insulin), insulin is secreted at a basal level (0.5 – 1 U/h) between meals and throughout the night. This basal insulin or background insulin accounts for 40—50% of total daily insulin and modulates the rate of glucose output (hepatic gluconeogenesis: 5 μmol/kg/min) overnight and during prolonged periods between meals.1 This also allows for sufficient glucose level for cerebral energy production at bedtime. There is not a single FDA-approved controlled release delivery system available to deliver insulin continuously for more than a day following a single injection to meet the needs of basal insulin. Repeated administration of exogenous insulin exposes the body to fluctuating blood glucose levels which causes serious damage to nerves and blood vessels throughout the body resulting in serious microvascular and macrovascular complications. Diabetic individuals with fluctuating blood glucose are at higher risks of development of cardiovascular diseases, hypertension, stroke, kidney failure, nerve damage, limb amputations and reduced quality of life.2,3 Accordingly, there is a huge unmet need for cheap, effective and patient compliant diabetes treatment strategies.
In this study, we propose a thermosensitive copolymer based in situ depot forming system for controlled release of insulin at basal rate for a prolonged period following a single subcutaneous injection. Thermosensitive triblock copolymer poly(D,L-lactide)-poly(ethylene glycol)-poly(D,L-lactide) (PLA-PEG-PLA, 1500-1500-1500, 4500 Da) was selected based on previous research.4,5 Briefly, PLA-PEG-PLA copolymer is biocompatible and biodegradable and its degradation products lactic acid and glycolic acid are naturally eliminated from the body.6,7 It is easily soluble in water and therefore avoids use of toxic organic solvents in the formulation. The chief characteristic of this smart copolymer is its thermosensitive nature. It is in a solution form at room temperature and below (≤ 25 °C) which allows for easy suspension of any drug/peptide in it by simple mixing. Upon injection at a subcutaneous site the copolymer undergoes phase transition, owing to the slightly higher body temperature (phase-transition temperature ≥ 26 °C), to instantaneously form a gel depot incorporating the therapeutic in the gel matrix and then releasing it at a sustained rate.8 However, stability and burst release are major concerns for controlled delivery of protein/peptide-based therapeutics from polymeric formulations. Additionally, release of drug incorporated in thermosensitive copolymer matrix is effected by a combination of diffusion through copolymer matrix and slow hydrolytic degradation of the copolymer which are affected by size and nature of the drug incorporated.9,10
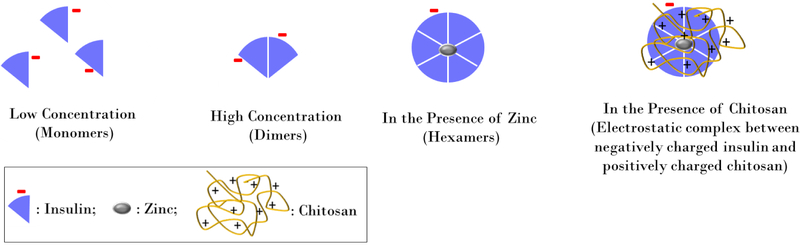
On the other hand, since insulin is a hydrophilic peptide hormone, sensitive to denaturation when stored at physiological temperature for a long duration, its thermal stability and release characteristics must be carefully modified before incorporating it in a controlled release system intended for prolonged use. Physiologically, zinc plays an important role in biosynthesis and storage of insulin within pancreatic β cells.11 Furthermore, insulin (isoelectric point ~5.3) is capable of forming electrostatic complexes with chitosan (CS) (pKa 6.5 – 7.0) owing to their negative and positive charges at physiological pH, respectively (Figure 1).12-14 These intrinsic properties of insulin to form hexamers in the presence of zinc and electrostatic complexes with CS were explored in this research to optimize stability and release profile of insulin. Different chain lengths of CS were investigated to study the effect increasing size of CS-zinc-insulin complexes on the stability and release profile of insulin through thermosensitive copolymeric depot-based delivery system. Modification of insulin with CS and zinc is expected to minimize burst release as well as preserve its stability during the entire duration of release and storage. Effect of zinc and different chain lengths of CS on themostability of insulin was determined using nano differential scanning calorimetry (DSC). Further characterization of CS-zinc-insulin electrostatic complexes was performed using isothermal calorimetry (ITC). Effect of CS chain length on release profile of insulin through copolymer matrix was determined using in vitro release model (Figure 2). Hydrolytic degradation of copolymer incorporating different formulations was assessed using size exclusion chromatography (SEC-HPLC) to understand the effect of CS chain length on the degradation profile of copolymer matrix at different time intervals. Stability of insulin during release and storage was determined using nano DSC and circular dichroism (CD) spectroscopy. Finally, since management of a chronic disease like diabetes requires prolonged treatment, safety and biocompatibility of the formulation are a major concern. Therefore, in vitro biocompatibility of the formulations was evaluated using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay in human embryonic kidney (HEK 293) cell line.
Figure 1.
Schematic representation of modification of insulin using zinc and chitosan.
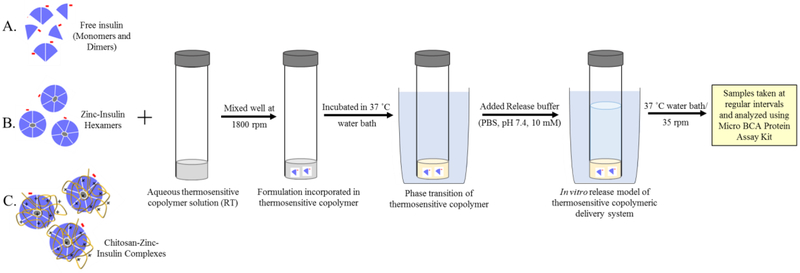
Figure 2.
Schematic representation of formulation and in vitro release study model for thermosensitive copolymeric-depot based delivery system incorporating either (A) free insulin, (B) zinc-insulin hexamers, or (C) chitosan-zinc-insulin complexes.
2. Material and methods
2.1. Materials
Human recombinant insulin (Cell Prime™ r-insulin) was purchased from EMD Millipore Corporation (Billerica, MA, USA). Anhydrous zinc acetate was procured from Alfa Aesar, (MA, USA). Chitosan (average molecular weight 5, 30, 50, and 200 kDa, ~90% degree of deacetylation) was obtained from Glentham Life Sciences (WIL, UK). 3,6-Dimethyl-1,4-dioxane-2,5-dione (D,L-lactide) was purchased from TCI America (Portland, OR, USA). Polyelthylene glycol (1500 Da), polystyrene standards, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Stannous Octoate was procured from Pfaltz and Bauer Inc. (Waterbury, CT, USA). MicroBCA protein assay kit was obtained from Pierce Biotechnology Inc. (Rockford, IL, USA). Human embryonic kidney (HEK 293) cell line, Dulbecco’s modified Eagle’s medium (DMEM) and phosphate buffered saline (PBS) were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). All other reagents were analytical grade and used without further modification.
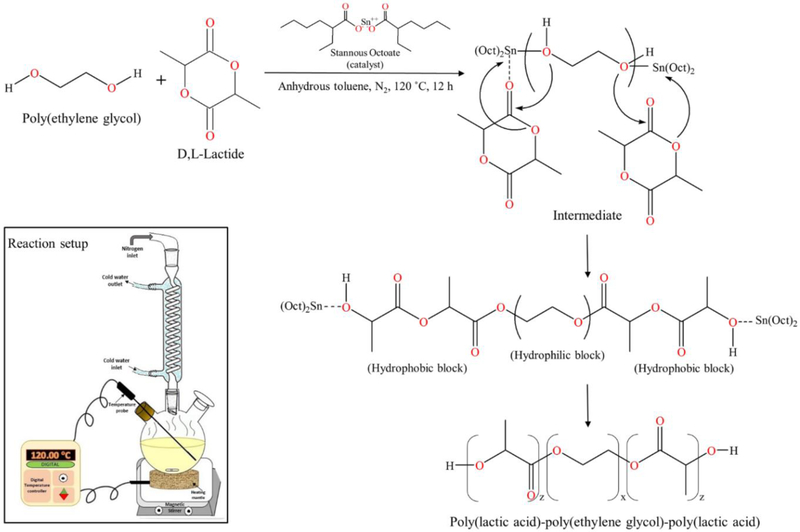
2.2. Synthesis and Characterization of Thermosensitive Triblock Copolymer
Thermosensitive triblock copolymer poly(D,L-lactide)-poly(ethylene glycol)-poly(D,L-lactide) (PLA-PEG-PLA, 1500-1500-1500, 4500 Da) was synthesized using ring opening polymerization of D,L-lactide catalyzed by stannous octoate using PEG (1500 Da) as an initiator, in anhydrous atmosphere.15 Briefly, PEG (9.99 g) was dissolved in anhydrous toluene (40 mL) in a three-necked round bottom flask at 90 °C in nitrogen atmosphere for 0.5 h. D,L-lactide (19.98 g) was added to the solution and temperature was increased to 120 °C. Reactants were allowed to dissolve completely to form a homogenous mixture followed by addition of stannous octoate (0.03% w/w, ~9 μL). Reaction was carried out under continuous stirring at 120 °C for 12 h.
