Abstract
A bioresponse theranostic nanoparticle to enhance cancer diagnostics and control cancer metastasis is highly desirable. This study developed such a bioresponse theranostic nanoparticle for synergistic photoimmunotherapy. In particular, this nanoparticle was constructed by embedding indocyanine green (ICG) into Mn2+-doped amorphous calcium carbonate (ACC(Mn)) nanoparticle, followed by loading of the Toll-like-receptor-7 agonist imiquimod (IMQ). The IMQ@ACC(Mn)-ICG/PEG nanoparticle responds to the acidic pH of the tumor microenvironment (TME) and co-delivers ICG and IMQ into the tumor. Selective phototherapy was achieved upon activation by a near-infrared laser. In the presence of IMQ and arising from phototherapeutically treated tumor cells, tumor-associated antigens give rise to a strong antitumor immune response. Reversal of the immunosuppressive TME via H+ scavenging of the tumor though ACC nanoparticles effectively inhibits tumor metastases. Moreover, ICG and Mn2+ also serve as an advanced contrast agent for cancer multimode imaging. Overall, this bioresponse nanoparticle provides a promising approach for cancer theranostics with promising potential for future clinical translation.
Introduction
Phototherapy has received increasing acceptance as an effective therapeutic approach for cancer treatment, due to its minimal invasiveness compared to traditional cancer treatment methods.1–3 However, in conventional phototherapy, most photo-conversion agents still afflicted with a number of limitations such as poor in vivo stability, toxic side effects, and low photo conversion efficiency.4–7
Recently, various nanomaterials were developed for cancer phototherapy to overcome these obstacles.8–12 Inorganic nanomaterials, such as gold nanorods, CuS nanoparticles (NPs), and antimonene nanosheets, have strong absorption at infrared wavelengths ≥ 800 nm, and have been applied to increase the photo conversion efficiency for phototherapy.13–16 Furthermore, biocompatible organic nanomaterials, such as chlorin e6 (Ce6), zinc phthalocyanine (ZnPc), and indocyanine green (ICG),17–19 have been used to enhance the phototherapy efficiency of loaded conventional dyes. This further improved the in vivo stability and tumor accumulation efficiency.
However, the poor biocompatibility of inorganic nanomaterials and the low photo conversion efficiency of organic nanomaterials still impedes their application in clinical phototherapy. Therefore, exploring biocompatible, effective, and potent nanomaterials for phototherapy is particularly critical.
Metastasis and cancer recurrence are the main causes of cancer-related death.20 However, phototherapy is largely restricted to the treatment of localized tumors that are accessible to light irradiation. Over the past few years, cancer immunotherapy showed promising potential for inhibiting cancer recurrence and metastasis.21–25 Although significant progresses have thus been achieved in cancer immunotherapy, this form of therapy still suffers from a variety of limitations, such as poor therapeutic responses, significant individual difference, and severe adverse events.26–28 Recently, cancer immunotherapy in combination with phototherapy has been widely explored for cancer treatment.29–35 Phototherapy can stimulate an antitumor immune response by inducing the release of tumor-derived pro-inflammatory protein antigens by the dying tumor cells.36–39 Although this phototherapy-induced antitumor immunity alone is difficult to lead to systemic tumor rejection, combining phototherapy and immunotherapy offers the potential to achieve systemic antitumor response for cancer treatment.
To obtain robust antitumor efficacy in photoimmunotherapy, an appropriate nanomaterial is desirable for photo-conversion and the delivery of immunostimulants. Recently, stimuli-responsive nanomaterials have been extensively explored, with the aim to develop precision cancer nanomedicine with high specificity and efficacy.40–45 Compared to conventional nanomaterials, stimuli-responsive nanomaterials (i.e., light-responsive, temperature-responsive, or pH-responsive) not only deliver specific drugs to active sites, thus minimizing side effects, but also selectively and efficiently destroy tumors while sparing healthy tissues.46–49 Thus, developing a stimuli-responsive nanomaterial for photoimmunotherapy offers promising potential to achieve antitumor response in tumor photoimmunotherapy.
Amorphous calcium carbonate (ACC) NPs, which is decompose into Ca2+ and CO2 under acidic environment, have been extensively used for pH-responsive drug delivery in recent years.43, 47–49 For example, Xu et. al. developed a tunable pH-response ACC NP by doping of Sr2+ or Mg2+ ions for the delivery drugs with varying characteristic pH microenvironment.47 To enhance the drug penetration and reduce side effect of the drug, Wang et. al. developed a lipase-triggered water-responsive ACC NP, which triggered by the lipase in tumor tissue while preserve the unwanted drug leakage in circulation.49 Besides, ACC NPs can simultaneously load multiple molecules to achieve the multimodal cancer treatment. Dong et. al. prepared a CaCO3-PDA NP for multimodal imaging guided PDT, preloading both imaging and therapeutic molecules simultaneously. According to the above analysis, ACC NPs have proved to be an ideal nanomaterial for the fabrication of a stimuli-responsive platform for cancer therapy.
Furthermore, calcium carbonate NPs were also used for cancer immunotherapy.45, 46 Glycolytic metabolism of cancer cells in hypoxic tumor microenvironment (TME) causes the production of lactate and H+, which promote the differentiation of tumor-associated macrophages (TAMs) to M2-like phenotype.50, 51 M2-like TAMs can promote tumor growth and metastasis by increasing cancer cell proliferation, immunosuppression, and angiogenesis.52, 53 Calcium carbonate NPs have the ability of the clearance of H+ in the tumor tissue, inhibiting polarization of TAMs to M2-like phenotype. For instance, Chen et. al. developed a CaCO3 NPs-based bio-responsive gel, which could release anti-CD47 antibody in TME and modulate the acidic and inflamed tumor resection environment by scavenging H+.45 Taking advantages of those features, ACC NPs have shown promising applications in the fabrication of the bioresponse photoimmunotherapy nanoplatform. However, the ACC NPs used in the bioresponse photoimmunotherapy has not yet been reported, to the best of our knowledge.
In the study,a bioresponse theranostics NP was designes bases on Mn2+-drop amorphous calcium (ACC(Mn)) NP for pH-triggered controllable drug delivery, modulation of the TME, and selective photoimmunotherapy. This system was designed to achieve tumor-specific enhanced combination therapy, guided by multimodal imaging. ACC(Mn) NPs were obtained via gas diffusion, and the photosensitizer ICG was embedded in ACC(Mn) NPs in situ (Fig. 1). ACC(Mn)-ICG NPs were functionalized with a lipid bilayer, which were followed by the loading of the Toll-like-receptor-7 agonist imiquimod (IMQ). In this system, ACC(Mn) NPs serve as a multicarrier to co-deliver ICG and IMQ to the tumor. In response to the acidic TME, the break-up of ACC(Mn) NPs would lead to the release of the previously loaded drugs, concurrently giving rise to the reversal of immunosuppressive TME by scavenging H+ of the tumor. Thus, ICG can largely accumulate in tumor tissues, and enhanced phototherapy could be achieved under near-infrared (NIR) laser irradiation. This approach enhanced phototherapeutically elicited immunogenic cell death (ICD) with improved release of tumor-associated antigens. With the help of the immune adjuvant IMQ, these tumor-associated antigens induced robust antitumor immune responses. Together with the reversal of immunosuppressive TME, the ACC-based NPs elicited a strong antitumor immune response. Therefore, employing this multifunctional NPs with full biocompatibility enable the combination of phototherapy with immunotherapy. This combined system realized whole body systemic antitumor therapeutic outcomes triggered by enhanced photoimmunotherapy of local tumors, which promises to inhibit tumor metastases and prevent tumor relapse.
Fig. 1.
