Abstract
Background
Angiotensin-converting enzyme 2 (ACE2) is an endogenous counter-regulator of the renin–angiotensin hormonal cascade. We assessed whether plasma ACE2 concentrations were associated with greater risk of death or cardiovascular disease events.
Methods
We used data from the Prospective Urban Rural Epidemiology (PURE) prospective study to conduct a case-cohort analysis within a subset of PURE participants (from 14 countries across five continents: Africa, Asia, Europe, North America, and South America). We measured plasma concentrations of ACE2 and assessed potential determinants of plasma ACE2 levels as well as the association of ACE2 with cardiovascular events.
Findings
We included 10 753 PURE participants in our study. Increased concentration of plasma ACE2 was associated with increased risk of total deaths (hazard ratio [HR] 1·35 per 1 SD increase [95% CI 1·29–1·43]) with similar increases in cardiovascular and non-cardiovascular deaths. Plasma ACE2 concentration was also associated with higher risk of incident heart failure (HR 1·27 per 1 SD increase [1·10–1·46]), myocardial infarction (HR 1·23 per 1 SD increase [1·13–1·33]), stroke (HR 1·21 per 1 SD increase [1·10–1·32]) and diabetes (HR 1·44 per 1 SD increase [1·36–1·52]). These findings were independent of age, sex, ancestry, and traditional cardiac risk factors. With the exception of incident heart failure events, the independent relationship of ACE2 with the clinical endpoints, including death, remained robust after adjustment for BNP. The highest-ranked determinants of ACE2 concentrations were sex, geographic ancestry, and body-mass index (BMI). When compared with clinical risk factors (smoking, diabetes, blood pressure, lipids, and BMI), ACE2 was the highest ranked predictor of death, and superseded several risk factors as a predictor of heart failure, stroke, and myocardial infarction.
Interpretation
Increased plasma ACE2 concentration was associated with increased risk of major cardiovascular events in a global study.
Funding
Canadian Institute of Health Research, Heart & Stroke Foundation of Canada, and Bayer.
Introduction
The renin–angiotensin system is a hormonal cascade whose modulation has resulted in several effective cardiovascular disease therapeutics. Decades of research and clinical practice have focused on the pressor arm of renin–angiotensin system. Angiotensin-converting enzyme (ACE) cleaves angiotensin I to angiotensin II, which acts on the type 1 angiotensin II receptor. Recent evidence has also shed light on an important counterbalancing component of the renin–angiotensin system axis, through the action of ACE2. In brief, ACE2 cleaves angiotensin II into the heptapeptide angiotensin 1–7, which acts on the Mas receptor pathway, which is widely believed to exert protective effects, including vasodilation and inhibition of fibrosis.1, 2, 3 There is a global effort to better understand ACE2, the receptor via which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the betacoronavirus responsible for COVID-19, enters cells.4, 5, 6 ACE2 is a known regulator of cardiac function and dysregulation of this system is worth further examination, particularly given that a considerable proportion of individuals who are admitted to hospital for COVID-19 exhibit signs of cardiac damage with subsequent poor prognosis.7 Small clinical studies suggest that increased circulating ACE2 activity and concentration might serve as a marker of poor prognosis in individuals with various cardiovascular diseases, but no study has provided data in a large cohort drawn from the general population.8, 9, 10, 11, 12 The Prospective Urban Rural Epidemiology (PURE) study provides an opportunity to examine the association between ACE2 levels with future cardiovascular disease events and deaths in a prospective global community-based cohort. In this study, we aim to 1) understand the role of demographic and clinical characteristics as potential determinants of plasma ACE2 concentration; (2) describe the association of plasma ACE2 as a risk marker for cardiovascular disease and death; and (3) describe the relative importance of plasma ACE2 as a risk marker for cardiovascular disease events and deaths compared with established cardiovascular disease risk factors.
Research in context.
Evidence before this study
We performed a systematic search of MEDLINE for relevant articles published between Jan 1, 2000 (the year of discovery of angiotensin-converting enzyme 2 [ACE2]), and May 12, 2020, restricted to the English language. Our search terms included “ACE2”, “cardiovascular disease,” “genome wide association study”, and “Mendelian randomization”. We searched published articles by title and abstract to identify relevant studies and additionally hand searched reference lists of eligible studies. We considered studies that assessed the relationship between plasma ACE2 concentration and cardiovascular disease. Although the search does not represent an exhaustive list of all available research, existing evidence from small clinical studies suggest there is an association of increased ACE2 in the plasma and poorer cardiovascular disease outcomes in those with pre-existing disease. There are no robust data on the importance of plasma ACE2 in general populations.
Added value of this study
This study provides the largest epidemiological analysis of the circulating biomarker ACE2 in a general population. 10 753 people were analysed from a large global cohort using a nested case-cohort design. Our study population includes participants from 14 countries and seven distinct ancestral groups over 9·4 years of follow-up. We find that increased circulating ACE2 is strongly associated with increased risk of death, cardiovascular disease, and diabetes. Notably, circulating ACE2 is the highest-ranked predictor of death when compared among a set of clinical risk factors (smoking, diabetes, blood pressure, lipids, and body-mass index [BMI]) and supersedes other common risk factors as a predictor of myocardial infarction, stroke, and heart failure risk. With the exception of incident heart failure events, the independent relationship of ACE2 with clinical endpoints remained robust even after adjustment for brain natriuretic protein (BNP). ACE2 levels are higher in men, older people, those with a smoking history, diabetes, higher BMI, higher blood pressure, and higher blood lipids. There are also wide variations in concentration across ancestral groups (with south Asians having the lowest levels of plasma ACE2 and east Asians having the highest levels in our sample). Plasma ACE2 is a heritable trait and our examination of common genetic variants through a genome-wide association study uncovered two loci at genome-wide significance. One locus was near the ACE2 gene and the other was near the HNF1A gene, which previous literature suggests induces higher cellular ACE2 expression levels in pancreatic islet cells. We also provide evidence that plasma ACE2 has important metabolic implications as evidenced by its relationship with BMI and association with incident diabetes.
