Abstract
Navajo Nation residents are at risk for exposure to uranium and other co-occurring metals found in abandoned mine waste. The Navajo Birth Cohort Study (NBCS) was initiated in 2010 to address community concerns regarding the impact of chronic environmental exposure to metals on pregnancy and birth outcomes. The objectives of this paper were to 1) evaluate maternal urine concentrations of key metals at enrollment and delivery from a pregnancy cohort; and 2) compare the NBCS to the US general population by comparing representative summary statistical values. Pregnant Navajo women (N=783, age range 14–45 years) were recruited from hospital facilities on the Navajo Nation during prenatal visits and urine samples were collected by trained staff in pre-screened containers. The U.S. Centers for Disease Control and Prevention (CDC), National Center for Environmental Health’s (NCEH) Division of Laboratory Sciences (DLS) analyzed urine samples for metals. Creatinine-corrected urine concentrations of cadmium decreased between enrollment (1st or 2nd trimester) and delivery (3rd trimester) while urine uranium concentrations were not observed to change. Median and 95th percentile values of maternal NBCS urine concentrations of uranium, manganese, cadmium, and lead exceeded respective percentiles for National Health and Nutrition Evaluation Survey (NHANES) percentiles for women (ages 14–45 either pregnant or not pregnant.) Median NBCS maternal urine uranium concentrations were 2.67 (enrollment) and 2.8 (delivery) times greater than the NHANES median concentration, indicating that pregnant Navajo women are exposed to metal mixtures and have higher uranium exposure compared to NHANES data for women. This demonstrates support for community concerns about uranium exposure and suggests a need for additional analyses to evaluate the impact of maternal metal mixtures exposure on birth outcomes.
Keywords: Uranium, metals, metal mixtures, maternal biomonitoring, urine, Navajo Birth Cohort Study
1. INTRODUCTION
More than 500 uranium mines have been abandoned on the Navajo Nation since the cessation of mining in the 1980s. These abandoned mines contain complex mixtures of metals and metalloids (hence forth referred to as metals for simplicity) including arsenic, uranium, lead, manganese, mercury, and others (Eichstaedt 1994). The presence of metal mixtures at these abandoned uranium mines places Navajo Nation residents at risk for exposure. Participation in traditional practices may also increase contact with environmental metals at these abandoned sites (Harper et al. 2012). Other non-mining sources of metals includes cadmium from smoking (Richter et al. 2009) and consumption of meat (Olmedo et al. 2017), as well as coal burning for home heating and power generation in the region (Bunnell et al. 2010). Additionally, arsenic and uranium are detected commonly in groundwater on the Navajo Nation and may be present in unregulated and regulated water sources (Corlin et al. 2016; Credo et al. 2019; Hoover et al. 2018; Hoover et al. 2017; Ingram et al. 2020; Jones et al. 2020). Previous work with Navajo communities in New Mexico used surveys and a Bayesian multivariate t-model to assess non-occupational uranium exposures. Survey data and self-reported health outcomes from 1,304 people were modeled and it was found that exposure to abandoned mine waste was associated with elevated risk of developing hypertension, diabetes, and kidney disease (Hund et al. 2015). Additionally, serum inflammatory markers from 273 Navajo men and women living in the Eastern Agency (New Mexico portion of Navajo Nation) were inversely associated with residential proximity to abandoned waste piles and induction of a pathway leading to development of atherosclerosis and cardiovascular disease (Harmon et al. 2017). These previous studies recruited an older population of Navajo Nation residents (mean age 56 years) who lived on the New Mexico portion of Navajo Nation and investigated chronic disease and uranium exposure. Other studies relied on medical record abstraction and reported that negative birth outcomes were associated with proximal residential location to abandoned uranium mine wastes in Shiprock, NM (Shields et al. 1992). Navajo community members who live near abandoned mines remain concerned about impacts on infant and child health (Lewis et al. 2015).
Many of the metals found in abandoned uranium mine waste are known to influence birth outcomes negatively. Oral and subcutaneous uranium exposure in animals is associated with reduced growth in offspring (Domingo 2001). Information about direct toxicity of natural uranium in a pregnant human population remains limited, however (Craft et al. 2004). Arsenic exposure is associated with a reduction in birth weight (Quansah et al. 2015), and manganese exposure is associated with low birth weight (Xia et al. 2016b), increased fetal mortality (Hafeman et al. 2007), and intellectual impairment in children (Bouchard et al. 2011). Maternal lead exposure is associated with anthropomorphic changes in newborns (Wang et al. 2017; Xie et al. 2013) and thallium exposure is associated with increased risk of low birth weight (Xia et al. 2016a). Cadmium exposure has been associated with fetal growth restrictions due to changes in placental trophoblast cells (Yang et al. 2006). Prenatal mercury exposure is also a concern because there is ample evidence it may affect fetal growth (Karagas et al. 2012; Murcia et al. 2016) and the development of neurodevelopment disorders (Counter and Buchanan 2004; Davidson et al. 2004). There is also limited evidence suggesting that co-exposure to lead and manganese or cadmium and arsenic could exacerbate neurotoxicity in children (Sanders et al. 2015). Although previous work demonstrated that abandoned uranium mine waste exposure is associated with an increased risk for developing multiple chronic diseases among older Navajo community members (Hund et al. 2015), there remains limited research investigating maternal exposure to metals found in abandoned uranium mine waste. A more developed understanding of maternal exposure to uranium, arsenic, cadmium, lead, manganese, mercury, and thallium will inform population studies investigating metals exposure and birth outcomes.
The Navajo Birth Cohort Study (NBCS) was initiated in 2010 to address community concerns regarding the impact of chronic environmental exposure to uranium and other co-occurring metals. The research team, led by the University of New Mexico Health Sciences Center Community Environmental Health Program, included partnerships with Navajo Nation Department of Health, Navajo Area Indian Health Service, Southwest Research Information Center, the US Centers for Disease Control and Prevention (CDC), and the Agency for Toxic Substances and Disease Registry (ATSDR). The NBCS provides a platform to assess maternal urine metal concentrations during pregnancy among a cohort with a range of exposures to uranium and co-occurring metals.
The objectives of this paper were to use the NBCS to 1) evaluate maternal urine concentrations of key metals at enrollment and delivery from a pregnancy cohort; and 2) compare the NBCS to the US general population by comparing representative summary statistical values. This information may also inform development of policies to address risks to the Navajo population from exposures to key metals in the waste.
2. MATERIALS AND METHODS
2.1. Study population
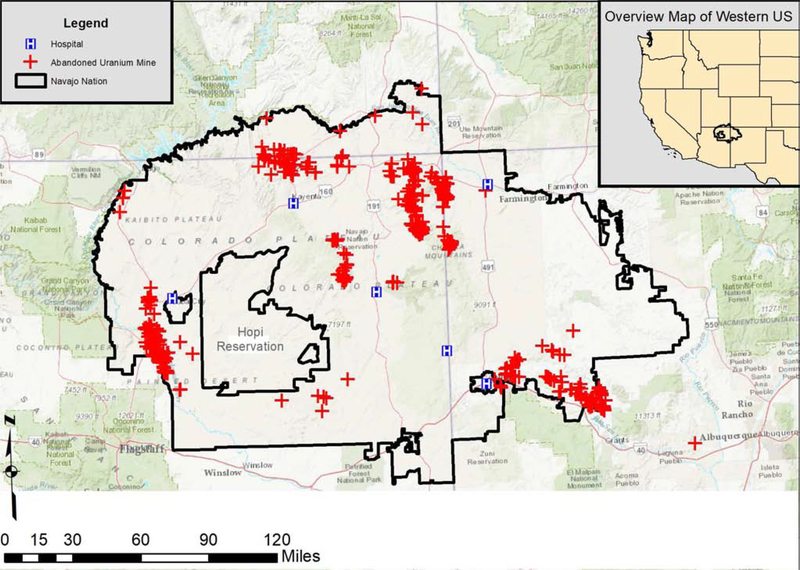
The sample population for this paper included 783 pregnant Navajo women recruited between February 2013 and June 2018 from locations across the Navajo Nation (Figure 1). Navajo staff recruited women during prenatal visits at participating Indian Health Service or Public Law 638 hospitals on the Navajo Nation. Inclusion criteria included 1) a clinically confirmed pregnancy; 2) maternal age between 14 and 45 years; 3) residence on the Navajo Nation for a minimum of five years; 4) willingness to deliver at one of six participating Indian Health Service or Public Law 638 hospital facilities; and 5) willingness to allow follow-up evaluations during the baby’s first year of life. At time of enrollment, a survey was administered to maternal participants to obtain information about age, employment status, educational attainment, annual household income, smoking status, home heating options and fuels, drinking water source, if they eat any fruits or vegetables they grow, and other information.
Figure 1.
Study area map illustrating the location of abandoned uranium mines (red X), Navajo Nation boundaries and the location of enrollment facilities.
Medical records were also reviewed by trained staff for pre-pregnancy height and weight. All women provided written informed consent and consent was obtained from a guardian for women who were minors at time of enrollment. While enrollment targeted women during their 1st trimester (<13 weeks gestation), women were also enrolled during 2nd (14–28 weeks gestation) and 3rd trimesters (>29 weeks) gestation. Statistical analysis was limited to enrollment samples collected during the 1st or 2nd trimester and delivery samples. Delivery samples were urine samples collected after 36 weeks gestational age or when a pregnant mother was admitted to the hospital for labor. The University of New Mexico Institutional Review Board and the Navajo Nation Human Research Review Board approved the study protocol. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
2.2. Biological sample collection
Spot urine samples were collected in sterile 50 mL pre-screened urine collection cups provided by the National Center for Environmental Health’s Division of Laboratory Sciences (DLS). Hospital laboratory personnel used a pre-screened transfer pipette to aliquot 1.8 mL of urine into separate 2.0 mL Nalgene cryovials for multi-element metals, total arsenic, and creatinine analyses. Urine samples were stored at −80°C in a freezer until University of New Mexico (UNM) staff picked up and transferred samples on dry ice to UNM freezer storage facilities where they were stored at −80°C. Staff reviewed and validated Chain of Custody forms at each stage of collection and storage. UNM staff shipped samples overnight on dry ice to the DLS and, once received, samples were stored at ≤ −20°C until preparation for analysis. Sample collection and storage protocols match CDC recommendations and NHANES protocols.
