Abstract
Lower prediagnostic circulating 25-hydroxyvitamin D (25[OH]D)-considered the best marker of total vitamin D exposure-is associated with higher mortality risk among colorectal cancer (CRC) patients. However, it is unknown whether this association differs by the vitamin D-binding protein (GC) isoform Gc2 (encoded by GC rs4588*C>A, Thr436Lys), which may substantially affect vitamin D metabolism and modify associations of 25(OH)D with colorectal neoplasm risk. Prediagnostic 25(OH) D-mortality associations according to Gc2 isoform were estimated using multivariable Cox proportional hazards regression among 1281 CRC cases (635 deaths, 483 from CRC) from two large prospective cohorts conducted in the United States (Cancer Prevention Study-II) and Europe (European Prospective Investigation into Cancer and Nutrition). 25(OH)D measurements were calibrated to a single assay, season standardized, and categorized using Institute of Medicine recommendations (deficient [<30], insufficient [30 - <50], sufficient [≥50 nmol/L]). In the pooled analysis, multivariable-adjusted hazard ratios (HRs) for CRC-specific mortality associated with deficient relative to sufficient 25(OH)D concentrations were 2.24 (95% CI 1.44–3.49) among cases with the Gc2 isoform, and 0.94 (95% CI 0.68–1.22) among cases without Gc2 (Pinteraction = .0002). The corresponding HRs for all-cause mortality were 1.80 (95% CI 1.24–2.60) among those with Gc2, and 1.12 (95% CI 0.84–1.51) among those without Gc2 (Pinteraction = .004). Our findings suggest that the association of prediagnostic vitamin D status with mortality among CRC patients may differ by functional GC isoforms, and patients who inherit the Gc2 isoform (GC rs4588*A) may particularly benefit from higher circulating 25(OH)D for improved CRC prognosis.
Keywords: 25-hydroxyvitamin D, cohort studies, gene-environment interaction, single nucleotide polymorphism, survival analysis
1 |. INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer death among men and women combined globally.1 Vitamin D regulates several important signaling pathways relevant to cancer progression and prognosis, including proliferation, differentiation, angiogenesis, apoptosis, inflammation and metastasis.2 Circulating 25-hydroxyvitamin D (collective term for D2 and D3, 25[OH]D) is considered the best marker of total vitamin D exposure and is used clinically to assess vitamin D status.3 Lower 25(OH)D concentrations are associated with higher mortality risk among CRC patients in observational studies4–7; however, it is unknown whether this association differs depending on functional variants in the gene (GC, formerly known as group-specific component) encoding for the vitamin D-binding protein (GC, also known as DBP), which may impact vitamin D bioavailability and metabolism. Investigation of interaction between 25(OH)D and functional GC variants could be important for: (a) identifying subgroups of individuals in which adequate 25(OH)D may be particularly beneficial, and (b) providing biologic insight into vitamin D metabolism and CRC progression.8
Nearly 90% of circulating 25(OH)D is bound to the GC protein, which delivers vitamin D to target tissues and helps maintain stable 25(OH)D stores.9,10 The two missense variants GC rs4588 and rs7041 encode for three common protein isoforms-Gc1s, Gc1f and Gc2.11 We recently reported that associations of 25(OH)D concentrations with risk of incident, sporadic colorectal adenoma12 and CRC13 were stronger among individuals with the Gc2 isoform than among those with only Gc1 isoforms. Relative to the Gc1 isoforms (distinguished by the rs7041 genotype), the Gc2 isoform (determined by the rs4588 genotype) is associated with an approximately twofold to fourfold lower 25(OH)D binding affinity14 and twofold to threefold higher vitamin D-pathway induction by 25(OH)D in vitro15, providing biologic plausibility for these clinically relevant genotype-specific associations.
Accordingly, we hypothesized that the association of prediagnostic 25(OH)D concentrations with mortality risk among CRC patients would be stronger among individuals with the Gc2 isoform than among those without it. We investigated whether associations of 25(OH)D with CRC-specific and all-cause mortality differed by Gc2 isoform among 1281 CRC patients in two large prospective cohort studies in the United States (US) and Europe.
2 |. METHODS
2.1 |. Study population
We analyzed individual patient data from the European Prospective Investigation into Cancer and Nutrition (EPIC) and the Cancer Prevention Study-II (CPS-II) prospective cohort studies. Details of the study populations and data collection were published previously for EPIC16 and CPS-II.17 Briefly, EPIC recruited over 520 000 men and women from the general population in 10 western European countries from 1992 to 1998,18 and CPS-II recruited 184 194 men and women across 21 US states from 1992 to 1993.17 Blood samples were collected prior to cancer diagnosis from EPIC participants between 1992 and 1998, and from CPS-II participants between 1998 and 2001. Prediagnostic circulating 25(OH)D concentrations were measured for 1248 and 298 incident CRC cases for previous case-control studies with 1:1 matching nested in EPIC18 and CPS-II,19 respectively. Detailed descriptions of case selection and exclusions for these studies are described elsewhere.4,18,19 Of these 1546 CRC cases, we further excluded seven non-white CPS-II cases, 142 EPIC cases and 44 CPS-II cases with missing genotyping information, 25 EPIC cases with missing cause of death information and 38 EPIC cases and nine CPS-II cases with missing follow-up or vital status information, leaving 1281 CRC cases for these analyses. The EPIC and CPS-II studies were approved by their respective institutional review boards, and written informed consent was obtained from each subject.
