Abstract
Senescence Accelerated Mouse-Prone 8 (SAMP8) mice exhibit characteristics of premature aging, including hair loss, cognitive dysfunction, reduced physical activity, impaired metabolic homeostasis, cardiac dysfunction, and reduced lifespan. Interestingly, circadian disruption can induce or augment many of these same pathologies. Moreover, previous studies have reported that SAMP8 mice exhibit abnormalities in circadian wheel running behavior, indicating possible alterations in circadian clock function. These observations led to the hypothesis that 24h rhythms in behavior and/or circadian clock function are altered in SAMP8 mice, and that these alterations may contribute to perturbations in whole body metabolism. Here, we report that 6 month old SAMP8 mice exhibit a more prominent biphasic pattern in daily behaviors (food intake and physical activity) and whole body metabolism (energy expenditure, respiratory exchange ratio), relative to SAMR1 control mice. Consistent with a delayed onset of food intake at the end of the light phase, SAMP8 mice exhibit a phase delay (1.3-1.9 h) in 24h gene expression rhythms of major circadian clock components (bmal1, rev-erbα, per2, dbp) in peripheral tissues (liver, skeletal muscle, white adipose tissue [WAT], brown adipose tissue [BAT]). Forcing mice to consume food only during the dark period improved alignment of both whole body metabolism and oscillations in expression of clock genes in peripheral tissues between SAMP8 and SAMR1 mice. Next, interrogation of metabolic genes revealed altered expression of thermogenesis mediators (ucp1, pgc1α, dio2) in WAT and/or BAT in SAMP8 mice. Interestingly, SAMP8 mice exhibit a decreased tolerance to an acute (5h) cold challenge. Moreover, SAMP8 and SAMR1 mice exhibited differential responses to a chronic (1 wk) decrease in ambient temperature; the greatest response in whole body substrate selection was observed in SAMR1 mice. Collectively, these observations reveal differential behaviors (e.g., 24h food intake patterns) in SAMP8 mice that are associated with perturbations in peripheral circadian clocks, metabolism, and thermogenesis.
INTRODUCTION
Aging is characterized by a progressive decline in physiologic integrity, increased susceptibility to pathology, and ultimately death (Lopez-Otin 2013). Genomic alterations, impairments in proteostatic mechanisms, mitochondrial dysfunction, cellular senescence, and neurohumoral perturbations are all associated with aging (Lopez-Otin 2013). Specific examples include mutations in nuclear and mitochondrial DNA, variations in telomere length, epigenetic modifications, abnormal protein synthesis/folding/turnover, energetic deficits, reactive oxygen species generation, and inflammation (Lopez-Otin 2013). Many of these physiologic perturbations have also been implicated in the pathogenesis of age-onset diseases, such as cancer, cardiometabolic diseases (e.g., obesity), neurodegenerative diseases (e.g., Alzheimer’s disease), and cardiovascular diseases (e.g., hypertension) (Forstermann 2008; Fernandez-Sanchez 2011; Hanahan and Weinberg 2011; Lassmann 2011). To date, however, no single mechanism links aging with homeostatic decline and pathogenesis.
Virtually all aspects of biology vary as a function of time of day, thereby allowing organisms to maintain processes within a physiologic range despite dramatic fluctuations in environmental stimuli and stressors (e.g., light/dark cycle) and behaviors (e.g., sleep/wake and feeding/fasting cycles) (Edery 2000). What has become apparent is that biological processes do not simply respond to changes in the environment over the 24h period (i.e., adaptation), but also prepare for daily fluctuations before they occur (i.e., anticipation). The selective advantage of anticipation is conferred by circadian clocks, cell autonomous molecular mechanisms comprising a series of positive and negative feedback loops with a free running period of approximately 24h (Takahashi 2008). Circadian clocks modulate cellular and organ function through a series of transcriptional and post-translational mechanisms, involving temporal governance over fundamental processes such as signaling, ion homeostasis, cellular constituent turnover, cell cycle gating, and metabolism (Takahashi 2008). Genetic, environmental, and/or behavioral alterations leading to circadian clock disruption increases risk of numerous pathologies, many of which are observed with aging (e.g., diabetes mellitus, cognitive impairments, and cardiovascular disease) (Hood and Amir 2017). Indeed, mice with genetic deletion of a core circadian clock component (brain and muscle ARNT-like 1; BMAL1) exhibit a number of age-onset characteristics (e.g., hair loss, muscle wasting, joint calcification), as well as reduced lifespan (Bunger 2005). Moreover, such mouse models display increased susceptibility to numerous environmental stressors, resulting in pathologic outcomes (Evans and Davidson 2013). With aging, circadian regulation of biological processes declines, manifesting at the level of diminished time-of-day-dependent oscillations in numerous fundamental processes, including neuroendocrine stimulation, metabolism, and body temperature regulation (Riera and Dillin 2015; Hood and Amir 2017). Such observations have led to suggestions that a decline in circadian governance of cellular processes may play a causal role in the development of age-related diseases.
In an effort to enhance our understanding of age-related diseases, a number of animal models have been developed and employed. This includes a number of Senescence Accelerated Mouse-Prone (SAMP) lines, which were developed through backcrossing of AKR/J mice and subsequent phenotyping for age-related traits (Takeda 1981). Both SAMP and Senescence Accelerated Mouse-Resistant (SAMR) lines were identified, of which SAMP8 and SAMR1 lines are most commonly utilized for studying age-related pathologies (particularly neurological defects) (Nomura and Okuma 1999). Indeed, by 6 months of age, SAMP8 mice develop significant neurodegeneration, cardiovascular abnormalities, and impaired metabolic homeostasis (compared to age-matched SAMR1 controls) (Akiguchi 2017; Barquissau 2017; Karuppagounder 2017). Interestingly, 7-month old (but not 2-month old) SAMP8 mice exhibit fragmented 24h rhythms in wheel running, suggesting impairment of the central circadian clock within the suprachiasmatic nucleus (SCN) (Pang 2006). In contrast, circadian clocks in peripheral tissues have not been investigated in SAMP8 mice. The purpose of the present study was therefore to test the hypothesis that 24h rhythms in behavior and/or circadian clock function are altered in SAMP8 mice, and that these alterations potentially perturb metabolic homeostasis. Here, we report that 6 month old SAMP8 mice exhibit a more prominent biphasic 24h pattern in behaviors (food intake and physical activity) and whole body metabolism (energy expenditure, respiratory exchange ratio [RER]), relative to control (Senescence Accelerated Mouse-Resistant 1 [SAMR1]) mice. Consistent with a delayed onset of food intake at the end of the light (less active) period in SAMP8 mice, analysis of 24h gene expression profiles indicated that circadian clocks are phase delayed in SAMP8 peripheral tissues (liver, skeletal muscle, white adipose tissue [WAT], brown adipose tissue [BAT]) by 1.3-1.9h. Forcing mice to consume food only during the dark (more active) period improved alignment of both whole body metabolism and oscillations in expression of clock genes in peripheral tissue between the two mouse strains. Interrogation of metabolic genes revealed altered expression of thermogenesis mediators (ucp1, pgc1α, dio2) in WAT and/or BAT of SAMP8 mice, associated with a differential response to either an acute (5h) or a chronic (1 wk) decrease in ambient temperature. Collectively, these observations reveal differential behaviors (e.g., 24h food intake patterns) in SAMP8 mice that are associated with perturbations in peripheral circadian clocks, metabolism, and thermogenesis.
MATERIALS AND METHODS
Mice.