Implications of all the available evidence
Plasma ACE2 is strongly associated with death, cardiovascular disease, and metabolic abnormalities in a multiancestral global cohort drawn from the general population. The relationship of this non-canonical marker of hormonal dysregulation with cardiovascular events and death, independent of traditional cardiac risk factors and BNP, suggests that understanding and modulating this arm of the renin–angiotensin system might lead to new approaches to reducing cardiovascular disease.
Methods
Study design and participants
PURE is a large prospective study of individuals in 27 low-income, middle-income, and high-income countries (appendix p 2). Participant recruitment and selection is described in the appendix (p 2) and has been described in detail in previous papers.13 A biobanking initiative was developed for a subset of PURE participants to assess genomic and proteomic markers of chronic disease risk. Blood samples from participants were shipped from 14 countries (ie, Argentina, Bangladesh, Brazil, Canada, Chile, Colombia, Iran, Pakistan, Philippines, South Africa, Sweden, Tanzania, United Arab Emirates, and Zimbabwe) to the Population Health Research Institute (Hamilton, ON, Canada) and stored at −165°C. Samples were considered eligible if they belonged to individuals from the major self-reported ethnicity in the residing country (eg, European ancestry in Sweden). Samples were deemed ineligible if they were unsuitable for analysis or were non-fasting.
Briefly, we took a random sample from the pool of 55 246 eligible participants. This random sample is known as the subcohort. Because it is a random sample of the pool of eligible participants, it will include some participants with incident events of interest. We then also include all individuals who have incident events of interest that were not selected as part of the subcohort for analyses. The final sample consists of participants who were members of the subcohort and those who had incident events outside the subcohort (appendix p 9). Our outcome events of interest included death, myocardial infarction, stroke, heart failure, and diabetes. This study design permits cost-effective, unbiased assessment of the exposure–outcome relationship of the original cohort from which it was sampled. Details on case-cohort sampling methods used for PURE and the participant flow diagram are in the appendix (p 7).
The study was approved by research ethics committees at each study centre and at Hamilton Health Sciences (Hamilton, ON, Canada). All study participants provided written informed consent.
Protein measurement
Plasma concentration was measured using an immunoassay based on proximity extension assay technology (Olink PEA CVD-II panel; Uppsala, Sweden).14 A 1·8 mL aliquot of plasma from each PURE participant was transported to the Clinical Research Laboratory and Biobank in Hamilton, ON, Canada. Data generated are expressed as relative quantification on the log2 scale of normalised protein expression (NPX) values. Although NPX values are relative quantification units, the OLink platform has been extensively validated and previous work shows strong relationships between measurements from the multiplex OLink panel and singleplex assays of the same markers with absolute units.8, 14 Individual samples were excluded on the basis of quality controls for immunoassay and detection, as well as degree of haemolysis (appendix p 19). NPX values were rank-based normal transformed for further analyses.
Genotyping and genetic analysis
PURE participants suitable for proteomics analyses were genotyped on the Thermofisher Axiom Precision Medicine Research Array (appendix p 20). To assess the robustness of the clinical determinants of ACE2 emerging from cross-sectional analyses, we did two-sample Mendelian randomisation, a causal inference technique using genetic variants to approximate effects of an exposure (ie, clinical risk factors) on an outcome (ie, ACE2 levels). If an exposure is causally related to an outcome, genetic variants associated with the exposure should affect the outcome in a manner directionally consistent and proportional to the effect size on the exposure.15, 16 Well conducted Mendelian randomisation analyses yield estimates that are robust to residual confounding and reverse causation.17 To account for differences in genetic architecture that exist between ancestral groups, genetic analyses were done within each group and meta-analysed to obtain a final estimate (appendix p 21). Ancestral groups with less than 1000 individuals per group were excluded from genetic analysis to ensure stability of individual ancestral estimates (appendix p 21). We applied this same Mendelian randomisation approach to assess whether anti-hypertensive therapies (ACE inhibitors, calcium channel blockers, and β blockers) influenced circulating ACE2 levels. More details on the genetic analysis are in the appendix (p 20).
Statistical analysis
Means and SDs for continuous variables and numbers with proportions for baseline characteristics are presented for the subcohort and for each event outside the subcohort. A descriptive analysis was done using an ordinary least-squares regression with plasma ACE2 concentration as the outcome in a cross-sectional analysis at baseline. Mutually adjusted effects of the following independent variables on ACE2 levels were presented: age (in years), sex (male vs female), diabetes (yes vs no), smoking (never smoker vs current or former smoker), body-mass index (BMI; kg/m2), systolic blood pressure (mm Hg), LDL cholesterol (mmol/L), and geographic ancestry (African, Arab, east Asian, European, Latin, Persian, or south Asian ancestry). Each predictor was ranked on the basis of the magnitude of their likelihood ratio χ2 value comparing the full model to the reduced model without that predictor.18
Association of anti-hypertensive therapies (ACE inhibitors, calcium channel blockers, β blockers, diuretics, and angiotensin receptor blockers) on plasma ACE2 was assessed in an ordinary least-squares regression where each medication was dummy coded. Effects were mutually adjusted and additionally adjusted for age, sex, BMI, smoking, diabetes, adjusted blood pressure, and geographic ancestry in individuals with hypertension.
Modelling of cardiovascular events was done to account for oversampling because of the case-cohort design (appendix p 18). The following incident outcomes were analysed: total deaths, cardiovascular deaths, non-cardiovascular deaths, myocardial infarction, stroke, heart failure, and incident diabetes. The association measure was presented as a hazard ratio (HR) per 1 SD unit increase in the marker, adjusted for the following: age, sex, smoking, BMI, systolic blood pressure, non-HDL cholesterol, and geographic ancestry. Each outcome was also adjusted for diabetes status; however, in the diabetes analysis, individuals with confirmed diabetes status were excluded. For death and cardiovascular disease outcomes, ACE2 was then compared with other commonly used risk factors (smoking status, diabetes status, systolic blood pressure, non-HDL cholesterol, and BMI) and ranked on the basis of magnitude of the Wald χ2 value. Analyses were done with R, version 3.6.2 and SAS, version 9.3.
