Abstract
Background:
In the current climate of an ageing population, it is imperative to identify preventive measures for dementia.
Aim:
We implemented a multi-faceted index of cognitive reserve (CR) markers and investigated dementia incidence over 15 years of follow-up in a representative sample of the English population.
Methods:
Data were 12,280 participants aged 50+ from the English Longitudinal Study of Ageing, free from dementia at their baseline assessments being either wave 1 (2002–2003), 3 (2006–2007), or 4 (2008–2009), and followed up until wave 8 (2016–2017). CR index was constructed as a composite measure of education, occupation and leisure activities using a standardised questionnaire. Cox proportional hazards regression models were used to estimate the hazard ratios (HR) of dementia in relation to CR levels (low, medium and high) and its components (education, occupation and leisure activities).
Results:
During the follow-up period, 602 participants aged 56 to 99 developed dementia. Higher levels of CR (HR 0.65, 95% CI 0.48–0.89, p=0.008) were associated with a lower risk of dementia. An individual analysis of its components showed that higher levels of education (HR 0.56, 95% CI 0.36–0.88, p=0.012), occupation (HR 0.72, 95% CI 0.56–0.91, p=0.008) and leisure activities (HR 0.74, 95% CI 0.56–0.99, p=0.047) were predictive of a reduced dementia risk, with the first two components particularly protective in younger participants (<85 years old).
Conclusions:
This study showed a reduced risk of dementia for individuals with a higher level of CR, represented by higher education, complex occupations, and multifaceted level of leisure activities.
Data Information:
Available via UK Data Services (https://discover.ukdataservice.ac.uk).
Dementia represents one of the major contributors to disability and dependency amongst the elderly population, imposing significant challenges over society’s welfare and healthcare systems1. Consequently, it is crucial to identify preventive measures to maintain cognitive health and reduce dementia risk.
The concept of cognitive reserve (CR) has been proposed to account for individual differences in the susceptibility to cognitive impairment related to neuropathological damage. CR appears to provide the ability to mask neurocognitive deficits, providing a protective effect against dementia risk2. This theory suggests that individuals with higher CR show less cognitive and functional impairment because their cognitive networks are more efficient, capable and flexible3, 4. Hence, CR is often considered as a potential moderator between brain damage and exhibited cognitive impairment. Epidemiological evidence has shown that CR capacity may be modified through lifetime activities such as education, occupation, and leisure activities3. However, there are important limitations to the investigation of CR in population studies. First, there is a lack of consensus on the definition of CR and how to assess its determinants5. Second, most published studies in this area are either cross-sectional or based on small samples. Third, there is an imperative need to understand the role of modifiable CR markers in order to promote optimal cognitive health and support the prevention of dementia.
The present study sought to implement a detailed measurement of CR markers in a longitudinal study of ageing by applying a multi-faceted index in a population-based cohort of older adults in England. The overall aim of this study was to investigate the association between a CR index, derived by Nucci et al6, and its components in relation to subsequent dementia incidence. Based on the previous literature, it was hypothesised that:
An increased score of CR index will have a protective effect against dementia risk.
An increased level of each CR marker will be associated with reduced dementia risk.
Methods
Study Population.
The data were extracted from the English Longitudinal Study of Ageing (ELSA), which is a longitudinal panel study of a representative sample of initially non-institutionalised people living in England, aged 50 and older. ELSA was designed to collect data on a range of multidisciplinary topics relevant to the ageing process. Data collection was carried out every two years, with refreshment samples joining the study at different stages. The baseline for the present analysis was either wave 1 (2002–2003) for those who started the study at wave 1, or wave 3 (2006–2007) or 4 (2008–2009) for those who joined the study as refreshment samples. The latest wave available at the time of this analysis was wave 8 (2016–2017), ensuring a follow-up period of up to 15 years. Participants with dementia at their baseline assessments were excluded. See Figure 1 for analytical sample flow-chart.
Figure 1.
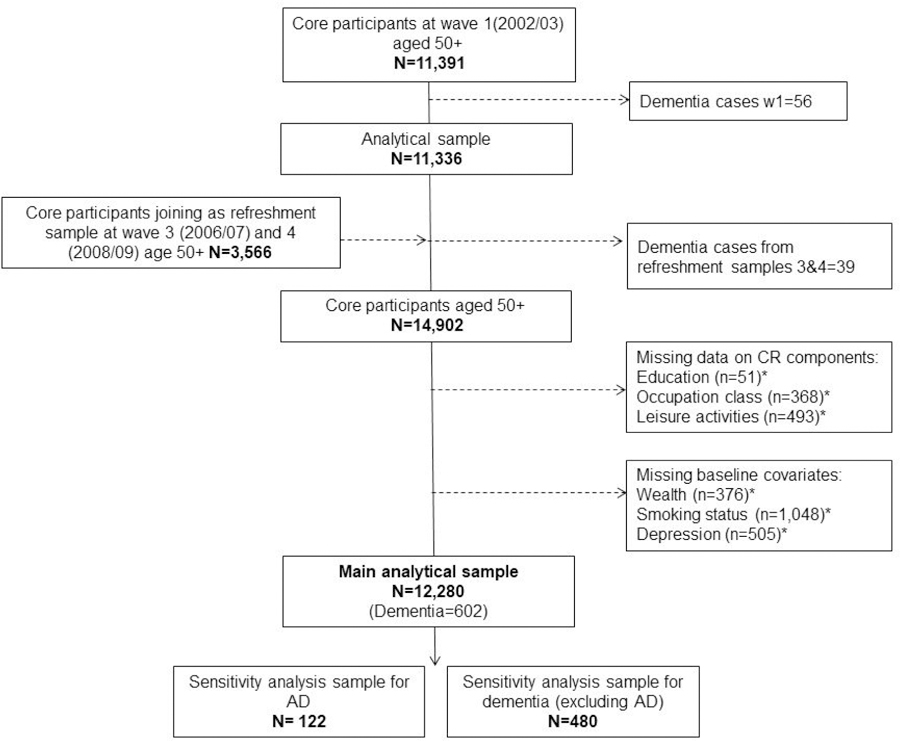
Flowchart of participants from the English Longitudinal Study of Ageing included in analyses
*Note. Numbers of excluded participants are non-mutually exclusive
Ethics.
