Abstract
The Met allele of the COMT Val158Met polymorphism slows metabolism and increases bioavailability of dopamine (DA) in the prefrontal cortex compared to the Val allele. Healthy Met-carriers outperform Val-carriers on executive function (EF) tests, yet this ‘advantage’ disappears in methamphetamine (METH) dependence. Met-carriers may be disproportionately vulnerable to METH-related perturbations of DA, yet it is unknown whether COMT modulates METH effects on CSF DA biomarkers. Participants were 75 METH+ and 47 METH- men who underwent neurocognitive testing, COMT genotyping, and lumbar puncture. CSF was assayed for DA and its metabolite, homovanillic acid (HVA). Separate linear models regressed DA, HVA, and HVA/DA ratios on COMT, METH and their interaction. Pearson correlations examined associations between DA and EF. Significant interactions indicated that METH+ had lower DA and higher HVA/DA ratios among Met/Met, but not Val/Met or Val/Val. Met/Met exhibited the highest DA levels among METH-, whereas DA levels were comparable between Met/Met and Val-carriers among METH+. Higher DA correlated with better EF in METH- Met/Met, but did not predict EF in the entire sample. DA was expectedly higher in METH- Met/Met, yet a discordant genotype-phenotype profile emerged in METH+ Met/Met, consistent with the notion that slow DA clearance exacerbates METH-associated DA dysregulation.
Keywords: dopamine, homovanillic acid, catechol-o-methyltransferase, methamphetamine, executive function, prefrontal cortex
1. Introduction
Repeated, heavy exposure to methamphetamine (METH) is associated with central nervous system (CNS) dysfunction across multiple neurotransmitter systems, including dopamine (DA), serotonin, GABA, and glutamate (Halpin et al., 2014). METH exerts particularly potent effects on the dopaminergic (DAergic) system by acting on the vesicular monoamine transporter (VMAT-2) and DA transporter (DAT) to stimulate release of DA from presynaptic vesicles and inhibit its reuptake, resulting in excessive levels of synaptic DA (Davidson et al., 2001). DA is highly active in frontostriatal pathways and DAergic excess disrupts frontal cortical circuitry that regulates motivation, self-control, decision-making and executive function, thereby perpetuating the cycle of addiction (Volkow et al., 2002). METH-dependence is linked to negative neurocognitive outcomes in domains supported by frontostriatal structures, including learning, memory, executive function, attention/working memory, and cognitive control (Salo et al., 2009; Scott et al., 2007). Cross-sectional brain imaging studies of METH exposure demonstrate lower gray matter volumes and greater white matter abnormalities in frontostriatal and limbic regions such as the striatum, amygdala, hippocampus, and the prefrontal cortex (PFC; Berman et al., 2008; Salo and Fassbender, 2011). Positron emission tomography (PET) studies, which have enabled in vivo and regionally-specific evaluation of DAergic activity, provide evidence of DAergic dysfunction in METH users across a number of molecular markers (e.g., DAT, D2 receptor availability, and VMAT-2) in both the striatum and PFC (Ashok et al., 2017; Sekine et al., 2001; Volkow et al., 2001a). Notably, this DAergic dysregulation correlates with markers of neurobehavioral dysfunction, including psychomotor and memory impairment, impulsivity, and psychiatric distress (Lee et al., 2009; Sekine et al., 2001; Sekine et al., 2003; Volkow et al., 2001b).
While adverse neurocognitive findings are frequently observed in METH users, METH use is not always associated with neurocognitive impairment and the reasons for this heterogeneity are unknown (Dean et al., 2013). Therefore, a major area of research interest lies in determining what factors may attenuate or exacerbate risk for METH-related CNS dysfunction and associated neurocognitive deficits. Thus far, investigations of a dose-dependent relationship with greater METH exposure leading to more severe neurocognitive deficits have resulted in null findings (Dean et al., 2013). Self-reported duration of METH use, frequency of use, length of abstinence, and cumulative lifetime exposure do not predict neurocognitive performance (Cherner et al., 2010b; Johanson et al., 2006). While parameters of drug exposure show little predictive value, individual differences in genetic and environmental factors may account for considerable variability in risk for METH-related neurocognitive deficits (Cherner et al., 2010a; Compton et al., 2005; Volkow, 2005). Identifying genetic variations that influence an individual’s vulnerability to METH effects can inform personalized approaches to mitigate METH-related neurocognitive impairment.
The catechol-O-methyltransferase (COMT) enzyme is implicated in DA neurotransmission in the PFC, and specifically assists in regulating clearance of DA from the synapse via metabolic degradation (Li et al., 2004; Meyer-Lindenberg et al., 2006). Functional variation in the COMT gene occurs at a single nucleotide polymorphism (SNP) resulting in a valine (Val) to methionine (Met) amino acid substitution (Val158Met). Compared to the Val allele, the Met allele is associated with reduced thermostability and enzymatic activity, leading to slower degradation of DA at the synapse and higher DA concentration in the PFC (Egan et al., 2001; Palmatier et al., 1999). In contrast, the Val allele is associated with more efficient DA catabolism and lower levels of synaptic DA. In healthy adults, Met-carriers outperform Val-carriers on tests of PFC-dependent neurocognition, including executive function and working memory (Barnett et al., 2007; Starr et al., 2007; Wishart et al., 2011). It is hypothesized that higher DA bioavailability conferred by the Met allele underlies this Met-associated neurocognitive ‘advantage’ in healthy adults. However, we have previously demonstrated that in chronic METH users (both HIV-seropositive and HIV-seronegative), whose PFC is repeatedly exposed to excessive levels of DA, the Met/Met genotype is no longer associated with better executive function and may in fact confer risk for executive dysfunction (Bousman et al., 2010; Cherner et al., 2019).
Although these findings suggest that the Met/Met genotype confers disproportionate risk to METH-related neurocognitive dysfunction, it remains unclear whether COMT genotype similarly modulates the effects of METH on biomarkers that directly measure the DAergic system. Thus, the present study evaluated cerebrospinal fluid (CSF) levels of DA and its metabolite, homovanillic acid (HVA), among a cohort of adult men stratified by METH-dependence and COMT genotype. Additionally, relationships between CSF DA and executive function were assessed to examine the role of CSF DA as an intermediary linking the interactive effects of METH and COMT on neurocognitive function.
2. Methods
2.1. Participants
Participants were 75 METH-dependent (METH+) and 47 METH-non-using comparison (METH-) men enrolled in the University of California, San Diego’s (UCSD) Translational Methamphetamine AIDS Research Center (TMARC), a NIDA-funded cohort study focusing on the CNS effects of HIV and METH. All participants gave written informed consent as approved by the UCSD Institutional Review Board. COMT genotyping in the parent study was restricted to male participants due to potential sexually-dimorphic effects of COMT (Tunbridge and Harrison, 2011) and inadequate numbers of female participants to support sex-stratified analyses. Exclusion criteria were: 1) DSM-IV diagnosis of other substance use dependence (except cannabis) within the last 5 years, or alcohol dependence within the last 12 months; 2) abuse of any substances (except alcohol and cannabis) other than METH within the last 12 months; 3) evidence of very recent METH use by positive urine toxicology results; 4) history of psychotic or mood disorder with psychotic features, neurological, or medical condition that may confound neuropsychological test results.