2.3. Sample preparation and analysis
Urine samples were measured for metals by the DLS using the same laboratory methods used in NHANES (Caldwell et al. 2005; CDC 2011; 2013; 2014; Jarrett et al. 2007; Jarrett et al. 2008). Total urine element concentrations of antimony, arsenic, barium, beryllium, cadmium, cesium, cobalt, iodine, lead, manganese, mercury, molybdenum, platinum, strontium, thallium, tin, tungsten, and uranium were measured using inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS). Two or three, depending on the method, custom-made, characterized urine bench quality control materials were inserted at the beginning and end of each analytical run, and the multi-rule quality control system (MRQCS), developed by Caudill et al. (2008), was used to determine if runs were in control. The limits of detection for these elements in urine ranged from 0.002 to 2.4 μg/L, depending on the analyte. Creatinine concentrations were determined using Enzymatic by Roche/Hitachi Modular P Chemistry Analyzer.
Subsequent statistical analysis focused on urine concentrations of total arsenic, cadmium, lead, manganese, mercury, thallium, and uranium because these elements may be found in abandoned mine waste and are associated with negative birth outcomes, as indicated in Section 1.
2.4. Statistical analysis
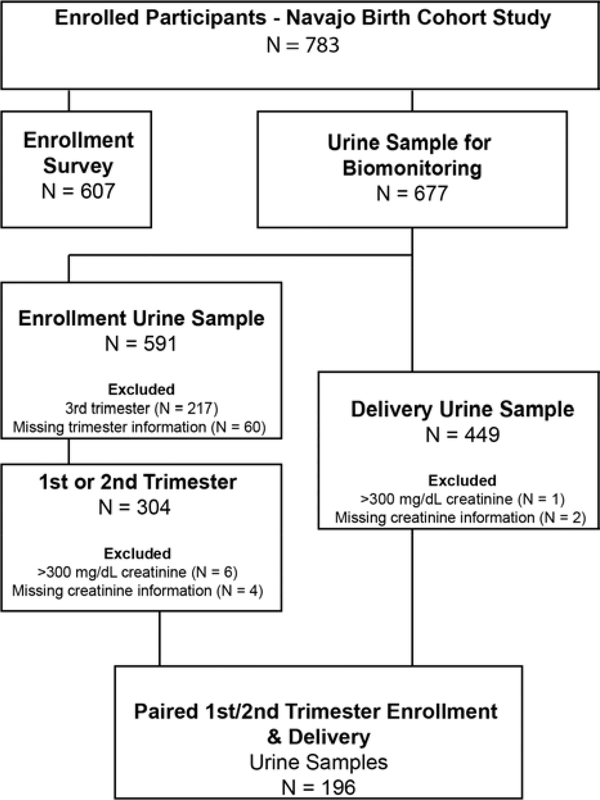
Urine metal concentrations were creatinine corrected to account for hydration status and flow rate differences among participants. Only urine samples with creatinine concentrations less than 300 mg/dL and greater than 0.3 mg/dL were included in this analysis (Figure 2). Figure 2 illustrates the available samples for this study and reasons samples may have been excluded from analysis. Spearman’s Rho correlation coefficient was calculated among urine metals at enrollment and delivery and between enrollment and delivery for individual urine metals. Additionally, because the biomonitoring data were non-normally distributed, the two-sided Mann-Whitney U Test was used to evaluate for differences in median urinary metal concentrations between enrollment and delivery. For the Mann-Whitney U test, concentrations less than the limit of detection (LOD) were imputed as the LOD/√(2).
Figure 2 –
Summary of surveys and biomonitoring samples selected for analysis
Descriptive statistics including sample size, percentage of samples below the LOD, range, median, geometric mean, and 95th percentile values were calculated for each urine metal at enrollment and delivery using the creatinine corrected results. Most urinary metals included some measurements less than LOD so robust Regression on Order Statistics (R-ROS), a left-censored data analysis method was used to generate the geometric mean, median, and 95th percentile for each urine metal. Left-censored methods have previously been shown to produce more stable and less biased measures of central tendency when compared to substitution methods (Helsel 2006; 2012). In the present analysis values for urine metal measurements less than the LOD were imputed using the R-ROS method and were not assumed to be zero. Calculations were completed using the R (v 3.6.2) Nondetects And Data Analysis (NADA) package (v 1.6–1) and confidence intervals were generated using the package boot (v 1.3–23)
Reference values were calculated using data from the National Health and Nutrition Evaluation Survey cycles 2011–12, 2013–14, and 2015–16. Data from these cycles were limited to women ages 14–45; demographic information including body mass index, smoking status, and educational achievement were extracted along with urine metal and creatinine measurements. Cycle results were combined by dividing the two-year weights by three (the number of cycles combined) to generate a new 6 year weight, as recommended by the CDC (CDC 2018). The 6-year weights were used to account for the complex survey design when calculating summary statistics. For simplicity women ages 14–45 sampled for NHANES cycles 2011–12, 2013–14, and 2015–16 are referred to as the US reference population in the present study.
3. RESULTS
3.1. Demographic summary
Women participating in the NBCS have a similar median age, income, and unemployment status compared to all women of the Navajo Nation (Table 1). Pre-pregnancy body mass index (BMI) results indicate that a higher percentage of women in the study were overweight and a lower percentage of study participants were obese relative to the Navajo Nation. Although a higher percentage of study women had a normal BMI than seen in the Navajo population, the percentage relative to women from the US reference population was lower.
Table 1.
Characteristics of pregnant women enrolled in the Navajo Birth Cohort (N=607)
| Characteristic | NBCS | Navajo Nation | NHANESa |
|---|---|---|---|
| Age – Median | 26 (25–27) | 32.0 (31.4–32.6)b | 29.5 (29.1–29.9) |
| Body Mass Index (BMI): | |||
| <=25 (Normal) | 28.2% (24.6–32.1%) | 17.6% (16.7–18.5%)c | 42.6% (40.4–44.8%) |
| 25–30 (Overweight) | 30.2% (26.4–34.0%) | 32.8% (31.7–33.9%)c | 24.2% (22.74–25.7%) |
| 30–35 (Obese) | 41.7% (37.6–45.9%) | 49.6% (48.5–50.7%)c | 33.3% (31.6–35.0%) |
| Education (Less than High School) | 21.7% (18.5–25.2%) | 18.4% (17.6–19.2%)d | 24.7% (22.8–26.6%) |
| Household Income <$20,000 annually | 65.7% (61.2–69.9%) | 42.6% (41.2–44.0%)e | 14.1% (12.2–16.0%) |
| Not working | 68.8% (64.9–72.5%) | 65% (64.5–65.5%)f | 35.0% (32.8–37.2%) |
| Smoking status | |||
| Ever smoker | 18.2% (15.3–21.8%) | 14.1% (13.1–15.1%)g | 31.3% (29.2–33.4%) |
| Never smoker | 81.8% (78.4–84.9%) | 85.9% (84.9–86.9%)g | 68.7% (66.6–70.8%) |
| Not reported | 5.3 % | - | - |
Note.
NHANES – National Health and Nutrition Evaluation Survey 2011–12, 2013–14, and 2015–16, females ages 14–45
Women only, from Table S0101, 2015 American Community Survey [5-year estimates], margin of error is a 90% Confidence Interval
From Table S1501, 2015 American Community Survey [5-year estimates], margin of error is a 90% Confidence Interval
From Table B19001C, 2015 American Community Survey [5-year estimates], margin of error is a 90% Confidence Interval
From Table S2301, 2015 American Community Survey [5-year estimates], margin of error is a 90% Confidence Interval
Never-smoker status among NBCS women was similar to all women on the Navajo Nation and more frequent than the US reference population. Compared to the US reference population, NBCS women were of similar age, have lower household incomes, higher rates of unemployment, higher BMI, and a higher frequency of never-smokers. No differences were observed when pre-pregnancy BMI, age, household income, employment status, or education attainment was compared among women who gave 0, 1 or 2 samples for metals analysis (Table 2).
Table 2.
Key demographic variables stratified by number of collected urine samples
| Characteristic | 0 Urine Samplesa | 1 Urine Sample | 2 Urine Samples |
|---|---|---|---|
| Median Age - Years (Median, SD) | 26.15 (6.36) | 27.50 (5.89) | 27.58 (5.96) |
| Median Body Mass Index (Median, SD): | 29.20 (6.55) | 29.06 (6.71) | 29.00 (6.46) |
| Proportion of participants with education less than high school (proportion, 95% CI) | 0.23 (0.11–0.41) | 0.24 (0.18–0.31) | 0.21 (0.17–0.25) |
| Proportion of participants with household income <$20,000 annually (proportion, 95% CI) | 0.86 (0.66–0.95) | 0.63 (0.54–0.71) | 0.65 (0.60–0.70) |
| Proportion of participants unemployed | 0.77 (0.59–0.89) | 0.69 (0.61–0.75) | 0.68 (0.63–0.73) |
| Proportion of participants classified as never smoker (proportion, 95% CI) | 0.24 (0.11–0.42) | 0.18 (0.13–0.25) | 0.18 (0.14–0.22) |
Notes -
Indicates that a woman agreed to participate in the study, completed an enrollment survey but did not provide a urine sample for metals analysis.
3.2. Urinary metals
Enrollment urine samples were collected from 304 pregnant women during the 1st or 2nd trimester and delivery samples were collected from 449 women. At enrollment 6 samples were excluded due to creatinine concentrations exceeding 300 mg/dL and creatinine measurements were missing for four additional samples. At delivery, 1 sample was excluded due to creatinine concentrations exceeding 300 mg/dL and creatinine measurements were missing for two additional samples (Figure 2). Of the 783 enrolled women, 22.4% did not provide an enrollment sample and 33.2% did not provide a delivery sample.
Creatinine corrected urinary metal concentrations for the select elements are shown in Table 3. Urinary concentrations of cadmium, lead, arsenic (total), thallium, and uranium were detected in more than 90% of enrollment samples. Manganese and mercury were detected in 65.5% and 48.8% of enrollment samples, respectively. At delivery arsenic (total), lead, thallium, and uranium were detected in more than 95% of samples. Cadmium, manganese, and mercury were detected in 76.9%, 68.9% and 40.4% of delivery samples, respectively.