Reactant mixture was then dried in a vacuum oven to evaporate toluene completely. Crude copolymer was then purified by dissolving it in ice cold water followed by heating to 80 °C to precipitate out the copolymer. Purification step was repeated 2 – 3 times to separate out unreacted monomers and catalyst. Finally, precipitated copolymer was lyophilized to get rid of residual water.
Structural composition of synthesized copolymer was determined using proton (1H) and carbon (13C) nuclear magnetic resonance (NMR) spectroscopy using deuterated chloroform (CDCl3) as a solvent (10 mg/0.6 mL). Tetramethylsilane (TMS) signal was used for calibration and its signal was taken as zero chemical shift. Bruker spectrometer operated at 400 MHz and 25 °C was used for measurements. 13C NMR spectra was also obtained to confirm the presence of PLA and PEG blocks.
Number average molecular weight (Mn), weight average molecular weight (Mw), and molecular weight distribution (polydispersity index, PDI) of synthesized copolymer (0.2% w/v in tetrahydrofuran (THF)) was determined using gel permeation chromatography (GPC) (Eco SEC HLC-8320-GPC system, Tosoh Bioscience, Japan) with a differential refractometer (DRI) detector. Separations were performed using 2 TSK gel SuperH3000 6.0 mm ID × 15 cm columns with an eluent flow rate of 0.35 ml min−1. Columns and detectors were thermostated at 40 °C, with eluent THF and injection volume 10 μL. Calibration was conducted using polystyrene standards (Agilent EasiVial PS-H 4ml).
Injectability of different aqueous copolymer concentrations was determined by injecting 0.5 mL copolymer into a glass tube using 25 G needle. Sol-gel transition temperature was noted by tube inversion method by immersing the tubes containing copolymer solution in a water bath at 8 °C, followed by raising the temperature 2 °C/step and observing phase transition of copolymer by inverting the tube horizontally.
2.3. Effect of Insulin Modification using Zinc and Chitosan
Effect of addition of zinc and CS on the thermostability and association state of insulin was investigated using nano DSC (TA Instruments, DE, USA). Zinc-insulin hexamers were prepared by adding homogenous solution of zinc ions (zinc acetate dissolved in 10 mM hydrochloric acid) to insulin solution in PBS (10 mM, pH 7.4) at 1:5 molar ratio of insulin hexamer to zinc ions. Insulin and zinc molecules were allowed to react for 10 minutes at room temperature with mild shaking. CS-zinc-insulin complexes were prepared by adding CS (5 moles of CS monomer unit per mole of protein) to zinc-insulin hexamers and incubating for 15 min with intermittent mixing. CS-zinc-insulin complexes containing CS of different chain lengths (5, 30, 50, 200 kDa) were prepared in identical manner. All samples were degassed for 10 minutes under vacuum before loading into nano DSC cells. Data was collected by scanning the samples from 10 to 110 °C at a scan rate of 1 °C/min. Reference scan (PBS, 10 mM, pH 7.4) was subtracted from sample scan during data analysis, performed using Nanoanalyze® software provided with the instrument. Transition curve were fitted using two-state scaled model following the “pseudo” V’ant Hoff method.
2.4. Characterization of Binding Interaction using Isothermal Calorimetry
Isothermal calorimetry was employed to determine the effect of CS chain length on binding constant, enthalpy of complex formation, and the stoichiometry of interaction between zinc-insulin hexamers and different chain lengths of CS. Interaction studies were performed using nano ITC (TA instruments, USA) with a cell volume of 1 mL at 25 °C temperature. All samples were degassed for 10 minutes under vacuum prior to use. The sample cell was filled with zinc-insulin hexamers dissolved in PBS, 10mM, pH 7.4 (2 mg/mL ~ 0.057 mM negatively charged hexameric units) and the reference cell was filled with buffer solution only.
CS solution (1.2 mg/mL ~ 6 mM of free amino groups) was introduced into the thermostated cell by means of a syringe and stirred at 250 rpm. Each titration integrated 25 subsequent 10 μL injections programmed to proceed at 400 s intervals. The heat of dilution was determined by titrating CS solution into PBS alone, and were subtracted from each experiment to obtain the net binding heat changes. Data was analyzed using Nanoanalyze® software (version 3.7.5) provided with the instrument.
2.5. Preparation of Delivery Systems
A simple and robust method for formulation of a delivery system is highly desired for achieving good reproducibility between batches as well as obtaining a cost-effective method for preparation of final product. Aqueous copolymeric delivery systems containing either free insulin, zinc-insulin hexamers, or CS-zinc-insulin complexes were prepared using zinc acetate as the zinc donor and CS of successive chain lengths: 5, 30, 50, and 200 kDa. Briefly, formulations were prepared by suspending either free insulin, zinc-insulin hexamers or CS-zinc-insulin complexes in aqueous copolymeric solution (35% w/v) followed by mild mixing on a vortex (Thermo Scientific, USA) at 1800 rpm for five minutes (Figure 2). Zinc-insulin hexamers were prepared by adding homogenous solution of zinc ions (zinc acetate dissolved in 10 mM hydrochloric acid) to insulin solution in PBS (10 mM, pH 7.4) at 1:5 molar ratio of insulin hexamer to zinc ions (Figure 1). Insulin and zinc molecules were allowed to react for 10 minutes at room temperature with mild shaking. CS-zinc-insulin complexes were prepared by adding CS (5 moles of CS monomer unit per mole of protein) to zinc-insulin hexamers and incubating for 15 min with intermittent mixing. CS-zinc-insulin complexes containing CS of different chain lengths (5, 30, 50, 200 kDa) were prepared in identical manner. Solutions containing zinc-insulin hexamers or CS-zinc-insulin complexes were centrifuged at 3,000 rpm for 10 minutes to collect the hexamers/complex as precipitate which were then suspended in aqueous copolymer solution (35% w/v). Supernatant following centrifugation were removed and analyzed to determine amount of unentrapped insulin quantified using micro BCA protein assay kit.
2.6. In Vitro Release Profile of Insulin
In vitro release profile of delivery systems in presence of insulin, zinc-insulin hexamers, and CS-zinc-insulin complexes was studied in detail using zinc acetate as the zinc donor and CS of successive chain lengths: 5, 30, 50, and 200 kDa. For studying in vitro release profile of insulin, 0.5 mL of the respective formulations were injected into borosilicate glass culture tubes and allowed to form gel by incubating at 37 °C water bath, shaken at 35 rpm (Figure 2). Pre-warmed PBS (10 mM, pH 7.4) was slowly added to the tubes as release medium. Aliquots were removed and replaced periodically, and amount of insulin released was quantified using micro BCA protein assay kit. Concentration correction was performed according to the method described by Hayton and Chen 16.
2.7. Mass Loss of Copolymer During In Vitro Release
Amount of copolymer lost during in vitro release was determined by removing release medium of delivery systems at fixed intervals (15, 30, and 45 days) followed by lyophilization of the remaining copolymer. Weight remaining of the delivery system was then calculated by weighing the copolymer and comparing it to the initial amount.
2.8. Reduction in Copolymer Molecular Weight During In Vitro Release
Molecular weight of the copolymer remaining was determined at fixed intervals (15, 30, and 45 days) using SEC-HPLC. Lyophilized copolymer residues were dissolved in THF and analyzed using Agilent Technologies’ (Santa Clara, CA, USA) 1120 series compact LC system coupled with Agilent 1200 series refractive index detector, thermostated at 35 °C. Polystyrene standards of different molecular weights were used for calibration. Analysis was performed using Waters styragel column (HR4E, 5 μm, 7.8 mm × 300 mm; Milford, MA, USA), THF as the carrier solvent, 1 mL/min flow rate, and 50 μL injection volume.
2.9. Stability of Insulin Released from Delivery System
Physicochemical characterization of samples containing insulin released in vitro from optimized formulation, CS (5 kDa)-zinc-insulin complexes incorporated in thermosensitive copolymeric depot, was performed at specific time points. All samples were centrifuged, filtered and degassed prior to analysis. Thermal transition of released insulin was recorded using nano DSC as specified earlier. Conformational stability analysis was performed using CD spectroscopy by scanning the samples in near-UV region (250-300 nm) and far-UV region (200-250 nm) to investigate the changes in tertiary and secondary structure, respectively. All spectra were recorded at a scan rate of 5 nm/min at 20 °C with a quartz cuvette (0.1 cm path length). Background interference was removed by subtracting scan of fresh PBS (10 mM, pH 7.4) in the same range. Freshly prepared insulin solution was used as standard. Spectra manager®2 software (Jasco, Tokyo, Japan) was used for spectrum analysis.
2.10. Storage Stability of Insulin in Formulation
Stability of insulin in optimized formulation, CS (5 kDa)-zinc-insulin complexes incorporated in thermosensitive copolymeric depot, during storage at 4 °C was also tested. Insulin was extracted from stored copolymer formulations (0.5 mL) using PBS and acetonitrile mixture (1:1 v/v) at specific intervals and tested using nano DSC and CD spectroscopy as discussed earlier.