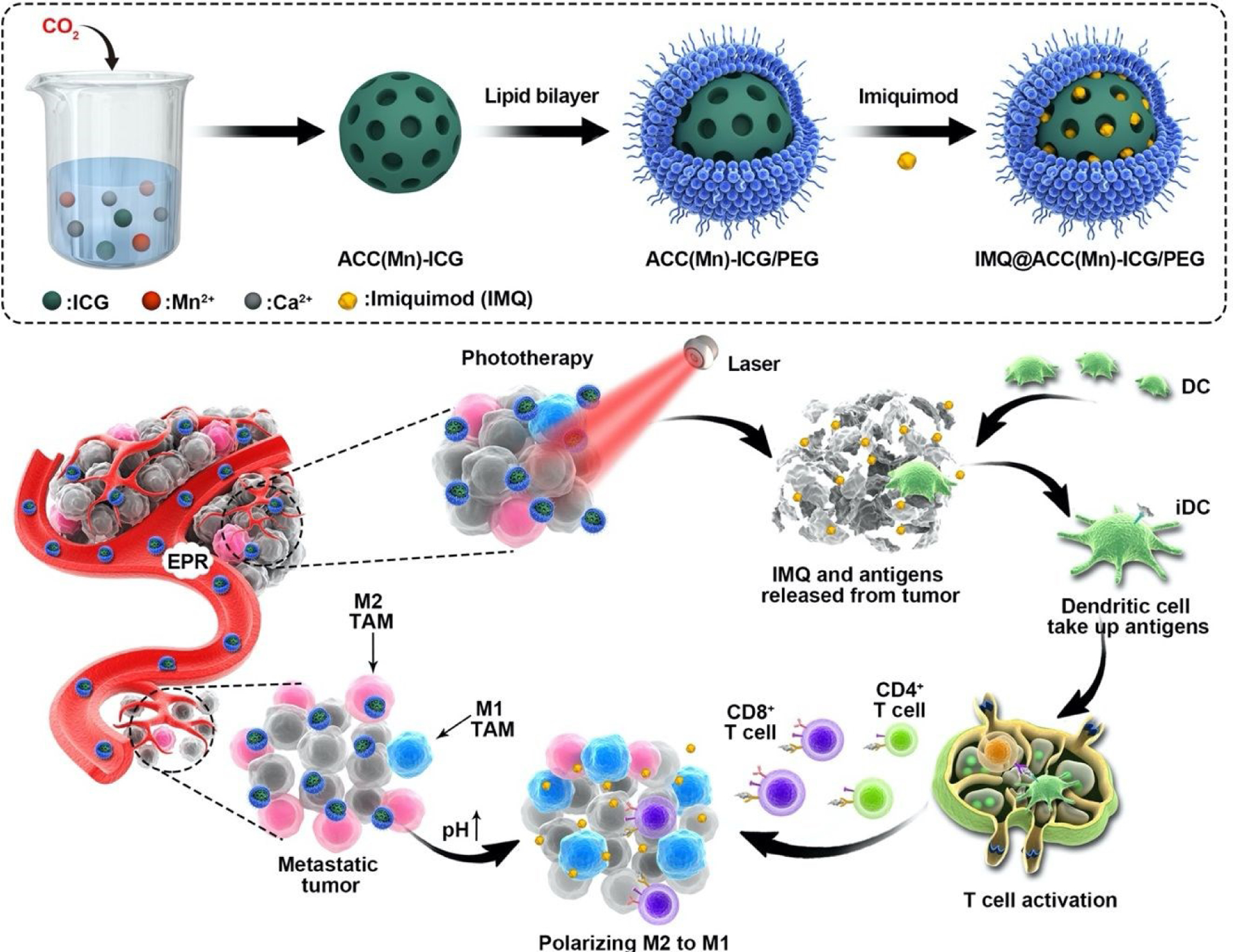
The scheme of the fabrication of IMQ@ACC(Mn)-ICG/PEG nanoparticles (NPs) and its photoimmunotherapy process for antitumor therapy.
Results and discussion
Fabrication and characterization of ACC(Mn)-ICG/PEG NPs
First, ACC(Mn)-ICG NPs were prepared via a gas diffusion approach according the methods in a previous study. (Fig. 1). During this process, ICG chelated with Ca2+ ions and was finally embedded in ACC in the form of a Ca-ICG complex. TEM images showed that ACC(Mn)-ICG NPs were monodispersed with a uniform size of ~43 nm in diameter (Fig. 2a; Fig. S1, Supporting Information). The corresponding chemical composition of ACC(Mn)-ICG NPs were further confirmed via element mapping of Ca, O, and Mn elements (Fig. 2b), and the doping concentration of Mn ions in ACC(Mn)-ICG NPs is ~5% (mol/mol) (Fig. S2, Supporting Information). Then, the ACC(Mn)-ICG NPs were modified with a self-assembled lipid bilayer to improve colloidal stability. DOPA molecules were capped on ACC(Mn)-ICG via Ca-phosphate interactions between ACC(Mn)-ICG NPs and DOPA molecules. Furthermore, a lipid monolayer, composed of DOPC, cholesterol, and DSPE-PEG2K, was further coated. The resulting ACC(Mn)-ICG/PEG NPs have excellent colloidal stability and an average diameter (measured via dynamic light scattering (DLS)) of 72.4 nm (Fig. 2c). As shown in Fig. 2d, ACC(Mn)/ICG-PEG NPs exhibited a similar absorption spectrum as free ICG. Furthermore, compared with free ICG, the corresponding characteristic absorption peaks of ACC(Mn)/ICG-PEG NPs has a red shift, which is due to the aggregation of ICG molecules.54, 55 The ICG loading efficiency was 56.8%, determined via UV-vis-NIR absorbance spectra (Fig. S3, Supporting Information).
Fig. 2.
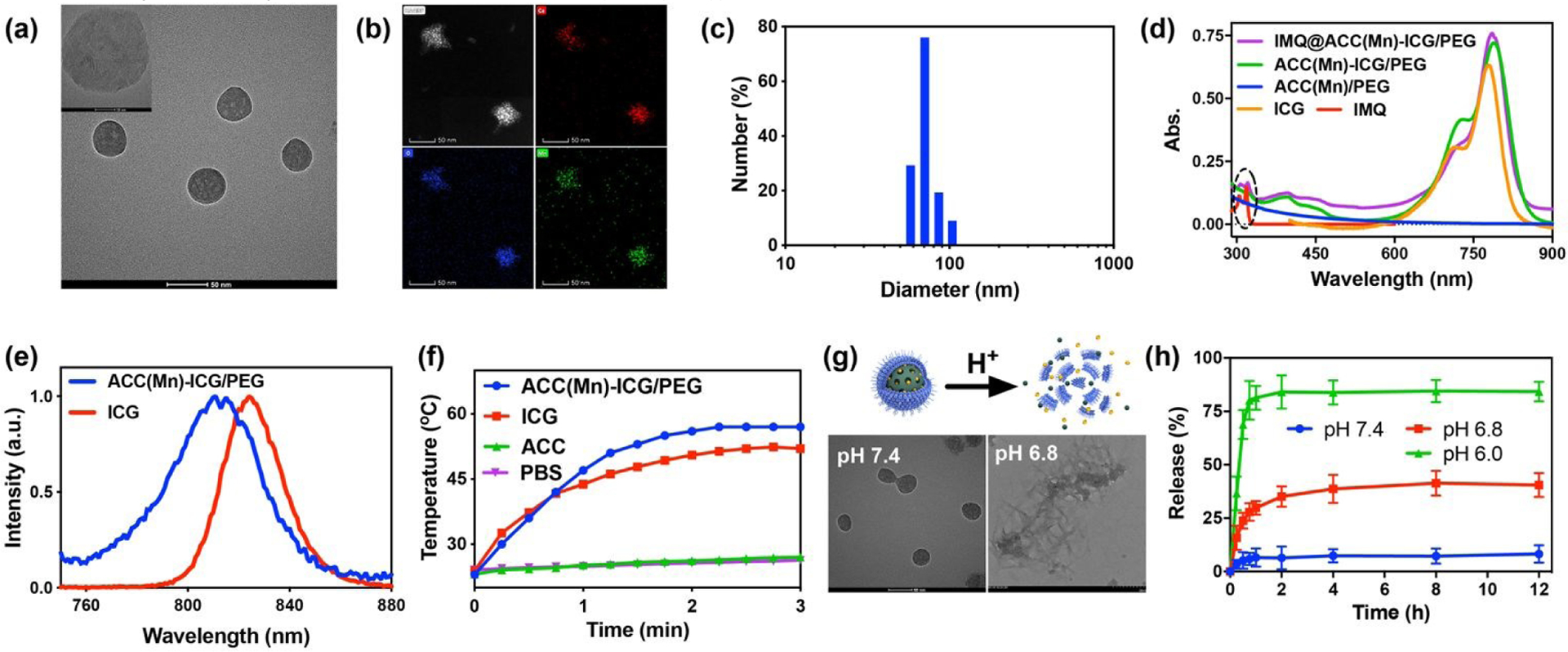
Synthesis and characterization of IMQ@ACC(Mn)-ICG/PEG NPs. (a) Transmission electron microscopy (TEM) image of ACC(Mn)-ICG NPs. Scale bar = 51 nm. (b) Representative scanning TEM images of ACC(Mn)-ICG NPs showing the calcium (red), oxygen (blue) and manganese (green). (c) Hydrodynamic size of ACC(Mn)-ICG/PEG NPs determined by dynamic light scattering. (d) UV-vis-NIR absorbance spectra of ACC(Mn)/PEG NPs, ICG, IMQ, ACC(Mn)-ICG/PEG NPs, and IMQ@ACC(Mn)-ICG/PEG NPs. (e) Fluorescence spectra of ICG and ACC(Mn)-ICG/PEG NPs. (f) Temperature elevations of PBS, ACC(Mn)/PEG NPs, ICG, and ACC(Mn)-ICG/PEG NPs under an 805 nm laser irradiation (0.75 W cm−2). (g) Scheme illustrating the acid-responsive decomposition of ACC(Mn)-ICG/PEG NPs and their corresponding TEM images after incubation in PBS with different pH values for 2 h. (h) Time-dependent release profiles of imiquimod (IMQ) from IMQ@ACC(Mn)-ICG/PEG NPs incubated in PBS at different pH values.
