Abstract
Skeletal muscle mass (SMM) plays an important role in health and physical performance. Its estimation is critical for the early detection of sarcopenia, a disease with high prevalence and high health costs. While multiple methods exist for estimating this body component, anthropometry and bioelectrical impedance analysis (BIA) are the most widely available in low- to middle-income countries. This study aimed to determine the correlation between muscle mass, estimated by anthropometry through measurement of calf circumference (CC) and skeletal mass index (SMI) by BIA. This was a cross-sectional and observational study that included 213 functional adults over 65 years of age living in the community. Measurements of height, weight, CC, and SMM estimated by BIA were made after the informed consent was signed. 124 women mean age 69.6 ± 3.1 years and 86 men mean age 69.5 ± 2.9 years had the complete data and were included in the analysis. A significant positive moderate correlation among CC and SMI measured by BIA was found (Pearson r= 0.57 and 0.60 for women and men respectively (p=0.0001)). A moderate significant correlation was found between the estimation of SMM by CC and by BIA. This suggests that CC could be used as a marker of sarcopenia for older adults in settings in lower-middle-income countries where no other methods of diagnosing muscle mass are available. Although the CC is not the unique parameter to the diagnosis of sarcopenia, it could be a useful procedure in the clinic to identify patients at risk of sarcopenia.
Keywords: skeletal muscle mass, bioelectrical impedance analysis, calf circumference, sarcopenia
Introduction
In 2010, a European Consensus defined sarcopenia (“sarx” for muscle, “penia” for loss) as low SMM plus low muscle function either as reduced strength or performance [1]. Recently, this geriatric syndrome has been recognized as a disease entity with the awarding of an ICD-10-CM (M62.84) code in September 2016. This designation enables the syndrome to be considered as a primary or secondary condition [2].
Sarcopenia has become a factor common to many of the chronic diseases of the elderly (heart failure, diabetes, type 2, obesity, COPD, CVD, and dementia, among others) [3,4,5]. The overall estimates of prevalence are in the range of 10–58% depending on the methods used and the proposed cutoff points. This prevalence is considered very high and highlights the need for early diagnosis. Sarcopenia is one of the most relevant public health problems in the elderly and is associated with a high rate of adverse outcomes and high healthcare costs [6]. In the United States, the mere costs of hospitalization, nursing home income, and home health care expenses amounted to USD 18.5 billion in 2000, representing approximately 1.5% of total health spending [7,8].
However, sarcopenia, although primarily described in older subjects, it is not exclusively a disease of elderly. It is also a condition that can occur in young people and different pathological conditions such as malnutrition associated to malignancy [9], rheumatoid arthritis [10], COPD patients [11] and other chronic inflammatory diseases. Moreover, a new type of sarcopenia has emerged in recent years named “sarcopenic obesity” (SO) that occurs when sarcopenia and obesity are simultaneously present in the same individual [12]; in which case, the risks of presenting other comorbidities are greater than in people who only have one of the two conditions [13]. This pathology is also presented by young people and is associated to nutrition and lifestyle factors. A national survey for 3937 middle-aged Koreans and older Korean individuals found that the SO group had a lower overall dietary quality, were more sedentary and had a greater number of adverse psychological conditions than the non-sarcopenic obesity group [14]. On the other hand, undernutrition can also be associated with loss of muscle mass. Beaudart et al. (2019) [15], studied the association between these two conditions in 336 Belgian men and women aged 72.5 ± 5.8 years and found that undernutrition was a strong predictor of sarcopenia and that these subjects had a fourfold increased risk of developing severe sarcopenia during a four-year follow-up.
According to the definition, the estimation of SMM is a critical component of sarcopenia. There are a variety of skeletal mass assessment tools; however, the choice for clinical practice depends largely on availability. Technologies such as Magnetic Resonance Imaging (MRI), Dual Energy X-ray Absorptiometry (DXA), Ultrasonography, and Computerized tomography, are not available in all clinical locations [16].