2.11. In Vitro Biocompatibility of Triblock Copolymer
Biocompatibility of the delivery system was investigated using MTT cell viability assay in human embryonic kidney cells (HEK-293). Briefly, aqueous copolymer solution (35% w/v, 0.5 mL) with and without CS (5 kDa) was incubated at 37 °C water bath and extracted into PBS (10 mM, pH 7.4, 5 mL) by incubating for 10 days. Extracts collected were successively diluted in serum-free DMEM and added to HEK-293 cells followed by incubation for 24, 48, and 72 hours. Post-incubation, 25 μL of MTT solution (5 mg/mL in DI water) was added to each well and allowed to react for 3 h. Unreacted MTT was carefully aspirated and cells were rinsed with cold PBS. Finally, 150 μL of dimethyl sulfoxide was added to dissolve the formazan crystals formed by viable cells and absorbance was recorded at 570 nm using a microplate reader. Cells without MTT treatment were considered as blank and were used to calibrate the spectrophotometer reading to zero absorbance. Cells treated with DMSO were taken as positive control. Long-term biocompatibility of degradation products of the copolymeric formulation was similarly evaluated by incubating aqueous copolymer solution (35% w/v, 0.5 mL) with and without CS (5 kDa) at 70 °C water bath and extracted into PBS (10 mM, pH 7.4, 5 mL) by incubating for 10 days. Since degradation rate of PLA based copolymers is higher at increased temperatures, long-term biocompatibility of this system was evaluated at a higher incubation temperature.17,18 Relative cell viability was calculated for treated cells by taking the cells without any treatment as negative control using the following equation:
Where, ASample is average absorbance of wells incubated with polymer extract dilutions and AControl is the average absorbance of the control wells incubated with serum-free DMEM.
2.12. Statistical Analysis
Data are expressed as mean ± SD. Statistical analyses were performed using two tailed unpaired student's t-test and one-way ANOVA with post-hoc Tukey HSD test. A p value of less than 0.05 was considered to be significant.
3. Results and discussion
3.1. Synthesis and Characterization of Thermosensitive Copolymer
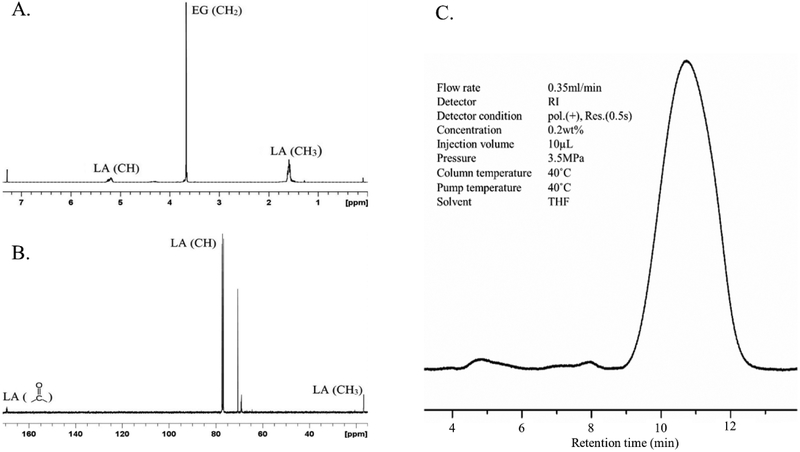
Ring opening polymerization is a widely used method for the synthesis of triblock copolymers of PLA, PLGA and PEG where the hydrophobic block is covalently linked to the hydrophilic block by an ester linkage.19 PLA1500-PEG1500-PLA1500 triblock copolymer was selected based on previous research.20 Copolymer was synthesized with ≥85% yield. Purified copolymer was characterized using 1H and 13C NMR (Figure 3). Signals corresponding to ─CH of LA at 5.20, ─CH3 of LA at 1.55, ─CH2 of PEG at 3.66, and ─CH2 of EG’s connecting unit to LA at 4.31 ppm were observed (Figure 3A). The signals corresponding to chemical groups ─CH and ─CH3 of LA and ─CH2 of ethylene glycol in 1H NMR were integrated and used to calculate the number average molecular weight (Mn) of the polymer.21 13C NMR spectrum showed the presence of the groups, ─C═O, ─CH and ─CH3 in the PLA block at 169.35, 77.23 and 16.67 ppm, respectively, while ─CH2 group in PEG block was found at 69.18 ppm (Figure 3B). NMR spectra confirmed structure of the synthesized copolymer.
Figure 3.
(A) Proton (1H) nuclear magnetic resonance spectrum, (B) Carbon (13C) nuclear magnetic resonance spectrum, and (C) Gel permeation chromatogram, of PLA-PEG-PLA triblock copolymer.
GPC was used to determine Mn, Mw and molecular weight distribution (PDI) of the synthesized copolymer. Retention time and PDI were found to be 11.025 min and 1.157, respectively, with a unimodal GPC trace (Figure 3C). Characteristics of the copolymer determined by NMR and GPC are summarized in table 1.
Table 1.
Characteristics of PLA-PEG-PLA copolymer determined using proton nuclear magnetic resonance spectroscopy and gel permeation chromatography.
| NMR | GPC | ||||
|---|---|---|---|---|---|
| Copolymer | Mwa | Mnb | Mwc | Mnd | Polydispersitye (Mwc/Mnd) |
| PLA-PEG-PLA | 4500 | 4660 | 5022 | 4339 | 1.147 |
Mwa: Theoretical molecular weight of copolymer
Mnb: Number average molecular weight determined by NMR
Mwc: Weight average molecular weight determined by GPC
Mnd: Number average molecular weight determined by GPC
Polydispersity indexe: Determined by GPC
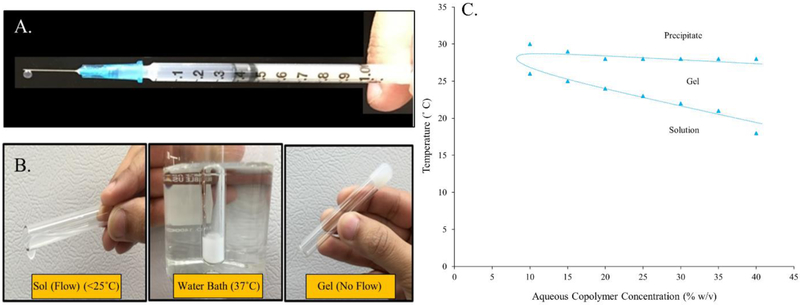
Aqueous solubility and injectability are two additional characteristics that make this copolymer a versatile delivery system. It avoids the use of toxic organic solvents in the formulation while allowing easy subcutaneous administration of formulation without a need for surgery. The aqueous copolymer solution (0.5 mL) was found to be easily injectable through a 25 G needle in the concentration range 10 – 35% w/v (Figure 4A). Phase transition of thermosensitive copolymers from an injectable solution form to a gel state is effected by an interplay between hydrophobic PLA blocks and hydrophilic PEG blocks. PLA-PEG-PLA copolymer shows a lower critical solution temperature (LCST) due to the hydrogen bonding between hydrophilic PEG blocks which is dominant at lower temperature, making the copolymer soluble. As the temperature increases above LCST, the hydrogen bonding gets weaker and hydrophobic interactions in the PLA blocks get stronger driving the transition to a gel/depot state.19 Phase transition of PLA-PEG-PLA copolymer is depicted in figure 4B. Sol-gel transition temperature of different aqueous copolymer concentrations (w/v) was in the range 18°C to 26°C, increasing with decreasing copolymer concentration (Figure 4C). Therefore, following subcutaneous injection, at physiological temperature the formulation would instantaneously transition into an in situ depot incorporating the therapeutic (insulin) in the copolymeric matrix, which is then released at a controlled rate owing to its slow diffusion through the copolymer matrix and gradual hydrolytic degradation of the copolymer.
Figure 4.
Picture images of aqueous PLA-PEG-PLA copolymer solution demonstrating (A) Injectability through a 25 G needle, (B) Phase transitioning ability in response to temperature, and (C) Graphical representation of sol-gel-precipitate transition of different aqueous copolymer.