The fluorescence of ACC(Mn)-ICG/PEG NPs in phosphate buffer saline (PBS) (pH 7.4) was investigated. As shown in Fig. 2e, ACC(Mn)-ICG/PEG NPs have a similar emission band as ICG, indicating that ACC-embedded ICG still retained its original optical property. The photothermal effect of ACC(Mn)-ICG/PEG NPs were studied under NIR laser irradiation. From the thermal imaging data, the temperature of ICG and ACC(Mn)-ICG/PEG NPs increased significantly from 23 °C to 58 °C under the NIR laser irradiation for 3 min, while no significant temperature increase was detected in ACC(Mn)/PEG NPs and PBS under the same conditions (Fig. 2f; Fig. S4, Supporting Information), suggesting that ACC-embedded ICG still maintained its original photothermal effect.
The pH-responsive decomposition behaviour of ACC(Mn)-ICG/PEG NPs was investigated by immersing the NPs in PBS at pH values of 6.8 and 7.4. ACC(Mn)-ICG/PEG NPs appear to be quite stable at pH 7.4 (Fig. 2g). Moreover, ACC(Mn)-ICG/PEG NPs did not show any obvious change in their morphologies after immersing in FBS solution (20%, v/v) or PBS buffer (pH 7.4) for 24 h (Fig. S5, Supporting Information), and the size of ACC(Mn)-ICG/PEG NPs did not show significant change after immersing in serum for 7 days (Fig. S6, Supporting Information). In contrast, when ACC(Mn)-ICG/PEG NPs were decomposed, they lost their origin morphology after immersed in PBS at pH 6.8 for 2 h. Furthermore, the changes of hydrodynamic sizes of ACC(Mn)-ICG/PEG NPs under different pH values were also assessed to verify the pH-responsive decomposition behaviours of ACC(Mn)-ICG/PEG (Fig. S7, Supporting Information). The obtained ACC(Mn)-ICG/PEG NPs with mesoporous structure and pH-responsive decomposition behaviours motivated the exploration of their pH-responsive drug delivery capacity. IMQ was utilized here as an immune adjuvant for ACC(Mn)-ICG/PEG based photoimmunotherapy. The loading efficiency of IMQ on ACC(Mn)-ICG/PEG NPs were 75.7% (Fig. S8, Supporting Information). The IMQ release from IMQ@ACC(Mn)-ICG/PEG NPs under different pH values (6.0, 6.8 and 7.4) was measured. Approximately 35.1% of the loaded IMQ was released from IMQ@ACC(Mn)-ICG/PEG NPs incubated in PBS at pH 6.8 for 2 h, which far exceeded the ~6.4% release from IMQ@ACC(Mn)-ICG/PEG NPs incubated in PBS at pH 7.4 for 2h (Fig. 2h), and indicated the pH-responsive decomposition of ACC(Mn)-ICG/PEG NPs.
We next studied the cellular uptake of ACC(Mn)-ICG/PEG NPs in 4T1 murine breast tumor cells. 4T1 cells were treated with PBS, ICG, and ACC(Mn)-ICG/PEG NPs for 4 h, and analysed via confocal laser scanning microscopy (CLSM) and flow cytometry. As shown in Fig. 3a and Fig. S9, the fluorescence intensity of ICG content in ACC(Mn)-ICG/PEG NPs group was significantly higher than those in ICG group. It is known that congregation of ICG, particularly through encapsulation, can reduce the fluorescence emission of ICG.54,55 However, the observed increase in fluorescence emission of ACC(Mn)-ICG/PEG NPs in Figs. 3a demonstrated the enhanced cellular update of ACC(Mn)-ICG/PEG NPs. Additionally, this enhanced cellular uptake of ACC(Mn)-ICG/PEG NPs were also demonstrated by flow cytometry results (Fig. 3b). These results indicate that ACC(Mn)-ICG/PEG NPs were efficiently internalized by 4T1 cells, which is expected to achieve improved therapeutic effects. Then, the intracellular reactive oxygen species (ROS) generation of ACC(Mn)-ICG/PEG NPs were evaluated via DCFH-DA staining. As displayed in Fig. 3c, d, an obviously higher ROS generation was observed in cells treated with ACC(Mn)-ICG/PEG NPs in compared with in cells treated with ICG.
Fig. 3.
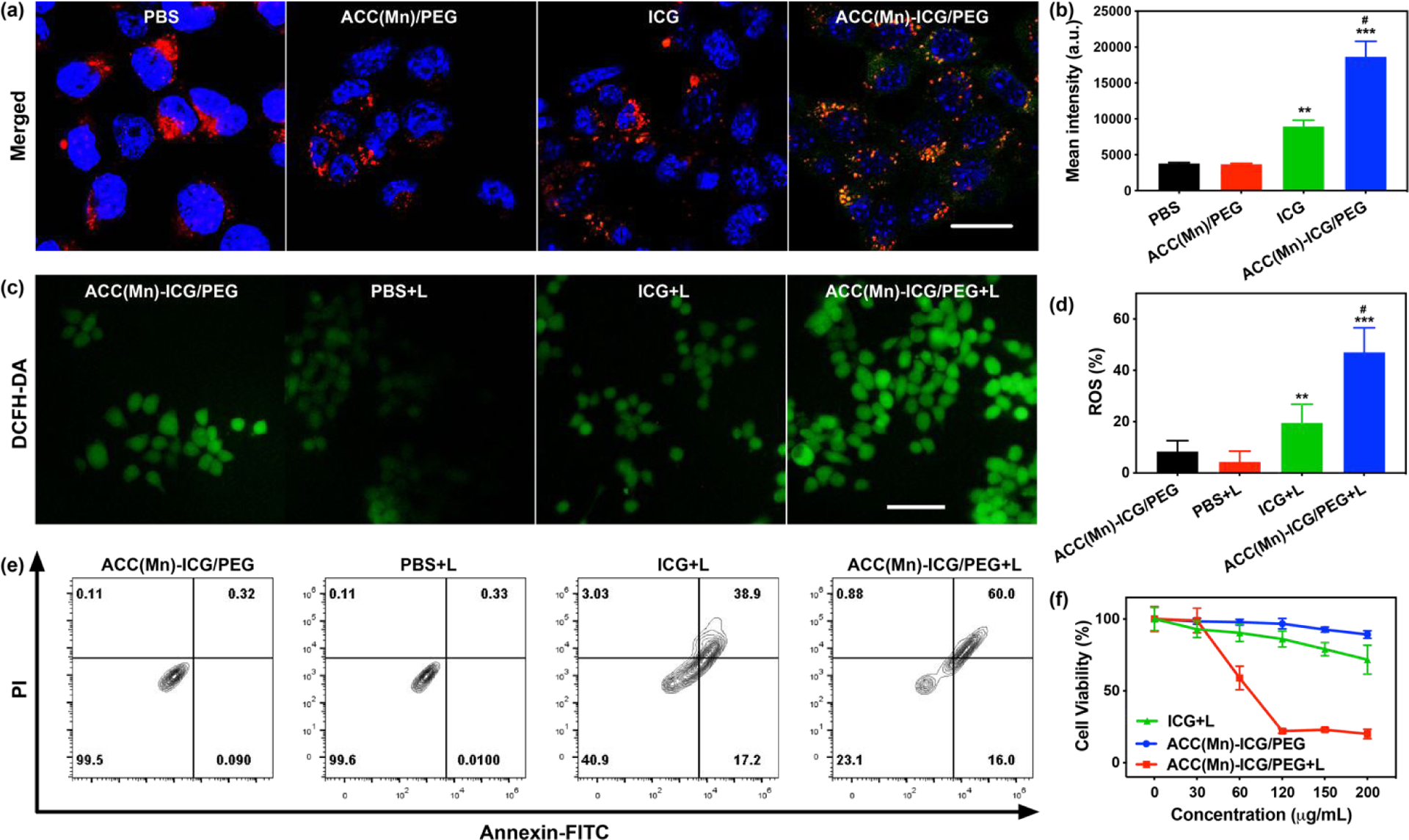
In vitro intracellular uptake and photocytotoxicity. (a) Fluorescence images of 4T1 cells after incubation with ICG (20 μg mL-1) and ACC(Mn)-ICG/PEG NPs (200 μg mL−1) for 4 h. (Scale bar = 20 μm) (b) Mean fluorescence intensity from flow cytometric analysis of 4T1 cells after incubation with ICG (20 μg mL−1) and ACC(Mn)-ICG/PEG NPs (200 μg mL−1) for 4 h. (**p < 0.01 vs PBS, ***p < 0.001 vs PBS, #p < 0.05 vs ICG). (c) Fluorescence images of ROS generation in 4T1 tumor cells (0.75 W cm−2 for 5 min). (Scale bar = 100 μm) (d) Flow cytometry quantification of the reactive oxygen species (ROS) generation in 4T1 tumor cells after various treatments. (**p < 0.01 vs PBS + laser, ***p < 0.001 vs PBS + laser, #p < 0.05 vs ICG + laser). (e) Apoptosis and necrosis analysis of 4T1 cells received the different treatment. (ICG: 20 μg mL, ACC(Mn)-ICG/PEG NPs: 200 μg mL−1; Laser: 805 nm, 0.75 W cm−2 for 5 min) (f) Viability of 4T1 tumor cells incubated with PBS, ICG, and ACC(Mn)-ICG/PEG NPs, followed by irradiation with an 805 nm laser (0.75 W cm−2 for 5 min). Data are expressed as mean ± SD (n = 4).
