Abstract
Neoadjuvant chemotherapy (NAC) is often the treatment of choice for borderline resectable and locally advanced invasive pancreatic ductal adenocarcinoma (PDAC); however, most cancers only partially respond to therapy. We hypothesized that the location of residual neoplastic cells in resected specimens following NAC could provide a clue as to the mechanisms of resistance. PDAC cells invade the stroma but can also invade back into and spread via the pancreatic ducts, which has been referred to as “cancerization of ducts” (COD). We compared the responsiveness to chemotherapy between PDAC cells in the stroma and PDAC cells in the duct. Pancreatic resections from a total of 174 PDAC patients (NAC, n = 97; immediate surgery, n = 77) were reviewed. On hematoxylin and eosin sections, COD was identified at the same prevalence in both groups (NAC: 50/97 cases, 52%; immediate surgery: 39/77 cases, 51%; p = 0.879, Fisher’s exact test). However, using quantitative image analysis of CK19 immunohistochemistry, we found that the proportion of cancer cells that were intraductal was significantly different between the NAC and immediate surgery groups (median; 12.7% versus 1.99%, p < 0.0001, Mann–Whitney U test). This proportion was highest in patients with marked therapy responses (36.2%) compared with patients with moderate or poor responses (7.2&7.9%). In summary, our data suggest that intraductal components in PDAC are less responsive to chemotherapy than the remainder of the tumor, which could have important implications for therapeutic resistance.
Keywords: pancreatic ductal adenocarcinoma, cancerization of ducts, neoadjuvant chemotherapy, CAP score, therapeutic resistance
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer with poor prognosis [1]. It is the third leading cause of cancer-related mortality in the United States and is predicted to cause 47,050 deaths in 2020 [2]. Moreover, PDAC is expected to become the second most common cause of cancer-related mortality by 2030 [3].
PDAC is characterized histologically by neoplastic gland-forming epithelial cells invading into dense fibrous stroma [4, 5]. PDACs can also grow from this stromal location into and along pre-existing pancreatic ducts and ductules [6–10]. This pattern of intraductal growth of malignant cells has been referred to as “cancerization of ducts” (COD) [10]. COD is defined by the presence of cytologically malignant intraductal neoplastic cells immediately adjacent to a carcinoma invading stroma, frequently with an abrupt transition between lesional and normal ductal epithelium. A recent study suggested that COD is extraordinarily common in PDAC, and indeed that it could be much more common than high-grade pancreatic intraepithelial neoplasia (PanIN) [10].
Neoadjuvant chemotherapy (NAC) has emerged as a promising treatment strategy for patients with borderline resectable and locally advanced PDAC [11–15]. NAC can downstage the disease, increase the rate of negative margin after resection (R0), and improve postoperative mortality rates [11–15]. The gold standard for the definitive evaluation of the response to NAC relies on postoperative histopathology [16–24]. However, the histopathological consequences of NAC are difficult to fully characterize [25, 26] because the pre-therapy histology is often limited to a small biopsy, making it impossible to determine if a histologic finding can be attributed to therapy or to sampling.
In this study, we hypothesized that responsiveness to chemotherapy differs between intraductal cancer cells (COD) and cancer cells growing in stroma. To test this hypothesis, we histologically reviewed surgical pancreatectomy specimens from a large cohort of PDAC patients treated with NAC followed by surgery and compared the findings to those in pancreata from patients who did not receive NAC. We also quantitatively analyzed the numbers of neoplastic cells inside and outside the pancreatic ducts. Our results suggest that neoplastic cells within ducts, compared to those within the stroma, are relatively resistant to therapy, a finding that has important implications for therapeutic resistance after chemotherapy.
Materials and methods
Case Selection
After we obtained approval from the institutional review board at the Johns Hopkins Hospital, we searched the pathology archives to identify patients who had undergone pancreatic resection with or without NAC for PDAC between 2014 and 2019. We evaluated histology and therapeutic effects in specific populations with strict exclusion criteria. We excluded the following patients: (1) patients with invasive carcinomas arising in intraductal papillary mucinous neoplasm of the pancreas (IPMN), as these lesions have prominent intraductal components that could confound quantification; (2) patients who had received less than two cycles of chemotherapy, as the histological effects of NAC may not be well developed at the time of resection; and (3) patients treated with experimental therapies (such as vaccine therapy) as their histological effects could differ from those of more standard NAC regimens. After exclusion based on these criteria, our study population comprised 97 patients who had been treated with NAC and 77 who had not received NAC. We reviewed the electronically stored clinical records to determine pathological characteristics and patient outcome. The pathological tumor, node, metastasis (TNM) stage was identified in accordance with the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Eighth Edition [16].
Identification of Duct Lesions
At our institution, the pancreas is serially sectioned for gross examination as part of specimen processing for clinical diagnosis. Representative sections of the ill-defined area of firmness, background tan-yellow lobulated pancreatic parenchyma, fatty connective tissue including lymph nodes, and surgical margins are submitted for histological evaluation (total >40 blocks). At least four representative blocks of tumor from each PDAC resection specimen were examined in this analysis.
In order to comprehensively identify COD in the PDACs in our cohort, all hematoxylin and eosin (H&E)-stained slides were jointly reviewed by three pathologists (K.F., E.D.T., and L.D.W.) for the morphological features of COD. Duct lesions were classified as COD or pancreatic intraepithelial neoplasia (PanIN) according to the previously established criteria [10]. Briefly, COD was defined as the intraductal spread of invasive carcinoma along the main or branched pancreatic duct. Intraductal lesions satisfying the following three criteria were diagnosed as COD rather than PanIN: (1) infiltrating carcinoma located in close proximity to a duct lesion; (2) abrupt transition from a highly atypical lesion to normal duct epithelium (Fig. 1); and (3) lesion cytology similar to that of the cancer cells in stroma. In only a few lesions it was difficult or impossible to distinguish COD from high-grade PanIN, even when we rigorously applied the diagnostic criteria. Such lesions were excluded from quantitative analysis. While most cases had a single slide with COD, we identified foci of COD on multiple slides in a subset of cases. When this occurred, we selected one representative slide with COD closest to the center of the PDAC mass. We found that the center of the PDAC mass was most representative, as it had the highest proportion of malignant cells (without large areas of non-neoplastic tissue) and was not confounded by growth of tumor into other structures (such as duodenum and common bile duct). The process for slide selection was performed consistently for all cases regardless of whether NAC treatment was administrated or not.
Fig 1. Cancerization of ducts (COD), or intraductal spread of invasive ductal adenocarcinoma.