2.2. Neuropsychiatric Assessment
Participants were evaluated for METH dependence, other substance use dependence, and Major Depressive Disorder (MDD) diagnoses using the Composite International Diagnostic Interview (CIDI; World Health Organization, 1998) or Structured Clinical Interview for DSM-IV (SCID-IV; Spitzer et al., 1995), as study methodology was developed prior to the release of the DSM-5. Participants were classified as METH+ if they met DSM-IV diagnostic criteria for lifetime METH dependence and met criteria for METH dependence or abuse within the past 18 months. Lifetime METH use parameters were assessed using a timeline follow-back interview. Current depressive symptoms were measured using the Beck Depression Inventory version two (BDI-II; Beck et al., 1996).
2.3. COMT Genotyping
All participants were genotyped for COMT Val158Met by standard procedures. DNA for genotyping was isolated from stored whole blood or peripheral blood mononuclear cells (PBMCs) using the Qiagen QIAamp DNA Mini Kit (Qiagen, Valencia, CA). COMT Val158Met (rs4680) SNP was assayed using an array that included SNPs within catecholaminergic and other addiction-relevant genes (Hodgkinson et al., 2008).
COMT distribution across the 122 study participants was 27 (22.1%) Met/Met, 62 (50.8%) Val/Met, and 33 (27.1%) Val/Val, resulting in the following six groups: METH- Met/Met (n=12), METH- Val/Met (n=23), METH- Val/Val (n=12), METH+ Met/Met (n=15), METH+ Val/Met (n=39), and METH+ Val/Val (n=21). COMT distribution was consistent with Hardy-Weinberg equilibrium in the full sample (χ2=0.04, p=0.84) and within each METH group (METH-: χ2 = 0.02, p = 0.88; METH+: χ2 = 0.16, p = 0.69).
2.4. Dopamine Biomarker Assays
CSF DA biomarker assays were performed using high-performance liquid chromatography (HPLC), as described in detail by Kumar et al. (Kumar et al., 2009). HPLC is a reliable method for quantifying DA and HVA concentrations in CSF; Kumar et al. reported an intra-assay coefficient of variance (% CV) of 5.4% and 10.9% for DA and HVA, respectively, as well as an interassay % CV of 7.1% and 11.5% for DA and HVA, respectively (Kumar et al., 2009). DA levels were expressed as pg/ml and HVA levels were expressed as ng/ml. HVA/DA ratios were examined as a proxy for the rate of metabolism of DA into HVA, with higher ratios suggesting faster metabolism. DA, HVA, and HVA/DA ratios were log-transformed to reduce skewness and allow for parametric analysis.
2.5. Neurocognitive Assessment
Participants completed a comprehensive, validated neurocognitive battery covering ability domains commonly impacted by METH use and HIV (Rippeth et al., 2004). Raw test scores were converted to demographically-corrected standard T scores (mean of 50 and standard deviation of 10) that adjusted for the effects of age, education, sex and race/ethnicity, as appropriate (Heaton et al., 2004; Heaton et al., 2003; Norman et al., 2011). These demographically-corrected individual test T scores were averaged within each neurocognitive ability domain to generate domain-specific T scores. Given the relevance of COMT to DAergic activity in the PFC (Meyer-Lindenberg et al., 2006) and our prior findings that COMT interacts with METH status to predict executive function, secondary analyses focused on the relationship between CSF DA and executive function T scores (Cherner et al., 2019). The executive function composite was composed of the Wisconsin Card Sorting Test 64-item-computerized version, Stroop Color-Word Test, and Trail Making Test Part B.
2.6. Neuromedical Assessment
All participants underwent a comprehensive neuromedical assessment, blood draw, and lumbar puncture. HIV disease was diagnosed by enzyme-linked immunosorbent assay with Western blot confirmation. Among HIV+ participants, HIV viral load in plasma was measured using reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN) and deemed undetectable at a lower limit of quantitation (LLQ) of 50 copies/ml. Hepatitis C virus (HCV) serostatus was diagnosed by standard clinical antibody detection.
2.7. Statistical Analysis
METH and COMT group differences on background characteristics (i.e., demographics, neuropsychiatric and medical characteristics) were examined using analysis of variance (ANOVA), Wilcoxon/Kruskal-Wallis tests, and Chi-square statistics as appropriate. First, univariable analyses were conducted to examine the main effects of METH status (METH+ vs. METH-) and COMT genotype (Met/Met vs. Val/Met vs. Val/Val) on CSF DA biomarkers (i.e., CSF DA, HVA, and the HVA/DA ratio). Next, separate multivariable linear regression analyses modelled CSF DA biomarkers as a function of COMT, METH status, and their interaction. COMT was dummy coded with the low enzymatic activity Met/Met genotype as the reference group. In order to probe models with significant interaction terms, we conducted follow-up analyses stratified by COMT genotype and separately, stratified by METH group. Cohen’s d statistics are presented for estimates of effect size for pairwise comparisons. To determine if the interactive effects of COMT and METH on DA biomarkers were attenuated by covariates, backward model selection guided by Akaike information criteria was applied such that final models considered covariates that differed by COMT or METH status at p-value < 0.10 (i.e., race/ethnicity, BDI-II, HCV serostatus, and lifetime substance use disorders [alcohol, cannabis, and cocaine]). HIV serostatus, age, antidepressant use, and time of lumbar puncture were also considered as covariates given their potential to influence DA levels. Last, group-stratified Pearson’s r correlations explored the relationship between CSF DA levels and executive function domain T scores. Given constraints in statistical power due to small METH × COMT cell size, analyses were not adjusted for multiple comparisons and are considered preliminary. All analyses were conducted using JMP Pro version 14.0.0 (JMP®, Version <12.0.1>. SAS Institute Inc., Cary, NC, 2018)
3. Results
3.1. Participant Characteristics
The full study sample was 66% non-Hispanic White with a mean age of 39.8 years (range: 19–57) and mean education of 13.0 years. Participant characteristics by METH group are presented in Table 1. METH+ participants reported higher BDI-II scores and were more likely to have lifetime non-METH substance use disorders (i.e., alcohol, cannabis, and cocaine) and HCV. Rates of HIV seropositivity did not differ between METH+ (n=30; 64%) and METH- (n=50; 67%) groups; however, HIV-seropositive METH+ individuals had higher rates of detectable virus in plasma than HIV-seropositive METH- individuals (63% vs. 37%). METH groups were comparable on all other HIV disease and treatment characteristics as well as demographic backgrounds. COMT groups were also comparable across demographic, neuropsychiatric, medical, and HIV characteristics, with the exception of race/ethnicity such that the prevalence of non-Hispanic White participants increased with each copy of the Met allele (χ2=16.0, p<.001; Val/Val [45%] vs. Val/Met [66%] vs. Met/Met [92%]).
Table 1.
