Abstract
Introduction:
The peptide hormone ghrelin regulates physiological processes associated with energy homeostasis such as appetite, insulin signaling, glucose metabolism, and adiposity. Ghrelin has also been implicated in a growing number of neurological pathways involved in stress response and addiction behavior. For ghrelin to bind the growth hormone secretagogue receptor 1a (GHS-R1a) and activate signaling, the hormone must first be octanoylated on a specific serine side chain. This key transformation is performed by the enzyme ghrelin O-acyltransferase (GOAT), and therefore GOAT inhibitors may be useful in treating disorders related to ghrelin signaling such as diabetes, obesity, and related metabolic syndromes.
Areas covered:
This report covers ghrelin and GOAT as potential therapeutic targets and summarizes work on GOAT inhibitors through the end of 2019, highlighting recent successes with both peptidomimetics and small molecule GOAT inhibitors as potent modulators of GOAT-catalyzed ghrelin octanoylation.
Expert opinion:
A growing body of biochemical and structural knowledge regarding the ghrelin / GOAT system now enables multiple avenues for identifying and optimizing GOAT inhibitors. We are at the beginning of a new era with increased opportunities for leveraging ghrelin and GOAT in the understanding and treatment of multiple health conditions including diabetes, obesity, and addiction.
Keywords: ghrelin, ghrelin O-acyltransferase, GHS-R1a receptor, membrane-bound O-acyltransferase, protein acylation, diabetes, obesity, metabolic syndrome, addiction, alcoholism
1. Introduction
Ghrelin is a 28-amino acid peptide hormone that is notable as the only known hunger-stimulating hormone in humans. Originally discovered in 1999 during the search for the endogenous ligand of the growth hormone secretagogue receptor (GHS-R1a), ghrelin stimulates the release of growth hormone (GH) upon receptor activation [1]. When compared to ghrelin isolated from rat stomach extracts by reverse-phase HPLC, a synthetic ghrelin peptide exhibited a shift in retention time indicating the presence of a hydrophobic modification on the naturally occurring hormone [1]. This modification was identified as octanoylation of the hydroxyl sidechain of serine-3, with this acylation found to be essential for ghrelin to activate GH secretion [1]. While originally identified in the stomach, ghrelin expression has also been reported in a wide range of other tissues, consistent with the variety of physiological effects attributed to ghrelin signaling [1, 2, 3, 4].
Ghrelin signaling impacts growth hormone release, appetite stimulation, and energy balance [1, 5, 6, 7, 8, 9, 10, 11, 12]. Activation of GHS-R1a stimulates the release of orexigenic peptides neuropeptide Y (NPY) and Agouti-related peptide (AgRP), leading to increased food intake [13, 14, 15, 16, 17, 18]. Mice lacking critical enzymes in the maturation pathway of ghrelin exhibit reduced GH release, causing fatal hypoglycemia when exposed to starvation conditions [19, 20]. Adiposity gains in NPY and GH deficient rodents have revealed a role for ghrelin signaling in energy balance outside release of NPY and GH. [6, 21]. Ghrelin has also been proposed to have roles in neurological functions including memory, addiction, and depression [22, 23, 24, 25, 26, 27]. Further correlations have been made between ghrelin and stress, as blocking ghrelin receptor activation has shown mediation of fear response in mice subjected to stress conditions [28, 29, 30]. The variety of physiological process impacted by ghrelin provide an opportunity to control multiple pathways by altering ghrelin signaling.
As the therapeutic potential of ghrelin and GHS-R1a signaling has been appreciated, the development of GHS-R1a antagonists and inverse agonists has received increasing attention. While a full discussion of this exciting research area is beyond the scope of this review on inhibitors targeting ghrelin acylation, we direct the reader to several recent reviews detailing studies of molecules developed to directly modulate GHS-R1a signaling activity [31, 32, 33, 34, 35, 36, 37].
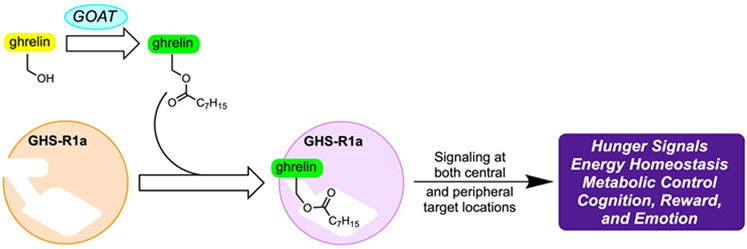
The search for the enzyme responsible for the lipidation of ghrelin led to the discovery of ghrelin O-acyltransferase (GOAT) in 2008 by two independent labs [38, 39]. A member of the membrane-bound O-acyltransferase (MBOAT) family of integral membrane proteins, GOAT acylates ghrelin with an octanoyl moiety that is unique among known proteins (Figure 1) [40, 41]. GOAT is most highly expressed in the stomach, but is also produced in the pancreas, with significant but lesser expression throughout the gastrointestinal tract [38, 39]. Expression of GOAT is also seen in the hypothalamus and pituitary, locations where ghrelin is also found [42].
Figure 1.
The Role of GOAT in Ghrelin Signaling
Ghrelin undergoes multiple processing steps before secretion as a mature peptide hormone. Initially translated as the 117 amino acid preproghrelin, the peptide is cleaved by signal peptidase to the prohormone proghrelin [43]. Proghrelin is then octanoylated by GOAT [38, 39], and acylated ghrelin is secreted after cleavage of the C-terminal portion of proghrelin to yield the mature 28 amino acid hormone [43]. Acyl ghrelin (AG) is cleaved by serum esterases to unacylated ghrelin (UAG), removing the ester-linked octanoyl chain and the ability to activate the GHS-R1a receptor [44, 45, 46, 47, 48]. While UAG does not activate GHS-R1a, there is evidence to suggest that it is still biologically active, playing a role in insulin secretion [22, 49, 50].
Due to their nature as integral membrane proteins, GOAT and other members of the MBOAT enzyme family have proven recalcitrant to structural studies. The membrane topologies of GOAT, Hhat, and several lipid- and small-molecule acylating MBOAT enzymes have been determined using selective permeabilization experiments [51, 52, 53, 54, 55, 56]. These studies indicate MBOAT family enzymes are topologically complex proteins with multiple transmembrane helices such as the eleven transmembrane domains in GOAT [51]. More recently, a computational structure of human GOAT and a crystal structure of the bacterial MBOAT alanyltransferase DltB have provided exciting insights into the catalytic strategies employed by these enzymes to effect transmembrane protein and lipid acylation [57, 58], and a cryo-EM structure of human sterol O-acyltransferase 1 (SOAT1) has provided insight into the mechanism and inhibition of this enzyme which is involved in cholesterol storage [59]. Access to these MBOAT structures opens a new avenue for structure-guided inhibitor design targeting GOAT.
2. Therapeutic targets for ghrelin
2.1. Obesity and eating disorders
Associated with its role as the “hunger hormone”, ghrelin signaling was linked to appetite control and stimulation shortly following its discovery [1, 6, 7, 40]. Ghrelin binding to GHS-R1a in the arcuate nucleus of the hypothalamus stimulates NPY and AgRP containing neurons, increasing food consumption and decreasing energy spending [13]. Increasing food consumption leading to increased weight gain is one possible effect of ghrelin signaling, but ghrelin can also modulate weight gain through the upregulation of adipogenesis independently from food intake [60, 61]. Despite the ability of ghrelin to influence weight gain, obese patients exhibit lower plasma ghrelin levels than controls [62, 63]. Interestingly, ghrelin levels do not fall after a meal in obese humans when compared to lean controls, indicating abnormal ghrelin signaling may be part of the pathophysiology of obesity [64]. Mice subjected to diet-induced obesity do not respond to exogenous ghrelin, failing to stimulate food intake, GH release, or NPY/AgRP signaling. Peptide-based inhibitors targeting the biosynthesis of acyl ghrelin have shown some promise in abating weight gain in mice, validating the potential therapeutic value of targeting ghrelin signaling in treating obesity [65]. However, a similar molecule that mimics UAG also impacts weight gain and rebound feeding following fasting in mice without directly impacting GOAT acylation activity [66]. This suggests additional complexity in the role and impact of ghrelin on these aspects of metabolic control that remains to be fully defined.
Perturbations in ghrelin signaling have also been investigated in eating disorders including anorexia nervosa and bulimia. Adolescents with anorexia nervosa have been shown to exhibit higher levels of ghrelin than controls, with a return to normal serum ghrelin concentrations after weight recovery [67, 68]. Higher ghrelin concentrations have been generally observed in patients with anorexia, while ghrelin measurements in bulimic patients have shown more variability between studies [69, 70, 71]. In these disorders, the impact of ghrelin upon behavior has been proposed to occur through the reward centers within the brain leading to reinforcement of restrictive behaviors regarding food intake and purging [72, 73, 74]. Somewhat counterintuitively, treatment with exogenous ghrelin or GHS-R1a agonists has been shown to increase hunger and food intake in patients with anorexia nervosa and cancer-induced cachexia [33, 75, 76, 77, 78]. A parallel study of peptide-based GHS-R1a receptor agonists and antagonists demonstrated treatment with both types of agents stimulated appetite in a rodent model [79], underscoring the necessity to develop a more refined molecular understanding of ghrelin signaling.