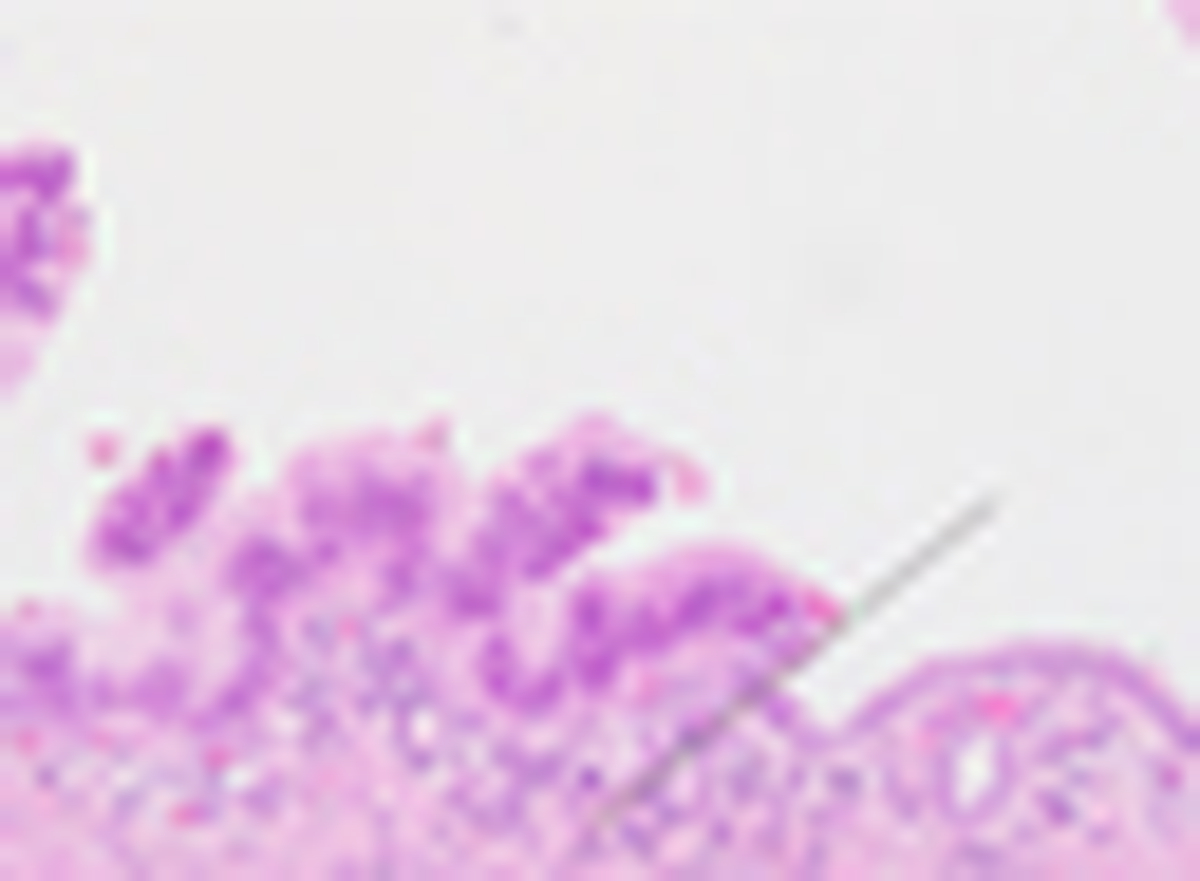
The black dotted line indicates the abrupt transition from intraductal pancreatic ductal adenocarcinoma (PDAC) cells to normal duct epithelium. Hematoxylin & eosin (H&E)-stained section; original magnification, 400×.
Evaluation of the Treatment Response
Pathologic treatment response, estimated from the extent of the residual tumor, was graded on the basis of the slide with the greatest amount of tumor and in accordance with the College of American Pathologists (CAP) criteria [21]: Score 0, no viable residual tumor, characterizing a pathologic complete response (pCR); Score 1, close to a CR, with minimal residual cancer in the form of single cells or rare small groups of cells; Score 2, a partial response (PR), where the residual cancer shows evident tumor regression but is more than single cells or rare small groups of cancer cells; or Score 3, poor or no response, characterized by extensive residual cancer showing no apparent tumor regression.
Immunohistochemistry
We performed immunohistochemical (IHC) labeling for cytokeratin 19 (CK19) on one representative slide per case with COD. Formalin-fixed, paraffin-embedded blocks were sectioned at 5 μm, deparaffinized, and subjected to antigen retrieval. Sections were then immunolabeled for CK19 (mouse monoclonal clone RCK108; 1:100 dilution; Dako, Denmark) using the BenchMark Ultra automated slide stainer (Ventana Medical Systems, AZ). We then scanned all the IHC slides at 20 × magnification using NanoZoomer XR (Hamamatsu Photonics, Japan). Regions of COD and carcinoma in stroma were separately annotated on each scanned IHC slide while concurrently reviewing the cellular morphology on the matched H&E slide using a microscope. This approach allowed distinction of normal ductal cells and PDAC, both of which are labeled by CK19 but have a strikingly different morphology on IHC and H&E stains. The number of CK19-positive cancer cells in each region was quantified using the HALO Image Analysis Platform (Indica labs, NW). Non-neoplastic CK19-positive epithelium was excluded from this quantification based on morphology.
Statistical Analysis
The data were analyzed using the Fisher’s exact test, paired t-test, or Mann–Whitney U test. A probability of p < 0.05 was considered to be significant. Overall survival was analyzed using the Kaplan-Meier method and log-rank test. Statistical analyses were performed using JMP software (version 10.0; SAS Institute, Cary, NC).
Results
Clinical and Pathological Characteristics
Of the 174 patients studied, 85 (52%) were male, 147 (84%) were Caucasian, and the median age was 68 years (range: 37–90; Table 1). The majority (78%) of PDACs were located in the head of the pancreas. Neoadjuvant chemotherapy plus surgery was administered to 97 of the patients (NAC group), and 77 were treated with immediate surgery (immediate surgery group). In the NAC group, 27 patients received gemcitabine-based therapy, 51 received FOLFIRINOX-based therapy, and 16 received another combination therapy. Responses to NAC on resection specimens were scored as follows: one patient (1%) with a CAP score of 0, 25 (26%) with a CAP score of 1, 53 (55%) with a CAP score of 2, and 18 (19%) with a CAP score of 3. The pathological stage was significantly lower in the NAC group compared to the immediate surgery group (p < 0.0001, Fisher’s exact test). The median size of tumors was 22 mm (range: 0–78 mm) in the NAC group and 30 mm (range: 5–75 mm) in the immediate surgery group (p < 0.0001, Mann–Whitney U test). In the patients in the NAC group, the pT-stage was less advanced (p < 0.0001, Fisher’s exact test), pN0 was more frequent (p < 0.0001, Fisher’s exact test), and lympho-vascular and perineural invasion were less frequent (p = 0.0149 and p < 0.0001, Fisher’s exact test) than for patients in the immediate surgery group. Fisher’s exact tests indicated that there were no significant differences in age, gender, ethnicity, tumor location, or final pancreatic margin between the two groups (Table 1). There was no statistically significant difference in overall survival between the immediate surgery group and NAC group (p = 0.650, log-rank test) although 3-year survival rate was lower in immediate surgery group (56% for immediate surgery group vs. 88% for NAC group). Patients with a CAP score of 1 had a longer overall survival compared to those with a CAP score of 2–3, though this did not reach statistical significance (3-year overall survival, 100% for Score 1 vs. 88% for Score 2 vs. 68% for Score 3; Score 1 vs Score 3, p = 0.0538, log-rank test).