Demographic and clinical characteristics by methamphetamine (METH) group
| METH− | METH+ | ||
|---|---|---|---|
| Variable | (n=47) | (n=75) | P |
| Demographics | |||
| Age (years), mean (SD) | 40.0 (10.04) | 39.8 (7.44) | 0.914 |
| Education (years), mean (SD) | 13.0 (1.92) | 12.9 (2.16) | 0.759 |
| Estimated premorbid verbal IQ, mean (SD) | 99.4 (12.00) | 97.2 (12.52) | 0.355 |
| Race/Ethnicity Non-Hispanic White, n (%) | 28 (59.6%) | 52 (68.4%) | 0.395 |
| Hispanic, n (%) | 8 (17.4%) | 12 (16.0%) | |
| Black, n (%) | 10 (21.7%) | 8 (10.7%) | |
| Other, n (%) | 1 (2.2%) | 2 (2.7%) | |
| Neuropsychiatric Characteristics | |||
| Lifetime Major Depressive Disorder, n (%)a | 25 (54.4%) | 36 (52.9%) | 0.883 |
| BDI-II score, median [IQR] | 7 [2, 13] | 15 [8, 21] | <0.001 |
| Antidepressant use, n (%) | 16 (34.0%) | 28 (37.3%) | 0.712 |
| Lifetime alcohol use disorder, n (%)a | 24 (52.2%) | 52 (76.5%) | 0.007 |
| Current alcohol use disorder, n (%)b | 2 (4.4%) | 0 (0.0%) | 0.158 |
| Lifetime cannabis use disorder, n (%)a | 14 (30.4%) | 36 (52.9%) | 0.017 |
| Current cannabis use disorder, n (%)b | 1 (2.2%) | 1 (1.5%) | 0.773 |
| Lifetime cocaine use disorder, n (%)a | 6 (13.0%) | 27 (39.7%) | 0.001 |
| Medical Characteristics | |||
| Hepatitis C virus seropositive, n (%) | 5 (10.6%) | 24 (32.0%) | 0.031 |
| HIV seropositive, n (%) | 30 (63.8%) | 50 (66.7%) | 0.825 |
| AIDS diagnosis, n (%) | 17 (56.7%) | 24 (48.0%) | 0.452 |
| Duration of HIV infection (years), median [IQR] | 9.1 [1.5, 12.0] | 6.9 [2.9, 12.7] | 0.824 |
| Current CD4 count (cells/mm3), median [IQR] | 470 [349, 653] | 401 [297, 675] | 0.228 |
| Nadir CD4 count (cells/mm3), median [IQR] | 201 [56, 324] | 218 [58, 381] | 0.676 |
| Detectable plasma viral load, n (%) | 11 (36.7%) | 30 (62.5%) | 0.026 |
| On ART, n (%) | 21 (72.4%) | 33 (66.0%) | 0.552 |
| Methamphetamine Use Parameters | |||
| Lifetime days of use | - | 1503 [669, 3611] | - |
| Lifetime grams consumed | - | 1357 [408, 2825] | - |
| Lifetime average daily use (grams/day) | - | 0.77 [0.38, 1.25] | - |
| Days since last use | - | 61 [16, 122] | - |
| Age of first use | - | 23.3 (7.62) | - |
ART= antiretroviral therapy; BDI-II= Beck Depression Inventory- II;
N = 117;
N = 115
3.2. METH, COMT, and Dopamine Biomarkers
CSF DA ranged from 0.2 to 256.1 pg/mL (median 34.0, interquartile range [IQR] 9.3–65.5) and CSF HVA ranged from 0.7 to 158.1 ng/mL (median 10.3, IQR 4.3–22.7). Table 2 presents estimates for the multivariable linear regression modelling CSF DA, HVA, and HVA/DA ratios as a function of METH, COMT, and their interaction. Univariably, CSF DA levels did not significantly differ by METH (t=0.14, p=.885) or COMT (F=1.96, p=.145). However, results indicated a significant interaction such that the effect of METH on DA significantly differed between Met/Met and Val/Met groups (METH × Val/Met (vs. Met/Met): p=.022). Figure 1 displays the results of the follow-up analyses whereby DA levels were significantly lower in METH+ individuals compared to METH- individuals only within the Met/Met group (d=−0.77, p=.050). DA levels did not significantly differ between METH+ and METH- individuals for the Val/Met and Val/Val groups (ps>.220). Among the METH- group, Met/Met individuals had significantly higher DA levels compared to Val/Val (d=0.82, p=.046) and trended toward higher DA levels compared to Val/Met individuals (d = 0.62, p = .082). Among the METH+ group, Val/Met individuals trended toward higher DA levels compared to Val/Val (d=0.53, p=.051).
Table 2.
Results of multiple linear regressions examining the interaction of methamphetamine (METH) status and COMT genotype on dopamine (DA), homovanillic acid (HVA) and HVA/DA ratios
| Outcome: CSF DA (log) | beta (SE) | 95% CI | p |
|---|---|---|---|
| METH+ (vs. METH−)a | −1.10 (0.55) | −2.20, 0.00 | 0.050 |
| Val/Met (vs. Met/Met)b | −0.89 (0.51) | −1.90, 0.12 | 0.082 |
| Val/Val (vs. Met/Met)b | −1.18 (0.58) | −2.34, −0.02 | 0.046 |
| METH+ x Val /Met | 1.56 (0.67) | 0.24, 2.89 | 0.021 |
| METH+ x Val/ Val | 1.09 (0.76) | −0.42, 2.59 | 0.154 |
| Outcome: CSF HVA (log) | beta (SE) | 95% CI | p |
| METH+ (vs. METH−)a | 0.13 (0.47) | −0.81, 1.07 | 0.778 |
| Val/Met (vs. Met/Met)b | −0.46 (0.44) | −1.33, 0.40 | 0.291 |
| Val/Val (vs. Met/Met)b | −0.35 (0.50) | −1.34, 0.64 | 0.482 |
| METH+ x Val /Met | −0.06 (0.57) | −1.19, 1.08 | 0.918 |
| METH+ x Val/ Val | −0.59 (0.65) | −1.88, 0.70 | 0.366 |
| Outcome: CSF HVA/DA (log) | beta (SE) | 95% CI | p |
| METH+ (vs. METH−)a | 1.24 (0.54) | 0.16, 2.32 | 0.025 |
| Val/Met (vs. Met/Met)b | 0.43 (0.50) | −0.56, 1.42 | 0.391 |
| Val/Val (vs. Met/Met)b | 0.83 (0.57) | −0.31, 1.97 | 0.152 |
| METH+ x Val /Met | −1.62 (0.66) | −2.93, −0.32 | 0.015 |
| METH+ x Val/ Val | −1.68 (0.75) | −3.15, −0.20 | 0.026 |
Represents effect of METH in Met/Met individuals only (reference group)
Represents effect of COMT in METH- individuals only (reference group)
Figure 1. METH+ individuals have lower CSF dopamine levels than METH- individuals only among COMT Met/Met genotype carriers.
