Abstract
Background/Aims:
Age-related cognitive decline is a pervasive problem in our aging population. To date, no pharmacological treatments to halt or reverse cognitive decline are available. Behavioral interventions, such as physical exercise and Mindfulness-Based Stress Reduction, may reduce or reverse cognitive decline, but rigorously designed randomized controlled trials are needed to test the efficacy of such interventions.
Methods:
Here, we describe the design of the Mindfulness, Education, and Exercise (MEDEX) study, an 18-month randomized controlled trial that will assess the effect of two interventions – mindfulness training plus moderate-to-vigorous intensity exercise or moderate-to-vigorous intensity exercise alone – compared with a health education control group on cognitive function in older adults. An extensive battery of biobehavioral assessments will be used to understand the mechanisms of cognitive remediation, by using structural and resting state functional magnetic resonance imaging, insulin sensitivity, inflammation, and metabolic and behavioral assessments.
Results:
We provide the results from a preliminary study (n=29) of non-randomized pilot participants who received both the exercise and Mindfulness-Based Stress Reduction interventions. We also provide details on the recruitment and baseline characteristics of the randomized controlled trial sample (n=585).
Conclusions:
When complete, the MEDEX study will inform the research community on the efficacy of these widely-available interventions improve cognitive functioning in older adults.
Keywords: Meditation, aerobic exercise, aging, elderly, intervention study
Introduction
Age-associated cognitive decline is a widespread, urgent, and growing public health issue worldwide, and most older adults experience deteriorating cognitive function. This deterioration particularly affects memory and cognitive control (also called executive function), both of which are key to participating and engaging in meaningful daily activities. As awareness of this issue has grown, more research is being conducted to develop novel interventions to remediate age-related cognitive decline. Behavioral interventions such as physical activity/exercise, mindfulness, sleep therapy,1 and cognitive training2 are being tested as ways to reverse or slow age-related cognitive decline. These approaches can potentially drive beneficial plasticity in the brain, particularly those regions involved in cognitive functions such as memory or executive function, and are widely available, inexpensive, and scalable. For example, mindfulness training is already available in every major city in the United States and can even be conducted online or via phone apps. The MEDEX study focuses on two such broadly available interventions: exercise and mindfulness.
The evidence supporting exercise as a promising intervention is particularly compelling. For many years, observational studies have shown links between physical activity, cognition, and brain health.3,4 More recently, randomized clinical trials have provided evidence that increasing physical activity can improve cognitive performance.5 However, many of these studies were conducted in a small number of participants and did not have a supervised or structured exercise intervention, and the participant populations varied among studies. Current research suggests that physical activity can improve cognitive function by increasing peripheral and central nervous system insulin sensitivity, aerobic capacity, Brain Derived Neurotrophic Factor,6,7 hippocampal volume, and synaptic density.8–15 All of these mechanisms support the need for translational research regarding neurobiological mechanisms leading to cognitive improvements with exercise training.
In recent years, mindfulness training has emerged as a novel intervention that can provide a variety of benefits, including boosting immunity16 and lowering blood pressure.17 Newer still is the idea that mindfulness training might remediate cognitive difficulties in older adults.18 Various types of mindfulness programs exist, but Mindfulness-Based Stress Reduction is readily adaptable for large-scale research because it involves an established protocol, following a once-weekly, eight-week program with planned lessons and content, is widely available in community settings, and is designed to be taught in a group format. In Mindfulness-Based Stress Reduction, participants are taught to train their attention on the present moment, rather than letting their mind wander to the past or future. It has been posited that Mindfulness-Based Stress Reduction improves cognitive function by two main mechanisms: (1) enhanced attentional processes,19 which produces changes in brain circuitry20–24 that are associated with age related decline;25 and (2) decreased stress and improved psychological health,26–33 which can have favorable physiological effects such as reducing hypothalamic pituitary adrenal-axis hyperactivity and inflammation, with downstream beneficial effects on brain health34 and cognitive functioning.35–41
The putative mechanisms involved in the therapeutic effects of physical activity and mindfulness interventions on cognitive function are distinct but related (Figure 1). Therefore, we hypothesized that the combination of exercise and mindfulness training would be more efficacious, by either additive or synergistic mechanisms, in remediating age-related cognitive decline than either therapy alone.42 There is growing interest in combining exercise and mindfulness training to increase their benefits,43,44 and some mind-body interventions (yoga, tai chi) are explicit combinations of physical activity and mindfulness.45
Figure 1.
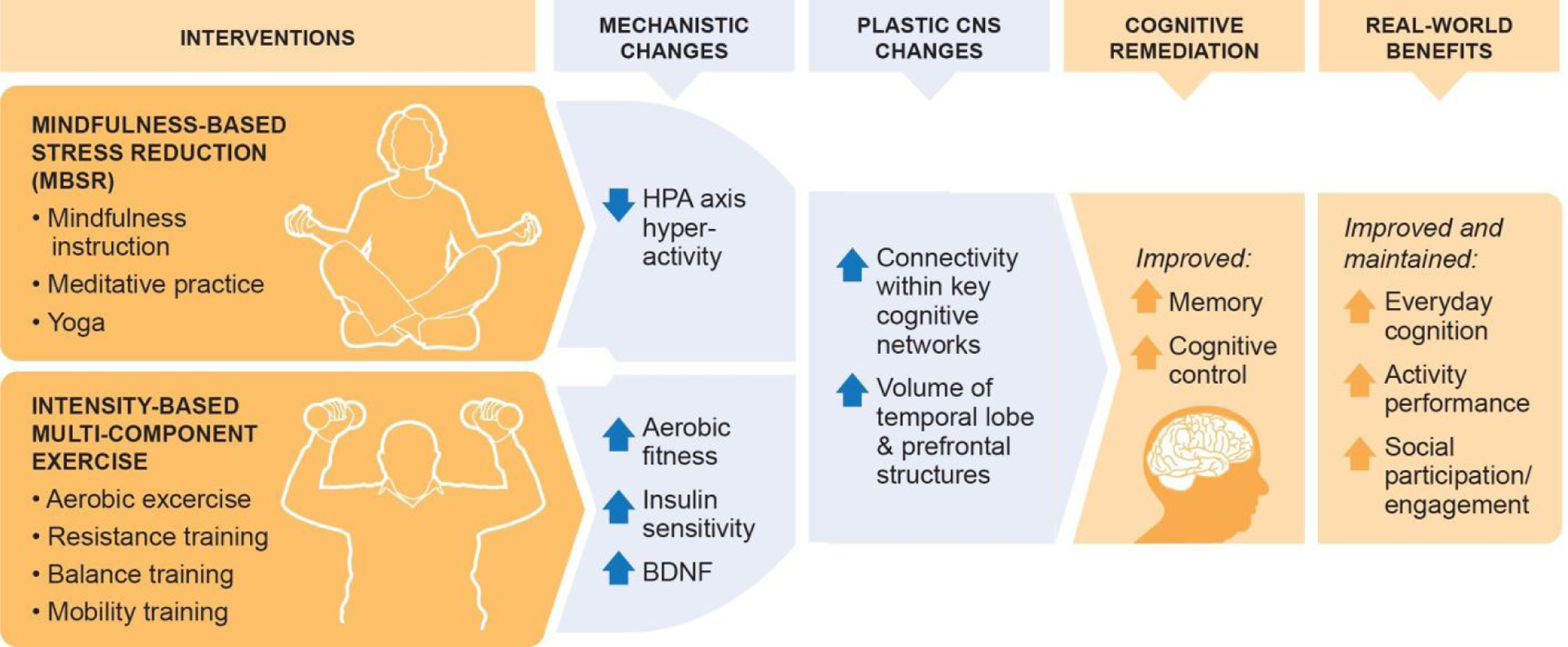
Rationale for the MEDEX Study
To further examine the mechanisms responsible for the beneficial effects of mindfulness and exercise on cognition, including effects on neurobiological and metabolic functioning, we designed a 2×2 factorial design randomized controlled trial (NCT02665481), in which participants were randomized to exercise only, Mindfulness-Based Stress Reduction only, exercise and Mindfulness-Based Stress Reduction, or a health education control condition (Figure 2). The 2×2 factorial design is optimal for testing the effects of each intervention and for testing the combined effects. Such a design is particularly appropriate for detection of interactive effects: synergistic effects (e.g., exercise is a therapeutic permissive for Mindfulness-Based Stress Reduction) or interfering effects (e.g., exercising reduces ability or motivation to engage in meditation).