Table 1:
Clinicopathologic Characteristics of Patients with Pancreatic Ductal Adenocarcinoma Treated With and Without Neoadjuvant Chemotherapy.
| Neoadjuvant therapy (N=97) | Immediate surgery (N=77) | All (N=174) |
P-value | |
|---|---|---|---|---|
| Age (median [range]) (y) | 68 [45–81] | 70 [37–90] | 68 [37–90] | 0.221 |
| Gender | ||||
| Male | 44 (45%) | 45 (58%) | 89 (52%) | 0.095 |
| Female | 53 (55%) | 32 (42%) | 85 (48%) | |
| Ethnicity | ||||
| Caucasian | 83 (86%) | 64 (83%) | 147 (84%) | 0.678 |
| Others | 14 (14%) | 13 (17%) | 27 (16%) | |
| Tumor size and location | ||||
| Size (median [range]) (mm)* | 22 [0–78] | 30 [5–75] | 27 [0–78] | <0.0001 |
| Head | 73 (75%) | 62 (81%) | 135 (78%) | 0.467 |
| Body or tail | 24 (25%) | 15 (19%) | 39 (22%) | |
| pT classification (AJCC 8th) | ||||
| pT0 | 1 (1%) | 0 (0%) | 1 (1%) | <0.0001 |
| pTis | 1 (1%) | 0 (0%) | 1 (1%) | |
| pT1 | 45 (46%) | 11 (14%) | 56 (32%) | |
| pT2 | 39 (40%) | 54 (70%) | 93 (53%) | |
| pT3 | 10 (10%) | 12 (16%) | 22 (13%) | |
| pT4 | 1 (1%) | 0 (0%) | 1 (1%) | |
| pN classification (AJCC 8th) | ||||
| pN0 | 53 (55%) | 12 (18%) | 65 (37%) | <0.0001 |
| pN1 | 30 (31%) | 38 (48%) | 68 (39%) | |
| pN2 | 14 (14%) | 27 (34%) | 41 (24%) | |
| pM classification (AJCC 8th) | ||||
| pM0 | 95 (98%) | 77 (100%) | 172 (99%) | 0.504 |
| pM1 | 2 (2%) | 0 (0%) | 2 (1%) | |
| pStage (AJCC 8th)** | ||||
| pStage 0 | 1 (1%) | 0 (0%) | 1 (1%) | <0.0001 |
| pStage I | 47 (48%) | 12 (16%) | 59 (34%) | |
| pStage II | 31 (32%) | 38 (49%) | 69 (40%) | |
| pStage III | 15 (15%) | 27 (35%) | 42 (24%) | |
| pStage IV | 2 (2%) | 0 (0%) | 2 (1%) | |
| Lymphovascular invasion | ||||
| Present | 36 (38%) | 43 (57%) | 79 (45%) | 0.0149 |
| Absent | 61 (62%) | 34 (43%) | 95 (55%) | |
| Perineural invasion | ||||
| Present | 66 (65%) | 72 (94%) | 138 (79%) | <0.0001 |
| Absent | 31 (35%) | 5 (6%) | 36 (21%) | |
| Histological grade | ||||
| G1 | NA | 5 (6%) | ||
| G2 | NA | 40 (52%) | ||
| G3 | NA | 30 (39%) | ||
| G4 | NA | 2 (3%) | ||
| Final pancreatic margin | ||||
| Positive | 8 (8%) | 12 (16%) | 20 (11%) | 0.155 |
| Negative | 89 (92%) | 65 (84%) | 154 (89%) | |
| Neoadjuvant therapy (NAC) | ||||
| NAC | 52 (54%) | NA | ||
| NAC+RT | 45 (46%) | NA | ||
| NAC regimen | ||||
| Gemcitabine based | 27 (28%) | NA | ||
| FOLFIRINOX based | 51 (53%) | NA | ||
| Combination | 16 (16%) | NA | ||
| Others | 3 (3%) | NA | ||
| Response to NAC (CAP score) | ||||
| Score 0 | 1 (1%) | NA | ||
| Score 1 | 25 (26%) | NA | ||
| Score 2 | 53 (55%) | NA | ||
| Score 3 | 18 (19%) | NA | ||
| Cancerization | ||||
| Present | 50 (52%) | 39 (51%) | 89 (51%) | 0.879 |
| Absent | 47 (48%) | 38 (49%) | 85 (49%) | |
Tumor size of invasive component
One pT0 case in NAC group was excluded according to the AJCC staging system.
Histological Changes after Chemotherapy
The neoplastic cells in the post-chemotherapy surgical specimens had morphological changes, such as nuclear enlargement, bizarre nuclei, cytoplasmic eosinophilia, and vacuolization. In the responsive area, the cancer tissue was replaced by massive fibrous, necrotic, or xanthogranulomatous lesions, with infiltrations of macrophages and lymphocytes. On H&E sections, COD was identified at the same prevalence in the NAC and immediate surgery groups (NAC: 50/97 cases, 52%; immediate surgery: 39/77 cases, 51%; p = 0.879, Fisher’s exact test). Interestingly, in the NAC group, we observed ten cases (10%) with extensive COD, with >50% of residual cancer cells in an intraductal location. In one exceptional case (NAC#83), which was pathologically staged as ypTis, residual cancer cells remained only within the ducts (Fig. 2).
Fig 2. Representative case with extensive cancerization of the ducts.