Table 3.
Summary statistics of select urinary metals (μg/g creatinine) by enrollment and delivery with NHANES comparison (females only, ages 14–45)
| Metal | Measure | NBCS - Enrollment | NBCS - Delivery | NHANES |
|---|---|---|---|---|
| Cd | GM (95% CI) | 0.219 (0.202 – 0.236) | 0.148 (0.138 – 0.159) | 0.133 (0.125–0.141) |
| Median (95% CI) | 0.225 (0.209 – 0.247) | 0.141 (0.132 – 0.154) | 0.132 (0.122–0.143) | |
| P95 (95% CI) | 0.609 (0.564 – 0.761) | 0.495 (0.425 – 0.613) | 0.543 (0.476–0.61) | |
| Range | 0.028 – 1.713 | 0.015 – 8.277 | 0.012–7.56 | |
| %<DL | 6.2 | 21.4 | 22.2 | |
| N | 304 | 449 | ||
| Detection Limit | 0.036 μg/L | |||
| Hg | GM (95% CI) | 0.155 (0.129 – 0.186) | 0.162 (0.137 – 0.193) | 0.263 (0.247–0.28) |
| Median (95% CI) | 0.123 (0.108 – 0.159) | 0.120 (0.109 – 0.157) | 0.243 (0.224–0.261) | |
| P95 (95% CI) | 1.114 (0.902 – 1.480) | 1.24 (1.01 – 1.43) | 1.489 (1.304–1.673) | |
| Range | 0.044 – 2.627 | 0.048 – 3.856 | 0.02–70.966 | |
| %<DL | 51.2 | 58.3 | 33.8 | |
| N | 299 | 446 | ||
| Detection Limit | 0.13 μg/L | |||
| Mn | GM (95% CI) | 0.189 (0.173 – 0.208) | 0.326 (0.296 – 0.362) | 0.145 (0.135–0.155) |
| Median (95% CI) | 0.172 (0.158 – 0.194) | 0.299 (0.277 – 0.343) | 0.139 (0.128–0.15) | |
| P95 (95% CI) | 0.744 (0.568 – 1.110) | 2.071 (1.74 – 3.55) | 0.565 (0.504–0.627) | |
| Range | 0.052 – 2.948 | 0.059 – 24.855 | 0.012–22.083 | |
| %<DL | 34.5 | 29.2 | 57.4 | |
| N | 304 | 448 | ||
| Detection Limit | 0.13 μg/L | |||
| Pb | GM (95% CI) | 0.282 (0.266 – 0.299) | 0.314 (0.296 – 0.334) | 0.265 (0.249–0.281) |
| Median (95% CI) | 0.261 (0.252 – 0.284) | 0.292 (0.275 – 0.316) | 0.252 (0.233–0.27) | |
| P95 (95% CI) | 0.78 (0.609 – 0.899) | 0.962 (0.817 – 1.34) | 0.876 (0.762–0.989) | |
| Range | 0.039 – 2.324 | 0.039 – 22.164 | 0.03–27.529 | |
| %<DL | 1.3 | 1.6 | 1.7 | |
| N | 304 | 449 | ||
| Detection Limit | 0.03 μg/L | |||
| As (Total) | GM (95% CI) | 5.834 (5.490 – 6.210) | 6.233 (5.91 – 6.59) | 6.991 (6.5–7.519) |
| Median (95% CI) | 5.449 (5.060 – 5.740 | 5.871 (5.59 – 6.20) | 5.791 (5.374–6.208) | |
| P95 (95% CI) | 13.983 (11.3 – 15.300) | 15.817 (14.0 – 17.4) | 48.646 (36.706–60.585) | |
| Range | 1.915 – 192.503 | 0.282 – 403.273 | 0.947–987.826 | |
| %<DL | 0 | 0.2 | 3.8 | |
| N | 302 | 448 | ||
| Detection Limit | 0.26 μg/L | |||
| Tl | GM (95% CI) | 0.159 (0.151 – 0.167) | 0.141 (0.136 – 0.147) | 0.176 (0.17–0.183) |
| Median (95% CI) | 0.158 (0.148 – 0.170) | 0.141 (0.135 – 0.151) | 0.174 (0.165–0.182) | |
| P95 (95% CI) | 0.332 (0.291 – 0.354) | 0.297 (0.272 – 0.319) | 0.453 (0.412–0.494) | |
| Range | 0.038 – 0.862 | 0.011 – 0.685 | 0.007–2.568 | |
| %<DL | 1 | 3.8 | 0.5 | |
| N | 304 | 449 | ||
| Detection Limit | 0.018 μg/L | |||
| U | GM (95% CI) | 0.018 (0.017 – 0.02) | 0.018 (0.017 – 0.02) | 0.006 (0.005–0.007) |
| Median (95% CI) | 0.017 (0.015 – 0.019) | 0.016 (0.016 – 0.018) | 0.006 (0.005–0.006) | |
| P95 (95% CI) | 0.064 (0.053 – 0.0861) | 0.069 (0.059 – 0.082) | 0.029 (0.023–0.035) | |
| Range | 0.003 – 1.789 | 0.004 – 0.584 | 0.001–1.012 | |
| %<DL | 2.6 | 2.9 | 20.7 | |
| N | 304 | 449 | ||
| Detection Limit | 0.002 μg/L | |||
Note: DL – Detection Limit; 95% CI - indicates the 95% confidence interval; NHANES – National Health and Nutrition Evaluation Survey Cycles 2011–12, 2013–14, and 2015–16 limited to females ages 14–45
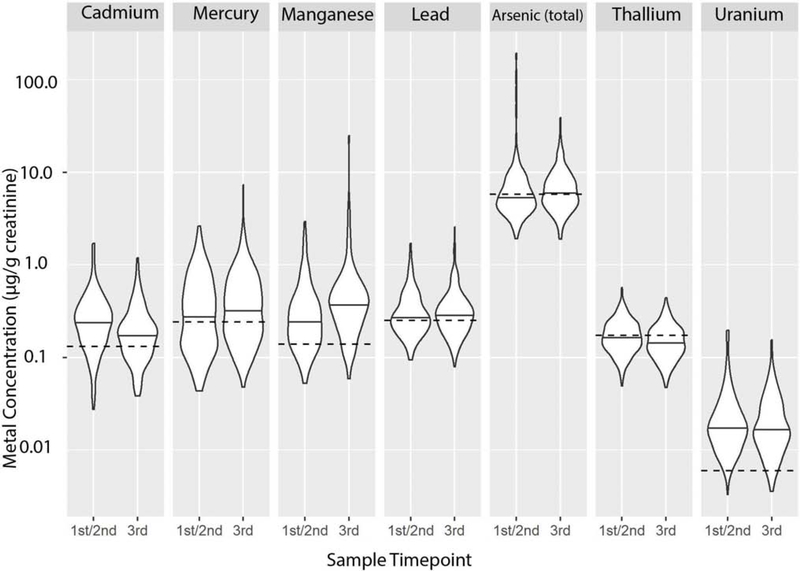
For NBCS enrollment and delivery samples the geometric mean, median, and 95th percentile values of cadmium, lead, manganese, and uranium exceeded the respective NHANES values for US reference population (Figure 3). In contrast, the geometric mean, median, and 95th percentile values of arsenic (total), mercury, and thallium for NBCS women were lower than the respective values for US reference population.
Figure 3.
Bean plot illustrating the distribution and median concentration for urine creatinine-corrected metal concentrations, stratified by enrollment (during 1st or 2nd trimester) and delivery (collected after 36 weeks gestational age or when a pregnant mother was admitted to the hospital for labor). Reference population (NHANES) median concentrations are plotted as a dashed line for each metal.
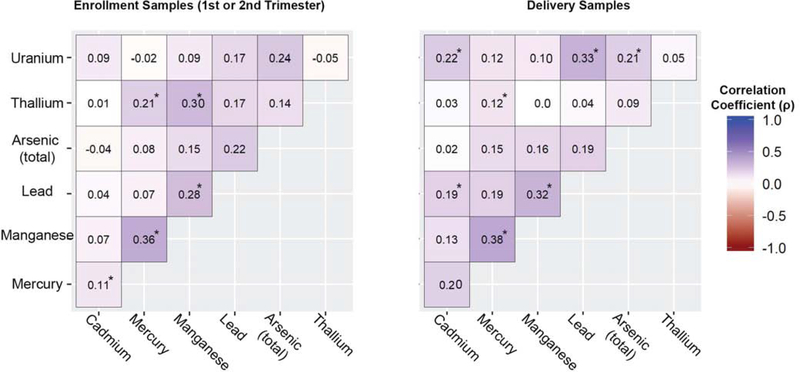
Generally, weak but statistically significant correlation coefficients were observed between metals at the enrollment and delivery time points (Figure 4). Spearman rho correlation coefficients were generated for creatinine corrected urine metal concentrations, units of μg/g-creatinine. At enrollment, the correlation coefficients ranged from −0.05 to 0.36. The strongest correlations were observed for mercury-manganese (ρ=0.36), thallium-manganese (ρ=0.30) followed by lead-manganese (ρ=0.28), thallium-mercury (ρ=0.21) and mercury-cadmium (ρ=0.11). Correlation values were similar for delivery samples ranging between 0.00 and 0.38. The strongest correlations were observed between mercury-manganese (ρ=0.38), uranium-lead (ρ=0.33), lead-manganese (ρ=0.32), uranium-cadmium (ρ=0.22), and uranium-arsenic (total) (ρ=0.21), and lead-cadmium (0.19).
Figure 4.
Correlation heat maps for seven metals at enrollment (1st or 2nd trimester) and delivery. Units are μg/g-creatinine. An * indicates a p-value <0.05.
3.3. Comparing observed enrollment/delivery differences to method variability
To evaluate how much of the differences we observed could be explained within normal method variability, we also compared the median un-corrected concentration changes with 3.0 times the standard deviation of repeated measurements of quality control materials at concentrations similar to the median concentrations observed in this study (Table 4). The NEHC lab only reports method variability in terms of raw metal concentrations, so raw concentrations from the NBCS were used for this analysis.