2.2 |. Follow-up
Follow-up for CRC incidence occurred during 1993–2004 in EPIC,4,18 and 1999–2007 in CPS-II.20 In EPIC, vital status and cancer incidence information was collected via linkage to regional and/or national mortality registries in all countries except France, Germany and Greece, where participants were followed using a combination of cancer/pathology registries, health insurance records and active follow-up, as described previously.4 Censoring dates for complete follow-up in EPIC occurred in 2012 (Netherlands, Greece), 2013 (France, Italy, Spain, UK, Denmark) and 2014 (Germany, Sweden). In CPS-II, CRC cases were followed through 2014, and vital status and cause of death information were collected via linkage to the National Death Index.20 CRC-attributable deaths were determined using the International Classification of Diseases for Oncology (ICD-O) 10th revision codes C18.0–18.7 and C19 for colon cancer (including C18.1 for appendix cancer), C20 for rectal cancer and C18.8–18.9 for overlapping/unspecified colorectal origin.
2.3 |. 25(OH)D Measurements
Total serum 25(OH)D (D2 and D3) was measured using the FDA-approved DiaSorin Liaison chemiluminescence immunoassay (CLIA) in CPS-II19 (Heartland Assays, Ames, Iowa), and the OCTEIA enzyme immunoassay (Immuno Diagnostic Systems, Boldon, UK) in EPIC.18 Inter-assay coefficients of variation were 5.2% in CPS-II and 5.7% in EPIC. EPIC 25(OH)D measurements were calibrated to the same assay used in CPS-II using a robust linear regression calculated by remeasuring a subset of 40 EPIC samples within each 25(OH)D decile using the DiaSorin CLIA, described previously.21 Each assay batch included the National Institute of Standards and Technology standard reference materials, for which the coefficients of variation were 16%, 9% and 9% at 17.7, 32.3 and 49.8 nmol/L, respectively.
2.4 |. Genotyping
Genotyping was performed using a custom GoldenGate Universalplex assay kit (Illumina, San Diego, California) in EPIC, and a custom Affymetrix genome-wide platform, the Axiom Correct Set (Affymetrix, Santa Clara, California), in CPS-II. Quality control measures for CPS-II22 and EPIC23 were reported previously. Individuals with the GC rs4588 CC, CA and AA genotypes were classified as having Gc1–1, Gc1–2 and Gc2–2 isoform combinations (or phenotypes), respectively.11 These genotypes perfectly predict the expected amino acid changes of the circulating protein isoforms as determined in previous proteomic analyses.24 In EPIC, GC rs3755967 was used as a proxy for rs4588 since these SNPs are in complete linkage disequilibrium (r2 = 1.0) in the HapMap Spanish and British Western European populations similar to EPIC’s (LDproxy, 1000 Genomes Project Phase 3). GC rs3755967 and rs4588 were in Hardy-Weinberg equilibrium in both studies.
2.5 |. Statistical analyses
To seasonally adjust 25(OH)D measurements, calibrated (EPIC) or newly measured (CPS-II) 25(OH)D values were regressed on week of blood draw using a cos/sin function, and residuals from the model were added to the study- and sex-specific mean among cases (details in references 19 and 21. The adjusted value may be interpreted as the predicted 25(OH)D concentration for a participant averaged over the entire year, accounting for study- and sex-specific seasonal variation in 25(OH)D.
CRC-specific mortality was the primary endpoint, and all-cause mortality was the secondary endpoint. Our primary exposure was circulating 25(OH)D categorized a priori according to clinical guidelines for vitamin D status set by the Institute of Medicine (IOM, now the National Academy of Medicine): <30 nmol/L (deficient), 30 to <50 nmol/L (insufficient), and ≥50 nmol/L (sufficient). For our primary analysis, effect modification by Gc2 was evaluated using a dominant inheritance model given the low frequency of Gc2–2 homozygotes. As a secondary analysis, we coded Gc2 using a codominant inheritance model as we would expect the 25(OH)D-CRC survival association to be stronger with an increasing number of Gc2-encoding alleles; here, 25(OH)D was dichotomized at 50 nmol/L to maximize statistical efficiency.
A Cox proportional hazards model, stratified by country of cancer diagnosis, was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for CRC-specific and all-cause mortality according to 25(OH)D concentrations and Gc2 isoform. Age between diagnosis and censorship or death was used as the time-scale, which may better control for age and reduce bias.25 Covariates included year of diagnosis (continuous), sex, tumor site (colon, rectum, missing/not specified), body mass index (BMI) (continuous), physical activity (quartiles 1–4, missing), smoking status (never, former, current, missing) and stage (I-IV, missing/not specified). Potential covariates were selected based on biological plausibility, causal structure, and previous literature; of those selected, education, dietary calcium and alcohol consumption were not included in the final model because they did not materially affect the estimated HRs. The proportional hazards assumption was evaluated by including a time-dependent covariate in the Cox model and by assessing the correlation between the Schoenfeld residuals and survival time.26 Estimates were calculated in each study separately and in a pooled analysis using aggregated data. Results presented hereafter are based on the pooled analysis unless otherwise stated. Multiplicative interaction between 25(OH)D and the Gc2 isoform was evaluated by comparing the pooled, adjusted Cox models with and without an interaction term using a likelihood ratio test.
To assess whether competing causes of death may have influenced the observed associations, adjusted cumulative incidence curves for CRC-specific mortality risk were estimated according to 25(OH)D and Gc2 isoform using Fine and Gray’s competing-risks regression.27
All statistical tests were two-sided; a P-value <.05 or a 95% confidence interval that excluded 1.0 was considered statistically significant. Analyses were performed in SAS version 9.4 (Cary, North Carolina).
3 |. RESULTS
3.1 |. Study population and follow-up
During follow-up of the 1281 CRC cases, 635 died, including 483 from CRC. Mean follow-up duration was 8.3 years in EPIC and 7.3 years in CPS-II. Characteristics of CRC cases according to IOM-defined vitamin D status categories are summarized in Table 1.