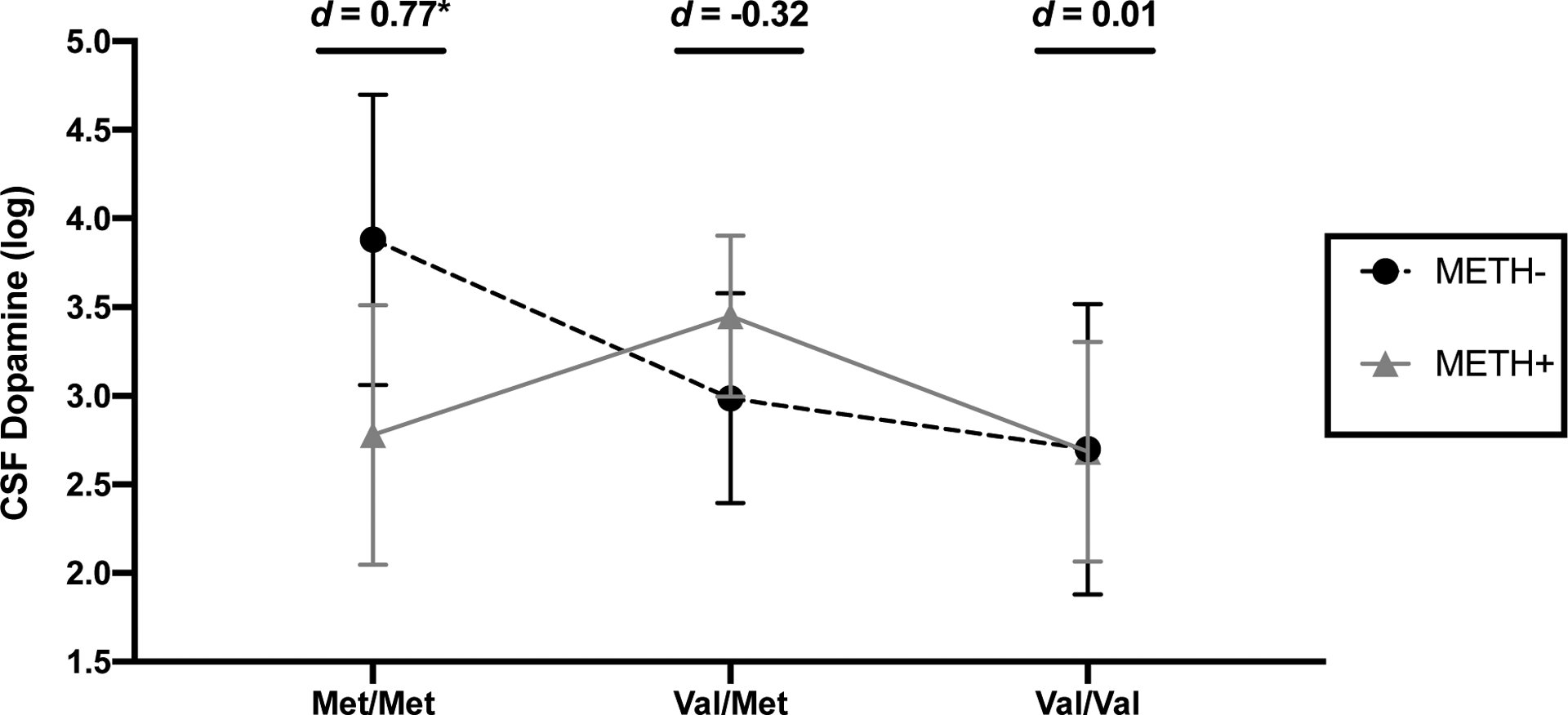
*p<.05. Error bars represent standard errors. Cohen’s d estimates reflect METH+ vs.METH- group differences within each COMT genotype.
Univariably, a trend-level omnibus effect of COMT on CSF HVA was detected (F=2.67, p=.073) such that Met/Met individuals had significantly higher HVA levels compared to Val/Val (d=0.59, p=.025) and trended toward higher HVA levels compared to Val/Met individuals (d=0.40, p=.083). HVA comparisons between Val/Met and Val/Val individuals as well as between METH groups did not reach statistical significance (ps>.390). Moreover, no significant interactive effects of COMT and METH were detected for HVA (ps>.290).
Univariably, CSF HVA/DA ratios did not significantly differ by METH (t=0.17, p=.868) or COMT (F=1.41, p=.249). However, results indicated significant interactions such that the effect of METH significantly differed in Met/Met individuals compared to both Val/Met (METH x Val/Met [vs. Met/Met]: p=.015) and Val/Val individuals (METH × Val/Val [vs. Met/Met]: p=.026). Follow-up analyses indicated that within the Met/Met group, HVA/DA ratios were significantly higher in METH+ individuals compared to METH- individuals (d=0.88, p=.025; Figure 2). Conversely, HVA/DA ratios did not significantly differ between METH+ and METH- individuals in the Val/Met and Val/Val groups (ps>.299). Among the METH- group, a stair-step pattern emerged in which Val/Val displayed the highest HVA/DA ratios followed by Val/Met then Met/Met (d range −0.59 to −0.28); however, no individual pairwise comparisons reached statistical significance (ps>.151). Among the METH+ group, Met/Met individuals displayed significantly higher HVA/DA ratios compared to Val/Met individuals (d=0.85, p=.006) and trended toward higher HVA/DA ratios compared to Val/Val individuals (d=0.60, p=.077).
Figure 2. METH+ individuals have higher CSF homovanillic acid (HVA)/dopamine ratios than METH- individuals only among COMT Met/Met genotype carriers.
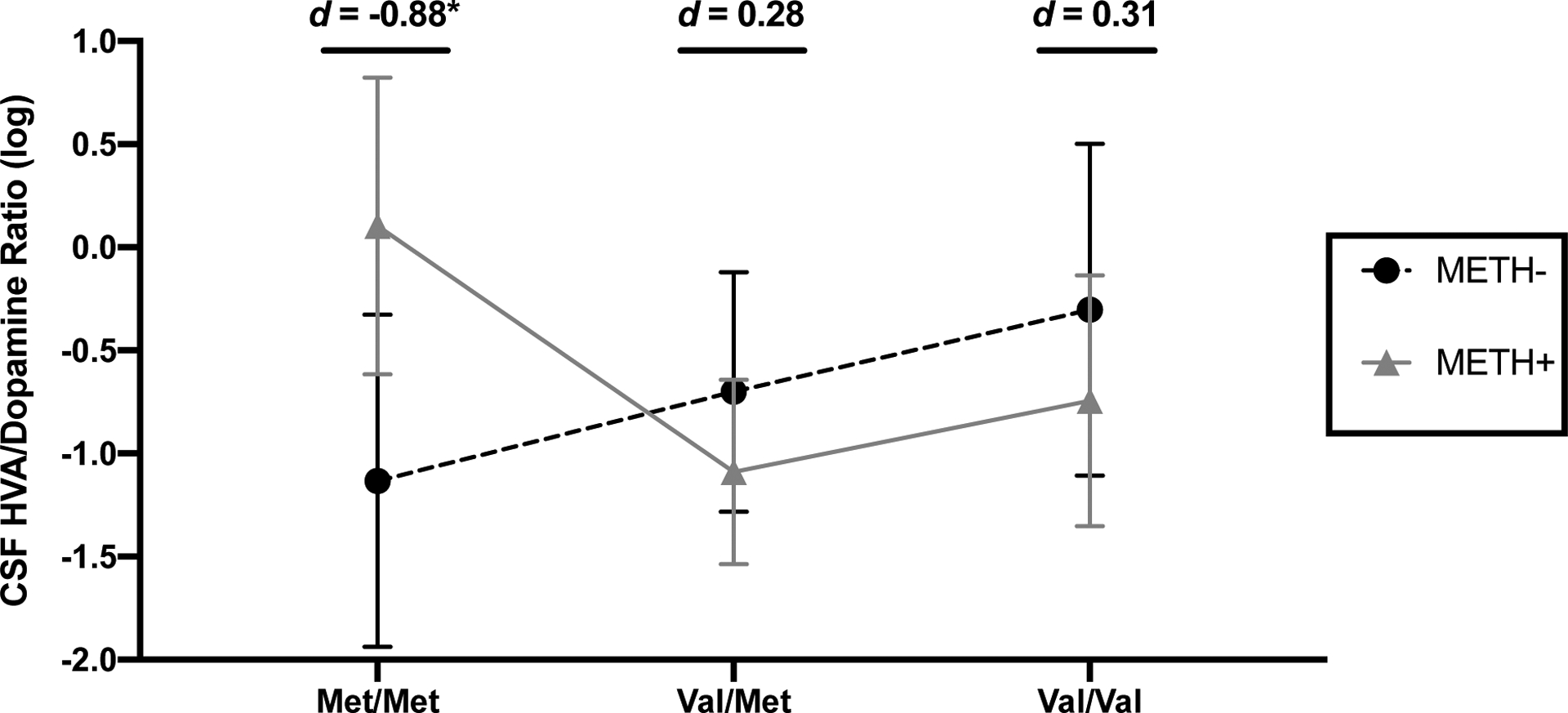
*p<.05. Error bars represent standard errors. Cohen’s d estimates reflect METH+ vs. METH- group differences within each COMT genotype.