Table 4.
Difference in concentrations between enrollment (1st or 2nd trimester) and delivery compared with laboratory method variability (3.0 times quality control standard deviation). All concentrations in μg/L.
| Metal | N | Median Change, (delivery – enrollment)† | Quality control Mean (3.0*SD) |
|---|---|---|---|
| Cd | 195 | −0.060 | 0.217 (0.049) |
| Hg | 193 | 0.000 | 0.329 (0.210) |
| Mn | 195 | 0.000 | 0.805 (0.225) |
| Pb | 196 | −0.050 | 0.441 (0.053) |
| As (total) | 192 | −0.600 | 10.12 (0.570) |
| Tl | 196 | −0.025 | 0.173 (0.025) |
| U | 195 | −0.0021 | 0.0174 (0.0034) |
Note.
pairwise difference
Changes in metal concentrations between enrollment and delivery (units = μg/L) exceeded or were equivalent to expected method variability for cadmium, arsenic (total), and thallium. Observed variability for mercury, manganese, lead, and uranium were less than expected method variability.
3.4. Correlation of paired enrollment/delivery urine samples
Pairwise enrollment and delivery samples (per participant) were available for 196 women. Paired maternal urinary concentrations (−μg/g creatinine) were significantly correlated for arsenic (total), cadmium, lead, thallium, mercury, and uranium (Table 5). Manganese concentrations were not significantly correlated between enrollment and delivery samples (ρ=0.133, p-value=0.091) when creatinine corrected concentrations were evaluated. When uncorrected concentrations were evaluated manganese was correlated (ρ=0.243, p-value=0.001). No other differences were observed when uncorrected urine concentrations were analyzed.
Table 5.
NBCS Pairwise Correlation (Spearman’s ρ) and median concentration change between enrollment (1st or 2nd trimester) and delivery
| Metal | Na | Correlation (p-value) | Concentration Δ in μg/g creatinine (p-value) | % Change |
|---|---|---|---|---|
| Cd | 195 | 0.540 (<0.001) | −0.060 (<0.001) | −27.4% |
| Hg | 193 | 0.502 (<0.001) | 0.010 (0.153) | +6.3% |
| Mn | 195 | 0.133 (0.091) | 0.109 (<0.001) | +41.7% |
| Pb | 196 | 0.435 (<0.001) | −0.003 (0.910) | −1.6% |
| As (total) | 192 | 0.339 (<0.001) | 0.64 (0.002) | +14.3% |
| Tl | 196 | 0.229 (<0.001) | −0.02 (0.002) | −14.6% |
| U | 195 | 0.452 (<0.001) | 0.000 (0.524) | −4.0% |
Note
number of paired enrollment and delivery samples; metal concentration presented in creatinine corrected units, μg/g creatinine
Paired differences between enrollment and delivery were also evaluated. Median creatinine-corrected concentrations of cadmium and thallium decreased from enrollment to delivery while creatinine-corrected concentrations of arsenic (total) increased from enrollment to delivery. The median concentrations of lead, mercury, and uranium were not different between time points.
4. DISCUSSION
The presence of abandoned mines on or near Native American reservations increases the risk for exposure to mine waste and metal mixtures. This paper emphasized maternal exposure to uranium, arsenic (total), cadmium, lead, manganese, mercury, and thallium because they are constituents of mine waste (US EPA 2006) and there is evidence suggesting these metals are associated with negative birth outcomes (Hafeman et al. 2007; Quansah et al. 2015; Wang et al. 2018; Wang et al. 2017; Xia et al. 2016a; Xia et al. 2016b; Xie et al. 2013; Zhang et al. 2015). The discussion first focuses on exposure and potential metal sources followed by a discussion of observed concentration changes in urine metals during pregnancy.
4.1. Uranium exposure
Few studies report urine uranium concentrations for the general population or among pregnant women (Callan et al. 2013; CDC 2018; Jain 2013). The median urine uranium concentration for NBCS women was 2.67–2.8 times higher than the US reference population. Similarly, the geometric mean and 95th percentile concentration for NBCS participants were also greater than respective NHANES values. These findings support long standing community concerns about uranium exposure (Lewis et al. 2015). Uranium was mined on the Navajo Nation from the mid-1940s until the late 1980s and previous work has identified uranium at elevated concentrations in abandoned uranium mines, waste piles, and water resources (Blake et al. 2015; Corlin et al. 2016; Credo et al. 2019; Hoover et al. 2018; Hoover et al. 2017) when compared to background sites in the region. Exposure to uranium may be linked with subsistence or traditional activities proximal to abandoned uranium mine sites (Lewis et al. 2017). Previous research in Spain indicated that dietary uranium exposure was predominately from fish and seafood as well as dairy products (Bellés et al. 2013); however, for Navajos, seafood and fish are not traditional dietary components and previous nutritional research indicated that these food items comprise less than 1% of dietary energy or protein (Ballew et al. 1997). Other exposure sources may include ingestion of uranium via drinking water, use of unregulated water sources (Ingram et al. 2020), inhalation of windblown dust (Zychowski et al. 2018), or ingestion of locally grown foods (Samuel-Nakamura 2013).
The NBCS uranium biomonitoring results support observations from other biomonitoring studies conducted in New Mexico, Arizona, and the Navajo Nation (Table 6). For example, the Grants Mineral Belt Uranium Biomonitoring Project surveyed 99 men and women who lived in an area proximal to the eastern portion of the Navajo Nation. While not directly comparable to NBCS results, this project reported an average urine uranium concentration of 0.067 μg/g creatinine (NMED 2011) which is greater than the median reported for the NBCS and higher than the NHANES values for the general population in the United States. Despite inclusion of some individuals with potential occupational exposure, results from the Grants Mineral Belt Uranium Biomonitoring Project similarly reflects uranium exposure among residents who live on or near the Navajo Nation. Another uranium exposure assessment study focused on Navajo households in the Arizona portion of Navajo Nation and reported a median urine uranium concentration of 0.031 μg/g creatinine (Murphy et al. 2009). This concentration is greater than the median reported for the NBCS. Despite different target populations, these studies consistently indicate greater uranium exposure for residents of the Navajo Nation when compared to nationally representative biomonitoring studies.
Table 6.
Comparison of urine uranium results among studies from the southwest USA
| Study | Sample Population | Measure of central tendency | Reported Value |
|---|---|---|---|
| NBCS | Pregnant women between 14–45 on the Navajo Nation | Median Geometric mean |
0.017 μg/g-creatinine 0.018 μg/g-creatinine |
| Grants Mineral Belt Uranium Biomonitoring Project (NMED (2011)) | 99 men and women living in the Grants Mineral Belt of western New Mexico | Mean | 0.067 μg/g-creatinine |
| CDC Biomonitoring (Murphy et al. 2009) | Adult Navajos who live in central area of the Reservation | Median | 0.031 μg/g-creatinine |
The NBCS results indicated that pregnant Navajo women are more exposed to uranium than women ages 14–45 in the US reference population. There are few comparable non-occupational exposure studies, especially those emphasizing pregnant women. Callan et al. (2013) investigated heavy metal exposure of pregnant women in Australia and reported a median creatinine corrected urine uranium concentration of 0.01 μg/g and 65.3% of samples less than the instrument limit of detection. For comparison, uranium was less than the limit of detection in less than 3.5% of NBCS samples. Reporting on results from a cohort of reproductive-aged Danish women, Rosofsky et al. (2017) reported that 68% of samples had detectable concentrations but a geometric mean of 0 ng/mL. Bloom et al. (2015) reported a median uncorrected urine uranium concentration of 0.003 μg/L for women with singleton deliveries that participated in the Longitudinal Investigation of Fertility and the Environment (LIFE) study in Michigan, USA and the US Gulf Coast. Comparison with these studies suggests that urine uranium concentrations among NBCS participants are higher than in other populations.
4.2. Exposure to other metals
Although NBCS urine arsenic (total) concentrations were less than values for the reference population, maternal arsenic exposure remains a concern because it is associated with a host of negative birth outcomes (Vahter 2009). Previous research suggests an association between negative birth outcomes and environmental arsenic when environmental concentrations exceed 50 μg/L (Kile et al. 2016; Milton et al. 2017; Quansah et al. 2015; Vahter 2009). We hypothesize that total arsenic concentration differences between NBCS participants and the US reference population may be driven by a number of factors including drinking water and diet. Drinking water is a potential source of arsenic exposure for residents of the Navajo Nation because it is regularly detected in unregulated water sources (Credo et al. 2019; Hoover et al. 2018; Hoover et al. 2017; Jones et al. 2020) and in some public water systems at concentrations exceeding the Safe Drinking Water Act Maximum Contaminant Level (MCL) of 10 μg/L. The Safe Drinking Water Act is the federal law enabling the US Environmental Protection Agency to regulate concentrations of specific chemicals in drinking water provided by public water systems. The maximum contaminant level (MCL) is the legally enforceable maximum concentration allowable under the regulatory power granted by the SDWA (Tiemann 2010). Contaminant specific MCLs provide benchmarks for comparison even for water sources that are not regulated by the SDWA. A review of the Enforcement and Compliance History Online (ECHO) data portal indicated 142 public water systems operating on the Navajo Nation in reporting year 2017. Twenty-three of these systems had health-based violations of the Safe Drinking Water, including seven systems with arsenic concentrations exceeding the MCL. Previous studies in Arizona, USA indicated that for individuals who drink water with arsenic concentrations that exceed the MCL (and do not consume seafood), 40% of total arsenic comes from drinking water. For people who use public water with arsenic concentrations less than the MCL, 7% of total arsenic intake is from water and the remainder is from diet (Kurzius-Spencer et al. 2013).