2.2. Prader-Willi Syndrome
Patients with Prader-Willi syndrome (PWS) exhibit multiple symptoms, including mild mental retardation, growth hormone deficiency, short stature, and behavioral issues. Obesity and excessive weight gain are common for those affected by PWS, and one unusual symptom of this disorder is insatiable appetite (hyperphagia) leading to chronic overeating [80, 81]. While the molecular mechanisms for many PWS symptoms are unknown, initial studies of PWS indicated the hallmark hyperphagic behavior was likely due to lack of satiation rather than increased hunger [81]. The discovery of ghrelin in 1999 offered a possibility that the hyperphagia and adiposity associated with PWS might result from dysregulated ghrelin signaling [1, 82]. Examining the levels of serum ghrelin associated with PWS revealed elevated ghrelin levels in both adults and children [82, 83, 84]. Complicating the connection between ghrelin and PWS symptoms, elevated ghrelin levels are detected in children prior to the onset of hyperphagia [85, 86]. As assays for AG have improved (primarily due to increased sensitivity and the development of methods for the stabilization of acylated ghrelin), increased ratios of AG to UAG have been detected in patients exhibiting hyperphagia or weight gain, while patients tested prior to the onset of these symptoms exhibit abnormally low AG to UAG ratios [87, 88]. These studies reconcile the anorexia shown by infants with the hyperphagia displayed by adults by demonstrating a reversal in the AG/UAG ratio, but the mechanism for this switch has yet to be deciphered.
Previous attempts to reduce ghrelin levels with somatostatin and its long-acting analogue octreotide have not led to reduction in the symptoms of PWS patients [89, 90]. A PWS mouse model, Snord116del has also exhibited lower sensitivity to GHS-R1a receptor targeted anorexic agents [91]. The inability of these molecules to affect cessation of PWS symptoms suggested alternative routes for targeting the ghrelin signaling pathway that do not depend on the GHS-R1a receptor, and the need to be able to change the AG/UAG ratio. Targeting the biosynthetic pathway of ghrelin, particularly the acylation catalyzed by GOAT, could provide such an option. However, it is important to note it remains to be conclusively demonstrated that elevated serum acyl ghrelin levels are a driving causal factor in PWS-associated hyperphagia. Clinical trials of GOAT inhibitors will offer the first insights into this important question (discussed below).
2.3. Addiction
Stimulation of the mesolimbic dopaminergic system, located in the ventral tegmental area (VTA) of the brain, results in dopamine release in local neurons. This release is associated with the reward pathways triggered through pleasurable behaviors like feeding or drugs of abuse [92, 93, 94, 95, 96]. Ghrelin injections into the VTA have been shown to release dopamine in a manner consistent with the activation of this reward pathway [97]. This ability for ghrelin to alter the brain response to pleasurable stimuli led to the examination of ghrelin in the context of altered seeking behaviors, including drug addiction.
A study performed with patients in hospital treatment for alcohol addiction reported that ghrelin levels were significantly increased in this cohort, even when corrected for BMI [98, 99]. Over the course of patient withdrawal, plasma ghrelin levels continued to increase but could not be correlated to craving experienced by the subjects [98]. Other studies have positively correlated ghrelin levels to alcohol cravings [100, 101, 102, 103]. Furthermore, recent fMRI studies have indicated that increased acyl ghrelin levels are significantly associated with alcohol cue-induced brain response, while an increase in total ghrelin level does not induce the same response [104, 105].
Pretreatment with synthetic ghrelin lowered the apparent threshold for cocaine to induce conditioned place preference (CPP) and locomotion in rats [106, 107, 108]. Ghrelin also has been shown to modulate the drug-seeking behavior of rats exposed to cocaine, increasing the response of the animals to cocaine-associated cues [109]. Locomotion and CPP have been shown to be reduced by the introduction of a GHS-R1a antagonist, indicating that the receptor plays a role in the exhibition of addictive behaviors [110]. Ghrelin has also been shown to have effects on the addiction pathway of nicotine and methamphetamine, as inhibition of GHS-R1a similarly reduces locomotion and CPP under treatment with these substances [110, 111, 112, 113].
The ghrelin signaling pathway may also play a role in the addiction to opioids. GHS-R1a antagonist pretreatment has been shown to reduce morphine-induced behavior in rats [114]. Measurement of endocannabinoids released under morphine challenge showed significant alteration following the introduction of a GHS-R1a antagonist, implicating ghrelin as an actor in opioid reinforcing mechanisms [115]. GHS-R1a antagonism has also been shown to reduce heroin seeking behaviors, as well as reduce fentanyl-induced dopamine release in rats [116, 117].
2.4. Type II diabetes
Type II Diabetes Mellitus (T2DM) is a disease closely linked with obesity characterized by dysregulation of the insulin signaling system. These problems may not be diagnosed until clinical hyperglycemia occurs due to the inability of islet β-cells to secrete enough insulin to counteract resistance [118, 119, 120]. Ghrelin has been linked to glucose regulation and energy balance through effects on insulin signaling [121, 122, 123, 124]. The treatment of human subjects with acyl ghrelin injection has been found to increase blood glucose levels and decrease insulin levels, consistent with rodent studies [9, 10, 125, 126]. Acylated ghrelin has been detected in the pancreatic islets, reducing Ca2+ signaling resulting in the inhibition of glucose-dependent insulin secretion [127]. Exogenous acyl ghrelin reduces the insulin response to glucose and indicates that endogenous ghrelin may play a role in physiologic insulin secretion. A combination of AG and UAG strongly improves insulin sensitivity in humans [49]. UAG correlates with lower insulin resistance in humans and lowering the ratio of AG to UAG may be important in increasing insulin action, possibly by increasing survival of islet β-cells [128, 129]. Injections of UAG and UAG analogues suppress the development of insulin intolerance in murine models of diabetes [130]. Ghrelin antagonists may be able to improve β-cell function by altering this ratio, providing a potential route towards treatment of T2DM [131]. GOAT provides a potentially useful target in the efforts to modulate the AG/UAG ratio, which could lead to a new treatment for diabetes.
3. Ghrelin O-acyltransferase inhibitors
As only acylated ghrelin binds to the growth hormone secretagogue receptor (GHS-R1a) and activates signaling, inhibition of the acylation step catalyzed by GOAT may provide a means to control ghrelin associated processes utilizing a small molecule GOAT inhibitor. While acylation is a common post-translational modification [132], the ghrelin peptide is modified on a serine side chain hydroxyl group with an unusual linear octanoic acid group [1]. The serine octanoylation present in ghrelin is the only known example of this post-translational modification, and ghrelin is the only known or predicted substrate for GOAT within the human proteome [38, 41]. The unique nature of the ghrelin-GOAT system suggests that inhibition of GOAT may provide a useful opportunity to develop a treatment for conditions impacted by ghrelin signaling such as diabetes and obesity with a reduced likelihood of side effects.
3.1. Peptide and peptidomimetic GOAT inhibitors
Given that the substrate for GOAT is a peptide, it is perhaps unsurprising that many of the initial GOAT inhibitors have been peptide based. The first study reporting GOAT inhibitors focused on interactions between GOAT and the product of GOAT-catalyzed acylation, octanoyl ghrelin (1), which was shown to inhibit GOAT with an IC50 of 7 μM [133]. Replacement of the serine ester linkage with a more hydrolytically stable amide bond provided peptide 2, which was an even more potent GOAT inhibitor with an IC50 of 0.2 μM. Further studies demonstrated a strong preference for the octanoyl sidechain, with chains of other lengths being significantly less potent [41]. Evaluation of shorter peptides showed the first five amino acids are the minimal sequence necessary for binding to GOAT, with [Dap3]octanoyl-ghrelin (1-5)-NH2 3 having an IC50 of 1 μM in a radioactivity filter-binding based assay. Subsequent studies of amide-linked octanoylated ghrelin mimics such as compounds 2 and 3 utilizing more advanced methods developed by other groups have demonstrated that this initial activity was underestimated, with IC50 values of 10 - 20 nM being a more accurate measure [41, 134, 135, 136].
In another study, the octanoylated amide in inhibitors like 3 was replaced with a triazole linkage [137, 138]. Triazoles are often utilized as ester and amide isosteres, and therefore it was anticipated that an appropriately functionalized triazole linked to a [Dap3]-ghrelin (1-5)-NH2 scaffold would be an effective GOAT inhibitor. Synthesis of a series of these compounds from the requisite azidoalanine containing peptide led to a new class of triazole based GOAT inhibitors like alkyltriazole 4 (Figure 2). These triazole based product mimetics show that the alkyl chain of the octanoate tolerates a number of hydrophobic groups, including the phenyl ring in GOAT inhibitor 4. The further modification of this hydrophobic sidechain may be a viable approach to increasing the potency of this class of GOAT inhibitors. Additionally, modification of the sidechain may provide GOAT inhibitors with increased stability in vivo and improved pharmacodynamic properties.
Figure 2.