Additional models employing backward selection of covariates were conducted to determine whether the interactive effects of COMT and METH on DA and HVA/DA ratios were better explained by clinical and demographic factors (i.e., age, race/ethnicity, HIV, HCV, BDI-II, lifetime substance use disorders [alcohol, cannabis, and cocaine], and time of lumbar puncture). For both DA and HVA/DA ratios, time of lumbar puncture was the only covariate that improved overall model fit and was therefore retained in regression models. Later time of day of lumbar puncture was significantly associated with lower DA (b=−5.23, p=.006) and higher HVA/DA ratios (b=3.68, p =.046). However, the inclusion of time of lumbar puncture did not attenuate the interactive effects of COMT and METH as all statistically significant terms from Table 2 remained statistically significant in these adjusted models.
3.3. Dopamine and Executive Function
Exploratory Pearson’s r correlations examined the relationship between CSF DA and executive function in the entire sample and within each METH × COMT group. DA was not significantly related to executive function in the entire study sample (r=.12, p=.20; Figure 3A), or within groups consisting of Val-allele carriers or METH+ individuals (rs<.24, ps>.26). However, among the METH- Met/Met group, higher DA levels were significantly correlated with higher (better) executive function (r=.70 [95% CI: .16 to .91], p=.026; Figure 3B).
Figure 3. CSF dopamine correlates with executive function only in METH- Met/Met.
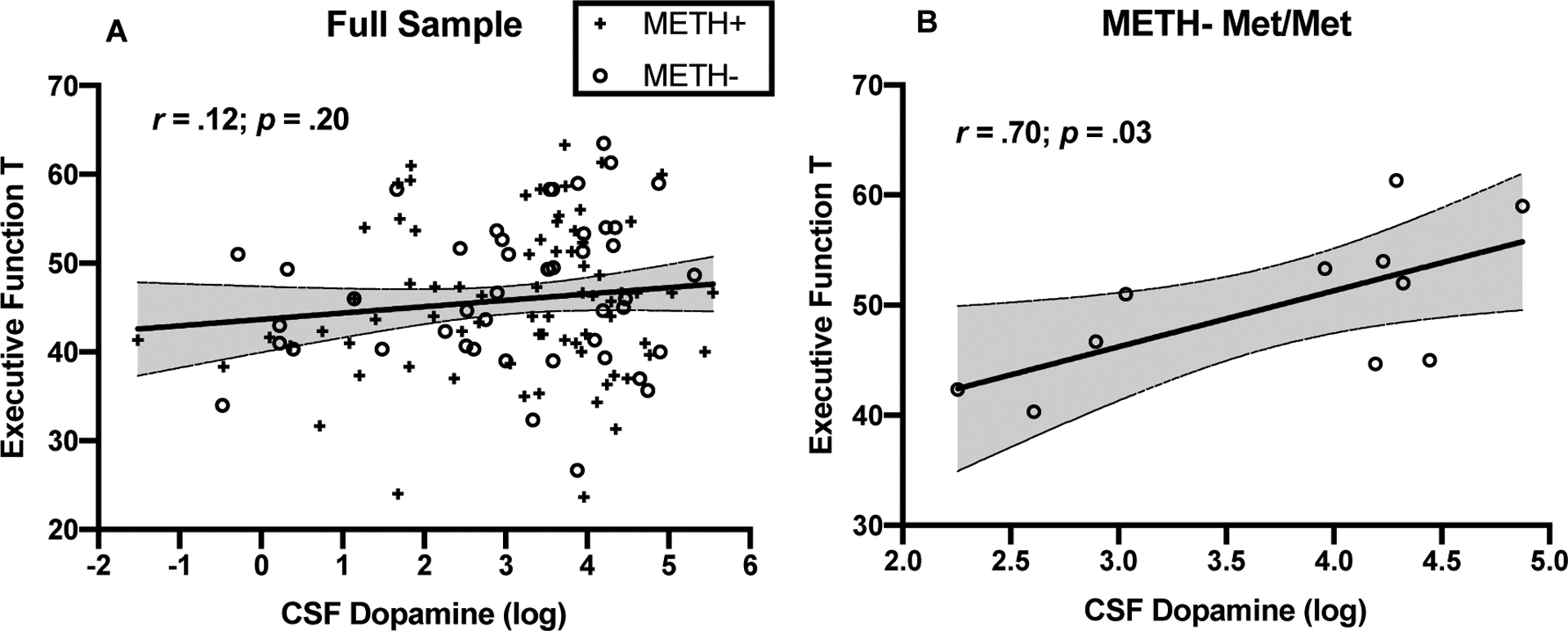
A) In the entire study sample, CSF dopamine levels do not significantly correlate with executive function T scores (r=.12, p=.20); B) Within the METH- Met/Met group, which had on average the highest levels of CSF dopamine, higher CSF dopamine significantly correlated with higher executive function T scores (r=.70, p=.03).