Figure 2.

Study design for the MEDEX study
The primary outcome measures were memory and cognitive control (executive function), derived from a set of well-validated cognitive assessment tools. We also conducted multiple assessments at baseline and endpoint that evaluated fitness, brain functional connectivity and structural integrity, insulin sensitivity, cortisol levels, body composition, daily activity, and sleep, as mediators and/or moderators of the interventions’ effects on cognitive function. We hope to answer the following questions: Does exercise and/or Mindfulness-Based Stress Reduction improve cognitive function in healthy older adults? What are the mechanisms? Who benefits the most? The specific aims and hypotheses for the ongoing randomized trial are as follows:
Aim 1.
Examine effects of Mindfulness-Based Stress Reduction, exercise, and their combination for remediation of memory and cognitive control, with secondary outcomes of everyday cognition, functional performance, and social participation and engagement.
H1:
Mindfulness-Based Stress Reduction and exercise will each produce benefits in healthy older adults’ cognitive performance, and combined Mindfulness-Based Stress Reduction + exercise will show greater cognitive improvements than either intervention alone.
Aim 2.
Examine mechanistic changes that underlie cognitive remediation from Mindfulness-Based Stress Reduction and exercise.
H2:
Decrease in peak cortisol accounts for improvements with Mindfulness-Based Stress Reduction.
Increased insulin sensitivity, aerobic fitness, and Brain Derived Neurotrophic Factor account for improvements with exercise.
Aim 3.
Examine whether the interventions cause changes in brain structure and functional connectivity and whether these plastic changes help explain the cognitive improvements.
H3:
Improved functional connectivity within and across specific cognitive networks and increased volume of hippocampal and lateral prefrontal regions account for improved cognitive function with the interventions.
Aim 4.
Examine predictors of variability in response to the interventions.
H4:
Baseline cortisol and insulin sensitivity will predict degree of cognitive remediation from Mindfulness-Based Stress Reduction and exercise; that is, high baseline cortisol will predict greater improvement from Mindfulness-Based Stress Reduction, while low insulin sensitivity will predict greater improvement from exercise.
We first carried out a small pilot study to determine the feasibility of our combined exercise and Mindfulness-Based Stress Reduction intervention prior to beginning the full-scale randomized, controlled trial. In this paper we (1) describe the protocol for the full-scale randomized, controlled trial, (2) provide results from the non-randomized pilot participants, and (3) describe the recruitment strategies, study implementation challenges, and baseline characteristics of the randomized, controlled trial sample.
Method
The MEDEX Study is conducted at two sites, Washington University in St. Louis and the University of California, San Diego. Inclusion criteria are: age 65–84, home-dwelling, self-reported cognitive complaints, sedentary lifestyle (defined as no current participation in structured exercise for the purpose of improving fitness, more than one day/week for 30 minutes or longer), and not currently practicing meditation. Exclusion criteria are: a diagnosis of dementia or other neurodegenerative disease; psychotic disorders or other unstable psychiatric conditions; serious medical conditions that would prohibit safe participation in the interventions (e.g., cardiovascular disease) or would interfere with assessments (e.g., ferromagnetic implants which would interfere with magnetic resonance imaging); language, hearing, or visual impairment that would prevent participation; alcohol abuse or illicit drug use within 6 months; current participation in cognitive training programs; and use of medications that would interfere with or confound the results of assessments (e.g., glucocorticoids or diabetes medications).
Our goals with respect to inclusion and exclusion criteria were to maximize generalizability of results, consistent with our goal of examining cognitive remediation strategies in healthy older adults. For example, we allowed for individuals with preclinical Alzheimer’s Disease as well as depression or anxiety in order to avoid drawing a “supernormal” population devoid of significant medical or mental health conditions. We were also attentive to the grant program announcement’s focus on interindividual heterogeneity and with findings that older adults with anxiety, depression, and pain are the most likely to participate in mind-body treatments.
Prior to randomization, each potential participant undergoes a screening battery of medical assessments. These include a medical records review and a maximal graded exercise cardiac stress test to determine safety for participation. Results outside of safety norms are referred back to participants’ primary care providers for further workup and treatment if indicated. Individuals with subsequent normal cardiac workup and clearance from the primary care provider are allowed to enroll.
Assessments
The primary outcomes for this study are memory and cognitive control (executive function) which are measured at baseline and at 3, 6, and 18 months after randomization. We focus on these specific outcomes for several reasons: first, the hypothesized mechanisms for cognitive effects of exercise and Mindfulness-Based Stress Reduction, reduction of chronic hypothalamic pituitary adrenal axis hyperactivity and improved insulin sensitivity, primarily affect frontal and hippocampal brain regions that are responsible for memory and cognitive control. Second, our pilot data provides support for effects on these domains. We administer an extensive battery of tests that will enable us to determine whether any changes are restricted to or more prominent in our hypothesized domains, relative to other cognitive functions.
Memory measures are immediate and delayed word list and paragraph recall using one 16-item list and two paragraphs developed at Washington University for repeated administrations during longitudinal studies (i.e., different list and paragraphs at each timepoint),18 and the Picture Sequence Memory test from National Institutes for Health Toolbox.46 We chose the verbal measures because they were reliable and sensitive to change in our pilot work. We chose the National Institutes for Health Toolbox measure to include a nonverbal test. Cognitive control measures are the Dimensional Change Card Sort, Flanker, and list-sorting working memory from National Institutes for Health Toolbox,47 and three computer-based tests: the Consonant-Vowel Odd-Even switching test,48 the Sustained Attention to Response Test,49 and the Stroop task.50 As recommended by National Institutes for Health, we chose Toolbox measures in order to enable combination of our results with others. The Consonant-Vowel Odd-Even switching test and Sustained Attention to Response Test were sensitive to change in our pilot work. The Consonant-Vowel Odd-Even switching test tests set-shifting by presenting respondents with a letter-number pair and requiring them to switch between deciding whether the letter is a consonant or vowel and deciding whether the number is odd or even. The Sustained Attention to Response Test is a go-no go test that measures sustained attention, working memory, and inhibitory control. In the version of the Stroop administered in this study, a computer displays four color words (red, blue, green, yellow) and four neutral words (bad, poor, deep, legal), in congruent, incongruent, and neutral conditions.51
In constructing the Memory and the Cognitive Control composite variables, a z-score is computed for each participant [(participant score – mean)/standard deviation] using the mean and standard deviation of that variable computed on all participants at baseline. The composite variable is then created by averaging the z-scores of all available variables that are associated with each composite.
A secondary cognitive outcome is the Observed Tasks of Daily Living-Revised,52 a performance-based test measuring functional cognition, or the ability to perform cognitively demanding instrumental activities of daily living, including managing finances and medications. We chose this measure with an eye to the clinical relevance and public health significance of our results; ultimately, ability to live independently in the real world is of more importance to older adults than is the ability to score well on cognitive tests in the laboratory.