Role of the funding source
Funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author and co-authors (SN, SY, MC, CR, SIB, SR, MP, and AW) had full access to the all the data in the study and had final responsibility for the decision to submit for publication.
Results
This study used samples collected from patients recruited between Jan 5, 2005, and Dec 31, 2006. From the pool of 55 246 eligible participants, we randomly selected 5693 patients, of whom 5084 patients were included as our subcohort after exclusions related to quality control, data quality, and missingness; 6373 individuals had at least one incident event of interest (including death, myocardial infarction, stroke, heart failure, and diabetes), of whom 5669 were included in analyses after exclusions (table 1 ; appendix pp 7–9). The final sample included 10 753 participants. Median follow-up was 9·42 years (IQR 8·74–10·48). Genetic analyses were limited to the following groups containing more than 1000 individuals: Latin (n=4058), European (n=3372), and Persian (n=1269).
Table 1.
PURE case-cohort participant characteristics
| Subcohort (n=5084) | Death*(n=1985) | Cardiovascular death*(n=561) | Myocardial Infarction*(n=882) | Stroke*( n=663) | Heart failure*(n=264) | Diabetes*(n=1715) | ||
|---|---|---|---|---|---|---|---|---|
| Age, years | 50·79 (9·58) | 58·34 (9·16) | 59·76 (8·24) | 56·82 (9·06) | 57·87 (8·74) | 58·45 (8·95) | 52·56 (9·17) | |
| Sex | ||||||||
| Female | 2149 (42·3%) | 1099 (55·4%) | 340 (60·6%) | 553 (62·7%) | 349 (52·6%) | 140 (53·0%) | 721 (42·0%) | |
| Male | 2935 (57·7%) | 886 (44·6%) | 221 (39·4%) | 329 (37·3%) | 314 (47·4%) | 124 (47·0%) | 994 (58·0%) | |
| Patients with diabetes | 500 (9·8%) | 408 (20·6%) | 146 (26·0%) | 203 (23·0%) | 153 (23·1%) | 68 (25·8%) | .. | |
| Systolic blood pressure | 134·68 (23·46) | 146·20 (26·42) | 151·97 (26·06) | 145·60 (24·73) | 152·33 (27·88) | 152·21 (26·75) | 140·58 (22·86) | |
| Smoking | 2010 (39·5%) | 1152 (58·0%) | 333 (59·4%) | 508 (57·6%) | 328 (49·5%) | 140 (53·0%) | 627 (36·6%) | |
| Body-mass index | 27·25 (5·45) | 27·32 (6·31) | 27·93 (6·43) | 28·13 (5·95) | 27·66 (5·65) | 29·16 (6·55) | 30·79 (5·46) | |
| Ethnicity | ||||||||
| African | 396 (7·8%) | 289 (14·6%) | 59 (10·5%) | 13 (1·5%) | 31 (4·7%) | 24 (9·1%) | 40 (2·3%) | |
| Arab | 129 (2·5%) | 27 (1·4%) | 6 (1·1) | 17 (1·9%) | 14 (2·1%) | 8 (3·0%) | 81 (4·7%) | |
| East Asian | 97 (1·9%) | 60 (3·0%) | 25 (4·5%) | 30 (3·4%) | 36 (5·4%) | 3 (1·1%) | 35 (2·0%) | |
| European | 1060 (20·8%) | 372 (18·7%) | 65 (11·6%) | 226 (25·6%) | 192 (29·0) | 77 (29·2%) | 322 (18·8%) | |
| Latin American | 2291 (45·1%) | 1043 (52·5%) | 320 (57·0%) | 378 (42·9%) | 270 (40·7%) | 137 (51·9%) | 696 (40·6%) | |
| Persian | 746 (14·7%) | 103 (5·2%) | 42 (7·5%) | 114 (12·9%) | 54 (8·1%) | 12 (4·5%) | 469 (27·3%) | |
| South Asian | 365 (7·2%) | 91 (4·6%) | 44 (7·8%) | 104 (11·8%) | 66 (10·0%) | 3 (1·1%) | 72 (4·2%) | |
| Non-HDL cholesterol, mmol/L | 3·91 (1·04) | 3·92 (1·15) | 4·07 (1·16) | 4·23 (1·09) | 4·14 (1·13) | 4·06 (1·20) | 4·22 (1·04) | |
| LDL, mmol/L | 3·14 (0·88) | 3·05 (0·95) | 3·11 (0·93) | 3·23 (0·95) | 3·19 (0·96) | 3·11 (1·00) | 3·21 (0·86) | |
| Triglyceride, mmol/L | 1·63 (1·29) | 1·81 (1·51) | 1·96 (1·39) | 2·00 (1·39) | 1·88 (1·77) | 1·80 (1·29) | 2·12 (1·51) | |
| History of hypertension | 2184 (43·0%) | 1265 (63·7%) | 425 (75·8%) | 553 (62·7%) | 464 (70·0) | 205 (77·7%) | 973 (56·7%) | |
| History of coronary heart disease | 188 (3·7%) | 192 (9·7%) | 104 (18·5%) | 137 (15·5%) | 62 (9·4%) | 44 (16·7%) | 85 (5·0%) | |
| On blood pressure-lowering medication | 1144 (22·5%) | 822 (41·4%) | 308 (54·9%) | 372 (42·2%) | 293 (44·2%) | 152 (57·6%) | 597 (34·8%) | |
| On cholesterol-lowering medication† | 345 (6·8%) | 219 (11·0%) | 71 (12·7%) | 140 (15·9%) | 106 (16·0%) | 46 (17·4%) | 231 (13·5%) | |
Includes cases that are within and outside of the subcohort; participants may have multiple incident events.