TABLE 1.
Selected characteristics of CRC cases according to prediagnostic vitamin D status in the EPIC and CPS-II cohorts (n = 1281)
| EPIC (n = 1043) | CPS-II (n = 238) | Pooled cohort (n = 1281) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D, nmol/L | 25(OH)D, nmol/L | 25(OH)D, nmol/L | ||||||||
| Characteristic | <30 (deficient) n = 331 |
30 to <50 (insufficient) n = 520 |
≥50 (sufficient) n = 192 | <30 (deficient) n = 35 |
30 to <50 (insufficient) n = 73 |
≥50 (sufficient) n = 130 |
<30 (deficient] n = 366 | 30 to <50 (insufficient) n = 593 |
≥50 (sufficient) n = 322 |
Pa |
| Age at diagnosis, mean (SD), years | 62.5 (7.4) | 62.0 (7.5) | 62.0 (6.8) | 74.2 (5.9) | 75.2 (5.7) | 74.5 (5.7) | 63.7 (8.0) | 63.6 (8.5) | 67.2 (8.9) | <.0001 |
| Women, % | 59 | 49 | 41 | 69 | 55 | 57 | 60 | 50 | 43 | <.0001 |
| Stage, % | ||||||||||
| I | 23 | 29 | 20 | 40 | 45 | 45 | 25 | 31 | 30 | |
| II | 24 | 17 | 22 | 20 | 18 | 20 | 24 | 17 | 21 | |
| III | 31 | 32 | 32 | 31 | 25 | 19 | 31 | 31 | 27 | |
| IV | 10 | 10 | 11 | 9 | 8 | 14 | 9 | 10 | 12 | .11 |
| Tumor location, % | ||||||||||
| Left colon | 36 | 35 | 27 | 40 | 25 | 24 | 36 | 34 | 27 | |
| Right colon | 35 | 31 | 30 | 46 | 63 | 65 | 36 | 35 | 44 | |
| Rectum | 24 | 28 | 33 | 14 | 10 | 11 | 23 | 26 | 24 | .08 |
| Body-mass index, mean (SD), kg/m2 | 27.0 (4.9) | 26.8 (4.1) | 26.0 (3.5) | 29.0 (7.2) | 26.8 (5.1) | 25.5 (4.1) | 27.2 (5.2) | 26.8 (4.2) | 25.8 (3.7) | <.0001 |
| Smoking status, % | ||||||||||
| Never | 44 | 42 | 35 | 40 | 42 | 49 | 44 | 41 | 41 | |
| Former | 23 | 37 | 43 | 57 | 45 | 42 | 27 | 38 | 43 | |
| Current | 31 | 22 | 22 | 3 | 4 | 3 | 28 | 20 | 15 | <.0001 |
| Physical activity quartilesb, % | ||||||||||
| 1 | 28 | 22 | 22 | 37 | 30 | 17 | 29 | 23 | 20 | |
| 2 | 20 | 25 | 24 | 26 | 26 | 25 | 21 | 25 | 24 | |
| 3 | 23 | 22 | 19 | 17 | 23 | 28 | 22 | 22 | 23 | |
| 4 | 26 | 25 | 28 | 20 | 19 | 29 | 25 | 24 | 28 | .05 |
Note: According to Institute of Medicine 2011 recommendations based on 25(OH)D blood concentrations. Column percentages (ie, within each vitamin D status category) are presented for categorical variables; percentages may not sum to 100 due to rounding and missing values.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CPS-II, Cancer Prevention Study-II; EPIC, European Prospective Investigation into Cancer and Nutrition; MET, metabolic equivalent.
P value calculated among the pooled sample using one-way analysis of variance for continuous variables and the χ2 test for categorical variables.
Study-specific quartiles based on recreational metabolic-equivalent hours (MET-hours) per week.
3.2 |. 25(OH)D and mortality according to Gc2
Associations of 25(OH)D concentrations with mortality among all participants and according to Gc2 isoform, assuming a dominant inheritance model, are summarized in Table 2. Relative to those with 25(OH)D concentrations considered sufficient by the IOM (≥50 nmol/L), CRC-specific mortality risk for those with concentrations considered deficient (<30 nmol/L) was statistically significantly 33% higher among all cases, 124% higher among cases with Gc2, and nonstatistically significantly 6% lower among cases without Gc2 (Pinteraction = .0002). There was a dose-response association trend between lower (poorer) vitamin D status and higher mortality risk among those with Gc2 (Ptrend = <.0001 and .0002 for CRC-specific and overall mortality, respectively), but not among those without Gc2 (Ptrend = .69 and .49 for CRC-specific and overall mortality, respectively). This pattern of effect modification by Gc2 was similar in both EPIC and CPS-II (Table S1).
TABLE 2.