3.2. Effect of Insulin Modification using Zinc and Chitosan
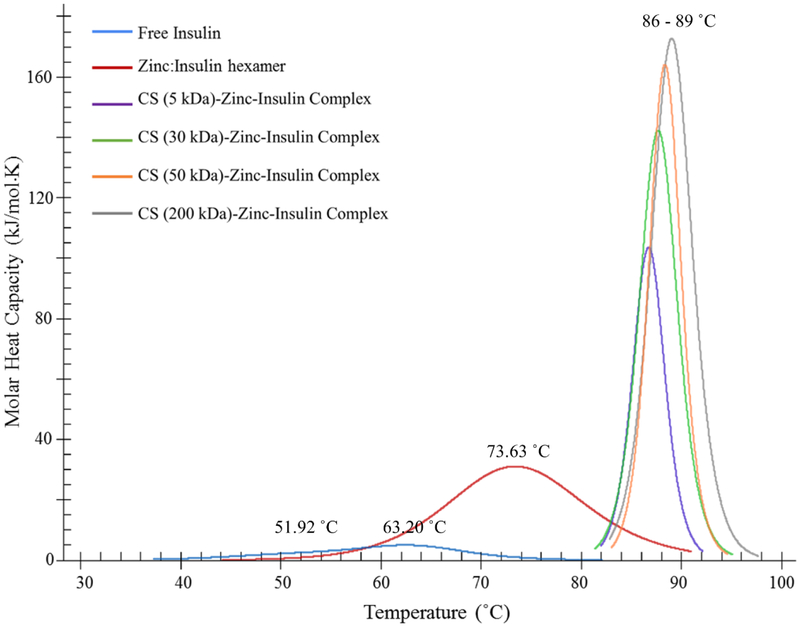
Thermo-stability and association state of insulin after addition of zinc and CS were investigated using nano DSC. Peaks in DSC thermogram represent transition midpoint temperature (Tm) of proteins. At this temperature, protein is half in its folded-stable state and half in its unfolded-denatured state. Higher Tm indicates higher stability of the protein. In the DSC thermogram obtained, biphasic denaturation was observed for free insulin with Tm1 ~51.92 °C and Tm2 ~63.20 °C suggesting presence of monomers and dimers, respectively (Figure 5). Further increase in Tm (~73.63 °C) was observed after addition of zinc to insulin due to hexamer formation. Furthermore, electrostatic complex formation between negatively charged zinc-insulin hexamers and positively charged CS resulted in a much higher Tm (86 – 89 °C) indicating increased thermostability of insulin when complexed with CS. Increasing chain length of CS demonstrated increasing Tm and transition enthalpy (ΔH) values indicating slightly higher thermal stability of insulin when complexed with longer chain length of CS. Tm and ΔH of free insulin, zinc-insulin hexamers and CS-zinc-insulin complexes prepared using different chain lengths of CS are summarized in table 2.
Figure 5.
Effect of addition of zinc and chitosan on thermal stability and association state of insulin determined using nano differential scanning calorimetry. [Insulin: 1 mg/mL; Zinc acetate: 1:5 molar ratio of insulin hexamer to zinc ions; Chitosan: 5 moles of CS monomer unit per mole of insulin monomer; in phosphate buffered saline (10 mM, pH 7.4)]
Table 2.
Midpoint transition temperature (Tm) and transition enthalpy (ΔH) of insulin, zinc-insulin hexamers and chitosan-zinc-insulin complexes prepared using different chain lengths of chitosan, in phosphate buffered saline at pH 7.4. Data are expressed as mean ± S.D, n = 3.
| Sample | Tm1 (°C) | Tm2 (°C) | ΔH (kJ/mol) |
|---|---|---|---|
| Insulin | 51.92 ± 0.21 | 63.20 ± 0.10 | 99.21 ± 0.34 |
| Zinc-Insulin hexamers | 73.63 ± 0.23 | - | 345.45 ± 0.51 |
| CS (5 kDa) –Zinc-Insulin complex | 86.73 ± 0.07 | - | 572.88 ± 4.33 |
| CS (30 kDa) –Zinc-Insulin complex | 87.69 ± 0.06 | - | 704.03 ± 1.12 |
| CS (50 kDa) –Zinc-Insulin complex | 88.36 ± 0.10 | - | 727.90 ± 0.97 |
| CS (200 kDa) –Zinc-Insulin complex | 89.04 ± 0.13 | - | 954.75 ± 3.69 |
3.3. Characterization of Binding Interaction using Isothermal Calorimetry
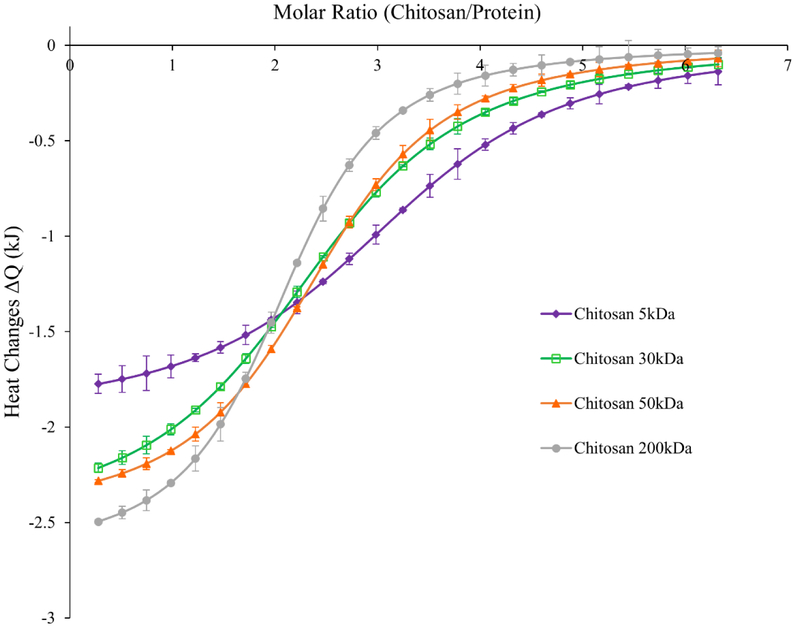
Electrostatic interactions between negatively charged zinc-insulin hexamers and positively charged CS are a major factor in maintaining the thermostability of insulin for a prolonged period. Isothermal analysis was employed to quantitatively assess binding affinity between zinc-insulin hexamers and increasing chain length of CS. Integrated net binding heat changes for the interaction using different CS chain lengths is shown in figure 6. Each injection of CS solution into zinc-insulin hexamer solution resulted in a sharp negative peak indicating an exothermic reaction. Subsequent injection of CS polymer into the cell resulted in decreased heat changes indicating gradual neutralization of zinc-insulin hexamers. Thermodynamic parameters of the studied interactions including binding constant (k), stoichiometry of binding (n), and enthalpy of complex formation (ΔH) are represented in table 3. The results suggest high binding affinity between longer chain length CS (200 kDa) and zinc-insulin hexamers indicated by higher binding constant (k) compared to shorter chain length CS (5 kDa). Consequently, this resulted in more exothermic heat changes (ΔH) from the interaction between longer chain length CS (200 kDa) and zinc-insulin hexamers compared to shorter chain lengths. This effect was similar to that observed using nano-DSC technique indicating higher transition enthalpy required for complexes prepared using longer chain length CS (200 kDa) (Table 2). This effect may be explained by slight increase in protonation constant (pKa) of CS with increasing chain length.14 Furthermore, interactions apart from electrostatic interactions between CS and zinc-insulin hexamers such as hydrophobic or non-specific interactions could be contributing to the binding between these molecules.22 Hence, different chain lengths of CS can have different effect on electrostatic interaction between CS and zinc-insulin hexamers which may ultimately play a role in determining the overall stability and release of insulin from such complexes.
Figure 6.
Integrated heats of interaction from calorimetric titrations of different chain lengths of chitosan into zinc-insulin hexamers in phosphate buffered saline (10 mM, pH 7.4).
Table 3.
Thermodynamic parameters of the binding interaction between different chain lengths of chitosan (titrant) and zinc-insulin hexamers (titrand) in 10 mM phosphate buffered saline at pH 7.4. Data are expressed as mean ± S.D, n = 3.