Statin or other cholesterol-lowering medication.
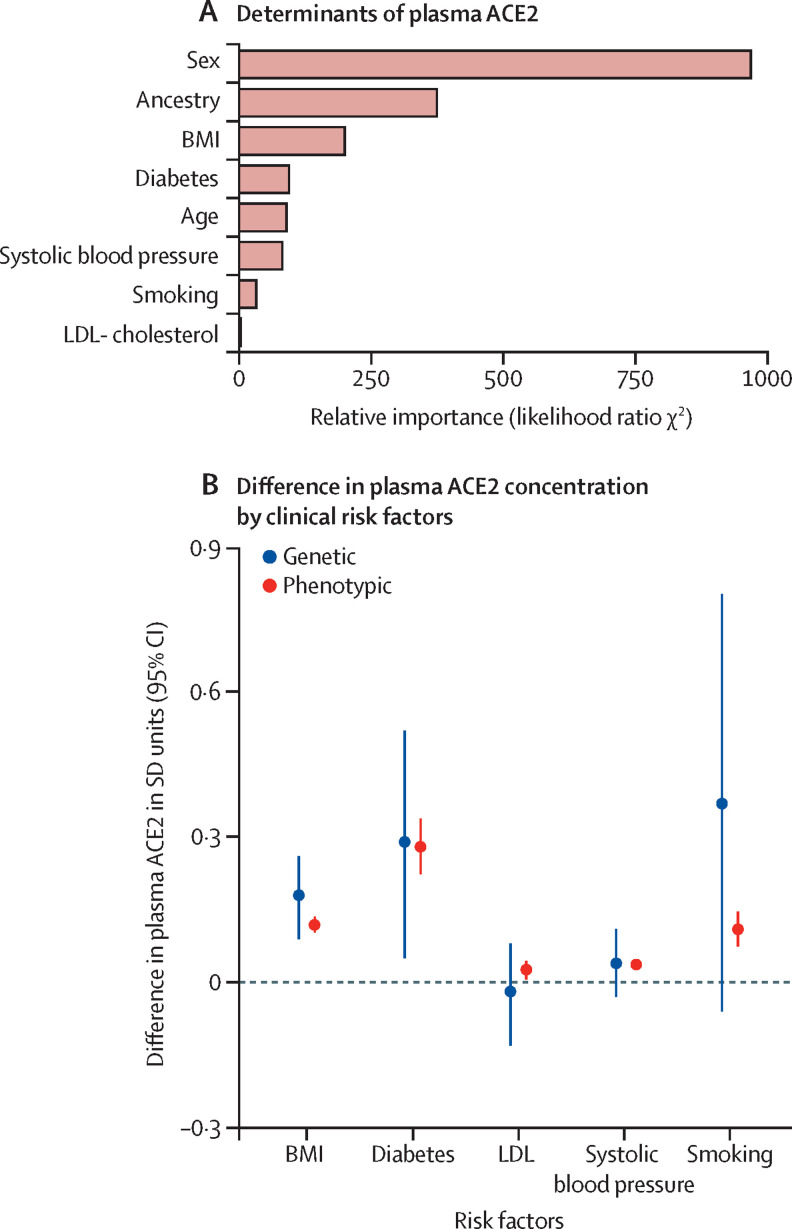
In analyses of the determinants of plasma ACE2 levels, sex accounted for the most variation, followed by geographic ancestry, BMI, diabetes status, age, systolic blood pressure, smoking status, and LDL cholesterol (figure 1A ). Men had higher plasma ACE2 than women and levels (0·58 SD units [95% CI 0·54–0·61] higher in men), and concentrations varied widely by geographic ancestry. There was an estimated 0·69 SD unit (95% CI 0·56–0·82) difference between the ancestral group with the lowest plasma ACE2 levels (south Asians) and those with the highest plasma ACE2 levels (east Asians; appendix p 33). Higher BMI, older age, diabetes, higher blood pressure, higher LDL cholesterol, and smoking were all associated with increased levels of circulating plasma ACE2. These findings were robust even after adjustment for coronary heart disease history (appendix p 26).
Figure 1.
Determinants of ACE2 concentration
(A) Ranked demographic and clinical determinants. (B) Phenotypic and genetic effects of clinical factors on plasma ACE2 concentration. BMI effect is reported per 5 kg/m2 increase. Blood pressure effect was reported per 10 mm Hg increase. LDL effect was reported per 1 mmol/L increase. Diabetes effect was reported versus those without diabetes for phenotypic analysis and in terms of 1 log odd unit increase for genotypic analysis. Smoking effect was reported in comparison to those without smoking history (phenotypic analysis) and in terms of 1 log odd unit increase (genotypic analysis). ACE2=angiotensin-converting enzyme 2. BMI=body-mass index.
To investigate whether the association between clinical risk factors and ACE2 levels were potentially causal, we did Mendelian randomisation analyses for the clinical risk factors identified in the previous analysis. Directionally concordant with the phenotypic associations, genetically higher BMI and greater risk of type 2 diabetes were associated with increased levels of plasma ACE2 (figure 1B). Conversely, although point estimates were similar to their phenotypic estimates, genetic predisposition to smoking, increased LDL cholesterol, and increased systolic blood pressure were not significantly associated with plasma ACE2 levels.
A cross-sectional analysis of common anti-hypertensive medications and their relationship with plasma ACE2 levels was done in a subset of the patients with hypertension (n=5216). We found no association between plasma ACE2 levels and use of ACE inhibitors, angiotensin-receptor blockers, β blockers, calcium channel blockers, or diuretics (appendix p 31). Results of our Mendelian randomisation-based approach using instrumentations of ACE inhibitors, β blockers, and calcium channel blockers were concordant with these null findings (appendix p 30).