Phototoxicity of ACC(Mn)-ICG/PEG NPs and ICD induced by ACC(Mn)-ICG/PEG NPs in vitro
The in vitro phototoxicity of ACC(Mn)-ICG/PEG NPs were studied via CCK-8 assay. After the 4T1 cells were cocultured with ACC(Mn)-ICG/PEG NPs of different concentrations in the dark for 24 h, only negligible cytotoxicity of ACC(Mn)-ICG/PEG NPs was observed (Fig. 3f), which verified that the ACC(Mn)-ICG/PEG NPs had excellent biocompatibility. In contrast, the vs PBS + laser, ***p < 0.001 vs PBS + laser, #p < 0.05 vs ICG + laser). (c) Detection of extracellular release of high mobility group 1 of 4T1 tumor cells after different treatments. (*p < 0.05 vs PBS + laser). Data are expressed as mean ± SD (n = 5). viability of 4T1 cells treated with ACC(Mn)-ICG/PEG NPs decreased significantly after laser irradiation for 5 min, compared to ICG + laser (Fig. 3f). The in vitro phototoxicity was further studied by apoptosis assay via flow cytometry. As shown in Fig. 3e, ACC(Mn)-ICG/PEG + laser treatment demonstrated high level of apoptosis (76%) compare to ICG or PBS + laser treatments. Moreover, the phototoxicity of ACC(Mn)-ICG/PEG NPs was further verified via confocal fluorescence imaging (Fig. S10, Supporting Information). These findings were consistent with the evaluation of cellular uptake, which further verified that the higher cellular uptake of ACC(Mn)-ICG/PEG NPs improved the therapeutic effects.
To study whether ACC(Mn)-ICG/PEG NPs based photoimmunotherapy can trigger ICD, we detected the calreticulin (CRT) exposure and high-mobility group box (HMGB1) release in 4T1 cells. As displayed in Fig. 4a, 4T1 cells treated with ACC(Mn)-ICG/PEG + laser had a much higher CRT exposure than those treated with ICG + laser. In contrast, there were no obvious CRT exposure in PBS + laser and ACC(Mn)-ICG/PEG NPs groups. Flow cytometry results showed that 4T1 cells were 47% CRT-positive ratio after treated with ACC(Mn)-ICG/PEG + laser, compared to those of PBS + laser, ACC(Mn)-ICG/PEG NPs, and ICG + laser (4%, 5%, and 14%, respectively). Meanwhile, HMGB1 release from 4T1 tumor cells was detected by enzyme-linked immune sorbent assay (ELISA) and similar tendency with CRT exposure was observed (Fig. 4c). Accordingly, ACC(Mn)-ICG/PEG NPs based phototherapy should elicit ICD and enhance the release of tumor-derived pro-inflammatory protein antigens from 4T1 tumor cells.
Fig. 4.
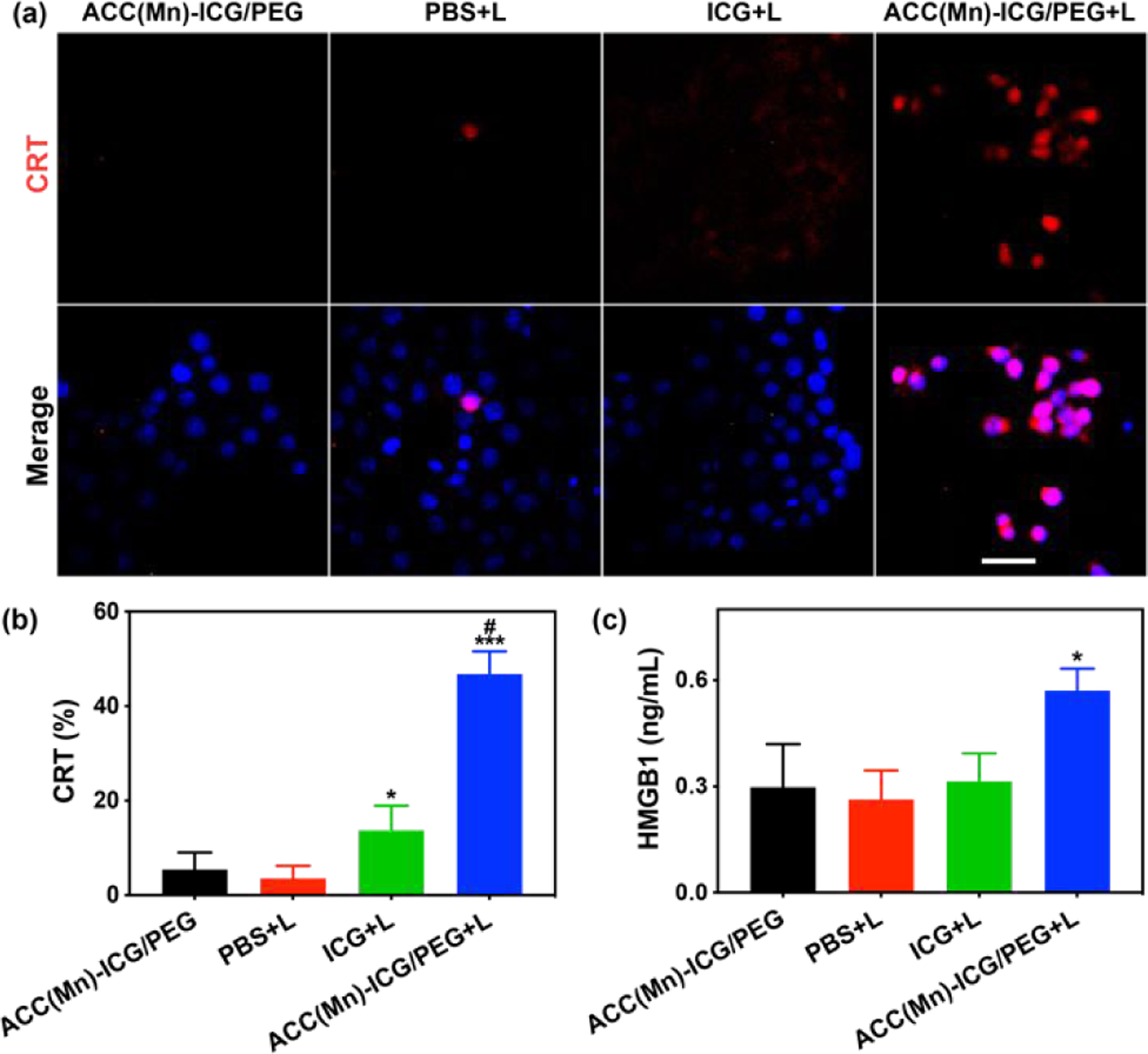
In vitro immunogenic cell death induced via photoimmunotherapy. (a) Fluorescent imaging of calreticulin (CRT) exposed on the surface of 4T1 tumor cells after treatment with PBS, ICG, and ACC(Mn)-ICG/PEG NPs under an 805 nm laser irradiation (0.75 W cm−2 for 5 min). (Scale bar = 100 μm) (b) Flow cytometry quantification of CRT on 4T1 tumor cells. (*p < 0.05 vs PBS + laser, ***p < 0.001 vs PBS + laser, #p < 0.05 vs ICG + laser). (c) Detection of extracellular release of high mobility group 1 of 4T1 tumor cells after different treatments. (*p < 0.05 vs PBS + laser). Data are expressed as mean ± SD (n = 5).
Multimodal imaging and biodistribution of ACC(Mn)-ICG/PEG nanoparticles in vivo
We next investigated the biodistribution of ACC(Mn)-ICG/PEG, NPs, using dual-modal imaging of orthotopic 4T1 tumors in mice. In vivo fluorescence imaging was performed first to trace the distribution of ACC(Mn)-ICG/PEG NPs. As shown in Fig. 5a the fluorescence intensity of the tumor site in ACC(Mn)-ICG/PEG NPs group was significantly stronger than that of the ICG group at the corresponding time points (1, 4, and 24h). Compared to the ICG, ACC(Mn)-ICG/PEG NPs showed an obvious long-term circulation (Fig. 5a; Fig. S11, Supporting Information). Moreover, the ex vivo imaging was further confirmed its remarkably high accumulation in tumor compared with that of ICG group (Fig. S12, Supporting Information), indicating that ACC(Mn)-ICG/PEG NPs circulated for a long time and achieved efficient tumor-specific accumulation compared to ICG. In addition, compared to the fluorescent signals of the liver, the kidney showed a stronger fluorescent signal in ACC(Mn)-ICG/PEG NPs. The strong fluorescent signal in the kidney can be ascribed to the degradation of ACC(Mn)-ICG/PEG NPs in the acidic environment of the tumor and then, ICG can be excreted in its free form. Next, a magnetic resonance (MR) imaging system was used to monitor the biodistribution of ACC(Mn)-ICG/PEG NPs by T1-weighted MR imaging. As shown in Fig. 5b, strong MR signal intensities were detected in the tumor after ACC(Mn)-ICG/PEG NPs injection, which gradually increased over time within 3 h. The signal changes in tumors or tissues at different time points were quantified with the time-dependent T1 relaxation times. The T1 values of the tumors showed a gradual decrease, which increased MR imaging signal enhancement over time and reached a high level 2 h post-treatment (Fig. S13, Supporting Information).