| Polymer | k × 107 (M−1) | n (mol) | ΔH (kJ mol−1) |
|---|---|---|---|
| Chitosan 5 kDa | 11.02 ± 0.11 | 2.74 ± 0.11 | −25.96 ± 0.88 |
| Chitosan 30 kDa | 16.87 ± 0.52 | 2.49 ± 0.03 | −33.10 ± 0.10 |
| Chitosan 50 kDa | 18.98 ± 0.38 | 2.47 ± 0.02 | −33.42 ± 0.16 |
| Chitosan 200 kDa | 23.25 ± 0.27 | 2.09 ± 0.04 | −35.88 ± 0.51 |
3.4. In Vitro Release Profile of Insulin
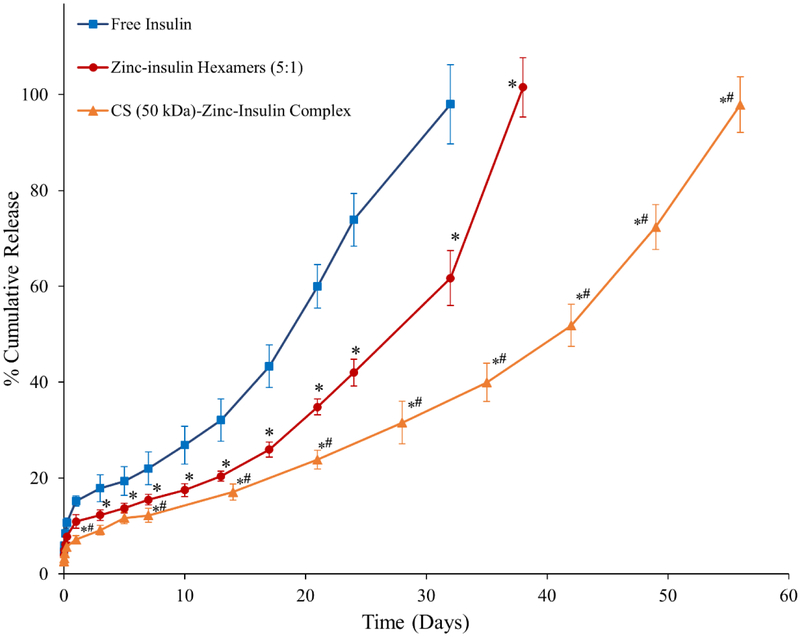
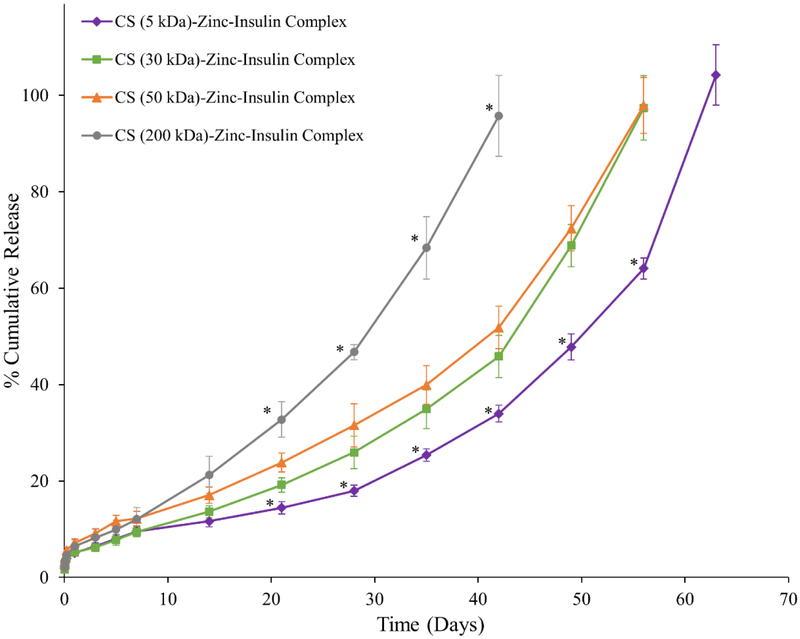
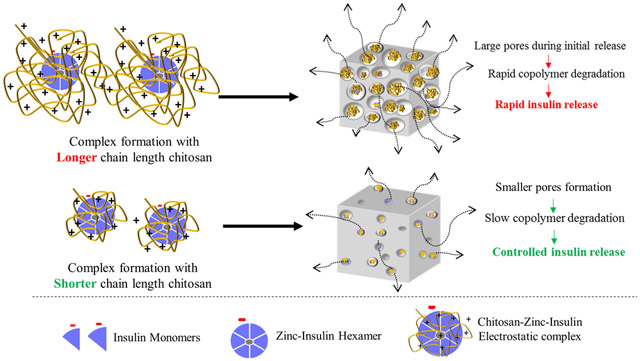
Cumulative release profile of insulin, zinc-insulin hexamers and CS (50 kDa)-zinc-insulin complexes incorporated in thermosensitive copolymer solution is shown in figure 7. Release through PLA/PEG based hydrogels can be affected by size and nature of the copolymer as well as the incorporated therapeutic. Initial burst release is a major concern of such delivery systems owing to the rapid release of therapeutic present on the depot surface. The release is further effected by a combination of diffusion through copolymer matrix and slow hydrolytic degradation of the copolymer.23 Addition of zinc and CS to free insulin significantly reduced initial burst release owing to the incorporation of less soluble zinc-insulin hexamers and CS-zinc-insulin complexes.24 Initially, the release from the copolymer is dominated by the slow diffusion of the incorporated therapeutic from the copolymeric matrix. Free insulin monomers were released at a faster rate compared to zinc-insulin hexamers and CS-zinc-insulin complexes. This was observed probably due to the restricted diffusion of larger zinc-insulin hexamers and complexes through the copolymer matrix. Formulation incorporating free insulin monomers and zinc-insulin hexamers also showed a high secondary burst release at 21 and 28 days, respectively. At this stage degradation of the hydrophobic PLA chains of the copolymer become dominant leading to a faster release.25 However, formulation containing CS showed low secondary burst release and released insulin at a controlled rate up to 60 days which is mainly due to the buffering action of CS. CS contains free NH2 groups which act like a “proton-sponge” resisting pH change in its microenvironment.26 This in turn lowers acid-catalyzed hydrolytic degradation of the copolymer further controlling the release of insulin for a longer duration. Promising improvement in stability and release profile of insulin upon formation of CS-zinc-insulin electrostatic complexes motivated us to investigate the effect of shorter and longer chain lengths of CS. CS chain length significantly affected the rate of insulin release (Figure 8) with largest (200 kDa) and smallest chain length (5 kDa) releasing insulin at the fastest and slowest rate, respectively, with best fit for zero-order release kinetics (r2 = 0.975 and 0.990, respectively). To further investigate the effect of different chain lengths of CS on the release profile of CS-zinc-insulin complexes through the copolymeric depot, hydrolytic degradation of the copolymer was studied by quantifying the percent mass loss and molecular weight of the copolymer remaining after regular intervals.
Figure 7.
Effect of addition of zinc and chitosan on in vitro release of insulin from 35% (w/v) PLA1500-PEG1500-PLA1500 copolymer, drug loading: 0.01% (w/v). Data is expressed as mean ± SD, n = 4. [Key: (■) free insulin, (●) zinc-insulin hexamers, (▲) chitosan (50 kDa)-zinc-insulin complex; *: significantly lower compared to free insulin; #: significantly lower compared to zinc-insulin hexamers; at p < 0.05]
Figure 8.
Effect of different chain lengths of chitosan on in vitro release of insulin from 35% (w/v) PLA1500-PEG1500-PLA1500 copolymer, drug loading: 0.01% (w/v). Data is expressed as mean ± SD, n = 4. [Key: (◆) chitosan (5 kDa)-zinc-insulin complex (■) chitosan (30 kDa)-zinc-insulin complex, (▲) chitosan (50 kDa)-zinc-insulin complex, , and (●) chitosan (200 kDa)-zinc-insulin complex; *: significantly different compared to chitosan (50 kDa)-zinc-insulin complex; at p < 0.05]
3.5. Hydrolytic Degradation Profile of Triblock Copolymer During In Vitro Release
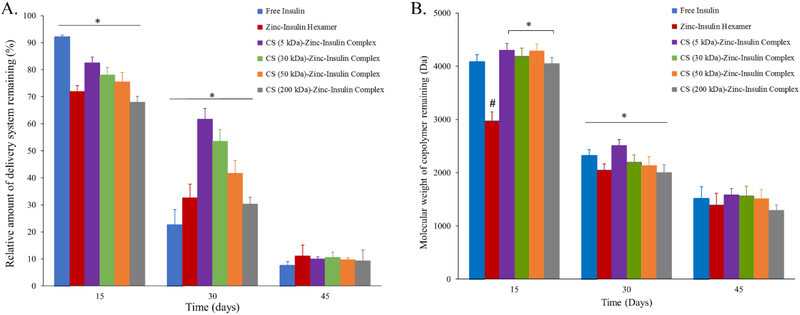
It has been well established that size and hydrophobicity of the incorporated molecule plays a major role in the degradation profile of PLA/PEG based copolymers consequently affecting release profile of the incorporated therapeutic.10,27 Therefore, effect of increasing chain length of CS on release profile of insulin via CS-zinc-insulin complexes incorporated in PLA1500-PEG1500-PLA1500 triblock copolymer was determined by comparing the degradation profile of copolymer between the different formulations. Quantification of the percent mass loss after hydrolytic degradation showed that thermosensitive copolymer incorporating CS-zinc-insulin complexes of higher chain length CS (200 kDa, large hydrophilic polymer) resulted in rapid mass loss of copolymer with time, implying that large sized complexes might have formed bigger pores and channels near the surface during initial release allowing rapid penetration of water molecules inside the gel, thus stimulating rapid degradation of copolymer (Figure 9) and consequently, higher rate of insulin release (Figure 8).27 However, thermosensitive copolymer incorporating CS-zinc-insulin complexes containing lower molecular weight CS (5 kDa) might have formed smaller CS-zinc-insulin complexes which resulted in comparatively smaller pore formation upon release and demonstrated slower degradation profile, evident from higher mass of copolymer remaining in the delivery system over time (Figure 9A). Simultaneously, copolymer molecular weight remaining after 30 days of in vitro release was significantly higher for copolymeric formulation containing smaller CS chain lengths (5 kDa) compared to longer chain lengths (Figure 9B). Furthermore, copolymer amount and molecular weight were found to decrease with increase in CS chain length indicating relationship between size of CS-zinc-insulin complexes formed and rate of hydrolytic degradation following initial release.
Figure 9.