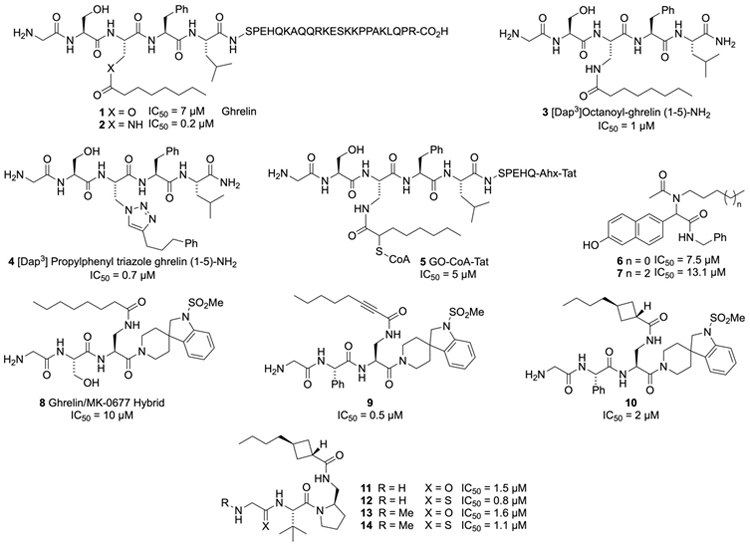
Peptide and peptidomimetic based inhibitors of GOAT
In their efforts to create a GOAT inhibitor, Cole and co-workers utilized a bisubstrate approach that has proven useful in developing inhibitors against other types of enzymes [65, 139, 140]. This resulted in the GO-CoA-Tat bisubstrate inhibitor 5 (Figure 2), which utilizes nonhydrolyzable linkages to connect the ghrelin peptide, octanoyl-CoA, and a Tat-derived peptide sequence which provides membrane permeability and cell penetration. GO-CoA-Tat inhibited GOAT with an IC50 of 5 μM in both HEK and HeLa cells that were stably transfected with the enzyme. GO-CoA-Tat was then tested in mice where it was shown to reduce acylated ghrelin levels in serum without changing the total amount of the ghrelin peptide in the blood. Further evaluation in mice on a medium-chain triglyceride (MCT)-rich high fat diet showed that GOAT inhibitor 5 prevented weight gain and improved glucose tolerance [65]. Providing further evidence these results reflect effects on ghrelin signaling, no similar beneficial metabolic effects were observed in mice where ghrelin levels had been lowered by genetic knockdown. Treatment of Siberian hamsters with GO-CoA-Tat (inhibitor 5) every 6 hours (the duration of effectiveness for the inhibitor) during food deprivation led to reduced food intake and an attenuation of food hoarding behavior during refeeding, which supports a role for ghrelin in food consumption and addictive behavior [141]. These studies provide the strongest evidence validating the pursuit of potent and bioavailable GOAT inhibitors for use as therapeutics. The success of these studies has spurred other groups to explore peptides as therapeutics targeting GOAT, but as these agents must be administered by injection there has been scant interest in peptides in the pharmaceutical industry [142].
Some smaller peptide based GOAT inhibitors were disclosed by Garner and Janda in 2011 [143]. They utilized a catalytic enzyme-linked click-chemistry assay (cat-ELCCA) to screen a library of small molecules for inhibition of GOAT [144], which resulted in the identification of the non-natural peptides 6 and 7 (Figure 2) as GOAT inhibitors with IC50 values of 7.5 and 13.1 μM, respectively. The alkyl chains which decorate the amides of these structures may be acting as surrogates for the octanoyl sidechain of ghrelin. These molecules provide easily accessible structures that can be used as tools to probe ghrelin acylation by GOAT, but no information is available on the behavior of these compounds in cell-based systems or in animals.
An alternative peptidomimetic approach to develop GOAT inhibitors was undertaken by Harran and co-workers [145, 146, 147, 148]. This work was performed in collaboration with the Brown and Goldstein groups at the University of Texas Southwest Medical Center. Harran hypothesized that a GHS-R1a receptor agonist could be modified into a potent GOAT inhibitor. The hybrid structure 8 bearing the ghrelin headgroup at the N-terminus combined with a spiroindoline sulfonamide derived from the ghrelin receptor agonist MK-0677 was initially investigated. Synthesis and testing of this molecule showed that spiroindoline 8 was indeed a GOAT inhibitor with an IC50 of 10 μM, and this compound was then used as a lead for further optimization. Initially the residues next to the terminal glycine were modified, which resulted in the discovery that a phenylglycine at this position had slightly better potency than the serine. Additionally, the octanamide side chain was modified which resulted in the discovery that rigidification of the alkyl chain by incorporating an alkyne provided the more potent GOAT inhibitor 9, with an IC50 of 0.5 μM. Concerns about the incorporation of the alkynoate, which is an excellent Michael acceptor and could react with glutathione and other thiols in the extracellular matrix, led to the evaluation of other rigid structures that were not capable of acting as alkylating agents. Cyclobutane 10 was found to be one of the better inhibitors from this study, with an IC50 of 2 μM. Further optimization of the spiroindoline sulfonamide showed that much of this structure was superfluous, and the molecular weight could be reduced and membrane permeability of the inhibitor could be enhanced by replacing this structure with a tert-leucine-proline diamine end group as in peptide 11. Studies with 11 showed the terminal glycine was being cleaved by proteases when the compound was dosed in mice. To slow this cleavage, methylation of the terminal amine was explored, which provided compound 13 which was just as active and more protease resistant. In addition, the glycine amide was also converted to the thioamide, leading to 12 and 14. These systems were more robust in vivo and still quite active, with thioamide 12 being the most potent GOAT inhibitor in this series with an IC50 of 0.8 μM. These optimized peptidomimetics were shown to readily cross cell membranes and lowered levels of octanoylated ghrelin in mice for up to 3 hours.
3.2. Terpenoid and steroid-based GOAT inhibitors
In addition to peptide-based structures, some terpenes have also been observed to inhibit GOAT (Figure 3). These were discovered by screening the Diversity IV library from the NCI utilizing a fluorescent peptide substrate as a reporter [135, 149, 150]. This screen resulted in the identification of a synthetic oleanate triterpenoid, 1-[2-cyano-3,12-dioxooleana-1,9(11)- dien-28-oyl]imidazole (CDDO-Im, 15) as the first reported small molecule inhibitor of human GOAT (hGOAT) activity, with an IC50 of 38 μM. This compound was part of a class of antiangiogenic and antitumor agents which primarily exert their effects through disrupting signaling in the Nrf2 and NF-κB pathways [151, 152, 153]. Further screening of CDDO derivatives found that the ethyl amide 16 and methyl ester 18 were even more potent hGOAT inhibitors. Interestingly, the free carboxylic acid 19 and the 3,3,3-trifluoroethyl ester 17 were significantly less active. Examination of 18 suggested the -cyanoenone may play key role in the pharmacophore as this functional group is an exceptional Michael acceptor, and functions as such to modify a cysteine residue in KEAP to inhibit Nrf2 signaling [154, 155]. In synthetic studies to define the features of the CDDO-family compounds essential for hGOAT inhibition, the smaller steroid-based -cyanoenone 20 supported the involvement of the Michael acceptor in blocking hGOAT activity as 20 is also a potent hGOAT inhibitor. Inhibition became less potent as the cyano group was exchanged for a less electron-withdrawing functional group (like the bromide 21 and the unfunctionalized enone 22), consistent with potency scaling with the electrophilic character of the inhibitor. The terpenoid/steroid scaffold also plays a role in hGOAT inhibition, as smaller cyclohexanone-based structures were significantly less potent. The cyanoenone compounds 18 and 20 were demonstrated to be reversible covalent inhibitors of hGOAT using pulse dilution experiments, providing a mode of action not observed with other GOAT inhibitors. These results were originally interpreted to implicate a functionally essential cysteine within hGOAT as their likely target, with the mouse orthologue mGOAT exhibiting resistance to cyanoenone 20 suggesting the inhibition mechanism may not involve catalytic residues [149]. Recent results demonstrating that cyanoenones also undergo Michael additions with histidine sidechains complicate this analysis [156], and studies are now in progress to determine which amino acids are modified by the cyanoenone GOAT inhibitors. Recently there have been reports of similar terpenoids without a Michael acceptor (like 23) lowering ghrelin levels in modified AGS‐GHRL8 cells which express both GOAT and produce octanoylated ghrelin in the presence of octanoic acid [157]. Given that a number of similar terpenoid structures were shown to have no effect on GOAT (unless they contain an electrophilic enone) [149], it is likely that these ghrelin lowering effects of 23 are due to interactions with other proteins or signaling pathways that influence ghrelin production or secretion rather than direct inhibition of GOAT.
Figure 3.