Fig. 5.
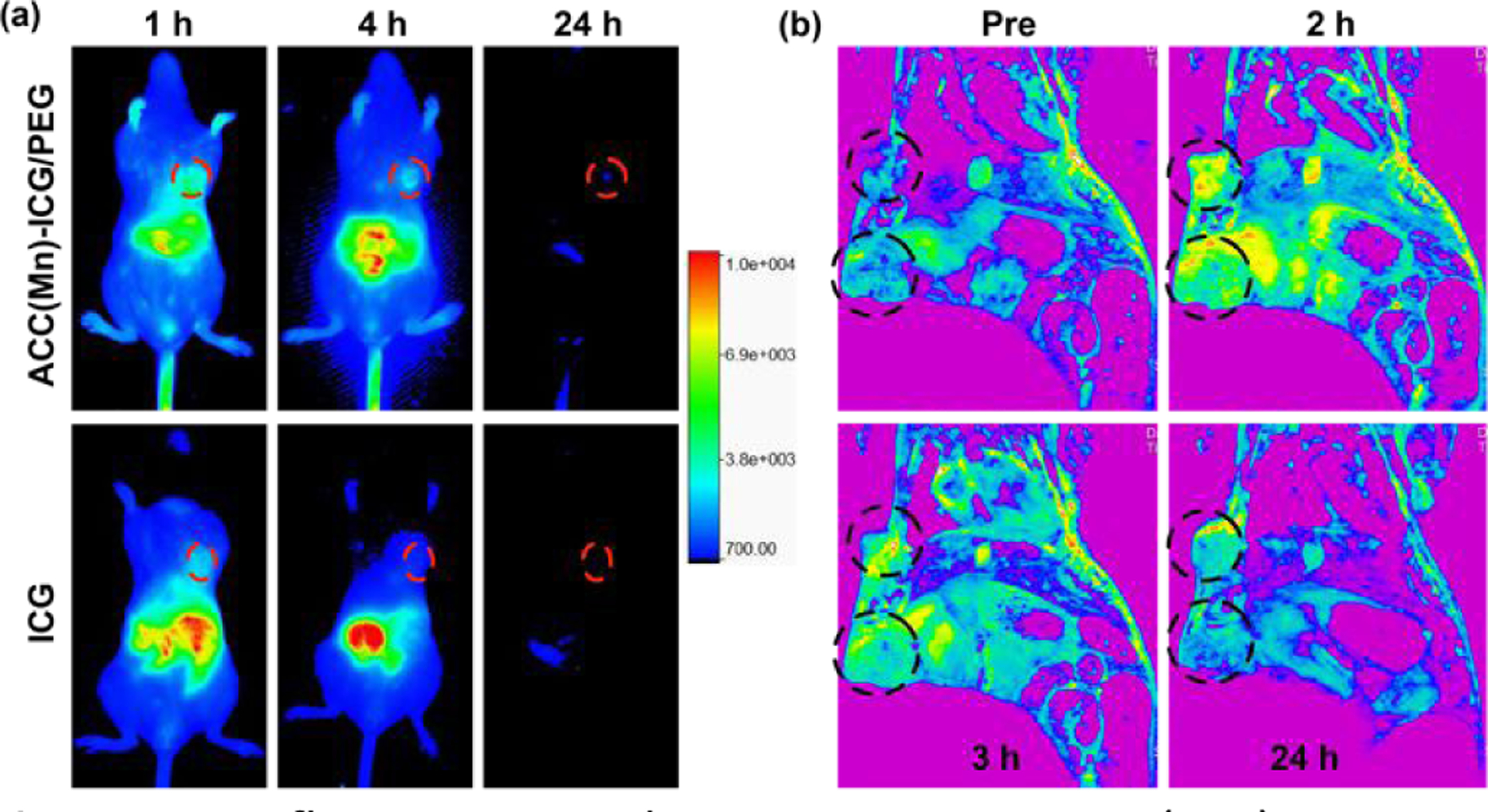
In vivo fluorescent and magnetic resonance (MR) imaging. (a) Typical in vivo fluorescence images of 4T1 tumor-bearing mice at indicated time points (1, 4, and 24) after intravenous injection of ICG (40 μg mL−1, 100 μL) and ACC(Mn)-ICG/PEG NPs (10 mg mL−1, 100 μL). Tumors are circled with red dashed lines. (b) Typical in vivo T1-weighted difference MR images of 4T1 tumor-bearing mice at indicated time points (0, 2, 3, and 24) after intravenous injection of ICG and ACC(Mn)-ICG/PEG NPs. Tumors are circled with black dashed lines.
Antitumor effects of ACC(Mn)-ICG/PEG NPs in vivo
To demonstrate the antitumor effects of ACC(Mn)-ICG/PEG NPs-based photoimmunotherapy in vivo, we utilized the orthotopic breast cancer model of 4T1 in BALB/c mice. Tumor-bearing mice were randomly divided into five groups, which were treated with 1) PBS + laser, 2) ICG + laser, 3) ACC(Mn)-ICG/PEG + laser, 4) IMQ@ACC(Mn)-ICG/PEG + laser, and 5) IMQ@ACC(Mn)-ICG/PEG. Thermal imaging showed that the temperature associated with the tumor treated by ACC(Mn)-ICG/PEG + laser rose to 56 °C within 4 min in comparison to a temperature of 45 °C when ICG + laser was used (Fig. S14, Supporting Information). This phototherapeutic effect was investigated by analysing the tumor tissues one day after treatment using hematoxylin-eosin (H&E) staining. As shown in Fig. 6a, the tumor structures from mice treated with IMQ@ACC(Mn)-ICG/PEG + laser was seriously damaged compared to those of ICG + laser and PBS + laser, confirming that IMQ@ACC(Mn)-ICG/PEG NPs provided excellent phototherapy effect. We next monitored the tumor growth after treatment.Mice that received ICG + laser or IMQ@ACC(Mn)-ICG/PEG treatment showed rapid tumor growth, with the median survival opf 31~33 days, which not significantly different compared to mice that received PBS + laser. In contrast, ACC(Mn)-ICG/PEG + laser and IMQ@ACC(Mn)-ICG/PEG + laser groups completely regressed the tumor and extended mice survival (Fig. 6b, c). Moreover, mice that received IMQ@ACC(Mn)-ICG/PEG + laser treatment lived significantly longer than ACC(Mn)-ICG/PEG + laser groups, and yielded a 34% survival rate (Fig. 6c), while all mice in the ACC(Mn)-ICG/PEG + laser group died within 43 day. Besides, there was no significant change in the body weight of mice and tissue sections, indicating the safety of IMQ@ACC(Mn)-ICG/PEG based photoimmunotherapy (Fig. S15, S16, Supporting Information). These results demonstrate that ACC(Mn)-ICG/PEG NPs served as a photo-immunoadjuvant augmented by additional loading of adjuvants for improved antitumor effect in vivo.
Fig. 6.