Diet is the dominant source of inorganic arsenic exposure for people using drinking water with arsenic concentrations less than the MCL (Kurzius-Spencer et al. 2014). It is well established that arsenic is present in food supplies in the United States and that common dietary sources of inorganic arsenic include rice, rice derived products, other cereals, vegetables, seafood, fruits, shellfish, and seaweed (Nachman et al. 2017). Of these foods, rice, most shellfish and seaweeds have very high concentrations of inorganic arsenic (as high as thousands of ng inorganic arsenic per g) while the other food types have lower concentrations (<20 ng inorganic arsenic per g) (Cubadda et al. 2017). Arsenic in seafood is generally in an organic form (arsenobetaine) that is considered low toxicity; however, certain seafood products may have 20–30% in an inorganic or methylated form that has greater toxicity (Kurzius-Spencer et al. 2013). This is particularly pronounced in like mollusks, crustaceans, and edible seaweed (Cubadda et al. 2017). Among the US general population, consumption of fish and seafood is a key driver of total urinary arsenic concentrations (Hughes et al. 2011; Navas-Acien et al. 2011). For Navajos, seafood and fish are not traditional dietary components and previous nutritional research indicated that these food items comprise less than 1% of dietary energy or protein (Ballew et al. 1997). De La Rosa et al. (2020) reported that NBCS women had lower seafood intake when compared to women 14–45 samples for NHANES. Consumption of other foods that may have higher arsenic accumulation, such as grains, are higher among NBCS participants compared to NHANES. Additional investigation is needed to evaluate the arsenic accumulation and type found in foods commonly consumed by NBCS participants – a broad challenge for dietary exposure assessments (Nachman et al. 2017) - and to conduct a more targeted dietary assessment to evaluate sources of arsenic intake and their correspondence with urine arsenic concentrations.
Total urine mercury is considered to represent predominately inorganic mercury exposure, which may include inorganic mercury from dental amalgams and demethylated fish-derived mercury (Sherman et al. 2013), and to a lesser extent, elemental mercury (Berglund et al. 2005). Previous research reported that recent seafood and fish consumption led to higher urine mercury concentrations among the US population (Jain 2017). NBCS participants reported less seafood intake compared the women in NHANES (De La Rosa et al. 2020), which may contribute to the lower overall urine mercury concentrations observed in this cohort when compared to NHANES. Dental health is of particular concern on the Navajo Nation because nearly 90% of preschoolers on the Navajo Nation experience dental decay and as much as 70% of individuals have untreated dental decay (Batliner et al. 2014). Previous studies have demonstrated that dental amalgams may help to explain urine mercury concentrations due to the presence of mercury in the amalgams (Carta et al. 2002); however, within the present study no data were available to assess the association between dental amalgams and urine mercury. Other potential mercury exposure sources for NBCS participants include the presence of three coal-burning power plants on and near the Navajo Nation (USEPA 2011), especially in areas where thermal inversions trap emissions closer to the surface (Bunnell et al. 2010).
Coal burning for home heating is another potential exposure source for mercury, cadmium, lead and other metals. Enrollment survey responses indicated that 40.3% of NBCS participants report burning coal for home heating. Unprocessed bituminous coal is widely available at a low cost on the Navajo Nation (Kirschbaum et al. 2000). Previous research demonstrated elevated concentrations of particulate matter (PM2.5) that contained cadmium, lead, manganese and mercury (Bunnell et al. 2010) and thallium (Dreesen et al. 1977) in Navajo homes where coal is burned for home heating.
Urine concentrations of cadmium are higher among NBCS mothers compared to the US reference population. Cigarette smoking is considered the primary non-dietary source of cadmium exposure for the US population (Richter et al. 2009). Tobacco has naturally high cadmium concentrations and urinary concentrations of cadmium of smokers are elevated when compared to non-smokers (ATSDR 2012; Tellez-Plaza et al. 2012). Nationally, a decreasing trend in urine cadmium concentrations has been observed since 1988, which may be due in part to decline smoking rates among the US population and smoking cessation programs (Tellez-Plaza et al. 2012). The majority of NBCS participants are never-smokers (smoked <100 cigarettes in their lifetime) however, which suggests that smoking may not be the primary source of cadmium exposure among participants. Although food is considered to be the most significant source of cadmium for non-occupational exposure (Faroon et al. 2012), it is unclear from the available data how locally grown foods contribute to cadmium exposure on the Navajo Nation. Enrollment survey results indicated that 16.1% of NBCS women reported growing fruits or vegetables for consumption. Locally grown vegetables may contribute to cadmium intake, as cadmium in the soil could be a significant source. However, there is limited investigation of soil cadmium concentrations on the Navajo Nation. Samuel-Nakamura and Hodge (2020) analyzed soil samples from locations in the Eastern Agency of Navajo Nation. They found soil cadmium concentrations of 0.50 mg/kg around plant roots and 0.512 mg/kg in topsoil, which is comparable to the crustal average of 0.1–0.5 mg/kg. Observations that median urine cadmium concentrations among pregnant Navajo women exceed women from NHANES suggests a need for follow up investigation to determine if adverse health outcomes are associated with the higher levels and a more thorough investigation of exposure routes to inform possible interventions to reduce exposure.
Urine lead measurements are less invasive than blood measurements but urine concentrations are subject to more biological variation than other biological fluids (Barbosa Jr et al., 2005). For this reason, urine is less preferred for evaluating lead exposure but has seen some use in long-term occupational exposure monitoring. Results from the present study indicate higher urine lead concentrations compared to NHANES; however, urine lead concentrations experience much biological variation so we suggest a follow up investigation emphasizing blood lead measurements. For individuals with lead exposure there are several potential sources on the Navajo Nation including ingesting water from some unregulated and regulated water sources (Hoover et al. 2018), inhaling coal dust from in-home burning of coal for heating (Bunnell et al 2010), and cigarette smoking (Richter et al 2009).
Overall thallium exposure for NBCS participants was lower compared to NHANES data, suggesting less exposure. There are several potential exposure sources for NBCS participants, including the environment and diet. Thallium is a metal that is regularly found in the environment at concentrations less than 1 part per million (Belzile and Chen 2017). Mining activities may lead to increased thallium concentrations and previous studies reported concentrations as high as 20,000 parts per million thallium in pyrite slag from antimony-thallium mines (Bačeva et al. 2014); however, previous research on Navajo Nation has not indicated extensive environmental testing for thallium in water sources across the Navajo Nation (Hoover et al. 2018), and in cases where thallium was measured it was infrequently detected in groundwater (US EPA 2006). Diet is another potential thallium source, including home grown fruits and green vegetables, and shellfish (Tyrrell et al. 2013). Survey results indicated that 16.1% of NBCS study participants indicated that they grow fruits or vegetables, suggesting this is a possible exposure source.
Urine manganese concentrations for NBCS participants were observed to be higher than the NHANES comparison group. Manganese is an essential element and potential neurotoxicant that has been associated lower birth weight (Eum et al 2014), diminished attention span, and lower intellectual growth in children (Wasserman et al., 2006; Wasserman et al., 2016). There are several possible sources of manganese exposure on the Navajo Nation. Hoover et al (2018) reported spatial clusters of unregulated water sources with water manganese concentrations regularly exceeding the Health-Based Screening Level of 300 μg/L. Manganese has also been identified in PM2.5 associated with soils on the Navajo Nation (Gonzalez–Maddux et al. 2014) and in coal burned in homes in the northern area of Navajo Nation (Bunnell et al. 2010). Additional investigation is needed to discern the dominant source (i.e., coal burning, regional power plants, diet, cigarette smoking) of cadmium, lead, thallium, and manganese exposure among NBCS participants and associations with negative birth outcomes and neurodevelopment.
4.3. Urine metals concentration changes during pregnancy
Collection of urine samples at two time points during pregnancy enabled evaluation of changes in urinary concentrations. There are few previous studies that investigated urine metal variability during pregnancy (Ashrap et al. 2020) and these cohorts were primarily urban populations (Fort et al. 2014). Available evidence from these studies suggests that some urine metal concentrations change during pregnancy. For example, Fort et al (2014) indicated a 9.4% increase in the median arsenic concentration from 1st to 3rd trimester and an 11.5% median decrease in urine cadmium from 1st to 3rd trimester.
The median concentration of cadmium in NBCS maternal samples decreased from enrollment to delivery trimester. This decrease has been observed in other pregnancy cohorts (Fort et al. 2014) and may be due to metallothioneins causing cadmium to accumulate in the placenta (Osman et al. 2000; Rudge et al. 2009). To investigate the role of smoking status on this observation we determined enrollment to delivery trends stratified into groups of never smokers and ever smokers and found a statistically significant decrease for both groups (data not shown). Nonetheless, we cannot rule out that the decrease may have been caused, in part, by participants changing their smoking habits during pregnancy, though we consider this to be less likely because 81.9% of NBCS maternal participants were never smokers before enrolling in the study. We observed a decrease in median, uncorrected urine cadmium (−0.060 μg/L) in agreement with the direction of change observed in the corrected concentrations.
The median creatinine corrected urine uranium results were not different between enrollment and delivery. The observed decrease (−0.0021 μg/L) using the uncorrected concentrations was less than the expected method variability (3.0 SD = 0.0034 μg/L). Pairwise individual-level evaluation of uranium from enrollment (1st or 2nd trimester) and delivery (3rd trimester) samples has not been reported for other pregnancy cohorts. Uranium concentrations were positively correlated between enrollment and delivery and median pairwise uranium concentrations were not different between the time points. Previous research using biomonitoring data from a subset of the NBCS cohort indicated no difference in urine uranium concentrations when 1st, 2nd, and 3rd trimester samples were compared (Dashner-Titus et al. 2018). The previous analysis focused on a subset of samples from the overall cohort and used enrollment samples only. No paired analysis was conducted to evaluate individual level changes in urine metal concentrations as pregnancy progresses. Additionally, the previous work compared NBCS urine metal concentrations to women sampled during NHANES 2011–12. In the present paper we expanded the reference group to include women ages 14–45 who participated in NHANES 2011–12, 2013–14, and 2015–16 so that the reference group would be temporally similar to the recruitment period for NBCS. The analysis presented in this paper is also different from previous publications of NBCS data because we investigated individual level differences for paired enrollment and delivery samples.