This study was approved by the National Research Ethics Committee. All participants provided informed consent to take part in this study.
Dementia.
In ELSA, dementia diagnosis was ascertained at each wave through a combination of self-report physician diagnosis of dementia or Alzheimer’s disease, and/or a higher score on the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)7. This questionnaire was completed by a family member or long-term caregiver. A score above the threshold of 3.38 in the IQCODE is considered indicative of pathological cognitive decline. This threshold has both high specificity (0.84) and sensitivity (0.82)8.
Cognitive Reserve Index questionnaire.
CR was measured with the Cognitive Reserve Index Questionnaire (CRIq) devised by Nucci and colleagues6, and designed to quantify various markers of CR, providing a standardised reflective measure of the CR acquired during a person’s lifetime. The CR Index is a composite measure of educational attainment, occupational class, and leisure activities. These three markers provide the CRIq with good reliability (α =0.62, 95% CI (0.56, 0.97))6. The data for each component were extracted from various self-completion questionnaires administered to each core member during their baseline assessment (Supplementary Table 1).
Education.
Education attainment was categorised into 4 groups, each representing an approximate amount of years spent in formal education - having a university degree or higher: 15 years; having completed A-levels or the equivalent (vocational specialisations, work-related training, and advanced level qualifications obtained after secondary education): 12 years; having completed education to School Certificate level, taken at age 15–16 years: 8 years; and lacking formal qualifications: 4 years.
Occupation.
Occupational class was categorised according to the National Statistic Socio-economic Classification (NS-SEC) into five categories: low skilled manual work, skilled manual work, skilled non-manual work, professional occupation, and highly responsible or intellectual occupation9. The original CRIq uses the number of years spent on work occupation and multiplies it by the level of the category6. However, in ELSA, the number of years was not specifically assessed, and therefore, we conducted our main analysis without this element.
Leisure activities.
Leisure activities were measured using 17 questions as per Nucci et al., which have shown good reliability (α = 0.73, 95% CI (0.70–0.76))6. This range of leisure activities was reflected by 4 levels of frequency: weekly (e.g. reading the newspaper), monthly (e.g. social activities), annual (e.g. journeys/trips) and fixed activities (e.g. pet care). In ELSA, pet tenancy information was only available at wave 5 (2010/2011), from where this information was extracted. Furthermore, participants were not asked whether they read books, so this question was not included. Finally, to calculate the raw score of this component, the original CRIq multiplies each of the leisure activities by the number of years participants engaged in the activities, however, in ELSA, we used a calculation of points based on each of the 16 activities performed without an estimation of years.
For the computation of occupation and leisure activities, linear regressions were carried out with baseline age as the predictor variable. The residuals of the two linear models were divided by the standard deviation of the sample, standardised and transposed to a scale with a mean of 100 and a standard deviation of 156.
Overall CR Index.
To derive an overall index of various markers of CR acquired throughout an individual’s lifespan, we averaged the scores of the 3 individual markers, standardised and transposed them to a scale with a mean of 100 and a standard deviation of 15 as per Nucci et al., (2012)6. We only included participants with complete data for age, education, occupation and at least two leisure activities items. In the present study, the overall CR index ranged between 60.6 and 150 and was classified according to the thresholds used by Nucci et al., (2012), (e.g. low [≤70], medium-low [71–84], medium [85–114], medium-high [115–129] and high [≥ 130]). However, given the limited number of participants captured into the highest and lowest categories in our sample, we re-grouped the top two highest and lowest categories, resulting into following three groups: low (≤84), medium (85–114) and high (≥115) (Supplementary Table 2).
Covariates.
Being female or unmarried has been identified as having an increased risk of developing dementia10, 11; hence, sex and marriage were included as covariates. Wealth quintiles were included since previous ELSA findings showed an association between lower wealth and dementia12. The measure of wealth reflects the accumulation of assets over the life course; the variable includes financial wealth, the value of properties, business assets and physical wealth minus any debt. The median wealth was approximately £120 for participants in the lowest quintile, and £180,000 for those in the highest quintile. The baseline median wealth for the overall sample included in these analyses was £15,10012. Furthermore, there is an established association between depression and dementia13; therefore, we included depressive symptoms ascertained with a score of 4 or higher on the 8-item Centre for Epidemiologic Studies Depression Scale (CES-D). Finally, because health behaviours and poor physical health are associated with an increased risk of dementia13, 14, smoking status, physician diagnosis of coronary heart disease (CHD, including myocardial infarction and angina), stroke, hypertension and diabetes were included as physical health covariates.
Statistical Analysis.
Descriptive statistics were examined using Pearson’s chi-squared tests to determine if there were significant differences in baseline characteristics between participants who developed dementia and those who remained dementia-free.
Cox proportional hazards regression models were used to estimate the hazard ratios (HR) and confidence intervals (CI) of dementia incidence in relation to CR index and its components. Age was used as the underlying time variable for the survival analyses considered here within the study period, ranging from wave 1 until the time of dementia onset or last wave of follow-up (wave 8) if not diagnosed with dementia. For individuals who died or dropped out, right censoring was applied considering the survival age at their last wave of data available. Attrition rates are presented in Supplementary Table 3.
Nine models were fit to the data. Model 1 examined the relationship between CR index and dementia incidence controlling for sex and marital status. Models 2 through 8 were based on Model 1, with additional adjustment for each of the wealth, smoking status, depressive symptoms, CHD, diabetes, stroke or hypertension, respectively; Model 9 adjusted for all covariates. The inflation factor was <2.01, suggesting no significant multicollinearity. Proportional hazards assumptions were checked for the CR index using Schoenfeld residuals. The CR index (p=0.179) and occupation (p=0.475) sub-component met the proportional hazards assumption. However, these assumptions were not fulfilled for education and leisure activities. Separate models were carried out before and after the age of 80 for education and before and after the age of 85 for leisure activities.