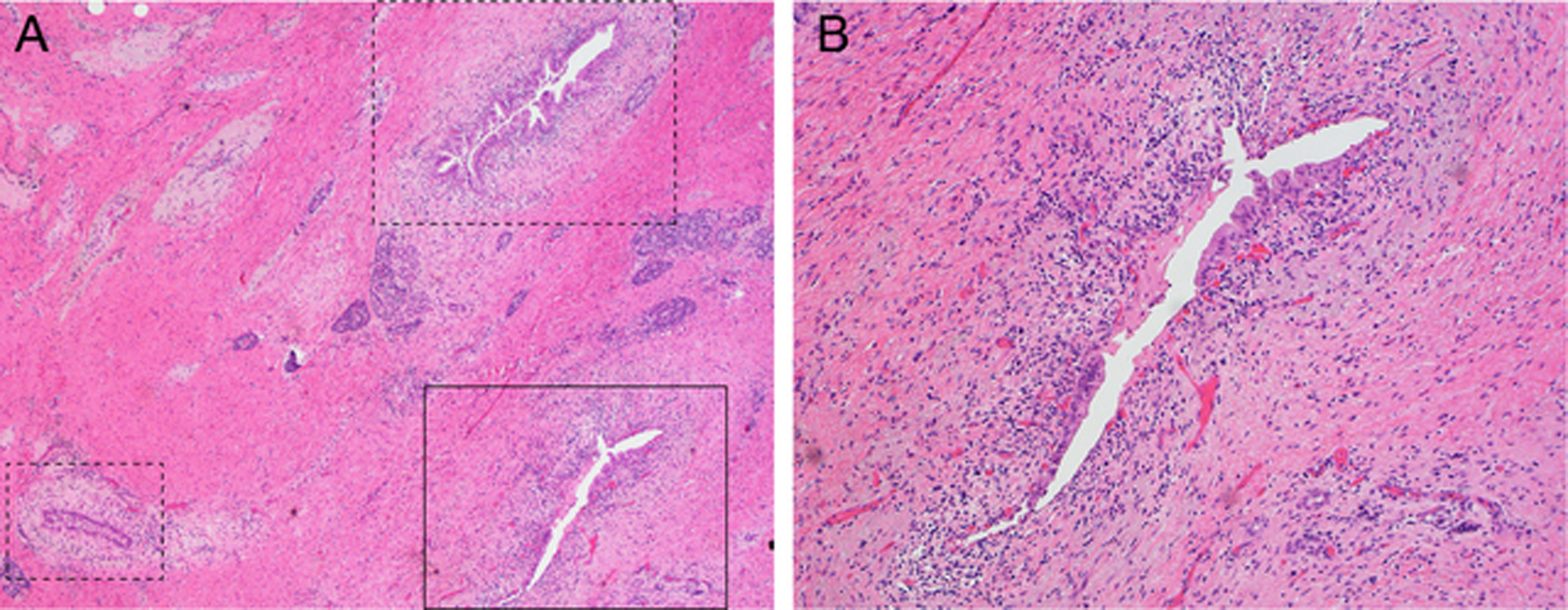
(A) Cancer cells invading stroma are not detected in this case (Case #83). Black straight/dotted boxes indicate cancerization of the ducts. H&E-stained section; original magnification, 20×. (B) Enlarged view of black straight box. Original magnification, 100×.
These observations raised the possibility that the intraductal PDAC cells (COD cells) are less responsive to chemotherapy compared to PDAC cells in the stroma. To further evaluate this, we examined the proportion of cancer cells that were intraductal, i.e., the number of intraductal malignant cells divided by the total number of malignant cells. A schematic of our study design is shown in Figure 3. We compared this proportion between two large cohorts of resected PDAC specimens: the NAC group (n = 97, including 50 with COD) and the immediate surgery group (n = 77, including 39 with COD). Specifically, we performed immunolabeling for CK19 to reliably identify PDAC cells and then digitally quantified PDAC cells inside and outside the ducts for each case with COD using the HALO image analysis program.
Fig 3.
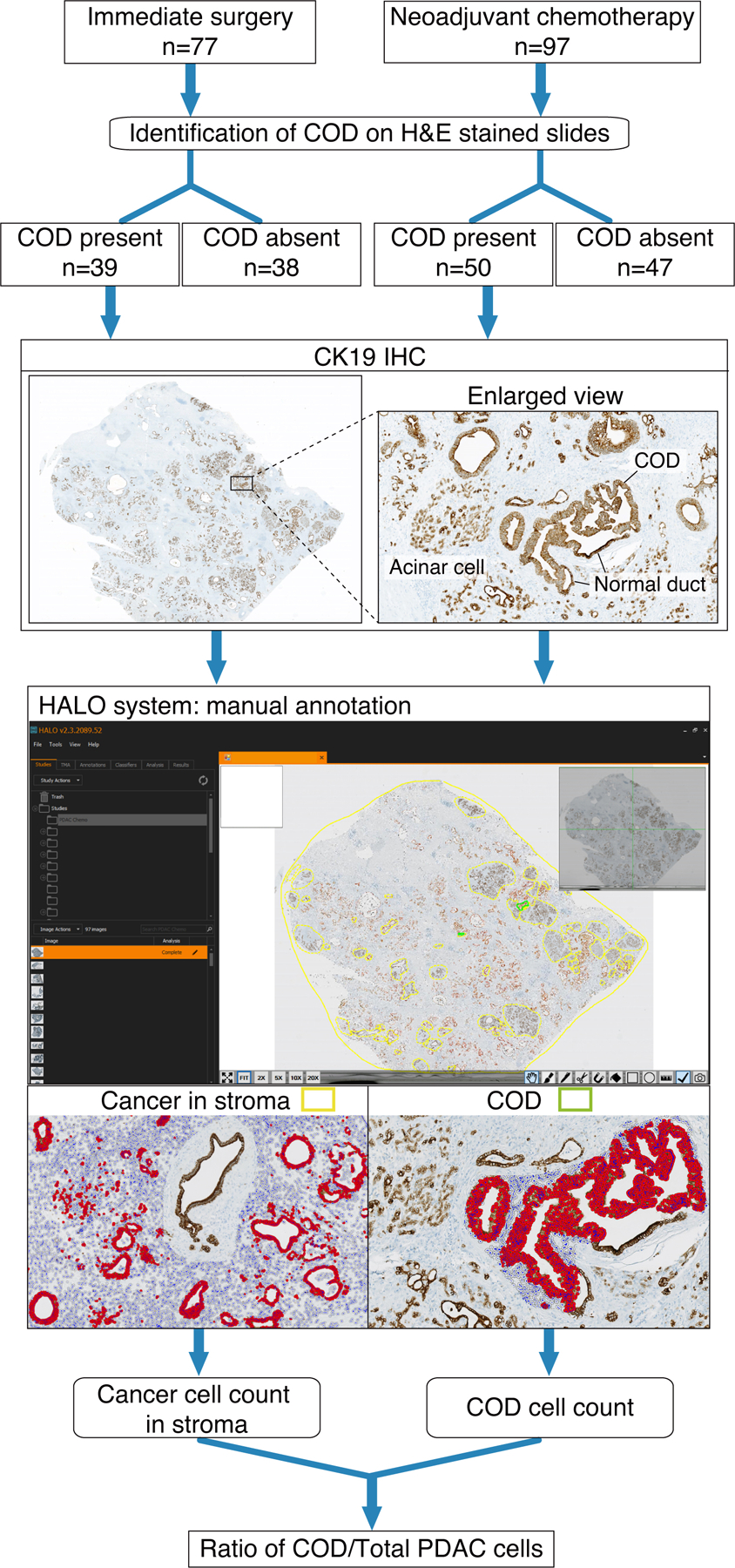
Flow diagram depicting the overall study design.