(A) Relative amount of delivery system remaining, and (B) Molecular weight of copolymer remaining, after hydrolytic degradation during in vitro release. Data expressed as mean ± SD, n = 4. [*: significantly different; #: significantly different from the rest; at p < 0.05]
3.6. Stability of Insulin Released from Delivery System
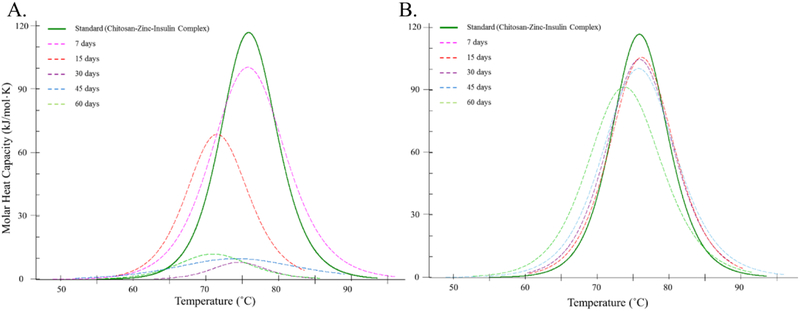
Insulin comprises of higher order structures to elicit biological response and any alteration in its primary, secondary, tertiary or quaternary structure greatly influences its activity.28,29 Hence, insulin aggregation is an important issue and major delimiting factor in preparation of long-term use therapies.29,30 Physicochemical characterization, such as determination of association state, folding/unfolding temperature and presence of secondary structural components (α helix, β sheets, and random coils) determined using nano-DSC and circular dichroism (CD) spectroscopy, respectively, can be used as a measure to assess in vitro thermal and conformational stability of insulin and other such proteins/peptide based therapeutics.4,5,31-33 DSC thermogram of samples of insulin released from in vitro depot-based release model demonstrated good thermostability of insulin over time. Tm values indicated that insulin is initially released from the delivery system in the form of CS-zinc-insulin complexes which later dissociated to zinc-insulin hexamers upon dilution (Figure 10A).4,5,34,35
Figure 10.
Nano-differential scanning calorimetry fitted thermogram of (A) Insulin released in vitro after 7, 15, 30, 45 and 60 days at 37 °C, and (B) Insulin extracted after 7, 15, 30, 45 and 60 days from copolymer formulation stored at 4 °C.
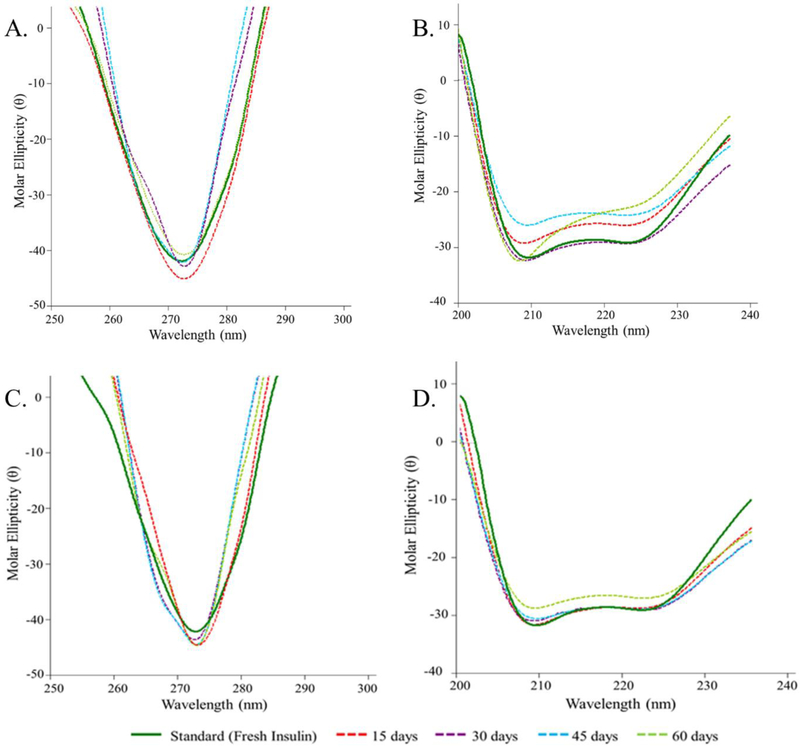
CD spectroscopy relies on the differential absorption of left and right circularly polarized light by insulin molecule. CD spectrum in the far UV region (250 – 200 nm) corresponds to peptide bond absorption which provides an estimation of secondary structural features of insulin.36 Standard insulin (freshly prepared solution) showed two minima at ~208 and 225 nm indicating presence of α helices (~43%) and β sheets (8%) (Table 4). Secondary structure of insulin released up to 60 days closely resembled its native structure with only slight attenuation of α helices (~35%) and β sheets (6%) confirming the ability of CS-zinc-insulin complexes in preserving conformational stability of insulin (Figure 11B). CD signal in the near UV region (300 – 250 nm) indicates the environment of aromatic amino acid side chains (phenylalanine, tryptophan, tyrosine) and provides an estimate of tertiary structure of a protein.36 Standard insulin showed a minima at ~273 nm representing tertiary structure of insulin (Figure 11A). No changes in the CD signal was observed up to 60 days indicating stable tertiary structure of insulin for the entire duration of release. These studies are coherent with previous studies from our lab performed using HPLC, MALDI-TOF mass spectrometry, DSC and CD spectroscopy demonstrating the ability of zinc and CS to protect chemical and conformational stability of insulin for a prolonged duration and consequently preserving the activity of insulin as demonstrated by the in vivo studies.4,5,15,31,37,38
Table 4.
Secondary structure estimation of insulin. Data expressed as mean ± SD, n = 4.
| Sample | Days | α Helix | β Sheets | β Turns | Random Coils |
|---|---|---|---|---|---|
| Standard (Fresh Insulin in PBS) | - | 42.3±5.1 | 7.5±2.7 | 24.9±3.6 | 25.3±1.1 |
| Insulin released in vitro from thermosensitive copolymer formulation incorporating chitosan (5 kDa)-zinc-insulin complexes at 37°C. | 15 | 48.8±2.7 | 8.3±1.0 | 22.6±4.2 | 20.3±4.8 |
| 30 | 47.5±3.9 | 7.9±7.1 | 23.4±3.5 | 21.1±6.4 | |
| 45 | 38.3±1.8 | 7.5±3.7 | 26.6±3.7 | 27.6±5.0 | |
| 60 | 34.9±1.9 | 5.9±1.6 | 27.3±2.0 | 31.9±1.4 | |
| Insulin extracted from thermosensitive copolymer formulation incorporating chitosan (5 kDa)-zinc-insulin complexes stored at 4°C. | 15 | 55.3±1.3 | 11.0±2.1 | 22.0±1.2 | 11.7±3.3 |
| 30 | 52.9±6.7 | 6.5±2.7 | 24.3±2.3 | 16.3±1.3 | |
| 45 | 54.3±4.2 | 6.0±1.2 | 24.0±1.0 | 15.8±2.9 | |
| 60 | 48.6±7.1 | 12.1±6.1 | 23.7±1.8 | 15.6±3.7 |
Figure 11.
(A) Near-UV and (B) Far-UV, circular dichroism spectrum of insulin released in vitro at 15, 30, 45 and 60 days. (C) Near-UV and (D) Far-UV circular dichroism spectrum of insulin extracted after 15, 30, 45 and 60 days from copolymer formulation stored at 4 °C.
3.7. Storage Stability of Insulin in Formulation
In amphiphilic copolymer PLA1500-PEG1500-PLA1500, the PEG chains orient themselves to form the outer hydrophilic shell of the micelles. This PEG layer facing the external aqueous environment reduces interactions of the incorporated therapeutic with foreign molecules resulting in increased stability and shelf life of such systems.19,39 Additionally, electrostatic complex formation between zinc-insulin hexamers and CS substantially increases the stability of insulin.4,31 In this study, insulin extracted from stored copolymer formulations analyzed using nano-DSC showed the presence of stable CS-zinc-insulin complex up to 60 days (Figure 10B). Additionally, tertiary and secondary structure of the extracted insulin was preserved during the entire duration of storage as determined using CD spectroscopy (Figure 11C, 11D).