Additionally, to assess the extent to which baseline risk factors explained the association of CR with dementia incidence, the percentage of excess risk mediated (PERM) was calculated for each one of the risk factors included: wealth, smoking status, depressive symptoms, coronary heart disease, diabetes, stroke and hypertension. The PERM15 was estimated as:
The baseline cross-sectional weights derived in ELSA were used in all analyses to ensure the sample is representative of the English population. We used STATA SE, Version 14 (StataCorp) to carry out all analyses. The statistical significance was considered at standard levels at or below 0.05.
Sensitivity Analyses.
Several sensitivity analyses were conducted. The first two investigated CR index in relation to dementia types (Alzheimer’s disease and other types of dementia). The third and fourth explored the categorisation of CR with different thresholds by dividing the CR into tertiles and quintiles. The fifth analysis used an overall CR index for which the occupation was calculated by multiplying the score corresponding to each level of working activity by the number of years estimated from age 40 until the age 65 (considered as the retirement age), or until the participant’s baseline age if they were younger than 65 at the beginning of the study. Finally, the sixth sensitivity analysis was conducted to further control for APOEe4 genotype and baseline alcohol consumption in a subset analytical sample due to a low number of observations for each (APOEe4 n=6,799 and alcohol consumption n=7,697). We also tested the interaction between APOEe4 and CR.
Results
Descriptive statistics.
The analytical sample was comprised of 12,280 individuals free form dementia at baseline, accounting for 114,234 person-years. The sample consisted of 5,626 men and 6,654 women, with a mean age of 63.66 ± 9.8 (SD) ranging from 50 to 100 years at baseline. At the time of the event or last wave of follow up, the mean age for all participants was 72.96 ± 9.7 years (SD), ranging from 52 to 108 years. From the overall sample, 602 participants were diagnosed with dementia, accounting for a 5% cumulative incidence during the 15-years follow-up period. The group diagnosed with dementia included 251 men (2%) and 351 women (2.8%) with the median age of 80.8 ± 8.2 years at the time of dementia diagnosis. Furthermore, from the total number of individuals diagnosed with dementia, 122 were diagnosed with AD.
Participants with missing information were less educated (57% vs. 39.7% lacking formal qualifications); however, the missing information according to sex, age, occupational class and leisure activities were fairly similar. Participants who developed dementia were significantly older, had lower CR index scores, less education, lower-ranked work occupations, engaged less in leisure activities and had less wealth than those who did not develop the condition (see Table 1).
Table 1.
Baseline characteristics of participants with and without dementia at follow-up
| No dementia (n=11,678) | Dementia (n=602) | p-value | |
|---|---|---|---|
| Age: 50–59 | 5,029 (43) | 51 (8) | 0.001 |
| 60–69 | 3,540 (30) | 154 (26) | |
| 70–79 | 2,281 (20) | 246 (41) | |
| ≥80 | 828 (7) | 151 (25) | |
| Sex: Men | 5,375 (46) | 251 (42) | 0.037 |
| Women | 6,303 (54) | 351 (58) | |
| CR Index | 100.8± 14.8 | 98.8 ± 14.5 | 0.001 |
| CR Index: Low | 3,612 (31) | 235 (39) | 0.001 |
| Medium | 3,933 (34) | 192 (32) | |
| High | 4,133 (35) | 175 (29) | |
| Education: 4 years | 4,323 (37) | 321 (53) | 0.001 |
| 8 years | 1,395 (12) | 84 (14) | |
| 12 years | 2,848 (24) | 105 (18) | |
| 15 years | 3,112 (27) | 92 (15) | |
| Occupation: Never worked | 138 (1) | 17 (3) | 0.001 |
| Low skilled manual work | 2,727 (23) | 171 (28) | |
| Skilled manual work | 2,027 (17) | 108 (18) | |
| Skilled non-manual work | 2,786 (24) | 145 (24) | |
| Professional occupation | 3,283 (29) | 140 (23) | |
| Intellectual occupation | 717 (6) | 21 (4) | |
| Leisure Activities: Low | 3,502 (30) | 217 (36) | 0.001 |
| Medium | 3,958 (34) | 210 (35) | |
| High | 4,218 (36) | 175 (29) | |
| Wealth: Q1 (Lowest) | 2,072 (18) | 156 (26) | 0.001 |
| Q2 | 2,301 (19) | 130 (22) | |
| Q3 | 2,332 (20) | 124 (21) | |
| Q4 | 2,399 (21) | 98 (16) | |
| Q5 (Highest) | 2,574 (22) | 94 (15) | |
| Smoking: No | 9,538 (82) | 526 (87) | 0.001 |
| Yes | 2,140 (18) | 76 (13) | |
| Marital status: Not Married | 7,890 (68) | 350 (58) | 0.001 |
| Married | 3,788 (32) | 252 (42) | |
| Depressive Symptoms: No | 9,810 (84) | 449 (75) | 0.001 |
| Yes | 1,868 (16) | 153 (25) | |
| CHD: No | 10,756 (92) | 487 (81) | 0.001 |
| Yes | 922 (8) | 115 (19) | |
| Diabetes: No | 11,048 (95) | 546 (91) | 0.001 |
| Yes | 630 (5) | 56 (9) | |
| Stroke No | 11,322 (97) | 554 (92) | 0.001 |
| Yes | 356 (3) | 48 (8) | |
| Hypertension: No | 8,246 (71) | 346 (57) | 0.001 |
| Yes | 3,432 (29) | 256 (43) |
N (%) and Means ± SD. CHD: Coronary Heart Disease, CR: Cognitive Reserve, Q: Quintile
Overall CR Index and dementia.