MEDEX is an explanatory clinical trial. Its goal is not only to clarify whether exercise and mindfulness remediate age-related cognitive decline but also to understand the mechanisms by which these interventions may do so, and who benefits the most based on their baseline profile.53 To address the underlying mechanisms, the MEDEX study includes numerous secondary measures, including biomarkers, metabolic/fitness variables, and behavioral/functional measures carried out at baseline, 6 months and 18 months.
Biomarkers include salivary cortisol, a measure of hypothalamic pituitary adrenal axis activity, collected at wake, 30 minutes post-wake, and bedtime for 3 consecutive days according to well-established procedures;54 and systemic markers of inflammation, assessed by using a Meso Scale Discovery electrochemiluminescence assay55 to determine 14 plasma markers of inflammation, including interleukin 1, interleukin 6, interleukin 17, Tumor Necrosis Factor, and eotaxin concentrations. We also store serum, plasma, and buffy coat samples at each time point as well as DNA for future analyses. We collect these data based in part on results from our pilot work, in which Mindfulness-Based Stress Reduction appears to alter stress-related biological pathways that contribute to cognitive changes in older adults, such as hypothalamic pituitary adrenal axis hyperactivity. In recent studies of Mindfulness-Based Stress Reduction, we showed that this mechanism contributes to cognitive remediation. We postulate that improvements in aerobic fitness and insulin sensitivity and increased Brain-Derived Neurotrophic Factor underlie exercise’s cognitive benefits in older adults.
Metabolic and fitness assessments include: 1) maximal graded exercise cardiac stress test to estimate cardiopulmonary fitness by using the length of time on the treadmill to reach 85% of the age-predicted estimate of maximum heart rate; 2) a 75 g 2-hour oral glucose tolerance test with sampling of insulin, glucose, and c-peptide at 0, 90, and 120 minutes to assess glucose tolerance and insulin sensitivity;56,57 3) Homeostatic model assessment of insulin resistance58 to provide a measure of insulin resistance; 4) dual energy X-ray absorptiometry to assess body fat mass and fat-free mass; 5) the Short Physical Performance Battery to assess walking speed, lower extremity strength (chair stands), and balance;59 and 6) a center of pressure test of total sway to assess balance.60
Behavioral and functional measures include the Cognitive and Affective Mindfulness Scale – Revised,61 Patient Reported Outcomes Measurement Information System self-report measures of Cognitive Concern, Anxiety, Depression, Insomnia, Satisfaction with Participation in Social Roles, and Ability to Participate in Social Roles.62
Physical activity is measured objectively using the ActiGraph Link (ActiGraph Inc., Pensacola, FL, USA), a tri-axial accelerometer that has previously been used among adults, children, and adolescents in the National Health and Nutrition Examination Survey63 and has consistently been shown to be both valid and reliable in measuring light, moderate, and vigorous physical activity.64–66 Participants wear the device continuously on their non-dominant wrist, except while bathing or swimming, for ten consecutive days.67–70 Data are processed so that absolute values from raw data (in accelerations) are transformed into “counts” (ActiGraph’s proprietary metric) per minute in the x, y, and z axes. These data are further aggregated into vector magnitude counts per minute. This metric incorporates intensity, frequency, and duration of acceleration and is recommended for assessing the total volume of physical activity in a 24-hour period.71 The ActiGraph is also used to distinguish sleep vs. wakefulness at night.72 Additional values derived include sleep latency, wake after sleep onset, and sleep quality.73 We also use a smartphone program to assess selected participant self-reports (such as positive and negative affect) daily for these 10 day periods (data from this program are not available for the pilot group).
We attempt to obtain neuroimaging data from all participants at baseline, 6 months, and 18 months. Structural neuroimaging data are collected via high-resolution (1×1×1mm) T1-weighted images. The magnetic resonance imaging protocols across the two sites were designed to be as similar as possible. The data is harmonized by comparing two control subjects that were scanned at both sites. Additionally, large amounts of control subject data are available from both sites that will enable us to perform a more complete comparison for differences across scanners. Data are analyzed with FreeSurfer;74 the key variables are hippocampal volume and prefrontal gray matter volumes, surface area, and thickness, corrected for whole brain volume. Structural magnetic resonance images are also reviewed by a study neuroradiologist who relays incidental findings to participants and/or their providers. Functional neuroimaging data are collected using multi-echo echoplanar imaging to measure the blood oxygen-level dependent signal while participants watch colorful, relaxing videos (no audio) of, for example, underwater scenes. Scans are analyzed by protocols developed at Washington University for isolating blood oxygen level dependent-related signal and motion correction75 and then the time courses in regions of interest are correlated with one another to reveal the coordinated activity of well-established large-scale networks. Finally, pseudo-continuous arterial spin labeling protocols are used to collect whole-brain and regional perfusion estimates.
Interventions
The goal of the interventions is for participants to develop a regular and sustained practice in exercise and/or mindfulness while benefiting optimally from these practices. Therefore, each intervention arm began with frequent, structured group sessions led by experts who trained and supervised participants, with increasing home practice increasing over time. The exercise intervention consists of twice-weekly group sessions for six months, followed by weekly group sessions for 12 months. All group sessions are led by experienced fitness instructors and include aerobic, progressive resistance training, and balance/mobility components. A manual was developed to ensure consistency across sites. Participants use treadmills, elliptical machines, and/or stationary bikes to achieve 65% of their peak heart rate at baseline, increasing to a rate of 70–85% of peak heart rate as tolerated over the course of the study. Strength training consists of performing one or two sets of 12–15 repetitions of each of 10 exercises on weight-lifting machines at a resistance of approximately 65% of their one-repetition maximum for the first 4–5 weeks, gradually increasing as tolerated to two sets at a resistance of up to 80% of one-repetition maximum, with 8–10 repetitions. Functional resistance training consists of whole body and compound muscle movements using body weight, dumbbells, and resistance bands. Participants also engage in dynamic movements to challenge postural stability and mobility. Home exercise is reinforced, to a goal of 150 minutes/week focused on aerobic and functional activities.
Mindfulness-Based Stress Reduction is conducted by instructors that have at least four years of experience and a personal mindfulness practice. The intervention is delivered according to the standard MBSR protocol,76 as developed by Jon Kabat-Zinn, Ph.D. After an orientation session, participants meet for eight 2.5 hour classes plus one four-hour retreat. They are taught mindful breathing, eating, listening, and everyday mindfulness; sitting, walking, and lying meditation; mindful movement (yoga); and loving-kindness meditation. They are assigned daily home practice to foster their learning. The only differences between the study classes and standard community Mindfulness-Based Stress Reduction classes are the shortening of the silent retreat from one day to a half day and the modification of some of the yoga exercises to account for age-related changes in flexibility and musculoskeletal conditions, based on feedback from older adults in previous studies by our research team.18 After the eight-week class is completed, participants attend instructor-led monthly booster sessions for the rest of the 18-month duration of the study. Although monthly boosters are not standard Mindfulness-Based Stress Reduction practice in most community settings, they are available at University of California San Diego’s Center for Mindfulness and are used to encourage continued practice. Daily home practice is reinforced, up to 45 minutes per day of formal meditative activities.
The health education control condition is based on a chronic disease self-management program developed at Stanford University77 and was used in a previous study by the authors.78 It is designed to be time-equivalent to Mindfulness-Based Stress Reduction, with 8 weeks of 2.5 hour weekly group classes followed by monthly group classes through the end of the study. This group-based intervention increases health-related knowledge and action. It improves disease management self-efficacy, but it does not teach mindfulness techniques and does not involve exercise. Instructors at both sites have backgrounds in social work, psychology, or nutrition and have experience leading health education groups.