3.8. In Vitro Biocompatibility of Triblock Copolymer
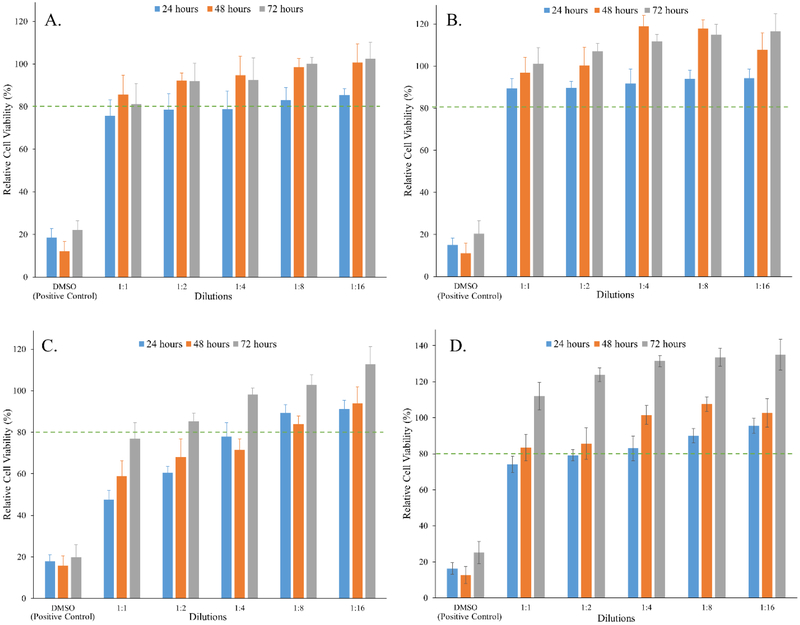
Biocompatibility is a complex phenomenon that identifies the interaction between implantable delivery system and the host tissue. Copolymer constituting PLA and PEG are inherently biodegradable and biocompatible owing to their spontaneous degradation into non-toxic products like lactic acid and glycolic acid which are naturally eliminated from the body.6,7,40 Additionally, PEG outer shell of such copolymeric micelles make them non-immunogenic.41 Furthermore, PLA-PEG-PLA copolymer shows pliability and mucomimetic characteristics which helps in minimizing mechanical irritation from such controlled release depots.42,43 CS is a biodegradable cationic copolymer derived from alkaline partial deacetylation of chitin. It is a linear polysaccharide consisting of randomly distributed β-(1, 4)-linked D-glucosamine and N-acetyl-D-glucosamine units. CS can be degraded by enzymes (chitinases) which hydrolyze glucosamine–glucosamine, glucosamine–N-acetyl-glucosamine and N-acetyl-glucosamine–N-acetyl-glucosamine linkages.44 The aim of this study was to assess the short- and long-term cytotoxic potential of residual degradation products of PLA1500-PEG1500-PLA1500 and CS polymer in vitro in HEK 293 cell line (ATCC, Rockville, MD, U.S.A.) by MTT cell viability assay. MTT assay is a colorimetric assay that quantifies the reduction of yellow MTT dye by mitochondrial succinate dehydrogenase enzyme present in metabolically active cells to insoluble purple formazan crystals. The formazan crystals formed are then solubilized using dimethyl sulfoxide (DMSO) and absorbance is measured spectrophotometrically at 570 nm wavelength. Only metabolically active cells have the ability to reduce MTT, therefore this activity can be taken as a marker of viable cells.26 HEK 293 human cell lineage is extensively used in cell biology and biotechnology. We chose this cell line as a model to support cyto-compatibility of our copolymeric formulation.45,46 Copolymeric degradation products with and without CS extracted at 37 °C showed concentration-dependent decrease of formazan production, with the most concentrated dilution (1:1 dilution) demonstrating ~75 and 90% relative cell viability, respectively, post 24 h of incubation (Figure 12A, 12B). Relative cell viability was found to increase with increasing dilution of the extracts. Furthermore, upon incubation for 48 – 72 h no significant difference (p < 0.05) was observed between the relative cell viability of copolymeric extracts compared to control (growth medium only). Similar effect was observed while studying long-term suitability of our delivery system tested by extracting degradation products with and without CS upon incubation at 70 °C temperature (Figure 12C, 12D). With increasing duration of incubation period at higher extract dilution the relative cell viability of HEK 293 cells demonstrated good cyto-compatibility compared to control (growth medium only). On the contrary, cell viability higher than control was observed upon longer incubation period with the copolymer extract, which could be possibly attributed to the increased energy metabolism and mitochondrial activity owing to an increased supply of copolymer degradation products which are consumed by the cells via the citric acid cycle.47 It was also observed that HEK-293 cells grow better in the presence of CS which can be attributed to the positive effect of CS on cell proliferation and attachment.48 CS with high degree of deacetylation has higher number of free primary amino groups promoting its adhesion to negatively charged cell surfaces owing to electrostatic interactions. This in turn also helps localize growth factors near the cell surface thereby promoting cell proliferation.48,49 Therefore, it can be concluded that copolymeric formulation with and without CS were relatively safe and showed minimal cytotoxicity.
Figure 12.
Graphical representation of in vitro cyto-compatibility of delivery systems depicted by percent relative cell viability after 24, 48 and 72 h at different dilutions of copolymer degradation products extracted by incubating copolymer formulation (A) without chitosan for 10 days at 37 °C, (B) with chitosan for 10 days at 37 °C, (C) without chitosan for 10 days at 70 °C, and (D) with chitosan for 10 days at 70 °C. Biocompatibility was evaluated in HEK-293 cell line using MTT assay. Data expressed as mean ± SD, n = 4.
4. Conclusions
Chitosan-zinc-insulin electrostatic complexes incorporated in thermosensitive triblock copolymer were prepared, characterized and optimized in this study for releasing insulin at a controlled rate in a stable form for a prolonged duration. Effect of increasing chain length of chitosan on the stability and release profile of chitosan-zinc-insulin complexes was studied in detail. Chitosan chain length was found to significantly affect both thermal stability and release of insulin from thermosensitive copolymeric depot-based delivery system. It was observed that chitosan-zinc-insulin complex formation using shorter chitosan chain lengths (5 kDa) released insulin at a controlled rate for up to two months, which could potentially replace 60 – 120 regular long-acting insulin injections. Complex formation with chitosan also preserved the conformational stability of insulin and protected insulin from aggregation/denaturation during the entire period of release and storage. Additionally, this formulation was found to be easily injectable, biodegradable, and biocompatible, which would help achieve improved patient compliance owing to low frequency of administration and thereby reduced injection site pain, inflammation and damage. Overall, the results exemplify CS (5 kDa)-zinc-insulin complexes incorporated into a thermosensitive copolymeric system as a promising alternative to conventional daily basal insulin therapy for efficiently managing blood glucose levels and prevention of diabetes complications.
Scheme 1.
Synthesis of triblock copolymer poly(D,L-lactide)-poly(ethylene glycol)-poly(D,L-lactide) (PLA-PEG-PLA) via ring opening polymerization of D,L-lactide with poly(ethylene glycol) catalyzed by stannous octoate. (Inset: Reaction setup)
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (NIH) grant# R15GM114701.
ABBREVIATIONS
- DM
diabetes mellitus
- PLA
poly(D,L-lactide)
- PEG
poly(ethylene glycol)
- CS
chitosan
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- DSC
differential scanning calorimetry
- ITC
isothermal calorimetry
- CD
circular dichroism
- UV
ultraviolet
- Tm
Midpoint transition temperature
- ΔH
transition enthalpy
- HEK 293
human embryonic kidney cell line
- 1H NMR
proton nuclear magnetic resonance
- GPC
gel permeation chromatography
- THF
tetrahydrofuran
- PDI
polydispersity index
- PBS
phosphate buffer saline
- DMSO
dimethyl sulfoxide
- DMEM
Dulbecco’s modified Eagle’s medium
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Wajngot A, Chandramouli V, Schumann WC, Ekberg K, Jones PK, Efendic S and Landau BR: ‘Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus’, Metabolism., 2001, 50, 47–52. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Dall TM, Beronjia K, Lin J, Semilla AP, Chakrabarti R, Hogan PF and Petersen MP: ‘Economic costs of diabetes in the U.S.’, Diabetes Care, 2017, 41, 917–928. [Google Scholar]
- 3.Zhang P and Gregg E: ‘Global economic burden of diabetes and its implications.’, lancet. Diabetes Endocrinol., 2017, 5, 404–405. [DOI] [PubMed] [Google Scholar]
- 4.Oak M and Singh J: ‘Chitosan-zinc-insulin complex incorporated thermosensitive polymer for controlled delivery of basal insulin in vivo’, J. Control. Release, 2012, 163, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oak M and Singh J: ‘Controlled delivery of basal level of insulin from chitosan-zinc-insulin-complex-loaded thermosensitive copolymer’, J. Pharm. Sci, 2012, 101, 1079–1096. [DOI] [PubMed] [Google Scholar]
- 6.Mei T, Zhu Y, Ma T, He T, Li L, Wei C and Xu K: ‘Synthesis, characterization, and biocompatibility of alternating block polyurethanes based on PLA and PEG’, J. Biomed. Mater. Res. - Part A, 2014, 102, 3243–3254. [DOI] [PubMed] [Google Scholar]
- 7.Kucharczyk P, Pavelková A, Stloukal P and Sedlarík V: ‘Degradation behaviour of PLA-based polyesterurethanes under abiotic and biotic environments’, Polym. Degrad. Stab, 2016, 129, 222–230. [Google Scholar]