CC has been considered a sensitive anthropometric parameter of muscle mass in the elderly [17]. On the other hand, BIA is considered an intermediate technique between these more accurate but more expensive methods and anthropometry that is cheaper but less reliable [18]. Moreover, it is necessary to define user-friendly tools in clinical practice. This should facilitate early detection of the disease and its inclusion in public health programs. Even more, it was recently shown that the limits for definitions must be ethnically sensitive, and different countries may need their separate cut off points [19].
Thus, the objective of this study was to evaluate the association between the estimation of SMM by SMI through BIA and CC by anthropometry. There are very few articles doing this comparison since most studies evaluate SMI by DXA to relate it to CC.
Materials and methods
Participants
The sample was estimated using registries of the National Administrative Department of Statistics; 1085 older individuals randomly selected were eligible and 213 agreed to participate. 3 patients did not have complete data and were excluded, thereby, 210 provided written consent and were included in the study developed during the months of March 2013 – February 2014.
The inclusion criteria were being between 55 and 75 years old and living in the community. The exclusion criteria were living at nursing homes, having a decompensated chronic disease, pacemakers, chronic kidney disease in hemodialysis, presence of edemas, metallic nonremovable pieces or prosthesis, diuretic consumption, limb amputation, hemiparesis or hemiplegia.
Anthropometric parameters
Weight (ICOB®) and height (Seca®) were measured by standardized protocols [20]. CC was measured to the nearest 0.1 cm in the standing position using a non-elastic, flexible plastic tape (Lord®). The tape was moved on the right calf along the length to find the maximal circumference according to the International Society for the Advancement of Kinenthropometry recommendations (ISAK) [21]. A low muscle mass assessed by CC was determined using the cutoff points from [22] which are <33 cm for women and <34 cm for men. Japanese population was chosen on the basis that anthropometry of Colombians is more like that of Asians, maybe because Native Americans come from at least three different Asian genetic influences [15].
SMI
Whole-body BIA measurements were performed according to a protocol previously published by [23] using a Hydra 4.200, Xitron Technologies®, San Diego (USA), device. For these measurements, verification was made of the previous fulfillment of the necessary conditions to carry out the measurements.
SMM was calculated using the Janssen formula [24]:
Where Ht is height in centimeters; R50 is BIA resistance in ohms; for gender, men = 1 and women = 0; and age is in years.
Afterward the SMI was calculated by the equation:
The cut-off points for low muscle mass by this technique were defined as an SMI of less than −2 standard deviations (SD) of the mean value for Colombian young adults, as defined previously from [25]; 6.42 and of 8.39 kg/m2 for women and men respectively.
Statistical analyses
Before analyzing the data, a Kolmogorov-Smirnov test was performed, which showed a normal distribution of the data. Qualitative variables were analyzed using absolute and relative frequencies and mean and SD for quantitative variables. Pearson's correlation coefficient was used to evaluate the associations between SMI and CC. A t-test was applied for correlation coefficients. P-values of less than 0.05 were considered to indicate statistical significance. The data obtained were analyzed using SPSS 21.0 (SPSS, Chicago, IL).
Informed consent
Informed consent has been obtained from all individuals included in this study.
Ethical approval
The research related to human use has complied with all relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration. The study protocol was reviewed and approved by the Ethics Committee of the Universidad de Caldas.
Results
124 women mean age 69.6 ± 3.1 years and 86 men mean age 69.5 ± 2.9 years were evaluated. The characteristics of the subjects are shown in Table 1. Figures 1 and 2 depict a direct positive correlation between SMI and CC for women and men respectively, although slightly higher in men. For women, the Pearson's correlation coefficient between CC and SMI was 0.57 (p-value <0.0001). For men, Pearson's correlation coefficient was 0.6 (p-value <0.0001).
Table 1.
Subject Characteristics
| Variables | Men (n=86) | Women (n=124) | Total (n=210) |
|---|---|---|---|
| Weight (kg) | 68.1 (9.3) | 59.6 (10.4) | 63.0 (10.7) |
| Height (m) (SD) | 1.66 (6.0) | 1.51 (5.9) | 1.57 (9.3) |
| BMI (kg/m2) | 24.6 (2.8) | 25.8 (3.9) | 25.3 (3.6) |
| CC (cm) (SD) | 35.2 (2.5) | 33.3 (2.9) | 34.1 (2.9) |
| SMM (kg) | 27.5 (3.5) | 16.7 (2.5) | 21.1 (6.0) |
| SMI (kg/m2) | 9.94 (1.0) | 7.2 (0.9) | 8.3 (1.6) |
Fig.1.