The current study utilized male SAMP8 and SAMR1 mice (Harlan Laboratories Inc). All mice were 6 months old (at the time of euthanasia), and were housed at the Animal Resource Program at the University of Alabama at Birmingham (UAB), under temperature-, humidity-, and light- controlled conditions. A strict 12h light/12h dark cycle regime was enforced (lights on at 06:00h; zeitgeber time [ZT] 0). The average light intensity during lights on was 325 lux (3.3ft above the floor). The light/dark cycle was maintained throughout these studies; as such, physiologic diurnal variations were investigated in mice (as opposed to circadian rhythms). The purpose of maintaining the light/dark cycle was that prior studies indicate that SAMP8 mice exhibit fragmented 24h wheel running patterns when housed under a standard light/dark cycle (Pang 2006). All mice had free access to water. When in the fed state, mice were provided a standard rodent chow (Labdiet NIH-31 Irradiated). At the time of tissue and blood collection, mice were anesthetized with pentobarbital. All animal experiments were approved by the Institutional Animal Care and Use Committee of UAB. All experimental protocols conform to international ethical standards dealing with biological rhythm research (Portaluppi 2010).
Chronic Behavioral/Environmental Protocols and Non-Invasive Mouse Monitoring.
Two behavioral/environmental interventions were performed in a chronic manner (i.e., 1-2 wk) and were enforced by a computer-controlled Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments Inc., Columbus, OH). For the time-of-day-dependent food access study, mice were allowed access to food either during the entire 24h period (i.e., ad libitum fed mice) or only during the 12h dark period (i.e., dark period [DP] fed mice); these feeding regimes were enforced by the CLAMS for a 2 week period, thereby allowing sufficient equilibration time. For the temperature study, mice were housed in the CLAMS for a 2 wk period at 28°C (i.e., close to thermoneutrality), followed by 1 wk at 22°C (i.e., below room temperature); during this time, all mice were fed in an ad libitum manner. The CLAMS also continuously assessed food intake, physical activity (beam breaks), energy expenditure (indirect calorimetry), and RER, as described previously (Bray 2013). In all studies, mice were singly housed and acclimatized to their housing conditions for at least 1 wk before the start of the experimental protocol.
Acute Temperature Challenge.
To evaluate the thermogenic response of mice to an acute cold challenge, mice were exposed to 4°C in a cold room starting at ZT6. Core body temperature was measured by using a rectal thermometer (MicroTherma 2T Hand Held Thermometer with RET3 probe) at 1h intervals, up to 5h. During this cold challenge, food was removed. At the end of the cold challenge, the mice were euthanized, followed immediately by tissue collection.
Quantitative RT-PCR.
RNA was extracted from tissues by use of standard procedures (Chomczynski and Sacchi 1987). Candidate gene expression analysis was performed by quantitative RT-PCR, through use of methods described previously (Gibson 1996; Heid 1996). Specific Taqman assays were designed for each gene from mouse and rat sequences available in GenBank. Sequence information has been published previously for the majority of the Taqman assays: bmal1 (Young 2014), per2 (Young 2001), rev-erbα (Young 2014), dbp (Young 2014), lpk (Bray 2013), accα (Bray 2013), glut4 (Young 2001), pdk4 (Tsai 2010), mcpt1 (Young 2001), mcad (Young 2001), atgl (Tsai 2010), lipe (Tsai 2010), pgc1α (Stavinoha 2004), and dio2 (Peliciari-Garcia 2016). Additional primer and probe information is as follows: glk (5’-ATGGCTCCGTGTACAAGCTG-3’ [forward], 5’-ATTCGATGAAGGTGATTTCGC-3’ [reverse], 5’-FAM-CGAGCTTCAAGGAGCGGTTTCACG-TAMRA-3’ [probe]), lgp (5’-GCTTGCAGCCTATGGATACG-3’ [forward], 5’- GACGAGCCTTCTCCCAAGG-3’ [reverse], 5’-FAM-CAGTCATCTGCCTCCTCTACCTGCCA-TAMRA-3’ [probe]), and ucp1 (5’-AAGCGTACCAAGCTGTGCGA-3’ [forward], 5’-AGAAAAGAAGCCACAAACCCTT-3’ [reverse], 5’-FAM-CCATGTACACCAAGGAAGGACCGACG-TAMRA-3’ [probe]). Quantitative RT-PCR data are presented as the fold change from the trough value in a specified group.
Immunohistochemistry.
Adipose tissue was fixed in 4% paraformaldehyde in phosphate buffered saline at 4°C overnight and then washed with PBS and stored in 70% ethanol at room temperature before paraffin imbedding and immunohistochemistry analysis. Anti-UCP1 antibody (Abcam, San Francisco, CA) was applied to sections at a final concentration of 1μg/ml in 1.5% goat serum, and the slides were incubated overnight at 4°C. A Leica DMRB equipped with a Leica DFC480 digital camera system was used to acquire images, which were processed by using Xnview software.
Statistical analysis.
Statistical analyses were performed using one- and two- way ANOVA, as well as repeated measures ANOVA, as described previously (Bray 2008; Bray 2013; Peliciari-Garcia 2018). Briefly, analyses were performed with Graph Pad Prism (GraphPad Software version 6.01, San Diego, CA, USA) to investigate main effects of time and/or nutritional status, followed by Bonferroni post hoc analyses for pair-wise comparisons (indicated in Figures). Cosinor analyses were performed for assessment of mesor (average value over 24-h period), amplitude (peak-to-mesor difference), and acrophase (timing of peak; ZT), as described previously (Peliciari-Garcia 2018). When appropriate, Student’s t-test was also applied to these parameters. In all analyses, the null hypothesis of no model effects was rejected at p<0.05 (2-tailed).
RESULTS
Diurnal Rhythms in Behavior and Metabolism in SAMR1 and SAMP8 Mice.
At 6 months of age, body weight of SAMP8 mice is significantly lower than that of SAMR1 mice (Table 1). We therefore sought to non-invasively assess diurnal rhythms in behavior (physical activity, food intake) and metabolism (energy expenditure, RER) in ad libitum fed SAMP8 and SAMR1 mice. Accordingly, mice were housed in CLAMS cages for 3 wk (1-wk equilibration period, followed by 2 wk assessment period). SAMR1 mice exhibited a biphasic pattern in food intake, with an initial trough at approximately ZT5 (light period) and a secondary trough at approximately ZT23 (dark period; Figure 1Ai). A biphasic pattern in food intake was also observed in SAMP8 mice, although the timing of the troughs differed. In SAMP8 mice, the troughs occurred at approximately ZT6 and ZT21 (Figure 1Ai). Diurnal rhythms in food intake were mirrored closely by patterns in physical activity, energy expenditure, and RER (Figure 1Aii-iv). In contrast, no significant differences in total daily averages of these 4 variables were observed between SAMR1 and SAMP8 mice (Table 1). Collectively, these data suggest that SAMP8 mice have a differential biphasic pattern in behaviors and whole body metabolism (relative to SAMR1 mice), with an apparent phase delay during the light (less active) period, and an apparent phase advance during the dark (more active) period.
Table 1.