- 8.Jeong B, Bae YH and Kim SW: ‘Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers.’, J. Control. Release, 2000, 63, 155–63. [DOI] [PubMed] [Google Scholar]
- 9.Jeong B, Bae YH, Lee DS and Kim SW: ‘Biodegradable block copolymers as injectable drug-delivery systems’, Nature, 1997, 388, 860–862. [DOI] [PubMed] [Google Scholar]
- 10.Sandor M, Enscore D, Weston P and Mathiowitz E: ‘Effect of protein molecular weight on release from micron-sized PLGA microspheres’, J. Control. Release, 2001, 76, 297–311. [DOI] [PubMed] [Google Scholar]
- 11.Dunn MF: ‘Zinc-ligand interactions modulate assembly and stability of the insulin hexamer - A review’, BioMetals, 2005, 18, 295–303. [DOI] [PubMed] [Google Scholar]
- 12.Wintersteiner O and Abramson A: ‘Isoelectric Point of Insulin’, J. Biol. Chem, 1933, 99, 741–753. [Google Scholar]
- 13.Lee DW, Lim C, Israelachvili JN and Hwang DS: ‘Strong adhesion and cohesion of chitosan in aqueous solutions’, Langmuir, 2013, 29, 14222–14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang QZ, Chen XG, Liu N, Wang SX, Liu CS, Meng XH and Liu CG: ‘Protonation constants of chitosan with different molecular weight and degree of deacetylation’, Carbohydr. Polym, 2006, 65, 194–201. [Google Scholar]
- 15.Al‐Tahami K, Oak M, Mandke R and Singh J: ‘Basal level insulin delivery: In vitro release, stability, biocompatibility, and in vivo absorption from thermosensitive triblock copolymers’, J. Pharm. Sci, 2011, 100, 4790–4803. [DOI] [PubMed] [Google Scholar]
- 16.Hayton WL, Chen T and William TC Hayton L: ‘Correction of Perfusate Concentration for Sample Removal’, J. Pharm. Sci, 1982, 71, 820–821. [DOI] [PubMed] [Google Scholar]
- 17.Lyu S and Untereker D: ‘Degradability of polymers for implantable biomedical devices.’, Int. J. Mol. Sci, 2009, 10, 4033–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felfel RM, Poocza L, Gimeno-Fabra M, Milde T, Hildebrand G, Ahmed I, Scotchford C, Sottile V, Grant DM and Liefeith K: ‘In vitro degradation and mechanical properties of PLA-PCL copolymer unit cell scaffolds generated by two-photon polymerization’, Biomed. Mater., 2016, 11, 015011. [DOI] [PubMed] [Google Scholar]
- 19.Ulery BD, Nair LS and Laurencin CT: ‘Biomedical applications of biodegradable polymers’, J. Polym. Sci. Part B Polym. Phys, 2011, 49, 832–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oak M and Singh J: ‘Controlled delivery of basal level of insulin from chitosan-zinc-insulin-complex-loaded thermosensitive copolymer.’, J. Pharm. Sci, 2012, 101, 1079–96. [DOI] [PubMed] [Google Scholar]
- 21.Jeong B, Han Bae Y and Wan Kim S: ‘Biodegradable thermosensitive micelles of PEG-PLGA-PEG triblock copolymers’, Colloids Surfaces B Biointerfaces, 1999, 16, 185–193. [Google Scholar]
- 22.Azevedo JR, Sizilio RH, Brito MB, Costa AMB, Serafini MR, Araújo AAS, Santos MRV, Lira AAM and Nunes RS: ‘Physical and chemical characterization insulin-loaded chitosan-TPP nanoparticles’, J. Therm. Anal. Calorim, 2011, 106, 685–689. [Google Scholar]
- 23.Jeong B, Bae YH and Kim SW: ‘Drug release from biodegradable injectable thermosensitive hydrogel of PEG – PLGA – PEG triblock copolymers’, J. Control. Release, 2000, 63, 155–163. [DOI] [PubMed] [Google Scholar]
- 24.Li YV: ‘Zinc and insulin in pancreatic beta-cells’, Endocrine, 2014, 45, 178–189. [DOI] [PubMed] [Google Scholar]
- 25.Al-Tahami K, Oak M, Mandke R and Singh J: ‘Basal level insulin delivery: In vitro release, stability, biocompatibility, and in vivo absorption from thermosensitive triblock copolymers’, J. Pharm. Sci, 2011, 100, 4790–4803. [DOI] [PubMed] [Google Scholar]
- 26.Sharma D and Singh J: ‘Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymer and Their Nanomicelles for Nonviral Gene Delivery Applications’, Bioconjug. Chem, 2017, 28, 2772–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oak M, Mandke R, Lakkadwala S, Lipp L and Singh J: ‘Effect of molar mass and water solubility of incorporated molecules on the degradation profile of the triblock copolymer delivery system’, Polymers (Basel)., 2015, 7, 1510–1521. [Google Scholar]
- 28.Hua QX, Jia W and Weiss MA: ‘Conformational dynamics of insulin’, Front. Endocrinol. (Lausanne)., 2011, 2, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brange J and Langkjoer L: ‘Insulin structure and stability.’, Pharm. Biotechnol, 1993, 5, 315–50. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Yan Y, Seeman D, Sun L and Dubin PL: ‘Multimerization and aggregation of native-state insulin: Effect of zinc’, Langmuir, 2012, 28, 579–586. [DOI] [PubMed] [Google Scholar]
- 31.Manoharan C and Singh J: ‘Evaluation of polyanhydride microspheres for basal insulin delivery: Effect of copolymer composition and zinc salt on encapsulation, in vitro release, stability, in vivo absorption and bioactivity in diabetic rats’, J. Pharm. Sci, 2009, 98, 4237–4250. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y and Singh J: ‘Thermosensitive drug delivery system of salmon calcitonin: In vitro release, in vivo absorption, bioactivity and therapeutic efficacies’, Pharm. Res, 2010, 27, 272–284. [DOI] [PubMed] [Google Scholar]
- 33.Tang Y and Singh J: ‘Biodegradable and biocompatible thermosensitive polymer based injectable implant for controlled release of protein’, Int. J. Pharm, 2009, 365, 34–43. [DOI] [PubMed] [Google Scholar]
- 34.Gliemann J and Gammeltoft S: ‘The Biological Activity and the Binding Affinity of Modified Insulins Determined on Isolated Rat Fat Cells’, Diabetologia, 1974, 10, 105–113. [DOI] [PubMed] [Google Scholar]
- 35.Ellis MJ, Darby SC, Jones RH and Sonksen PH: ‘In Vitro Bioactivity of Insulin Analogues: Lipogenic and Anti-Lipolytic Potency and Their Interaction with the Effect of Native Insulin’, Diabetologia, 1978, 15, 403–410. [DOI] [PubMed] [Google Scholar]
- 36.Kelly SM and Price NC: ‘The Use of Circular Dichroism in the Investigation of Protein Structure and Function’, Curr. Protein Pept. Sci, 2000, 1, 349–384. [DOI] [PubMed] [Google Scholar]
- 37.Manoharan C and Singh J: ‘Addition of Zinc Improves the Physical Stability of Insulin in the Primary Emulsification Step of the Poly(lactide-co-glycolide) Microsphere Preparation Process’, Polymers (Basel)., 2015, 7, 836–850. [Google Scholar]
- 38.Manoharan C and Singh J: ‘Insulin loaded PLGA microspheres: Effect of zinc salts on encapsulation, release, and stability’, J. Pharm. Sci, 2009, 98, 529–542. [DOI] [PubMed] [Google Scholar]
- 39.Makadia HK and Siegel SJ: ‘Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier’, Polymers (Basel)., 2011, 3, 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsawy MA, Kim KH, Park JW and Deep A: ‘Hydrolytic degradation of polylactic acid (PLA) and its composites’, Renew. Sustain. Energy Rev, 2017, 79, 1346–1352. [Google Scholar]
- 41.Veronese FM and Pasut G: ‘PEGylation, successful approach’, 2005, 10, 1451–1458. [DOI] [PubMed] [Google Scholar]
- 42.Wake MC, Gupta PK and Mikos AG: ‘Fabrication of pliable biodegradable polymer foams to engineer soft tissues.’, Cell Transplant., 1996, 5, 465–73. [DOI] [PubMed] [Google Scholar]
- 43.Bonacucina G, Cespi M, Mencarelli G, Giorgioni G and Palmieri GF: ‘Thermosensitive self-assembling block copolymers as drug delivery systems’, Polymers (Basel)., 2011, 3, 779–811. [Google Scholar]
- 44.Kean T and Thanou M: ‘Biodegradation, biodistribution and toxicity of chitosan’, Adv. Drug Deliv. Rev, 2010, 62, 3–11. [DOI] [PubMed] [Google Scholar]
- 45.Basarkar A and Singh J: ‘Poly (lactide-co-glycolide)-polymethacrylate nanoparticles for intramuscular delivery of plasmid encoding interleukin-10 to prevent autoimmune diabetes in mice’, Pharm. Res, 2009, 26, 72–81. [DOI] [PubMed] [Google Scholar]
- 46.Werner ME, Cummings ND, Sethi M, Wang EC, Sukumar R, Moore DT and Wang AZ: ‘Preclinical evaluation of genexol-pm, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer’, Int. J. Radiat. Oncol. Biol. Phys, 2013, 86, 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ignatius AAA and Claes LEE: ‘In vitro biocompatibility of bioresorbable polymers: poly(L, DL-lactide) and poly(L-lactide-co-glycolide)’, Biomaterials, 1996, 17, 831–839. [DOI] [PubMed] [Google Scholar]
- 48.Teng D: in Chitosan-Based Hydrogels: Functions and Applications, eds. Yao K, Li J, Yao F and Yin Y, CRC Press, 2017, p. 521. [Google Scholar]
- 49.Kweon D-K, Song S-B and Park Y-Y: ‘Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator’, Biomaterials, 2003, 24, 1595–1601. [DOI] [PubMed] [Google Scholar]