Multivariable-adjusted associations of prediagnostic vitamin D status with CRC-specific and all-cause mortality among all CRC cases and according to vitamin D-binding protein (GC) isoform, assuming a dominant inheritance model, in the EPIC and CPS-II cohorts combined (n = 1281)
| Circulating 25(OH)D concentrations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥50 nmol/L (sufficient) | 30 to <50 nmol/L (insufficient) | <30 nmol/L (deficient) | |||||||||
| Outcome and GC strata | No. total | No. died | HR (95% CI)c | No. total | No. died | HR (95% CI)c | No. total | No. died | HR (95% CI)c | Ptrenda | Pinteractionb |
| CRC-specific mortality | |||||||||||
| All CRC cases | 322 | 106 | 1.00 (Ref) | 593 | 241 | 1.09 (0.83–1.43) | 366 | 136 | 1.33 (1.03–1.72) | .02 | |
| No Gc2 (GC rs4588*CC) | 187 | 72 | 1.00 (Ref) | 309 | 114 | 1.11 (0.78–1.57) | 164 | 70 | 0.94 (0.68–1.22) | .69 | |
| Gc2 (GC rs4588*CA or AA) | 135 | 34 | 1.00 (Ref) | 284 | 127 | 1.29 (0.81–2.06) | 202 | 66 | 2.24 (1.44–3.49) | <.0001 | .0002 |
| All-cause mortality | |||||||||||
| All CRC cases | 322 | 146 | 1.00 (Ref) | 593 | 301 | 1.13 (0.90–1.43) | 366 | 188 | 1.36 (1.09–1.70) | .005 | |
| No Gc2 (GC rs4588*CC) | 187 | 93 | 1.00 (Ref) | 309 | 148 | 1.26 (0.93–1.72) | 164 | 93 | 1.12 (0.84–1.51) | .49 | |
| Gc2 (GC rs4588*CA or AA) | 135 | 53 | 1.00 (Ref) | 284 | 153 | 1.09 (0.75–1.61) | 202 | 95 | 1.80 (1.24–2.60) | .0002 | .004 |
Note: According to the Institute of Medicine 2011 recommendations.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; CPS-II, Cancer Prevention Study-II; CRC, colorectal cancer; GC, vitamin D-binding protein; EPIC, European Prospective Investigation into Cancer and Nutrition; HR, hazard ratio.
Ptrend calculated by using vitamin D status as a continuous variable in the model.
Pinteraction between vitamin D status and GC isoform calculated using a likelihood ratio test.
From multivariable Cox proportional hazards models, adjusted for year of diagnosis (continuous), sex, tumor site (colon, rectum, missing/not specified), BMI (continuous), physical activity (Quartiles 1–4, missing), smoking status (never, former, current, missing) and stage (I-IV, missing/not specified), and stratified by country.
Associations of 25(OH)D concentrations with CRC-specific and all-cause mortality among all participants and according to Gc2 isoform, assuming a codominant inheritance model, are summarized in Table 3. Relative to those with 25(OH)D concentrations considered sufficient, CRC-specific mortality risk for those with nonsufficient concentrations (<50 nmol/L) was close to the null among Gc1–1 cases, statistically significantly 54% higher among Gc1–2 cases, and nonstatistically significantly 150% higher among Gc2–2 cases (Pinteraction = .003). Estimated all-cause mortality risk for those with nonsufficient relative to sufficient 25(OH)D concentrations varied from 6% to 33% higher among Gc1–1, Gc1–2 and Gc2–2 cases, but did not statistically significantly differ by Gc2 (Pinteraction = .09). The pattern of effect modification by number of Gc2-encoding alleles for CRC-specific mortality was similar in EPIC and CPS-II (Table S2).
TABLE 3.
Multivariable-adjusted associations of prediagnostic vitamin D status with CRC-specific and all-cause mortality among all CRC cases and according to vitamin D-binding protein (GC) isoform, assuming a codominant inheritance model, in the EPIC and CPS-II cohorts combined (n = 1281)
| Outcome and GC strata | Circulating 25(OH)D concentrations | ||||||
|---|---|---|---|---|---|---|---|
| ≥50 nmol/L (sufficient) | <50 nmol/L (nonsufficient) | ||||||
| Number of total | Number of died | HR (95% CI)b | Number of total | Number of died | HR (95% CI)b | Pinteractiona | |
| CRC-specific mortality | |||||||
| All CRC cases | 322 | 106 | 1.00 (Ref) | 959 | 377 | 1.22 (0.97–1.52) | |
| Gc1–1 (GC rs4588*CC) | 187 | 72 | 1.00 (Ref) | 473 | 184 | 0.96 (0.72–1.29) | .003 |
| Gc1–2 (GC rs4588*CA) | 120 | 32 | 1.00 (Ref) | 390 | 149 | 1.54 (1.02–2.32) | |
| Gc2–2 (GC rs4588*AA) | 15 | 2 | 1.00 (Ref) | 96 | 44 | 2.50 (0.56–11.1) | |
| All-cause mortality | |||||||
| All CRC cases | 322 | 146 | 1.00 (Ref) | 959 | 489 | 1.21 (1.00–1.47) | |
| Gc1–1 (GC rs4588*CC) | 187 | 93 | 1.00 (Ref) | 473 | 241 | 1.06 (0.83–1.37) | .09 |
| Gc1–2 (GC rs4588*CA) | 120 | 48 | 1.00 (Ref) | 390 | 194 | 1.33 (0.94–1.86) | |
| Gc2–2 (GC rs4588*AA) | 15 | 5 | 1.00 (Ref) | 96 | 54 | 1.13 (0.41–3.05) | |
Note: According to the Institute of Medicine 2011 recommendations.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; CPS-II, Cancer Prevention Study-II; CRC, colorectal cancer; GC, vitamin D-binding protein; EPIC, European Prospective Investigation into Cancer and Nutrition; HR, hazard ratio.
Pinteraction between vitamin D status and GC isoform calculated using a likelihood ratio test.
From multivariable Cox proportional hazards models adjusted for age at diagnosis, year of diagnosis, sex, tumor site (colon, rectum, missing/not specified), BMI (continuous), physical activity (quartiles 1–4, missing), smoking status (never, former, current, missing) and stage (I-IV, missing/not specified) and stratified by country.