There was a statistically significant difference in the proportion of residual cancer cells located in the ducts between the NAC and immediate surgery groups (median: 12.7% vs. 1.99%, p < 0.0001, Mann–Whitney U test) (Fig. 3A). When the NAC group was divided into four subgroups based on the CAP scores (0–3) representing the pathological response to NAC, the proportion of COD cells was highest (median: 36.2%) in the score 1 subgroup, and much lower in the score 2 (median: 7.21%, p = 0.0020 vs. score 1, Mann–Whitney U test) and score 3 (median: 7.91%, p = 0.0162 vs. score 1) subgroups (Fig. 3B). One case with score 0 was not included in these analyses since no viable cancer cells were identified in either stroma or duct in any examined slides (pCR). In the other cases, the residual cancer cells in the stroma exhibited various morphological changes, including distortion of glandular architecture, cellular enlargement due to increased cytoplasm, eosinophilic change and cytoplasmic vacuolization. These nuclear/cytoplasmic changes were less apparent in the intraductal cancer cells than in the cancer cells in the stroma (Fig. 4). In order to quantify proliferation, we also counted mitoses in COD and stromal cancer cells in scanned slides from 24 cases (NAC n = 16; immediate surgery n= 8) in our cohort. This analysis showed that the mean proportion of cells in mitosis was similar for COD cells and stromal cancer cells in both NAC and immediate surgery groups, with no statistically significant difference in the proportion of mitotic cells between these two regions (p > 0.5, paired t-test; Supplementary Table 1). This suggests that the proliferation rate of malignant cells is similar in the stromal and intraductal locations.
Fig 4. Proportion of cancer cells which are intraductal.
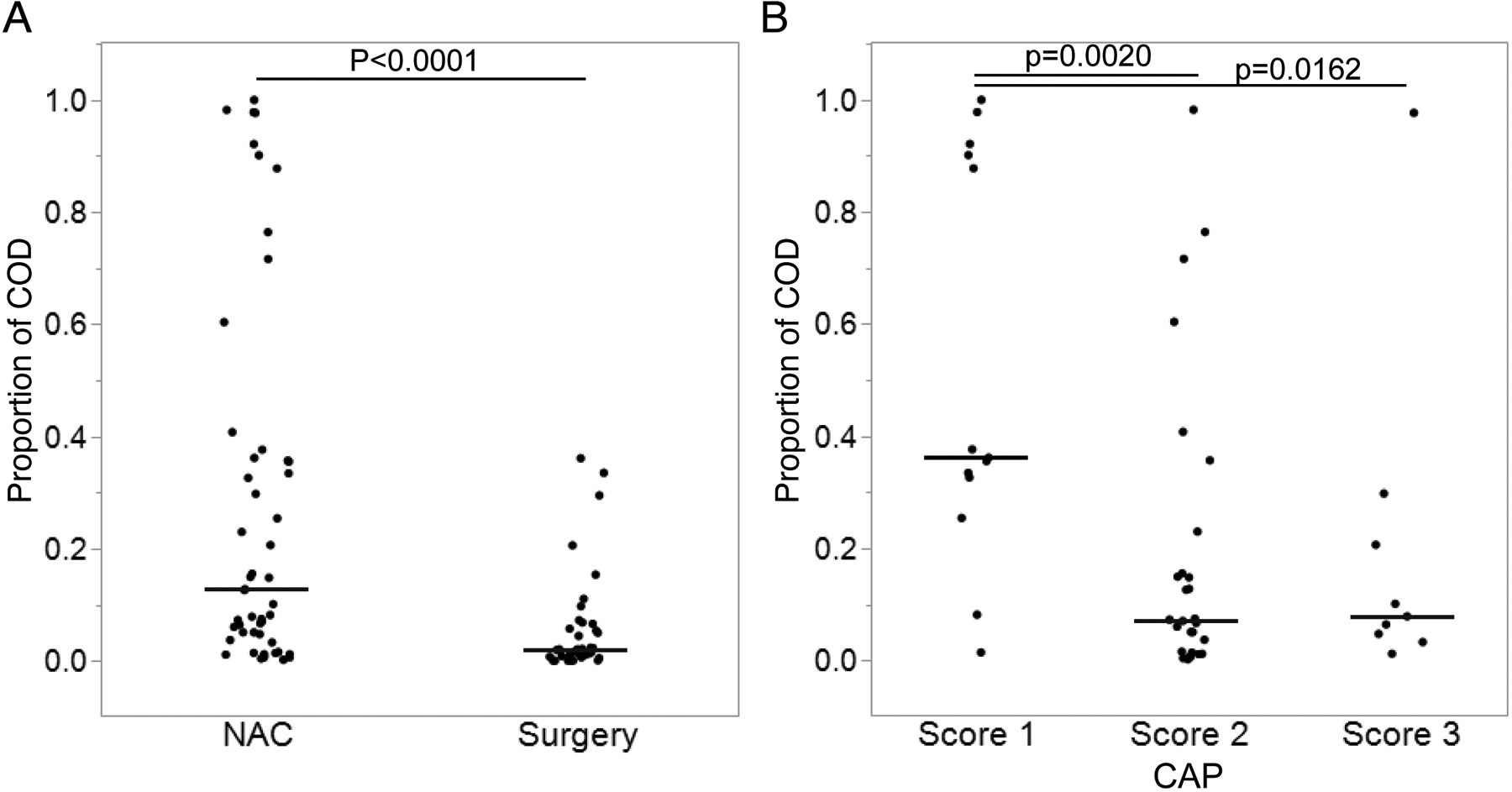
Data are presented as scatter dot plot with lines representing median. (A) Neoadjuvant chemotherapy (NAC) group versus Immediate surgery group. (B) College of American Pathologists (CAP) score 1–3.
In this retrospective study, the patients in the NAC group included those treated with NAC alone (n = 52, 54%) as well as those treated with NAC combined with radiation therapy (RT) (n = 45, 46%) (Table 1). In addition, our cohort included NAC using multiple different regimens (gemcitabine-based, FOLFILINOX-based, combination of these two, and others) (Table 1). We therefore investigated the correlation between the proportion of intraductal cancer cells and neoadjuvant treatment approach, but we found no significant differences between NAC and NAC+RT (p = 0.741, Mann–Whitney U test), or between distinct NAC regimens (p = 0.732, Mann–Whitney U test) (Supplementary Figure 1).