Common genetic variants accounted for a substantial proportion of the variation in plasma ACE2 concentrations with heritability estimates of 44% (95% CI 0·14–0·74) for those with Latin ethnicity, 33% (0·11–0·55) for those with European ethnicity, and 66% (0–1) for those with Persian ethnicity. A genome-wide meta-analysis identified two genetic loci associated with ACE2 concentrations at genome-wide significance (p<5 × 10−8). These include an X chromosome-encoded variant (rs5936022; chrX:15726446-C-T; β=0·14 [95% CI 0·12–0·17]; p=2·2 × 10−28), which is 106 kilobases upstream of the ACE2 locus. The ACE2-increasing allele of this variant is also associated with increased ACE2 expression in the tibial artery, tibial nerve, and multiple brain regions.19, 20 A variant located 1 kilobase upstream the HNF1A locus (rs2464190; chr12:121415390-T-C; β=–0·16 [95% CI −0·18 to −0·13]; p=5·1 × 10−26) was also significantly associated with plasma ACE2 (appendix p 37). No additional single-nucleotide polymorphisms were identified on conditional and joint analysis.
In our analysis adjusted for demographic attributes and clinical risk factors (table 2 ), plasma ACE2 concentration was associated with higher risk of overall death (HR 1·35 per SD [95% CI 1·29–1·43]), including cardiovascular deaths (HR 1·40 per SD [1·27–1·54]) and non-cardiovascular deaths (HR 1·34 per 1 SD increase [1·27–1·43]). Plasma ACE2 concentration was also associated with greater risk of heart failure, myocardial infarction, stroke, and diabetes.
Table 2.
Association of plasma ACE2 and BNP with outcomes
| ACE2: model 1 | ACE2: model 2 | ACE2: model 3 | ACE2: model 3, additionally adjusted for BNP | BNP: model 3 | BNP: model 3, additionally adjusted for ACE2 | |
|---|---|---|---|---|---|---|
| All-cause mortality | 1·55 (1·48–1·63) | 1·41 (1·34–1·49) | 1·35 (1·29–1·43) | 1·31 (1·25–1·38) | 1·37 (1·31–1·44) | 1·33 (1·27–1·40) |
| Cardiovascular death | 1·71 (1·57–1·87) | 1·52 (1·38–1·68) | 1·40 (1·27–1·54) | 1·29 (1·16–1·42) | 1·93 (1·76–2·12) | 1·85 (1·69–2·03) |
| Non-cardiovascular death | 1·50 (1·41–1·58) | 1·38 (1·30–1·46) | 1·34 (1·27–1·43) | 1·32 (1·25–1·41) | 1·19 (1·13–1·26) | 1·16 (1·10–1·23) |
| Myocardial infarction | 1·43 (1·34–1·54) | 1·37 (1·27–1·48) | 1·23 (1·13–1·33) | 1·17 (1·08–1·27) | 1·41 (1·31–1·51) | 1·38 (1·28–1·48) |
| Stroke | 1·33 (1·23–1·44) | 1·32 (1·21–1·45) | 1·21 (1·10–1·32) | 1·17 (1·07–1·28) | 1·38 (1·26–1·50) | 1·35 (1·24–1·48) |
| Heart failure | 1·52 (1·33–1·73) | 1·43 (1·25–1·65) | 1·27 (1·10–1·46) | 1·14 (0·99–1·32) | 2·15 (1·88–2·47) | 2·10 (1·83–2·41) |
| Diabetes* | 1·48 (1·41–1·55) | 1·68 (1·59–1·77) | 1·44 (1·36–1·52) | 1·46 (1·38–1·54) | 0·87 (0·83–0·92) | 0·85 (0·80–0·89) |
Data are hazard ratios (95% CI) per 1 SD increase in ACE2 (1 SD of ACE2=0·73 normalised protein expression units). Model 1 is the unadjusted Cox model. Model 2 is controlled for age, sex, and ancestry. Model 3 is controlled for age, sex, ancestry, systolic blood pressure, non-HDL cholesterol, smoking, and diabetes. ACE2=angiotensin-converting enzyme 2. BNP=brain natriuretic peptide.
Patients with diabetes at baseline were excluded from analysis for these results.
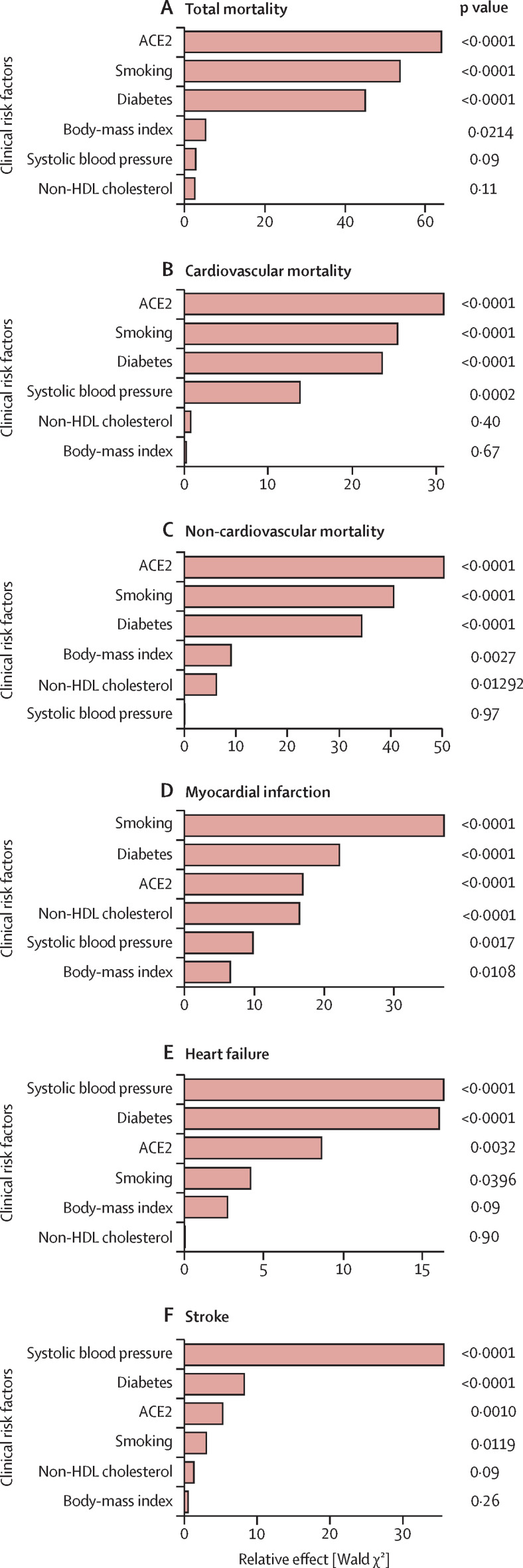
ACE2 was compared with clinical risk factors (diabetes, BMI, smoking status, non-HDL cholesterol, and systolic blood pressure) in its relationship with each outcome. Models were adjusted for clinical risk factors, age, sex, and ancestry. Compared with these clinical risk factors, ACE2 was the highest-ranked predictor of total deaths (figure 2A ; appendix p 34), cardiovascular deaths (figure 2B), and non-cardiovascular deaths (figure 2C), the third-highest ranked predictor of myocardial infarction (after smoking, diabetes, and similar to non-HDL cholesterol; figure 2D), and the third-highest ranked predictor of both stroke and heart failure (after systolic blood pressure and diabetes; figure 2E, F). As a complementary analysis, we did a minimally adjusted analysis of continuous modifiable risk factors (blood pressure, BMI, and non-HDL cholesterol; appendix p 24) In this analysis, ACE2 has the strongest association (HR 1·52 per SD [95% CI 1·38–1·68]) with cardiovascular deaths of these factors. The second strongest risk factor was systolic blood pressure (HR 1·32 per SD [1·22–1·43]). In a resampling-based analysis, ACE2 consistently emerged among the top predictors of total deaths along with diabetes and smoking status (appendix p 39). We examined whether associations between ACE2 and events were consistent in different subpopulations by doing a subgroup analysis for each risk factor. Although male sex is associated with higher levels of ACE2 concentration, our subgroup analysis suggests there is no differential effect of ACE2 between sexes in terms of cardiovascular outcomes (HR for women was 1·21 per SD [95% CI 1·09–1·34]; HR for men was 1·23 per SD [1·11–1·37]; pinteraction=0·65; appendix p 22). Additionally, there was no evidence for heterogeneous effects of ACE2 on major adverse cardiovascular outcomes after correction for multiple hypothesis testing in terms of age, BMI, ancestry, and diabetes (appendix p 23).
Figure 2.