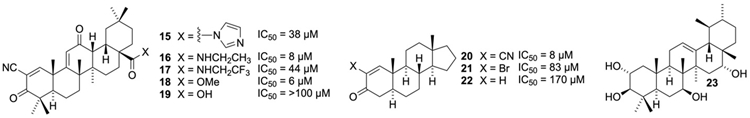
Terpenoid GOAT inhibitors and related structures
3.3. Small molecule GOAT inhibitors from the pharmaceutical industry – screening and optimization
Pharmaceutical companies have also been active in screening for small molecule inhibitors of GOAT. For example, researchers at Eli Lilly reported a series of aminopyrimidines as GOAT inhibitors (Figure 4). Initially, the furanopyrimidine 24 was found through a screening campaign utilizing an ELISA assay to measure GOAT acylation activity. This initial hit was not competitive with ghrelin, but instead was competitive with the octanoyl-CoA acyl donor. This suggests that compound 24 targets the acyl donor binding site within GOAT rather than the ghrelin binding site that presumably binds the ghrelin derived peptidomimetics discussed above. Deletion of a methyl group led to a significant loss in activity as shown for pyrimidine 25, while replacement of the methyl with larger ethyl or isopropyl groups (26 and 27) yielded inactive derivatives. The initial lead 24 showed poor plasma stability, as the furan was not inert and generated many reactive metabolites under physiological conditions [158, 159]. Deletion of the furan and replacement of the oxygen with a chlorine at the R2 position of 28 or with a trifluoromethyl at R1 and a methyl group at R2 relieved these issues. Further optimization of the benzoic acid containing substituent led to structures like 28, with a piperidine linker connecting the 4-aminopyrimidine and the diamide [158, 159, 160, 161, 162]. These studies eventually culminated in the discovery of LY3073084 29 (also known as GLWL-01), which demonstrated excellent PK/PD properties, good renal clearance and an IC50 of 69 nM against hGOAT [163]. This compound is currently being evaluated clinically for a number of disorders [164, 165, 166].
Figure 4.
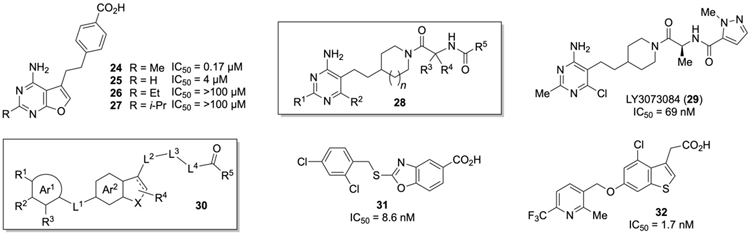
Heterocyclic GOAT inhibitors from Eli Lilly and Takeda
Several GOAT inhibitors with significantly different structures have also been disclosed from workers at Takeda Pharmaceutical. These GOAT inhibitors are based on an aromatic or heteroaromatic group (Ar1, 30, Figure 4) linked through a benzothiophene and benzofuran to a carboxylic acid [134, 167]. Work towards these compounds began with a screen of ~500,000 small molecules using a homogeneous time-resolved fluorescence (HTRF) assay. This initial screen provided 301 compounds which were then evaluated by ELISA to remove false positives, followed by dose response assays and substrate competition assays to determine the best inhibitors. This approach identified the 2-thiobenzoxazole 31 as a lead compound, which was then optimized to the benzothiophene 32 [134]. Like the inhibitors reported by the Lilly group, both 31 and 32 were octanoyl-CoA competitive and noncompetitive when evaluated against des-acyl ghrelin. While the oxazole 31 showed poor oral availability in mice, benzothiophene 32 exhibited good oral bioavailability and was able to lower acylated ghrelin levels in mice (dose of 3 mg/kg) while the total amount of ghrelin remained unchanged. The authors noted that more in vivo studies were ongoing with these molecules.
With the published successes in peptidomimetic and small molecule inhibition of GOAT, several other groups at pharmaceutical companies have now disclosed their results in the screening and optimization of GOAT inhibitors (Figure 5). Inhibitors similar to those disclosed from the Takeda group were recently revealed from a group at GlaxoSmithKline [168, 169], with the same benzothiophene acetic acid core as shown in 33. These inhibitors are distinguished by the incorporation of a functionalized bicyclic pyridocyclopentyl ether (as shown for 34 and 35). Interestingly, the stereochemistry of the ether was not particularly influential in the bioactivity of these systems. This series appears to be more potent than the Takeda compounds, with the most potent compounds providing IC50 values of <50 nM against GOAT. Yet another series of benzothiophene based GOAT inhibitors was recently disclosed, this time with the general structure of 36 (Figure 5) [170]. These inhibitors keep the heterocyclic linkage to the 6-position of the benzothiophene, but substitute an ester linkage for the ether, and also replace the acetic acid at the 3-position of the benzothiophene with an ether linkage at the 2-position to a functionalized pyridine. The most active compounds have fluorine and methoxy substituents on this pyridine ring, as shown for 37 and 38.
Figure 5.
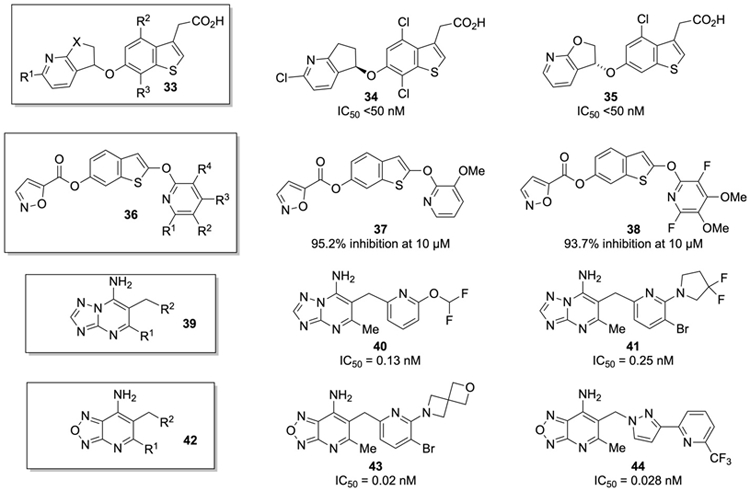
Heterocyclic Small Molecule GOAT Inhibitors from Other Patents
The most potent GOAT inhibitors reported were recently disclosed by workers at Boehringer Ingelheim. They first disclosed a series of 7-amino-[1,2,4]triazolo[1,5,-a]pyrimidines with the base structure of 39 as GOAT inhibitors [171, 172, 173]. The most active members of this series were substituted with a methyl group at the 5-position of the pyrimidine with a benzylic pyridyl group at the 6 position as shown for 40 and 41 (Figure 5). These inhibitors usually had the pyridyl group decorated with a fluorinated ether or amine at the 2-position of the pyridine. Inhibitors of this type exhibited picomolar inhibitory activity against hGOAT as measured with an ELISA assay. Later it was reported that 7-amino-[1,2,5]oxadiazolo[3,4,-b]pyridines based on the structure of 42 were even more potent GOAT inhibitors [174, 175]. The most active compounds in this report were structures like 43 and 44 with IC50 values of 0.02 nM and 0.028 nM, respectively. In the future it will be interesting to determine if these significantly more potent GOAT inhibitors can exert their activity in animal models of obesity and diabetes.
4. Expert opinion
In the two decades since the discovery of ghrelin, ghrelin-dependent signaling pathways have been increasingly considered enticing targets for the development of new pharmaceuticals for treatment of disorders ranging from diabetes to obesity to addiction. Utilizing GOAT inhibitors to specifically target this pathway has now become a reality, with multiple classes of potent GOAT inhibitors developed both in academia and industry. The next step in the deployment of these agents for therapeutic use will require judicious choices for the conditions targeted in clinical studies, with diabetes and addiction/alcoholism amongst the most promising areas based on currently available data. As these inhibitors proceed towards the clinic, they can be employed in a variety of contexts to investigate the role of ghrelin in signaling pathways and disease states. Beyond informing our growing appreciation for the impact of ghrelin signaling across multiple physiological contexts, each new connection found through these studies can offer a potential avenue for therapeutic exploration. With the first GOAT inhibitors now entering human trials,[164, 165, 166] we anticipate a growing number of these agents supporting further clinical studies in the coming years.
The recent creation of a structural model of GOAT now offers new opportunities for understanding how reported GOAT inhibitors function and should accelerate understanding of how the most potent GOAT inhibitors interact with the enzyme.[58] The growing body of biochemical and structural knowledge regarding the ghrelin / GOAT system can now enable virtual inhibitor screening, rational design of substrate- and product mimetic inhibitors, efficient optimization of known GOAT inhibitors, and offers options for the creation of mechanism-based GOAT inhibitors. Further development in GOAT activity assays will be needed to enable economical high-throughput screening to support these continuing inhibitor development efforts, providing new options compared to the ELISA-based screens reported for industrial programs. Working in concert, these approaches will allow researchers to rapidly apply these molecular tools to further elucidate ghrelin-dependent signaling pathways and explore interconnections between ghrelin and other metabolism-regulating hormones such as insulin. These studies will advance our knowledge of how GOAT and ghrelin perform their essential roles in controlling metabolic regulation and neuroendocrinology. Looking past ghrelin and GOAT, the successful elucidation of the catalytic strategies employed by GOAT and inhibitor development targeting this acyltransferase will inform parallel studies of other MBOAT family members that are considered validated cancer drug targets [176, 177, 178].