Body weight, as well as food intake, physical activity, energy expenditure, and RER in ad libitum and dark phase restricted fed SAMR1 and SAMP8 mice. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). Body weight was assessed after 2 wk of the restricted feeding (or ad libitum) protocol was enforced. Food intake, physical activity, energy expenditure, and RER were monitored continuously using a CLAMS; restricted feeding was enforced in an automated manner by a CLAMS. Data are reported as mean ± SEM for 15-26 mice per experimental group.
| SAMR1 Ad Libitum | SAMP8 Ad Libitum | SAMR1 Restricted Fed | SAMP8 Restricted Fed | |
|---|---|---|---|---|
| Body Weight (g) | 34.6 ± 0.6 | 29.1 ± 0.9* | 34.9 ± 0.5 | 30.2 ± 0.9* |
| 24h Cumulative Food Intake (g/24-hr) | 3.93 ± 0.10 | 4.10 ± 0.14 | 3.82 ± 0.13 | 4.08 ± 0.14 |
| 24h Cumulative Physical Activity (beam breaks; A.U.) | 31889 ± 1782 | 35857 ± 2063 | 34004 ± 2264 | 32835 ± 2089 |
| 24h Average Energy Expenditure (kcals/hr) | 0.464 ± 0.013 | 0.440 ± 0.015 | 0.467 ± 0.011 | 0.452 ± 0.015 |
| 24h Average Respiratory Exchange Ratio | 0.870 ± 0.005 | 0.858 ± 0.007 | 0.859 ± 0.007 | 0.849 ± 0.004 |
| 12h Light Phase Cumulative Food Intake (g/12h) | 1.22 ± 0.07 | 1.11 ± 0.09 | 0.33 ± 0.04# | 0.25 ± 0.04# |
| 12h Light Phase Cumulative Physical Activity (beam breaks; A.U.) | 10938 ± 652 | 13818 ± 1030 | 11182 ± 1037 | 10339 ± 954 |
| 12h Light Phase Average Energy Expenditure (kcals/hr) | 0.421 ± 0.012 | 0.394 ± 0.018 | 0.396 ± 0.010 | 0.374 ± 0.013 |
| 12h Light Phase Average Respiratory Exchange Ratio | 0.828 ± 0.005 | 0.815 ± 0.007 | 0.775 ± 0.005# | 0.776 ± 0.005# |
| 12hr Dark Phase Cumulative Food Intake (g/12h) | 2.71 ± 0.06 | 2.99 ± 0.13 | 3.49 ± 0.12# | 3.81 ± 0.13# |
| 12h Dark Phase Cumulative Physical Activity (beam breaks; A.U.) | 20951 ± 1304 | 22038 ± 1478 | 22822 ± 1636 | 22495 ± 1506 |
| 12h Dark Phase Average Energy Expenditure (kcals/hr) | 0.507 ± 0.014 | 0.484 ± 0.015 | 0.538 ± 0.014 | 0.531 ± 0.017 |
| 12h Dark Phase Average Respiratory Exchange Ratio | 0.912 ± 0.006 | 0.902 ± 0.008 | 0.943 ± 0.008# | 0.922 ± 0.005 |
p<0.05 for pair wise comparison between strains subjected to the same feeding protocol.
p<0.05 for pair wise comparison between feeding protocols within the same strain.
It is noteworthy that minimal (<9% daily average) food intake was measured during the light phase in the restricted feeding groups; this was primarily due to the temporal resolution of the assessments performed (15-min intervals/duration), some of which spanned the light-to-dark and dark-to-light transitions
Figure 1.

Diurnal variations in food intake (i), physical activity (ii), energy expenditure (iii), and RER (iv) in ad libitum fed (A; solid line) and dark phase restricted fed (B; dashed line) SAMR1 and SAMP8 mice. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). All parameters were monitored continuously using a CLAMS; restricted feeding was enforced in an automated manner by a CLAMS. Data are reported as mean ± SEM, at 15 min intervals (i.e., average of last 2 d of a 2 wk period), for 15-17 mice per experimental group. Food intake, physical activity, and energy expenditure units are g/15 min, beam breaks/15 min, and kcals/hr, respectively. Main effects for time and/or mouse strain are reported at the top of the figure panels.
Behaviors such as food intake and physical activity can dramatically influence whole body metabolism. We therefore hypothesized that perturbations in behaviors might contribute toward differences in metabolism. To test this hypothesis, we standardized the timing of food intake by subjecting both mouse strains to restricted feeding during the dark (more active) period. In general, the biphasic nature of all whole body parameters investigated was either attenuated or abolished in both strains of mice, and the 24h patterns in these parameters tended to exhibit greater similarity between the two strains during dark period restricted feeding than during ad libitum feeding (Figure 1B). However, some subtle differences remained. For example, during the first 15 min of food presentation at the start of the dark period, SAMR1 mice consumed approximately twice as much food than did SAMP8 mice (Figure 1Bi). Thereafter, the rate of food intake was similar, except toward the end of the dark period, when SAMR1 mice ate less food (Figure 1Bi). Similarly, physical activity was higher in SAMR1 mice (relative to SAMP8 mice) during the early portion of the dark period, but was lower towards the end of the dark period (Figure 1Bii). Interestingly, the onset of physical activity prior to food availability (termed food anticipatory activity; FAA) was more prominent in SAMR1 versus SAMP8 mice (Figure 1Bii). Both energy expenditure and RER exhibited oscillations with a periodicity of 24h (p<0.05); overall, these rhythms were fairly similar between the two strains of mice. Collectively, these data suggest that dark period restricted feeding improved the alignment of diurnal rhythms in food intake, physical activity, energy expenditure, and RER between SAMP8 and SAMR1 mice (relative to ad libitum feeding).
Diurnal Rhythms in Expression of Clock Genes in Peripheral Tissues.
Circadian clocks regulate various biological processes, including metabolism (Kohsaka and Bass 2007; Bailey 2014). Furthermore, clock function can be influenced by behaviors, such as food intake (Damiola 2000; Bray 2013). Accordingly, we next decided to investigate the expression of genes encoding circadian clock components (bmal1, rev-erbα, per2) and output (dbp) in metabolically-relevant tissues (liver, skeletal muscle, WAT, BAT) isolated from SAMR1 and SAMP8 mice at 4h intervals over the 24h period. Out of the 16 assessments (i.e., 4 genes in 4 tissues), a significant phase delay (i.e., larger acrophase value) was observed in SAMP8 mice (relative to SAMR1 mice) for 10 comparisons (Figure 2 and Table 2). The average acrophase difference for the four genes assessed between SAMR1 and SAMP8 mice was 1.33-h, 1.89h, 1.65h, and 1.91h, for liver, skeletal muscle, WAT, and BAT, respectively (Figure 4). In addition, gene- and tissue -specific differences in amplitude were also observed. For example, a significant increase in amplitude was observed for per2 in skeletal muscle isolated from SAMP8 mice (Figure 2Bii). Interestingly, the mesor (average daily expression) of rev-erbα tended to be higher in three of four tissues investigated (i.e., skeletal muscle, WAT, and BAT; Figure. 2Biii, 2Ciii, and 2Diii, respectively). Collectively, these data suggest that peripheral clocks are phase delayed in SAMP8 mice (in addition to tissue- and gene- specific differences in amplitude/mesor).
Figure 2.


Diurnal variations in expression of bmal1 (i), per2 (ii), rev-erbα (iii), and dbp (iv) in liver (A), skeletal muscle (B), WAT (C), and BAT (D) isolated from ad libitum fed (solid line) SAMR1 and SAMP8 mice. Tissues were isolated from mice at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, followed by assessment of gene expression by RT-PCR. Data are reported as mean ± SEM, for 4-5 mice per experimental group. Main effects for time and/or mouse strain are reported at the top of the figure panels. *, p<0.05 for pair wise comparison within a ZT.
Table 2.