3.3 |. Competing risks regression and cumulative incidence curves
Using multivariable-adjusted competing-risks regression, we observed a dose-response association of lower 25(OH)D concentrations with higher CRC-specific mortality among those with the Gc2 isoform, but not among those without Gc2 (Figure 1). Among individuals with Gc2, the estimated risk dying from CRC within 5 years of diagnosis was approximately 15% if vitamin D sufficient, 20% if vitamin D insufficient and 30% if vitamin D deficient prior to diagnosis, controlling for all other covariates and accounting for competing causes of death.
FIGURE 1.
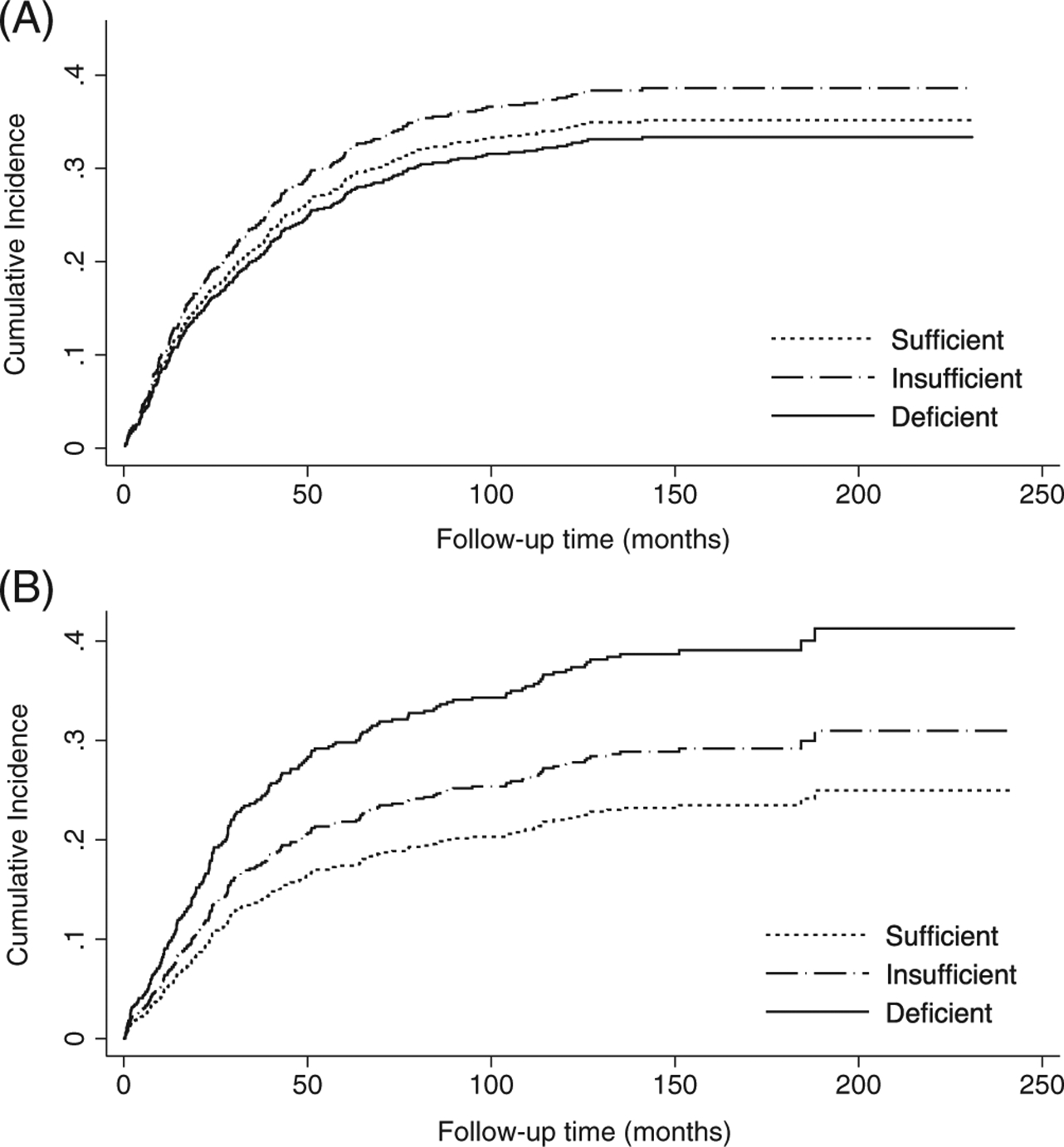
Adjusted cumulative incidence curves for CRC-specific mortality according to vitamin D status-using Institute of Medicine recommended 25-hydroxyvitamin D cut-points-in the combined EPIC and CPS-II cohort (n = 1281) among, A, patients without Gc2 (GC rs4588*CC) and B, patients with Gc2 (GC rs4588*CA or AA). Cumulative incidence curves were estimated using Fine and Gray’s competing-risks regression models adjusted for age at diagnosis (continuous), year of diagnosis (continuous), sex, tumor site (colon, rectum, missing/not specified), BMI (continuous), physical activity (Quartiles 1–4, missing), smoking status (never, former, current, missing), stage (I-IV, missing/not specified) and country. 25(OH)D concentrations <30, 30 to <50 and ≥50 nmol/L categorized as deficient, insufficient and sufficient, respectively, based on the Institute of Medicine guidelines
3.4 |. Subgroup and sensitivity analyses
The association of 25(OH)D concentrations <50 relative to ≥50 nmol/L with CRC-specific mortality among individuals with and without the Gc2 isoform did not statistically significantly differ according to sex, stage, tumor site or calcium intake; however, the observed effect-modification pattern by Gc2 was slightly more pronounced among rectal cancer cases, Stages I-II cases, and individuals with above-median dietary calcium intake (Table S3). In sensitivity analyses, our effect-modification findings were slightly stronger when we excluded metastatic CRC cases (Table S4) or cases diagnosed within 1 or 3 years of their prediagnostic blood draw (Table S5). There was also a similar pattern of effect modification by Gc2 when we categorized 25(OH)D using study-specific 25(OH)D tertile cut-points (Table S6), further supporting the robustness of our findings.