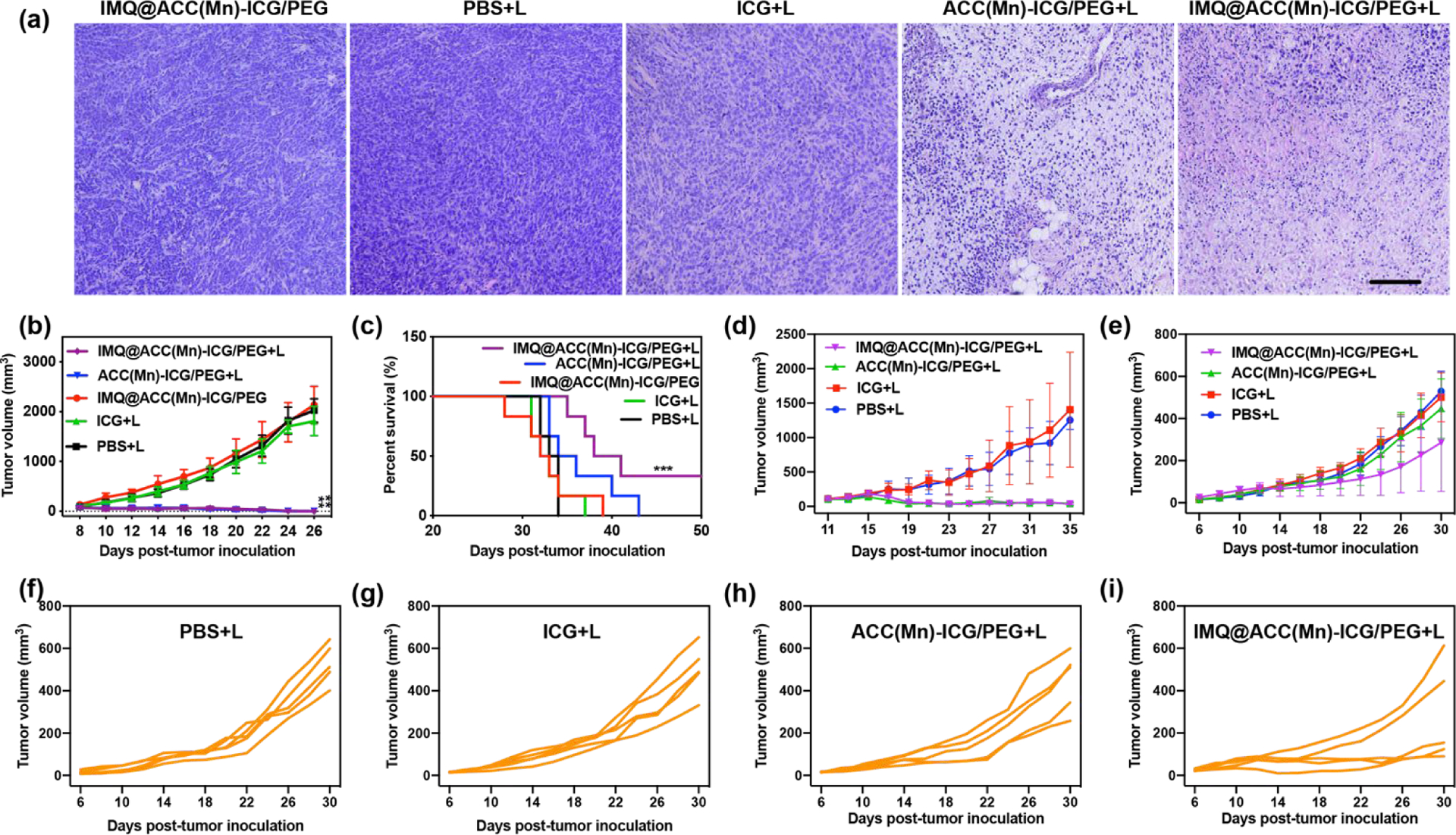
In vivo antitumor effect of IMQ@ACC(Mn)-ICG/PEG NPs-based photoimmunotherapy. (a) Representative H&E-stained images of 4T1 tumors after different treatments as indicated. Scale bar = 100 μm. (b) Average tumor-growth curves of different treatment groups of mice with orthotopic 4T1 tumors (n = 6, **p < 0.01 vs PBS). (c) Survival curves of different treatment groups of mice bearing orthotopic 4T1 tumors (n = 6, ***p < 0.01 vs PBS + laser). (d, e) Growth curves of primary tumors and distant tumors of mice after various treatments. (n = 5, ***p < 0.001 vs PBS + laser). (f-i) Distant tumor growth curves of individual mouse after various treatments. Data are expressed as mean ± SD.
We next evaluated the abscopal effect of IMQ@ACC(Mn)-ICG/PEG based phototherapy using bilateral tumor model of breast cancer 4T1 in the flank regions of BALB/c mice (Fig. S17). The right tumor was defined as the primary tumor and the left tumor was defined as the distant tumor. After the primary tumor reached ~100 mm3, the mice received a single injection of PBS, ICG, ACC(Mn)-ICG/PEG, and IMQ@ACC(Mn)-ICG/PEG into the primary tumor, and then treated with the laser irradiation (805 nm, 0.75 W/cm2 for 10 min). As shown in Figure 6d, ACC(Mn)-ICG/PEG + laser and IMQ@ACC(Mn)-ICG/PEG + laser group completely regressed the primary tumors, while ICG + laser group failed to inhibit the tumor growth. Moreover, mice that received IMQ@ACC(Mn)-ICG/PEG + laser treatment significantly inhibited the growth of distant tumors compared to the ACC(Mn)-ICG/PEG + laser group (Fig. 6e–i). These results suggest that ACC(Mn)-ICG/PEG served as photo-immunoadjuvants to effectively activate the immune system and inhibit tumor metastasis.
Furthermore, the potential in vivo toxicity of NPs was analysed by hematology analysis and blood biochemistry. After i.v. injection of IMQ@ACC(Mn)-ICG/PEG NPs, the blood biochemistry and hematology exhibited no significantly changed compared with the indexes of control group (Fig. S19, Supporting Information). In addition, the pharmacokinetic property of ACC(Mn)/ICG-PEG NPs was reflected by its half-life of blood of mice, which was calculated as 68.1 mins (Fig. S20, Supporting Information). Thus, our results demonstrated that there were no apparent toxicities induced by IMQ@ACC(Mn)-ICG/PEG NPs at our tested condition in vivo.
Antitumor immune responses induced by IMQ@ACC(Mn)-ICG/PEG NPs in vivo
Having observed strong antitumor efficacy of ACC(Mn)-ICG/PEG based photoimmunotherapy, we next investigated the IMQ@ACC(Mn)-ICG/PEG NPs induced immune responses via flow cytometry, ELISA, and immunofluorescence staining assay. A bilateral 4T1 model was developed by subcutaneously injecting 4T1 cells into both the left and right flank regions of mice, and the right tumor was designated as the primary tumor while the left tumor was designated as the distant tumor. After the tumor reached ~100 mm3, the primary tumors were treated with PBS + laser, ICG + laser, ACC(Mn)-ICG/PEG + laser, and IMQ@ACC(Mn)-ICG/PEG + laser. Firstly, we investigated whether ACC(Mn)-ICG/PEG based photoimmunotherapy could promote dendritic cells (DCs) maturation in vivo. Lymph nodes (LNs) were collected on day 3 after laser treatment. LN cells were obtained, stained with co-stimulatory molecules (CD11c and CD86), and analyzed via flow cytometry. As displayed in Fig. 7a, b, IMQ@ACC(Mn)-ICG/PEG + laser significantly promoted DC maturation in LNs, by 1.6-fold, 1.4-fold, and 1.3-fold compared to the PBS + laser group, ACC + laser group, and ACC(Mn)-ICG/PEG + laser group, respectively. The increase of DC maturation was also reflected by the CD11+ expression levels within the treated tumors (Fig. S21).
Fig. 7.
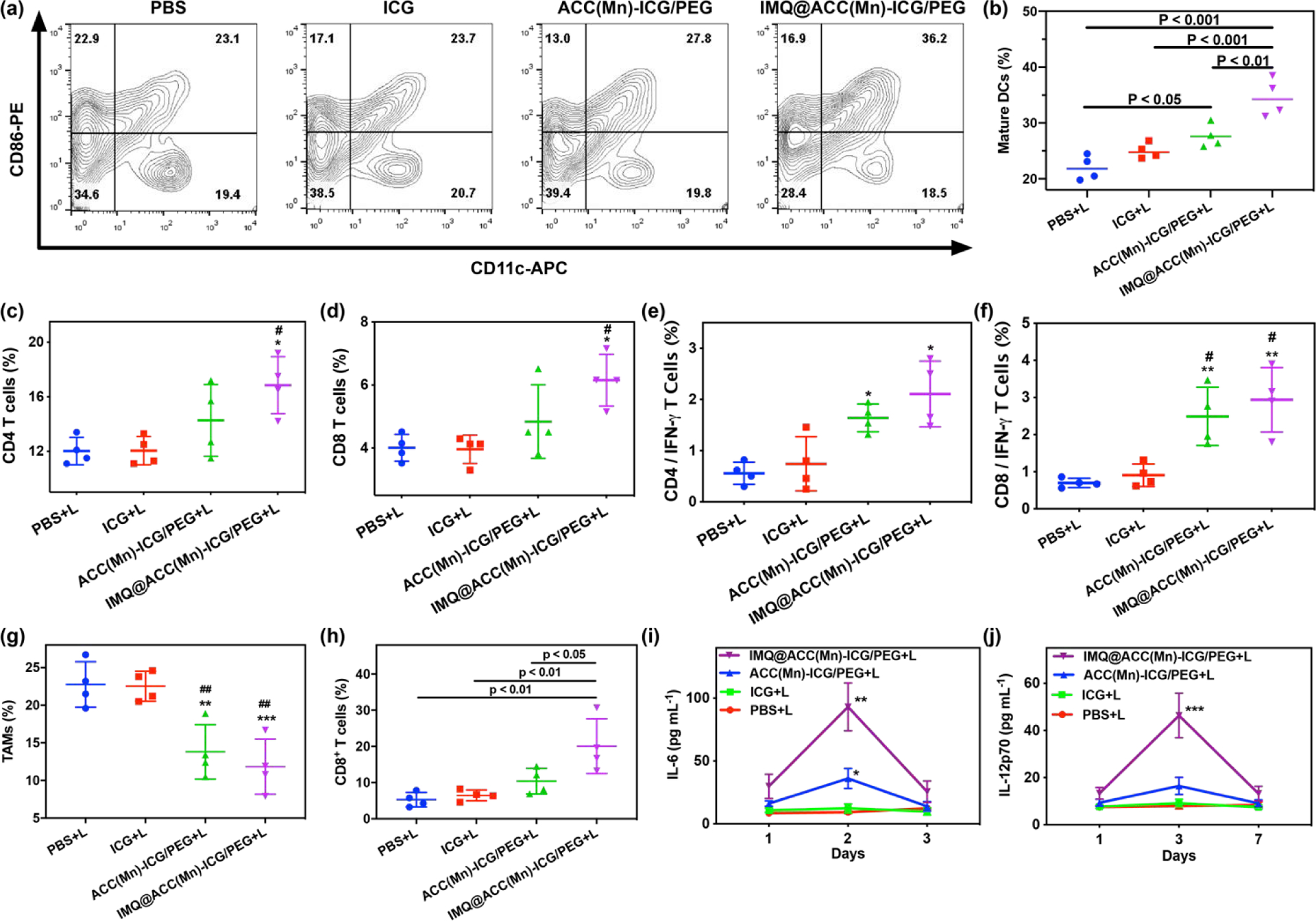
Antitumor immunity of IMQ@ACC(Mn)-ICG/PEG based photoimmunotherapy. (a, b) DC maturation induced by IMQ@ACC(Mn)-ICG/PEG based photoimmunotherapy treatment of mice-bearing 4T1 tumors. (c, d) Flow cytometric analysis of the relative abundance of CD8+ and CD4+ T-cell subpopulations in spleens. (*p < 0.05 vs PBS + laser, #p < 0.05 vs ICG + laser). (e, f) Flow cytometric analysis of IFN-γ secreting T cells in the spleens after being stimulated ex vivo with 4T1 lysates for 24 h. (*p < 0.05 vs PBS + laser, ***p < 0.001 vs PBS + laser, ##p < 0.01 vs ICG + laser). (g) Flow cytometric analysis of the relative abundance of M2-like TAM-cell subpopulations in tumors. (**p < 0.01 vs PBS + laser, ***p < 0.001 vs PBS + laser, ##p < 0.01 vs ICG + laser). (h) Flow cytometric analysis of the relative abundance of CD8+ T-cell subpopulations in distant tumors. (i, j) Cytokine levels in sera from mice isolated at 1, 3, and 7 days post different treatments, respectively. (**p < 0.01 vs PBS + laser, ***p < 0.001 vs PBS + laser). Data are expressed as mean ± SD (n = 4).