Cosinor analysis of circadian clock genes in Liver, Skeletal muscle, white adipose tissue (WAT), and brown adipose tissue (BAT, isolated from SAMR1 and SAMP8 subjected to either ad libitum feeding or dark phase restricted feeding.
| SAMR1 Ad Libitum | SAMP8 Ad Libitum | SAMR1 Restricted Fed | SAMP8 Restricted Fed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mesor | Amplitude | Acrophase | Mesor | Amplitude | Acrophase | Mesor | Amplitude | Acrophase | Mesor | Amplitude | Acrophase | |
| Liver | ||||||||||||
| bmal1 | 9.93±0.25 | 10.39±0.36 | 21.07±0.08 | 10.17±0.51 | 10.92±0.72 | 22.31±0.15* | 10.71±0.99 | 10.54±1.40 | 22.10±0.30* | 10.88±0.44 | 11.62±0.62 | 22.49±0.12 |
| dbp | 44.80±15.09 | 67.09±21.34 | 7.44±1.13 | 38.73±9.09 | 55.10±12.85 | 9.56±0.53 | 51.24±16.84 | 81.02±23.81 | 9.11±1.07 | 32.57±7.93 | 47.17±11.21 | 10.08±0.54 |
| rev-erbα | 32.26±9.41 | 48.06±13.31 | 5.39±1.03 | 31.93±7.72 | 44.71±10.92 | 6.51±0.56 | 29.05±8.45 | 41.13±11.94 | 6.46±1.07 | 28.18±9.87 | 41.85±13.95 | 7.24±1.16 |
| per2 | 3.63±0.18 | 2.80±0.26 | 13.23±0.21 | 2.86±0.25 | 2.12±0.36 | 14.08±0.38 | 3.43±0.43 | 3.19±0.61 | 15.40±0.44* | 2.97±0.24 | 2.51±0.34 | 15.53±0.31 |
| Skeletal Muscle | ||||||||||||
| bmal1c | 2.62±0.09 | 1.87±0.12 | 20.31±0.15 | 2.85±0.06 | 2.11±0.08 | 22.16±0.09* | 2.89±0.09 | 2.29±0.13 | 22.00±0.13* | 3.04±0.17 | 2.28±0.24 | 23.30±0.24@,# |
| dbpc | 4.42±0.32 | 4.13±0.46 | 7.09±0.25 | 4.61±0.31 | 4.66±0.43 | 9.40±0.21* | 4.83±0.90 | 5.60±1.28 | 8.56±0.52 | 4.47±0.54 | 4.60±0.76 | 9.30±0.38 |
| rev-erbα | 2.85±0.23 | 2.36±0.33 | 2.43±0.32 | 3.34±0.30 | 2.39±0.42 | 4.12±0.40 | 2.62±0.25 | 2.03±0.35 | 4.43±0.39 | x | x | x |
| per2 | 2.43±0.12 | 1.05±0.17 | 9.59±0.37 | 3.12±0.22 | 2.29±0.31* | 11.31±0.31* | 2.56±0.09 | 1.60±0.12 | 10.47±0.18 | 2.81±0.19 | 1.68±0.26 | 11.57±0.36 |
| WAT | ||||||||||||
| bmal1 | 7.05±0.88 | 8.09±1.24 | 21.48±0.35 | 7.94±0.75 | 8.91±1.06 | 23.53±0.27* | 7.67±0.71 | 8.65±1.00 | 22.36±0.26 | 7.85±0.46 | 7.93±0.66 | 23.00±0.19 |
| dbp | 11.18±1.62 | 12.71±2.29 | 8.57±0.41 | 11.68±1.44 | 13.57±2.03 | 11.19±0.34* | 11.91±2.02 | 13.54±2.85 | 10.06±0.48 | 12.81±2.08 | 13.77±2.94 | 11.30±0.49 |
| rev-erbα | 9.80±0.41 | 9.85±0.58 | 6.56±0.14 | 12.74±1.27 | 13.76±1.80 | 8.15±0.30* | 10.08±1.32 | 10.60±1.87 | 7.07±0.40 | 11.09±0.14 | 11.04±0.19 | 9.13±0.04*# |
| per2 | 2.50±0.27 | 1.86±0.38 | 13.10±0.47 | 2.47±0.10 | 1.63±0.14 | 13.45±0.20 | 2.52±0.16 | 1.78±0.23 | 14.49±0.30 | 2.69±0.23 | 1.90±0.32 | 14.37±0.39 |
| BAT | ||||||||||||
| bmal1 | 6.29±0.52 | 6.48±0.73 | 21.49±0.26 | 7.24±0.31 | 7.48±0.44 | 23.11±0.13* | 7.50±0.72 | 8.59±1.02 | 23.03±0.27* | 7.63±0.57 | 8.09±0.80 | 24.00±0.23 |
| dbp | 12.58±2.32 | 15.46±3.28 | 8.09±0.49 | 13.11±1.35 | 15.56±1.91 | 10.36±0.28* | 13.47±2.52 | 17.48±3.56 | 9.34±0.47 | 11.65±2.14 | 14.01±3.02 | 10.34±0.49 |
| rev-erbα | 7.37±1.28 | 9.09±1.81 | 5.22±0.46 | 9.74±1.00 | 10.81±1.41 | 7.01±0.30 | 7.10±0.63 | 8.10±0.89 | 6.34±0.25 | x | x | x |
| per2 | 5.08±0.09 | 3.96±0.13 | 11.55±0.08 | 5.27±0.63 | 4.67±0.89 | 13.50±0.44* | 5.47±0.64 | 5.47±0.90 | 13.52±0.38* | 5.35±0.68 | 5.30±0.96 | 14.47±0.42 |
Bonferroni’s post hoc test
P<0.05 vs SAMR1 Ad Libitum, SAMP8 Ad Libitum, and SAMR1 Restricted Fed respectively.
no significant rhythmicity observed following cosinor analysis.
Cosinor analyses were performed for assessment of mesor (average value over 24-h period), amplitude (peak-to-mesor difference), and acrophase (timing of peak; ZT). Original data for gene expression are shown in Figures 2 and 3 as fold change from SAMR1 trough; accordingly units for mesor and amplitude are considered arbitrary units. Acrophase is the ZT at which expression peaks; units are therefore hours.
Figure 4.

Average phase difference in expression of circadian clock component/output genes in peripheral tissue between SAMR1 and SAMP8 mice during ad libitum (AL) fed and dark phase (DP) restricted fed conditions. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). Tissues were isolated from mice at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, followed by assessment of gene expression by RT-PCR. Phase differences were determined between SAMR1 and SAMP8, for those genes that significantly fit a cosine curve (see Table 2). Data are reported as mean ± SEM, for 3-4 genes that fit a cosine curve per tissue. *, p<0.05 for independent t-tests within a tissue.
The timing of food intake following a fast can influence the phase of peripheral circadian clocks (Damiola 2000; Bray 2013). Given that ad libitum fed SAMP8 mice exhibited an apparent delay in onset of food intake during the light (less active) period (Figure 1Ai), we hypothesized that the phase delay in oscillations of clock genes in peripheral tissues was secondary to feeding behaviors. To test this hypothesis, we assessed the expression of circadian clock genes in peripheral tissues isolated from dark period restricted fed SAMR1 and SAMP8 mice. Out of the 16 assessments (i.e., 4 genes in 4 tissues), a significant phase delay (i.e., later acrophase value) was observed in restricted fed SAMR1 mice (relative to ad libitum fed SAMR1 mice) for 5 comparisons (Figures 2-3 and Table 2). In contrast, restricted feeding only significantly phase delayed bmal1 in skeletal muscle of SAMP8 mice (relative to ad libitum fed SAMP8 mice; Figures 2-3 and Table 2). The average acrophase difference of the four genes assessed between restricted fed SAMR1 and restricted fed SAMP8 mice was 0.57h, 1.05h, 0.96h, and 0.97h, for liver, skeletal muscle, WAT, and BAT, respectively (Figure 4). Collectively, dark period restricted feeding appeared to selectively phase shift oscillations in expression of clock genes in peripheral tissues of SAMR1 mice, resulting in improved alignment with the expression of these genes in SAMP8 mice.