In the NBCS there is limited evidence of urine concentration change between enrollment and delivery for manganese, thallium, mercury, lead, or arsenic. The median creatinine concentration change for thallium decreased from enrollment to delivery (−0.020 μg/g) but the uncorrected concentration change (−0.025 μg/L) was equivalent to expected method variability (3.0 SD = 0.025 μg/L). Furthermore, the median creatinine concentration change of lead indicated a decrease (−0.003 μg/g) that was also reflected in the uncorrected concentrations (−0.050 μg/L); however, this change was less than the reported method variability (3.0 SD = 0.053 μg/L). The median creatinine corrected urine manganese increased (+0.109 μg/g) from enrollment to delivery, but no change was observed when uncorrected concentrations were evaluated (0.000 μg/L). The observed increase in creatinine corrected concentrations was less than expected method variability (3.0 SD = 0.225 μg/L). Similarly, urine mercury concentrations increased (+0.010 μg/g) when the creatinine corrected values were evaluated but no changed was observed using the raw concentrations (0.000 μg/L). The method variability for urine mercury was 0.210 μg/L. For urine arsenic (total) we observed a median increase of 0.64 μg/g creatinine using corrected concentrations but a median decrease of −0.60 μg/L when uncorrected concentrations were used, which is slightly greater than method variability (3.0 SD = 0.570 μg/L). We interpret these observations with caution because the results seem influenced by creatinine correction. This suggests that creatinine correction of urine metal concentrations may have unintended impacts on results (Barr et al. 2004). More generally these results suggest that it is critical to include a measure of method variability when evaluating concentration differences among paired samples.
Observed changes in urine concentrations of metals between time points may be caused by metabolic changes experienced during pregnancy. Pregnancy is known to increase glomerular filtration rate (Cheung and Lafayette 2013) and cause plasma volume expansion (Hytten and Paintin 1963), both of which may result in altered metal and metalloid filtration from blood into urine. For example, metabolic changes during pregnancy may influence urine manganese concentrations, which has been observed in other birth cohorts (Arbuckle et al. 2016). Pregnant women and their infants may see an increase in blood manganese concentrations due to fetal demands for manganese during pregnancy (Spencer 1999). Changes in urine metal/metalloid concentrations may also be caused by diet changes during pregnancy. No information is available for the present study to evaluate diet changes during pregnancy. The food frequency analysis previously completed and reported by De La Rosa et al. (2020) relied on one-time data collection to characterize general patterns of food consumption throughout pregnancy. A follow up study could be conducted to evaluate diet changes during pregnancy, which would provide additional information to interpret changes in urine metal concentrations.
Correlations among creatinine corrected urine metals were generally weak, as evidenced by the correlation plots (Figure 4). Statistically significant correlations were observed among metals at both timepoints. Generally low and many insignificant correlations is consistent with other investigations. Ashrap et al (2020) reported a lead-manganese correlation of ρ=0.26, which is similar to the present study (ρ=0.28) and a strong correlation between cadmium and lead (ρ=0.55). The cadmium-lead correlation observed in the present study was not as strong (ρ=0.19) at delivery but statistically significant. These differences could be due to the use of creatinine corrected values and not specific gravity correction or raw values as presented in other publications. The significantly correlated values for some of the metals may indicate common sources of exposure, such as drinking water, sociodemographic, diet, or other exposures. More focused exposure assessments for these metals may help further explain common sources, suggested by the correlation values.
4.4. Limitations
The recruitment protocol for this study enabled women to enroll at any point during their pregnancy. As a result, there is variability in the number of samples available for analysis by time point. While all urine samples were collected at the hospital during appointments between normal clinic hours and therefore represent a restricted 8-hour period, the data have not been analyzed with respect to morning versus afternoon collections; diurnal variations in urine metal and creatinine concentrations cannot be ruled out. Additionally, seasonal effects might influence metals exposure and was not explicitly assessed in this paper. NBCS sample collection occurred throughout the year with samples collected during all seasons so seasonal effects should be minimized. We also assume that metals exposure during the pregnancy period is generally consistent because few participants reported moving during this period and previous research demonstrated the mean duration of residence to exceed 36 years in some Navajo communities. The recruitment procedure emphasized women who sought prenatal care at one of six hospital facilities on the Navajo Nation and willingness to delivery at one of the six participating facilities was an inclusion criterion. While there is potential for selection bias due to this inclusion criterion, the participating hospital facilities are the largest on the Navajo Nation and account for the majority of births. Lastly, the reference population used in this study was limited to women ages 14–45 from NHANES cycles 2011–12, 2013–14, and 2015–16. The population was not limited to pregnant women which likely contributes to some of the differences observed between the NBCS study population and the reference population.
This study also did not consider residential proximity to abandoned uranium mines and its relationship with urine metal concentrations. Previous research on the Navajo Nation found positive associations between residential proximity to abandoned uranium mines and circulating biomarkers of inflammation potential (Harmon et al. 2017). A similar approach could be applied to evaluate associations between residential proximity and urine metal concentrations in this cohort because home locations were collected for approximately half of the study participants. De la Rosa et al (2020) reported food frequency questionnaire results for a subset of NBCS participants. These questionnaires were administered late in the third trimester and were designed to assess typical diet throughout pregnancy. Information is not available to evaluate dietary changes throughout pregnancy, though we cannot rule it out as a possible explanation for changes in urine metal concentrations between 1st and 3rd trimester. Lastly, dietary assessment of metals exposure faces a broader challenge of characterizing the occurrence and form of metals in food products. Although new technologies are available to support this effort, dietary exposure assessment previously completed, including those on the Navajo Nation, have not quantified the metals occurrence in many of the foods consumed by the study population. This remains an area for future research to evaluate the role of diet in metals exposure. Despite this limitation, the study results are consistent with other investigations that reported some changes in urine metal concentrations during pregnancy, without considering dietary changes. Follow up investigations could be designed to specifically address the relationship between diet changes, metabolism changes, and urine metal concentration changes throughout pregnancy.
4.5. Conclusions
The Navajo Birth Cohort Study was initiated to address community concerns about exposure to uranium and co-occurring metals. Biomonitoring results from NBCS women indicated higher uranium exposure for pregnant Navajo women compared to women ages 14–45 in the US general population and lower exposure to other toxic metals and metalloids, such as mercury and arsenic. These results support longstanding community concerns about uranium exposure and provides evidence of exposure to multiple metals – many of which have previously been associated with negative birth outcomes. Furthermore, comparison of urine metal concentrations from two time points during pregnancy indicated that concentrations of some metals do change as pregnancy progresses while other remain stable This suggests that for some elements gestational age at time of sample collection may impact measured urine concentrations. These findings suggest a continued need to refine our understanding of the environmental and dietary sources of metals exposure to inform strategies that reduce exposure among pregnant women and residents of the Navajo Nation.
Highlights.
Navajo Nation residents may be exposed to uranium (U) and co-occurring metals
Median urine U value for pregnant Navajo women is 2.8 times higher than NHANES
Urine cadmium and lead are higher among pregnant Navajo women compared to NHANES
Urine metal concentrations may be impacted by gestational age at time of collection
5. ACKNOWLEDGEMENTS
Thank you to Bernadette Pacheco, Navajo Area Indian Health Service, and P.L. 638 laboratory staff for processing biological samples for analysis, and to Jennifer Ong and Elena O’Donald for biomonitoring data management and quality control. The NBCS Study team includes Qeturah Anderson*, Lorraine Barton, David Begay*, Delila Begay*, Francine Begay*, Mae-Gilene Begay*, Nikki Begay*, Priscilla Begay*,Dorena Bennally*, Malcolm Benally*, Benita Brown*, Courtney Burnette, Miranda Cajero, Carla Chavez, Beth Chee*, LaShelly Crank*, Ruofei Du, Danielle Duarte, Esther Erdei, Adrienne Ettinger, Myra Francisco*, Lisa Kear, CJ Laselute*, Lynda Lasiloo, Ji-Hyun Lee, Johnnye Lewis (PI), Li Luo, Debra MacKenzie, Chris Miller, Curtis Miller, Anita Muneta*, Olivia Musket*, Teddy Nez*, Sara Nozadi, Elena O’Donald, Jennifer Ong, Bernadette Pacheco, Sandy Ramone*, Johnna Rogers*, Carolyn Roman, Anna Rondon*, Diedre Sam*, Melissa Samuel*, Abigail Sanders*, Chris Shuey, Becky Smith, Charlotte Swindal, Marcia Tapaha*, Roxanne Thompson*, Doris Tsinnijinnie*, Monique Tsosie*, Shasity Tsosie*, Chris Vining*, Josey Watson*, Maria Welch* and Berlintia Yazzie (* indicates a Navajo team member). Additional thanks to the University of New Mexico Community Environmental Health Program, Navajo Nation Department of Health, and the Southwest Research and Information Center for providing additional support critical for completion of this research. Funding support provided for the Navajo Birth Cohort Study by U01 TS000135-05 and training support provided by K12GM088021-08 Academic Science Education and Research Training (ASERT) Program. Additional thanks to Gulcheckhra Shakirova, Nolan Hilliard, Rebecca Hunt, Reba Williams, and Shannon Sullivan Cigan of the National Center for Environmental Health, Centers for Disease Control and Prevention, Adam Baer, Megan McMichael, Darlyn Mercado Saldivia, Danielle Stukes, of the Battelle Memorial Institute, and Jennifer Ysseldyke Fong Sam of the Oak Ridge Institute of Standards and Education for performing the total elemental and arsenic speciation testing on these biological samples. Thank you to Pam G. Olive MT (ASCP) of the Battelle Memorial Institute for testing urine samples for creatinine. Figure 1 Map created by Rusty Butler, Environmental Studies Program, Montana State University Billings.
Footnotes
6. CONFLICT OF INTEREST
The authors declare no conflict of interest
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph H Hoover, Montana State University Billings, Billings, MT United States.
Esther Erdei, University of New Mexico Health Sciences Center.
David Begay, University of New Mexico Health Sciences Center.
Melissa Gonzales, University of New Mexico Health Sciences Center.
Jeffery M Jarrett, Centers for Disease Control and Prevention.
Po-Yung Cheng, Centers for Disease Control and Prevention.
Johnnye Lewis, University of New Mexico Health Sciences Center.