We next compared the proportion of COD cells by tumor stage in immediate surgery and NAC groups. There was no significant difference in the proportion of COD cells between tumor stages in the immediate surgery group (pStage 0–2 vs. pStage 3, p = 0.394, Mann–Whitney U test) or NAC group (pStage0–2 vs. pStage 3–4, p = 0.325, Mann–Whitney U test) (Supplementary Figure 2). In addition, in three NAC cases that would have been resectable by immediate surgery, we observed a higher mean proportion of COD cells compared to resectable cases undergoing immediate surgery (0.10 in NAC resectable cases vs 0.05 in immediate surgery resectable cases) (Supplementary Figure 3). Taken together, these data suggest that clinical and pathological stage were not related to the proportion of COD cells, and even when matched for resectability, NAC cases have a higher proportion of COD cells.
Correlation between the Presence of COD and Clinicopathological Features
There were no statistically significant associations between the presence of COD and tumor size, tumor location, TNM stage, perineural invasion, or neoadjuvant treatment (Table 2). COD was, however, associated with the presence of lympho-vascular invasion (present in 55% of cases with COD vs. 35% of cases without COD, p = 0.0100, Fisher’s exact) and a positive surgical margin (present in 17% of cases with COD vs. 6% of cases without COD, p = 0.0313). In the COD group, this included 13/89 (15%) patients with cancer cells in the stroma at the margin, as well as 2/89 (2%) patients with intraductal cancer cells at the margin. In contrast, in the group without COD, all positive margins (5/85 or 6% of the group) had cancer cells in the stroma at the margin. Despite the significant difference in the prevalence of positive surgical margins, we observed no significant difference in overall survival between cases with COD and without COD (hazard ratio, 0.847; p = 0.778, log-rank test).
Table 2:
Clinicopathologic Characteristics of Patients with Pancreatic Ductal Adenocarcinoma With and Without Cancerization.
| COD Present (N=89) |
COD absent (N=85) |
All (N=174) |
P-value | |
|---|---|---|---|---|
| Tumor size and location | ||||
| Size (median [range]) (mm)* | 30 [0–75] | 25 [0–78] | 27 [0–78] | 0.287 |
| Head | 65 (73%) | 70 (82%) | 135 (78%) | 0.151 |
| Body or tail | 24 (27%) | 15 (18%) | 39 (22%) | |
| pT classification (AJCC 8th) | ||||
| pT0 | 0 (1%) | 1 (1%) | 1 (1%) | 0.345 |
| pTis | 1 (1%) | 0 (0%) | 1 (1%) | |
| pT1 | 25 (28%) | 31 (36%) | 56 (32%) | |
| pT2 | 51 (57%) | 42 (49%) | 93 (53%) | |
| pT3 | 12 (13%) | 10 (12%) | 22 (13%) | |
| pT4 | 0 (0%) | 1 (1%) | 1 (1%) | |
| pN classification (AJCC 8th) | ||||
| pN0 | 32 (36%) | 33 (39%) | 65 (37%) | 0.341 |
| pN1 | 32 (36%) | 36 (42%) | 68 (39%) | |
| pN2 | 25 (28%) | 16 (19%) | 41 (24%) | |
| pM classification (AJCC 8th) | ||||
| pM0 | 88 (99%) | 84 (99%) | 172 (99%) | 1.00 |
| pM1 | 1 (1%) | 1 (1%) | 2 (1%) | |
| pStage (AJCC 8th)** | ||||
| pStage 0 | 1 (1%) | 0 (0%) | 1 (1%) | 0.532 |
| pStage I | 30 (34%) | 29 (34%) | 59 (34%) | |
| pStage II | 32 (36%) | 37 (44%) | 69 (40%) | |
| pStage III | 25 (28%) | 17 (20%) | 42 (24%) | |
| pStage IV | 1 (1%) | 1 (1%) | 2 (1%) | |
| Lymphovascular invasion | ||||
| Present | 49 (55%) | 30 (35%) | 79 (45%) | 0.0100 |
| Absent | 40 (45%) | 55 (65%) | 95 (55%) | |
| Perineural invasion | ||||
| Present | 73 (82%) | 65 (76%) | 138 (79%) | 0.455 |
| Absent | 16 (18%) | 20 (24%) | 36 (21%) | |
| Final pancreatic margin | ||||
| Positive | 15 (17%) | 5 (6%) | 20 (11%) | 0.0313 |
| Negative | 74 (83%) | 80 (94%) | 154 (89%) | |
| Neoadjuvant therapy | ||||
| NAC | 27 (30%) | 25 (29%) | 52 (30%) | 0.99 |
| NAC+RT | 23 (26%) | 22 (26%) | 45 (26%) | |
| No treatment | 39 (44%) | 38 (45%) | 77 (45%) | |
Tumor size of invasive component
One pT0 case in NAC group was excluded according to the AJCC staging system.
Discussion
The goal of this study was to better understand the location of residual PDAC cells following NAC. We focused on two different components of PDAC cells: cancer cells in ducts (COD cells) and cancer cells invading stroma. We observed a significant increase in the proportion of cancer cells in an intraductal location in post-NAC surgical specimens compared to specimens from PDAC patients who underwent immediate surgery. In addition, the proportion of intraductal cancer cells was significantly higher in cases where the chemotherapy had a marked effect (i.e., CAP score of 1). This strongly suggests that the intraductal component of PDAC is less responsive to chemotherapy than the component in the stroma.
The underlying mechanism by which the intraductal PDAC cells resist chemotherapy is unknown. One possible explanation is that molecular changes unique to COD cells drive their lack of response to chemotherapy. While multiple possible molecular mechanisms for therapeutic resistance have been proposed (for example, loss of TP53 function activating JAK2-STAT3 signaling [27], expression of the gemcitabine transporter ENT1 and the solute carrier protein ZIP4 [28]), there is no evidence to date that these are differentially present in intraductal cancer cells. For example, similar patterns of TP53 and SMAD4 loss are seen in intraductal cancer cells and cancer in the stroma [10]. Still, molecular changes that accumulate after intraductal spread (including those caused by the distinct tumor microenvironment) could contribute to the relative resistance to chemotherapy in these COD cells [29].
It is also possible that a physical obstacle is created when neoplastic cells grow within ducts: the basement membrane and the distance of the cancer cells from capillary vessels may be a barrier to the delivery of chemotherapeutic agents. Invasive carcinoma cells are accompanied by surrounding angiogenesis, and nutrients are supplied via capillary vessels around the periphery of the tumor. In contrast, intraductal cancer cells are covered by the original basement membrane and are supplied only by perfusion from capillary vessels outside the ducts. Thus, it is possible that the chemotherapeutic agents have limited delivery to intraductal cancer cells, accounting for their decreased response to treatment. Intriguingly, previous work has focused on the impact of the stroma on chemotherapeutic response in PDAC, but our study suggests that cancer cells within the pancreatic ducts, not those in the stroma, are the least responsive to chemotherapy [30–32].