4. Discussion
METH-dependence and COMT are known to alter DAergic pathways and PFC function. To our knowledge, the present study is among the first to examine how the interaction of these genetic and environmental factors impacts levels of DA and its metabolite, HVA, in the CNS. METH was associated with lower DA levels, accompanied by higher HVA/DA ratios, only among Met/Met individuals, suggesting that COMT genotype may underlie inter-individual differences in vulnerability to METH effects on DAergic tone. Among METH- individuals, Met/Met genotype exhibited significant (vs. Val/Val) or trend-level (vs. Val/Met) medium-to-large effects on higher DA levels and small-to-medium effects (statistically non-significant) on lower HVA/DA ratios (proxy for slower DA metabolism), consistent with the known functional effects of the COMT enzyme (Chen et al., 2004; Slifstein et al., 2008). However, this Met/Met-related DAergic ‘advantage’ disappeared in METH+ individuals, which parallels our prior findings demonstrating the absence of an executive function ‘advantage’ in METH+ Met/Met individuals (Bousman et al., 2010; Cherner et al., 2019). Notably, the strongest associations between DA and executive function occurred within METH- Met/Met individuals, who, on average, exhibited the highest levels of DA. Our findings highlight the conditional influence of COMT on neurobiological and behavioral markers of PFC function in METH use, particularly in the context of HIV, with potential relevance to other neuropsychiatric conditions characterized by DA and PFC dysfunction.
The discordant COMT genotype/DA endophenotype profile in METH+ individuals suggests that genetically-driven metabolism of synaptic DA can alter the extent to which chronic METH exposure disrupts DAergic activity. Preclinical data demonstrate that the repeated overstimulation of DA release into the synaptic cleft due to serial METH exposure results in the auto-oxidation of DA and subsequent production of reactive oxygen species that are toxic to monoaminergic terminals in the PFC, striatum, and hippocampus (McDonnell-Dowling and Kelly, 2017; Moszczynska and Callan, 2017). In an examination of DA biomarkers in post-mortem brain tissues, Kish et al. found 50–61% reductions in striatal DA levels in chronic METH users compared to age-matched controls, yet did not observe any group differences in brain concentrations of DA metabolites, including HVA (Kish et al., 2017). The preservation of DA metabolites in the context of low DA suggests that although complete DA neuronal death is unlikely in METH use, compared to the severe loss of DA neurons in Parkinson’s disease, other mechanisms such as compromised storage of vesicular DA may explain why METH reduces DA but not HVA levels (Kish et al., 2017; Pifl et al., 2014). Although we did not observe a main effect of METH on DA biomarkers, these post-mortem results agree with our findings in METH+ Met/Met individuals, who exhibited substantially lower CSF DA and higher CSF HVA/DA ratios compared to METH- Met/Met individuals.
Our results extend our previous finding that the typically-observed positive effect of the Met allele on executive function is absent among METH users (Cherner et al., 2019). Specifically, COMT and METH use related to CSF DA levels, which in turn related to executive function. As such, this study helps to bridge the gap in the numerous neuropsychological studies that used COMT as a proxy for DA levels, which may be particularly complex in medical and neuropsychiatric conditions associated with DA dysregulation such as HIV. Interestingly, it was only in the group with the highest levels of DA, the METH- Met/Met group, that we observed a strong association between higher DA levels and better executive function. Associations between DA levels and cortical function are typically interpreted within the framework of the inverted-U hypothesis. DA levels at the peak of the curve (middle of curve) are optimal for PFC-dependent neurocognition, whereas DA levels that are supraoptimal (right side of curve) or suboptimal (left side of curve) lead to poorer performance on these tasks (Cai and Arnsten, 1997; Cools and D’Esposito, 2011; Mattay et al., 2003; Zahrt et al., 1997). Our findings are in line with previous findings in the general population that the Met/Met genotype and resulting higher levels of DA are optimal for PFC-dependent neurocognition and, even within this genotype group, higher DA levels are advantageous for PFC-dependent neurocognition. However, lower DA levels that result from the Val allele in METH- individuals and from stimulant-induced injury in METH+ individuals, may be too far left of the curve to detect neurocognitive benefits of higher DA. Taken together, this pattern of results may suggest a threshold effect whereby the neurocognitive benefits of DA are only observed when DA levels reach a certain level and DA levels in METH users fall below this threshold.
It is important to consider our results and their interpretation in the context of our sample, which partially consisted of individuals with comorbid conditions that can impact the DAergic system (e.g., HIV disease). The majority of our sample was HIV+ (66%). HIV is known to cause DA dysregulation though the release of neurotoxic viral proteins on DA neurons (Bennett et al., 1995; Itoh et al., 2000), and this DA dysregulation is associated with neurocognitive deficits (Kumar et al., 2011). Thus, METH use can be particularly detrimental to brain and neurocognitive health in HIV+ individuals due to the compounding effects of METH and HIV on the DAergic system and the related mechanisms of oxidative stress, neuroinflammation, and blood brain barrier permeability (Kumar et al., 2011; Silverstein et al., 2011). Consistent with our own findings in METH- individuals without HIV disease (Cherner et al., 2019), previous studies reported a positive effect of the Met allele on PFC-dependent neurocognition in HIV+ individuals (Bousman et al., 2010; Saloner et al., 2019; Sundermann et al., 2015); however, this effect was absent in HIV+ men with METH dependence (Bousman et al., 2010).
Interestingly, our AIC regression analysis did not identify HIV serostatus or other comorbidities (e.g., depression, lifetime alcohol and non-METH substance use disorders) as relevant predictors of DA in this sample, suggesting that METH use and COMT genotype are more salient modulators of the DAergic system in our study sample. Nevertheless, it is possible that the background of DA dysfunction that occurs with HIV disease and other comorbid conditions in our sample can shift the inverted-U curve and, thereby, influence the effects of COMT and METH on neurocognitive function. For example, neuroinflammatory-mediated dampening of DAergic signaling in cortico-striatal circuitry is a putative pathogenic mechanism of depression (Felger, 2017; Felger and Miller, 2012), which may partially underlie the higher levels of depressive symptoms in the METH+ group. These DAergic driven depressive symptoms including apathy and anhedonia may also exhibit a reciprocal relationship with addictive behaviors (Leventhal et al., 2008), particularly in METH+ individuals with extensive histories of polysubstance use (Rawson, 2013). With respect to other factors, accounting for time of lumbar puncture significantly improved overall model fit but did not attenuate the effects of COMT and METH on DA and HVA/DA ratios. This is consistent with the literature examining diurnal fluctuations in CSF and plasma markers of catecholaminergic function and the influence of DAergic circuitry on clock gene expression (Janssens et al., 2019; Korshunov et al., 2017; Verwey et al., 2016). Given the potential for time of CSF sampling to influence catecholamine levels, it is important that studies aim to standardize the time of CSF collection across participants and/or adjust for time of collection in analyses.
We acknowledge several limitations to these data. Cell sizes were small for a gene by environment interaction analysis, which precluded correction for multiple comparisons. Accordingly, these data should be considered preliminary and require independent confirmation. Nevertheless, our sample size was sufficient to model each COMT allelic variant independently and the large magnitude of effects yielded statistically significant COMT by METH group differences in CSF DA. Our neurocognitive analyses also limited multiple comparisons by conservatively focusing on the domain of executive function; importantly, our findings were consistent with prior studies that used the same well-validated tests of executive function. DA biomarkers in CSF provide a novel in vivo window into DA function in the CNS, yet they only represent a global measurement of DA and cannot formally test hypotheses about DA function in specific brain regions. Thus, future PET imaging studies are warranted to determine regional susceptibilities (e.g., PFC vs. striatum) to the impact of COMT on METH-related DAergic injury. Understanding genetic risk for prefrontal dysfunction is of clinical relevance for our study sample of predominantly HIV+ men, who are at risk for developing CNS complications and neuropsychiatric disorders. However, our results may not generalize to women given previously reported sexually dimorphic effects of COMT genotype on COMT enzymatic activity and risk of psychiatric disorders, as well as the reciprocal relationship between the regulation of COMT and estrogens (Tunbridge and Harrison, 2011). Thus, future studies should replicate our analyses in other clinical and non-clinical samples with adequate representation of women. Similarly, the absence of genetic ancestry markers is a limitation and studies that incorporate these markers in sufficiently sized samples to allow for stratified analyses within ethnic groups would increase confidence in these findings.