Figure 3.


Diurnal variations in expression of bmal1 (i), per2 (ii), rev-erbα (iii), and dbp (iv) in liver (A), skeletal muscle (B), WAT (C), and BAT (D) isolated from dark phase restricted fed (dashed line) SAMR1 and SAMP8 mice. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). Tissues were isolated from mice at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, followed by assessment of gene expression by RT-PCR. Data are reported as mean ± SEM, for 4-5 mice per experimental group. Main effects for time and/or mouse strain are reported at the top of the figure panels. *, p<0.05 for pair wise comparison within a ZT.
Diurnal Variations in Expression of Metabolic Genes in Peripheral Tissues.
Peripheral circadian clocks influence metabolism through a number of transcriptional (and post-translational) mechanisms (Cox and Takahashi 2019). We therefore investigated 24h rhythms in the expression of candidate genes encoding for metabolically-relevant proteins, in the peripheral tissues of ad libitum and dark phase restricted fed SAMR1 and SAMP8 mice. In the liver, glycolytic genes encoding for glucokinase (glk) and liver pyruvate kinase (lpk) exhibited significant time-of-day-dependent variations in tissues isolated from ad libitum fed mice (Figure 5Ai-ii). Similar to the circadian clock genes, oscillations in glk mRNA appeared to be phase advanced in SAMR1 versus SAMP8 livers during ad libitum feeding; dark period restricted feeding improved alignment between the two mouse strains (Figure 5Bi). In contrast, 24h patterns in lpk mRNA were similar between SAMR1 and SAMP8 livers, independent of the feeding regime (Figures 5Aii and 5Bii). The glycogenolytic gene encoding for liver glycogen phosphorylase (lgp) exhibited a time-of-day-dependent variation in both SAMR1 and SAMP8 mouse livers; a significant cosine oscillation was observed only for ad libitum fed SAMP8 mice (Figure 5Aiii); during dark period restricted feeding, lgp mRNA levels appeared to selectively increase at ZT15 only in SAMR1 livers (Figure 5Biii). The lipogenic gene encoding for acetyl-CoA carboxylase alpha (accα) did not exhibit significant time-of-day-dependent variations in SAMR1 and SAMP8 livers (Figure 5Aiv). Interesting, independent of feeding status, accα mRNA levels were significantly lower in SAMP8 than in SAMR1 livers (Figure 5Aiv and 5Biv). Next, genes encoding for proteins involved in glucose utilization (glut4 [glucose transporter 4], pdk4 [pyruvate dehydrogenase 4]) and fatty acid oxidation (mcpt1 [muscle carnitine palmitoyltransferase 1], mcad [medium chain acyl-CoA dehydrogenase]) were investigated in skeletal muscle. Somewhat surprisingly, no significant differences were observed for these metabolic genes between SAMR1 and SAMP8 mice, independent of feeding status (Figure 6). In WAT, genes encoding for proteins known to regulate lipolysis (atgl [adipose triglyceride lipase], lipe [hormone sensitive lipase]) and mitochondrial function (pgc1α [peroxisome proliferator activated receptor coactivator 1α], ucp1 [uncoupling protein 1]) were assessed. In ab libitum fed mice, lipe expression exhibited a time-of-day-dependent variation in WAT (which was absent during dark phase restricted feeding; Figure 7Aii and 7Bii). In contrast, atgl, ucp1, and pgc1α all exhibited significant strain effects in WAT, with increased, decreased, and decreased (respectively) expression in SAMP8 mice. During dark period restricted feeding, decreased ucp1 and pgc1α expression in SAMP8 mouse WAT persisted (Figure 7Aiii-iv and 7Biii-iv). In contrast, ucp1 and pgc1α expression levels did not exhibit strain differences in BAT when assessed throughout the day (Figure 8Ai-ii and 8Bi-ii). Similarly, glut4 expression was not significantly different between SAMR1 and SAMP8 mice in BAT (Figure 8Aiii and 8Biii). However, dio2 (deiodinase 2; the encoded protein influences thermogenesis through modulation of T3 levels) expression was significantly higher in BAT isolated from SAMP8 mice during the dark phase, a difference that was augmented during dark period restricted feeding (Figure 8Aiv and 8Biv). Collectively, these data reveal tissue-specific differences in metabolic gene expression in SAMR1 versus SAMP8 mice.
Figure 5.

Diurnal variations in expression of glk (i), lpk (ii), lpg (iii), and accα (iv) in liver isolated from ad libitum (A; solid line) and dark phase (B; dashed line) restricted fed SAMR1 and SAMP8 mice. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). Tissues were isolated from mice at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, followed by assessment of gene expression by RT-PCR. Data are reported as mean ± SEM, for 4-5 mice per experimental group. Main effects for time and/or mouse strain are reported at the top of the figure panels. *, p<0.05 for pair wise comparison within a ZT.
Figure 6.

Diurnal variations in expression of glut4 (i), pdk4 (ii), mcpt1 (iii), and mcad (iv) in skeletal muscle isolated from ad libitum (A; solid line) and dark phase (B; dashed line) restricted fed SAMR1 and SAMP8 mice. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). Tissues were isolated from mice at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, followed by assessment of gene expression by RT-PCR. Data are reported as mean ± SEM, for 4-5 mice per experimental group. Main effects for time and/or mouse strain are reported at the top of the figure panels. *, p<0.05 for pair wise comparison within a ZT.
Figure 7.

Diurnal variations in expression of atgl (i), lipe (ii), pgc1α (iii), and ucp1 (iv) in WAT isolated from ad libitum (A; solid line) and dark phase (B; dashed line) restricted fed SAMR1 and SAMP8 mice. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). Tissues were isolated from mice at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, followed by assessment of gene expression by RT-PCR. Data are reported as mean ± SEM, for 4-5 mice per experimental group. Main effects for time and/or mouse strain are reported at the top of the figure panels. *, p<0.05 for pair wise comparison within a ZT.
Figure 8.

Diurnal variations in expression of pgc1α (i), ucp1 (ii), glut4 (iii), and dio2 (iv) in BAT isolated from ad libitum (A; solid line) and dark phase (B; dashed line) restricted fed SAMR1 and SAMP8 mice. Dark phase restricted fed mice were allowed access to chow only between ZT12 (light-to-dark phase transition) and ZT24 (dark-to-light phase transition). Tissues were isolated from mice at ZT3, ZT7, ZT11, ZT15, ZT19, and ZT23, followed by assessment of gene expression by RT-PCR. Data are reported as mean ± SEM, for 4-5 mice per experimental group. Main effects for time and/or mouse strain are reported at the top of the figure panels. *, p<0.05 for pair wise comparison within a ZT.
Impaired Thermogenesis in SAMP8 mice.