Adherence and fidelity
One challenge in behavioral interventions is measuring adherence to the intervention. Measuring adherence is critical, as it is the only way to quantify the “dose” of the intervention that a given participant receives. These data are essential to understand how much exercise and/or Mindfulness-Based Stress Reduction one needs to do to reap the cognitive benefits. There are two facets of adherence in the MEDEX study: attendance at classes, and completion of at-home practice. Tracking class attendance is straightforward; tracking at-home practice is less so. Simply asking that participants do a certain amount of the activity each day or week is not enough; participants must report what they are actually doing so that we can account for noncompliance, breaks in adherence due to sickness and injuries, etc. However, regularly reporting activity can be burdensome to participants, and using paper logs can result in inaccurate data if participants try to fill it out days later. The MEDEX study responded to this challenge with the help of technology and feedback from the pilot participants. Study participants are asked to record their at-home practice on an app that can be either downloaded to their smartphone or used on an electronic tablet provided by the study. The app rings every day at the same time, and participants have a window of 7 hours to answer the survey. The survey asks how many minutes of exercise and/or Mindfulness-Based Stress Reduction they have completed that day; this serves as both a measurement and reinforcement of adherence.
To ensure that instructors were carrying out the interventions with good fidelity across sites, the exercise instructors had regular phone calls to discuss and agree on techniques, while Mindfulness-Based Stress Reduction and Health Education instructors meet weekly with a supervisor. In the randomized, controlled trial, fidelity to the intervention is monitored by video recording Mindfulness-Based Stress Reduction groups. Mindfulness-Based Stress Reduction experts at the Center for Mindfulness Research and Practice at Bangor University in Wales are rating these recordings according to mindfulness-based teaching assessment criteria.
Results
Prior to starting the randomized, controlled trial, the study team determined that it was necessary to first test the feasibility of recruiting and maintaining groups of older adults simultaneously in exercise and mindfulness training. Therefore, in an open trial, 29 older adults, 14 in St. Louis and 15 in San Diego, received simultaneous Mindfulness-Based Stress Reduction and exercise interventions for 18 months. Participants were 71.8 years old (SD = 5.7) and 65.5% were women (Table 1). They were predominantly Caucasian (93.1%, with the remaining 6.9% LatinX), and 72.4% had at least a Bachelor’s degree.
Table 1.
Demographics for the pilot sample and randomized controlled trial sample
| Pilot (N = 29) | RCT (N = 585) | |
|---|---|---|
| Age (Years), Mean (SD) | 71.8 (5.7) | 71.5 (4.8) |
| Gender, n (%) | ||
| Female | 19 (65.5) | 424 (72.5) |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 27 (93.1) | 546 (93.3) |
| Race, n (%) | ||
| Unknown/Not Reported | 1 (3.4) | 6 (1.0) |
| CIRS-G Total Score, Mean (SD) | 7.7 (4.3) | 6.8 (2.9) |
For biomarkers, actigraphy, and neuroimaging data, outliers were removed if they fell outside of 2 interquartile ranges from the first and third quartiles. For neuroimaging measures, data are presented for regional brain size only, as analysis of functional and perfusion images is still ongoing. Differences between baseline and 6 months as well as baseline and 18 months in each variable were explored by using a marginal model with time as a fixed effect and with an unstructured covariance structure. Estimated marginal means and standard errors are also provided (Table 2). Level of significance was set to 5%. All analyses were conducted in SPSS V24.
Table 2.
Results from the pilot study
| VARIABLES | Baseline | 3 Months | 6 Months | 18 Months | Marginal Model Results | |
|---|---|---|---|---|---|---|
| Baseline/6 Months | Baseline/18 Months | |||||
| Memory Composite | 0.00 (0.14) | 0.38 (0.15) | 0.53 (0.16) | 0.36 (0.19) | F[l,24.00]=38.99, p<0.001* | F[l,23.13]=9.35, p=0.01* |
| Paragraph Recall-Immediate | 44.59 (1.77) | 45.23 (1.55) | 48.05 (1.74) | 46.24 (2.40) | F[1,24.46]=3.63, p=0.07 | F[1,23.01]=0.76, p=0.39 |
| Paragraph Recall-Delay | 38.79 (1.97) | 41.94 (1.54) | 45.38 (1.52) | 40.70 (2.75) | F[1,26.62]=26.65, p<0.01* | F[1,23.10]=0.52, p=0.48 |
| Word List Learning-Immediate | 30.45 (1.44) | 34.30 (1.41) | 35.17 (1.68) | 35.01 (1.74) | F[1,24.10]=17.41, p<0.001* | F[1,23.10]=14.57, p=0.001* |
| Word List Learning-Delay | 6.90 (0.51) | 8.12 (0.69) | 8.37 (0.70) | 7.91 (0.69) | F[1,24.01]=10.55, p=0.003* | F[1,23.00]=5.80, p=0.02* |
| Picture Sequence Memory Test Raw Score | 14.93 (1.24) | 17.65 (1.59) | 18.40 (1.67) | 17.50 (1.75) | F[1,45.70]=2.79, p=0.10 | F[1,39.72]=1.44, p=0.24 |
| Cognitive Control Composite | 0.00 (0.09) | 0.09 (0.10) | 0.14 (0.10) | 0.22 (0.12) | F[1,24.21]=7.03, p=0.01* | F[1,23.01]=7.85, p=0.01* |
| Flanker Computed Score | 7.99 (0.13) | 7.99 (0.14) | 8.00 (0.15) | 7.94 (0.19) | F[1,24.29]=0.04, p=0.84 | F[1,23.51]=0.16, p=0.69 |
| List Sorting Working Memory Raw Score | 15.59 (0.59) | 16.44 (0.52) | 16.15 (0.61) | 16.71 (0.58) | F[1,24.37]=1.69, p=0.21 | F[1,26.02]=3.63,p=0.07 |
| Dimensional Change Card Sort Computed Score | 7.59 (0.15) | 7.62 (0.15) | 7.77 (0.15) | 7.85 (0.16) | F[1,25.63]=2.51, p=0.13 | F[1,24.53]=4.05, p=0.06 |
| CVOE Switch Trial Accuracy | 0.89 (0.03) | 0.91 (0.03) | 0.93 (0.02) | 0.93 (0.03) | F[1,28.39]=1.86, p=0.18 | F[1,27.01]=1.54, p=0.23 |
| CVOE Standardized Global Switch Cost | 1.36 (0.07) | 1.35 (0.13) | 1.51 (0.04) | 1.49 (0.07) | F[1,28.52]=3.35, p=0.08 | F[1,26.72]=2.38, p=0.14 |