The association between CR index and dementia incidence across 15 years of follow-up are presented in Table 2a. Model 1 of the Cox regression showed that the HR of the medium level of CR was 0.65 (95% CI 0.53–0.81, p<0.001), when compared to the low level after adjustment for sex and marital status, and decreased by 17% after adjustment for wealth. Similarly, in the minimally adjusted model, the HR of the high level of CR was 0.48 (95% CI 0.36–0.64, p<0.001), when compared to the low level and decreased by 23% after adjustment for wealth. The fully adjusted model showed a 27% decreased risk of dementia for those in the medium level (Model 9, adjusted HR 0.73, 95% CI 0.59–0.92, p<0.008), with an overall attenuation of 23% after adjusting for all risk factors. Individuals in the high level showed a 35% decreased risk of dementia (Model 9, adjusted HR 0.65, 95% CI 0.48–0.89, p=0.008), with an overall attenuation of 33% after adjusting for all risk factors, which suggests an additive effect of covariates. We also explored the interaction between covariates and found an interaction between wealth and CHD (p=0.04), which accounted for some of the difference in the overall PERM. Figure 2 presents the smoothed hazard function of each level of CR index.
Table 2a.
Hazard ratios from Multivariate Cox regressions models indicating the incidence of dementia by levels of CR Index, using an adaptation of the previously published thresholds (Nucci et al., 2012)
| CR Index | Hazard ratio (95% CI) | p-value | PERM | |
|---|---|---|---|---|
| Model 1 | Low | 1[Reference] | ||
| (Sex + Marital status) | Medium | 0.65(0.53–0.81) | <0.001 | … |
| High | 0.48(0.36–0.64) | <0.001 | … | |
| Model 2 | Low | 1[Reference] | ||
| (Model 1 + Wealth) | Medium | 0.71(0.56–0.88) | 0.002 | 17% |
| High | 0.60(0.44–0.81) | 0.001 | 23% | |
| Model 3 | Low | 1[Reference] | ||
| (Model 1 + Smoke) | Medium | 0.67(0.53–0.83) | <0.001 | 6% |
| High | 0.50(0.37–0.67) | <0.001 | 4% | |
| Model 4 | Low | 1[Reference] | ||
| (Model 1 + Depressive symptoms) | Medium | 0.68(0.55–0.85) | 0.001 | 9% |
| High | 0.53(0.40–0.71) | <0.001 | 10% | |
| Model 5 | Low | 1[Reference] | ||
| (Model 1 + CHD) | Medium | 0.66(0.53–0.82) | <0.001 | 3% |
| High | 0.49(0.37–0.65) | <0.001 | 2% | |
| Model 6 | Low | 1[Reference] | ||
| (Model 1 + Diabetes) | Medium | 0.66(0.53–0.82) | <0.001 | 3% |
| High | 0.49(0.37–0.65) | <0.001 | 2% | |
| Model 7 | Low | 1[Reference] | ||
| (Model 1 + Stroke) | Medium | 0.66(0.53–0.82) | <0.001 | 3% |
| High | 0.49(0.37–0.65) | <0.001 | 2% | |
| Model 8 | Low | 1[Reference] | ||
| (Model 1 + Hypertension) | Medium | 0.66(0.53–0.81) | <0.001 | 3% |
| High | 0.49(0.37–0.64) | <0.001 | 2% | |
| Model 9 | Low | 1[Reference] | ||
| (Model 1 + All covariates) | Medium | 0.73(0.59–0.92) | 0.008 | 23% |
| High | 0.65(0.48–0.89) | 0.008 | 33% |
CHD: Coronary Heart Disease, CR: Cognitive Reserve, PERM: Percentage of excess risk mediated
Figure 2.
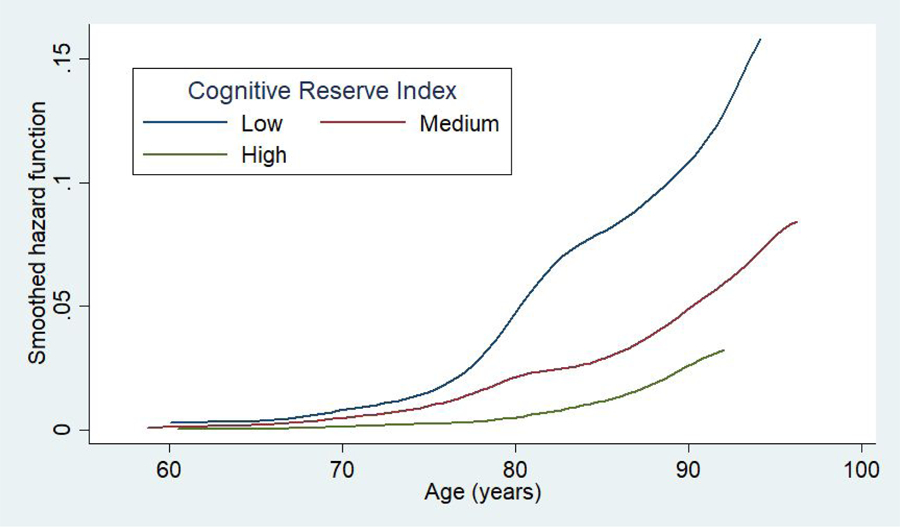
Adjusted smoothed hazard estimates by levels of cognitive reserve index in the English Longitudinal Study of Ageing
CR markers and dementia.
As presented in Table 2b, in the fully adjusted model, increased years of education predicted a lower risk of dementia for the 50 to 79 age group (Model 9, adjusted HR 0.56, 95% CI 0.36–0.88, p=0.012), but not for the 80+ age group.
Table 2b.