4 |. DISCUSSION
Our findings suggest that prediagnostic vitamin D deficiency relative to sufficiency, based on IOM recommendations, may be associated with higher mortality risk among CRC patients, but only among those with the common Gc2-encoding GC rs4588*A functional variant that may affect 25(OH)D binding affinity, bioavailability and vitamin D-pathway activation.11,28 This association was stronger for CRC-specific mortality, which may have been due to non-vitamin D-related deaths in the all-cause mortality group. To the best of our knowledge, this is the first study to investigate the association of 25(OH)D concentrations with mortality among CRC patients by GC vitamin D-binding protein isoform.
Findings from observational studies suggest an association of circulating 25(OH)D concentrations-including those measured before diagnosis4,5 and after diagnosis7-with CRC-specific mortality. Furthermore, findings from some studies indicate that 25(OH)D may be a clinically relevant prognostic factor and add value to predictive survival models for CRC patients.7,29 However, our findings suggest that the utility of 25(OH)D as prognostic factor among CRC patients in the US and Europe may critically depend on inherited genotypes encoding common, functional GC isoforms. If our findings are confirmed, they would support GC genotyping, which could be easily and affordably obtained in clinical settings, for guiding vitamin D-related therapy and survival stratification.
Evidence from randomized clinical trials (RCTs) of vitamin D supplementation improving survival of CRC patients is limited. In a US phase-II, multicenter RCT with 139 patients with advanced or metastatic CRC, those randomized to high-dose (4000 IU/day) relative to low-dose (400 IU/day) vitamin D supplementation had longer progression-free survival (HR = 0.64, one-sided 95% CI 0–0.90, P = .02), which was the primary outcome, although no significant treatment effect was observed for overall survival.30 Importantly, findings from a larger RCT (n = 2259) suggest that the effects of vitamin D supplementation on increasing 25(OH)D concentrations31 and reducing colorectal adenoma risk32 are stronger among individuals with the Gc2-encoding variant. Specifically, the effect of vitamin D supplementation on adenoma risk was statistically significantly 18% lower with each Gc2-encoding-rs4588 variant inherited (interaction relative risk = 0.82, 95% CI 0.69–0.98, Pinteraction = .03).32 These findings are consistent with ours, and collectively suggest that future trials should consider potential differences in supplementation effects according to Gc2 isoform. If confirmed, this effect modification could be important clinically, and for public health, given the high prevalence of the Gc2-encoding allele (40%−50% with European ancestry33) and vitamin D concentrations <50 nmol/L in the US and Europe (26%−76%, depending on age and country3,34).
The Gc2 isoform is encoded by the missense GC rs4588*C>A variant resulting in a Threonine (Gc1)→Lysine (Gc2) amino acid substitution at residue 436.11 Although the physiologic consequences of the isoforms have not been fully elucidated, the Gc2-encoding variant is strongly associated with lower circulating 25(OH)D concentrations and higher odds of vitamin D insufficiency.35–37 This association may be mediated by lower GC protein concentration (20%−30% lower among Gc2 homozygotes relative to Gc1 homozygotes in studies that did not use the isoform-biased monoclonal R&D assay24,38–40) since GC mediates the renal reabsorption of 25(OH)D and prolongs its circulating half-life.41 Gc2 may also have lower 25(OH)D binding affinity than Gc1 isoforms,14 which, in addition to lower circulating GC, could lead to higher levels of bioavailable and free 25(OH)D available to target tissues.9,14,24,28 This may underlie the higher induction of vitamin D target genes by 25(OH)D in cultured monocytes and colon cancer cell lines with Gc2 relative to cells cultured with Gc1 isoforms.15,42 Importantly, normal and neoplastic colon tissues express the vitamin D-receptor (VDR) and are able to locally convert 25(OH)D to the VDR-activating 1,25(OH)2D form, which may play an important role in CRC progression via modulating pathways involved in cell proliferation, inflammation, angiogenesis and metastasis.2,43 Taken together, we hypothesize that individuals with the Gc2 isoform may particularly benefit from higher 25(OH)D concentrations as these concentrations may lead to higher vitamin D-pathway activation and may be needed to compensate for Gc2 individuals’ reduced capacity to maintain adequate 25(OH)D concentrations.
Supporting this hypothesis are other studies that reported a similar pattern of effect modification by Gc2 in relation to 25(OH)D and risk of colorectal neoplasms. In a pooled US case-control study of individuals of European ancestry, 25(OH)D concentrations ≥50 relative to <50 nmol/L were associated with lower risk of incident, sporadic colorectal adenoma, but only among those with Gc2 (OR among Gc1–2/Gc2–2 = 0.51, 95% CI 0.33–0.81; OR among Gc1–1 = 1.11, 95% CI 0.68–1.82; Pinteraction = .05).12 Additionally, in a pooled nested case-control study using EPIC, CPS-II and Nurses’ Health Study data (n = 3359), 25(OH)D concentrations ≥50 relative to <30 nmol/L were associated with a statistically significant 53% lower CRC risk among individuals with Gc2, but nonstatistically significant 12% lower risk among individuals without Gc2 (Pheterogeneity = .01).13
Our study strengths include its prospective design, long follow-up, and use of data from two independently conducted cohort studies of participants in the US and 10 European countries. Additional strengths include using seasonally adjusted 25(OH)D concentrations (limiting exposure misclassification) and calibrating 25(OH)D measurements to a standard assay to permit estimating hazards using absolute clinical cut-points.