| SART No-Go Accuracy | 0.92 (0.02) | 0.93 (0.02) | 0.93 (0.02) | 0.95 (0.02) | F[1,27.26]=0.07, p=0.79 | F[1,20.02]=0.29, p=0.60 |
| SART Go Trial CoV | 0.24 (0.02) | 0.21 (0.01) | 0.20 (0.01) | 0.22 (0.01) | F[1,25.07]=5.15, p=0.03* | F[1,11.61]=0.76, p=0.40 |
| Stroop Switch Trial Accuracy | 0.66 (0.03) | 0.70 (0.04) | 0.74 (0.04) | 0.72 (0.04) | F[1,23.22]=8.69, p=0.007* | F[1,23.20]=3.07, p=0.09 |
| Stroop Standardized Switch Cost | 0.18 (0.05) | 0.27 (0.04) | 0.23 (0.05) | 0.20 (0.05) | F[1,26.67]=0.83, p=.37 | F[1,25.341=0.32, p=0.58 |
| Sensory Variables | ||||||
| Mars Letter Contrast Sensitivity Test Contrast Sensitivity | 1.69 (0.03) | 1.70 (0.04) | F[1,25.20]=0.08, p=0.78 | |||
| Ishihara Color Vision Test | ||||||
| Number of Red-Green Plates Correctly Identified | 13.03 (0.47) | 13.12 (0.48) | F[1,23.09]=0.40, p=0.53 | |||
| Number of Blue-Yellow Plates Correctly Identified | 1.76 (0.11) | 1.92 (0.08) | F[1,27.65]=3.15, p=0.09 | |||
| Electronic Visual Acuity Score | 22.28 (2.99) | 23.10 (3.93) | F[1,23.58]=0.17, p=0.69 | |||
| Biomarkers (log10) | ||||||
| Peak Cortisol | 0.98 (0.06) | 0.98 (0.05) | 0.92 (0.05) | F[1,21.00]=0.001, p=0.98 | F[1,21.16]=0.51, p=0.48 | |
| IL-1 alpha | −1.13 (0.08) | −1.07 (0.10) | −1.00 (0.07) | F[1,21.79]=0.27, p=0.61 | F[1,18.23]=1.87, p=0.19 | |
| IL-1 beta | −2.42 (0.18) | −2.28 (0.15) | −1.97 (0.12) | F[1,26.41]=0.89, p=0.35 | F[1,26.01]=5.39, p=0.03* | |
| IL-6 | −0.12 (0.05) | −0.13 (0.05) | −0.18 (0.04) | F[1,25.23]=0.04, p=0.85 | F[1,24.71]=1.58, p=0.22 | |
| IL-17A | 0.12 (0.05) | 0.10 (0.05) | 0.08 (0.05) | F[1,26.05]=0.22, p=0.64 | F[1,23.83]=0.73, p=0.40 | |
| TNF-alpha | 0.33 (0.03) | 0.36 (0.03) | 0.36 (0.02) | F[1,24.26]=1.89, p=0.18 | F[1,21.41]=2.51, p=0.13 | |
| TNF-beta | −0.62 (0.07) | −0.66 (0.09) | −0.69 (0.06) | F[1,24.32]=0.24, p=0.63 | F[1,26.96]=0.83, p=0.37 | |
| Eotaxin | 1.92 (0.04) | 1.92 (0.04) | 1.96 (0.03) | F[1,25.28]=0.01, p=0.91 | F[1,25.40]=1.37, p=0.25 | |
| Eotaxin-3 | 0.66 (0.08) | 0.72 (0.07) | 0.72 (0.07) | F[1,24.41]=0.59, p=0.45 | F[1,22.67]=0.54, p=0.47 | |
| Metabolic/Fitness | ||||||
| HOMA-IR (log) | 0.36 (0.06) | 0.33 (0.05) | 0.39 (0.05) | F[1,25.91]=0.62, p=0.44 | F[1,23.61]=1.08, p=0.31 | |
| OGIS | 368.41 (12.34) | 379.89 (11.82) | 360.05 (11.58) | F[1,24.88]=1.43, p=0.24 | F[1,19.07]=1.17, p=0.29 | |
| ISI | 3.71 (0.39) | 3.49 (0.34) | 3.09 (0.30) | F[1,17.47]=0.74, p=0.40 | F[1,19.83]=8.53, p=0.009* | |
| Lean Body Mass (kg) | 44.98 (1.96) | 44.99 (1.95) | 44.94 (1.99) | F[1,23.95]=0.001, p=0.98 | F[1,23.00]=0.06, p=0.81 | |
| Percent Fat (Region) | 38.58 (1.33) | 38.10 (1.38) | 38.23 (1.47) | F[1,24.03]=1.15, p=0.30 | F[1,23.02]=0.38, p=0.55 | |
| SPPB | ||||||
| 25-ft Walk; Fastest of 2 Regular Time Walks | 6.27 (0.16) | 6.32 (0.21) | 6.57 (0.23) | F[1,24.79]=0.07, p=0.79 | F[1,23.33]=2.27, p=0.15 | |
| Chair Stands Score (0–4) | 2.48 (0.20) | 3.20 (0.13) | 2.80 (0.23) | F[1,28.84]=16.07, p<0.001* | F[1,25.68]=1.77, p=0.20 | |
| Balance Tests Total Score (0–4) | 3.76 (0.11) | 3.66 (0.15) | 3.66 (0.14) | F[1,25.86]=0.43, p=0.52 | F[1,24.95]=0.59, p=0.45 | |
| COP-Derived Total Sway for Better Balance Tests (cm) (log) | 1.50 (0.04) | 1.54 (0.04) | 1.53 (0.03) | F[1,24.04]=3.53, p=0.07 | F[1,24.31]=1.88, p=0.18 | |
| Behavioral Functional Raw Scores | ||||||
| CAMS-R | 36.35 (1.11) | 37.49 (0.92) | 38.89 (1.12) | 37.88 (1.05) | F[1,25.37]=8.67, p=0.007* | F[1,24.92]=1.96, p=0.17 |
| Cognitive Function | 65.55 (2.30) | 66.03 (2.36) | 67.11 (2.62) | 66.71 (2.71) | F[1,24.08]=0.86, p=0.36 | F[1,23.08]=0.22, p=0.64 |
| Anxiety | 15.00 (0.87) | 13.96 (0.79) | 13.96 (1.02) | 14.03 (1.02) | F[1,24.48]=1.63, p=0.21 | F[1,23.48]=1.09, p=0.31 |
| Depression | 13.35 (0.76) | 13.28 (0.99) | 13.36 (1.02) | 12.56 (0.87) | F[1,24.02]=0.003, p=0.96 | F[1,23.44]=1.71, p=0.20 |
| Insomnia | 20.62 (1.16) | 19.76 (1.20) | 19.88 (1.40) | 19.87 (1.36) | F[1,24.31]=0.42, p=0.52 | F[1,22.99]=0.21, p=0.65 |
| Satisfaction with Social Roles | 30.79 (1.11) | 31.20 (1.06) | 32.12 (1.03) | 32.80 (0.98) | F[1,26.53]=1.55, p=0.22 | F[1,25.90]=3.99, p=0.06 |
| Participation in Social Roles | 28.97 (1.04) | 29.35 (1.09) | 29.66 (1.05) | 29.42 (1.16) | F[1,25.20]=0.37, p=0.55 | F[1,23.16]=0.39, p=0.54 |
| OTDL-R | 19.31 (0.56) | 21.80 (0.54) | 21.89 (0.53) | F[1,24.011=15.44, p=0.001* | F[1,23.14]=16.71, p<0.001* | |
| Actigraph Data | ||||||
| VM-CPM | 1883.77 (104.53) | 1854.93 (112.56) | 1763.61 (91.75) | F[1,23.20]=0.20, p=0.66 | F[1,24.21]=3.58, p=0.07 | |
| Latency (log) | 0.64 (0.06) | 0.60 (0.07) | 0.74 (0.08) | F[1,25.05]=0.73, p=0.40 | F[1,19.34]=2.28, p=0.15 | |
| Total Sleep Time | 393.82 (9.78) | 390.97 (9.51) | 409.63 (10.37) | F[1,24.43]=0.01, p=0.91 | F[1,23.14]=2.97, p=0.10 | |
| Neuroimaging (Avg RH/LH) | ||||||
| Hippocampal Volume | 3450.31 (83.48) | 3451.42 (77.62) | 3421.55 (85.46) | F[1, 19.73]=0.01, p=0.91 | F[1, 18.25]=0.70, p=0.42 | |
| Gray Matter Volume | 30810.83 (778.33) | 30509.65 (730.39) | 30497.45 (736.45) | F[1, 17.02]=2.16, p=0.16 | F[1,18.19]=2.04, p=0.17 | |
| Surface Area | 10945.75 (289.02) | 11120.43 (334.13) | 10981.31 (331.65) | F[1,17.46]=1.75, p=0.20 | F[1, 18.82]=0.09, p=0.77 | |
| Thickness | 2.82 (0.03) | 2.76 (0.04) | 2.79 (0.04) | F[1,17.01]=1.88, p=0.19 | F[1, 19.85]=0.86, p=0.37 | |
Statistics are estimated marginal means and standard errors
CVOE=Consonant Vowel Odd Even; SART=Sustained Attention Response Test; IL=Interleukin; TNF=Tumor necrosis factor; HOMA-IR= Homeostatic model assessment of insulin resistance; OGIS=Oral glucose insulin sensitivity; ISI=Insulin sensitivity index; COP=Center of pressure; CAMS-R=Cognitive and Affective Mindfulness Scale-Revised; OTDL-R=Revised Observed Tasks of Daily Living; VM-CPM=Vector magnitude counts per minute.