Hazard ratios from Multivariate Cox regressions models indicating the incidence of dementia by each marker of CR Index
| Education | (years) | Age 50–79, N=9,155 | Age 80+ , N=3,125 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | PERM | HR (95% CI) | p-value | PERM | ||
| Model 1 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.93(0.64–1.37) | 0.749 | … | 0.89(0.65–1.22) | 0.490 | … | |
| 12 | 0.82(0.59–1.14) | 0.249 | … | 0.84(0.61–1.17) | 0.319 | … | |
| 15 | 0.44(0.29–0.66) | <0.001 | … | 1.04(0.77–1.42) | 0.761 | … | |
| Model 2 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.98(0.67–1.45) | 0.954 | 71% | 0.94(0.68–1.29) | 0.711 | 45% | |
| 12 | 0.93(0.67–1.31) | 0.708 | 61% | 0.93(0.67–1.30) | 0.698 | 56% | |
| 15 | 0.54(0.34–0.83) | 0.006 | 18% | 1.23(0.90–1.68) | 0.187 | 475% | |
| Model 3 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.94(0.64–1.38) | 0.765 | 14% | 0.89(0.65–1.23) | 0.508 | 0% | |
| 12 | 0.83(0.60–1.15) | 0.269 | 6% | 0.86(0.62–1.18) | 0.364 | 13% | |
| 15 | 0.44(0.29–0.67) | <0.001 | 0% | 1.05(0.78–1.43) | 0.717 | 25% | |
| Model 4 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.99(0.67–1.46) | 0.994 | 86% | 0.92(0.67–1.26) | 0.619 | 27% | |
| 12 | 0.90(0.64–1.24) | 0.530 | 44% | 0.88(0.64–1.22) | 0.475 | 25% | |
| 15 | 0.49(0.33–0.74) | 0.001 | 9% | 1.09(0.80–1.49) | 0.558 | 125% | |
| Model 5 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.94(0.64–1.38) | 0.768 | 14% | 0.89(0.65–1.22) | 0.508 | 0% | |
| 12 | 0.85(0.62–1.18) | 0.344 | 17% | 0.85(0.62–1.18) | 0.353 | 6% | |
| 15 | 0.46(0.30–0.69) | <0.001 | 4% | 1.05(0.77–1.43) | 0.735 | 25% | |
| Model 6 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.95(0.65–1.40) | 0.828 | 29% | 0.89(0.65–1.22) | 0.491 | 0% | |
| 12 | 0.84(0.61–1.16) | 0.304 | 11% | 0.85(0.61–1.17) | 0.324 | 6% | |
| 15 | 0.45(0.30–0.67) | <0.001 | 2% | 1.05(0.77–1.42) | 0.740 | 25% | |
| Model 7 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.94(0.64–1.38) | 0.770 | 14% | 0.89(0.65–1.23) | 0.509 | 0% | |
| 12 | 0.83(0.60–1.14) | 0.263 | 6% | 0.85(0.62–1.18) | 0.354 | 6% | |
| 15 | 0.45(0.30–0.67) | <0.001 | 2% | 1.05(0.77–1.43) | 0.723 | 25% | |
| Model 8 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 0.94(0.64–1.38) | 0.782 | 14% | 0.89(0.65–1.22) | 0.484 | 0% | |
| 12 | 0.84(0.61–1.17) | 0.315 | 11% | 0.84(0.61–1.17) | 0.319 | 0% | |
| 15 | 0.45(0.30–0.68) | <0.001 | 2% | 1.04(0.77–1.41) | 0.776 | 0% | |
| Model 9 | 4 | 1[Reference] | 1[Reference] | ||||
| 8 | 1.04(0.70–1.53) | 0.833 | 157% | 0.96(0.70–1.33) | 0.843 | 64% | |
| 12 | 0.99(0.70–1.40) | 0.969 | 94% | 0.98(0.71–1.37) | 0.951 | 88% | |
| 15 | 0.56(0.36–0.88) | 0.012 | 21% | 1.27(0.93–1.74) | 0.125 | 575% | |
| Occupation | Age 50+ , N=12,280 | ||||||
| HR (95% CI) | p-value | PERM | |||||
|
| |||||||
| Model 1 | Low | 1[Reference] | |||||
| Med. | 0.64(0.53–0.78) | <0.001 | … | ||||
| High | 0.60(0.48–0.74) | <0.001 | … | ||||
| Model 2 | Low | 1[Reference] | |||||
| Med. | 0.68(0.56–0.84) | <0.001 | 11% | ||||
| High | 0.70(0.55–0.88) | 0.003 | 25% | ||||
| Model 3 | Low | 1[Reference] | |||||
| Med. | 0.65(0.54–0.79) | <0.001 | 3% | ||||
| High | 0.61(0.49–0.76) | <0.001 | 3% | ||||
| Model 4 | Low | 1[Reference] | |||||
| Med. | 0.66(0.55–0.80) | <0.001 | 6% | ||||
| High | 0.63(0.51–0.79) | <0.001 | 8% | ||||
| Model 5 | Low | 1[Reference] | |||||
| Med. | 0.64(0.53–0.78) | <0.001 | 0% | ||||
| High | 0.60(0.48–0.75) | <0.001 | 0% | ||||
| Model 6 | Low | 1[Reference] | |||||
| Med. | 0.65(0.53–0.78) | <0.001 | 3% | ||||
| High | 0.60(0.48–0.75) | <0.001 | 0% | ||||
| Model 7 | Low | 1[Reference] | |||||
| Med. | 0.65(0.53–0.78) | <0.001 | 3% | ||||
| High | 0.60(0.48–0.75) | <0.001 | 0% | ||||
| Model 8 | Low | 1[Reference] | |||||
| Med. | 0.64(0.53–0.78) | <0.001 | 0% | ||||
| High | 0.60(0.48–0.74) | <0.001 | 0% | ||||
| Model 9 | Low | 1[Reference] | |||||
| Med. | 0.70(0.57–0.85) | <0.001 | 17% | ||||
| High | 0.72(0.56–0.91) | 0.008 | 30% | ||||
| Leisure | Age 50–84, N=10,692 | Age 85+, N=1,588 | |||||
| HR (95% CI) | p-value | PERM | HR (95% CI) | p-value | PERM | ||
|
| |||||||
| Model 1 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.82(0.64–1.03) | 0.099 | … | 0.79(0.56–1.12) | 0.190 | … | |
| High | 0.57(0.44–0.73) | <0.001 | … | 0.70(0.48–1.01) | 0.060 | … | |
| Model 2 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.88(0.69–1.13) | 0.341 | 33% | 0.80(0.57–1.13) | 0.211 | 5% | |
| High | 0.69(0.52–0.91) | 0.009 | 28% | 0.73(0.50–1.07) | 0.113 | 10% | |
| Model 3 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.82(0.64–1.04) | 0.104 | 0% | 0.81(0.57–1.14) | 0.235 | 10% | |
| High | 0.57(0.44–0.74) | <0.001 | 0% | 0.73(0.50–1.07) | 0.111 | 10% | |
| Model 4 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.87(0.68–1.11) | 0.277 | 28% | 0.81(0.57–1.14) | 0.229 | 10% | |
| High | 0.64(0.49–0.83) | 0.001 | 16% | 0.73(0.50–1.06) | 0.102 | 10% | |
| Model 5 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.81(0.64–1.03) | 0.099 | 6% | 0.79(0.56–1.12) | 0.193 | 0% | |
| High | 0.57(0.44–0.74) | <0.001 | 0% | 0.70(0.48–1.02) | 0.064 | 0% | |