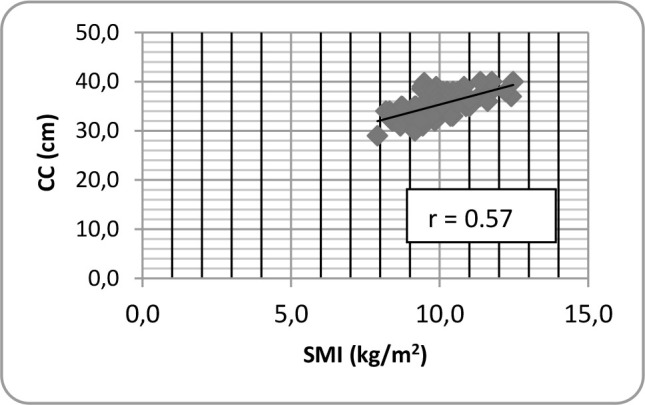
Pearson correlation between calf circumference (CC) and SMI for women.
Fig.2.
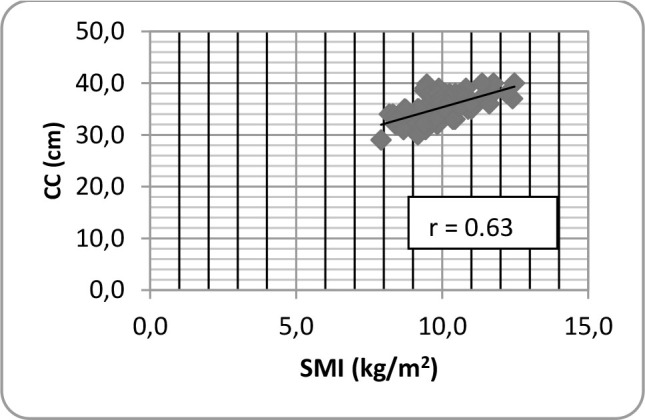
Pearson correlation between calf circumference (CC) and SMI for men.
Discussion
This study showed that there was a significant positive moderate correlation between CC and SMI measured by BIA (r=0.57 and 0.60 for women and men, respectively (p=0.0001). Quinonez-Olivas et al., 2016 [26], evaluated 105 Mexican patients with a mean age of 76 years (±7.3) and showed a lower positive correlation between SMI and CC than in the present study (r=0.31; p=0.000). However, they considered that CC could be a reliable measure to assess muscle mass in older adults in geriatric ambulatory clinics.
On the other hand, Handayani et al., 2018 [27], examined 96 elderly healthy women aged 60 years or more, independent in their daily activities. They found a Spearman correlation of 0.43 (p< 0.05).
More recently, Santos et al. (2019) [28], evaluated DEXA and calf circumference data from 15,293 adults surveyed in the 1999–2006 NHANES. They found a higher correlation (r= 0.79 for males and 0.74 for females) between calf circumference and appendicular skeletal mass as measured by DXA. This finding was not only in older adults like those in the present study but also in adults of early and middle age.
Average CC for women was 33.3 (±2.9) cm and 35.2 (±2.5) cm for men. The difference between men and women is interesting in this study. Women had a higher body mass index, however, their CC was lower than that of men, which would suggest that they may have more visceral fat and less skeletal muscle mass, placing them at higher risk of developing sarcopenic obesity, as suggested by [29].
Moreover, other Asian countries such as Malaysia, show slightly different cut off points from their Japanese neighbors (32.0 ±4.2 cm in men and 30.5 ±4.6 cm in women) [30]. The European Consensus established a lower limit (31 cm) for CC, which would also lead to different results from those reported in this study [31].
Skeletal mass for our participants was 16.7 kg for women and 27.5 kg for men and SMI was 7.2 and 9.9 kg/m2, respectively. The above study from Handayani et al., 2018 [32], found a women's mean muscle mass of 14.2 kg and SMI of 6.6 kg/m2. However, these women were living in a nursing home for at least 2 years and probably were more sedentary.