Given the observation that pgc1α and ucp1 mRNA levels are decreased in WAT of SAMP8 (compared to SAMR1) mice, yet dio2 mRNA levels are increased in BAT of SAMP8 mice, we next sought to investigate whether differences in thermogenesis existed between the two strains of mice. Body temperature decreased to a greater extent in SAMP8 than in SAMR1 mice, when the mice were placed at 4°C for a 5h period (ZT6 to ZT11; Fig. 9A). At ZT11, BAT and inguinal WAT (iWAT) were collected from cold exposed mice, as well as from room temperature control littermates; all mice were fasted from ZT6 to ZT11. Similar to BAT collected from ad libitum fed mice at ZT11 (Figure 8A), neither ucp1 nor dio2 was significantly different in BAT collected from fasted SAMP8 versus SAMR1 mice housed at room temperature (RT; Figure 9B). However, pgc1α was significantly higher in BAT of fasted SAMP8 mice (relative to SAMR1 mice; Figure 9B). Cold exposure increased expression of ucp1 only in BAT isolated from SAMR1 mice (Figure 9B). In contrast, cold exposure increased expression of pgc1α and dio2 in BAT of both mouse strains; induction of these genes was higher in SAMP8 BAT (compared to SAMR1 mice; Figure 9B). Next, thermogenesis-related genes were assessed in iWAT from SAMP8 and SAMR1 mice following cold exposure. Consistent with assessments in epididymal WAT from ad libitum fed mice (Figure 7A), the expression of these genes tended to be lower in SAMP8 iWAT (Figure 9C). Cold exposure tended to increase these genes in SAMR1 iWAT, but not SAMP8 iWAT (Figure 9C). Moreover, immunohistochemistry revealed that cold exposure increased UCP1 levels in SAMR1, but not SAMP8, iWAT (Figure 9D). Collectively, these observations suggest decreased cold tolerance in SAMP8 mice, which may be due to attenuated browning or “beiging” of WAT.
Figure 9.

Response of SAMR1 and SAMP8 mice to an acute cold challenge. Following equilibration at room temperature, SAMR1 and SAMP8 mice were exposed to 4-5°C, for up to 5h (ZT6 to ZT11); rectal temperature was assessed hourly (A). Food was removed during the cold challenge. After the 5h cold exposure (CE), mice were euthanized, followed by gene expression analysis in BAT (B) and iWAT (C), as well as immunohistochemistry analysis of UCP1 (D); littermates housed at room temperature (RT) served as controls. Data are reported as mean ± SEM, for 11-13 mice per experimental group (cold challenge), 6 mice per experimental group (gene expression), or 2 mice per experimental group (immunohistochemistry). *, p<0.05 for pair wise comparison between strains exposed to the same temperature. $, p<0.05 for pair wise comparison between temperatures within the same strain.
Metabolic Responses of SAMP8 and SAMR1 Mice to Chronic Alterations in Ambient Temperature.
Given that SAMP8 mice appear to be acutely intolerant to cold exposure, we next investigated whether these mice exhibit a differential response to chronic changes in ambient temperature, at the level of whole body metabolism. More specifically, SAMR1 and SAMP8 mice were housed for a 2 wk period at 28°C (i.e., close to thermoneutrality), followed immediately by a 1 wk period at 22°C (i.e., cold challenge), during which time behaviors and whole body metabolism were monitored. Ambient temperature did not dramatically influence either food intake or physical activity in SAMR1 and SAMP8 mice (Figure 10Ai-ii and 10Bi-ii). As anticipated, the reduction in ambient temperature resulted in increased energy expenditure in mice; this temperature-induced response was comparable in the two strains of mice (Figure 10Aiii and 10Biii). Interestingly, a reduction in ambient temperature significantly reduced RER in SAMR1 mice, both during the light period, and toward the end of the dark period (Figure 10Aiv and 10Ci). In contrast, exposure of SAMP8 mice to 22°C did not significantly influence RER (Figure 10Biv and 10Cii). Collectively, these data revealed a differential response of SAMP8 (compared to SAMR1) mice to a reduction in ambient temperature, at the level of whole body substrate selection (i.e., RER).
Figure 10.
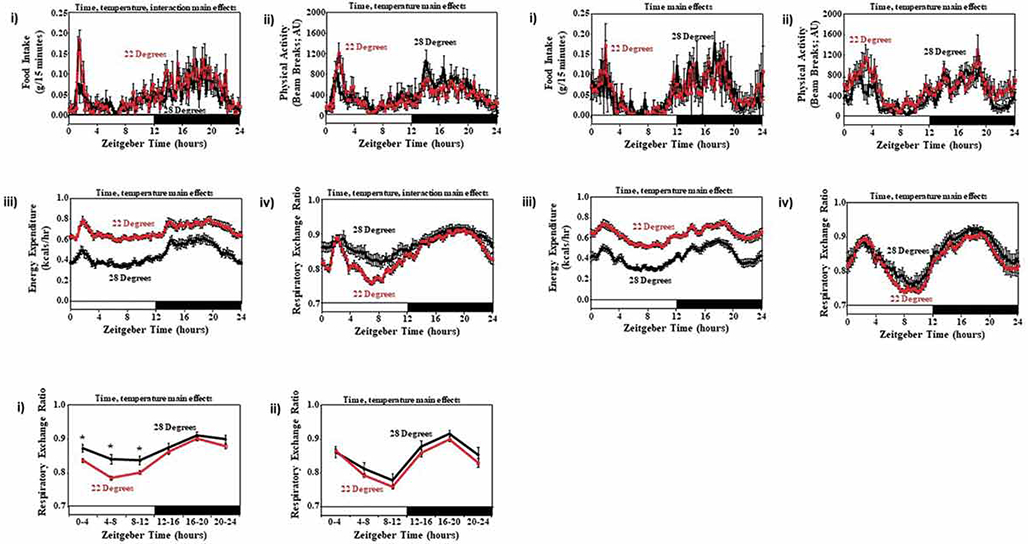
Diurnal variations in food intake (i), physical activity (ii), energy expenditure (iii), and RER (iv) in SAMR1 (A) and SAMP8 (B) mice housed at 28° and 22° C. All parameters were monitored continuously using a CLAMS. Data are reported as mean ± SEM, at 15 min intervals (i.e., average of last 2 d of a 1-2 wk period), for 9 mice per experimental group; food intake, physical activity, and energy expenditure units are grams/15 min, beam breaks/15 min, and kcals/hour, respectively. Average RER over a 4h time interval was also calculated in SAMR1 (i) and SAMP8 (ii) mice (C). Main effects for time and/or temperature are reported at the top of the figure panels. *, p<0.05 for pair wise comparison within a ZT.
DISCUSSION
The purpose of the present study was to investigate 24h rhythms in behaviors and whole body metabolism in a mouse model of accelerated aging. Here, we report that SAMP8 mice exhibit a differential 24h biphasic pattern in behaviors and whole body metabolism (relative to SAMR1 control mice), with an apparent delay during the light (less active) period, and an apparent advance during the dark (more active) period (Figure 1). Consistent with earlier onset of food intake in SAMR1 mice during the light phase (relative to SAMP8 mice; Figure 1A), oscillations in expression of circadian clock genes were phase advanced in SAMR1 peripheral tissues by 1.3 to 1.9h (Figure 2). Importantly, strain-specific differences in behavioral, metabolic, and circadian clock gene rhythms were attenuated by restricting food intake only to the dark (more active) period (Figures 1 and 4). Next, interrogation of metabolic genes in peripheral tissues revealed numerous strain-specific differences, some of which might influence thermogenesis (Figures 5-8). Acutely, SAMP8 mice exhibit decreased cold tolerance (Figure 9); whereas, SAMR1 mice exhibit greater cold-induced alterations in substrate selection (Figure 10). Collectively, these studies reveal behavioral, diurnal, and thermogenic alterations in SAMP8 mice.