| Model 6 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.82(0.65–1.04) | 0.111 | 0% | 0.79(0.56–1.11) | 0.189 | 0% | |
| High | 0.58(0.45–0.74) | <0.001 | 2% | 0.70(0.48–1.01) | 0.063 | 0% | |
| Model 7 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.83(0.65–1.05) | 0.126 | 6% | 0.79(0.56–1.12) | 0.192 | 0% | |
| High | 0.58(0.45–0.75) | <0.001 | 2% | 0.70(0.48–1.01) | 0.061 | 0% | |
| Model 8 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.82(0.64–1.03) | 0.099 | 0% | 0.79(0.56–1.12) | 0.196 | 0% | |
| High | 0.57(0.44–0.74) | <0.001 | 0% | 0.69(0.48–1.01) | 0.058 | 3% | |
| Model 9 | Low | 1[Reference] | 1[Reference] | ||||
| Med. | 0.92(0.72–1.18) | 0.542 | 56% | 0.83(0.59–1.17) | 0.303 | 19% | |
| High | 0.74(0.56–0.99) | 0.047 | 40% | 0.79(0.53–1.17) | 0.249 | 30% | |
HR: Hazard Ratio, CHD: Coronary Heart Disease, CR: Cognitive Reserve, PERM: Percentage of excess risk mediated. Model 1 adjusted for sex and marital status. Models 2–8 were based on Model 1, and each adjusted for wealth, smoking status, depressive symptoms, CHD, diabetes, stroke or hypertension, respectively. Model 9 adjusted for all covariates.
For the entire analytical sample, higher occupational class predicted lower risk of dementia in the fully adjusted model with individuals in the medium level indicating a 30% decreased risk of dementia in comparison to those in the lower level (Model 9, adjusted HR 0.70, 95% CI 0.57–0.85 p<0.001) and participants in the high level showing a 28% decreased risk of dementia in comparison to the lower level (Model 9, adjusted HR 0.72, 95% CI 0.56–0.91 p=0.008). The overall attenuation after adjusting for all risk factors was 17% for the medium level and 30% for the higher level.
For the 50 to 84 age group, individuals in the higher levels of leisure activity indicated a 26% decreased dementia risk in comparison to those in the lower level (Model 9, adjusted HR 0.74, 95% CI 0.56–0.99, p=0.047) with an attenuation of 40% after adjusting for all risk factors. For the older age group, leisure activities showed no significant association with dementia incidence.
Sensitivity Analyses.
Sensitivity analyses 1 and 2 showed a significant impact of higher CR on dementia but not on Alzheimer’s disease (Supplementary Tables 4 and 5). Sensitivity analysis 3 and 4 indicated that different thresholds of CR do not affect the relationship found between CR and dementia incidence (see Supplementary Tables 6 and 7). Sensitivity analysis 5 showed no significant relationship between occupation (estimated from age 40) and dementia for the fully adjusted model (see Supplementary Table 8). Finally, the models controlling independently for alcohol consumption and APOEe4 in sensitivity analysis 6 showed significant associations between CR and dementia. However, in the fully adjusted models, the relationship between CR and dementia becomes non-significant (see Supplementary Table 9). The interaction between CR and APOEe4 was found to be non-significant (p=0.155).
Discussion
This study investigated the association between various markers of CR by applying a multi-faceted index and subsequent dementia incidence in a representative sample of the English population aged 50 years and older. In multivariable analyses, increased levels of a CR Index were negatively and independently associated with dementia incidence when compared to the lowest level. A further sensitivity analysis exploring different thresholds confirmed these results. Our findings suggest a higher risk of dementia for individuals with lower CR, evaluated with several markers such as educational attainment, occupational class, and engagement in leisure activities. Wealth explained 17 to 23% of excess dementia diagnosis in individuals with medium and high levels of CR index. Investigation of CR markers suggested that low occupational class is associated with higher dementia risk for the entire analytical sample. Education and leisure activities were found to be independently associated with reduced dementia risk only for younger individuals in this cohort.
This study provided a standardised and structuralised index of CR, which is replicable and broadly consistent with previous epidemiologic analyses that have found evidence of the protective effects of CR markers16. For instance, longitudinal findings from the Cognitive Function and Ageing Study17 indicated that a composite score of education, occupation and social engagement, similar to the one used in the present study, was protective of dementia, with those with a high score having a 40% decreased risk of developing dementia in comparison to those with a low score18. One of the most widespread models regarding the mechanism of action for CR, suggests that increased levels of CR markers moderate between brain pathology and cognitive function2. The level of CR can be increased through the engagement in intellectually demanding activities, such as education, non-manual occupations and leisure activities19. It has been suggested that the protective effects of these activities accumulate and act continuously at different stages across life, possibly leading to an increase in the amount of CR5, 20.