Indicates a significant result at the 5% level
Pilot participants attended 79% of exercise sessions and 84% of Mindfulness-Based Stress Reduction sessions during the acute phase of the study. Both primary outcomes (memory composite score and the cognitive control composite score) improved over the course of participation (Table 2). The main effect of time was significant at both 6 and 18 months. Significant improvements were noted at 6 months for delayed paragraph recall, immediate and delayed list recall, and picture sequence memory. At 18 months, immediate and delayed list recall remained significantly improved. With respect to the individual cognitive control tests, the Sustained Attention to Response Test Go Trial coefficient of variance and Stroop switch trial accuracy were significantly improved at 6 months. No changes in individual cognitive control tests were statistically significant at 18 months. We also found significant increases in Observed Tasks of Daily Living-Revised scores at 6 and 18 months.
With respect to secondary outcomes and markers, we found improvements in chair stands and mindfulness at 6 months and in plasma IL-1beta concentration at 18 months, without correction for multiple comparisons. No other changes in biomarkers, metabolic/fitness measures, activity levels, or brain volumes were statistically significant.
The pilot group participants also provided feedback that we used to hone our materials and processes in preparation for the randomized, controlled trial. For example, they recorded their at-home practice (e.g., of exercise) on weekly paper logs but turned in these logs sporadically, sometimes with data mislabeled or missing. Participants told us: 1) we were asking questions that were too specific (e.g., we asked how many minutes they spent doing strength exercises, and how many minutes they spent doing functional exercises. They did not understand the difference between these two questions.); 2) they were forgetting to fill out the logs in real time and could not remember what activities they had done in order to fill it out later; and 3) they were losing the logs. Therefore, for the randomized, controlled trial we shortened the number of questions (i.e., we made strength and functional one category) and implemented an electronic system that sent auditory prompts, was answered in real time, and was less likely to get lost since it was completed on a smartphone or tablet.
Randomized controlled trial
We completed recruitment for the randomized, controlled trial in January 2019. The intended sample size was 580, but each site exceeded their target slightly, resulting in a randomized n=585 (291 in St. Louis and 294 in San Diego). Demographic information appears in Table 1; participants were 71.5 years old (SD=4.8), 73% were women and 75% were non-Hispanic White. Currently, the last participants are in the maintenance phase, with final data collection to be completed in July 2020.
Design features of the randomized controlled trial
This was a factorial design trial: all participants were simultaneously randomized to receive or not receive exercise, and to receive or not receive Mindfulness-Based Stress Reduction. This design is considered ideal for randomized, controlled trials testing multiple interventions, as it allows for using the entire randomized, controlled trial sample to test each intervention.79 This study design also allows researchers to examine whether there is an interactive effect of the two interventions – for example, does engaging in Mindfulness-Based Stress Reduction plus exercise have an additive effect (based on complementary mechanisms), a synergistic effect, or an interfering effect (based on competing demands or ceiling effects). Factorial design studies may be particularly useful for testing combination interventions in areas such as age-related cognitive decline, in which it is unlikely that the effect size of a single intervention modality will be sufficient in the aggregate and in the real world many individuals will be participating in more than one intervention at a time (for example, making physical activity and dietary changes). Factorial designs also have several challenges.80 In our case, one challenge was whether participants would have reduced adherence to the combination of exercise and Mindfulness-Based Stress Reduction, undermining the design; however, our pilot data showed that this did not appear to be a problem. It also raised the question of the appropriate control intervention in the case of participants randomized to no exercise and no Mindfulness-Based Stress Reduction. Ultimately, we settled on Health Education, based on the principle of providing an intervention to all participants to reduce expectancy, and controlling for time and attention.
Recruitment into the randomized, controlled trial
The challenges of recruiting older adults into clinical trials are well-documented.81–84 Because of the large sample size and relatively tight inclusion/exclusion criteria of the randomized, controlled trial, we generated a robust recruitment plan in order to meet overall and minority-specific recruitment goals. In the later years of the study, we were able to receive a supplement from the funding institute so that we could hire recruitment specialists to help meet these goals. Key recruitment strategies that worked were media stories (both newspaper and television), online sources, targeted mailings, word of mouth, flyers, and research participant registries (Table 3). We were able to meet our recruitment goal on time, with an adequate level of racial and ethnic diversity, given that this is an older adult sample with requirements to be an English speaker.
Table 3.
Recruitment by source in the randomized controlled trial (total n=585 randomized)
| Recruitment tactic | Number of randomized participants from this source | Percentage of sample from this source |
|---|---|---|
| Online sources (i.e., website, Facebook, Trialspark) | 146 | 25.48% |
| Press (TV, Newspaper, Magazine, etc…) | 145 | 25.31% |
| Recruitment Letter | 89 | 15.53% |
| Word of Mouth | 79 | 13.79% |
| Flyer | 47 | 8.20% |
| Other/unknown | 37 | 6.46% |
| University-wide research registry | 21 | 3.66% |
| Referred from different study | 8 | 1.40% |
| Physician Referral | 1 | 0.17% |
Conclusions
MEDEX is a large-scale, explanatory, randomized controlled trial designed to test the efficacy and mechanisms of two readily-available non-pharmacological interventions -- Mindfulness-Based Stress Reduction and exercise -- alone and in combination, for remediating age-related cognitive decline in adults aged 65 and older. In addition to the primary outcome variables of memory and cognitive control, we are investigating biomarker, metabolic/fitness, behavioral/functional, activity, and neuroimaging variables in order to test potential mechanisms of change and secondary outcomes. To our knowledge, this study represents one of the largest exercise studies and the largest study of a mindfulness intervention to date in older healthy adults. When complete, it should provide an understanding of whether and in whom these commonly-available behavioral interventions benefit cognitive functioning.
Benefits of conducting pilot testing
We conducted an open-label pilot study involving 29 adults from both sites receiving both active interventions to determine whether it was feasible to carry out a combination behavioral intervention study of this scope. We found that it is feasible to simultaneously engage study participants in both Mindfulness-Based Stress Reduction and exercise interventions, and with high retention rates. Anecdotally, we also found that carrying out a pilot study was beneficial for improving study procedures.
We also found preliminary evidence supporting our hypotheses that the combination of exercise and Mindfulness-Based Stress Reduction improves memory and cognitive control over time (Table 1). We further found improvements in a performance-based measure of instrumental activities of daily living, the Observed Test of Daily Living-Revised. These preliminary findings will be robustly tested in our full-scale randomized, controlled trial that includes a comparator condition. The results may support the efficacy of two readily available behavioral interventions for preserving cognitive function and, more importantly, independence, with aging.
Limitations of the study
Some limitations of the MEDEX study bear mention. First, the Short Physical Performance Battery may not be the most sensitive to change due to ceiling effects in a sample of community-dwelling healthy older adults. Similarly, a measure of dynamic balance would have been a helpful addition to the sway test measure of static balance employed. Additionally, the sample is relatively young, mainly white, and quite healthy. Thus, results may not generalize to the overall population of older adults with cognitive concerns.