The first European Consensus on definition and diagnosis of sarcopenia did not recommend anthropometry for routine diagnosis of this condition [1]; however, in the new consensus [27], the researchers tried to facilitate the early detection of sarcopenia and consequently its timely treatment. On this occasion, they admit that the CC may be an alternative for clinical environments where there are no facilities such as BIA to estimate muscle mass. This is how the findings of this study, with a moderate correlation between the SMI and the thigh circumference, lead us to recommend this alternative in low-middle income countries such as Colombia, wherein many occasions and clinical contexts there is only a measuring tape.
A European survey aimed to assess the usage of tools for the assessment of muscle mass, muscle strength, and physical performance for the diagnosis of sarcopenia was completed by 255 clinicians from 55 countries across 5 continents. The authors found that only 53.3% of the responders assess muscle mass in their daily practice with different tools among which the most used was the CC in 57.5% of the cases, followed by DXA (45.9%), skinfold thickness (30.8%), BIA (22.6%), ultrasonography 18.5%, MRI (16.4%), CT-scan (14.4%) and other not specified (8.9%) [32]. The authors call for the need of standardizing the tools and the cut-off values.
The cross-sectional design of the study constitutes a limitation since it was only possible to establish an association between the muscle mass estimated by BIA and CC. Thus, subsequent studies with a larger number of subjects and more elaborate designs are required to validate the usefulness of CC in sarcopenia diagnosis and to have cut off points representing the national population for this parameter to establish the true usefulness of CC as a tool for the early detection of sarcopenia.
As the general population tends to age worldwide, an increase in the costs for their health care is expected. This situation makes us think about the need to timely prevent sarcopenia that starts from earlier ages in life. Concomitant, obesity, and malnutrition that can lead to muscle weakness, must be subject to public health authorities. A prevention strategy could be carried out by improving the quality of the diet and physical activity of young people [14] as well as an earlier diagnosis using simple and inexpensive tools, such as CC measurement.
Conclusion
Although the CC is not the unique parameter to aid in the diagnosis of sarcopenia, it does seem to have a significant correlation with SMM and it could be a useful procedure in the clinic to identify patients at risk of sarcopenia if we stick to the moderate association that was found between the SMI and CC. This makes us suggest CC later as a substitute marker for muscle mass evaluation for older adults in settings in low-middle income countries where no other muscle mass diagnostic methods are available. However, the diagnosis can vary depending on the reference cut of point used and it would be important to validate the existing cut-off points in the literature and build own values for each region or country.
Acknowledgments
We thank the volunteers who participated in this study. Thanks also to Drs Ana Milena López and David González who designed and participated in the main study that led to this new analysis of the data collected.
Footnotes
Statements of Authorship
This study was performed by all authors who also read and approved the final version of this manuscript.
Conflict of interest
Authors state no conflict of interest.