Then, the systemic immune effects induced by ACC(Mn)-ICG/PEG based photoimmunotherapy were investigated by analysis of the population and phenotype of T lymphocytes in the spleen. T cells in spleens were collected on 7 day after various treatments, and analyzed via flow cytometry. The results showed significantly increased CD8+ T cells and CD4+ T cells population in the IMQ@ACC(Mn)-ICG/PEG + laser treatment group compared to the other treatment groups (Fig. 7c, d). In addition, to assess whether IMQ@ACC(Mn)-ICG/PEG + laser treatment can elicit systemic T-cell activation, the ex vivo production of antitumor cytokine interferon-γ (IFN-γ) was investigated. As shown in Fig. 7e, f, IMQ@ACC(Mn)-ICG/PEG + laser group had the highest percentage of IFN-γ-secreting CD8+ and CD4+ T cells compared to other groups. We next analyzed the population of M2-like TAMs and the tumor-infiltrating T CaCO3 to scavenge H+, the number of M2-like TAMs in primary tumors dramatically decreased in the IMQ@ACC(Mn)-ICG/PEG + laser treatment group (Fig. 7g; Fig. S24, Supporting Information). Additionally, more infiltrating CD8+ T cells were found in the IMQ@ACC(Mn)-ICG/PEG + laser group compared to other treatment groups (Fig. 7h; Fig. S26, Supporting Information). Immunofluorescence staining also showed a significantly higher expression of CD8+ T cells and an obvious decrease of the percentage of regulatory T cells in the secondary tumors of the IMQ@ACC(Mn)-ICG/PEG + laser treatment group compared to the other groups (Fig. S27, Supporting Information). Furthermore, the cytokines secreted during the ACC(Mn)-ICG/PEG based photoimmunotherapy were measured by ELISA. As displayed in Fig. 7i, j, IMQ@ACC(Mn)-ICG/PEG + laser induced a much higher level of IL-6 and IL-12 compared to other groups. There results indicate that ACC(Mn)-ICG/PEG based photoimmunotherapy can elicit systemic antitumor immune effects.
Experimental
Materials
CaCl2 (96%), MnCl2 4H2O, NH4HCO3 (99%), indocyanine green (ICG), cholesterol, dichloromethane (DCM), and ethanol were purchased from Sigma-Aldrich (USA). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000] (DSPE-PEG2K), DOPA, and DOPC were purchased from Avanti Polar Lipids (USA). Unless otherwise mentioned, all chemicals were used as received and without further purification.
Cell line and animals
The 4T1 cell line was obtained from the American Type Culture Collection (Rockville, USA), and this line was authenticated via morphology, karyotyping, and PCR-based approaches as well as tested for mycoplasma. 4T1 cells were cultured in RPMI 1640 (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 U ml−1 penicillin, and 100 μg mL−1 streptomycin (Gibco). Cell cultures were maintained below 50% confluence and early passage cultures (between four and nine) were utilized for the experiments. Female BALB/c mice (6 weeks, 18–20 g) were provided by Harlan Sprague Dawley Co. (USA). Mice were housed in the animal facility of the Department of Comparative Medicine at the University of Oklahoma Health Sciences Center (OUHSC, Oklahoma, USA). All experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the NIH and approved by the OUHSC Institutional Animal Care and Use Committee.
Synthesis of ICG embedded amorphous calcium carbonate (ACC(Mn)-ICG) NPs
CaCl2 (100 mg) and ICG (0.4 mL, 10 mg mL−1, in aqueous solution) were dissolved in 50 mL absolute ethanol. The mixture was transferred into a glass bottle and covered by parafilm with several pores. Then, the bottle was left in a desiccator along with two glass bottles of ammonia bicarbonate (NH4HCO3) at room temperature. After two days of vapor diffusion, the products were centrifuged at 8,000 rpm and re-dissolved in ethanol solutions containing MnCl2. After 4 h of vigorous stirring, the ACC(Mn)-ICG NPs were collected and purified by repeated centrifugation at 8,000 rpm. Finally, the obtained ACC(Mn)-ICG NPs were dispersed in ethanol and kept at room temperature until further use.
Preparation of DOPA modified ACC(Mn)-ICG/DOPA NPs
ACC(Mn)-ICG NPs were dispersed in DCM (5 mL, 4 mg mL−1), followed by mixing with DOPA at a concentration of 4 mg mL−1. Subsequently, the resulting mixture was sonicated for ~5 min. The obtained ACC(Mn)-ICG/DOPA NPs were precipitated by adding 5 mL ethanol, collected by centrifugation, and finally re-dispersed in DCM.
Preparation of ACC(Mn)-ICG/PEG NPs
ACC(Mn)-ICG/DOPA NPs were dispersed in DCM (5 mL, 4 mg mL−1), followed by mixing with DOPC, cholesterol, and DSPE-PEG2K at concentrations of 4, 2, and 8 mg mL−1, respectively. Subsequently, the resulting mixture was sonicated for ~5 min, and the solvent of DCM was fully removed via rotary evaporation. The residual solid was hydrated by adding 10 mL purified water and sonicated for ~5 min. The resulting ACC(Mn)-ICG/PEG NPs were collected via centrifugation at 13,000 rpm for 10 min, and re-dispersed in 4 mL PBS.
IMQ loading
IMQ is a basic poorly soluble molecule. Due to the weakly alkaline of the ACC(Mn)-ICG/PEG NPs, IMQ can precipitate from the water to the pores of the ACC(Mn)-ICG/PEG NPs. IMQ (0.3 mg mL−1) was dissolved in methanol and the mixture was added to ACC(Mn)-ICG/PEG solutions (5 mg mL−1) and stirred at room temperature overnight. Then, excess IMQ molecules were removed via ultrafiltration (Millipore) with a molecular weight cut off at 200 kDa. The amount of loaded IMQ was quantitatively evaluated via UV–vis-NIR spectrophotometer. The loading capacity of IMQ was calculated using the following equation: loading efficiency of IMQ (%) = ((weight of IMQ) - (weight of unloaded IMQ)) / (weight of IMQ) × 100%.
Conclusions
A bioresponse theranostic NP was successfully constructed by embedding ICG in ACC NPs. After lipid modification, the ACC(Mn)-ICG/PEG NPs showed good physiological stability and exhibited pH-dependent degradation. The ACC(Mn)-ICG/PEG NPs strongly accumulates in tumor tissues and significantly ablates tumors after FL/MR imaging-guided laser irradiation. Furthermore, with the loading of IMQ, ACC(Mn)-ICG/PEG NPs could effectively promote the cancer immunity. Administration of the IMQ@ACC(Mn)-ICG/PEG NPs, followed by NIR laser-triggered activation, demonstrated efficient destruction of primary tumors and inhibition of metastasis by simultaneously boosting specific immune responses. This bioresponse theranostic nanoparticle based biodegradable ACC could become an effective tool for cancer theranostics and offer substantial potential for future clinical translation.