From a clinical perspective, our findings bear relevance for the treatment of METH-dependence. DA agonists have been considered as a means of stabilizing DA function and promoting abstinence from METH use (Verrico et al., 2013). In a clinical trial examining the incremental efficacy of modafinil in treating METH-dependence, on top of contingency management and cognitive behavioral therapy, relapse rates were lower in modafinil vs. placebo in Val/Val homozygotes, yet modafinil did not reduce relapse rates in Met-carriers (Heinzerling et al., 2012). Additionally, low baseline D2 receptor availability and blunted striatal DA release in response to methylphenidate predicts future relapse in METH users (Wang et al., 2012). Thus, the combination of chronic METH exposure and the Met allele may result in enough DAergic injury to render treatment-seeking METH users non-responsive to the pharmacological effects of DA agonists. Given the putative contribution of DAergic deficits to relapse even after the most effective psychosocial interventions (Venniro et al., 2017), approaches that target the endogenous production of DA (e.g., exercise (Robertson et al., 2016)) may confer neurobehavioral benefits and inform precision treatments for Met-carriers who are non-responsive to pharmacotherapy.
Taken together, the present study highlights the influence of genetically-driven differences in DA metabolism on the effects of chronic METH use on DA and DA-related executive function. Our observation of lower, albeit statistically non-significant, levels of CSF DA and significantly higher CSF HVA/DA ratios in METH+ Met/Met individuals compared to METH+ Val/Met individuals challenges the widely-held assumption that the Met-allele translates into higher levels of bioavailable DA. Such an assumption may be more appropriate in healthier samples, which is supported by the stair-step pattern of higher CSF DA with each additional Met allele in the METH- group; however, careful consideration should be given when forming hypotheses and interpreting data regarding the role of COMT in clinical populations that are characterized by DA dysfunction (e.g., METH use, schizophrenia, Parkinson’s disease, attention-deficit/hyperactivity disorder). This conditional relationship between COMT, METH, and DAergic activity may help explain why some studies have failed to find consistent independent effects of COMT and METH on neurobehavioral outcomes.
Highlights.
Methamphetamine (METH) alters dopamine (DA) integrity and executive function (EF)
METH-related EF deficits are greatest in Met/Met carriers of COMT Val158Met gene
METH+ have lower CSF DA levels than METH- only in Met/Met, but not Val-carriers
CSF DA is highest in METH- Met/Met and only correlates with better EF in this group
Slow DA clearance conferred by Met/Met exacerbates METH-related DA injury
Acknowledgment
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD), the Sanford-Burnham Medical Discovery Institute (SBMDI), and the University of California, Irvine (UCI). The TMARC comprises: Administrative Coordinating Core (ACC) – Executive Unit: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Mariana Cherner, Ph.D.; Associate Center Managers – Erin E. Morgan, Ph.D. and Jared Young, Ph.D.; Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC – Statistics Unit: Florin Vaida, Ph.D. (Unit Chief), Ian S. Abramson, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Behavioral Assessment and Medical (BAM) Core – Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, M.D. (Core Co-Director/NLU Chief), Ronald J. Ellis, M.D., Ph.D.; BAM Core – Neuropsychiatric Unit (NPU): Robert K. Heaton, Ph.D. (Core Co-Director/NPU Chief), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson (NPU Manager); Neuroimaging (NI) Core: Gregory G. Brown, Ph.D. (Core Director), Thomas T. Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John R. Hesselink, M.D., Mary Jane Meloy, Ph.D., Craig E.L. Stark, Ph.D.; Neuroscience and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Marcus Kaul, Ph.D., Virawudh Soontornniyomkij, M.D.; Pilot and Developmental (PAD) Core: Mariana Cherner, Ph.D. (Core Director), Stuart A. Lipton, M.D., Ph.D.; Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark A. Geyer, Ph.D., Jared W. Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Susan F. Tapert, Ph.D., Assawin Gongvatana, Ph.D.; Project 3: Erin E. Morgan, Ph.D. (Project Director), Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director).; Project 5: Marcus Kaul, Ph.D. (Project Director).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Funding
This research was supported by grants from the National Institute on Drug Abuse (NIDA) R01DA26334: COMT Genotype and Risky Decision Making in HIV and Methamphetamine Dependence (PI: Mariana Cherner); P50DA026306: Translational Methamphetamine AIDS Research Center (TMARC; PI: Igor Grant); K23DA037793: Biomarkers of Blood Brain Barrier Injury in Methamphetamine Use and HIV Disease (PI: Jennifer E. Iudicello); and R01DA047879: Identification of Biomarkers of CNS injury and Resilience Related to HIV-1 and Methamphetamine (PIs: Jennifer E. Iudicello and Scott L. Letendre). Stipend support to RS is funded by National Institute of Aging (NIA) award F31AG064989. Stipend support to CWW is funded by NIDA award T32DA031098. The NIDA and NIA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None.