The MEDEX study is a large randomized, controlled trial that will evaluate the potential benefits and mechanisms of two commonly available interventions for age-related cognitive decline: Mindfulness-Based Stress Reduction and exercise. We have shown that this study is feasible and has resulted in a large sample of older adults. We anticipate our findings will affect our understanding of how to remediate age-related cognitive decline. Insights from MEDEX could inform the community whether, how, and in whom exercise and mindfulness training each provide benefits for cognitive functioning, potentially with greater benefits from the combination of both interventions. In particular, we expect to demonstrate that exercise and mindfulness training impact physiological processes related to aging,85 such as insulin sensitivity, inflammation, and stress hormone levels.86–88 We will also test whether brain structural and functional changes mediate the cognitive benefits of the interventions on neuropsychological performance and behavioral measures of every day functioning. This study has considerable clinical implications because of its potential to establish the use of behavioral interventions to preserve cognitive function and independence in older adults.
References
- 1.Dzierzewski JM, Dautovich N and Ravyts S. Sleep and Cognition in Older Adults. Sleep Med Clin 2018; 13(1): 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lange AG, Brathen ACS, Rohani DA, et al. The effects of memory training on behavioral and microstructural plasticity in young and older adults. Hum Brain Mapp 2017; 38(11): 5666–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson KI, Raji CA, Lopez OL, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 2010; 75(16): 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan S, Wallis M, Polit D, et al. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing 2014; 43(5): 623–629. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Fiocco AJ, Lindquist K, et al. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology 2009; 72(23): 2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson KI, Gildengers AG and Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci 2013; 15(1): 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Pinilla F and Hillman C. The influence of exercise on cognitive abilities. Compr Physiol 2013; 3(1): 403–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark PJ, Brzezinska WJ, Thomas MW, et al. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 2008; 155(4): 1048–1058. [DOI] [PubMed] [Google Scholar]
- 9.Creer DJ, Romberg C, Saksida LM, et al. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A 2010; 107(5): 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006; 14(3): 345–356. [DOI] [PubMed] [Google Scholar]
- 11.Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004; 124(1): 71–79. [DOI] [PubMed] [Google Scholar]
- 12.Izumi Y, Yamada KA, Matsukawa M, et al. Effects of insulin on long-term potentiation in hippocampal slices from diabetic rats. Diabetologia 2003; 46(7): 1007–1012. [DOI] [PubMed] [Google Scholar]
- 13.van Praag H, Shubert T, Zhao C, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 2005; 25(38): 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivar C, Potter MC and van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci 2013; 15: 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voss MW, Vivar C, Kramer AF, et al. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 2013; 17(10): 525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black DS and Slavich GM. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann N Y Acad Sci 2016; 1373(1): 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes JW, Fresco DM, Myerscough R, et al. Randomized controlled trial of mindfulness-based stress reduction for prehypertension. Psychosom Med 2013; 75(8): 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenze EJ, Hickman S, Hershey T, et al. Mindfulness-based stress reduction for older adults with worry symptoms and co-occurring cognitive dysfunction. Int J Geriatr Psychiatry 2014; 29(10): 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, Davidson RJ. Mental training enhances attentional stability: neural and behavioral evidence. J Neurosci 2009; 29(42): 13418–13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci 2007; 2(4): 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res 2011; 191(1): 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang YY, Lu Q, Fan M, et al. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci U S A 2012; 109(26): 10570–10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang YY, Lu Q, Geng X, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci U S A 2010;107(35): 15649–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells RE, Yeh GY, Kerr CE, et al. Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: a pilot study. Neurosci Lett 2013; 556: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews-Hanna JR, Snyder AZ, Vincent JL, et al. Disruption of large-scale brain systems in advanced aging. Neuron 2007; 56(5): 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deyo M, Wilson KA, Ong J, et al. Mindfulness and rumination: does mindfulness training lead to reductions in the ruminative thinking associated with depression? Explore (NY) 2009; 5(5): 265–271. [DOI] [PubMed] [Google Scholar]
- 27.Goldin PR and Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 2010; 10(1): 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain S, Shapiro SL, Swanick S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med 2007; 33(1): 11–21. [DOI] [PubMed] [Google Scholar]
- 29.Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry 1992; 149(7): 936–943. [DOI] [PubMed] [Google Scholar]
- 30.Lengacher CA, Johnson-Mallard V, Barta M, et al. Feasibility of a mindfulness-based stress reduction program for early-stage breast cancer survivors. J Holist Nurs 2011; 29(2): 107–117. [DOI] [PubMed] [Google Scholar]
- 31.Matousek RH and Dobkin PL. Weathering storms: a cohort study of how participation in a mindfulness-based stress reduction program benefits women after breast cancer treatment. Curr Oncol 2010; 17(4): 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JJ, Fletcher K and Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 1995;17(3):192–200. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro SL, Oman D, Thoresen CE, et al. Cultivating mindfulness: effects on well-being. J Clin Psychol 2008; 64(7): 840–862. [DOI] [PubMed] [Google Scholar]
- 34.Ashton NJ, Hye A, Leckey CA, et al. Plasma REST: a novel candidate biomarker of Alzheimer’s disease is modified by psychological intervention in an at-risk population. Transl Psychiatry 2017; 7(6): e1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brand S, Holsboer-Trachsler E, Naranjo JR, et al. Influence of mindfulness practice on cortisol and sleep in long-term and short-term meditators. Neuropsychobiology 2012; 65(3): 109–118. [DOI] [PubMed] [Google Scholar]
- 36.Branstrom R, Kvillemo P and Akerstedt T. Effects of mindfulness training on levels of cortisol in cancer patients. Psychosomatics 2013; 54(2): 158–164. [DOI] [PubMed] [Google Scholar]
- 37.Carlson LE, Speca M, Faris P, et al. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun 2007; 21(8): 1038–1049. [DOI] [PubMed] [Google Scholar]
- 38.Carlson LE, Speca M, Patel KD, et al. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology 2004; 29(4): 448–474. [DOI] [PubMed] [Google Scholar]
- 39.Lengacher CA, Kip KE, Barta M, et al. A pilot study evaluating the effect of mindfulness-based stress reduction on psychological status, physical status, salivary cortisol, and interleukin-6 among advanced-stage cancer patients and their caregivers. J Holist Nurs 2012; 30(3): 170–185. [DOI] [PubMed] [Google Scholar]
- 40.Matousek RH, Pruessner JC and Dobkin PL. Changes in the cortisol awakening response (CAR) following participation in mindfulness-based stress reduction in women who completed treatment for breast cancer. Complement Ther Clin Pract 2011; 17(2): 65–70. [DOI] [PubMed] [Google Scholar]
- 41.Nyklicek I, Mommersteeg PM, Van Beugen S, et al. Mindfulness-based stress reduction and physiological activity during acute stress: a randomized controlled trial. Health Psychol 2013; 32(10): 1110–1113. [DOI] [PubMed] [Google Scholar]
- 42.Heisz JJ, Clark IB, Bonin K, et al. The effects of physical exercise and cognitive training on memory and neurotrophic factors. J Cogn Neurosci 2017; 29(11): 1895–1907. [DOI] [PubMed] [Google Scholar]
- 43.de Bruin EI, Formsma AR, Frijstein G, et al. Mindful2Work: effects of combined physical exercise, yoga, and mindfulness meditations for stress relieve in employees. A proof of concept study. Mindfulness (N Y) 2017; 8(1): 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards MK and Loprinzi PD. Affective responses to acute bouts of aerobic exercise, mindfulness meditation, and combinations of exercise and meditation: a randomized controlled intervention. Psychol Rep 2019; 122(2): 465–484. [DOI] [PubMed] [Google Scholar]