We currently are experiencing an exciting inflection point in the investigation of the ghrelin/GOAT system. The challenging integral membrane nature of GOAT has impeded investigation of this intriguing enzyme, but after many years of steady progress we now have the foundational structural and functional insights needed to accelerate studies towards molecular agents to modulate GOAT activity. Furthermore, the unexpected finding that ghrelin is a unique substrate for GOAT within the human proteome (an “one enzyme – one substrate” system) suggests the potential to develop GOAT inhibitors with minimal potential for undesired off-target pharmacodynamic effects.[41] In the next decade, we anticipate a subset of the growing family of GOAT inhibitors will be explored as treatments for a range of diseases including diabetes and obesity. In most cases, this will involve GOAT inhibition in coordination with other established treatments to increase the efficacy of existing therapies through exploitation of ghrelin’s linkage to other signaling pathways within the body. Once considered an “undruggable” target, we now can confidently pronounce the beginning of a new era for leveraging GOAT inhibition towards understanding the effects of ghrelin signaling in multiple disorders and disease states.
Article highlights.
Ghrelin is a peptide hormone implicated in food intake, metabolic regulation, stress response, and addictive behaviors.
To be biologically active, ghrelin must be modified with an eight-carbon acyl group by ghrelin O-acyltransferase (GOAT).
Development of potent peptidomimetic and small molecule GOAT inhibitors offers the opportunity to modulate ghrelin signaling by blocking GOAT acylation activity.
GOAT inhibitors have recently entered preclinical and clinical studies.
Ghrelin and GOAT provide potential unexploited therapeutic avenues for treating diabetes, obesity, and addiction.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (GM134102, JL Hougland and JD Chisholm), the American Diabetes Association (1-16-JDF-042, JL Hougland), and Syracuse University.
Footnotes
Declaration of interest
JL Hougland and JD Chisholm have patent interests in GOAT inhibitors discussed in this review. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1. *.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660.Identification of ghrelin, including discovery of serine octanoylation modification and its requirement for ghrelin biological activity.
- 2.Korbonits M, Bustin SA, Kojima M, et al. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86(2):881–887. [DOI] [PubMed] [Google Scholar]
- 3.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. [DOI] [PubMed] [Google Scholar]
- 4.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mrna of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87(6):2988–2991. [DOI] [PubMed] [Google Scholar]
- 5.Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85(12):4908–4911. [DOI] [PubMed] [Google Scholar]
- 6.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. [DOI] [PubMed] [Google Scholar]
- 7.Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(11):2540–2547. [DOI] [PubMed] [Google Scholar]
- 8.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. [DOI] [PubMed] [Google Scholar]
- 9.Egido EM, Rodriguez-Gallardo J, Silvestre RA, et al. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol. 2002;146(2):241–244. [DOI] [PubMed] [Google Scholar]
- 10.Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–921. [DOI] [PubMed] [Google Scholar]
- 11.McFarlane MR, Brown MS, Goldstein JL, et al. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014;20(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abizaid A, Hougland JL. Ghrelin signaling: GOAT and GHS-R1a take a leap in complexity. Trends Endocrinol Metab. 2020;31(2):107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. [DOI] [PubMed] [Google Scholar]
- 14.Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide y and agouti-related protein. Endocrinology. 2004;145(6):2607–2612. [DOI] [PubMed] [Google Scholar]
- 15.Kohno D, Sone H, Tanaka S, et al. AMP-activated protein kinase activates neuropeptide Y neurons in the hypothalamic arcuate nucleus to increase food intake in rats. Neurosci Lett. 2011;499(3):194–198. [DOI] [PubMed] [Google Scholar]
- 16.Kohno D, Sone H, Minokoshi Y, et al. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun. 2008;366(2):388–392. [DOI] [PubMed] [Google Scholar]
- 17.Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279(13):12005–12008. [DOI] [PubMed] [Google Scholar]
- 18.Anderson KA, Ribar TJ, Lin F, et al. Hypothalamic camkk2 contributes to the regulation of energy balance. Cell Metab. 2008;7(5):377–388. [DOI] [PubMed] [Google Scholar]
- 19.Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107(16):7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie TY, Ngo ST, Veldhuis JD, et al. Effect of deletion of ghrelin-O-acyltransferase on the pulsatile release of growth hormone in mice. J Neuroendocrinol. 2015;27(12):872–886. [DOI] [PubMed] [Google Scholar]
- 21.Tschop M, Statnick MA, Suter TM, et al. GH-releasing peptide-2 increases fat mass in mice lacking NPY: Indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology. 2002;143(2):558–568. [DOI] [PubMed] [Google Scholar]
- 22.Broglio F, Gottero C, Prodam F, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89(6):3062–3065. [DOI] [PubMed] [Google Scholar]
- 23.Diano S, Farr SA, Benoit SC, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9(3):381–388. [DOI] [PubMed] [Google Scholar]
- 24.Hsu TM, Suarez AN, Kanoski SE. Ghrelin: A link between memory and ingestive behavior. Physiol Behav. 2016;162:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrenho D, Santos SD, Carvalho AL. The role of ghrelin in regulating synaptic function and plasticity of feeding-associated circuits. Front Cell Neurosci. 2019;13:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panagopoulos VN, Ralevski E. The role of ghrelin in addiction: A review. Psychopharmacology (Berl). 2014;231(14):2725–2740. [DOI] [PubMed] [Google Scholar]
- 27.Lutter M, Sakata I, Osborne-Lawrence S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer RM, Burgos-Robles A, Liu E, et al. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry. 2014;19(12):1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmatz ES, Stone L, Lim SH, et al. Central ghrelin resistance permits the overconsolidation of fear memory. Biol Psychiatry. 2017;81(12):1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousufzai M, Harmatz ES, Shah M, et al. Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans. Transl Psychiatry. 2018;8(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron KO, Bhattacharya SK, Loomis AK. Small molecule ghrelin receptor inverse agonists and antagonists. J Med Chem. 2014;57(21):8671–8691. [DOI] [PubMed] [Google Scholar]
- 32.McGovern KR, Darling JE, Hougland JL. Progress in small molecule and biologic therapeutics targeting ghrelin signaling. Mini-Rev Medl Chem. 2016;16(6):465–480. [DOI] [PubMed] [Google Scholar]
- 33.Collden G, Tschop MH, Muller TD. Therapeutic potential of targeting the ghrelin pathway. Int J Mol Sci. 2017;18(4): 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howick K, Griffin BT, Cryan JF, et al. From belly to brain: Targeting the ghrelin receptor in appetite and food intake regulation. Int J Mol Sci. 2017;18(2): 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avau B, Carbone F, Tack J, et al. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil. 2013;25(9):720–732. [DOI] [PubMed] [Google Scholar]
- 36.Chollet C, Meyer K, Beck-Sickinger AG. Ghrelin--a novel generation of anti-obesity drug: Design, pharmacomodulation and biological activity of ghrelin analogues. J Pept Sci. 2009;15(11):711–730. [DOI] [PubMed] [Google Scholar]
- 37.Costantino L, Barlocco D. New perspectives on the development of antiobesity drugs. Future Med Chem. 2015;7(3):315–336. [DOI] [PubMed] [Google Scholar]
- 38. *.Yang J, Brown MS, Liang G, et al. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396.Identification of GOAT as ghrelin modifying enzyme.
- 39. *.Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105(17):6320–6325.Identification of GOAT as ghrelin modifying enzyme.
- 40.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85(2):495–522. [DOI] [PubMed] [Google Scholar]
- 41.Darling JE, Zhao F, Loftus RJ, et al. Structure-activity analysis of human ghrelin O-acyltransferase reveals chemical determinants of ghrelin selectivity and acyl group recognition. Biochemistry. 2015;54(4):1100–1110. [DOI] [PubMed] [Google Scholar]
- 42.Gahete MD, Cordoba-Chacon J, Salvatori R, et al. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol. 2010;317(1–2):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Cao Y, Voogd K, et al. On the processing of proghrelin to ghrelin. J Biol Chem. 2006;281(50):38867–38870. [DOI] [PubMed] [Google Scholar]
- 44.Delhanty PJ, Huisman M, Julien M, et al. The acylated (AG) to unacylated (UAG) ghrelin ratio in esterase inhibitor-treated blood is higher than previously described. Clin Endocrinol (Oxf). 2015;82(1):142–146. [DOI] [PubMed] [Google Scholar]
- 45.De Vriese C, Gregoire F, Lema-Kisoka R, et al. Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology. 2004;145(11):4997–5005. [DOI] [PubMed] [Google Scholar]
- 46.Satou M, Sugimoto H. The study of ghrelin deacylation enzymes. Methods Enzymol. 2012;514:165–179. [DOI] [PubMed] [Google Scholar]
- 47.Brimijoin S, Chen VP, Pang YP, et al. Physiological roles for butyrylcholinesterase: A BCHE-ghrelin axis. Chem Biol Interact. 2016;259(Pt B):271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen VP, Gao Y, Geng L, et al. Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int J Obes (Lond). 2017;41(9):1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gauna C, Meyler FM, Janssen JA, et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab. 2004;89(10):5035–5042. [DOI] [PubMed] [Google Scholar]
- 50.Gauna C, Kiewiet RM, Janssen JAMJL, et al. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol-Endoc M. 2007;293(3):E697–E704. [DOI] [PubMed] [Google Scholar]
- 51.Taylor MS, Ruch TR, Hsiao PY, et al. Architectural organization of the metabolic regulatory enzyme ghrelin O-acyltransferase. J Biol Chem. 2013;288(45):32211–32228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matevossian A, Resh MD. Membrane topology of hedgehog acyltransferase. J Biol Chem. 2015;290(4):2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konitsiotis AD, Jovanovic B, Ciepla P, et al. Topological analysis of hedgehog acyltransferase, a multipalmitoylated transmembrane protein. J Biol Chem. 2015;290(6):3293–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joyce CW, Shelness GS, Davis MA, et al. ACAT1 and ACAT2 membrane topology segregates a serine residue essential for activity to opposite sides of the endoplasmic reticulum membrane. Mol Biol Cell. 2000;11(11):3675–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin S, Lu X, Chang CC, et al. Human acyl-coenzyme A:cholesterol acyltransferase expressed in chinese hamster ovary cells: Membrane topology and active site location. Mol Biol Cell. 2003;14(6):2447–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pagac M, de la Mora HV, Duperrex C, et al. Topology of 1-acyl-sn-glycerol-3-phosphate acyltransferases SLC1 and ALE1 and related membrane-bound O-acyltransferases (MBOATs) of saccharomyces cerevisiae. J Biol Chem. 2011;286(42):36438–36447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. **.Ma D, Wang Z, Merrikh CN, et al. Crystal structure of a membrane-bound O-acyltransferase. Nature. 2018;562(7726):286–290.First crystal structure of a bacterial MBOAT family acyltransferase.