References
- 1.Cruz-Jentoft A, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F. et al. Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. https://doi.org/10.1093/ageing/afq034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falcon LJ, Harris MO. Sarcopenia and the New ICD-10-CM Code: Screening, Staging, and Diagnosis Considerations. Aging in Motion. [Internet]. [Updated 2016 apr 28; cited 2019 jun 7]. Available from: http://aginginmotion.org/news/2388-2/ [PMC free article] [PubMed]
- 3.Korkmaz M, Eyigor S. Association between sarcopenia and rheumatological diseases. World J Rheumatol. 2019;9(1):1–8. https://doi.org/10.5499/wjr.v9.i1.1 . [Google Scholar]
- 4.Beretta MV, Dantas-Filho FF, Ecce-Freiberg R, Feldman JV, Nery, Ticiana C, Rodrigues C. Sarcopenia and Type 2 diabetes mellitus as predictors of 2-year mortality after hospital discharge in a cohort of hospitalized older adults. Diabetes Res and Clin Pract. 2019;159 doi: 10.1016/j.diabres.2019.107969. 107969. https://doi.org/10.1016/j.diabres.2019.107969 . [DOI] [PubMed] [Google Scholar]
- 5.Kamal-Mansour KM, Goulart C, Carvalho J, Soares de Carvalho LC, Trimer R, Borghi-Silva A, Gonçalves da Silva AL. Pulmonary function and functional capacity cut-off point to establish sarcopenia and dynapenia in patients with COPD. J Bras Pneumol. 2019;45(6) doi: 10.1590/1806-3713/e20180252. e20180252. https://doi.org/10.1590/1806-3713/e20180252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. https://doi.org/10.1186/s40200-017-0302-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaudart C, Rizzoli R, Bruyère O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72(1):45. doi: 10.1186/2049-3258-72-45. https://doi.org/10.1186/2049-3258-72-45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruyère O, Beaudart C, Ethgen O, Reginster JY, Locquet M. The health economics burden of sarcopenia: A systematic review. Maturitas. 2019;119:61–9. doi: 10.1016/j.maturitas.2018.11.003. https://doi.org/10.1016/j.maturitas.2018.11.003 . [DOI] [PubMed] [Google Scholar]
- 9.Gammone M, Ficoneri C, D'Orazio N. Assessment of body composition in oncologic patients: Experimental survey on the role of bioimpedentiometric analysis. Journal of Electrical Bioimpedance. 2019;10(1):90–5. doi: 10.2478/joeb-2019-0013. https://doi.org/10.2478/joeb-2019-0013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barone M, Viggiani MT, Anelli MG, Fanizzi R, Lorusso O, Lopalco G. et al. Sarcopenia in Patients with Rheumatic Diseases: Prevalence and Associated Risk Factors. Journal of clinical medicine. 2018;7(12):504. doi: 10.3390/jcm7120504. https://doi.org/10.3390/jcm7120504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pineda-Zuluaga MC, Gonzalez-Correa CH, Sepulveda-Gallego LE. Sarcopenia in Patients with Chronic Obstructive Pulmonary Disease and Evaluation of Raw Bioelectrical Impedance Analysis Data. Springer Nature Singapore Pte Ltd. 2020;72:92–98. https://doi.org/10.1007/978-981-13-3498-6_14 . [Google Scholar]
- 12.Lombardo M., Boaria A., Aulisa G, Padua E, Annino G, Pratesi A., Caprio M, Iellamo F, Bellia A. Sarcopenic obesity: etiology and lifestyle therapy. Eur Rev Med Pharmacol Sci. 2019;23(16):7152–62. doi: 10.26355/eurrev_201908_18761. https://doi.org/10.26355/eurrev_201908_18761 . [DOI] [PubMed] [Google Scholar]
- 13.Koliaki C, Liatis S, Dalamaga M, Kokkinos A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr Obes Rep. 2019;8(4):458–71. doi: 10.1007/s13679-019-00359-9. https://doi.org/10.1007/s13679-019-00359-9 . [DOI] [PubMed] [Google Scholar]
- 14.Yoo S, Kim DY, Lim H. Sarcopenia in relation to nutrition and lifestyle factors among middle-aged and older Korean adults with obesity. Eur J Nutr. 2020;10 doi: 10.1007/s00394-020-02179-3. https://doi.org/10.1007/s00394-020-02179-3 . [DOI] [PubMed] [Google Scholar]
- 15.Beaudart C, Rolland Y, Cruz-Jentoft AJ, Bauer JM, Sieber C, Cooper C. et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice. Calcif Tissue Int. 2019;105(1):1–14. doi: 10.1007/s00223-019-00545-w. https://doi.org/10.1007/s00223-019-00545-w . [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Physical Status: The Use and the Interpretation of Anthropometry, Report of a WHO Expert Committee. [Internet] [Updated 1995; cited 2020 may 1]. Available from: https://apps.who.int/iris/handle/10665/37003 . [PubMed]
- 17.Kuriyan R. Body composition techniques. Indian J Med Res. 2018;148(5):648–58. doi: 10.4103/ijmr.IJMR_1777_18. https://doi.org/10.4103/ijmr.IJMR_1777_18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo J, Arai H, Ng TP, Sayer AA, Wong M, Syddall H. et al. Ethnic and geographic variations in muscle mass, muscle strength and physical performance measures. Eur Geriatr Med. 2014;5:155–64. https://doi.org/10.1016/j.eurger.2014.04.003 . [Google Scholar]
- 19.Lohman TG, Roche AF, Martorell R. Anthropometric reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 20.Kawakami R, Murakami H, Sanada K, Tanaka N, Sawada SS, Tabata I. et al. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int. 2015;15(8):969–976. doi: 10.1111/ggi.12377. https://doi.org/10.1111/ggi.12377 . [DOI] [PubMed] [Google Scholar]
- 21.Norton KI. Standards for Anthropometry Assessment. In Kinanthropometry and Exercise Physiology. Routledge. 2020:68–137. https://doi.org/10.4324/9781315385662-4 .