7. REFERENCES
- Safe Drinking Water Act of 1974. United States: 40 C.F.R. [Google Scholar]
- Arbuckle TE; Liang CL; Morisset A-S; Fisher M; Weiler H; Cirtiu CM; Legrand M; Davis K; Ettinger AS; Fraser WD Maternal and fetal exposure to cadmium, lead, manganese and mercury: the MIREC study. Chemosphere 2016;163:270–282 [DOI] [PubMed] [Google Scholar]
- Ashrap P; Watkins DJ; Mukherjee B; Boss J; Richards MJ; Rosario Z; Vélez-Vega CM; Alshawabkeh A; Cordero JF; Meeker JD Predictors of urinary and blood Metal (loid) concentrations among pregnant women in Northern Puerto Rico. Environmental Research 2020;183:109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological profile for cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2012 [PubMed] [Google Scholar]
- Bačeva K; Stafilov T; Šajn R; Tănăselia C; Makreski P Distribution of chemical elements in soils and stream sediments in the area of abandoned Sb–As–Tl Allchar mine, Republic of Macedonia. Environmental research 2014;133:77–89 [DOI] [PubMed] [Google Scholar]
- Ballew C; White LL; Strauss KF; Benson LJ; Mendlein JM; Mokdad AH Intake of nutrients and food sources of nutrients among the Navajo: findings from the Navajo Health and Nutrition Survey. The Journal of nutrition 1997;127:2085S–2093S [DOI] [PubMed] [Google Scholar]
- Barr DB; Wilder LC; Caudill SP; Gonzalez AJ; Needham LL; Pirkle JL Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environmental health perspectives 2004;113:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batliner T; Wilson AR; Tiwari T; Glueck D; Henderson W; Thomas J; Braun P; Cudeii D; Quissell D; Albino J Oral health status in N avajo N ation H ead S tart children. Journal of public health dentistry 2014;74:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellés M; Linares V; Perelló G; Domingo JL Human dietary exposure to uranium in Catalonia, Spain. Biological trace element research 2013;152:1–8 [DOI] [PubMed] [Google Scholar]
- Belzile N; Chen Y-W Thallium in the environment: a critical review focused on natural waters, soils, sediments and airborne particles. Applied Geochemistry 2017;84:218–243 [Google Scholar]
- Berglund M; Lind B; Björnberg KA; Palm B; Einarsson Ö; Vahter M Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environmental Health 2005;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JM; Avasarala S; Artyushkova K; Ali A-MS; Brearley AJ; Shuey C; Robinson WP; Nez C; Bill S; Lewis J; Hirani C; Pacheco JSL; Cerrato JM Elevated Concentrations of U and Co-occurring Metals in Abandoned Mine Wastes in a Northeastern Arizona Native American Community. Environ Sci Technol 2015;49:8506–8514 [DOI] [PubMed] [Google Scholar]
- Bloom MS; Louis GMB; Sundaram R; Maisog JM; Steuerwald AJ; Parsons PJ Birth outcomes and background exposures to select elements, the Longitudinal Investigation of Fertility and the Environment (LIFE). Environmental research 2015;138:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF; Sauvé S; Barbeau B; Legrand M; Brodeur M-È; Bouffard T; Limoges E; Bellinger DC; Mergler D Intellectual impairment in school-age children exposed to manganese from drinking water. Environmental health perspectives 2011;119:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell JE; Garcia LV; Furst JM; Lerch H; Olea RA; Suitt SE; Kolker A Navajo coal combustion and respiratory health near Shiprock, New Mexico. Journal of environmental and public health 2010;2010:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KL; Hartel J; Jarrett JM; Jones RL Inductively Coupled Plasma Mass Spectrometry to Measure Multiple Toxic Elements in Urine in NHANES 1999–2000. Atomic Spectroscopy 2005;26 [Google Scholar]
- Callan AC; Hinwood AL; Ramalingam M; Boyce M; Heyworth J; McCafferty P; Odland JØ Maternal exposure to metals - Concentrations and predictors of exposure. Environmental Research 2013;126:111–117 [DOI] [PubMed] [Google Scholar]
- Carta P; Flore C; Ibba A; Tocco M; Aru G; Mocci F; Sanna F Urinary and blood markers of internal mercury dose in workers from a chlorakali plant and in subjects not occupationally exposed: relation to dental amalgam and fish consumption. La Medicina del lavoro 2002;93:176–183 [PubMed] [Google Scholar]
- Caudill SP; Schleicher RL; Pirkle JL Multi-rule quality control for the age-related eye disease study. Statistics in medicine 2008;27:4094–4106 [DOI] [PubMed] [Google Scholar]
- CDC. Urine iodine and mercury by ICP-DRC-MS Laboratory Procedure Manual DLS 3002.1. Atlanta, GA: Division of Laboratory Sciences US Centers for Disease Control; 2011 [Google Scholar]
- CDC. Albumin & Creatinine - Urine Laboratory Procedure Manual. Atlanta, GA: Division of Laboratory Sciences US Centers for Disease Control; 2013 [Google Scholar]
- CDC. Urine Multi-Element ICP-DRC-MS Laboratory Procedure Manual DLS 3018.6. Atlanta, GA: Division of Laboratory Sciences US Centers for Disease Control; 2014 [Google Scholar]
- CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, ( March 2018). in: U.S. Department of Health and Human Services Centers for Disease Control and Prevention, ed. Atlanta, GA; 2018 [Google Scholar]
- Cheung KL; Lafayette RA Renal physiology of pregnancy. Advances in chronic kidney disease 2013;20:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlin L; Rock T; Cordova J; Woodin M; Durant JL; Gute DM; Ingram J; Brugge D Health effects and environmental justice concerns of exposure to uranium in drinking water. Current Environmental Health Reports 2016;3:434–442 [DOI] [PubMed] [Google Scholar]
- Counter SA; Buchanan LH Mercury exposure in children: a review. Toxicology and applied pharmacology 2004;198:209–230 [DOI] [PubMed] [Google Scholar]
- Craft ES; Abu-Qare AW; Flaherty MM; Garofolo MC; Rincavage HL; Abou-Donia MB Depleted and natural uranium: chemistry and toxicological effects. Journal of Toxicology and Environmental Health, Part B 2004;7:297–317 [DOI] [PubMed] [Google Scholar]
- Credo J; Torkelson J; Rock T; Ingram JC Quantification of elemental contaminants in unregulated water across western Navajo Nation. International journal of environmental research and public health 2019;16:2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubadda F; Jackson BP; Cottingham KL; Van Horne YO; Kurzius-Spencer M Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Science of the total environment 2017;579:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashner-Titus EJ; Hoover J; Li L; Lee J-H; Du R; Liu KJ; Traber MG; Ho E; Lewis J; Hudson LG Metal exposure and oxidative stress markers in pregnant Navajo Birth Cohort Study participants. Free radical biology & medicine 2018;124:484–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW; Myers GJ; Weiss B Mercury exposure and child development outcomes. Pediatrics 2004;113:1023–1029 [PubMed] [Google Scholar]
- De La Rosa VY; Hoover J; Du R; Jimenez EY; MacKenzie D; Team NS; Lewis J Diet quality among pregnant women in the Navajo Birth Cohort Study. Maternal & Child Nutrition 2020:e12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL Reproductive and developmental toxicity of natural and depleted uranium: a review. Reproductive Toxicology 2001;15:603–609 [DOI] [PubMed] [Google Scholar]
- Dreesen DR; Gladney ES; Owens JW; Perkins BL; Wienke CL; Wangen LE Comparison of levels of trace elements extracted from fly ash and levels found in effluent waters from a coal-fired power plant. Environ Sci Technol 1977;11:1017–1019 [Google Scholar]
- Eichstaedt P If You Poison Us edêds. Santa Fe, NM: Red Crane Books; 1994 [Google Scholar]
- Faroon O; Ashizawa A; Wright S; Tucker P; Jenkins K; Ingerman L; Rudisill C Toxicological profile for cadmium. 2012; [PubMed]
- Fort M; Cosían-Tomás M; Grimly JO; Querol X; Casas M; Sunnier J Assessment of exposure to trace metals in a cohort of pregnant women from an urban center by urine analysis in the first and third trimesters of pregnancy. Environmental Science and Pollution Research 2014;21:9234–9241 [DOI] [PubMed] [Google Scholar]
- Gonzalez–Maddux C; Marcotte A; Upadhyay N; Herckes P; Williams Y; Haxel G; Robinson M Elemental composition of PM2. 5 in Shiprock, New Mexico, a rural community located near coal–burning power plants and abandoned uranium mine tailings sites. Atmospheric Pollution Research 2014;5:511–519 [Google Scholar]
- Hafeman D; Factor-Litvak P; Cheng Z; van Geen A; Ahsan H Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environmental health perspectives 2007;115:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon ME; Lewis J; Miller C; Hoover J; Ali A-MS; Shuey C; Cajero M; Lucas S; Zychowski K; Pacheco B; Erdei E; Ramone S; Nez T; Gonzales M; Campen MJ Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities. Journal Of Exposure Science And Environmental Epidemiology 2017;27:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper BL; Harding A; Harris S; Berger P Subsistence exposure scenarios for tribal applications. Hum Ecol Risk Assess 2012;18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel D Fabricating data: How substituting values for nondetects can ruin results, and what can be done about it. Chemosphere 2006;65:2434–2439 [DOI] [PubMed] [Google Scholar]
- Helsel D Statistics for Censored Environment Data Using Minitab and R.2nd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2012 [Google Scholar]
- Hoover J; Coker E; Barney Y; Shuey C; Lewis J Spatial Clustering of Metal and Metalloid Mixtures in Unregulated Water Sources on the Navajo Nation - Arizona, New Mexico, Utah, USA. Science of the Total Environment 2018;633:1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J; Gonzales M; Shuey C; Barney Y; Lewis J Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo Nation, USA. Exposure and Health 2017;9:113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF; Beck BD; Chen Y; Lewis AS; Thomas DJ Arsenic exposure and toxicology: a historical perspective. Toxicological Sciences 2011;123:305–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund L; Bedrick EJ; Miller C; Huerta G; Nez T; Ramone S; Shuey C; Cajero M; Lewis J A Bayesian framework for estimating disease risk due to exposure to uranium mine and mill waste on the Navajo Nation. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2015;178:1069–1091 [Google Scholar]
- Hytten F; Paintin D Increase in plasma volume during normal pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1963;70:402–407 [DOI] [PubMed] [Google Scholar]