- 45.Chan JSY, Deng K, Wu J, et al. Effects of meditation and mind-body exercises on older adults’ cognitive performance: a meta-analysis. Gerontologist 2019; 59(6): e782–e790. [DOI] [PubMed] [Google Scholar]
- 46.Dikmen SS, Bauer PJ, Weintraub S, et al. Measuring episodic memory across the lifespan: NIH toolbox picture sequence memory test. J Int Neuropsychol Soc 2014; 20(6): 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH toolbox. Neurology 2013; 80(11 Suppl 3): S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson JD, Balota DA, Duchek JM, et al. White matter integrity and reaction time intraindividual variability in healthy aging and early-stage Alzheimer disease. Neuropsychologia 2012; 50(3): 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Schie MK, Thijs RD, Fronczek R, et al. Sustained attention to response task (SART) shows impaired vigilance in a spectrum of disorders of excessive daytime sleepiness. J Sleep Res 2012; 21(4): 390–395. [DOI] [PubMed] [Google Scholar]
- 50.Balota DA, Tse CS, Hutchison KA, et al. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: the power of errors in stroop color naming. Psychol Aging 2010; 25(1): 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duchek JM, Balota DA, Thomas JB, et al. Relationship between Stroop performance and resting state functional connectivity in cognitively normal older adults. Neuropsychology 2013; 27(5): 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diehl M, Marsiske M, Horgas AL, et al. The revised observed tasks of daily living: a performance-based assessment of everyday problem solving in older adults. J Appl Gerontol 2005; 24(3): 211–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015; 350: h2147. [DOI] [PubMed] [Google Scholar]
- 54.Rosnick CB, Wetherell JL, White KS, et al. Cognitive-behavioral therapy augmentation of SSRI reduces cortisol levels in older adults with generalized anxiety disorder: A randomized clinical trial. J Consult Clin Psychol 2016; 84(4): 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefura WP, Graham C, Lotoski L, et al. Improved methods for quantifying human chemokine and cytokine biomarker responses: ultrasensitive ELISA and meso scale electrochemiluminescence assays. Methods Mol Biol 2019; 2020: 91–114. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda M and DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22(9): 1462–1470. [DOI] [PubMed] [Google Scholar]
- 57.Mari A, Pacini G, Murphy E, et al. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001; 24(3): 539–548. [DOI] [PubMed] [Google Scholar]
- 58.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28(7): 412–419. [DOI] [PubMed] [Google Scholar]
- 59.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49(2): M85–M94. [DOI] [PubMed] [Google Scholar]
- 60.O’Connor SM, Baweja HS and Goble DJ. Validating the BTrackS Balance Plate as a low cost alternative for the measurement of sway-induced center of pressure. J Biomech 2016; 49(16): 4142–4145. [DOI] [PubMed] [Google Scholar]
- 61.Feldman G, Hayes A, Kumar S, et al. Mindfulness and emotion regulation: the development and initial validation of the cognitive and affective mindfulness scale-revised (CAMS-R). J Psychopathol Behav Assess 2007; 29: 177–190. [Google Scholar]
- 62.Riley WT, Pilkonis P and Cella D. Application of the National Institutes of Health patient-reported outcome measurement information system (PROMIS) to mental health research. J Ment Health Policy Econ 2011; 14(4): 201–208. [PMC free article] [PubMed] [Google Scholar]
- 63.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008; 40(1): 181–188. [DOI] [PubMed] [Google Scholar]
- 64.John D and Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc 2012; 44(1 Suppl 1): S86–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robusto KM and Trost SG. Comparison of three generations of ActiGraph activity monitors in children and adolescents. J Sports Sci 2012; 30(13): 1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warren JM, Ekelund U, Besson H, et al. Assessment of physical activity - a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2010; 17(2): 127–139. [DOI] [PubMed] [Google Scholar]
- 67.Bassett DR, Troiano RP, McClain JJ, et al. Accelerometer-based physical activity: total volume per day and standardized measures. Med Sci Sports Exerc 2015; 47(4): 833–838. [DOI] [PubMed] [Google Scholar]
- 68.Freedson P, Bowles HR, Troiano R, et al. Assessment of physical activity using wearable monitors: recommendations for monitor calibration and use in the field. Med Sci Sports Exerc 2012; 44(1 Suppl 1): S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Troiano RP, McClain JJ, Brychta RJ, et al. Evolution of accelerometer methods for physical activity research. Br J Sports Med 2014; 48(13): 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tudor-Locke C, Barreira TV, Schuna JM Jr, et al. Improving wear time compliance with a 24-hour waist-worn accelerometer protocol in the International Study of Childhood Obesity, Lifestyle and the Environment (ISCOLE). Int J Behav Nutr Phys Act 2015; 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cole RJ, Kripke DF, Gruen W, et al. Automatic sleep/wake identification from wrist activity. Sleep 1992; 15(5): 461–469. [DOI] [PubMed] [Google Scholar]
- 72.Sadeh A, Sharkey KM and Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17(3): 201–207. [DOI] [PubMed] [Google Scholar]
- 73.Full KM, Kerr J, Grandner MA, et al. Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health 2018; 4(2): 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fischl B FreeSurfer. Neuroimage 2012; 62(2): 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Power JD, Mitra A, Laumann TO, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014; 84: 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santorelli SF, Meleo-Meyer F and Koerbel L. Mindfulness-based stress reduction (MBSR) authorized curriculum Guide. University of Massachusetts Medical School: Center for Mindfulness in Medicine, Health Care, and Society, 2017. [Google Scholar]
- 77.Lorig K, Holman H and Sobel D. Living a healthy life with chronic conditions: self-management of heart disease, arthritis, diabetes, depression, asthma, bronchitis, emphysema and other physical and mental health conditions. Boulder, Colorado: Bull Publishing, 2012. [Google Scholar]
- 78.Wetherell JL, Hershey T, Hickman S, et al. Mindfulness-based stress reduction for older adults with stress disorders and neurocognitive difficulties: a randomized controlled trial. J Clin Psychiatry 2017; 78(7): e734–e743. [DOI] [PubMed] [Google Scholar]
- 79.Collins LM, Dziak JJ and Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods 2009; 14(3): 202–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker TB, Smith SS, Bolt DM, et al. Implementing clinical research using factorial designs: a primer. Behav Ther 2017; 48(4): 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boles M, Getchell WS, Feldman G, et al. Primary prevention studies and the healthy elderly: evaluating barriers to recruitment. J Community Health 2000; 25(4): 279–292. [DOI] [PubMed] [Google Scholar]
- 82.Knechel NA. The challenges of enrolling older adults into intervention studies. Yale J Biol Med 2013; 86(1): 41–47. [PMC free article] [PubMed] [Google Scholar]
- 83.Sanders ML, Stuckenschneider T, Devenney KE, et al. Real world recruiting of older subjects with mild cognitive impairment for exercise trials: community readiness is pivotal. J Alzheimers Dis 2018; 62(2): 579–581. [DOI] [PubMed] [Google Scholar]
- 84.Schneider LS. Recruitment methods for United States Alzheimer disease prevention trials. J Nutr Health Aging 2012; 16(4): 331–335. [DOI] [PubMed] [Google Scholar]
- 85.Fiocco AJ, Krieger L, D’Amico D, et al. A systematic review of existing peripheral biomarkers of cognitive aging: Is there enough evidence for biomarker proxies in behavioral modification interventions?: An initiative in association with the nutrition, exercise and lifestyle team of the Canadian Consortium on Neurodegeneration in Aging. Ageing Res Rev 2019; 52: 72–119. [DOI] [PubMed] [Google Scholar]
- 86.Frazier HN, Ghoweri AO, Anderson KL, et al. Broadening the definition of brain insulin resistance in aging and Alzheimer’s disease. Exp Neurol 2019; 313: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nichols MR, St-Pierre MK, Wendeln AC, et al. Inflammatory mechanisms in neurodegeneration. J Neurochem 2019; 149(5): 562–581l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ouanes S and Popp J. High cortisol and the risk of dementia and Alzheimer’s disease: a review of the literature. Front Aging Neurosci 2019; 11: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]