Supplementary Material
Acknowledgements
The acknowledgements come at the end of an article after the conclusions and before the notes and references. We gratefully acknowledge the National Key R&D Program of China (2018YFC0910602), the U.S. National Institutes of Health (RS20132225-106, R01 CA205348, and S10 OD023508), the Oklahoma Center for Advancement of Science and Technology (HR16-085), the National Natural Science Foundation of China (61775145, 61525503, 61620106016, 61835009, and 81727804), the China Postdoctoral Science Foundation (2018M633116), the Guangdong Province Key Area R&D Program (2019B110233004), the Project of Department of Education of Guangdong Province (2015KGJHZ002, and 2016KCXTD007), and the Natural Science Foundation of Fujian Province (2016J05060) for their financial support of this research.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Zhao LY, Liu YM, Chang R, Xing RR and Yan XH, Adv. Funct. Mater, 2019, 29, 1806877. [Google Scholar]
- 2.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW and Hasan T, Chem. Rev, 2010, 110, 2795–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng L, Wang C, Feng LZ, Yang K and Liu Z, Chem. Rev, 2014, 114, 10869–10939. [DOI] [PubMed] [Google Scholar]
- 4.Lucky SS, Soo KC and Zhang Y, Chem. Rev, 2015, 115, 1990–2042. [DOI] [PubMed] [Google Scholar]
- 5.Chu KF and Dupuy DE, Nat. Rev. Cancer, 2014, 14, 199–208. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee DK, Fong LS and Zhang Y, Adv. Drug Deliv. Rev, 2008, 60, 1627–1637. [DOI] [PubMed] [Google Scholar]
- 7.Paszko E, Ehrhardt C, Senge MO, Kelleher DP and Reynolds JV, Photodiagn. Photodyn, 2011, 8, 14–29. [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Li Z, Zhao Y, Yang J, Yang Y, Pendharkar AI, Zhang Y, Kelmar S, Chen L, Wu W, Zhao J and Han G, Adv. Mater, 2017, 29, e1604789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan W, Yung B, Huang P and Chen X, Chem. Rev, 2017, 117, 13566–13638. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Chi C, Wang S, Wang L, Liang P, Liu F, Shang W, Wang W, Zhang F, Li S, Shen H, Yu X, Liu H and Tian J, Adv Mater, 2017, 29, 1700448. [DOI] [PubMed] [Google Scholar]
- 11.Lan G, Ni K, Xu Z, Veroneau SS, Song Y and Lin W, J. Am. Chem. Soc, 2018, 140, 5670–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR and Lin W, Nat. Commun, 2016, 7, 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao W, Ji X, Zhu X, Li L, Wang J, Zhang Y, Saw PE, Li W, Kong N, Islam MA, Gan T, Zeng X, Zhang H, Mahmoudi M, Tearney GJ and Farokhzad OC, Adv Mater, 2018, 30, e1802061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu M, Wang D, Liang W, Liu L, Zhang Y, Chen X, Sang DK, Xing C, Li Z, Dong B, Xing F, Fan D, Bao S, Zhang H and Cao Y, P. Natl. Acad. Sci. USA, 2018, 115, 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Y, Cheng Y, Feng Y, Jian H, Wang L, Ma X, Li X and Zhang H, Nano Lett, 2018, 18, 886–897. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Song J, Zhou F, Hoover AR, Murray C, Zhou B, Wang L, Qu J and Chen WR, Adv. Sci, 2019, 0, 1802157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Xu L, Liang C, Wang C, Peng R and Liu Z, Nat. Commun, 2016, 7, 13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Liu L, Liang R, Luo Z, He H, Wu Z, Tian H, Zheng M, Ma Y and Cai L, ACS Nano, 2018, 12, 8633–8645. [DOI] [PubMed] [Google Scholar]
- 19.Shan W, Chen R, Zhang Q, Zhao J, Chen B, Zhou X, Ye S, Bi S, Nie L and Ren L, Adv Mater, 2018, 30, e1707567. [DOI] [PubMed] [Google Scholar]
- 20.Rankin EB and Giaccia AJ, Science, 2016, 352, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T and Anderson DG, Nat. Rev. Cancer, 2011, 12, 39–50. [DOI] [PubMed] [Google Scholar]
- 22.Couzin-Frankel J, Science, 2013, 342, 1432–1433. [DOI] [PubMed] [Google Scholar]
- 23.Hartshorn CM, Bradbury MS, Lanza GM, Nel AE, Rao J, Wang AZ, Wiesner UB, Yang L and Grodzinski P, ACS Nano, 2018, 12, 24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Ye Y, Hu Q, Bellotti A and Gu Z, Adv. Mater, 2017, 29, 1606036–n/a. [DOI] [PubMed] [Google Scholar]
- 25.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y and Santamaria P, ACS Nano, 2015, 9, 16–30. [DOI] [PubMed] [Google Scholar]
- 26.Ledford H, Nature, 2013, 497, 544. [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Kantoff PW, Wooster R and Farokhzad OC, Nat. Rev. Cancer, 2017, 17, 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardoll DM, Nat. Rev. Cancer, 2012, 12, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castano AP, Mroz P and Hamblin MR, Nat. Rev. Cancer, 2006, 6, 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding B, Shao S, Yu C, Teng B, Wang M, Cheng Z, Wong KL, Ma P and Lin J, Adv. Mater, 2018, 30, e1802479. [DOI] [PubMed] [Google Scholar]
- 31.Im S, Lee J, Park D, Park A, Kim YM and Kim WJ, ACS Nano, 2019, 13, 476–488. [DOI] [PubMed] [Google Scholar]
- 32.Luo L, Zhu C, Yin H, Jiang M, Zhang J, Qin B, Luo Z, Yuan X, Yang J, Li W, Du Y and You J, ACS Nano, 2018, 12, 7647–7662. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, He L, Dong H, Liu Y, Wang K, Li A, Ren T, Shi D and Li Y, Adv. Sci, 2018, 5, 1700805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Jing D, Wang L, Sun Y, Li JJ, Hill B, Yang F, Li Y and Lam KS, Nano Lett, 2018, 18, 7092–7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu KD, He CB, Guo NN, Chan C, Ni KY, Lan GX, Tang HD, Pelizzari C, Fu YX, Spiotto MT, Weichselbaum RR and Lin WB, Nat. Biomed. Eng, 2018, 2, 600–+. [DOI] [PubMed] [Google Scholar]
- 36.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P and Vandenabeele P, Nat. Rev. Cancer, 2012, 12, 860–875. [DOI] [PubMed] [Google Scholar]
- 37.Krysko O, Love Aaes T, Bachert C, Vandenabeele P and Krysko DV, Cell Death Dis, 2013, 4, e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubin MM, Artyomov MN, Mardis ER and Schreiber RD, J. Clin. Invest, 2015, 125, 3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schumacher TN and Schreiber RD, Science, 2015, 348, 69–74. [DOI] [PubMed] [Google Scholar]
- 40.Ling D, Park W, Park SJ, Lu Y, Kim KS, Hackett MJ, Kim BH, Yim H, Jeon YS, Na K and Hyeon T, J Am Chem Soc, 2014, 136, 5647–5655. [DOI] [PubMed] [Google Scholar]
- 41.Yang GB, Liu JJ, Wu YF, Feng LZ and Liu Z, Coordin. Chem. Rev, 2016, 320, 100–117. [Google Scholar]
- 42.Min KH, Min HS, Lee HJ, Park DJ, Yhee JY, Kim K, Kwon IC, Jeong SY, Silvestre OF, Chen X, Hwang YS, Kim EC and Lee SC, ACS Nano, 2015, 9, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong ZL, Feng LZ, Zhu WW, Sun XQ, Gao M, Zhao H, Chao Y and Liu Z, Biomaterials, 2016, 110, 60–70. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Ji X, Tong WWL, Askhatova D, Yang T, Cheng H, Wang Y and Shi J, Angew. Chem. Int. Edit, 2018, 57, 1510–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, Wang J, Wen D, Zhang Y, Lu Y, Yang G, Jiang C, Wang J, Dotti G and Gu Z, Nat. Biotechnol, 2019, 14, 89–97. [DOI] [PubMed] [Google Scholar]
- 46.Ruan H, Hu Q, Wen D, Chen Q, Chen G, Lu Y, Wang J, Cheng H, Lu W and Gu Z, Adv Mater, 2019, 31, e1806957. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Yan Y, Tan J, Yang D, Jia X, Wang L, Xu Y, Cao S and Sun S, Adv. Funct. Mater, 2019, 0, 1808146. [Google Scholar]
- 48.Dong Z, Feng L, Hao Y, Chen M, Gao M, Chao Y, Zhao H, Zhu W, Liu J, Liang C, Zhang Q and Liu Z, J. Am. Chem. Soc, 2018, 140, 2165–2178. [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Chen S, Wang Y, Liu X, Hu F, Sun J and Yuan H, Adv. Mater, 2018, 30, e1706407. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Marchesi F, Malesci L Laghi, Allavena P, Nat. Rev. Clin. Oncol, 2017, 14, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngambenjawong C, Gustafson HH, Pun SH, Adv. Drug Del. Rev, 2017, 114, 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R, Nature, 2014, 513, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Y, Aimetti AA, Langer R, Gu Z, Nat. Rev. Mater 2016, 2, 16075. [Google Scholar]
- 54.Yu J, Javier D, Yaseen MA, Nitin N, Richards-Kortum R, Anvari B and Wong MS, J. Am. Chem. Soc 2010, 132, 1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirstein S and Daehne S, Int. J. Photoenergy 2006, 020363. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