An alternative explanation for our findings is that differences in tumor aggressiveness between the NAC and immediate surgery groups underlie the differing proportions of intraductal cancer cells between the two groups. This explanation posits that PDACs receiving NAC are clinically more advanced and thus more invasive, allowing more extensive access to the duct system and leading to a higher proportion of COD cells in this group. According to this explanation, tumors in the NAC group have a higher proportion of COD cells not because such cells are less responsive to NAC but instead because these tumors are more invasive at baseline.
However, several lines of evidence in our study argue against this explanation. First, we report a similar prevalence of COD in the NAC and immediate surgery groups as shown in Table 1. Thus, it is not the ability to access the duct system that differs between the NAC and immediate surgery groups but rather the number of cells in the duct compared to the stroma. Second, as shown in Figure 4, the proportion of COD cells was highest in tumors with a CAP score of 1 which had a marked response to NAC. This shows that the most responsive tumors in the NAC group (rather than the least response/most aggressive) have the highest proportion of COD cells. Third, we show that the proportion of COD cells was similar between pStage 0–2 and pStage 3 in the immediate surgery group, arguing against the hypothesis that more advanced tumors have more extensive intraductal invasion. Finally, the proportion of COD cells in resectable PDACs receiving NAC was higher than in immediate surgery cases. Thus, there was a higher proportion of COD cells in NAC cases matched with immediate surgery cases for resectability, though the number of cases in this comparison was too small to reach statistical significance. Taken together, the data in our study do not support the explanation that the proportion of COD cells increases with increasing stage. Instead our data show that the receipt of NAC (rather than tumor stage) is the key factor driving the proportion of intraductal cancer cells. Thus, our results suggest that that the distinct proportions of COD cells in the NAC and immediate surgery groups are due to differential response of the COD and stromal cancer cells to NAC.
Our results also have implications for grading of response to NAC in PDAC. Currently, several histopathologic grading schemes have been proposed for assessing tumor regression in PDAC after NAC [18–24]. Our results underscore that cases with residual cancer cells remaining only within the ducts should not be classified as a complete response, as these intraductal neoplastic cells represent invasive rather than in situ carcinoma. Moreover, as the proportion of intraductal cancer cells is high in some patients with marked therapy responses, extensive residual COD after NAC can be a hallmark of marked (though not complete) response.
One limitation of our study is the inability to compare the histology of paired pre- and post-NAC samples in the same patient. However, such a comparison is not possible, as comprehensive analysis of COD requires examination of resection specimens, which are only available at a single time point from each patient. To overcome this difficulty, we examined a large cohort of PDAC patients (>170) and compared COD in resection specimens from those treated with NAC to those treated with immediate surgery. Although not directly comparing pre- and post-NAC samples from the same patient, our large cohort should minimize the impact of interpatient variability in prevalence and behavior of COD. In addition, as described in the Materials and Methods, the entire pancreas was not examined microscopically in all cases in this retrospective study. Nonetheless, a total of >40 slides per case, including at least four representative tumor blocks, were reviewed in our analysis. Although we cannot exclude the possibility of unsampled COD in a small subset of cases, our cohort provided adequate statistical power to detect the difference in therapy response between intraductal and stromal cancer cells in cases with COD.
In conclusion, our pathologic analysis of PDACs reveals a difference in responsiveness to NAC between PDAC cells in the duct (COD cells) and PDAC cells in the stroma. Even if NAC has a marked effect on tumor size, residual cancer cells may remain viable within the duct system and could then contribute to tumor regrowth. Further research is needed to clarify the prognostic significance of such residual intraductal components and the underlying mechanisms of this phenomenon.
Supplementary Material
Fig 5. Morphological changes in the nucleus and cytoplasm of residual cancer cells following treatment with NAC.
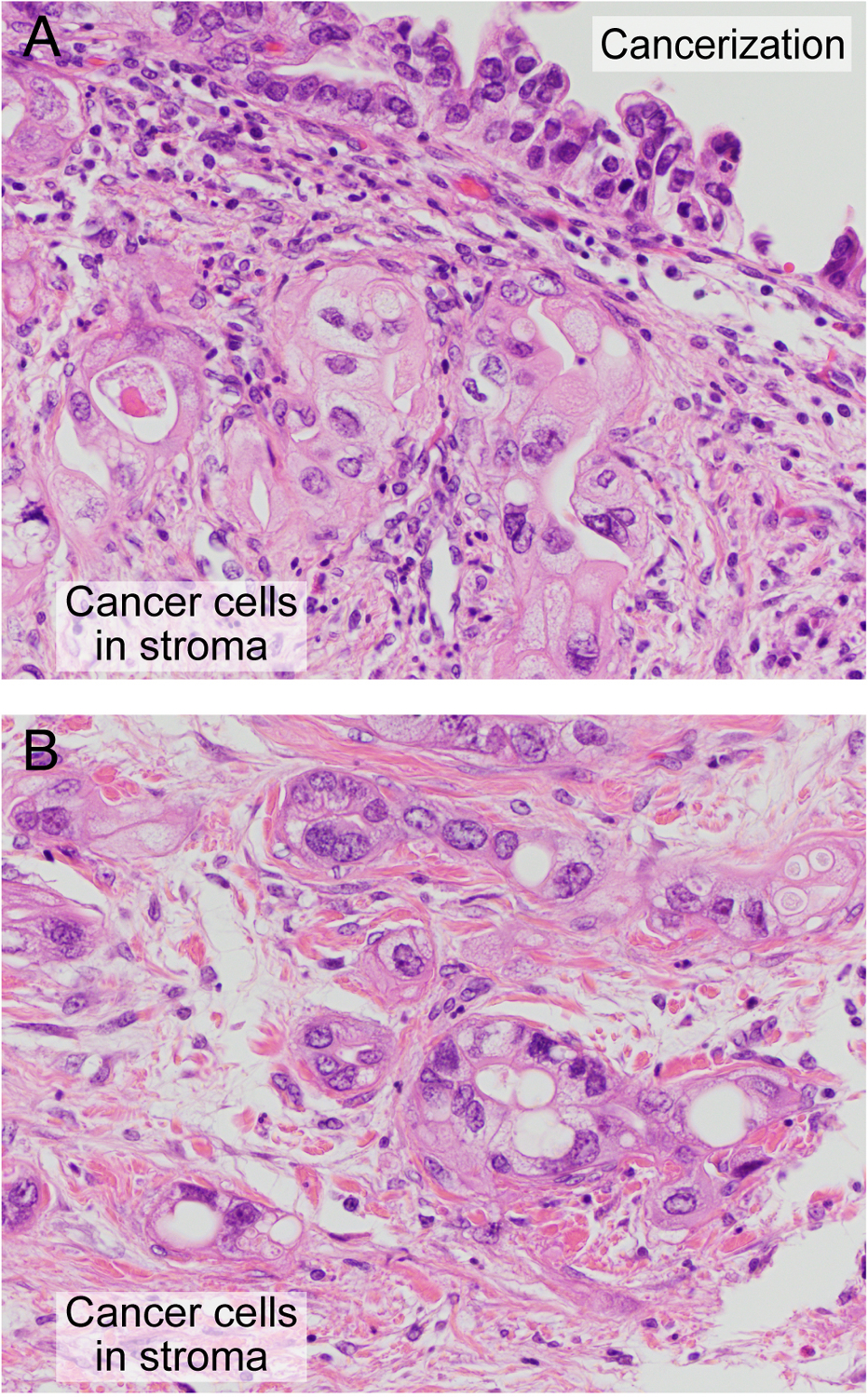
(A) PDAC cells in the stroma and PDAC cells in the duct (COD cells). (B) PDAC cells in the stroma adjacent to panel (A). Original magnification, 400×, all fields.