- 22.Reich D, Patterson N, Campbell D, Tandon A, Mazieres S, Ray N. et al. Reconstructing Native American population history. Nature. 2012;488(7411):370–4. doi: 10.1038/nature11258. https://doi.org/10.1038/nature11258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Correa CH, Caicedo-Eraso JC. Bioelectrical impedance analysis (BIA): a proposal for standardization of the classical method in adults. Journal of Physics: Conference Series Journal of Physics: Conference Series. 407 conference 1. https://doi.org/10.1088/1742-6596/407/1/012018 . [Google Scholar]
- 24.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–71. doi: 10.1152/jappl.2000.89.2.465. https://doi.org/10.1152/jappl.2000.89.2.465 . [DOI] [PubMed] [Google Scholar]
- 25.Villada-Gómez JS, González-Correa CH, Marulanda-Mejía F. Puntos de corte provisionales para el diagnóstico de sarcopenia en ancianos de Caldas, Colombia. Biomédica. 2018;38:521–6. doi: 10.7705/biomedica.v38i4.4302. https://doi.org/10.7705/biomedica.v38i4.4302 . [DOI] [PubMed] [Google Scholar]
- 26.Quinonez-Olivas CG, Salinas-Martínez R, Ortiz-Jiménez XA, Gámez-Treviño DG, Guajardo-Álvarez G, González-García B. Muscle Mass Measured Using Bioelectrical Impedance Analysis, Calf Circumference and Grip Strength in Older Adults. Medicina Universitaria. 2016;18(72):158–162. https://doi.org/10.1016/j.rmu.2016.06.005 . [Google Scholar]
- 27.Handayani M, Sadewa AH, Farmawati Rochmah W. Anthropometric Prediction Equations for Estimating Muscle Mass of Elderly Women. KEMAS. 2018;14(2):195–204. https://doi.org/10.15294/kemas.v14i2.14073 . [Google Scholar]
- 28.Santos LP, Gonzalez MC, Orlandi SP, Bielemann RM., Barbosa-Silva TG, Heymsfield SB. New Prediction Equations to Estimate Appendicular Skeletal Muscle Mass Using Calf Circumference: Results From NHANES 1999–2006. Journal of Parenteral and Enteral Nutrition. 2019;43:998–1007. doi: 10.1002/jpen.1605. https://doi.org/10.1002/jpen.1605 . [DOI] [PubMed] [Google Scholar]
- 29.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: etiology, epidemiology and treatment strategies. Nature Rev Endo. 2018;14(9):513–537. doi: 10.1038/s41574-018-0062-9. https://doi.org/10.1038/s41574-018-0062-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harith S, Shahar S, Adznam SN. Determination of CC Cut-Off Values for Malaysian Elderly and its Predictive Value in Assessing Risk of Malnutrition. MJN. 2016;22(3):375–87. [Google Scholar]
- 31.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. https://doi.org/10.1093/ageing/afy169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruyère O, Beaudart C, Reginster JY, Buckinx F, Schoene D, Hirani V, Cooper C, Kanis JA, Rizzoli R, McCloskey E, Cederholm T, Cruz-Jentoft A, Freiberger E. Assessment of muscle mass, muscle strength and physical performance in clinical practice: An international survey. Eur Geriatr Med. 2016;7(3):243–6. https://doi.org/10.1016/j.eurger.2015.12.009 . [Google Scholar]