Prior studies highlight chronobiological abnormalities during aging at multiple levels. In humans, both decreased amplitude and phase advances have been observed for 24h oscillations in multiple parameters known to be under circadian control, including waking time, core body temperature, and plasma levels of cortisol and melatonin (Hood and Amir 2017). At a molecular level, 24h oscillations in circadian clock components (e.g., per1 and per2 transcripts) exhibited both a reduced amplitude and phase advance in the orbitofrontal cortex of adults over 60 y of age (Chen 2016). Reduced amplitude and phase advances have also been reported during aging in animal models. For example, aged mice (>24 months) exhibit both decreases in amplitude (e.g., lung) and phase advances (e.g., heart) of circadian clock components (compared to young controls) (Yamazaki 2002; Durgan 2011). Interestingly, disruption of circadian clocks (e.g., genetic deletion of BMAL1) not only abolishes normal 24h governance of physiological processes (such as behaviors, neurohumoral levels, and cellular processes), but is also associated with onset of several age-related disorders (including cardiometabolic disorders, neurological impairment, and cardiovascular dysfunction) (Bunger 2000; Bunger 2005; Marcheva 2010; Lefta 2012). As the median age of the worldwide population increases, there is a clear need for greater understanding of the mechanistic links between aging-associated circadian abnormalities and the etiology of age-related diseases.
Senescence accelerated mice (SAM) arose from spontaneous mutations in AKR/J mice; SAMP mice are aging prone, while SAMR mice age at a normal rate (Takeda 1981). Prior studies have utilized SAMP mice when studying Alzheimer’s disease, cardiovascular disease, and inflammatory diseases, reporting profound alterations in cellular signaling, mitochondrial function, ER stress, and immunoreactivity (Morley 2012; Sreedhar 2016; Karuppagounder 2017). Interestingly, all these processes are circadian regulated, and circadian disruption leads to an accelerated aging phenotype (Bunger 2005; Takahashi 2008). Moreover, it has been suggested previously that daily rhythms in critical biological processes become attenuated with age, and that age-onset pathologies may be prevented through strategies designed to reinstate normal rhythmicity (Riera and Dillin 2015). However, little is known about 24h rhythm disturbances in SAMP8 mice. Interestingly, circadian rhythms in wheel running behavior become fragmented in SAMP8 mice by 7-months of age (while SAMP8 mice at 2-months of age exhibit normal behavior), suggestive of central clock impairment (Pang 2006). However, the extent to which daily rhythms in common behaviors (e.g., food intake), metabolism, and/or the molecular clock are altered in SAMP8 mice was previously unknown. Here, we report that 6-month old SAMP8 mice housed under standard light/dark (i.e., diurnal) conditions, exhibit biphasic patterns in food intake, physical activity, energy expenditure, and RER (an indicator of substrate utilization; Figure 1A). These biphasic rhythms differed from those in age-matched SAMR1 mice. Interrogation of known circadian clock component (bmal1, rev-erbα, per2) and output (dbp) genes in peripheral tissues (liver, skeletal muscle, WAT, and BAT) revealed an average phase delay in the clock by approximately 1.7h in SAMP8 mice (Figures 2 and 4). Interestingly, heterozygous tau mutant hamsters, which harbor an intrinsic clock with a periodicity of approximately 22h (i.e., 2h shorter), exhibit age-onset cardiovascular and renal disease (Martino 2008). Thus, subtle differences in circadian clock timing (similar to those observed in SAMP8 mice) may be sufficient to contribute towards previously reported pathologies. However, in surprising contrast to aged human and rodents (which typically exhibit reduced amplitudes and phase advances in 24h oscillations), 6-month old SAMP8 mice exhibit phase delays in circadian clock components in the absence of amplitude alterations.
Circadian clocks are markedly plastic in nature, allowing them to respond to fluctuations in environmental and behavioral cues. In doing so, circadian clocks are entrained/reset in response to alterations in light/dark (e.g., seasonal and geographical changes), sleep/wake (e.g., physical activity), and fasting/feeding cycles (Golombek and Rosenstein 2010). Concerning the latter, circadian clocks within peripheral tissues are particularly sensitive to the timing of food intake (Damiola 2000). Given that food intake (following the light period fast) is phase delayed in SAMP8 mice (Figure 1), we hypothesized that this may be responsible for the phase delay in the peripheral circadian clocks. Consistent with this concept, forcing SAMP8 and SAMR1 mice to consume food only during the dark period improved alignment of peripheral circadian clocks (which were only phase delayed by an average of 0.9h in SAMP8 mice during restricted feeding; Figures 3-4). Interestingly, time-of-day-restricted feeding interventions have been shown to reduce the risk of various cardiometabolic and cardiovascular diseases in rodents and humans (Longo and Panda 2016). Whether prolonged dark phase restricted feeding in SAMP8 mice reduces susceptibility to pathologies requires further investigation. However, during the time-of-day-restricted feeding intervention, it appears that circadian clocks in peripheral tissues of SAMR1 mice phase shift such that they are in better alignment with SAMP8 mice (while time-of-day-restricted feeding has little effect on the phase of peripheral circadian clocks in SAMP8 mice).
With the exception of the posttranslational peroxiredoxin clock, the mammalian circadian clock mechanism consists of a series of transcriptional positive and negative feedback loops (Takahashi 2008; O’Neill and Reddy 2011). As such, output from the circadian clock mechanism often involves changes in mRNA species that impact cellular processes (Storch 2002). Given that differences in diurnal variations were observed for both circadian clock components and whole body metabolism (as evidenced by energy expenditure and RER) between age-matched SAMP8 and SAMR1 mice, candidate genes with known critical metabolic functions were investigated in peripheral tissues. As shown in Figures 5-8, both strain- and time-of-day-dependent differences in metabolic genes were observed, often in tissue-specific manners. With regards to time-related differences between strains, glk mRNA diurnal variations appeared to be phase delayed in SAMP8 livers (similar to the phase delay in circadian clock components; Figure 5A). A number of metabolic genes also appear to exhibit augmented diurnal variations in SAMP8 peripheral tissues (compared to SAMR1 controls); these included lgp in liver (Figure 5A), pdk4 in skeletal muscle (Figure 6A), atgl in WAT (Figure 7A), and dio2 in BAT (Figure 8A). These observations are somewhat surprising, given that aging is typically associated with a dampening of metabolic 24-hr rhythms (Hood and Amir 2017). Concerning strain-dependent differences, SAMP8 mice exhibited decreased expression of accα in the liver, decreased expression of pgc1α and ucp1 in WAT, and increased expression of dio2 in BAT. Consistent with the decreased accα expression (an important lipogenesis gene (Kim 1997)), body weight was 16% lower in SAMP8 mice than in SAMR1 control mice (Table 1). Given that genes such as pgc1α, ucp1, and dio2 have established roles in thermogenesis (Silva 2006), SAMP8 and SAMR1 mice were cold challenged (both acutely and chronically). Similar to other models of aging (Darcy and Tseng 2019), SAMP8 mice exhibit cold intolerance (Figure 9), which may be secondary to insufficient beiging of WAT (as suggested by attenuated cold-induced induction of pgc1α mRNA and UCP1 protein in iWAT; Figire 9). It is noteworthy that increased dio2 expression in SAMP8 BAT is not consistent with impaired thermogenesis; DIO2 promotes BAT thermogenic activity, by increasing local T3 levels (Christoffolete 2004). The possibility exists that impairments downstream of T3, such as mitochondrial dysfunction, supersede the effects of DIO2. Interestingly, prior studies have reported that the circadian clock component REV-ERBα attenuates thermogenesis (Gerhart-Hines 2013); the results shown in Figure 2 and Table 2 suggest that rev-erbα expression was higher in three out of four SAMP8 peripheral tissues investigated. We speculate that chronically high levels of rev-erbα in SAMP8 mice may contribute toward impaired thermogenesis.