Evidence from systematic reviews and a meta-analysis suggest that education contributes to CR and that low educational attainment increases the risk of dementia21. However, the present study found an independent relationship between increased education and reduced dementia risk for the 50 to 79 age group, but not in the older group. This is in accordance with a recent study carried out in ELSA, showing that socioeconomic disadvantage, and lower wealth rather than low education, was a strong indicator of dementia incidence for individuals born earlier in the 20th century12. These results hint to some potential cohort effects and variation in older individuals educated around the Second World War, when education in England was particularly restrictive (e.g. schools evacuated, teachers in shortage). Further research is needed to clarify the impact of education on CR and dementia risk in other intergenerational cohorts with larger population samples to fully disentangle these effects in population subgroups born across different decades.
Furthermore, the current study investigated the independent association between occupation levels as another marker of CR index and dementia risk, which was found to be significant. Our results are in concordance with findings from the Whitehall II study22, indicating that higher employment grade, ordered by increasing salary, was protective of cognitive function23. Other studies that have measured occupational complexity for jobs dealing with data, people or things, have also indicated a protective effect of work against dementia24. A systematic review of 14 studies investigating the long-term effects of the workplace on dementia concluded that there is evidence for the protective effects of complex occupations dealing with people and data25. However, the contribution of occupation to CR remains highly debated with studies yielding conflicting results2, 26. A more recent systematic review examining 34 studies found inconclusive results for the association between work activity and dementia risk21. This inconsistency might be caused by the different measures used to assess occupation in different studies.
Leisure activities have been found to be a robust predictor of dementia, with large longitudinal studies showing a lower incidence of dementia ranging between 33 to 52% for those who engage in various leisure activities2,27,28. We also found that participants who engaged in leisure activities showed a 26% reduced risk of dementia when compared to those who did not engaged, especially in those aged 50 to 84 years. Other recent longitudinal findings from the Kungsholmen Project in Sweden29 indicated that an aggregated measure of late-life leisure activities showed an increased protective effect against dementia in comparison to early life and adulthood activities, which included educational and occupational components30. Furthermore, a systematic review exploring 15 longitudinal studies on social networks and leisure activity and their association with dementia risk, concluded that social, mental and physical activity could have protective effects against dementia diagnosis by contributing to CR31. Therefore, it is plausible that leisure activities involving mental stimulation and those involving physical activity contribute simultaneously to a reduced risk of dementia through neuroprotection by increasing synaptogenesis and enhancing the brain’s vasculature32.
However, in our study, leisure activities showed no significant association with dementia incidence for the 85+ age group; this might be explained by the low number of participants in this age group, reduced engagement in leisure activities or increased neurodegeneration in older age that may surpass CR capacity.
Our sensitivity analysis exploring dementia type indicated that heightened levels of CR are negatively and independently associated with the incidence of dementia, but not Alzheimer’s disease. We also tested the role of APOEe4 in a sensitivity analysis, and despite a decreased power, this further adjustment did not affect the relationship between CR and dementia in the basic adjusted model.
To summarise, our findings support the theory that various CR markers, such as occupation and leisure activities may have the ability to decrease the risk of dementia. To the best of our knowledge, this is the first study to implement a multi-faceted index of CR to examine its overall effect and the role of each specific marker (e.g. education, occupation and leisure activities) on dementia risk in a representative sample of the English population. Our findings support the theory that CR can mitigate the symptomatology of neurodegenerative disorders and can potentially buffer dementia onset3.
However, some methodological issues should be taken into consideration when interpreting our findings. The first is related to the CRIq implementation in ELSA; this study did not include the number of years spent in work or performing various leisure activities. We partially addressed this aspect by including an estimation of years worked according to baseline age in one of our sensitivity analyses; however, this approach was not appropriate to estimate the element of time for leisure activities. The second limitation is related to the distribution of participants across the levels of CR index (see Supplementary Table 2). A third limitation constitutes the use of self-reported dementia diagnosis, which might have resulted in a slight underestimation of the number of participants with dementia in this study. There is also a lack of ethnic variability in the study, as well as a potential attrition bias due to the longitudinal nature of the study. Lastly, further analyses could take into consideration the time-varying element of both exposures and covariates to better understand how changes in these factors could affect dementia risk.
However, this study has multiple strengths. We used a large population-based longitudinal study in England to assess multiple markers of CR in relation to dementia risk, contributing to the current demand for consistent and replicable methods5,19,21. We provide evidence for the suitability of assessing a multifaceted index of CR by using a standardised questionnaire ascertaining multiple markers rather than a singular proxy (e.g. education).
Our findings support the hypothesis that CR represents a complex and multifaceted construct, which may have a synergistic influence on dementia risk. Education, occupation and leisure activities were found to be independently related to a reduced risk of dementia, contributing to the accumulation of CR across the lifespan. Given that CR capacity appears to be determined earlier in life and continues to be enhanced throughout life, this work emphasises the importance of long-life learning and investing in social networks or leisure activities. Our findings also highlight the feasibility of obtaining a standardised, structuralised and replicable index of CR in a longitudinal study of ageing. Considering that CR is malleable throughout life, public health interventions focusing on increasing brain and mental resilience are recommended in order to contribute to successful ageing and reduced dementia risk.
Supplementary Material
Acknowledgements
The authors would like to thank Professor Massimo Nucci, University of Padova, Italy for providing the formulas for the derivation of the CRIq and Dr Eddy Davelaar, Birkbeck College, University of London, UK for implementing the algorithms that were applied to the raw data.
Funding
The work was supported by the National Institute on Aging (grants RO1AG7644-01A1 & RO1AG017644); Economic and Social Research Council (grant ES/S013830/1) and Alzheimer Society (grant 477, AS-PhD-18b-022). The English Longitudinal Study of Ageing is funded by the National Institute on Aging (Grant RO1AG7644) and by a consortium of UK government departments coordinated by the Economic and Social Research Council and the Office for National Statistics.