References
- Ashok AH, Mizuno Y, Volkow ND, Howes OD, 2017. Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis. JAMA Psychiatry 74 (5), 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Muller U, 2007. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry 12 (5), 502–509. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G, 1996. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX, Psychology Corporation. [Google Scholar]
- Bennett BA, Rusyniak DE, Hollingsworth CK, 1995. HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res 705 (1–2), 168–176. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED, 2008. Abuse of amphetamines and structural abnormalities in brain. Annals of the New York Academy of Sciences 1141, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Glatt SJ, Atkinson JH, Grant I, Tsuang MT, Everall IP, 2010. Impact of COMT Val158Met on executive functioning in the context of HIV and methamphetamine. Neurobehav HIV Med 2010, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai JX, Arnsten AF, 1997. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther 283 (1), 183–189. [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR, 2004. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75 (5), 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Bousman C, Everall I, Barron D, Letendre S, Vaida F, Atkinson JH, Heaton R, Grant I, 2010a. Cytochrome P450–2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: preliminary findings. J Int Neuropsychol Soc 16 (5), 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, Vaida F, Atkinson JH, Grant I, Heaton RK, Group H, 2010b. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend 106 (2–3), 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Watson CW, Saloner R, Halpin LE, Minassian A, Murray SS, Vaida F, Bousman C, Everall I, 2019. Adverse effect of catechol-O-methyltransferase (COMT) Val158Met met/met genotype in methamphetamine-related executive dysfunction. Addict Behav 98, 106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Conway KP, Colliver JD, 2005. Developments in the epidemiology of drug use and drug use disorders. American Journal of Psychiatry 162 (8), 1494–1502. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M, 2011. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69 (12), e113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH, 2001. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Research Reviews 36 (1), 1–22. [DOI] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED, 2013. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38 (2), 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR, 2001. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 98 (12), 6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, 2017. The Role of Dopamine in Inflammation-Associated Depression: Mechanisms and Therapeutic Implications. Curr Top Behav Neurosci 31, 199–219. [DOI] [PubMed] [Google Scholar]
- Felger JC, Miller AH, 2012. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Frontiers in neuroendocrinology 33 (3), 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK, 2014. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci 97 (1), 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I, 2004. Revised Comprehensive Norms for an Expanded Halstead Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Psychological Assessment Resources, Inc, Lutz, FL. [Google Scholar]
- Heaton RK, Taylor MJ, Manly J, 2003. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III, Clinical interpretation of the WAIS-III and WMS-III. Academic Press, San Diego, CA, US, pp. 181–210. [Google Scholar]
- Heinzerling KG, McCracken JT, Swanson AN, Ray LA, Shoptaw SJ, 2012. COMT Val158Met, BDNF Val66Met, and OPRM1 Asn40Asp and methamphetamine dependence treatment response: preliminary investigation. J Clin Psychopharmacol 32 (1), 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D, 2008. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol 43 (5), 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S, 2000. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol 99 (4), 376–384. [DOI] [PubMed] [Google Scholar]
- Janssens J, Atmosoerodjo SD, Vermeiren Y, Absalom AR, den Daas I, De Deyn PP, 2019. Sampling issues of cerebrospinal fluid and plasma monoamines: Investigation of the circadian rhythm and rostrocaudal concentration gradient. Neurochemistry international 128, 154–162. [DOI] [PubMed] [Google Scholar]
- Johanson C-E, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, 2006. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 185 (3), 327–338. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Boileau I, Callaghan RC, Tong J, 2017. Brain dopamine neurone ‘damage’: methamphetamine users vs. Parkinson’s disease - a critical assessment of the evidence. Eur J Neurosci 45 (1), 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov KS, Blakemore LJ, Trombley PQ, 2017. Dopamine: A Modulator of Circadian Rhythms in the Central Nervous System. Frontiers in cellular neuroscience 11, 91–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M, 2009. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol 15 (3), 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M, 2011. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol 17 (1), 26–40. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA, 2009. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29 (47), 14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, Zimmerman M, 2008. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. The American journal on addictions 17 (3), 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chen CK, Hu X, Ball D, Lin SK, Chen W, Sham PC, Loh el W, Murray RM, Collier DA, 2004. Association analysis of the DRD4 and COMT genes in methamphetamine abuse. Am J Med Genet B Neuropsychiatr Genet 129B (1), 120–124. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR, 2003. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A 100 (10), 6186–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell-Dowling K, Kelly JP, 2017. The Role of Oxidative Stress in Methamphetamine-induced Toxicity and Sources of Variation in the Design of Animal Studies. Curr Neuropharmacol 15 (2), 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, Mattay VS, Egan M, Weinberger DR, 2006. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry 11 (9), 867–877, 797. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Callan SP, 2017. Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment. J Pharmacol Exp Ther 362 (3), 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr., Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK, Group H, 2011. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol 33 (7), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK, 1999. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biological psychiatry 46 (4), 557–567. [DOI] [PubMed] [Google Scholar]
- Pifl C, Rajput A, Reither H, Blesa J, Cavada C, Obeso JA, Rajput AH, Hornykiewicz O, 2014. Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J Neurosci 34 (24), 8210–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, 2013. Current research on the epidemiology, medical and psychiatric effects, and treatment of methamphetamine use. Journal of food and drug analysis 21 (4), S77–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I, 2004. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc 10 (1), 1–14. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA, Cooper CB, Brown AK, Mandelkern MA, London ED, 2016. Effect of Exercise Training on Striatal Dopamine D2/D3 Receptors in Methamphetamine Users during Behavioral Treatment. Neuropsychopharmacology 41 (6), 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Fassbender C, 2011. Structural, functional and spectroscopic MRI studies of methamphetamine addiction, Brain Imaging in Behavioral Neuroscience. Springer, pp. 321–364. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH, 2009. Drug abstinence and cognitive control in methamphetamine-dependent individuals. Journal of substance abuse treatment 37 (3), 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, Marquine MJ, Sundermann EE, Hong S, McCutchan JA, Ellis RJ, Heaton RK, Grant I, Cherner M, 2019. COMT Val158Met Polymorphism, Cardiometabolic Risk, and Nadir CD4 Synergistically Increase Risk of Neurocognitive Impairment in Men Living With HIV. J Acquir Immune Defic Syndr 81 (5), e148–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I, 2007. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17 (3), 275–297. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N, 2001. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry 158 (8), 1206–1214. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Mori N, 2003. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry 160 (9), 1699–1701. [DOI] [PubMed] [Google Scholar]
- Silverstein PS, Shah A, Gupte R, Liu X, Piepho RW, Kumar S, Kumar A, 2011. Methamphetamine toxicity and its implications during HIV-1 infection. J Neurovirol 17 (5), 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Frankle WG, Weinberger DR, Laruelle M, Abi-Dargham A, 2008. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry 13 (8), 821–827. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M, 1995. Structured Clinical Interview for DSM-IV. American Psychiatric Press, Washington, DC. [Google Scholar]
- Starr JM, Fox H, Harris SE, Deary IJ, Whalley LJ, 2007. COMT genotype and cognitive ability: a longitudinal aging study. Neuroscience letters 421 (1), 57. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Bishop JR, Rubin LH, Little DM, Meyer VJ, Martin E, Weber K, Cohen M, Maki PM, 2015. Genetic predictor of working memory and prefrontal function in women with HIV. J Neurovirol 21 (1), 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, 2011. Importance of the COMT gene for sex differences in brain function and predisposition to psychiatric disorders. Curr Top Behav Neurosci 8, 119–140. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, Chiamulera C, Morales M, Shaham Y, 2017. The Anterior Insular Cortex-->Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron 96 (2), 414–427.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Haile CN, Newton TF, Kosten TR, De La Garza R 2nd, 2013. Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin Investig Drugs 22 (12), 1549–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey M, Dhir S, Amir S, 2016. Circadian influences on dopamine circuits of the brain: regulation of striatal rhythms of clock gene expression and implications for psychopathology and disease. F1000Research 5, F1000 Faculty Rev-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, 2005. What do we know about drug addiction? American Journal of Psychiatry 162 (8), 1401–1402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N, 2001a. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158 (12), 2015–2021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN, 2001b. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158 (3), 377–382. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Goldstein RZ, 2002. Role of Dopamine, the Frontal Cortex and Memory Circuits in Drug Addiction: Insight from Imaging Studies. Neurobiology of Learning and Memory 78 (3), 610–624. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS, 2012. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17 (9), 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Roth RM, Saykin AJ, Rhodes CH, Tsongalis GJ, Pattin KA, Moore JH, McAllister TW, 2011. COMT Val158Met Genotype and Individual Differences in Executive Function in Healthy Adults. J Int Neuropsychol Soc 17 (1), 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 1998. Composite Diagnositic International Interview (CIDI, version 2.1) World Health Organization, Geneva, Switzerland. [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF, 1997. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17 (21), 8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]