Ranking risk factors by their effect on death and cardiovascular disease
(A) Total deaths, (B) cardiovascular deaths, (C) non-cardiovascular deaths, (D) myocardial infarction, (E) heart failure, and (F) stroke. ACE2=angiotensin-converting enzyme 2.
We did an additional analysis comparing the association of ACE2 and brain natriuretic peptide (BNP) with deaths and cardiovascular events (table 2). When additionally adjusted for BNP, increased ACE2 remains associated with greater risk of death, myocardial infarction, stroke, and diabetes. The relationship of ACE2 with heart failure was directionally consistent but attenuated upon adjustment for BNP.
Discussion
We present the first large-scale epidemiological analysis of blood ACE2 levels as a marker of cardiovascular disease. Our study uses a community-based, prospective (median follow-up time 9·42 years [IQR 8·74–10·48]) design to clarify the importance of the counter-regulatory axis of renin–angiotensin system in determining cardiovascular disease endpoints. We found higher levels of plasma ACE2 are associated with greater risk of death, cardiovascular and non-cardiovascular deaths, stroke, myocardial infarction, diabetes, and heart failure independent of age, sex, ancestry, and traditional cardiac risk factors. The results, including the relationship with all-cause deaths and all cardiovascular disease events, except for heart failure, remained robust even after adjustment for BNP. Blood ACE2 concentration, in comparison to established risk factors, was the highest ranked predictor of death, cardiovascular and non-cardiovascular deaths, and superseded many other common risk factors in explaining variation in stroke, myocardial infarction, and heart failure. We observed that male sex, higher blood pressure, smoking, higher BMI, and older age were all associated with higher levels of circulating ACE2 concentration. To our knowledge, our Mendelian randomisation analysis is the first to show a potential causal role for adiposity in determining ACE2 concentration.
Past work of plasma ACE2 assessed prognostic implications of circulating ACE2 in patients with established cardiovascular disease. Many of the previous studies examining blood ACE2 in cardiovascular disease in humans examined ACE2 catalytic activity, particularly by means of a quenched fluorescent substrate assay. Previous examinations of ACE2 concentration have chiefly been measured in urine samples; however, there has been increased use of assays measuring blood ACE2 concentration. The relationship between blood ACE2 concentration and ACE2 activity might require further study in different patient populations; however, a previous investigation showed a strong correlation between catalytic activity and concentration.21 Furthermore, studies measuring ACE2 activity show concordant patterns to ours in terms of the factors determining higher circulating concentrations as well as how ACE2 relates to clinical events prospectively. Specifically, among individuals with obstructive coronary disease, atrial fibrillation, heart failure, and aortic stenosis, increased activity of ACE2 corresponded with an increased risk of impaired functional status and adverse cardiac events.8, 9, 10, 11, 12 This implies that the increase of circulating ACE2 in these populations acts as a marker of disease or its severity. However, a previous investigation suggested that ACE2 delivered in a recombinant manner reduces deleterious angiotensin II and upregulates protective angiotensin 1–7 in a prospective heart failure cohort.22 Our findings with a highly sensitive protein assay suggest that plasma ACE2 is worth examining further as a marker of dysregulated renin–angiotensin system, even in an apparently healthy population, and suggests that increased ACE2 is associated with increased risk of cardiovascular disease and death. Our findings of factors associated with plasma ACE2 are likewise notable. Sex explained the most variation in circulating ACE2 levels. This is consistent with numerous previous studies showing marked differences in circulating ACE2 activity and concentration between men and women.8, 11, 12, 23 Differences in ACE2 expression between sexes have also been reported in various human tissues, including adipose tissue, the heart, and the renal cortex.20 Given that the gene encoding ACE2 is located on the X chromosome, X chromosome inactivation escape might also play a part in observed differences in ACE2 between men and women. However, biological implications of increased ACE2 concentrations in men and their relationships to sex-differential predisposition to cardiovascular disease remain poorly understood, although our subgroup analysis suggests there is little evidence for heterogenous effect of ACE2 between sexes. Because of the global nature of our cohort, we were able to detect variations in plasma ACE2 levels by geographic ancestry, which is consistent with observations that different ancestral groups might have marked variation in plasma protein concentration.24 Future efforts in examining circulating ACE2 should consider this variation, particularly when using reference panels not developed for local populations.