- 58. **.Campana MB, Irudayanathan FJ, Davis TR, et al. The ghrelin O-acyltransferase structure reveals a catalytic channel for transmembrane hormone acylation. J Biol Chem. 2019;294(39):14166–14174.Creation and validation of a structural model for human GOAT; first structure of an eukaryotic MBOAT family member.
- 59.Guan C, Niu Y, Chen S-C, et al. Inhibition mechanism of human sterol O-acyltransferase 1 by competitive inhibitor. bioRxiv. 2020; 10.1101/2020.01.07.897124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez-Tilve D, Heppner K, Kirchner H, et al. Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J. 2011;25(8):2814–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hopkins AL, Nelson TA, Guschina IA, et al. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R1a activity: Evidence for target cell-induced acylation. Sci Rep. 2017;7:45541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87(1):240–244. [DOI] [PubMed] [Google Scholar]
- 63.Tschop M, Weyer C, Tataranni PA, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. [DOI] [PubMed] [Google Scholar]
- 64.English PJ, Ghatei MA, Malik IA, et al. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87(6):2984–2987. [DOI] [PubMed] [Google Scholar]
- 65. **.Barnett BP, Hwang Y, Taylor MS, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330(6011):1689–1692.Development and validation of GO-CoA-Tat, a bisubstrate mimetic GOAT inhibitor; first evidence for GOAT inhibition leading to improved glucose tolerance and reduced weight gain in rodents.
- 66.Wellman MK, Patterson ZR, MacKay H, et al. Novel regulator of acylated ghrelin, cf801, reduces weight gain, rebound feeding after a fast, and adiposity in mice. Front Endocrinol. 2015;6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soriano-Guillen L, Barrios V, Campos-Barros A, et al. Ghrelin levels in obesity and anorexia nervosa: Effect of weight reduction or recuperation. J Pediatr. 2004;144(1):36–42. [DOI] [PubMed] [Google Scholar]
- 68.Misra M, Miller KK, Herzog DB, et al. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2004;89(4):1605–1612. [DOI] [PubMed] [Google Scholar]
- 69.Misra M, Klibanski A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014;2(7):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fabbri AD, Deram S, Kerr DS, et al. Ghrelin and eating disorders Arch Clin Psychiatry. 2015;42(2):52–62. [Google Scholar]
- 71.Schorr M, Miller KK. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat Rev Endocrinol. 2017;13(3):174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smitka K, Papezova H, Vondra K, et al. The role of 'mixed' orexigenic and anorexigenic signals and autoantibodies reacting with appetite-regulating neuropeptides and peptides of the adipose tissue-gut-brain axis: Relevance to food intake and nutritional status in patients with anorexia nervosa and bulimia nervosa. Int J Endocrinol. 2013;2013:483145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berner LA, Brown TA, Lavender JM, et al. Neuroendocrinology of reward in anorexia nervosa and bulimia nervosa: Beyond leptin and ghrelin. Mol Cell Endocrinol. 2019;497:110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monteleone AM, Castellini G, Volpe U, et al. Neuroendocrinology and brain imaging of reward in eating disorders: A possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt B):132–142. [DOI] [PubMed] [Google Scholar]
- 75.Hotta M, Ohwada R, Akamizu T, et al. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: A pilot study. Endocr J. 2009;56(9):1119–1128. [DOI] [PubMed] [Google Scholar]
- 76.Neary NM, Small CJ, Wren AM, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: Acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(6):2832–2836. [DOI] [PubMed] [Google Scholar]
- 77.Haruta I, Fuku Y, Kinoshita K, et al. One-year intranasal application of growth hormone releasing peptide-2 improves body weight and hypoglycemia in a severely emaciated anorexia nervosa patient. J Cachexia Sarcopenia Muscle. 2015;6(3):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strasser F, Lutz TA, Maeder MT, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: A randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98(2):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hassouna R, Labarthe A, Zizzari P, et al. Actions of agonists and antagonists of the ghrelin/GHS-R pathway on GH secretion, appetite, and cFos activity. Front Endocrinol (Lausanne). 2013;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holm VA, Cassidy SB, Butler MG, et al. Prader-willi syndrome: Consensus diagnostic criteria. Pediatrics. 1993;91(2):398–402. [PMC free article] [PubMed] [Google Scholar]
- 81.Lindgren AC, Barkeling B, Hagg A, et al. Eating behavior in Prader-Willi syndrome, normal weight, and obese control groups. J Pediatr. 2000;137(1):50–55. [DOI] [PubMed] [Google Scholar]
- 82.DelParigi A, Tschop M, Heiman ML, et al. High circulating ghrelin: A potential cause for hyperphagia and obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2002;87(12):5461–5464. [DOI] [PubMed] [Google Scholar]
- 83.Haqq AM, Farooqi IS, O'Rahilly S, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88(1):174–178. [DOI] [PubMed] [Google Scholar]
- 84.Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader-Willi syndrome. Nat Med. 2002;8(7):643–644. [DOI] [PubMed] [Google Scholar]
- 85.Feigerlova E, Diene G, Conte-Auriol F, et al. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(7):2800–2805. [DOI] [PubMed] [Google Scholar]
- 86.Kweh FA, Miller JL, Sulsona CR, et al. Hyperghrelinemia in Prader-Willi syndrome begins in early infancy long before the onset of hyperphagia. Am J Med Genet A. 2015;167A(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuppens RJ, Diene G, Bakker NE, et al. Elevated ratio of acylated to unacylated ghrelin in children and young adults with Prader-Willi syndrome. Endocrine. 2015;50(3):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beauloye V, Diene G, Kuppens R, et al. High unacylated ghrelin levels support the concept of anorexia in infants with Prader-Willi syndrome. Orphanet J Rare Dis. 2016;11(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haqq AM, Stadler DD, Rosenfeld RG, et al. Circulating ghrelin levels are suppressed by meals and octreotide therapy in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88(8):3573–3576. [DOI] [PubMed] [Google Scholar]
- 90.De Waele K, Ishkanian SL, Bogarin R, et al. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader-Willi syndrome. Eur J Endocrinol. 2008;159(4):381–388. [DOI] [PubMed] [Google Scholar]
- 91.Lin D, Wang Q, Ran H, et al. Abnormal response to the anorexic effect of GHS-R inhibitors and exenatide in male snord116 deletion mouse model for Prader-Willi syndrome. Endocrinology. 2014;155(7):2355–2362. [DOI] [PubMed] [Google Scholar]
- 92.Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36(2):229–240. [DOI] [PubMed] [Google Scholar]
- 93.Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: A microdialysis study. Pharmacol Biochem Be. 1996;53(1):221–226. [DOI] [PubMed] [Google Scholar]
- 94.Volkow ND, Wang GJ, Maynard L, et al. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord. 2003;33(2):136–142. [DOI] [PubMed] [Google Scholar]