The authors acknowledge the following sources of funding:
NIH/NCI P50 CA62924; NIH/NIDDK K08 DK107781; Sol Goldman Pancreatic Cancer Research Center; Buffone Family Gastrointestinal Cancer Research Fund; Carol S. and Robert M. Long Pancreatic Cancer Research Fund; Kaya Tuncer Career Development Award in Gastrointestinal Cancer Prevention; AGA-Bernard Lee Schwartz Foundation Research Scholar Award in Pancreatic Cancer; Sidney Kimmel Foundation for Cancer Research Kimmel Scholar Award; AACR-Incyte Corporation Career Development Award for Pancreatic Cancer Research; American Cancer Society Research Scholar Grant; Emerson Collective Cancer Research Fund; Rolfe Pancreatic Cancer Foundation; Joseph C Monastra Foundation; The Gerald O Mann Charitable Foundation (Harriet and Allan Wulfstat, Trustees); Susan Wojcicki and Denis Troper.
Abbreviations
- PDAC
Pancreatic ductal adenocarcinoma
- COD
Cancerization of ducts
- PanIN
Pancreatic intraepithelial neoplasia
- NAC
Neoadjuvant chemotherapy
- RT
Radiation therapy
- CAP
College of American Pathologists
- IHC
Immunohistochemistry
- pStage
Pathological Stage
- pCR
Pathological complete response
- PR
Partial response
Footnotes
Conflict of Interest
LDW receives research support from Applied Materials. The other authors declare no conflict of interest.
References
- 1.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet. 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014; 74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, Klimstra DS. Adenocarcinoma of the pancreas. Semin Diagn Pathol. 2014;31:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hackeng WM, Hruban RH, Offerhaus GJ, et al. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol. 2016;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klöppel G, Bommer G, Ruckert K, et al. Intraductal proliferation in the pancreas and its relationship to human and experimental carcinogenesis. Virchows Arch A Pathol Anat Histol. 1980;387:221–233. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki S, Suda K, Nobukawa B, et al. Intraductal spread of pancreatic cancer. Clinicopathologic study of 54 pancreatectomized patients. Pancreatology. 2002;2:407–412. [DOI] [PubMed] [Google Scholar]
- 8.Basturk O, Hong SM, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii M, Kimura Y, Sugita S, et al. Surgical and oncological impact of main pancreatic duct spread in invasive ductal adenocarcinoma: A clinicopathological study of 184 resected cases. Pancreatology. 2015;15:681–687. [DOI] [PubMed] [Google Scholar]
- 10.Hutchings D, Waters KM, Weiss MJ, et al. Cancerization of the Pancreatic Ducts: Demonstration of a Common and Under-recognized Process Using Immunolabeling of Paired Duct Lesions and Invasive Pancreatic Ductal Adenocarcinoma for p53 and Smad4 Expression. Am J Surg Pathol. 2018;42:1556–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. [DOI] [PubMed] [Google Scholar]
- 12.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. [DOI] [PubMed] [Google Scholar]
- 13.Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol. 2015;22:3512–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackert T, Sachsenmaier M, Hinz U, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg. 2016;264:457–63. [DOI] [PubMed] [Google Scholar]
- 15.Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakar S, Pawlik TM, Allen PJ, et al. Exocrine pancreas In: Amin M, ed. AJCC Cancer Staging Manual, 8th ed. New York, NY: Springer; 2016:337–347. [Google Scholar]
- 17.Cong L, Liu Q, Zhang R, et al. Tumor size classification of the 8(th) edition of TNM staging system is superior to that of the 7(th) edition in predicting the survival outcome of pancreatic cancer patients after radical resection and adjuvant chemotherapy. Sci Rep. 2018;8:10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa O, Ohhigashi H, Teshima T, et al. Clinical and histopathological appraisal of preoperative irradiation for adenocarcinoma of the pancreatoduodenal region. J Surg Oncol. 1989;40:143–151. [DOI] [PubMed] [Google Scholar]
- 19.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakar S, Shi C, Adsay V, et al. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. College of American Pathologists. 2017 [Google Scholar]
- 22.Lee SM, Katz MH, Liu L, et al. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol. 2016;40:1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee D, Katz MH, Foo WC, et al. Prognostic Significance of New AJCC Tumor Stage in Patients With Pancreatic Ductal Adenocarcinoma Treated With Neoadjuvant Therapy. Am J Surg Pathol. 2017;41:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SS, Ko AH, Nakakura EK, et al. Comparison of Tumor Regression Grading of Residual Pancreatic Ductal Adenocarcinoma Following Neoadjuvant Chemotherapy Without Radiation: Would Fewer Tier-Stratification Be Favorable Toward Standardization? Am J Surg Pathol. 2019;43:334–340. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee D, Katz MH, Rashid A, et al. Pancreatic intraepithelial neoplasia and histological changes in non-neoplastic pancreas associated with neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma. Histopathology. 2013;63:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii-Nishimura Y, Nishiyama R, Kitago M, et al. Two Cases of Pathological Complete Response to Neoadjuvant Chemoradiation Therapy in Pancreatic Cancer. Keio J Med. 2015;64:26–31. [DOI] [PubMed] [Google Scholar]
- 27.Wörmann SM, Song L, Ai J, et al. Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology. 2016;151:180–193. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Zhang Y, Yang J, et al. ZIP4 Increases Expression of Transcription Factor ZEB1 to Promote Integrin α3β1 Signaling and Inhibit Expression of the Gemcitabine Transporter ENT1 in Pancreatic Cancer Cells. Gastroenterology. 2020;158:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makohon-Moore AP, Matsukuma K, Zhang M, et al. Precancerous neoplastic cells can move through the pancreatic ductal system. Nature. 2018;561:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neesse A, Algül H, Tuveson DA, et al. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64:1476–84. [DOI] [PubMed] [Google Scholar]
- 32.DuFort CC, DelGiorno KE, Hingorani SR. Mounting Pressure in the Microenvironment: Fluids, Solids, and Cells in Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2016;150:1545–1557.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