Our finding that plasma ACE2 had a strong association with incident diabetes is noteworthy, in part because of its compelling biological basis. For example, diabetic ACE2 knockout mice show increased fibrosis and weakened ACE inhibitor response as it relates to hypertension and renal protection.25 Specimens of kidney tissue from individuals with diabetic nephropathy have lower renal ACE2 expression than tissue from individuals with two healthy kidneys.26 Diabetes models likewise show alterations in activity of endogenous ACE2 regulators, such as ADAM-17.27, 28 The Mendelian randomisation associations of BMI and diabetes with ACE2 blood concentration strengthen the case for ACE2 as a metabolic marker. The HNF1A variant (rs2464190) shown to be associated with ACE2 levels is a master regulator of metabolism; other studies have linked this specific variant with susceptibility to coronary artery disease and type 2 diabetes.29, 30 Furthermore, HNF1A is probably a direct regulator of ACE2 levels because its promoter region contains three HNF1A binding sites, and within pancreatic islet cells, HNF1A induces higher cellular ACE2 expression levels.28
The ACE2 receptor facilitates viral entry for SARS-CoV-2. In patients with COVID-19, the ACE2 receptors might play a role in leading to cardiovascular complications such as thrombosis, cardiac injury, and heart failure. ACE2 is a possible link between SARS-CoV-2 and the cardiac presentations described in findings that have emerged from global data during the COVID-19 pandemic.31 Recent discussion surrounding SARS-CoV-2 has centred on altering hypertension medication management to account for concerns that ACE inhibitors or angiotensin II receptor blockers might increase viral entry through ACE2.31, 32 Our findings, as well as parallel analyses examining the effects of ACE inhibitors or angiotensin II receptor blockers on ACE2, do not support altering antihypertensive treatment regimens for the sole purpose of modifying ACE2.33, 34, 35 However, well conducted randomised controlled trials will be needed to make more definitive claims about the role of ACE inhibitors and angiotensin receptor blockers in COVID-19 prognosis.
Our study has some limitations. In our cross-sectional analysis, we were unable to fully account for unmeasured confounding and reverse causality with regards to how demographic and clinical factors determine ACE2 concentrations. However, our Mendelian randomisation analyses support BMI as a potentially modifiable risk determinant of ACE2 concentrations. Our Mendelian randomisation approach did not support the hypotheses that smoking, blood pressure, or lipids have an effect on plasma ACE2 concentration. Second, although our analysis shows ancestry accounts for variability in plasma ACE2 levels in our sample, the study design and methods used were not suitable for distinguishing genetic from environmental effects or addressing clinical implications of the observed differences in ACE2 levels between groups. Third, plasma ACE2 requires special consideration with regards to biological interpretation. Although increased cell-bound ACE2 exerts protective effects against cellular proliferation, hypertrophy, oxidative damage, and vasoconstriction, the mechanism by which levels rise in the plasma remains an area of active research. It is likely that a complex interaction between cellular expression, enzymatic cleavage, and impaired plasma clearance affects plasma concentrations. We note that a genetic variant associated with plasma ACE2 levels (rs5936022) in our study is associated with increased expression in the heart, brain, and vasculature, suggesting that increased blood levels also reflect increased ACE2 synthesis. However, limitations in our biological understanding of plasma ACE2 still preclude inference on function at the tissue level. Fourth, although we assessed risk factors for their causal effect on ACE2 concentrations using Mendelian randomisation, our genome-wide association study only detected one variant near the ACE2 gene at genome-wide significance, despite an overall heritability of 33–66%. Future Mendelian randomisation-based analyses of ACE2 as a causal marker of cardiovascular disease outcomes will require further power to robustly detect suitable instruments.
Plasma concentration of ACE2 shows an independent association with cardiovascular disease, including death, myocardial infarction, stroke, heart failure, and diabetes in a global population-based study. Compared with established clinical risk factors, ACE2 consistently emerges as a strong predictor of cardiovascular disease or death. Regardless of cause, plasma ACE2 might present a readily measurable indicator of renin–angiotensin system dysregulation. Our primary means of modulating the renin–angiotensin system cascade has focused on therapies dampening the pressor arm using agents such as ACE inhibitors and angiotensin receptor blockers. Modulation of ACE2 and the counter-balancing arm might represent an important therapeutic frontier, and clinical trials are underway to this effect.