- Ingram JC; Jones L; Credo J; Rock T Uranium and arsenic unregulated water issues on Navajo lands. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 2020;38:031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB Effect of pregnancy on levels of urinary metals for females aged 17–39 years old: Data from National Health and Nutrition Examination Survye 2003–2010. Journal of Toxicology and Environmental Health, Part A: Current Issues 2013;76:86–97 [DOI] [PubMed] [Google Scholar]
- Jain RB Trends in and factors affecting the observed levels of urinary inorganic and total blood mercury among US children, adolescents, adults, and senior citizens over 2005–2012. Environmental toxicology and pharmacology 2017;56:268–281 [DOI] [PubMed] [Google Scholar]
- Jarrett JM; Jones RL; Caldwell KL; Verdon CP Total urine arsenic measurements using inductively coupled plasma mass spectrometry with a dynamic reaction cell. Atomic Spectroscopy 2007;28:113 [Google Scholar]
- Jarrett JM; Xiao G; Caldwell KL; Henahan D; Shakirova G; Jones RL Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS,. J Anal At Spectrom 2008;23:962–967 [Google Scholar]
- Jones M; Credo J; Ingram J; Baldwin J; Trotter R Jr; Propper C Arsenic concentrations in ground and surface waters across Arizona including native lands. Journal of Contemporary Water Research & Education 2020;169:44–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR; Choi AL; Oken E; Horvat M; Schoeny R; Kamai E; Cowell W; Grandjean P; Korrick S Evidence on the human health effects of low-level methylmercury exposure. Environmental health perspectives 2012;120:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML; Cardenas A; Rodrigues E; Mazumdar M; Dobson C; Golam M; Quamruzzaman Q; Rahman M; Christiani DC Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a Bangladeshi cohort. Epidemiology (Cambridge, Mass) 2016;27:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum MA; Roberts LN; Biewick LR Geologic Assessment of Coal in the Colorado Plateau: Arizona, Colorado, New Mexico, and Utah: US Geological Survey; 2000 [Google Scholar]
- Kurzius-Spencer M; Burgess JL; Harris RB; Hartz V; Roberge J; Huang S; Hsu C-H; O’Rourke MK Contribution of diet to aggregate arsenic exposures—an analysis across populations. Journal of exposure science & environmental epidemiology 2014;24:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M; O’rourke MK; Hsu C-H; Hartz V; Harris RB; Burgess JL Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. Journal of exposure science & environmental epidemiology 2013;23:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J; Gonzales M; Burnette C; Benally M; Seanez P; Shuey C; Nez H; Nez C; Nez S Environmental exposures to metals in native communities and implications for child development: Basis for the Navajo Birth Cohort Study. Journal of Social Work in Disability & Rehabilitation 2015:1–25 [DOI] [PubMed] [Google Scholar]
- Lewis J; Hoover J; MacKenzie D Mining and Environmental Health Disparities in Native American Communities. Current Environmental Health Reports 2017:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton A; Hussain S; Akter S; Rahman M; Mouly T; Mitchell K A review of the effects of chronic arsenic exposure on adverse pregnancy outcomes. International journal of environmental research and public health 2017;14:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia M; Ballester F; Enning AM; Iñiguez C; Valvi D; Basterrechea M; Rebagliato M; Vioque J; Maruri M; Tardon A Prenatal mercury exposure and birth outcomes. Environmental research 2016;151:11–20 [DOI] [PubMed] [Google Scholar]
- Murphy M; Lewis L; Sabogal RI; Bell C Survey of unregulated drinking water sources on the Navajo Nation. Annual Meeting of the American Public Health Association Philadelphia, PA; 2009 [Google Scholar]
- Nachman KE; Ginsberg GL; Miller MD; Murray CJ; Nigra AE; Pendergrast CB Mitigating dietary arsenic exposure: current status in the United States and recommendations for an improved path forward. Science of the Total Environment 2017;581:221–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A; Francesconi KA; Silbergeld EK; Guallar E Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environmental research 2011;111:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMED. Grants Mineral Belt Uranium Biomonitoring Project Summary. New Mexico Environment Department; 2011 [Google Scholar]
- Olmedo P; Grau-Perez M; Fretts A; Tellez-Plaza M; Gil F; Yeh F; Umans JG; Francesconi KA; Goessler W; Franceschini N Dietary determinants of cadmium exposure in the Strong Heart Family Study. Food and chemical toxicology 2017;100:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman K; Åkesson A; Berglund M; Bremme K; Schütz A; Ask K; Vahter M Toxic and essential elements in placentas of Swedish women. Clinical biochemistry 2000;33:131–138 [DOI] [PubMed] [Google Scholar]
- Quansah R; Armah FA; Essumang DK; Luginaah I; Clarke E; Marfoh K; Cobbina SJ; Nketiah-Amponsah E; Namujju PB; Obiri S Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environmental health perspectives 2015;123:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood D; Lanier AP; Renner C; Smith J; Tom-Orme L; ML S. Differences in cigarette and smokeless tobacco use among American Indian and Alaska Native people living in Alaska and the Southwest United States. Nicotine & Tobacco Research 2010;12:791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter PA; Bishop EE; Wang J; Swahn MH Tobacco smoke exposure and levels of urinary metals in the US youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999–2004. International journal of environmental research and public health 2009;6:1930–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosofsky A; Janulewicz P; Thayer KA; McClean M; Wise LA; Calafat AM; Mikkelsen EM; Taylor KW; Hatch EE Exposure to multiple chemicals in a cohort of reproductive-aged Danish women. Environmental research 2017;154:73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge CV; Röllin HB; Nogueira CM; Thomassen Y; Rudge MC; Odland JØ The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. Journal of Environmental Monitoring 2009;11:1322–1330 [DOI] [PubMed] [Google Scholar]
- Samuel-Nakamura C Uranium and other heavy metals in the plant-animal-human food chain near abandoned mining sites and structures in an American Indian community in northwestern New Mexico. School of Nursing: UCLA; 2013 [Google Scholar]
- Samuel-Nakamura C; Hodge FS Occurrence and Risk of Metal (loid) s in Thelesperma megapotamicum Tea Plant. Plants 2020;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP; Henn BC; Wright RO Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature. Current environmental health reports 2015;2:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS; Blum JD; Franzblau A; Basu N New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ Sci Technol 2013;47:3403–3409 [DOI] [PubMed] [Google Scholar]
- Shields LM; Wiese W; Skipper B; Charley B; Banally L Navajo birth outcomes in the Shiprock uranium mining area. Health Physics 1992;63:542–551 [DOI] [PubMed] [Google Scholar]
- Slattery ML; Ferucci ED; Murtaugh MA; Edwards S; Ma K-N; Etzel RA; Tom-Orme L; Lanier AP Associations among body mass index, waist circumference, and health indicators in American Indian and Alaska Native adults. American Journal of Health Promotion 2010;24:246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A Whole blood manganese levels in pregnancy and the neonate. Nutrition 1999;15:731–734 [DOI] [PubMed] [Google Scholar]
- Tellez-Plaza M; Navas-Acien A; Caldwell KL; Menke A; Muntner P; Guallar E Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environmental health perspectives 2012;120:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann M Safe drinking water act (SDWA): A summary of the act and its major requirements. Washington, DC: Congressional Research Service; 2010 [Google Scholar]
- Tyrrell J; Melzer D; Henley W; Galloway TS; Osborne NJ Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001–2010. Environment international 2013;59:328–335 [DOI] [PubMed] [Google Scholar]
- US EPA. Abandoned Uranium Mines (AUM) on the Navajo Nation. San Francisco, CA: US EPA: Region 9; 2006 [Google Scholar]
- USEPA. 2011 NATA: Assessment Results. US Environmental Protection Agency; 2011 [Google Scholar]
- Vahter M Effects of arsenic on maternal and fetal health. Annual review of nutrition 2009;29:381–399 [DOI] [PubMed] [Google Scholar]
- Wang H; Lfti J; Zhang X; Zhu P; Hao J-H; Tao F-B; Xu D-X Maternal serum arsenic level during pregnancy is positively associated with adverse pregnant outcomes in a Chinese population. Toxicology and applied pharmacology 2018:114–119 [DOI] [PubMed] [Google Scholar]
- Wang J; Gao Z-Y; Yan J; Ying X-L; Tong S-L; Yan C-H Sex differences in the effects of prenatal lead exposure on birth outcomes. Environmental Pollution 2017;225:193–200 [DOI] [PubMed] [Google Scholar]
- Xia W; Du X; Zheng T; Zhang B; Li Y; Bassig BA; Zhou A; Wang Y; Xiong C; Li Z; Yao Y; Hu J; Zhou Y; Liu J; Xue W; Ma Y; Pan X; Peng Y; Xu S A Case-Control Study of Prenatal Thallium Exposure and Low Birth Weight in China. Environ Health Perspect 2016a;124:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W; Zhou Y; Zheng T; Zhang B; Bassig BA; Li Y; Wise JP; Zhou A; Wan Y; Wang Y Maternal urinary manganese and risk of low birth weight: a case–control study. BMC public health 2016b;16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X; Ding G; Cui C; Chen L; Gao Y; Zhou Y; Shi R; Tian Y The effects of low-level prenatal lead exposure on birth outcomes. Environmental pollution 2013;175:30–34 [DOI] [PubMed] [Google Scholar]
- Yang K; Julan L; Rubio F; Sharma A; Guan H Cadmium reduces 11β-hydroxysteroid dehydrogenase type 2 activity and expression in human placental trophoblast cells. American Journal of Physiology-Endocrinology and Metabolism 2006;290:E135–E142 [DOI] [PubMed] [Google Scholar]
- Zhang B; Xia W; Li Y; Bassig BA; Zhou A; Wang Y; Li Z; Yao Y; Hu J; Du X; Zhou Y; Liu J; Xue W; Ma Y; Pan X; Peng Y; Zheng T; Xu S Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case–control study in China. Reproductive Toxicology 2015;57:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychowski KE; Kodali V; Harmon M; Tyler C; Sanchez B; Ordonez Suarez Y; Herbert G; Wheeler A; Avasarala S; Cerrato JM Respirable Uranyl-Vanadate Containing Particulate Matter Derived from a Legacy Uranium Mine Site Exhibits Potentiated Cardiopulmonary Toxicity. Toxicological Sciences 2018;164:101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]