Footnotes
Declaration of interest
All the authors declare no financial relationships with any organisations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the proposed work.
Data Availability
The English Longitudinal Study of Ageing (ELSA) was developed by a team of researchers based at University College London, the Institute for Fiscal Studies and the National Centre for Social Research. The data are linked to the UK Data Archive and freely available through the UK data services and can be accessed here: https://discover.ukdataservice.ac.uk.
References:
- 1.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009;374(9696):1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y Cognitive reserve. Neuropsychologia 2009;47(10):2015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11(11):1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci 2013;17(10):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Ageing, Neuropsychol Cogn 2016;23(1):40–60. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M, Mapelli D, Mondini S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Ageing Clin Exp Res 2012;24(3):218–26. [DOI] [PubMed] [Google Scholar]
- 7.Jorm A A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994;24(1):145–53. [DOI] [PubMed] [Google Scholar]
- 8.Quinn TJ, Fearon P, Noel-Storr AH, Young C, McShane R, Stott DJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within community-dwelling populations. Cochrane Libr 2014;10(4). [DOI] [PubMed] [Google Scholar]
- 9.Office for National Statistics. The National Statistics Socio-Economic Classification (NS-SEC) [Internet] [cited 2017. Aug 20]. Available from: https://www.ons.gov.uk/
- 10.Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer’s Disease incidence data from the Kungsholmen Project, Stockholm. Neurology 1997;48(1):132–8. [DOI] [PubMed] [Google Scholar]
- 11.Håkansson K, Rovio S, Helkala EL, Vilska AR, Winblad B, Soininen H, et al. Association between mid-life marital status and cognitive function in later life: population-based cohort study. BMJ Br Med J 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadar D, Lassale C, Davies H, Llewellyn DJ, Batty GD, Steptoe A. Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-Year follow-up in the English Longitudinal Study of Ageing. JAMA Psychiatry 2018;75(7):723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13(8):788–94. [DOI] [PubMed] [Google Scholar]
- 14.Wolters FJ, Segufa RA, Darweesh SK, Bos D, Ikram MA, Sabayan B, et al. Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimer’s Dement 2018;1–12. [DOI] [PubMed]
- 15.Elovainio M, Hakulinen C, Pulkki-Råback L, Virtanen M, Josefsson K, Jokela M, et al. Contribution of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Heal 2017;2(6):e260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marioni RE, van den Hout A, Valenzuela MJ, Brayne C, Matthews FE. Active cognitive lifestyle associates with cognitive recovery and a reduced risk of cognitive decline. J Alzheimer’s Dis 2012;28(1):223–30. [DOI] [PubMed] [Google Scholar]
- 17.Brayne C, McCracken C, Matthews FE. Cohort profile: The Medical Research Council Cognitive Function and Ageing Study (CFAS). Int J Epidemiol 2006;35: 1140–5. [DOI] [PubMed] [Google Scholar]
- 18.Valenzuela M, Brayne C, Sachdev P, Wilcock G, Matthews F. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol 2011;173(9):1004–12. [DOI] [PubMed] [Google Scholar]
- 19.Harrison SL, Sajjad A, Bramer WM, Ikram MA, Tiemeier H, Stephan BC. Exploring strategies to operationalize cognitive reserve: a systematic review of reviews. J Clin Exp Neuropsychol 2015;37(3):253–64. [DOI] [PubMed] [Google Scholar]
- 20.Dekhtyar S, Wang HX, Scott K, Goodman A, Koupil I, & Herlitz A A life-course study of cognitive reserve in dementia—from childhood to old age. Am J Geriatr Psychiatry 2015;23(9):885–96. [DOI] [PubMed] [Google Scholar]
- 21.Chapko D, McCormack R, Black C, Staff R, Murray A. Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia–a systematic literature review. Aging Ment Health 2017;13(1):1–12. [DOI] [PubMed] [Google Scholar]
- 22.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. International Journal of Epidemiology. Int J Epidemiol 2005;34(2):251–6. [DOI] [PubMed] [Google Scholar]
- 23.Xue B, Cadar D, Fleischmann M, Stansfeld S, Carr E, Kivimäki M, et al. Effect of retirement on cognitive function: The Whitehall II cohort study. Eur J Epidemiol 2017;1–13. [DOI] [PMC free article] [PubMed]
- 24.Kröger E, Andel R, Lindsay J, Benounissa Z, Verreault R, Laurin D. Is complexity of work associated with risk of dementia? The Canadian Study of Health and Aging. Am J Epidemiol 2008;167(7):820–30. [DOI] [PubMed] [Google Scholar]
- 25.Then F, Luck T, Luppa M, Thinschmidt M, Deckert S, Nieuwenhuijsen K, et al. Systematic review of the effect of the psychosocial working environment on cognition and dementia. Occup Environmental Med 2013;71(5). [DOI] [PubMed] [Google Scholar]
- 26.Helmer C, Letenneur L, Rouch I, Richard-Harston S Barberger-Gateau P, Fabrigoule C, Orgogozo JM, et al. Occupation during life and risk of dementia in French elderly community residents. J Neurol Neurosurg Psychiatry 2001;71(3):303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 2001;57(12):2236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson RS, De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287(6):742–8. [DOI] [PubMed] [Google Scholar]
- 29.Fratiglioni L, Viitanen M, Bäckman L, Sandman PO, Winblad B. Occurrence of dementia in advanced age: the study design of the Kungsholmen Project. Neuroepidemiology 1992;11(1):29–36. [DOI] [PubMed] [Google Scholar]
- 30.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol 2002;155(12):1081–7. [DOI] [PubMed] [Google Scholar]
- 31.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 2004;3(6):343–53. [DOI] [PubMed] [Google Scholar]
- 32.Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging 2002;23(5):941–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