- 95.Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35(1–2):227–263. [DOI] [PubMed] [Google Scholar]
- 96.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–1449. [DOI] [PubMed] [Google Scholar]
- 97.Jerlhag E, Egecioglu E, Dickson SL, et al. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12(1):6–16. [DOI] [PubMed] [Google Scholar]
- 98.Kraus T, Schanze A, Groschl M, et al. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res. 2005;29(12):2154–2157. [DOI] [PubMed] [Google Scholar]
- 99.Kim DJ, Yoon SJ, Choi B, et al. Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol. 2005;40(1):76–79. [DOI] [PubMed] [Google Scholar]
- 100.Leggio L, Ferrulli A, Cardone S, et al. Ghrelin system in alcohol-dependent subjects: Role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17(2):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akkisi Kumsar N, Dilbaz N. Relationship between craving and ghrelin, adiponectin, and resistin levels in patients with alcoholism. Alcohol Clin Exp Res. 2015;39(4):702–709. [DOI] [PubMed] [Google Scholar]
- 102.Hillemacher T, Kraus T, Rauh J, et al. Role of appetite-regulating peptides in alcohol craving: An analysis in respect to subtypes and different consumption patterns in alcoholism. Alcohol Clin Exp Res. 2007;31(6):950–954. [DOI] [PubMed] [Google Scholar]
- 103.Addolorato G, Capristo E, Leggio L, et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res. 2006;30(11):1933–1937. [DOI] [PubMed] [Google Scholar]
- 104.Koopmann A, Bach P, Schuster R, et al. Ghrelin modulates mesolimbic reactivity to alcohol cues in alcohol-addicted subjects: A functional imaging study. Addict Biol. 2019;24(5):1066–1076. [DOI] [PubMed] [Google Scholar]
- 105.Bach P, Bumb JM, Schuster R, et al. Effects of leptin and ghrelin on neural cue-reactivity in alcohol addiction: Two streams merge to one river? Psychoneuroendocrinology. 2019;100:1–9. [DOI] [PubMed] [Google Scholar]
- 106.Davis KW, Wellman PJ, Clifford PS. Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept. 2007;140(3):148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wellman PJ, Hollas CN, Elliott AE. Systemic ghrelin sensitizes cocaine-induced hyperlocomotion in rats. Regul Pept. 2008;146(1–3):33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jang JK, Kim WY, Cho BR, et al. Microinjection of ghrelin in the nucleus accumbens core enhances locomotor activity induced by cocaine. Behav Brain Res. 2013;248:7–11. [DOI] [PubMed] [Google Scholar]
- 109.Tessari M, Catalano A, Pellitteri M, et al. Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats. Addict Biol. 2007;12(1):22–29. [DOI] [PubMed] [Google Scholar]
- 110.Jerlhag E, Egecioglu E, Dickson SL, et al. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl). 2010;211(4):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Havlickova T, Charalambous C, Lapka M, et al. Ghrelin receptor antagonism of methamphetamine-induced conditioned place preference and intravenous self-administration in rats. Int J Mol Sci. 2018;19(10):2925–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jerlhag E, Engel JA. Ghrelin receptor antagonism attenuates nicotine-induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend. 2011;117(2–3):126–131. [DOI] [PubMed] [Google Scholar]
- 113.Wellman PJ, Clifford PS, Rodriguez J, et al. Pharmacologic antagonism of ghrelin receptors attenuates development of nicotine induced locomotor sensitization in rats. Regul Pept. 2011;172(1–3):77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sustkova-Fiserova M, Jerabek P, Havlickova T, et al. E.15 - ghrelin receptor antagonism attenuates morphine-induced accumbal dopamine release and behavioral stimulation in rats. 15th Biennial Meeting of the European Behavioural Pharmacology Society; 2013. September 6–9, 2013; La Rochelle. [Google Scholar]
- 115.Sustkova-Fiserova M, Jerabek P, Havlickova T, et al. Ghrelin and endocannabinoids participation in morphine-induced effects in the rat nucleus accumbens. Psychopharmacology (Berl). 2016;233(3):469–84. [DOI] [PubMed] [Google Scholar]
- 116.D'Cunha TM, Chisholm A, Hryhorczuk C, et al. A role for leptin and ghrelin in the augmentation of heroin seeking induced by chronic food restriction. Psychopharmacology (Berl). 2020;237(3):787–800. [DOI] [PubMed] [Google Scholar]
- 117.Sustkova-Fiserova M, Puskina N, Havlickova T, et al. Ghrelin receptor antagonism of fentanyl-induced conditioned place preference, intravenous self-administration, and dopamine release in the nucleus accumbens in rats. Addict Biol. 2019; 10.1111/adb.12845:e12845. [DOI] [PubMed] [Google Scholar]
- 118.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferrannini E, Mari A. Beta-cell function in type 2 diabetes. Metabolism. 2014;63(10):1217–1227. [DOI] [PubMed] [Google Scholar]
- 120.DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 121.Heppner KM, Tong J. Mechanisms in endocrinology: Regulation of glucose metabolism by the ghrelin system: Multiple players and multiple actions. Eur J Endocrinol. 2014;171(1):R21–32. [DOI] [PubMed] [Google Scholar]
- 122.Delhanty PJ, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides. 2011;32(11):2309–18. [DOI] [PubMed] [Google Scholar]
- 123.van der Lely AJ. Ghrelin and new metabolic frontiers. Horm Res. 2009;71 Suppl 1:129–133. [DOI] [PubMed] [Google Scholar]
- 124.van der Lely AJ, Tschop M, Heiman ML, et al. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–457. [DOI] [PubMed] [Google Scholar]
- 125.Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083–5086. [DOI] [PubMed] [Google Scholar]
- 126.Yada T, Damdindorj B, Rita RS, et al. Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Diabetes Obes Metab. 2014;16 Suppl 1:111–117. [DOI] [PubMed] [Google Scholar]
- 127.Dezaki K, Hosoda H, Kakei M, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: Implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. [DOI] [PubMed] [Google Scholar]
- 128.Barazzoni R, Zanetti M, Ferreira C, et al. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(10):3935–3940. [DOI] [PubMed] [Google Scholar]
- 129.Granata R, Settanni F, Julien M, et al. Des-acyl ghrelin fragments and analogues promote survival of pancreatic beta-cells and human pancreatic islets and prevent diabetes in streptozotocin-treated rats. J Med Chem. 2012;55(6):2585–2596. [DOI] [PubMed] [Google Scholar]
- 130.Delhanty PJ, Huisman M, Baldeon-Rojas LY, et al. Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J. 2013;27(4):1690–1700. [DOI] [PubMed] [Google Scholar]
- 131.Tong J, Prigeon RL, Davis HW, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59(9):2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang H, Zhang X, Chen X, et al. Protein lipidation: Occurrence, mechanisms, biological functions, and enabling technologies. Chem Rev. 2018;118(3):919–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang J, Zhao T-J, Goldstein JL, et al. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci U S A. 2008;105(31):10750–10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. **.Yoneyama-Hirozane M, Deguchi K, Hirakawa T, et al. Identification and characterization of a new series of ghrelin O-acyl transferase inhibitors. SLAS Discovery. 2018;23(2):154–163.Small molecule GOAT inhibitors and assay development by Takeda Pharmaceuticals.