Our study has several limitations. The CPS-II sample size was small; however, the direction of the HRs within strata and the pattern of effect modification were consistent across studies, supporting the validity and reproducibility of our findings. Larger studies are needed to yield more precise estimates among individuals with the rare Gc2–2 genotype. There may have been some misclassification of vitamin D status related to using the DiaSorin immunoassay; however, this assay is one of the most commonly used in clinical settings, and is highly concordant (r2 > 0.95) with liquid chromatography-mass spectrometry.44 Thus, we would expect this misclassification to be small and comparable to that found in real-world clinical practice. Additionally, while 25(OH)D was measured only once prior to diagnosis, estimated within-person correlations for repeated 25(OH)D measures taken 1 to 11 years apart were 0.53 to 0.81 in other studies, suggesting that single 25(OH)D measurements may be a relatively valid marker of longterm vitamin D status.45,46 Furthermore, using 25(OH)D measurements prior to diagnosis limits the concern for reverse causality (eg, patients with aggressive tumors may be sicker and thus develop lower 25(OH)D concentrations near diagnosis) and our results were similar when we excluded patients diagnosed within 3 years of 25(OH)D measurement. We lacked data on CRC treatment, but adjusted for year of cancer diagnosis and stratified by country to account for potential temporal or geographic treatment differences. 25(OH)D may be a marker of an overall healthier lifestyle that could influence survival; however, we adjusted for BMI, smoking and physical activity, and further adjusting for factors, such as alcohol intake and education, did not materially affect our results. Adjusting for these potential shared risk factors for CRC risk and survival also reduces the possibility of a spurious association due to collider-stratification bias.47 We did not collect tumor microenvironment data, such as degree and type of tumor-infiltrating lymphocytes-important histologic prognostic features of CRC.48 Given the putative immunomodulatory functions of vitamin D,2,15 future research is warranted to investigate whether and how vitamin D and GC isoforms may influence, or interact with, immune cells in the CRC tumor microenvironment. Finally, our findings among Europeans and US whites with European ancestry may not be generalizable to other races or populations.
In conclusion, our findings, together with previous literature, suggest that the association of prediagnostic 25(OH)D with mortality risk among CRC patients may differ by common, inherited genotypes encoding GC vitamin D-binding protein isoforms, such that CRC patients with the Gc2 isoform may particularly benefit from a sufficient vitamin D status.
Supplementary Material
What’s new?
Vitamin D regulates molecular pathways that are relevant to cancer progression. In patients with colorectal cancer (CRC), low serum levels of vitamin D have been associated with increased mortality. A protein called GC binds vitamin D and delivers it to tissues. In this study, the authors found that the increased mortality risk with low vitamin D was only seen in those CRC patients who carry a genetic variant of GC called Gc2. These results may help to identify a vulnerable subgroup of CRC patients, who may particularly benefit from vitamin D supplementation.
ACKNOWLEDGEMENTS
This research was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (F30 CA236231 to D. C. G.; R03 CA183016 to V. F.), the Anne and Wilson P. Franklin Foundation (to R. M. B.) and the World Cancer Research Fund (WCRF) (WCRF 2011-443; to M. J.). The American Cancer Society funds the creation, maintenance and updating of the Cancer Prevention Study-II (CPS-II) cohort. The coordination of the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort is financially supported by the European Commission (Directorate-General for Health and Consumers, DG-SANCO) and the International Agency for Research on Cancer (IARC). The national EPIC cohorts are supported by: Danish Cancer Society (Kræftens Bekæmpelse) (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale and Institut National de la Santé et de la Recherche Médicale (INSERM) (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum (DKFZ) and Bundesministerium für Bildung und Forschung (Germany); Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro (AIRC) and AIRE-ONLUS Ragusa, AVIS Ragusa, Sicilian Government (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), ZonMw and Statistics Netherlands (The Netherlands); Health Research Fund (FIS), Regional Governments of Andalucía, Asturias, Basque Country and Murcia, Instituto de Salud Carlos III (ISCIII) (RD06/0020) and the Catalan Institute of Oncology (Spain); Swedish Cancer Society (Cancerfonden), Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK (C570/A16491 for EPIC-Oxford) and the Medical Research Council (1000143 for EPIC-Norfolk and MR/M012190/1 for EPIC-Oxford, UK).
Bundesministerium für Bildung und Forschung; Cancer Research UK, Grant/Award Number: C570/A16491; Cancerfonden; Catalan Institute of Oncology; Centre International de Recherche sur le Cancer; Deutsches Krebsforschungszentrum; Directorate-General for Health and Consumers; Dutch Ministry of Public Health, Welfare and Sports; Health Research Fund (Fondo de Investigaciones Sanitarias, FIS); Hellenic Health Foundation; Institut Gustave Roussy; Institut National de la Santé et de la Recherche Médicale; Instituto de Salud Carlos III, Grant/Award Number: RD06/0020; Kræftens Bekæmpelse; Ligue Contre le Cancer; Medical Research Council, Grant/Award Numbers: 1000143, MR/M012190/1; Mutuelle Générale de l’Education Nationale; National Cancer Institute, Grant/Award Numbers: F30 CA236231, R03 CA183016; Netherlands Cancer Registry (NKR); Regional Government of Skåne and Västerbotten; Regional Governments of Andalucía, Asturias, Basque Country, and Murcia; Sicilian Government; Statistics Netherlands; Swedish Scientific Council; World Cancer Research Fund, Grant/Award Number: WCRF 2011-443; ZonMw
Funding information
AIRE-ONLUS Ragusa; American Cancer Society; Anne and Wilson P. Franklin Foundation; Associazione Italiana per la Ricerca sul Cancro; AVIS Ragusa;
Abbreviations:
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CI
confidence interval
- CLIA
chemiluminescence immunoassay
- CPS-II
Cancer Prevention Study-II
- CRC
colorectal cancer
- EPIC
European Prospective Investigation into Cancer and Nutrition
- GC
group-specific component
- GC
vitamin D-binding protein
- HR
hazard ratio
- RCT
randomized clinical trial
- US
United States
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: IARC Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA ACCESSIBILITY
Data are available by application to the EPIC Steering Committee (https://epic.iarc.fr/access/) and the American Cancer Society’s Behavioral & Epidemiology Research Group (https://www.cancer.org/content/dam/cancer-org/research/epidemiology/cancer-prevention-study-data-access-policies.pdf).