Acknowledgments
Acknowledgments
The PURE study is an investigator-initiated study that is funded by the Population Health Research Institute, the Canadian Institutes of Health Research (CIHR), Heart and Stroke Foundation of Ontario, support from CIHR's Strategy for Patient Oriented Research, through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care and through unrestricted grants from several pharmaceutical companies (with major contributions from AstraZeneca [Canada], Sanofi-Aventis [France and Canada], Boehringer Ingelheim [Germany and Canada], Servier, and GlaxoSmithKline), and additional contributions from Novartis and King Pharma and from various national or local organisations in participating countries as follows: Argentina—Fundacion ECLA; Bangladesh—Independent University, Bangladesh and Mitra and Associates; Brazil—Unilever Health Institute, Brazil; Canada—Public Health Agency of Canada and Champlain Cardiovascular Disease Prevention Network; Chile—Universidad de la Frontera; Colombia—Colciencias (grant number 6566–04–18062); South Africa—The North-West University, SANPAD (SA and Netherlands Programme for Alternative Development), National Research Foundation, Medical Research Council of South Africa, The South Africa Sugar Association, Faculty of Community and Health Sciences; Sweden—grants from the Swedish State under the Agreement concerning research and education of doctors, the Swedish Heart and Lung Foundation, the Swedish Research Council, the Swedish Council for Health, Working Life and Welfare, King Gustaf V's and Queen Victoria Freemasons Foundation, AFA Insurance, Swedish Council for Working Life and Social Research, Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning, grant from the Swedish State under (LäkarUtbildningsAvtalet) Agreement, and grant from the Västra Götaland Region; and United Arab Emirates—Sheikh Hamdan Bin Rashid Al Maktoum Award for Medical Sciences, Dubai Health Authority, Dubai. The PURE biomarker project was supported by Bayer and the CIHR. The biomarker project was led by PURE investigators at the Population Health Research Institute (Hamilton, Canada) in collaboration with Bayer scientists. Bayer directly compensated the Population Health Research Institute for measurement of the biomarker panels, scientific, methodological, and statistical work. Genetic analyses were supported by CIHR (G-18–0022359) and Heart and Stroke Foundation of Canada (application number 399497) in the form of funding to GP.
Contributors
SN and GP conceived the study. SN conducted data analyses and wrote the first draft of the manuscript. GP supervised all the analyses, assumes responsibility for analyses, and assumes responsibility for data interpretation. GP conceived and organised the PURE biomarker and genetics study. SY is the principal investigator of PURE, planned the biobank, stored blood and urine samples, and reviewed and commented on drafts of the manuscript. MC organised the genotyping pipeline, conceived and conducted the genetic data analyses, helped in writing the manuscript, reviewed the drafts, and commented on it. CR conducted data analyses and reviewed and commented on the manuscript. AW and MP constructed the dataset, including the biomarker measurements in each participant, and reviewed and commented on the drafts of the manuscript. SIB reviewed and commented on the data analysis. SR coordinated the worldwide study and reviewed and commented on drafts of the manuscript. MvE and KL reviewed and commented on drafts of the manuscript.
Declaration of interests
SY is supported by the Heart and Stroke Foundation/Marion W Burke Chair in Cardiovascular Disease. MC is supported by a Canadian Institute of Health Research doctoral award and has received consulting fees from Bayer. MP is supported by the EJ Moran Campbell Internal Career Research Award from McMaster University. MvE and KL are employees of Bayer. MvE owns stock in Bayer. GP is supported by the CISCO Professorship in Integrated Health Systems. SN, CB, SR, SIB, and AW declare no competing interests.
Supplementary Material
References
- 1.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 2.Oudit GY, Penninger JM. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr Heart Fail Rep. 2011;8:176–183. doi: 10.1007/s11897-011-0063-7. [DOI] [PubMed] [Google Scholar]
- 3.Mercure C, Yogi A, Callera GE, et al. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 4.Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–e00220. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramchand J, Patel SK, Kearney LG, et al. Plasma ACE2 activity predicts mortality in aortic stenosis and is associated with severe myocardial fibrosis. JACC Cardiovasc Imaging. 2020;13:655–664. doi: 10.1016/j.jcmg.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 11.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epelman S, Shrestha K, Troughton RW, et al. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S. The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J. 2009;158:1–7.e1. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 16.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrell FE. Springer; New York: 2001. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis; pp. 205–207. [Google Scholar]
- 19.Carithers LJ, Ardlie K, Barcus M, et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv Biobank. 2015;13:311–319. doi: 10.1089/bio.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonsdale J, Thomas J, Salvatore M, et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Cong M, Wang N, et al. Association of angiotensin-converting enzyme 2 gene polymorphism and enzymatic activity with essential hypertension in different gender: a case-control study. Medicine. 2018;97 doi: 10.1097/MD.0000000000012917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol. 2017;69:805–819. doi: 10.1016/j.jacc.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 23.Roberts MA, Velkoska E, Ierino FL, Burrell LM. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol Dial Transplant. 2013;28:2287–2294. doi: 10.1093/ndt/gft038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjaarda J, Gerstein HC, Kutalik Z, et al. Influence of genetic ancestry on human serum proteome. Am J Hum Genet. 2020;106:303–314. doi: 10.1016/j.ajhg.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tikellis C, Bialkowski K, Pete J, et al. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- 26.Mizuiri S, Hemmi H, Arita M, et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Lambert DW, Yarski M, Warner FJ, et al. Tumor necrosis factor-α convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen KB, Chhabra KH, Nguyen VK, Xia H, Lazartigues E. The transcription factor HNF1α induces expression of angiotensin-converting enzyme 2 (ACE2) in pancreatic islets from evolutionarily conserved promoter motifs. Biochim Biophys Acta. 2013;1829:1225–1235. doi: 10.1016/j.bbagrm.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellanné-Chantelot C, Carette C, Riveline J-P, et al. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes. 2008;57:503–508. doi: 10.2337/db07-0859. [DOI] [PubMed] [Google Scholar]
- 30.Reiner AP, Gross MD, Carlson CS, et al. Common coding variants of the HNF1A gene are associated with multiple cardiovascular risk phenotypes in community-based samples of younger and older European-American adults: the Coronary Artery Risk Development in Young Adults Study and The Cardiovascular Health Study. Circ Cardiovasc Genet. 2009;2:244–254. doi: 10.1161/CIRCGENETICS.108.839506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill D, Arvanitis M, Carter P, et al. ACE inhibition and cardiometabolic risk factors, lung ACE2 and TMPRSS2 gene expression, and plasma ACE2 levels: a Mendelian randomization study. medRxiv. 2020 doi: 10.1101/2020.04.10.20059121. published online April 14. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1624. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.