- 135.Darling JE, Prybolsky EP, Sieburg M, et al. A fluorescent peptide substrate facilitates investigation of ghrelin recognition and acylation by ghrelin O-acyltransferase. Anal Biochem. 2013;437(1):68–76. [DOI] [PubMed] [Google Scholar]
- 136.Hougland J, Darling J, inventors; Syracuse University, USA, assignee. Fluorescence assay for ghrelin O-acyltransferase activity United States patent US 20140212904 B2. 2015. August 25. [Google Scholar]
- 137.Zhao F, Darling JE, Gibbs RA, et al. A new class of ghrelin O-acyltransferase inhibitors incorporating triazole-linked lipid mimetic groups. Bioorg Med Chem Lett. 2015;25(14):2800–2803. [DOI] [PubMed] [Google Scholar]
- 138.Hougland J, inventor; Syracuse University, USA, assignee. Inhibitors targeting human ghrelin O-acyltransferase Unites States patent US 20150018520 A1. 2015. May 17. [Google Scholar]
- 139.Cole PA, Barnett BP, Hwang Y, et al. , inventors; The Johns Hopkins University, USA, assignee. Methods for synthesis and uses of peptide-coenzyme a conjugates as inhibitors of ghrelin O-acyltransferase as potential therapeutic agents for obesity and diabetes World patent WO2010039461A2. 2010. April 8. [Google Scholar]
- 140.Cole PA, Barnett BP, Hwang Y, et al. , inventors; The Johns Hopkins University, USA, assignee. Methods for synthesis and uses of inhibitors of ghrelin O-acyltransferase as potential therapeutic agents for obesity and diabetes. United States patent US 20110257086 A1. 2011. October 20. [Google Scholar]
- 141.Teubner BJW, Garretson JT, Hwang Y, et al. Inhibition of ghrelin O-acyltransferase attenuates food deprivation-induced increases in ingestive behavior. Horm Behav. 2013;63(4):667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bowers CY, Coy DH, Hocart SJ, et al. , inventors; The Administrators of the Tulane Educational Fund, USA; McGill University, assignee. Methods of inhibiting the ghrelin/growth hormone secretagogue receptor pathway and uses thereof World patent WO 2010132580 A2. 2010. November 18. [Google Scholar]
- 143.Garner AL, Janda KD. A small molecule antagonist of ghrelin O-acyltransferase (GOAT). Chem Commun. 2011;47(26):7512–7514. [DOI] [PubMed] [Google Scholar]
- 144.Garner AL, Janda KD. Cat-elcca: A robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT). Angew Chem, Int Ed. 2010;49(50):9630–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hollibaugh RA. Defining a minimal pharmacophore to selectively inhibit mboat4 [dissertation]. Los Angeles (CA): UCLA; 2016. [Google Scholar]
- 146.Hollibaugh RA, Liu H, Elmajian N, et al. , editors. Small molecule inhibitors of ghrelin O-acyl transferase. 243rd ACS National Meeting & Exposition; 2012. March 25-29, 2012; San Diego, CA: American Chemical Society. [Google Scholar]
- 147.Harran PG, Brown MS, Goldstein JL, et al. , inventors; University of Texas, USA, assignee. Small molecule inhibitors of ghrelin O-acyltransferase and therapeutic use thereof United States patent US 20100086955 A1. 2010. April 8. [Google Scholar]
- 148.Harran PG, Hollibaugh RA, Liu H, inventors; University of California, USA, assignee. Small lipopeptidomimetic inhibitors of ghrelin O-acyl transferase World patent WO 2016044467 A1. 2016. March 24. [Google Scholar]
- 149.McGovern-Gooch KR, Mahajani NS, Garagozzo A, et al. Synthetic triterpenoid inhibition of human ghrelin O-acyltransferase: The involvement of a functionally required cysteine provides mechanistic insight into ghrelin acylation. Biochemistry. 2017;56(7):919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sieburg MA, Cleverdon ER, Hougland JL. Biochemical assays for ghrelin acylation and inhibition of ghrelin O-acyltransferase. Methods Mol Biol. 2019;2009:227–241. [DOI] [PubMed] [Google Scholar]
- 151.Yates MS, Tauchi M, Katsuoka F, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of nrf2-regulated genes. Mol Cancer Ther. 2007;6(1):154–162. [DOI] [PubMed] [Google Scholar]
- 152.Yore MM, Liby KT, Honda T, et al. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-κb activation through direct inhibition of iκb kinase β. Mol Cancer Ther. 2006;5(12):3232–3239. [DOI] [PubMed] [Google Scholar]
- 153.Honda T, Rounds BV, Gribble GW, et al. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1998;8(19):2711–2714. [DOI] [PubMed] [Google Scholar]
- 154.Wong MH, Bryan HK, Copple IM, et al. Design and synthesis of irreversible analogues of bardoxolone methyl for the identification of pharmacologically relevant targets and interaction sites. J Med Chem. 2016;59(6):2396–2409. [DOI] [PubMed] [Google Scholar]
- 155.Liby KT, Sporn MB. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64(4):972–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. *.Jakob CG, Upadhyay AK, Donner PL, et al. Novel modes of inhibition of wild-type isocitrate dehydrogenase 1 (IDH1): Direct covalent modification of His315. J Med Chem. 2018;61(15):6647–6657.Key report which shows a-cyanoenones can react with histidines as well as cysteine sidechains.
- 157.Nguyen HT, Nakajima K, Uto T, et al. Bioactive triterpenes from the root of salvia miltiorrhiza bunge. Phytother Res. 2017;31(9):1457–1460. [DOI] [PubMed] [Google Scholar]
- 158.Galka CS, Hembre EJ, Honigschmidt NA, et al. , inventors; Eli Lilly and Company, USA, assignee. Preparation of n-acylamino acid derivatives as ghrelin O-acyl transferase inhibitors World patent WO 2016168225 A1. 2016. October 20. [Google Scholar]
- 159.Galka CS, Hembre EJ, Honigschmidt NA, et al. , inventors; Eli Lilly and Company, USA, assignee. Preparation of 5-[2-[1-[n-acyl-l-alanyl]piperidin-4-yl]ethyl]-2-(trifluoromethyl)-6-methylpyrimidin-4-amine as ghrelin O-acyl transferase inhibitors World patent WO 2016168222 A1. 2016. October 20. [Google Scholar]
- 160.Martinez-Grau MA, inventor; Eli Lilly and Company, USA, assignee. Ghrelin O-acyl transferase inhibitor United States patent US 20150133474 A1. 2015. May 14. [Google Scholar]
- 161.Ruano G, Galka C, Hembre E, et al. Ghrelin O-acyl transferase (GOAT) inhibitors: Optimization of the 6-chloro-2-methyl-5-[2-(4-piperidyl)ethyl]pyrimidin-4-amine scaffold 252nd ACS National Meeting & Exposition; 2016. August 21-25, 2016; Philadelphia, PA: American Chemical Society. [Google Scholar]
- 162.Martinez-Grau M, Dominguez C, Galka C, et al. Discovery and evaluation of the first small molecules targeting GOAT inhibition in vivo 252nd ACS National Meeting & Exposition; 2016. August 21-25, 2016; Philadelphia, PA: American Chemical Society. [Google Scholar]
- 163.Hembre E, Brier R, Chen Y, et al. Discovery of ly3073084, a novel non-peptide small molecule ghrelin-O-acyl transferase (GOAT) inhibitor 252nd ACS National Meeting & Exposition; 2016. August 21-25, 2016; Philadelphia, PA: American Chemical Society. [Google Scholar]
- 164.GLWL Research Inc. A study of GLWL-01 in patients with Prader-Willi syndrome. ClinicalTrials.gov Identifier: NCT03274856: GLWL Research Inc.; 2017–2019. [Google Scholar]
- 165.Batterham R, Zakeri R. Investigating the role of ghrelin in regulating appetite and energy intake in patients following bariatric surgery (bari-insight) ClinicalTrials.gov Identifier: NCT03641417: University College, London; 2018–2019. [Google Scholar]
- 166.Leggio L Ghrelin signaling via GOAT inhibition in alcohol use disorder. ClinicalTrials.gov Identifier: NCT03896516: National Institute on Alcohol Abuse and Alcoholism (NIAAA); 2019–2020. [Google Scholar]
- 167.Takakura N, Banno Y, Terao Y, et al. , inventors; Takeda Pharmaceutical Company Limited, Japan, assignee. Preparation of benzothiophenyl- and benzofuranacetic acid derivatives as inhibitors of GOAT for treating obesity World patent WO 2013125732 A1. 2013. August 29. [Google Scholar]
- 168.Bandyopadhyay A, Cheung M, Eidam HS, et al. , inventors; GlaxoSmithKline Intellectual Property Development Limited, UK, assignee. Preparation of ghrelin O-acyltransferase inhibitors for treatment of metabolic disorders India patent IN 201811004277A. 2019. August 9. [Google Scholar]
- 169.Bandyopadhyay A, Cheung M, Eidam HS, et al. , inventors; GlaxoSmithΚline Intellectual Property Development Limited, UK, assignee. Preparation of ghrelin O-acyltransferase inhibitors for treatment of metabolic disorders World patent WO 2019149959 A1. 2019. August 8. [Google Scholar]
- 170.Wang L, inventor; Peop. Rep. China, assignee. Ghrelin O-acyltransferase (GOAT) inhibitor and its applications in obesity and diabetes China patent CN 108516972A. 2018. September 11. [Google Scholar]
- 171.Trieselmann T, Godbout C, Hoenke C, et al. , inventors; Boehringer Ingelheim International GmbH, Germany, assignee. Preparation of benzyl-, (pyridin-3-yl)methyl- or (pyridin-4-yl)methyl-substituted oxadiazolopyridine derivatives as ghrelin O-acyl transferase (GOAT) inhibitors World patent WO 2019149657 A1. 2019. August 8. [Google Scholar]
- 172.Trieselmann T, Godbout C, Vintonyak V, inventors; Boehringer Ingelheim International GmbH, Germany, assignee. Preparation of heterocyclyl-substituted oxadiazolopyridine derivatives for use as ghrelin O-acyl transferase inhibitors World patent WO 2019149659 A1. 2019. August 8. [Google Scholar]
- 173.Trieselmann T, Godbout C, Hoenke C, et al. , inventors; Boehringer Ingelheim International GmbH, Germany, assignee. Preparation of triazolopyrimidine derivatives as ghrelin O-acyl transferase (GOAT) inhibitors World patent WO 2019149660 A1. 2019. August 8. [Google Scholar]
- 174.Godbout C, Trieselmann T, Vintonyak V, inventors; Boehringer Ingelheim International GmbH, Germany, assignee. Preparation of oxadiazolopyridine derivatives for use as ghrelin O-acyl transferase (GOAT) inhibitors World patent WO 2018024653 A1. 2018. February 8. [Google Scholar]
- 175.Godbout C, Trieselmann T, Vintonyak V, inventors; Boehringer Ingelheim International GmbH, Germany, assignee. Preparation of heterocyclyl-substituted oxadiazolopyridine derivatives for use as ghrelin O-acyl transferase inhibitors World patent WO 2019149658 A1. 2019. August 8. [Google Scholar]
- 176.Resh MD. Fatty acylation of proteins: The long and the short of it. Prog Lipid Res. 2016;63:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ho SY, Keller TH. The use of porcupine inhibitors to target wnt-driven cancers. Bioorg Med Chem Lett. 2015;25(23):5472–5476. [DOI] [PubMed] [Google Scholar]
- 178.Resh MD. Palmitoylation of proteins in cancer. Biochem Soc Trans. 2017;45(2):409–416. [DOI] [PubMed] [Google Scholar]