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 4.Fedirko V, Riboli E, Tjønneland A, et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European populations. Cancer Epidemiol Biomarkers Prev. 2012;21:582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984–2991. [DOI] [PubMed] [Google Scholar]
- 6.Wesa KM, Segal NH, Cronin AM, et al. Serum 25-hydroxy vitamin D and survival in advanced colorectal cancer: a retrospective analysis. Nutr Cancer. 2015;67:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol 2014;32:2430–2439. [DOI] [PubMed] [Google Scholar]
- 8.van der Weele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 9.Bikle DD, Gee E, Halloran B, Kowalaski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. [DOI] [PubMed] [Google Scholar]
- 10.Safadi FF, Thornton P, Magiera H, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik S, Fu L, Juras DJ, et al. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci. 2013;50:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs DC, Fedirko V, Um C, Gross MD, Thyagarajan B, Bostick RM. Associations of circulating 25-hydroxyvitamin D3 concentrations with incident, sporadic colorectal adenoma risk according to common vitamin D binding protein isoforms. Am J Epidemiol. 2018;187:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs D, Song M, McCullough M, et al. Association of circulating vitamin D with colorectal cancer depends on vitamin D-binding protein isoforms: a pooled nested case-control study. JNCI Cancer Spectrum. 2020;4:pkz083 10.1093/jncics/pkz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet. 1993;92:183–188. [DOI] [PubMed] [Google Scholar]
- 15.Chun RF, Lauridsen AL, Suon L, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy-and 1, 25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riboli E, Hunt K, Slimani N, et al. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. [DOI] [PubMed] [Google Scholar]
- 17.Calle EE, Rodriguez C, Jacobs EJ, et al. The American cancer society cancer prevention study II nutrition cohort. Cancer. 2002;94:2490–2501. [DOI] [PubMed] [Google Scholar]
- 18.Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. 2018;111:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang B, McCullough ML, Gapstur SM, et al. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the cancer prevention study-II nutrition cohort. J Clin Oncol. 2014;32:2335–2343. [DOI] [PubMed] [Google Scholar]
- 21.Gail MH, Wu J, Wang M, et al. Calibration and seasonal adjustment for matched case-control studies of vitamin D and cancer. Stat Med. 2016;35:2133–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacher FR, Schmit SL, Jiao S, et al. Genome-wide association study of colorectal cancer identifies six new susceptibility loci. Nat Commun. 2015;6:7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedirko V, Mandle HB, Zhu W, et al. Vitamin D-related genes, blood vitamin D levels and colorectal cancer risk in western european populations. Nutrients. 2019;11:1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielson CM, Jones KS, Chun RF, et al. Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101:2226–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiébaut AC, Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23: 3803–3820. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbaum DG, Klein M. Survival analysis. Vol 3 Cham: Springer; 2010. [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 28.Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D: analysis by mathematical modeling. PLoS One. 2012;7:e30773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaughan-Shaw P, Zgaga L, Ooi L, et al. Low plasma vitamin D is associated with adverse colorectal cancer survival after surgical resection, independent of systemic inflammatory response. Gut. 2019;69: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng K, Nimeiri HS, McCleary NJ, et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. 2019;321:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry EL, Rees JR, Peacock JL, et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab. 2014;99:E2133–E2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry EL, Peacock JL, Rees JR, et al. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol. 2017;3:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamboh M, Ferrell R. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986;72:281–293. [DOI] [PubMed] [Google Scholar]
- 34.Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, O’Reilly PF, Aschard H, et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun. 2018;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. The Lancet. 2010;376:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47:753–756. [PubMed] [Google Scholar]
- 39.Daiger SP, Miller M, Chakraborty R. Heritability of quantitative variation at the group-specific component (Gc) locus. Am J Hum Genet. 1984;36:663. [PMC free article] [PubMed] [Google Scholar]
- 40.Constans J, Arlet P, Viau M, Bouissou C. Unusual sialilation of the serum DBP associated with the Gc 1 allele in alcoholic cirrhosis of the liver. Clin Chim Acta. 1983;130:219–230. [DOI] [PubMed] [Google Scholar]
- 41.Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012;30:445–456. [DOI] [PubMed] [Google Scholar]
- 42.Hibler EA, Hu C, Jurutka PW, Martinez ME, Jacobs ET. Polymorphic variation in the GC and CASR genes and associations with vitamin D metabolite concentration and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2012;21: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3–1α-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370–2376. [DOI] [PubMed] [Google Scholar]
- 44.Arneson WL, Arneson DL. Current methods for routine clinical laboratory testing of vitamin D levels. Lab Med. 2013;44:e38–e42. [Google Scholar]
- 45.Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2010;19:927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scarmo S, Afanasyeva Y, Lenner P, et al. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: a nested case-control study. Breast Cancer Prev. 2013;15:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2009;39:417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