In summary, the current study reveals altered diurnal variations in both behaviors and whole body metabolism in a mouse model of accelerated aging (i.e., SAMP8 mice). Moreover, changes in feeding behavior (i.e., phase delay in food intake following the light phase fast) likely contribute toward alterations observed in peripheral tissue circadian clocks. Unlike aging in humans and other animal models, SAMP8 mice do not exhibit either a dampening or a phase advance in 24h oscillations in circadian clock and metabolic genes/processes; instead a phase delay is typically observed. We further speculate that impaired cold tolerance in SAMP8 may be due to REV-ERBα−mediated attenuation of thermogenesis. Future studies are required to determine the mechanisms by which 24h rhythms in behaviors (such as food intake) are altered in SAMP8 mice and/or whether circadian clock component differences observed in SAMP8 mice contribute to the accelerated aging phenotype and increased susceptibility to various pathologies.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Aging (R01AG043972 [DBA]; R03AG058078 [JK]) and the National Heart Lung, and Blood Institute (R01HL142216 [MEY]; R01 HL128695 [JK]). We would like to thank Maximiliano Grenett, Sabrina Moon, Hayden Bickerton, Amanda Feagans, Luisa Szimmtenings, and Stephanie Reeds for technical assistance.
Footnotes
DISCLOSURES
None.
REFERENCES
- Akiguchi I, Pallas M, Budka H, Akiyama H, Ueno M, Han J, Yagi H, Nishikawa T, Chiba Y, Sugiyama H, et al. 2017. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda's legacy and future directions. Neuropathology. 37(4):293–305. eng. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Udoh US, Young ME. 2014. Circadian regulation of metabolism. J Endocrinol. 222(2):R75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquissau V, Capel F, Dardevet D, Feillet-Coudray C, Gallinier A, Chauvin MA, Rieusset J, Morio B. 2017. Reactive oxygen species enhance mitochondrial function, insulin sensitivity and glucose uptake in skeletal muscle of senescence accelerated prone mice SAMP8. Free radical biology & medicine. 113:267–279. eng. [DOI] [PubMed] [Google Scholar]
- Bray M, Shaw C, Moore M, Garcia R, Zanquetta M, Durgan D, Jeong W, Tsai J, Bugger H, Zhang D, et al. 2008. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function; metabolism; and gene expression. Am J Physiol Heart Circ Physiol. 294:H1036–H1047. [DOI] [PubMed] [Google Scholar]
- Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. 2013. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes (Lond). 37(6):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. 2005. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 41(3):122–32. eng. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. 2000. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 103(7):1009–17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, McClung CA. 2016. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A. 113(1):206–11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 162(1):156–9. eng. [DOI] [PubMed] [Google Scholar]
- Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, et al. 2004. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 53(3):577–84. eng. [DOI] [PubMed] [Google Scholar]
- Cox KH, Takahashi JS. 2019. Circadian clock genes and the transcriptional architecture of the clock mechanism. Journal of molecular endocrinology. 63(4):R93–r102. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le M N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. 2000. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14:2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy J, Tseng Y-H. 2019. ComBATing aging-does increased brown adipose tissue activity confer longevity? Geroscience. 41(3):285–296. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, et al. 2011. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int. 28(3):187–203. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery I 2000. Circadian rhythms in a nutshell. Physiol Genomics. 3:59–74. [DOI] [PubMed] [Google Scholar]
- Evans JA, Davidson AJ. 2013. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 119:283–323. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, Morales-Gonzalez JA. 2011. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 12(5):3117–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U 2008. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nature clinical practice Cardiovascular medicine. 5(6):338–49. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, et al. 2013. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature. 503(7476):410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson UE, Heid CA, Williams PM. 1996. A novel method for real time quantitative RT-PCR. Genome Res. 6(10):995–1001. eng. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. 2010. Physiology of circadian entrainment. Physiol Rev. 90(3):1063–102. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144(5):646–74. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. 1996. Real time quantitative PCR. Genome Res. 6(10):986–94. eng. [DOI] [PubMed] [Google Scholar]
- Hood S, Amir S. 2017. The aging clock: circadian rhythms and later life. J Clin Invest. 127(2):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder V, Arumugam S, Babu SS, Palaniyandi SS, Watanabe K, Cooke JP, Thandavarayan RA. 2017. The senescence accelerated mouse prone 8 (SAMP8): A novel murine model for cardiac aging. Ageing Res Rev. 35:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH. 1997. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr. 17:77–99. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Bass J. 2007. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 18(1):4–11. [DOI] [PubMed] [Google Scholar]
- Lassmann H 2011. Mechanisms of neurodegeneration shared between multiple sclerosis and Alzheimer's disease. J Neural Transm (Vienna). 118(5):747–52. [DOI] [PubMed] [Google Scholar]
- Lefta M, Campbell KS, Feng HZ, Jin JP, Esser KA. 2012. Development of dilated cardiomyopathy in Bmal1-deficient mice. Am J Physiol Heart Circ Physiol. 303(4):H475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Panda S. 2016. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 23(6):1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell. 153(6):1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. 2010. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 466(7306):627–31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ. 2008. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 294(5):R1675–83. eng. [DOI] [PubMed] [Google Scholar]
- Morley JE, Armbrecht HJ, Farr SA, Kumar VB. 2012. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer's disease. Biochim Biophys Acta. 1822(5):650–6. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Okuma Y. 1999. Age-related defects in lifespan and learning ability in SAMP8 mice. Neurobiology of aging. 20(2):111–5. eng. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Reddy AB. 2011. Circadian clocks in human red blood cells. Nature. 469(7331):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang KC, Miller JP, Fortress A, McAuley JD. 2006. Age-related disruptions of circadian rhythm and memory in the senescence-accelerated mouse (SAMP8). Age. 28(3):283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peliciari-Garcia RA, Bargi-Souza P, Young ME, Nunes MT. 2018. Repercussions of hypo and hyperthyroidism on the heart circadian clock. Chronobiol Int. 35(2):147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peliciari-Garcia RA, Prévide RM, Nunes MT, Young ME. 2016. Interrelationship between 3,5,3´-triiodothyronine and the circadian clock in the rodent heart. Chronobiology international. 33(10):1444–1454. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27(9-10):1911–29. eng. [DOI] [PubMed] [Google Scholar]
- Riera CE, Dillin A. 2015. Tipping the metabolic scales towards increased longevity in mammals. Nature cell biology. 17(3):196–203. [DOI] [PubMed] [Google Scholar]
- Silva JE. 2006. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 86(2):435–64. [DOI] [PubMed] [Google Scholar]
- Sreedhar R, Giridharan VV, Arumugam S, Karuppagounder V, Palaniyandi SS, Krishnamurthy P, Quevedo J, Watanabe K, Konishi T, Thandavarayan RA. 2016. Role of MAPK-mediated endoplasmic reticulum stress signaling in the heart during aging in senescence-accelerated prone mice. Biofactors. 42(4):368–75. [DOI] [PubMed] [Google Scholar]
- Stavinoha M, RaySpellicy J, Hart-Sailors M, Mersmann H, Bray M, Young M. 2004. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol. 287:E878–E887. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. 2002. Extensive and divergent circadian gene expression in liver and heart. Nature. 417(6884):78–83. eng. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 9(10):764–75. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, et al. 1981. A new murine model of accelerated senescence. Mechanisms of ageing and development. 17(2):183–94. [DOI] [PubMed] [Google Scholar]
- Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, et al. 2010. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 285(5):2918–29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. 2002. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 99(16):10801–6. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M, Razeghi P, Cedars A, Guthrie P, Taegtmeyer H. 2001. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 89:1199–1208. [DOI] [PubMed] [Google Scholar]
- Young M, Razeghi P, Taegtmeyer H. 2001. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 88:1142–1150. [DOI] [PubMed] [Google Scholar]
- Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, et al. 2014. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms. 29(4):257–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


